Key Points
Question
Is there an association between migraine with aura and cardiovascular disease (CVD) incidence rates in women, relative to that of other major vascular risk factors?
Findings
In this cohort study that included 27 858 female health professionals aged at least 45 years, the adjusted incidence rate of major CVD was 3.36 per 1000 person-years for women who reported migraine with aura and 2.11 per 1000 person-years for women who reported migraine without aura or no migraine, a difference that was statistically significant.
Meaning
Among female health professionals aged at least 45 years, self-reported migraine with aura was associated with increased incidence rates of CVD, but the clinical importance of this finding remains to be determined.
Abstract
Importance
Migraine with aura is known to increase the risk of cardiovascular disease (CVD). The absolute contribution of migraine with aura to CVD incidence in relation to other CVD risk factors remains unclear.
Objective
To estimate the CVD incidence rate for women with migraine with aura relative to women with other major vascular risk factors.
Design, Setting, and Participants
Female health professionals in the US (the Women’s Health Study cohort) with lipid measurements and no CVD at baseline (1992-1995) were followed up through December 31, 2018.
Exposures
Self-reported migraine with aura compared with migraine without aura or no migraine at baseline.
Main Outcomes and Measures
The primary outcome was major CVD (first myocardial infarction, stroke, or CVD death). Generalized modeling procedures were used to calculate multivariable-adjusted incidence rates for major CVD events by risk factor status that included all women in the cohort.
Results
The study population included 27 858 women (mean [SD] age at baseline, 54.7 [7.1] years), among whom 1435 (5.2%) had migraine with aura and 26 423 (94.8%) did not (2177 [7.8%] had migraine without aura and 24 246 [87.0%] had no migraine in the year prior to baseline). During a mean follow-up of 22.6 years (629 353 person-years), 1666 major CVD events occurred. The adjusted incidence rate of major CVD per 1000 person-years was 3.36 (95% CI, 2.72-3.99) for women with migraine with aura vs 2.11 (95% CI, 1.98-2.24) for women with migraine without aura or no migraine (P < .001). The incidence rate for women with migraine with aura was significantly higher than the adjusted incidence rate among women with obesity (2.29 [95% CI, 2.02-2.56]), high triglycerides (2.67 [95% CI, 2.38-2.95]), or low high-density lipoprotein cholesterol (2.63 [95% CI, 2.33-2.94]), but was not significantly different from the rates among those with elevated systolic blood pressure (3.78 [95% CI, 2.76-4.81]), high total cholesterol (2.85 [95% CI, 2.38-3.32]), or family history of myocardial infarction (2.71 [95% CI, 2.38-3.05]). Incidence rates among women with diabetes (5.76 [95% CI, 4.68-6.84]) or who currently smoked (4.29 [95% CI, 3.79-4.79]) were significantly higher than those with migraine with aura. The incremental increase in the incidence rate for migraine with aura ranged from 1.01 additional cases per 1000 person-years when added to obesity to 2.57 additional cases per 1000 person-years when added to diabetes.
Conclusions and Relevance
In this study of female health professionals aged at least 45 years, women with migraine with aura had a higher adjusted incidence rate of CVD compared with women with migraine without aura or no migraine. The clinical importance of these findings, and whether they are generalizable beyond this study population, require further research.
This cohort study uses Women’s Health Study data to compare the association of migraine with aura vs other major vascular risk factors (diabetes, hypertension, dyslipidemia, smoking, BMI, CVD family history) with incidence of cardiovascular disease.
Introduction
Migraine is a chronic, intermittent primary headache disorder characterized by moderate to severe pain and specific associated features, including nausea/vomiting, photophobia, and phonophobia.1 About one-third of individuals with migraine also report aura symptoms, which are transient neurological symptoms (most often visual disturbances) prior to headache onset caused by changes in brain activity.2 Migraine, particularly migraine with aura, has been consistently associated with increased risk of overall and specific cardiovascular disease (CVD) events on the relative scale,3,4 and migraine has been included in a risk score for CVD.5
Potential reasons for the increased risk of CVD observed among individuals with migraine with aura include impairments of the endovascular function,6 genetic predisposition to both migraine and CVD,7 inflammatory processes,8 and others.9 Migraine and, particularly, migraine with aura have also been associated with other vascular risk factors, such as hypertension,10 smoking,11 lipid levels,12 and body composition.13
When consequences of the association of migraine with CVD on the population level are discussed, it has been argued that the association of migraine with aura on CVD incidence on the absolute scale is small and that other vascular risk factors have much stronger associations.14 Thus, the aim of this study was to compare the adjusted incidence rate of CVD between women with migraine with aura, women with migraine without aura or no migraine, and women with other major vascular risk factors.
Methods
Participants
Study participants were from the Women’s Health Study, a large randomized, placebo-controlled trial designed to examine the effects of aspirin and vitamin E on the primary prevention of CVD and cancer. The design, methods, and main results of the trial have been published previously.15 Briefly, at baseline (1992-1995), 39 876 female health professionals in the US aged 45 years or older without a history of CVD or cancer were randomized to receive low-dose aspirin (vs placebo) or vitamin E (vs placebo). Since the end of the trial in March 2004, women continue to be followed up on an observational basis. The Women’s Health Study was approved by the institutional review board at Brigham and Women’s Hospital and all participants provided written informed consent.
