Abstract
Reductions in positive affect are a salient feature of preschool-onset major depressive disorder. Yet, little is known about the psychophysiological correlates of this blunted positive affect and whether reduced physiological responding to pleasant stimuli may differentiate depressed and healthy young children. 120 four-to-seven year old children with current depression and 63 psychiatrically healthy 4-to-7 year old children completed a simple picture-viewing task of pleasant and neutral pictures while event-related potentials (ERPs) were recorded. The early-childhood version of the Kiddie Schedule for Affective Disorders and Depression was used to establish psychiatric diagnoses. A one-way ANCOVA was used to test for group differences in response to pleasant and neutral pictures. Young children with depression showed a reduced response to pleasant vs. neutral pictures (LPP), after controlling for children’s age (F(1,180) = 4.15, p = 0.04, η2 = 0.02). The LPP for the children with preschool-onset depression (M = 0.99, SE = 0.65) was significantly smaller than the LPP in the healthy group of young children (M = 3.27, SE = 0.90). This difference did not vary as a function of depression or anhedonia severity within the group with depression or the healthy children. Similar to older children and adolescents with depression, young children with depression display reductions in responsivity to pleasant stimuli as indexed by the LPP. These findings extend prior findings indicating a blunted response to pleasant stimuli in preschool- onset depression. Given the greater neuroplasticity of emotional response and regulation, these findings suggest clinical attention to emotional response to pleasure is an important target in preschool-onset depression. Clinical trial registration information: A Randomized Control Trial of PCIT-ED for Preschool Depression; http://clinicaltrials.gov/;NCT02076425.
Keywords: Late positive potential (LPP), Early childhood, Depression, ERP
Introduction
Public health recognition of, and validation for, Preschool-Onset Major Depressive Disorder (PO-MDD) has increased significantly over the last 2 decades. PO-MDD has been shown to have a chronic and relapsing course with functional impairment and is related to poor developmental outcomes in emotional, social, and cognitive domains (Bufferd et al. 2012; Hankin 2015; Luby et al. 2002, 2009b, 2014; Whalen et al. 2016, 2017). Depression is also increasingly recognized as a disorder of diminished positive affect (Forbes and Dahl 2005, 2012; McCabe 2018). Consistent with findings in adolescent and adult depression, a reduction of positive affect is a salient feature of PO-MDD (Barch et al. 2018; Luby and Belden 2008; Luby et al. 2003). For instance, reduced positive affect at age 3 predicts later depressive cognitive styles when measured at age 7 (Hayden et al. 2006). Other work has shown that preschoolers with PO-MDD were 17 times more likely than healthy peers, 7 times more likely than disruptive disordered peers, and 5 times more likely than anxiety disordered peers to exhibit behavioral manifestations of anhedonia (Luby et al. 2009a). Reductions in positive affect associated with PO-MDD may be particularly important in younger children, given that joyful play exploration is a central and normative developmental milestone to facilitate emotional well being and competence across multiple domains.
Although there is a growing body of research that describes alterations in brain structure, function, and connectivity in school age children with a history of PO-MDD (Belden et al. 2015; Luking et al. 2011; Pagliaccio et al. 2014), much less work has been done to examine aberrations in neural processes of acutely depressed preschool children. In particular, despite the established link between having a history of PO-MDD and alterations in affective-oriented neurobiology later in childhood (Barch et al. 2012; Gaffrey et al. 2010; Luby et al. 2016; Suzuki et al. 2012) there remains a dearth of research examining emotion-focused neural functioning in young children with acute PO-MDD. An important exception to this work by Gaffrey and colleagues demonstrated alterations in amygdala activation in response to fear faces and increased amygdala reactivity to both gain and loss on a gambling task using task-based fMRI in acutely depressed preschoolers (Gaffrey et al. 2013, 2018). In addition, work by Belden and colleagues using a subgroup of children from the current study found reduced responses to reward in PO-MDD using event-related potentials (Belden et al. 2016). The identification of early biomarkers associated with behavioral manifestations of PO-MDD could be critical to illuminating the mechanisms of risk and guiding the form of interventions most likely to be effective (Kujawa and Burkhouse 2017). Thus, the goal of the current study was to examine an additional potential biomarker related to children’s engagement with emotional stimuli.
Approaches to Examining Atypical Neural Functioning Associated with PO-MDD
Recent findings indicate that deficits in reward processing at both a neural and behavioral level are associated with risk for the onset of depression and the persistence and recurrence of already existing depression among children, adolescents, and adults. Event-related potentials (ERPs) provide an excellent way to study neural activity related to basic affective processes as well as the specific abnormalities underlying early childhood onset psychiatric disorders. In particular, the late positive potential (LPP) is a positive deflection in the ERP waveform that indicates dynamic and sustained engagement with emotional stimuli, including positively and negatively valenced scenes, faces, and words (Hajcak and Dennis 2009; Hajcak et al. 2010; Schupp et al. 2003; Weinberg and Hajcak 2011). The LPP is typically maximal between 250 and 1000 ms following stimulus onset (Foti et al. 2009; Hajcak et al. 2010) and is thought to arise from reciprocal activation of frontal and occipital-parietal regions (Cuthbert et al. 2000). Enhanced LPP amplitudes are reliably observed in response to emotional images, including positive and negatively valenced pictures, compared with neutral images (Cuthbert et al. 2000; Hajcak and Dennis 2009; Hua et al. 2014), even in children as young as 4 years of age (Hua et al. 2014). Moreover, the LPP elicited by emotional images is highly related to autonomic and self-reported indices of arousal (Cuthbert et al. 2000). Such findings suggest that the LPP indexes engagement of attentional resources by motivational systems in service of basic survival behaviors (Lang et al. 1997). The LPP is thought to reflect more the sustained engagement with the emotional content of the stimuli, as it is typically lasts throughout stimulus presentation and can even persist for up to 1000 ms after the offset of the emotional stimulus without habituating (Hajcak et al. 2010).
Variation in LPP magnitudes has been related to depression and anxiety from childhood through adulthood (DeCicco et al. 2014; Dennis and Hajcak 2009; Foti et al. 2010; Grunewald et al. 2018; Kujawa et al. 2015; MacNamara et al. 2016; Solomon et al. 2012). Further, variation in LPP magnitudes has also been related to risk for these disorders in children and adolescents by virtue of having a depressed or anxious parent (Kayser et al. 2017; Kujawa et al. 2012a; Nelson et al. 2015b; Speed et al. 2016; van den Heuvel et al. 2015). Several studies find a reduced LPP to pleasant (Benning and Oumeziane 2017; Foti et al. 2010; Grunewald et al. 2018; Levinson et al. 2018; Mardaga and Iakimova 2014; Weinberg et al. 2016) and unpleasant stimuli (Foti et al. 2010; Grunewald et al. 2018; Kayser et al. 2000; Kujawa et al. 2015; MacNamara et al. 2016; Weinberg et al. 2016) among individuals with depression or depressive symptoms when compared to either healthy controls or those with fewer depressive symptoms. Findings indicating reduced LPPs to emotional stimuli in both adults and children appear to be consistent with Rottenberg’s emotion context insensitivity (ECI) hypothesis (Rottenberg et al., 2005). For instance, Weinberg and colleagues (Weinberg et al. 2016) found a reduced LPP to pleasant and threatening images in adults with an onset of depression during adolescence when compared to adults with an onset of depression during adulthood. A reduced LPP to emotional stimuli has also been shown among individuals at-risk for depression (Kayser et al. 2017; Kujawa et al. 2012a, b; Nelson et al. 2015b, individuals with a history of suicide attempts (Weinberg et al. 2017), and individuals with low positive affect—a significant clinical feature of depression (Kessel et al. 2017; Weinberg and Sandre 2018).
By contrast, several others have shown the opposite pattern, namely a greater LPP to emotional stimuli among those with depression and/or depressive symptoms (Auerbach et al. 2015; Burkhouse et al. 2017; Chronaki et al. 2018; Dennis and Hajcak 2009; Jaworska et al. 2012; Shestyuk and Deldin 2010; Webb et al. 2017), remitted depression (Burkhouse et al. 2017), and at-risk for depression (Speed et al. 2016). For example, Auerbach et al. (2015) found that depressed female adolescents (13–18 years) showed enhanced LPP to negative but not positive self-referential words when compared to healthy adolescents, who exhibited an opposite pattern (e.g., enhanced LPP to positive words). This finding was also replicated in depressed and remitted-depressed female adolescents (ages 11–18 years) who exhibited enhanced LPPs across all types of emotional facial expressions (Burkhouse et al. 2017).
