Abstract
Zinc is an essential element for all forms of life, and one in every ten human proteins is a zinc protein. Zinc has catalytic, structural and signalling functions and its correct homeostasis affects many cellular processes. Zinc deficiency leads to detrimental consequences, especially in tissues with high demand such as skeletal muscle. Zinc cellular homeostasis is tightly regulated by different transport and buffer protein systems. Specifically, in skeletal muscle, zinc has been found to affect myogenesis and muscle regeneration due to its effects on muscle cell activation, proliferation and differentiation. In relation to skeletal muscle, exercise has been shown to modulate zinc serum and urinary levels and could directly affect cellular zinc transport. The oxidative stress induced by exercise may provide the basis for the mild zinc deficiency observed in athletes and could have severe consequences on health and sport performance. Proteostasis is induced during exercise and zinc plays an essential role in several of the associated pathways.
Keywords: Zinc regulation, Zinc homeostasis, Exercise, Skeletal muscle, Physical performance
Highlights
-
•
Zinc deficiency could be a crucial issue in sport performance for athletes.
-
•
Exercise could modulate zinc serum and cellular homeostasis.
-
•
Zinc is part of proteostatic systems critical during exercise.
1. Introduction
1.1. Zinc in human health and disease
The first indication of the importance of zinc for biological systems dates back to 1869, when Jules Raulin demonstrated that zinc is required for the growth of Aspergillus niger [1]. Decades later, it was shown that zinc was also essential in plants [2] and animals ([3]), including humans [4]. Zinc is the second most abundant ‘trace’ metal in the human body after iron, with an average 70 kg human containing 2–3 g of zinc, with only 0.1% being replenished daily through dietary intake [5]. However, it is not homogenously distributed in the body, with the majority contained in skeletal muscle (~60%) and bone (30%) [6,7]. The tissue concentration of total zinc is also highly variable, as zinc is abundant in skeletal muscle, bone, prostate and pancreas (200 μg/g) [7], whereas heart, brain and plasma contain a lower concentration (1–23 μg/g). Only ~0.1% of bodily zinc is contained in plasma, where it is predominantly bound by albumin (~80%) and α2-macroglobulin (~20%) [8,9].
Zinc has structural and catalytic roles in proteins and cellular signalling functions [10,11]. Confirmation as a structural cofactor in proteins came in 1938 when insulin was crystallised with zinc [12], while the first catalytic function of zinc was described for carbonic anhydrase in 1939 [13]. Zinc has since been shown to be a cofactor in all six enzyme classes [14]. Additionally, zinc has been shown to regulate the activity of different proteins, for instance in protein tyrosine phosphatases [15]. The effects of cellular zinc signalling are an active research area given its wide implications in cell signalling.
The identification of zinc-finger domains in Xenopus laevis transcription factor TFIIIA [16,17] laid the foundation to search genomes for zinc proteins. Indeed, bioinformatics analyses predict that zinc proteins make up 4–5% of bacterial and archaeal proteomes [18], while mammals express more than 3000 zinc proteins, accounting for at least 9–10% of the total proteome [19]. Considering the vast amount of proteins that bind this cation, it is not surprising that zinc is involved in multiple physiological processes, including nucleic acid and protein synthesis, transcriptional regulation, glucose metabolism and hormone production, storage and secretion [10].
Zinc deficiency has detrimental consequences for many aspects of human health [7] and affects two billion humans worldwide [20]. Severe zinc deficiency leads to generalized poor growth concomitant with multiple disorders including loss of appetite, immune abnormalities, dwarfism, hypogonadism, mental lethargy and rough skin [4,21]. Due to its proliferative nature, the immune system is particularly susceptible to zinc deficiency [20]. Zinc regulates several crucial processes in innate immunity, including phagocytosis, intracellular killing and cytokine production, while in the adaptive immune system, zinc deficiency results in reduced T cell development and function and B cell antibody production [22]. Zinc deficiency has been also associated with development of cardiovascular diseases [23], anemia [24,25] and damages in neurogenesis during early development [26]. Zinc supplementation can improve these symptoms, but it must be accompanied by copper, otherwise the excess zinc can lead to copper deficiency [27,28]. Importantly, plasma zinc concentration cannot be used as a reliable marker of zinc status because it does not necessarily correlate with cellular zinc concentration [29]. Under multiple disorders associated with zinc deficiency, clinical improvement of these disorders associated with zinc administration could be used as indicator of zinc status and patient recovery due to plasma zinc could not be totally reliable [20,24,25,30].
1.2. Zinc cellular homeostasis
The human body does not have a tissue reservoir for zinc, nor does it have a protein analogous to ferritin, which stores iron and releases it when needed [31]. Therefore, an adequate dietary supply is required to prevent zinc deficiency unless a genetic condition is the root of the problem [32]. In this sense, zinc deficiency can result as symptom of diseases which impair either intestinal control of zinc uptake, for example in acrodermatitis enteropathica or cystic fibrosis [33], or intestinal release, for example in inflammatory bowel diseases such as Crohn's disease [34]. The myriad of biological processes in which zinc is required makes zinc homeostasis to be tightly controlled at the organism, tissue and cellular levels. Dietary zinc absorption is regulated by the duodenum and jejunum [35], while excretion of excess zinc is achieved through gastrointestinal secretion, sloughing of mucosal cells and integument, and renal excretion [36]. Zinc ingestion is dependent on the quantity and bioavailability of zinc in food [28]. Foods rich in zinc include red meat and pulses, while cereals containing non-digestible plant ligands such as phytate reduce zinc bioavailability [37,38].
Bone zinc accounts for approximately 29% of whole-body zinc, but the largest zinc pool in the body is muscle, which contains approximately 57% [9]. However, zinc is not homogeneously distributed in all muscles: it has been reported that red oxidative muscle can contain up to four-fold zinc than white glycolytic muscles [39]. The zinc pool mainly responsible for this difference is located in myofibrils and the nucleus. Intriguingly, muscle zinc levels are maintained under conditions of dietary zinc deficiency [6] except in soleus, a red muscle with high zinc content [40]. Interestingly, although growth is severely retarded in rats on a zinc-deficient diet, they do undergo an increase in muscle mass, which therefore requires an increase in total muscle zinc content [41]. This is possible because the organism can mobilize zinc from tissues with lower requirements, mainly bone, to others with higher requirements, especially muscle. This does not imply that the bone is the body reservoir for zinc, but that the organism is able prioritize in cases of extreme necessity [41].
Zinc complexes are second only to copper in their relative stability as described by the Irving-Williams series of transition divalent metal ions [42]. The high affinity of structural and catalytic protein-zinc complexes is one of the reasons for the large disparity between cellular total and free zinc; while the average total cellular zinc content is hundreds of micromolar, the free cytosolic zinc concentration is approximately picomolar [43,44]. The tight control of cytosolic zinc(II) ion concentrations in mammalian cells underpins the significance of zinc as a signalling ion akin to calcium and magnesium [45]. At the cellular level, the main components of zinc homeostasis regulation are zinc-binding proteins, zinc sensors, and zinc transporters. The critical function of this repertoire is to be able to deal with both long and short-term zinc stimuli [11,46].
1.3. Zinc-binding proteins and zinc sensors
Metallothioneins (MTs) are small, cysteine-rich cytosolic proteins [47] that in mammals can bind up to seven zinc(II) ions with differing affinity, linking the zinc occupancy of MTs with the zinc status of the cell. Indeed, during cytosolic zinc deficiency the zinc bound to low affinity sites in MTs will become displaced and available, whereas during high cytosolic zinc concentrations, zinc will occupy these low affinity sites, reducing the cytosolic free zinc. Thus, MTs can function as temporary storage for zinc [44,48,49]. Therefore, a major cytosolic store of zinc is bound by cysteine residues, meaning that cellular zinc homeostasis is inextricably linked to the redox state of the cell [11], despite zinc itself being redox neutral. In humans, MT-1 and MT-2 are ubiquitously expressed and their expression levels respond to different stimuli such as metal ions, cytokines, glucocorticoids and oxidative stress. MT-3 and MT-4 are found only in the central nervous system and epithelia respectively [47,50]. Special mention should be made to metal response element-binding transcription factor-1 or MTF-1, to date the only zinc sensor described in mammals [48]. When intracellular zinc concentration is high, MTF-1 suffers a conformational change upon zinc binding to its zinc-fingers. This allows MTF-1 binding to dedicated regulatory elements called MREs (metal response elements) located at the promoters of specific genes inducing their expression. MTs are among these genes and therefore, they can respond rapidly to changes in cellular zinc status [51,52].
1.4. Zinc transporters
Zinc transporters are proteins which enable the flux of Zn2+ across biological membranes. The two families of zinc transporters expressed in mammals are the Zrt/Irt-like proteins (ZIPs; SLC39A) and the zinc transporters (ZnTs; SLC30A), which function to transport zinc into and out of the cytosol respectively [53]. In humans there are 14 ZIPs and 10 ZnTs, each with specific tissue expression and subcellular localisation [54].
2. Zinc and skeletal muscle
2.1. Effects of zinc deficiency in skeletal muscle
As stated above, zinc levels are maintained in skeletal muscle even if zinc intake is low. However, zinc deficiency does have a negative impact on muscle function, highlighting the biological relevance of small changes in the status of this cation. The first evidence towards this fact was reported in the late 197 0s, when a study found that zinc deficiency per se in post-weanling rats had little effect on the number and size of fibers, except for the soleus muscle, which showed an overall reduction in weight as a result of increased fiber loss [40]. The authors hypothesized that soleus muscles might be more susceptible to changes in zinc concentration because of their higher zinc content in comparison to more glycolytic muscles [40]. This result was confirmed by a second study, which also found that the proportion of fast to slow fiber types was increased in rats fed on a zinc-deficient diet with respect to ad libitum or pair-fed controls on a normal zinc diet [55]. This suggests that this phenotype would not be merely caused by the reduced food intake induced by inadequate zinc levels. Importantly, rat neonate soleus muscles contain a high proportion of fast fibers that turn into the slow type as the animal grows [55]. Thus, the authors hypothesized that zinc deficiency could specifically cause a defect in development or differentiation [55]. Similarly, the diaphragms of the zinc-deficient group also showed altered fiber type composition compatible with a perturbation in development [55]. It is important to mention that the development of the mature muscle fiber type composition partially depends on motor neuron input during pre- and post-natal development [56,57], and zinc may impinge in this process. Indeed, motor neurons differentiated from human induced stem cells under low zinc levels exhibit altered synaptic function [58]. Whether zinc also plays a role in motor neuron development in vivo remains to be elucidated. Still, animal models of zinc deficiency show apparent stiffness of the joints and abnormal gait, specially the growing guinea pig [59] and chick ([60]), as well as severely deficient rats [61]. These phenotypes could derive from defects in muscle, nerve or the neuromuscular junction (NMJ) alone or in combination. Thus, the alterations in fiber type composition observed upon dietary zinc deficiency in rats might be partially due to defects in motor neuron-muscle communication.
Zinc deficiency reduces the activity of some, but not all, metalloenzymes [10]. Further, the same metalloenzyme could be affected in some tissues but not in others. For example, lactic dehydrogenase activity was found to be significantly reduced in heart, kidney and gastrocnemius muscle of zinc deficient rats, whereas no changes were observed in pancreas or lung [62]. In the same experiment, the activity of mitochondrial glutamic dehydrogenase was not altered in any of the tissues analysed [62]. Thus, zinc deficiency does not affect all zinc-proteins equally. The decrease in the activity of zinc metalloenzymes could be due to the absence of appropriate amounts of zinc to use as a cofactor, or simply because the concentration of these proteins is reduced, either due to a reduction in their gene expression or to an increase in their degradation rate [63]. Therefore, even if zinc levels are mostly maintained in muscle in conditions of dietary zinc deficiency, muscle function is impaired to some extent due to the decline in the activity of specific zinc proteins.
