Abstract
Introduction
Frail older people are known to have low rates of advance care planning (ACP). Many frail patients prefer less aggressive treatment, but these preferences are often not known or respected. Frail patients often have multiple hospital admissions, potentially providing opportunities for ACP.
Objective
To systematically review the literature concerning ACP with frail older people in the acute hospital, with particular reference to: (1) Does ACP improve outcomes? (2) What are the views of patients, relatives and healthcare professionals regarding ACP? (3) Does ACP currently occur? (4) What are the facilitators and barriers to ACP?
Design
Systematic literature review and narrative synthesis. Electronic search of MEDLINE, CINAHL, ASSIA, PsycINFO and Embase databases from January 1990 to May 2019 inclusive. Studies in the acute setting of populations with a mean age >75 years, not focused on a disease-specific terminal condition were included.
Results
16 133 articles were retrieved, 14 met inclusion criteria. No studies used an objective measure of frailty. One randomised controlled trial (RCT) found that ACP improves outcomes for older patients. Although 74%–84% of capacitous older inpatients are receptive to ACP, rates of ACP are 0%–5%; the reasons for this discrepancy have been little studied. The nature of ACP in clinical practice is unknown thus the extent to which it reflects the RCT intervention cannot be assessed. The outcomes that are important to patients are poorly understood and family and physician experiences have not been explored.
Conclusions
A better understanding of this area could help to improve end-of-life care for frail older people.
PROSPERO registration number
CRD42017080246.
Keywords: communication, chronic conditions, hospital care, prognosis
Introduction
Frailty is associated with major adverse health outcomes, including falls, delirium, and death, as well as greater use of hospital services.1 2 In the USA, patients with frailty are more than twice as likely to die in the intensive care unit than patients with cancer and the families of frail patients are less likely to rate their end-of-life care as excellent.3 Frail older people frequently prefer less aggressive medical care than they receive, but this is often not accurately recorded in their records.4 5 One large multicentre prospective study found that for those patients who prefer comfort care only, this was accurately documented in just 16% of cases.5
Patients with frailty, defined by requiring admission to a nursing home, who do not have cancer or organ failure have been identified as having a distinct dying trajectory.6 This ‘frail dying trajectory’ is characterised by higher levels of disability in the last year of life and unpredictability around the timing of death,6 and is estimated to be the most common type of dying trajectory, accounting for approximately 40% of deaths.7 Patients with frailty have been identified as having palliative care needs at similar levels to people with cancer,4 but differences in attitudes towards dying and talking about death have been noted between frail patients and those with cancer or organ failure, in part related to unpredictability of death and dying.8
ACP has been found to be beneficial in a range of patient groups and settings and to increase the likelihood of patients receiving end-of-life care in accordance with their wishes.9–11 However, evidence for the effect of ACP on outcomes such as psychological well-being or satisfaction with healthcare is mixed.9 11 Policy across North America, Europe and Australia endorses ACP as part of good medical care.12–14 In the USA, the Centres for Medicare and Medicaid Services pay providers for ACP in hospital and outpatient settings.15
A previous systematic review of ACP with frail older patients in any setting found that the majority of patients (61%–91%) would like to discuss end-of-life preferences but only a minority (2%–29%) have the opportunity for such discussions with a healthcare professional.16 There has been no systematic review of frail older people in the hospital inpatient setting, who may be regarded as a particularly ‘in need’ group, given their increased risk of morbidity and mortality.17 Moreover, admission to an acute hospital may be seen as a potential opportunity for ACP given the higher use of hospital services by frail patients.2
To our knowledge, this is the first systematic review of ACP with frail older people in the acute hospital setting. Our review questions, with respect to this patient population, are:
Does ACP improve outcomes?
What are the views of patients, relatives and healthcare professionals regarding ACP?
Does ACP currently occur?
What are the facilitators and barriers to ACP?
Methods
Search strategy and selection criteria
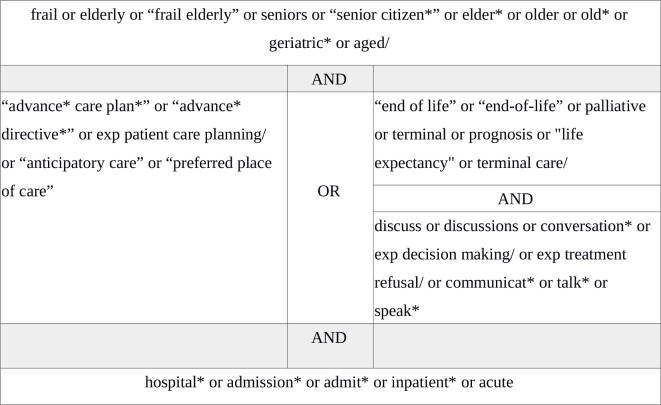
The protocol was developed using guidance from the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.18 An electronic search of MEDLINE, CINAHL, ASSIA, PsycINFO and Embase databases from 1 January 1990 to 31 May 2019 was developed in collaboration with a Medical Librarian (VP), using Medical Subject Headings and synonyms (figure 1). Reference lists of included studies were searched.
Figure 1.
MEDLINE search strategy.
Definition of terms
Frailty is defined as ‘a state of increased vulnerability to poor resolution of homeostasis following a stress, which increases the risk of adverse outcomes including falls, delirium and disability’.1 This definition is usually operationalised either phenotypically (eg, Fried’s Frailty Phenotype)19 or by assessment of accrual of cumulative deficits (eg, Frailty Index).20
Advance care planning (ACP) is a process that supports people in ‘understanding and sharing their personal values, life goals and preferences regarding future medical care. The goal of ACP is to help ensure that people receive medical care that is consistent with their values, goals and preferences during serious and chronic illness’.21 An Advance Directive (AD) is a legal document that specifies treatments a patient wishes or does not wish to receive if they lose capacity.22 ACP may lead to the creation of an AD.
