Abstract
Objective
To determine whether earlier hospital discharge is feasible and safe in selected patients with subarachnoid hemorrhage (SAH) using an outpatient “fast-track” protocol.
Patients and Methods
We conducted a prospective quality improvement cohort study with the primary feasibility end point of patients with SAH deemed safe for discharge by treating team consensus. All patients received detailed education and outpatient transcranial Doppler monitoring; caregivers could contact the on-call team 24-7. Primary safety end points were adverse events after discharge and hospital readmission.
Results
From January 1, 2010, to January 1, 2015, our center had 377 SAH diagnoses, of which 200 were included in the final cohort, 36 qualifying for fast-track early discharge. The 30-day readmission rate for fast-track patients was 11.0% (4 of 36) compared with 11.4% (18 of 164) for non–fast-track patients. The rate of delayed cerebral ischemia and stroke was 3% (1 of 36) in the fast-track group vs 25.0% (41 of 164) for the non–fast-track group. Adverse events occurred in 11.0% (4 of 36) of the fast-track group compared with 26.0% (43 of 164) in the non–fast-track group. The mean length of stay was reduced 60% from 15 days to 6.6 days in the fast-track group.
Conclusion
Although our fast-track group was relatively small, data suggested early feasibility and safety in a carefully selected group of patients with SAH. Direct and indirect financial benefits of early discharge over a 5-year period were an estimated savings at least $864,000 in overall costs. A comparative effectiveness study is planned to replicate and validate these results using a larger multicenter design.
Abbreviations and Acronyms: DCI, delayed cerebral ischemia; ICU, intensive care unit; LOS, length of stay; MCA, middle cerebral artery; mFS, modified Fisher scale; QI, quality improvement; SAH, subarachnoid hemorrhage; TCD, transcranial Doppler ultrasonography; TDABC, time-driven activity-based cost; WFNS, World Federation of Neurological Surgeons
Aneurysmal subarachnoid hemorrhage (SAH), caused by a ruptured cerebral aneurysm, is a neurosurgical emergency that warrants transfer to a neurosurgical stroke center that can provide high-level care. Subarachnoid hemorrhage was associated with an historically high mortality rate of about 40% by 1 month in one study.1 It causes death and disability in 18,000 patients each year in North America, with annual costs in the billions.1
Standard of care management of SAH includes transfer of affected patients to a higher-level neurosurgical center that can endovascularly coil or neurosurgically clip the aneurysm to prevent rebleeding. Most patients are admitted to a neurologic intensive care unit (ICU) for close neuromonitoring and management. Vasospasm and delayed cerebral ischemia (DCI) are delayed neuroinflammatory states after SAH that peak 4 to 14 days after initial aneurysm rupture. As a result, most centers caring for these patients monitor them carefully, requiring prolonged hospital length of stay (LOS) of 14 to 21 days.2 Longer LOS leads to elevated health care costs for both patients and hospitals. Further, the cost of an ICU stay varies depending on the intensity of care but ranges from $1500 to $10,000 per day due to equipment and supply, bed and board, nursing and other personnel, medications, and mechanical ventilation costs.3 For example, a 14-day neurologic ICU stay can easily translate into an estimated cost of $42,000 (ie, $3000/d × 14). Therefore, to reduce overall hospital costs, we hypothesized that a selected group of patients with SAH could be discharged home with outpatient monitoring via transcranial Doppler ultrasonography (TCD) to identify cerebral vasospasm.2 We also believed there may be other indirect patient benefits from earlier hospital discharge and reduced LOS, such as lower nosocomial infection risk from Clostridium difficile colitis and methicillin-resistant Staphylococcus aureus, which would further lessen health care costs.4 Hospitals are under increasing pressure to avoid unnecessary patient harm while maintaining quality and lowering costs. Furthermore, Hospital Consumer Assessment of Healthcare Providers and Systems reporting adds more pressure and complexity to health care systems because these ratings are tied to reimbursement from postdischarge patient surveys, and, in theory, earlier discharge might improve patient satisfaction. Therefore, we hypothesized that earlier discharge (ie, fast track) in selected patients with SAH would be safe and feasible compared with other patients with SAH, with the potential to provide direct and indirect health economic benefits.
Patients and Methods
After institutional review board approval, we conducted a prospective, single-center quality improvement (QI) study at a Joint Commission–certified comprehensive stroke center at Mayo Clinic in Jacksonville, Florida. We reviewed the clinical, radiographic, and laboratory data of all patients with SAH and compared outcomes for the selected fast-track group vs the remainder of the SAH cohort (non–fast track). Our primary hypothesis was that fast track would be feasible and safe compared with other discharged patients with SAH by monitoring readmissions at 30 days and 1 year after discharge. Secondary end points included decrease of LOS by at least 50% in patients with SAH and exploratory economic cost savings and nosocomial infection reduction associated with earlier discharge.