There may be several reasons for the inconsistencies in findings across studies. First, four of the studies finding a greater LPP to emotional stimuli used females only, and more specifically females in early-to-late adolescence (Auerbach et al. 2015; Burkhouse et al. 2017; Speed et al. 2016; Webb et al. 2017). There are well-documented increases in depression during adolescence, specifically for adolescent females, who report twice as many depressive episodes as male adolescents, suggesting that this population may be unique with regard to the neutral correlates and associated clinical features of depression beginning during this time. Further, several of these studies used self-referential words as stimuli (Auerbach et al. 2015; Shestyuk and Deldin 2010; Speed et al. 2016), which may be more likely to elicit the feelings of worthlessness and helplessness occurring in depression (depressogenic self-view) rather than more general reduced positive affectivity.
The LPPs among children with PO-MDD have yet to be examined. If similar alterations (reductions) in the LPP are seen in children with PO-MDD as compared to those seen in older children and adolescents with depression, it will provide further evidence for the neurobiological continuity of depression across the lifespan and may help guide early detection and intervention. Given the limited number of longitudinal studies in this area, it is difficult to determine whether enhanced or blunted LPP may be a risk factor for depression, a core feature of the pathology, or an alteration that develops in conjunction with other dysregulated processes such as sleep or neuroendocrine disruptions. There appears to be support from the cross-sectional studies reviewed above for each of these possibilities.
Taken together, research suggests that the LPP may be a developmentally appropriate marker of reduced responding to pleasant stimuli, a known risk factor for and correlate of depression in older child and adolescent samples (Grunewald et al. 2018; Kujawa et al. 2012a, b; Levinson et al. 2018; Nelson et al. 2015a, b). Replicating this work in younger samples is particularly important because maturation of brain regions involved in emotion regulation and cognitive control are rapidly developing during the preschool years and setting the stage for how children process emotional information. Detecting reductions in responding to pleasant stimuli during this age may help tailor interventions toward altering this specific process. Therefore, we hypothesized that: (1) PO-MDD would be associated with reduced responding to pleasant stimuli as indexed by the LPP when compared to healthy young children; and (2) depression, anhedonia severity, and motivation to approach goal oriented outcomes as measured by clinical interviews and parental-reports would be significantly related to reduced responses to pleasant stimuli (LPP) when compared to less severely depressed children. Given the prior findings with adolescent females, on a more exploratory basis, we examined the role of sex on group differences in the LPP and depression, anhedonia severity, and motivation to approach goal oriented outcomes.
Method
Participants
One hundred twenty-six depressed young children and their caregivers provided informed consent to participate in the current ERP study. Depressed preschool participants were recruited from a randomized clinical trial study (PCIT-ED; Parent-Child Interaction Therapy-Emotion Development (Luby et al. 2018)) for early childhood onset major depressive disorder. Caregivers and their children were invited to participate in the current study during their baseline assessment for PCIT-ED. Thus, ERP data was collected from children prior to randomization into the treatment study (e.g., 18-week treatment vs.18-week waitlist) and prior to the commencement of any therapy sessions. An additional group of healthy preschoolers (n = 83) were recruited as a comparison group and assessed once, with healthy status based on low scores on the Child Behavior Checklist. They were matched to the PO-MDD group as best as possible on child gender, age, ethnicity, and SES. The institutional review board at Washington University in St. Louis approved all procedures.
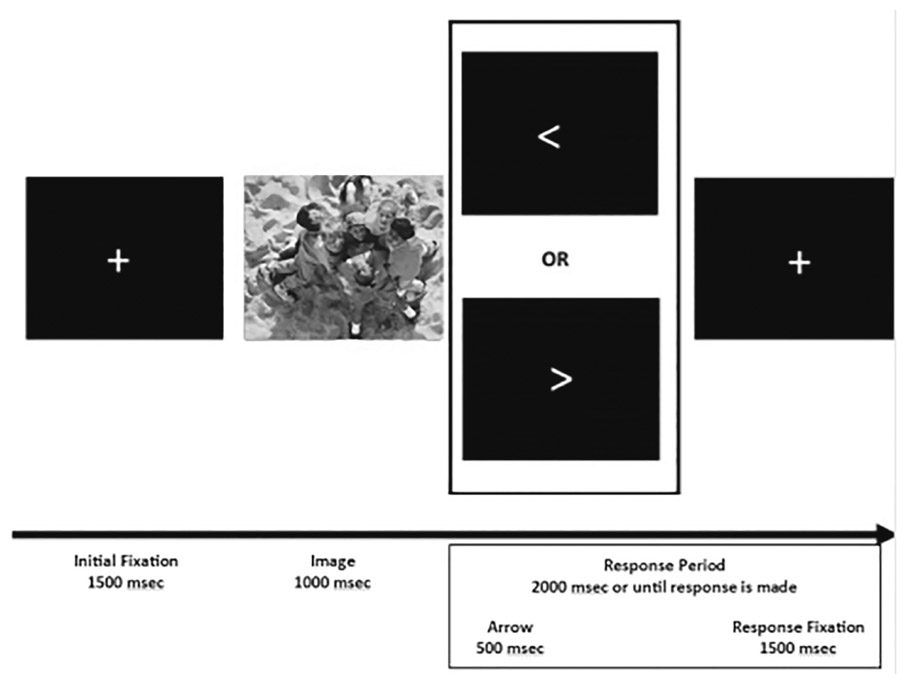
Children participated in one EEG session that included two ERP tasks: a Door Guessing Task measuring reward processing and reported previously (Belden et al. 2016) and a Picture task measuring responding to pleasant stimuli (LPP; described here). All children completed the Doors task prior to the Picture task. The PO-MDD group also completed two additional EEG sessions: one during treatment and one following treatment. The data from these additional testing sessions is not included in the present report (see Barch et al., 2018). The following criteria were implemented to determine inclusion of LPP data for the analyses. First, children had to have at least 20 usable ERP segments (>50% of the presented trials) from electrode sites O1, Oz, and O2 for each condition (e.g., pleasant and neutral). Second, the task used to elicit the LPP incorporated a simple response task between each stimulus presentation (i.e., children had to press a button that matched the direction of an arrow presented on the screen) in order to keep children engaged and attentive to the presentation of the stimuli (see Fig. 1). Thus, to be included in analyses children had to have responded on at least half of the trials.
Fig. 1.
Timing of the Picture Viewing Task
IAPS photo ID’s: 2514, 2580, 5390, 5395, 5500, 5731, 5740, 5900, 7000, 7002, 7009, 7010, 7026, 7038, 7039, 7090, 7100, 7130, 7175, 7190, 1463, 1710, 1750, 1811, 2070, 2091, 2092, 2224, 2340, 2345, 2347, 7325, 7330, 7400, 8031, 8200, 8370, 8461, 8496, 8497
Of the total 209 children who consented to an ERP assessment, four children (PO-MDD = 3; Healthy = 1) did not complete an ERP task due to scheduling issues. Of the remaining 205 children, n = 41 children (PO-MDD = 24; Healthy = 17) were excluded because they had less than 20 usable ERP segments in one of the conditions. Thus, the final sample consisted of 164 children: 99 children with PO-MDD and 65 healthy young children. Children excluded for poor quality EEG were younger than children who were included (see Table S1, available online) but did not differ from those included on the basis of sex, race, family income-to-needs ratio, or depression severity.
Behavioral and Diagnostic Measures
Behavioral Activation/Inhibition
The caregivers of children in both groups completed the Behavioral Activation and Inhibition Scales (BIS/BAS; Carver and White 1994). The BIS/BAS is a valid and reliable measure of children’s appetitive motives (BAS), in which the goal is to move toward something desired, as well as aversive motives (BIS), in which the goal is to move away from something unpleasant. We examined each subscale using the recently revised and validated scoring for children (Pagliaccio et al., 2015).
Child Behavior Checklist
The caregivers of all children in the healthy comparison group completed The Child Behavior Checklist (CBCL (Achenbach and Edelbrock 1983; Achenbach and Rescorla 2000). This widely used and well-validated measure assesses internalizing and externalizing behavioral problems using age-based norms. To be included in the healthy group T-scores for internalizing and externalizing pathology had to be within the average range (e.g, ≥60).