Indeed, after the realization that zinc is necessary for the correct functioning of thousands of proteins in the organism, most experiments in the postgenomics era rely on the removal or downregulation of specific genes to unravel their function. Only in this manner, specific molecular mechanisms can be deciphered. This is in contrast with the majority of the experiments described above in the 70s and 80s, which characterize the general effects dietary zinc deficiency exerts on muscle biology and function as a whole. In accordance, these type of experiments are only applied nowadays when the experimenter is interested in the final outcome of performance of the organism, as in the case of the effect of zinc diet on exercise (see below).
2.2. Zinc functions in relation to skeletal muscle
Ex vivo muscle testing has been extensively used over decades to assess muscle properties, such as contractility, more accurately than with experiments in vivo. With this technique, it was found that zinc reversibly blocks synaptic transmission at the NMJ [64], and prolongs the active state of the frog's sartorius muscle but has no effect on its maximal intensity [65]. Additionally, it increases the absolute refractory period of the most excitable fibers: it takes them four times longer to initiate a second contraction than in the absence of zinc in the medium [65]. Considering the shape of the action potentials, the authors hypothesized that zinc probably delays the exit of K+ ions during repolarization [65], which was later confirmed in the same experimental setting [66]. We now know that zinc regulates the activity of several ion channels, including acetylcholine signalling [67], causing the activation or inhibition of the corresponding ion currents, depending on zinc concentration and binding location [68,69].
Zinc has been shown to play a major role in myogenesis in vitro. For example, in the C2C12 mouse skeletal muscle cell line it was shown that addition of zinc in the growth medium promoted the proliferation and activation of myoblasts [70,71], as well as the differentiation and maturation of myofibers later in the process [70]. This raises the interesting possibility that the release of zinc from muscle upon damage in vivo could contribute to the activation and proliferation of muscle satellite cells to repair the damage [71]. Mechanistically, zinc would exert these functions through a cascade of events that initiates with the entry into the cell through dedicated ZIP transporters. Once in the cytosol, it would induce the phosphorylation and subsequent activation of Zip7, a zinc transporter localized to the endoplasmic reticulum. Activated ZIP7 releases Zn2+ from the ER, promoting Akt phosphorylation and myogenic differentiation [70].
More molecular insight into how zinc impinges on myogenesis came recently from Padilla-Benavides’ group, who sought to fully characterize the zinc content and the expression of zinc transporters throughout the course of differentiation of C2C12 cells [72]. They showed that zinc content is dynamic during myoblast differentiation: it initially decreases but starts recovering after 12 hpd (hours post-differentiation) until it reaches a maximum in mature myotubes at 72 hpd. This dynamism is also observed in the expression of zinc transporters. Regarding the influx transporters, while the expression patterns of Zip5 and Zip6 exhibit a single discrete peak at 12 hpd, Zip3 and Zip8 are upregulated throughout the whole process. Of note, it has been shown that Zip8-mediated zinc influx promotes MTF-1 transcriptional activity, which in turn upregulates the expression of matrix metalloproteinases (MMPs) in chondrocytes [73]. The same mechanism could take place during myogenesis as MMPs are required for myotube fusion [74]. In relation to ZnT efflux proteins, it is interesting to note that the expression of ZnT4, which is localized in endosomes and secretory vesicles, and of ZnT7, which is localized in the Golgi apparatus, peak at 12 hpd. Importantly, the activity of the insulin-signalling pathway is downregulated in mouse skeletal muscle deficient for ZnT7 [75]. Furthermore, ZnT8 was the sole transporter to become downregulated after the onset of myogenesis, and its expression was only restored after differentiation [76]. Interestingly, ZnT8 is involved in the regulation of insulin secretion in pancreatic beta cells [76]. Hence, data on ZnT7 and ZnT8 can provide a link between zinc and insulin signalling pathways during myogenesis. Intriguingly, Zip11 accumulates late, at 72 hpd [76]. This is the only zinc transporter known to be localized to the nuclear membrane (it can also be found in the Golgi apparatus) , so it would be interesting to explore the potential influence of zinc inside the nucleus in mature myotubes in the future.
Further evidence accumulates for zinc biology contributing to myogenesis and the control of muscle mass. The ablation of MT-1 and MT-2 in mice causes a hypertrophic phenotype that is more pronounced in fast-twitch muscles and that correlates with an increase in muscle strength [77]. Yet, total intracellular zinc content remained constant in these mice [77] MT-1 and MT-2 are also upregulated in skeletal muscle atrophy models [78] and in sarcopenia [77], where total intracellular zinc content is increased as well. It is important to mention that none of these studies measured the concentration of free intracellular zinc. A change in zinc content could lead to the deregulation of specific signalling pathways and explain the observed phenotypes. Indeed, while the measurement of total intracellular zinc (free zinc and that bound to proteins) is relatively straightforward, the quantification of the free zinc pool, which relies on fluorescent approaches, is much more challenging [45]. Although these data suggest that MTs and zinc control muscle mass, the exact mechanisms remain unknown due to the complex interplay between zinc binding to MTs, which depends on zinc concentration and the redox state of the cell, the fact that zinc induces the expression of these proteins, the potential appearance of compensatory mechanisms for zinc buffering when the expression of MTs is compromised and the difficulty in measuring free intracellular zinc content. On the other hand, it was recently shown that the zinc transporter Zip14 is upregulated in cancer-associated atrophic muscles (cachexia), which leads to an excess total intracellular zinc content [79]. By employing muscle progenitor cells in vitro, the authors demonstrated that the Zip14-mediated increase in zinc uptake directly promoted muscle wasting through myosin heavy chain loss and impaired regeneration [79]. Altogether, these findings suggest that MT-1/2 and Zip14 could be used as targets to revert or ameliorate those conditions that involve loss of skeletal muscle mass.
Regarding cellular metabolism, zinc acts as an insulin mimetic agent leading to increased total glucose consumption in mouse, rat and human skeletal muscle cells in vitro [80,81]. Mechanistically, Zip7 could contribute to this function. Indeed, its expression is induced by glucose and repressed in conditions mimicking insulin resistance in C2C12, as well as in vivo in mice fed on a high fat diet [82]. Further, Zip7 levels have important consequences for cellular metabolism. Its silencing in vitro causes the downregulation of the expression of genes involved in glucose and glycogen metabolism, as well as to a decrease in Akt-mediated signalling [82,83]. As a result, in the absence of Zip7, cells become insulin resistant. On the contrary, Zip7 overexpression triggers the induction of the expression genes involved in insulin signalling and glucose metabolism, including insulin itself [82,83]. Taking these data together, Zip7 contributes to glycemic control in skeletal muscle cells. However, whether Zip7 expression levels really influence zinc cytoplasmic content remains to be explored.
Cardiotoxin (CTX) is a cytolytic toxin from snake venom that is widely used to study muscle regeneration in animal models. CTX selectively kills myofibers while leaving satellite cells unaffected [84]. After CTX-induced injury, satellite cells are activated, proliferate, differentiate and fuse to restore muscle structure and function. In mice , both marginal zinc deficiency or excessive zinc intake (8 or 190 mg Zn2+/kg diet, respectively, relative to a zinc-adequate diet of 35 mg Zn2+/kg diet) delay muscle recovery from CTX-induced injury [85]. The authors hypothesized that this delay was the result of the reduction in muscle protein synthesis observed under a zinc-deficient or zinc-supplemented diet [63]. However, given that zinc promotes the proliferation and activation of C2C12 cells in vitro [70,71], it would be worth exploring whether satellite cells are affected in this setting. Altogether, this finding could have profound implications for the general population but especially for athletes, who often incur muscle injuries: it is important to take appropriate amounts of zinc to prevent the impairment of muscle regeneration.
3. Zinc and its importance in exercise
Skeletal muscle is a tissue whose main function is contraction, force and movement production and involves a complex mix of compositions, structures and functions. It is constituted of several bundles of highly vascularized and innervated myofibers [86]. Skeletal muscle has been defined as a malleable tissue that is modulated by exercise where reactive oxygen and nitrogen species regulate adaptation training responses [87]. Physical exercise is considered a critical tool to prevent and treat muscle abnormalities [88]. Additionally, exercise is considered to be a polypill for health maintenance and recovery due to its potential to reduce risks and damage from chronic diseases, its easy availability and lack of adverse effects [89]. Furthermore, exercise helps to maintain muscle mass promoting healthy aging and reduced sarcopenia and has been proven to be a perfect toolkit for prevention and treatment of a wide variety of diseases [90].
3.1. Modulation of serum and urine zinc by exercise
Exercise has been proven to activate different metabolic pathways modulating the levels of many metabolites and minerals including zinc (Table 1). Acute aerobic endurance and muscular strength exercise decrease zinc serum levels while increasing urinary zinc, especially in case of exercise until exhaustion [[91], [92], [93]]. These changes in zinc levels have been found during the recovery period, so it could be related with the muscle repairing processes that take part during this phase. However, immediately after exercise, increased zinc serum levels can be observed [95].
Table 1.
Effect of exercise on zinc homeostasis in serum and urine.
| Sport modality | Type of intervention | Subjects | ZN status in serum | ZN status in urine | Time of measurement | Reference |
|---|---|---|---|---|---|---|
| Aerobic exercise bout | Acute | – | Decreased | Increased | Before and 4 h following exercise cessation | [91] |
| Aerobic endurance and muscular strength | Acute | Trained participants | Decreased in both cases | Increased in both cases | Before and after exercise | [92] |
| Acute physical activity until exhaustion | Acute | Middle- and long-distance runners and sedentary subjects | Decreased | Increased | Before and after exercise test | [93] |
| 30–40 min hard exercise on a cycle ergometer | Acute | Twelve healthy male athletes | Decreased | – | Before and after exercise test | [94] |
| Immediately after a bout of aerobic exercise | Acute | – | Immediately increased after exercise. | – | Immediately after exercise | [94,95] |
| Run 6 mi near their maximal pace | Acute | Nine male runners (23–46 yr) | Decreased after exercise (2h) | Increased (2 h later more than two-fold and more than 1.5 fold 24h later) | 2h (serum and urine) and 24 h later (urine) | [96] |
| Acute exercise at 90% of VO2 max to exhaustion | Acute | Eight moderately trained and five untrained male runners | Decreased after 2h | No differences | 2 h post exercise | [97] |
| Acute exhaustive exercise | Acute | 12 healthy, sedentary men, 25–35 years of age | Decreased | – | 2, 5, 10, 15, 30, 45, 60, 75, 90 and 120 min after exercise | [98] |
| Brief intensive exercise or more prolonged road-running exercise | Acute | Seven untrained volunteers and seven trained volunteers | Decreased | Increased | Post exercise | [99] |
| 3-weeks training program | Chronic | Elite swimmers amateur swimmers and sedentary individuals | Increased after test and decreased 1h post-test. | . | Immediately after and 1 h post-test. | [100] |
| Middle distance runners and untrained subject. | Chronic | Middle distance runners and untrained, non-sportsmen participant | Increased in athletes | No significant changes | Six months after basal measurements | [101] |
| 30 min of moderate-intensity running for 6 weeks | Chronic | Wistar rats | Increased | – | After 6 weeks of training | [102] |
| 3 months endurance or endurance-strength training | Chronic | Abdominally obese women | Decreased | Increased | Basal and after 3 months | [103] |
In contrast, after a chronic exercise intervention zinc serum levels were increased in humans [101] and animal models [102]. It is obvious that modulation of zinc levels by exercise are influenced by the duration of exercise intervention (chronic or acute). Even living an active or sedentary lifestyle influences basal zinc serum levels [104].