Blood samples were collected in tubes containing ethylenediaminetetraacetic acid prior to randomization and stored in vapor-phase liquid nitrogen (−170 °C). Total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were measured in a core laboratory certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization program.16 Women with missing information on migraine status or whose cholesterol or triglyceride levels could not be measured were excluded.
Women were sent follow-up questionnaires, which included questions about demographics, lifestyle, and health information, twice during the first year of the trial and yearly thereafter. For these analyses, we used data through December 31, 2018. As of that date, follow-up was more than 97% complete.
Migraine Assessment
On the baseline questionnaire, participants were asked about migraine and associated features. In particular, they were asked “Have you ever had migraine headaches?’’ Women who answered positively were asked “In the past year, have you had migraine headaches?’’ Women who experienced migraine headaches within the past year were asked about the characteristics of their headaches, including aura or any indication that a migraine was coming. Agreement of self-reported migraine with International Classification of Headache Disorders II criteria was excellent.17 Based on this self-reported information, participating women were classified as either having migraine with aura or not. Women who indicated migraine without aura or a history of migraine but no migraine headaches in the year prior to baseline have not been shown to have increased risk of any CVD events in prior studies using data from the Women’s Health Study.18,19 Therefore, these women, as well as women with no history of migraine, were included in the reference group. Thus, the reference group was composed of women who reported migraine without aura and those who did not report migraine in the year prior to baseline (including women with and without a history of migraine).
Cardiovascular Outcome Ascertainment
During the follow-up of the Women’s Health Study, participants self-reported cardiovascular events. Medical records were then obtained and reviewed by an end point committee of physicians. Nonfatal stroke was confirmed if the participant had a new focal neurologic deficit of sudden onset that persisted for more than 24 hours, and was classified into its major subtypes (ischemic vs hemorrhagic) based on the available clinical and diagnostic information with an excellent interrater agreement.20 The occurrence of myocardial infarction was confirmed if symptoms met World Health Organization criteria and if the event was associated with abnormal levels of cardiac enzymes or diagnostic electrocardiogram results. Cardiovascular deaths were confirmed by review of autopsy reports, death certificates, medical records, or information obtained from next of kin or family members.
The primary outcome for this study was major CVD incidence, a combined end point defined as the first of any of the following events: nonfatal stroke (ischemic and hemorrhagic), nonfatal myocardial infarction, or death due to CVD. The association with the individual end points of first stroke, first myocardial infarction, and death due to CVD was also evaluated. Because migraine with aura was associated with both ischemic and hemorrhagic stroke in the Women’s Health Study,18,21 we did not evaluate associations with major stroke subtypes separately in this study.
Statistical Analyses
The mean and frequency of vascular risk factors and main baseline characteristics of women who reported migraine with aura were compared with those of women who did not report migraine with aura (including women who reported migraine without aura and those who reported no migraine in the year prior to baseline).
Crude and adjusted incidence rates per 1000 person-years of the CVD outcomes were calculated for women with migraine with aura and women with other vascular risk factors. Adjusted incidence rates were computed using multivariable regression models including potential confounding factors as independent variables. The vascular risk factors were classified in 2 ways: first as dichotomous and second, if possible, as polychotomous variables. In addition to having migraine with aura vs not (including women who reported migraine without aura and those who reported no migraine in the year prior to baseline), we evaluated the following main vascular risk factors: diabetes (yes vs no), smoking (current vs noncurrent or current, past, never), blood pressure (≥160 mm Hg vs <160 mm Hg or <120, 120-129, 130-139, 140-159, ≥160 mm Hg), total cholesterol (≥280 mg/dL vs <280 mg/dL or <160, 160-199, 200-239, 240-279, ≥280 mg/dL), HDL cholesterol (≤40 mg/dL vs >40 mg/dL or ≥60, 50-59, 40-49, <40 mg/dL), triglycerides (≥194 mg/dL vs <194 mg/dL or <77, 77-104, 105-137, 138-193, ≥194 mg/dL), family history of myocardial infarction prior to age 60 (yes vs no), and body mass index (≥30 vs <30 or <25, 25-29, 30-34, ≥35).
The following potential confounding factors were included in all multivariable models: age (5-year categories), alcohol use (≥1 drink per week vs <1 drink per week), exercise (≥1 time per week vs <1 time per week), hypertensive treatment (yes vs no), ever use of hormones (yes vs no), and premenopausal status at baseline (yes vs no).
Three different modeling approaches were used. In the first, adjusted incidence rates of the CVD outcomes were calculated for the levels of the dichotomous vascular risk factors. The adjusted incidence rate for each of the individual vascular risk factors was estimated when each of the respective other vascular risk factors and the confounding factors were set to the mean level observed in the entire study population.
In the second approach, the polychotomous categorization of the main vascular risk factors was used. If the main vascular risk factor did not have a polychotomous categorization available, the dichotomous categorization was used (migraine with aura, diabetes, and family history of myocardial infarction prior to age 60 y). The adjusted incidence rates of the CVD outcomes were calculated for each level of the vascular risk factors, while the levels of the respective other vascular risk factors and the confounding factors were set to the mean level observed in the entire study population. Accordingly, the adjusted incidence rates of the dichotomous factors may differ between the 2 models.
