Abstract
Antioxidant supplements are commonly consumed by endurance athletes to minimize exercise-induced oxidative stress, with the intention of enhancing recovery and improving performance. There are numerous commercially available nutritional supplements that are targeted to athletes and health enthusiasts that allegedly possess antioxidant properties. However, most of these compounds are poorly investigated with respect to their in vivo redox activity and efficacy in humans. Therefore, this review will firstly provide a background to endurance exercise-related redox signalling and the subsequent adaptations in skeletal muscle and vascular function. The review will then discuss commonly available compounds with purported antioxidant effects for use by athletes. N-acetyl cysteine may be of benefit over the days prior to an endurance event; while chronic intake of combined 1000 mg vitamin C + vitamin E is not recommended during periods of heavy training associated with adaptations in skeletal muscle. Melatonin, vitamin E and α-lipoic acid appear effective at decreasing markers of exercise-induced oxidative stress. However, evidence on their effects on endurance performance are either lacking or not supportive. Catechins, anthocyanins, coenzyme Q10 and vitamin C may improve vascular function, however, evidence is either limited to specific sub-populations and/or does not translate to improved performance. Finally, additional research should clarify the potential benefits of curcumin in improving muscle recovery post intensive exercise; and the potential hampering effects of astaxanthin, selenium and vitamin A on skeletal muscle adaptations to endurance training. Overall, we highlight the lack of supportive evidence for most antioxidant compounds to recommend to athletes.
1. Introduction
Intense exercise and muscle contraction increase production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and promote oxidative stress in skeletal muscle [[1], [2], [3], [4], [5], [6], [7], [8]]. Excessive ROS levels are deleterious to cells [9,10] through increased damage and modifications to cellular proteins, lipids and DNA [11]. Moreover, elevated ROS levels have been implicated in the pathogenesis of numerous chronic diseases, including cardiovascular disease [[12], [13], [14]] and type 2 diabetes [15]. On the other hand, emerging evidence shows that several ROS and RNS produced in physiological amounts are important signalling molecules, acting through mechanisms such as post-translational redox modifications of cysteine thiols on proteins [16,17]. Recent research has highlighted the importance of redox signalling in physiological processes, including normal molecular and cellular responses to exercise [10]. In particular, redox-signalling pathways have been implicated in the acute and chronic responses of skeletal muscle to endurance exercise, including skeletal muscle glucose uptake and insulin sensitivity [18,19]; induction of endogenous antioxidant enzymes [6,20,21]; mitochondrial biogenesis [[22], [23], [24]]; and muscle contraction force [[25], [26], [27]]. There is also growing evidence of redox regulation of vascular function, with relevance to exercise and its adaptations that require further examination [[28], [29], [30], [31]]. Factors such as the magnitude and/or duration of altered rates of ROS generation/exposure, the (sub)cellular origin of ROS production [32] and the proximity of reactive protein thiols to sites of ROS/RNS production [33] may impact on redox signalling events that either promote healthful effects or promote disease.
Antioxidants play important roles in regulating ROS levels through direct free radical scavenging mechanisms, through regulation of ROS/RNS-producing enzymes, and/or via adaptive electrophilic-like mechanisms. Acute and chronic endurance exercise tends to increase expression and activity of endogenous antioxidant enzymes in skeletal muscle [6,20,21], in turn enabling an improved capacity to moderate adverse effects of ROS. To enhance the capacity of skeletal muscle to neutralize ROS produced during exercise, athletes regularly consume exogenous antioxidant supplements [34,35]. Benefits of antioxidant supplements might relate to an improvement in cellular redox state and decreased oxidative modifications to DNA, lipids and proteins. Some evidence shows an ameliorating effect of antioxidants on muscle recovery following intense muscle-damaging exercise [[36], [37], [38], [39]]. ROS have also been implicated in premature muscular fatigue during sustained muscle contraction and exercise [[40], [41], [42]]. Therefore, the use of exogenous antioxidants might help to delay muscular fatigue and improve endurance exercise performance.
Despite the potential benefits of antioxidant supplementation in exercising humans, growing research has implicated hampering effects of exogenous antioxidant supplementation on some acute and chronic responses of skeletal muscle to exercise [22,24,26,43,44]. These impairments in adaptive changes within skeletal muscle are presumably a result of an attenuation of normal redox-signalling pathways in muscle by antioxidants [10]. In particular, antioxidant supplementation has been found in some studies to impair adaptive responses to endurance exercise training [24,43,44]. There are numerous compounds with purported antioxidant properties that are available commercially over the counter or via online vendors for athletes and other health consumers. However, for many of these compounds, there is a lack a critical evaluation of their efficacy and benefits. Thus, there is a need to further evaluate the current evidence on potential benefits and risks of these compounds for athletes.
The present review aims to firstly discuss endurance exercise-related skeletal muscle redox signalling, with key focus areas of (a) sites of ROS production and their temporal changes with respect to exercise; and (b) mechanisms of ROS as a signal in skeletal muscle adaptations to exercise, including adaptations such as mitochondrial biogenesis, antioxidant enzyme induction and vascular functional changes. The second half of the review will focus on current evidence on effects of commercially available compounds with purported antioxidant effects on oxidative stress, endurance exercise-related outcomes and muscle recovery post exercise. Where possible, we have focussed on evidence arising from studies using healthy human participants given the potential applicability of such findings to human athletic endeavours. Moreover, we have focussed, where possible, on effects that are specific to skeletal muscle.
2. Endurance exercise and skeletal muscle redox signalling
2.1. Redox signalling mechanisms in skeletal muscle
ROS (such as superoxide [O2●─] and hydrogen peroxide [H2O2]) and RNS (such as nitric oxide [NO] and peroxynitrite [ONOO─] can participate in redox signalling in cells either directly or indirectly [45]. Transient and reversible post translational chemical modifications of reactive cysteine thiol residues on proteins through processes such as S-nitrosylation, S-glutathionylation, sulfenylation and disulphide formation are important redox modifications through which cells respond to altered levels of ROS and RNS [17,27,46]. For instance, protein S-nitrosylation can promote cellular effects such as altered regulation of enzyme activities, altered receptor and transporter activities, altered gene transcription and translation, and protein-protein interactions [47]. S-glutathionylation of a protein can result in its activation or deactivation, which may be important in the regulation of cell signalling mediators [17,48]. Post-translational redox modifications such as S-nitrosylation and S-glutathionylation may play important molecular signalling roles in skeletal muscle given their intricate associations with key proteins linked to muscle contraction and exercise adaptations [[49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65]].
In addition to ROS and RNS, antioxidants are involved in redox signalling in cells. Antioxidants can regulate thiol redox state and therefore regulate redox signalling pathways [66]. Endogenous antioxidants, which include enzymes and small molecules such as thioredoxins (Trx), sulfiredoxins (Srx), glutathione reductases (GR), peroxiredoxins (Prdx) and glutathione (GSH) are fundamental to the control of redox signalling networks [67]. Additionally, exogenous compounds with antioxidant properties such as polyphenols, vitamin C and vitamin E that are consumed either in the diet or as dietary supplements may also participate in and interact with these redox signalling networks. Exercise-related redox signalling in skeletal muscle induced by ROS/RNS and regulated by antioxidants, might include acute and/or chronic alterations in gene expression, modified kinase and phosphatase activities, and altered levels of molecular chaperones, transporters, proteasomes, and transcription factors [27,68].
2.2. Sites of ROS production before, during and post endurance exercise
Total intracellular ROS levels reflect the net rates of formation and removal across numerous individual subcellular sites. In the context of physiological stressors such as exercise, these have most often been studied under defined steady state bioenergetic conditions, i.e. either at rest or during exercise/contraction [69,70]. The following section not only summarises the known sites of ROS formation and removal under these conditions, but also focusses on the dynamic temporal shifts in site-specific ROS generation and removal that occur during the transition periods between steady state bioenergetic situations. Specifically, these are the transitions from i) rest to work, ii) work to rest, and iii) the early post-exercise recovery period.
In resting skeletal muscle, intracellular ADP concentrations are low relative to ATP since energy supply exceeds demand. This low ADP/ATP ratio serves to supress mitochondrial oxidative phosphorylation (OXPHOS) activity [72], while also promoting a highly reduced redox environment (i.e. high NADH/NAD+). This ‘reducing pressure’ caused by surplus electron flow into the ubiquinone (Q) complex in the electron transport chain (ETC) can promote the leakage of unpaired electrons from the ETC [[73], [74], [75]]. Reduction of molecular oxygen by a single electron results in the formation of the superoxide anion (O2·-). While O2·- is the primary form of ROS, it is rapidly dismutated either spontaneously or enzymatically to the more stable hydrogen peroxide (H2O2). The formation of O2·- occurs from at least 11 distinct sites within mitochondria at complexes I, II and III, four dehydrogenases in the matrix compartment and others in the intermembrane space including glycerol-3-phosphate and dihydroorotate dehydrogenases [76]. In addition, NADPH-oxidase (NOX)-4, an H2O2 generating enzyme, has been shown to be localised within skeletal muscle mitochondria [70,77]. Under in vitro conditions mimicking ‘rest’, the main contributing sites of mitochondrial O2·- production are the complex-I ubiquinone reducing site (IQ, ~23% of total O2·-) and the flavin site (IF, ~20%), the complex III ubiquinol oxidising site (IIIQO, ~15%), the flavin in complex-II (IIF, ~24%), and a site involved in the fatty acid β-oxidation pathway (EF, ~13%) [69].
In addition to these mitochondrial sites of ROS formation, non-mitochondrial NOX enzymes localised to the cytosol and sarcolemma also contribute to the overall cellular O2·- formation at rest. Recent advances in pharmacological tools to study redox biology have revealed that in mouse myoblast cells, O2·- from mitochondria contributes to ~45% and non-mitochondrial NOX enzymes contribute to ~40% of the total cellular O2·- generation under basal conditions, respectively [78,79].
In addition to the various sites of ROS formation, specific subcellular distributions of various antioxidant enzymes enable precise spatial regulation of ROS scavenging for the maintenance of intracellular redox homeostasis [80]. These include superoxide dismutase which uses a copper/zinc or manganese ion as the active site, of which isoform 1 (SOD1) is cytosolic and intermembrane space localised, while isoform 2 (SOD2) is located in mitochondrial matrix. Glutathione (GSH), a cysteine-containing tripeptide functions both as a direct scavenger of H2O2 and also as a substrate for the NADPH-dependent glutathione peroxidases localised in the cytosol (GPx 1 and 2) and the mitochondria (GPx 1 and 4). Thioredoxins and peroxiredoxins comprise another key NADPH-dependent scavenging pathway both in the cytosol (Trx 1 and Prdx 1 and 2) and mitochondrial compartment (Trx 2 and Prdx 3 and 5). The lower Km for H2O2 of Prdx compared with GPx is critical for fine-tuning physiological H2O2 fluxes [81,82].
2.3. ROS during rest-to-work transition
At the onset of myofibril contraction, ATP hydrolysis increases up to 100-fold from rest [83]. In order to maintain energy homeostasis, mitochondrial oxidative phosphorylation is upregulated within seconds, primarily in response to the increasing ADP:ATP ratio [84]. Consequently, a rapid decrease in reducing-pressure occurs at the electron entry points of the mitochondrial ETC, thereby attenuating the majority of O2·- generation from these mitochondrial ETC sites [69] (Fig. 1). Thus, although mitochondria have the capacity to generate large quantities of O2·- under specific bioenergetic conditions, native O2·- generation rates in vivo are dynamic and approximately inversely proportional to the oxidative phosphorylation rate. Indeed, in isolated mitochondrial preparations in vitro, it is well established that when ADP is added to stimulate oxidative phosphorylation to induce the classical experimental respiratory ‘state-3’, rates of mitochondrial O2·-/H2O2 emission decrease dramatically [69,85] (Fig. 1). Notably, under in vitro conditions mimicking those of intense exercise, it was shown that total mitochondrial O2·-/H2O2 production is only about 15% of rest values, which is mostly attributable to site IF [69]. Thus, on the basis of fundamental bioenergetic principles as well as experimental evidence, mitochondria are negligible contributors to total muscle ROS generation during exercise. Mitochondria can also consume O2·-/H2O2 produced within the matrix compartment or H2O2 that diffuses into mitochondria from cytosolic origins [86]. It is therefore possible that mitochondria could consume more ROS than is generated during energetic conditions similar to exercise [87]. Despite this, total levels of skeletal muscle ROS are well known to increase in muscle fibres during in vitro contractions [3], and in vivo during exercise in rodents and humans [2,5,88,89]. Primary sources of ROS formation during skeletal muscle contraction include the NOXs, which are ‘professional’ O2·- generating enzymes (i.e. no other known function), along with a minor contribution from xanthine oxidase [[89], [90], [91], [92], [93]]. In addition, exercise-induced calcium fluxes may contribute to increased activity of calcium-dependent phospholipase A2 (PLA2) which forms arachidonic acid and subsequently activates the O2·- generating enzyme, lipoxygenase [94]. Other potential sources of O2·- that have not yet been studied in the context of exercise include the protein p66shc which is known to translocate to the mitochondrial intermembrane space where it can oxidize cytochrome-c to form H2O2 [95], and pyruvate dehydrogenase which has been shown to generate ROS under in vitro conditions similar to exercise [96].
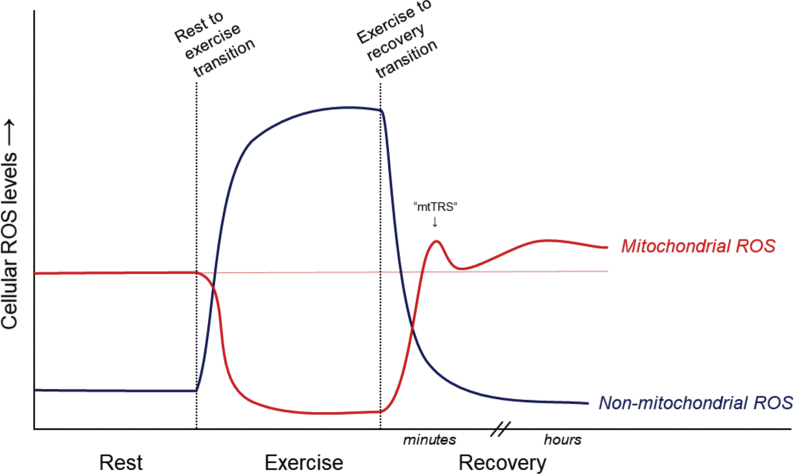
Fig. 1.
Proposed relative contributions of mitochondrial and non-mitochondrial sources of ROS to overall cellular ROS levels in skeletal muscle during and in the minutes and hours following a single session of endurance exercise. mtTRS, mitochondrial transition spike (refer to text and reference [71]).
2.4. ROS during transition from work-to-rest
After the cessation of myofibril contraction that occurs during exercise, energy demand and ATP synthesis declines at an exponential rate returning to near-resting values within the first minute [97]. During these changing energetic conditions, a short-lived surge in ROS has been described, termed the ‘mitochondrial ROS transition spike’(mTRS) (Fig. 1), as demonstrated in vitro during the transition from state 3 (ADP stimulated oxidative phosphorylation) to state 4 (non-phosphorylating) [71]. The underlying mechanism for this phenomenon was reported to involve the nicotinamide nucleotide transhydrogenase (NNT). This work by Sharaf et al. [71] showed that when the rate of oxidative respiration slows during transition from state 3 to 4, the increased proton motive force promotes O2·- formation from complex I. This presents as the upward phase of the ROS spike. Simultaneously, the slowing energy demand during state 3 to 4 transition also allows NADH levels to increase (due to slowing complex-I activity), which drives NNT activity to restore NADPH levels, thereby fueling the NADPH-dependent glutathione (GSH) and thioredoxin (Trx) antioxidant systems. This enhances the rate of ROS removal to begin restoring redox homeostasis, which presents as the downward phase of the ROS transition spike (Fig. 1). Whether this occurs in skeletal muscle at the cessation of exercise/contraction remains to be investigated.
2.5. ROS in the early post-exercise recovery period
In the hours following exercise, when muscle bioenergetic demands have returned to near resting/basal levels, ROS formation returns to being primarily of mitochondrial origin and has been shown to be elevated for at least 3 h post exercise [85]. Various mechanisms could contribute to this, because exercise causes homeostatic challenges within the cellular milleu and various exercise-induced factors may modify macromolecules. For instance, elevation in the circulation and oxidation of free fatty acids (FFA) in the post exercise period [98] could modulate the production of ROS [99] by increasing H2O2 emission at complex-II and the electron-transferring flavoprotein (ETF) in skeletal muscle mitochondria [100]. Inflammation resulting from microtrauma to sarcomeres due to unaccustomed and/or eccentric exercise [101] leads to infiltration of neutrophils that generate bursts of superoxide via NOX activation [102]. Further, a number of proinflammatory cytokines released from skeletal muscle (i.e.: ‘myokines’) during contraction [103] such as interleukin-6 (IL-6) can persist in the circulation after exercise and continue to stimulate ROS generating enzymes including xanthine oxidase, lipoxygenase and NOXs [104].
Other mechanisms causing post-exercise increases in ROS generation might involve post translational modifications (PTMs) such as phosphorylation, O-GlcNAcylation or acetylation to mitochondrial proteins [105]. Notably, oxidative PTMs such as S-glutathionylation and S-nitrosylation, are known to affect activity of protein complexes within the ETS and can occur as a result of exercise [85,[106], [107], [108]]. Localised accumulation of H2O2 occurs via reversible inactivation of Prdx antioxidant enzyme activity as a result of oxidation by H2O2 itself [81], which may occur in response to exercise [109]. Moreover, the abundance of the Prdx1 protein was also shown to be lower 3 h post exercise [85], thereby allowing localised increases in H2O2 at the subcellular level. Another factor that may impact post-exercise ROS homeostasis is changes in mitochondrial morphology. Exercise induces changes in mitochondrial membrane morphology and this is known to impact respiratory function [110], and thereby ROS formation rates [111]. Moreover, some of the molecular machinery responsible for mitochondrial dynamics/morphological changes are redox sensitive [[110], [111], [112], [113]]. Mitochondrial quality is regulated by mitochondrial autophagy processes (termed ‘mitophagy’), via ubiquitin kinase PINK1-Parkin pathway [114] or via redox sensitive Nix and Bnip3 [115,116] which may play a role in mitochondrial respiratory function and ROS generation in the post-exercise period. Similarly, antioxidant proteins in the mitochondria may be tagged for autophagy in the face of oxidative stress via stress-activated pathways and proteolysis decreasing their abundance and enzymatic function, thus impacting rates of ROS removal [117]. Taken together, these factors may explain the acute changes in skeletal muscle mitochondrial ROS that have been observed in the hours following acute bouts of exercise [85]. The major implications of elevated post-exercise mitochondrial ROS are that they could modulate redox sensitive cysteine residues throughout the entire proteome [118] and alter the expression of exercise-responsive genes and adaptive responses [119,120].
In summary, under basal/resting conditions the sites of ROS formation in skeletal muscle are primarily mitochondrial, yet these become negligible during exercise (Fig. 1). Total muscle ROS formation rates increase sharply during exercise primarily due to the contributions of cytosolic and sarcolemmal ROS-forming enzymatic systems. The concept of the mitochondrial ROS ‘transition spike’ represents an interesting avenue for further investigation during the transition from exercise to recovery/rest. Finally, further studies are warranted to identify and functionally characterise post-translational protein modifications that cause or are a consequence of changes in rates of ROS formation and removal in response to exercise.
3. Mechanisms of ROS as a signal in skeletal muscle adaptations to endurance exercise
3.1. Role of redox signalling in mitochondrial biogenesis
Increased mitochondrial volume and functional capacity is a hallmark adaptation to exercise training in skeletal muscle. Peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC1-α) is considered the master regulator in programming the transcriptional response to an aerobic exercise stimulus [121]. PGC-1α activation is achieved by signals from upstream kinases including such as AMPK and p38 MAPK [122,123]. This initiates transcription of downstream targets that lead to upregulation of mitochondrial genes and proteins [124]. Importantly, mitochondrial biogenesis appears to be a redox sensitive process [125,126]. This may be associated with the redox sensitive AMPK regulation of PGC-1α [127], although, it was recently shown by mutation of a putative redox sensitive Cys residue in the AMPKα subunit, that the response to H2O2 is indirect [128]. On the other hand, it was demonstrated in the absence of confounding bioenergetic variables, that O2·-/H2O2 specifically formed within the mitochondrial matrix activates p38 MAPK [129]. Also, recent studies using knockout mice have demonstrated that O2·-/H2O2 formed during exercise by NOX2 is required for the normal post exercise increases in p38 MAPK phosphorylation [89]. Allopurinol, a xanthine oxidase inhibitor, was shown to decrease muscle ROS production in rats which blunted exercise induced PGC1-α gene expression and downstream mitochondrial biogenesis related transcriptional activity [130]. In contrast, we did not identify changes in PGC-1α gene expression in allopurinol supplemented rats following 60 min of acute exercise, although components of upstream stress signalling (ERK and p38 MAPK phosphorylation) as well as downstream gene expression (Tfam) were blunted [90]. It is possible that allopurinol influences only some aspects of these redox sensitive responses. On the other hand, it was shown that PGC-1α mRNA expression was augmented after exercise in rats treated with diethyl maleate which depletes glutathione - a primary endogenous antioxidant pool in skeletal muscle [131]. This is also consistent with a previous finding that glutathione depletion induces a redox-dependant activation of PGC-1α via p53 binding to the promotor region of the PPARGC1A gene [132].
