Summary
Leukocyte common antigen-related receptor tyrosine phosphatases (LAR-RPTPs) are evolutionarily conserved presynaptic organizers. The synaptic role of vertebrate LAR-RPTPs in vivo, however, remains unclear. In the current study, we analyzed the synaptic role of PTPσ using newly generated, single conditional knockout (cKO) mice targeting PTPσ. We found that the number of synapses was reduced in PTPσ cKO cultured neurons in association with impaired excitatory synaptic transmission, abnormal vesicle localization, and abnormal synaptic ultrastructure. Strikingly, loss of presynaptic PTPσ reduced neurotransmitter release prominently at excitatory synapses, concomitant with drastic reductions in excitatory innervations onto postsynaptic target areas in vivo. Furthermore, loss of presynaptic PTPσ in hippocampal CA1 pyramidal neurons had no impact on postsynaptic glutamate receptor responses in subicular pyramidal neurons. Postsynaptic PTPσ deletion had no effect on excitatory synaptic strength. Taken together, these results demonstrate that PTPσ is a bona fide presynaptic adhesion molecule that controls neurotransmitter release and excitatory inputs.
Subject Areas: Biological Sciences, Molecular Neuroscience, Cellular Neuroscience
Graphical Abstract
Highlights
-
•
Conditional PTPσ KO produces specifically impaired presynaptic functions
-
•
Presynaptic PTPσ regulates glutamate release efficiency
-
•
Presynaptic PTPσ does not transsynaptically regulate postsynaptic receptor responses
Biological Sciences; Molecular Neuroscience; Cellular Neuroscience
Introduction
Distinct molecular assemblies at presynaptic nerve terminals and postsynaptic densities are responsible for the fast and precise transmission of neural information (Südhof, 2018). These structures act by coordinating the regulation of bidirectional signals across synaptic clefts, determining the properties of individual synapses, including the computation of neural information (Südhof, 2018). Several synaptic cell-adhesion molecules are thought to act not only as physical connectors across synaptic clefts but also as trans-synaptic signaling hubs (Missler et al., 2012, Südhof, 2017, Südhof, 2018).
Leukocyte common antigen-related receptor tyrosine phosphatases (LAR-RPTPs) are evolutionarily conserved key synaptic organizers expressed in presynaptic active zones (AZs) (Han et al., 2019, Südhof, 2012, Um and Ko, 2013). Invertebrate LAR-RPTP orthologs (dLAR in Drosophila melanogaster and PTP-3 in Caenorhabditis elegans) were shown to be expressed in axons/growth cones, playing critical roles in axon guidance, dendritic growth, and synapse formation (Ackley et al., 2005, Chagnon et al., 2004, Han et al., 2019). In contrast, vertebrate LAR-RPTPs, consisting of three members (LAR, PTPσ, and PTPδ), are present in both dendritic spines and axons of cultured neurons (Han et al., 2018, Takahashi et al., 2012, Wyszynski et al., 2002). Analogous to Nrxns, LAR-RPTPs bind postsynaptic ligands, which do not overlap with Nrxn ligands, to induce presynaptic differentiation (Bomkamp et al., 2019, Choi et al., 2016, Han et al., 2018, Li et al., 2015, Takahashi et al., 2011, Yim et al., 2013, Yoshida et al., 2011). Constitutive KO mice of individual or multiple LAR-RPTP exhibit pleiotropic abnormalities in both the peripheral and central nervous systems and impairment in certain aspects of synapse development and function (Elchebly et al., 1999, Horn et al., 2012, McLean et al., 2002, Thompson et al., 2003, Uetani et al., 2000, Uetani et al., 2006, Wallace et al., 1999). In contrast, loss of PTPσ and/or PTPδ, using short-hairpin-mediated knockdown (KD)-mediated manipulations, impairs structural and functional development, as well as the LAR-RPTP ligand-induced formation of artificial synapses (Bomkamp et al., 2019, Dunah et al., 2005, Han et al., 2018, Takahashi et al., 2012, Yim et al., 2013). Intriguingly, PTPσ and PTPδ serve as functional receptors for presynaptic assembly at specific synapse types (i.e., PTPσ for excitatory synapses, such as Slitrks, TrkC, and SALMs, and PTPδ for inhibitory synapses, such as Slitrk3, and excitatory synapses, such as IL1RAPL1) (Choi et al., 2016, Han et al., 2018, Li et al., 2015, Takahashi et al., 2012, Valnegri et al., 2011, Yim et al., 2013, Yoshida et al., 2011). Although these findings clearly indicated that LAR-RPTPs may be central components in both pre- and postsynaptic neurons that organize various aspects of synapse development, a sophisticated approach using conditional knockout (cKO) deficient in LAR-RPTPs is required to precisely assess the synaptic role of vertebrate LAR-RPTPs in vivo. A recent study showed that all three LAR-RPTPs regulate postsynaptic responses mediated by N-methyl-D-aspartate receptors (NMDA-type glutamate receptors) through trans-synaptic mechanism(s), but this study did not examine the presynaptic role of individual LAR-RPTPs (Sclip and Südhof, 2020).
In the present study, we generated mutant mice carrying PTPσ cKO alleles. We found that, in keeping with the KD effects (Han et al., 2018), conditional genetic deletions of PTPσ specifically impaired excitatory synaptic transmission. Moreover, deletion of PTPσ resulted in an abnormal vesicular organization in presynaptic boutons. Furthermore, PTPσ loss from hippocampal CA1 pyramidal neurons selectively impaired innervation and neurotransmitter release at excitatory, but not inhibitory, synapses formed on subicular pyramidal neurons. Strikingly, loss of presynaptic PTPσ in hippocampal CA1 neurons did not alter postsynaptic glutamate receptor-mediated responses in subicular neurons. These results suggest that PTPσ is essential for the regulation of presynaptic functions in vivo, distinct from the roles of presynaptic Nrxns.
Results
Generation of PTPσ cKO Mice
Previous studies employing constitutive KO mice have precluded investigations of the mechanisms of action of LAR-RPTPs because of the pleiotropic phenotypes that are likely unrelated to their synaptic roles (Um and Ko, 2013). Transgenic mice with deletion of PTPσ were generated by crossing PTPσf/f mice, with exon 4 flanked by loxP sites with a Cre recombinase driver line under control of the Nestin promoter (Nestin-Cre) (Figure S1). RNAscope-based fluorescence in situ hybridization showed that the expression patterns of mRNAs encoding all three LAR-RPTP family members overlap in both the mPFC and the hippocampus (Figure S2A). PTPσ and PTPδ mRNAs were detected in CaMKIIα-positive glutamatergic neurons and in Gad1-positive GABAergic neurons of adult mouse brains (Figures S2B and S2C).
To evaluate the cellular effects of endogenous PTPσ deletions, hippocampal neurons were cultured from PTPσ cKO mice. Neurons cultured for 3–4 days in vitro (DIV) were infected with lentiviruses expressing EGFP-fused nuclear Cre recombinase, which results in a global loss of PTPσ in all neurons due to high infection efficiency, or with a non-functional mutant version of Cre recombinase (ΔCre). Global expression of Cre recombinase caused a specific and nearly complete loss of PTPσ mRNA expression and completely eliminated PTPσ protein expression in PTPσ-cKO neurons analyzed at DIV13–14 (Figures S3A–S3C). Complete elimination of PTPσ protein was also confirmed by immunoblot analyses of lysates of PTPσ-cKO brains (Figure S3D). cKO mice in which PTPσ was deleted from the entire brain were viable and fertile, although a modest reduction of body size was observed (Figure S3E). In addition, PTPσ-cKO brains showed normal gross morphology, as revealed by staining for the neuron-specific marker NeuN (Figure S4A) and for Nissl (Figure S4B). Quantitative immunoblot analysis of PTPσ-deficient brains showed comparable expression of presynaptic AZ and postsynaptic density proteins (Figures S5A and S5B).