In a third approach, adjusted incidence rates of the CVD outcomes were calculated again for the levels of the dichotomous vascular risk factors. However, in this model, each vascular risk factor was analyzed not as a single main factor but with the observable combination of all possible other vascular risk factors and controlling for the confounding factors as before.
For the model with dichotomous vascular risk factors, t tests were used to test whether the adjusted incidence rates of CVD for women who reported migraine with aura differed from those who did not (including women who reported migraine without aura and those who reported no migraine in the year prior to baseline). We also tested whether the adjusted incidence rates of CVD for women with migraine with aura differed from the adjusted incidence rates of CVD for women with the other vascular risk factors. In the model that included polychotomous risk factors, we used P values for trend across risk factor categories, when applicable, or χ2 tests.
In secondary analyses, the third modeling approach was used. The adjusted incidence rate of the CVD outcomes for different combinations of all individual vascular risk factors with and without adding migraine with aura were calculated for each vascular risk factor combination observed in the study population. Each combination of risk factors was compared when migraine with aura was added vs not having migraine with aura (including women who reported migraine without aura and those who did not report migraine in the year prior to baseline).
Adjusted incidence rates were defined as the least square mean from the regression model, which assumed that observed CVD events had a Poisson distribution and that the logarithm of the expected number of events can be modeled by a linear combination of the risk factors.22,23,24 Using SAS software, version 9.4 (SAS Institute), the GENMOD procedure was used to calculate the least square mean of the Poisson regression model25 and the NLMIXED procedure was used to calculate the P values of the contrast of the adjusted incidence rates.26
Less than 1.7% of the study population had missing information on any variable. Missing information on any of the vascular risk factors was imputed by assigning the median value of that variable. For participants with missing information on other factors, a missing variable indicator was created and included in the multivariable models.
A sensitivity analysis was conducted in which the estimation of the adjusted incidence rates of CVD for migraine with aura and the other vascular risk factors were repeated using multiple imputation to account for missing information. Further details are described in the eAppendix in the Supplement. In another sensitivity analysis, only women who did not report migraine at or prior to baseline were included in the reference group and the primary analysis was repeated.
Two-tailed P values were calculated, and a P value of less than .05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points and analyses should be interpreted as exploratory.
Results
Blood samples were collected in tubes containing ethylenediaminetetraacetic acid from 28 345 women. Total cholesterol and triglyceride analyses were performed on 27 938 of the blood samples. A total of 79 women with missing information on migraine status on the baseline questionnaire and 1 woman who reported a history of myocardial infarction prior to baseline during follow-up were excluded.
The study population included 27 858 women (mean [SD] age at baseline, 54.7 (7.1) years), including 1435 (5.2%) who had migraine with aura and 26 423 who did not (2177 [7.8%] had migraine without aura and 24 246 [87.0%] did not report migraine in the year prior to baseline). The baseline characteristics of select vascular risk factors for women with migraine with aura and women without migraine with aura are summarized in Table 1. Women with migraine with aura were slightly younger, had higher triglycerides levels, and more frequently reported a family history of premature myocardial infarction. They also reported drinking less alcohol and were more likely to have ever used postmenopausal hormones.
Table 1. Baseline Characteristics of Participants in a Study of the Association of Migraine With Aura With Incident Cardiovascular Disease in Women (N = 27 858).
| Characteristic | No. (%) | |
|---|---|---|
| Migraine with aura (n = 1435) | No migraine with aura (n = 26 423)a | |
| Age, mean (SD), y | 53.2 (6.1) | 54.8 (7.1) |
| Diabetes | 24 (1.7) | 658 (2.5) |
| Smoking | ||
| Current | 151 (10.5) | 3090 (11.7) |
| Past | 525 (36.6) | 9685 (36.6) |
| Systolic blood pressure, mean (SD), mm Hg | 122.7 (13.1) | 123.7 (13.8) |
| Cholesterol, mean (SD), mg/dL | ||
| Total | 211.9 (41.7) | 211.8 (41.8) |
| HDL | 53.1 (14.4) | 53.8 (15.1) |
| Triglycerides, median (IQR), mg/dL | 126.0 (90.0-184.0) | 118.0 (83.0-175.0) |
| Family history of myocardial infarction prior to age 60 y | 231 (16.3) | 3714 (14.3) |
| BMI, mean (SD) | 25.9 (4.9) | 25.9 (5.0) |
| BMI ≥30 | 244 (17.0) | 4695 (17.8) |
| Ever use of postmenopausal hormones | 847 (59.0) | 13468 (51.0) |
| Alcohol use at least once per week | 554 (38.6) | 11294 (42.8) |
| Exercise at least once per week | 597 (41.6) | 11420 (43.2) |
| Hypertensive treatment | 184 (12.8) | 3540 (13.4) |
| Premenopausal | 390 (27.3) | 7263 (27.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HDL, high-density lipoprotein; IQR, interquartile range.
SI conversion factors: To convert total and HDL cholesterol to mmol/L, multiply by 0.0259; to convert triglycerides to mmol/L, divide by 88.57.
All women who did not report migraine with aura in the year prior to baseline, including women who reported migraine without aura and those who did not report migraine in the year prior to baseline.
