Abstract
Cellular energy demands are readily changed during physical exercise resulting in adaptive responses by signaling proteins of metabolic processes, including the NAD+ dependent lysine deacetylase SIRT1. Regular exercise results in systemic adaptation that restores the level of SIRT1 in the kidney, liver, and brain in patients with neurodegenerative diseases, and thereby normalizes cellular metabolic processes to attenuate the severity of these diseases. In skeletal muscle, over-expression of SIRT1 results in enhanced numbers of myonuclei improves the repair process after injury and is actively involved in muscle hypertrophy by up-regulating anabolic and downregulating catabolic processes. The present review discusses the different views of SIRT1 dependent deacetylation of PGC-α.
Graphical abstract
1. Introduction
Every attachment of the myosin heavy chain to actin requires ATP, and during high-intensity exercise, sufficient production ATP is a huge metabolic challenge for skeletal muscle. The transfer of electrons in the mitochondrial electron transport chain, as well as other cytosolic and mitochondrial molecules are obligatory to ATP production. NAD is a key factor of electron transfer, and the ratio of reduced and oxidized (NADH: NAD+) forms of NAD reflects redox homeostasis, which is dependent on cellular compartments and the rate of metabolism. The lactate-dehydrogenase system reflects the NAD+:NADH ratio, which in mitochondria is about 100- to 1000-fold higher than in the cytosol. In addition, the NAD+:NADH ratio does not necessarily change parallel with changes in metabolism [1]. However, an increase in the NAD+:NADH ratio in the sarcolemma results in enhanced expression of SIRT1 mRNA and protein levels [2]. Therefore it is not surprising that physical exercise, which significantly changes metabolism, also alters the NAD+:NADH ratio and leads to a potent induction of SIRT1. SIRT1 is a mammalian homolog of the sir2 gene discovered in yeast twenty years ago as a mating-type regulator [3]. All of seven orthologs of sirtuins are dependent on NAD+. Most of the sirtuins have a powerful lysine deacetylating capacity and are associated with cellular metabolism. Sirtuins are directly, or indirectly through signaling pathways, associated with the regulation of gluconeogenesis, fat oxidation, apoptosis, autophagy, mitochondrial biogenesis, DNA repair and redox homeostasis [4,5]. In addition, sirtuins have been shown to exert life-extending effects for organisms like yeast, worms, and flies [5]. However, the role of sirtuins on the maximal life span of mammals is under debate.
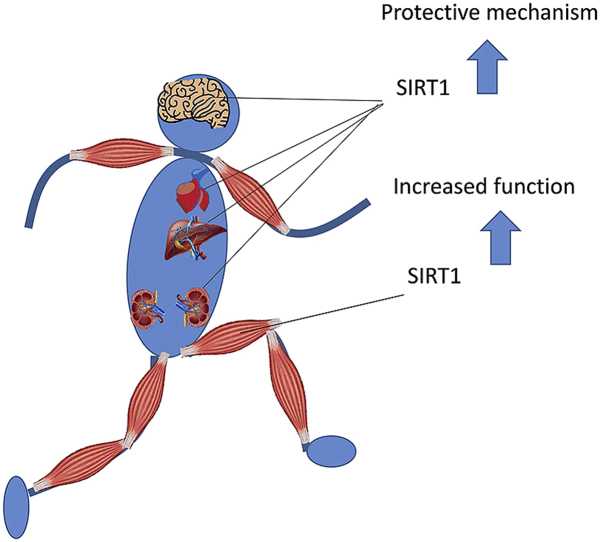
It is suggested that a significant part of the health promoting and age retarding effects of regular physical exercise at the cellular level could be mediated by sirtuin proteins [5]. In the present review, we summarize our current knowledge on how SIRT1 is involved in exercise-induced adaptation in the liver, kidney, brain, heart and skeletal muscle. Moreover, we also examine to role of SIRT1 in endurance and muscular strength. While we acknowledge there are important biological roles for the entire sirtuin family, the present review focuses on SIRT1.
2. SIRT1 forward messages of exercise to liver and kidneys
During heavy acute exercise, the renal blood delivery can decrease to 25% of resting values [6], and after exercise, due to the increased glomerular permeability, protein, and urea are excreted. However, the positive effects of regular exercise on renal function is well established [[7], [8], [9]].
Kidneys are heavily affected by metabolic diseases including diabetes, especially in aging individuals. Aerobic exercise is often utilized to attenuate the diabetic nephropathy in a diabetic rat model. Streptozotocin injection resulted in proteinuria, increased collagen, decreased SIRT1 and PGC-1α levels and mitochondrial dysfunction in renal tissue [10]. On the other hand, aerobic exercise training significantly attenuated these abnormalities in the kidney [10].
In another diabetic model, C57BLKS/J mice were subjected to moderate intensity treadmill running for 8 weeks, and renal and hepatic histology was studied with biological markers [11]. Exercise prevented the downregulation of SIRT1 in the kidney of these diabetic mice and reduced acetylation of transcription factor NF-κB. The exercise-induced adaptive response included the up-regulation of citrate synthase, subunits of mitochondrial complexes as well as PGC1α protein levels [11]. As a consequence of these molecular adaptations, regular training decreased creatinine, urea and triglyceride levels in this diabetic model [11].
Similar to the kidneys, the liver also experiences a significant drop in blood flow during acute exercise, however because of the central role of the liver in lipid and carbohydrate metabolism, the regular exercise-mediated adaptation of this organ has a particular importance. An acute bout of exercise, especially vigorous exercise, which results in significant metabolic challenge to the body, is associated with powerful activation of cellular signaling pathways in the liver. Indeed, it has been reported that the mitogen-activated protein kinase (MAPK) signaling pathway, significant transcriptional activation of Fos/Jun-family, growth arrest, and energy depletion associated genes, are all upregulated during heavy acute bouts of exercise [12].
To gain more information on endurance associated adaptation, a mouse model has been developed by phenotypic selection. High endurance capacity mice had a 3.8-fold higher running capacity than control mice [13]. When the liver metabolism-associated biochemical assays were compared, it turned out that protein levels of SIRT1, acetyl-CoA-synthetase, acetyl-CoA-carboxylase, phosphoenolpyruvate carboxykinase, and glutamate-dehydrogenase were higher in the liver of mice with a high running capacity than in control mice [13]. Based on this finding, it was suggested that mice with higher endurance have higher levels of gluconeogenesis and lipogenesis [13]. In accordance with this, it has been shown that 36 weeks of treadmill running resulted in elevation of the activity and content of SIRT1 as well as PGC-1α levels and improved redox balance in the liver compared to control animals [14]. However, it has been also reported that in a transgenic mouse model with overexpression of SIRT1 in the liver, the increased SIRT1 did not guarantee improved glucose metabolism and improved insulin sensitivity [15], showing that the exercise-mediated adaptation is very complex and not just dependent upon a single protein.