Conditional PTPσ KO Reduces the Number of Excitatory Synapses
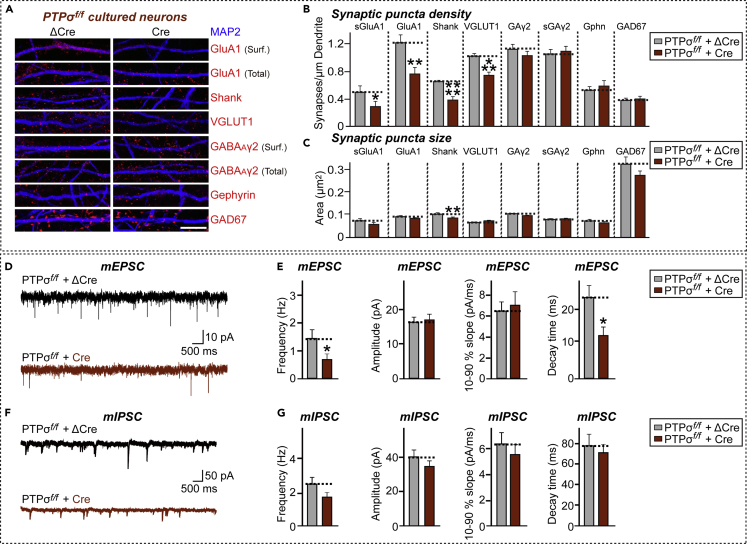
To assess the synaptic role of PTPσ, cultured hippocampal PTPσ-cKO neurons were infected with lentiviruses expressing either ΔCre (Control) or wild-type Cre recombinase at DIV3–4 and the neurons were stained with antibodies to various excitatory and inhibitory synaptic markers at DIV14–16 (Figures 1A–1C). The density of excitatory, but not of inhibitory, synaptic puncta was significantly reduced, as measured by staining of PTPσ-deficient neurons with antibodies to GluA1 (both surface and total), pan-Shank, and VGLUT1 (∼30%–40%) (Figures 1A and 1B). There were no marked changes in the density of inhibitory synaptic puncta on PTPσ KO neurons (Figures 1A and 1B). Moreover, measurements of the apparent sizes of synaptic puncta, reflecting a combination of antigen concentration and true synapse size, showed a small but significant reduction in the sizes of pan-Shank+ puncta on PTPσ-deficient neurons (Figures 1A and 1C). These results are consistent with the previously reported PTPσ KD effect (Han et al., 2018).
Figure 1.
Conditional KO of PTPσ Impairs Excitatory Synapse Development and Transmission in Cultured Hippocampal Neurons
(A) PTPσ cKO in cultured hippocampal neurons specifically reduces excitatory synapse density. Double immunofluorescence analysis of MAP2 (blue) and the indicated synaptic markers (red) in mature cultured neurons (DIV14) derived from PTPσf/f mice infected with lentiviruses expressing ΔCre or Cre at DIV3. Synaptic markers assayed included surface GluA1 (sGluA1), total GluA1, Shank, and VGLUT1 as excitatory synaptic markers, and surface GABAAγ2 (sGAγ2), total GABAAγ2, Gephyrin (Gphn), and GAD67 as inhibitory synaptic markers. Scale bar: 10 μm.
(B and C) Quantification of images in (A), measuring the density (B) and area (C) of the indicated synaptic marker puncta. Data are means ± SEMs (n denotes number of analyzed neurons; ΔCre/PTPσ cKO/sGluA1, n = 16; Cre/PTPσ cKO/sGluA1, n = 17; ΔCre/PTPσ cKO/GluA1, n = 16; Cre/PTPσ cKO/GluA1, n = 15; ΔCre/PTPσ cKO/Shank, n = 16; Cre/PTPσ cKO/Shank, n = 16; ΔCre/PTPσ cKO/VGLUT1, n = 15; Cre/PTPσ cKO/VGLUT1, n = 16; ΔCre/PTPσ cKO/sGABAARγ2, n = 15; Cre/PTPσ cKO/sGABAARγ2, n = 15; ΔCre/PTPσ cKO/GABAARγ2, n = 16; Cre/PTPσ cKO/GABAARγ2, n = 16; ΔCre/PTPσ cKO/Gephyrin, n = 16; Cre/PTPσ cKO/Gephyrin, n = 15; ΔCre/PTPσ cKO/GAD67, n = 16; and Cre/PTPσ cKO/GAD67, n = 16. Mann-Whitney U test; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001).
(D and E) Representative mEPSC traces (D) and quantification of frequencies, amplitudes, and kinetics (E) of mEPSCs recorded from hippocampal cultured neurons derived from PTPσf/f mice infected with lentiviruses expressing inactive (ΔCre) or active (Cre) Cre recombinase. Data are means ± SEMs (n denotes number of analyzed neurons; ΔCre, 20 and Cre, 15; unpaired t test; ∗p < 0.05).
(F and G) Representative mIPSC traces (F) and quantification of frequencies, amplitudes, and kinetics (G) of mIPSCs recorded from hippocampal cultured neurons derived from PTPσf/f mice infected with lentiviruses expressing ΔCre or Cre. Data are means ± SEMs (n denotes number of analyzed neurons; ΔCre, 18 and Cre, 17; unpaired t test)
Conditional PTPσ KO Impairs Excitatory Synaptic Transmission
To examine whether the reduced number of synapses in PTPσ-deficient neurons were accompanied by corresponding effects on the transmission of respective synapse types, hippocampal dissociated cultured neurons were assessed electrophysiologically (Figures 1D–1G). Lentivirus-mediated global loss of PTPσ specifically reduced the frequency (but not amplitude) of excitatory, but not inhibitory, synaptic transmission, as shown by measurement of miniature excitatory postsynaptic currents (mEPSCs) and miniature inhibitory postsynaptic currents (mIPSCs) (Figures 1E and 1G). These results are consistent with the PTPσ KD effect on excitatory synaptic transmission (Dunah et al., 2005, Han et al., 2018, Ko et al., 2015). Notably, PTPσ KO induced a significant decrease in mEPSC decay time (peak to 10%), implying a change in subunit composition of AMPA-type glutamate receptors (Jonas, 2000).