During a mean follow-up of 22.6 years (629 353 total person-years), 1666 major CVD events, 887 strokes (fatal and nonfatal), 629 myocardial infarctions (fatal and nonfatal), and 391 deaths due to CVD occurred. The crude overall incidence rate per 1000 person-years was 2.87 for major CVD, 1.52 for total stroke, 1.07 for myocardial infarction, and 0.62 for deaths due to CVD.
With the exception of obesity, all major risk factors were statistically significantly associated with major CVD incidence (eTable 1 in the Supplement). Results for the individual cardiovascular outcomes are summarized in eTables 2, 3, and 4 in the Supplement.
The adjusted incidence rate of major CVD was 3.36 cases per 1000 person-years (95% CI, 2.72-3.99) among women with migraine with aura (adjusted for other dichotomous vascular risk factors and confounding variables) and 2.11 per 1000 person-years (95% CI, 1.98-2.24) for women with migraine without aura or no migraine (P value comparing the adjusted incidence rates of migraine without aura vs migraine with aura <.001) (Table 2). Compared with having migraine with aura, only the presence of 2 other risk factors led to significantly higher adjusted incidence rates of major CVD: diabetes (adjusted incidence rate, 5.76 [95% CI, 4.68-6.84]; P value comparing the incidence rate to that of migraine with aura <.001) and current smoking (adjusted incidence rate, 4.29 [95% CI, 3.79-4.79]; P value comparing the incidence rate with that of migraine with aura = .02). A similar pattern was observed for myocardial infarction and CVD death (Table 2). Women with migraine with aura did not have a significantly different rate of major CVD compared with women who had the highest level of systolic blood pressure and total cholesterol and family history of myocardial infarction. In contrast, the incidence rate of major CVD was significantly higher for women with migraine with aura than the incidence rate among those with obesity (2.29 [95% CI, 2.02-2.56]; P value comparing the incidence rate with that of migraine with aura = .001) or those who had unfavorable lipid profiles (triglycerides ≥194 mg/dL: 2.67 [95% CI, 2.38-2.95]; P value comparing the incidence rate with that of migraine with aura = .043; HDL cholesterol <40 mg/dL: 2.63 [95% CI, 2.33-2.94]; P value comparing the incidence rate with that of migraine with aura = .04) (Table 2).
Table 2. Incidence Rates of Cardiovascular Disease (CVD) per 1000 Person-Years According to Vascular Risk Factors in a Study of the Association of Migraine With Aura With Incident CVD (N = 27 858)a.
| Vascular risk factor | No. of events/total No. | Person- years | Crude rate | Adjusted rate (95% CI)a | P valueb |
|---|---|---|---|---|---|
| Major CVD (n = 1666) | |||||
| Migraine with aura | 110/1435 | 29 956 | 3.67 | 3.36 (2.72-3.99) | |
| Migraine without aura or no migraine | 1556/26423 | 550 656 | 2.83 | 2.11 (1.98-2.24) | <.001 |
| Diabetes | 134/682 | 11 387 | 11.77 | 5.76 (4.68-6.84) | <.001 |
| Current smoking | 313/3241 | 60 511 | 5.17 | 4.29 (3.79-4.79) | .02 |
| Systolic blood pressure ≥160 mm Hg | 58/318 | 5636 | 10.29 | 3.78 (2.76-4.81) | .48 |
| Total cholesterol ≥280 mg/dL | 163/1645 | 33 094 | 4.93 | 2.85 (2.38-3.32) | .19 |
| HDL cholesterol <40 mg/dL | 413/4822 | 95 299 | 4.33 | 2.63 (2.33-2.94) | .04 |
| Triglycerides ≥194 mg/dL | 517/5546 | 110 583 | 4.68 | 2.67 (2.38-2.95) | .043 |
| Family history of myocardial infarction prior to age 60 y | 267/3945 | 82 544 | 3.24 | 2.71 (2.38-3.05) | .07 |
| Body mass index ≥30 | 360/4939 | 100 284 | 3.59 | 2.29 (2.02-2.56) | .001 |
| Total stroke (n = 887) | |||||
| Migraine with aura | 57/1435 | 30 329 | 1.88 | 1.78 (1.31-2.24) | |
| Migraine without aura or no migraine | 830/26423 | 555 289 | 1.50 | 1.17 (1.07-1.26) | .01 |
| Diabetes | 55/682 | 11 934 | 4.61 | 2.50 (1.79-3.21) | .09 |
| Current smoking | 138/3241 | 61 724 | 2.24 | 1.92 (1.58-2.25) | .61 |
| Systolic blood pressure ≥160 mm Hg | 28/318 | 5796 | 4.83 | 1.94 (1.19-2.69) | .71 |
| Total cholesterol ≥280 mg/dL | 81/1645 | 33 650 | 2.41 | 1.53 (1.17-1.88) | .39 |