Patient Selection
A consensus patient care protocol was created a priori by the departments of neurologic surgery, neurology, and critical care medicine to determine patient eligibility for SAH fast-track discharge because we could find no evidence of any other early discharge programs in the literature (see National Library of Medicine Search Engine Results section). Fast-track SAH consensus inclusion criteria included all patients with SAH (perimesencephalic and aneurysmal) who were monitored up to hospital day 7 by standard of care, had no evidence of active vasospasm clinically or by daily TCD, had no unstable or concerning medical issues that the medical or neurology attending physician believed would make discharge unsuitable or would lead to readmission, and had a caregiver willing to be with the patient for at least a week while the patient intermittently returned to get outpatient TCD monitoring as part of the protocol (Table 1). We excluded SAH from traumatic etiologies. Given the preliminary safety and feasibility of this model presented in 2011 by external reviewers and combined with internal QI review, our institutional review board deemed our study a minimal-risk QI project, with the assumption that safeguards would be tracked on a prospective basis by the comprehensive stroke center and principal investigators (C.I.C., W.D.F.).
Table 1.
Fast-Track SAH Consensus Criteria, Outpatient Safety Monitoring, Education, and Interventions
| Outpatient—safety and feasibility monitoring method | Goal and intervention |
|---|---|
| Fast-track discharge consensus criteria are met and agreed upon by medical and neurosurgical attending physicians days leading up to discharge | No medical/surgical issues or adverse events would prevent safe and early discharge No EVD or vasospasm by clinical and TCD/CTA criteria by day of discharge Available caregiver support for outpatient TCD appointments |
| Education: stroke, TIA packet, and symptom diary | Warning signs of neurologic deficits by patient or caregiver to bring patient to emergency department for immediate evaluation. Also, severity of headache and other deficits would be noted |
| TCD monitoring | Monday, Wednesday, and Friday schedule for TCD for monitoring vasospasm. Vascular neurologist would review and if abnormal, would trigger call to patient to check symptomatology or come into clinic or emergency department for evaluation |
| Fluid I/O monitoring | Measured fluid intake and outputs (urine), as well as daily weight logged into a diary as information for a medical professional if needed or symptoms emerged. No specific laboratory tests were part of the protocol |
| Neurology/neurosurgery team | All postdischarge care team contact information and business cards of the neurovascular surgeon, neurocritical care/vascular neurologist, APRN/PA, and nursing team would be provided in a folder for the patient, as well as discharge summary with anticipated follow-up visits and TCD appointment dates |
| 24-7 Telephone call availability to physician and cerebrovascular nursing | 24-7 Call line to on-call neurology team for patient/caregiver for any symptom clarification or needs (eg, prescription) Outpatient cerebrovascular nurse call line available for routine questions Monday through Friday, 8 am-5 pm |
ARNP/PA = advanced registered nurse practitioner/physician assistant; CTA = computed tomographic angiography; EVD = external ventricular drain; I/O = intake and output; SAH = subarachnoid hemorrhage; TCD = transcranial Doppler ultrasonography; TIA = transient ischemic attack.
Safety and Feasibility End Points
The primary feasibility end point was identification of a selected group of patients with SAH who could be discharged between day 7 and day 14 after SAH. Based on National Library of Medicine search engine methodology (see National Library of Medicine Search Engine Results section), we could not find any data on the safety of early discharge after SAH, and thus, it was critical to combine feasibility with safety end points. Primary safety end points were monitoring adverse events in the fast-track group, such as DCI or vasospasm, vs the non–fast-track group for similar event and 30-day readmission rates in both groups, since details that led to readmission would be used to refine the feasibility and safety of early discharge (eg, vasospasm and stroke, salt-wasting hyponatremia, dehydration).
Patient Protection
All patients with SAH were identified through the Mayo Clinic Comprehensive Stroke Center database from 2015. The International Classification of Diseases, Ninth Revision, Diagnosis-Related Group code 430 was used until the International Classification of Diseases, Tenth Revision code I60.9 was released.5 All adult patients with atraumatic SAH diagnosed from January 1, 2010, to January 1, 2015, were identified, and fast-track patients within the same time period were included in the study. Readmission rates were determined from data collected from the earliest patient to 1 year after the last patient (January 1, 2016) of the same study group. Patients who met inclusion criteria by day 7 after SAH were considered for fast-track discharge. Risks vs benefits of early discharge were discussed with all patients and caregivers using a provided safety checklist; discussion and consent were documented in the electronic medical record with other discharge materials. All patients who had SAH on initial computed tomography or imaging (Supplemental Figure A, available online at http://www.mcpiqojournal.org) but a negative result on initial cerebral angiography underwent a second formal cerebral angiography (Supplemental Figure C) as necessary before discharge to ensure there was no occult aneurysm, arteriovenous malformation, or severe vasospasm (Supplemental Figure B and C) to preclude discharge. Patients also required removal of external ventricular drain (Supplemental Figure E), if present, by the treating neurosurgeon at least 24 hours before any consideration of fast-track discharge.
TCD Monitoring Methods
Daily TCDs of the proximal cerebral circulation were performed to assess for vasospasm. Once consensus criteria were met for safe discharge, a caregiver (family member or medical surrogate) was required to stay with the patient near our hospital for at least 1 week to complete up to 3 outpatient TCDs, depending on the SAH on the day of discharge. For example, if a patient was discharged on SAH day 7, they would have 3 outpatient TCDs scheduled every other day (ie, Monday, Wednesday, Friday) as an outpatient. We used the same ultrasound machines (Sonosite M-Turbo, FUJIFILM SonoSite, Inc) for both inpatient and outpatient TCD, with US Food and Drug Administration–approved TCD software package6 and P21x transducer probe on the transtemporal window. We also used the same set of American College of Radiology–certified ultrasound technicians during our study.