Non-alcoholic fatty liver disease (NAFLD) encompasses a disease spectrum that can progress from significant uptake of carbohydrates that leads to enhanced lipogenesis in the liver, which could result in increased storage of hepatic triacylglycerol or nonalcoholic steatohepatitis [16]. NAFLD is also associated with insulin resistance and oxidative stress [17]. Due to its systemic effects [18,19], exercise has all of the means to prevent or decrease the deleterious consequences of NAFLD. Indeed, 8 weeks of treadmill running decreased the NAFLD induced by a high fat diet by suppression of lipolysis, enhanced mitochondrial biogenesis in the liver, and fatty acid oxidation [20]. These changes were the result of the activation of SIRT1 mediated cellular pathways, especially by the induction of AMPK [20]. It is suggested that SIRT1 can attenuate the poor phosphorylation of AMPK and its downstream target acetyl-CoA carboxylase and elevation in the expression of fatty acid synthase, hence the storage of lipids [21].
Based on the knowledge of the important role of SIRT1 in the treatment of NAFLD, a study was done where nicotinamide riboside (NR) was administered to boost NAD+ levels to examine the effects of pharmacological intervention [22]. NR attenuated the deleterious effects of NAFLD by activating SIRT1 and SIRT3 mediated mitochondrial unfolded protein responses leading to hepatic β-oxidation and mitochondrial complex content and activity [22].
Overall, the data suggest that SIRT1 controls cellular metabolism, through the regulation of lipid metabolism since it inhibits hepatic de novo lipogenesis by deacetylation of carbohydrate response element binding protein and sterol regulatory element binding protein-1c [23,24] and increases fatty acid β-oxidation via deacetylating PPARα/PGC-1α [25,26] and reduces inflammation through deacetylating NF-κB [27].
3. Is SIRT1 involved in the neuroprotective effects of exercise?
The neuroprotective effects of regular exercise are very well-established [28], which among others includes improved function [29], increased resistance to oxidative stress [30,31], increased generation of brain derived neurotrophic factor (BDNF) [32], neurogenesis [33], and increased mitochondrial biogenesis [34]. Interestingly enough, activation of SIRT1 mediated cellular pathways can result in all of the above mentioned neuroprotective effects [[35], [36], [37], [38], [39]].
Gomez-Pinilla and Ying were one of the first investigators, who showed that exercise increases SIRT1 content in the hippocampus, which was associated with increased levels of phosphorylated AMPK [40]. When the effects of exercise training on young and aged cerebella was studied, data revealed a positive correlation between protein acetylation and the gripping test, which suggests that the age-dependent decrease in relative activity of SIRT1 in the cerebellum impairs motor function [41]. Aging is associated with increased levels of oxidative stress, and 8-oxo-7,8-dihydroguanine (8-oxoG) is considered to be a major mutagenic DNA base lesion [42]. Data revealed that aging increases the levels of 8-oxoG, which could be due to decreased acetylation of 8-oxoguanine DNA glycosylase-1 (OGG1) [43]. Meanwhile, it turned out that OGG1 has the potential to directly modulate gene expression [44]. Oxidative stress directly or cytokine (e.g., TNF-α) induced increase in ROS levels resulted in enrichment of OGG1 primarily in the regulatory regions of genes [44]. Hence, the regulation of OGG1 appears to have a complex role for cellular function, and we have shown that the deacetylation, and therefore the activity of OGG1, is regulated by SIRT1 [33].
It is suggested that most of the neuroprotective effects of exercise are mediated by the induction of brain-derived neurotrophic factor (BDNF) [[45], [46], [47]]. Upon the translocation and DNA binding of the transcription factor cyclic AMP response element-binding protein (CREB), transcript levels of Bdnf gene are increased. Mature and pro-BDNF are then transferred to axons/presynaptic terminals and dendrites and released in response to glutamate receptor activation [48]. Released BDNF bounds to tropomyosin-related kinase B (TrkB) receptors, which can lead to the activation of intracellular signaling pathways leading to activation of transcription factors that regulate expression of proteins involved in neuronal survival, plasticity, cellular energy balance and mitochondrial biogenesis [49,50]. It turns out that SIRT1 may also be an activator of BDNF production, by deacetylating the transcriptional coactivator of 1 (TORC1), which activates CREB resulting in enhanced transcription of BDNF in a mammalian Huntington's disease model [35]. Therefore, some of the neuroprotective effects of exercise induced SIRT1 activation can be mediated by BDNF. In another animal model, where melatonin was used to attenuate the lipopolysaccharide (LPS) induced oxidative stress, melatonin activated the SIRT1/Nrf2 (nuclear factor-erythroid 2-related factor 2) signaling pathway, which suppressed the LPS-induced ROS generation [36].
There could be several mechanisms by which exercise activates Bdnf expression. One recently reported pathway could be through lactate-SIRT1- Fndc-5- axis. Exercising muscles produce lactate, which readily crosses the blood brain barrier. It is reported, voluntary exercise, which leads to increased levels of hippocampal lactate compared to control mice, resulted in lactate-induced BDNF levels and improved spatial learning [51]. Intraperitoneal injection of lactate also resulted in enhanced Bdnf expression in the hippocampus. Moreover, SIRT1 levels were also increased by lactate. Silencing SIRT1 by shRNAs and administration of SIRT1 inhibitor, sirtinol, on the other hand prevented the lactate-associated induction of Bdnf expression, strongly suggesting that it is SIRT1 mediated [51]. Moreover, this study also suggested that SIRT1 activates the PGC-1α/FNDC-5 pathway and leads to the induction of hippocampal Bdnf expression and enhanced learning and memory [51]. Physiological functions like memory or learning are regulated by a variety of cellular pathways, but cellular metabolism associated pathways in which SIRT1 could be an important player, are most likely involved.
4. The role of SIRT1 in endurance
Since endurance capacity is strongly dependent on cardiovascular function and mitochondrial biogenesis [52] we outline the role of SIRT1 in the function of cardiac and skeletal muscle. Oxidative stress and aging are often associated with activation of inflammation and apoptosis via NFκB and FOXO3 pathways, and the deacetylation of these transcription factors by SIRT1 has beneficial effects on cellular survival [53]. Indeed, exercise training along with resveratrol treatment, which is a potent activator of SIRT1, increased SIRT1 and PI3K-Akt pathways and suppressed FOXO3 in aging hearts [53].