Inclusion and exclusion criteria
A preliminary scoping review found no potential papers which identified patients using an objective measurement of frailty. In view of this, the inclusion and exclusion criteria were developed to capture studies of mixed populations that would include a significant proportion of frail patients, as measured by a validated operationalisation of frailty, drawing on a previous systematic review in this area16 and reflecting other disease-specific reviews that have included mixed populations.23 A mean age criterion of 75 years was chosen in view of the strong correlation between frailty and older age (70% of hospitalised patients over the age of 75 years have been found to be frail),24 and this was combined with the absence of focus on a disease-specific terminal condition, for example, malignancy, organ failure or dementia. ACP was considered to be discussions with patients about their personal values, life goals and preferences regarding future medical care.21 Studies focusing on resuscitation decisions in isolation or on the goals-of-care for the current hospital admission without planning for future episodes were excluded. Studies on ADs were included. Articles published prior to 1990 were excluded because the existing constructs of ACP only came into existence in the mid-1990s to late 1990s and so studies prior to 1990 were not felt to be directly relevant to current practice.25 Articles that were not published in English were excluded because resources were not available for translation. Table 1 shows the full list of inclusion and exclusion criteria.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria | |
| Patient characteristics | Mean age >75 years. | Mean age <75 years. |
| Focus on a disease-specific terminal condition, eg, malignancy, organ failure or dementia. | ||
| Content of advance care planning conversations | Discussions with patients about their personal values, life goals and preferences regarding future medical care. | Discussions concerning resuscitation or goals-of-care for current admission. |
| Discussions of end-of-life plans or advance care plans or advance directives. | Appointment of a healthcare proxy without other elements of advance care planning. | |
| Assisted suicide or euthanasia. | ||
| Discharge planning. | ||
| Care in the last few days of life. | ||
| Setting | Acute inpatient setting, including data from several settings where acute hospital data are presented separately. | Outpatient clinics, general practitioner clinic, care home or rehabilitation setting. |
| All global healthcare systems. | ||
| Publication characteristics | All research methods presenting new empirical data. | Types of article: opinion pieces, guidelines, individual case reports, study proposals/protocols, conference abstracts, PhD theses, grey literature and non-peer-reviewed journals. |
| Articles not published in English. | ||
| Articles published prior to 1990. |
After exclusion of duplicate and irrelevant titles, abstracts were independently assessed for eligibility by two reviewers (SAH and AB) with disagreement resolved by consensus. Full-texts of potentially relevant papers were assessed by SAH with independent second review by AB where eligibility was unclear.
Data extraction, quality appraisal and data synthesis
Data relevant to the review questions was extracted into a study-specific data extraction sheet. Included papers were independently weighted by two reviewers using Gough’s Weight of Evidence (WoE) criteria (table 2).26 Gough’s WoE has been widely used in systematic reviews of heterogeneous literature. It was selected because in addition to appraising the quality of the evidence (WoE A), it assesses the appropriateness (WoE B) and relevance (WoE C) of the study to the review questions, and thus guides how much emphasis should be given to a particular study when answering a review question. For component A of the WoE criteria, which assesses study quality in its own terms, the reviewers referred to the relevant EQUATOR network reporting guidelines for the study design in question. For each WoE component (A–D), papers were weighted as high (H), medium (M) or low (L) quality. Any discrepancies in weightings were discussed and consensus achieved.
Table 2.
Gough’s Weight of Evidence (WoE) criteria*26
| WoE A | This is a generic and thus non-review-specific judgement about the coherence and integrity of the evidence in its own terms. That may be the generally accepted criteria for evaluating the quality of this type of evidence by those who generally use and produce it. |
| WoE B | This is a review-specific judgement about the appropriateness of that form of evidence for answering the review question, that is the fitness for purpose of that form of evidence. For example, the relevance of certain research designs such as experimental studies for answering questions about process. |
| WoE C | This is a review-specific judgement about the relevance of the focus of the evidence for the review question. For example, a research study may not have the type of sample, the type of evidence gathering or analysis that is central to the review question or it may not have been undertaken in an appropriate context from which results can be generalised to answer the review question. There may also be issues of propriety of how the research was undertaken such as the ethics of the research that could impact on its inclusion and interpretation in a review (Pawson et al 2003). |
| WoE D | WoE A, B and C are combined to form an overall assessment WoE D of the extent that a study contributes evidence to answering a review question. |
*Reprinted with permission from Routledge, original copyright 2007.
Data synthesis used a narrative approach, chosen to allow synthesis of both quantitative and qualitative evidence and involved three iterative steps27:
Development of a preliminary synthesis: for each research question, a textual description of the relevant studies was generated from the data extraction sheets. Studies were grouped into clusters where relevant, for example, according to the methodological approach. Thematic analysis was performed.
Exploring relationships in the data: conceptual mapping was used to identify commonalities and differences and to map relationships between themes.
Assessing the robustness of the synthesis: each stage of the synthesis was informed by the Gough’s WoE framework. Papers rated as high quality were considered more credible and relevant. Papers judged to be of low quality were included in the synthesis but the low quality of the evidence was highlighted to identify gaps in knowledge and need for further research.
Results
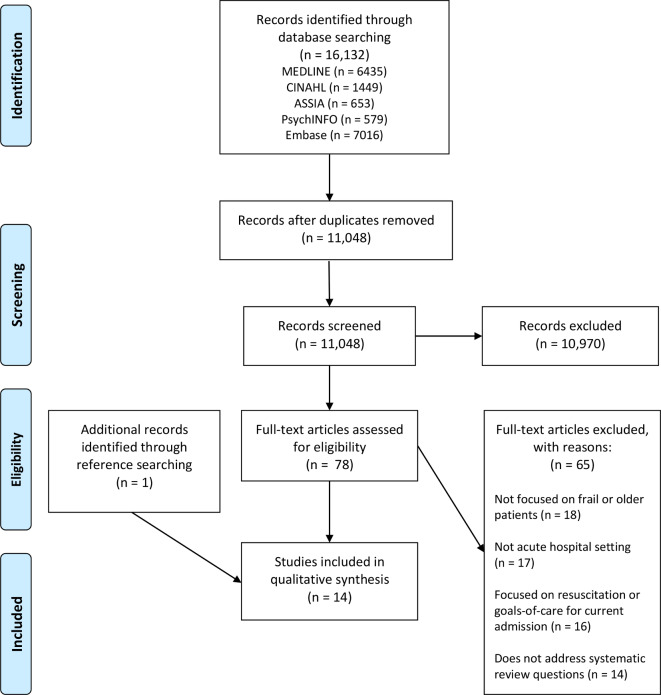
Of 16 133 records identified, 14 studies were included in the final synthesis. The PRISMA flow diagram (adapted from Moher et al 18) is presented in figure 2.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
Ten papers reported information on patients (combined n=114 785, range of n=17–113 612, mean age=78 years). Four studies reported information on healthcare professionals. Five studies were conducted in the USA, four each in Australia and Europe and one in Canada. Eight studies were cross-sectional observational studies; four were interventional; one randomised controlled trial (RCT), one pre-post study, one a non-randomised trial and one an interrupted time series. Two studies were qualitative; one semi-structured interview study and one focus-group study.