Mayo Clinic TCD-defined vasospasm severity was classified as mild, moderate, and severe vasospasm by middle cerebral artery (MCA) M1 segment mean flow velocities of 120 cm/s, 150 cm/s, and 200 cm/s, respectively, with Lindegaard ratio values (ipsilateral MCA-M1/ internal carotid artery). Lindegaard ratios greater than 3 were considered legitimate vasospasm not due to hyperdynamic cardiac state (eg, elevated heart rate). The treating neurointensivist and neurosurgeon factored all clinical and TCD data information in the overall safety and consensus of safety for discharge of patients.
Outpatient Diary and Safety Monitoring
Before discharge, a safety checklist or education and discharge packet (Table 1) was given to the patient and caregiver by a certified stroke educator (L.H.M.) or delegate (neurologic ICU or neurosurgery advanced practice professional) on the day of discharge. The packet included a symptom diary to record and act upon signs and symptoms and a pocket stroke wallet card (Supplemental Figure F). Patients with SAH were required to maintain a stroke symptom diary and log of their intake and output of fluids. This diary was to be kept by the patient so that in the event a complication would arise, healthcare professionals would have ready access to this information. No specific laboratory tests were ordered as part of the protocol. Prescriptions of all required medications (eg, nimodipine) were filled before discharge to ensure that the patient had all medications required.
Primary and Secondary Outcomes
We measured LOS from admission to discharge and monitored readmissions and adverse events, such as vasospasm, by TCD (Supplemental Figure D). Readmission rates with diagnosis were used to identify patients who had to be readmitted for adverse effects of SAH or other medical conditions. The goal was a readmission rate in the fast-track group equivalent to that of the non–fast-track group. We evaluated cost savings by comparing costs of hospitalization (provided by our institution’s billing department) for the 2 study groups.
Statistical Analyses
Patient demographic data (age, sex) and SAH severity scale score for the fast-track and non–fast-track groups were analyzed using SAS statistical software, version 9.4 (SAS Institute). We utilized the Student t test for continuous variable comparison of age on outcomes, χ2 test for categorical variables, and rank sum test for LOS and modified Fisher scale (mFS) score and considered P≤.05 statistically significant.
Secondary Health Economic Analysis
Health economic modeling was based on a published average ICU cost per day, which can be as high as $3000,3,7 multiplied by the number of days saved in the fast-track group compared with the mean LOS to discharge in the remainder of the cohort. Time-driven activity-based cost (TDABC) modeling was also applied using Harvard Business School methodology8 to understand downstream economic benefits from earlier discharge, including reduced nursing, equipment, and other costs. Three investigators (W.D.F., B.L.T., R.S.K.) performed the health care economic mathematical modeling for effects of LOS reduction on financials. Estimated cost savings was extrapolated from 2 equations:
| (1) |
where the fast-track LOS (SAHft = 7 days [rounded up from 6.6]) was subtracted from the non–fast-track LOS (SAHnft = 15 days) to determine the change in LOS (LOSΔ = 8 days).
| (2) |
where the cost per day (CPD) was multiplied by LOSΔ multiplied by the total number of SAHft patients.
Results
Mayo Clinic Cohort: Fast-Track and Non–Fast-Track SAH Patients
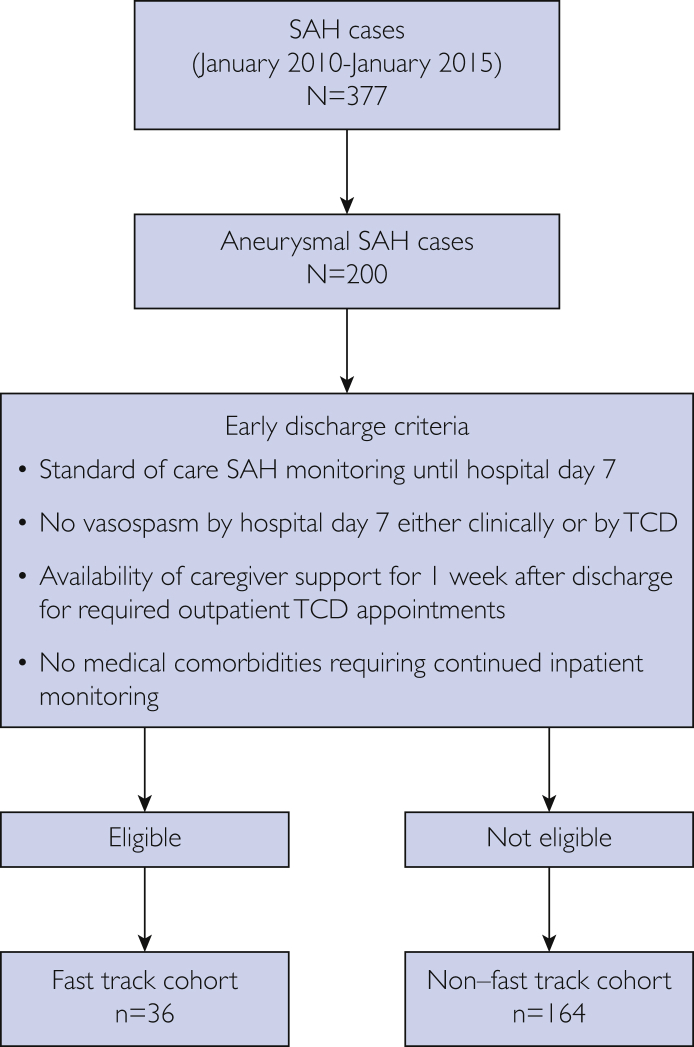
Over the 5-year study period, we identified 377 SAH cases at our center, but only 200 patients had SAH identified as being potentially aneurysmal or suspected aneurysmal in nature. Of these 200 patients, 36 (18.0%) met the consensus early discharge criteria (fast-track group) (Figure 1). The demographic and baseline characteristics for the fast-track and non–fast-track groups are summarized in Table 2. The mean ± SD age was 49.8±12.6 years in the fast-track group compared with 55.9±15.8 in the non–fast-track group. Of the 36 fast-track patients, 27 (75.0%) were men, yet the majority of patients in the non–fast-track group were women. The non–fast-track group had a higher mean ± SD mFS score of 3.6±0.8 compared with 2.7±0.9 in the fast-track group. There were no significant differences in rates of smoking (P=.92) and hyperlipidemia (P=.25), but hypertension was more common in the non–fast-track group (P=.004). World Federation of Neurological Surgeons (WFNS) grading did not exceed 2 in any fast-track patient. Of the 36 patients in the fast-track group, 5 (7%) received endovascular treatment of the aneurysm. Thirty-one cases (93%) were considered to be perimesencephalic SAH.