Conditional KO of PTPσ Reduces Synaptic Localization of Excitatory Synaptic Vesicles in Presynaptic Boutons
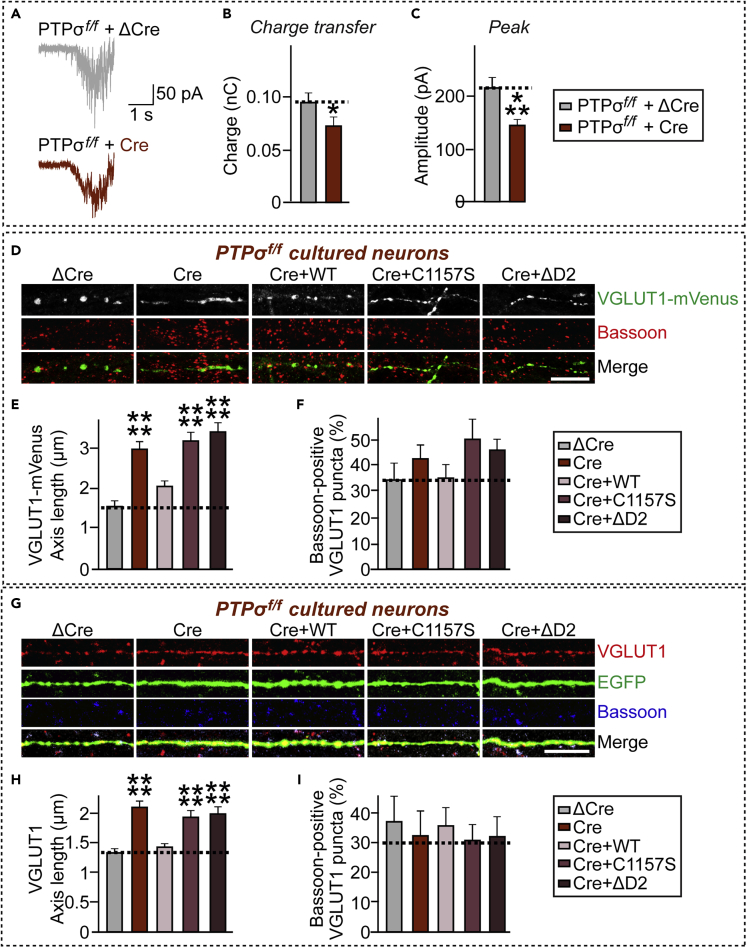
It is possible that deletion of PTPσ in presynaptic neurons alters localization of synaptic vesicles, resulting in reduced frequency of mEPSCs (Figure 1). Thus, we next asked whether PTPσ could regulate the size of readily releasable vesicles (RRPs) (Zucker and Regehr, 2002). We examined whether RRP size was changed at excitatory synapses by stimulating release of the entire RRP using a well-established hypertonic sucrose solution (500 mOsm) and quantifying RRP size by integrating the total charge transfer during the first 2 s of the release (Rosenmund and Stevens, 1996). Strikingly, the number of RRPs was significantly reduced in PTPσ-cKO neurons, as indicated by reductions of ∼14.7% in charge transfer and ∼31.2% in peak amplitude (Figures 2A–2C). This provides a hypothetical explanation for the positive regulation of neurotransmitter release by PTPσ at excitatory synapses.
Figure 2.
PTPσ Deletion Reduces Vesicle Localization in Excitatory Presynaptic Boutons
(A) Representative traces of AMPAR-EPSCs evoked by single 2-s pulse of 0.5 M sucrose delivered at a 1-min interval, recorded from hippocampal cultured neurons derived from PTPσf/f mice infected with lentiviruses expressing inactive (ΔCre) or active (Cre) Cre recombinase.
(B and C) Bar graphs showing charge transfer (B) and peak amplitudes (C) of sucrose-evoked EPSCs, estimated as the synaptic charge transfer integrated over 30 s. Recordings were performed in the presence of 1 μM tetrodoxin and 50 μM picrotoxin. Data are means ± SEMs (n denotes number of analyzed neurons; ΔCre, 26 and Cre, 29; ∗p < 0.05; ∗∗∗p < 0.001; unpaired t test).
(D) Representative images of cultured neurons (DIV10) derived from PTPσf/f mice infected with lentiviruses expressing ΔCre or Cre at DIV3 and transfected with VGLUT1-mVenus (green) at DIV8. Anti-Bassoon (red) was used to mark the presynaptic active zone. Scale bar: 10 μm.
(E) Quantification of synaptic vesicle diffusion from images in (D), determined by measuring the average length of the major axis of VGLUT1-mVenus fluorescence in transfected axons. Data are means ± SEMs (n denotes the number of analyzed neurons; ΔCre, n = 19; Cre, n = 18; Cre+/PTPσ WT, n = 15; Cre+/PTPσ C1157S, n = 15; and Cre+/PTPσ ΔD2, n = 17; ∗∗∗∗p < 0.0001; ANOVA with a non-parametric Kruskal-Wallis test).
(F) Quantification of VGLUT1-mVenus fluorescence enrichment at presynaptic active zone for the images in (D). Data are means ± SEMs (n denotes the number of analyzed neurons; ΔCre, n = 19; Cre, n = 18; Cre+/PTPσ WT, n = 15; Cre+/PTPσ C1157S, n = 15; and Cre+/PTPσ ΔD2, n = 17; ANOVA with a non-parametric Kruskal-Wallis test).
(G) Representative images of cultured neurons (DIV10) derived from PTPσf/f mice infected with lentiviruses expressing ΔCre or Cre at DIV3 and transfected with EGFP (green) at DIV8. Anti-Bassoon (blue) was used to mark the presynaptic active zone. Scale bar: 10 μm.
(H) Quantification of synaptic vesicle diffusion from images in (G), determined by measuring the average length of the major axis of VGLUT1 fluorescence in transfected axons. Data are means ± SEMs (n denotes the number of analyzed neurons; ΔCre, n = 14; Cre, n = 12; Cre+/PTPσ WT, n = 13; Cre+/PTPσ C1157S, n = 15; and Cre+/PTPσ ΔD2, n = 13; ∗∗∗∗p < 0.0001; ANOVA with a non-parametric Kruskal-Wallis test).
(I) Quantification of VGLUT1 fluorescence enrichment at presynaptic active zone for the images in (G). Data are means ± SEMs (n denotes the number of analyzed neurons; ΔCre, n = 14; Cre, n = 12; Cre+/PTPσ WT, n = 13; Cre+/PTPσ C1157S, n = 15; and Cre+/PTPσ ΔD2, n = 13; ANOVA with a non-parametric Kruskal-Wallis test).
To better understand the role of PTPσ in organizing presynaptic functions at the excitatory synapse (see Figure 1), we infected cultured hippocampal PTPσ-cKO neurons with ΔCre-EGFP or Cre-EGFP-expressing lentiviruses, transfected the neurons with mVenus-fused VGLUT1 (an excitatory synapse-specific vesicle marker) 5 days after the infections, and stained neurons with antibodies to Bassoon (a presynaptic AZ marker) 2 days after the transfections (Figures 2D–2F). We found that expression of VGLUT1-mVenus in PTPσ-cKO neurons resulted in a more diffuse pattern of mVenus-fused vesicular markers compared with control neurons infected with ΔCre-EGFP, indicating that ablation of PTPσ inhibits the synaptic localization of synaptic vesicles at excitatory presynaptic boutons (Figures 2D and 2E). No changes in Bassoon localization at presynaptic boutons were observed in PTPσ-cKO neurons (Figures 2D, 2F, and 2I). To further dissect the mechanism by which PTPσ regulates vesicle localization at excitatory synapses, we designed three lentiviruses expressing PTPσ variants, based on validated HA epitope-tagged PTPσ variants that were previously used in cultured neurons (Han et al., 2018). These lentiviruses expressed PTPσ wild-type (WT), a PTPσ deletion mutant lacking the D2 domain (ΔD2), or a PTPσ point mutant defective in tyrosine phosphatase activity (C1157S). Lentiviral expression of PTPσ WT, but not other PTPσ variants, completely reversed the diffuse distribution pattern of VGLUT1-mVenus fluorescence (Figures 2D and 2E) and puncta immunoreactive to anti-VGLUT1 antibodies (Figures 2G and 2H) in PTPσ-cKO neurons, producing a punctate pattern. In addition, synaptic localization of vesicles was not rescued by expression of PTPσ intracellular mutants, suggesting that PTPσ requires D2 domain-mediated molecular interactions and tyrosine phosphatase activity to appropriately direct excitatory synaptic vesicles into presynaptic boutons. Collectively, these results suggest that PTPσ is involved in presynaptic assembly by organizing vesicle localization at excitatory synapses using intracellular mechanisms.