| HDL cholesterol <40 mg/dL | 193/4822 | 97 013 | 1.99 | 1.37 (1.14-1.59) | .11 |
| Triglycerides ≥194 mg/dL | 239/5546 | 112 615 | 2.12 | 1.30 (1.10-1.49) | .06 |
| Family history of myocardial infarction prior to age 60 y | 136/3945 | 83 507 | 1.63 | 1.42 (1.18-1.67) | .17 |
| Body mass index ≥30 | 178/4939 | 101 445 | 1.76 | 1.24 (1.04-1.44) | .03 |
| Myocardial infarction (n = 629) | |||||
| Migraine with aura | 43/1435 | 30 387 | 1.42 | 1.21 (0.84-1.58) | |
| Migraine without aura or no migraine | 586/26423 | 555 777 | 1.05 | 0.77 (0.69-0.85) | .02 |
| Diabetes | 65/682 | 11 674 | 5.57 | 2.58 (1.86-3.29) | .001 |
| Current smoking | 150/3241 | 61 325 | 2.45 | 1.92 (1.58-2.25) | .004 |
| Systolic blood pressure ≥160 mm Hg | 20/318 | 5787 | 3.46 | 1.23 (0.66-1.79) | .96 |
| Total cholesterol ≥280 mg/dL | 68/1645 | 33 563 | 2.03 | 1.10 (0.81-1.38) | .62 |
| HDL cholesterol <40 mg/dL | 191/4822 | 96 484 | 1.98 | 1.10 (0.90-1.30) | .58 |
| Triglycerides ≥194 mg/dL | 233/5546 | 112 134 | 2.08 | 1.14 (0.95-1.33) | .74 |
| Family history of myocardial infarction prior to age 60 y | 114/3945 | 83 403 | 1.37 | 1.08 (0.88-1.29) | .54 |
| Body mass index ≥30 | 135/4939 | 101 398 | 1.33 | 0.73 (0.58-0.87) | .01 |
| Death due to cardiovascular disease (n = 391) | |||||
| Migraine with aura | 22/1435 | 32 695 | 0.67 | 0.46 (0.26-0.65) | |
| Migraine without aura or no migraine | 369/26423 | 596 658 | 0.62 | 0.28 (0.23-0.33) | .07 |
| Diabetes | 40/682 | 13 431 | 2.98 | 0.85 (0.54-1.16) | .03 |
| Current smoking | 77/3241 | 68 457 | 1.13 | 0.69 (0.52-0.87) | .06 |
| Systolic blood pressure ≥160 mm Hg | 20/318 | 6542 | 3.06 | 0.54 (0.28-0.79) | .62 |
| Total cholesterol ≥280 mg/dL | 40/1645 | 36 762 | 1.09 | 0.36 (0.23-0.49) | .38 |
| HDL cholesterol<40 mg/dL | 93/4822 | 106 409 | 0.87 | 0.31 (0.22-0.39) | .14 |
| Triglycerides ≥194 mg/dL | 124/5546 | 123 244 | 1.01 | 0.34 (0.26-0.42) | .25 |
| Family history of myocardial infarction prior to age 60 y | 54/3945 | 89 926 | 0.60 | 0.35 (0.25-0.46) | .33 |
| Body mass index ≥30 | 96/4939 | 110 248 | 0.87 | 0.38 (0.28-0.48) | .45 |
Abbreviation: HDL, high-density lipoprotein.
Adjusted for migraine with aura, the vascular risk factors (dichotomous) listed in the table, age, alcohol use, exercise, hypertensive treatment, ever use of hormones, and premenopausal status.
P value from a t test examining whether the adjusted incidence rate among those with migraine with aura differs statistically from the adjusted incidence rate of the other vascular risk factor. For example, for major CVD, the P value for current smoking (P = .02) indicates that the adjusted incidence rate of current smoking (4.29) is statistically different from the adjusted incidence rate of migraine with aura (3.36).
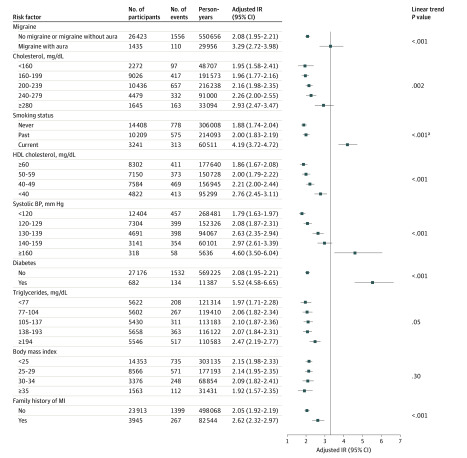
The adjusted incidence rates of migraine with aura and the polychotomous categorization of the other vascular risk factors is shown in the Figure for major CVD and in eFigures 1, 2, and 3 in the Supplement for the other vascular outcomes.
Figure. Adjusted Incidence Rates for Major Cardiovascular Disease Events per 1000 Person-Years in a Study of the Association of Migraine With Aura With Incident Cardiovascular Disease in Women.
The vertical line indicates the adjusted incidence rate for migraine with aura. Values to the left of the vertical line indicate lower and values to the right indicate higher adjusted incident rates of major cardiovascular disease compared with the incidence rate of migraine with aura. Adjusted for all factors listed as well as age (5-year groups), alcohol use, exercise, hypertensive treatment, ever use of hormones, and premenopausal status. Because 2 different approaches were used for categorizing covariates in the multivariable models, the estimated adjusted incidence rates differ slightly in the Figure (in which some covariates were categorized as polychotomous) and in Table 2 (in which all covariates were categorized as dichotomous).
aP value was calculated using a χ2 test. HDL indicates high-density lipoprotein; BP, blood pressure; MI, myocardial infarction.