Figure 1.
Participant flowchart. SAH = subarachnoid hemorrhage; TCD = transcranial Doppler ultrasonography.
Table 2.
| Characteristic | Non–fast track (n=164) | Fast track (n=36) | Total (N=200) | P value |
|---|---|---|---|---|
| Age (y) | .03 | |||
| Mean ± SD | 55.9±15.8 | 49.8±12.6 | 54.8±15.5 | |
| Median (range) | 54.0 (18.0-99.0) | 52.0 (19.0-78.0) | 53.0 (18.0-99.0) | |
| IQR | 45.5-67.0 | 41.0-56.0 | 45.0-65.0 | |
| Sex | <.001 | |||
| Female | 98 (59.8) | 9 (25.0) | 107 (53.5) | |
| Male | 66 (40.2) | 27 (75.0) | 93 (46.5) | |
| Length of stay (d) | <.001 | |||
| Mean ± SD | 15.0±18.2 | 6.6±3.8 | 13.5±16.8 | |
| Median (range) | 14.0 (0.0-196.0) | 6.0 (2.0-17.0) | 10.5 (0.0-196.0) | |
| IQR | 5.5-19.0 | 3.0-8.0 | 5.0-18.0 | |
| Modified Fisher scale score | <.001 | |||
| Missing | 25 | 0 | 25 | |
| 0 | 2 (1.4) | 2 (5.6) | 4 (2.3) | |
| 1 | 4 (2.9) | 0 (0.0) | 4 (2.3) | |
| 2 | 6 (4.3) | 10 (27.8) | 16 (9.1) | |
| 3 | 30 (21.6) | 20 (55.6) | 50 (28.6) | |
| 4 | 97 (69.8) | 4 (11.1) | 101 (57.7) | |
| Mean ± SD | 3.6±0.8 | 2.7±0.9 | 3.4±0.9 | |
| Median (range) | 4.0 (0.0-4.0) | 3.0 (0.0-4.0) | 4.0 (0.0-4.0) | |
| IQR | 3.0-4.0 | 2.0-3.0 | 3.0-4.0 | |
| Smoker | .92 | |||
| Missing | 3 | 0 | 3 | |
| No | 124 (77.0) | 28 (77.8) | 152 (77.2) | |
| Yes | 37 (23.0) | 8 (22.2) | 45 (22.8) | |
| Hypertension | .004 | |||
| No | 89 (54.3) | 29 (80.6) | 118 (59.0) | |
| Yes | 75 (45.7) | 7 (19.4) | 82 (41.0) | |
| Hyperlipidemia | .25 | |||
| No | 144 (87.8) | 34 (94.4) | 178 (89.0) | |
| Yes | 20 (12.2) | 2 (5.6) | 22 (11.0) |
IQR = interquartile range.
Data are presented as No. (percentage) of patients unless indicated otherwise.
Safety and Adverse Event Monitoring
Safety data included readmission and adverse event rates. Readmission rate at 30 days for the non–fast-track group was 11.4% (18 of 164) compared with 11.0% in the fast-track group (Table 3). There were no readmissions among fast-track patients locally at 1 year after discharge, which may suggest a trend toward continued QI of the methods. Other safety monitoring measures included outpatient TCD evidence of vasospasm, follow-up imaging of DCI and stroke, and adverse effects from SAH. Two fast-track patients had MCA-M1 vasospasm (1 mild, 1 moderate) on TCD, but when the patients were called, they were completely asymptomatic; education was reiterated that if any focal symptoms developed, they were to come to emergency department immediately. These 2 patients never required emergency department visits and had no DCI or adverse events detected by TCD or radiography. The rate of vasospasm-related DCI and stroke was 25.0% (43 of 164) and 3% (1 of 36) in the non–fast-track and fast-track groups, respectively, but the non–fast-track group may have been inherently a sicker population. The rate of other adverse events, which in the fast-track group included lumbar radiculopathy, likely from blood in the caudal sac, and cerebral salt wasting presenting as headache in the emergency department, was 26.0% (43 of 164) and 11.0% (4 of 36) in the non–fast-track and fast-track groups, respectively. These adverse events were likely somewhat attributable to education as radiculopathy can be a common symptom of SAH and another patient was under the impression that he had to drink excessive amounts of water only to stay hydrated. One patient was lost to follow-up. Of the remainder of patients, all complied with the outpatient TCD appointments per the protocol. Fluid diary compliance was not tracked, although the fast-track patient who was seen in the emergency department for hyponatremia did comply with the diary, which was available for health care provider review.