3.2. Role of redox signalling in antioxidant enzyme induction
The increased ROS during contraction is also partly responsible for the induction of antioxidant enzymes. This occurs via the induction of several distinct but interrelated pathways to mediate many of the redox adaptations to exercise, including activation of mitochondrial biogenesis, the Keap1-Nrf2-ARE pathway and nuclear factor κB (NFκB) signaling. These pathways are regulated, in part, by the activation of several redox-sensitive kinases including AMPK, the MAP kinases p38 MAPK, JNK and ERK1/2, which are all increased to some extent during skeletal muscle contraction [93,[133], [134], [135]]. Both AMPK and p38 MAPK activate the mitochondrial biogenesis pathway via PGC-1α, with PGC-1α required for the induction of superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase [136]. The redox sensitive transcription factor NFκB is also increased during muscle contraction and is known to regulate SOD2 transcription [137].
One of the major pathways for exercise-induced antioxidant induction is the Keap1-Nrf2-ARE pathway, which is dependent on the induction of the redox sensitive transcription factor Nuclear factor erythroid-derived 2-like 2 (Nrf2, also referred to as NFE2L2 (but not to be confused with nuclear respiratory factor 2)). Merry and Ristow [138] have shown using Nrf2 knockout mice that Nrf2 is required for the increases in antioxidant gene expression of SOD1, SOD2 and catalase normally observed following a single bout of exercise and also SOD activity following endurance training. Briefly, during unstressed conditions, Nrf2 remains bound to Keap1 in the cytosol. In response to oxidative stress, cysteine residues are modified on Keap1, resulting in the dissociation of the complex and the translocation of Nrf2 to the nucleus. Upon entering the nucleus, Nrf2 activates the transcription of antioxidant enzymes through its binding to the antioxidant response element (ARE). For more detailed information on the activation and regulation of Nrf2 mediated redox adaptations to exercise, see review by Done and Traustadottir [139]. However, ROS induced cysteine modifications on Keap1 are not the only avenue of Nrf2 activation, since the redox sensitive kinases such as AMPK, ERK and JNK are also known to activate Nrf2 [140,141]. Interestingly, AMPK activation by AICAR in vitro, also directly phosphorylates Nrf2 in the cytosol, leading to its nuclear accumulation and ARE binding in vitro [140]. This activation of Nrf2 independent of oxidative stress, suggests that during exercise the energetic stress (AMP/ATP ratio) and/or oxidative stress could be acting alone or together to regulate redox adaptions to exercise training.
3.3. Redox regulation of blood flow: implications for exercise, health and disease
The vascular system is essential for nutrient and gas exchange between blood and peripheral tissues such as skeletal muscle. Contracting skeletal muscle can lead to a 100-fold increase in metabolic demand, which is offset by balancing vascular tone with haemodynamics within the central (cardiac), macro- (large arteries) and micro-vascular (resistance arteries, peripheral arterioles and capillaries) systems to meet metabolic demand of the working muscles [142,143]. However, even minor alterations in blood flow can lead to profound effects in various tissue including the heart, brain, skin, liver, and skeletal muscle, affecting components of exercise capacity and performance including cognitive function and fatigue, substrate metabolism, thermoregulation, and muscular contraction and force production [142,144,145]. Although blood flow is considered to be one of the primary rate-limiting steps for exercise performance and capacity, the precise mechanisms controlling vascular function, both at rest and during exercise, in both health and disease, have yet to be completely elucidated [143]. Considering the well-established and previously discussed relationship between skeletal muscle contraction and ROS production [41], redox regulation of vascular function has emerged as a potentially important mechanism contributing to exercise capacity, performance and adaptation, and overall health and disease [143,144,[146], [147], [148]].
3.4. ROS-mediated vascular pathophysiology
Several studies have reported ROS production and subsequent oxidative stress in the vasculature tissue itself during conditions of increased blood flow or vascular shear stress [144,[149], [150], [151], [152]]. Predominant sources of vascular derived oxidative stress include mitochondria, NOX, xanthine oxidase (XO), and uncoupled endothelial nitric oxide synthase (eNOS), which can be found in endothelial cells, vascular smooth muscle cells and fibroblasts [153]. ROS-mediated regulation of blood flow is predominantly known for its pathophysiological role in promoting vasoconstriction, aberrant vascular remodeling, inflammation, and fibrosis, which can both acutely and chronically lead to impaired cardiac, macro and microvascular function [149,[153], [154], [155], [156], [157], [158], [159], [160], [161], [162], [163], [164], [165]].
3.5. ROS-mediated vascular dysfunction in healthy individuals
Antioxidant treatment (e.g., vitamin C, E, α-lipoic acid, and Mito-Q10) has been reported to acutely restore or improve measures of endothelial function, vascular and myogenic tone, cardiac hypertrophy, and myocardial function in a variety of vascular derived conditions that are characterised by chronic low-grade oxidative stress including type 2 diabetes, chronic hypertension, atherosclerosis, cardiovascular disease, and ageing [[165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181]]. However, excess ROS can also lead to transient vascular dysfunction in healthy populations. For example, increased systemic oxidative stress and/or decreased antioxidant capacity following acute hyperlipidemia and hyperglycemia in healthy humans and rodents coincides with macro- and micro-vascular endothelial dysfunction [154,156,182], effects which can be largely prevented via acute co-infusion of vitamin C, N-acetylcysteine, and apocynin [154,156]. In the absence of an oxidative stress insult (e.g., nutrient overload) or chronically elevated levels of oxidative stress (e.g., oxidative stress associated disease), antioxidant treatment in healthy individuals rarely leads to improved vascular function [183], with the vast majority of evidence supporting little to no benefit [165,166,[168], [169], [170], [171],173,174]. As such, acute antioxidant treatment for improving vascular function appears to be only effective under conditions of acute and/or chronic oxidative stress associated vascular pathophysiology.
3.6. ROS-mediated vascular physiology
Despite a clear role for ROS in vascular dysfunction, ROS is paradoxically required for flow mediated dilatation (FMD), a measure of arterial endothelial function and its ability to dilate in response to increased blood flow or shear stress. For example, H2O2 is required for normal FMD in isolated coronary resistance arteries [184,185] and becomes the predominant mechanism for FMD, rather than NO, in isolated visceral adipose tissue arterioles from patients with coronary disease [186,187]. In both young and old rodents, treatment of soleus muscle arterioles with the O2●─ and H2O2 scavengers tempol and catalase, respectively, prevents FMD [164]. Likewise, Liu, Zhao [187] reported that shear stress induced O2●─ production from vascular-derived mitochondria respiration contributes to FMD of isolated human coronary resistance arteries. ROS-mediated vascular function may explain why acute antioxidant treatment (vitamin C, 1000 mg; vitamin E, 600 IU; alpha-lipoic acid, 600 mg) in healthy young humans leads to impaired brachial artery FMD [175]. ROS also play a role in healthy vascular adaptations including endothelial cell and vascular smooth muscle cell proliferation, differentiation and migration [[188], [189], [190]] and the upregulation of eNOS expression in endothelial cells [30]. Angiogenesis and vascular adaptations occur predominantly through ROS-stimulated vascular endothelial growth factor expression, but also indirectly through activating redox-sensitive signaling pathways including p38 MAPK, PGC-1α, eNOS, and hypoxia inducible factor (HIF)-1α [191,192].
Current findings support a clear role for redox regulation of macro and microvascular function in various tissues including skeletal muscle, heart, and the pulmonary system. During conditions of excessive oxidative stress (such as during nutrient overload or oxidative stress associated disease) ROS appear to contribute to vascular dysfunction, whereas in the absence of excessive oxidative stress, such as observed in healthy young humans, ROS are likely beneficial and even necessary for normal vascular function.
3.7. ROS-mediated vascular function, exercise capacity and training adaptations
Redox regulation of exercise-induced blood flow, and the subsequent effects on exercise capacity, performance and muscle function, has received limited empirical investigation. Nevertheless, current research supports a role for ROS in regulating exercise/contraction induced blood flow, and exercise capacity, performance and vascular adaptation [28,157,193].
The majority of evidence supporting redox regulation of vascular function during exercise comes from antioxidant treatment studies, and much like basal redox regulation of vascular function, the effects depend on the context and redox environment. For example, antioxidant ingestion prior to hand-grip exercise (300 mg of α-lipoic acid, 500 mg of vitamin C, and 200–400 IU of vitamin E) decreases ROS production as measured by electron paramagnetic resonance spectroscopy, and restores exercise-induced brachial vasodilation in sedentary older adults [29]. In contrast, the same antioxidant treatment in younger individuals leads to impaired exercise-induced brachial artery dilation [28]. Vitamin C infusion has been robustly shown to improve forearm blood flow in older individuals during hand grip exercise [[194], [195], [196], [197]], which is largely abolished with NOS inhibition; however, the direct role of free radical quenching by vitamin C during these conditions remains contentious [195,197]. Furthermore, exercise-induced total leg blood flow in chronic obstructive pulmonary disease patients is improved with prior ingestion of an antioxidant cocktail (300 mg of α-lipoic acid, 500 mg of vitamin C, and 200–400 IU of vitamin E) [198]. In contrast, others have reported no benefit of antioxidant treatment on contraction-induced blood flow in young healthy individuals [196,198]. Similar findings have been reported using mitochondrial specific antioxidant treatment (MitoQ) which prevents cardiac dysfunction and exercise intolerance in cardiomyocyte-specific NOX4 knockout rodents, but has no additive benefits on cardiac function and exercise capacity in healthy control animals [157]. In humans, daily (10 mg) oral ingestion of MitoQ in young healthy men failed to augment, or alter, adaptations to 3 weeks of endurance training including exercise capacity, muscle mitochondrial capacity, and training-induced increases in numerous types of circulating angiogenic cells [31]. Taken together, these findings support that some vascular responses to exercise may be improved with acute antioxidant treatment in populations where chronic oxidative stress and perturbed redox homeostasis is likely to be present. In contrast, in healthy individuals where redox homeostasis is likely to be retained, vascular responses to exercise are unlikely to be improved by antioxidant treatment, and in some cases may even lead to perturbed redox homeostasis and impaired vascular function.
Exercise also alters redox status in other tissues such as the heart which can directly affect cardiac and vascular function and exercise capacity. For example, wild type mice that undergo acute exercise stress over two consecutive days exhibit increased myocardial ROS, as measured by EPR, and upregulation of the Keap1-Nrf2-ARE antioxidant signaling pathway in the heart [199]. It is likely that this exercise-induced redox response is directly involved in cardiac function, as cardiomyocyte-specific NOX4 and Nrf2 knockout mice exhibit impaired cardiac performance and exercise capacity which is largely restored by Nrf2 activators or mitochondrial targeted antioxidant treatment [157]. Furthermore, six weeks of moderate-intensity exercise training in rodents upregulates myocardial Nrf2 and Nrf2-antioxidant genes and protects against cardiac dysfunction and injury incurred through isoproterenol induced oxidative stress [200]. These findings support exercise-induced myocardial ROS, and subsequent upregulation of antioxidant activity, to directly affect cardiac function and exercise capacity. Other examples of how exercise can lead to improved vascular function via promoting a shift in the redox balance has been reported in humans. For example, a prior bout of acute moderate or high-intensity interval exercise in healthy humans improves brachial artery FMD responses to a high-fat meal ingested 16–18 h later, with improved FMD strongly correlating with increased plasma total anti-oxidant capacity [155]. Likewise, acute hyperglycemia-induced oxidative stress and impaired macro and microvascular endothelial dysfunction, which can be prevented by either N-acetylcysteine or apocynin treatment in rodents [154], can also be prevented by a prior bout of aerobic exercise in humans [201]. These findings support the growing ideology of exercise as a natural antioxidant which can improve vascular dysfunction during conditions of elevated oxidative stress.
Exercise training is also known to promote long-term antioxidant effects that can be beneficial for vascular function and adaptation. For example, 6-weeks of knee extensor exercise training decreases systemic oxidative stress, improves blood pressure and flow-mediated and contraction-mediated arterial dilation in older individuals [29,202]. On the other hand, these training-induced improvements in vascular function were prevented with acute antioxidant treatment prior to testing [29,202]. Similarly, intravenous vitamin C infusion (bolus infusion of 0.06 g/kg over 20 min followed by 0.02 g/kg over 60 min) improves brachial artery FMD in sedentary older individuals, whereas no benefits were reported in endurance-exercise trained older adults [203]. Exercise training in both young and aged rodents improves FMD in isolated soleus arterioles, which is blunted by acute antioxidant co-treatment with tempol, catalase, and tempol plus catalase [164]. The same group reported increased aortic and myocardial eNOS expression following 3 weeks of training in wild-type mice whereas this training effect is absent in transgenic littermates overexpressing human catalase [204]. These findings support in vitro experiments showing that H2O2 treatment can directly increase eNOS expression in endothelial cells [205].
3.8. Summary
Research investigating ROS-mediated regulation of vascular function support the notion that ROS play both a physiological and pathophysiological role. In certain situations in which ROS are elevated and redox homeostasis is perturbed, vascular dysfunction can ensue. Importantly, much like the effects of antioxidant supplementation on training adaptations in healthy and/or trained individuals, current findings support the general consensus that antioxidant treatment is unlikely to provide meaningful benefits, and may even impair vascular function and adaptations in healthy and trained individuals both at rest, and after acute and chronic exercise training. This may not be surprising, as ROS are now known to be necessary for normal vascular function, both in the acute setting and for long-term vascular health. Likewise, exercise leads to the upregulation of antioxidant defenses which maintain vascular function, at rest and during exercise. As such, continual disruption of the redox environment via antioxidant treatment during exercise may impair beneficial training vascular adaptations in both healthy and clinical populations.
4. Antioxidant supplementation and endurance exercise
This section of the review will provide an overview of various redox-modulating compounds that are available commercially with potential application to athletes. This section will focus on their antioxidant effects and their effects on exercise-related outcomes. As already discussed, there is the potential for antioxidants to have negative effects on exercise-related outcomes. Where such evidence exists, this will be discussed, with a focus on their effects in skeletal muscle. It is beyond the scope of this paper to provide a comprehensive review of each antioxidant compound with respect to their bioavailability, antioxidant mechanisms and clinical safety profile. Therefore, for a more detailed discussion of these factors, it is recommended that readers seek out review articles on the specific antioxidants.
Table 1 summarises the redox-related effects of antioxidant compounds in skeletal muscle (and systemically, where skeletal muscle data is unavailable) in humans, particularly with respect to exercise. Effects of these antioxidant compounds on skeletal muscle mitochondrial biogenesis, exercise performance, vascular function and post-exercise muscle recovery (focussing on recovery of muscle strength, delayed onset of muscle soreness (DOMs) and systemic markers of muscle damage such as creatine kinase [CK] and lactate dehydrogenase [LDH]) are also summarized in Table 1.
Table 1.
Reported effects of antioxidant compounds on exercise-related redox markers, mitochondrial biogenesis, vascular function and performance outcomes 18.
| Antioxidant compound (Oral doses used) | Oxidative stress | Antioxidant enzyme levels | Mitochondrial biogenesis | Vascular function | Endurance performance/VO2 max | Post-exercise muscle recovery (Muscle strength, DOMs, CK, LDH) |
|---|---|---|---|---|---|---|
| Anthocyanins (80–547 mg/day) |
Chronic studies (6-21d)Systemic measures: ↓ [[206], [207], [208]] ↔ [[209], [210], [211]] |
Chronic studies (8-21d)Systemic measures: ↑ [207] ↔ [210] |
N/A |
Chronic studies (7d) ↑ [212] (resting only) ↑ [213] (during exercise) |
Chronic studies (3—21d) ↑ [212,214,215] ↔ [210,216,217] |
Chronic studies (7-8d) Beneficial [211] No impact [206,207] |
| Astaxanthin (4–20 mg/day) |
Chronic studies (21-90d)Systemic measures: ↓ [218] ↔ [[219], [220], [221], [222], [223]] |
Chronic studies (21-90d)Systemic measures: ↑ [223] ↔ [222] |
N/A | N/A |
Chronic studies (28d) ↔ [222] |
Chronic studies (21-90d) Beneficial [219,223] No impact [221,224] |
| Catechins (30–1800 mg/day) |
Acute studiesSystemic measures: ↔ [225] (resting only) Chronic studies (14-90d)Systemic measures: ↓ [226] ↔ [[227], [228], [229]] In Skeletal Muscle ↓ [230] |
Acute studiesSystemic measures: ↑ [225] (resting only) Chronic studies (14-28d)Systemic measures: ↔ [[227], [228], [229]] |
Chronic studies (28-90d) ↑ [229] (SDH protein expression) [230] ↔ [229] (CS, Cytochrome C protein expression) |
Acute studies ↑ [231] Chronic studies (56-84d) ↑ [232] ↑ [233] (resting only) |
Chronic studies (2-90d) ↑ [230] ↔ [232,[234], [235], [236]] |
Acute studies No impact [237] Chronic studies (2-90d) Beneficial [227,228] |
| Curcumin (50–2120 mg/day) |
Chronic studies (4d)Systemic measures ↔ [238] |
N/A | N/A |
Chronic studies (56d) ↑ [239](resting only) |
N/A |
Acute studies Beneficial [38] Chronic studies (4-56d) Beneficial [[36], [37], [38]] |
| Quercetin (250–1000 mg per day – most studies used 1000 mg/day) |
Chronic studies (21-24d)Systemic measures ↔ [240,241] |
Chronic studies (21-24d)Systemic measures ↔ [240,241] |
Chronic studies (14-24d) ↔ [242,243] |
N/A |
Chronic studies (7-42d) ↑ [[243], [244], [245], [246], [247]] ↔ [240,242,248,249] |
Chronic studies (9-24d) Beneficial [242,250] No impact [249] |
| Resveratrol (150–600 mg/day) |
Chronic studies (56d)In Skeletal Muscle Attenuated ↓ [251] |
Chronic studies (84d)In Skeletal Muscle ↔ [252] |
Chronic studies (24-84d) ↑ [252] ↔ [251] Attenuated [253] |
Chronic studies (56 d) Attenuated [254] |
Chronic studies (28-180d) ↑ [252] ↔ [251,253,255] Attenuated [254] |
Chronic studies (7d) No impact [256] |
| Vitamin C (400–3000 mg/day) |
Acute studiesSystemic measures ↓ [257] ↔ [39] Chronic studies (8-17d)Systemic measures ↓ [[258], [259], [260]] ↔ [261,262] |
Chronic studies (42d)In Skeletal Muscle ↔ [263,264] (resting only) |
Chronic studies (42d)In Skeletal Muscle ↔ [264] (resting only) |
Acute studies (Intravenous or Oral) ↑ [[194], [195], [196], [197],265] (ref [196] in older adults only) ↔ [196] (ref [196] in younger adults) ↔ [203,266] Chronic studies (30d) ↔ [203] |
Chronic studies (7-56d) ↔ [22,261,267] ↓ [210] |
Acute studies No impact [39] Chronic studies (3-17d) Beneficial [258,259,268] No impact [262,269,270] Detrimental [271] |
| Alpha-lipoic acid (600–1200 mg/day) |
Chronic studies (3-10d)Systemic measures ↓ [[272], [273], [274], [275]] |
Chronic studies (3-10d)Systemic measures ↑ [[273], [274], [275]] |
N/A |
Acute studies ↔ [276] |
N/A |
Chronic studies (3-10d) Beneficial [272] No impact [273,274] |
| Coenzyme Q10 (90–300 mg/day) |
Chronic studies (20-56d)Systemic measures ↓ [277] ↔ [[278], [279], [280], [281]] |
Chronic studies (28d)Systemic measures ↔ [278] |
N/A |
Chronic studies (30d) ↑ [282,283] |
Chronic studies (8-180d) ↑ [280,[282], [283], [284], [285], [286]] ↔ [31,279,281,[287], [288], [289], [290]] ↓ [291,292] |
Chronic studies (20-56d) Beneficial [277] No impact [278,279,293,294] |
| Vitamin A/β-carotene (Vitamin A: 300 mg/day; β-carotene 30 mg/day) |
Chronic studies (28-30d)Systemic measures ↔ [295,296] |
Chronic studies (28d)Systemic measures ↔ [295] |
N/A | N/A |
Chronic studies (30d) ↔ [296] |
N/A |
| Vitamin E (450-1200 IU) |
Chronic studies (14-48d)Systemic measures ↓ [[297], [298], [299], [300], [301]] ↑ [302] In Skeletal Muscle ↓ [300] |
Chronic studies (42d)Systemic measures ↑ [302] ↔ [300] |
N/A | N/A |
Chronic studies (42-180d) ↑ [303,304] ↔ [300,301,305,306] |
Chronic studies(30-150d) Beneficial [301] No impact [268,307,308] |
| Melatonin (acute doses 2.5–6 mg; chronic doses 9–100 mg/day) |
Acute studiesSystemic measures ↓ [309] Chronic studies (3-28d)Systemic measures ↓ [[310], [311], [312]] |
Acute studiesSystemic measures ↑ [309] Chronic studies (3-28d)Systemic measures ↑ [[310], [311], [312]] |
N/A |
Acute studies ↑ [313] (Systolic BP decrease post-exercise only) |
Acute studies ↔ [314,315] |
Chronic studies (28d) Beneficial [311] |
| N-acetyl cysteine (acute: oral 1800 mg - 150 mg/kg (chronic: 1200 mg/day – 250 mg/kg/day |
Acute studiesIn Skeletal Muscle (Intravenous) ↓ [19] |
Acute studiesIn Skeletal Muscle (Intravenous) ↑ [316] Attenuated ↑ [317] |
Acute studies (Intravenous) ↔ [317] |
Acute studies (Intravenous) ↑ [318] (MAP in older adults) ↔ [318] (Blood flow, vascular conductance; and MAP in young adults) Acute studies (Oral) ↔ [319] |
Acute studies (Intravenous) ↑ [40,316,320,321] (ref [321]-at low frequency only) ↔ [40,321,322] (ref [321]-at higher frequencies) (Oral) ↑ [[323], [324], [325]] (ref [325] at 80% VO2 max only) ↔ [319,325] (ref [325] at >80% VO2max) Chronic studies (4-9d) ↑ [[326], [327], [328]] ↓ [329] |
Chronic studies (14d) Beneficial [228] |
| Selenium (180–240 μg/day |
Chronic studies (21 d)Systemic measures ↓ [330] (in overweight only) |
Chronic studies (21–70 d)Systemic measures ↑ [331] ↔ [330] In Skeletal Muscle ↑ [332] ↔ [331] |
Chronic studies (70d) ↔ [331] Attenuated [333] |
N/A |
Chronic (70d) ↔ [331] |
N/A |
| Zinc (20–30 mg/day) |
Chronic studies (6-84d)Systemic measures ↓ [334,335] |
Chronic studies (84d)Systemic measures ↔ [335] |
N/A | N/A |
Chronic studies (7-42d) ↔ [336,337] |
N/A |
(↑ = Increased; ↔ = No effect; ↓ = Decreased; DOMs – delayed onset muscle soreness; CK – creatine kinase; LDH – lactate dehydrogenase; SDH – succinate dehydrogenase; CS – citrate synthase; MAP – mean arterial pressure).
