Abstract
The recent and ongoing outbreak of coronavirus disease (COVID‐19) is a huge global challenge. The outbreak, which first occurred in Wuhan City, Hubei Province, China and then rapidly spread to other provinces and to more than 200 countries abroad, has been declared a global pandemic by the World Health Organization. Those with compromised immune systems and/or existing respiratory, metabolic or cardiac problems are more susceptible to the infection and are at higher risk of serious illness or even death. The present review was designed to report important functional food plants with immunomodulatory and anti‐viral properties. Data on medicinal food plants were retrieved and downloaded from English‐language journals using online search engines. The functional food plants herein documented might not only enhance the immune system and cure respiratory tract infections but can also greatly impact the overall health of the general public. As many people in the world are now confined to their homes, inclusion of these easily accessible plants in the daily diet may help to strengthen the immune system and guard against infection by SARS‐CoV‐2. This might reduce the risk of COVID‐19 and initiate a rapid recovery in cases of SARS‐CoV‐2 infection.
Keywords: COVID‐19, immunomodulators, medicinal plants, respiratory tract infections, SARS‐CoV
Abbreviations
- CD4+
cluster of differentiation 4
- Con A
concanavalin A
- COVID‐19
coronavirus disease
- CoVs
coronaviruses
- DT
dendritic cells
- HMGB1
high‐mobility‐group box1
- IFN‐γ
interferon gamma
- IL‐4
interleukin‐4
- MAPK
mitogen‐activated protein kinase
- MERS
Middle East respiratory syndrome
- NK
natural killer cells
- PBL
peripheral blood lymphocytes
- RSV
respiratory syncytial virus
- SARS
severe acute respiratory syndrome coronavirus
- T‐cells
thymus cells
- TF2B
3‐isotheaflavin‐3‐gallate
- Th1 type
thymus helper type 1
- Th2 type
thymus helper type 2
- TNF‐α
tumor necrosis factor alpha
- WBC
white blood cell
- WHO
World Health Organization
1. INTRODUCTION
Coronaviruses (CoVs) are enveloped, positive‐sense, single‐stranded RNA viruses belonging to the family Coronaviridae. CoVs can affect the respiratory and digestive systems of animals and humans but were not considered as seriously infectious to humans until the outbreak of severe acute respiratory syndrome (SARS) in 2002 and 2003 in Guangdong, China (Guan, et al., 2003) and Middle East respiratory syndrome (MERS) in the Middle East in 2013 (Hemida, et al., 2013). SARS infected more than 8,000 people and caused the deaths of nearly 800 people, with a fatality rate of 9.6%. Similarly, MERS was confirmed in more than 2,500 people, causing the deaths of more than 850 people, with a mortality rate of 35%. These two outbreaks led to extensive research in understanding CoVs. The origins of SARS‐CoV and MERS‐CoV are thought to be from bats, and they were transmitted to humans through civets and camels, respectively (Cui, Li, & Shi, 2019; Hu et al., 2017). Due to the high occurrence, varied distribution, great genetic diversity and recurrent recombination of their genomes and their elevated human–animal interfaces, CoVs are very likely to emerge periodically in humans.
The ongoing pandemic occurred approximately 7 years after the outbreak of MERS‐CoV. The International Committee on Taxonomy of Viruses named the virus as SARS‐CoV‐2, which causes coronavirus disease (COVID‐19). On 30 December 2019, a first four pneumonia cases were reported by Wuhan Municipal Health Commission in Wuhan City, Hubei Province, China (Zhu et al., 2020). All of these cases were linked to the Huanan Seafood Wholesale Market, a live animal and seafood market in Jianghan District, Wuhan. The local government afterwards took prompt sanitation and disinfection measures to prevent the infection from spreading. The symptoms of the cases documented were: fever, breathing problems, coughing, SARS, and kidney failure in severe cases (WHO, 2020). The number of cases increased rapidly, and many more were identified in other cities of China and now in more than 200 countries (Figure 1). The latest report issued by World Health Organization (WHO) confirmed more than 3.8 million cases and more than 260,000 deaths worldwide. Li, et al. (2020) reported that a large proportion of infected patients were above the age of 60 years, and most of them were male, which suggests that these people are not immune competent. Moreover, it was also reported that most of the patients who died of SARS‐CoV‐2 were already suffering from several other medical problems (Hong Kong Centre for Health Protection, 2020). This clearly indicates that a healthy immune system and general good health are crucial for alleviating the risk factors associated with COVID‐19 and for increasing the chances of survival and recovery. Moreover, a healthy immune system helps the host to control and prevent several pathogenic infections (Chaplin, 2010).
FIGURE 1.
Global distribution of COVID‐19. Source: Center for Systems Science and Engineering (CSSE, Johns Hopkins University, 28 April 2020) [Colour figure can be viewed at wileyonlinelibrary.com]
In the current situation, measures to control infection risk are crucial for disease management. Several preventive measures have been advised for general public health including, hand and respiratory hygiene and safe food practices (in relation to raw animal products) in order to reduce the risk from and transmission of SARS‐CoV‐2 (WHO, 2020). In the current situation, there is a great need to propose effective preventive therapeutic measures that might alleviate the risk of COVID‐19 infection until a vaccine or other antiviral agents are designed.
Plants have been used for centuries in almost all cultures worldwide as traditional medicines to cure many chronic infections, including viral diseases (Salehi et al., 2019a; Sharifi‐Rad, et al., 2019; Salehi, et al., 2020). In recent decades, scientists have been attempting to scientifically validate the health‐improving potential of functional and nutraceutical foods (Sharifi‐Rad, et al., 2018; Salehi et al., 2019b; Salehi et al., 2019c). The aim of the present review is to document common and easily accessible functional food plants that can modulate the immune system and are biologically active against several medical problems arising from respiratory tract infections. We hypothesise that functional food plants may help individuals to overcome the infection by: (a) modulating the body's immune system, (b) generating antiviral activity against the infection, and (c) reducing other respiratory problems. This review will provide guidelines to the general public to include important medicinal food plants in their daily diet for strengthening and improving their immune system and overall health.
2. METHODOLOGY
The data on medicinal food plants were retrieved and downloaded using different search engines, including Web of Science, PubMed, Google Scholar and Scopus. The present review includes only those articles that were published in English‐language journals that meet required quality standards in relation to information. Moreover, only those medicinal plants that are commonly used as vegetables, fruits, spices and are active against respiratory tract infections were selected. Different keywords were used for the data search, including ‘ethnomedicinal usage of food plants’, ‘immunomodulatory action of functional food plants’ and ‘antiviral and biological activities’. The data were organised and analysed in Microsoft Excel (2010) software and then summarised in to tables and figures. For the taxonomic treatment of the documented plant species, the online botanical databases ‘The Plant List’ (Royal Botanic Gardens, Kew, UK and Missouri Botanical Garden, USA) and ‘Tropicos’ (Missouri Botanical Garden, USA) were used.
3. DISCUSSION
Plants have been cultivated and used by humans for a very long time, providing not only food but also medicines for the treatment of chronic infections. Large numbers of ethnomedicines are currently being evaluated for their therapeutic properties. The medicinal properties of plants are due to the chemicals present in the different plant parts, which act in the same manner as conventional medical drugs. Recently, there has been a growing interest in using medicinal plants for modulating the human immune system, and researchers have suggested that different classes of compounds, including alkaloids, flavonoids, terpenoids and polysaccharides, possess immunomodulatory properties with fewer side effects than allopathic drugs. (Wadood, et al., 2013).
3.1. Immunomodulatory functional food plants
The human immune response is the body's most important defence mechanism against disease, and the survival of humans is greatly dependent on this system of fighting against foreign pathogenic microorganisms, including viruses. The possible immunomodulatory function of plants is a recent concept in the field of phytomedicines. Immunomodulators not only enhance humoral and cell‐mediated immunity but also activate non‐specific immune responses such as activation of the natural killer (NK) cells, macrophages, granulocytes and complement systems, which enhance resistance to infections non‐specifically. Activation of these important immune cells results in the production of various molecules such as interferons (IFNs), cytokines and chemokines involved in the enhancement of immune responses. In most SARS autopsies, the numbers of several immune cells present in the spleen, including lymphocytes [cluster of differentiation (CD)4+, CD8+, CD20+], dendritic cells (DT) and NK cells, decreased (Zhan, et al., 2006). Other studies have shown that the size of macrophages increased by more than 100%, and macrophages and T‐lymphocytes have been reported to be infected with high viral loads (Farcas, et al., 2005; Gu, et al., 2005).
In this review, we have documented 20 functional food plants with immunomodulatory and antiviral properties, including liquorice (Glycyrrhiza glabra L.), garlic (Allium sativum L.), tea (Camellia sinensis [L.] Kuntze), ginger (Zingiber officinale Roscoe), turmeric (Curcuma longa L.), pomegranate (Punica granatum L.), black pepper (Piper nigrum L.) and several others (Table 1). These plants have been reported to induce the immune system in several ways.