Zinc levels fluctuations during exercise could be related with changes in the antioxidant response stimulated by exercise. Zinc is involved in the antioxidant response to exercise through changes in expression of copper zinc superoxide dismutase (CuZnSOD) [105] and this response could be modulated by exercise and resting [105,106]. The antioxidant response promoted by exercise could vary in function of the sport modality due to differences in erythrocyte zinc, CuZnSOD activity and MT [107]. Zinc could be playing an essential role in antioxidant response to exercise by inhibiting free radical production, increasing antioxidant activity and preventing muscle exhaustion [108]. Zinc administration has been examined in pulmonary rehabilitation [109] in combination with exercise obtaining an increase in muscle strength and a tendency of higher type I fibre proportion. Zinc administration in combination with exercise could improve lipid profile decreasing total cholesterol, LDL-cholesterol and triglycerides and increase HDL cholesterol [110].
It has been proposed that zinc deficiency could be leading to higher levels of lipid peroxidation in different tissues while zinc administration produces an increase in glutathione levels in different tissues resulting in a higher antioxidant capacity [111]. Moreover, zinc deficiency has been related with a lower DNA integrity, more oxidative stress and altered DNA repair capacity [103]. Particularly in sport performance zinc deficiency has been associated with a lower peak oxygen uptake, carbon dioxide output and respiratory exchange ratio [112] and with a reduction in total work capacity of skeletal muscle [113]. Zinc deficiency in physically active men leads to a reduction carbonic anhydrase activity in red blood cells, impairing metabolic responses during exercise, and contributing to a lower physical performance observed in conditions of dietary zinc deficiency [112]. Zinc depletion in humans leads to a decrease in total work capacity for knee and shoulder extensors and flexors and a reduction in physical performance that correlates with decreased plasma zinc [113]. Zinc levels could have been maintained in muscle due to the compensatory effects from high zinc content tissues where zinc does not play a key role such as bone, highlighting the capacity of the organism to prioritize and mobilize zinc from different tissues to others [41]. The reason by which muscle performance was not maintained under zinc depletion could be related to lactic acid accumulation due to lactate dehydrogenase activity being reduced under zinc depletion leading to lactic acid increase and muscle fatigue [113]. Zinc deficiency is common in athletes in different training periods [114].
3.2. Effectiveness of zinc supplementation on exercise
Zinc deficiency constitutes a crucial issue for professional athletes because it could affect sport performance and health. Although zinc deficiency is a serious issue, there is not experimental evidence that support the possible benefits on zinc administration beyond recommended dietary intakes as a potential supplement for athletes, thus extra zinc for athletes would not report more beneficial effects [115,116] or enhance training adaptations [117]. Consequently, athletes should be discouraged to ingest extra zinc supplementation because studies that determine the effect of zinc supplementation in athletes beyond their recommended daily intakes levels in sport performance are lacking. The effect of extra zinc administration in this specific situation remains unclear further long human trials studies are required [51]. In agreement, it has been shown that the organism is able to make some adjustments in zinc excretion, in order to maintain a balanced supply to all tissues according to their specific demands, therefore extra zinc would be excreted [118]. Moreover, overdose of zinc could trigger side effects including nausea, abdominal cramping, vomiting, tenesmus, diarrhea, copper deficiency, even teratogenic or lethal effect during early embryogenesis [28].
Taking all these data into account, zinc could be involved in antioxidant response activated by exercise, therefore zinc deficiency in athletes could have consequences for metabolic adaptations modulated by exercise and it could induce negative effects to sport performance, exercise adaptation and affecting the long-term health of the athlete.
3.3. Modulation on cellular zinc levels by exercise
Exercise could modulate changes in serum and urine zinc levels. However, it should also be considered that these changes in zinc serum levels do not necessarily mean the same regulation in cellular free zinc levels. Both finding appropriate zinc biomarkers and measuring the approximately picomolar intracellular free zinc2+ concentrations are ongoing challenges in zinc biochemistry [45]).
Particularly in cardiac cells [119], exercise induces RNA levels of metallothioneins (MT1 and MT2), while mice lacking MT1/2 exhibit cardiac dysfunction during exercise. Exercise could also stimulate MT levels in aortic tissues [120], even an increase has been reported specifically in muscle due to exercise [121].
Moreover, the effect of exercise on zinc homeostasis extend beyond muscle, stimulation of MT1 and MT2 by exercise has been shown using an in vitro model applying electric pulse stimulation in hepatocytes [122]. Additionally, exercise improved the spatial learning abilities of rats [123]. Interestingly, an upregulation of different ZnTs (ZnT2, ZnT4, ZnT5, ZnT6 and ZnT7) in hippocampus was reported indicating a potential effect of exercise on zinc cellular homeostasis [123]. Exercise could enhance the transport of cytosolic zinc to cellular organelles. Moreover, they also reported an increase in zinc intracellular transporters, specifically ZIP transporters (ZIP6 and ZIP7) was found, while MT1 and MT3 levels were also increased in hippocampus [123]. Additionally, exercise has been shown to promote activation of MTs, especially MT1, MT2 and MT3 in mouse spinal cord [124]. After two weeks of running 30 min per day, an increase in mRNA and proteins levels of MTs in spinal cord were found compared with sedentary controls [124]. Another study reported an increase in MTs levels in response to exercise in rat renal tubes suggesting a protective role against oxidative stress and apoptosis in proximal tubular cells [125].
Exercise is associated with oxidative stress production in muscle producing lipids and proteins oxidation [126]. As it has been discussed above, exercise could lead to a cytosolic zinc levels increase that could also promote zinc accumulation in mitochondria [127]. An abnormal mitochondrial zinc accumulation associated with over-exercise could be associated with higher oxidative stress production resulting in mitochondrial dysfunction in vascular smooth muscle cells [127,128]. Zinc accumulation could be regulating mitochondrial function affecting ZnTs, ZIPs and MTs homeostasis [127]. Consequently, zinc levels changes related to exercise could be involved in oxidative stress production.
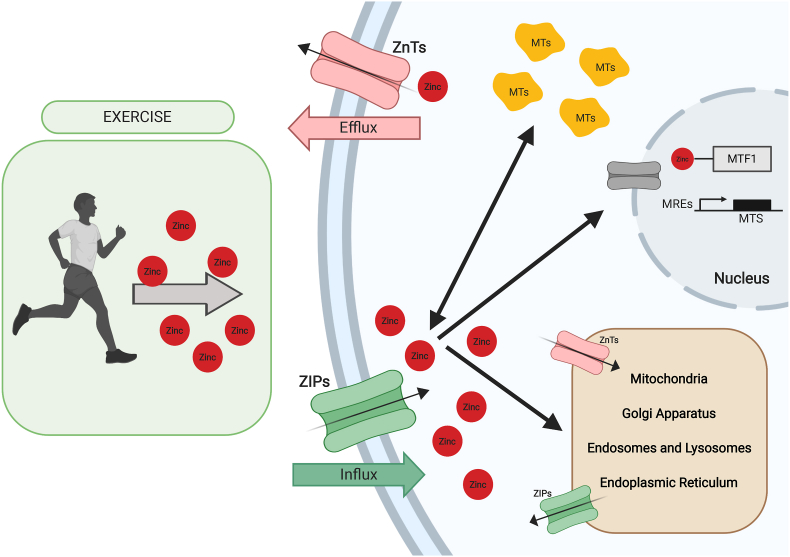
On balance, exercise could stimulate zinc cellular pathways promoting zinc uptake into the cell and zinc storage in different proteins and cellular organelles (through ZnTs and MTs regulation) as it is shown in Fig. 1 thus this process is altered in athletes that present zinc deficiency leading to alterations that could affect health and sport performance.
Fig. 1.
Cellular Zinc homeostasis modulated by exercise.
An overview of Zinc cellular homeostasis modulated by exercise. Exercise induces Zn2+ celular input through ZIP transporters, upregulating Zn2+ input to nucleus and different organelles. High celular Zn2+ levels incite MTs Zn accumulation.
4. Zinc and protein degradation
4.1. Proteostasis
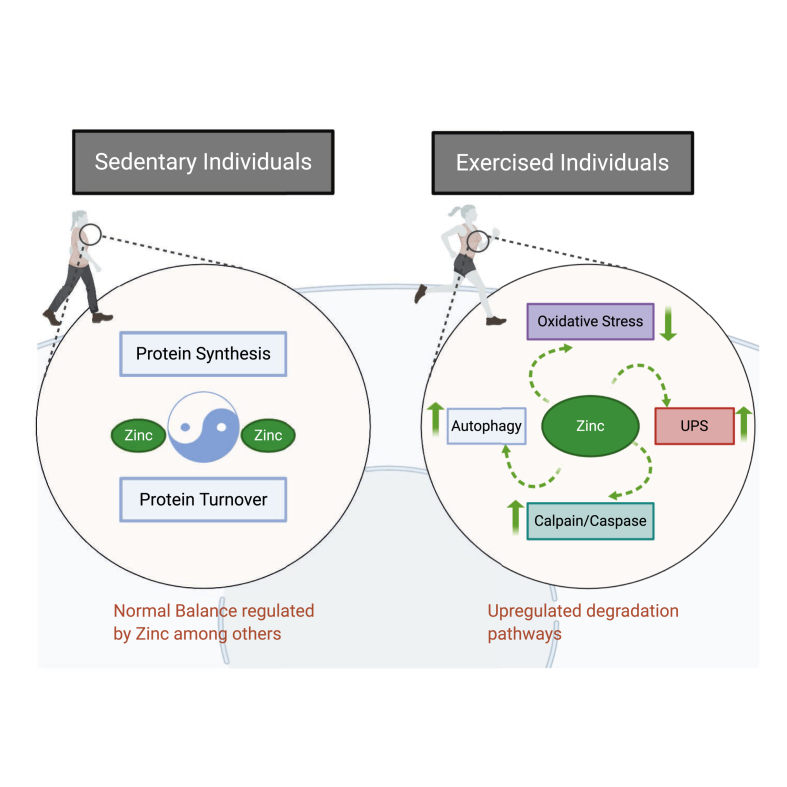
Proteostasis is the collective term for the mechanisms mediating the correct synthesis, folding, trafficking and degradation of proteins. These networks are comprised of various protein chaperones, degradation complexes and stress-response pathways. The balance between protein synthesis and protein degradation determines whether muscle increases or decreases in mass. A key component of muscle metabolism is the correct recognition and repair or degradation of misfolded or otherwise damaged proteins, allowing muscle remodelling, training adaptation and increased muscle mass and as Fig. 2 illustrates exercise could influence protein turnover. Proteolysis provides amino acid precursors for maintenance of proteostasis and is mediated through the autophagy-lysosome, calpain, caspase, and ubiquitin-proteasome system (UPS). These systems are interlinked and act together to regulate muscle proteolysis in response to nutrition and mechanical force during exercise [129]. Moderate exercise training improves muscle atrophy induced by impaired skeletal muscle autophagy and proteostasis [130]. Additionally, exercise enhanced protein turnover even when mechanisms to maintain proteostasis and protein degradation are impaired [131].
Fig. 2.
Proposed and reviewed effects of exercise on the proteasomal machinery. The bibliography reviewed suggests that there is more protein turnover with exercise to facilitate recovery and zinc may be implicated in the exercise-induced up regulation of degradation pathways.