One way to study the role of targeted proteins on cellular function is by ablation of the protein. When SIRT1 was ablated in the cardio-myocyte and the heart was subjected to ischemia/reperfusion, the ejection fraction of SIRT1 KO mice was impaired [54]. Moreover, SIRT1 deficiency significantly compromised substrate metabolism in cardiac muscle [54]. In accordance with this, when trained and untrained rats were challenged by ischemia/reperfusion, trained rats displayed significantly decreased injury, and exercise activated the SIRT1 and SIRT3 pathways and reduced p53 mediated apoptosis and oxidative damage [55].
p53 is one of the most well studied targets of SIRT1. Accumulating evidence suggests that p53 acts as a threshold regulator of cellular homeostasis [56], since under mild cellular stress p53 induces cell cycle arrest to allow cells to repair damage, while greater stress results in growth arrest or apoptosis. In harsh stress conditions that cause irreparable damage, p53 activates a number of pro-apoptotic genes to terminate the cell [57]. Studies on p53 knockout (KO) mice showed decreases in the levels of mitochondrial content, marked reduction of PGC-1α content and exercise capacity [58]. Hence p53 is an important protein for exercise induced adaptation. This transcription factor controls metabolism via down-stream targets like cytochrome c oxidase 2 (SCO2), which regulates the cytochrome c oxidase (COX) [59] and phosphate-activated mitochondrial glutaminase (GLS2) [60]. One of the important targets of p53 is TP53-induced glycolysis and apoptosis regulator (TIGAR), which decreases cellular fructose-2,6-bisphosphate levels, glycolysis and ROS [61]. Ablation of TIGAR resulted in decreased running capacity in mice and decreased mitochondrial content and function in the skeletal muscle. These effects were attenuated by resveratrol treatment by promoting SIRT1 and the PGC-1α axis [62]. Data suggests that TIGAR translocation into the mitochondria is important for increasing the endurance capacity of fast-twitch fibers, and this process involves SIRT1 and PGC-1α [62].
Endurance exercise have been shown to increase mitochondrial biogenesis [63] and SIRT1 content and activity [64,65], and it has also been shown that SIRT1 deacetylates PGC-1α [26]. Therefore, it has been suggested that SIRT1, especially nuclear SIRT1, can cause mitochondrial biogenesis by deacetylation of PGC-1α [66]. However, the study of Nemoto et al. [26], actually suggests that deacetylation of PGC-1α results in reduction of oxygen consumption at least in PC12 cells. Along with this observation, it has been shown that mice lacking SIRT1 deacetylase activity in skeletal muscle showed similar adaptive responses to exercise as wild type animals, indicating that exercise can induce mitochondrial biogenesis independently from SIRT1 [67].
The complexity of this phenomenon is further emphasized by nutritional manipulations, especially by resveratrol supplementation. We supplemented the diet of rats with low and high running capacity with resveratrol [68,69]. The same amount of resveratrol decreased endurance in low-running capacity and increased it in high running capacity rats, and differently affected the levels of AMPK, SIRT1, PGC-1α and mitochondrial transcription factor A [68,69]. On the other hand, when resveratrol and piperine were administered to subjects during wrist flexor exercise training and the mitochondrial capacity (probably oxidative capacity) was monitored by near-infrared spectroscopy, the results revealed that the treated group had a greater increase at mitochondrial capacity [70]. When resveratrol was supplemented to patients with peripheral artery diseases, their walking performance, as assessed by a 6-min walking test, was very similar to the placebo group, suggesting that resveratrol treatment was not effective [71]. Resveratrol was supplemented to subjects who carried out high intensity exercise training, and the results did not show significant performance enhancing effects of this treatment [72]. In addition, when resveratrol was supplemented to aged man to test the possible exercise enhancing effects, results revealed that resveratrol supplementation did not increase the protein levels of SIRT1 and actually eliminated the beneficial effects of exercise training on cardiovascular health parameters [73]. Conversely, there are a number of studies in which flavonoids, like myricetin [74], taheebo [75], rutin [76], resveratrol [68,77], or quercin [78] were administered to animals, and in most of these studies, the treatment increased levels of SIRT1 and PGC-1α as well as enhanced exercise performance. However, the parallel increase of PGC-1α and SIRT1 from exercise training, does not necessarily indicate a functional link.
Higashida and co-workers showed that training with resveratrol supplementation does not have performance enhancing effects on rats, and SIRT1 actually inhibits PGC-1α by deacetylation [77]. The authors argued that in cell culture, resveratrol supplementation actually activates AMPK in a manner that results in decreased production of ATP, which in turn activates PGC-1α. Induction of AMPK then results in enhanced levels of SIRT1. They further suggest that acetylation of PGC-1α activates this co-activator, which fits well with earlier reports [26,79]. However, other investigators suggest that deacetylation of PGC-1α leads to mitochondrial biogenesis, and therefore increased activity of SIRT1 directly leads to better mitochondrial function [[80], [81], [82]].
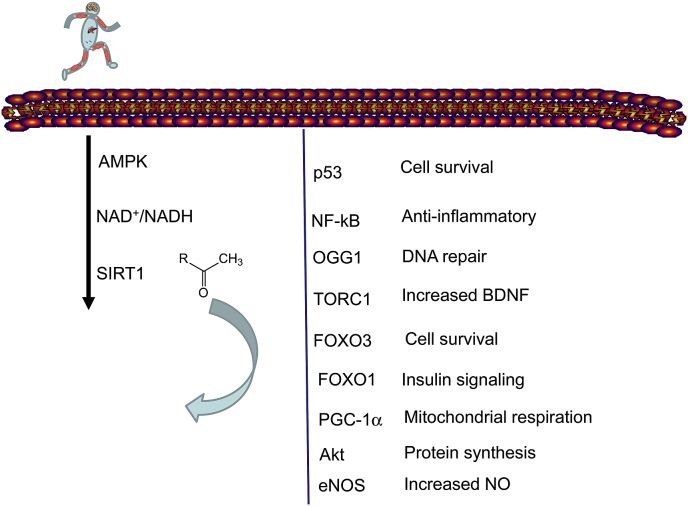
Regardless of the differing evaluations of the role of acetylation/deacetylation on the activity of PGC-1α, it appears that the exercise mediated induction of SIRT1 has a complex effect on cellular function, which importantly involves metabolic processes and cellular survival (Fig. 1) [83,84]. One possible explanation for the different views on SIRT1 mediated deacetylation of PGC-1α could be due to possible differences in the effects of site-specific lysine acetylation. It is known that the site-specific acetylation of lysine residues on histone proteins could have different downstream effects [85]. Therefore, it cannot be excluded that certain residues of PGC-1α that are acetylated may result in activation and acetylation of other residue(s) may also cause inhibition of the activity of this co-activator. This selectivity works well in the regulation of histones, so is makes sense that it would work for other proteins as well. If this is the case, it would in part explain the different outcomes of SIRT1 and PGC-1α associated studies.