Table 3.
Readmission Rates at 30 Days and Adverse Events
| Variable | Non–fast track | Fast track |
|---|---|---|
| 30-Day readmission rates | 11.4% (18 of 164) | 11.0% (4 of 36) |
| Adverse events | Vasospasm-related DCI (25% [41 of 164]) Cerebral salt wasting (26% [41 of 164]) |
Vasospasm-related DCI (3% [1 of 36]) Cerebral salt wasting (11% [4 of 36]) Lumbar radiculopathy (3% [1 of 36]) |
DCI = delayed cerebral ischemia.
Secondary Health Economic Data
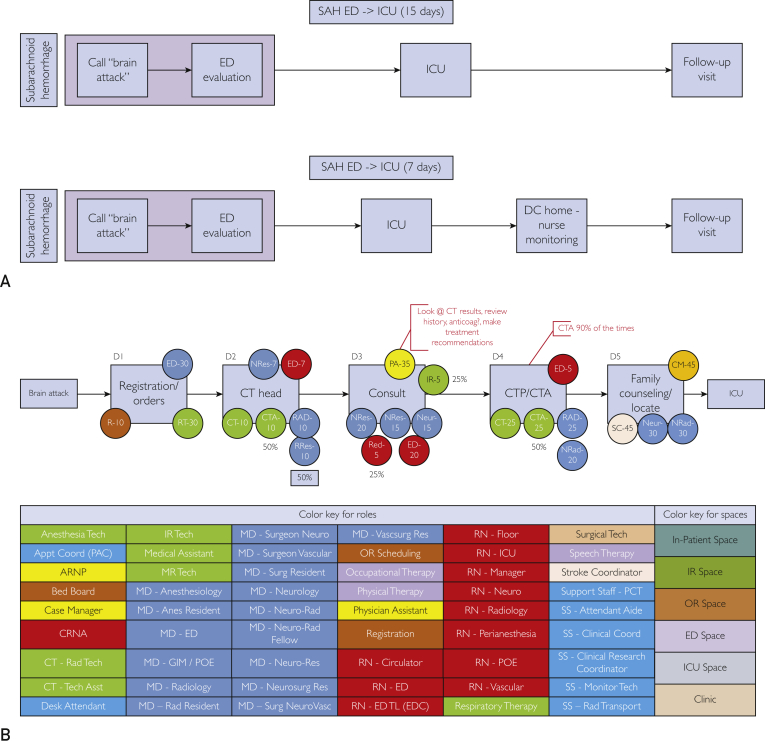
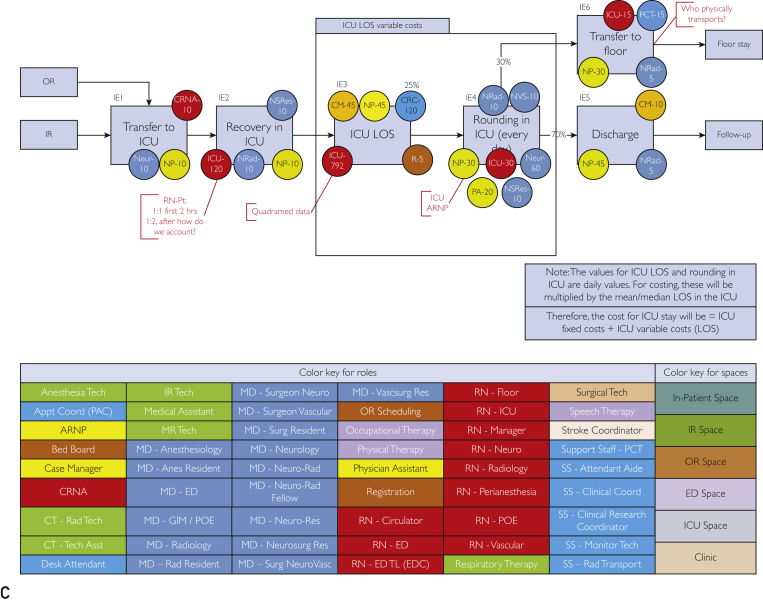
The mean ± SD LOS for the non–fast-track group was 15±18.2 days. The fast-track group had a much shorter mean ± SD LOS of 6.6±3.8 days, reducing LOS by approximately 60% compared with the non–fast-track group. The estimated cost savings was extrapolated from 2 equations where the fast track LOS (SAHft =7 days [rounded up from 6.6]) was subtracted from the non–fast-track LOS (SAHnft = 15 days) to determine the change in LOS (LOSΔ = 8 days) where the cost per day (CPD) was multiplied by LOSΔ multiplied by the total number of SAHft patients (n=36). The result was an $864,000 estimated total savings over 5 years. The TDABC maps are shown in Figure 2. The TDABC method accounts for costs along each time point. In the SAHft group, we did not include overhead costs of the nursing staff because the call volume was relatively low and did not require additional incremental resources or nurse staffing.
