Graphical abstract
Keywords: Coronavirus, SARS-CoV-2, Chloroquine, Severe acute respiratory syndrome, Antiviral agents, Biosensor
Abstract
The emergence and rapid spread of coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a potentially fatal disease, is swiftly leading to public health crises worldwide. The origin of SARS-CoV-2 infection was first reported in people exposed to a seafood market in Wuhan City, China in December 2019. It has been suggested that the infection is likely to be of zoonotic origin and transmitted to humans through a not-yet-known intermediary. As of 22 May 2020, the World Health Organization reported that there were approximately 4,995,996 confirmed cases and 327,821 deaths. SARS-CoV-2 is transmitted via inhalation or direct contact with droplets from infected people. It has an incubation period ranging from 2 to ≥14 days. The rate of spread of SARS-CoV-2 is greater than that for severe acute respiratory syndrome coronavirus and Middle East respiratory coronavirus. The symptoms are similar to influenza (i.e. breathlessness, sore throat and fatigue) and infected cases are isolated and treated. Infection is mild in most cases, but in elderly (>50 years) patients and those with cardiac and respiratory disorders, it may progress to pneumonia, acute respiratory distress syndrome and multi-organ failure. People with strong immunity or those who have developed herd immunity are asymptomatic. The fatality rate ranges from 3% to 4%. Recommended methods for diagnosis of COVID-19 are molecular tests (e.g. polymerase chain reaction) on respiratory secretions, chest scan and common laboratory diagnosis. Currently, treatment is essentially supportive, and the role of antiviral agents is yet to be established as a vaccine is not yet available. This review will focus on epidemiology, symptoms, transmission, pathogenesis, ongoing available treatments and future perspectives of SARS-CoV-2.
1. Introduction
Since the 19th Century, pathogenic viral outbreaks and their complex interactions with humans and animals (species jump) have resulted in cross-species transmission, posing a great threat to human health and safety [1], [2]. With rapid globalization and human activities, pathogenic transmission across continents has escalated and resulted in several pandemics, especially viral pandemics [3], [4]. Over the last two decades, there has been an upsurge in newly identified coronaviruses, such as Middle East respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia [5], haemorrhagic fever viruses (Lassa, Ebola) in West Africa, and novel coronaviruses including severe acute respiratory syndrome coronavirus (SARS-CoV) and highly pathogenic influenza (avian influenza A H7N9, pandemic H1N1) in China [4], [6]. These viral pandemics have resulted in substantial numbers of deaths. For example, SARS-CoV originated in bats and crossed over to humans via palm civets (host) in Guangdong Province, China; there were 8422 reported cases including 916 deaths (mortality rate 11%) in 26 countries [7]. MERS-CoV also originated in bats, with dromedary camels as the intermediate host; there were 2494 reported cases including 858 deaths (mortality rate 34%) in 27 countries [8]. Similarly, 28,637 cases of Ebola infection were notified including 11,315 deaths (mortality rate ≤40%) [9]. These pandemic situations have caused significant mortality and economic losses, and it is critical to prevent the spread of emerging viruses. This review sheds light on the current SARS-CoV-2 pandemic, epidemiology, global status and possible treatment strategies with future potential.
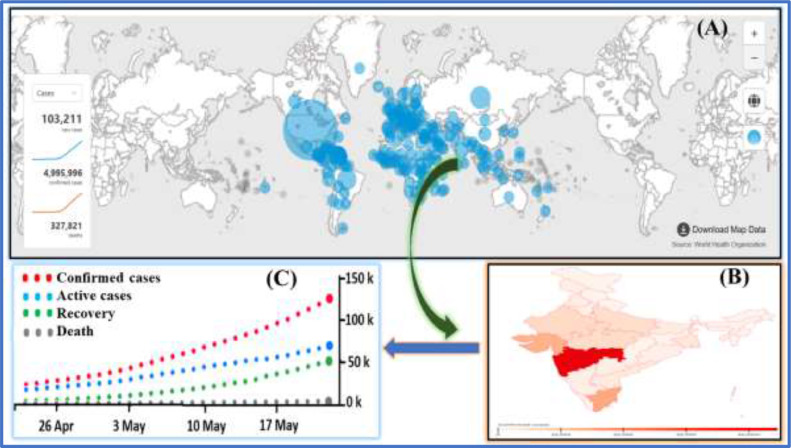
Coronaviruses (subfamily Coronavirinae, order Nidovirales) are common human pathogens. They are enveloped, positive-sense, single-stranded RNA viruses that belong to the family Coronavirdiae, and are known to cause acute respiratory, hepatic and neurological diseases with varying severity in humans as well as animals [10], [11], [12]. Coronaviruses are divided into four genera: alphacoronavirus (αCoV), betacoronavirus (βCoV), gammacoronavirus (γCoV) and deltacoronavirus (δCoV) [13]. Among them, two novel βCoVs (i.e. SARS-CoV and MERS-CoV) have been reported with high mortality rates, as discussed above. The evolutionary analysis has notified that αCoVs and βCoVs are mainly found in bats and rodents, whereas δCoVs and γCoVs are mainly found in birds [11]. Coronaviruses are able to cross species barriers repeatedly and are important human pathogens. In December 2019, an outbreak of pneumonia-like illness caused by a novel coronavirus occurred in Wuhan, Hubei Province and spread throughout China to the rest of the world [14]. In February 2020, the World Health Organization (WHO) named the disease ‘coronavirus disease 2019’ (COVID-19) [15] and the International Committee on Virus Taxonomy named the virus ‘severe acute respiratory syndrome coronavirus-2’ (SARS-CoV-2) [16]. A group of virologists in China suggested renaming SARS-CoV-2 as ‘human coronavirus 2019’ (HCoV-19) to distinguish it from SARS-CoV and for consistency with WHO's name for the disease [17]. On 11 March 2020, WHO characterized SARS-CoV-2 as a pandemic situation [18]. On 22 May 2020, there were 4,995,996 confirmed cases of SARS-CoV-2 including 327,821 deaths in 216 countries [19], and the number is increasing worldwide (Fig. 1 A). In China, the total number of confirmed cases reported is 84,520 including 4645 deaths. The USA has reported the highest number of cases (1,525,186) and deaths (91,527) (mortality rate >12%). Fig. 1B shows the spread of COVID-19 in India, which is a neighbouring country to China, where the number of active cases (118,447) is increasing on a daily basis (Fig. 1C) but with a low death rate (~3.0%). This review will discuss the epidemiology, transmission, pathogenesis, clinical manifestations, ongoing available treatments and future perspectives of SARS-CoV-2. By summarizing all information in one place, it is hoped that this review will be useful to the wider audience.
Fig. 1.
(A) World map of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) pandemic (source: World Health Organization, accessed on 22 May 2020, time: GMT+5.30). (B) Occurrence of SARS-CoV-2 across different states of India on 22 May 2020. (C) One-month data of active, cured and deceased cases of SARS-CoV-2 across India on 22 May 2020 (source: Ministry of Health and Family Welfare, Government of India, accessed on 22 May 2020, time: GMT+5.30; https://www.covid19india.org/).
2. Origin and spread of SARS-CoV-2 (COVID-19)
Coronaviruses are enveloped, positive-sense RNA viruses with a diameter of 60–140 nm. These viruses are characterized by club-like spike projections of protein on the surface, with a crown-like (latin for crown is ‘coronam’, hence named ‘coronavirus’) appearance under the electron microscope [20]. In CoVs, the unusually large viral RNA genome (~30 kb) has a unique replication strategy due to the presence of a 5′ cap structure and a 3′ poly (A) tail; these allow it to act as an mRNA for translation of the replicase polyproteins. SARS-CoV-2 has strong affinity to bind with human cell receptors, which differentiates it from other coronaviruses [21]. Coronaviruses are known to cause a variety of diseases in mammals and birds, ranging from enteritis in cows and pigs, to upper respiratory disease in chickens, to potentially fatal respiratory infections in humans [21]. Previously, four different families of viruses – HKU1, 229E, NL63 and OC43 – were identified with transmission in humans leading to mild respiratory disease [22]. On 8 December 2019, adults in Wuhan, Hubei Province, China reported severe pneumonia of unknown cause in local hospitals, and the common exposure of these initial cases was a seafood market trading live animals in Huanan [22]. A surveillance system was activated immediately, and respiratory samples of diseased patients were collected for aetiological investigations. On 31 December 2019, WHO notified this incident as an outbreak, and the Huanan seafood market was closed on 1 January 2020. Based on virological investigations, on 7 January 2020, the virus outbreak was identified as a coronavirus infection that had >70% similarity with SARS-CoV and >95% homology with bat coronavirus. Environmental samples from Huanan seafood market were also reported to be positive for SARS-CoV-2 [23]. It was observed that the number of cases increased exponentially; however, some cases did not have direct exposure to the seafood market, suggesting that human-to-human transmission had occurred [24]. The first case was reported on 8 December 2019, but on tracing the virus back to where it originated and learning more about its spread, it was found that the first case was hospitalized on 17 November 2019. Massive migration of the Chinese population during Chinese New Year spread the epidemic globally, and cases were reported in other countries and on other continents. Transmission to healthcare professionals treating infected patients was first reported between 20 and 23 January 2020. To prevent further transmission, Wuhan was placed under lockdown, with restrictions place on 11 million people in terms of entry to and exit from the province.