The adjusted incidence rate of major CVD for individual vascular risk factors with and without adding migraine with aura is shown in Table 3. The adjusted incidence rate after adding migraine with aura to each vascular risk factor was higher than only having the vascular risk factor. The incremental increase in the incidence rate ranged from 1.01 additional cases per 1000 person-years, when migraine with aura was added to body mass index, to 2.57 additional cases per 1000 person-years, when migraine with aura was added to diabetes.
Table 3. Adjusted Incidence Rate of Major Cardiovascular Disease per 1000 Person-Years of Vascular Risk Factors With and Without the Addition of Migraine With Aura (N = 27 858)a.
| Risk factor | Adjusted incidence rate (95% CI)a | P valuec | |
|---|---|---|---|
| With migraine with aura | Without migraine with aurab | ||
| Diabetes | 6.92 (5.05-8.79) | 4.35 (3.48-5.22) | <.001 |
| Current smoking | 5.50 (4.29-6.72) | 3.46 (3.02-3.90) | <.001 |
| Systolic blood pressure ≥160 mm Hg | 4.46 (2.98-5.94) | 2.80 (2.02-3.58) | .001 |
| Total cholesterol ≥280 mg/dL | 3.40 (2.55-4.24) | 2.13 (1.76-2.51) | <.001 |
| HDL cholesterol <40 mg/dL | 3.22 (2.50-3.93) | 2.02 (1.75-2.30) | <.001 |
| Triglycerides ≥194 mg/dL | 3.29 (2.58-4.01) | 2.07 (1.81-2.33) | <.001 |
| Family history of myocardial infarction prior to age 60 y | 3.30 (2.57-4.04) | 2.07 (1.80-2.35) | <.001 |
| Body mass index ≥30 | 2.71 (2.11-3.32) | 1.71 (1.48-1.93) | <.001 |
Abbreviation: HDL, high-density lipoprotein.
Adjusted for the vascular risk factors (dichotomous) shown in the table plus age, alcohol use, exercise, hypertensive treatment, ever use of hormones, and premenopausal status.
Includes women who reported migraine without aura and those who did not report migraine in the year prior to baseline.
P value shows the contrast of each factor without adding migraine with aura vs adding migraine with aura.
The adjusted incidence rate of major CVD for the maximum combination of vascular risk factors with and without adding migraine with aura is shown in Table 4. With a combination of all vascular risk factors, except for a family history of premature myocardial infarction, the incidence rate of major CVD increased from 39.55 cases per 1000 person-years to 62.96 cases per 1000 person-years when migraine with aura was added.
Table 4. Adjusted Incidence Rate of Major Cardiovascular Disease per 1000 Person-Years for Combinations of Major Vascular Risk Factors With and Without the Addition of Migraine With Aura (N = 27 858)a.
| Risk factor combination | Adjusted rate (95% CI)a | P valuec | |
|---|---|---|---|
| With migraine with aura | Without migraine with aurab | ||
| Current smoking, total cholesterol ≥280 mg/dL, HDL cholesterol <40 mg/dL, triglycerides ≥194 mg/dL, family history of MI | 15.90 (11.19-20.62) | 9.99 (7.62-12.37) | <.001 |
| Diabetes, SBP ≥160 mm Hg, total cholesterol ≥280 mg/dL, triglycerides ≥194 mg/dL, BMI ≥30 | 22.79 (13.64-31.95) | 14.32 (9.23-19.41) | .002 |
| Diabetes, SBP ≥160 mm Hg, total cholesterol, HDL cholesterol <40 mg/dL, triglycerides ≥194 mg/dL, BMI ≥30 | 28.97 (17.38-40.56) | 18.20 (11.75-24.65) | .002 |
| Diabetes, current smoking, SBP ≥160 mm Hg, HDL cholesterol <40 mg/dL, triglycerides ≥194 mg/dL, BMI ≥30 | 46.95 (28.71-65.18) | 29.49 (19.50-39.48) | .002 |
| Diabetes, current smoking, total cholesterol ≥280 mg/dL, HDL cholesterol <40 mg/dL, triglycerides ≥194 mg/dL, family history of MI, BMI ≥30 | 46.58 (30.61-62.54) | 29.26 (20.81-37.70) | .001 |
| Diabetes, current smoking, SBP ≥160 mm Hg, total cholesterol ≥280 mg/dL, HDL cholesterol <40 mg/dL, triglycerides ≥194 mg/dL, BMI ≥30 | 62.96 (37.13-88.80) | 39.55 (25.13-53.97) | .002 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HDL, high-density lipoprotein; MI, myocardial infarction; SBP, systolic blood pressure.
Adjusted for the vascular risk factors (dichotomous) shown in the table plus age, alcohol use, exercise, hypertensive treatment, ever use of hormones, and premenopausal status.
Includes women who reported migraine without aura and who reported no migraine in the year prior to baseline.
P value shows the contrast of the combination of vascular risk factors without adding migraine with aura vs adding migraine with aura.
The results from the sensitivity analysis in which missing values were imputed using multiple imputations is shown in eTable 5 in the Supplement. The results were similar to those of the primary analysis. Results were similar when women who reported migraine without aura or any history of migraine at baseline were excluded from the reference group, indicating that this group did not have different rates of major CVD compared with women who did not report migraine in the year prior to baseline (eTable 6 in the Supplement).