TABLE 1.
Immunomodulatory and anti‐viral functional food plants
| Plant | Family | English Name | Part Used | Formulation | Compounds | References |
|---|---|---|---|---|---|---|
| Allium cepa L. | Amaryllidaceae | Onion | Bulb | Crushed & mixed with honey | Quercetin, thiosulfinates, and anthocyanins | Mirabeau and Samson, 2012; Gansukh et al., 2016 |
| Allium sativum L. | Amaryllidaceae | Garlic | Bulb | Crushed & mixed with honey | Diallyl disulphide, alliin, polyphenols, proteins (QR‐1, QR‐ 2, and QR‐3) | Ishikawa et al., 2006; Clement et al., 2009; Liu et al., 2009; Mehrbod et al., 2009; Zamani et al., 2009; Sahoo and Banik 2018; Anywar et al., 2019; |
| Berberis vulgaris L. | Berberidaceae | Barberry | Fruit, stem and root | Boiled extract and poultice | Berbamine, berberine | Wu et al., 2011; Shin et al., 2015; Kalmarzi et al., 2019 |
| Camellia sinensis (L.) Kuntze | Theaceae | Tea Plant | Leaf | Boiled and drunk | Catechins, quercetin, gallic acid, theaflavin‐3,3ˊ‐digallate | Zvetkova et al., 2001; Chen et al., 2005; Chattopadhyay et al., 2011; Kumar et al., 2012; Lee et al., 2014; Karimi et al., 2016; Reygaert et al., 2018 |
| Carica papaya L. | Caricaceae | Papaya | Fruit and leaves | Leaves are ground to prepare juice; fruit can be directly eaten | Caricaxanthin, violaxanthin, zeaxanthin, carpaine, dehydrocarpaine I and II and cardenolide | Kala, 2012; Pandey et al., 2016; Radhakrishnan et al., 2017; |
| Citrus aurantium L. | Rutaceae | Bitter orange | Fruit and Peel | Dried peel or fruit juice | Polysaccharides, polyphenolic compounds | Shen et al., 2017; Mannucci et al., 2018 |
| Curcuma longa L. | Zingiberaceae | Turmeric | Rhizome | Pounded, tincture, powder | Curcumin | Srivastava et al., 2011; Catanzaro et al., 2018; Momtazi‐Borojeni et al., 2018; Anywar et al., 2019 |
| Ficus carica L. | Moraceae | Fig | Fruit, leaves | Decoction with honey | Terpenoids, anthocyanins, steroids | Idolo et al., 2010; Patil et al., 2010c; Aref et al., 2011a, b |
| Glycine max (L.) Merr. | Fabaceae | Soybean | Seeds | Cooked or roasted | Isoflavones, flavonoids, phytosterols, organic acid and saponins | Hayashi et al., 1997; Kinjo et al., 1999; Anitha et al., 2015 |
| Glycyrrhiza glabra L. | Fabaceae | Liquorice | Root | Dried roots extracted. The extract is vacuum dried to a dark paste, or maybe dried to a powder | Glycyrrhizin | Jeong and Kim, 2002; Cinatl et al., 2003; Raphael and Kuttan, 2003; Asl & Hosseinzadeh 2008; Seki et al., 2008; Michaelis et al., 2010; Smirnov et al., 2012 |
| Lycium barbarum L. | Solanaceae | Wolfberry | Fruit | Fresh fruit directly eaten | Polysaccharide‐protein complexes, phenolic compounds | Tang et al., 2012; Cheng et al., 2015; Byambasuren et al., 2019 |
| Mangifera indica L. | Anacardiaceae | Mango | Bark, leaves, roots, fruits, and flowers | Boiling or powdering of bark, leaves, root and flowers, while fruit can be directly eaten | Flavonoids, xanthones (Mangiferin), phenolic acids, triterpenes | Makare et al., 2001; Garrido et al., 2005; Rawi et al., 2019 |
| Morus alba L. | Moraceae | Mulberry | Fruit leaf, root | Fruit juice, leaves and root bark decoction or tea | Carotene, vitamin B1, folic acid, folinic acid, vitamin D, polyhydroxylated alkaloids, glycoprotein, Anthocyanins, benzofurans, stilbenes | Singh et al., 2013; Grienke et al., 2016. Wei et al., 2016; Kim and Chung, 2018 |
| Nigella sativa L. | Ranunculaceae | Black Cumin | Seeds | Roasted anend eat | Quinones, alkaloids, saponins | Ahmad et al., 2013; Koshak et al., 2018 |
| Piper longum L. | Piperaceae | Long pepper | Fruit and root | Decoction | Piperine | Koul & Kapil, 1993; Tripathi et al., 1999; Sunila and Kuttan, 2004; Kumar et al., 2010 |
| Piper nigrum L. | Piperaceae | Black pepper | fruit | Dried and used as spice | Piperine | Majeed and Prakash, 2000; Chaudhry and Tariq, 2006 |
| Prunus domestica L. | Rosaceae | Plum | Fruit | Eaten fresh | Anthocyanins, protocatechuic acid | Kayano et al., 2002;Walle et al., 2003; Rasne et al., 2018 |
| Psidium guajava L. | Myrtaceae | Guava | Fruit, shoots, leaves | Fruit can be directly eaten. Decoction and poultice of leaves and shoots | Phenolic, flavonoid, carotenoid, terpenoid and triterpenes | Gutierrez et al., 2008; Sriwilaijaroen et al., 2012; Ravi and Divyashree, 2014 |
| Punica granatum L. | Lythraceae | Pomegranate | Fruit, Seeds, Bark | Fruit juice, decoction of seeds, dried bark | Anthocyanins, fatty acids, alkaloids, vitamins | Bhowmik et al., 2013; Howell and Souza, 2013; Moradi et al., 2017 |
| Zingiber officinale Roscoe | Zingiberaceae | Ginger | Root | Dried or roasted and eaten with honey | Essential oil, crude fiber, proteins, fatty oils, carbohydrates | Sahoo and Banik, 2018; Mahboubi, 2019 |
Note: 1) Immunomodulatory properties of these plants are for overall body immune system, not for any particular disease, infection or organ, 2) while antiviral properties are mostly against respiratory tract infectious viruses (references are provided for all plants, but only some of them are discussed in this article)
For example, liquorice has been used as a medicinal and flavouring herb since ancient times. Traditionally, the dried roots are first crushed and then boiled to prepare an extract. The extract can be dried to a dark paste or powder and taken orally to treat different types of chronic infections (Asl & Hosseinzadeh, 2008). The root contains a saponin named glycyrrhizin, which is responsible for immunomodulation, antiviral and other biological activities (Seki et al., 2008). Innate immunity and adaptive immunity both greatly depend on the activity and function of the white blood cell (WBC) count, and it is also evident that most of the immune cells are produced from haematopoietic stem cells of bone marrow. Raphael and Kuttan (2003) reported that mice treated with glycyrrhizic acid showed an increased in WBCs, bone marrow cellularity and α‐esterase positive cells. In addition, they analysed the humoral immune response by measuring the production of antibodies and the number of antibody‐producing cells in the spleen, which were found to have increased in the treated mice. Moreover, the in vitro results showed that glycyrrhizin promotes the growth response of splenic T‐lymphocytes to anti‐CD3 monoclonal antibodies or concanavalin A (Con A) through improvement of interleukin‐2 (IL‐2) and IL‐2 receptor (IL‐2R) expression (Zhang, et al., 1993). Glycyrrhizin affects some post‐receptor stages of signal transduction because the increased production of IL‐2 by glycyrrhizin was also found in spleen cells. Dai et al. (2018) reported an increase in lipopolysaccharide‐induced IL‐12 p70 and p40 protein production in glycyrrhizin‐treated mice by peritoneal macrophages due to up‐regulation of IL‐12 p35 and p40 messenger RNAs, which was associated with enhanced NF‐kB (a protein complex that controls DNA transcription) activation, cytokine production and cell survival. It was further found that glycyrrhizin can interfere with immune responses by acting on dendritic cells (DCs). The results of flow cytometry analysis showed that in the mice spleen glycyrrhizin can up‐regulate the expressions of maturation markers (MHC‐II, CD40 and CD86) on DCs, which led to enhanced production of IL‐12 by these cells. In addition, glycyrrhizin enhanced the production of T‐cells and cytokines (IFN‐c and IL‐10) and reduced the production of IL‐12 (Bordbar, Karimi, & Amirghofran, 2012). Liquorice polysaccharides were also found to possess immunomodulatory properties as investigated in in‐vivo conditions. Low molecular weight polysaccharides showed increased thymus/spleen index, T‐lymphocytes (CD4+ and CD8 + 0) populations, IL‐2, IL‐6, IL‐7 levels and decreased tumor necrosis factor alpha (TNF‐α) levels (Ayeka, Bian, Githaiga, & Zhao, 2017). Extensive research on liquorice shows that it has strong immunomodulation activity and could be most helpful in enhancing the body's immune system against pathogenic infections.