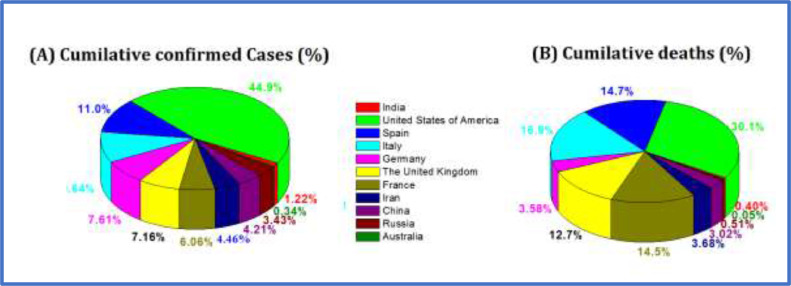
Despite the extensive preventive measures, the spread of SARS-CoV-2 in countries outside China was reported in populations with no history of travel, suggesting that local human-to-human transmission had started [25]. Considering the severity of the pandemic, countries evacuated their citizens from Wuhan and other ‘hot’ SARS-CoV-2 zones using special flights, and started testing for the virus or placing these people in isolation for 14 days for the safety of asymptomatic people. Importantly, the number of new cases reported in China has reduced recently, but numbers have increased exponentially in other countries [19]. Fig. 2 represents the percentage distribution of total confirmed cases and deaths in various countries on 25 April 2020. These numbers may underestimate the total numbers of infected or confirmed cases and deaths due to limitations of surveillance and testing in developing countries. Although the probable origin of SARS-CoV-2 is bats, the intermediary animal through which it crossed over to humans is uncertain. Pangolins and snakes are the current suspects [11], [22].
Fig. 2.
Percentage of cases of coronavirus disease 2019 in 11 main countries affected. Dates are: India (30 January–25 April 2020); USA (30 January–26 April 2020); Spain (31 January–25 April 2020); Italy (29 January–25 April 2020); Germany (28 January–25 April 2020); UK (31 January–25 April 2020); France (24 January–25 April 2020); Iran (19 January–25 April 2020); China (11 January–26 April 2020); Russia (31 January–25 April 2020); and Australia (25 January–26 April 2020) [19].
3. Symptoms and diagnosis
Usually, the symptoms of COVID-19 appear after an incubation period of 2–14 days, with an average period of 5.2 days [26]. Most commonly, the onset of COVID-19 is marked by fever, dry cough, fatigue and muscle pain, with other symptoms such as headache, lymphopenia and dyspnoea. Some people may have diarrhoea or nausea 1–2 days before infection [27], [28], [29]. Patients may face difficulties in breathing 5 days after the onset of infection, and acute respiratory distress syndrome (ARDS) on day 8. If the patient's condition worsens, they may experience abdominal distress and pneumonia, with other functional failures depending on their immune and health status [30]. The length of time from onset of infection until death ranges from 6 to 41 days, with an average of 14 days [31]. This period is dependent on several factors such as age and health, and is shorter for patients with comorbidities and aged >70 years [31].
The Diagnosis and Treatment Programme (6th version) of the National Health Commission of the People's Republic of China defined the diagnosis of viral pneumonia based on radiological features as one of the diagnostic criteria for COVID-19 [32]. Accurate diagnosis using chest computer tomography (CT) may play an important role in the management of patients infected with SARS-CoV-2, especially when no scientifically proven treatment exists. Clinical features revealed by chest CT scan are indicated as pneumonia in infected patients. Other abnormal features, such as ARDS, acute cardiac injury, RNAaemia and ground-glass opacities, that lead to death have also been reported [24]. The major notable symptom is the presence of multiple peripheral ground glass opacities in the subpleural regions of both lungs, which induce both systemic and localized immune responses leading to increased inflammation [33]. Importantly, there are similarities in symptoms between COVID-19 and earlier reported βCoV infections, such as dry cough, fever, dyspnoea and bilateral ground glass opacities [24]. However, COVID-19 also exhibits other clinical features targeting the lower respiratory region, such as rhinorrhoea, sneezing and sore throat [29], [34].
Additionally, investigation of chest radiographs of patients revealed infiltrate in the upper lobe of the lung which is associated with increasing dyspnoea with hypoxaemia [35]. The above discussions suggest that chest CT should be the preferred modality for diagnosis of COVID-19; however,the use of CT to diagnose COVID-19 is controversial. Detailed CT scan features for COVID-19 have been reported [27], [36]. Patients infected with SARS-CoV-2 usually develop gastrointestinal symptoms such as diarrhoea, whereas a low percentage of patients with MERS-CoV or SARS-CoV experienced similar gastrointestinal distress. Therefore, it is important to test faecal and urine samples to exclude a potential alternative route of transmission, especially among healthcare workers, patients etc.
4. Transmission of SARS-CoV-2
Following the first case of transfer of SARS-CoV-2 from animal to human, several cases of local transmission and, most seriously, community transmission, have been reported, leading to the pandemic status [18]. Based on the cases of infected people exposed at Wuhan seafood market, where live animals are sold routinely, it was suggested that the origin of SARS-CoV-2 could be zoonotic. Various efforts and retrosearching have been undertaken to identify a reservoir host or intermediate carrier from where the infection might be transmitted to humans. Two species of snakes have been identified as possible reservoirs of SARS-CoV-2; however, to date, there is no consistent evidence for a coronavirus reservoir other than mammals and birds [29], [37].
According to reports, human-to-human transmission of SARS-CoV-2 is possible when an individual is in the incubation stage or showing symptoms, whereas some individuals are contagious and remain asymptomatic (i.e. superspreaders). Transmission occurs via inhalation of respiratory droplets (>10 µm) of exhaled virus from an infected person (within 1 m). The virus remains airborne for a prolonged period. Transmission also occurs via contact with infected surfaces, such as skin-to-skin, and through touching an infected inanimate object then mediating it through the mouth, nose or eyes [4], [30]. SARS-CoV-2 is reported to survive for several hours on contaminated metal surfaces and sterile sponges. Latex surgical gloves, if not changed after handling an infected patient, increase the opportunity for transmission via touch. The relative contributions of large respiratory droplets, smaller airborne aerosols (<5 µm) and direct surface contacts to the transmissibility of SARS-CoV-2 need to be evaluated to enable effective control of transmission and infection.
The faecal transmission route should also be considered, as SARS-CoV-2 has been detected in stool samples of infected patients [31]. Previously, it was reported that SARS-CoV can survive in stool samples for up to 4 days [6]. In other studies, the presence of coronaviruses has been reported in water, sewage, and pure or pasteurized settled sewage, and these viruses can remain infectious for 3 days to 3 weeks [38]. The presence of stool from an infected patient in wastewater may generate a further route of transmission via the generation of virus-laden aerosols during wastewater treatment. For example, a contaminated faulty sewage system in a high-rise housing estate in Hong Kong in 2003 was linked to a SARS outbreak in the surrounding area [39]. Therefore, aerosols arising from contaminated sewage represent a potential cause of transmission of SARS-CoV-2 and needs further investigation.
Airborne dust is another possible route of transmission in respiratory diseases. Earlier reports have shown that transmission of infectious diseases is linked to the presence of micro-organisms in airborne particulate matter (PM)/airborne dust [40]. Air pollution is common in some countries (e.g. India, China, Saudi Arabia, Iran), and airborne PM matter or dust may influence the rate of transmission of viral infections. Inhalation of virus-laden fine PM could transport the virus into deep alveolar tissue and tracheobronchial regions, and increase the chance of infection [4]. Surface adsorption of SARS-CoV-2 on PM and airborne dust can contribute to long-range transport of the virus. Thus, an investigation of the survival of SARS-CoV-2 in airborne PM/dust will help to explain their role in transmission.
Recently, Chen et al. conducted a small study on pregnant women who were confirmed to have COVID-19. Although there is no evidence for transmission of viral infection from mother to child, the cloud of uncertainty persists [41]. Understanding this factor is very important as pregnant mothers are more susceptible to infection with respiratory pathogens and severe pneumonia. All pregnant mothers in the study by Chen et al. underwent a caesarean delivery, so it remains unclear whether viral transmission can occur during vaginal birth.
5. Pathogenesis of SARS-CoV-2 and clinical manifestations
Recognition and receptor binding on host cells is the first step of viral infection, followed by fusion with the cell membrane [29]. It is reasoned that the lung epithelial cells are the primary target of the virus. Thus, it has been reported that human-to-human transmission of SARS-CoV occurs by the receptor-binding domain of the SARS-CoV spike protein binding to angiotensin-converting enzyme 2 (ACE2) receptors [20], [22]. Importantly, the sequence of the receptor-binding domain of the SARS-CoV-2 spike protein is similar to that of SARS-CoV. This suggests that entry into the host cells is most likely via the ACE2 receptor [20], as depicted in Fig. 3 . The SARS-CoV-2 virus or virion contains four different proteins (envelope, spike protein, membrane and nucleoside) and an RNA strand. The spike protein helps to establish contact with human cells, and has a region which recognizes ACE2 receptors in human cells, especially respiratory cells. As the virion binds with ACE2 protein, another TMPRSS2 protein (on the exterior cell wall) helps to open the spike protein and cleave the membrane, allowing entry of the virion. Next, the virion releases its RNA strand, which is translated into protein and forms more RNA strands. During cell development (Fig. 3), one strand of the virion enters the Golgi bodies and evolving new virion those come out of cell where membrane and envelop proteins are present. One virion particle has the ability to make several hundred new virions in this way, each of which is capable of infecting new cells.
Fig. 3.
Schematic representation of the genesis and transmission of severe acute respiratory syndrome coronavirus-2 in humans.
The pathogenesis of SARS-CoV-2 leading to acute respiratory disorders and pneumonia-like symptoms seems to be particularly complex and is responsible for initiating an excessive immune reaction in the host. The cytopathic effect and cytokine storm are related to the clinical condition of patients with COVID-19 [42]. Levels of pro-inflammatory cytokines and chemokines, including interleukin 6 (IL-6), tumour necrosis factor and macrophage inflammatory proteins, are elevated and play an important role in the immunopathology of patients with COVID-19 [43]. During the period of infection, cytokines are expressed in lung epithelial cells, leukocytes and dendritic cells via the activation of pattern recognition receptors (including Toll-like receptors TLR3, TLR7 and TLR8), retinoic acid-inducible gene I and the NOD-like receptor family members. Also, direct virus-induced tissue damage and the synergistic cytokine effect cause extensive tissue damage and multiple organ dysfunction. An abundance of ACE2 protein on lung alveolar epithelial cells and enterocytes of the small intestine helps to explain the routes of infection and disease manifestations. IL-6 activates leukocytes, promotes differentiation of B lymphocytes and alters cell replication. IL-6 also stimulates the production of acute phase proteins, and plays a role in the thermoregulation and anti-inflammatory response [44]. Furthermore, deficiency of IL-6 and antiIL-6R monoclonal antibody leads to the persistence of influenza-like infection. Additionally, lymphocytes lack ACE2 receptors and compromise T lymphocytes. Most patients with COVID-19 have similar symptoms, such as fever, malaise and influenza-like features; however, some patients rapidly develop ARDS and multiple organ failure. Clinical examination reveals a reduced peripheral lymphocyte count and lymphocytopenia (due to reduction of CD4 T and CD8 T cells). Importantly, ACE2 regulates blood pressure, and an attack of SARS-CoV-2 on ACE2 receptors in endothelial cells results in coagulation, hypotension, cardiac injury, kidney dysfunction and secondary infections [42].
6. Therapeutics and treatment options
To date, no specific antiviral treatment or vaccine is available for treatment of COVID-19. Molecular interaction between virus cell receptors and host cells, mainly with surface spike glycoprotein(s), is particularly important for the development of antiviral treatment. Treatment of severe influenza still presents multiple challenges. Several trials are currently underway, aiming to discover a potential antiviral treatment for COVID-19. At present, the only option available is the use of broad-spectrum antiviral drugs [nucleoside analogues and human immunodeficiency virus (HIV)-protease inhibitors] in combination with antimalarial drugs and some potential antibiotics. All possible treatment options for the management of COVID-19 have been reviewed and are detailed below.
6.1. Hydroxychloroquine/chloroquine
Recent publications [45], [46], [47] have focused attention on the potential benefits of chloroquine (CQ) and hydroxychloroquine (HCQ) for the treatment of COVID-19. CQ is an amine acidotropic form of quinine, and has been used worldwide for decades as a front-line drug for the treatment and prophylaxis of malaria [48]. HCQ is a 4-aminoquiniline analogue of CQ with a hydroxyl group at the end of the CQ side chain. The pharmacokinetics of HCQ are similar to those of CQ, and it can be used for long periods with improved tolerability. The sulphate form of HCQ is available for oral administration with a higher dose than CQ for rapid gastrointestinal absorption and renal elimination. Clinical indications and toxic doses of these drugs are slightly different [45]. Regrettably, the efficacy of CQ/HCQ has declined gradually due to the continual emergence of CQ-resistant strains of Plasmodium falciparum. However, the activity of these drugs is not limited to malaria, as they have also been used in the treatment of autoimmune disorders (HIV) and viral infections associated with inflammation, and have broad-spectrum activity against a range of microbial (viral, bacterial and fungal) infections and inhibit their replication cycles [49], [50], [51], [52]. During long-term therapy, the clinical safety of HCQ is better than that of CQ, allowing a higher daily dose; however HCQ has fewer issues with drug–drug interactions. The broad-spectrum antiviral effects and clinical response of CQ and HCQ in SARS-CoV warrant particular attention for repurposing this drug for use in the treatment of COVID-19 [45], [50].
6.1.1. Antiviral potential of HCQ/ CQ against SARS-CoV-2
Due to the broad spectrum of activity of HCQ/CQ against viruses, including most coronaviruses, particularly SARS-CoV, and as coronaviruses enter cells through the endolysosomal pathway [53], it is reasonable, given the current public health emergency and the absence of any known efficient therapy, to further investigate the possible effects of CQ against SARS-CoV-2. In 2005, Vincent et al. reported that CQ had a strong antiviral effect on SARS-CoV infection of primate cells treated pre or post infection, suggesting both a prophylactic and therapeutic advantage [54]. In-vitro studies described that lethal infection of newborn mice with recombinant HCoV-O43 could be avoided by administering CQ via the mother's milk. CQ has also proved to be effective against MERS-CoV [48], although in-vitro results against MERS-CoV remain controversial.
In addition to several antiviral agents, CQ and HCQ have been proposed as treatments that could reduce viral transmission. In-vitro studies have shown that CQ and HCQ can inhibit SARS-CoV-2 transmission via alkalinization of the intracellular phagolysosome [55], which prevents virion fusion and uncoating, thereby decreasing viral spread. In a recent report, the Chinese National Centre for Biotechnology Development indicated that CQ is one of the potential drug candidates for the treatment of COVID-19. It has been investigated in hospitals in Beijing, Hunan Province and Guangdong Province. According to preliminary reports [56], [57], approximately 100 patients with COVID-19 treated with CQ experienced a more rapid decline in fever and improvement in lung function. The improved computed tomography (CT) images and shortening of recovery time compared with control groups, with no serious adverse effects, led to the inclusion of CQ in the treatment guidelines for COVID-19 by the Chinese Medical Advisory Board. As a result, CQ was probably the first drug to be used in China and elsewhere as the front-line treatment of severe COVID-19.
Under the Ministry of Health and Family Welfare, the Indian Council of Medical Research recommended chemoprophylaxis and therapeutic use of HCQ in patients with COVID-19, with a dosage of 400 mg twice on day 1, then 400 mg once per week thereafter for asymptomatic healthcare workers treating infected patients and for asymptomatic household contacts of confirmed cases [58]. For the treatment of diagnosed cases, no clinical information is available, except for some pre-clinical examination results, such as reduction of viral load by administering HCQ in combination with azithromycin [47]. On the basis of these preliminary findings, CQ and HCQ have been prescribed to treat patients with COVID-19 to reduce the length of hospital stay and improve the SARS-CoV-2-related pneumonia. WHO has also recommended that healthcare providers should use CQ [59] and HCQ [60] in adults and adolescents who weigh ≥50 kg. Recently published guidelines from the Surviving Sepsis Campaign on the management of critically infected patients concluded that there is a lack of evidence to offer any recommendations on standalone use of these drugs in patients admitted to the intensive care unit (ICU) [61]. It seems that discrepancies about the use of antimalarial drugs in the clinical management of patients in the ICU with severe COVID-19 require exhaustive research. This evidence, or the lack thereof, hardly justifies state-endorsed, widespread use of HCQ for prophylaxis. Long-term use of these drugs for the treatment of malaria demonstrates their safety during acute administration to humans; however, one cannot ignore the minor risk of macular retinopathy, prolongation of the QT interval in arrhythmic patients, and renal and hepatic impairment, which depends on the cumulative dose [59], [60]. A survey of the adverse effects of CQ/HCQ treatment in patients with COVID-19 with multiple organ dysfunction (e.g. cardiac injury and kidney malfunction) needs further evaluation. Currently, at least 10 clinical trials are testing CQ as a treatment for COVID-19 [62].
6.1.2. Mode of action of CQ/HCQ
CQ/HCQ has multiple mechanisms of action that may be useful in the treatment of COVID-19 [45], [63]:
-
•
HCQ inhibits entry of the virus into the host cells by binding to the host cell surface receptors by disabling ACE2 terminal glycosylation, leading to changes in surface morphology [54]. The association between SARS-CoV-2 and the host cells is disrupted as ACE2 is needed for the virus to attach to the host cells. CQ also inhibits quinone reductase-2, which is a structural neighbour of UDP-N-acetylglucosamine 2-epimerases [64] which are involved in the biosynthesis of sialic acids (acidic monosaccharides) present at the extremities of sugar chains present on cell transmembrane proteins, and one of the important components required for ligand recognition.
-
•
HCQ/CQ can impair the early stages of viral replication by increasing the endosomal pH required for the virus–host cell infusion. The inhibition likely involves endocytosis and annulment of virus–endosome fusion.
-
•
CQ can also interfere with the post-translational modification of viral proteins, which involves two enzyme proteases and glycosyltransferases. The process occurs within the endoplasmic reticulum/trans-Golgi network vesicles and may require a low pH. It localizes the M proteins in the Golgi complex beyond the site of virion budding, which suggests that this drug may inhibit replication of the SARS-CoV-2 virion.
-
•
HCQ acts as an ionophoric agent for Zn2+ ions and thus increases the influx of Zn2+ ions into the cytoplasm of the host cells regardless of whether the host target cells are infected or not [62]. Zn2+ ions adhere to the RNA-dependent RNA polymerase enzyme of the virus and stop SARS-CoV-2 intracellular polymerization.
-
•
HCQ not only attenuates the inflammatory response but also decreases the level of cytokines, which is generally higher in patients infected with SARS-CoV-2. In one study, conducted by Yao et al., it was reported that HCQ is more potent than CQ in the inhibition of SARS-CoV-2 [65]. In addition, CQ can inhibit IL-1β mRNA expression in THP-1 cells and reduce the release of IL-1β and tumour necrosis factor.