4.2. Autophagy
Autophagy is a system of protein degradation that is the primary mechanism through which larger proteins, protein aggregates and organelles are degraded by the cathepsin protease family in the lumen of lysosomes. These proteases can degrade specific muscle proteins (e.g. troponin T, myosin heavy chain and tropomyosin) [132]. While animal models suggest that the majority of muscle protein degradation occurs via the UPS [129], autophagy is purported to be responsible for degrading membrane-bound receptor proteins which are critical for muscle remodelling [133]. Exercise has shown to increase markers of autophagy and autophagy capacity in skeletal muscle during the recovery period, therefore exercise could modulate autophagy [133]. During autophagy, translocation of substrates into the lysosome lumen is mediated through macroautophagy, microautophagy or chaperone-mediated autophagy.
Zinc is critical for basal and induced autophagy. In vitro studies have shown that supplementing culture medium with zinc enhances basal [134,135] and induced autophagy in cultured cells, particularly induced by various exogenous stressors including hydrogen peroxide in astrocytes [136], ethanol in human hepatoma cells [135], and tamoxifen in MCF7 breast cancer cells [137]. These studies also showed a decrease in basal and induced autophagy when cells were incubated with the zinc chelator TPEN or Chelex-100.
Zinc is involved in regulating both the initiation of autophagy (early autophagy) and the protein degradation in the lumen of the autolysosome (late autophagy). The mechanisms through which zinc may regulate early autophagy are not fully elucidated.
However, zinc regulation of phosphorylation of extracellular-signal-regulated kinases (ERK1/2) is required for its effects on autophagy [135,137], zinc supplementation in cells induced ERK activation, autophagy and cell death while chelation of zinc2+ blocks the increase in ERK phosphorylation reducing cell death and autophagic vacuoles formation [137]. Particularly, zinc administration that promotes ERK activity could modulate Beclin expression inducing autophagy [138]. ERK1/2 has been shown to promote the dissociation of the mTORC1 from the ULK1/2 complex through the Beclin1-PI3K complex [139], where PI3K is specifically induced by zinc in a dose dependent manner [140]. Exercise has shown to increase phosphorylation of AMPKThr172 and ULKSer317 in skeletal muscle along with different markers of autophagy while phosphorylation of mTORSer2448 and ULKSer757 was unaffected in skeletal muscle [133]. Particularly, zinc administration in macrophages has shown to decrease mTOR phosphorylation levels and activate apoptosis markers [141]. Moreover, an increase or a decrease in a zinc finger protein in breast cancer cells actives or inhibits autophagy associated to cell death through activation or inhibition of mTOR pathway [142]. Exercise stimulates zinc incorporation into the cell as it has been discussed above promoting ERK activation and autophagy induction through ULK1/2 complex and mTORC1 dissociation.
Both Beclin1-PI3K and ULK1/2 are involved in the formation of the phagophore in mammalian cells [143,144]. Beclin1 protein levels were increased in skeletal muscle following moderate intensity exercise [133,145].
Peroxisome proliferator-activated receptor alpha (PPARα) is a nuclear receptor that works as a transcription factor that is activated in energy deficiency conditions. Exercise can upregulate autophagy markers such as Beclin1 and LC311 and induce upregulation of PPARα triggering autophagy [146]. In addition, zinc administration can stimulate PPARα under autophagy activation conditions inducing lipolysis by autophagy-mediated lipophagy through the MTF-1 DNA binding at PPARα promoter region [144].
There are also predicted to be several MTF1 binding sites in the promoter regions of ATG7 and DFCP1 [147]. Regulation by MTF1 implicates the redox-sensitive zinc binding protein metallothionein as linking oxidative stress with autophagy induction [147]. Autophagy related gene 7 (ATG7) is involved in cell degradation and recycling, the knocked-out mice for this autophagy gene develop muscular atrophy and show that autophagy is necessary during exercise to eliminate damaged mitochondria and affected muscle fibers [148] and ATG7 autophagy function could be modulated by zinc [149]. DFCP1 is involved in membrane trafficking and cell signalling during autophagy, particularly in phagophore formation from omegasome compartments [150].
In late autophagy, there is evidence that zinc is necessary for lysosomal protein degradation. Zinc-deficient rats accumulated lipofuscin in the retina, a marker of incomplete lysosomal degradation of photoreceptor proteins [151]. Lipofuscin accumulation was also noted in astrocytes extracted from MT3 knockout mice [136]. These cells also had reduced activity of acid phosphatase and neuraminidase and reduced expression of cathepsin D and L [136]. Additionally, zinc depletion induced by TPEN treatment of hepatoma cells impaired degradation of lysosomal cargo [135]. Even, high zinc doses improved autophagy in mice exposed to ethanol while zinc deficiency impaired autophagy proposing the idea that zinc is necessary for a proper autophagy [149]. Conversely, in yeast, non-specific autophagy was found to be induced under conditions of zinc depletion [49]). The authors found that the autophagic response was a protective mechanism to recycle Zn2+ ions for use in the cell, suggesting that the relationship between cellular zinc homeostasis and autophagy is a two-way street [49,152].
5. Calpain/caspase
In addition, the calpain and caspase families both also contribute to muscle proteolysis [153,154]. Both families cleave larger myofibrillar proteins into smaller actomyosin fragments, which are then able to be degraded by the UPS [154,155]. These families have muscle-specific specialisation with calpain-3 (aka p94, CAPN3) highly expressed in skeletal muscle [156] and caspase-3 activity increased in skeletal muscle following a modest, short-term energy deficit [157]. In muscle, protein and gene expression levels of calpain/caspase proteolytic system components such as calpain 1 and cathepsin D have been found to be increased after exercise [158]. Additionally, calpain 3 has been identified as a required element for muscle growth after exercise [159]. In mouse muscle caspase-3 cleaves the Rpt2 and Rpt6 subunits of the 19S proteasome, increasing proteasomal activity [160]. Interestingly, caspases-3, -6, and -8 are inhibited by physiologically relevant concentrations of Zn2+ (i.e. low nanomolar) and bind inhibitory Zn2+ with differing stoichiometries and at different sites [161], suggesting that Zn2+ may be liberated from caspase-3 following an energy deficit. In retinal pigment epithelial cells, zinc deficiency produces a decrease in cells viability with an activation of calpain-1, caspase-9 and caspase 3 [162] while zinc administration generates a normalization in elevated calpain and caspase 3 activities [163]. Additionally, zinc chelator used in retinal pigment epithelium human cells to induce zinc deficiency lead to calpain activation [164]. Besides, zinc administration could promote caspase activation [165]. On balance, muscle proteolysis from calpain and caspase are related with zinc.
The UPS, another system of protein turnover, is responsible for degradation of more than 80% of intracellular proteins [166], and it is the primary mechanism through which actomyosin fragments are degraded in healthy muscle. Specific motifs in protein substrates are covalently tagged with activated ubiquitin by a ubiquitin (E3) ligase, further ubiquitin molecules are conjugated to the first and the protein is transferred to the 26S proteasome for degradation [166,167]. The proteasome then utilises multiple enzymatic and non-enzymatic tools to unfold, deubiquitinate and lyse the target protein. Hundreds of different E3 ligases provide specificity through motif recognition. The muscle-specific E3 ligases are MAFbx and muscle RING finger-1 (MuRF-1), which are upregulated in response to stressors including oxidative stress and malnutrition [167].
Most of the proteins are degraded by a large multi-subunit 26S proteasome that is constituted of two supercomplexes; a regulatory supercomplex (19S) and a core supercomplex (20S). The regulatory particle (RP) modifies and directs the proteins to the core particle (CP) where the substrates are degraded [168]. Zinc influences on the turnover of proteins that present susceptibility to proteasome-dependent proteolysis [168,169]. Several proteins in the 26S proteasome require zinc for their structure and function [170]. The 19S RP recognises polyubiquitinated proteins and the deubiquitinating enzymes Rpn11 and Ubp6 remove the polyubiquitin chain [170]. Rpn11 is a zinc metalloproteinase and mutation of the zinc-coordinating residues in the active site render the enzyme inactive [171]. Additionally, a member of the ubiquitous ZFAND protein family, ZFAND5/ZNF216 contains two zinc finger domains, is induced during muscle atrophy, and stimulates overall UPS protein degradation [172].
6. Zinc and oxidative stress
Oxidative stress and inflammation are associated with proteostasis induction and exercise and they are hallmarks of metabolic disorders including obesity, metabolic syndrome, diabetes mellitus type II and cardiovascular diseases. Zinc is a metal ion that binds to numerous proteins in the cell contributing to their stability and to enzymes regulating their catalytic activities. This connection with proteins makes zinc an important factor involved in multiple metabolic and transcriptomic pathways mostly related to prevent oxidative stress and inflammation [173].
The antioxidant function of zinc is related to multiple activities [174]. At an initial step, zinc upregulates the antioxidant system through the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) transcription factor [175] and prevents the production of reactive oxygen and nitrogen species [176,177]. Although Zn2+ ions do not have a direct action on redox reactions, Zn2+ is a cofactor of cytosolic Cu, Zn-superoxide dismutase (SOD1) and can also activate glutathione peroxidase (GPx) providing enzyme mechanisms of antioxidant protection against toxic molecules [178,179]. Furthermore, regular exercised individuals have shown higher serum levels of SOD-3 and endothelial nitric oxide synthase (eNOS) than aged-matched sedentary individuals, suggesting a protective role of exercise in antioxidant defence [180]. Additionally, zinc inhibits the activation of NF-кB through the activation of (PPARs-α) and zinc-finger protein A20 [181]. These regulatory functions of transcription factors by zinc are determinant in its anti-inflammatory properties causing a decrease of pro-inflammatory cytokine production [182]. Interestingly, low levels of zinc have been associated with increased inflammatory markers in either obese humans with metabolic syndrome or patients with type II diabetes, and inflammatory markers were decreased with zinc supplementation [100,183,184].
7. Conclusion
In conclusion, zinc deficiency could have serious consequences on health, particularly in athletes affecting sport performance. While the evidence presented here argues the benefits of meeting daily zinc requirements, there is no evidence to support that excess zinc ingestion would be beneficial beyond daily recommended intakes. In relation to skeletal muscle, zinc could be involved in skeletal muscle synthesis and regeneration that take part after exercise. More significantly, zinc seems to be involved in the different proteostatic systems modulated after exercise, but further studies would be necessary to support it. Zinc-proteins and their relation to exercise and physical performance is an understudied area of research and further investigation into the requirement of zinc for the benefits of exercise is warranted.