There have been no studies investigating ACP with frail patients in the acute setting which have characterised participants using a operational measure of frailty or a measure of functional status. Four papers provided information about discharge destination or place of residence prior to admission and one of these also provided data on number of comorbidities. The remaining six studies that reported information on patients provided no information about need for assistance with activities of daily living nor level of comorbidity. All four studies that reported information on healthcare professionals asked about views on ACP in the hospital setting with older patients in general and were not specific to frailty. Further study characteristics are shown in table 3. The main findings from each of the included papers is summarised in table 4.
Table 3.
Summary of characteristics of included papers
| Study | Design | Aim | Sample | Weight of evidence* |
| Barnato et al 38 | Observational: cross-sectional | Explore uptake of the ACP billing code in the USA | 113 612 inpatients aged >65 years in 250 US hospitals. Mean age 78 years, diagnosis: 3% cancer, 23% organ failure, 4% dementia, 70% other; 49% requiring home healthcare, care home or inpatient care at discharge or deceased. | High (H, H, H) |
| Black41 | Observational: cross-sectional | Describe social workers' AD communications with hospitalised older patients | 29 social workers in 6 US hospitals. | Low (L, L, L) |
| Black34 | Qualitative: focus groups | Compare nurses' and social workers' roles in AD communication with older patients | 6 nurses and 5 social workers in one US hospital selected from practice areas with high volumes of older patients. | Low (L, M, L) |
| Black and Emmet35 | Observational: cross-sectional | Describe nurses' communication about ADs with hospitalised older patients | 74 nurses in 2 US hospitals working primarily with geriatric patients. | Low (L, L, L) |
| Bristowe et al 29 | Interventional: non-randomised trial | Compare experience of care when supported by an intervention including ACP with standard care | Next of kin of 95 medical inpatients in UK hospitals who died <100 days after discharge. Mean age 77 years, 31% cancer diagnosis, 69% non-cancer, no information regarding need for help with personal care. | Medium (L, M, M) |
| Cantillo et al 39 | Interventional: interrupted time series | Design, implement and evaluate an ACP programme, focusing on hospitalised older patients | Inpatients aged >80 years with 2 admissions in the last 6 months, or aged >90 years, or discharged to hospice services in 4 hospitals in Hawai'i. No further information on sample characteristics. | Low (M, L, L) |
| Cheang et al 30 | Observational: cross-sectional | Assess prevalence of ACP; to explore the feasibility of an ACP screening interview. | 100 consecutive inpatients aged >80 years at an Australian hospital. Mean age 87 years, diagnosis: 3% cancer, 3% organ failure, 6% stroke, 88% other diagnoses; 67% requiring care home or inpatient care at discharge. | Medium (M, H, M) |
| Detering, 201028 | Interventional: RCT | Assess whether ACP with older inpatients improves outcomes | 309 medical inpatients aged >80 years at an Australian hospital. Median age 84–85 years, admission diagnosis 32%–34% cardiac, 30%–33% respiratory, 8%–14% falls, 24%–25% other. No information regarding need for help with personal care. | High (H, H, M) |
| Detering et al 40 | Observational: cross-sectional | Assess feasibility and acceptability of ACP in older non-English-speaking patients | 112 inpatients aged >65 years at a teaching hospital in Australia. Median age 81–82 years, diagnosis 35%–39% cardiopulmonary, 17%–24% cancer, 8%–13% neurological, 29%–35% other. No information regarding need for help with personal care. | Low (M, L, L) |
| Peck et al 33 | Qualitative: semi-structured interviews | Determine the barriers and facilitators to ACP engagement in hospital | 17 inpatients: 2 aged >85 with an acute medical problem, 15 whose doctor would not be surprised if they died in <6 months in one Canadian hospital. Mean age 75 years. No information on diagnosis nor need for help with personal care. | High (H, H, M) |
| Pérez et al 36 | Observational: cross-sectional | Determine opinions of hospital doctors and nurses on ADs | 283 hospital physicians and nurses in Spain. | Low (M, L, L) |
| Schiff et al 31 | Observational: cross-sectional | Determine older inpatients’ knowledge about ADs | 74 medical inpatients aged >65 years at two UK hospitals. Mean age 81 years. No information on diagnosis; 50% ‘received home help’, no other information on need for help with personal care. | Medium (M, M, M) |
| Schiff et al 32 | Observational: cross-sectional | Evaluate an ACP document for older inpatients | 99 inpatients aged >60 years on geriatric wards in two UK hospitals. Mean age 81 years. No information on diagnosis nor need for help with personal care. | Medium (M, M, M) |
| Scott et al 37 | Interventional: before and after study | To develop, implement and assess an ACP programme in an Australian hospital | 381 medical inpatients with estimated life expectancy of <12 months. Mean age 78 years, mean number of comorbidities 3.9, 37% living in care home on admission. | Medium (M, M, H) |
*Weight of evidence D (weight of evidence A, weight of evidence B, weight of evidence C, where L=low, M=medium, H=high).
ACP, advance care planning; AD, Advance Directive; RCT, randomised controlled trial.
Table 4.