Figure 2.
Time-driven activity-based cost maps for standard subarachnoid hemorrhage non–fast-track and fast-track groups and costs along the different models. A, Pathway patient takes from time of entry into the facility until discharge. B, Flow of the emergency department (ED) evaluation, the staffing assigned, and average time they spend doing the task for each of the processes. Color key is located at the bottom to show the positions, as well as the spaces within the facility where these activities occur. C, Flow of intensive care unit (ICU) stay from point of entry until discharge. Staffing assigned and time spent doing that task for each of the processes is identified by the color circles attached to each task. anes = anesthesiology; anticoag = anticoagulation; appt = appointment; asst = assistant; ARNP = advanced registered nurse practitioner; CM = case manager; coord = coordinator; CRC = clinical research coordinator; CRNA = certified registered nurse anesthetist; CT = computed tomography; CTA = computed tomographic angiography; CTP = computed tomographic perfusion; D = day; DC = discharge; EDC = emergency discharge; GIM = general internal medicine; IR = interventional radiology; LOS = length of stay; MD = medical doctor; MR = magnetic resonance imaging; Neur/neuro = neurology; neurosurg = neurosurgery; NeuroVasc = neurovascular; NP = nurse practioner; NRad = neuroradiologist; NRes = neurology resident; NSRes = neurosurgery resident; NVS = neurovascular surgeon; OR = operating room; OT = occupational therapy; PA = physician assistant; PCT = patient care technician; POE = postoperative evaluation; PT = physical therapy; R = radiologist; RAD/rad = radiology; res = resident; RN = registered nurse; RRes = radiology resident; RT = respiratory therapy; SAH = subarachnoid hemorrhage; SS = clinical coodinator; surg = surgery; tech = technologist; TL = team leader; vascsurg = vascular surgery.
National Library of Medicine Search Engine Results
Using the search term aneurysmal subarachnoid hemorrhage resulted in 27,718 publications; when early discharge was added to the search, we received 150 results (0.5%). However, we did not find a relevant article related to an early discharge home strategy in patients with SAH. One article referenced an average ICU LOS of 12.7 days and total hospital LOS of 17 days but not for an early discharge strategy.9 The search term combination aneurysmal subarachnoid hemorrhage and fast track produced only one article about the emotional and psychological well-being of patients with SAH after discharge, unrelated to early discharge strategy.10 To be sure that some SAH cases were not being discharged early because of perimesencephalic SAH pattern, we searched for this term and subarachnoid hemorrhage, which yielded 324 hits, and when combined with early discharge only 2 hits returned, none of which were relevant to early SAH discharge strategy. Another article noted that insurance status was often a determinant of discharge status and ability but did not compare early discharge with current standard and incorporated all stroke patient discharges.11 One article focused on the economic cost savings of early aneurysm treatment compared with delayed treatment.12 A study by Alaraj et al13 investigating reduction of LOS and costs associated with SAH found that LOS has been declining from about 21 days to about 14 days, with similar reduction in costs. These investigators did not mention early discharge but did suggest that a physician-led daily huddle might help reduce LOS.13
Searching aneurysmal subarachnoid hemorrhage and readmission yielded 34 hits, with only 1 relevant article about creation of a step-down unit for patients with SAH; however, this step only shortened LOS by 2 days.14 Searching aneurysmal subarachnoid hemorrhage and safety yielded 710 hits, but combining with early discharge produced only 13 results unrelated to early discharge strategy. Therefore, the sensitivity, specificity, and precision for detecting any relevant articles related to any previous SAH early discharge strategy was close to 0%, suggesting that our study is the first to prospectively evaluate this process.
Discussion
To our knowledge, ours is the first prospective analysis of the potential safety and feasibility of an early discharge strategy in patients with SAH, a complex patient population typically admitted to the ICU, which is a high-cost setting. Our study’s main limitations are the small size of the fast-track group and potential selection bias toward that population, which had lower WFNS grades (SAHnft mean = 4.5 vs SAHft mean = 1). However, with SAH, there is a distribution of so-called good (WFNS 0-3) and poor (WFNS 4-5) clinical grades, and initial WFNS grade is predictive of LOS and ultimate clinical outcome.15 This should not negate the importance of our work in shortening LOS in SAH, which has not been done before, and so dramatically (6.6 vs 15 days), with considerable reduction in overall patient costs. Another limitation includes a priori patient selection for this study. As the first study to discharge these carefully selected SAH patients, no typical selection criteria existed. As this important work is validated in the future in multiple sites, more rigorous criteria can be established. For example, patient inclusion criteria could also include Fisher or WFNS grading or necessity of external ventricular drain placement. We did, however, include all patients who could participate in the protocol with similar results in readmission data. The vast majority of fast-track patients had a perimesencephalic SAH pattern, but interestingly, most had a mFS score of 3. Despite this severity score, and without stricter inclusion/exclusion criteria, we were able to discharge these patients faster and without increased readmission rates. Strengths of our project include our preliminary safety and feasibility data supporting that some patients with SAH can be discharged almost a week earlier, with similar rates of readmission and adverse events after discharge. Further, “bending down the cost curve” in health care is extremely challenging, especially in sick and complicated ICU patients, yet this preliminary work reveals feasibility when a carefully selected and consensus protocol is applied with an overarching plan to distribute care to the outpatient continuum with adequate caregiver support. It is interesting to note that the overwhelming majority of fast-track patients were male. There was no lack of caregiver support for women who may have qualified for fast-track status. Further studies could expound upon sex differences in these groups to perhaps provide a physiologic explanation.