Funding
This work was supported by the Association Française contre les Myopathies (AFM) through fellowship grant #22450 to C.V.-G., and Spanish Ministry of Education, Culture and Sports [FPU16/03264] for J.D.H.C.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Raulin J. Etudes chimiques sur la végétation. Ann. Sci. Nat. Bot. Biol. Veg. 1869;11:92–299. [Google Scholar]
- 2.Sommer A.L., Lipman C.B. Evidence on the indispensable nature of zinc and boron for higher green plants. Plant Physiol. 1926;1(3):231–249. doi: 10.1104/pp.1.3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Todd W.R., Elvehjem C.A., Hart E.B. Zinc in the nutrition of the rat. Am. J. Physiol. 1934;107:146–156. [Google Scholar]
- 4.Prasad A., Halsted J.A., Nadimi M. Syndrome of iron deficiency anemia, hepatsplenomegaly, hypogonadism, dwarfism, and geophagia. Am. J. Med. 1961;31(4):532–546. doi: 10.1016/0002-9343(61)90137-1. [DOI] [PubMed] [Google Scholar]
- 5.Wastney M.E., Aamodt R.L., Rumble W.F., Henkin R.I. Kinetic analysis of zinc metabolism and its regulation in normal humans. Am. J. Physiol. 1986;251(2 Pt 2):R398–R408. doi: 10.1152/ajpregu.1986.251.2.R398. [DOI] [PubMed] [Google Scholar]
- 6.Jackson M.J., Jones D.A., Edwards R.H. Tissue zinc levels as an index of body zinc status. Clin. Physiol. 1982;2(4):333–343. doi: 10.1111/j.1475-097x.1982.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 7.Rink L. IOS Press; Amsterdam, The Netherlands: 2011. Zinc in Human Health; p. 596. [Google Scholar]
- 8.Barnett J.P., Blindauer C.A., Kassaar O., Khazaipoul S., Martin E.M., Sadler P.J., Stewart A.J. Allosteric modulation of zinc speciation by fatty acids. Biochim. Biophys. Acta. 2013;1830(12):5456–5464. doi: 10.1016/j.bbagen.2013.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Jackson M.J. Physiology of zinc: general aspects. In: Mills C.F., editor. In Zinc in Human Biology. Springer-Verlag; London: 1989. pp. 1–14. [Google Scholar]
- 10.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv. Nutr. 2013;4(1):82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maret W., Li Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009;109(10):4682–4707. doi: 10.1021/cr800556u. [DOI] [PubMed] [Google Scholar]
- 12.Scott D.A., Fisher A.M. The insulin and the zinc content of normal and diabetic pancreas. J. Clin. Invest. 1938;17(6):725–728. doi: 10.1172/JCI101000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keilin D., Mann T. Carbonic anhydrase. Nature. 1939;144(3644):442–443. [Google Scholar]
- 14.Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73(1):79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 15.Brautigan D.L., Bornstein P., Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J. Biol. Chem. 1981;256(13):6519–6522. https://www.ncbi.nlm.nih.gov/pubmed/6165721 Retrieved from. [PubMed] [Google Scholar]
- 16.Hanas J.S., Hazuda D.J., Bogenhagen D.F., Wu F.Y., Wu C.W. Xenopus transcription factor A requires zinc for binding to the 5 S RNA gene. J. Biol. Chem. 1983;258(23):14120–14125. https://www.ncbi.nlm.nih.gov/pubmed/6196359 Retrieved from. [PubMed] [Google Scholar]
- 17.Miller J., McLachlan A.D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. https://www.ncbi.nlm.nih.gov/pubmed/4040853 Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreini C., Banci L., Bertini I., Rosato A. Zinc through the three domains of life. J. Proteome Res. 2006;5(11):3173–3178. doi: 10.1021/pr0603699. [DOI] [PubMed] [Google Scholar]
- 19.Andreini C., Bertini I., Rosato A. Metalloproteomes: a bioinformatic approach. Acc. Chem. Res. 2009;42(10):1471–1479. doi: 10.1021/ar900015x. [DOI] [PubMed] [Google Scholar]
- 20.Prasad A.S. Discovery of human zinc deficiency: its impact on human health and disease. Adv. Nutr. 2013;4(2):176–190. doi: 10.3945/an.112.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livingstone C. Zinc: physiology, deficiency, and parenteral nutrition. Nutr. Clin. Pract. 2015;30(3):371–382. doi: 10.1177/0884533615570376. [DOI] [PubMed] [Google Scholar]
- 22.Maywald M., Wessels I., Rink L. Zinc signals and immunity. Int. J. Mol. Sci. 2017;18(10) doi: 10.3390/ijms18102222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi S., Liu X., Pan Z. Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018;39(7):1120–1132. doi: 10.1038/aps.2018.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atasoy H.I., Bugdayci G. Zinc deficiency and its predictive capacity for anemia: unique model in school children. Pediatr. Int. 2018;60(8):703–709. doi: 10.1111/ped.13603. [DOI] [PubMed] [Google Scholar]
- 25.Palacios A.M., Hurley K.M., De-Ponce S., Alfonso V., Tilton N., Lambden K.B.…Black M.M. Zinc deficiency associated with anaemia among young children in rural Guatemala. Matern. Child Nutr. 2020;16(1) doi: 10.1111/mcn.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adamo A.M., Liu X., Mathieu P., Nuttall J.R., Supasai S., Oteiza P.I. Early developmental marginal zinc deficiency affects neurogenesis decreasing neuronal number and altering neuronal specification in the adult rat brain. Front. Cell. Neurosci. 2019;13:62. doi: 10.3389/fncel.2019.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandstead H.H. Requirements and toxicity of essential trace elements, illustrated by zinc and copper. Am. J. Clin. Nutr. 1995;61(3 Suppl):621S–624S. doi: 10.1093/ajcn/61.3.621S. [DOI] [PubMed] [Google Scholar]
- 28.Maret W., Sandstead H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006;20(1):3–18. doi: 10.1016/j.jtemb.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Wieringa F.T., Dijkhuizen M.A., Fiorentino M., Laillou A., Berger J. Determination of zinc status in humans: which indicator should we use? Nutrients. 2015;7(5):3252–3263. doi: 10.3390/nu7053252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalid N., Ahmed A., Bhatti M.S., Randhawa M.A., Ahmad A., Rafaqat R. A question mark on zinc deficiency in 185 million people in Pakistan--possible way out. Crit. Rev. Food Sci. Nutr. 2014;54(9):1222–1240. doi: 10.1080/10408398.2011.630541. [DOI] [PubMed] [Google Scholar]
- 31.MacKenzie E.L., Iwasaki K., Tsuji Y. Intracellular iron transport and storage: from molecular mechanisms to health implications. Antioxidants Redox Signal. 2008;10(6):997–1030. doi: 10.1089/ars.2007.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackland M.L., Michalczyk A. Zinc deficiency and its inherited disorders -a review. Genes Nutr. 2006;1(1):41–49. doi: 10.1007/BF02829935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClain C.J. Zinc metabolism in malabsorption syndromes. J. Am. Coll. Nutr. 1985;4(1):49–64. doi: 10.1080/07315724.1985.10720066. [DOI] [PubMed] [Google Scholar]
- 34.Solomons N.W., Rosenberg I.H., Sandstead H.H., Vo-Khactu K.P. Zinc deficiency in Crohn's disease. Digestion. 1977;16(1-2):87–95. doi: 10.1159/000198059. [DOI] [PubMed] [Google Scholar]
- 35.Steel L., Cousins R.J. Kinetics of zinc absorption by luminally and vascularly perfused rat intestine. Am. J. Physiol. 1985;248(1 Pt 1):G46–G53. doi: 10.1152/ajpgi.1985.248.1.G46. [DOI] [PubMed] [Google Scholar]
- 36.Hambidge M., Krebs N.F. Interrelationships of key variables of human zinc homeostasis: relevance to dietary zinc requirements. Annu. Rev. Nutr. 2001;21:429–452. doi: 10.1146/annurev.nutr.21.1.429. [DOI] [PubMed] [Google Scholar]
- 37.Sandstead H.H., Smith J.C., Jr. Deliberations and evaluations of approaches, endpoints and paradigms for determining zinc dietary recommendations. J. Nutr. 1996;126(9 Suppl):2410S–2418S. doi: 10.1093/jn/126.suppl_9.2410S. [DOI] [PubMed] [Google Scholar]
- 38.Tang N., Skibsted L.H. Zinc bioavailability from phytate-rich foods and zinc supplements. Modeling the effects of food components with oxygen, nitrogen, and sulfur donor ligands. J. Agric. Food Chem. 2017;65(39):8727–8743. doi: 10.1021/acs.jafc.7b02998. [DOI] [PubMed] [Google Scholar]
- 39.Cassens R.G., Hoekstra W.G., Faltin E.C., Briskey E.J. Zinc content and subcellular distribution in red vs. white porcine skeletal muscle. Am. J. Physiol. 1967;212(3):688–692. doi: 10.1152/ajplegacy.1967.212.3.688. [DOI] [PubMed] [Google Scholar]
- 40.O'Leary M.J., McClain C.J., Hegarty P.V. Effect of zinc deficiency on the weight, cellularity and zinc concentration of different skeletal muscles in the post-weanling rat. Br. J. Nutr. 1979;42(3):487–495. doi: 10.1079/bjn19790140. [DOI] [PubMed] [Google Scholar]
- 41.Giugliano R., Millward D.J. Growth and zinc homeostasis in the severely zinc-deficient rat. Br. J. Nutr. 1984;52(3):545–560. doi: 10.1079/bjn19840122. [DOI] [PubMed] [Google Scholar]
- 42.Irving H., Williams R.J.P. Order of stability of metal complexes. Nature. 1948;162:746–747. [Google Scholar]
- 43.Krezel A., Maret W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016;611:3–19. doi: 10.1016/j.abb.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krezel A., Maret W. Dual nanomolar and picomolar zinc(II) binding properties of metallothionein. J. Am. Chem. Soc. 2007;129(35):10911–10921. doi: 10.1021/ja071979s. [DOI] [PubMed] [Google Scholar]
- 45.Maret W. Analyzing free zinc(II) ion concentrations in cell biology with fluorescent chelating molecules. Metall. 2015;7(2):202–211. doi: 10.1039/c4mt00230j. [DOI] [PubMed] [Google Scholar]
- 46.Kimura T., Kambe T. The functions of metallothionein and ZIP and ZnT transporters: an overview and perspective. Int. J. Mol. Sci. 2016;17(3):336. doi: 10.3390/ijms17030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasak M., Meloni G. Chemistry and biology of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011;16(7):1067–1078. doi: 10.1007/s00775-011-0799-2. [DOI] [PubMed] [Google Scholar]
- 48.Andrews G.K. Cellular zinc sensors: MTF-1 regulation of gene expression. Biometals. 2001;14(3-4):223–237. doi: 10.1023/a:1012932712483. [DOI] [PubMed] [Google Scholar]
- 49.Giles N.M., Watts A.B., Giles G.I., Fry F.H., Littlechild J.A., Jacob C. Metal and redox modulation of cysteine protein function. Chem. Biol. 2003;10(8):677–693. doi: 10.1016/s1074-5521(03)00174-1. [DOI] [PubMed] [Google Scholar]
- 50.Wessels I., Maywald M., Rink L. Zinc as a gatekeeper of immune function. Nutrients. 2017;9(12) doi: 10.3390/nu9121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heffernan S.M., Horner K., De Vito G., Conway G.E. The role of mineral and trace element supplementation in exercise and athletic performance: a systematic review. Nutrients. 2019;11(3) doi: 10.3390/nu11030696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dong G., Chen H., Qi M., Dou Y., Wang Q. Balance between metallothionein and metal response element binding transcription factor 1 is mediated by zinc ions (review) Mol. Med. Rep. 2015;11(3):1582–1586. doi: 10.3892/mmr.2014.2969. [DOI] [PubMed] [Google Scholar]
- 53.Lichten L.A., Cousins R.J. Mammalian zinc transporters: nutritional and physiologic regulation. Annu. Rev. Nutr. 2009;29:153–176. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 54.Kambe T., Tsuji T., Hashimoto A., Itsumura N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015;95(3):749–784. doi: 10.1152/physrev.00035.2014. [DOI] [PubMed] [Google Scholar]