6.2. Ivermectin
Ivermectin is a synthetic derivative of macrocyclic lactones commonly known as avermectins that possesses broad-spectrum antiparasitic activity. Ivermectin has been approved by the US Food and Drug Administration as an antiparasitic agent. In recent years, it has shown potent antiviral activity against a broad range of viruses, such as influenza A and dengue virus (DENV) [65], [66], [67]. It is known to inhibit the interaction between the HIV-1 integrase protein (IN) and the importin (IMP) α/β heterodimer responsible for importing the integrase protein [66], [68]. Ivermectin also inhibits IN nuclear import and HIV-1 and DENV replication [69]. It has been reported that this drug potentially inhibits the nuclear import of host cell and viral proteins, as observed in the case of SV40 large tumour antigen protein of simian virus and non-structural protein 5 of DENV [68], [69], [70], [71]. However, ivermectin did not show any potential efficiency against Zika virus. A clinical trial in Thailand (2014–2017) against DENV infection found that a single dose of ivermectin can result in a significant reduction in viral NS1 protein in serum, but no change in clinical benefits was notified [72].
6.2.1. Ivermectin potential against SARS-CoV-2
A series of clinical trials are underway to identify potential therapies or a vaccine for COVID-19. A group of researchers [72] from Monash University, Australia reported that ivermectin has shown broad-spectrum in-vitro antiviral activity against SARS-CoV-2. In an in-vitro experiment, they added a single dose of ivermectin to Vero-hSLAM cells infected with SARS-CoV-2 for 2 h. After analysing the cell pellets and supernatant for 3 days using reverse transcriptase polymerase chain reaction (RT-PCR) to analyse the replica of SARS-CoV-2 RNA, a 93% reduction in viral RNA in the supernatant was quantified in the sample treated with ivermectin compared with the control (DMSO treated), and a 99.9% reduction in cell-associated viral RNA. Efficiency increased to ~5000 fold by 48 h, and no toxicity was observed [67], [69]. In the above study, a serial dilution method showed similar efficiency with IC50 of ivermectin of ~2 µM.
6.2.2. Mode of action of ivermectin
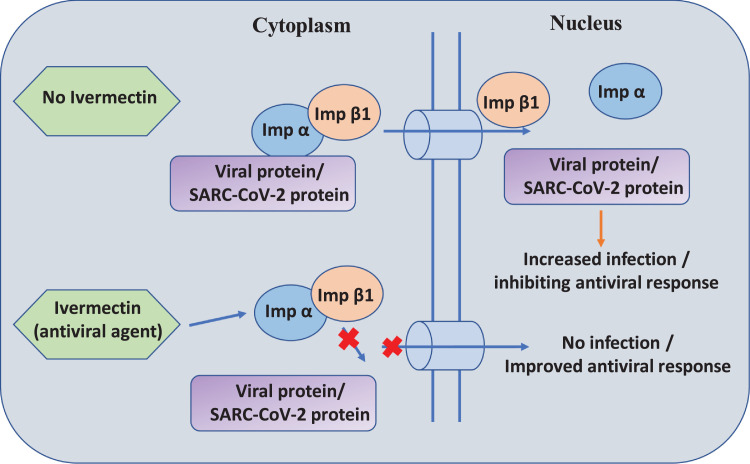
Earlier studies on SARS-CoV protein revealed a potential role for IMP α/β1 in signal-dependent nucleocytoplasmic shutting of the SARS-CoV nucleocapsid protein [73], which potentially impacts host cell division [74]. Additionally, SARS-CoV protein (ORF6) antagonizes the antiviral activity of the STAT1 transcription factor sequestering IMP α/β1 on the membrane of rough endoplasmic reticulum/Golgi bodies [75]. Ivermectin's nuclear transport inhibitory activity may be effective against SARS-CoV-2. Caly et al. hypothesized the inhibition of SARS-CoV-2 by ivermectin by the inhibition of IMP α/β1-mediated nuclear import of viral proteins (Fig. 4 ), as shown for other RNA viruses [72]. Further identification and confirmation of this mechanism in SARS-CoV-2 infection still needs to be evaluated and various clinical trials are ongoing.
Fig. 4.
Proposed antiviral mechanism of action of ivermectin against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). In the absence of ivermectin, IMP α/β1 binds to the coronavirus cargo protein in the cytoplasm, and crosses the membrane barrier through the nuclear pore complex into the nucleus where the complex dissociates and the viral cargo infects and reduces the host cell's antiviral response. In the presence of ivermectin, ivermectin binds to and destabilizes the IMP α/β1 heterodimer, preventing the binding of IMP α/β1 to the viral protein; this prevents the cargo protein from crossing the membrane barrier to enter the nucleus, thereby preventing infection and improving the antiviral response [69].
6.3. Lopinavir/ritonavir (protease inhibitors) and antiviral drugs
Lopinavir/ritonavir are antiretroviral drugs with protease inhibitor action, widely used for the treatment of HIV, and recently suggested as potential candidates for the treatment of COVID-19 [76], [77]. Previous studies confirmed the effectiveness of lopinavir/ritonavir against other coronaviruses [66], [78]. An open-label, non-randomized study showed reduced risk of severe hypoxia or death in 41 patients with SARS-CoV infection treated with lopinavir/ritonavir compared with 111 patients treated with ribavirin alone [78]. However, there is no evidence from randomized trials for the efficacy of lopinavir/ritonavir for treatment of SARS-CoV or MERS-CoV infection [79].
6.3.1. Potential mechanism of action
SARS-CoV-2 enters the host cells and replicates, producing strands which contain multiple copies of RNA which accumulate at the periphery of the host cells to be cleaved and released [80]. 3-Chymotrypsin-like protease (3CLpro) is an enzyme with a crucial role in processing viral RNA [81]. Lopinavir/ritonavir are protease inhibitors that inhibit the action of 3CLpro and thereby disrupt the process of viral replication and release from host cells [81]. Recent evidence suggests that these drugs have in-vitro antiviral activity against SARS-CoV-2 [76], [77], [82]. Most importantly, coronavirus proteases, including 3CLpro, do not have a C2-symmetric pocket, which is a target site for HIV protease inhibitors. This brings into question the potential potency of HIV protease inhibitors for the treatment of coronaviruses [83], and shows that C2 pockets are not important for antiviral action.
6.3.2. Clinical efficiency of protease inhibitors/antiviral drugs against SARS-CoV-2
In Wuhan, an open-label randomized clinical trial was conducted on 199 adult patients hospitalized with SARS-CoV-2 pneumonia-like symptoms [84], treated with lopinavir 400 mg/100 mg twice per day for 14 days (n=99) or standard care (n=100). The baseline characteristics were similar in both groups, and most patients were severely unwell and required urgent clinical attention. Even after 28 days of treatment, the intention-to-treat analysis revealed no positive symptomatic relief in the primary outcome between the two groups. Moreover, there was some evidence that lopinavir can reduce mortality, shorten ICU stay and reduce time to discharge from hospital by 1 day. However, no clinical improvement was observed after 28 days in patients treated with lopinavir. No difference in viral clearance was found between the two groups, and conditions worsened due to the appearance of adverse side effects. The ELACOI (Efficacy of Lopinavir Plus Ritonavir and Arbidol Against Novel Coronavirus Infection) study was conducted on 44 patients with COVID-19 [85], divided into three groups: lopinavir, umifenovir and control. No difference was observed in the primary results. No improvement in pyrexia, cough or lung CT was observed after 7 and 14 days, and the clinical condition of patients deteriorated further [81]. Other studies [86], [87] conducted on hospitalized patients with COVID-19 treated with lopinavir and other protease inhibitors showed no potential improvement in clinical status, and patients exhibited a high rate of unfavourable outcomes with no difference in time for viral clearance compared with patients who did not receive this treatment.
An unpublished in-vitro study [88] reported that darunavir (HIV-1 protease inhibitor) was not active against SARS-CoV-2; however, on 4 February 2020, a group of researchers in China [89] reported that, in an in-vitro experiment, darunavir 300 μM inhibited SARS-CoV-2 infection, and the inhibition efficiency was calculated to be 280-fold compared with the untreated group. In-vitro studies using mouse models treated with remdesivir (a nucleoside analogue) found stronger evidence for antiviral MERS-CoV activity compared with lopinavir [90]. Wang et al. reported that remdesivir blocked SARS-CoV-2 infection with EC50 (effective concentration to inhibit 50% of cells) of 0.77 µM with a high selectivity index (>129.87) [91]. Holshue et al. reported that remdesivir exhibited promising results in the treatment of patients with COVID-19 in the USA [92]. In China, a randomized, placebo-controlled, double-blind, multicentre phase III clinical trial was started on 5 February 2020 to evaluate the efficacy of remdesivir in the treatment of COVID-19 [89]. Infected patients received an initial intravenous infusion of 200 mg of remdesivir with subsequent doses of 100 mg for 9 consecutive days, and the control group was treated in a similar manner with a placebo regimen. The outcomes of the trial are expected by the end of April 2020.
In-vitro antiviral efficacy of remdesivir/lopinavir against SARS-CoV-2 on Vero-E6 cells at 50% effective concentration was reported [82]. Ribavirin/favipiravir, which are in clinical trials, showed no viral inhibition even at higher concentrations (i.e. 100 μM). A synergistic effect of remdesivir and emetine could help to achieve 64.9% inhibition in viral yield. This proves that the combinational therapy may help to reduce the effective concentration of drugs and increase clinical benefits. Several groups of researchers are working on this class of drugs; however, due to insufficient evidence, only lopinavir is used to treat patients with COVID-19. Various dosage regimens of CQ, HCQ and other antiviral agents are undergoing investigation for treatment for chemoprophylaxis and therapeutic use, and these are summarized in Table 1 .
Table 1.
Recommended guidelines for various dosage regimens for the treatment of coronavirus disease 2019 [52], [55], [86].
| Drug | Administration | Dosage | Duration of treatment |
|---|---|---|---|
| Chloroquine phosphate | Oral | 500 mg twice per day | <10 days |
| Hydrochloroquine | Oral | For asymptomatic healthcare personnel, 400 mg twice on day 1, then 400 mg once per week | For 7 weeks |
| For quarantining individuals following contact with positive cases, 400 mg twice on day 1, then 400 mg once per week | For 3 weeks | ||
| Lopinavir/ ritonavir | Oral | 200 mg twice per day (2 capsules of 50 mg each time) | <10 days |
| Ribavirin | Intravenous infusion | 500 mg two or three times per day in combination with lopinavir/ritonavir or interferon-α | <10 days |
| Interferon-α | Vapour inhalation | 5 million U twice per day | <10 days |
| Arbidol | Oral | 200 mg three times per day | <10 days |
6.4. Other potential drug candidates/therapies
6.4.1. Baricitinib
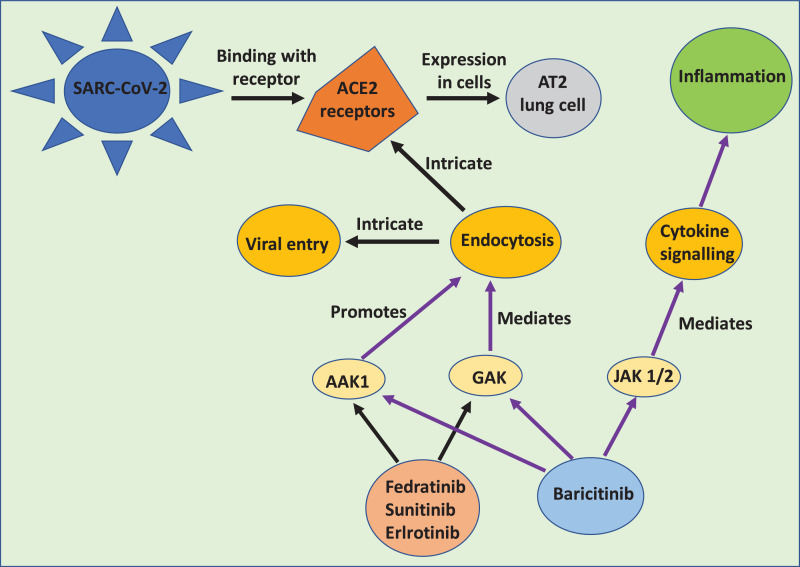
BenevolentAI is a target identification platform and knowledge graph which is a large repository of structured medical information with abundant connections extracted from the available scientific literature by a machine learning process [93]. Based on a BenevolentAI graph (Fig. 5 ), Richardson et al. searched for approved drug candidates with potential efficiency for inhibition of propagation of SARS-CoV-2 [94]. Herein, baricitinib was identified as a potential candidate that may reduce the capability of SARS-CoV-2 to infect lung cells. Baricitinib is a high-affinity AP2-associated protein kinase-1 (AAK1)-inhibiting drug with Janus kinase 1/2 (JAK 1/2) inhibition, which also binds to cyclin G-associated kinase (a regulator of endocytosis) [95]. A plasma concentration of baricitinib at therapeutic dose (i.e. 2 mg or 4 mg single dose) is sufficient to inhibit AAK1. It has been clinically tested on a selected population with COVID-19 using an appropriate patient population to reduce viral entry and inflammation in patients with a MuLBSTA score, and also predict viral mortality [96]. Due to the high affinity of baricitinib for AAK1 and JAK, its ability to ameliorate allied chronic inflammation in interferonopathies and pharmacokinetic properties make it a potential candidate for combination with direct-acting antivirals (lopinavir or ritonavir and remdesivir) to combat SARS-CoV-2 [97].
Fig. 5.
BenevolentAI knowledge graph integrating biomedical data from existing structured and unstructured sources. Constructed on the basis of a fleet of algorithms to establish new relationships suggesting new treatment methods for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). AAK1, AP2-associated protein kinase-1; JAK 1/2, Janus kinase 1/2; GAK, cyclin-G-associated kinase [90], [91].
6.4.2. Interferon-α
According to WHO, the antiviral drug interferon (IFN)-α is recommended for the treatment of COVID-19 [98]. IFN-α is a broad-spectrum antiviral agent usually used to treat hepatitis [99], and it has been reported to inhibit SARS-CoV reproduction in vitro [100]. The recommended treatment regimen in adults is vapour inhalation of IFN-α at a dose of 5 million U dispersed in 2 mL of sterile water twice per day. If IFN-α is administered in combination with other antiviral drugs or nucleoside analogues, it could be more effective for the treatment of COVID-19. Currently, IFN-β1a and IFN-α2b are under investigation as potential candidates for the treatment of patients with COVID-19. IFNs can improve the immune system by turning on dormant parts, and aligning them with the defence mechanism against SARS-CoV-2. Moreover, as IFNs improve the immune system, the influenza-like symptoms of COVID-19 may worsen as naturally occurring IFNs are responsible for influenza-like symptoms. For instance, if a patient is on a ventilator or undergoing asthmatic treatment, the administration of IFN-based medicine could be catastrophic. WHO is investigating different IFNs to treat COVID-19, but no treatments exist to date. An open randomized clinical study has been launched in China on the efficacy and safety of IFN-α2β for treatment of COVID-19 in 328 adult patients; the results are due in June 2020 [101].
6.4.3. Arbidol
Arbidol is a potent broad-spectrum antiviral agent that has activity against enveloped and non-enveloped viruses [102]. Arbidol is a well-known antiviral agent in Russia, China and some Western countries. It was developed by the Russian Research Chemical Pharmaceutical Institute. Since 1990, arbidol has been used as an over-the-counter medicine for prophylaxis and treatment of ARDS, including influenza [102]. Arbidol exhibits a molecular mechanism of action against influenza viruses (influenza viruses A and B) that differs from other antiviral drugs. The antiviral mechanism of action of arbidol involves inhibition of virus-mediated fusion with the target membrane, which blocks virus entry into the target cells [103]. According to the literature, arbidol has been approved in Russia and China for the treatment of SARS, influenza and other viruses [104]. Recently, Deng et al. investigated combination therapy of arbidol with lopinavir/ritonavir using a small sample [105], and a small number of studies have confirmed the successful recovery of patients treated with arbidol and lopinavir. Zhu et al. undertook a study on 50 patients divided into two groups: lopinavir/ritonavir 400 mg/100 mg two times per day for 7 days (n=34 patients), and arbidol 0.2 g three times per day (n=16 patients). Data were analysed retrospectively [106]. After 14 days of treatment, no viral load was detected in the arbidol-treated group; however, the viral load was 44.1% in the lopinavir/ritonavir-treated group. No significant side effects were observed in either group. This combination therapy has been recommended by the National Health Commission and National Administration of Traditional Chinese Medicine for the treatment of COVID-19 [104], with limited clinical data available. An in-vitro study suggested that arbidol can significantly inhibit SARS-CoV-2 infection at a concentration of 10–30 µM [85].
6.4.4. Convalescent plasma or blood plasma therapy
Convalescent plasma therapy (CPT) is akin to passive immunization, and has been proposed as a preventive measure for SARS-CoV-2 infection. The principle of therapy is very simple, and based on the premise that the plasma of a patient who has recovered from COVID-19 contains antibodies which are highly specific and have the ability to fight SARS-CoV-2 [107]. When antibodies obtained from recovered patients are ingested by a patient undergoing treatment, they will start fighting the infection in the second patient. This is a potential treatment measure for those who are critically affected by the virus, and may be preventative in individuals at high risk of contracting the virus (e.g. healthcare workers, immediate families of patients and other high-risk contacts).
Previously, CPT was employed successfully in the treatment of SARS [108], H1N1 [109], MERS [107] and H5N1 [110], with promising efficacy and safety. A meta-analysis of 32 studies of SARS-CoV infection and severe influenza showed that CPT led to a significant reduction in mortality compared with placebo [111]. A pilot study on 10 patients with SARS-CoV-2 infection was conducted in China to explore the feasibility of CPT [107]. A single transfusion of convalescent plasma 200 mL into a patient with severe COVID-19 was well tolerated, and clinical symptoms improved with an increase in oxyhaemoglobin saturation within 3 days of treatment. Rapid neutralization of viraemia and no significant side effects were also observed. The US Food and Drug Administration has issued guidelines to healthcare providers and investigators to further investigate the efficacy of CPT from individuals who have recovered from COVID-19 [112], [113], and also issued guidelines for donors of convalescent plasma antibodies [112]. CPT may not be effective in every case, due to multiple organ failure or immunodeficiency, so a clinical trial is required to determine its feasibility and efficacy. In another report, an uncontrolled case series of five critically ill patients with COVID-19 and ARDS were treated with CPT containing neutralizing antibodies, and this led to an improvement in their clinical status [114]. In India, the Sree Chitra Tirunal Institute for Medical Sciences and Technology has received approval for the use of CPT for the treatment of patients with COVID-19 [115].
6.4.5. Vaccination for SARS-CoV-2
To date, there is no evidence of a vaccine for SARS-CoV-2. After WHO's response to the SARS-CoV-2 outbreak, a research and development blueprint has been initiated to accelerate the development of vaccines and therapeutics for the SARS-CoV-2 outbreak [116]. Under WHO's coordination, several groups of scientists and experts with diverse backgrounds around the world are working on the development of a vaccine against SARS-CoV-2. According to one report, Bacille Calmette-Guérin (BCG) vaccine may be a potential candidate; however, WHO does not recommend the use of BCG vaccine for SARS-CoV-2 as little evidence is available on its potency [117]. Two clinical trials are addressing this question, and WHO will evaluate the evidence and announce when it is available. Several vaccines are undergoing trials by various organizations and research groups, including ChAdOx1 nCoV-19 (University of Oxford, 510 estimated participants, completion due May 2021) [118], mRNA-1273 vaccine (Modern and Vaccine Research Centre; targets the spike protein of coronaviruses) [119] and other forms of vaccine [120].
7. Miscellaneous
Numerous attempts to identify a potential treatment are underway worldwide. Other individual drugs or classes of drugs have been found to be effective in in-vitro experiments against similar viruses, such as fusion peptide (EK1) [122], RNA synthesis inhibitors (e.g. TDF, 3TC), antibiotics (azithromycin) [46], anti-inflammatory drugs (e.g. hormones), neuraminidase inhibitors (e.g. oral oseltamivir) and immunomodulators [36]. Additionally, Chinese medicine, such as ShuFengJieDu capsules and Lianhuaqingwen capsules, have shown potential in the prevention and treatment of new respiratory infectious diseases such as influenza A (H1N1) [121]. These drugs in combination with antiviral agents can decrease the time required for viral shedding, or improve patients’ health conditions. Nonetheless, the efficacy and safety of these drugs in COVID-19 need further evaluation and confirmation by clinical experiments.
WHO has launched ‘Solidarity’, an international clinical trial to find an effective treatment for COVID-19 [122]. Four treatment options are being evaluated to assess their efficacy and safety against viral infection, and other potential drug candidates may enter the regimen at a later date. Until and unless there are satisfactory outcomes from the various trials across the world, WHO and other regulatory bodies – such as the Indian Council of Medical Research and the European Union – are cautious about physicians and healthcare professionals recommending or administering unproven treatments to patients with COVID-19. The agencies are also concerned by individuals who are self-medicating with any types of medication which are thought to be effective (e.g. CQ) and causing serious side effects.
Very recently, a group of researchers from the Indian Institute of Technology, Delhi, India and Advanced Industrial Science & Technology, Tsukuba, Japan revealed that Ashwagandha (Withania somnifera, family solanaceae) and honeybee propolis contain bioactive agents such as withanolides (from Ashwagandha) and caffeic acid phenethyl ester (CAPE, from a mixture of honeybee propolis and fungal resin) that interact with a highly conserved protein, Mpro, of SARS-CoV-2 [123] using molecular docking tools. They found that Wi-N (withanone) and CAPE bind to the substrate-binding pocket of Mpro of SARS-CoV-2 with equivalent efficacy and binding energies as an N3 protease inhibitor. Superimposition confirmed that all the ligands exhibited similar binding mechanisms, and the binding free energies calculated using MM/GBSA for the N3 inhibitor, CAPE and Wi-N were also comparable. This study predicted that these natural compounds have the potential to inhibit the enzymes essential for virus survival, and will help in initial screening of anti-SARS-CoV-2 drugs.
8. Conclusions and future perspectives to control the SARS-CoV-2 pandemic
Since December 2019, SARS-CoV-2 has posed an ongoing threat to the human race. This viral strain infects host cells through ACE2 to cause COVID-19, and also causes damage to the respiratory tract and myocardium, although the specific mechanisms are still uncertain. Patients infected with SARS-CoV-2 have an adverse prognosis, and patients already suffering from cardiovascular disease, ARDS and low immunity deserve particular attention. Based on the spread of the epidemic in several countries, WHO declared this infection to be a pandemic. SARS-CoV-2 has an incubation period of 2–14 days, and the infection is usually confirmed by PCR and chest scan. To date, no medical treatment or vaccine is available to treat COVID-19. Treatment with antiviral agents is essentially supportive, and higher doses have adverse effects. Therefore, there is an immediate need for medical treatment or a vaccine for COVID-19. The BenevolentAI knowledge graph [93] shows features of integrated biomedical data which could be a possible link for identifying treatment. The detection and quantification of biomarkers, such as cytokinins and IFNs, is important and useful in early screening of infection. The viral strands have different shelf lives on different surfaces. The presence of SARS-CoV-2 in human faecal matter has been confirmed, and it is known that the virus can survie in facal matter for a long duration. In underdeveloped nations, where faecal decomposition take place in open areas, the rate of contact with the virus may increase. Hence, faecal screening of patients with COVID-19 is a promising challenge to understand transmission. The outer part of SARS-CoV-2 is made up of lipids that can be disrupted by surfactant or handwashing. As discussed above, CPT or blood plasma therapy could be a potential approach [107] in the treatment of COVID-19, as patients who have recovered from COVID-19 have developed antibodies to SARS-CoV-2. Administration of these antibodies into an infected patient will help to provide a quick response against the infection. However, parameters of clinical diagnosis will be required in this procedure, such as patient's clinical status and diagnosis of other disorders.
Natural resources such as plants and resinous matter with antiviral characteristics are promising candidates for screening as lead molecules for drug design and development for the treatment of COVID-19. These existing natural resources will save time and be cost-effective in the current scenario of an international health emergency and lack of treatment modalities.
In future, the development of a biosensor (a device that can transform the biochemical signal into a measurable signal) with the ability to detect SARS-CoV-2 or a biomarker associated with COVID-19 [124], wearable devices with the ability to detect clinical symptoms/physiological signs related to the onset of SARS-CoV-2 (e.g. fever, cough and fatigue [125], and development of vaccine could help to achieve early detection and management of SARS-CoV-2. Based on the authors’ experience [126], [127] and other literature [128], [129], a biosensor could be a promising solution to address fast screening of patients infected with SARS-CoV-2, and an alternative to the routinely employed RT-PCR technique. In one strategy, an optical biosensor could detect the presence of SARS-CoV-2 in exhaled air, sneeze droplets or a swab sample from an individual, where biochemical signal generation is converted into a measurable optical signal such as colour change, coagulation or visual signal generation. For example, fluorescently labelled biorecognition elements (nucleic acid, antibody, peptide) can be immobilized on the surface of nanoparticles (with the ability to quench the fluorescence, such as titanium dioxide nanoparticles, carbon nanotubes, graphene etc). In the absence of an antigen (virus in the present case), the fluorescence of labelled bioreceptors will be quenched due to fluorescence energy transfer between fluorescently labelled bioreceptors and nanoparticles. In the presence of an antigen, the high-affinity molecular interaction between the antigen and the bioreceptors will lead to conformational changes in the structure of bioreceptors, and result in fluorescence recovery due to desorption from the nanoparticle surface or a decrease in the Förster resonance energy transfer phenomenon [126]. This signal can be analysed qualitatively for the presence of virus molecules by a switch on/off system [128], or could calculate these signals quantitatively against the concentration of antigen [129]. In another strategy, an aptasensor and immunosensor could be designed; for example, by capturing virion particles using immobilized aptamer/antibody on a gold quartz crystal surface mounted on an electrochemical quartz crystal nanobalance. The binding of virion molecules to receptors will result in a change in resonant energy or mass over the crystal surface, and the surface can subsequently be regenerated with a regenerating agent (e.g. 4.0 % w/v glycine or 0.10 M glycine), where lowering the pH will break the binding interaction or regenration by addition of 1.0% SDS [130], [131]. Secondly, the addition of a surfactant (e.g. CTAB) to the antigen eluted fraction will result in cell lysis by disrupting the cell membrane of virion molecules, and the subsequent addition of gold nanoparticles will allow colorimetric detection based on CTAB-induced aggregation of AuNPs [132]. These strategies have been proposed based on the authors’ experience and the available literature; however, the reliability and reproducibility of these strategies cannot be predicted until experiments are undertaken.
The current molecular diagnosis used for the detection of SARS-CoV-2 using RT-PCR takes 3–4 h, including the various steps of preparation of viral RNA, incubation, washing etc. Therefore, there is an obvious need for highly sensitive, fast and effective diagnostic methods that could detect these antigens directly in the complex matrix with little or no sample preparation. Recently, two biosensing platforms based on dual functional plasmonic photothermal biosensors coupled with surface plasmon resonance [133] and a field-effect transistor (FET)-based biosensor [134] were reported for detection of SARS-CoV-2 in clinical samples such as human nasopharyngeal swab specimens. The dual-functional LSPR biosensor [133] exhibited high sensitivity towards SARS-CoV-2 sequences, offered a lower detection limit (0.22 pM), and allowed precise detection of the specific target in a multigene mixture. The FET-based biosensor exhibited detection limits of 1 fg mL−1 and 100 fg mL−1 SARS-CoV-2 spike proteins in phosphate-buffered saline and clinical transport medium, respectively. Additionally, it successfully detected 1.6 × 101 pfu mL−1 SARS-CoV-2 in culture medium and 2.42 × 102 copies mL−1 in clinical samples. The above detection platforms are reliable and easy to implement for the improvement of diagnostic accuracy, and require little or no sample pre-treatment. These platforms could relieve the pressure on PCR-based methods.
Funding: None.
Competing interests: None declared.
Ethical approval: None.
References
- 1.Reperant LA, Cornaglia G, Osterhaus ADME. In: One health: the human–animal–environment interfaces in emerging infectious diseases: the concept and examples of a one health approach. Mackenzie JS, Jeggo M, Daszak P, Richt JA, editors. Springer; Berlin: 2013. The importance of understanding the human–animal interface; pp. 49–81. [Google Scholar]
- 2.Reperant LA. Osterhaus ADME. AIDS, avian flu, SARS, MERS, Ebola, Zika… what next? Vaccine. 2017;35:4470–4474. doi: 10.1016/j.vaccine.2017.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, Dai S, Wang M, Hu Z, Wang H, Deng F. Virus like particle-based vaccines against emerging infectious disease viruses. Virol Sin. 2016;31:279–287. doi: 10.1007/s12250-016-3756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qu G, Li X, Hu L, Jiang G. An imperative need for research on the role of environmental factors in transmission of novel coronavirus (COVID-19) Environ Sci Technol. 2020;54:3730–3732. doi: 10.1021/acs.est.0c01102. [DOI] [PubMed] [Google Scholar]
- 5.De Groot RJ, Baker SC, Baric RS, Brown CS, Drosten C, Enjuanes L, et al. Commentary: Middle East respiratory syndrome coronavirus (MERS-CoV): announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber DJ, Rutala WA, Fischer WA, Kanamori H, Sickbert-Bennett EE. Emerging infectious diseases: focus on infection control issues for novel coronaviruses (severe acute respiratory syndrome-CoV and Middle East respiratory syndrome-CoV), hemorrhagic fever viruses (Lassa and Ebola), and highly pathogenic avian influenza viruses, A (H5N1) and A (H7N9) Am J Infect Control. 2016;44:e91–100. doi: 10.1016/j.ajic.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. SARS statistics. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Geneva: WHO. Revised on 26 Sep 2003. Available at:https://www.who.int/csr/sars/country/table2004_04_21/en/ [accessed 13 April 2020].