K-SADS-EC
The K-SADS-EC is a semi-structured clinical interview for DSM-5 disorders adapted for use in children aged 3.0–6.11. This measure generates both categorical and dimensional measures of DSM-5 Axis I disorders with good test-retest reliability and construct validity (Gaffrey and Luby 2012). The presence and severity of MDD and other Axis I comorbidities were assessed in the PO-MDD group only via parental report. All K-SADS-EC interviews were conducted by master’s level clinicians, videotaped, reviewed for reliability, and calibrated for accuracy. Satisfactory inter-rater reliability was established prior to onset of the study and kappa’s during the study were maintained on a monthly basis with overall kappa’s of K = 0.74 for MDD and all diagnoses K = 0.88. A MDD core symptom score and Anhedonia sum score were derived from this interview. The MDD score was the number of core MDD symptoms endorsed on the K-SADS-EC. The Anhedonia score was the sum of boredom, anhedonia, and amotivation items from the K-SADS-EC. Amotivation items were developed for this version of the KSADS using more detailed questions from the Preschool Age Psychiatric Assessment (PAPA; See Gaffrey et al. 2018 for more details). Most children in the PO-MDD group (n = 78) had a comorbid diagnosis, including: separation anxiety (SAD; n = 15), social phobia (n = 6), specific phobia (n = 6), generalized anxiety disorder (GAD; n = 28), obsessive compulsive disorder (n = 1), attention deficit hyperactivity disorder (ADHD; n = 23), oppositional defiant disorder (ODD; n = 43), and conduct disorder (CD; n = 1). Given this, we controlled for the presence of the most common co-morbid diagnoses in our sample: GAD, ADHD, and ODD in our primary analyses.
Preschool Feelings Checklist: Screening and Scale Versions (PFC and PFC-Scale)
The PFC, a validated screening checklist with favorable sensitivity, was used to ascertain young children at high risk for MDD for enrollment into the larger randomized control trial (Luby et al. 2004). The PFC-Scale, a 23-item Likert scale, adapted from the PFC, was administered to measure depression severity via caregiver report in both the PO-MDD and healthy groups. Example questions from the PFC include: “My child frequently appears sad or says he/she is sad,” and “My child blames him/herself for things.” The PFC-Scale was used as a dimensional assessment of depression severity.
Income-to-Needs
The income-to-needs ratio was computed as the total family income at baseline divided by the federal poverty level, based on family size at enrollment for both PO-MDD and healthy subjects.
Stimulus Materials and Picture-Viewing Task
After a research assistant (RA) provided a brief description of the task to child participants, electroencephalography (EEG) sensors were attached. Once electrodes were attached and acceptable impedance ranges were achieved, the RA provided detailed, age appropriate task instructions. Children were told that they would see lots of pictures and that they were to simply look at the pictures that appeared on the screen. Children were told that that an arrow pointing to the left or the right would appear on the screen after each picture and instructed to press the left or right button on the game controller that matched the direction of the arrow. Forty developmentally appropriate pictures were selected from the International Affective Picture System (IAPS Lang et al. 1995); of these 20 photos depicted pleasant/affectively positive scenes (e.g., smiling faces, candy) and 20 pictures depicted neutral scenes (e.g., neutral faces, household object such as a towel).1 The pleasant stimuli had a mean valence of 7.49 (SD = 1.51) and arousal of 5.00 (SD = 2.27). The neutral stimuli had a mean valence of 5.27 (SD = 1.26) and arousal of 3.03 (SD = 1.91). Although valence and arousal ratings both use a scale of one to nine, lower ratings for valence correspond to more unpleasant ratings, whereas lower ratings for arousal indicate less arousing stimuli. These pictures have been validated for children in this age range and used in previous ERP studies (DeCicco et al. 2012; Hajcak and Dennis 2009; Hua et al. 2014; Solomon et al. 2012). However, children this age have difficulty understanding the self-assessment mannequin rating (Lang et al., 1993; Solomon et al. 2012) and we were unable to obtain subjective valence and arousal ratings.
The task was administered on an Intel Core 2 Duo computer, using Presentation software (Neurobehavioral Systems, Inc.; Berkeley, CA) to control the presentation and timing of all stimuli. Each picture was displayed in color and occupied the entirety of a 20-in. monitor. Prior to each picture, a fixation cross was presented on the screen for 1500 ms. Pictures were than displayed for 1000 ms, followed by a left or right arrow (i.e., < or >) that appeared on the screen for 500 ms or until children responded with the controller. Children were placed approximately 60 cm from the screen and each picture occupied approximately 40 degrees of visual angle horizontally and vertically. Subjects first viewed a practice series of 10 pictures to familiarize them with the task procedure. After the practice children performed 80 trials, with each picture presented twice, once in the first half and once in the second half of the task. The order of the trials was randomly determined for each child. Children were offered a break after they completed 40 trials, the halfway point in the task.
Psychophysiological Recording and Data Reduction
The continuous EEG was recorded using the Brain Vision ActiCHamp 32 active electrode system (BrainVision LLC). Recordings were taken from 32 scalp electrodes based on the 10/20 system (FP1, F3, F7, FC1, FC5, FT9, C3, T7, CP1, CP5, TP9, P3, P7, O1, Fz, Cz, Pz, Oz, FP2, F4, F8, FC2, FC6, FT10, C4, T8, CP2, CP6, P4, P8, TP10, O2). A ground electrode was located at FPz. The electrooculogram (EOG) generated from blinks and eye movements was recorded from five facial electrodes: two approximately 1 cm above and below the subject’s left eye, one approximately 1 cm to the left of the left eye, and one approximately 1 cm to the right of the right eye. All bioelectric signals were digitized on a standard laboratory computer using PyCoder software (Brain Products). The EEG was sampled at 500 Hz and referenced to Cz. Off-line analysis was performed using Brain Vision Analyzer software (version 2.0, Brain Products, Germany). EEG data were re-referenced offline to the average of TP9 and TP102 (located adjacent to the mastoids) and bandpass filtered (Butterworth) with half-power cutoffs at 0.1 and 30 Hz. The EEG was corrected for EOG artifacts using the procedure from Gratton and colleagues (Gratton et al. 1983). Physiological artifacts were removed using a semiautomatic procedure where epochs containing a voltage >50 μV between sample points, a voltage difference of 175 μV within a segment, or a maximum voltage difference of < 0.50 μV within 100-ms intervals were rejected. At the initial start of the task, impedances were lowered at all sites to below 10 kΩ. Given that children with less than 20 usable ERP segments in either condition were removed from the analysis, no grand mean outliers (>3SD above the mean) were identified.
ERPs were constructed by separately averaging trials for each of the two picture types (pleasant and neutral). The average activity in the 200 ms window prior to picture onset served as the baseline. The LPP was evaluated where emotion modulation was maximal, at the average of the O1, Oz, and O2 recording sites. In order to determine where emotion modulation was maximal, we visually inspected the grand average ERP waveforms and referenced previous research using a similar task and sample (Chronaki et al. 2018; Hua et al. 2014; Kujawa et al. 2012a, bThe LPP was defined as the mean amplitude activity in the 400–600 ms window following stimulus onset (Hajcak and Dennis 2009; Hua et al. 2014; Solomon et al. 2012). Given that LPP is thought to reflect more sustained neural responding, lasting throughout stimulus presentation and even up to 1000 ms after stimulus offset, we also examined children’s LPP to pleasant vs. neutral pictures in a later window from 600 to 1000 ms using the same visual inspection procedure and time windows from prior research described above. Between and within group differences in children’s LPP activity were statistically evaluated using SPSS (Version 24.0).
Data Analysis Plan
The current study used t-tests and chi-square analyses to examine diagnostic group differences in relation to children’s demographic and clinical characteristics that may impact the ERP waveforms. First, repeated measures analyses of variance (RM ANOVAs) were conducted to explore group differences in behavioral data related to the ERP task, including response rates, accuracy, reaction time, and internal consistency. These analyses were conducted with and without the covariates (see supplemental material) found to be significant in prior t-tests and chi square analyses. All pairwise comparisons were Bonferroni adjusted. For EEG data, RM ANOVAs were conducted to compare the two groups across the two conditions (pleasant and neutral) including and excluding the covariates found to be significant in the above analyses. To further test for diagnostic group differences in children’s LPP mean amplitude, we created a difference wave subtracting the ERP to neutral pictures from the ERP to positive pictures (raw difference score). In addition to the more common difference score approach described above, we also calculated residual difference scores (Levinson et al. 2018; Weinberg et al. 2016). Residualized difference scores were used in addition to raw difference scores as residualized difference scores possess superior psychometric properties compared with raw difference scores in some situations (Cohen et al. 2014; Cronbach and Furby 1970; Weinberg et al. 2015) and may better isolate condition-specific activity. In order to create the residualized difference scores, a regression was conducted where the mean amplitude of the neutral response predicted the mean amplitude of the pleasant response (Pleasantresid). This residual score reflects variation in the response to pleasant stimuli not predicted by the neutral response. We did this separately for the 400–600 ms (early LPP) and 600–1000 ms (late LPP) segments. ANCOVAs were used to test for group and sex differences in children’s LPP amplitude using the residual scores including and excluding the covariates of interest (See supplemental material). We also examined whether individual differences in MDD, anhedonia severity, and motivation to approach goal oriented outcomes more specifically were correlated with individual differences in LPP to positive pictures. SEs are reported for post-hoc mean comparisons (Field 2017).