Garlic has been one of the most popular herbal remedies since ancient times. It is believed that freshly crushed garlic mixed with honey, or without, can strengthen the immune system, as well as having antiviral and other biological properties, which might be due to the presence of several bioactive sulphur‐containing compounds including sulphoxide, proteins and polyphenols (Anywar et al., 2019; Sahoo & Banik, 2018). Several studies have suggested interesting beneficial effects of garlic on the immune cells and on immunity in general. For example, Kuttan (2000) reported the immunostimulatory effects of sulphur compounds (diallyl sulphide, diallyl disulphide and allyl methyl sulphide) in mice. Amongst the compounds studied, mice treated with diallyl disulphide showed higher numbers of WBCs (17,900 cells/mm3) and antibody titers than mice treated with other compounds. These compounds also significantly improved the bone marrow cellularity, the number of α‐esterase positive cells and the number of plaque‐forming cells in the spleen. Other studies have reported that garlic protein fraction 4 (F4) improved the cytotoxicity of human peripheral blood lymphocytes (PBL) against NK sensitive (K562) and NK‐resistant (M14) cell lines. F4 further improved IL‐2‐induced and Con A‐induced proliferation and their receptor expressions of PBL (Ishikawa et al., 2006). Moreover, liquid garlic extract and protein fraction showed modulatory effects on macrophages and T‐lymphocyte functions. These findings were further supported by the identification of three protein components of ∼13 kD (QR‐1, QR‐2 and QR‐3) from garlic extract exhibiting mitogenic activity on certain immune cells that include, lymphocytes, mast cells and basophils (Clement et al., 2010). Other studies have shown that garlic oil has dual effects on T‐helper cells; for example, at low concentrations, the response of T‐cells was elevated towards that of the Th1 type, whereas at high concentrations, the response triggered the Th2 type (Liu et al., 2009). However, another study reported that oral garlic administration supported the Th2 response by increasing IL‐4 production in the spleen lymphocytes of rats (Zamani et al., 2009). In addition, using peripheral blood monocytes, aged garlic extract up‐regulated IL‐10 by inhibiting the production of pro‐inflammatory cytokines such as TNF‐α and IL‐6, whereas it decreased IL‐12 production, which could further down‐regulate pro‐inflammatory cytokines such as interferon gamma IFN‐γ and IL‐2 produced by T‐cells (Gazzinelli, Oswald, James, & Sher, 1992; Hodge, Hodge, & Han, 2002). The immunostimulatory potential of garlic could be beneficial in clinical applications because it can boost both innate and specific cell immunity as well as can enhance host resistance.
Similarly, other documented plants also possess immunostimulatory properties, such as curcumin derived from C. longa, which can interact with several types of immune cells, including dendritic cells, B‐ and T‐lymphocytes, macrophages and cytokines (Catanzaro et al., 2018; Momtazi‐Borojeni et al., 2018) and enhance the defence mechanism of the host. A black tea (C. sinensis) decoction in cultured human peripheral mononuclear cells showed increased lympho‐proliferative action at 72 h (Chattopadhyay et al., 2012). In human mononuclear cell cultures, green tea (also from C. sinensis) extract showed increased production of neopterin (potential marker for activation of cell‐mediated immunity) in unstimulated peripheral mononuclear cells, whereas a reduction in neopterin was observed in cells stimulated with Con A, phytohemagglutinin and gamma interferon, confirming the immunomodulatory properties of green tea (Zvetkova et al., 2001). These immunostimulatory properties of black tea and green tea are due to the presence of (−)‐epigallocatechin gallate, quercetin and gallic acid in the leaves (Kumar et al., 2012). Furthermore, Patil, Bhangale, and Patil (2011) found that oral administration of ethanolic leaves extracts of the common fig (Ficus carica L.) ameliorated humoral and cell‐mediated immune responses. Traditionally, this plant has also been used against several respiratory, gastrointestinal, inflammatory and cardiovascular problems (Duke, Bogenschutz‐Godwin, Du Celliar, & Duke, 2002). Moreover, methanolic extracts of mango (Mangifera indica L.) bark (rich in mangiferin) enhanced delayed type hypersensitivity and humoral antibody titers, confirming its possible immunomodulatory properties (Makare et al., 2001). In vivo oral administration of hexane leaves extract of mango increased the WBC count and the size of the thymus and spleen, indicating immunomodulation in WBCs and bone marrow haematopoietic cells (Shailajan, Menon, Kulkarni, & Tiwari, 2016). Other plants reported in this review, including pomegranate (P. granatum), black pepper (P. nigrum), long pepper (Piper longum L.), black caraway (also known as black cumin; Nigella sativa L.), barberry (Berberis vulgaris L.), papaya (also known as pawpaw; Carica papaya L.), white mulberry (Morus alba L.), Chinese boxthorn (also known as the Himalayan goji; Lycium barbarum L.), bitter (or Seville) orange (Citrus aurantium L.), European plum (Prunus domestica L.) and soybean (or soya bean; Glycine max [L.] Merr.) all possess immunomodulatory activity in one way or another, supporting our first hypothesis that these functional food plants boost the immune response of the host and could be used as a preventive measure against COVID‐19. As mentioned earlier, in SARS autopsies, reductions in the concentrations of various immune cells were observed. Moreover, clinical evidence has shown the infection of T‐lymphocytes and macrophages/monocytes in circulating blood, spleen, lungs and lymph nodes in SARS autopsies (Zhan et al., 2006; Farcas et al., 2005). These immune cells are involved in both the innate and adaptive immune systems, and any infection in these cells would result in a weakened immune system in SARS patients. These findings further strengthen our hypothesis and give an insight into the possible role of these reported phytocompounds in clinical applications against COVID‐19.
3.2. Antiviral functional food plants
According to WHO, respiratory tract infections are the leading cause of mortality amongst all infectious diseases. Viral diseases are life‐threatening due to their rapid outbreak in developing as well as developed countries, whereas treating them is a huge challenge due to, easy adaptation, resistant viral pathogens, and the emergence of new viral strains and the ineffectiveness of antibiotics (Amber, Adnan, Tariq, & Mussarat, 2017). The first genome sequence of SARS‐CoV‐2 has been compared with those of SARS‐CoV and MERS‐CoV, and the results show that SARS‐CoV‐2 shares a closer genome sequence homology with SARS‐CoV, mostly in ORF1a and S‐proteins (which facilitate the attachment of the virus to the host receptor). Later, it was found that SARS‐CoV‐2 showed an 85% identity with SARS‐like CoV (bat‐SL‐CoVZC45, MG772933.1). Following these observations, SARS‐CoV‐2 has been clustered with beta‐coronavirus genera, including SARS and SARS‐like coronaviruses (Xu, et al., 2020; Zhu, et al., 2020). The initial symptoms of SARS‐CoV‐2 infection include fever, coughing, sneezing and shortening of breath, whereas severe symptoms include lower respiratory tract infection or pneumonia (Li et al., 2020). In histopathological investigations, the lungs of SARS patients showed diffuse alveolar damage, collapse of alveoli, extensive oedema, fibrous tissue in the alveolar spaces, hyaline membrane formation, desquamation of alveolar epithelial cells, leading to acute lung damage or pneumonia (Gu & Korteweg, 2007).