Fig. 1.
Exercise activated SIRT1 regulates wide range of adaptive response.
Exercise induced metabolic challenge increase the activity of AMPK phosphorylation, which leads to SIRT1 activation. SIRT1 deacetylates key proteins, which play important role in cellular adaptation to exercise training.
5. Resistance training and SIRT1
There are only a few reports on SIRT1 and resistance training. However, SIRT1 plays an important role in the physiology of skeletal muscle, which includes development, repair, hypertrophy and metabolism. Resistance training generally aims to increase the strength and size of skeletal muscle. An early paper from Fulco et al. [86] suggested that Sir2 is a redox sensitive modulator of gene expression and differentiation of the skeletal muscle. Matured myofibers can include hundreds of myonuclei, and each myonucleus regulates the gene products for a given area of cytoplasm, termed the myonuclear domain (MND) [87]. Greater number of myonuclei results in smaller MND and faster, better transport of gene products to the given area. Slow twitch fibers have smaller MND to feed the large number of mitochondria for producing the necessary gene products. Moreover, there is also a need to increase the number of myonuclei during resistance training, which aims to cause muscle hypertrophy. It has been reported that PGC-1α not only regulates mitochondrial biogenesis, but it also controls the number of myonuclei in the fibers, and thus the size of the MND as well [88]. Overexpression of PGC-1α in the extensor digitorum longus resulted in increased number of myonuclei resulting in decreased volumes of MND [88]. Moreover, over-expression of the gene SIRT1 caused the myonuclear number in the tibialis anterior muscle to significantly increase, with the concomitant decrease in MND size, while ablation of SIRT1 caused decreased myonuclear numbers [89]. Interestingly, neither over-expression nor deletion of the SIRT1 gene changed the levels of mitochondrial markers [89]. Therefore, it is suggested that SIRT1 mediated changes in myonuclear number is independent from PGC-1α.
Activated satellite cells play an important role in muscle repair and can be responsible for increases in myonuclear number. Interestingly, ablation of the SIRT1 gene lead to impaired muscle regeneration and revealed impaired activation of genes responsible for muscle development [90]. Nitric oxide (NO) leads to the proliferation of satellite cells [91], and inhibition of NO production by l-NAME results in decreased levels of SIRT1 in both slow- and fast twitch skeletal muscles [92]. Moreover, it has been reported that SIRT1 up-regulates eNOS [93]. We have shown that SIRT1 protein levels and activity are increased during overload induced hypertrophy of the plantaris muscle, and this increase was paralleled by an increase in NO content by deacetylation of eNOS [94]. Akt deacetylation by SIRT1 on the other hand, also suppresses catabolic processes via down‐regulation of FOXO1 [95]. Hence, SIRT1 activation during overload induced hypertrophy resulted in up-regulation of anabolic and downregulation of catabolic pathways (Fig. 2). In addition, Koltai et al. [96] have shown that overload induced hypertrophy results in changes in muscle‐specific microRNA (myomiR) expression, and it was reported that microRNA‐1 and ‐133a levels were negatively correlated with muscle mass and SIRT1 expression.
Fig. 2.
SIRT1 regulates myonuclear numbers.
Over-expression of SIRT1 results in increased myonuclear number in skeletal muscle, and SIRT1 is actively involved in hypertrophy of skeletal muscle by enhancing NO levels to stimulate satellite cell proliferation and pro-synaptic pathways. The inhibition of FOXO1 curbs cellular catabolism.
One of the striking effects of aging is sarcopenia, loss of muscle mass and strength, which is associated with decreased levels and activity of SIRT1 [65,97,98]. It has been reported that one of the reasons for the different adaptive responses of young and old mice to resistance training is due to different levels of Poly [ADP-ribose] polymerase (PARP-1) acetylation by histone acetyltransferase General control of amino acid synthesis protein 5-like 2, GCN5 [98]. Aging decreases SIRT1 activity, which causes hyper-acetylation of PARP-1 and the consequent decrease of NAD+ and suppression of SIRT1 activity [98]. Acetylation of PARP-1 results in NF-κB dependent gene activation [99] and enhanced inflammation, which is one of the hallmarks of sarcopenia. The SIRT1 dependent adaptive response, which attenuates the aging process, also involves endothelial function in the skeletal muscle. It has been recently reported that exercise increases the levels of enthothelial NAD+ and SIRT1, which activates VEGF-associated angiogenesis [100], resulting in better supply of oxygen and food to the aging skeletal muscle.
6. Conclusion
Exercise results in massive changes in cellular metabolism and alteration of NAD+:NADH ratios. However, the activity of SIRT1 is not just dependent on NAD+ levels. Exercise mediated increases in the activity of SIRT1 is systemic, and it is observed in many organs. Regular exercise restores levels of SIRT1 in the kidney and liver in patients with neurodegenerative diseases, and therefore normalizes cellular metabolic processes and attenuates the severity of the diseases. The available data on the parallel increases in the activity of SIRT1 and PGC-1α in skeletal muscle during endurance training does not suggest a causative relationship. Overexpression of SIRT1 does not cause increased mitochondrial biogenesis, but without question, SIRT1 is an important adaptive protein for endurance training as it controls metabolic pathways. SIRT1 levels also significantly increase the number of myonuclei, the levels of NO and concomitant satellite cell proliferation. SIRT1 has emerged as an active regulator of muscle repair and hypertrophy. Regular exercise rejuvenates aging skeletal muscle partly because it has powerful stimulating effects on SIRT1. Despite the significant knowledge on the role of SIRT1 in cellular signaling, there is still much to be learned. It cannot be excluded that different site-specific acetylation/deacetylation of different lysine residues has different effects on the activity of proteins like PGC-1α.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
This study was supported by OTKA (112810) and National Excellence Program (126823), and Scientific Excellence Program TUDFO/51757/2019-ITM, at the University of Physical Education, Innovation and Technology Ministry, Hungary, grants awarded to Z.R.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101467.