Results
Demographic and Clinical Differences in Diagnostic Groups
As seen in Table 1, PO-MDD and healthy control groups did not differ significantly in relation to familial income (t(162) = −0.43; p = 0.67), sex (X21 = 0.11; p = 0.74), or race (X23 = 0.02; p = 0.99). There was a significant group difference in child’s age at assessment (t162 = 2.19; p = 0.03), and thus age (in addition to co-morbid GAD, ADHD, and ODD; See supplemental analyses) was used as a covariate in analyses. As expected, children in the PO-MDD group (M = 40.00; SD = 11.21) had significantly higher depression severity scores compared to healthy control children (M = 9.69; SD = 6.81) on PFC-S, t(162) = 19.54, p < 0.00. Despite sex not differing significantly between the two groups, given previous ERP research highlighting sex differences in adolescents with depression, we included sex as a potential predictor in our analyses below.
Table 1.
Demographic and clinical differences between groups of children completing an ERP assessment
| Healthy control children (n = 65) | Depressed children (n = 99) | Group comparison statistic | |
|---|---|---|---|
| Age y, mean (SD) | 5.43 (0.85) | 5.73 (0.82) | t162 = 2.19; p = 0.03 |
| Gender (% male) | 63 | 66 | X21 = 0.11; p = 0.74 |
| Race | X23 = 0.02; p = 0.99 | ||
| %White | 78 | 79 | |
| %African American | 11 | 10 | |
| %Other | 11 | 11 | |
| Income to Needs Ratio, mean (SD) | 3.02 (1.19) | 2.94 (1.30) | t(162) = −0.43; p = 0.67 |
| PFC Scale Score, mean (SD) | 9.69(6.81) | 40.00 (11.21) | t(162) = 19.54, p < 0.00 |
| KSADS Anhedonia Sum Score | 0.05(0.21) | 1.39 (1.09) | t(162) = 11.40, p < 0.00 |
| KSADS MDD Core Symptoms | 0.68 (0.89) | 5.84 (1.56) | t(162) = 20.92, p < 0.00 |
| BAS Drive Score | 18.54(4.03) | 20.48(5.20) | t(155) = 2.48, p = 0.01 |
| BAS Reward Responsiveness Score | 25.30(2.26) | 22.75(3.41) | t(155) = −5.15, p < 0.00 |
Diagnostic Group and Sex Differences in Behavioral Data when Covarying for Age, GAD, ODD, and ADHD
Repeated measures ANOVAs were conducted to determine if there were behavioral differences between diagnostic groups and sex in response rates, accuracy, and reaction time after controlling for covariates of interest (Tables 2 and 3).
Table 2.
Raw and residualized Event-Related Potential (ERP) mean scores during pleasant and neutral picture viewing
| ERP | Healthy control children (n = 65) |
Depressed children (n = 99) |
||||||
|---|---|---|---|---|---|---|---|---|
| Amplitude μV | Amplitude μV | |||||||
| Early: 400–600 ms | Mean | SD | Min | Max | Mean | SD | Min | Max |
| Raw O1OzO2 Pleasant | 47.37 | 13.37 | 16.79 | 81.68 | 47.87 | 13.79 | 13.56 | 86.08 |
| Residualized O1OzO2 Pleasant | 1.76 | 9.01 | −14.50 | 32.13 | −1.16 | 7.11 | −21.80 | 17.56 |
| Raw O1OzO2 Neutral | 43.85 | 13.36 | 19.48 | 89.18 | 45.30 | 13.90 | 4.08 | 78.67 |
| Raw O1OzO2 | 4.21 | 6.52 | −12.48 | 27.26 | 2.39 | 5.70 | −19.12 | 19.58 |
| Pleasant-Neutral Difference | ||||||||
| Score | ||||||||
| Late: 600–1000 ms | Mean | SD | Min | Max | Mean | SD | Min | Max |
| Raw O1OzO2 Pleasant | 34.86 | 12.27 | 11.01 | 66.62 | 36.18 | 12.61 | 10.36 | 70.87 |
| Residualized O1OzO2 Pleasant | −0.87 | 5.34 | −12.78 | 10.93 | −0.82 | 5.90 | −16.65 | 19.21 |
| Raw O1OzO2 Neutral | 31.39 | 11.91 | 10.86 | 70.39 | 32.89 | 12.20 | −1.53 | 66.86 |
| Raw O1OzO2 | 3.47 | 5.32 | −9.46 | 14.40 | 3.29 | 5.93 | −17.54 | 22.20 |
| Pleasant-Neutral Difference | ||||||||
| Score | ||||||||
Table 3.
Correlations between Event-Related Potential (ERP) Mean scores during pleasant and neutral picture viewing and MDD symptoms, Anhedonia symptoms, PFC symptom severity and BAS
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Neutral 400–600 ms |
– | |||||||||||||
| 2. Pleasant 400–600 ms |
0.89*** | – | ||||||||||||
| 3. Neutral 600–1000 ms |
0.91*** | 0.81*** | – | |||||||||||
| 4. Pleasant 600–100 ms |
0.84*** | 0.89*** | 0.89*** | – | ||||||||||
| 5. Pleasant – Neutral Difference Score 400–600 ms |
−0.25** | 0.22** | −0.24** | 0.10 | – | |||||||||
| 6. Pleasant – Neutral Difference Score 600–1000 ms |
−0.09 | 0.24** | −.17* | 0.29*** | 0.74*** | – | ||||||||
| 7. Residualized Pleasant 400–600 ms |
0.00 | 0.36 | −0.03 | 0.22** | 0.76*** | 0.55*** | – | |||||||
| 8. Residualized Pleasant 600–1000 ms |
.20* | 0.53 | 0.16* | 0.58*** | 0.66*** | 0.95*** | 0.54*** | – | ||||||
| 9. Sex | −0.09 | −0.08 | −0.04 | −0.09 | 0.01 | −0.11 | −0.06 | −0.13 | – | |||||
| 10. MDD core symptoms | 0.08 | 0.08 | 0.05 | 0.06 | −0.00 | 0.02 | −0.09 | 0.03 | −0.06 | – | ||||
| 11. PFC Scale Score | 0.04 | 0.05 | 0.06 | 0.09 | 0.01 | 0.07 | −0.09 | 0.09 | −0.05 | 0.72*** | – | |||
| 12. Anhedonia Sum Score | 0.02 | −0.03 | −0.07 | −0.10 | −0.11 | −0.08 | −0.15 | −0.10 | −0.04 | 0.69*** | 0.56*** | – | ||
| 13. BAS Drive | 0.16* | 0.14 | 0.15 | 0.17* | −0.05 | 0.05 | −0.10 | 0.10 | 0.04 | 0.01 | 0.15 | −0.13 | – | |
| 14. BAS Reward Responsiveness | 0.02 | 0.02 | −0.03 | −0.01 | 0.00 | 0.06 | 0.04 | 0.05 | 0.03 | −0.31** | −0.48*** | −0.35*** | 0.32*** | – |
p < 0.05;
p < 0.01;
p < 0.000
Response Rate
There were no main effects of diagnostic group or time indicating that response rate did not differ in the sample as a whole or by diagnostic group following pleasant vs. neutral trials or during the first vs. second half of the task. There was a main effect of valence (F1,154 = 3.89, p = 0.05, η2 = 0.03); however post-hoc comparisons of pleasant and neutral stimuli did not reveal a significant difference (F1,154 = 2.19, p = 0.14, η2 = 0.01). Despite a non-significant interaction between diagnostic group and valance, pairwise within group analyses indicated that PO-MDD children (F1,97 = 6.25, p = 0.01 η2 = 0.04) missed significantly more trials following a pleasant image (M = 2.52, SE = 0.27) than following a neutral image (M = 2.16, SE = 0.25), whereas healthy control children missed an equivalent amount of trials following pleasant (M = 2.02, SE = 0.47) and neutral (M = 1.87, SE = 0.44) images.