4.1. Polyphenols
Polyphenols, such as those discussed below, have potent free radical scavenging and/or metal chelation effects in vitro [[338], [339], [340], [341], [342], [343], [344]]. However, the in vivo relevance of these mechanisms is debatable due to their (1) generally poor oral bioavailability with transient low accumulation only [[345], [346], [347], [348], [349]]; (2) relatively poor uptake into peripheral tissues like skeletal muscle [[350], [351], [352], [353]]; and (3) competition for ROS/RNS with endogenous antioxidants. Indirect antioxidant mechanisms of polyphenols might include induction of endogenous antioxidant gene expression [[354], [355], [356], [357]] and regulation of ROS/RNS producing enzymes [[358], [359], [360], [361]] and redox-related transcription factors [[362], [363], [364], [365]]. Ingested polyphenols undergo extensive metabolism and modification in the liver and intestines. Metabolites of polyphenols may exert important redox-related effects given their typically much higher concentrations reached in the body compared to parent compounds [[366], [367], [368]]. However, redox-related effects of polyphenol metabolites have generally been poorly studied to date. A recent meta-analysis of RCTs involving predominantly trained males concluded that polyphenol supplementation (average dose 688 mg/day) for at least 7 days can improve exercise performance by ~1.9% [246]. Potential ergogenic mechanisms proposed by the authors include enhanced mitochondrial biogenesis, improved vascular function and blood flow, and enhanced fat oxidation [246]. With few exceptions, polyphenols are believed to be largely safe when consumed at high doses, including within the ranges used in the studies described below [246,[369], [370], [371], [372], [373], [374]].
4.1.1. Anthocyanins
Evidence is limited and mixed in terms of systemic antioxidant effects of anthocyanins (ACNs) in relation to exercise in humans (Table 1). Moreover, antioxidant effects of ACNs have not been clearly explored in skeletal muscle of either humans or animals. Findings are equivocal with respect to benefits of ACN supplementation on endurance performance, VO2max and post-exercise muscle recovery (Table 1). It is possible that underlying mechanisms relating to improved performance following ACN supplementation might include improved endothelial function and alterations in blood flow. Morgan et al. [212] found improved muscle tissue oxygenation at rest after 7 days of supplementation with Montmorency cherry supplementation in trained cyclists; although this did not occur during exercise, despite an improved time trial performance. In another study, 7 days of New Zealand blackcurrant supplementation was found to decrease blood pressure and total peripheral resistance; but increase femoral artery diameter, during submaximal isometric contraction of the knee extensors [213]. Further research is required to better elucidate mechanisms of performance enhancement with ACNs. Studies investigating ACNs with exercise in humans have been limited by small sample sizes, use of predominantly male-only trained participants, and supplementation periods of less than 6 weeks. Furthermore, some studies have used supplements that contain additional polyphenols or other antioxidant compounds, which makes it difficult to clearly isolate the effects of ACNs. Mechanistic insights into skeletal muscle adaptations to training in response to ACNs have not currently been undertaken in humans or animals, requiring future investigation to elucidate any potential effects.
4.1.2. Astaxanthin
Evidence is limited and unclear with respect to benefits of astaxanthin (ASX) supplementation on systemic oxidative stress markers in humans (Table 1). In rodents, ASX has been found to increase skeletal muscle ASX concentration [375,376] and decrease skeletal muscle markers of oxidative stress [[376], [377], [378]]. In one study, ASX hampered exercise-induced expression of Nrf2 and induction of endogenous antioxidant enzymes at higher doses [378]. While evidence in rodent studies shows improved endurance performance after chronic ASX supplementation [375,377,379], a study in athletes [222] found no ergogenic effect after chronic supplementation. A study in rodents [380] reported enhanced fat oxidation rate and sparing of muscle glycogen during exercise, which is suggestive of a possible ergogenic mechanism of action of ASX. Beneficial effects on post-exercise muscle recovery are equivocal in the limited human studies published (Table 1). Overall, studies in rodents implicate endurance performance enhancement with ASX, although with possible hampering of some skeletal muscle training adaptations. Given these findings and a lack of studies in human participants, future research should focus on effects of ASX on endurance performance and training-related adaptations in skeletal muscle of humans.
4.1.3. Catechins
Catechins are naturally found in high concentrations in green tea, red wine, berries, cocoa and chocolate. Evidence is equivocal overall in terms of effects of supplemental catechins on systemic antioxidant effects in relation to exercise in humans (Table 1). However, cocoa flavanols have been shown to produce small positive effects on markers of oxidative stress across untrained and trained participants [226]. Limited human [230] and rodent [381,382] data is equivocal with respect to beneficial effects of catechin supplementation on oxidative stress in skeletal muscle with or without concomitant exercise training. Evidence of improvements in endurance performance is also equivocal in rodent studies after catechin supplementation [[383], [384], [385], [386], [387]], while evidence is not supportive of its ergogenic effects across untrained and trained human participants (Table 1). Catechin supplementation may improve vascular function, although these changes don't appear to translate to improvements in exercise performance. Studies in overweight/obese individuals have found an attenuated blood pressure response to submaximal exercise after acute cocoa flavanol supplementation [231] and a reduced blood pressure after chronic cocoa flavanol supplementation (without additive effects of exercise training) [233]. Studies have also shown increased FMD after acute [231,233] and chronic [233] cocoa flavanol supplementation in overweight/obese humans. A recent meta-analysis concluded that cocoa flavanol supplementation improves FMD, although not exercise performance, across untrained and trained participants [226]. In healthy rats, catechin supplementation (4 mg epicatechin/kg/day for 3 wks) modestly improved mean arterial blood pressure at rest and during exercise, but had no effects on exercise capacity, VO2 peak, exercising skeletal muscle blood flow and vascular conductance, or contraction induced muscle microvascular function [196].
Increased skeletal muscle β-oxidation activity and whole-body fat oxidation (and decreased carbohydrate oxidation) were shown in rodents after catechin supplementation [383]. Human studies appear mixed with regards to substrate oxidation [225,[234], [235], [236],388], although a recent meta-analysis reported significantly decreased RER and increased energy expenditure after catechin supplementation of between 300 and 600 mg/day for 2–12 weeks [389]. Rodent studies appear to indicate improved mitochondrial biogenesis after catechin supplementation [384,385], while studies in humans are limited and mixed [229,230]. Studies on muscle recovery post muscle damaging exercise have also produced mixed findings in humans, and evidence is equivocal (Table 1). Given a potential risk of elevated liver injury with high catechin doses (i.e. >800 mg EGCG/day [390]), a lack of an unclear threshold dose of safety [390], and a lack of convincing exercise-related benefits, green tea-based catechin supplements are potentially undesirable choices of antioxidant supplements for athletes. Modest cocoa flavanol supplementation may, however, be potentially beneficial for overweight/obese athletes or exercisers who want to improve their vascular health.
4.1.4. Curcumin
Investigations into either skeletal muscle-related or systemic antioxidant effects of curcumin are lacking in exercise studies in humans. In rodents, acute and chronic curcumin supplementation has been shown to decrease skeletal muscle markers of oxidative stress [361,362,364,365,391]. Studies investigating effects of curcumin supplementation on endurance performance in humans are lacking. Limited studies in rodents show an improvement in exercise capacity after chronic curcumin supplementation [364,391,392]. Moreover, limited rodent data suggests enhanced mitochondrial biogenesis with curcumin supplementation when combined with exercise training [393]. There is a paucity of studies investigating effects of curcumin on exercise-related vascular function in humans. Curcumin supplementation (150 mg/day for 8 weeks) was found to increase FMD in post-menopausal women to a similar extent as did moderate aerobic exercise training [239]. However, the combined effects of curcumin plus exercise training were not explored in this study [239]. Studies in humans show an attenuated decline in muscle recovery post-muscle damaging eccentric exercise after curcumin supplementation (Table 1), although further investigation is required to confirm these findings. There is a need for further investigation of curcumin supplementation in exercising humans given some potentially promising findings in animals with respect to performance outcomes and mitochondrial biogenesis.
4.1.5. Quercetin
There is currently limited evidence of any systemic changes in oxidative stress markers or antioxidant capacity when quercetin is supplemented along with exercise training in athletes (Table 1). Moreover, limited findings in rodents have not been supportive of improvements in oxidative stress or antioxidant enzyme activation in skeletal muscle when undertaking exercise training [394]. Some evidence shows enhanced markers of mitochondrial biogenesis in muscle after quercetin and exercise training in rodents [394,395]; although effects of quercetin on exercise performance are equivocal in animal studies [394,395]. There appears to be no effect of quercetin supplementation on mitochondrial biogenesis in humans undertaking exercise training [242,243] and effects on exercise performance appear small and inconsistent (Table 1). Published meta-analyses [[245], [246], [247]] have concluded small (0.74%–5%) but significant improvements in endurance exercise performance in human studies involving quercetin supplementation; although effects appear to be largely limited to untrained individuals [247]. Moreover, studies are also currently limited by small sample sizes and use of male participants only. Effects on muscle recovery post muscle-damaging exercise are equivocal in humans (Table 1). There is currently limited convincing evidence to recommend quercetin to trained athletes given a lack of reported improvements in oxidative stress markers along with small, possibly trivial, endurance exercise performance benefits that are limited mostly to untrained individuals.
4.1.6. Resveratrol
Evidence in rodent skeletal muscle has shown improvements in oxidative stress [[396], [397], [398], [399], [400], [401]] and induction of endogenous antioxidant enzymes [396,397,399,400] after resveratrol supplementation was combined with exercise. However, no effects on oxidative stress or antioxidant levels were observed in skeletal muscle of older humans undertaking exercise training [252]. Similarly, while studies in rodents are mostly supportive of endurance performance benefits of resveratrol [398,[401], [402], [403], [404], [405], [406]], limited studies in humans are not currently supportive of its ergogenic effects (Table 1). In humans, findings are equivocal with respect to the impact of resveratrol on exercise-related skeletal muscle mitochondrial adaptations in both younger [253] and older [251,252] individuals. Limited studies have explored the impact of resveratrol on exercise-related vascular function. Gliemann et al. [254] reported that chronic resveratrol supplementation (250 mg/day for 8 weeks) hampered the exercise-training induced blood pressure reduction in older adults. Further exercise studies in humans, including those using more athletic populations and real-world athletic performance tests, are required to clarify the impact of resveratrol. Future studies might also attempt to investigate a possible dose-related impact of resveratrol supplementation, given that no studies in humans involving exercise have utilized high doses of ≥2g/day that result in much higher systemic levels of resveratrol and its metabolites [366,407]. A potential limitation of using higher doses (>2g/day) is an increased risk of adverse effects such as nausea, flatulence, abdominal discomfort, diarrhoea [408].
4.2. Water-soluble antioxidants
4.2.1. Vitamin C
Acute and chronic vitamin C supplementation have been shown to have mixed effects on systemic markers of exercise-related oxidative stress (Table 1). Studies using healthy individuals (without exercise) found no effect of vitamin C supplementation on resting skeletal muscle antioxidant enzyme levels [234,263] or markers of mitochondrial biogenesis [234]. In rodents, vitamin C supplementation was shown to hamper exercise-induced antioxidant enzyme gene expression and mitochondrial biogenesis in skeletal muscle [22]. Interestingly, studies in animal models have tended to favour null [409] or even ergolytic [22,410] endurance-related effects of vitamin C. In humans, there is no convincing evidence of endurance performance enhancement following vitamin C supplementation (Table 1). Findings implicate improvements in vascular function during exercise with acute vitamin C supplementation in older adults; although limited evidence is supportive of benefits in younger adults or with chronic oral supplementation (Table 1). Observed effects of vitamin C on vascular function may be related to an improved NO bioavailability and antioxidant-related mechanisms [411]. Effects of vitamin C supplementation on muscle recovery post muscle-damaging exercise are equivocal in humans (Table 1). Evidence is not currently supportive of any ergogenic effects of vitamin C supplementation in athletes. A lack of clear effects observed could relate to poor direct in vivo redox-modulating effects of vitamin C during exercise [45]. Studies examining the effects of vitamin C supplementation alone on skeletal muscle adaptations to exercise training are lacking, and require further investigation given hampering effects found in rodents; and hampering effects found when vitamin C is combined with vitamin E in some human studies [44,412,413].
4.3. Lipid-soluble antioxidants
4.3.1. Alpha lipoic acid
Limited studies in humans show improvements in systemic markers of oxidative stress and antioxidant capacity following muscle-damaging exercise with short-term ALA supplementation (Table 1). Furthermore, rodent studies show improvements in oxidative stress markers in skeletal muscle following chronic ALA supplementation [[414], [415], [416], [417], [418]], although with mixed effects on endogenous antioxidant levels/activities [414,415,419,420]. Animal studies have shown mixed effects of ALA supplementation on endurance exercise performance [414,415,417,420,421] and mitochondrial biogenesis markers in muscle [414,415,419,420], however, there is currently a lack of evidence on these outcomes in humans. There is also a lack of studies in humans that have investigated vascular function following ALA supplementation without concomitant ingestion of other antioxidants (Table 1). Findings with respect to recovery from muscle damaging exercise are equivocal in humans (Table 1). Current evidence is suggestive of improved exercise-related oxidative stress following ALA supplementation in humans and rodents. However, given a lack of evidence available on effects of ALA on endurance performance and skeletal muscle adaptations in humans, ALA supplementation cannot be recommended at present for athletes.
4.3.2. Coenzyme Q10
Most studies in humans have failed to demonstrate improvements in systemic markers of oxidative stress or antioxidant enzymes after chronic coenzyme Q10 (CoQ10) supplementation with exercise in participants ranging from untrained to highly trained (Table 1). Moreover, limited studies in rodents have found CoQ10 does not alter skeletal muscle markers of oxidative stress or concentrations of endogenous antioxidants when combined with exercise training [422,423]. Effects of exogenous CoQ10 in human skeletal muscle remain unclear, particularly considering that supplementation appears ineffective at increasing muscle CoQ10 concentration [287,288,292,424]. CoQ10 supplementation has beneficial effects on endothelial function [425] which might contribute to functional improvements in patients with cardiovascular diseases. CoQ10 supplementation (300 mg/day for 1 month) in patients with coronary heart disease resulted in improvements in VO2max as well as endothelial-dependent vasodilation and endothelium-bound extracellular SOD activity [282]. CoQ10 (100 mg/day for 1 month) improved VO2peak and endothelium-dependent dilation of the brachial artery in patients with chronic heart failure, particularly when combined with regular exercise training [283]. Supplementation with mitochondrial-targeted CoQ10, MitoQ (10 mg/day for 3 weeks), was shown to have no effect on exercise-training induced improvements in VO2 max or oxidative capacity in healthy young adults [31]. A lack of effect in the latter study might relate to specific exercise-training-related variables (e.g. training intensity and study duration) and/or the targeting of mitochondrial rather than non-mitochondrial sources of ROS. Studies investigating effects of CoQ10 supplementation on endurance performance have produced mixed findings in humans using dosage regimens ranging from 100 to 300 mg/day for 8 days to 6 months (Table 1), with findings suggestive of limited benefit in healthy individuals. Furthermore, most studies are not supportive of beneficial effects of CoQ10 on post-exercise muscle recovery (Table 1). Overall there appears to be no convincing evidence to recommend CoQ10 supplements to enhance endurance performance or post-exercise muscle recovery in athletes.
4.3.3. Vitamin A/β-carotene
The current limited evidence available does not appear to support beneficial effects of chronic supplementation with either preformed vitamin A (e.g. retinol) or β-carotene on systemic markers of exercise-induced oxidative stress [295,296]. In rodents, chronic supplementation with retinyl palmitate in combination with exercise training increased skeletal muscle oxidative stress and decreased exercise-induced induction of endogenous antioxidant enzymes [426]. This finding implicates an impairment in exercise-induced antioxidant adaptations in skeletal muscle with exogenous vitamin A. There is a notable lack of studies investigating effects of vitamin A/β-carotene on either exercise performance or skeletal muscle mitochondrial biogenesis in both humans and animals. Given the overall lack of data in humans along with impairments in some exercise-induced muscle adaptations found in rodents, future research should further investigate effects of vitamin A and β-carotene as stand-alone supplements in human studies involving exercise training.
4.3.4. Vitamin E
Vitamin E supplementation has been shown to decrease systemic markers of exercise-related oxidative stress (Table 1); although increased post-exercise hydroperoxides were reported in one study in triathletes [302]. Vitamin E supplementation also decreased intense eccentric-exercise-induced conjugated dienes in skeletal muscle of humans [300]. Rodent studies indicate hampering effects of chronic vitamin E supplementation on the induction of antioxidant enzymes and mitochondrial biogenesis with exercise training in skeletal muscle [23,427]. Current evidence is equivocal with respect to benefits of vitamin E supplementation on endurance outcomes in humans (Table 1). The most supportive evidence for vitamin E appears to be for performance outcomes at altitude in trained participants [303,304]. Acute vitamin E supplementation was also found to improve endurance exercise-induced acetylcholine-mediated vasodilation in aortas of healthy rats; although chronic supplementation with vitamin E had no additional effects over exercise training alone on vascular function [428]. There is a lack of studies investigating effects of vitamin E (in the absence of other additional antioxidants) on exercise-related vascular function in humans. Evidence for beneficial effects of vitamin E supplementation on muscle recovery post muscle damaging exercise are lacking (Table 1). Despite a considerable number of studies investigating effects of combined vitamin C and E on skeletal muscle exercise adaptations (see below), there is an absence of studies investigating vitamin E alone on skeletal muscle adaptations to exercise training in humans. Given the limited evidence of ergogenic effects of vitamin E for athletes, potentialsafety risks associated with commonly consumed doses [429,430], and hampering effects shown in animal studies on exercise adaptations in muscle; there is currently no clear basis for recommending vitamin E supplements for athletes.