All of these investigations clearly indicate that this virus causes respiratory distress, and, bearing this in mind, several common and easily accessible functional food plants have been documented in this review that possess immunomodulatory, antiviral (especially for respiratory tract infections) and other biological activities. For example, glycyrrhizin isolated from G. glabra was tested on SARS‐CoV‐infected patients admitted to the Clinical Centre of Frankfurt University, Germany (Cinatl et al., 2003). The results of this study showed that glycyrrhizin was the most effective inhibitor of SARS‐CoV replication, with a selectivity index of 67 in Vero cells, compared with other tested compounds. Furthermore, this compound inhibited not only replication but also the adsorption and penetration of the virus. Although the exact mechanism by which glycyrrhizin inhibits SARS‐CoV adsorption, penetration and replication is unclear, a literature review revealed that it affects cellular signalling pathways, including protein kinase C, transcription factors and casein kinase II. Also, glycyrrhizin enhances production and expression of nitrous oxide (NO) in macrophages, which remarkably inhibits virus replication (Jeong & Kim, 2002; Lin, et al., 1997). In addition, glycyrrhizin possesses potent activity against influenza A virus (H5N1), which is also an emerging virus and, like SARS‐CoV, targeting the lungs. These viruses also have certain pathological similarities and differences. Previous studies have reported that a 100 μg/ml concentration of glycyrrhizin reduced the capacity of H5N1 to affect chemokine and interleukin (IL‐6) production, as well as H5N1‐induced apoptosis (Michaelis et al., 2010). H5N1 replication was found to be enhanced at the high‐mobility‐group box1 (HMGB1) DNA binding site, but glycyrrhizin inhibited the polymerase activity of H5N1 by affecting HMGB1 binding to DNA (Smirnov et al., 2012). Therefore, this compound could be considered as a potent antiviral agent and should be given serious attention. Moreover, the 3C‐like protease of SARS‐CoV is an important target for drug discovery and development because it is involved in proteolytic procession during maturation of the virus. Chen et al. (2005) tested different compounds derived from C. sinensis, including tannic acid, 3‐isotheaflavin‐3‐gallate (TF2B) and several catechins, on 3CL protease activity. It has been suggested that catechins do not show inhibitory potential; however, both tannic acid (IC50 = 7 μM) and TF2B (IC50 = 3 μM) were found to be potent inhibitors. These results suggest the potent role of the tea plant against SARS‐CoV infection, but further investigation of its possible inhibitory action on the replication of CoV in cell culture might strengthen its claim to antiviral activity. Similar to SARS‐CoV and influenza viruses, respiratory syncytial virus (RSV) also causes acute respiratory infections and is considered a major threat to people of different ages globally. Berberine, an alkaloid isolated from B. vulgaris, has been tested and found to significantly reduce RSV replication in epithelial cells by decreasing synthesis of mRNA and viral proteins. Such inhibition might be due to suppression of RSV‐induced phosphorylation of p38 mitogen‐activated protein kinase, which is very important for successful infection of RSV (Shin et al., 2015). Furthermore, curcumin derived from C. longa decreased the yield of influenza virus by more than 90% in cell culture at 30 μM concentration, which might have been because it affected the synthesis of viral proteins such as haemagglutinin, neuraminidase and matrix protein (Chen et al., 2010). Curcumin was also found to be effective against RSV, by inhibiting its replication and budding in the nasal epithelial cells of humans, and it also improved epithelial barrier activity (Obata, et al., 2013). Similarly, other reported immunomodulatory functional food plants in this review also exhibit elevated antiviral activity, particularly against viruses causing respiratory tract problems, and so should be considered as potential antiviral agents.
3.3. Other biological properties
The medicinal food plants described in this review not only possess immunomodulatory and antiviral properties but also exhibit several other biological actions, including antibacterial, antifungal, anti‐inflammatory, antioxidant, anti‐nephroprotective and anticancer activity (Figure 2). Curcumin derived from C. longa was tested against Staphylococcus aureus surface protein attaching transpeptidase (Sortase A) and showed inhibitory activity at an IC50 of 13.8 mg/ml concentration. However, curcumin did not show an inhibitory effect on the growth of the bacteria, but both pathogenesis and acute infection were decreased due to inhibition of Sortase A (Park, et al., 2005). S. aureus is a multi‐drug‐resistant bacterium causing respiratory tract problems, including pneumonia and skin infections. C. longa is also very effective against several other groups of pathogenic bacteria and fungi, including Escherichia spp., Pseudomonas spp., Aspergillus spp. and Candida spp. (Praditya, et al., 2019). It has been shown that oral administration of C. longa can reduce inflammation by inhibiting the synthesis of inflammatory prostaglandin and neutrophil functions effectively (Cronin, 2003). An ethanolic extract of F. carica was found to be a potent antipyretic (reducing body temperature) in a dose‐dependent manner (100, 200 and 300 mg/kg; Badgujar, Patel, Bandivdekar, & Mahajan, 2014). An ethanolic extract of F. carica was further reported to be active against carrageenan‐induced rat paw oedema, and hence possess anti‐inflammatory properties (Patil and Patil, 2011). F.icarica is also very potent against several pathogenic microbial strains, different cancer cell lines and other biological problems of humans (Badgujar et al., 2014). In vitro investigation revealed that an aqueous extract of G. glabra showed increased inhibition of bacteria causing respiratory tract infections, including S. aureus and S. pyogenes (Damle, 2014) cultures, which may have been due to the presence of glycyrrhizin. Furthermore, glycyrrhizic acid displays potent hepatoprotective activity by reducing free radical production and lipid peroxidation (Jeong, et al., 2002). Piperine extracted from P. nigrum also showed antimicrobial properties by inhibiting the growth of S. aureus due to its high activity against the NorA efflux pump, which contributes to microbial resistance to antibiotics (Sangwan et al., 2008). Black pepper extracts were also found to be very potent against other bacterial infections caused by E. coli, Pseudomonas spp., Bacillus spp., and so forth (Butt et al., 2013). All of the documented plants in this review have a large range of pharmacological properties, and so could be very useful for maintaining good health.
FIGURE 2.
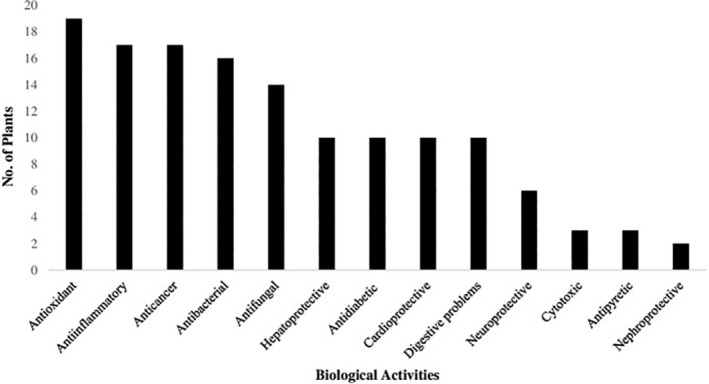
Biological activities of functional immunomodulatory and antiviral food plants
In SARS cases, a number of co‐infections were reported being caused by S. aureus, Streptococcus spp., Pseudomonas aeruginosa, Aspergillus spp. and Klebsiella spp. (Chong, et al., 2004; Hwang, et al., 2005). Moreover, as mentioned earlier, most of the patients who died as a result of COVID‐19 already had several other health‐related problems. Therefore, the functional food plants documented in this review would not only enhance the immune system and cure respiratory tract infections but would also greatly impact the overall health of the general public. This could possibly reduce the risk of COVID‐19 and initiate a rapid recovery in cases of SARS‐CoV‐2 infection. In the current situation of COVID‐19, most of the people in China and other countries are restricted to their homes; therefore, these functional food plants, which are easily accessible, could serve them as a cheap way of maintaining a healthy immune system and general good health. It is highly recommended to include these medicinal food plants in the daily diet as a preventive measure for effective health management.
3.4. Toxicology of functional food plants
In contrast of the positive effects, some functional food plants also possess toxic effects to the human body if taken in an excessive amount. For example; liquorice and glycyrrhizin lead to certain harmful impacts on human health if the consumption level is higher and the duration of consumption is prolonged. It is well known that licorice produces less side effects if taken orally, rather than by intraperitoneal or intravenous injection, but even oral administration for several weeks or longer can cause toxic effects (Hosseinzadeh, et al., 2005; Nassiri Asl & Parvardeh, 2015). Previous studies reported that the most important side effects of licorice and glycyrrhizin are hypertension and hypokalemic‐induced secondary disorders, with the acceptable daily intake reported for glycyrrhizin as 0.2 mg/kg/day (Nazari, Rameshrad, & Hosseinzadeh, 2017; Snafi‐Al, 2018). Moreover, a low dose of liquorice and glycyrrhizin can be toxic for people having cardiovascular, kidney and blood pressure problems. Clinical evidence also suggests that the daily intake of low doses this plant species can cause serious complications in pregnant women and breast‐feeding children (Choi, et al., 2013; Cuzzolin, et al., 2010).
While the Food and Drug Administration considers garlic as safe for humans, the species can induce several complications (diarrhoea, dizziness, nausea, vomiting, headache and flatulence) if ingested in high doses especially on an empty stomach. Oral or intraperitoneal administration of garlic at 50 mg/kg concentration per day did not show any toxic effects on lung and liver tissues, whereas 250–1,000 mg/kg concentration per day resulted in severe deformities in the lung and liver tissues of the rats (Rana, Pal, Vaiphei, Sharma, & Ola, 2011). In another study, garlic extracts at the concentration of 300–600 mg for 3 weeks resulted in delayed growth in male and female rats (Mikaili, Maadirad, Moloudizargari, Aghajanshakeri, & Sarahroodi, 2013). The recommended doses of the garlic are 4 g (raw form) or 7.2 g (aged garlic extract) or one tablet of dried garlic powder twice or thrice per day for adults (Tattelman, 2005). Curcumin derived from C. longa, does not cause any severe toxicity at a dose of up to 8 g per day, over a short period of time. However, human based studies showed that curcumin at doses ranging from 0.9 to 3.6 g per day for 1–4 months can cause nausea and diarrhoea (Somasundaram, et al., 2002). In addition, higher amount of green tea consumption can pose hepatotoxic and gastrointestinal problems if taken on an empty stomach (Bedrood et al., 2018). Similarly, other functional food plants reported in this review also possess variety of toxic effects on living system if taken in higher amount and for longer period of time. Hence, it is really important to have an updated knowledge on the risk‐benefits of functional food plants.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGEMENT
The authors are most grateful to the Chinese government for taking substantial measures to control transmission of the virus. The authors also extend their thanks and appreciation to the many health workers for their unfailing efforts in taking care of infected patients. This work was financially supported by Joint Funds of the National Natural Science Foundation of China and the Government of Xinjiang Uygur Autonomous Region of China (U1603233 and U1903102), National Natural Science Foundation of China (NSFC‐41977050 and NSFC‐41877012), and President's International Fellowship Initiative (PIFI) for Postdoctoral Researchers, Chinese Academy of Sciences (2020PB0002), China.