Accuracy
Once covariates were included in the model, there were no main effects of diagnostic group, valence, or time indicating that accuracy did not differ in the sample as a whole or by diagnostic group following pleasant vs. neutral trials or during the first vs. second half of the task. However, there was a significant 3-way interaction between valence, time, and diagnostic group (F1,154 = 4.22, p = 0.04, η2 = 0.03). Within-group analyses indicated that there was a significant time by valence interaction within the PO-MDD children (F1,97 = 4.19, p = 0.04, η2 = 0.03) and within the healthy control group (F1,62 = 4.11, p = 0.04, η2 = 0.03). PO-MDD children were less accurate during the second half of the task following pleasant pictures (M = 66.47, SE = 2.54) compared to neutral pictures (M = 70.15, SE = 2.48). There were no differences in pleasant versus neutral accuracy during the first half of the task. A similar pattern was found for healthy control children who were also less accurate during the second half of the task following pleasant pictures (M = 69.59, SE = 4.45) compared to neutral pictures (M = 75.99, SE = 4.35). There were no differences in pleasant versus neutral accuracy during the first half of the task. However, only healthy control children evidenced a significant within-valence increase in their accuracy(F1,62 = 4.95, p = 0.03, η2 = 0.03), such that they were significantly more accurate following neutral pictures during the second half of the task (M = 75.99, SE = 4.345 when compared to their accuracy during the first half of the task(M = 68.24, SE = 4.33).
Reaction Time
Once covariates were included in the model, there were no main effects of diagnostic group, valence, or time or interactions with valence or time indicating that reaction times did not differ in the sample as a whole or by diagnostic group following pleasant vs. neutral trials or during the first vs. second half of the task.
Internal Consistency
In order to calculate internal consistency, we divided responses into odd versus even trials and calculated accuracy and reaction times for odd and even trials separately. These values were corrected using the Spearman Brown formula. Accuracy (r = 0.90) and reaction times (r = 0.98) were highly correlated between odd and even trials for the entire sample, as well as within the PO-MDD group (accuracy: r = 0.89; reaction time: r = 0.98, and healthy control group (accuracy: r = 0.92; reaction time: r = 0.99).
Diagnostic Group and Sex Differences in the LPP Using Average of O1/Oz/O2 Sites when Covarying for Age, GAD, ODD, and ADHD
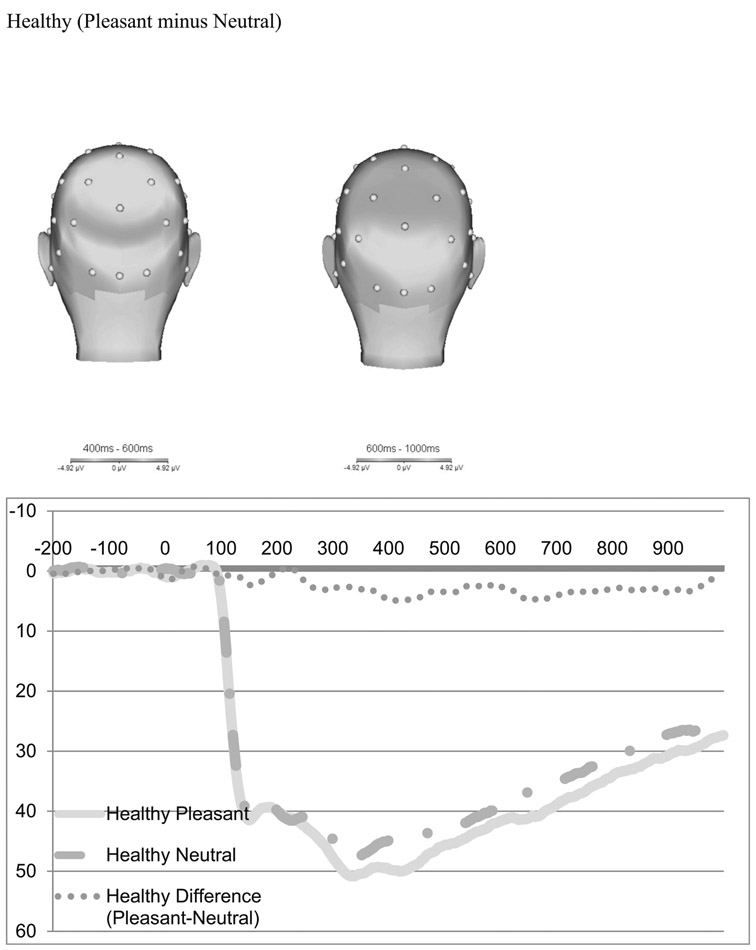
The scalp distribution of the difference between pleasant and neutral trials in the time-range of the LPP is presented in Fig. 2 for healthy controls (top) and PO-MDD (bottom). Consistent with previous work in young children, pleasant pictures were associated with a relative positivity over occipital recording sites (Hua et al. 2014; Kujawa et al. 2012a, b). Figure 2 also present the ERPs for pleasant and neutral trials for each group, as well as the pleasant minus neutral difference score. The scalp topographies and ERP waveforms suggest that PO-MDD children were characterized by a lack of differentiation in electrocortical reactivity to pleasant versus neutral stimuli.
Fig. 2.
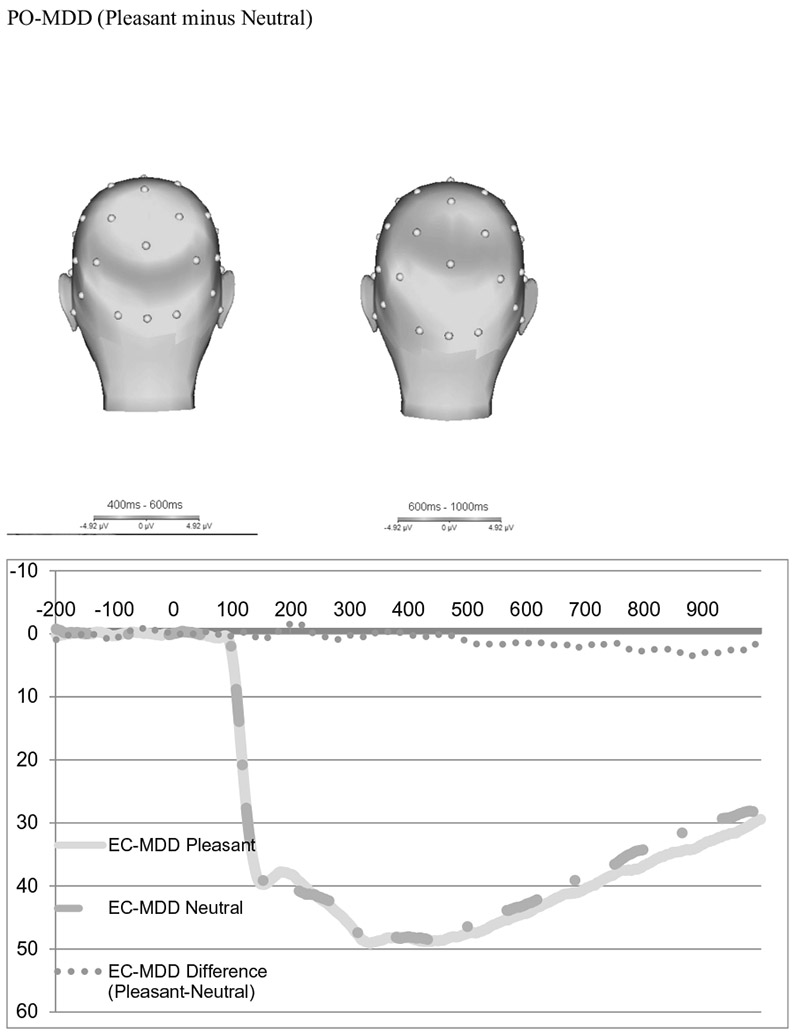
Scalp distribution of the difference between pleasant and neutral trials in the time-range of the LPP and ERPs of pleasant and neutral trials
We conducted paired samples t tests to determine whether the positive images significantly differed from the neutral images across the sample. From 400 to 600 ms (early), the LPP was more positive following pleasant images (M = 47.67 μV) than neutral images (M = 44.72 μV; t(163) = 5.88; p < 0.00). From 600 to 1000 ms (late) there was also a significant average difference between positive and neutral images, with the average positive images (M = 35.69 μV) higher than neutral images (M = 32.33 μV; t(163) = 7.44; p < 0.00). Although these mean amplitudes seem higher than previous work using preschool-age children (e.g., Hua et al. 2014; Solomon et al. 2012), there are several differences in the calculations between samples that contribute to this difference. First, Hua et al. (2014) and Solomon et al. (2012) used an extended time window of 300–700 ms for the LPP, compared to our window of 400–600 ms. In addition, Hua and colleagues (Hua et al. 2014) as well as Solomon and colleagues (Solomon et al. 2012) also combined the posterior electrodes of Pz, P3, P4 along with those used here Oz, O1 and O2. Our LPP amplitudes appear to be consistent with several other studies using preschool and child samples using similar time windows and electrodes (e.g., DeCicco et al. 2014; Kujawa et al. 2012a, b, 2013).