4.4. Both water- and lipid-soluble antioxidants
4.4.1. Melatonin
Evidence from acute and chronic supplementation studies in humans shows that melatonin is beneficial in reducing systemic exercise-induced oxidative stress and improving antioxidant capacity in athletes (Table 1). Furthermore, studies in rodents have shown acute and chronic melatonin supplementation to be effective at attenuating acute exercise-associated oxidative stress in muscle [[431], [432], [433]] and improving endogenous antioxidant concentrations [[432], [433], [434]]. However, no evidence currently exists on antioxidant effects of melatonin in skeletal muscle of humans with exercise. There is evidence to support improved endurance performance outcomes in rodent studies [[435], [436], [437], [438]], which might be related to increased fat oxidation and glycogen conservation [437,439]. Findings are mixed in rodents with respect to effects of melatonin on skeletal muscle markers of exercise-induced mitochondrial biogenesis [437,438]. Studies using acute melatonin supplementation have failed to report benefits on time trial performance in human participants [314,315]. However, overall, there is a paucity of studies that have investigated the effects of melatonin on exercise performance, mitochondrial adaptations in muscle and muscle recovery outcomes in humans (Table 1). Studies are also lacking with respect to effects of melatonin on exercise-related vascular function, although one study in humans found a post-exercise systolic hypotensive effect after acute melatonin (2.5 mg) ingestion and intermittent exercise under modest heat stress [313]. Given the demonstrated antioxidant effects of melatonin in human and animal studies with exercise, future investigations should investigate potential ergogenic effects of chronic melatonin supplementation in athletes, along with any effects on skeletal muscle exercise adaptations in humans.
4.4.2. N-acetyl cysteine
N-acetyl cysteine (NAC) infusion was found to increase muscle GSH during fatiguing exercise in endurance-trained humans [316] and decrease GSSG while increasing post-exercise GSH/GSSG in muscle of healthy participants [19]. However, exercise-induced gene expression of SOD2 was attenuated in skeletal muscle with NAC infusion in healthy participants [317]. Despite these findings using infusion of NAC, there is a lack of evidence currently in human skeletal muscle using oral NAC supplementation. Acute NAC infusion was found to decrease mean arterial pressure during low intensity exercise in both active and sedentary older adults; although it had no effect during exercise in sedentary young adults [318]. In the same study, NAC infusion had no effect on exercise leg blood flow or vascular conductance in either young or older adults [318]. Acute oral NAC (70 mg/kg) also had no effect on brachial artery blood flow or tissue oxygenation indices during a sustained intense hand grip exercise in young healthy adults [319]. Study findings in humans tend to favour an improvement in exercise performance following acute or chronic NAC supplementation (Table 1). Thus, NAC might be a beneficial supplement for endurance athletes. However, at least a few factors may limit the practical efficacy of NAC supplementation in athletes. Firstly, studies using performance tasks characteristic of real-world athletic events have yielded mixed results using NAC supplementation [[327], [328], [329]]. Second, NAC is not well tolerated at high doses, with potential dose-related side effects that mostly include gastrointestinal disturbance and metallic taste sensation; while anaphylactoid reactions have been reported rarely. Findings of Ferreira et al. [440] implicate an acute oral dose of 70 mg/kg as a threshold of intake to avoid adverse effects in individuals undertaking fatiguing exercise. However, it should be noted that there is evidence of improved tolerance with new flavoured effervescent formulations [441]. Thirdly, according to the 2020 World Anti-Doping Agency (WADA) Prohibited List, methods of intravenous infusion >100 ml per 12 h period are banned [442], therefore potentially restricting intravenous NAC use in athletes.
4.4.3. Combined vitamin C and E
Studies using combined doses of 500 mg/day vitamin C and 400 IU/day E have shown no negative effects on the adaptive responses of skeletal muscle to endurance training in humans, such as increased mitochondrial proteins and antioxidant enzymes [[443], [444], [445]]. However, there are some human studies that have reported blunting of skeletal muscle adaptations to endurance training using 1 g/day vitamin C in combination with vitamin E [44,412,413]. Ristow et al. [44] reported 1 g/day vitamin C in combination with 400 IU/day vitamin E in humans attenuated mRNA responses in several markers of mitochondrial biogenesis and antioxidant enzymes following endurance training. Paulsen et al. [413] observed that following training in humans, the increase in COX IV protein abundance and the cytosolic (but not whole cell) levels of PGC-1α are attenuated with 1 g/day vitamin C combined with 260 IU/day E. Our group found that 1 g/day vitamin C in combination with 400 IU/day vitamin E in humans attenuates the increase in skeletal muscle TFAM protein abundance and SOD enzyme activity [412]. However, there were other skeletal muscle adaptations that were not attenuated, such as increased CS activity [412]. Collectively, there appears to be convincing evidence that 1 g/day vitamin C in combination with vitamin E in humans hampers some skeletal muscle adaptations to endurance training. Despite these effects, there is no evidence from these studies that combined 1 g/day vitamin C and vitamin E supplementation prevents improvements in VO2 max or endurance performance in humans. A recent systematic review appears to confirm an absence of performance-modulating effects of vitamin C and/or E on these outcomes in adults [446].
A combination of 500 mg/day vitamin C and 1200 IU/day vitamin E was found to enhance the rate of recovery of maximal knee extensor voluntary isometric contraction force after intense repetitive fatiguing eccentric knee extension exercises in humans [447]. In contrast, another study in young healthy adults found that chronic supplementation of combined 1g/day vitamin C and 400 IU/day vitamin E did not alter recovery of muscle function or muscle damage markers following an acute damaging exercise bout in trained or untrained individuals [448]. There is currently a lack of clear evidence to support the use of combined vitamin C and vitamin E for athletic performance. A lack of clear effects observed could relate to poor direct in vivo redox-modulating effects of vitamin C and vitamin E during exercise [45]. However, given some potential hampering effects on skeletal muscle adaptations, it might be advisable to avoid this supplementation strategy during extended periods of training that induce adaptations.
4.5. Antioxidant minerals
4.5.1. Selenium
Limited studies in humans have shown decreased exercise-related serum lipid peroxidation markers in overweight participants with low selenium (Se) levels [330]. Effects of Se supplementation on antioxidant concentrations are mixed in humans (Table 1). There have only been limited investigations of Se supplementation on exercise performance in humans and animals, with no beneficial effects shown in humans [331]. Studies investigating effects of Se supplementation in combination with exercise training on mitochondrial biogenesis have shown mixed findings in humans (Table 1). A recent systematic review that investigated 5 studies of Se supplementation on exercise effects concluded that there is some evidence that Se supplementation may blunt some muscle mitochondrial adaptations to exercise; although Se supplementation might also decrease post-exercise (systemic) oxidative stress in overweight individuals with lower Se levels [337]. The systematic review also concluded that there is a current lack of studies that have looked at clear effects of Se supplementation on performance outcomes. Thus, at present evidence is not supportive of Se supplementation in athletes. There is currently limited evidence available on effects of selenium supplementation with exercise in humans. However, given the potential of hampering of some exercise adaptations along with possible safety issues at high oral doses [449,450], Se supplementation is not currently recommended for athletes.
4.5.2. Zinc
Limited evidence available in humans shows some beneficial effects of zinc (Zn) supplementation on systemic markers of exercise-induced oxidative stress (Table 1). Zn supplementation in rodents (injected IP) was found to increase GSH concentration but have no effect on muscle lipid peroxidation in skeletal muscle [451]. There have been limited studies of Zn supplementation on endurance performance in humans (Table 1). A recent systematic review [337] summarized 9 studies involving Zn supplementation and athletic performance. The review concluded inconsistent findings linking Zn supplementation to changes in VO2 max and athletic performance. It was concluded in the review that it is unlikely that Zn as a standalone supplement will contribute to improved athletic performance. It should be noted however, that few studies have directly assessed the effects of Zn supplements on athletic performance outcomes without the concomitant intake of other nutrients or antioxidant compounds. There is currently limited supportive evidence to recommend Zn supplementation in athletes.
4.6. Personalised antioxidant supplementation
Based on our discussion it is apparent that there is currently limited supportive evidence for the use of antioxidant supplements in athletes and otherwise healthy exercisers to enhance recovery or performance. However, there may be circumstances relevant to an individual that may warrant targeted antioxidant supplementation approaches. If an athlete is deficient in a particular micronutrient (e.g. vitamin E, C, zinc or selenium), then supplementation might be appropriate to restore redox homeostasis and subsequent exercise capacity [452]. A recent study found that NAC supplementation (1200 mg twice daily for 30 days) improved glutathione status, VO2 max and exercise performance in individuals with low (but not moderate or high) baseline erythrocyte glutathione concentrations [453]. Along with these restorative functional improvements, decreases in markers of oxidative stress and increases in some markers of antioxidant activity were also reported [453]. Further work needs to be done in this area, and a detailed discussion of personalised antioxidant strategies is beyond the scope of this review. However, interested readers are directed to a recent review on this topic [454].
4.7. Summary
The current review paper has provided a discussion on endurance exercise-related redox signalling and the subsequent adaptations in skeletal muscle and vascular function. It has also summarized the impact of supplementation with diverse antioxidant compounds on these exercise-related outcomes, in addition to endurance performance. Overall, there is a lack of supportive evidence for most compounds reviewed to recommend to athletes (Table 2). A lack of effects for some antioxidants reviewed might relate to their inability to exert direct and potent redox-modulating effects in vivo; while for others, evidence on performance-related outcomes and knowledge of their redox-modulating effects in vivo is currently scarce.
Table 2.
Summary and recommendations regarding antioxidant supplementation for people undertaking endurance training.
| Antioxidant compound | Evidence summary – exercise-related effects |
|---|---|
| Anthocyanins |
|
| Astaxanthin |
|
| Catechins |
|
| Curcumin |
|
| Quercetin |
|
| Resveratrol |
|
| Vitamin C |
|
| Alpha-lipoic acid |
|
| Coenzyme Q10 |
|
| Vitamin A/β-carotene |
|
| Vitamin E |
|
| Melatonin |
|
| N-acetylcysteine |
|
| Vitamin C + E |
|
| Selenium |
|
| Zinc |
|
Declaration of competing interest
The authors have none to declare.
Acknowledgements
Dr Parker was supported by a NHMRC/National Heart Foundation Early Career Fellowship (APP1157930). Dr Trewin was supported by a Faculty of Health Dean's Postdoctoral Research Fellowship, Deakin University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101471.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Alessio H.M., Goldfarb A.H., Cutler R.G. MDA content increases in fast- and slow-twitch skeletal muscle with intensity of exercise in a rat. Am. J. Physiol. 1988;255(6 Pt 1):C874–C877. doi: 10.1152/ajpcell.1988.255.6.C874. [DOI] [PubMed] [Google Scholar]
- 2.Davies K.J. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982;107(4):1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 3.Reid M.B. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J. Appl. Physiol. 1985;73(5):1797–1804. doi: 10.1152/jappl.1992.73.5.1797. 1992. [DOI] [PubMed] [Google Scholar]
- 4.Reid M.B. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J. Appl. Physiol. 1985;73(5):1805–1809. doi: 10.1152/jappl.1992.73.5.1805. 1992. [DOI] [PubMed] [Google Scholar]
- 5.Jackson M.J., Edwards R.H., Symons M.C. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim. Biophys. Acta. 1985;847(2):185–190. doi: 10.1016/0167-4889(85)90019-9. [DOI] [PubMed] [Google Scholar]
- 6.McArdle A. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am. J. Physiol. Cell Physiol. 2001;280(3):C621–C627. doi: 10.1152/ajpcell.2001.280.3.C621. [DOI] [PubMed] [Google Scholar]
- 7.Silveira L.R. Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic. Biol. Med. 2003;35(5):455–464. doi: 10.1016/s0891-5849(03)00271-5. [DOI] [PubMed] [Google Scholar]
- 8.Pattwell D.M. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic. Biol. Med. 2004;37(7):1064–1072. doi: 10.1016/j.freeradbiomed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 9.Forman H.J., Davies K.J., Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Cabrera M.C. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic. Biol. Med. 2015;86:37–46. doi: 10.1016/j.freeradbiomed.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Nordberg J., Arner E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31(11):1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 12.Vendrov A.E. Antioxid Redox Signal; 2015. NOX4 NADPH Oxidase-dependent Mitochondrial Oxidative Stress in Aging-Associated Cardiovascular Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown D.I., Griendling K.K. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 2015;116(3):531–549. doi: 10.1161/CIRCRESAHA.116.303584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youn J.Y. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes. 2014;63(7):2344–2355. doi: 10.2337/db13-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folli F. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011;7(5):313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- 16.Powers S.K., Talbert E.E., Adhihetty P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 2011;589(Pt 9):2129–2138. doi: 10.1113/jphysiol.2010.201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biswas S., Chida A.S., Rahman I. Redox modifications of protein–thiols: emerging roles in cell signaling. Biochem. Pharmacol. 2006;71(5):551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 18.Merry T.L., McConell G.K. Do reactive oxygen species regulate skeletal muscle glucose uptake during contraction? Exerc. Sport Sci. Rev. 2012;40(2):102–105. doi: 10.1097/JES.0b013e318245837b. [DOI] [PubMed] [Google Scholar]
- 19.Trewin A.J. Effect of N-acetylcysteine infusion on exercise-induced modulation of insulin sensitivity and signaling pathways in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2015;309(4):E388–E397. doi: 10.1152/ajpendo.00605.2014. [DOI] [PubMed] [Google Scholar]
- 20.Alessio H.M., Goldfarb A.H. Lipid peroxidation and scavenger enzymes during exercise: adaptive response to training. J. Appl. Physiol. 1985;64(4):1333–1336. doi: 10.1152/jappl.1988.64.4.1333. 1988. [DOI] [PubMed] [Google Scholar]
- 21.Vincent H.K. Short-term exercise training improves diaphragm antioxidant capacity and endurance. Eur. J. Appl. Physiol. 2000;81(1–2):67–74. doi: 10.1007/PL00013799. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Cabrera M.C. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008;87(1):142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 23.Strobel N.A. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2011;43(6):1017–1024. doi: 10.1249/MSS.0b013e318203afa3. [DOI] [PubMed] [Google Scholar]
- 24.Paulsen G. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J. Physiol. 2014;592(Pt 8):1887–1901. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid M.B., Moody M.R. Dimethyl sulfoxide depresses skeletal muscle contractility. J. Appl. Physiol. 1985;76(5):2186–2190. doi: 10.1152/jappl.1994.76.5.2186. 1994. [DOI] [PubMed] [Google Scholar]
- 26.Paulsen G. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J. Physiol. 2014;592(Pt 24):5391–5408. doi: 10.1113/jphysiol.2014.279950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson M.J. Redox regulation of adaptive responses in skeletal muscle to contractile activity. Free Radic. Biol. Med. 2009;47(9):1267–1275. doi: 10.1016/j.freeradbiomed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Richardson R.S. Exercise-induced brachial artery vasodilation: role of free radicals. Am. J. Physiol. Heart Circ. Physiol. 2007;292(3):H1516–H1522. doi: 10.1152/ajpheart.01045.2006. [DOI] [PubMed] [Google Scholar]
- 29.Donato A.J. Exercise-induced brachial artery vasodilation: effects of antioxidants and exercise training in elderly men. Am. J. Physiol. Heart Circ. Physiol. 2010;298(2):H671–H678. doi: 10.1152/ajpheart.00761.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai H. Induction of endothelial NO synthase by hydrogen peroxide via a Ca(2+)/calmodulin-dependent protein kinase II/janus kinase 2-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2001;21(10):1571–1576. doi: 10.1161/hq1001.097028. [DOI] [PubMed] [Google Scholar]
- 31.Shill D.D. Mitochondria-specific antioxidant supplementation does not influence endurance exercise training-induced adaptations in circulating angiogenic cells, skeletal muscle oxidative capacity or maximal oxygen uptake. J. Physiol. 2016;594(23):7005–7014. doi: 10.1113/JP272491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mason S., Wadley G.D. Skeletal muscle reactive oxygen species: a target of good cop/bad cop for exercise and disease. Redox Rep. 2014;19(3):97–106. doi: 10.1179/1351000213Y.0000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winterbourn C.C., Hampton M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008;45(5):549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Braun H. Dietary supplement use among elite young German athletes. Int. J. Sport Nutr. Exerc. Metabol. 2009;19(1):97–109. doi: 10.1123/ijsnem.19.1.97. [DOI] [PubMed] [Google Scholar]
- 35.Petroczi A. Nutritional supplement use by elite young UK athletes: fallacies of advice regarding efficacy. J Int Soc Sports Nutr. 2008;5:22. doi: 10.1186/1550-2783-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jager R., Purpura M., Kerksick C.M. Eight weeks of a high dose of curcumin supplementation may attenuate performance decrements following muscle-damaging exercise. Nutrients. 2019;11(7) doi: 10.3390/nu11071692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicol L.M. Curcumin supplementation likely attenuates delayed onset muscle soreness (DOMS) Eur. J. Appl. Physiol. 2015;115(8):1769–1777. doi: 10.1007/s00421-015-3152-6. [DOI] [PubMed] [Google Scholar]
- 38.Tanabe Y. Attenuation of indirect markers of eccentric exercise-induced muscle damage by curcumin. Eur. J. Appl. Physiol. 2015;115(9):1949–1957. doi: 10.1007/s00421-015-3170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson D. Muscle soreness and damage parameters after prolonged intermittent shuttle-running following acute vitamin C supplementation. Int. J. Sports Med. 2001;22(1):68–75. doi: 10.1055/s-2001-11358. [DOI] [PubMed] [Google Scholar]
- 40.Reid M.B. N-acetylcysteine inhibits muscle fatigue in humans. J. Clin. Invest. 1994;94(6):2468–2474. doi: 10.1172/JCI117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powers S.K., Jackson M.J. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008;88(4):1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamb G.D., Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J. Physiol. 2011;589(Pt 9):2119–2127. doi: 10.1113/jphysiol.2010.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrison D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015;89:852–862. doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 44.Ristow M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. U. S. A. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cobley J.N. Influence of vitamin C and vitamin E on redox signaling: implications for exercise adaptations. Free Radic. Biol. Med. 2015;84:65–76. doi: 10.1016/j.freeradbiomed.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 46.Lo Conte M., Carroll K.S. The redox biochemistry of protein sulfenylation and sulfinylation. J. Biol. Chem. 2013;288(37):26480–26488. doi: 10.1074/jbc.R113.467738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marozkina N.V., Gaston B. S-Nitrosylation signaling regulates cellular protein interactions. Biochim. Biophys. Acta. 2012;1820(6):722–729. doi: 10.1016/j.bbagen.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dalle-Donne I. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem. Sci. 2009;34(2):85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Sun J. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc. Natl. Acad. Sci. U. S. A. 2001;98(20):11158–11162. doi: 10.1073/pnas.201289098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellinger A.M. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 2009;15(3):325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yasukawa T. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J. Biol. Chem. 2005;280(9):7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 52.Carvalho-Filho M.A. S-nitrosation of the insulin receptor, insulin receptor substrate 1, and protein kinase B/Akt: a novel mechanism of insulin resistance. Diabetes. 2005;54(4):959–967. doi: 10.2337/diabetes.54.4.959. [DOI] [PubMed] [Google Scholar]