Yang F, Zhang Y, Tariq A, et al. Food as medicine: A possible preventive measure against coronavirus disease (COVID‐19). Phytotherapy Research. 2020;34:3124–3136. 10.1002/ptr.6770
Yang Fan, Yue Zhang, and Akash Tariq contributed equally to this study.
Funding information National Natural Science Foundation of China, Grant/Award Numbers: NSFC‐41877012, NSFC‐41977050, U1903102, U1603233; Chinese Academy of Sciences, Grant/Award Number: 2020PB0002; Government of Xinjiang Uygur Autonomous Region of China, Grant/Award Numbers: U1603233, U1903102
Contributor Information
Akash Tariq, Email: akash.malik786@mails.ucas.ac.cn.
Xiaolan Jiang, Email: jxlljy2011@163.com.
REFERENCES
- Ahmad, A. , Husain, A. , Mujeeb, M. , Khan, S. A. , Najmi, A. K. , Siddique, N. A. , … Anwar, F. (2013). A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac J Trop Biomed, 3, 337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Rawi, A. A. S. , Al‐Dulaimi, H. S. H. , & Al‐Rawi, M. A. A. (2019). Antiviral Activity of Mangifera Extract on Influenza Virus Cultivated in Different Cell Cultures. J Pure Appl Microbiol, 13, 455–458. [Google Scholar]
- Amber, R. , Adnan, M. , Tariq, A. , & Mussarat, S. (2017). A review on antiviral activity of the Himalayan medicinal plants traditionally used to treat bronchitis and related symptoms. The Journal of Pharmacy and Pharmacology, 69, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitha, T. , Parameswari, K. , Kishori, B. , & Usha, R. (2015). Evaluation of in vitro immunomodulatory effects of soybean (Glycine max L) extracts on mouse immune system. Int J Pharma Sci Res, 6, 2112–2120. [Google Scholar]
- Anywar, G. , Kakudidi, E. , Byamukama, R. , Mukonzo, J. , Schubert, A. , & Oryem‐Origa, H. (2019). Medicinal plants used by traditional medicine practitioners to boost the immune system in people living with HIV/AIDS in Uganda. European Journal of Integrative Medicine. 10.1016/j.eujim.2019.101011 [DOI] [Google Scholar]
- Aref, H. L. , Gaaliche, B. , Fekih, A. , Mars, M. , Aouni, M. , Chaumon, J. P. , & Said, K. (2011b). In vitro cytotoxic and antiviral activities of Ficus carica latex extracts. Nat Prod Res, 25, 310–319. [DOI] [PubMed] [Google Scholar]
- Aref, H. L. , Habib, M. , Hanen, L. , Said, K. , & Selmi, B. (2011a). Partial characterization of a novel amylase activity isolated from Tunisian Ficus carica latex. Pharm Biol, 49, 1158–1166. [DOI] [PubMed] [Google Scholar]
- Asl, M. N. , & Hosseinzadeh, H. (2008). Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytotherapy Research, 22, 709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayeka, P. A. , Bian, Y. , Githaiga, P. M. , & Zhao, Y. (2017). The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor‐bearing mice. BMC Complementary and Alternative Medicine, 17, 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgujar, H. B. , Patel, V. V. , Bandivdekar, A. H. , & Mahajan, R. T. (2014). Traditional uses, phytochemistry and pharmacology of Ficus carica: A review. Pharmaceutical Biology, 52, 1487–1503. [DOI] [PubMed] [Google Scholar]
- Bedrood, Z. , Rameshrad, M. , & Hosseinzadeh, H. (2018). Toxicological effects of Camellia sinensis (green tea): A review. Phytotherapy Research, 32, 1163–1180. [DOI] [PubMed] [Google Scholar]
- Bhowmik, D. , Gopinath, H. , Kumar, P. , Duraivel, S. , Aravind, V. , & Kumar, K. P. S. (2013). Medicinal Uses of Punica granatum and Its Health Benefits. J Pharmacogn Phytochem, 1, 2278–4136. [Google Scholar]
- Bordbar, N. , Karimi, M. H. , & Amirghofran, Z. (2012). The effect of glycyrrhizin on maturation and T cell stimulating activity of dendritic cells. Cellular Immunology, 280, 44–49. [DOI] [PubMed] [Google Scholar]
- Butt, M. S. , Pasha, I. , Sultan, M. T. , Randhawa, M. A. , Saeed, F. , & Ahmed, W. (2013). Black pepper and health claims: A comprehensive treatise. Critical Reviews in Food Science and Nutrition, 53, 875–886. [DOI] [PubMed] [Google Scholar]
- Byambasuren, S. E. , Wang, J. , & Gaudel, G. (2019). Medicinal value of wolfberry (Lycium barbarum L.). J Med Plants Stud, 7, 90–97. [Google Scholar]
- Catanzaro, M. , Corsini, E. , Rosini, M. , Rachhi, M. , & Lanni, C. (2018). Immunomodulators inspired by nature: A review on Curcumin and Echinacea. Molecules, 23, 27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry, M. N. A. , & Tariq, P. (2006). Bactericidal activity of black pepper, bay leaf, aniseed and coriander against oral isolates. Pak J Pharma Sci, 19, 214–218. [PubMed] [Google Scholar]
- Cheng, J. , Zhou, Z. , Sheng, H. P. , He, L. J. , Fan, X. W. , He, Z. X. , … Zhou, S. F. (2015). An evidence‐based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des Devel Ther, 9, 33–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin, D. D. (2010). Overview of the immune response. The Journal of Allergy and Clinical Immunology, 125, S3–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay, C. , Chakrabarti, N. , Chatterjee, M. , Mukherjee, S. , Sarkar, K. , & Chaudhuri, R. (2012). Black tea (Camellia sinensis) decoction shows immunomodulatory properties on an experimental animal model and in human peripheral mononuclear cells. Pharmacognosy Research, 4, 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. N. , Lin, C. P. C. , Huang, K. K. , Chen, W. C. , Heish, H. P. , Liang, P. H. , & Hsu, J. T. A. (2005). Inhibition of SARS‐CoV 3C‐like protease activity by Theaflavin‐3,3‐digallate (TF3). eCAM, 2, 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, D. Y. , Shien, J. H. , Tiley, L. , Chiou, S. S. , Chiou, S. Y. , Jye, T. , & Hsu, W. L. (2010). Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chemistry, 119, 1346–1351. [Google Scholar]
- Choi, J. S. , Han, J. Y. , Ahn, H. K. , Ryu, H. M. , Kim, M. Y. , Chung, J. H. , & Koren, G. (2013). Fetal and neonatal out‐comes in women reporting ingestion of licorice (Glycyrrhiza uralensis) during pregnancy. Planta Medica, 79, 97–101. [DOI] [PubMed] [Google Scholar]
- Chong, P. Y. , Chui, P. , Ling, A. E. , Franks, T. J. , Tai, D. Y. H. , Leo, Y. S. , … Travis, W. D. (2004). Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: Challenges in determining a SARS diagnosis. Archives of Pathology & Laboratory Medicine, 128, 195–204. [DOI] [PubMed] [Google Scholar]
- Cinatl, J. , Morgenstern, B. , Bauer, G. , Chandra, P. , Rabenau, H. , & Doerr, H. W. (2003). Glycyrrhizin, an active component of liquorice roots, and replication of SARS‐associated coronavirus. Lancet, 361, 2045–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement, F. , Pramod, S. N. , & Venkatesh, Y. P. (2010). Identity of the immunomodulatory proteins from garlic (Allium sativum) with the major garlic lectins or agglutinins. International Immunopharmacology, 10, 316–324. [DOI] [PubMed] [Google Scholar]
- Cronin, J. R. (2003). Curcumin: Old spice is a new medicine. Alternative and Complementary Therapies, 9, 34–38. [Google Scholar]
- Cui, J. , Li, F. , & Shi, Z. L. (2019). Origin and evolution of pathogenic coronaviruses. Nature Reviews. Microbiology, 17, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzolin, L. , Francini‐Pesenti, F. , Verlato, G. , Joppi, M. , Baldelli, P. , & Benoni, G. (2010). Use of herbal products among 392 Italian pregnant women: Focus on pregnancy outcome. Pharmacoepidemiology and Drug Safety, 19, 1151–1158. [DOI] [PubMed] [Google Scholar]