We acknowledge that not all health systems can assemble a collection of outpatient resources and teams required (outpatient TCD) to carefully follow up an SAH patient population. However, the size of ultrasound devices is decreasing due to the Moore law; we believe that many of the strategies we employed could be adopted and diffused to other hospitals in the United States, and even globally, using telemedicine and mobile health strategies.
There are tremendous financial implications of scaling our fast-track model to other hospitals and across the US health care systems. American health care expenditures are currently estimated at $2.3 trillion and are increasing because of an aging population with more comorbidities.16 The costs of SAH alone are estimated at between $1.7 billion and $5.6 billion,1,17 and individual patient costs are estimated at $228,030, almost double the cost for patients with intracerebral hemorrhage or ischemic stroke.17 According to the Brain Aneurysm Foundation, approximately 30,000 patients suffer SAH each year, 18.0% (5400) of whom, per our results, would qualify for early discharge, with a potential financial savings of $216 million ($864,000/36 = $24,000 per patient). We realize that not all patients with SAH are eligible for the fast-track strategy; however, even using our conservative estimate of 18.0% of 30,000 SAH cases nationally, and applying similar ICU LOS cost savings ($3000/d), 8 days saved would equate to a $158,400,000 cost savings for the SAH patients in the US health care system. Recently, Stelfox et al18 reported similar ICU discharge to home safety and feasibility among a large cohort of 6732 ICU patients in a multicenter Canadian ICU system. They reported discharge from the ICU to home in 922 patients (13.7%) that was feasible and relatively safe for general medical conditions, with similar readmission rates.18
Our center is currently exploring a multicenter comparative effectiveness study to scale this model to other hospitals using similar inclusion and safety monitoring criteria to validate our preliminary data. In the future, we are also considering creation of an “Ultra–Fast-Track” SAH model with a potential discharge time of less than 7 days by using the same care process but focused on individualizing or using precision medicine for those patients with low mFS (1-2), who are at even lower risk for DCI and vasospasm and have no cerebrospinal fluid diversion requirements.
Conclusion
An early fast-track discharge strategy appears feasible and relatively safe in a selected group of patients with SAH compared with a larger SAH cohort when outpatient multidisciplinary monitoring is implemented. Given the financial implications for health care for patients with SAH nationally, a larger comparative effectiveness study is being planned.
Acknowledgments
We thank Ms Carol Raper, Mayo Clinic, Quality Management Services, Jacksonville, FL, and the Mayo Clinic ultrasonography technicians who performed the outpatient imaging for our patients.
Footnotes
Dr Hasan is now with the Department of Neurology, Ochsner Louisiana State University Health Sciences Center, Shreveport, LA.
Grant Support: This study was supported in part by the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Potential Competing Interests: The authors report no competing interests.
Supplemental material can be found online at http://www.mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
Supplemental Online Material
Vasospasm is a Delayed, Inflammatory Arterial Narrowing that Peaks Between Days 4 and 12 After SAH.8 A, Head CT with diffuse SAH pattern of blood or white areas in the basal cistern. B, Aneurysmal rupture of an MCA aneurysm followed by arterial narrowing or vasospasm (insert). Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved. C, Cerebral angiogram in patient with MCA vasospasm. D, TCD measured mean flow velocity. CT = computed tomography; EDV = end-diastolic velocity; MCA = middle cerebral artery; MI = middle cerebral artery first segment; P21 = probe type; PI = pulsatility index; PSV = peak systolic velocity; PW = pulse wave; RI = resistance index; SAH = subarachnoid hemorrhage; S/D = standard deviation; TAP = time average peak (similar to mean flow); TCD = transcranial Doppler. E, External Ventricular Drain Method of Monitoring Intracranial Pressure and Drainage of Cerebrospinal Fluid. Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved. F, Mayo Clinic Education Stroke Wallet Card. Provided at discharge with other educational material, intake and output, weight, discharge follow-up, and team contact information. This wallet card is a decision aid tool for all stroke patients and caregivers for warning signs, similar to National Institutes of Health heart attack warning wallet card,9 which gets patients to the emergency department faster and helps communicate information rapidly, especially for unresponsive or noncommunicative patients, and to assist caregivers from our Knowledge Evaluation Research Unit.10 Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.
References
- 1.Mukhtar T.K., Molyneux A.J., Hall N., et al. The falling rates of hospital admission, case fatality, and population-based mortality for subarachnoid hemorrhage in England, 1999-2010. J Neurosurg. 2016;125(3):698–704. doi: 10.3171/2015.5.JNS142115. [DOI] [PubMed] [Google Scholar]
- 2.Diringer M.N., Bleck T.P., Claude Hemphill J., III, et al. Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society's multidisciplinary consensus conference. Neurocrit Care. 2011;15(2):211–240. doi: 10.1007/s12028-011-9605-9. [DOI] [PubMed] [Google Scholar]
- 3.Dasta J.F., McLaughlin T.P., Mody S.H., Piech C.T. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 4.Dasenbrock H.H., Rudy R.F., Smith T.R., et al. Hospital-acquired infections after aneurysmal subarachnoid hemorrhage: a nationwide analysis. World Neurosurg. 2016;88:459–474. doi: 10.1016/j.wneu.2015.10.054. [DOI] [PubMed] [Google Scholar]
- 5.2020 ICD-10-CM Diagnosis Code I60.9: nontraumatic subarachnoid hemorrhage, unspecified. ICD10Data.com website. https://www.icd10data.com/ICD10CM/Codes/I00-I99/I60-I69/I60-/I60.9 Published 2020. Accessed May 7, 2020.