- 53.Nogueira L. Myosin is reversibly inhibited by S-nitrosylation. Biochem. J. 2009;424(2):221–231. doi: 10.1042/BJ20091144. [DOI] [PubMed] [Google Scholar]
- 54.Aquilano K., Baldelli S., Ciriolo M.R. Nuclear recruitment of neuronal nitric-oxide synthase by alpha-syntrophin is crucial for the induction of mitochondrial biogenesis. J. Biol. Chem. 2014;289(1):365–378. doi: 10.1074/jbc.M113.506733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samengo G. Age-related loss of nitric oxide synthase in skeletal muscle causes reductions in calpain S-nitrosylation that increase myofibril degradation and sarcopenia. Aging Cell. 2012;11(6):1036–1045. doi: 10.1111/acel.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salanova M., Schiffl G., Blottner D. Atypical fast SERCA1a protein expression in slow myofibers and differential S-nitrosylation prevented by exercise during long term bed rest. Histochem. Cell Biol. 2009;132(4):383–394. doi: 10.1007/s00418-009-0624-y. [DOI] [PubMed] [Google Scholar]
- 57.Salanova M. Nitrosative stress in human skeletal muscle attenuated by exercise countermeasure after chronic disuse. Redox Biol. 2013;1:514–526. doi: 10.1016/j.redox.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colussi C. HDAC2 blockade by nitric oxide and histone deacetylase inhibitors reveals a common target in Duchenne muscular dystrophy treatment. Proc. Natl. Acad. Sci. U. S. A. 2008;105(49):19183–19187. doi: 10.1073/pnas.0805514105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Humphries K.M., Deal M.S., Taylor S.S. Enhanced dephosphorylation of cAMP-dependent protein kinase by oxidation and thiol modification. J. Biol. Chem. 2005;280(4):2750–2758. doi: 10.1074/jbc.M410242200. [DOI] [PubMed] [Google Scholar]
- 60.Reddy S. Inactivation of creatine kinase by S-glutathionylation of the active-site cysteine residue. Biochem. J. 2000;347 Pt 3:821–827. [PMC free article] [PubMed] [Google Scholar]
- 61.Anselmo A.N., Cobb M.H. Protein kinase function and glutathionylation. Biochem. J. 2004;381(Pt 3):e1–2. doi: 10.1042/BJ20040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cruz C.M. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 2007;282(5):2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zmijewski J.W. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J. Biol. Chem. 2010;285(43):33154–33164. doi: 10.1074/jbc.M110.143685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salsman S.J., Hensley K., Floyd R.A. Sensitivity of protein tyrosine phosphatase activity to the redox environment, cytochrome C, and microperoxidase. Antioxidants Redox Signal. 2005;7(7–8):1078–1088. doi: 10.1089/ars.2005.7.1078. [DOI] [PubMed] [Google Scholar]
- 65.Pastore A., Piemonte F. S-Glutathionylation signaling in cell biology: progress and prospects. Eur. J. Pharmaceut. Sci. 2012;46(5):279–292. doi: 10.1016/j.ejps.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Bindoli A., Fukuto J.M., Forman H.J. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxidants Redox Signal. 2008;10(9):1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Levonen A.-L. Redox regulation of antioxidants, autophagy, and the response to stress: implications for electrophile therapeutics. Free Radic. Biol. Med. 2014;71:196–207. doi: 10.1016/j.freeradbiomed.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janssen-Heininger Y.M. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goncalves R.L. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem. 2015;290(1):209–227. doi: 10.1074/jbc.M114.619072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakellariou G.K. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxidants Redox Signal. 2013;18(6):603–621. doi: 10.1089/ars.2012.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharaf M.S., Stevens D., Kamunde C. Mitochondrial transition ROS spike (mTRS) results from coordinated activities of complex I and nicotinamide nucleotide transhydrogenase. Biochim. Biophys. Acta Bioenerg. 2017;1858(12):955–965. doi: 10.1016/j.bbabio.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 72.Wang Z. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am. J. Clin. Nutr. 2010;92(6):1369–1377. doi: 10.3945/ajcn.2010.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robb E.L. Control of mitochondrial superoxide production by reverse electron transport at complex I. J. Biol. Chem. 2018;293(25):9869–9879. doi: 10.1074/jbc.RA118.003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Onukwufor J.O., Berry B.J., Wojtovich A.P. Physiologic implications of reactive oxygen species production by mitochondrial complex I reverse electron transport. Antioxidants. 2019;8(8) doi: 10.3390/antiox8080285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berry B.J. Use the protonmotive force: mitochondrial uncoupling and reactive oxygen species. J. Mol. Biol. 2018;430(21):3873–3891. doi: 10.1016/j.jmb.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong H.S. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J. Biol. Chem. 2017;292(41):16804–16809. doi: 10.1074/jbc.R117.789271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferreira L.F., Laitano O. Regulation of NADPH oxidases in skeletal muscle. Free Radic. Biol. Med. 2016;98:18–28. doi: 10.1016/j.freeradbiomed.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong H.S., Benoit B., Brand M.D. Mitochondrial and cytosolic sources of hydrogen peroxide in resting C2C12 myoblasts. Free Radic. Biol. Med. 2019;130:140–150. doi: 10.1016/j.freeradbiomed.2018.10.448. [DOI] [PubMed] [Google Scholar]
- 79.Goncalves R.L.S. The use of site-specific suppressors to measure the relative contributions of different mitochondrial sites to skeletal muscle superoxide and hydrogen peroxide production. Redox Biol. 2019;28:101341. doi: 10.1016/j.redox.2019.101341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dey S., Sidor A., O'Rourke B. Compartment-specific control of reactive oxygen species scavenging by antioxidant pathway enzymes. J. Biol. Chem. 2016;291(21):11185–11197. doi: 10.1074/jbc.M116.726968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rhee S.G. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 2005;17(2):183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 82.Munro D. The thioredoxin and glutathione-dependent H2O2 consumption pathways in muscle mitochondria: involvement in H2O2 metabolism and consequence to H2O2 efflux assays. Free Radic. Biol. Med. 2016;96:334–346. doi: 10.1016/j.freeradbiomed.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 83.Hochachka P.W., McClelland G.B. Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J. Exp. Biol. 1997;200(Pt 2):381–386. doi: 10.1242/jeb.200.2.381. [DOI] [PubMed] [Google Scholar]
- 84.Wilson D.F. Oxidative phosphorylation: unique regulatory mechanism and role in metabolic homeostasis. J. Appl. Physiol. 1985;122(3):611–619. doi: 10.1152/japplphysiol.00715.2016. 2017. [DOI] [PubMed] [Google Scholar]
- 85.Trewin A.J. Acute HIIE elicits similar changes in human skeletal muscle mitochondrial H2O2 release, respiration and cell signaling as endurance exercise even with less work. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315(5):R1003–R1016. doi: 10.1152/ajpregu.00096.2018. [DOI] [PubMed] [Google Scholar]
- 86.Kamunde C., Sharaf M., MacDonald N. H2O2 metabolism in liver and heart mitochondria: low emitting-high scavenging and high emitting-low scavenging systems. Free Radic. Biol. Med. 2018;124:135–148. doi: 10.1016/j.freeradbiomed.2018.05.064. [DOI] [PubMed] [Google Scholar]
- 87.Treberg J.R. Differentiating between apparent and actual rates of H2O2 metabolism by isolated rat muscle mitochondria to test a simple model of mitochondria as regulators of H2O2 concentration. Redox Biol. 2015;5:216–224. doi: 10.1016/j.redox.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bailey D.M. Regulation of free radical outflow from an isolated muscle bed in exercising humans. Am. J. Physiol. Heart Circ. Physiol. 2004;287(4):H1689–H1699. doi: 10.1152/ajpheart.00148.2004. [DOI] [PubMed] [Google Scholar]
- 89.Henriquez-Olguin C. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat. Commun. 2019;10(1):4623. doi: 10.1038/s41467-019-12523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wadley G.D. Xanthine oxidase inhibition attenuates skeletal muscle signaling following acute exercise but does not impair mitochondrial adaptations to endurance training. American Journal of Physiology-Endocrinology and Metabolism. 2013;304(8):E853–E862. doi: 10.1152/ajpendo.00568.2012. [DOI] [PubMed] [Google Scholar]
- 91.Veskoukis A. Effects of xanthine oxidase inhibition on oxidative stress and swimming performance in rats. Appl. Physiol. Nutr. Metabol. 2008;33(6):1140–1154. doi: 10.1139/H08-102. [DOI] [PubMed] [Google Scholar]
- 92.Brown G.C., Borutaite V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion. 2012;12(1):1–4. doi: 10.1016/j.mito.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 93.Gomez-Cabrera M.C. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. 2005;567(Pt 1):113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zuo L. Lipoxygenase-dependent superoxide release in skeletal muscle. J. Appl. Physiol. 2004;97(2):661–668. doi: 10.1152/japplphysiol.00096.2004. [DOI] [PubMed] [Google Scholar]
- 95.Giorgio M. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122(2):221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 96.Quinlan C.L. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. J. Biol. Chem. 2014;289(12):8312–8325. doi: 10.1074/jbc.M113.545301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson D.F. Regulation of metabolism: the work-to-rest transition in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2016;310(8):E633–e642. doi: 10.1152/ajpendo.00512.2015. [DOI] [PubMed] [Google Scholar]
- 98.Kiens B., Richter E.A. Utilization of skeletal muscle triacylglycerol during postexercise recovery in humans. Am. J. Physiol. Endocrinol. Metabol. 1998;275(2):E332–E337. doi: 10.1152/ajpendo.1998.275.2.E332. [DOI] [PubMed] [Google Scholar]
- 99.Schönfeld P., Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic. Biol. Med. 2008;45(3):231–241. doi: 10.1016/j.freeradbiomed.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 100.Perevoshchikova I.V. Sites of superoxide and hydrogen peroxide production during fatty acid oxidation in rat skeletal muscle mitochondria. Free Radic. Biol. Med. 2013;61:298–309. doi: 10.1016/j.freeradbiomed.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Buford T.W. Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl. Physiol. Nutr. Metabol. 2009;34(4):745–753. doi: 10.1139/H09-067. [DOI] [PubMed] [Google Scholar]
- 102.Childs A. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic. Biol. Med. 2001;31(6):745–753. doi: 10.1016/s0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 103.Steensberg A. IL-6 and TNF-α expression in, and release from, contracting human skeletal muscle. American Journal of Physiology-Endocrinology and Metabolism. 2002;283(6):E1272–E1278. doi: 10.1152/ajpendo.00255.2002. [DOI] [PubMed] [Google Scholar]
- 104.Flohé L. Redox regulation of NF-kappa B activation. Free Radic. Biol. Med. 1997;22(6):1115–1126. doi: 10.1016/s0891-5849(96)00501-1. [DOI] [PubMed] [Google Scholar]
- 105.Stram A.R., Payne R.M. Post-translational modifications in mitochondria: protein signaling in the powerhouse. Cell. Mol. Life Sci. 2016;73(21):4063–4073. doi: 10.1007/s00018-016-2280-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dröse S., Brandt U., Wittig I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim. Biophys. Acta Protein Proteonomics. 2014;1844(8):1344–1354. doi: 10.1016/j.bbapap.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 107.Engelhard J. In situ kinetic trapping reveals a fingerprint of reversible protein thiol oxidation in the mitochondrial matrix. Free Radic. Biol. Med. 2011;50(10):1234–1241. doi: 10.1016/j.freeradbiomed.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 108.Kramer P.A. Fatiguing contractions increase protein S-glutathionylation occupancy in mouse skeletal muscle. Redox Biol. 2018;17:367–376. doi: 10.1016/j.redox.2018.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wadley A.J., Aldred S., Coles S.J. An unexplored role for Peroxiredoxin in exercise-induced redox signalling? Redox Biol. 2016;8:51–58. doi: 10.1016/j.redox.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Picard M. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J. Appl. Physiol. 2013;115(10):1562–1571. doi: 10.1152/japplphysiol.00819.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trewin A.J., Berry B.J., Wojtovich A.P. Exercise and mitochondrial dynamics: keeping in shape with ROS and AMPK. Antioxidants. 2018;7(1) doi: 10.3390/antiox7010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fan X., Hussien R., Brooks G.A. H 2 O 2-induced mitochondrial fragmentation in C 2 C 12 myocytes. Free Radic. Biol. Med. 2010;49(11):1646–1654. doi: 10.1016/j.freeradbiomed.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mailloux R.J., Jin X., Willmore W.G. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox biology. 2014;2:123–139. doi: 10.1016/j.redox.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lazarou M. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang J., Ney P.A. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009;16(7):939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kubli D.A. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2008;295(5):H2025–H2031. doi: 10.1152/ajpheart.00552.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jung T., Höhn A., Grune T. The proteasome and the degradation of oxidized proteins: Part II–protein oxidation and proteasomal degradation. Redox biology. 2014;2:99–104. doi: 10.1016/j.redox.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brigelius-Flohé R., Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxidants Redox Signal. 2011;15(8):2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pilegaard H. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am. J. Physiol. Endocrinol. Metabol. 2000;279(4):E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- 120.Perry C.G. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J. Physiol. 2010;588(23):4795–4810. doi: 10.1113/jphysiol.2010.199448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fernandez-Marcos P.J., Auwerx J. Regulation of PGC-1α, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 2011;93(4):884S–890S. doi: 10.3945/ajcn.110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Akimoto T. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway. J. Biol. Chem. 2005;280(20):19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 123.Wu Z. Transducer of regulated CREB-binding proteins (TORCs) induce PGC-1α transcription and mitochondrial biogenesis in muscle cells. Proc. Natl. Acad. Sci. Unit. States Am. 2006;103(39):14379–14384. doi: 10.1073/pnas.0606714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garnier A. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. Faseb. J. 2005;19(1):43–52. doi: 10.1096/fj.04-2173com. [DOI] [PubMed] [Google Scholar]
- 125.Piantadosi C.A., Suliman H.B. Redox regulation of mitochondrial biogenesis. Free Radic. Biol. Med. 2012;53(11):2043–2053. doi: 10.1016/j.freeradbiomed.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miranda S. Oxidative stress and upregulation of mitochondrial biogenesis genes in mitochondrial DNA-depleted HeLa cells. Biochem. Biophys. Res. Commun. 1999;258(1):44–49. doi: 10.1006/bbrc.1999.0580. [DOI] [PubMed] [Google Scholar]
- 127.Irrcher I., Ljubicic V., Hood D.A. Interactions between ROS and AMP kinase activity in the regulation of PGC-1 transcription in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2009;296(1):C116. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 128.Hinchy E.C. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J. Biol. Chem. 2018;293(44):17208–17217. doi: 10.1074/jbc.RA118.002579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Trewin A.J. Mitochondrial reactive oxygen species generated at the complex-II matrix or intermembrane space microdomain have distinct effects on redox signaling and stress sensitivity in Caenorhabditis elegans. Antioxidants Redox Signal. 2019;31(9):594–607. doi: 10.1089/ars.2018.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kang C. Exercise activation of muscle peroxisome proliferator-activated receptor-[gamma] coactivator-1 [alpha] signaling is redox sensitive. Free Radic. Biol. Med. 2009;47(10):1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 131.Strobel N.A. Altering the redox state of skeletal muscle by glutathione depletion increases the exercise‐activation of PGC‐1α. Physiological reports. 2014;2(12):e12224. doi: 10.14814/phy2.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Aquilano K. p53 orchestrates the PGC-1α-mediated antioxidant response upon mild redox and metabolic imbalance. Antioxidants Redox Signal. 2013;18(4):386–399. doi: 10.1089/ars.2012.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Irrcher I., Ljubicic V., Hood D.A. Interactions between ROS and AMP kinase activity in the regulation of PGC-1{alpha} transcription in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2009;296(1):C116–C123. doi: 10.1152/ajpcell.00267.2007. [DOI] [PubMed] [Google Scholar]
- 134.Sandstrom M.E. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J. Physiol. 2006;575(Pt 1):251–262. doi: 10.1113/jphysiol.2006.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wadley G.D. Xanthine oxidase inhibition attenuates skeletal muscle signaling following acute exercise but does not impair mitochondrial adaptations to endurance training. Am. J. Physiol. Endocrinol. Metabol. 2013;304(8):E853–E862. doi: 10.1152/ajpendo.00568.2012. [DOI] [PubMed] [Google Scholar]
- 136.St-Pierre J. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 137.Ji L.L., Gomez-Cabrera M.C., Vina J. Role of nuclear factor kappaB and mitogen-activated protein kinase signaling in exercise-induced antioxidant enzyme adaptation. Appl. Physiol. Nutr. Metabol. 2007;32(5):930–935. doi: 10.1139/H07-098. [DOI] [PubMed] [Google Scholar]
- 138.Merry T.L., Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016;594(18):5135–5147. doi: 10.1113/JP270654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Done A.J., Traustadottir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Joo M.S. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol. Cell Biol. 2016;36(14):1931–1942. doi: 10.1128/MCB.00118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sun Z., Huang Z., Zhang D.D. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PloS One. 2009;4(8):e6588. doi: 10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Joyner M.J., Casey D.P. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol. Rev. 2015;95(2):549–601. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gliemann L. Regulation of skeletal muscle blood flow during exercise. Current Opinion in Physiology. 2019;10:146–155. [Google Scholar]
- 144.Trinity J.D., Broxterman R.M., Richardson R.S. Regulation of exercise blood flow: role of free radicals. Free Radic. Biol. Med. 2016;98:90–102. doi: 10.1016/j.freeradbiomed.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Secher N.H., Seifert T., Van Lieshout J.J. Cerebral blood flow and metabolism during exercise: implications for fatigue. J. Appl. Physiol. 1985;104(1):306–314. doi: 10.1152/japplphysiol.00853.2007. 2008. [DOI] [PubMed] [Google Scholar]
- 146.Henriquez-Olguin C. Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2. Redox Biol. 2019;24:101188. doi: 10.1016/j.redox.2019.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Knock G. NADPH oxidase in the vasculature: expression, regulation and signalling pathways; role in normal cardiovascular physiology and its dysregulation in hypertension. Free Radic. Biol. Med. 2019;145:385–427. doi: 10.1016/j.freeradbiomed.2019.09.029. [DOI] [PubMed] [Google Scholar]
- 148.Touyz R.M., Briones A.M. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens. Res. 2011;34(1):5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 149.Duarte J.A. Endothelium-derived oxidative stress may contribute to exercise-induced muscle damage. Int. J. Sports Med. 1993;14(8):440–443. doi: 10.1055/s-2007-1021207. [DOI] [PubMed] [Google Scholar]
- 150.Laurindo F.R. Vascular free radical release. Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ. Res. 1994;74(4):700–709. doi: 10.1161/01.res.74.4.700. [DOI] [PubMed] [Google Scholar]