- Dai, J. , Gu, L. , Su, Y. , Wang, Q. , Zhao, Y. , Chen, X. , … Li, K. (2018). Inhibition of curcumin on influenza a virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF‐kB pathways. International Immunopharmacology, 54, 177–187. [DOI] [PubMed] [Google Scholar]
- Damle, M. (2014). Glycyrrhiza glabra (Liquorice) ‐ a potent medicinal herb. International Journal of Herbal Medicine, 2, 132–136. [Google Scholar]
- Duke, J. A. , Bogenschutz‐Godwin, M. J. , Du Celliar, J. , & Duke, P. K. (2002). Handbook of medicinal herbs (2nd ed., pp. 314–315). Boca Raton: CRC Press. [Google Scholar]
- Farcas, G. A. , Poutanen, S. M. , Mazzulli, T. , Willey, B. M. , Butany, J. , Asa, S. L. , … Kain, K. C. (2005). Fatal severe acute respiratory syndrome is associated with multiorgan involvement by coronavirus. The Journal of Infectious Diseases, 191, 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gansukh, E. , Muthu, M. , Paul, D. , Ethiraj, G. , Chun, S. C. , & Gopal, G. (2017). Nature nominee quercetin's anti‐influenza combat strategy‐Demonstrations and remonstrations. Rev Med Virol, 27, e1930. [DOI] [PubMed] [Google Scholar]
- Garrido, G. , Gonzalez, D. , Lemus, Y. , Garcia, D. , Lodeiro, L. , & Quintero, G. (2004). In vivo and in vitro anti‐inflammatory activity of Mangifera indica L. extract (VIMANG). Pharmacol Res, 50, 143–149. [DOI] [PubMed] [Google Scholar]
- Gazzinelli, R. T. , Oswald, I. P. , James, S. L. , & Sher, A. (1992). IL‐10 inhibits parasite killing and nitrogen oxide production by IFN—activated macrophages. Journal of Immunology, 148, 1792–1796. [PubMed] [Google Scholar]
- Grienke, U. , Ritcher, M. , Walter, E. , Hoffmann, A. , Kirchmair, J. , Makarov, V. , … Rollinger, J. M. (2016). Discovery of prenylated flavonoids with dual activity against influenza virus and Streptococcus pneumonia. Sci Rep, 6, 27156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, R. M. P. , Mitchell, S. , & Solis, R. S. (2008). Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol, 117, 1–27. [DOI] [PubMed] [Google Scholar]
- Gu, J. , Gong, E. C. , Zhang, B. , Zheng, J. , Gao, Z. , Zhong, Y. , … Leong, A. S. Y. (2005). Multiple organ infection and the pathogenesis of SARS. The Journal of Experimental Medicine, 202, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, J. , & Korteweg, C. (2007). Pathology and pathogenesis of severe acute respiratory syndrome. The American Journal of Pathology, 170, 1136–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, Y. , Zheng, B. J. , He, Y. Q. , Liu, X. L. , Zhuang, Z. X. , Cheung, C. L. , … Guan, Y. J. (2003). Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science, 302, 276–278. [DOI] [PubMed] [Google Scholar]
- Hayashi, K. , Hayashi, H. , Hiraoka, N. , & Ikeshiro, Y. (1997). Inhibitory activity of soyasaponin II on virus replication in vitro. Planta Med, 63, 102–105. [DOI] [PubMed] [Google Scholar]
- Hemida, M. G. , Perera, R. A. , Wang, P. , Alhammadi, M. A. , Siu, L. Y. , Li, M. , … Peiris, M. (2013). Middle East respiratory syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveillance, 18, 21–27. [DOI] [PubMed] [Google Scholar]
- Hodge, G. , Hodge, S. , & Han, P. (2002). Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: Potential therapeutic use in the treatment of inflammatory bowel disease. Cytometry, 48(4), 209–215. [DOI] [PubMed] [Google Scholar]
- Hong Kong Centre for Health Protection . (2020). Countries/areas with reported cases of novel. Retrieved from https://www.chp.gov.hk/files/pdf/statistics_of_the_cases_novel_coronavirus_infection_en.pdf
- Hosseinzadeh, H. , Nassiri Asl, M. , & Parvardeh, S. (2005). The effects of carbenoxolone, a semisynthetic derivative of glycyrrhizinic acid, on peripheral and central ischemia‐reperfusion injuries in the skeletal muscle and hippocampus of rats. Phytomedicine, 12, 632–637. [DOI] [PubMed] [Google Scholar]
- Hu, B. , Zeng, L. P. , Yang, X. L. , Ge, X. Y. , Zhang, W. , Li, B. , … Shi, Z. L. (2017). Discovery of a rich gene pool of bat SARS‐ related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathogens, 13, e1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AB, Dsouza DH. 2013. The Pomegranate: Effects on Bacteria and Viruses That Influence Human Health. Evid‐Based Complem Altern Med 10.1155/2013/606212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, D. M. , Chamberlain, D. W. , Poutanen, S. M. , Low, D. E. , Asa, S. L. , & Butany, J. (2005). Pulmonary pathology of severe acute respiratory syndrome in Toronto. Modern Pathology, 18, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idolo, M. , Motti, R. , & Mazzoleni, S. (2010). Ethnobotanical and phytomedicinal knowledge in a long‐history protected area the Abruzzo, Lazio and Molise National Park (Italian Apennines). J Ethnopharmacol, 127, 379–395. [DOI] [PubMed] [Google Scholar]
- Ishikawa, H. , Saeki, T. , Otani, T. , Suzuki, T. , Shimozuma, K. , Nishino, H. , … Morimoto, K. (2006). Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. The Journal of Nutrition, 136, 816S–820S. [DOI] [PubMed] [Google Scholar]
- Jeong, H. G. , & Kim, J. Y. (2002). Induction of inducible nitric oxide synthase expression by 18‐glycyrrhetinic acid in macrophages. FEBS Letters, 513, 208–212. [DOI] [PubMed] [Google Scholar]
- Jeong, H. G. , You, H. J. , Park, S. J. , Moon, A. R. , Chung, Y. C. , Kang, S. K. , & Chun, H. K. (2002). Hepatoprotective effects of 18β‐ glycyrrhetinic acid on carbon tetrachloride‐induced liver injury: Inhibition of cytochrome P450 2E1 expression. Pharmacological Research, 46, 221–227. [DOI] [PubMed] [Google Scholar]
- Kala, C. P. (2012). Leaf Juice of Carica papaya L.: A Remedy of Dengue Fever. Med Aromat Plants, 1, 6. [Google Scholar]
- Kalmarzi, R. N. , Naleini, S. N. , Ashtary‐Larky, D. , Peluso, I. , Jouybari, L. , Rafi, A. , … Kooti, W. 2019. Anti‐Inflammatory and Immunomodulatory Effects of Barberry (Berberis vulgaris) and Its Main Compounds. Oxid Med Cell Longev 10.1155/2019/6183965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi, A. , Moradi, M. T. , Alidadi, S. , & Hashemi, L. (2016). Anti‐adenovirus activity, antioxidant potential, and phenolic content of black tea (Camellia sinensis Kuntze) extract. J Complement Integr Med, 13, 357–363. [DOI] [PubMed] [Google Scholar]
- Kayano, S. , Kikuzaki, H. , Fukutsuka, N. , Mitani, T. , & Nakatani, N. (2002). Antioxidant activity of prune (Prunus Domestica L.) Constituents and a new synergist. J Agric Food Chem, 50, 3708–3712. [DOI] [PubMed] [Google Scholar]
- Kim, H. , & Chung, M. S. (2018). Antiviral Activities of Mulberry (Morus alba) Juice and Seed against Influenza Viruses. Evid‐Based Complem Altern Med. 10.1155/2018/2606583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjo, J. , Udayama, M. , Hatakeyama, M. , Ikeda, T. , Sohno, Y. , & Yoshiki, Y. (1999). Hepatoprotective effects of oleanene glucuronides in several edible beans. Nat Med, 53, 141–144. [Google Scholar]
- Koshak, A. E. , Yousif, N. M. , Fiebich, B. L. , Koshak, E. A. , & Heinrich, M. (2018). Comparative Immunomodulatory Activity of Nigella sativa L. Preparations on Proinflammatory Mediators: A Focus on Asthma. Front Pharmacol, 9, 1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koul, I. B. , & Kapil, A. (1993). Evaluation of the liver protective potential of Piperine, an active principle of black pepper and long pepper. Planta Med, 59, 413–417. [DOI] [PubMed] [Google Scholar]