- 8.World Health Organization. Statistics on Middle East respiratory syndrome. Geneva: WHO. Published on Nov 2019. Available at:https://www.who.int/emergencies/mers-cov/en/ [accessed 13 April 2020].
- 9.World Health Organization . WHO; Geneva: 2015. Ebola situation report 21 October 2015.https://apps.who.int/ebola/current-situation/ebola-situation-report-21-october-2015 Available at: [accessed 13 April 2020] [Google Scholar]
- 10.Zumla A, Chan JF, Azhar EI, Hui DS, Yuen K-Y. Coronaviruses – drug discovery and therapeutic options. Nat Rev Drug Discov. 2016;15:327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis M, Vandeweerd V, Verbeke R, Van der Vliet D. COVID-19 overview of information available to support the development of medical countermeasures and interventions against COVID-19. Transdisciplinary Insights – Living Papers. 2020:1–173. Published on 20 April 2020. [Google Scholar]
- 12.Woo PC, Lau SK, Huang Y, Yuen KY. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med. 2009;234:1117–1127. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- 13.Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . WHO; Geneva: 2020. Novel coronavirus (2019-nCoV) situation report 22.https://apps.who.int/iris/handle/10665/330991 Available at: [accessed 13 April 2020] [Google Scholar]
- 16.Gorbalenya AE, Baker SC, Baric RS, de Groot RJ, Drosten C, Gulyaeva AA, et al. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Micro. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang S, Shi Z, Shu Y, Song J, Gao GF, Tan W, et al. A distinct name is needed for the new coronavirus. Lancet. 2020;395:949. doi: 10.1016/S0140-6736(20)30419-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization . WHO; Geneva: 2020. Coronavirus disease 2019 (COVID-19) situation report 51.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 Available at: [accessed 13 April 2020] [Google Scholar]
- 19.World Health Organization . WHO; Geneva: 2020. Coronavirus disease (COVID-19) pandemic.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 Available at: [accessed 25 April 2020] [Google Scholar]
- 20.Peiris JSM. In: Medical microbiology. 18th ed. Greenwood D, Barer M, Slack R, Irving W, editors. Churchill Livingstone; Edinburgh: 2012. Coronaviruses; pp. 587–593. [Google Scholar]
- 21.Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singhal T. A review of coronavirus disease-2019 (COVID-19) Indian J Pediatr. 2020;87:281–286. doi: 10.1007/s12098-020-03263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization . WHO; Geneva: 2020. Report of the WHO–China joint mission on coronavirus disease 2019 (COVID-19). Published on 28 Feb 2020.https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf Available at: [accessed 14 April 2020] [Google Scholar]
- 24.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019‐nCoV) in Wuhan, China. J Med Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlos WG, Dela Cruz CS, Cao B, Pasnick S, Jamil S. Novel Wuhan (2019-nCoV) coronavirus. Am J Respir Crit Care Med. 2020;201:7–8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- 29.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization . WHO; Geneva: 2020. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations: scientific brief, 27 March 2020.https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations Available at: [accessed 13 April 2020] [Google Scholar]
- 31.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Health Commission of the People's Republic of China website . National Health Commission of the People's Republic of China; 2020. Diagnosis and treatment of novel coronavirus infection (trial version 6)www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml Available at: . [accessed 14 April 2020] [Google Scholar]
- 33.Lei J, Li J, Li X, Qi X. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:18. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, Al-Rabiah FA, Al-Hajjar S, Al-Barrak A, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phan LT, Nguyen TV, Luong QC, Nguyen TV, Nguyen HT, Le HQ, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 37.Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019‐nCoV) infections: challenges for fighting the storm. Eur J Clin Invest. 2020;50:e13209. doi: 10.1111/eci.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casanova L, Rutala WA, Weber DJ, Sobsey MD. Survival of surrogate coronaviruses in water. Water Res. 2009;43:1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu IT, Li Y, Wong TW, Tam W, Chan AT, Lee JH, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y-R, Cao Q-D, Hong Z-S, Tan Y-Y, Chen S-D, Jin H-J, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. StatPearls Publishing; Statpearls: 2020. Features, evaluation and treatment coronavirus (COVID-19)https://www.ncbi.nlm.nih.gov/books/NBK554776/ Available at: [accessed 15 June 2020] [PubMed] [Google Scholar]
- 45.Devaux CA, Rolain J-M, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. In press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. Malaria. Lancet. 2014;383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 49.Boelaert JR, Piette J, Sperber K. The potential place of chloroquine in the treatment of HIV-1-infected patients. J Clin Virol. 2001;20:137–140. doi: 10.1016/s1386-6532(00)00140-2. [DOI] [PubMed] [Google Scholar]
- 50.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases. Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee S-J, Silverman E, Bargman JM. The role of antimalarial agents in the treatment of SLE and lupus nephritis. Nat Rev Nephrol. 2011;7:718. doi: 10.1038/nrneph.2011.150. [DOI] [PubMed] [Google Scholar]
- 52.Rolain J-M, Colson P, Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burkard C, Verheije MH, Wicht O, van Kasteren SI, van Kuppeveld FJ, Haagmans BL, et al. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. Published on 22 Aug 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taccone FS, Gorham J, Vincent JL. Hydroxychloroquine in the management of critically ill patients with COVID-19: the need for an evidence base. Lancet Respir Med. 2020;8:539–541. doi: 10.1016/S2213-2600(20)30172-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 57.Guangdong Provincial Department of Science and Technology and Guangdong Provincial Health Commission Multi-center Collaborative Group of Chloroquine Phosphate for the Treatment of New Coronavirus Pneumonia. Expert Consensus for Chloroquine Phosphate for the Treatment of New Coronavirus Pneumonia [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia] Chin J Tuberc Respir Dis. 2020;43:185–188. [Google Scholar]
- 58.Rathi S, Ish P, Kalantri A, Kalantri S. Hydroxychloroquine prophylaxis for COVID-19 contacts in India. Lancet Infect Dis. 2020;S1473-3099(20) doi: 10.1016/S1473-3099(20)30313-3. 30313–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.US Food and Drug Administration . FDA; White Oak, MD: 2020. Data sheet for health care providers emergency use authorization (EUA) of chloroquine sulfate supplied from the strategic national stockpile for treatment of COVID-19 in certain hospitalized patients. EUA chloroquine phosphate health care provider fact sheet. [Google Scholar]
- 60.US Food and Drug Administration . FDA; White Oak, MD: 2020. Data sheet for health care providers emergency use authorization (EUA) of hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of COVID-19 in certain hospitalized patients. EUA chloroquine phosphate health care provider fact sheet. [Google Scholar]
- 61.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:1–34. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotech. 2020;38:379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- 63.Baidya A, Shankar A, Ahmed R, Das AK. Relevance and role of hydroxychloroquine in prophylaxis and therapy of COVID-19. J Med Sci Clin Res. 2020;8:94–101. [Google Scholar]
- 64.Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 65.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020:ciaa237. doi: 10.1093/cid/ciaa237. 09 March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Götz V, Magar L, Dornfeld D, Giese S, Pohlmann A, Höper D, et al. Influenza A viruses escape from MxA restriction at the expense of efficient nuclear vRNP import. Sci Rep. 2016;6:23138. doi: 10.1038/srep23138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tay M, Fraser JE, Chan W, Moreland NJ, Rathore AP, Wang C, et al. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor ivermectin. Antiviral Res. 2013;99:301–306. doi: 10.1016/j.antiviral.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 68.Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443:851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wagstaff KM, Rawlinson SM, Hearps AC, Jans DA. An AlphaScreen®-based assay for high-throughput screening for specific inhibitors of nuclear import. J Biomol Screen. 2011;16:192–200. doi: 10.1177/1087057110390360. [DOI] [PubMed] [Google Scholar]
- 70.Kosyna FK, Nagel M, Kluxen L, Kraushaar K, Depping R. The importin α/β-specific inhibitor ivermectin affects HIF-dependent hypoxia response pathways. Biol Chem. 2015;396:1357–1367. doi: 10.1515/hsz-2015-0171. [DOI] [PubMed] [Google Scholar]
- 71.van der Watt PJ, Chi A, Stelma T, Stowell C, Strydom E, Carden S, et al. Targeting the nuclear import receptor Kpnβ1 as an anticancer therapeutic. Mol Can Ther. 2016;15:560–573. doi: 10.1158/1535-7163.MCT-15-0052. [DOI] [PubMed] [Google Scholar]
- 72.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Timani KA, Liao Q, Ye L, Zeng Y, Liu J, Zheng Y, et al. Nuclear/nucleolar localization properties of C-terminal nucleocapsid protein of SARS coronavirus. Virus Res. 2005;114:23–34. doi: 10.1016/j.virusres.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wurm T, Chen H, Hodgson T, Britton P, Brooks G, Hiscox JA. Localization to the nucleolus is a common feature of coronavirus nucleoproteins, and the protein may disrupt host cell division. J Virol. 2001;75:9345–9356. doi: 10.1128/JVI.75.19.9345-9356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gunalan V, Mirazimi A, Tan YJ. A putative diacidic motif in the SARS-CoV ORF6 protein influences its subcellular localization and suppression of expression of co-transfected expression constructs. BMC Res Notes. 2011;4:446. doi: 10.1186/1756-0500-4-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Costanzo M, De Giglio MAR, Roviello GN. SARS CoV-2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem. 2020;27:4536–4541. doi: 10.2174/0929867327666200416131117. [DOI] [PubMed] [Google Scholar]
- 77.Nutho B, Mahalapbutr P, Hengphasatporn K, Pattaranggoon NC, Simanon N, Shigeta Y, et al. Why are lopinavir and ritonavir effective against the newly emerged coronavirus 2019? Atomistic insights into the inhibitory mechanisms. Biochemistry. 2020;59:1769–1779. doi: 10.1021/acs.biochem.0c00160. [DOI] [PubMed] [Google Scholar]
- 78.Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dorward J, Gbinigie K. Centre for Evidence Based Medicine; Oxford: 2020. There is currently no strong evidence for the efficiency of lopinavir/ritonavir in treatment of COVID-19.https://www.cebm.net/covid-19/lopinavir-ritonavir-a-rapid-review-of-the-evidence-for-effectiveness-in-treating-covid/ Available at: [accessed 20 April 2020] [Google Scholar]
- 80.Vastag B. Old drugs for a new bug. JAMA. 2003;290:1695–1696. doi: 10.1001/jama.290.13.1695. [DOI] [PubMed] [Google Scholar]
- 81.Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300:1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 82.Choy K-T, Wong AY-L, Kaewpreedee P, Sia S-F, Chen D, Hui KPY, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat Rev Drug Dis. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 84.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y, Xie Z, Lin W, Cai W, Wen C, Guan Y, et al. An exploratory randomized controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) MedRxiv. 2020; Published on 15 April 2020. [Google Scholar]
- 86.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, et al. COVID‐19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;0:1–11. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 87.Hu L, Chen S, Fu Y, Gao Z, Long H, Ren HW, et al. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. MedRxiv. 2020; doi: 10.1093/cid/ciaa539. Published on 30 March 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnson & Johnson. Lack of evidence to support use of darunavir-based treatments for SARS-CoV-2. 2020. Available at: https://www.jnj.com/lack-of-evidence-to-support-darunavir-based-hiv-treatments-for-coronavirus [accessed 4 April 2020].