Summary of main findings of included papers
| Study | Aim | Main findings |
| Barnato et al 38 | Explore uptake of the ACP billing code in the USA | 5.4% of all admissions involved a billed ACP conversation. The average age among patients with a billed ACP conversation was higher, and the prevalence of cancer, heart failure and dementia was higher in this group. ACP rates varied from 0% to 35% at the hospital-level and 0% to 93% at the physician-level. Most ACP discussions were held by 25% of physicians while a third of physicians never billed for ACP. |
| Black41 | Describe social workers' communication about ADs with hospitalised older patients | Social workers play an active role in AD communication. The majority felt the amount of time they spend is inadequate. |
| Black34 | Compare nurses' and social workers' roles in AD communication with older patients | Both nurses and social workers felt their role was to primarily help educate patients about ADs, including their benefits, and also to ensure that families understand a patient's wishes. Nurses were particularly focused on explaining outcomes of particular treatment options, such as cardiopulmonary resuscitation, so that patients could make informed decisions. |
| Black and Emmet35 | Describe nurses' communication about ADs with hospitalised older patients | Aspects of communication that nurses reported most frequently were disclosure of information and initiation of topic. Nurses with their own AD were more likely to initiate the topic with patients. |
| Bristowe et al 29 | Compare experience of care when supported by an intervention including ACP with standard care | Relatives of patients in the intervention group reported that patients were significantly more likely to have spoken to their doctor about their poor prognosis and to know they may die. Relatives were less likely to feel the information they had received was clear and understandable. |
| Cantillo et al 39 | Design, implement and evaluate an ACP programme, focusing on hospitalised older patients | The programme interventions included ACP facilitators, clinician and public education, standardised electronic documentation. During the programme, ACP increased from 29% to 87%. No data provided about ACP rates prior to commencement of programme. |
| Cheang et al 30 | Assess prevalence of ACP; to explore the feasibility of an ACP screening interview | No patients had an ACP in their current medical notes. All patients were at least somewhat comfortable discussing ACP and 82% of patients were very comfortable; 79% of patients said they would be comfortable having further discussions about ACP. |
| Detering et al 28 | Assess whether ACP with older inpatients improves outcomes | Patients in the intervention group reported higher satisfaction with their hospital admission. Among patients who died within 6 months of the intervention (n=56), end-of-life wishes were more likely to be known and respected, family members had lower anxiety, depression and stress and family members were more likely to be satisfied with the quality of the death. |
| Detering et al 40 | Assess feasibility and acceptability of ACP in older non-English-speaking patients | In patients from a non-English-speaking background, the use of formal interpreters was associated with higher rates of advance care directive completion (p<0.005). |
| Peck et al 33 | Determine the barriers and facilitators to ACP engagement in hospital | Some patients felt hospital was an appropriate time to discuss ACP while others felt it was the wrong time. Some patients were motivated to engage in ACP to achieve certain goals while other patients described focusing their energy on living in the moment and found that engaging in ACP stripped them of this possibility. Some patients felt comfortable discussing death, and making plans in the face of uncertainty, while others felt they could not engage in ACP because they did not know what would happen in the future, or felt that death was unlikely. |
| Pérez et al 36 | Determine opinions of hospital doctors and nurses on ADs | 43% favoured AD discussions with all ‘elderly’ inpatients, however most doctors did not have an accurate understanding of ADs and had never discussed them with patients. |
| Schiff et al 31 | Determine older inpatients’ knowledge about ADs | 74% expressed interest in writing an AD. Of those interested in writing an AD, 50% wanted to ensure their wishes were known and 44% wanted to relieve burden on family. |
| Schiff et al 32 | Evaluate an ACP document for older inpatients | In patients administered the ACP tool, 31% completed an ACP; 22% of patients did not open the information; 84% of patients who completed the feedback questionnaire felt the ACP tool addressed an area of healthcare that was important. Reasons for not completing an ACP included feeling the content was not relevant/they did not wish to discuss end of life care, and wishing to consider further. |
| Scott et al 37 | To develop, implement and assess an ACP programme in an Australian hospital | Pre-ACP intervention implementation, 0.6% of patients completed an ACP in hospital, post- ACP intervention, 41% of patients completed an ACP in hospital. Of those approached by a clinician to discuss ACP, 77% completed an ACP. Clinicians did not discuss ACP with 47% of eligible patients. Reasons included discharge prior to discussion, and patient/family felt unable to participate. |
ACP, advance care planning; AD, Advance Directive.
Three papers were rated as providing high-quality evidence, five as medium quality and six as low quality. Table 5 gives a summary of the quality of evidence broken down by review subquestion.
Table 5.
Quality of available evidence, broken down by review subquestion
| Review subquestion | High-quality papers (n=3) | Medium-quality papers (n=5) | Low-quality papers (n=6) |
| 1. Does ACP improve outcomes? (n=2) | Detering et al 28 (n=1) | Bristowe et al 29 (n=1) | (n=0) |
| 2. What are the views of patients, relatives and healthcare professionals regarding ACP? (n=8) | Peck et al 33 (n=1) | Cheang et al,30 Schiff et al,31 Schiff et al 32 (n=3) | Black,41 Black,34 Black and Emmet,35 Perez et al 36 (n=4) |
| 3. Does ACP currently occur? (n=4) | Barnato et al,38 Detering et al 28 (n=2) | Cheang et al,30 Scott et al 37 (n=2) | (n=0) |
| 4. What are the facilitators and barriers to ACP? (n=11) | Barnato et al 38 Detering et al,28 Peck et al 33 (n=3) | Cheang et al,30 Scott et al 37 (n=2) | Black,41 Black,34 Black and Emmet,35 Cantillo et al,39 Detering et al,40 Perez et al 36 (n=6) |
ACP, advance care planning.
The results are presented by review questions.
Does ACP improve outcomes? (n=2)
One high-quality single-centre RCT assessed the effect of an ACP intervention with medical inpatients aged 80 years or older (n=309).28 The intervention group received formal ACP from a trained facilitator at some point during their admission, with additional input as needed from treating doctors to ensure that patients understood their illness, treatment options and likely prognosis. Patients in the intervention group reported higher satisfaction with their hospital admission; among patients who died within 6 months of the intervention (n=56), end-of-life wishes were more likely to be known and respected, family members had lower anxiety, depression and stress and family members were more likely to be satisfied with the quality of the death. The effect on patient quality of life was not assessed.