Authors’ contributions
ZR, KS, AP, RG, ZP, IB, and EK contributed by searching and discussions on the relevant literature. ZR drafted the final version of the paper, but all contributed to the manuscript writing. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Williamson D.H., Lund P., Krebs H.A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem. J. 1967;103:514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gambini J., Gomez-Cabrera M.C., Borras C., Valles S.L., Lopez-Grueso R., Martinez-Bello V.E., Herranz D., Pallardo F.V., Tresguerres J.A.F., Serrano M., Viña J. Free [NADH]/[NAD+] regulates sirtuin expression. Arch. Biochem. Biophys. 2011;512:24–29. doi: 10.1016/j.abb.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Klar A.J., Fogel S., Macleod K. MAR1-a regulator of the HMa and HMalpha loci in Saccharomyces Cerevisiae. Genetics. 1979;93:37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dali‐Youcef N., Lagouge M., Froelich S., Koehl C., Schoonjans K., Auwerx J., Sirtuins The ‘ magnificent seven ’, function, metabolism and longevity. Ann. Med. 2007;39:335–345. doi: 10.1080/07853890701408194. [DOI] [PubMed] [Google Scholar]
- 5.Radak Z., Koltai E., Taylor A.W., Higuchi M., Kumagai S., Ohno H., Goto S., Boldogh I. Redox-regulating sirtuins in aging, caloric restriction, and exercise. Free Radic. Biol. Med. 2013;58:87–97. doi: 10.1016/j.freeradbiomed.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Poortmans J.R. Exercise and renal function. Sport. Med. 1984;1:125–153. doi: 10.2165/00007256-198401020-00003. [DOI] [PubMed] [Google Scholar]
- 7.Qiu Z., Zheng K., Zhang H., Feng J., Wang L., Zhou H. Physical exercise and patients with chronic renal failure: a meta-analysis. BioMed Res. Int. 2017;2017:1–8. doi: 10.1155/2017/7191826. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Martens C.R., Kirkman D.L., Edwards D.G. The vascular endothelium in chronic kidney disease: a novel target for aerobic exercise. Exerc. Sport Sci. Rev. 2016;44:12–19. doi: 10.1249/JES.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greco A., Paroni G., Seripa D., Addante F., Dagostino M.P., Aucella F. Frailty, disability and physical exercise in the aging process and in chronic kidney disease. Kidney Blood Press. Res. 2014;39:164–168. doi: 10.1159/000355792. [DOI] [PubMed] [Google Scholar]
- 10.Tang L.X., Wang B., Wu Z.K. Aerobic exercise training alleviates renal injury by interfering with mitochondrial function in type-1 diabetic mice. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2018;24:9081–9089. doi: 10.12659/MSM.912877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H.W., Kao H.H., Wu C.H. Exercise training upregulates SIRT1 to attenuate inflammation and metabolic dysfunction in kidney and liver of diabetic db/db mice. Nutr. Metab. 2019;16:22. doi: 10.1186/s12986-019-0349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoene M., Weigert C. The stress response of the liver to physical exercise. Exerc. Immunol. Rev. 2010;16:163–183. [PubMed] [Google Scholar]
- 13.Brenmoehl J., Walz C., Renne U., Ponsuksili S., Wolf C., Langhammer M., Schwerin M., Hoeflich A. Metabolic adaptations in the liver of born long-distance running mice. Med. Sci. Sports Exerc. 2013;45:841–850. doi: 10.1249/MSS.0b013e31827e0fca. [DOI] [PubMed] [Google Scholar]
- 14.Bayod S., del Valle J., Lalanza J.F., Sanchez-Roige S., de Luxán-Delgado B., Coto-Montes A., Canudas A.M., Camins A., Escorihuela R.M., Pallàs M. Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp. Gerontol. 2012;47:925–935. doi: 10.1016/j.exger.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Boutant M., Kulkarni S.S., Joffraud M., Raymond F., Métairon S., Descombes P., Cantó C. SIRT1 gain of function does not mimic or enhance the adaptations to intermittent fasting. Cell Rep. 2016;14:2068–2075. doi: 10.1016/j.celrep.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Del Campo J., Gallego-Durán R., Gallego P., Grande L. Genetic and epigenetic regulation in nonalcoholic fatty liver disease (NAFLD) Int. J. Mol. Sci. 2018;19:911. doi: 10.3390/ijms19030911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bessone F., Razori M.V., Roma M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019;76:99–128. doi: 10.1007/s00018-018-2947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radak Z., Torma F., Berkes I., Goto S., Mimura T., Posa A., Balogh L., Boldogh I., Suzuki K., Higuchi M., Koltai E. Exercise effects on physiological function during aging. Free Radic. Biol. Med. 2019;132:33–41. doi: 10.1016/j.freeradbiomed.2018.10.444. [DOI] [PubMed] [Google Scholar]
- 19.Radak Z., Chung H.Y., Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic. Biol. Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Cho J., Lee I., Kim D., Koh Y., Kong J., Lee S., Kang H. Effect of aerobic exercise training on non-alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. J. Exerc. Nutr. Biochem. 2014;18:339–346. doi: 10.5717/jenb.2014.18.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou X., Xu S., Maitland-Toolan K.A., Sato K., Jiang B., Ido Y., Lan F., Walsh K., Wierzbicki M., Verbeuren T.J., Cohen R.A., Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gariani K., Menzies K.J., Ryu D., Wegner C.J., Wang X., Ropelle E.R., Moullan N., Zhang H., Perino A., Lemos V., Kim B., Park Y.K., Piersigilli A., Pham T.X., Yang Y., Ku C.S., Koo S.I., Fomitchova A., Cantó C., Schoonjans K., Sauve A.A., Lee J.Y., Auwerx J. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190–1204. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noriega L.G., Feige J.N., Canto C., Yamamoto H., Yu J., Herman M.A., Mataki C., Kahn B.B., Auwerx J. CREB and ChREBP oppositely regulate SIRT1 expression in response to energy availability. EMBO Rep. 2011;12:1069–1076. doi: 10.1038/embor.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Defour A., Dessalle K., Castro Perez A., Poyot T., Castells J., Gallot Y.S., Durand C., Euthine V., Gu Y., Béchet D., Peinnequin A., Lefai E., Freyssenet D. Sirtuin 1 regulates SREBP-1c expression in a LXR-dependent manner in skeletal muscle. PloS One. 2012;7 doi: 10.1371/journal.pone.0043490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashida S., Arimoto A., Kuramoto Y., Kozako T., Honda S.I., Shimeno H., Soeda S. Fasting promotes the expression of SIRT1, an NAD+-dependent protein deacetylase, via activation of PPARα in mice. Mol. Cell. Biochem. 2010;339:285–292. doi: 10.1007/s11010-010-0391-z. [DOI] [PubMed] [Google Scholar]