- 151.McNally J.S. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am. J. Physiol. Heart Circ. Physiol. 2003;285(6):H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- 152.Takabe W. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxidants Redox Signal. 2011;15(5):1379–1388. doi: 10.1089/ars.2010.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weseler A.R., Bast A. Oxidative stress and vascular function: implications for pharmacologic treatments. Curr. Hypertens. Rep. 2010;12(3):154–161. doi: 10.1007/s11906-010-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Loader J. Effects of sugar-sweetened beverage consumption on microvascular and macrovascular function in a healthy population. Arterioscler. Thromb. Vasc. Biol. 2017;37(6):1250–1260. doi: 10.1161/ATVBAHA.116.308010. [DOI] [PubMed] [Google Scholar]
- 155.Tyldum G.A. Endothelial dysfunction induced by post-prandial lipemia: complete protection afforded by high-intensity aerobic interval exercise. J. Am. Coll. Cardiol. 2009;53(2):200–206. doi: 10.1016/j.jacc.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Beckman J.A. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001;103(12):1618–1623. doi: 10.1161/01.cir.103.12.1618. [DOI] [PubMed] [Google Scholar]
- 157.Hancock M. Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise. Elife. 2018;7 doi: 10.7554/eLife.41044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Faria A., Persaud S.J. Cardiac oxidative stress in diabetes: mechanisms and therapeutic potential. Pharmacol. Ther. 2017;172:50–62. doi: 10.1016/j.pharmthera.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 159.Seals D.R., Jablonski K.L., Donato A.J. Aging and vascular endothelial function in humans. Clin. Sci. (Lond.) 2011;120(9):357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Forstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017;120(4):713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 161.Son S.M. Role of vascular reactive oxygen species in development of vascular abnormalities in diabetes. Diabetes Res. Clin. Pract. 2007;77(Suppl 1):S65–S70. doi: 10.1016/j.diabres.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 162.Wright E., Jr., Scism-Bacon J.L., Glass L.C. Oxidative stress in type 2 diabetes: the role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006;60(3):308–314. doi: 10.1111/j.1368-5031.2006.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Velmurugan G.V. Defective Nrf2-dependent redox signalling contributes to microvascular dysfunction in type 2 diabetes. Cardiovasc. Res. 2013;100(1):143–150. doi: 10.1093/cvr/cvt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sindler A.L. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J. Physiol. 2009;587(Pt 15):3885–3897. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Perticone F. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50(1):159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- 166.Taddei S. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97(22):2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- 167.Natali A. Effect of vitamin C on forearm blood flow and glucose metabolism in essential hypertension. Arterioscler. Thromb. Vasc. Biol. 2000;20(11):2401–2406. doi: 10.1161/01.atv.20.11.2401. [DOI] [PubMed] [Google Scholar]
- 168.Sherman D.L. Pharmacological concentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension. 2000;35(4):936–941. doi: 10.1161/01.hyp.35.4.936. [DOI] [PubMed] [Google Scholar]
- 169.Schneider M.P. Superoxide scavenging effects of N-acetylcysteine and vitamin C in subjects with essential hypertension. Am. J. Hypertens. 2005;18(8):1111–1117. doi: 10.1016/j.amjhyper.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 170.Timimi F.K. Vitamin C improves endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. J. Am. Coll. Cardiol. 1998;31(3):552–557. doi: 10.1016/s0735-1097(97)00536-6. [DOI] [PubMed] [Google Scholar]
- 171.Ting H.H. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Clin. Invest. 1996;97(1):22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schinzari F. Generalized impairment of vasodilator reactivity during hyperinsulinemia in patients with obesity-related metabolic syndrome. Am. J. Physiol. Endocrinol. Metab. 2010;299(6):E947–E952. doi: 10.1152/ajpendo.00426.2010. [DOI] [PubMed] [Google Scholar]
- 173.Hirai N. Insulin resistance and endothelial dysfunction in smokers: effects of vitamin C. Am. J. Physiol. Heart Circ. Physiol. 2000;279(3):H1172–H1178. doi: 10.1152/ajpheart.2000.279.3.H1172. [DOI] [PubMed] [Google Scholar]
- 174.Jablonski K.L. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J. Appl. Physiol. 1985;103(5):1715–1721. doi: 10.1152/japplphysiol.00533.2007. 2007. [DOI] [PubMed] [Google Scholar]
- 175.Wray D.W. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension. 2012;59(4):818–824. doi: 10.1161/HYPERTENSIONAHA.111.189456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gioscia-Ryan R.A. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J. Physiol. 2014;592(12):2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Graham D. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54(2):322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 178.Lopes R.A. Vol. 66. 2015. pp. 1240–1250. (Downregulation of Nuclear Factor Erythroid 2–Related Factor and Associated Antioxidant Genes Contributes to Redox-Sensitive Vascular Dysfunction in Hypertension). 6. [DOI] [PubMed] [Google Scholar]
- 179.Teramoto K. Acute effect of oral vitamin C on coronary circulation in young healthy smokers. Am. Heart J. 2004;148(2):300–305. doi: 10.1016/j.ahj.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 180.McNulty P.H. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. 2007;102(5):2040–2045. doi: 10.1152/japplphysiol.00595.2006. [DOI] [PubMed] [Google Scholar]
- 181.Kaufmann P.A. vol. 102. 2000. pp. 1233–1238. (Coronary Heart Disease in Smokers). 11. [DOI] [PubMed] [Google Scholar]
- 182.Tripathy D. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52(12):2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 183.Alexopoulos N. The acute effect of green tea consumption on endothelial function in healthy individuals. Eur. J. Cardiovasc. Prev. Rehabil. 2008;15(3):300–305. doi: 10.1097/HJR.0b013e3282f4832f. [DOI] [PubMed] [Google Scholar]
- 184.Phillips S.A., Hatoum O.A., Gutterman D.D. The mechanism of flow-induced dilation in human adipose arterioles involves hydrogen peroxide during CAD. Am. J. Physiol. Heart Circ. Physiol. 2007;292(1):H93–H100. doi: 10.1152/ajpheart.00819.2006. [DOI] [PubMed] [Google Scholar]
- 185.Zinkevich N.S. Roles of NADPH oxidase and mitochondria in flow-induced vasodilation of human adipose arterioles: ROS-induced ROS release in coronary artery disease. Microcirculation. 2017;24(6) doi: 10.1111/micc.12380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Miura H. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ. Res. 2003;92(2):e31–40. doi: 10.1161/01.res.0000054200.44505.ab. [DOI] [PubMed] [Google Scholar]
- 187.Liu Y. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ. Res. 2003;93(6):573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 188.Yasuda M. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 1999;64(4):249–258. doi: 10.1016/s0024-3205(98)00560-8. [DOI] [PubMed] [Google Scholar]
- 189.Stone J.R., Collins T. The role of hydrogen peroxide in endothelial proliferative responses. Endothelium. 2002;9(4):231–238. doi: 10.1080/10623320214733. [DOI] [PubMed] [Google Scholar]
- 190.Yamaoka-Tojo M. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species--dependent endothelial migration and proliferation. Circ. Res. 2004;95(3):276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- 191.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc. Res. 2006;71(2):226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 192.Chinsomboon J. The transcriptional coactivator PGC-1α mediates exercise-induced angiogenesis in skeletal muscle. 2009;106(50):21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Radak Z. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxidants Redox Signal. 2013;18(10):1208–1246. doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Richards J.C. Acute ascorbic acid ingestion increases skeletal muscle blood flow and oxygen consumption via local vasodilation during graded handgrip exercise in older adults. Am. J. Physiol. Heart Circ. Physiol. 2015;309(2):H360–H368. doi: 10.1152/ajpheart.00209.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Crecelius A.R. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am. J. Physiol. Heart Circ. Physiol. 2010;299(5):H1633–H1641. doi: 10.1152/ajpheart.00614.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kirby B.S. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J. Physiol. 2009;587(Pt 9):1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Trinity J.D. Ascorbic acid improves brachial artery vasodilation during progressive handgrip exercise in the elderly through a nitric oxide-mediated mechanism. Am. J. Physiol. Heart Circ. Physiol. 2016;310(6):H765–H774. doi: 10.1152/ajpheart.00817.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rossman M.J. Oral antioxidants improve leg blood flow during exercise in patients with chronic obstructive pulmonary disease. Am. J. Physiol. Heart Circ. Physiol. 2015;309(5):H977–H985. doi: 10.1152/ajpheart.00184.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Muthusamy V.R. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic. Biol. Med. 2012;52(2):366–376. doi: 10.1016/j.freeradbiomed.2011.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Shanmugam G. Exercise mediated Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling. Frontiers in cardiovascular medicine. 2019;6 doi: 10.3389/fcvm.2019.00068. 68-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Varsamis P. Transient endothelial dysfunction induced by sugar-sweetened beverage consumption may be attenuated by a single bout of aerobic exercise. Microvasc. Res. 2018;115:8–11. doi: 10.1016/j.mvr.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 202.Wray D.W. Oral antioxidants and cardiovascular health in the exercise-trained and untrained elderly: a radically different outcome. Clin. Sci. (Lond.) 2009;116(5):433–441. doi: 10.1042/CS20080337. [DOI] [PubMed] [Google Scholar]
- 203.Eskurza I. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J. Physiol. 2004;556(Pt 1):315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lauer N. Critical involvement of hydrogen peroxide in exercise-induced up-regulation of endothelial NO synthase. Cardiovasc. Res. 2005;65(1):254–262. doi: 10.1016/j.cardiores.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 205.Drummond G.R. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ. Res. 2000;86(3):347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- 206.Bell P.G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients. 2014;6(2):829–843. doi: 10.3390/nu6020829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Howatson G. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports. 2010;20(6):843–852. doi: 10.1111/j.1600-0838.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 208.Bloedon T.K. Impact of anthocyanin-rich whole fruit consumption on exercise-induced oxidative stress and inflammation: a systematic review and meta-analysis. Nutr. Rev. 2019;77(9):630–645. doi: 10.1093/nutrit/nuz018. [DOI] [PubMed] [Google Scholar]
- 209.McCormick R. Effect of tart cherry juice on recovery and next day performance in well-trained Water Polo players. J Int Soc Sports Nutr. 2016;13:41. doi: 10.1186/s12970-016-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Braakhuis A.J., Hopkins W.G., Lowe T.E. Effects of dietary antioxidants on training and performance in female runners. Eur. J. Sport Sci. 2014;14(2):160–168. doi: 10.1080/17461391.2013.785597. [DOI] [PubMed] [Google Scholar]
- 211.Bell P.G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl. Physiol. Nutr. Metabol. 2015;40(4):414–423. doi: 10.1139/apnm-2014-0244. [DOI] [PubMed] [Google Scholar]
- 212.Morgan P.T., Barton M.J., Bowtell J.L. Montmorency cherry supplementation improves 15-km cycling time-trial performance. Eur. J. Appl. Physiol. 2019;119(3):675–684. doi: 10.1007/s00421-018-04058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cook M.D. Blackcurrant alters physiological responses and femoral artery diameter during sustained isometric contraction. Nutrients. 2017;9(6) doi: 10.3390/nu9060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cook M.D. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur. J. Appl. Physiol. 2015;115(11):2357–2365. doi: 10.1007/s00421-015-3215-8. [DOI] [PubMed] [Google Scholar]
- 215.Murphy C.A., Cook M.D., Willems M.E.T. Effect of New Zealand blackcurrant extract on repeated cycling time trial performance. Sports. 2017;5(2) doi: 10.3390/sports5020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Clifford T., Mitchell N., Scott A. The influence of different sources of polyphenols on submaximal cycling and time trial performance. Journal of Athletic Enhancement. 2013;2:S10. [Google Scholar]
- 217.Willems M.E. Beneficial physiological effects with blackcurrant intake in endurance athletes. Int. J. Sport Nutr. Exerc. Metabol. 2015;25(4):367–374. doi: 10.1123/ijsnem.2014-0233. [DOI] [PubMed] [Google Scholar]
- 218.Karppi J. Effects of astaxanthin supplementation on lipid peroxidation. Int. J. Vitam. Nutr. Res. 2007;77(1):3–11. doi: 10.1024/0300-9831.77.1.3. [DOI] [PubMed] [Google Scholar]
- 219.Baralic I. Effect of astaxanthin supplementation on paraoxonase 1 activities and oxidative stress status in young soccer players. 2013;27(10):1536–1542. doi: 10.1002/ptr.4898. [DOI] [PubMed] [Google Scholar]
- 220.Baralic I. Effect of astaxanthin supplementation on salivary IgA, oxidative stress, and inflammation in young soccer players. Evid Based Complement Alternat Med. 2015;2015:783761. doi: 10.1155/2015/783761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Klinkenberg L.J. Effect of antioxidant supplementation on exercise-induced cardiac troponin release in cyclists: a randomized trial. PloS One. 2013;8(11) doi: 10.1371/journal.pone.0079280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Res P.T. Astaxanthin supplementation does not augment fat use or improve endurance performance. Med. Sci. Sports Exerc. 2013;45(6):1158–1165. doi: 10.1249/MSS.0b013e31827fddc4. [DOI] [PubMed] [Google Scholar]
- 223.Djordjevic B. Effect of astaxanthin supplementation on muscle damage and oxidative stress markers in elite young soccer players. J. Sports Med. Phys. Fit. 2012;52(4):382–392. [PubMed] [Google Scholar]
- 224.Bloomer R.J. Astaxanthin supplementation does not attenuate muscle injury following eccentric exercise in resistance-trained men. Int. J. Sport Nutr. Exerc. Metabol. 2005;15(4):401–412. doi: 10.1123/ijsnem.15.4.401. [DOI] [PubMed] [Google Scholar]
- 225.Sugita M. Influence of green tea catechins on oxidative stress metabolites at rest and during exercise in healthy humans. Nutrition. 2016;32(3):321–331. doi: 10.1016/j.nut.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 226.Decroix L. Cocoa flavanol supplementation and exercise: a systematic review. Sports Med. 2018;48(4):867–892. doi: 10.1007/s40279-017-0849-1. [DOI] [PubMed] [Google Scholar]
- 227.da Silva W. Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage. Physiol. Behav. 2018;194:77–82. doi: 10.1016/j.physbeh.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 228.Kerksick C.M., Kreider R.B., Willoughby D.S. Intramuscular adaptations to eccentric exercise and antioxidant supplementation. Amino Acids. 2010;39(1):219–232. doi: 10.1007/s00726-009-0432-7. [DOI] [PubMed] [Google Scholar]
- 229.Schwarz N.A. (-)-Epicatechin supplementation inhibits aerobic adaptations to cycling exercise in humans. Front Nutr. 2018;5:132. doi: 10.3389/fnut.2018.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Taub P.R. Beneficial effects of dark chocolate on exercise capacity in sedentary subjects: underlying mechanisms. A double blind, randomized, placebo controlled trial. Food Funct. 2016;7(9):3686–3693. doi: 10.1039/c6fo00611f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Berry N.M. Impact of cocoa flavanol consumption on blood pressure responsiveness to exercise. Br. J. Nutr. 2010;103(10):1480–1484. doi: 10.1017/S0007114509993382. [DOI] [PubMed] [Google Scholar]
- 232.Ota N., Soga S., Shimotoyodome A. Daily consumption of tea catechins improves aerobic capacity in healthy male adults: a randomized double-blind, placebo-controlled, crossover trial. Biosci. Biotechnol. Biochem. 2016;80(12):2412–2417. doi: 10.1080/09168451.2016.1224638. [DOI] [PubMed] [Google Scholar]
- 233.Davison K. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int. J. Obes. 2008;32(8):1289–1296. doi: 10.1038/ijo.2008.66. [DOI] [PubMed] [Google Scholar]
- 234.Martin B.J. No effect of short-term green tea extract supplementation on metabolism at rest or during exercise in the fed state. Int. J. Sport Nutr. Exerc. Metabol. 2014;24(6):656–664. doi: 10.1123/ijsnem.2013-0202. [DOI] [PubMed] [Google Scholar]
- 235.Dean S., Braakhuis A., Paton C. The effects of EGCG on fat oxidation and endurance performance in male cyclists. Int. J. Sport Nutr. Exerc. Metabol. 2009;19(6):624–644. doi: 10.1123/ijsnem.19.6.624. [DOI] [PubMed] [Google Scholar]
- 236.Stellingwerff T. The effect of acute dark chocolate consumption on carbohydrate metabolism and performance during rest and exercise. Appl. Physiol. Nutr. Metabol. 2014;39(2):173–182. doi: 10.1139/apnm-2013-0152. [DOI] [PubMed] [Google Scholar]
- 237.Peschek K. The effects of acute post exercise consumption of two cocoa-based beverages with varying flavanol content on indices of muscle recovery following downhill treadmill running. Nutrients. 2013;6(1):50–62. doi: 10.3390/nu6010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Drobnic F. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva(R)): a randomised, placebo-controlled trial. J Int Soc Sports Nutr. 2014;11:31. doi: 10.1186/1550-2783-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Akazawa N. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr. Res. 2012;32(10):795–799. doi: 10.1016/j.nutres.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 240.Quindry J.C. Oral quercetin supplementation and blood oxidative capacity in response to ultramarathon competition. Int. J. Sport Nutr. Exerc. Metabol. 2008;18(6):601–616. doi: 10.1123/ijsnem.18.6.601. [DOI] [PubMed] [Google Scholar]
- 241.McAnulty S.R. Chronic quercetin ingestion and exercise-induced oxidative damage and inflammation. Appl. Physiol. Nutr. Metabol. 2008;33(2):254–262. doi: 10.1139/H07-177. [DOI] [PubMed] [Google Scholar]
- 242.Nieman D.C. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med. Sci. Sports Exerc. 2009;41(7):1467–1475. doi: 10.1249/MSS.0b013e318199491f. [DOI] [PubMed] [Google Scholar]
- 243.Nieman D.C. Quercetin's influence on exercise performance and muscle mitochondrial biogenesis. Med. Sci. Sports Exerc. 2010;42(2):338–345. doi: 10.1249/MSS.0b013e3181b18fa3. [DOI] [PubMed] [Google Scholar]
- 244.Davis J.M. The dietary flavonoid quercetin increases VO(2max) and endurance capacity. Int. J. Sport Nutr. Exerc. Metabol. 2010;20(1):56–62. doi: 10.1123/ijsnem.20.1.56. [DOI] [PubMed] [Google Scholar]
- 245.Kressler J., Millard-Stafford M., Warren G.L. Quercetin and endurance exercise capacity: a systematic review and meta-analysis. Med. Sci. Sports Exerc. 2011;43(12):2396–2404. doi: 10.1249/MSS.0b013e31822495a7. [DOI] [PubMed] [Google Scholar]
- 246.Somerville V., Bringans C., Braakhuis A. Polyphenols and performance: a systematic review and meta-analysis. Sports Med. 2017;47(8):1589–1599. doi: 10.1007/s40279-017-0675-5. [DOI] [PubMed] [Google Scholar]
- 247.Pelletier D.M., Lacerte G., Goulet E.D. Effects of quercetin supplementation on endurance performance and maximal oxygen consumption: a meta-analysis. Int. J. Sport Nutr. Exerc. Metabol. 2013;23(1):73–82. doi: 10.1123/ijsnem.23.1.73. [DOI] [PubMed] [Google Scholar]
- 248.MacRae H.S., Mefferd K.M. Dietary antioxidant supplementation combined with quercetin improves cycling time trial performance. Int. J. Sport Nutr. Exerc. Metabol. 2006;16(4):405–419. doi: 10.1123/ijsnem.16.4.405. [DOI] [PubMed] [Google Scholar]
- 249.Cureton K.J. Dietary quercetin supplementation is not ergogenic in untrained men. J. Appl. Physiol. 1985;107(4):1095–1104. doi: 10.1152/japplphysiol.00234.2009. 2009. [DOI] [PubMed] [Google Scholar]
- 250.Bazzucchi I. The effects of quercetin supplementation on eccentric exercise-induced muscle damage. Nutrients. 2019;11(1) doi: 10.3390/nu11010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Olesen J. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J. Physiol. 2014;592(8):1873–1886. doi: 10.1113/jphysiol.2013.270256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Alway S.E. Resveratrol enhances exercise-induced cellular and functional adaptations of skeletal muscle in older men and women. J Gerontol A Biol Sci Med Sci. 2017;72(12):1595–1606. doi: 10.1093/gerona/glx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Scribbans T.D. Resveratrol supplementation does not augment performance adaptations or fibre-type-specific responses to high-intensity interval training in humans. Appl. Physiol. Nutr. Metabol. 2014;39(11):1305–1313. doi: 10.1139/apnm-2014-0070. [DOI] [PubMed] [Google Scholar]
- 254.Gliemann L. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J. Physiol. 2013;591(20):5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.McDermott M.M. Effect of resveratrol on walking performance in older people with peripheral artery disease: the RESTORE randomized clinical trial. JAMA Cardiol. 2017;2(8):902–907. doi: 10.1001/jamacardio.2017.0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Laupheimer M.W. Resveratrol exerts no effect on inflammatory response and delayed onset muscle soreness after a marathon in male athletes.: a randomised, double-blind, placebo-controlled pilot feasibility study. Transl Med UniSa. 2014;10:38–42. [PMC free article] [PubMed] [Google Scholar]