- Kumar, S. , Kamboj, S. , & Sharma, S. (2011). Overview for Various Aspects of the Health Benefits of Piper Longum Linn. Fruit. J Acupunct Meridian Stud, 4, 134–140. [DOI] [PubMed] [Google Scholar]
- Kumar, D. , Arya, V. , Kaur, R. , Bhat, Z. A. , Gupta, V. K. , & Kumar, V. (2012). A review of immunomodulators in the Indian traditional health care system. Journal of Microbiology, Immunology, and Infection, 45, 165–184. [DOI] [PubMed] [Google Scholar]
- Kuttan, G. (2000). Immunomodulatory effect of some naturally occurring Sulphur‐containing compounds. Journal of Ethnopharmacology, 72, 93–99. [DOI] [PubMed] [Google Scholar]
- Lee, H. S. , Jun, J. H. , Jung, E. H. , Koo, B. A. , & Kim, Y. S. (2014). Epigallocatechin‐3‐gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP‐9 and VEGF activation. Molecules, 19, 12150–12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Q. , Guan, X. , Wu, P. , Wang, X. , Zhou, L. , Tong, Y. , … Feng, Z. (2020). Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. New England Journal of Medicine, 382, 1199–1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Y. L. , Huang, Y. L. , Ma, S. H. , Yeh, C. T. , Chiou, S. Y. , & Liao, C. L. (1997). Inhibition of Japanese encephalitis virus infection by nitric oxide: Antiviral effect of nitric oxide on RNA virus replication. Journal of Virology, 71, 5227–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. T. , Su, H. M. , Lii, C. K. , & Sheen, L. Y. (2009). Effect of supplementation with garlic oil on activity of T1 and T2 lymphocytes from rats. Planta Medica, 75, 205–210. [DOI] [PubMed] [Google Scholar]
- Mahboub, M. (2019). Zingiber officinale Rosc. essential oil, a review on its composition and bioactivity. Clin. Phytoscience, 5, 6. [Google Scholar]
- Majeed, P. L. (2000). The Medicinal Uses of Pepper. Int Pepper News, 1, 23–31. [Google Scholar]
- Makare, N. , Bodhankar, S. , & Rangari, V. (2001). Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. Journal of Ethnopharmacology, 78, 133–137. [DOI] [PubMed] [Google Scholar]
- Mannucci, C. , Calapai, F. , Cardia, L. , Inferrera, G. , Arena, G. , Pietro, M. D. , … Calapai, G. (2018). Clinical Pharmacology of Citrus aurantium and Citrus sinensis for the Treatment of Anxiety. Evid‐Based Complem Altern Med. 10.1155/2018/3624094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrbod, P. , Amini, E. , & Tavassoti‐Kheiri, M. (2009). Antiviral Activity of Garlic Extract on Influenza Virus. Iran J Virol, 3, 19–23. [Google Scholar]
- Michaelis, M. , Geiler, J. , Naczk, P. , Sithisarn, P. , Ogbomo, H. , Altenbrandt, B. , … Cinatl, J. (2010). Glycyrrhizin inhibits highly pathogenic H5N1 influenza a virus–induced pro‐inflammatory cytokine and chemokine expression in human macrophages. Medical Microbiology and Immunology, 199, 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabeau, T. Y. , & Samson, E. S. (2012). Effect of Allium Cepa and Allium Sativum on Some Immunological Cells in Rats. Afr J Tradit Complement Altern Med, 9, 374–379. [PMC free article] [PubMed] [Google Scholar]
- Mikaili, P. , Maadirad, S. , Moloudizargari, M. , Aghajanshakeri, S. , & Sarahroodi, S. (2013). Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iranian Journal of Basic Medical Sciences, 16, 1031–1048. [PMC free article] [PubMed] [Google Scholar]
- Moradi, M. T. , Karimi, A. , Lorigooini, Z. , Lorigooini, Z. , Pourgheysari, B. , Alidadi, S. , & Hashemi, L. (2017). In vitro Anti influenza virus activity, antioxidant potential and total phenolic content of twelve Iranian medicinal plants. Marmara Pharm J, 21, 843–851. [Google Scholar]
- Momtazi‐Borojeni, A. A. , Haftcheshmeh, S. M. , Esmaeili, S. A. , Johnston, T. P. , Abdollahi, E. , & Sahebkar, A. (2018). Curcumin: A natural modulator of immune cells in systemic lupus erythematosus. Autoimmunity Reviews, 17, 125–135. [DOI] [PubMed] [Google Scholar]
- Nazari, S. , Rameshrad, M. , & Hosseinzadeh, H. (2017). Toxicological effects of Glycyrrhiza glabra (licorice): A review. Phytotherapy Research, 31, 1635–1650. [DOI] [PubMed] [Google Scholar]
- Obata, K. , Kojima, T. , Masaki, T. , Okabayashi, T. , Yokota, S. , Hirakawa, S. , … Sawada, N. (2013). Curcumin prevents replication of respiratory syncytial virus and the epithelial responses to it in human nasal epithelial cells. PLoS One, 8, e70225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, S. , Peter, J. , Cabot, P. , Shaw, N. , & Amitha, K. (2016). Anti‐inflammatory and immunomodulatory properties of Carica papaya. J Immunotoxicol, 13, 590–602. [DOI] [PubMed] [Google Scholar]
- Park, B. S. , Kim, J. G. , Kim, M. R. , Lee, S. E. , Takeoka, G. R. , Oh, K. B. , & Kim, J. H. (2005). Curcuma longa L. constituents inhibit sortase a and Staphylococcus aureus cell adhesion to fibronectin. Journal of Agricultural and Food Chemistry, 53, 9005–9009. [DOI] [PubMed] [Google Scholar]
- Patil, V. V. , Bhangale, S. C. , & Patil, V. R. (2011). Studies on immunomodulatory activity of Ficus carica . International Journal of Pharmacy and Pharmaceutical Sciences, 2, 97–99. [Google Scholar]
- Patil, V. V. , & Patil, V. R. (2011). Evaluation of anti‐inflammatory activity of Ficus carica Linn leaves. Indian J Nat Prod Resour, 2, 151–155. [Google Scholar]
- Praditya, D. , Kirchhoff, L. , Brüning, J. , Rachmawati, H. , Steinmann, J. , & Steinmann, E. , et al. (2019). Anti‐infective properties of the Golden spice Curcumin. Frontiers in Microbiology, 10, 912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan, N. , Lam, K. W. , & Norhaizan, M. E. (2017). Molecular docking analysis of Carica papaya Linn constituents as antiviral agent. Int Food Res J, 24, 1819–1825. [Google Scholar]
- Rana, S. V. , Pal, R. , Vaiphei, K. , Sharma, S. K. , & Ola, R. P. (2011). Garlic in health and disease. Nutrition Research Reviews, 24, 60–71. [DOI] [PubMed] [Google Scholar]
- Raphael, T. J. , & Kuttan, G. (2003). Effect of naturally occurring triterpenoids glycyrrhizic acid, ursolic acid, oleanolic acid and nomilin on the immune system. Phytomedicine, 10, 483–489. [DOI] [PubMed] [Google Scholar]
- Rasne, A. , Sonwane, V. , Somani, R. , & Kumthekar, P. (2018). Evaluation of Immunomodulatory Activity of Protocatechuic Acid. J Res Notes, 1, 1007. [Google Scholar]
- Ravi, K. , & Divyashree, P. (2014). Psidium guajava: A review on its potential as an adjunct in treating periodontal disease. Pharmacogn Rev, 8, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawi, A. A. S. , AL Dulaimi HSH, & AL Rawi, M. A. A. (2019). Antiviral Activity of Mangifera Extract on Influenza Virus Cultivated in Different Cell Cultures. J Pure Appl Microbiol, 13, 455–458. [Google Scholar]
- Reygaert WC. 2018. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. BioMed Res Int 10.1155/2018/9105261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo, B. M. , & Banik, B. K. (2018). Medicinal plants: Source for immunosuppressive agents. Immunology: Current Research, 2, 106. [Google Scholar]
- Salehi, B. , Konovalov, D. A. , Fru, P. , Kapewangolo, P. , Peron, G. , Ksenija, M. S. , … Sharifi‐Rad, J. (2020). Areca catechu—From farm to food and biomedical applications. Phytotherapy Research, 1–19. 10.1002/ptr.6665 [DOI] [PubMed] [Google Scholar]
- Salehi, B. , Krochmal‐Marczak, B. , Skiba, D. , Patra, J. K. , Das, J. K. , Das, G. , … Martorell, M. (2019a). Convolvulus plant—A comprehensive review from phytochemical composition to pharmacy. Phytotherapy Research, 34, 315–328. [DOI] [PubMed] [Google Scholar]