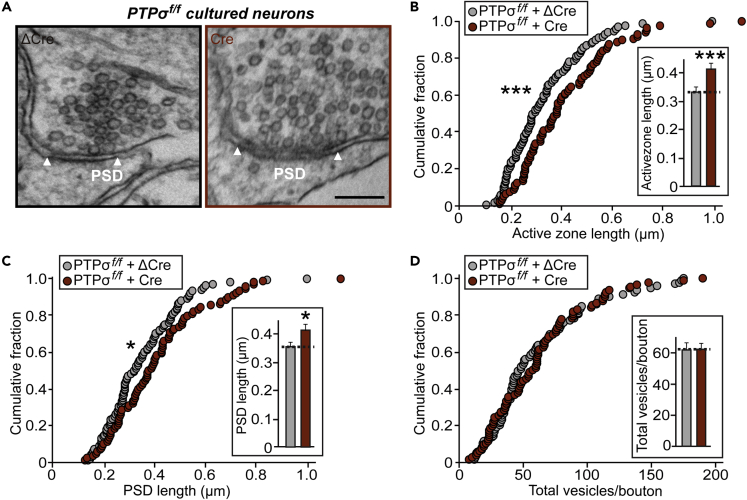
Conditional KO of PTPσ Alters Active Zone Architectures
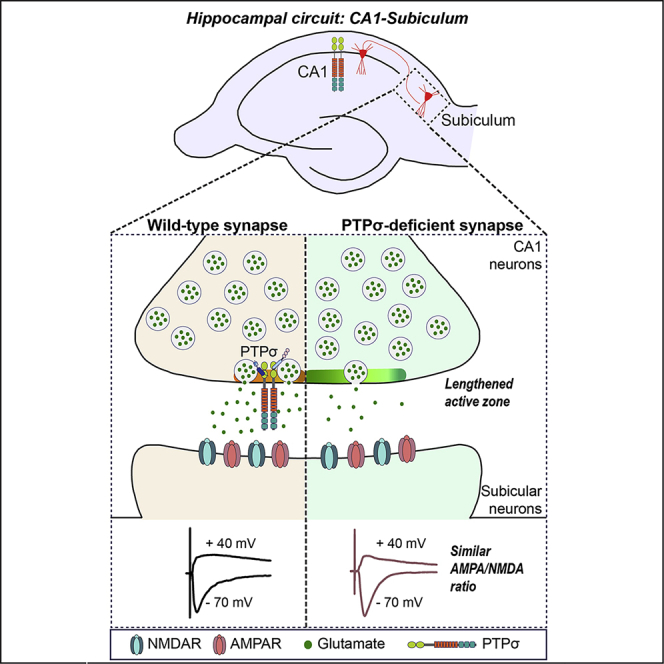
To further understand whether PTPσ-cKO affects synaptic structures, we examined presynaptic terminals and postsynaptic densities in cultured cells and imaged chemically fixed hippocampal neurons using transmission electron microscopy (TEM), as previously described (Acuna et al., 2016) (Figure 3). TEM analyses of cultured neurons showed that PTPσ-deficient and control presynaptic terminals contained similar numbers of total vesicles (Figures 3A and 3D). Surprisingly, AZ length was increased by ∼30% (Figures 3A and 3B), similar to the doubling in AZ size in Caenorhabditis elegans mutants lacking ptp-3A, an ortholog of the type IIa RPTP gene (Ackley et al., 2005, Han et al., 2019). Consistent with this, a corresponding increase (∼15%) in PSD length was observed (Figures 3A and 3C). These results suggest that PTPσ is crucial in controlling the structural organization of both presynaptic AZs and PSDs.
Figure 3.
PTPσ Deletion Induces Abnormal Organization of Synaptic Structures
(A) Representative electron micrographs of hippocampal neurons cultured from PTPσf/f mice infected with lentiviruses expressing ΔCre (control) or Cre.
(B and C) PTPσ deletion increases length of synaptic membranes. Cumulative distribution of the lengths of AZ (B) and PSD (C) for the indicated genotypes. Data are means ± SEMs (n denotes the number of analyzed neurons; ΔCre, 100 and Cre, 88; ∗p < 0.05; ∗∗∗p < 0.001; Mann-Whitney U test).
(D) Total numbers of vesicles per bouton in control and PTPσ-deficient synapses. Data are means ± SEMs (n denotes the number of analyzed neurons; ΔCre, 100 and Cre, 88).
Conditional PTPσ KO in CA1 Neurons Reduces Excitatory Presynaptic Innervation onto Postsynaptic Subicular Neurons and Excitatory Neurotransmitter Release
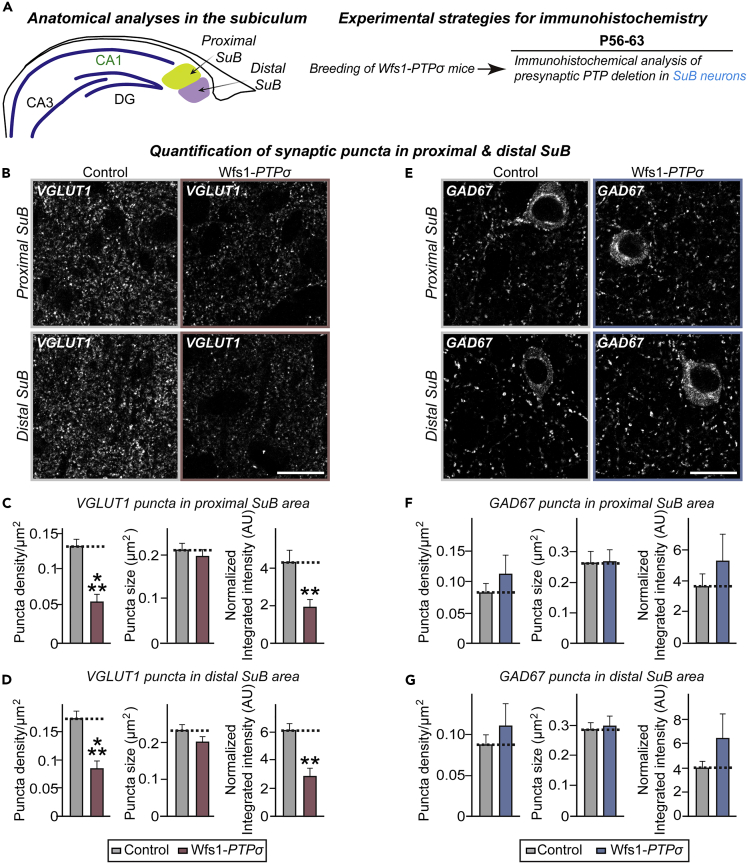
Although the synaptic roles of invertebrate orthologs of type IIa RPTPs have been primarily studied from the perspective of their presynaptic structure and function (Chagnon et al., 2004, Um and Ko, 2013), PTPσ appears to be expressed at both presynaptic and postsynaptic neurons (Dunah et al., 2005, Han et al., 2018) (Figure S5C). Because the functional locus of a specific synaptic protein cannot be precisely determined in cultured neurons, a Cre driver line under the control of a Wolfram syndrome 1 homolog (Wfs1) promoter was utilized (Kitamura et al., 2014, Madisen et al., 2010). The presence of Wfs1-positive neurons, including in the dorsal CA1 and layer II/III of the mPFC, was confirmed by robust tdTomato expression in the Ai9 reporter mouse line (Luuk et al., 2008, Madisen et al., 2010) (Figure S6A). Immunohistochemical analysis of Wfs1 expression in the mPFC and hippocampal CA1 showed strong Wfs1-immunoreactive signals in Tbr1-positive excitatory neurons but not GAD67-positive GABAergic neurons (Figure S6B). Thus, Wfs1-PTPσ mice (obtained by crossing PTPσf/f mice with a Wfs1-Cre driver line) allowed us to investigate the effects of selective loss of PTPσ at presynaptic loci in a given neural circuit. The pre- and postsynaptic effects of PTPσ deletions were analyzed by focusing on presynaptic CA1 neurons of the hippocampus at synapses formed onto postsynaptic pyramidal neurons in the subiculum. Wfs1-PTPσ mice were viable and fertile and comparable in size with control mice (Figure S6C). Moreover, NeuN and Nissl staining of Wfs1-PTPσ brains showed normal gross morphology (Figures S6D and S6E). Anatomical changes at synapses formed by presynaptic CA1 region neurons on postsynaptic subicular neurons were evaluated by quantitative immunofluorescence analyses (Figure 4A), which showed that the density and integrated intensity of VGLUT1 puncta were significantly reduced in subicular neurons (Figures 4B–4D). However, the density and intensity of GAD67 puncta in the corresponding brain regions were comparable in Wfs1-PTPσ and control mice (Figures 4E–4G). Adeno-associated viruses (AAVs) expressing Cre recombinase (AAV-Cre) or inactive Cre recombinase (AAV-ΔCre) were stereotactically injected into ventral hippocampal CA1 (vCA1) of PTPσf/f mice. Subsequent quantitative immunohistochemical analyses showed decreased excitatory (but not GABAergic) innervations onto subicular neurons from PTPσf/f mice infected with AAV-Cre (Figure S7).