- 26.Nemoto S., Fergusson M.M., Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1α. J. Biol. Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 27.Lee J.H., Song M.Y., Song E.K., Kim E.K., Moon W.S., Han M.K., Park J.W., Kwon K.B., Park B.H. Overexpression of SIRT1 protects pancreatic β-cells against cytokine toxicity by suppressing the nuclear factor-κB signaling pathway. Diabetes. 2009;58:344–351. doi: 10.2337/db07-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radak Z., Ihasz F., Koltai E., Goto S., Taylor A.W., Boldogh I. The redox-associated adaptive response of brain to physical exercise. Free Radic. Res. 2014;48:84–92. doi: 10.3109/10715762.2013.826352. [DOI] [PubMed] [Google Scholar]
- 29.Radák Z., Kaneko T., Tahara S., Nakamoto H., Pucsok J., Sasvári M., Nyakas C., Goto S. Regular exercise improves cognitive function and decreases oxidative damage in rat brain. Neurochem. Int. 2001;38:17–23. doi: 10.1016/S0197-0186(00)00063-2. [DOI] [PubMed] [Google Scholar]
- 30.Siamilis S., Jakus J., Nyakas C., Costa A., Mihalik B., Falus A., Radak Z. The effect of exercise and oxidant-antioxidant intervention on the levels of neurotrophins and free radicals in spinal cord of rats. Spinal Cord. 2009;47:453–457. doi: 10.1038/sc.2008.125. [DOI] [PubMed] [Google Scholar]
- 31.Radak Z., Taylor A.W., Ohno H., Goto S. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc. Immunol. Rev. 2001;7:90–107. [PubMed] [Google Scholar]
- 32.Radak Z., Toldy A., Szabo Z., Siamilis S., Nyakas C., Silye G., Jakus J., Goto S. The effects of training and detraining on memory, neurotrophins and oxidative stress markers in rat brain. Neurochem. Int. 2006;49:387–392. doi: 10.1097/MCA.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 33.Sarga L., Hart N., Koch L.G., Britton S.L., Hajas G., Boldogh I., Ba X., Radak Z. Aerobic endurance capacity affects spatial memory and SIRT1 is a potent modulator of 8-oxoguanine repair. Neuroscience. 2013;252:326–336. doi: 10.1016/j.neuroscience.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marosi K., Felszeghy K., Mehra R.D., Radák Z., Nyakas C. Are the neuroprotective effects of estradiol and physical exercise comparable during ageing in female rats? Biogerontology. 2012;13:413–427. doi: 10.1007/s10522-012-9386-3. [DOI] [PubMed] [Google Scholar]
- 35.Jeong H., Cohen D.E., Cui L., Supinski A., Savas J.N., Mazzulli J.R., Yates J.R., Bordone L., Guarente L., Krainc D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat. Med. 2012;18:159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah S.A., Khan M., Jo M.H., Jo M.G., Amin F.U., Kim M.O. Melatonin stimulates the SIRT1/nrf2 signaling pathway counteracting lipopolysaccharide (LPS)-Induced oxidative stress to rescue postnatal rat brain. CNS Neurosci. Ther. 2017;23:33–44. doi: 10.1111/cns.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L., Zhang Q., Cai Y., Sun D., He X., Wang L., Yu D., Li X., Xiong X., Xu H., Yang Q., Fan X. Resveratrol counteracts lipopolysaccharide-induced depressivelike behaviors via enhanced hippocampal neurogenesis. Oncotarget. 2016;7:56045–56059. doi: 10.18632/oncotarget.11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma C.Y., Yao M.J., Zhai Q.W., Jiao J.W., Yuan X.B., Poo M.M. SIRT1 suppresses self-renewal of adult hippocampal neural stem cells. Development. 2014;141:4697–4709. doi: 10.1242/dev.117937. [DOI] [PubMed] [Google Scholar]
- 39.Wang S.J., Zhao X.H., Chen W., Bo N., Wang X.J., Chi Z.F., Wu W. Sirtuin 1 activation enhances the PGC-1á/mitochondrial antioxidant system pathway in status epilepticus. Mol. Med. Rep. 2015;11:521–526. doi: 10.3892/mmr.2014.2724. [DOI] [PubMed] [Google Scholar]
- 40.Gomez-Pinilla F., Ying Z. Differential effects of exercise and dietary docosahexaenoic acid on molecular systems associated with control of allostasis in the hypothalamus and hippocampus. Neuroscience. 2010;168:130–137. doi: 10.1016/j.neuroscience.2010.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marton O., Koltai E., Nyakas C., Bakonyi T., Zenteno-Savin T., Kumagai S., Goto S., Radak Z. Aging and exercise affect the level of protein acetylation and SIRT1 activity in cerebellum of male rats. Biogerontology. 2010;11:679–686. doi: 10.1007/s10522-010-9279-2. [DOI] [PubMed] [Google Scholar]
- 42.Radak Z., Boldogh I. 8-Oxo-7,8-dihydroguanine: links to gene expression, aging, and defense against oxidative stress. Free Radic. Biol. Med. 2010;49:587–596. doi: 10.1016/j.freeradbiomed.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koltai E., Zhao Z., Lacza Z., Cselenyak A., Vacz G., Nyakas C., Boldogh I., Ichinoseki-Sekine N., Radak Z. Combined exercise and insulin-like growth factor-1 supplementation induces neurogenesis in old rats, but do not attenuate age-associated DNA damage. Rejuvenation Res. 2011;14:585–596. doi: 10.1089/rej.2011.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hao W., Qi T., Pan L., Wang R., Zhu B., Aguilera-Aguirre L., Radak Z., Hazra T.K., Vlahopoulos S.A., Bacsi A., Brasier A.R., Ba X., Boldogh I. Effects of the stimuli-dependent enrichment of 8-oxoguanine DNA glycosylase1 on chromatinized DNA. Redox Biol. 2018;18:43–53. doi: 10.1016/j.redox.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedersen B.K. Physical activity and muscle–brain crosstalk. Nat. Rev. Endocrinol. 2019;15:383–392. doi: 10.1038/s41574-019-0174-x. [DOI] [PubMed] [Google Scholar]
- 46.Wang R., Holsinger R.M.D. Exercise-induced brain-derived neurotrophic factor expression: therapeutic implications for Alzheimer's dementia. Ageing Res. Rev. 2018;48:109–121. doi: 10.1016/j.arr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Liu P.Z., Nusslock R. Exercise-mediated neurogenesis in the hippocampus via BDNF. Front. Neurosci. 2018;12:52. doi: 10.3389/fnins.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dean C., Liu H., Staudt T., Stahlberg M.A., Vingill S., Bückers J., Kamin D., Engelhardt J., Jackson M.B., Hell S.W., Chapman E.R. Distinct subsets of syt-IV/BDNF vesicles are sorted to axons versus dendrites and recruited to synapses by activity. J. Neurosci. 2012;32:5398–5413. doi: 10.1523/JNEUROSCI.4515-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marosi K., Mattson M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metabol. 2014;25:89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedersen B.K., Pedersen M., Krabbe K.S., Bruunsgaard H., Matthews V.B., Febbraio M.A. Role of exercise-induced brain-derived neurotrophic factor production in the regulation of energy homeostasis in mammals. Exp. Physiol. 2009;94:1153–1160. doi: 10.1113/expphysiol.2009.048561. [DOI] [PubMed] [Google Scholar]