Discussion
In this large cohort study of women aged at least 45 years, women with migraine with aura had a higher incidence rate of self-reported major CVD events compared with women without migraine or with migraine without aura, after adjusting for other major vascular risk factors and confounding. Although diabetes and current smoking were associated with higher rates of major CVD, migraine with aura was more strongly associated with the rate of major CVD than obesity or unfavorable lipid levels, and had a similar association as with elevated systolic blood pressure or high total cholesterol. The association patterns were similar for specific CVD events.
The association of migraine and migraine with aura on overall and specific CVD events has been evaluated using relative risk in many clinic- and population-based studies.3,4,27,28 Although there was an indication of some heterogeneity across studies, the relative risk estimates ranged from 1.5 for migraine overall to more than 2 for migraine with aura. In a 2018 nationwide population-based study from Denmark, Adelborg and colleagues4 estimated the relative hazard of CVD according to migraine status. Migraine with aura was associated with myocardial infarction (hazard ratio, 1.74 [95% CI, 1.44-2.11]), ischemic stroke (hazard ratio, 2.49 [95% CI, 2.16-2.86]), and hemorrhagic stroke (hazard ratio, 1.82 [95% CI, 1.34-2.48]).4 In contrast to data from the Women’s Health Study,18 migraine without aura was also associated with an increased risk of CVD in the study by Adelborg and colleagues,4 but the effect size was smaller compared with migraine with aura. A previous study using Women’s Health Study data observed similar incidence rates of major CVD for migraine with aura as seen in this study after adjustment for age.18 However, to the best of our knowledge, no studies have compared the estimated adjusted incidence rates of CVD by migraine status with the adjusted incidence rates of CVD due to other cardiovascular risk markers.
Several biological mechanisms have been proposed to link migraine, particularly migraine with aura, with increased risk of CVD events. These mechanisms have included elevated vascular biomarkers8,29 leading to endothelial dysfunction, other vascular pathology,30 open patent foramen ovale, genetic predisposition,7 or migraine-specific or other pain medication use.31 However, with regard to medication use, not only patients with migraine with aura use such treatment. Cortical spreading depolarization, the electrophysical mechanisms involved in migraine aura, can play a role in stroke32 and potentially in other vascular events.33 Nevertheless, the precise mechanisms linking migraine with aura to CVD incidence are still not well understood.
Strengths of this study include the large number of participants and outcome events, available information on various vascular risk factors, confirmation of CVD events by medical record review, and the homogeneity of the study population, which reduced potential biases, including differences in health care access.
Limitations
This study has several limitations. First, information on migraine and vascular risk factors were self-reported, and misclassification is possible. Second, migraine with aura and vascular risk factors were assessed at baseline, and changes in these risk factors over time were not taken into account. Third, detailed information on management of migraine or vascular risk factors, which may influence CVD risk, was not available. Fourth, this study was conducted in a cohort of female health professionals and these results may not be generalizable to other settings, particularly to men. Fifth, this study did not explore how changes in migraine frequency over time may modify the association between migraine with aura and CVD incidence. Additionally, no information on when migraine started for the participating women was available.
Conclusions
In this study of female health professionals aged at least 45 years, women with migraine with aura had a higher adjusted incidence rate of CVD compared with women with migraine without aura or no migraine. The clinical importance of these findings, and whether they generalize beyond this study population, require further research.
eAppendix
References
- 1.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders 3rd ed. Cephalalgia; 2018. [DOI] [PubMed] [Google Scholar]
- 2.Charles A, Hansen JM. Migraine aura: new ideas about cause, classification, and clinical significance. Curr Opin Neurol. 2015;28(3):255-260. doi: 10.1097/WCO.0000000000000193 [DOI] [PubMed] [Google Scholar]
- 3.Mahmoud AN, Mentias A, Elgendy AY, et al. . Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ Open. 2018;8(3):e020498. doi: 10.1136/bmjopen-2017-020498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adelborg K, Szépligeti SK, Holland-Bill L, et al. . Migraine and risk of cardiovascular diseases: Danish population based matched cohort study. BMJ. 2018;360:k96. doi: 10.1136/bmj.k96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. doi: 10.1136/bmj.j2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liman TG, Neeb L, Rosinski J, et al. . Peripheral endothelial function and arterial stiffness in women with migraine with aura: a case-control study. Cephalalgia. 2012;32(6):459-466. doi: 10.1177/0333102412444014 [DOI] [PubMed] [Google Scholar]