A one-way ANCOVA to test for diagnostic group and sex differences in the pleasant-neutral difference score while controlling for children’s age and the presence of GAD, ADHD, and ODD co-morbid diagnoses. Separate ANCOVA’s were conducted for each time-window of interest (e.g., “early” 400–600 ms and “late” 600–1000 ms). There was a significant effect of group on the pleasant-neutral difference score from 400 to 600 ms, F(1,160) = 3.69, p = 0.05, η2 = 0.02. The LPP for the PO-MDD group (M = 2.78, SE = 0.83) was significantly smaller than the LPP for the healthy group (M = 5.20, SE = 1.44). No significant group effect was found for the pleasant-neutral difference score from 600 to 1000 ms (F(1,164 2.32, p = 0.13, η2 = 0.02). No significant sex differences were found for the pleasant-neutral differences score from 400 to 600 ms (F(1,164 0.15, p = 0.70, η2 = 0.00) or 600–1000 ms (F(1,164 1.02, p = 0.31, η2 = 0.01).
Similar ANCOVA’s were conducted to test for diagnostic group and sex differences using the residual scores for response to pleasant stimuli, while controlling for children’s age and co-morbid diagnoses. There was a significant effect of group on the residualized response to pleasant stimuli from 400 to 600 ms, F(1,164) = 5.38, p = 0.02, η2 = 0.03. The mean residualized score for the PO-MDD group (M = −1.82, SE = 1.05) was significantly smaller than mean residualized score for the healthy group (M = 1.86, SE = 1.83). No group effect was found for the residualized response to pleasant (F(1,164 = 1.59, p = 0.21, η2 = 0.01) stimuli from 600 to 1000 ms. No significant sex differences were found for the pleasant-neutral differences score from 400 to 600 ms(F(1,164 0.72, p = 0.40, η2 = 0.01) or 600–1000 ms (F(1,164 1.83, p = 0.18, η2 = 0.01).
Accuracy
Given the behavioral results above indicating that PO-MDD children were less accurate in their responding, we conducted a repeated measures ANCOVA including only correct trials. This decreased our sample size from 164 to 107, as 57 subjects (24 healthy control; 33 PO-MDD) did not have enough correct trials with usable data using the parameters outlined above. Results of the RM ANCOVA indicated a main effect of time (F1,100 = 5.30, p = 0.02, η2 = 0.05) and a valence by time interaction(F1,100 = 4.37, p = 0.04, η2 = 0.04). Specifically, neutral images (M = 30.44, SE = 2.30) elicited a smaller response during the 600–1000 ms segment compared to the 400–600 ms segment (M = 42.91, SE = 2.68). There were no main effects or interactions with diagnostic group.
We also conducted the same ANCOVA’s described above to test for diagnostic group and sex differences using the pleasant minus neutral difference score and residual score for response to pleasant stimuli, while controlling for children’s age and comorbid diagnoses using only correct trials. When only correct trials were included, there was no main effect of diagnostic group on the pleasant-minus neutral difference score (F1,100 = 0.09 p = 0.77, η2 = 0.00) or residualized response to pleasant stimuli (F1,100 = 0.004 p = 0.95, η2 = 0.00).
Internal Consistency
The mean amplitude of the LPP for odd trials was 46.44 (SD = 13.74) and the mean amplitude of the LPP for even trials was 46.37 (SD = 13.76). The LPP was highly correlated between odd and even trials across the whole sample (Spearman-Brown corrected r = 0.96), as well as within the PO-MDD (Spearman-Brown corrected r = 0.96) and healthy control groups (Spearman-Brown corrected r = 0.97).
Correlations between LPP and MDD/Anhedonia Severity and BIS/BAS
Partial Correlations
Partial correlations (controlling for child age and diagnostic group) were conducted to determine whether LPP amplitudes were associated with core MDD symptoms, anhedonia sum score, PFC scale score, BIS/BAS, or sex (Table 4). Core MDD symptoms, anhedonia sum score and PFC scale scores were not correlated with pleasant-neutral difference score during the early or late segment, the residualized response to pleasant or neutral stimuli during the early or late segment, or the residualized pleasant minus neutral difference score during the early or late segment. BAS drive scores were positively correlated with mean amplitudes for Pleasant during the early segment (r = 0.18, p = 0.05), Neutral during the early segment (r = .18, p = 0.05), and Pleasant during the late segment (r = 0.21, p = 0.03). Sex was negatively correlated with mean amplitudes for Pleasant during the late segment (r = −0.19, p = 04), such that boys evidenced greater mean amplitudes than girls.
Table 4.
Partial Correlations between Event-Related Potential (ERP) Mean scores during pleasant and neutral picture viewing and MDD symptoms, Anhedonia symptoms, PFC symptom severity and BAS
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. MDD Core Symptoms | – | |||||||||||||
| 2. PFC Scale Score | 0.33*** | – | ||||||||||||
| 3. Anhedonia Sum Score | 0.59*** | 0.36*** | – | |||||||||||
| 4. BAS Drive | −0.12 | −0.07 | −0.19* | – | ||||||||||
| 5. BAS Reward Responsiveness | −0.14 | −0.34*** | −0.26** | 0.44*** | – | |||||||||
| 6. Sex | −0.07 | −0.09 | −0.02 | 0.1 | 0.03 | – | ||||||||
| 7. Pleasant 400–600 ms |
0.05 | 0.05 | −0.07 | 0.18* | −0.03 | −0.15 | – | |||||||
| 8. Neutral 400–600 ms |
0.03 | 0 | −0.01 | 0.17 | 0.01 | −0.13 | 0.9*** | – | ||||||
| 9. Pleasant – Neutral Difference Score 400–600 ms |
0.04 | 0.1 | −0.12 | 0.02 | −0.08 | −0.03 | 0.16 | −0.28** | – | |||||
| 10. Residualized Pleasant 400–600 ms |
0.06 | 0.1 | −0.1 | −0.05 | −0.09 | −0.16 | 0.43*** | 0.09 | 0.73*** | – | ||||
| 11. Pleasant 600–100 ms |
0.02 | 0.09 | −0.15 | 0.21* | −0.02 | −0.17 | 0.89*** | 0.82*** | 0.11 | 0.34*** | – | |||
| 12. Neutral 600–1000 ms |
0 | 0.04 | −0.1 | 0.18* | −0.03 | −0.09 | 0.81*** | 0.9*** | −0.26** | 0.05 | 0.88*** | – | ||
| 13. Pleasant – Neutral Difference Score 600–1000 ms |
0.04 | 0.11 | −0.1 | 0.07 | 0.03 | −0.16 | 0.19* | −0.14 | 0.74*** | 0.59*** | 0.28** | −0.21* | – | |
| 14. Residualized Pleasant 600–100 ms |
0.04 | 0.12 | −0.14 | 0.13 | 0.02 | −0.2* | 0.45*** | 0.13 | 0.68*** | 0.61*** | 0.56*** | 0.1 | 0.95*** | – |
p < 0.05;
p < 0.01;
p < 0.000
Discussion
In this study, we found that children with preschool onset Major Depressive Disorder (PO-MDD) exhibited reduced responding to pleasant stimuli as indexed by the early LPP during a picture-viewing task when compared to healthy control children. This finding held when controlling for age, sex, and co-morbid diagnoses. It appears that this finding was driven by responses to pleasant stimuli, as group differences emerged to the pleasant – neutral difference score as well as the residualized response to pleasant stimuli. However, the pleasant - neutral difference score finding was no longer significant when covariates were removed from our model (see supplemental material). Further, analyses using only correct trials failed to find a diagnostic group effect on the pleasant-neutral difference score or residualized response to pleasant stimuli. This suggests there may be some variation in the attentional resources devoted to the task that may be influencing both task accuracy and LPPs.
A reduced attentional allocation to positive stimuli as index by the LPP in the children with PO-MDD is consistent with findings from older child, adolescent, and adult samples (Foti et al. 2010; Grunewald et al. 2018; Kujawa et al. 2015; Nelson et al. 2015a, b). In particular, our findings converge with work in adult samples that retrospectively assessed age of depression onset and demonstrated that an earlier onset was associated with a more blunted LPP (Weinberg et al. 2016). These findings provide additional evidence for electrocortical deficits in response to pleasant stimuli in children with PO-MDD and add to the growing literature on the neurophysiology of depression in this young age group (Belden et al. 2016; Grunewald et al. 2018). There were no significant relationships between children’s responding to pleasant stimuli and core MDD symptoms, anhedonia sum score, and/or PFC scale score (measures of depression severity), regardless of whether covariates were included. However, BAS drive scores were positively correlated with mean amplitudes for pleasant and neutral images.