- 51.El Hayek L., Khalifeh M., Zibara V., Abi Assaad R., Emmanuel N., Karnib N., El-Ghandour R., Nasrallah P., Bilen M., Ibrahim P., Younes J., Abou Haidar E., Barmo N., Jabre V., Stephan J.S., Sleiman S.F. Lactate mediates the effects of exercise on learning and memory through sirt1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF) J. Neurosci. 2019;39:2369–2382. doi: 10.1523/JNEUROSCI.1661-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powers S.K. Exercise: teaching myocytes new tricks. J. Appl. Physiol. 2017;123:460–472. doi: 10.1152/japplphysiol.00418.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin C.H., Lin C.C., Ting W.J., Pai P.Y., Kuo C.H., Ho T.J., Kuo W.W., Chang C.H., Huang C.Y., Lin W.T. Resveratrol enhanced FOXO3 phosphorylation via synergetic activation of SIRT1 and PI3K/Akt signaling to improve the effects of exercise in elderly rat hearts. Age. 2014;36:9705. doi: 10.1007/s11357-014-9705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L., Quan N., Sun W., Chen X., Cates C., Rousselle T., Zhou X., Zhao X., Li J. Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc. Res. 2018;114:805–821. doi: 10.1093/cvr/cvy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Donniacuo M., Urbanek K., Nebbioso A., Sodano L., Gallo L., Altucci L., Rinaldi B. Cardioprotective effect of a moderate and prolonged exercise training involves sirtuin pathway. Life Sci. 2019;222:140–147. doi: 10.1016/j.lfs.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Beyfuss K., Hood D.A. A systematic review of p53 regulation of oxidative stress in skeletal muscle. Redox Rep. 2018;23:100–117. doi: 10.1080/13510002.2017.1416773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vousden K.H., Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 58.Bartlett J.D., Close G.L., Drust B., Morton J.P. The emerging role of p53 in exercise metabolism. Sport. Med. 2014;44:303–309. doi: 10.1007/s40279-013-0127-9. [DOI] [PubMed] [Google Scholar]
- 59.Matoba S., Kang J.G., Patino W.D., Wragg A., Boehm M., Gavrilova O., Hurley P.J., Bunz F., Hwang P.M. p53 regulates mitochondrial respiration. Science. 2006;312(80-):1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki S., Tanaka T., V Poyurovsky M., Nagano H., Mayama T., Ohkubo S., Lokshin M., Hosokawa H., Nakayama T., Suzuki Y., Sugano S., Sato E., Nagao T., Yokote K., Tatsuno I., Prives C. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7461–7466. doi: 10.1073/pnas.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bensaad K., Tsuruta A., Selak M.A., Vidal M.N.C., Nakano K., Bartrons R., Gottlieb E., Vousden K.H. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 62.Geng J., Wei M., Yuan X., Liu Z., Wang X., Zhang D., Luo L., Wu J., Guo W., Qin Z.H. TIGAR regulates mitochondrial functions through SIRT1-PGC1α pathway and translocation of TIGAR into mitochondria in skeletal muscle. Faseb. J. 2019;33:6082–6098. doi: 10.1096/fj.201802209R. [DOI] [PubMed] [Google Scholar]
- 63.Holloszy J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 64.Koltai E., Szabo Z., Atalay M., Boldogh I., Naito H., Goto S., Nyakas C., Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech. Ageing Dev. 2010;131:21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koltai E., Hart N., Taylor A.W., Goto S., Ngo J.K., Davies K.J.A., Radak Z. Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:R127–R134. doi: 10.1152/ajpregu.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gurd B.J., Yoshida Y., McFarlan J.T., Holloway G.P., Moyes C.D., Heigenhauser G.J.F., Spriet L., Bonen A. Nuclear SIRT1 activity, but not protein content, regulates mitochondrial biogenesis in rat and human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R67–75. doi: 10.1152/ajpregu.00417.2010. [DOI] [PubMed] [Google Scholar]
- 67.Philp A., Chen A., Lan D., Meyer G.A., Murphy A.N., Knapp A.E., Olfert I.M., McCurdy C.E., Marcotte G.R., Hogan M.C., Baar K., Schenk S. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) deacetylation following endurance exercise. J. Biol. Chem. 2011;286:30561–30570. doi: 10.1074/jbc.M111.261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hart N., Sarga L., Csende Z., Koltai E., Koch L.G., Britton S.L., Davies K.J.A., Kouretas D., Wessner B., Radak Z. Resveratrol enhances exercise training responses in rats selectively bred for high running performance. Food Chem. Toxicol. 2013;61:53–59. doi: 10.1016/j.fct.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hart N., Sarga L., Csende Z., Koch L.G., Britton S.L., Davies K.J.A., Radak Z. Resveratrol attenuates exercise-induced adaptive responses in rats selectively bred for low running performance. Dose-Response. 2014;12:57–71. doi: 10.2203/dose-response.13-010.Radak. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Polley K.R., Jenkins N., O'Connor P., McCully K. Influence of exercise training with resveratrol supplementation on skeletal muscle mitochondrial capacity. Appl. Physiol. Nutr. Metabol. 2015;41:26–32. doi: 10.1139/apnm-2015-0370. [DOI] [PubMed] [Google Scholar]
- 71.McDermott M.M., Leeuwenburgh C., Guralnik J.M., Tian L., Sufit R., Zhao L., Criqui M.H., Kibbe M.R., Stein J.H., Lloyd-Jones D., Anton S.D., Polonsky T.S., Gao Y., De Cabo R., Ferrucci L. Effect of resveratrol onwalking performance in older people with peripheral artery disease the restore randomized clinical trial. JAMA Cardiol. 2017;2:902–907. doi: 10.1001/jamacardio.2017.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olesen J., Gliemann L., Biensø R., Schmidt J., Hellsten Y., Pilegaard H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J. Physiol. 2014;592:1873–1886. doi: 10.1113/jphysiol.2013.270256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gliemann L., Schmidt J.F., Olesen J., Biensø R.S., Peronard S.L., Grandjean S.U., Mortensen S.P., Nyberg M., Bangsbo J., Pilegaard H., Hellsten Y. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 2013;591:5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jung H.Y., Lee D., Ryu H.G., Choi B.H., Go Y., Lee N., Lee D., Son H.G., Jeon J., Kim S.H., Yoon J.H., Park S.M., Lee S.J.V., Lee I.K., Choi K.Y., Ryu S.H., Nohara K., Yoo S.H., Chen Z., Kim K.T. Myricetin improves endurance capacity and mitochondrial density by activating SIRT1 and PGC-1α. Sci. Rep. 2017;7:6237. doi: 10.1038/s41598-017-05303-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yada K., Suzuki K., Oginome N., Ma S., Fukuda Y., Iida A., Radak Z. Single dose administration of taheebo polyphenol enhances endurance capacity in mice. Sci. Rep. 2018;8:14625. doi: 10.1038/s41598-018-33029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su K.Y., Yu C.Y., Chen Y.W., Huang Y.T., Chen C.T., Wu H.F., Sophia Chen Y.L. Rutin, a flavonoid and principal component of Saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int. J. Med. Sci. 2014;11:528–537. doi: 10.7150/ijms.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Higashida K., Kim S.H., Jung S.R., Asaka M., Holloszy J.O., Han D.H. Effects of resveratrol and SIRT1 on PGC-1α activity and mitochondrial biogenesis: a reevaluation. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davis J.M., Murphy E.A., Carmichael M.D., Davis B. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R1071–R1077. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- 79.Puigserver P., Adelmant G., Wu Z., Fan M., Xu J., O'Malley B., Spiegelman B.M. Activation of PPARγ coactivator-1 through transcription factor docking. Science. 1999;286(80-):1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- 80.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 81.Cantó C., Jiang L.Q., Deshmukh A.S., Mataki C., Coste A., Lagouge M., Zierath J.R., Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metabol. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cantó C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C., Elliott P.J., Puigserver P., Auwerx J. AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharples A.P., Hughes D.C., Deane C.S., Saini A., Selman C., Stewart C.E. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell. 2015;14:511–523. doi: 10.1111/acel.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taheripak G., Bakhtiyari S., Rajabibazl M., Pasalar P., Meshkani R. Protein tyrosine phosphatase 1B inhibition ameliorates palmitate-induced mitochondrial dysfunction and apoptosis in skeletal muscle cells. Free Radic. Biol. Med. 2013;65:1435–1446. doi: 10.1016/j.freeradbiomed.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 85.Shahbazian M.D., Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu. Rev. Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 86.Fulco M., Schiltz R.L., Iezzi S., King M.T., Zhao P., Kashiwaya Y., Hoffman E., Veech R.L., Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol. Cell. 2003;12:51–62. doi: 10.1016/S1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 87.Hall Z.W., Ralston E. Nuclear domains in muscle cells. Cell. 1989;59:771–772. doi: 10.1016/0092-8674(89)90597-7. [DOI] [PubMed] [Google Scholar]
- 88.Ross J.A., Pearson A., Levy Y., Cardel B., Handschin C., Ochala J. Exploring the role of PGC-1α in defining nuclear organisation in skeletal muscle fibres. J. Cell. Physiol. 2017;232:1270–1274. doi: 10.1002/jcp.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ross J.A., Levy Y., Svensson K., Philp A., Schenk S., Ochala J. SIRT1 regulates nuclear number and domain size in skeletal muscle fibers. J. Cell. Physiol. 2018;233:7157–7163. doi: 10.1002/jcp.26542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ryall J.G., Dell'Orso S., Derfoul A., Juan A., Zare H., Feng X., Clermont D., Koulnis M., Gutierrez-Cruz G., Fulco M., Sartorelli V. The NAD+-dependent sirt1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell. 2015;16:171–183. doi: 10.1016/j.stem.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderson J.E. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol. Biol. Cell. 2000;11:1859–1874. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suwa M., Nakano H., Radak Z., Kumagai S. Effects of nitric oxide synthase inhibition on fiber-type composition, mitochondrial biogenesis, and SIRT1 expression in rat skeletal muscle. J. Sports Sci. Med. 2015;14:548–555. [PMC free article] [PubMed] [Google Scholar]
- 93.Csiszar A., Labinskyy N., Pinto J.T., Ballabh P., Zhang H., Losonczy G., Pearson K., De Cabo R., Pacher P., Zhang C., Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H13–20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mattagajasingh I., Kim C.S., Naqvi A., Yamamori T., Hoffman T.A., Jung S.B., DeRicco J., Kasuno K., Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stitt T.N., Drujan D., Clarke B.A., Panaro F., Timofeyva Y., Kline W.O., Gonzalez M., Yancopoulos G.D., Glass D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell. 2004;14:395–403. doi: 10.1016/S1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 96.Koltai E., Bori Z., Chabert C., Dubouchaud H., Naito H., Machida S., Davies K.J., Murlasits Z., Fry A.C., Boldogh I., Radak Z. SIRT1 may play a crucial role in overload-induced hypertrophy of skeletal muscle. J. Physiol. 2017;595:3361–3376. doi: 10.1113/JP273774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ziaaldini M.M., Koltai E., Csende Z., Goto S., Boldogh I., Taylor A.W., Radak Z. Exercise training increases anabolic and attenuates catabolic and apoptotic processes in aged skeletal muscle of male rats. Exp. Gerontol. 2015;67:9–14. doi: 10.1016/j.exger.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 98.Mohamed J.S., Wilson J.C., Myers M.J., Sisson K.J., Alway S.E. Dysregulation of SIRT-1 in aging mice increases skeletal muscle fatigue by a PARP-1-dependent mechanism. Aging. 2014;6:820–834. doi: 10.18632/aging.100696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hassa P.O., Haenni S.S., Buerki C., Meier N.I., Lane W.S., Owen H., Gersbach M., Imhof R., Hottiger M.O. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-κB-dependent transcription. J. Biol. Chem. 2005;280:40450–40464. doi: 10.1074/jbc.M507553200. [DOI] [PubMed] [Google Scholar]
- 100.Das A., Huang G.X., Bonkowski M.S., Longchamp A., Li C., Schultz M.B., Kim L.J., Osborne B., Joshi S., Lu Y., Treviño-Villarreal J.H., Kang M.J., tyng Hung T., Lee B., Williams E.O., Igarashi M., Mitchell J.R., Wu L.E., Turner N., Arany Z., Guarente L., Sinclair D.A. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173:74–89. doi: 10.1016/j.cell.2018.02.008. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.