Figure 4.
Wfs1-PTPσ KO Mice Exhibit Decreased Excitatory Synaptic Innervation in Postsynaptic Subicular Pyramidal Neurons
(A) Schematic depiction of anatomical analyses in the subiculum. Each subiculum was divided into the proximal and distal subiculum.
(B and E) Representative immunofluorescence images of the proximal and distal SuB of Control and Wfs1-PTPσ mice using VGLUT1 (B) or GAD67 (E). Scale bar: 20 μm.
(C and F) Quantification of the density, size, and integrated intensity of VGLUT1-positive (C) and GAD67-positive (F) synaptic puncta in the proximal SuB. Data are means ± SEMs (n denotes the number of analyzed brain mice; 8 mice per group; ∗∗p < 0.01 and ∗∗∗p < 0.001; Mann-Whitney U test).
(D and G) Quantification of the density, size, and integrated intensity of VGLUT1-positive (D) and GAD67-positive (G) synaptic puncta in the distal SuB. Data are means ± SEMs (n denotes the number of analyzed mice; 8 mice per group; ∗∗p < 0.01, ∗∗∗p < 0.001; Mann-Whitney U test). See also Figures S7 and S8.
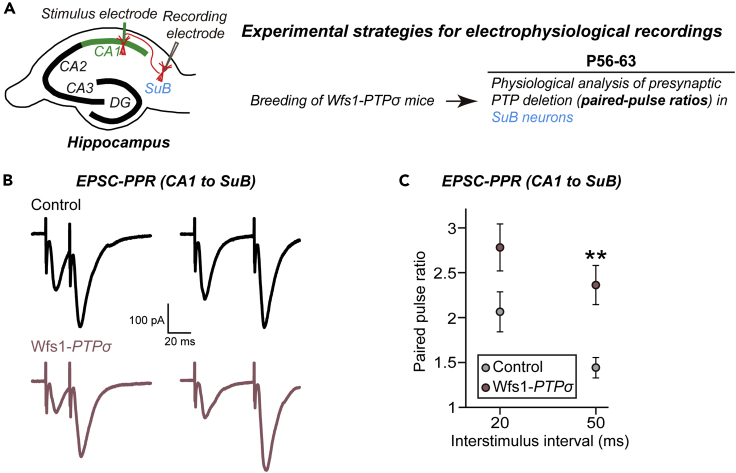
To further corroborate these anatomical observations and identify any possible presynaptic changes, PPRs in subicular neurons were measured (Figure 5A). EPSC-PPRs were significantly increased in hippocampal CA1-to-subicular synapses (Figures 5B and 5C). Taken together, these results suggest that PTPσ is a critical modulator of presynaptic innervations and neurotransmitter release at excitatory synapses.
Figure 5.
Presynaptic Deletion of PTPσ Impairs Neurotransmitter Release at Excitatory Synapses of Postsynaptic Subicular Pyramidal Neurons
(A) Experimental strategies for electrophysiological recordings in hippocampal SuB neurons of Wfs1-PTPσ mice.
(B) Representative traces of paired pulse ratios (PPRs) of EPSCs in synapses of CA1–SuB at two different interstimulus intervals (20 and 50 ms for EPSC-PPRs).
(C) EPSC-PPRs in synapses of CA1–SuB as a function of the indicated interstimulus intervals. Data are means ± SEMs (∗∗p < 0.01; two-tailed Student's t test).
Conditional PTPσ Deletions Exert No Postsynaptic Effect
Next, the effect of presynaptic deletion of PTPσ on basal synaptic transmission was analyzed in Wfs1-PTPσ mice. Unexpectedly, there was a no reduction in frequency or amplitude of spontaneous EPSCs (sEPSCs) in subicular pyramidal neurons (Figures S8A–S8C). A recent study using triple conditional KO mice lacking all three LAR-RPTPs showed decreased NMDA receptor-mediated responses in hippocampal CA1 neurons (Sclip and Südhof, 2020). Thus, we tested whether PTPσ deletion also regulated postsynaptic responses via a trans-synaptic mechanism. To test this, we assessed the ratio of AMPA- to NMDA-receptor mediated EPSCs (i.e., AMPA/NMDA ratio) by stimulating Schaffer collateral axons of hippocampal CA3 neurons or axons of hippocampal CA1 neurons and measuring postsynaptic responses in hippocampal CA1 neurons or subicular neurons (Figures 6 and 7). There was no change in the AMPA/NMDA ratio at PTPσ-deficient CA1 pyramidal neurons or subicular neurons innervated by PTPσ-deficient CA1 pyramidal neurons (Figures 6 and 7). In addition, there was no change in the frequency or amplitude of mEPSCs in PTPσ-deficient CA1 pyramidal neurons (Figure S8). These results suggest that PTPσ primarily functions presynaptically and does not trans-synaptically regulate postsynaptic responses in vivo.
Figure 6.
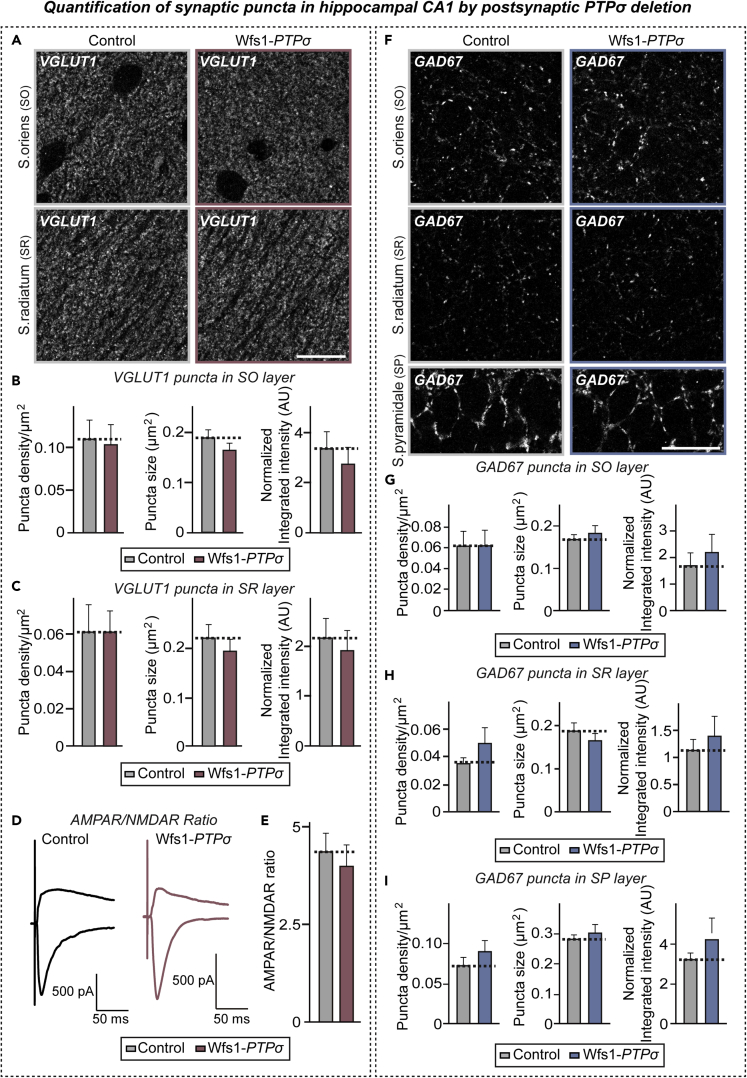
Postsynaptic Deletion of PTPσ Exerts No Effects on Excitatory Synapse Organization in Hippocampal CA1 Region
(A and F) Representative immunofluorescence images of the stratum oriens (SO) and stratum pyramidal (SP) layer of control and Wfs1-PTPσ mice using VGLUT1 (A) or GAD67 (F). Scale bar: 20 μm.
(B and C) Quantification of the density, size, and integrated intensity of VGLUT1-positive synaptic puncta in the SO (B) and SR (C) layers of the hippocampal CA1 region. Data are means ± SEMs (n = 8 mice per group; Mann-Whitney U test).
(D) Representative traces of evoked EPSCs at holding potentials of −70 and +40 mV in control and Wfs1-PTPσ mice.
(E) AMPAR/NMDAR ratios at CA3–CA1 synapses, calculated by dividing the EPSC peak amplitude at 10 ms (−70 mV) by the EPSC amplitude at 130 ms (+40 mV). Data are means ± SEMs (n = 14 cells from 4–5 mice; two-tailed Student's t test).
(G–I) Quantification of the density, size, and integrated intensity of GAD67-positive synaptic puncta in the SO (G), SR (H), and SP (I) layers of the hippocampal CA1 region. Data are means ± SEM (n = 8 mice per group; Mann-Whitney U test).
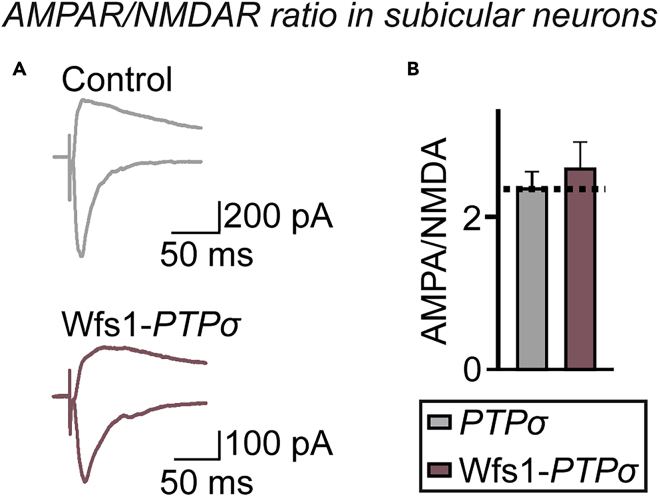
Figure 7.
Presynaptic Deletion of PTPσ in Hippocampal CA1 Area Exerts No Altered Postsynaptic Responses in Subicular Neurons
(A) Representative traces of evoked EPSCs at holding potentials of −70 and +40 mV in control and Wfs1-PTPσ mice.
(B) AMPAR/NMDAR ratios at CA1–Sub synapses, calculated by dividing the EPSC peak amplitude at 10 ms (−70 mV) by the EPSC amplitude at 130 ms (+40 mV). Data are means ± SEMs (n denotes the number of analyzed neurons; Control, 15 cells/4 mice and Wfs1-PTPσ, 12 cells/4 mice; two-tailed Student's t test).
Discussion
Prior investigations using constitutive KO mice lacking one or two LAR-RPTPs, or shRNA-mediated KD approaches, showed that LAR-RPTPs are significant regulators of various aspects of nervous system development (Chagnon et al., 2004, Han et al., 2016, Han et al., 2019, Takahashi and Craig, 2013, Um and Ko, 2013). These studies showed that LAR-RPTPs affect the development and/or maturation of synapses as well as have potential compensatory adaptations and possibly unintentional off-target phenomena during development. Therefore, the present study involved the generation of PTPσ floxed mice, with these conditional KO mice analyzed to determine the synaptic roles of PTPσ. Our in vitro data demonstrated that PTPσ KO specifically reduced the numbers of excitatory synapses and basal excitatory synaptic transmission, in agreement with previous KD studies (Han et al., 2018, Ko et al., 2015) (Figure 1). Moreover, PTPσ KO decreased RRP size and synaptic localization of excitatory synaptic vesicles (Figure 2). Furthermore, PTPσ KO altered ultrastructural features (Figure 3). Our in vivo results further suggest that PTPσ functions presynaptically at excitatory synapses involving hippocampal CA1–subiculum connections in the hippocampus by modulating glutamate release (Figures 4 and 5). Compared with the pervasive loss-of-function consequences of Nrxns in various cell types across diverse brain areas and neural circuits (Südhof, 2017), the effects of conditional deletion of PTPσ were relatively marginal. These findings were unexpected, as members of the LAR-RPTP family bind to various synapse organizers crucial for discrete aspects of synapse development (Südhof, 2017, Um and Ko, 2013).
A rich body of previous studies have shown that invertebrate LAR-RPTPs have presynaptic roles in controlling AZ assembly and proper vesicle localization (Han et al., 2019). A few studies of vertebrate LAR-RPTP function have revealed their critical roles in postsynaptic neurons, including their regulation of spine morphogenesis development and their stabilization of surface AMPA-type glutamate receptors (Dunah et al., 2005, Wyszynski et al., 2002). Because several LAR-RPTP ligands have been identified as putative postsynaptic organizers in rodent neurons, LAR-RPTPs were thought to act in presynaptic neurons in a manner similar to Nrxns (Südhof, 2012). Taken together with our previous results (Han et al., 2018), our current findings demonstrate that PTPσ KO and PTPσ KD have similar overall effects on synapse density and transmission in vitro, indicating the importance of confirmatory analyses using a sophisticated system and approaches in elucidating PTPσ function in vivo. It is possible, however, that the approaches employed in this study were not sufficiently sensitive to detect subtle changes in synapse properties. Moreover, the selection of experimental preparations may preclude detection of synaptic roles of PTPσ at in vivo synapses. In addition, PTPδ may functionally compensate for PTPσ loss, but not vice versa, whereas PTPσ may have peripheral roles in the operation of specific neural circuits in which PTPδ is expressed. The use of cKO models to study the canonical and non-canonical roles of PTPσ in vivo at various stages of synapse development in other classes of neurons is warranted and may provide a more sophisticated understanding of how LAR-RPTPs act as a multivalent signaling platform in presynaptic neurons.