- 55.Maltin C.A., Duncan L., Wilson A.B., Hesketh J.E. Effect of zinc deficiency on muscle fibre type frequencies in the post-weanling rat. Br. J. Nutr. 1983;50(3):597–604. doi: 10.1079/bjn19830131. [DOI] [PubMed] [Google Scholar]
- 56.Chal J., Pourquie O. Making muscle: skeletal myogenesis in vivo and in vitro. Development. 2017;144(12):2104–2122. doi: 10.1242/dev.151035. [DOI] [PubMed] [Google Scholar]
- 57.Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J. Neurol. Sci. 1976;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- 58.Pfaender S., Föhr K., Lutz A.-K., Putz S., Achberger K., Linta L.…Grabrucker A.M. Cellular zinc homeostasis contributes to neuronal differentiation in human induced pluripotent stem cells. Neural Plast. 2016 doi: 10.1155/2016/3760702. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O'Dell B.L., Becker J.K., Emery M.P., Browning J.D. Production and reversal of the neuromuscular pathology and related signs of zinc deficiency in Guinea pigs. J. Nutr. 1989;119(2):196–201. doi: 10.1093/jn/119.2.196. [DOI] [PubMed] [Google Scholar]
- 60.O'Dell B.L., Newberne P.M., Savage J.E. Significance of dietary zinc for the growing chicken. J. Nutr. 1958;65(4):503–518. doi: 10.1093/jn/65.4.503. [DOI] [PubMed] [Google Scholar]
- 61.Hurley L.S. Zinc deficiency in the developing rat. Am. J. Clin. Nutr. 1969;22(10):1332–1339. doi: 10.1093/ajcn/22.10.1332. [DOI] [PubMed] [Google Scholar]
- 62.Huber A.M., Gershoff S.N. Effects of dietary zinc on zinc enzymes in the rat. J. Nutr. 1973;103(8):1175–1181. doi: 10.1093/jn/103.8.1175. [DOI] [PubMed] [Google Scholar]
- 63.Grider A., Mouat M.F., Scrimgeour A.G. Consumption of a moderately zinc-deficient and zinc-supplemented diet affects soluble protein expression in rat soleus muscle. J. Nutr. Biochem. 2007;18(11):753–759. doi: 10.1016/j.jnutbio.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 64.Sandow A., Bien S.M. Blockade of neuromuscular transmission by zinc. Nature. 1962;193:689–690. doi: 10.1038/193689a0. [DOI] [PubMed] [Google Scholar]
- 65.Isaacson A., Sandow A. Effects of zinc on responses of skeletal muscle. J. Gen. Physiol. 1963;46:655–677. doi: 10.1085/jgp.46.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stanfield P.R. The effect of zinc ions on the gating of the delayed potassium conductance of frog sartorius muscle. J. Physiol. 1975;251(3):711–735. doi: 10.1113/jphysiol.1975.sp011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ragozzino D., Giovannelli A., Degasperi V., Eusebi F., Grassi F. Zinc permeates mouse muscle ACh receptor channels expressed in BOSC 23 cells and affects channel function. J. Physiol. 2000;529(Pt 1):83–91. doi: 10.1111/j.1469-7793.2000.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harrison N.L., Gibbons S.J. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994;33(8):935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 69.Noh S., Lee S.R., Jeong Y.J., Ko K.S., Rhee B.D., Kim N., Han J. The direct modulatory activity of zinc toward ion channels. Integr. Med. Res. 2015;4(3):142–146. doi: 10.1016/j.imr.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mnatsakanyan H., Serra R.S.I., Rico P., Salmeron-Sanchez M. Zinc uptake promotes myoblast differentiation via Zip7 transporter and activation of Akt signalling transduction pathway. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-32067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohashi K., Nagata Y., Wada E., Zammit P.S., Shiozuka M., Matsuda R. Zinc promotes proliferation and activation of myogenic cells via the PI3K/Akt and ERK signaling cascade. Exp. Cell Res. 2015;333(2):228–237. doi: 10.1016/j.yexcr.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Paskavitz A.L., Quintana J., Cangussu D., Tavera-Montanez C., Xiao Y., Ortiz-Miranda S.…Padilla-Benavides T. Differential expression of zinc transporters accompanies the differentiation of C2C12 myoblasts. J. Trace Elem. Med. Biol. 2018;49:27–34. doi: 10.1016/j.jtemb.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim J.H., Jeon J., Shin M., Won Y., Lee M., Kwak J.S.…Chun J.S. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156(4):730–743. doi: 10.1016/j.cell.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 74.Alameddine H.S. The matrix metalloproteinase and tissue inhibitors of metalloproteinase balance in physiological and pathological remodeling of skeletal muscles. In: Chakraborti S., Dhalla N., editors. In Proteases in Physiology and Pathology. Springer; Singapore: 2017. [Google Scholar]
- 75.Huang L., Kirschke C.P., Lay Y.A., Levy L.B., Lamirande D.E., Zhang P.H. zinct7-null mice are more susceptible to diet-induced glucose intolerance and insulin resistance. J. Biol. Chem. 2012;287(40):33883–33896. doi: 10.1074/jbc.M111.309666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wijesekara N., Dai F.F., Hardy A.B., Giglou P.R., Bhattacharjee A., Koshkin V.…Wheeler M.B. Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia. 2010;53(8):1656–1668. doi: 10.1007/s00125-010-1733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Summermatter S., Bouzan A., Pierrel E., Melly S., Stauffer D., Gutzwiller S.…Fournier B. Blockade of metallothioneins 1 and 2 increases skeletal muscle mass and strength. Mol. Cell Biol. 2017;37(5) doi: 10.1128/MCB.00305-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lecker S.H., Jagoe R.T., Gilbert A., Gomes M., Baracos V., Bailey J.…Goldberg A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. Faseb. J. 2004;18(1):39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 79.Wang G., Biswas A.K., Ma W., Kandpal M., Coker C., Grandgenett P.M.…Acharyya S. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat. Med. 2018;24(6):770–781. doi: 10.1038/s41591-018-0054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Norouzi S., Adulcikas J., Sohal S.S., Myers S. Zinc stimulates glucose oxidation and glycemic control by modulating the insulin signaling pathway in human and mouse skeletal muscle cell lines. PloS One. 2018;13(1) doi: 10.1371/journal.pone.0191727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu Y., Lu H., Yang H., Li C., Sang Q., Liu X.…Sun Z. Zinc stimulates glucose consumption by modulating the insulin signaling pathway in L6 myotubes: essential roles of Akt-GLUT4, GSK3beta and mTOR-S6K1. J. Nutr. Biochem. 2016;34:126–135. doi: 10.1016/j.jnutbio.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Norouzi S., Adulcikas J., Henstridge D.C., Sonda S., Sohal S.S., Myers S. The zinc transporter Zip7 is downregulated in skeletal muscle of insulin-resistant cells and in mice fed a high-fat diet. Cells. 2019;8(7) doi: 10.3390/cells8070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Myers S.A., Nield A., Chew G.S., Myers M.A. The zinc transporter, Slc39a7 (Zip7) is implicated in glycaemic control in skeletal muscle cells. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0079316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hardy D., Besnard A., Latil M., Jouvion G., Briand D., Thepenier C.…Chretien F. Comparative study of injury models for studying muscle regeneration in mice. PloS One. 2016;11(1) doi: 10.1371/journal.pone.0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jinno N., Nagata M., Takahashi T. Marginal zinc deficiency negatively affects recovery from muscle injury in mice. Biol. Trace Elem. Res. 2014;158(1):65–72. doi: 10.1007/s12011-014-9901-2. [DOI] [PubMed] [Google Scholar]
- 86.Mukund K., Subramaniam S. Skeletal muscle: a review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Sys. Biol. Med. 2020;12(1) doi: 10.1002/wsbm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Camera D.M., Smiles W.J., Hawley J.A. Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic. Biol. Med. 2016;98:131–143. doi: 10.1016/j.freeradbiomed.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 88.Gomes M.J., Martinez P.F., Pagan L.U., Damatto R.L., Cezar M.D.M., Lima A.R.R.…Okoshi M.P. Skeletal muscle aging: influence of oxidative stress and physical exercise. Oncotarget. 2017;8(12):20428–20440. doi: 10.18632/oncotarget.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fiuza-Luces C., Garatachea N., Berger N.A., Lucia A. Exercise is the real polypill. Physiology. 2013;28(5):330–358. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 90.Pedersen B.K., Saltin B. Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports. 2015;25(Suppl 3):1–72. doi: 10.1111/sms.12581. [DOI] [PubMed] [Google Scholar]
- 91.Chu A., Petocz P., Samman S. Plasma/serum zinc status during aerobic exercise recovery: a systematic review and meta-analysis. Sports Med. 2017;47(1):127–134. doi: 10.1007/s40279-016-0567-0. [DOI] [PubMed] [Google Scholar]
- 92.Granell J. Zinc and copper changes in serum and urine after aerobic endurance and muscular strength exercise. J. Sports Med. Phys. Fit. 2014;54(2):232–237. https://www.ncbi.nlm.nih.gov/pubmed/24509996 Retrieved from. [PubMed] [Google Scholar]
- 93.Maynar M., Munoz D., Alves J., Barrientos G., Grijota F.J., Robles M.C., Llerena F. Influence of an acute exercise until exhaustion on serum and urinary concentrations of molybdenum, selenium, and zinc in athletes. Biol. Trace Elem. Res. 2018;186(2):361–369. doi: 10.1007/s12011-018-1327-9. [DOI] [PubMed] [Google Scholar]
- 94.Aruoma O.I., Reilly T., MacLaren D., Halliwell B. Iron, copper and zinc concentrations in human sweat and plasma; the effect of exercise. Clin. Chim. Acta. 1988;177(1):81–87. doi: 10.1016/0009-8981(88)90310-5. [DOI] [PubMed] [Google Scholar]
- 95.Chu A., Petocz P., Samman S. Immediate effects of aerobic exercise on plasma/serum zinc levels: a meta-analysis. Med. Sci. Sports Exerc. 2016;48(4):726–733. doi: 10.1249/MSS.0000000000000805. [DOI] [PubMed] [Google Scholar]
- 96.Anderson R.A., Polansky M.M., Bryden N.A. Acute effects on chromium, copper, zinc, and selected clinical variables in urine and serum of male runners. Biol. Trace Elem. Res. 1984;6(4):327–336. doi: 10.1007/BF02989240. [DOI] [PubMed] [Google Scholar]
- 97.Anderson R.A., Bryden N.A., Polansky M.M., Deuster P.A. Acute exercise effects on urinary losses and serum concentrations of copper and zinc of moderately trained and untrained men consuming a controlled diet. Analyst. 1995;120(3):867–870. doi: 10.1039/an9952000867. [DOI] [PubMed] [Google Scholar]
- 98.Volpe S.L., Lowe N.M., Woodhouse L.R., King J.C. Effect of maximal exercise on the short-term kinetics of zinc metabolism in sedentary men. Br. J. Sports Med. 2007;41(3):156–161. doi: 10.1136/bjsm.2006.030346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Rij A.M., Hall M.T., Dohm G.L., Bray J., Pories W.J. Changes in zinc metabolism following exercise in human subjects. Biol. Trace Elem. Res. 1986;10(2):99–105. doi: 10.1007/BF02795562. [DOI] [PubMed] [Google Scholar]
- 100.Donath M.Y., Boni-Schnetzler M., Ellingsgaard H., Halban P.A., Ehses J.A. Cytokine production by islets in health and diabetes: cellular origin, regulation and function. Trends Endocrinol. Metabol. 2010;21(5):261–267. doi: 10.1016/j.tem.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 101.Maynar M., Bartolome I., Alves J., Barrientos G., Grijota F.J., Robles M.C., Munoz D. Influence of a 6-month physical training program on serum and urinary concentrations of trace metals in middle distance elite runners. J. Int. Soc. Sports. Nutr. 2019;16(1):53. doi: 10.1186/s12970-019-0322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Somboonwong J., Traisaeng S., Saguanrungsirikul S. Moderate-intensity exercise training elevates serum and pancreatic zinc levels and pancreatic zincT8 expression in streptozotocin-induced diabetic rats. Life Sci. 2015;139:46–51. doi: 10.1016/j.lfs.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 103.Song Y., Leonard S.W., Traber M.G., Ho E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J. Nutr. 2009;139(9):1626–1631. doi: 10.3945/jn.109.106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rakhra G., Masih D., Vats A., Verma S.K., Singh V.K., Rana R.T.…Singh S.N. Effect of physical activity and age on plasma copper, zinc, iron, and magnesium concentration in physically active healthy males. Nutrition. 2017;43–44:75–82. doi: 10.1016/j.nut.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 105.Kwon I., Song W., Jang Y., Choi M.D., Vinci D.M., Lee Y. Elevation of hepatic autophagy and antioxidative capacity by endurance exercise is associated with suppression of apoptosis in mice. Ann. Hepatol. 2020;19(1):69–78. doi: 10.1016/j.aohep.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 106.Koury J.C., de Oliveira C.F., Portella E.S., Oliveira A.V., Jr., Donangelo C.M. Effect of the period of resting in elite judo athletes: hematological indices and copper/zinc-dependent antioxidant capacity. Biol. Trace Elem. Res. 2005;107(3):201–211. doi: 10.1385/bter:107:3:201. [DOI] [PubMed] [Google Scholar]
- 107.Koury J.C., de Olilveria A.V., Jr., Portella E.S., de Olilveria C.F., Lopes G.C., Donangelo C.M. Zinc and copper biochemical indices of antioxidant status in elite athletes of different modalities. Int. J. Sport Nutr. Exerc. Metabol. 2004;14(3):358–372. doi: 10.1123/ijsnem.14.3.358. [DOI] [PubMed] [Google Scholar]
- 108.Bicer M., Gunay M., Baltaci A.K., Uney K., Mogulkoc R., Akil M. Effect of zinc supplementation on lipid peroxidation and lactate levels in rats with diabetes induced by streptozotocin and subjected to acute swimming exercise. Bratisl. Lek. Listy. 2012;113(4):199–205. doi: 10.4149/bll_2012_046. [DOI] [PubMed] [Google Scholar]
- 109.Gouzi F., Maury J., Heraud N., Molinari N., Bertet H., Ayoub B.…Hayot M. Additional effects of nutritional antioxidant supplementation on peripheral muscle during pulmonary rehabilitation in COPD patients: a randomized controlled trial. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/5496346. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cinar V., Akbulut T., Kilic Y., Ozdal M., Sarikaya M. The effect of 6-week zinc supplement and weight training on the blood lipids of the sedentaries and athletes. Cell. Mol. Biol. 2018;64(11):1–5. https://www.ncbi.nlm.nih.gov/pubmed/30213281 Retrieved from. [PubMed] [Google Scholar]
- 111.Ozturk A., Baltaci A.K., Mogulkoc R., Oztekin E., Sivrikaya A., Kurtoglu E., Kul A. Effects of zinc deficiency and supplementation on malondialdehyde and glutathione levels in blood and tissues of rats performing swimming exercise. Biol. Trace Elem. Res. 2003;94(2):157–166. doi: 10.1385/BTER:94:2:157. [DOI] [PubMed] [Google Scholar]
- 112.Lukaski H.C. Low dietary zinc decreases erythrocyte carbonic anhydrase activities and impairs cardiorespiratory function in men during exercise. Am. J. Clin. Nutr. 2005;81(5):1045–1051. doi: 10.1093/ajcn/81.5.1045. [DOI] [PubMed] [Google Scholar]
- 113.Van Loan M.D., Sutherland B., Lowe N.M., Turnlund J.R., King J.C. The effects of zinc depletion on peak force and total work of knee and shoulder extensor and flexor muscles. Int. J. Sport Nutr. 1999;9(2):125–135. doi: 10.1123/ijsn.9.2.125. [DOI] [PubMed] [Google Scholar]
- 114.Giolo De Carvalho F., Rosa F.T., Marques Miguel Suen V., Freitas E.C., Padovan G.J., Marchini J.S. Evidence of zinc deficiency in competitive swimmers. Nutrition. 2012;28(11-12):1127–1131. doi: 10.1016/j.nut.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 115.McClung J.P. Iron, zinc, and physical performance. Biol. Trace Elem. Res. 2019;188(1):135–139. doi: 10.1007/s12011-018-1479-7. [DOI] [PubMed] [Google Scholar]
- 116.Davison G., Marchbank T., March D.S., Thatcher R., Playford R.J. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. Am. J. Clin. Nutr. 2016;104(2):526–536. doi: 10.3945/ajcn.116.134403. [DOI] [PubMed] [Google Scholar]
- 117.Wilborn C.D., Kerksick C.M., Campbell B.I., Taylor L.W., Marcello B.M., Rasmussen C.J.…Kreider R.B. Effects of zinc magnesium aspartate (ZMA) supplementation on training adaptations and markers of anabolism and catabolism. J. Int. Soc. Sports. Nutr. 2004;1(2):12–20. doi: 10.1186/1550-2783-1-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.King J.C., Shames D.M., Woodhouse L.R. Zinc homeostasis in humans. J. Nutr. 2000;130(5S Suppl):1360S–1366S. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- 119.Lighthouse J.K., Burke R.M., Velasquez L.S., Dirkx R.A., Jr., Aiezza A., 2nd, Moravec C.S., Small E.M. Exercise promotes a cardioprotective gene program in resident cardiac fibroblasts. JCI Insight. 2019;4(1) doi: 10.1172/jci.insight.92098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bobillier Chaumont S., Maupoil V., Jacques Lahet J., Berthelot A. Effect of exercise training on metallothionein levels of hypertensive rats. Med. Sci. Sports Exerc. 2001;33(5):724–728. doi: 10.1097/00005768-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 121.Saxena S., Shukla D., Saxena S., Khan Y.A., Singh M., Bansal A.…Jain S.K. Hypoxia preconditioning by cobalt chloride enhances endurance performance and protects skeletal muscles from exercise-induced oxidative damage in rats. Acta Physiol. 2010;200(3):249–263. doi: 10.1111/j.1748-1716.2010.02136.x. [DOI] [PubMed] [Google Scholar]
- 122.Evers-van Gogh I.J., Alex S., Stienstra R., Brenkman A.B., Kersten S., Kalkhoven E. Electric pulse stimulation of myotubes as an in vitro exercise model: cell-mediated and non-cell-mediated effects. Sci. Rep. 2015;5 doi: 10.1038/srep10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ni H., Li C., Feng X., Cen J.N. Effects of forced running exercise on cognitive function and its relation to zinc homeostasis-related gene expression in rat hippocampus. Biol. Trace Elem. Res. 2011;142(3):704–712. doi: 10.1007/s12011-010-8793-z. [DOI] [PubMed] [Google Scholar]
- 124.Hashimoto K., Hayashi Y., Inuzuka T., Hozumi I. Exercise induces metallothioneins in mouse spinal cord. Neuroscience. 2009;163(1):244–251. doi: 10.1016/j.neuroscience.2009.05.067. [DOI] [PubMed] [Google Scholar]
- 125.Podhorska-Okolow M., Dziegiel P., Dolinska-Krajewska B., Dumanska M., Cegielski M., Jethon Z.…Zabel M. Expression of metallothionein in renal tubules of rats exposed to acute and endurance exercise. Folia Histochem. Cytobiol. 2006;44(3):195–200. https://www.ncbi.nlm.nih.gov/pubmed/16977800 Retrieved from. [PubMed] [Google Scholar]
- 126.Powers S.K., Radak Z., Ji L.L. Exercise-induced oxidative stress: past, present and future. J. Physiol. 2016;594(18):5081–5092. doi: 10.1113/JP270646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Salazar G. NADPH oxidases and mitochondria in vascular senescence. Int. J. Mol. Sci. 2018;19(5) doi: 10.3390/ijms19051327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nam E., Han J., Suh J.M., Yi Y., Lim M.H. Link of impaired metal ion homeostasis to mitochondrial dysfunction in neurons. Curr. Opin. Chem. Biol. 2018;43:8–14. doi: 10.1016/j.cbpa.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 129.Jackman R.W., Kandarian S.C. The molecular basis of skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. 2004;287(4):C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 130.Campos J.C., Baehr L.M., Gomes K.M.S., Bechara L.R.G., Voltarelli V.A., Bozi L.H.M.…Ferreira J.C.B. Exercise prevents impaired autophagy and proteostasis in a model of neurogenic myopathy. Sci. Rep. 2018;8(1):11818. doi: 10.1038/s41598-018-30365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Musci R.V., Hamilton K.L., Miller B.F. Targeting mitochondrial function and proteostasis to mitigate dynapenia. Eur. J. Appl. Physiol. 2018;118(1):1–9. doi: 10.1007/s00421-017-3730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bechet D., Tassa A., Taillandier D., Combaret L., Attaix D. Lysosomal proteolysis in skeletal muscle. Int. J. Biochem. Cell Biol. 2005;37(10):2098–2114. doi: 10.1016/j.biocel.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 133.Brandt N., Gunnarsson T.P., Bangsbo J., Pilegaard H. Exercise and exercise training-induced increase in autophagy markers in human skeletal muscle. Phys. Rep. 2018;6(7) doi: 10.14814/phy2.13651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hung H.H., Huang W.P., Pan C.Y. Dopamine- and zinc-induced autophagosome formation facilitates PC12 cell survival. Cell Biol. Toxicol. 2013;29(6):415–429. doi: 10.1007/s10565-013-9261-2. [DOI] [PubMed] [Google Scholar]
- 135.Liuzzi J.P., Yoo C. Role of zinc in the regulation of autophagy during ethanol exposure in human hepatoma cells. Biol. Trace Elem. Res. 2013;156(1-3):350–356. doi: 10.1007/s12011-013-9816-3. [DOI] [PubMed] [Google Scholar]
- 136.Lee S.J., Koh J.Y. Roles of zinc and metallothionein-3 in oxidative stress-induced lysosomal dysfunction, cell death, and autophagy in neurons and astrocytes. Mol. Brain. 2010;3(1):30. doi: 10.1186/1756-6606-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hwang J.J., Kim H.N., Kim J., Cho D.H., Kim M.J., Kim Y.S.…Koh J.Y. Zinc(II) ion mediates tamoxifen-induced autophagy and cell death in MCF-7 breast cancer cell line. Biometals. 2010;23(6):997–1013. doi: 10.1007/s10534-010-9346-9. [DOI] [PubMed] [Google Scholar]
- 138.Bian X., Teng T., Zhao H., Qin J., Qiao Z., Sun Y.…Xu Z. Zinc prevents mitochondrial superoxide generation by inducing mitophagy in the setting of hypoxia/reoxygenation in cardiac cells. Free Radic. Res. 2018;52(1):80–91. doi: 10.1080/10715762.2017.1414949. [DOI] [PubMed] [Google Scholar]