- 6.510(k) Submission for SonoSite Ultrasound Systems. https://www.accessdata.fda.gov/cdrh_docs/pdf10/K101757.pdf Published 2010. Accessed September 13, 2018.
- 7.Rappleye E. Average cost per inpatient day across 50 states. Becker's Healthcare website. https://www.beckershospitalreview.com/finance/average-cost-per-inpatient-day-across-50-states.html Published May 19, 2015. Accessed September 13, 2018.
- 8.Kaplan R.S., Anderson S.R. Time-driven activity-based costing. Harvard Business Review website. https://hbr.org/2004/11/time-driven-activity-based-costing Published November 2004. Accessed September 13, 2018. [PubMed]
- 9.Yu W., Kavi T., Majic T., et al. Treatment modality and quality benchmarks of aneurysmal subarachnoid hemorrhage at a comprehensive stroke center. Front Neurol. 2018;9:152. doi: 10.3389/fneur.2018.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarvis A., Talbot L. Multiprofessional follow up of patients after subarachnoid haemorrhage. Br J Nurs. 2004;13(21):1262–1267. doi: 10.12968/bjon.2004.13.21.17117. [DOI] [PubMed] [Google Scholar]
- 11.Cho J.S., Hu Z., Fell N., Heath G.W., Qayyum R., Sartipi M. Hospital discharge disposition of stroke patients in Tennessee. South Med J. 2017;110(9):594–600. doi: 10.14423/SMJ.0000000000000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonig A., Shallwani H., Natarajan S.K., et al. Better outcomes and reduced hospitalization cost are associated with ultra-early treatment of ruptured intracranial aneurysms: a US nationwide data sample study. Neurosurgery. 2018;82(4):497–505. doi: 10.1093/neuros/nyx241. [DOI] [PubMed] [Google Scholar]
- 13.Alaraj A., Hussein A.E., Esfahani D.R., Amin-Hanjani S., Aletich V.A., Charbel F.T. Reducing length of stay in aneurysmal subarachnoid hemorrhage: a three year institutional experience. J Clin Neurosci. 2017;42:66–70. doi: 10.1016/j.jocn.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 14.Chartrain A.G., Awad A.J., Sarkiss C.A., et al. A step-down unit transfer protocol for low-risk aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2017;43(5):E15. doi: 10.3171/2017.8.FOCUS17448. [DOI] [PubMed] [Google Scholar]
- 15.Rosen D.S., Macdonald R.L. Subarachnoid hemorrhage grading scales: a systematic review. Neurocrit Care. 2005;2(2):110–118. doi: 10.1385/NCC:2:2:110. [DOI] [PubMed] [Google Scholar]
- 16.US health care expenditures [search results]. WolframAlpha website. http://www.wolframalpha.com/input/?i=us+health+care+expenditures
- 17.Taylor T.N., Davis P.H., Torner J.C., Holmes J., Meyer J.W., Jacobson M.F. Lifetime cost of stroke in the United States. Stroke. 1996;27(9):1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 18.Stelfox H.T., Soo A., Niven D.J., et al. Assessment of the safety of discharging select patients directly home from the intensive care unit: a multicenter population-based cohort study. JAMA Intern Med. 2018;178(10):1390–1399. doi: 10.1001/jamainternmed.2018.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vasospasm is a Delayed, Inflammatory Arterial Narrowing that Peaks Between Days 4 and 12 After SAH.8 A, Head CT with diffuse SAH pattern of blood or white areas in the basal cistern. B, Aneurysmal rupture of an MCA aneurysm followed by arterial narrowing or vasospasm (insert). Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved. C, Cerebral angiogram in patient with MCA vasospasm. D, TCD measured mean flow velocity. CT = computed tomography; EDV = end-diastolic velocity; MCA = middle cerebral artery; MI = middle cerebral artery first segment; P21 = probe type; PI = pulsatility index; PSV = peak systolic velocity; PW = pulse wave; RI = resistance index; SAH = subarachnoid hemorrhage; S/D = standard deviation; TAP = time average peak (similar to mean flow); TCD = transcranial Doppler. E, External Ventricular Drain Method of Monitoring Intracranial Pressure and Drainage of Cerebrospinal Fluid. Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved. F, Mayo Clinic Education Stroke Wallet Card. Provided at discharge with other educational material, intake and output, weight, discharge follow-up, and team contact information. This wallet card is a decision aid tool for all stroke patients and caregivers for warning signs, similar to National Institutes of Health heart attack warning wallet card,9 which gets patients to the emergency department faster and helps communicate information rapidly, especially for unresponsive or noncommunicative patients, and to assist caregivers from our Knowledge Evaluation Research Unit.10 Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.