Our results suggest that PTPσ contributes to the organization of trans-synaptic nanomolecular complexes for efficient glutamate release (Figure 6). Investigation of common/redundant functions of LAR-RPTPs using conditional triple KOs of PTPσ, PTPδ, and LAR is required to provide further insights into the major observations of the current study (Sclip and Südhof, 2020). In addition, it remains to be determined whether LAR-RPTPs and other presynaptic adhesion molecules (e.g., Nrxns) cooperate in the presynaptic assembly in vivo (Roppongi et al., 2020).
Impaired glutamate release efficiency observed in PTPσ cKO mice has been observed in several prior studies reporting loss of function of Nrxn1α (Etherton et al., 2009), liprin-α2 (Spangler et al., 2013), and RIM1 (Kaeser et al., 2008). PTPσ KO drastically suppressed RRP size in cultured neurons that is controlled by positional priming (Neher and Sakaba, 2008) (Figure 2). These results suggest that PTPσ KO may disorganize AZ structure (Figure 3) and function by decoupling key nanomolecular machinery in AZs, a possibility that remains to be tested using high-resolution imaging or electron microscopy analyses.
Our genetic manipulations using the Wfs1-Cre driver line allowed determination of the presynaptic or postsynaptic role of PTPσ in excitatory neurons (Figure 4, Figure 5, Figure 6, Figure 7). PTPσ deletion in hippocampal CA1 reduced structural innervation of excitatory inputs and increased EPSC-PPRs in subicular neurons, without changing the frequency or amplitude of sEPSCs (Figures 5 and S8). Intriguingly, PTPσ deletion in hippocampal CA1 failed to elicit any changes in Schaffer collateral evoked excitatory synaptic transmission or basal synaptic transmission, indicating that PTPσ is not involved in trans-synaptic regulation of postsynaptic responses in the hippocampal CA1-subicular synapses in vivo. Given that deletion of all three LAR-RPTPs significantly decreases postsynaptic NMDA-type receptor responses in hippocampal Schaffer collateral neural circuits (Sclip and Südhof, 2020), it is possible that PTPδ and LAR might be selectively involved in controlling this synapse property in hippocampal CA1-subicular synapses. It is equally likely that LAR-RPTPs specifically regulate postsynaptic NMDA-type receptor responses in only a subset of neural circuits (i.e., CA3-CA1 synapses). It is also worth addressing whether alterations in AMPA-type glutamate receptor subunit composition occur in vivo at synapses of PTPσ-deficient neurons and, if so, what their significance might be. Future studies are warranted to systematically address these possibilities. In sum, our results clearly demonstrate that PTPσ is a key presynaptic factor in tuning presynaptic properties by organizing the neurotransmitter release machinery at excitatory synapses.
Limitations of the Study
Although the current study clearly demonstrated a presynaptic role for PTPσ in regulating neurotransmitter release at hippocampal CA1-subicular synapses, it remains to be determined whether this role extends generally to other hippocampal neural circuits and circuits in other brain areas. In addition, it is unclear how PTPσ and other LAR-RPTP members (PTPδ and LAR) exert their differential canonical functions in presynaptic neurons. Furthermore, differences in the requirement for PTPσ in controlling postsynaptic responses at different hippocampal synapses need to be more rigorously probed. Lastly, the pathological significance and mechanisms of coupling of PTPσ (and by extension, LAR-RPTPs) to intracellular machineries warrant further systematic investigation.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by Jaewon Ko (jaewonko@dgist.ac.kr)
Materials Availability
All unique/stable reagents generated in this study are available from the Lead Contact upon reasonable request and with a completed Materials Transfer Agreement.
Data and Code Availability
This study did not generate datasets.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Jinha Kim (DGIST) for her technical assistance, Drs. Susumu Tonegawa (MIT, USA) and Albert Chen (DUKE-NUS, Singapore) for the gift of Wfs1-Cre and Nestin-Cre driver lines, respectively. This study was supported by grants from the Korea Healthcare Technology R & D Project, funded by the Ministry for Health and Welfare Affairs, Republic of Korea (Grant HI17C0080 to J.K.) and the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (2019R1A2B5B02069324 to J.K.).
Author Contributions
S.-Y.C. and J.K. conceived the project; K.A.H., H.-Y.L., D.L., J.S., T.H.Y., C.L., and X.L. performed the experiments; K.A.H., H.-Y.L., D.L., J.S., T.H.Y., C.L., J.-S.R., J.W.U., S.-Y.C., and J.K. analyzed the data; S.-Y.C. and J.K. wrote the manuscript with input from the other authors.
Declaration of Interests
The authors declare no competing interests.
Published: June 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101203.
Contributor Information
Se-Young Choi, Email: sychoi@snu.ac.kr.
Jaewon Ko, Email: jaewonko@dgist.ac.kr.
Supplemental Information
References
- Ackley B.D., Harrington R.J., Hudson M.L., Williams L., Kenyon C.J., Chisholm A.D., Jin Y. The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J. Neurosci. 2005;25:7517–7528. doi: 10.1523/JNEUROSCI.2010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuna C., Liu X., Südhof T.C. How to make an active zone: unexpected universal functional redundancy between RIMs and RIM-BPs. Neuron. 2016;91:792–807. doi: 10.1016/j.neuron.2016.07.042. [DOI] [PubMed] [Google Scholar]
- Bomkamp C., Padmanabhan N., Karimi B., Ge Y., Chao J.T., Loewen C.J.R., Siddiqui T.J., Craig A.M. Mechanisms of PTPsigma-mediated presynaptic differentiation. Front. Synaptic Neurosci. 2019;11:17. doi: 10.3389/fnsyn.2019.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagnon M.J., Uetani N., Tremblay M.L. Functional significance of the LAR receptor protein tyrosine phosphatase family in development and diseases. Biochem. Cell Biol. 2004;82:664–675. doi: 10.1139/o04-120. [DOI] [PubMed] [Google Scholar]
- Choi Y., Nam J., Whitcomb D.J., Song Y.S., Kim D., Jeon S., Um J.W., Lee S.G., Woo J., Kwon S.K. SALM5 trans-synaptically interacts with LAR-RPTPs in a splicing-dependent manner to regulate synapse development. Sci. Rep. 2016;6:26676. doi: 10.1038/srep26676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah A.W., Hueske E., Wyszynski M., Hoogenraad C.C., Jaworski J., Pak D.T., Simonetta A., Liu G., Sheng M. LAR receptor protein tyrosine phosphatases in the development and maintenance of excitatory synapses. Nat. Neurosci. 2005;8:458–467. doi: 10.1038/nn1416. [DOI] [PubMed] [Google Scholar]
- Elchebly M., Wagner J., Kennedy T.E., Lanctot C., Michaliszyn E., Itie A., Drouin J., Tremblay M.L. Neuroendocrine dysplasia in mice lacking protein tyrosine phosphatase sigma. Nat. Genet. 1999;21:330–333. doi: 10.1038/6859. [DOI] [PubMed] [Google Scholar]