A non-randomised trial of medium quality surveyed the relatives of patients who had died within 100 days of discharge, and compared relatives of patients who had received an intervention including ACP with those who had been on wards where the intervention had not been implemented.29 Relatives of patients in the intervention group reported that patients were significantly more likely to have spoken to their doctor about their poor prognosis and to know they may die, however relatives were less likely to feel the information they had received was clear and understandable.
What are the views of patients, relatives and healthcare professionals regarding ACP? (n=8)
Three small questionnaire studies, all of medium quality, asked older inpatients about their views on ACP and found 74%–84% were receptive to ACP (combined n=157).30–32 One small study asked patients for their reasons for wanting to participate in ACP: 50% wanted to ensure their wishes were known and 44% wanted to relieve burden on family (n=50).31 None of the patients in these studies had participated in ACP with a healthcare professional.
One high-quality interview study asked patients about their experiences of ACP initiated at the end of an acute hospital admission.33 Some patients felt this was an appropriate time to discuss ACP and that the context reinforced the importance of planning for the future. However, others felt that this was the wrong time, for example, “I don’t need to talk about this today, I want to focus on getting well”. Some patients were motivated to engage in ACP to achieve certain goals, such as a home death or lessening the decision-making burden on their family, while other patients described focusing their energy on living in the moment and found that engaging in ACP stripped them of this possibility. Some patients felt comfortable discussing death, and making plans in the face of uncertainty, while others felt they could not engage in ACP because they did not know what would happen in the future, or felt that death was unlikely.
There has been no research into the views or experiences of relatives or informal caregivers.
Four studies, three of low quality and one of medium quality, investigated the experiences of healthcare professionals.34–37 Nurses and social workers described their role as primarily educating older inpatients on ADs.34 35 A survey of Spanish hospital physicians and nurses (n=283) found 43% favoured AD discussions with all ‘elderly’ inpatients, however most doctors did not have an accurate understanding of ADs and had never discussed them with patients.36 Healthcare professionals had mixed views on the most appropriate setting for ACP.36 37
Does ACP currently occur? (n=4)
Four studies reported very low rates of ACP (0%–5.4%) with older inpatients.28 30 37 38 Two of these studies were of high quality and two of medium quality and together included 114 033 patients. These studies used the presence of a documented advance care plan or ACP billing codes to determine rates of ACP. The form and content of ACP with frail older patients in routine clinical practice outside of the research setting is unknown.
One high-quality retrospective cross-sectional study of 113 612 patients across 250 hospitals analysed ACP rates in patients aged over 65 years by hospital and physician. ACP rates varied from 0% to 35% at the hospital-level and 0% to 93% at the physician-level. Most ACP discussions were held by 25% of physicians while a third of physicians never billed for ACP.38
What are the facilitators and barriers to ACP? (n=11)
Three interventional studies reported postintervention ACP rates of 77%–87% in capacitous patients.28 37 39 This, combined with the wide variation in ACP rates at the hospital and physician-level outlined above,38 suggests there are important barriers and facilitators at the hospital and healthcare professional-level.
Hospital and healthcare professional-level factors
Interventions implemented to increase ACP include dedicated ACP facilitators, healthcare professional education, electronic prompts and standardised ACP documentation.28 37–39 Using combinations of these interventions, three studies reported high rates of ACP.28 37 39 However, average ACP rates of just 5% were observed in a national group of hospitals which had implemented mandatory ACP training, personal financial incentives for facilitating ACP and electronic prompts.38
Patients describe that good physician communication skills, including demonstrating they have time for ACP, and adequate ACP information are important facilitators.33 Absence of privacy is a barrier to ACP in hospital.33 The use of an interpreter is an important facilitator in patients from a non-English-speaking background.40 ACP documents made prior to admission are often not available or identified during an admission.30
The evidence concerning healthcare professionals’ perceptions of ACP barriers and facilitators is limited. Nurses and social workers feel they have inadequate time for AD conversations in hospital, especially if patients have ‘unrealistic’ expectations about their illness.34 41 Nurses who themselves have an AD are more likely to initiate conversations with patients.35 There is no evidence on the views of physicians, although there is some low-quality evidence that physicians’ understanding of ADs is poor.36
Patient-level factors
Even in an environment geared to promoting ACP, 13%–23% of capacitous patients decline ACP.28 37 39 One interventional study documented the reasons patients did not participate: 15% of patients felt their prognosis did not warrant ACP, 15% did not want to discuss ACP and 7% felt their family understood their wishes. For 58% this was attributed to ‘various capacity constraints’ including poor health literacy, cultural taboos, limited English and patient illness.37 A qualitative study of patient’s experiences of ACP in hospital found significant variation in patients’ attitudes to ACP33 (see question 2). The likelihood of achieving completed ACP documentation during an inpatient admission correlates strongly with the presence of family members at the ACP discussion.28
Three studies identified lack of decision-making capacity as a barrier for 42%–57% of older inpatients.28 30 37 Notably, only one study followed up patients after discharge prior to death and reported ‘numerous participants did not remember the conversation about ACP while in hospital’, while other participants remembered the conversation but felt too sick to engage properly with the discussion at the time.33 All of the patients in this study had been assessed to have capacity at the time of ACP in hospital.
Although many studies point to possible barriers and facilitators, only one study was designed to investigate this question, through exploring patients’ experiences.33 There have been no process evaluations of ACP interventions, and no studies including physicians. The evidence to answer this review question is limited.
Discussion
Summary of key findings
This is the first systematic review of ACP with frail older inpatients. It demonstrates that there have been no studies of ACP with frail patients in the acute setting that have used an operational measure of frailty or a measure of functional status to characterise study participants. As a result, this systematic review reports on studies with a mean age of >75 years and no disease-specific diagnosis. Such studies are likely to include a high proportion of frail patients,24 but the number of frail patients in each study and their level of frailty is unknown.