- 7.Winsvold BS, Bettella F, Witoelar A, et al. ; International Headache Genetics Consortium . Shared genetic risk between migraine and coronary artery disease: a genome-wide analysis of common variants. PLoS One. 2017;12(9):e0185663. doi: 10.1371/journal.pone.0185663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tietjen GE, Khubchandani J, Herial N, et al. . Migraine and vascular disease biomarkers: a population-based case-control study. Cephalalgia. 2018;38(3):511-518. doi: 10.1177/0333102417698936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sacco S, Kurth T. Migraine and the risk for stroke and cardiovascular disease. Curr Cardiol Rep. 2014;16(9):524. doi: 10.1007/s11886-014-0524-1 [DOI] [PubMed] [Google Scholar]
- 10.Rist PM, Winter AC, Buring JE, Sesso HD, Kurth T. Migraine and the risk of incident hypertension among women. Cephalalgia. 2018;38(12):1817-1824. doi: 10.1177/0333102418756865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagen K, Åsberg AN, Stovner L, et al. . Lifestyle factors and risk of migraine and tension-type headache: follow-up data from the Nord-Trøndelag Health Surveys 1995-1997 and 2006-2008. Cephalalgia. 2018;38(13):1919-1926. doi: 10.1177/0333102418764888 [DOI] [PubMed] [Google Scholar]
- 12.Rist PM, Tzourio C, Kurth T. Associations between lipid levels and migraine: cross-sectional analysis in the epidemiology of vascular ageing study. Cephalalgia. 2011;31(14):1459-1465. doi: 10.1177/0333102411421682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelaye B, Sacco S, Brown WJ, Nitchie HL, Ornello R, Peterlin BL. Body composition status and the risk of migraine: a meta-analysis. Neurology. 2017;88(19):1795-1804. doi: 10.1212/WNL.0000000000003919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burch RC, Rayhill ML. Migraine and vascular disease. BMJ. 2016;353:i2806. doi: 10.1136/bmj.i2806 [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Cook NR, Lee I-M, et al. . A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352(13):1293-1304. doi: 10.1056/NEJMoa050613 [DOI] [PubMed] [Google Scholar]
- 16.Mora S, Lee I-M, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295(12):1412-1419. doi: 10.1001/jama.295.12.1412 [DOI] [PubMed] [Google Scholar]
- 17.Schürks M, Buring JE, Kurth T. Agreement of self-reported migraine with ICHD-II criteria in the Women’s Health Study. Cephalalgia. 2009;29(10):1086-1090. doi: 10.1111/j.1468-2982.2008.01835.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurth T, Gaziano JM, Cook NR, Logroscino G, Diener H-C, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296(3):283-291. doi: 10.1001/jama.296.3.283 [DOI] [PubMed] [Google Scholar]
- 19.Kurth T, Schürks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: prospective cohort study. BMJ. 2008;337:a636. doi: 10.1136/bmj.a636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atiya M, Kurth T, Berger K, Buring JE, Kase CS; Women’s Health Study . Interobserver agreement in the classification of stroke in the Women’s Health Study. Stroke. 2003;34(2):565-567. doi: 10.1161/01.STR.0000054159.21017.7C [DOI] [PubMed] [Google Scholar]
- 21.Kurth T, Kase CS, Schürks M, Tzourio C, Buring JE. Migraine and risk of haemorrhagic stroke in women: prospective cohort study. BMJ. 2010;341:c3659. doi: 10.1136/bmj.c3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flanders WD, Kleinbaum DG. Basic models for disease occurrence in epidemiology. Int J Epidemiol. 1995;24(1):1-7. doi: 10.1093/ije/24.1.1 [DOI] [PubMed] [Google Scholar]
- 23.Bender R. Introduction to the use of regression models in epidemiology. Methods Mol Biol. 2009;471:179-195. doi: 10.1007/978-1-59745-416-2_9 [DOI] [PubMed] [Google Scholar]
- 24.Joffe MM, Greenland S. Standardized estimates from categorical regression models. Stat Med. 1995;14(19):2131-2141. doi: 10.1002/sim.4780141907 [DOI] [PubMed] [Google Scholar]
- 25.Chu LC, Xie F Using SAS to calculate incidence and prevalence rates in a dynamic population. Paper presented at: Western Users of SAS Software Conference; September 5-7, 2012; Long Beach, California. [Google Scholar]
- 26.Chou NT, Steenhard D A flexible count data regression model using SAS PROC NLMIXED. Paper presented at: SAS Global Forum; March 22-25, 2009; Washington, DC. [Google Scholar]
- 27.Kurth T, Winter AC, Eliassen AH, et al. . Migraine and risk of cardiovascular disease in women: prospective cohort study. BMJ. 2016;353:i2610. doi: 10.1136/bmj.i2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liman TG, Bachelier-Walenta K, Neeb L, et al. . Circulating endothelial microparticles in female migraineurs with aura. Cephalalgia. 2015;35(2):88-94. doi: 10.1177/0333102414529671 [DOI] [PubMed] [Google Scholar]
- 30.Magalhães JE, Barros IML, Pedrosa RP, Sampaio Rocha-Filho PA. Migraine and markers of carotid atherosclerosis in middle-aged women: a cross-sectional study. Headache. 2019;59(1):77-85. doi: 10.1111/head.13460 [DOI] [PubMed] [Google Scholar]
- 31.Schmidt M, Sørensen HT, Pedersen L. Diclofenac use and cardiovascular risks: series of nationwide cohort studies. BMJ. 2018;362:k3426. doi: 10.1136/bmj.k3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4):439-447. doi: 10.1038/nm.2333 [DOI] [PubMed] [Google Scholar]
- 33.Ripa P, Ornello R, Pistoia F, Carolei A, Sacco S. Spreading depolarization may link migraine, stroke, and other cardiovascular disease. Headache. 2015;55(1):180-182. doi: 10.1111/head.12436 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix