Abstract
Objective
To perform a systematic review and meta-analysis evaluating the prevalence of gastrointestinal (GI) symptoms and mortality in patients with coronavirus disease 2019 (COVID-19) diagnosed.
Methods
A systematic search of MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Scopus was performed from December 1, 2019 to May 7, 2020. Observational studies including adults with COVID-19 infection and reporting GI symptoms were included. The primary outcome was assessing the weighted pooled prevalence (WPP) of GI symptoms in patients with COVID-19 infection. Secondary outcomes were WPP of overall mortality, and mortality in patients with COVID-19 infection with GI symptoms.
Results
A total of 78 studies with 12,797 patients were included. Among GI symptoms (at onset of illness in 6, at admission in 17, data given separately for both in 3, and data unavailable in 52 studies), the WPP of diarrhea was 12.4% (95% CI, 8.2% to 17.1%), I2=94%; nausea and/or vomiting, 9.0% (95% CI, 5.5% to 12.9%), I2=93%; loss of appetite, 22.3% (95% CI, 11.2% to 34.6%, I2=94%; and abdominal pain, 6.2% (95% CI, 2.6% to 10.3%), I2=92%. Mortality among patients with GI symptoms (0.4%; 95% CI, 0% to 1.1%; I2=74%) was similar to overall mortality (2.1%; 95% CI, 0.2% to 4.7%; I2=94%), P=.15. Most studies had high risk of bias and overall quality of evidence was low to very low for all outcomes.
Conclusion
Gastrointestinal symptoms are seen in up to 1 in 5 patients with COVID-19 infection. More high-quality evidence is needed to confirm these findings and explore factors causing mortality in these patients.
Abbreviations and Acronyms: C-NERC, COVID National Emergency Response Center; C-NIRST, Coronavirus Disease 2019 National Incident Room Surveillance Team; COVID-19, coronavirus disease 2019; GI, gastrointestinal; GRADE, Grading of Recommendations, Assessment, Development and Evaluations; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; WPP, weighted pooled prevalence
Coronavirus disease 2019 (COVID-19) is an infection caused by the novel coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first case of COVID-19 infection was reported in December 2019 in Wuhan, China. Since then, the disease has been declared a pandemic, affecting more than 4,700,000 people and causing more than 300,000 deaths globally (as of May 21, 2020).1 , 2 Similar to other coronaviruses, SARS-CoV-2 primarily affects the pulmonary system, but multisystem involvement has been reported. The spectrum of disease includes asymptomatic colonization; mild disease with fever, cough, and fatigue; and severe disease characterized by dyspnea, hypoxemia, acute respiratory distress syndrome, need for mechanical ventilation, and death.3
The presence of gastrointestinal (GI) manifestations in COVID-19–infected patients has been noted in several reports recently, with 16% to 50% of patients reporting one or more GI symptom at presentation or during the illness.4 Recognition of these symptoms has important implications for the identification of individual cases and would influence testing and isolation strategies, which are continually evolving based on emerging data. One recent meta-analysis of 4243 patients found the pooled prevalence of all GI symptoms to be 17.6%5; however, most of the data included in the analysis were from Asia, limiting its generalizability. Another meta analysis of 47 studies reported the symptoms of diarrhea and nausea/vomiting to be present in 7.7% and 7.8% patients with COVID 19 infection, respectively. However, the study did not report mortality rates among these patients.6
Given the rapidly growing literature regarding GI symptoms in patients with COVID-19 infection, we conducted an updated systematic review and meta-analysis to assess the prevalence of GI symptoms in patients with COVID-19 infection and determine whether mortality is influenced by the presence of GI symptoms in these patients.
Methods
All procedures used in this meta-analysis were consistent with the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (Appendix, available online at http://www.mayoclinicproceedings.org).7
Selection Criteria
The studies considered in this meta-analysis were observational studies that included adults with confirmed COVID-19 infection and reported clinical characteristics, including GI symptoms. Studies not reporting the presence or absence of GI symptoms (because nonreporting during this pandemic would not equate to lack of GI symptoms) and individual case reports were excluded.
Data Sources and Search Strategy
A comprehensive search of several databases from December 1, 2019 to May 7, 2020, excluding animal studies, was conducted. The databases included Ovid MEDLINE and Epub ahead of print, In-Process; Other Non-Indexed Citations and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus.
The search strategy was designed and conducted by an experienced librarian (L.H.) with input from the study’s principal investigators. Controlled vocabulary supplemented with keywords was used to search for studies describing GI manifestations of COVID-19 infection.
The actual strategy listing all search terms used and how they are combined is available in Supplemental Table 1 (available online at http://www.mayoclinicproceedings.org).
Two authors (R.T. and S.S.) independently reviewed titles and abstracts of the identified studies, and those that did not answer the research question of interest were excluded. The remaining articles were reviewed to determine inclusion criteria fulfillment. Reference lists of articles with information on the topic were also reviewed for additional pertinent studies. A flow diagram of included studies is shown in Figure 1 .
Figure 1.
Flow diagram of study selection process.
Data Abstraction
Data were independently abstracted to a predetermined collection form by 2 investigators (R.T. and S.S.). Data collected for each study included study setting and design, month and year of publication, location, number of patients, patients with GI symptoms, symptom onset (symptoms assessed at onset of illness or at admission to the hospital), severity of COVID-19 infection, duration of follow-up, and mortality. Severe infection was defined as admission to the intensive care unit or need for mechanical ventilation. Conflicts in data abstraction were resolved by consensus, referring to the original article.
Methodological Quality of Included Studies
Most studies included were case series, hence an appropriate risk-of-bias tool was applied (Supplemental Table 2, available online at http://www.mayoclinicproceedings.org).8 Risk of bias was assessed based on 4 domains: selection, ascertainment, causality, and reporting. An overall judgment of risk of bias was made based on factors deemed to be most critical for the systematic review (selection criteria, ascertainment of outcome, and follow-up duration).
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework was used to interpret the findings of the study. The principles of the GRADE system have been adopted by the Cochrane Collaboration for evaluating the quality of evidence for the outcomes reported in systematic reviews. For systematic reviews, the GRADE approach defines the quality of the body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. The GRADE framework classifies the quality of evidence in 1 of 4 levels: high, moderate, low, and very low. Quality of a body of evidence involves consideration of the study design of included studies, methodological quality, directness of evidence, heterogeneity, inconsistency of results, and risk of publication bias.9 , 10
Data for symptom prevalence are expected to be of low quality because the evidence primarily arises from observational studies. This is particularly true in the context of an ongoing pandemic. Due to the clinical importance of the study question, such studies were included in the systematic review and meta-analysis.
Outcomes Assessed
Our primary analysis focused on assessing the weighted pooled prevalence (WPP) of GI symptoms in patients with COVID-19 infection, occurring any time during the course of illness. Secondary outcomes were the WPPs of mortality in all COVID-19–infected patients and in those with GI symptoms.
Statistical Analyses
We calculated the WPP with corresponding 95% CI for each symptom. We used the inverse variance heterogeneity model of meta-analysis with corresponding 95% CI for the overall and subgroup analyses. The inverse variance heterogeneity model is a modification of fixed-effects models that accounts for between-study heterogeneity, retains the individual weights of the studies, and decreases the variance in estimates.11 Freeman-Tukey double arcsine transformation was used to avoid giving more weight to studies with prevalence estimates that are too large or too small. We assessed heterogeneity within groups using the I 2 statistic, which estimates the proportion of total variation across studies that is due to heterogeneity in study patients, design, or interventions rather than chance; I 2 values greater than 50% suggest substantial heterogeneity.12 Publication bias was assessed visually using funnel plots if more than 10 studies were included in the analysis. Subgroup analyses were done for symptoms and mortality by onset of symptoms (at onset of illness) and for all all outcomes by study location (China vs non-China). Sensitivity analyses were done by excluding outlier studies and studies with high risk of bias. Calculations were performed and graphs were constructed using MetaXL meta-analysis software (version 5.3; EpiGear International Pty Ltd).
Results
Search Results
The described database search strategy revealed 827 unique studies, 10 studies were identified from other sources; titles and abstracts were screened, and relevant articles were obtained. Of the potentially relevant articles, 759 were excluded for various reasons (Figure 1), leaving 78 studies that were included in this meta-analysis (Table 4 , 5 , 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88).
Table.
Characteristics of Included Studiesa
| Serial No. | Reference, year | Country | Study Period | Type of Study | N | Age (y)b | Female (%) | Severity of Infection | Hospitalization | Follow-up (d) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | An et al,13 2020 | China | 1/17/20-1/24/20 | Case series | 9 | 35.8 (28-45) | 44 | NS | ( ) | 10-17 |
| 2 | Chan et al,14 2020 | China | 1/10/20-1/15/20 | Case series | 6 | 50 (20-66) | 50 | ( ) | All | ( ) |
| 3 | Chang et al,15 2020 | China | 1/16/20-1/29/20 | Case series | 13 | 34 (34-48) | 23.1 | ( ) | No | 20 |
| 4 | Chen et al,16 2020 | China | 1/1/20-1/20/20 | Case series | 99 | 55 (21-82) | 32 | ( ) | All | 16-25 |
| 5 | Chen et al,17 2020 | China | ( ) | Case series | 9 | 54-25 | 77.8 | NS | All | 13 |
| 6 | Chen et al,18 2020 | China | 1/1/20-3/11/20 | Case series | 145 | 47.5±14.6 | 54.5 | B | All | Up to 69 |
| 7 | Cheung et al,5 2020 | Hong Kong | 2/2/20-2/29/20 | Case series | 59 | 58.5 (22-96) | 54.2 | ( ) | ( ) | ( ) |
| 8 | Cholankeril et al,19 2020 | US | 3/4/20-3/24/20 | Case series | 116 | 50 (35-67) | 46.5 | ( ) | All | ( ) |
| 9 | C-NERC,20 2020 | South Korea | 1/10/20-2/14/20 | Case series | 28 | 42.6 (20-73) | 46.1 | ( ) | All | 12.7 (range, 8-19) |
| 10 | C-NIRST,21 2020 | Australia | Until 3/14/20 | Case series (national database) | 295 | 47 (0-94) | 50 | ( ) | 225 | Up to 3/14/20 |
| 11 | Fan H et al,22 2020 | China | 12/30/19-2/16/20 | Case series | 101 | 65 (24-83) | 36.6 | S | All | ( ) |
| 12 | Fernandez-Ruiz et al,23 2020 | Spain | 3/5/20-3/23/20 | Case series | 17 | 71 (38-80) | 24 | ( ) | All | ( ) |
| 13 | Gritti et al,24 2020 | Italy | 3/11/20-3/24/20 | Case series | 21 | 64 (48-75) | 14.3 | ( ) | All | 8 (median) |
| 14 | Guan et al,25 2020 | China | 12/11/19-1/29/20 | Retrospective cohort | 1099 | 47 (35-58) | 41.9 | B | All | 13 |
| 15 | Hajifathalian et al,26 2020 | US | 3/4/20-4/16/20 | Case series | 1059 | 61±18 | 42.3 | B | 768 | Up to 34 |
| 16 | Han et al,27 2020 | China | 2/13/20-2/29/20 | Retrospective cohort | 206 | 62.5±32.5 | 55.8 | NS | All (for monitoring, quarantine) | 19-35 |
| 17 | Hsih et al,28 2020 | Taiwan | 1/20/20-2/19/20 | Case series | 2 | 45 (39-51) | 50 | ( ) | All | 35 |
| 18 | Huang et al,29 2020 | China | 12/16/19-1/2/20 | Prospective cohort | 41 | 49 (41-58)c | 27 | NS | All | ( ) |
| 19 | Huang et al,30 2020 | China | 1/21/20-2/1/20 | Case series | 11 | NA | 72.7 | ( ) | ( ) | ( ) |
| 20 | Huang et al,31 2020 | China | NA | Case series | 2 | 73.5 (73-74) | 100 | NS | All | 9 |
| 21 | Huang et al,32 2020 | China | 12/21/19-1/8/20 | Case series | 34 | 56.2 (26-88) | 58.8 | B | 33 | ( ) |
| 22 | Jin et al,33 2020 | China | 1/17/20-2/8/20 | Case series | 651 | 46.4±14.19 | 49.1 | B | All | ( ) |
| 23 | Kim ES et al,34 2020 | Korea | 1/19/20-2/17/20 | Case series | 28 | 40 (20-73) | 46.4 | B | All | Median time of off-isolation/discharge was 18.5 d after symptom onset (range, 11-27) |
| 24 | Klopfenstein et al,35 2020 | France | 3/1/20-3/17/20 | Case series | 114 | 56±18 | 59 | ( ) | ( ) | ( ) |
| 25 | Kluytmans et al,36 2020 | the Netherlands | 3/7/20-3/12/20 | Case series | 86 | 49 (22-66) | 83 | ( ) | 2 | 8 (range, 1-20) |
| 26 | Kuang et al,37 2020 | China | 1/1/20-2/10/20 | Retrospective cohort | 944 | 47 (21-96) | 49.6 | ( ) | All | ( ) |
| 27 | Liu et al,38 2020 | China | 12/30/19-1/24/20 | Case series | 137 | 57 (20-83) | 54.4 | ( ) | All | ( ) |
| 28 | Kujawski et al,39 2020 | US | 1/20/20-2/2/20 | Case series | 12 | 53 (21-68) | 33.3 | B | 7 | 1-14 |
| 29 | Lechien et al,40 2020 | Europe | ( ) | Case series | 417 | 36.9 (19-77) | 63.1 | ( ) | ( ) | ( ) |
| 30 | Li et al,41 2020 | China | 1/20-2/20 | Case series | 83 | 45±12.3) | 47 | B | All | ( ) |
| 31 | Lin et al,42 2020 | China | 1/17/20-2/15/20 | Case series | 95 | 45.3 | 52.6 | ( ) | ( ) | ( ) |
| 32 | Liu et al,43 2020 | China | 1/11/20-1/20/20 | Case series | 12 | 49 (10-72) | 33 | B | All | ( ) |
| 33 | Luo et al,4 2020 | China | 1/1/20-2/20/20 | Case series | 1141 | 53.8 | 44 | B | All | ( ) |
| 34 | Nobel et al,44 2020 | US | 3/10/20-3/21/20 | Case control | 278 | ( ) | 48 | B | 207 | 8 |
| 35 | Pan et al,45 2020 | China | 1/12/20-2/6/20 | Retrospective cohort | 21 | 35 (21-59) | 71.4 | NS | All | 26 |
| 36 | Pan et al,46 2020 | China | 1/18/20-2/28/20 | Retrospective cohort | 103 | 52 | 47.5 | B | All | 20-61 |
| 37 | Pung et al,47 2020 | Singapore | ( )-2/15/20 | Case series | 17 | 40 (36-51) | 59 | ( ) | All | ≥22 |
| 38 | Redd et al,48 2020 | US | Until 4/2/20 | Retrospective cohort | 318 | 63.4±16.6 | 45.3 | B | All | ( ) |
| 39 | Ren et al,49 2020 | China | 12/18/19-12/29/19 | Case series | 5 | 53.6 (41-65) | 40 | S | All | 17 |
| 40 | Shi et al,50 2020 | China | 12/20/19-1/23/20 | Retrospective cohort | 81 | 49.5 (25-81) | 48 | NS | All | ( ) |
| 41 | Shi et al,51 2020 | China | 1/20/20-2/10/20 | Retrospective cohort | 416 | 64 (21-95) | 50.7 | ( ) | All | ( ) |
| 42 | Shu et al,52 2020 | China | 2/13/20-2/29/20 | Retrospective cohort | 545 | 50 (38-58) | 51.6 | NS | All | ( ) |
| 43 | Siegel et al,53 2020 | US | 2/20 | Case series | 3 | 38.6 (26-50) | 0 | ( ) | All | 9 |
| 44 | Song et al,54 2020 | China | 1/20/20-1/27/20 | Case series | 51 | 49±16 | 50.9 | ( ) | All | 5 |
| 45 | Spiteri et al,55 2020 | Italy | 1/24/20-2/21/20 | Case series | 38 | 42 (2-81) | 34.2 | ( ) | 35 | Up to 20 |
| 46 | Tabata et al,56 2020 | Japan | 2/11/20-2/25/20 | Case series | 104 | 68 (25-93) | 53.3 | B | 10 | 3-15 (median, 10) |
| 47 | Wan et al,57 2020 | China | 2/12/20-3/6/20 | Case series | 230 | 47.5 (7-90) | 44 | B | All | ( ) |
| 48 | Wang et al,58 2020 | China | 1/1/20-1/28/20 | Case series | 138 | 56 (22-92) | 45.7 | B | All | ( ) |
| 49 | Wang et al,59 2020 | China | 1/21/20-2/5/20 | Case series | 18 | 39 (29-55) | 5.5 | B | All | 3-18 |
| 50 | Wang et al,60 2020 | China | 1/31/20-2/12/20 | Case series | 26 | 42.0 (33.5-53.3) | 57 | ( ) | All | ( ) |
| 51 | Wang et al,61 2020 | China | 1/1/20-2/6/20 | Case series | 339 | 69 (65-76) | 49.8 | B | All | 28 |
| 52 | Wang et al,62 2020 | China | 2/7/20-2/12/20 | Case series | 1012 | 50 (16-89) | 48.2 | ( ) | All | 24 |
| 53 | Wang et al,63 2020 | China | 1/21/20-1/24/20 | Case series | 4 | 47.5 (19-63) | 25 | ( ) | All | ( ) |
| 54 | Wei et al,64 2020 | China | 1/19/20-2/7/20 | Case series | 84 | 37 (24-74) | 66.6 | ( ) | All | 13-32 |
| 55 | Wolfel et al,65 2020 | Germany | 1/23/20-( ) | Case series | 17 | 40 (36-51) | 58.8 | ( ) | All | ( ) |
| 56 | Wu et al,66 2020 | China | 1/22/20-2/14/20 | Retrospective cohort | 80 | 46.1 (30.7-61.5)c | 51.3 | ( ) | All | 24 |
| 57 | Wu et al,67 2020 | China | 1/20-2/20 | Case series | 80 | 44±11 | 47.5 | ( ) | All | ( ) |
| 58 | Wu et al,68 2020 | China | 1/16/20-3/15/20 | Case series | 74 | ( ) | 24.3 | ( ) | All | ( ) |
| 59 | Xia et al,69 2020 | China | ( ) | Case series | 10 | 56.5±11.16 | 40 | ( ) | All | ( ) |
| 60 | Xiao et al,70 2020 | China | 2/1/20-2/14/20 | Case series | 73 | 43 (10 mo-78 y) | 65.7 | B | All | 26 |
| 61 | Xie et al,71 2020 | China | 2/2/20-2/23/20 | Case series | 79 | 60.0 (48.0-66.0) | 44.3 | B | All | 11.9 |
| 62 | Xiong et al,72 2020 | China | 1/11/20-2/5/20 | Case series | 42 | 49.5 (26-75) | 40.5 | NS | All | 22 |
| 63 | Xu et al,73 2020 | China | 1/23/20-2/4/20 | Case series | 90 | 50 (18-86) | 56.6 | B | All | ( ) |
| 64 | Xu et al,74 2020 | China | 1/10/20-1/26/20 | Case series | 62 | 41 (32-52) | 44 | NS | All | 17 |
| 65 | Yang F et al,75 2020 | China | 1/1/20-4/15/20 | Case series | 52 | 63 (34-98) | 46.2 | B | All | 41 |
| 66 | Yang et al,76 2020 | China | 12/24/19-1/26/20 | Case series | 52 | 59.7±13.3 | 33 | S | All | 15-48 |
| 67 | Young et al,77 2020 | Singapore | 1/23/20-2/3/20 | Case series | 18 | 47 (31-73) | 50 | NS | All | 23-34 |
| 68 | Yu et al,78 2020 | China | 1/20/20-1/23/20 | Case series | 4 | 74.5 (65-88) | 50 | B | ( ) | 18 |
| 69 | Zhang et al,79 2020 | China | 1/2/20-2/10/20 | Case series | 221 | 55.0 (39.0-66.5) | 51 | B | All | 6-45 |
| 70 | Zhang et al,80 2020 | China | 12/19-2/16/20 | Case series | 140 | 57 (25-87) | 50 | B | All | ( ) |
| 71 | Zhang et al,81 2020 | China | 1/27/20-2/10/20 | Case series | 14 | 41 (18-87) | 50 | ( ) | All | 1-14 |
| 72 | Zhao et al,82 2020 | China | 1/2/20-2/5/20 | Retrospective cohort | 19 | 48 (27-56) | 42.1 | ( ) | All | ( ) |
| 73 | Zhao et al,83 2020 | China | ( ) | Case series | 101 | 44.4 (17-75) | 44.5 | B | All | ( ) |
| 74 | Zhao et al,84 2020 | China | 1/16/20-2/10/20 | Case series | 91 | 46 () | 46.2 | B | All | 1-26 |
| 75 | Zhou et al,85 2020 | China | 12/29/19-1/31/20 | Retrospective cohort | 191 | 56 (46-67) | 38 | S | All | ( ) |
| 76 | Zhou et al,86 2020 | China | 1/16/20-1/30/20 | Case series | 62 | 52.8 (30-77) | 37.1 | ( ) | All | 14 |
| 77 | Zhou et al,87 2020 | China | 12/20/19-2/9/20 | Case series | 254 | 50.6 (15-87) | 54.7 | ( ) | All | ( ) |
| 78 | Zou et al,88 2020 | China | 1/7/20-1/26/20 | Case series | 18 | 59 (26-76) | 50 | B | All | ( ) |
( ) = missing; All = if all were hospitalized, number represents patients who were hospitalized; B = both; C-NERC = COVID-19 National Emergency Response Center; C-NIRST = COVID-19 National Incident Room Surveillance Team; NA = not available; NS = nonsevere; S = severe.
Represented as median (range) or mean ± SD, unless otherwise specified.
Represented as median (interquartile range).
Methodological Quality of Included Studies
The risk of bias of included studies is shown in Supplemental Table 2. Risk of bias was high in 48 studies, medium in 24 studies, and low in 6 studies.
Characteristics of Included Studies
The 78 included studies reported a total of 12,767 patients.4 , 5 , 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88 Of these studies, 57 were performed in mainland China; 6 in the United States; 1 in Australia; 1 in Europe; 1 in France; 1 in Germany; 1 in the Netherlands; 1 in Hong Kong; 2 in Italy; 1 in Spain; 1 in Japan; 1 in Korea; 1 in South Korea; 1 in Taiwan; and 2 in Singapore (Table). Among the studies performed in China, 24 were from Wuhan City in Hubei province; 4 included data from the entire Hubei province; 1 from Jingzhou city; 2 from Beijing City; 3 from Chongqing city; 3 from Shanghai; 3 from Zhuahi City; 2 from Nanjing province; 1 from Anhai province; 3 from Zhejiang province; 3 from Guangdong province; 1 from Zhengzou province; 1 from Hunan province; 1 from Jiangsu province; 1 from Shenzun province; and 1 from all provinces in China. The earliest study recruitment period started from December 11, 2019, and the last date of patient enrollment was April 16, 2020. Twelve studies were retrospective cohort studies, 1 was a prospective cohort study, 1 was a case-control study, and the remaining 64 were case series (Table).
The age distribution among the included patients ranged from 10 months to 96 years, and 58.4% (7455 of 12,767) were female. Of the included studies, 64 included only patients who were hospitalized (in 1 study, patients with mild symptoms were included, but they were hospitalized for monitoring and quarantine), 10 studies included both inpatients and outpatients, 1 included outpatients only, and 3 studies did not mention the location of patients. Thirty-four studies reported the severity of COVID-19 infection in patients. Among these studies, 3 included patients with severe disease only, 24 included patients with both severe and nonsevere disease, and 7 included patients with nonsevere disease only. The follow-up period was variable and ranged from 1 to 69 days; only 41 studies provided information on follow-up period.
Twelve studies (total 140 patients) reported the presence or absence of preexisting GI disease. The GI comorbid conditions reported were gastroesophageal reflux disease in 80, irritable bowel syndrome in 5, inflammatory bowel disease in 4, peptic ulcer disease in 9, Helicobacter pylori infection in 10, chronic liver disease in 16, hepatitis B virus infection in 1, fatty liver disease in 1, and other GI conditions in 14. The GI symptoms at onset of illness were assessed in 6 studies, at admission to the hospital in 17, data given separately for both in 3 studies and were not specified in 52 studies (Supplemental Table 3, available online at http://www.mayoclinicproceedings.org). Only 1 study reported data for how many patients had GI symptoms with and without pulmonary symptoms. In this study, 23.3% (48 of 206) of patients had GI symptoms only, 43.2% (89 of 206) had pulmonary symptoms only, and 33.5% (69 of 206) had both.
Prevalence of GI Symptoms
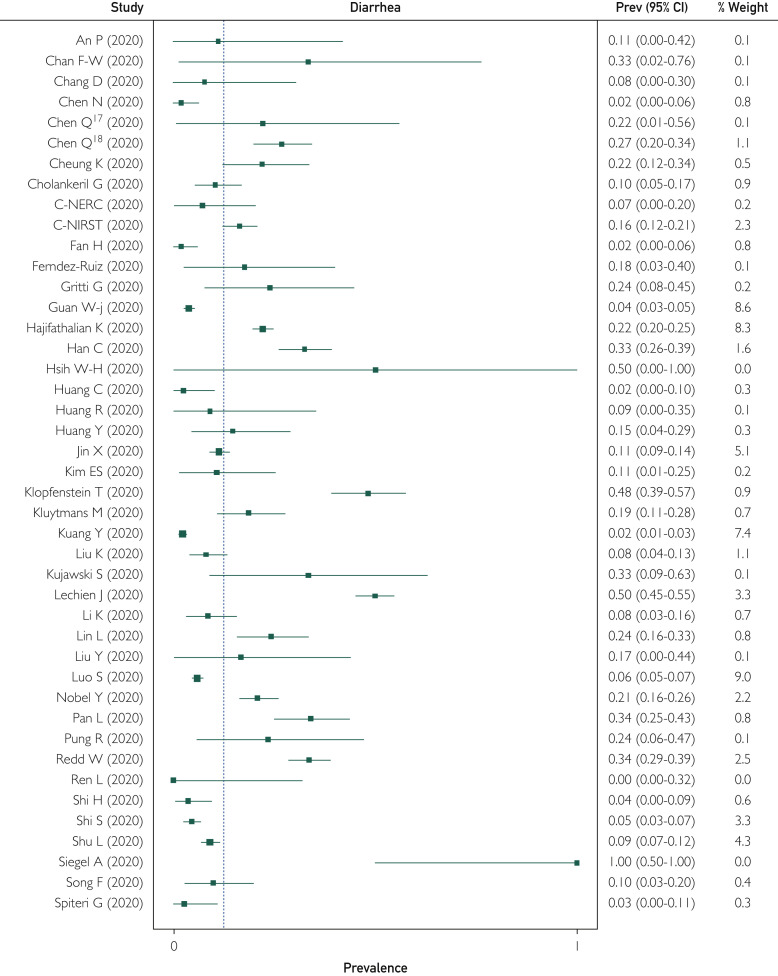
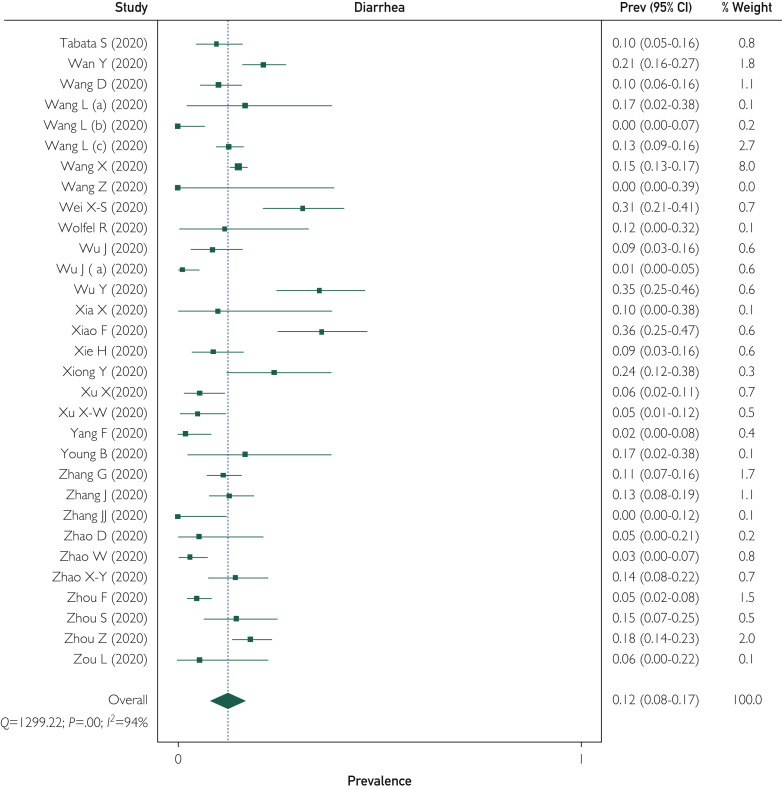
Of the included studies, 74 reported the prevalence of diarrheal symptoms in patients with COVID-19 infection ranging from 0% to 100%. Overall, of the 12,688 patients, 1773 reported diarrhea; the WPP was 12.4% (95% CI, 8.2% to 17.1%). There was significant heterogeneity among the studies, with I 2=94% (Figure 2 ). Publication bias was seen on visual inspection of a funnel plot (Supplemental Figure 1, available online at http://www.mayoclinicproceedings.org). There was 1 outlier study (Siegel et al53) with only 3 patients, all of whom had diarrhea (inclusion was patients presenting with predominantly GI symptoms). On removing this study, the WPP was unchanged (12.4%; 95% CI, 8.2% to 17.1%; I 2=94%; P=.90).
Figure 2.
Forest plot shows weighted pooled prevalence (Prev) of diarrhea in patients with coronavirus disease 2019 infection. C-NERC = COVID National Emergency Response Center; C-NIRST = Coronavirus Disease 2019 National Incident Room Surveillance Team; Q = Cochrane's Q test.
The prevalence of nausea and/or vomiting with COVID-19 infection was reported in 42 studies. Among a total of 9696 patients, 988 reported nausea and/or vomiting with a WPP of 9.0% (95% CI, 5.5% to 12.9%). There was significant heterogeneity among the studies with I 2=93% (Supplemental Figure 2, available online at http://www.mayoclinicproceedings.org). Removing the outlier study, the WPP was unchanged (9.0%; 95% CI, 5.5% to 12.9%; I 2=93%).
Other GI symptoms reported were loss of appetite and abdominal pain, reported in 20 and 27 studies, respectively. Among the 20 studies reporting loss of appetite, prevalence ranged from 1% to 100%; overall, 744 of 3201 patients with COVID-19 infection reported loss of appetite with a WPP of 22.3% (95% CI, 11.2% to 34.6%; I 2=94%; Supplemental Figure 3, available online at http://www.mayoclinicproceedings.org).
Among the 27 studies reporting on abdominal pain, prevalence ranged from 0% to 50%; overall, 418 of 5896 patients with COVID-19 infection reported abdominal pain, with a WPP of 6.2% (95% CI, 2.6% to 10.3%; I 2=92%; Supplemental Figure 4, available online at http://www.mayoclinicproceedings.org).
Subgroup and Sensitivity Analyses
For diarrhea, 9 studies reported symptoms at onset of illness, with a WPP of 8.1% (95% CI, 1.3% to 16.6%; I 2=95%). This was similar to the WPP from 19 studies that reported diarrhea at hospital admission (10.2%; 95% CI, 4.0% to 17.4%; I 2=95%; P=.68). For nausea/vomiting, 5 studies reported symptoms at illness onset with a WPP of 12.1% (95% CI, 9.4% to 14.9%; I 2=26%). This was similar to the estimate from 10 studies reporting symptoms at admission (WPP=8.3%; 95% CI, 0.3% to 19.2%; I 2=96%; P=.45).
Loss of appetite at illness onset was reported in 4 studies (WPP=28.9%; 95% CI, 11.5% to 48.1%; I 2=84%), which was similar to the estimate from the 3 studies reporting it at admission (WPP=16.3%; 95% CI, 0% to 39.8%; I 2=80%; P=.36). Four studies each reported abdominal pain at illness onset (WPP=4.1%; 95% CI, 1.5% to 7.3%; I 2=50%) and at admission (WPP=7.3%; 95% CI, 0% to 18.6%; I 2=67%), with the estimates being statistically similar (P=.53).
For diarrhea, 53 studies were from China, while 21 studies were from outside China. The WPP of diarrhea was higher in the non-China subgroup (25.3%; 95% CI, 14.8% to 36.5%; I 2=92% vs 9.2%; 95% CI, 5.8% to 12.9%; I 2=91%; P=.01). For nausea/vomiting, the WPP from the 12 studies in the non-China subgroup (17.4%; 95% CI, 11.3% to 24%; I 2=96%) was higher than that from the 30 studies in the China subgroup (6.4%; 95% CI, 3.4% to 9.8%; I 2=90%; P<.001]. The WPP of loss of appetite was similar in both subgroups (non-China: 4 studies; WPP=27.3%; 95% CI, 13.9% to 41.7%; I 2=85% vs China: 16 studies; WPP=21.4%; 95% CI, 7.7% to 36.9%; I 2=95%; P=0.57). The WPP of abdominal pain was higher in the non-China subgroup (13 studies; WPP=12%; 95% CI, 3.4% to 22.3%; I 2=91% vs China subgroup: 14 studies; WPP=3.5%; 95% CI, 2.3% to 4.8%; I 2=53%), though this was not statistically significant (P=.08).
On performing sensitivity analyses by removing studies with high risk of bias, estimates did not change and heterogeneity did not decrease for any symptom (all P>.05).
Mortality Among Patients With COVID-19 Infection
A total of 42 studies reported overall mortality among patients with COVID-19 infection, which ranged from 0% to 100%. One study included only patients who had died of COVID-19 infection and hence was excluded from this analysis. Of the 8122 patients in the remaining 41 studies, 320 died with a WPP for overall mortality of 2.1% (95% CI, 0.2% to 4.7%). There was significant heterogeneity among the studies with an I 2=94% (Figure 3 ). Cause of death (regardless of whether attributable to COVID-19 infection) was not specified in any of the studies. Publication bias was seen on visual inspection of a funnel plot (Supplemental Figure 5, available online at http://www.mayoclinicproceedings.org).
Figure 3.
Forest plot shows weighted pooled rate of overall mortality in coronavirus disease 2019 infection. C-NIRST = Coronavirus Disease 2019 National Incident Room Surveillance Team; Prev = prevalence; Q = Cochrane's Q test.
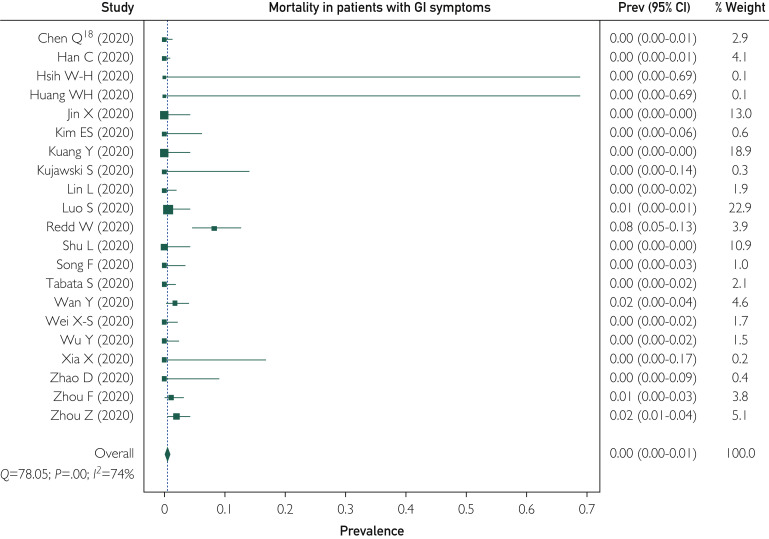
Twenty-one studies reported mortality as an outcome among patients with GI symptoms. Of a total of 4983 patients, 34 died with a WPP of 0.4% (95% CI, 0% to 1.1%). There was significant heterogeneity among the studies with I 2=74% (Figure 4 ). Mortality among patients with GI symptoms was similar to the overall mortality (P=.15).
Figure 4.
Forest plot shows weighted pooled rate of mortality in coronavirus disease 2019 infection with gastrointestinal (GI) symptoms. Prev = prevalence; Q = Cochrane's Q test.
Subgroup analyses by study location provided similar estimates of overall mortality (non-China subgroup: 13 studies; WPP=5.8%; 95% CI, 0.9% to 11.9%; I 2=88% vs China subgroup: 28 studies; WPP=1.2%; 95% CI, 0% to 3.5%; I 2=94%; P=.12). Mortality estimates were similar in patients presenting with GI symptoms (non-China subgroup: 5 studies; WPP=3.8%; 95% CI, 0% to 12.3%; I 2=78% vs China subgroup: 16 studies; WPP=0.3%; 95% CI, 0% to 0.7%; I 2=56%; P=.27).
On performing sensitivity analyses by removing studies with high risk of bias, estimates and between-study heterogeneity did not decrease for either mortality in patients with GI symptoms or overall mortality.
Quality of Evidence
Per the GRADE framework, the quality of evidence for the prevalance of GI symptom outcome was low because of study design (observational studies only), lack of consistency of methodology, presence of publication bias, and significant heterogeneity in all effect estimates. The quality of evidence was considered very low for mortality outcome because of study design (observational studies only), lack of consistency of methodology, presence of publication bias, significant heterogeneity, and inconsistency among reported results. Additionally, none of the studies had adjusted for any potential confounding factors for mortality outcome (Supplemental Table 4, available online at http://www.mayoclinicproceedings.org).
Discussion
In this systematic review and meta-analysis, we found that GI symptoms were present in up to 1 in 5 patients with COVID-19 infection. The highest prevalence was for loss of appetite (approximately one-fifth of patients), whereas the other symptoms occurred in up to 10% of patients. Mortality among patients with GI symptoms was similar to overall mortality. This must be interepreted with caution because variable follow-up, lack of uniform criteria for COVID-19–attributable mortality, and lack of adjustment for cofounders would affect estimates of mortality. The WPPs of diarrhea and nausea/vomiting were higher in the subgroup of studies conducted outside China compared with studies from China, likely due to increasing awareness and reporting of GI manifestations as the pandemic progressed. There was substantial heterogeneity for all estimates, and publication bias was present. Overall, the quality of evidence was low for outcomes of prevelance of GI symptoms and very low for mortality outcomes.
Several reports have described the occurrence of GI symptoms and possible feco-oral transmission in patients with COVID-19 infection.4 , 29 , 68 A previous systematic review of 6 studies reported diarrhea, nausea, vomiting, and abdominal pain in less than 10% of patients, which is similar to our results.89 One recent meta-analysis of 47 studies with 10,890 patients estimated a pooled prevelance of diarrhea to be 7.7%; nausea/vomiting, 7.8%; and abdominal pain, 2.7%. The study also pooled the prevalence of diarrhea among studies from countries other than China only and found the prevelance of diarrhea in non-China studies to be higher, with a pooled prevelance of 18.3%.6 Another meta-analysis of 29 studies reported a pooled prevalence of digestive symptoms of 15%, with nausea or vomiting, diarrhea, and loss of appetite being the 3 most common symptoms.90 The prevalence of most GI symptoms in our study and the subgroup analyses by study location are similar to prior meta-analyses. Among all GI symptoms, the prevalance of loss of appetite in our study was higher than the other GI symptoms. Loss of appetite is commonly seen with febrile illness, which could contribute to the higher rates. However, fewer included studies reported this symptom, which could affect our estimates.
Several important factors should be considered while interpreting results from our study. Until the date of this review, we are still in the mid-phase of the pandemic, with data reported predominantly being from China. Only a quarter of the included studies are from reports outside China. Additionally, the quality of data collection during pandemics is not robust due to the possibility of inadequate documentation of symptoms. The studies included in this meta-analysis primarily included hospitalized patients. Patients with mild disease who were not admitted to the hospital were not included. Patients with mild to moderate symptoms constitute a majority (81%) of those infected with SARS-CoV-2.3 Exclusion of these patients is likely to affect estimates of symptom prevalence. In the absence of published reports of symptom analyses in this cohort of patients, it is difficult to assess the direction in which our estimates would change and therefore our results are not necessarily generalizable to outpatients.
Another important factor is that GI symptoms may be underreported. Increasing awareness, comprehensive symptom questionnaires, and prospective study design would likely provide more reliable estimates of GI symptoms in future studies. In the studies included, there was also limited information for GI-specific laboratory tests, endoscopy reports, histopathology reports, and imaging. Angiotensin-converting enzyme 2 is one of the receptors to which coronaviruses bind. Angiotensin-converting enzyme 2 is expressed in the lung, and within the GI tract, it is expressed in the small intestine, large intestine, and cholangiocytes.91 This may facilitate the spread of the virus through the GI tract and could explain the occurrence of GI symptoms in COVID-19 infection. Future studies should explore these aspects, which would shed light on the pathophysiology of GI involvement in COVID-19 infections.
We found mortality in patients with GI symptoms to be similar to overall mortality. Of note, mortality reported here is all-cause mortality. Deaths in patients with COVID-19 could be due to the infection itself or to underlying comorbid conditions. A uniform definition for COVID-19–attributable mortality and a standardized time frame (eg, within 30 days) would be essential in assessing the impact of the infection and its differing presentations on mortality. Studies included in the systematic review did not assess the effect of different factors such as age and comorbid conditions on mortality. Moreover, several studies did not report or had limited follow-up, which would affect mortality estimates. By doing a sensitivity analysis based on risk of bias (which incorporates follow-up duration as a quality indicator), we could partly adjust for limited follow-up.
Strengths of this study include a comprehensive search strategy with studies from different countries and assessment of several GI symptoms. However, the study has several limitations. Most of the included studies are retrospective and thus at high risk of bias. There is also evidence of significant heterogeneity and publication bias. Several included studies are from Wuhan, China, making it difficult to exclude the possibility of overlapping patients in different studies. As mentioned, several factors affect mortality estimates. Only 12 included studies had information regarding comorbid GI conditions, which precluded assessment of whether symptoms were attributable to the infection or underlying disease. Only 1 study mentioned whether patients had GI symptoms only or if they had GI symptoms with respiratory symptoms. Hence, we could not assess whether GI symptoms present alone or with other symptoms. Finally, most studies were in the hospital setting. Thus, there was underrepresentation of mild to moderate cases and cases within the community.
Conclusion
Up to 1 in 5 patients with COVID-19 infection have GI symptoms. Clinicians should be aware of the possibility that patients with COVID-19 infection can have GI symptoms and keep a low threshold for testing for the infection. Our study highlights the need for high-quality prospectively collected data, with inclusion of patients in the community setting and exploration of the causes underlying mortality.
Footnotes
Potential Competing Interests: The authors declare no competing interests.
Supplemental Online Material
Supplemental material can be found online at http://www.mayoclinicproceedings.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.World Health Organization Coronavirus disease 2019 (COVID-19): situation report-121. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200520-covid-19-sitrep-121.pdf Accessed May 21, 2020.
- 2.Johns Hopkins University of Medicine, Coronavirus Resource Center. COVID-19 case tracker. 2020. https://coronavirus.jhu.edu/ Accessed May 21, 2020.
- 3.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. https://doi.org/10.1001/jama.2020.2648 [published online ahead of print February 24, 2020]. JAMA. [DOI] [PubMed]
- 4.Luo S., Zhang X., Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19) Clin Gastroenterol Hepatol. 2020;18(7):1636–1637. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung K.S., Hung I.F., Chan P.P. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. https://doi.org/10.1053/j.gastro.2020.03.065 [published online ahead of print April 3, 2020]. Gastroenterology. [DOI] [PMC free article] [PubMed]
- 6.Sultan S., Altayar O., Siddique S.M. on behalf of the American Gastroenterological Association∗. AGA Institute rapid review of the GI and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. https://doi.org/10.1053/j.gastro.2020.05.001 [published online ahead of print May 11, 2020]. Gastroenterology. [DOI] [PMC free article] [PubMed]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Collaborators Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyatt G.H., Oxman A.D., Vist G.E., GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murad M.H. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc. 2017;92(3):423–433. doi: 10.1016/j.mayocp.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Doi S.A.R., Barendregt J.J., Khan S., Thalib L., Williams G.M. Advances in the meta-analysis of heterogeneous clinical trials I: The inverse variance heterogeneity model. Contemp Clin Trials. 2015;45(pt A):130–138. doi: 10.1016/j.cct.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Easterbrook P.J., Berlin J.A., Gopalan R., Matthews D.R. Publication bias in clinical research. Lancet. 1991;337(8746):867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 13.An P., Chen H., Jiang X. Clinical features of 2019 novel coronavirus pneumonia presented gastrointestinal symptoms but without. https://ssrn.com/abstract=3532530 fever onset [published online ahead of print February 6, 2020]. Lancet. or. Accessed May 21, 2020. [DOI]
- 14.Chan J.F.-W., Yuan S., Kok K.-H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang D., Lin M., Wei L. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323(11):1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q., Quan B., Li X. A report of clinical diagnosis and treatment of nine cases of coronavirus disease 2019. J Med Virol. 2020;92(6):683–687. doi: 10.1002/jmv.25755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Q., Zheng Z., Zhang C. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou. https://doi.org/10.1007/s15010-020-01432-5 Zhejiang, China [published online ahead of print April 28, 2020]. Infection. [DOI] [PMC free article] [PubMed]
- 19.Cholankeril G., Podboy A., Aivaliotis V.I. High prevalence of concurrent gastrointestinal manifestations in patients with SARS-CoV-2: early experience from California. https://doi.org/10.1053/j.gastro.2020.04.008 [published online ahead of print April 10, 2020]. Gastroenterology. [DOI] [PMC free article] [PubMed]
- 20.COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res Perspect 2020. 2020;11(1):8–14. doi: 10.24171/j.phrp.2020.11.1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID-19 National Incident Room Surveillance Team COVID-19, Australia: Epidemiology Report 7 (reporting week ending 19:00 AEDT 14 March 2020) https://doi.org/10.33321/cdi.2020.44.23 [published online ahead of print March 19, 2020]. Commun Dis Intell (2018) [DOI] [PubMed]
- 22.Fan H., Zhang L., Huang B. Retrospective analysis of clinical features in 101 death cases with COVID-19. [preprint posted March 17, 2020]. medRxiv. [DOI]
- 23.Fernandez-Ruiz M., Andres A., Loinaz C. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. https://doi.org/10.1111/ajt.15929 [published online ahead of print April 16, 2020]. Am J Transplant. [DOI] [PMC free article] [PubMed]
- 24.Gritti G., Raimondi F., Ripamonti D. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. [preprint posted May 22, 2020]. MedRxiv. [DOI]
- 25.Guan W.J., Ni Z.Y., Hu Y. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajifathalian K., Krisko T., Mehta A., WCM-GI research group Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical implications. https://doi.org/10.1053/j.gastro.2020.05.010 [published online ahead of print May 7, 2020]. Gastroenterology. [DOI] [PMC free article] [PubMed]
- 27.Han C., Duan C., Zhang S. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115(6):916–923. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsih W.-H., Cheng M.-Y., Ho M.-W. Featuring COVID-19 cases via screening symptomatic patients with epidemiologic link during flu season in a medical center of central Taiwan. J Microbiol Immunol Infect. 2020;53(3):459–466. doi: 10.1016/j.jmii.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang R., Xia J., Chen Y., Shan C., Wu C. A family cluster of SARS-CoV-2 infection involving 11 patients in Nanjing, China. Lancet Infect Dis. 2020;20(5):534–535. doi: 10.1016/S1473-3099(20)30147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang W.H., Teng L.C., Yeh T.K. 2019 Novel coronavirus disease (COVID-19) in Taiwan: reports of two cases from Wuhan, China. J Microbiol Immunol Infect. 2020;53(3):481–484. doi: 10.1016/j.jmii.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y., Tu M., Wang S. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis. https://doi.org/10.1016/j.tmaid.2020.101606 [published online ahead of print February 27, 2020] Travel Med Infect Dis. [DOI] [PMC free article] [PubMed]
- 33.Jin X., Lian J.S., Hu J.H. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim E.S., Chin B.S., Kang C.K., Korea National Committee for Clinical Management of COVID-19 Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 2020;35(13):e142. doi: 10.3346/jkms.2020.35.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klopfenstein T., Kadiane-Oussou N.J., Royer P.Y., Toko L., Gendrin V., Zayet S. Diarrhea: an underestimated symptom in coronavirus disease 2019. Clin Res Hepatol Gastroenterol. 2020;44(3):282–283. doi: 10.1016/j.clinre.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kluytmans M., Buiting A., Pas S. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020. [preprint posted March 31, 2020]. medRxiv. [DOI]
- 37.Kuang Y., Zhang H., Zhou R. Epidemiological and clinical characteristics of 944 cases of 2019 novel coronavirus infection of non-COVID-19 exporting city, Zhejiang, China [preprint posted February 28, 2020] https://ssrn.com/abstract=3543604
- 38.Lui K., Fang Y.-Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kujawski S.A., Wong K.K., Collins J.P. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States [preprint posted March 12, 2020]. MedRxiv. [DOI]
- 40.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. https://doi.org/10.1007/s00405-020-05965-1 [published online ahead of print April 6, 2020]. Eur Arch Otorhinolaryngol. [DOI] [PMC free article] [PubMed]
- 41.Li K., Wu J., Wu F. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55(6):327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin L., Jiang X., Zhang Z. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nobel Y.R., Phipps M., Zucker J. Gastrointestinal symptoms and COVID-19: case-control study from the United States. https://doi.org/10.1053/j.gastro.2020.04.017 [published online ahead of print April 12, 2020]. Gastroenterology. [DOI] [PMC free article] [PubMed]
- 45.Pan F., Ye T., Sun P. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020;295(3):200370. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan L., Mu M., Ren H. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pung R., Chiew C.J., Young B.E., Singapore 2019 Novel Coronavirus Outbreak Research Team Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395(10229):1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redd W.D., Zhou J.C., Hathorn K.E. Prevalence and characteristics of gastrointestinal symptoms in patients with SARS-CoV-2 infection in the United States: a multicenter cohort study. https://doi.org/10.1053/j.gastro.2020.04.045 [published online ahead of print April 22, 2020]. Gastroenterology. [DOI] [PMC free article] [PubMed]
- 49.Ren L.L., Wang Y.M., Wu Z.Q. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J (Engl) 2020;133(9):1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. https://ssrn.com/abstract=3552844https://doi.org/10.1001/jamacardio.2020.0950 [published online ahead of print March 25, 2020]. JAMA Cardiol. or. Accessed May 21, 2020. [DOI] [PMC free article] [PubMed]
- 52.Shu L., Wang X., Li M. Clinical characteristics of 545 cases confirmed COVID-19 in Wuhan Stadium Cabin Hospital. Accessed. [DOI] [PMC free article] [PubMed]
- 53.Siegel A., Chang P.J., Jarou Z.J. Lung base findings of coronavirus disease (COVID-19) on abdominal CT in patients with predominant gastrointestinal symptoms. https://doi.org/10.2214/AJR.20.23232 [published online ahead of print April 17, 2020]. AJR Am J Roentgenol. [DOI] [PubMed]
- 54.Song F., Shi N., Shan F. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295(1):210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spiteri G., Fielding J., Diercke M. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25(9):2000178. doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tabata S., Imai K., Kawano S. The clinical characteristics of COVID-19: a retrospective analysis of 104 patients from the outbreak on board the Diamond Princess cruise ship in Japan [preprint posted April 7, 2020]. medRxiv. [DOI]
- 57.Wan Y., Li J., Shen L. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol. 2020;5(6):534–535. doi: 10.1016/S2468-1253(20)30118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L., Duan Y., Zhang W. Epidemiologic and clinical characteristics of 26 cases of COVID-19 arising from patient-to-patient transmission in Liaocheng, China. Clin Epidemiol. 2020;12:387–391. doi: 10.2147/CLEP.S249903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L., Gao Y.H., Lou L.L., Zhang G.J. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur Respir J. 2020;55(4):2000398. doi: 10.1183/13993003.00398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L., He W., Yu X. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X., Fang J., Zhu Y. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. https://doi.org/10.1016/j.cmi.2020.03.032 [published online ahead of print April 2, 2020]. Clin Microbiol Infect. [DOI] [PMC free article] [PubMed]
- 63.Wang Z., Chen X., Lu Y., Chen F., Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment [erratum in Biosci Trends. 2020;14(1):E1] Biosci Trends. 2020;14(1):64–68. doi: 10.5582/bst.2020.01030. [DOI] [PubMed] [Google Scholar]
- 64.Wei X.-S., Wang X., Niu Y.-R. Clinical characteristics of SARS-CoV-2 infected pneumonia with diarrhea [preprint posted March 10, 2020] https://ssrn.com/abstract=3546120 or. Accessed May 21, 2020. [DOI]
- 65.Wolfel R., Corman V.M., Guggemos W. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 66.Wu J., Liu J., Zhao X. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. [published online ahead of print February 29, 2020]. Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 67.Wu J., Wu X., Zeng W. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest Radiol. 2020;55(5):257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Y., Guo C., Tang L. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia X.Y., Wu J., Liu H.L., Xia H., Jia B., Huang W.X. Epidemiological and initial clinical characteristics of patients with family aggregation of COVID-19. J Clin Virol. 2020;127:104360. doi: 10.1016/j.jcv.2020.104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao F., Tang M., Zheng X., Liu Y., Li X., Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xie H., Zhao J., Lian N., Lin S., Xie Q., Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40(6):1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiong Y., Sun D., Liu Y. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest Radiol. 2020;55(6):332–339. doi: 10.1097/RLI.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu X., Yu C., Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu X.-W., Wu X.-X., Jiang X.-G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series [erratum in BMJ. 2020:368:m792] BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang F., Shi S., Zhu J., Shi J., Dai K., Chen X. Clinical characteristics and outcomes of cancer patients with COVID-19. https://doi.org/10.1002/jmv.25972 [published online ahead of print May 5, 2020]. J Med Virol. [DOI] [PubMed]
- 76.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore [published online ahead of print March 9, 2020; correction in JAMA. 2020;323(15):1510]. JAMA, 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed]
- 78.Yu P., Zhu J., Zhang Z., Han Y. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis. 2020;221(11):1757–1761. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang G., Hu C., Luo L. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang J., Wang S., Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J Med Virol. 2020;92(6):680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. https://doi.org/10.1111/all.14238 [published online ahead of print February 19, 2020]. Allergy. [DOI] [PubMed]
- 82.Zhao D., Yao F., Wang L. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. https://doi.org/10.1093/cid/ciaa247 [published online ahead of print March 12, 2020]. Clin Infect Dis. [DOI] [PMC free article] [PubMed]
- 83.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214(5):1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 84.Zhao X.Y., Xu X.X., Yin H.S. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20(1):311. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214(6):1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 87.Zhou Z., Zhao N., Shu Y., Han S., Chen B., Shu X. Effect of gastrointestinal symptoms on patients infected with COVID-19. Gastroenterology. 2020;158(8):2294–2297. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miri S.M., Roozbeh F., Omranirad A., Alavian S.M. Panic of buying toilet papers: a historical memory or a horrible truth? Systematic review of gastrointestinal manifestations of COVID-19. Hepat Mon. 2020;20(3):e102729. [Google Scholar]
- 90.Mao R., Qiu Y., He J.S. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao Q.Y., Chen Y.X., Fang J.Y. 2019 Novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21(3):125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.