- 257.Yimcharoen M. Effects of ascorbic acid supplementation on oxidative stress markers in healthy women following a single bout of exercise. J Int Soc Sports Nutr. 2019;16(1):2. doi: 10.1186/s12970-019-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Thompson D. Prolonged vitamin C supplementation and recovery from demanding exercise. Int. J. Sport Nutr. Exerc. Metabol. 2001;11(4):466–481. doi: 10.1123/ijsnem.11.4.466. [DOI] [PubMed] [Google Scholar]
- 259.Bryer S.C., Goldfarb A.H. Effect of high dose vitamin C supplementation on muscle soreness, damage, function, and oxidative stress to eccentric exercise. Int. J. Sport Nutr. Exerc. Metabol. 2006;16(3):270–280. doi: 10.1123/ijsnem.16.3.270. [DOI] [PubMed] [Google Scholar]
- 260.Goldfarb A.H. Vitamin C supplementation affects oxidative-stress blood markers in response to a 30-minute run at 75% VO2max. Int. J. Sport Nutr. Exerc. Metabol. 2005;15(3):279–290. doi: 10.1123/ijsnem.15.3.279. [DOI] [PubMed] [Google Scholar]
- 261.Nieman D.C. Influence of vitamin C supplementation on oxidative and immune changes after an ultramarathon. J. Appl. Physiol. 1985;92(5):1970–1977. doi: 10.1152/japplphysiol.00961.2001. 2002. [DOI] [PubMed] [Google Scholar]
- 262.Thompson D. Post-exercise vitamin C supplementation and recovery from demanding exercise. Eur. J. Appl. Physiol. 2003;89(3–4):393–400. doi: 10.1007/s00421-003-0816-4. [DOI] [PubMed] [Google Scholar]
- 263.Khassaf M. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J. Physiol. 2003;549(Pt 2):645–652. doi: 10.1113/jphysiol.2003.040303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Mason S.A. High-dose vitamin C supplementation increases skeletal muscle vitamin C concentration and SVCT2 transporter expression but does not alter redox status in healthy males. Free Radic. Biol. Med. 2014;77:130–138. doi: 10.1016/j.freeradbiomed.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 265.Meade R.D. Local infusion of ascorbate augments NO-dependent cutaneous vasodilatation during intense exercise in the heat. J. Physiol. 2015;593(17):4055–4065. doi: 10.1113/JP270787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Limberg J.K. Exercise-mediated vasodilation in human obesity and metabolic syndrome: effect of acute ascorbic acid infusion. Am. J. Physiol. Heart Circ. Physiol. 2014;307(6):H840–H847. doi: 10.1152/ajpheart.00312.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Roberts L.A. Vitamin C consumption does not impair training-induced improvements in exercise performance. Int. J. Sports Physiol. Perform. 2011;6(1):58–69. doi: 10.1123/ijspp.6.1.58. [DOI] [PubMed] [Google Scholar]
- 268.Jakeman P., Maxwell S. Effect of antioxidant vitamin supplementation on muscle function after eccentric exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1993;67(5):426–430. doi: 10.1007/BF00376459. [DOI] [PubMed] [Google Scholar]
- 269.Thompson D. Prolonged vitamin C supplementation and recovery from eccentric exercise. Eur. J. Appl. Physiol. 2004;92(1–2):133–138. doi: 10.1007/s00421-004-1064-y. [DOI] [PubMed] [Google Scholar]
- 270.Connolly D.A. The effects of vitamin C supplementation on symptoms of delayed onset muscle soreness. J. Sports Med. Phys. Fit. 2006;46(3):462–467. [PubMed] [Google Scholar]
- 271.Close G.L. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br. J. Nutr. 2006;95(5):976–981. doi: 10.1079/bjn20061732. [DOI] [PubMed] [Google Scholar]
- 272.Zembron-Lacny A. Physical activity and alpha-lipoic acid modulate inflammatory response through changes in thiol redox status. 2013;69(3):397–404. doi: 10.1007/s13105-012-0221-8. [DOI] [PubMed] [Google Scholar]
- 273.Zembron-Lacny A., Ostapiuk J., Szyszka K. Effects of sulphur-containing compounds on plasma redox status in muscle-damaging exercise. Chin. J. Physiol. 2009;52(5):289–294. doi: 10.4077/cjp.2009.amh026. [DOI] [PubMed] [Google Scholar]
- 274.Zembron-Lacny A. Assessment of the antioxidant effectiveness of alpha-lipoic acid in healthy men exposed to muscle-damaging exercise. J. Physiol. Pharmacol. 2009;60(2):139–143. [PubMed] [Google Scholar]
- 275.Zembron-Lacny A. The comparison of antioxidant and hematological properties of N-acetylcysteine and alpha-lipoic acid in physically active males. Physiol. Res. 2009;58(6):855–861. doi: 10.33549/physiolres.931590. [DOI] [PubMed] [Google Scholar]
- 276.Sharman J.E. Alpha-lipoic acid does not acutely affect resistance and conduit artery function or oxidative stress in healthy men. Br. J. Clin. Pharmacol. 2004;58(3):243–248. doi: 10.1111/j.1365-2125.2004.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Kon M. Reducing exercise-induced muscular injury in kendo athletes with supplementation of coenzyme Q10. Br. J. Nutr. 2008;100(4):903–909. doi: 10.1017/S0007114508926544. [DOI] [PubMed] [Google Scholar]
- 278.Okudan N., Belviranli M., Torlak S. Coenzyme Q10 does not prevent exercise-induced muscle damage and oxidative stress in sedentary men. J. Sports Med. Phys. Fit. 2018;58(6):889–894. doi: 10.23736/S0022-4707.17.07146-8. [DOI] [PubMed] [Google Scholar]
- 279.Ostman B. Coenzyme Q10 supplementation and exercise-induced oxidative stress in humans. Nutrition. 2012;28(4):403–417. doi: 10.1016/j.nut.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 280.Bonetti A. Effect of ubidecarenone oral treatment on aerobic power in middle-aged trained subjects. J. Sports Med. Phys. Fit. 2000;40(1):51–57. [PubMed] [Google Scholar]
- 281.Bloomer R.J. Impact of oral ubiquinol on blood oxidative stress and exercise performance. Oxid Med Cell Longev. 2012;2012:465020. doi: 10.1155/2012/465020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Tiano L. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: a double-blind, randomized controlled study. Eur. Heart J. 2007;28(18):2249–2255. doi: 10.1093/eurheartj/ehm267. [DOI] [PubMed] [Google Scholar]
- 283.Belardinelli R. Coenzyme Q10 and exercise training in chronic heart failure. Eur. Heart J. 2006;27(22):2675–2681. doi: 10.1093/eurheartj/ehl158. [DOI] [PubMed] [Google Scholar]
- 284.Mizuno K. Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition. 2008;24(4):293–299. doi: 10.1016/j.nut.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 285.Alf D., Schmidt M.E., Siebrecht S.C. Ubiquinol supplementation enhances peak power production in trained athletes: a double-blind, placebo controlled study. Sports Nutr. Rev. J. 2013;10 doi: 10.1186/1550-2783-10-24. 24-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Deichmann R.E., Lavie C.J., Dornelles A.C. Impact of coenzyme Q-10 on parameters of cardiorespiratory fitness and muscle performance in older athletes taking statins. Phys Sportsmed. 2012;40(4):88–95. doi: 10.3810/psm.2012.11.1991. [DOI] [PubMed] [Google Scholar]
- 287.Cooke M. Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J Int Soc Sports Nutr. 2008;5:8. doi: 10.1186/1550-2783-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhou S. Muscle and plasma coenzyme Q10 concentration, aerobic power and exercise economy of healthy men in response to four weeks of supplementation. J. Sports Med. Phys. Fit. 2005;45(3):337–346. [PubMed] [Google Scholar]
- 289.Porter D.A. The effect of oral coenzyme Q10 on the exercise tolerance of middle-aged, untrained men. Int. J. Sports Med. 1995;16(7):421–427. doi: 10.1055/s-2007-973031. [DOI] [PubMed] [Google Scholar]
- 290.Weston S.B. Does exogenous coenzyme Q10 affect aerobic capacity in endurance athletes? Int. J. Sport Nutr. 1997;7(3):197–206. doi: 10.1123/ijsn.7.3.197. [DOI] [PubMed] [Google Scholar]
- 291.Laaksonen R. Ubiquinone supplementation and exercise capacity in trained young and older men. Eur. J. Appl. Physiol. Occup. Physiol. 1995;72(1–2):95–100. doi: 10.1007/BF00964121. [DOI] [PubMed] [Google Scholar]
- 292.Malm C. Effects of ubiquinone-10 supplementation and high intensity training on physical performance in humans. Acta Physiol. Scand. 1997;161(3):379–384. doi: 10.1046/j.1365-201X.1997.00198.x. [DOI] [PubMed] [Google Scholar]
- 293.Kaikkonen J. Effect of combined coenzyme Q10 and d-alpha-tocopheryl acetate supplementation on exercise-induced lipid peroxidation and muscular damage: a placebo-controlled double-blind study in marathon runners. Free Radic. Res. 1998;29(1):85–92. doi: 10.1080/10715769800300101. [DOI] [PubMed] [Google Scholar]
- 294.Kizaki K. Effect of reduced coenzyme Q10 (ubiquinol) supplementation on blood pressure and muscle damage during kendo training camp: a double-blind, randomized controlled study. J. Sports Med. Phys. Fit. 2015;55(7–8):797–804. [PubMed] [Google Scholar]
- 295.Patlar S., Baltaci A.K., Mogulkoc R. Effect of vitamin A administration on free radicals and lactate levels in individuals exercised to exhaustion. Pak. J. Pharm. Sci. 2016;29(5):1531–1534. [PubMed] [Google Scholar]
- 296.Sumida S. Effect of a single bout of exercise and beta-carotene supplementation on the urinary excretion of 8-hydroxy-deoxyguanosine in humans. Free Radic. Res. 1997;27(6):607–618. doi: 10.3109/10715769709097864. [DOI] [PubMed] [Google Scholar]
- 297.Dillard C.J. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1978;45(6):927–932. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- 298.Sumida S. Exercise-induced lipid peroxidation and leakage of enzymes before and after vitamin E supplementation. Int. J. Biochem. 1989;21(8):835–838. doi: 10.1016/0020-711x(89)90280-2. [DOI] [PubMed] [Google Scholar]
- 299.Meydani M. Protective effect of vitamin E on exercise-induced oxidative damage in young and older adults. Am. J. Physiol. 1993;264(5 Pt 2):R992–R998. doi: 10.1152/ajpregu.1993.264.5.R992. [DOI] [PubMed] [Google Scholar]
- 300.Keong C.C., Singh H.J., Singh R. Effects of palm vitamin e supplementation on exercise-induced oxidative stress and endurance performance in the heat. J. Sports Sci. Med. 2006;5(4):629–639. [PMC free article] [PubMed] [Google Scholar]
- 301.Rokitzki L. alpha-Tocopherol supplementation in racing cyclists during extreme endurance training. Int. J. Sport Nutr. 1994;4(3):253–264. doi: 10.1123/ijsn.4.3.253. [DOI] [PubMed] [Google Scholar]
- 302.McAnulty S.R. Effect of alpha-tocopherol supplementation on plasma homocysteine and oxidative stress in highly trained athletes before and after exhaustive exercise. J. Nutr. Biochem. 2005;16(9):530–537. doi: 10.1016/j.jnutbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 303.Simon-Schnass I., Pabst H. Influence of vitamin E on physical performance. Int. J. Vitam. Nutr. Res. 1988;58(1):49–54. [PubMed] [Google Scholar]
- 304.Kobayaski Y. University of New Mexico; 1974. Effect of Vitamin E on Aerobic Work Performance in Man during Acute Exposure to Hypoxic Hypoxia. [Google Scholar]
- 305.Lawrence J.D. Effects of alpha-tocopherol acetate on the swimming endurance of trained swimmers. Am. J. Clin. Nutr. 1975;28(3):205–208. doi: 10.1093/ajcn/28.3.205. [DOI] [PubMed] [Google Scholar]
- 306.Sharman I.M., Down M.G., Sen R.N. The effects of vitamin E and training on physiological function and athletic performance in adolescent swimmers. Br. J. Nutr. 1971;26(2):265–276. doi: 10.1079/bjn19710033. [DOI] [PubMed] [Google Scholar]
- 307.Helgheim I. The effects of vitamin E on serum enzyme levels following heavy exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1979;40(4):283–289. doi: 10.1007/BF00421520. [DOI] [PubMed] [Google Scholar]
- 308.Beaton L.J. Contraction-induced muscle damage is unaffected by vitamin E supplementation. Med. Sci. Sports Exerc. 2002;34(5):798–805. doi: 10.1097/00005768-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 309.Maldonado M.D. Melatonin administrated immediately before an intense exercise reverses oxidative stress, improves immunological defenses and lipid metabolism in football players. Physiol. Behav. 2012;105(5):1099–1103. doi: 10.1016/j.physbeh.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 310.Ortiz-Franco M. Effect of melatonin supplementation on antioxidant status and DNA damage in high intensity trained athletes. Int. J. Sports Med. 2017;38(14):1117–1125. doi: 10.1055/s-0043-119881. [DOI] [PubMed] [Google Scholar]
- 311.Leonardo-Mendonca R.C. The benefit of a supplement with the antioxidant melatonin on redox status and muscle damage in resistance-trained athletes. Appl. Physiol. Nutr. Metabol. 2017;42(7):700–707. doi: 10.1139/apnm-2016-0677. [DOI] [PubMed] [Google Scholar]
- 312.Ochoa J.J. Melatonin supplementation ameliorates oxidative stress and inflammatory signaling induced by strenuous exercise in adult human males. J. Pineal Res. 2011;51(4):373–380. doi: 10.1111/j.1600-079X.2011.00899.x. [DOI] [PubMed] [Google Scholar]
- 313.Atkinson G. Effects of melatonin on the thermoregulatory responses to intermittent exercise. J. Pineal Res. 2005;39(4):353–359. doi: 10.1111/j.1600-079X.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 314.Brandenberger K.J. Consumption of a 5-mg melatonin supplement does not affect 32.2-km cycling time trial performance. J. Strength Condit Res. 2018;32(10):2872–2877. doi: 10.1519/JSC.0000000000001955. [DOI] [PubMed] [Google Scholar]
- 315.Atkinson G. Effects of daytime ingestion of melatonin on short-term athletic performance. Ergonomics. 2005;48(11–14):1512–1522. doi: 10.1080/00140130500100967. [DOI] [PubMed] [Google Scholar]
- 316.Medved I. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J. Appl. Physiol. 1985;97(4):1477–1485. doi: 10.1152/japplphysiol.00371.2004. 2004. [DOI] [PubMed] [Google Scholar]
- 317.Petersen A.C. Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle. Acta Physiol. 2012;204(3):382–392. doi: 10.1111/j.1748-1716.2011.02344.x. [DOI] [PubMed] [Google Scholar]
- 318.Nyberg M. Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J. Physiol. 2012;590(21):5361–5370. doi: 10.1113/jphysiol.2012.239053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Smith J.R. Acute supplementation of N-acetylcysteine does not affect muscle blood flow and oxygenation characteristics during handgrip exercise. Physiological reports. 2016;4(7) doi: 10.14814/phy2.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.McKenna M.J. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J. Physiol. 2006;576(Pt 1):279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Travaline J.M. Effect of N-acetylcysteine on human diaphragm strength and fatigability. Am. J. Respir. Crit. Care Med. 1997;156(5):1567–1571. doi: 10.1164/ajrccm.156.5.96-09133. [DOI] [PubMed] [Google Scholar]
- 322.Medved I. N-acetylcysteine infusion alters blood redox status but not time to fatigue during intense exercise in humans. J. Appl. Physiol. 1985;94(4):1572–1582. doi: 10.1152/japplphysiol.00884.2002. 2003. [DOI] [PubMed] [Google Scholar]
- 323.Matuszczak Y. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve. 2005;32(5):633–638. doi: 10.1002/mus.20385. [DOI] [PubMed] [Google Scholar]
- 324.Kelly M.K. Effects of N-acetylcysteine on respiratory muscle fatigue during heavy exercise. Respir. Physiol. Neurobiol. 2009;165(1):67–72. doi: 10.1016/j.resp.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 325.Corn S.D., Barstow T.J. Effects of oral N-acetylcysteine on fatigue, critical power, and W' in exercising humans. Respir. Physiol. Neurobiol. 2011;178(2):261–268. doi: 10.1016/j.resp.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 326.Koechlin C. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am. J. Respir. Crit. Care Med. 2004;169(9):1022–1027. doi: 10.1164/rccm.200310-1465OC. [DOI] [PubMed] [Google Scholar]
- 327.Slattery K.M. Effect of N-acetylcysteine on cycling performance after intensified training. Med. Sci. Sports Exerc. 2014;46(6):1114–1123. doi: 10.1249/MSS.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 328.Cobley J.N. N-Acetylcysteine's attenuation of fatigue after repeated bouts of intermittent exercise: practical implications for tournament situations. Int. J. Sport Nutr. Exerc. Metabol. 2011;21(6):451–461. doi: 10.1123/ijsnem.21.6.451. [DOI] [PubMed] [Google Scholar]
- 329.Trewin A.J. N-acetylcysteine alters substrate metabolism during high-intensity cycle exercise in well-trained humans. Appl. Physiol. Nutr. Metabol. 2013;38(12):1217–1227. doi: 10.1139/apnm-2012-0482. [DOI] [PubMed] [Google Scholar]
- 330.Savory L.A. Selenium supplementation and exercise: effect on oxidant stress in overweight adults. Obesity. 2012;20(4):794–801. doi: 10.1038/oby.2011.83. [DOI] [PubMed] [Google Scholar]
- 331.Margaritis I. Effects of endurance training on skeletal muscle oxidative capacities with and without selenium supplementation. J. Trace Elem. Med. Biol. 1997;11(1):37–43. doi: 10.1016/S0946-672X(97)80008-9. [DOI] [PubMed] [Google Scholar]
- 332.Tessier F. Muscle GSH-Px activity after prolonged exercise, training, and selenium supplementation. Biol. Trace Elem. Res. 1995;47(1–3):279–285. doi: 10.1007/BF02790128. [DOI] [PubMed] [Google Scholar]
- 333.Zamora A.J. Mitochondria changes in human muscle after prolonged exercise, endurance training and selenium supplementation. Eur. J. Appl. Physiol. Occup. Physiol. 1995;71(6):505–511. doi: 10.1007/BF00238552. [DOI] [PubMed] [Google Scholar]
- 334.Singh A., Failla M.L., Deuster P.A. Exercise-induced changes in immune function: effects of zinc supplementation. J. Appl. Physiol. 1985;76(6):2298–2303. doi: 10.1152/jappl.1994.76.6.2298. 1994. [DOI] [PubMed] [Google Scholar]
- 335.de Oliveira Kde J. Effect of zinc supplementation on the antioxidant, copper, and iron status of physically active adolescents. Cell Biochem. Funct. 2009;27(3):162–166. doi: 10.1002/cbf.1550. [DOI] [PubMed] [Google Scholar]
- 336.Saeedy M., Bijeh N., Shoorideh Z. Effect of six weeks of high intensity interval training and zinc supplement on serum creatine kinase and uric acid levels in futsal players. 2016;5:19–27. [Google Scholar]
- 337.Heffernan S.M. The role of mineral and trace element supplementation in exercise and athletic performance: a systematic review. Nutrients. 2019;11(3) doi: 10.3390/nu11030696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Huang W. Antioxidant and anti-inflammatory effects of blueberry anthocyanins on high glucose-induced human retinal capillary endothelial cells. Oxid Med Cell Longev. 2018;2018:1862462. doi: 10.1155/2018/1862462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Goto S. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta. 2001;1512(2):251–258. doi: 10.1016/s0005-2736(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 340.Morel I. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem. Pharmacol. 1993;45(1):13–19. doi: 10.1016/0006-2952(93)90371-3. [DOI] [PubMed] [Google Scholar]
- 341.Ferrali M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997;416(2):123–129. doi: 10.1016/s0014-5793(97)01182-4. [DOI] [PubMed] [Google Scholar]
- 342.Takahama U., Hirota S., Oniki T. Quercetin-dependent scavenging of reactive nitrogen species derived from nitric oxide and nitrite in the human oral cavity: interaction of quercetin with salivary redox components. Arch. Oral Biol. 2006;51(8):629–639. doi: 10.1016/j.archoralbio.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 343.Leonard S.S. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003;309(4):1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 344.Lorenz P. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide. 2003;9(2):64–76. doi: 10.1016/j.niox.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 345.Netzel M. Bioactive anthocyanins detected in human urine after ingestion of blackcurrant juice. 2001;20(2):7. [PubMed] [Google Scholar]
- 346.Bub A. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001;40(3):113–120. doi: 10.1007/s003940170011. [DOI] [PubMed] [Google Scholar]
- 347.Schiborr C. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014;58(3):516–527. doi: 10.1002/mnfr.201300724. [DOI] [PubMed] [Google Scholar]
- 348.Wenzel E., Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005;49(5):472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 349.Mercke Odeberg J. Oral bioavailability of the antioxidant astaxanthin in humans is enhanced by incorporation of lipid based formulations. Eur. J. Pharmaceut. Sci. 2003;19(4):299–304. doi: 10.1016/s0928-0987(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 350.Swezey R.R. Absorption, tissue distribution and elimination of 4-[(3)h]-epigallocatechin gallate in beagle dogs. Int. J. Toxicol. 2003;22(3):187–193. doi: 10.1080/10915810305101. [DOI] [PubMed] [Google Scholar]
- 351.Sakakibara H. Distribution and excretion of bilberry anthocyanins [corrected] in mice. J. Agric. Food Chem. 2009;57(17):7681–7686. doi: 10.1021/jf901341b. [DOI] [PubMed] [Google Scholar]
- 352.Mullen W. Determination of flavonol metabolites in plasma and tissues of rats by HPLC-radiocounting and tandem mass spectrometry following oral ingestion of [2-(14)C]quercetin-4'-glucoside. J. Agric. Food Chem. 2002;50(23):6902–6909. doi: 10.1021/jf020598p. [DOI] [PubMed] [Google Scholar]
- 353.Andres-Lacueva C. Distribution of resveratrol metabolites in liver, adipose tissue, and skeletal muscle in rats fed different doses of this polyphenol. J. Agric. Food Chem. 2012;60(19):4833–4840. doi: 10.1021/jf3001108. [DOI] [PubMed] [Google Scholar]
- 354.Yang Y. Astaxanthin-rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J. Nutr. 2011;141(9):1611–1617. doi: 10.3945/jn.111.142109. [DOI] [PubMed] [Google Scholar]