Although 74%–84%30–32 of capacitous older patients are receptive to ACP in the acute hospital, only 0%–5% of patients participate in ACP.28 30 37 38 There is evidence from a single RCT that ACP with hospitalised older patients improves outcomes for patients who die within 6 months.28
Despite RCT evidence, there is a wide variation in ACP rates but the reasons for this are not understood.38 The experiences of patients who have participated in ACP are diverse, suggesting more complexity than is captured by the RCT findings.33 The nature of ACP in clinical practice is not known and thus the extent to which it replicates the RCT intervention cannot be assessed and caution should be exercised when predicting its benefits. Superficially, similar interventions have had variable success at achieving higher ACP rates. Family and physician experiences have not been explored, which might provide insight into barriers and facilitators of ACP with frail older inpatients.
Implications for practice, policy and future research
This systematic review provides evidence for physicians that they could usefully offer ACP to older hospitalised patients, or assist ACP facilitators to do so. Notably, while a large majority of patients expressed an interest in participating in ACP, experiences of those who had done so were mixed: many patients are ‘ambivalent’ to ACP, simultaneously experiencing benefits and unpleasant feelings.42
Clinicians need to be aware that 13%–23% of older patients would prefer not to participate in ACP, and that patients may have limited recollection of ACP conversations held in hospital. Furthermore, there is evidence in other patient groups of a ‘hospitalisation dip’, where patients initially prefer less aggressive treatment following a hospital admission but revert to wanting more life-sustaining treatments over time.43 Clinicians could usefully consider arranging a postdischarge review of ACP discussions and documents made in hospital.
At an organisational level, this review provides some evidence that hospitals should seek to support ACP practice and that hospital-level factors may account for a significant amount of variation in ACP rates. Further evidence is needed on the best way to increase ACP but multicomponent interventions including ACP facilitators, healthcare professional and patient education and standardised documentation have been successful in some sites.
Policy already advocates offering patients ACP12–14: this systematic review provides evidence to endorse this, while also demonstrating that there remain considerable areas of uncertainty. Policy could usefully emphasise the need for postdischarge review of ACP discussions held in hospital.
This systematic review highlights several important areas where further research is needed. First and foremost, research using a validated operational measure of frailty is needed. Better characterisation of the study population would improve the generalisability of the research findings. It would also allow insight into whether level of frailty affects attitudes to or outcomes from ACP, and may help to explain some of the diversity of patients’ experiences of ACP.
Second, there have been no process evaluations of ACP interventions with older inpatients. ACP is a complex intervention and a process evaluation would help to identify its mechanisms so that these ‘key ingredients’ can be replicated in other settings and similar outcomes achieved. A process evaluation would also assess intervention fidelity (how the intervention was actually delivered on the ground) and context (factors external to the intervention that effect its implementation or outcome),44 thereby aiding understanding of barriers and facilitators to ACP. Without a process evaluation, it is difficult to be confident that implementing ACP interventions in other settings will lead to the same beneficial outcomes measured in the single RCT. This is particularly the case given that RCTs of ACP in other contexts have shown various levels of success.10 11 Furthermore, there is an identified lack of research on ACP implementation and process evaluations in all patient groups and settings,45 limiting the ability to extrapolate from other areas.
Third, there is limited evidence regarding why rates of ACP with hospitalised older patients are so low or the nature of ACP that currently occurs in clinical practice. Nor are the reasons for the wide variation in practice understood, at the hospital-level or individual physician-level. Understanding this better would help to design effective strategies for increasing ACP. Experiences of healthcare professionals might shed light on these issues.
Fourth, questions remain around outcomes from ACP. There is limited evidence regarding what older patients would like to achieve through ACP, no data on whether ACP affects quality of life and limited data on patients who do not die within 6 months of receiving ACP. The diversity of patient experience suggests that there is more complexity than is captured by the findings of the single RCT: a better understanding of what type of conversations are helpful, in what way they are helpful, in which circumstances, and for whom, is urgently needed. The experiences of relatives and informal caregivers have not been adequately investigated, although there is evidence for reduced stress, anxiety and depression in bereaved relatives.
The relationship between discussion of prognosis and ACP is of notable interest. The only RCT in this review did not include discussion of prognosis, which was instead left to treating doctors ‘as needed’.28 The frequency and impact of these conversations is not known. In contrast, the other outcome study did include discussion of prognosis, although the intervention group was less likely to feel they had received clear information.29 Discussion of prognosis has been identified as a key barrier to transition to palliative care in the acute hospital46 and physicians find discussion of uncertain prognosis difficult.46 47 It remains uncertain whether discussion of prognosis a necessary part of ACP with frail older patients. Although a majority of frail older patients would like to participate in ACP,16 30 32 there is less evidence regarding their preferences for discussing prognosis and the evidence that does exist is mixed.48 49 Unpicking the relationship between ACP and discussion of prognosis may help guide clinicians’ practice and also address a potential barrier to ACP.
Strengths and limitations
There have been no studies that have looked specifically at ACP with frail older patients in the inpatient setting, and nor have there been any studies that have used an operational measure of frailty. The prevalence and severity of frailty in the studies included in this systematic review is therefore unknown. As a result, the extent to which the study findings representative of frail older people is unknown, however the study findings are generalisable to older patients without a disease-specific terminal diagnosis in the acute hospital setting.
Frail older patients are difficult to target in a search strategy. Broad search terms were therefore adopted, resulting in low specificity and many titles to screen: good search sensitivity is demonstrated by only one additional paper being identified by reference searching. Both health and social care databases were included since the topic is cross-disciplinary.
This review included a wide interpretation of ACP and aimed to use thematic analysis and narrative synthesis to investigate commonalities and differences. The advantage of this approach is the review includes a broader evidence base; the disadvantage is the results are heterogeneous and different studies are not directly comparable. This review distinguished between goals-of-care conversations and ACP but certain studies included elements of both making it difficult to separate the evidence.29 39
Study quality was variable as the weight of evidence scoring reflects. There is a risk of participation bias: patients who are receptive to ACP maybe more likely to agree to participate in a study about ACP. However, most studies had good response rates, helping to mitigate this risk. A grey literature search was not undertaken thus there is a risk of publication bias. All studies were conducted in Western cultures and studies not published in English were excluded, limiting extrapolation to other settings.