Interesting behavioral differences emerged between groups. Children with PO-MDD were less accurate throughout the task, and more specifically, less accurate following a pleasant picture. In addition, children with PO-MDD were more likely to not respond to trials following the presentation of a pleasant picture. These differences were maintained once the covariates of age, ADHD, ODD, GAD, and sex were included. Other work using the IAPS pictures in slightly older, healthy children has also found decreases in accuracy and increases in errors when presenting emotional as opposed to neutral stimuli (Kujawa et al. 2012a, b). Perhaps the emotional stimuli were more distracting to children with PO-MDD and thus, decreased their accuracy in responding. This speculation fits with prior research on adults with depression who demonstrate decreased attention to positive stimuli (Lawrence et al. 2004; Joormann and Gotlib 2007; Suslow et al. 2010). Further, healthy control children evidenced an increase in their accuracy to neutral pictures during the second half of the task. This may suggest that healthy children were able to more flexibly adapt to the task demands and allocate attentional resources in order to increase performance. Interestingly, there is some work that suggests that depression may be characterized by psychological inflexibility, and the findings presented here fit with this hypothesis in that the healthy children were able to improve their performance over the course of the task, whereas the PO-MDD children did not (Gilbert et al. 2018). Additional work using a more novel trial by trial analysis of the links between LPP, reaction time and accuracy (e.g., brain-behavior coupling) may help to clarify the nature of these findings (Weinberg and Hajcak 2011; Stange et al. 2017).
There may also be additional reasons for the variation in findings when including only correct trials, in that there were no differences based on the presence of depression when only correct trials were included in the analyses. Children with more incorrect trials were also more likely to be younger and female. It may be that the use of the game controller was more difficult or novel for these groups, such that rather than the difference being driven solely by lack of attention to the stimuli, it may also be due to lack of familiarity and comfort with controller used to record responses. The children may have been paying attention but have incorrect recorded a response, as there were no differences in reaction time between children included and excluded based on incorrect trials. For some younger children, the lack of correct trials could also be due to confusion surrounding left/right. Given that this study assessed one of the youngest age groups of children and particularly depressed children, these possibilities may need to be further explored in future work.
Despite adolescent research showing sex differences in the LPP, we did not find any significant differences between preschool males and females in neural responding. Other work using children in the age range has also failed to find sex differences using a similar task and in similar regions, such as electrodes O1, O2, and Oz (e.g., Solomon et al. 2012). Taken together, it is likely that sex differences, particularly those related to the onset and maintenance of depression may emerge later in development, possibly during puberty when sex differences in depression rates diverge. Behaviorally, males and females did differ in the number of missed trials. Females, but not males missed significantly more trials during the second half of the task than the first half of the task. This may be due to females paying less attention or losing interest in the task faster than males.
In our sample, we found that LPP amplitudes were positively correlated with scores on a trait measure empirically related to approach systems (BAS). Specifically, BAS drive scores, which measure the persistent pursuit of desired goals were associated with higher amplitudes to both pleasant and neutral pictures during the task. Although a secondary focus of the current report, these finding suggest that there may be a relationship between positive-approach oriented affect and LPP amplitudes in very young children, regardless of diagnostic status. Anhedonia, reduced interest and pleasure in almost all daily activities is a core feature of MDD was hypothesized to be related to reduced neural responses to pleasant and rewarding stimuli. However, we did not find evidence suggesting that individual differences in anhedonia severity were related to reductions in neural responding to pleasant stimuli, regardless of whether covariates were included. This is inconsistent with findings demonstrating associations between anhedonia and a blunted LPP in adults (Benning and Oumeziane 2017; Weinberg et al. 2016). However, there is no evidence to our knowledge in children that demonstrates these associations. Interestingly, research suggests that low positive affect, one component of anhedonia longitudinally predicts blunted LPP (Kessel et al. 2016). Further, in a large sample of adolescents, low positive emotionality, an aspect of temperament thought to be associated with depression, was related to reduced LPP amplitudes (Speed et al. 2016) whereas negative emotionality was not. As such, in early childhood samples, we speculate that the LPP is not state-dependent, but rather, is a trait biomarker of PO-MDD that shows greater associations to more stable characteristics, such as positive affectivity more generally.
Given the greater neuroplasticity of emotional response and regulation, these findings indicate clinical attention to emotional response to pleasure as an important target in early childhood depression (Kujawa et al. 2012a, b). Additional research on the LPP in young children, particularly those with and at-risk for MDD, will help to clarify the role of the LPP in early-onset MDD. For instance, it may be that the LPP simply reflects a general vulnerability to depression, but that it is not associated with depression severity. Longitudinal studies may offer a unique opportunity to capture change and/or stability in the LPP that might occur following remission of depression in young children.
The finding of blunted response to pleasant stimuli in early childhood depression only occurred during the early LPP window from 400 to 600 ms. This matches with recent findings showing that young children (ages 6–11 years) with higher levels of anxiety and depression displayed larger LPP amplitudes to angry as opposed to neutral faces, but only during the LPP time window from 520 to 610 ms (Chronaki et al. 2018). Converging evidence indicates that the LPP is most evident from 300 to 750 ms and becomes less stable at later time windows (Kujawa et al. 2012a, b; Schupp et al. 2006; Solomon et al. 2012). Our findings extend this evidence to a sample of younger children.
Previously, our group has shown that preschoolers with MDD display reductions in responsivity to rewards using a different reward task and focusing on a different ERP component (RewP) than described here. The RewP is a frontocentral deflection peaking 250–300 milliseconds after feedback that indexes responses to positive outcomes in a range of tasks using reward, such as gambling, and the RewP reflects the initial, binary evaluation of outcomes as either better or worse than expected. The finding was driven by a blunted response to reward and consistent with prior research using adolescent and adult samples with MDD. The RewP measured in our prior study reflects processing that occurs in response to the presentation of a rewarding stimulus. In contrast, the LPP measured here tracks the sustained processing of emotional content in the presence of an emotional stimuli. These are unique markers of emotional and cognitive processing that relate to different systems of dysfunction in depression. Although similar, the findings presented in this manuscript suggest a more basic difference between the groups of children in terms of overall responding to pleasant stimuli. That is not only are preschool children exhibiting alterations in their reward responding, they are also exhibiting reduced responding to pleasant stimuli more generally. Further, these findings also highlight the behavioral differences between the groups, as children with PO-MDD were less accurate following the appearance of a pleasant picture. Both sets of findings fit with clinical presentations of preschool-onset depression that highlight the absence of (or significantly diminished) positive affect, a striking deviation from what is expected during this developmental period.
Limitations
This study has several limitations worthy of consideration. The positive stimuli presented to children were isolated pictures and may not fully translate to the positive stimuli children often encounter in their day-to-day lives. More complex emotional stimuli may be more effective at eliciting the LPP responses. In addition, we were unable to examine LPP’s to other emotionally salient stimuli, such as threat, anger, or sadness, which are also likely relevant for understanding the pathophysiology of PO-MDD and to fully test the emotion context insensitivity theory. Thus, we cannot be sure that our findings are specific to pleasant, as opposed to other forms of emotionally evocative stimuli. Children in our study were too young to rate the valance and arousal of the pictures that we used and therefore, it is unclear whether the children found the pictures to be of the same valance or arousal as the established norms. Further, our study used only visual stimuli (e.g., pictures), however it would be interesting for future research to include the types of verbal and linguistic stimuli, as well as gaze aversion used in studies with older adolescents and adults with MDD (Auerbach et al. 2015), particularly given the different patterns of results these studies have found regarding the LPP and depression. Although the use of ERP to measure LPP is becoming increasingly common and has several advantages, this method also has some limitations. ERPs have excellent temporal resolution for measuring real-time neural response and are highly feasible to use in young children. Further, they provide an important objective measure of responding to pleasant stimuli that is not dependent on parent- or selfreport. However, ERPs do not provide the same spatial resolution as measures such as fMRI and we cannot pinpoint which specific brain regions may be altered in the PO-MDD group. Finally, it appears that both groups habituated in their neural responding during the second half of the task, where both the PO-MDD and healthy control groups exhibited reduced amplitudes during the second block of pictures. Additional work focusing on the duration of ERP tasks may prove to be beneficial for researchers working with young children.