- Salehi, B. , Upadhyay, S. , Orhan, I. K. , Jugran, A. K. , Jayaweera, S. L. D. , & Dias, D. A. (2019b). Therapeutic potential of α‐and β‐Pinene: A miracle gift of nature. Biomolecules, 9, 738. 10.3390/biom9110738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi, B. , Zakaria, Z. A. , Gyawali, R. , Ibrahim, S. A. , Rajkovic, J. , Shinwari, Z. K. , … Setzer, W. N. (2019c). Piper species: A comprehensive review on their Phytochemstry, biological activities and applications. Molecules, 24, 1364. 10.3390/molecules24071364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan, P. L. , Koul, J. L. , Koul, S. , Reddy, M. V. , Thota, N. , Khan, I. A. , … Qazi, G. N. (2008). Piperine analogs as potent Staphylococcus aureus NorA efflux pump inhibitors. Bioorganic & Medicinal Chemistry, 16, 9847–9857. [DOI] [PubMed] [Google Scholar]
- Seki, H. , Ohyama, K. , Sawai, S. , Mizutani, M. , Ohnishi, T. , Sudo, H. , … Muranaka, T. , et al. (2008). Licorice β‐amyrin 11‐oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proceedings of the National Academy of Sciences of the United States of America, 105, 14204–14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shailajan, S. , Menon, S. , Kulkarni, S. , & Tiwari, B. (2016). Standardized extract of Mangifera indica L. leaves as an antimycobacterial and immunomodulatory agent. Pharmacogn Commun, 6, 137–147. [Google Scholar]
- Sharifi‐Rad, M. , Roberts, T. H. , Matthews, K. R. , Bezerra, C. F. , Morais‐Braga, M. F. B. , Coutinho, H. D. M. , … Sharifi‐Rad, J. (2018). Ethnobotany of the genus Taraxacum—Phytochemicals and antimicrobial activity. Phytotherapy Research, 32, 2131–2145. [DOI] [PubMed] [Google Scholar]
- Sharifi‐Rad, J. , Melgar‐Lalanne, G. , Hernández‐Álvarez, A. J. , Taheri, Y. , Shaheen, S. , Kregiel, D. , … Martins, N. (2019). Malva species: Insights on its Chemical Composition towards Pharmacological Applications. Phytotherapy Research, 34, 564–567. [DOI] [PubMed] [Google Scholar]
- Shen, H. Y. , Jiang, J. G. , Li, M. Q. , Zheng, C. Y. , & Zhu, W. (2017). Structural characterization and immunomodulatory activity of novel polysaccharides from Citrus aurantium Linn. variant amara. Engl J Funct Foods, 35, 352–362. [Google Scholar]
- Shin, H. B. , Choi, M. S. , Minyi, C. , Lee, J. , Lee, N. J. , & Inn, K. S. (2015). Inhibition of respiratory syncytial virus replication and virus‐induced p38 kinase activity by berberine. International Immunopharmacology, 27, 65–68. [DOI] [PubMed] [Google Scholar]
- Singh, R. , Bagachi, A. , Semwal, A. , Kaur, S. , & Bhardwaj, A. (2013). Traditional uses, phytochemistry and pharmacology of Morus alba Linn: A review. J Med Plants Res, 7, 461–469. [Google Scholar]
- Srivastava, R. M. , Singh, S. , Dubey, S. K. , Misra, K. , & Khar, A. (2011). Immunomodulatory and therapeutic activity of curcumin. Int Immunopharmacol, 11, 331–341. [DOI] [PubMed] [Google Scholar]
- Sriwilaijaroen, N. , Fukumoto, S. , Kumagai, K. , Hiramatsu, H. , Odagiri, T. , Tashiro, M. , & Suzuki, Y. (2012). Antiviral effects of Psidium guajava Linn. (guava) tea on the growth of clinical isolated H1N1 viruses: its role in viral hemagglutination and neuraminidase inhibition. Antiviral Res, 94, 139–146. [DOI] [PubMed] [Google Scholar]
- Smirnov, V. S. , Zarubaev, V. V. , Anfimov, P. M. , & Shtro, A. A. (2012). Effect of a combination of glutamyl‐tryptophan and glycyrrhizic acid on the course of acute infection caused by influenza (H3H2) virus in mice. Voprosy Virusologii, 57, 7–23. [PubMed] [Google Scholar]
- Snafi‐Al, A. E. (2018). Glycyrrhiza glabra: A phytochemical and pharmacological review. IOSR Journal of Pharmacy (IOSRPHR), 8, 01–17. [Google Scholar]
- Somasundaram, S. , Edmund, N. A. , Moore, D. T. , Small, G. W. , Shi, Y. Y. , & Orlowski, R. Z. , et al. (2002). Dietary curcumin inhibits chemotherapy‐induced apoptosis in models of human breast cancer. Cancer Research, 62, 3868–3875. [PubMed] [Google Scholar]
- Sunila, E. S. , & Kuttan, G. (2004). Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J Ethnopharmacol, 90, 339–346. [DOI] [PubMed] [Google Scholar]
- Tang, W. M. , Chan, E. , Kwok, C. Y. , Lee, Y. K. , Wu, J. H. , Wan, C. W. , … Chan, S. W. (2012). A review of the anticancer and immunomodulatory effects of Lycium barbarum fruit. Inflammopharmacol, 20, 307–314. [DOI] [PubMed] [Google Scholar]
- Tattelman, E. (2005). Health effects of garlic. American Family Physician, 72, 103–106. [PubMed] [Google Scholar]
- Tripathi, D. M. , Gupta, N. , Lakshmi, V. , Saxena, K. C. , & Agrawal, A. K. (1999). Antigiardial and immunostimulatory effect of Piper longum on giardiasis due to Giardia lamblia. Phytother Res, 13, 561–565. [DOI] [PubMed] [Google Scholar]
- Wadood, A. , Ghufran, M. , Jamal, S. B. , Naeem, M. , Khan, A. , Ghaffar, R. , & Asnad. (2013). Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochemistry and Analytical Biochemistry, 2, 1–4. [Google Scholar]
- Walle T, Alston T, Browning A, Reed S, Walle UK. 2003. Effect of dietary flavonoids on oral cancer cell proliferation: bioactivation by saliva and antiproliferative mechanisms. Second Annual AACR International Conference Frontiers in Cancer Prevention Research, Phoenix, AZ.
- Wei, H. , Zhu, J. , Liu, X. Q. , Feng, W. H. , Wang, Z. M. , & Yan, L. H. (2016). Review of bioactive compounds from root barks of Morus plants (Sang‐Bai‐Pi) and their pharmacological effects. Cogent Chem, 2, 1212320. [Google Scholar]
- WHO . 2020. Pneumonia of unknown cause in China. Retrieved from https://www.who.int/csr/don/05‐january‐2020‐pneumonia‐of‐unkown‐cause‐china/en/
- Wu, Y. , Li, J. , Kim, Y. , Wu, J. , Wang, Q. , & Hao, Y. (2011). In Vivo and in Vitro Antiviral Effects of Berberine on Influenza Virus. Chin J Integr Med, 17, 444–452. [DOI] [PubMed] [Google Scholar]
- Xu, X. , Chen, P. , Wang, J. , Feng, J. , Zhou, H. , Li, X. , … Hao, P. (2020). Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Science China. Life Sciences, 63, 457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani, A. , Vahidinia, A. , & Ghannad, M. S. (2009). The effect of garlic consumption on TH1/TH2 cytokines in phytohemagglutinin (PHA) activated rat spleen lymphocytes. Phytotherapy Research, 23, 579–581. [DOI] [PubMed] [Google Scholar]
- Zhan, J. , Deng, R. , Tang, J. , Zhang, B. , Tang, Y. , Wang, J. K. , … Gu, J. (2006). The spleen as a target in severe acute respiratory syndrome. The FASEB Journal, 20, 2321–2328. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. H. , Isobe, K. , Nagase, F. , Terunuma, H. , Kasai, H. , Iwakula, Y. , … Ito, M. (1993). Glycyrrhizin as a promoter of the late signal transduction for interleukin‐2 production by splenic lymphocytes. Immunology, 79, 528–534. [PMC free article] [PubMed] [Google Scholar]
- Zhu, N. , Zhang, D. , Wang, W. , Li, X. , Yang, B. , Song, J. , … Tan, W. (2020). A novel coronavirus from patients with pneumonia in China, 2019. The New England Journal of Medicine, 382, 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvetkova, E. , Wirleitner, B. , Tram, N. T. , Schennach, H. , & Fuchs, D. (2001). Aqueous extracts of Crinum latifolium L. and Camellia sinensis show immunomodulatory properties in human peripheral blood mononuclear cells. International Immunopharmacology, 1, 2143–2150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