Conclusions
Frailty causes many deaths, yet frail patients receive poor end-of-life care.3 This systematic review highlights that ACP in the acute hospital can improve outcomes for older patients and relatives, yet ACP rates remain low. However, mixed patient experiences and variable success in increasing ACP rates despite apparently similar interventions point to the complexity of ACP in clinical practice. This complexity needs to be better understood if we are to understand the prevailing mismatch between RCT evidence for ACP and clinical practice, thereby improving end-of-life care for frail older people, the ‘disadvantaged dying’.50
Footnotes
Twitter: @saahopkins
Contributors: All authors contributed to study design. SAH, VP and SB designed the search strategy. SAH, AB and screened abstracts, extracted data and assessed evidence quality. SAH and SB analysed and interpreted the data. SAH drafted the manuscript. All authors provided critical feedback and revisions on the manuscript. All authors approved the final version for submission.
Funding: SH is supported by the Addenbrooke's Charitable Trust and the Evelyn Trust. SB is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East of England at Cambridgeshire and Peterborough NHS Foundation Trust. He is also supported by the NIHR School for Primary Care Research. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Disclaimer: The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Clegg A, Young J, Iliffe S, et al. . Frailty in elderly people. Lancet 2013;381:752–62. 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilardi F, Scarcella P, Proietti MG, et al. . Frailty as a predictor of mortality and hospital services use in older adults: a cluster analysis in a cohort study. Eur J Public Health 2018;28:842–6. 10.1093/eurpub/cky006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wachterman MW, Pilver C, Smith D, et al. . Quality of end-of-life care provided to patients with different serious illnesses. JAMA Intern Med 2016;176:1095–102. 10.1001/jamainternmed.2016.1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stow D, Spiers G, Matthews FE, et al. . What is the evidence that people with frailty have needs for palliative care at the end of life? A systematic review and narrative synthesis. Palliat Med 2019;33:399–414. 10.1177/0269216319828650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heyland DK, Barwich D, Pichora D, et al. . Failure to engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med 2013;173:778–87. 10.1001/jamainternmed.2013.180 [DOI] [PubMed] [Google Scholar]
- 6. Lunney JR, Lynn J, Foley DJ, et al. . Patterns of functional decline at the end of life. JAMA 2003;289:2387–92. 10.1001/jama.289.18.2387 [DOI] [PubMed] [Google Scholar]
- 7. The gold standards framework centre, the GSF prognostic indicator guidance, 2011. Available: https://www.goldstandardsframework.org.uk/cd-content/uploads/files/General%20Files/Prognostic%20Indicator%20Guidance%20October%202011.pdf [Accessed 20 Jan 2020].
- 8. Kendall M, Carduff E, Lloyd A, et al. . Different experiences and goals in different advanced diseases: comparing serial interviews with patients with cancer, organ failure, or frailty and their family and professional carers. J Pain Symptom Manage 2015;50:216–24. 10.1016/j.jpainsymman.2015.02.017 [DOI] [PubMed] [Google Scholar]
- 9. Houben CHM, Spruit MA, Groenen MTJ, et al. . Efficacy of advance care planning: a systematic review and meta-analysis. J Am Med Dir Assoc 2014;15:477–89. 10.1016/j.jamda.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 10. Brinkman-Stoppelenburg A, Rietjens JAC, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med 2014;28:1000–25. 10.1177/0269216314526272 [DOI] [PubMed] [Google Scholar]
- 11. Weathers E, O'Caoimh R, Cornally N, et al. . Advance care planning: a systematic review of randomised controlled trials conducted with older adults. Maturitas 2016;91:101–9. 10.1016/j.maturitas.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 12. Dying in America: improving quality and honoring individual preferences near the end of life, 2015. Institute of medicine of the National academies. Available: https://www.nap.edu/catalog/18748/dying-in-america-improving-quality-and-honoring-individual-preferences-near [Accessed 16 Oct 2019]. [PubMed]
- 13. Quality statement 3: assessment, care planning and review, end of life care for adults, quality Standards, 2017. National Institute for health and care excellence. Available: https://www.nice.org.uk/guidance/qs13/chapter/Quality-statement-3-Assessment-care-planning-and-review [Accessed 16 Oct 2019].
- 14. Position statement on end of life care and advance care planning, 2014. Austrialian Medical association. Available: https://ama.com.au/sites/default/files/documents/AMA_position_statement_on_end_of_life_care_and_advance_care_planning_2014.pdf [Accessed 16 Oct 2019].
- 15. Advance Care Planning Medicare Learning Network Fact Sheet, Centers for Medicare and Medicaid services, 2019. Available: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/Downloads/AdvanceCarePlanning.pdf [Accessed 16 Oct 2019].
- 16. Sharp T, Moran E, Kuhn I, et al. . Do the elderly have a voice? Advance care planning discussions with frail and older individuals: a systematic literature review and narrative synthesis. Br J Gen Pract 2013;63:e657–68. 10.3399/bjgp13X673667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gill TM, Gahbauer EA, Han L, et al. . The role of intervening hospital admissions on trajectories of disability in the last year of life: prospective cohort study of older people. BMJ 2015;350:h2361 10.1136/bmj.h2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fried LP, Tangen CM, Walston J, et al. . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–57. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 20. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001;1:323–36. 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sudore RL, Lum HD, You JJ, et al. . Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage 2017;53:821–32. 10.1016/j.jpainsymman.2016.12.331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Advance Care Planning: Healthcare Directives National Institute on aging. Available: https://www.nia.nih.gov/health/advance-care-planning-healthcare-directives [Accessed 14 Oct 2019].