- 355.Yang Y. Astaxanthin lowers plasma TAG concentrations and increases hepatic antioxidant gene expression in diet-induced obesity mice. Br. J. Nutr. 2014;112(11):1797–1804. doi: 10.1017/S0007114514002554. [DOI] [PubMed] [Google Scholar]
- 356.Spanier G. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J. Physiol. Pharmacol. 2009;60(Suppl 4):111–116. [PubMed] [Google Scholar]
- 357.Tung B.T. Modulation of endogenous antioxidant activity by resveratrol and exercise in mouse liver is age dependent. J Gerontol A Biol Sci Med Sci. 2014;69(4):398–409. doi: 10.1093/gerona/glt102. [DOI] [PubMed] [Google Scholar]
- 358.Steffen Y. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008;469(2):209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 359.Centeno-Baez C., Dallaire P., Marette A. Resveratrol inhibition of inducible nitric oxide synthase in skeletal muscle involves AMPK but not SIRT1. Am. J. Physiol. Endocrinol. Metab. 2011;301(5):E922–E930. doi: 10.1152/ajpendo.00530.2010. [DOI] [PubMed] [Google Scholar]
- 360.Palipoch S. Hepatoprotective effect of curcumin and alpha-tocopherol against cisplatin-induced oxidative stress. BMC Compl. Alternative Med. 2014;14:111. doi: 10.1186/1472-6882-14-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Kawanishi N. Curcumin attenuates oxidative stress following downhill running-induced muscle damage. Biochem. Biophys. Res. Commun. 2013;441(3):573–578. doi: 10.1016/j.bbrc.2013.10.119. [DOI] [PubMed] [Google Scholar]
- 362.He H.J. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes. 2012;3(5):94–104. doi: 10.4239/wjd.v3.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Cimino F. Anthocyanins protect human endothelial cells from mild hyperoxia damage through modulation of Nrf2 pathway. Genes Nutr. 2013;8(4):391–399. doi: 10.1007/s12263-012-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Wafi A.M. Curcumin improves exercise performance of mice with coronary artery ligation-induced HFrEF: Nrf2 and antioxidant mechanisms in skeletal muscle. J. Appl. Physiol. 1985;126(2):477–486. doi: 10.1152/japplphysiol.00654.2018. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Receno C.N. Effects of prolonged dietary curcumin exposure on skeletal muscle biochemical and functional responses of aged male rats. Int. J. Mol. Sci. 2019;20(5) doi: 10.3390/ijms20051178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Brown V.A. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70(22):9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Czank C. Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a (13)C-tracer study. Am. J. Clin. Nutr. 2013;97(5):995–1003. doi: 10.3945/ajcn.112.049247. [DOI] [PubMed] [Google Scholar]
- 368.Moon J.H. Identification of quercetin 3-O-beta-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic. Biol. Med. 2001;30(11):1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 369.Spiller G.A., Dewell A. Safety of an astaxanthin-rich Haematococcus pluvialis algal extract: a randomized clinical trial. J. Med. Food. 2003;6(1):51–56. doi: 10.1089/109662003765184741. [DOI] [PubMed] [Google Scholar]
- 370.Lao C.D. Dose escalation of a curcuminoid formulation. BMC Compl. Alternative Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Harwood M. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007;45(11):2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 372.Shoskes D.A. Quercetin in men with category III chronic prostatitis: a preliminary prospective, double-blind, placebo-controlled trial. Urology. 1999;54(6):960–963. doi: 10.1016/s0090-4295(99)00358-1. [DOI] [PubMed] [Google Scholar]
- 373.Yiu E.M. An open-label trial in Friedreich ataxia suggests clinical benefit with high-dose resveratrol, without effect on frataxin levels. J. Neurol. 2015;262(5):1344–1353. doi: 10.1007/s00415-015-7719-2. [DOI] [PubMed] [Google Scholar]
- 374.Almeida L. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009;53(Suppl 1):S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 375.Aoi W. Comparison of the effect of non-esterified and esterified astaxanthins on endurance performance in mice. J. Clin. Biochem. Nutr. 2018;62(2):161–166. doi: 10.3164/jcbn.17-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Aoi W. Astaxanthin limits exercise-induced skeletal and cardiac muscle damage in mice. Antioxidants Redox Signal. 2003;5(1):139–144. doi: 10.1089/152308603321223630. [DOI] [PubMed] [Google Scholar]
- 377.Polotow T.G. Astaxanthin supplementation delays physical exhaustion and prevents redox imbalances in plasma and soleus muscles of Wistar rats. Nutrients. 2014;6(12):5819–5838. doi: 10.3390/nu6125819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zhou Y. High-dose astaxanthin supplementation suppresses antioxidant enzyme activity during moderate-intensity swimming training in mice. Nutrients. 2019;11(6) doi: 10.3390/nu11061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Ikeuchi M. Effects of astaxanthin supplementation on exercise-induced fatigue in mice. Biol. Pharm. Bull. 2006;29(10):2106–2110. doi: 10.1248/bpb.29.2106. [DOI] [PubMed] [Google Scholar]
- 380.Aoi W. Astaxanthin improves muscle lipid metabolism in exercise via inhibitory effect of oxidative CPT I modification. Biochem. Biophys. Res. Commun. 2008;366(4):892–897. doi: 10.1016/j.bbrc.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 381.Nagasawa T. Induction of oxidatively modified proteins in skeletal muscle by electrical stimulation and its suppression by dietary supplementation of (-)-epigallocatechin gallate. Biosci. Biotechnol. Biochem. 2000;64(5):1004–1010. doi: 10.1271/bbb.64.1004. [DOI] [PubMed] [Google Scholar]
- 382.Pence B.D. Effects of exercise and dietary epigallocatechin gallate and beta-alanine on skeletal muscle in aged mice. Appl. Physiol. Nutr. Metabol. 2016;41(2):181–190. doi: 10.1139/apnm-2015-0372. [DOI] [PubMed] [Google Scholar]
- 383.Murase T. Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288(3):R708–R715. doi: 10.1152/ajpregu.00693.2004. [DOI] [PubMed] [Google Scholar]
- 384.Nogueira L. (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. J. Physiol. 2011;589(Pt 18):4615–4631. doi: 10.1113/jphysiol.2011.209924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Lee I. (-)-Epicatechin combined with 8 weeks of treadmill exercise is associated with increased angiogenic and mitochondrial signaling in mice. Front. Pharmacol. 2015;6:43. doi: 10.3389/fphar.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Copp S.W. (-)-Epicatechin administration and exercising skeletal muscle vascular control and microvascular oxygenation in healthy rats. Am. J. Physiol. Heart Circ. Physiol. 2013;304(2):H206–H214. doi: 10.1152/ajpheart.00714.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Bhattacharya T.K. Exercise but not (-)-epigallocatechin-3-gallate or beta-alanine enhances physical fitness, brain plasticity, and behavioral performance in mice. Physiol. Behav. 2015;145:29–37. doi: 10.1016/j.physbeh.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Ichinose T. Effect of endurance training supplemented with green tea extract on substrate metabolism during exercise in humans. Scand. J. Med. Sci. Sports. 2011;21(4):598–605. doi: 10.1111/j.1600-0838.2009.01077.x. [DOI] [PubMed] [Google Scholar]
- 389.Kapoor M.P. Physiological effects of epigallocatechin-3-gallate (EGCG) on energy expenditure for prospective fat oxidation in humans: a systematic review and meta-analysis. J. Nutr. Biochem. 2017;43:1–10. doi: 10.1016/j.jnutbio.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 390.Younes M. Scientific opinion on the safety of green tea catechins. EFSA Journal. 2018;16(4) doi: 10.2903/j.efsa.2018.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Chaudhary P. High altitude mediated skeletal muscle atrophy: protective role of curcumin. Biochimie. 2019;156:138–147. doi: 10.1016/j.biochi.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 392.Huang S.L. Curcuminoids modulate the PKCdelta/NADPH oxidase/reactive oxygen species signaling pathway and suppress matrix invasion during monocyte-macrophage differentiation. J. Agric. Food Chem. 2015;63(40):8838–8848. doi: 10.1021/acs.jafc.5b04083. [DOI] [PubMed] [Google Scholar]
- 393.Ray Hamidie R.D. Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism. 2015;64(10):1334–1347. doi: 10.1016/j.metabol.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 394.Casuso R.A. Oral quercetin supplementation hampers skeletal muscle adaptations in response to exercise training. Scand. J. Med. Sci. Sports. 2014;24(6):920–927. doi: 10.1111/sms.12136. [DOI] [PubMed] [Google Scholar]
- 395.Davis J.M. Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296(4):R1071–R1077. doi: 10.1152/ajpregu.90925.2008. [DOI] [PubMed] [Google Scholar]
- 396.Ryan M.J. Suppression of oxidative stress by resveratrol after isometric contractions in gastrocnemius muscles of aged mice. J Gerontol A Biol Sci Med Sci. 2010;65(8):815–831. doi: 10.1093/gerona/glq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Jackson J.R., Ryan M.J., Alway S.E. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol A Biol Sci Med Sci. 2011;66(7):751–764. doi: 10.1093/gerona/glr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Sin T.K. Effects of long-term resveratrol-induced SIRT1 activation on insulin and apoptotic signalling in aged skeletal muscle. Acta Diabetol. 2015;52(6):1063–1075. doi: 10.1007/s00592-015-0767-3. [DOI] [PubMed] [Google Scholar]
- 399.Muhammad M.H., Allam M.M. Resveratrol and/or exercise training counteract aging-associated decline of physical endurance in aged mice; targeting mitochondrial biogenesis and function. J. Physiol. Sci. 2018;68(5):681–688. doi: 10.1007/s12576-017-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Tung B.T. Organ and tissue-dependent effect of resveratrol and exercise on antioxidant defenses of old mice. Aging Clin. Exp. Res. 2015;27(6):775–783. doi: 10.1007/s40520-015-0366-8. [DOI] [PubMed] [Google Scholar]
- 401.Murase T. Suppression of the aging-associated decline in physical performance by a combination of resveratrol intake and habitual exercise in senescence-accelerated mice. Biogerontology. 2009;10(4):423–434. doi: 10.1007/s10522-008-9177-z. [DOI] [PubMed] [Google Scholar]
- 402.Hart N. Resveratrol enhances exercise training responses in rats selectively bred for high running performance. Food Chem. Toxicol. 2013;61:53–59. doi: 10.1016/j.fct.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Dolinsky V.W. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J. Physiol. 2012;590(11):2783–2799. doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Lagouge M. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 405.Ringholm S. Effect of lifelong resveratrol supplementation and exercise training on skeletal muscle oxidative capacity in aging mice; impact of PGC-1alpha. Exp. Gerontol. 2013;48(11):1311–1318. doi: 10.1016/j.exger.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 406.Kan N.W. Effects of resveratrol supplementation and exercise training on exercise performance in middle-aged mice. Molecules. 2016;21(5) doi: 10.3390/molecules21050661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Boocock D.J. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 408.Ramirez-Garza S.L. Health effects of resveratrol: results from human intervention trials. Nutrients. 2018;10(12) doi: 10.3390/nu10121892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Gohil K. Vitamin E deficiency and vitamin C supplements: exercise and mitochondrial oxidation. J. Appl. Physiol. 1985;60(6):1986–1991. doi: 10.1152/jappl.1986.60.6.1986. 1986. [DOI] [PubMed] [Google Scholar]
- 410.Marshall R.J. Supplemental vitamin C appears to slow racing greyhounds. J. Nutr. 2002;132(6 Suppl 2) doi: 10.1093/jn/132.6.1616S. 1616s-21s. [DOI] [PubMed] [Google Scholar]
- 411.Huang A. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J. Biol. Chem. 2000;275(23):17399–17406. doi: 10.1074/jbc.M002248200. [DOI] [PubMed] [Google Scholar]
- 412.Morrison D. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic. Biol. Med. 2015;89:852–862. doi: 10.1016/j.freeradbiomed.2015.10.412. [DOI] [PubMed] [Google Scholar]
- 413.Paulsen G. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind, randomised, controlled trial. J. Physiol. 2014;592(8):1887–1901. doi: 10.1113/jphysiol.2013.267419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Saengsirisuwan V. Interactions of exercise training and lipoic acid on skeletal muscle glucose transport in obese Zucker rats. J. Appl. Physiol. 1985;91(1):145–153. doi: 10.1152/jappl.2001.91.1.145. 2001. [DOI] [PubMed] [Google Scholar]
- 415.Saengsirisuwan V. Effects of exercise training and antioxidant R-ALA on glucose transport in insulin-sensitive rat skeletal muscle. J. Appl. Physiol. 1985;92(1):50–58. doi: 10.1152/japplphysiol.000617.2001. 2002. [DOI] [PubMed] [Google Scholar]
- 416.Chae C.H., Shin C.H., Kim H.T. The combination of alpha-lipoic acid supplementation and aerobic exercise inhibits lipid peroxidation in rat skeletal muscles. Nutr. Res. 2008;28(6):399–405. doi: 10.1016/j.nutres.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 417.Khanna S. Alpha-lipoic acid supplementation: tissue glutathione homeostasis at rest and after exercise. J. Appl. Physiol. 1985;86(4):1191–1196. doi: 10.1152/jappl.1999.86.4.1191. 1999. [DOI] [PubMed] [Google Scholar]
- 418.Kinnunen S. alpha-Lipoic acid modulates thiol antioxidant defenses and attenuates exercise-induced oxidative stress in standardbred trotters. Free Radic. Res. 2009;43(8):697–705. doi: 10.1080/10715760903037673. [DOI] [PubMed] [Google Scholar]
- 419.Kinnunen S. alpha-Lipoic acid supplementation enhances heat shock protein production and decreases post exercise lactic acid concentrations in exercised standardbred trotters. Res. Vet. Sci. 2009;87(3):462–467. doi: 10.1016/j.rvsc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 420.Saengsirisuwan V. Interactions of exercise training and alpha-lipoic acid on insulin signaling in skeletal muscle of obese Zucker rats. Am. J. Physiol. Endocrinol. Metab. 2004;287(3):E529–E536. doi: 10.1152/ajpendo.00013.2004. [DOI] [PubMed] [Google Scholar]
- 421.Rousseau A.S. alpha-Lipoic acid up-regulates expression of peroxisome proliferator-activated receptor beta in skeletal muscle: involvement of the JNK signaling pathway. Faseb. J. 2016;30(3):1287–1299. doi: 10.1096/fj.15-280453. [DOI] [PubMed] [Google Scholar]
- 422.Kon M. Effect of Coenzyme Q10 supplementation on exercise-induced muscular injury of rats. Exerc. Immunol. Rev. 2007;13:76–88. [PubMed] [Google Scholar]
- 423.Belviranli M., Okudan N. Effect of coenzyme Q10 alone and in combination with exercise training on oxidative stress biomarkers in rats. Int. J. Vitam. Nutr. Res. 2018;88(3–4):126–136. doi: 10.1024/0300-9831/a000261. [DOI] [PubMed] [Google Scholar]
- 424.Svensson M. Effect of Q10 supplementation on tissue Q10 levels and adenine nucleotide catabolism during high-intensity exercise. Int. J. Sport Nutr. 1999;9(2):166–180. doi: 10.1123/ijsn.9.2.166. [DOI] [PubMed] [Google Scholar]
- 425.Gao L. Effects of coenzyme Q10 on vascular endothelial function in humans: a meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221(2):311–316. doi: 10.1016/j.atherosclerosis.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 426.Petiz L.L. Vitamin A oral supplementation induces oxidative stress and suppresses IL-10 and HSP70 in skeletal muscle of trained rats. Nutrients. 2017;9(4) doi: 10.3390/nu9040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Venditti P. Vitamin E supplementation modifies adaptive responses to training in rat skeletal muscle. Free Radic. Res. 2014;48(10):1179–1189. doi: 10.3109/10715762.2014.937341. [DOI] [PubMed] [Google Scholar]
- 428.Hoffman R.M., Olesiak M., Harris M.B. Effects of vitamin E supplementation and exercise on endothelial function in rat aortas. Faseb. J. 2013;27(1_supplement) lb661-lb661. [Google Scholar]
- 429.Miller E.R., 3rd Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 430.Kappus H., Diplock A.T. Tolerance and safety of vitamin E: a toxicological position report. Free Radic. Biol. Med. 1992;13(1):55–74. doi: 10.1016/0891-5849(92)90166-e. [DOI] [PubMed] [Google Scholar]
- 431.Alonso M., Collado P.S., Gonzalez-Gallego J. Melatonin inhibits the expression of the inducible isoform of nitric oxide synthase and nuclear factor kappa B activation in rat skeletal muscle. J. Pineal Res. 2006;41(1):8–14. doi: 10.1111/j.1600-079X.2006.00323.x. [DOI] [PubMed] [Google Scholar]
- 432.Hara M. Tissue changes in glutathione metabolism and lipid peroxidation induced by swimming are partially prevented by melatonin. Pharmacol. Toxicol. 1996;78(5):308–312. doi: 10.1111/j.1600-0773.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- 433.Hara M. Administration of melatonin and related indoles prevents exercise-induced cellular oxidative changes in rats. Biol. Signals. 1997;6(2):90–100. doi: 10.1159/000109113. [DOI] [PubMed] [Google Scholar]
- 434.Borges Lda S. Melatonin decreases muscular oxidative stress and inflammation induced by strenuous exercise and stimulates growth factor synthesis. J. Pineal Res. 2015;58(2):166–172. doi: 10.1111/jpi.12202. [DOI] [PubMed] [Google Scholar]
- 435.Beck W.R. Melatonin has an ergogenic effect but does not prevent inflammation and damage in exhaustive exercise. Sci. Rep. 2015;5:18065. doi: 10.1038/srep18065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Beck W.R., Scariot P.P., Gobatto C.A. Melatonin is an ergogenic aid for exhaustive aerobic exercise only during the wakefulness period. Int. J. Sports Med. 2016;37(1):71–76. doi: 10.1055/s-0035-1559698. [DOI] [PubMed] [Google Scholar]
- 437.Mendes C. Adaptations of the aging animal to exercise: role of daily supplementation with melatonin. J. Pineal Res. 2013;55(3):229–239. doi: 10.1111/jpi.12065. [DOI] [PubMed] [Google Scholar]
- 438.Rahman M.M. Melatonin supplementation plus exercise behavior ameliorate insulin resistance, hypertension and fatigue in a rat model of type 2 diabetes mellitus. Biomed. Pharmacother. 2017;92:606–614. doi: 10.1016/j.biopha.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 439.Mazepa R.C. Melatonin increases muscle and liver glycogen content in nonexercised and exercised rats. Life Sci. 2000;66(2):153–160. doi: 10.1016/s0024-3205(99)00573-1. [DOI] [PubMed] [Google Scholar]
- 440.Ferreira L.F., Campbell K.S., Reid M.B. N-acetylcysteine in handgrip exercise: plasma thiols and adverse reactions. Int. J. Sport Nutr. Exerc. Metabol. 2011;21(2):146–154. doi: 10.1123/ijsnem.21.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Greene S.C. Effervescent N-acetylcysteine tablets versus oral solution N-acetylcysteine in fasting healthy adults: an open-label, randomized, single-dose, crossover, relative bioavailability study. Curr. Ther. Res. Clin. Exp. 2016;83:1–7. doi: 10.1016/j.curtheres.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Agency W.A.-d. 2020. The World Anti-doping Code International Standard Prohibitied List January 2020. [Google Scholar]
- 443.Yfanti C. Antioxidant supplementation does not alter endurance training adaptation. Med. Sci. Sports Exerc. 2010;42(7):1388–1395. doi: 10.1249/MSS.0b013e3181cd76be. [DOI] [PubMed] [Google Scholar]
- 444.Yfanti C. Role of vitamin C and E supplementation on IL-6 in response to training. J. Appl. Physiol. 1985;112(6):990–1000. doi: 10.1152/japplphysiol.01027.2010. 2012. [DOI] [PubMed] [Google Scholar]
- 445.Yfanti C. Effect of antioxidant supplementation on insulin sensitivity in response to endurance exercise training. Am. J. Physiol. Endocrinol. Metab. 2011;300(5):E761–E770. doi: 10.1152/ajpendo.00207.2010. [DOI] [PubMed] [Google Scholar]
- 446.Clifford T. The effects of vitamin C and E on exercise-induced physiological adaptations: a systematic review and Meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019:1–11. doi: 10.1080/10408398.2019.1703642. [DOI] [PubMed] [Google Scholar]
- 447.Shafat A. Effects of dietary supplementation with vitamins C and E on muscle function during and after eccentric contractions in humans. Eur. J. Appl. Physiol. 2004;93(1–2):196–202. doi: 10.1007/s00421-004-1198-y. [DOI] [PubMed] [Google Scholar]
- 448.Theodorou A.A. No effect of antioxidant supplementation on muscle performance and blood redox status adaptations to eccentric training. Am. J. Clin. Nutr. 2011;93(6):1373–1383. doi: 10.3945/ajcn.110.009266. [DOI] [PubMed] [Google Scholar]
- 449.Yang G.Q. Endemic selenium intoxication of humans in China. Am. J. Clin. Nutr. 1983;37(5):872–881. doi: 10.1093/ajcn/37.5.872. [DOI] [PubMed] [Google Scholar]
- 450.Duffield-Lillico A.J. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J. Natl. Cancer Inst. 2003;95(19):1477–1481. doi: 10.1093/jnci/djg061. [DOI] [PubMed] [Google Scholar]
- 451.Ozturk A. Effects of zinc deficiency and supplementation on malondialdehyde and glutathione levels in blood and tissues of rats performing swimming exercise. Biol. Trace Elem. Res. 2003;94(2):157–166. doi: 10.1385/BTER:94:2:157. [DOI] [PubMed] [Google Scholar]
- 452.Paschalis V. Low vitamin C values are linked with decreased physical performance and increased oxidative stress: reversal by vitamin C supplementation. Eur. J. Nutr. 2016;55(1):45–53. doi: 10.1007/s00394-014-0821-x. [DOI] [PubMed] [Google Scholar]
- 453.Paschalis V. N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione. Free Radic. Biol. Med. 2018;115:288–297. doi: 10.1016/j.freeradbiomed.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 454.Margaritelis N.V. Antioxidants in personalized nutrition and exercise. Adv Nutr. 2018;9(6):813–823. doi: 10.1093/advances/nmy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.