- 89.Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 90.Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Segler MH, Preuss M, Waller MP. Planning chemical syntheses with deep neural networks and symbolic AI. Nature. 2018;555:604–610. doi: 10.1038/nature25978. [DOI] [PubMed] [Google Scholar]
- 94.Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sorrell FJ, Szklarz M, Azeez KRA, Elkins JM, Knapp S. Family-wide structural analysis of human numb-associated protein kinases. Structure. 2016;24:401–411. doi: 10.1016/j.str.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guidelines for the prevention, diagnosis, and treatment of novel coronavirus-induced pneumonia. 5th ed. Available at:http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a/files/ab6bec7f93e64e7f998d802991203cd6.pdf[accessed 21 April 2020].
- 99.Tan EL, Ooi EE, Lin CY, Tan HC, Ling AE, Lim B, et al. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg Infect Dis. 2004;10:581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jianping J, Randomized, open, blank control study on the efficacy and safety of recombinant human interferon α1β; in the treatment of patients with new type of coronavirus infection in Wuhan. Source- U.S. National Library of Medicine. Published on 03 March 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04293887 [accessed 21 April 2020].
- 102.Boriskin YS, Leneva IA, Pecheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15:997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 103.Zhao C, Zhao Y, Chai H, Gong P. Synthesis and in vitro anti-hepatitis B virus activities of some ethyl 5-hydroxy-1H-indole-3-carboxylates. Bioorg Med Chem. 2006;14:2552–2558. doi: 10.1016/j.bmc.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 104.Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, et al. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. J Zhejiang Univ (Med Sci) 2020;49:147–157. doi: 10.3785/j.issn.1008-9292.2020.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, et al. Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. 2020;81:e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu Z, Lu Z, Xu T, Chen C, Yang G, Zha T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020;81:e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Nat Acad Sci. 2020;4:168. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cheng Y, Wong R, Soo Y, Wong W, Lee C, Ng M, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ko JH, Seok H, Cho SY, Ha YE, Baek JY, Kim SH, et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23:617–622. doi: 10.3851/IMP3243. [DOI] [PubMed] [Google Scholar]
- 110.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 111.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.US Food and Drug Administration . FDA; White Oak, MD: 2020. Recommendations for investigational COVID-19 convalescent plasma.https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma Available at: [accessed 15 June 2020] [Google Scholar]
- 113.Tanne JH, Hayasaki E, Zastrow M, Pulla P, Smith P, Rada AG. COVID-19: how doctors and healthcare systems are tackling coronavirus worldwide. BMJ. 2020;368:1090. doi: 10.1136/bmj.m1090. [DOI] [PubMed] [Google Scholar]
- 114.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Venkateswaran TV, Singh J, India to explore novel blood plasma therapy for COVID-19. New Delhi. Published on 10 April 2020. Available at: https://www.thehindubusinessline.com/news/science/india-to-explore-novel-blood-plasma-therapy-for-covid-19/article31311466.ece [accessed 22 April 2020].
- 116.Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, et al. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.World Health Organization . WHO; Geneva: 2020. Bacille Calmette-Guérin (BCG) vaccination and COVID-19.https://www.who.int/news-room/commentaries/detail/bacille-calmette-guérin-(bcg)-vaccination-and-covid-19 Available at: [accessed 22 April 2020] [Google Scholar]
- 118.University of Oxford: a study of a candidate COVID-19 vaccine (COV001). Source- U.S. National Library of Medicine. Published on 27 March 2020. Available at: https://clinicaltrials.gov/ct2/show/NCT04324606 [accessed 22 April 2020]
- 119.Hodgson J. The pandemic pipeline. Nat Biotechnol. 2020;38:523–532. doi: 10.1038/d41587-020-00005-z. [DOI] [PubMed] [Google Scholar]
- 120.Duddu P. Coronavirus treatment: vaccines/drugs in the pipeline for COVID-19. Available at: https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/. Published on 16 April 2020. [accessed 22 April 2020].
- 121.Ji S, Bai Q, Wu X, Zhang D-W, Wang S, Shen JL, et al. Unique synergistic antiviral effects of Shufeng Jiedu Capsule and oseltamivir in influenza A viral-induced acute exacerbation of chronic obstructive pulmonary disease. Biomed Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109652. [DOI] [PubMed] [Google Scholar]
- 122.World Health Organization . WHO; Geneva: 2020. “Solidarity” clinical trial for COVID-19 treatments.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments Available at: [accessed 25 April 2020] [Google Scholar]
- 123.Kumar V, Dhanjal JK, Kaul SC, Wadhwa R, Sundar D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (Mpro) of SARS-CoV-2 and inhibit its activity. J Biomol Struct Dyn. 2020:1–7. doi: 10.1080/07391102.2020.1772108. (Published on 01 June 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morales-Narváez E, Dincer C. The impact of biosensing in a pandemic outbreak: COVID-19. Biosens Bioelectron. 2020;163 doi: 10.1016/j.bios.2020.112274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu G, Li J, Meng Z, Yu Y, Li Y, Tang X, et al. Learning from large-scale wearable device data for predicting epidemics trend of COVID-19. Discrete Dyn Nat Soc. 2020;2020 [Google Scholar]
- 126.Sharma A, Hayat A, Mishra RK, Catanante G, Shahid SA, Bhand S, et al. Design of a fluorescence aptaswitch based on the aptamer modulated nano-surface impact on the fluorescence particles. RSC Adv. 2016;6:65579–65587. [Google Scholar]
- 127.Nawaz MH, Hayat A, Catanante G, Latif U, Marty JL. Development of a portable and disposable NS1 based electrochemical immunosensor for early diagnosis of dengue virus. Anal Chim Acta. 2018;1026:1–7. doi: 10.1016/j.aca.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 128.Khan A, Rao TS. In: Nanomaterials for air remediation. Abdeltif A, Assadi AA, Nguyen-Tri P, Nguyen TA, Rtimi S, editors. Elsevier; Amsterdam: 2020. Nanobiosensors for virus detection in the environment; pp. 61–87. [Google Scholar]
- 129.Huang R, Xi Z, Deng Y, He N. Fluorescence based aptasensors for the determination of hepatitis B virus antigen. Sci Rep. 2016;6:31103. doi: 10.1038/srep31103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mishra GK, Sharma A, Bhand S. Ultrasensitive detection of streptomycin using flow injection analysis-electrochemical quartz crystal nanobalance (FIA-EQCN) biosensor. Biosens Bioelectron. 2015;67:532–539. doi: 10.1016/j.bios.2014.09.033. [DOI] [PubMed] [Google Scholar]
- 131.Dutra RF, Kubota LT. An SPR immunosensor for human cardiac troponin T using specific binding avidin to biotin at carboxymethyldextran-modified gold chip. Clin Chim Acta. 2007;376:114–120. doi: 10.1016/j.cca.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 132.Du G, Wang L, Zhang D, Ni X, Zhou X, Xu H, et al. Colorimetric aptasensor for progesterone detection based on surfactant-induced aggregation of gold nanoparticles. Anal Biochem. 2016;514:2–7. doi: 10.1016/j.ab.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 133.Qiu G, Gai Z, Tao Y, Schmitt J, Kullak-Ublick GA, Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14:5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- 134.Seo G, Lee G, Kim MJ, Baek SH, Choi M, Ku KB, et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]