- 23. Schichtel M, Wee B, Perera R, et al. . The effect of advance care planning on heart failure: a systematic review and meta-analysis. J Gen Intern Med 2019. 10.1007/s11606-019-05482-w. [Epub ahead of print: 12 Nov 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Richards SJG, D'Souza J, Pascoe R, et al. . Prevalence of frailty in a tertiary Hospital: a point prevalence observational study. PLoS One 2019;14:e0219083 10.1371/journal.pone.0219083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sabatino CP. The evolution of health care advance planning law and policy. Milbank Q 2010;88:211–39. 10.1111/j.1468-0009.2010.00596.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gough D. Weight of evidence: a framework for the appraisal of the quality and relevance of evidence. Res Pap Educ 2007;22:213–28. 10.1080/02671520701296189 [DOI] [Google Scholar]
- 27. Popay J, Roberts H, Sowden A, et al. . Guidance on the conduct of narrative synthesis in systematic reviews, 2016. Available: https://www.lancaster.ac.uk/shm/research/nssr/research/dissemination/publications.php [Accessed 14 Mar 2019].
- 28. Detering KM, Hancock AD, Reade MC, et al. . The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010;340:c1345 10.1136/bmj.c1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bristowe K, Carey I, Hopper A, et al. . Patient and carer experiences of clinical uncertainty and deterioration, in the face of limited reversibility: a comparative observational study of the AMBER care bundle. Palliat Med 2015;29:797–807. 10.1177/0269216315578990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheang F, Finnegan T, Stewart C, et al. . Single-centre cross-sectional analysis of advance care planning among elderly inpatients. Intern Med J 2014;44:967–74. 10.1111/imj.12550 [DOI] [PubMed] [Google Scholar]
- 31. Schiff R, Rajkumar C, Bulpitt C. Views of elderly people on living wills: interview study. BMJ 2000;320:1640–1. 10.1136/bmj.320.7250.1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schiff R, Shaw R, Raja N, et al. . Advance end-of-life healthcare planning in an acute NHS hospital setting; development and evaluation of the expression of healthcare preferences (EHP) document. Age Ageing 2009;38:81–5. 10.1093/ageing/afn235 [DOI] [PubMed] [Google Scholar]
- 33. Peck V, Valiani S, Tanuseputro P, et al. . Advance care planning after hospital discharge: qualitative analysis of facilitators and barriers from patient interviews. BMC Palliat Care 2018;17:127 10.1186/s12904-018-0379-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Black K. Advance directive communication: nurses' and social workers' perceptions of roles. Am J Hosp Palliat Care 2006;23:175–84. 10.1177/1049909106289080 [DOI] [PubMed] [Google Scholar]
- 35. Black K, Emmet C. Nurses’ advance care planning communication: an investigation. Geriatr Nurs N Y N 2006;27:222–7. quiz 228 10.1016/j.gerinurse.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 36. Pérez M, Herreros B, Martín MD, et al. . Do Spanish Hospital Professionals Educate Their Patients About Advance Directives? : A Descriptive Study in a University Hospital in Madrid, Spain. J Bioeth Inq 2016;13:295–303. 10.1007/s11673-016-9703-7 [DOI] [PubMed] [Google Scholar]
- 37. Scott IA, Rajakaruna N, Shah D, et al. . Normalising advance care planning in a general medicine service of a tertiary hospital: an exploratory study. Aust Health Rev Publ Aust Hosp Assoc 2016;40:391–8. 10.1071/AH15068 [DOI] [PubMed] [Google Scholar]
- 38. Barnato AE, O'Malley AJ, Skinner JS, et al. . Use of advance care planning billing codes for hospitalized older adults at high risk of dying: a national observational study. J Hosp Med 2019;14:229–31. 10.12788/jhm.3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cantillo M, Corliss A, Ashton M, et al. . Honoring patient choices with advance care planning. J Hosp Palliat Nurs 2017;19:305–11. 10.1097/NJH.0000000000000359 [DOI] [Google Scholar]
- 40. Detering K, Sutton E, Fraser S, et al. . Feasibility and acceptability of advance care planning in elderly Italian and Greek speaking patients as compared to English-speaking patients: an Australian cross-sectional study. BMJ Open 2015;5:e008800 10.1136/bmjopen-2015-008800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Black K. Advance Directive communication with hospitalized elderly patients. J Gerontol Soc Work 2004;43:131–45. 10.1300/J083v43n02_09 [DOI] [Google Scholar]
- 42. Zwakman M, Jabbarian LJ, van Delden J, et al. . Advance care planning: a systematic review about experiences of patients with a life-threatening or life-limiting illness. Palliat Med 2018;32:1305–21. 10.1177/0269216318784474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ditto PH, Jacobson JA, Smucker WD, et al. . Context changes choices: a prospective study of the effects of hospitalization on life-sustaining treatment preferences. Med Decis Making 2006;26:313–22. 10.1177/0272989X06290494 [DOI] [PubMed] [Google Scholar]
- 44. Moore GF, Audrey S, Barker M, et al. . Process evaluation of complex interventions: medical Research Council guidance. BMJ 2015;350:h1258 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lund S, Richardson A, May C. Barriers to advance care planning at the end of life: an explanatory systematic review of implementation studies. PLoS One 2015;10:e0116629 10.1371/journal.pone.0116629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gott M, Ingleton C, Bennett MI, et al. . Transitions to palliative care in acute hospitals in England: qualitative study. BMJ 2011;342:d1773 10.1136/bmj.d1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. The AM, Hak T, Koëter G, van Der Wal G, et al. . Collusion in doctor-patient communication about imminent death: an ethnographic study. BMJ 2000;321:1376–81. 10.1136/bmj.321.7273.1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gott M, Ingleton C, Gardiner C, et al. . Transitions to palliative care for older people in acute hospitals: a mixed-methods study. Southampton (UK): NIHR journals library; 2013 Nov. (Health Services and Delivery Research, No. 1.11.) Chapter 6, In-depth post-discharge interviews with patients (phase 4). Available: https://www.ncbi.nlm.nih.gov/books/NBK259457/ [PubMed]
- 49. Waller A, Sanson-Fisher R, Nair BR, et al. . Preferences for end-of-life care and decision making among older and seriously ill inpatients: a cross-sectional study. J Pain Symptom Manage 2020;59:187–96. 10.1016/j.jpainsymman.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 50. Harris L. Continuing care. The disadvantaged dying. Nurs Times 1990;86:26–9. [PubMed] [Google Scholar]