- 139.Wang J., Whiteman M.W., Lian H., Wang G., Singh A., Huang D., Denmark T. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J. Biol. Chem. 2009;284(32):21412–21424. doi: 10.1074/jbc.M109.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Song W.J., Jeong M.S., Choi D.M., Kim K.N., Wie M.B. Zinc oxide nanoparticles induce autophagy and apoptosis via oxidative injury and pro-inflammatory cytokines in primary astrocyte cultures. Nanomaterials. 2019;9(7) doi: 10.3390/nano9071043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Roy R., Singh S.K., Chauhan L.K., Das M., Tripathi A., Dwivedi P.D. Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol. Lett. 2014;227(1):29–40. doi: 10.1016/j.toxlet.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 142.Li Y., Zhang L., Li K., Li J., Xiang R., Zhang J.…Wei Y. ZNF32 inhibits autophagy through the mTOR pathway and protects MCF-7 cells from stimulus-induced cell death. Sci. Rep. 2015;5:9288. doi: 10.1038/srep09288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kihara A., Kabeya Y., Ohsumi Y., Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2(4):330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wei C.C., Luo Z., Hogstrand C., Xu Y.H., Wu L.X., Chen G.H.…Song Y.F. Zinc reduces hepatic lipid deposition and activates lipophagy via zinc(2+)/MTF-1/PPARalpha and Ca(2+)/CaMKKbeta/AMPK pathways. Faseb. J. 2018 doi: 10.1096/fj.201800463. fj201800463. [DOI] [PubMed] [Google Scholar]
- 145.Liuzzi J.P., Guo L., Yoo C., Stewart T.S. Zinc and autophagy. Biometals. 2014;27(6):1087–1096. doi: 10.1007/s10534-014-9773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo R., Yu Q., Liong E.C., Fung M.L., Tipoe G.L. Cathepsin-B dependent autophagy ameliorates steatoheaptitis in chronic exercise rats. Histol. Histopathol. 2020 doi: 10.14670/HH-18-204. 18204. [DOI] [PubMed] [Google Scholar]
- 147.Kawamata T., Horie T., Matsunami M., Sasaki M., Ohsumi Y. Zinc starvation induces autophagy in yeast. J. Biol. Chem. 2017;292(20):8520–8530. doi: 10.1074/jbc.M116.762948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Filfan M., Sandu R.E., Zavaleanu A.D., GresiTa A., Glavan D.G., Olaru D.G., Popa-Wagner A. Autophagy in aging and disease. Rom. J. Morphol. Embryol. 2017;58(1):27–31. https://www.ncbi.nlm.nih.gov/pubmed/28523294 Retrieved from. [PubMed] [Google Scholar]
- 149.Liuzzi J.P., Narayanan V., Doan H., Yoo C. Effect of zinc intake on hepatic autophagy during acute alcohol intoxication. Biometals. 2018;31(2):217–232. doi: 10.1007/s10534-018-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nanao T., Koike M., Yamaguchi J., Sasaki M., Uchiyama Y. Cellular localization and tissue distribution of endogenous DFCP1 protein. Biomed. Res. 2015;36(2):121–133. doi: 10.2220/biomedres.36.121. [DOI] [PubMed] [Google Scholar]
- 151.Ding B., Zhong Q. Zinc deficiency: an unexpected trigger for autophagy. J. Biol. Chem. 2017;292(20):8531–8532. doi: 10.1074/jbc.H116.762948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Du J., Wang X., Miereles C., Bailey J.L., Debigare R., Zheng B.…Mitch W.E. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J. Clin. Invest. 2004;113(1):115–123. doi: 10.1172/JCI18330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Costelli P., Reffo P., Penna F., Autelli R., Bonelli G., Baccino F.M. Ca(2+)-dependent proteolysis in muscle wasting. Int. J. Biochem. Cell Biol. 2005;37(10):2134–2146. doi: 10.1016/j.biocel.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 154.Kinbara K., Sorimachi H., Ishiura S., Suzuki K. Muscle-specific calpain, p94, interacts with the extreme C-terminal region of connecting, a unique region flanked by two immunoglobulin C2 motifs. Arch. Biochem. Biophys. 1997;342(1):99–107. doi: 10.1006/abbi.1997.0108. [DOI] [PubMed] [Google Scholar]
- 155.Carbone J.W., Pasiakos S.M., Vislocky L.M., Anderson J.M., Rodriguez N.R. Effects of short-term energy deficit on muscle protein breakdown and intramuscular proteolysis in normal-weight young adults. Appl. Physiol. Nutr. Metabol. 2014;39(8):960–968. doi: 10.1139/apnm-2013-0433. [DOI] [PubMed] [Google Scholar]
- 156.Wang X.H., Zhang L., Mitch W.E., LeDoux J.M., Hu J., Du J. Caspase-3 cleaves specific 19 S proteasome subunits in skeletal muscle stimulating proteasome activity. J. Biol. Chem. 2010;285(28):21249–21257. doi: 10.1074/jbc.M109.041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Eron S.J., MacPherson D.J., Dagbay K.B., Hardy J.A. Multiple mechanisms of zinc-mediated inhibition for the apoptotic caspases-3, -6, -7, and -8. ACS Chem. Biol. 2018;13(5):1279–1290. doi: 10.1021/acschembio.8b00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Velez E.J., Azizi S., Lutfi E., Capilla E., Moya A., Navarro I.…Gutierrez J. Moderate and sustained exercise modulates muscle proteolytic and myogenic markers in gilthead sea bream (Sparus aurata) Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017;312(5):R643–R653. doi: 10.1152/ajpregu.00308.2016. [DOI] [PubMed] [Google Scholar]
- 159.Kramerova I., Torres J.A., Eskin A., Nelson S.F., Spencer M.J. Calpain 3 and CaMKIIbeta signaling are required to induce HSP70 necessary for adaptive muscle growth after atrophy. Hum. Mol. Genet. 2018;27(9):1642–1653. doi: 10.1093/hmg/ddy071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang J., Maldonado M.A. The ubiquitin-proteasome system and its role in inflammatory and autoimmune diseases. Cell. Mol. Immunol. 2006;3(4):255–261. https://www.ncbi.nlm.nih.gov/pubmed/16978533 Retrieved from. [PubMed] [Google Scholar]
- 161.Schwartz A.L., Ciechanover A. Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu. Rev. Pharmacol. Toxicol. 2009;49:73–96. doi: 10.1146/annurev.pharmtox.051208.165340. [DOI] [PubMed] [Google Scholar]
- 162.Nakajima E., Hammond K.B., Shearer T.R., Azuma M. Activation of the mitochondrial caspase pathway and subsequent calpain activation in monkey RPE cells cultured under zinc depletion. Eye. 2014;28(1):85–92. doi: 10.1038/eye.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Adebayo O.L., Sandhir R., Adenuga G.A. Protective roles of selenium and zinc against postnatal protein-undernutrition-induced alterations in Ca(2+)-homeostasis leading to cognitive deficits in Wistar rats. Int. J. Dev. Neurosci. 2015;43:1–7. doi: 10.1016/j.ijdevneu.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 164.Tamada Y., Walkup R.D., Shearer T.R., Azuma M. Contribution of calpain to cellular damage in human retinal pigment epithelial cells cultured with zinc chelator. Curr. Eye Res. 2007;32(6):565–573. doi: 10.1080/02713680701359633. [DOI] [PubMed] [Google Scholar]
- 165.Yan M., Hardin K., Ho E. Differential response to zinc-induced apoptosis in benign prostate hyperplasia and prostate cancer cells. J. Nutr. Biochem. 2010;21(8):687–694. doi: 10.1016/j.jnutbio.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Varshavsky A. The ubiquitin system, an immense realm. Annu. Rev. Biochem. 2012;81:167–176. doi: 10.1146/annurev-biochem-051910-094049. [DOI] [PubMed] [Google Scholar]
- 167.Bodine S.C., Baehr L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014;307(6):E469–E484. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Budenholzer L., Cheng C.L., Li Y., Hochstrasser M. Proteasome structure and assembly. J. Mol. Biol. 2017;429(22):3500–3524. doi: 10.1016/j.jmb.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kim I., Kim C.H., Kim J.H., Lee J., Choi J.J., Chen Z.A.…Ahn Y.S. Pyrrolidine dithiocarbamate and zinc inhibit proteasome-dependent proteolysis. Exp. Cell Res. 2004;298(1):229–238. doi: 10.1016/j.yexcr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 170.Tomko R.J., Jr., Hochstrasser M. Molecular architecture and assembly of the eukaryotic proteasome. Annu. Rev. Biochem. 2013;82:415–445. doi: 10.1146/annurev-biochem-060410-150257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Verma R., Aravind L., Oania R., McDonald W.H., Yates J.R., 3rd, Koonin E.V., Deshaies R.J. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science. 2002;298(5593):611–615. doi: 10.1126/science.1075898. [DOI] [PubMed] [Google Scholar]
- 172.Lee D., Takayama S., Goldberg A.L. ZFAND5/ZNF216 is an activator of the 26S proteasome that stimulates overall protein degradation. Proc. Natl. Acad. Sci. U. S. A. 2018;115(41):E9550–E9559. doi: 10.1073/pnas.1809934115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Prasad A.S., Bao B. Molecular mechanisms of zinc as a pro-antioxidant mediator: clinical therapeutic implications. Antioxidants. 2019;8(6) doi: 10.3390/antiox8060164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Olechnowicz J., Tinkov A., Skalny A., Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018;68(1):19–31. doi: 10.1007/s12576-017-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li B., Cui W., Tan Y., Luo P., Chen Q., Zhang C.…Cai L. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J. Cell Mol. Med. 2014;18(5):895–906. doi: 10.1111/jcmm.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hadwan M.H., Almashhedy L.A., Alsalman A.R. Study of the effects of oral zinc supplementation on peroxynitrite levels, arginase activity and NO synthase activity in seminal plasma of Iraqi asthenospermic patients. Reprod. Biol. Endocrinol. 2014;12:1. doi: 10.1186/1477-7827-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ogawa D., Asanuma M., Miyazaki I., Tachibana H., Wada J., Sogawa N.…Makino H. High glucose increases metallothionein expression in renal proximal tubular epithelial cells. Exp. Diabetes Res. 2011;2011 doi: 10.1155/2011/534872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bashandy S.A.E., Alaamer A., Moussa S.A.A., Omara E.A. Role of zinc oxide nanoparticles in alleviating hepatic fibrosis and nephrotoxicity induced by thioacetamide in rats. Can. J. Physiol. Pharmacol. 2018;96(4):337–344. doi: 10.1139/cjpp-2017-0247. [DOI] [PubMed] [Google Scholar]
- 179.Sefi M., Chaabane M., Elwej A., Bejaoui S., Marrekchi R., Jamoussi K.…Soudani N. Zinc alleviates maneb-induced kidney injury in adult mice through modulation of oxidative stress, genotoxicity, and histopathological changes. Environ. Sci. Pollut. Res. Int. 2020 doi: 10.1007/s11356-019-07175-7. [DOI] [PubMed] [Google Scholar]
- 180.Teixeira M., Gouveia M., Duarte A., Ferreira M., Simoes M.I., Conceicao M.…Ribeiro F. Regular exercise participation contributes to better proteostasis, inflammatory and vasoactive profiles in patients with hypertension. Am. J. Hypertens. 2019 doi: 10.1093/ajh/hpz160. [DOI] [PubMed] [Google Scholar]
- 181.Fernandez-Sanchez A., Madrigal-Santillan E., Bautista M., Esquivel-Soto J., Morales-Gonzalez A., Esquivel-Chirino C.…Morales-Gonzalez J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011;12(5):3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Prasad A.S. Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr. 2014;1:14. doi: 10.3389/fnut.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Amin M.N., Siddiqui S.A., Uddin M.G., Ibrahim M., Uddin S.M.N., Adnan M.T., ⋯ Islam M.S. Increased oxidative stress, altered trace elements, and macro-minerals are associated with female obesity. Biol. Trace Elem. Res. 2020 doi: 10.1007/s12011-019-02002-z. [DOI] [PubMed] [Google Scholar]
- 184.Jung S., Kim M.K., Choi B.Y. The relationship between zinc status and inflammatory marker levels in rural Korean adults aged 40 and older. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0130016. [DOI] [PMC free article] [PubMed] [Google Scholar]