- Etherton M.R., Blaiss C.A., Powell C.M., Südhof T.C. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc. Natl. Acad. Sci. U S A. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K.A., Jeon S., Um J.W., Ko J. Emergent synapse organizers: LAR-RPTPs and their companions. Int. Rev. Cell. Mol. Biol. 2016;324:39–65. doi: 10.1016/bs.ircmb.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Han K.A., Ko J.S., Pramanik G., Kim J.Y., Tabuchi K., Um J.W., Ko J. PTPsigma drives excitatory presynaptic assembly via various extracellular and intracellular mechanisms. J. Neurosci. 2018;38:6700–6721. doi: 10.1523/JNEUROSCI.0672-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K.A., Um J.W., Ko J. Intracellular protein complexes involved in synapse assembly in presynaptic neurons. Adv. Protein Chem. Struct. Biol. 2019;116:347–373. doi: 10.1016/bs.apcsb.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Horn K.E., Xu B., Gobert D., Hamam B.N., Thompson K.M., Wu C.L., Bouchard J.F., Uetani N., Racine R.J., Tremblay M.L. Receptor protein tyrosine phosphatase sigma regulates synapse structure, function and plasticity. J. Neurochem. 2012;122:147–161. doi: 10.1111/j.1471-4159.2012.07762.x. [DOI] [PubMed] [Google Scholar]
- Jonas P. The time course of signaling at central glutamatergic synapses. News Physiol. Sci. 2000;15:83–89. doi: 10.1152/physiologyonline.2000.15.2.83. [DOI] [PubMed] [Google Scholar]
- Kaeser P.S., Kwon H.B., Chiu C.Q., Deng L., Castillo P.E., Südhof T.C. RIM1alpha and RIM1beta are synthesized from distinct promoters of the RIM1 gene to mediate differential but overlapping synaptic functions. J. Neurosci. 2008;28:13435–13447. doi: 10.1523/JNEUROSCI.3235-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Pignatelli M., Suh J., Kohara K., Yoshiki A., Abe K., Tonegawa S. Island cells control temporal association memory. Science. 2014;343:896–901. doi: 10.1126/science.1244634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.S., Pramanik G., Um J.W., Shim J.S., Lee D., Kim K.H., Chung G.Y., Condomitti G., Kim H.M., Kim H. PTPsigma functions as a presynaptic receptor for the glypican-4/LRRTM4 complex and is essential for excitatory synaptic transmission. Proc. Natl. Acad. Sci. U S A. 2015;112:1874–1879. doi: 10.1073/pnas.1410138112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhang P., Choi T.Y., Park S.K., Park H., Lee E.J., Lee D., Roh J.D., Mah W., Kim R. Splicing-Dependent trans-synaptic SALM3-LAR-RPTP interactions regulate excitatory synapse development and locomotion. Cell Rep. 2015;12:1618–1630. doi: 10.1016/j.celrep.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luuk H., Koks S., Plaas M., Hannibal J., Rehfeld J.F., Vasar E. Distribution of Wfs1 protein in the central nervous system of the mouse and its relation to clinical symptoms of the Wolfram syndrome. J. Comp. Neurol. 2008;509:642–660. doi: 10.1002/cne.21777. [DOI] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Palmiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J., Batt J., Doering L.C., Rotin D., Bain J.R. Enhanced rate of nerve regeneration and directional errors after sciatic nerve injury in receptor protein tyrosine phosphatase sigma knock-out mice. J. Neurosci. 2002;22:5481–5491. doi: 10.1523/JNEUROSCI.22-13-05481.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missler M., Südhof T.C., Biederer T. Synaptic cell adhesion. Cold Spring Harb. Perspect. Biol. 2012;4:a005694. doi: 10.1101/cshperspect.a005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Roppongi R.T., Dhume S.H., Padmanabhan N., Silwal P., Zahra N., Karimi B., Bomkamp C., Patil C.S., Champagne-Jorgensen K., Twiley R.E. Neuron. 2020;106:108–125. doi: 10.1016/j.neuron.2020.01.003. [DOI] [PubMed] [Google Scholar]
- Rosenmund C., Stevens C.F. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Sclip A., Südhof T.C. LAR receptor phospho-tyrosine phosphatases regulate NMDA-receptor responses. Elife. 2020;9:e53406. doi: 10.7554/eLife.53406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler S.A., Schmitz S.K., Kevenaar J.T., de Graaff E., de Wit H., Demmers J., Toonen R.F., Hoogenraad C.C. Liprin-alpha2 promotes the presynaptic recruitment and turnover of RIM1/CASK to facilitate synaptic transmission. J. Cell Biol. 2013;201:915–928. doi: 10.1083/jcb.201301011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T.C. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T.C. Synaptic neurexin complexes: a molecular code for the logic of neural circuits. Cell. 2017;171:745–769. doi: 10.1016/j.cell.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T.C. Towards an understanding of synapse formation. Neuron. 2018;100:276–293. doi: 10.1016/j.neuron.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Craig A.M. Protein tyrosine phosphatases PTPdelta, PTPsigma, and LAR: presynaptic hubs for synapse organization. Trends Neurosci. 2013;36:522–534. doi: 10.1016/j.tins.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Arstikaitis P., Prasad T., Bartlett T.E., Wang Y.T., Murphy T.H., Craig A.M. Postsynaptic TrkC and presynaptic PTPsigma function as a bidirectional excitatory synaptic organizing complex. Neuron. 2011;69:287–303. doi: 10.1016/j.neuron.2010.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Katayama K., Sohya K., Miyamoto H., Prasad T., Matsumoto Y., Ota M., Yasuda H., Tsumoto T., Aruga J., Craig A.M. Selective control of inhibitory synapse development by Slitrk3-PTPdelta trans-synaptic interaction. Nat. Neurosci. 2012;15:389–398. doi: 10.1038/nn.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K.M., Uetani N., Manitt C., Elchebly M., Tremblay M.L., Kennedy T.E. Receptor protein tyrosine phosphatase sigma inhibits axonal regeneration and the rate of axon extension. Mol. Cell. Neurosci. 2003;23:681–692. doi: 10.1016/s1044-7431(03)00120-9. [DOI] [PubMed] [Google Scholar]
- Uetani N., Kato K., Ogura H., Mizuno K., Kawano K., Mikoshiba K., Yakura H., Asano M., Iwakura Y. Impaired learning with enhanced hippocampal long-term potentiation in PTPdelta-deficient mice. EMBO J. 2000;19:2775–2785. doi: 10.1093/emboj/19.12.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetani N., Chagnon M.J., Kennedy T.E., Iwakura Y., Tremblay M.L. Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J. Neurosci. 2006;26:5872–5880. doi: 10.1523/JNEUROSCI.0386-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Um J.W., Ko J. LAR-RPTPs: synaptic adhesion molecules that shape synapse development. Trends Cell Biol. 2013;23:465–475. doi: 10.1016/j.tcb.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Valnegri P., Montrasio C., Brambilla D., Ko J., Passafaro M., Sala C. The X-linked intellectual disability protein IL1RAPL1 regulates excitatory synapse formation by binding PTPdelta and RhoGAP2. Hum. Mol. Genet. 2011;20:4797–4809. doi: 10.1093/hmg/ddr418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M.J., Batt J., Fladd C.A., Henderson J.T., Skarnes W., Rotin D. Neuronal defects and posterior pituitary hypoplasia in mice lacking the receptor tyrosine phosphatase PTPsigma. Nat. Genet. 1999;21:334–338. doi: 10.1038/6866. [DOI] [PubMed] [Google Scholar]
- Wyszynski M., Kim E., Dunah A.W., Passafaro M., Valtschanoff J.G., Serra-Pages C., Streuli M., Weinberg R.J., Sheng M. Interaction between GRIP and liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34:39–52. doi: 10.1016/s0896-6273(02)00640-2. [DOI] [PubMed] [Google Scholar]
- Yim Y.S., Kwon Y., Nam J., Yoon H.I., Lee K., Kim D.G., Kim E., Kim C.H., Ko J. Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases. Proc. Natl. Acad. Sci. U S A. 2013;110:4057–4062. doi: 10.1073/pnas.1209881110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Yasumura M., Uemura T., Lee S.J., Ra M., Taguchi R., Iwakura Y., Mishina M. IL-1 receptor accessory protein-like 1 associated with mental retardation and autism mediates synapse formation by trans-synaptic interaction with protein tyrosine phosphatase delta. J. Neurosci. 2011;31:13485–13499. doi: 10.1523/JNEUROSCI.2136-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R.S., Regehr W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate datasets.