Clinical Implications
These findings warrant increased clinical attention to alterations in the processing of pleasant and rewarding stimuli as a core area of emotional impairment in PO-MDD. Despite our speculation that the LPP may be a trait biomarker of PO-MDD that shows greater associations to more stable characteristics, such as positive affectivity, clinical interventions can still target state-like or situational aspects of responses to pleasant stimuli. Given the importance of joyful play exploration at this developmental stage, this may be a key area to target in early interventions. Related, this information could be utilized to create personalized or targeted interventions depending on baseline trait positive affective responding. These findings extend the known deficits in responding to pleasant and rewarding stimuli seen among children and adolescents with depression, to children as young as preschool. The findings described here suggest that an increased focus on enhancing children’s responses to pleasant stimuli may be one fruitful pathway for intervention in young children with depression. Clinicians might choose to focus on enhancing children’s responses to pleasure and positive stimuli through tools such as savoring and positive rumination.
This study demonstrates that children with PO-MDD exhibit reduced accuracy and a reduced ERP response (LPP) to pleasant pictures when compared to psychiatrically healthy children. This is the youngest sample in which the LPP has been examined as a function of PO-MDD and these results add to the literature on the utility of ERP for understanding the underlying pathophysiology of depression in young children. These findings indicate that reduced responding to pleasant stimuli may be a key mechanism associated with PO-MDD.
Supplementary Material
Acknowledgements
The authors wish to thank the many parents and children who participated in the Parent-Child Interaction Treatment Emotion Development (PCIT-ED) study.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10802-019-00585-8) contains supplementary material, which is available to authorized users.
Conflict of Interest Dr. Barch consults for Pfizer. Dr. Luby receives royalties from Guildford Press. All other authors report no biomedical financial interests or potential conflicts of interest.
Ethics and Consent Statement This study was carried out in accordance with the recommendations of Washington University School of Medicine Institutional Review Board with written informed consent from all caregivers and verbal assent from all preschoolers. All caregivers gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Washington University School of Medicine Institutional Review Board
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The number of the IAPS pictures used were: pleasant (1463, 1710, 1750, 1811, 2070, 2091, 2092, 2224, 2340, 2345, 2347, 7325, 7330, 7400, 8031, 8200, 8370, 8461, 8496, 8497) and neutral (2514, 2580, 5390, 5395, 5500, 5731, 5740, 5900, 7000, 7002, 7009, 7010, 7026, 7038, 7039, 7090, 7100, 7130, 7175, 7190).
A repeated measures ANOVA was conducted that included the raw (unadjusted) pleasant and neutral LPP for the average of the two reference electrodes (TP9 and TP10) in order to demonstrate validity for this cohort. Results of the repeated measures ANOVA indicated a main effect of valence (F1,162 = 33.78, p = 0.000, η2 = 0.17). Specifically, mean amplitudes for pleasant trials (M = 11.33, SE = 0.28) were significantly more positive than mean amplitudes for neutral trials (M = 10.19, SE = 0.27). There were no main effects or interactions with diagnostic group (F1,162 = 0.01, p = 0.92, η2 = 0.000).
REFERENCES
- Achenbach TM, & Edelbrock CS (1983). Manual for the child behavior checklist and revised child behvaior profile. Department of Psychiatry: University of Vermont. [Google Scholar]
- Achenbach TM, & Rescorla LA (2000). Manual for the ASEBA preschool forms & profiles: An integrated system of multi-informant assessment; child behavior checklist for ages 1 1/2–5; language development survey. Caregiver-teacher report form: University of Vermont. [Google Scholar]
- Auerbach RP, Stanton CH, Proudfit GH, & Pizzagalli DA (2015). Self-referential processing in depressed adolescents: A high-density ERP study. Journal of Abnormal Psychology, 124(2), 233–245. 10.1037/abn0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Gaffrey MS, Botteron KN, Belden AC, & Luby JL (2012). Functional brain activation to emotionally valenced faces in school-aged children with a history of preschool-onset major depression. Biological Psychiatry, 72(12), 1035–1042. 10.1016/j.biopsych.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Whalen D, Gilbert K, Kelly D, Kappenman ES, Hajcak G, & Luby JL (2018). Neural indicators of anhedonia: Predictors and mechanisms of treatment change in a randomized clinical trial in early childhood depression. Biological Psychiatry, 85, 863–871. 10.1016/j.biopsych.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Belden AC, Pagliaccio D, Murphy ER, Luby JL, & Barch DM (2015). Neural activation during cognitive emotion regulation in previously depressed compared to healthy children: Evidence of specific alterations. Journal of the American Academy of Child & Adolescent Psychiatry, 54(9), 771–781. 10.1016/j.jaac.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden AC, Irvin K, Hajcak G, Kappenman ES, Kelly D, Karlow S, Luby JL, & Barch DM (2016). Neural correlates of reward processing in depressed and healthy preschool-age children. Journal of the American Academy of Child & Adolescent Psychiatry, 55, 1081–1089. 10.1016/j.jaac.2016.09.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning SD, & Oumeziane BA (2017). Reduced positive emotion and underarousal are uniquely associated with subclinical depression symptoms: Evidence from psychophysiology, self-report, and symptom clusters. Psychophysiology, 54(7), 1010–1030. 10.1111/psyp.12853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufferd SJ, Dougherty LR, Carlson GA, Rose S, & Klein DN (2012). Psychiatric disorders in preschoolers: Continuity from ages 3 to 6. American Journal of Psychiatry, 169(11), 1157–1164. 10.1176/appi.ajp.2012.12020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Owens M, Feurer C, Sosoo E, Kudinova A, & Gibb BE (2017). Increased neural and pupillary reactivity to emotional faces in adolescents with current and remitted major depressive disorder. Social Cognitive and Affective Neuroscience, 12(5), 783–792. 10.1093/scan/nsw184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology, 67(2), 319–333. 10.1037/0022-3514.67.2.319. [DOI] [Google Scholar]
- Chronaki G, Broyd SJ, Garner M, Benikos N, Thompson MJJ, Sonuga-Barke EJS, & Hadwin JA (2018). The moderating effect of self-reported state and trait anxiety on the late positive potential to emotional faces in 6–11-year-old children. Frontiers in Psychology, 9 10.3389/fpsyg.2018.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, West SG, & Aiken LS (2014). Applied multiple regression/correlation analysis for the behavioral sciences. 10.4324/9781410606266. [DOI]
- Cronbach LJ, & Furby L (1970). How we should measure “change”: Or should we? Psychological Bulletin, 74(1), 68–80. 10.1037/h0029382. [DOI] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, & Lang PJ (2000). Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology, 52(2), 95–111. 10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- DeCicco JM, Solomon B, & Dennis TA (2012). Neural correlates of cognitive reappraisal in children: An ERP study. Developmental Cognitive Neuroscience, 2(1), 70–80. 10.1016/j.dcn.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCicco JM, O’Toole LJ, & Dennis TA (2014). The late positive potential as a neural signature for cognitive reappraisal in children. Developmental Neuropsychology, 39(7), 497–515. 10.1080/87565641.2014.959171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, & Hajcak G (2009). The late positive potential: A neuro-physiological marker for emotion regulation in children. Journal of Child Psychology and Psychiatry, 50(11), 1373–1383. 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A (2017). Discovering statistics using IBM SPSS statistics: North American edition. Sage Publishers. [Google Scholar]
- Forbes EE, & Dahl RE (2005). Neural systems of positive affect: Relevance to understanding child and adolescent depression? Development and Psychopathology, 17(3), 827–850. 10.1017/S095457940505039X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, & Dahl RE (2012). Research review: Altered reward function in adolescent depression: What, when and how? Journal of Child Psychology and Psychiatry, 53(1), 3–15. 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G, & Dien J (2009). Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology, 46(3), 521–530. 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Olvet DM, Klein DN, & Hajcak G (2010). Reduced electrocortical response to threatening faces in major depressive disorder. Depression and Anxiety, 27(9), 813–820. 10.1002/da.20712. [DOI] [PubMed] [Google Scholar]
- Gaffrey MS, & Luby JL (2012). Kiddie Schedule for Affective Disorders and Schizophrenia - Early Childhood Version (K-SADS-EC). St Louis: Washington University School of Medicine. [Google Scholar]
- Gaffrey MS, Luby JL, Repovš G, Belden AC, Botteron KN, Luking KR, & Barch DM (2010). Subgenual cingulate connectivity in children with a history of preschool-depression. Neuroreport, 21(18), 1182–1188. 10.1097/WNR.0b013e32834127eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Barch DM, Singer J, Shenoy R, & Luby JL (2013). Disrupted amygdala reactivity in depressed 4- to 6-year-old children. Journal of the American Academy of Child & Adolescent Psychiatry, 52(7), 737–746. 10.1016/j.jaac.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Barch DM, Bogdan R, Farris K, Petersen SE, & Luby JL (2018). Amygdala reward reactivity mediates the association between preschool stress response and depression severity. Biological Psychiatry, 83(2), 128–136. 10.1016/j.biopsych.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert KE, Tonge NA, & Thompson RJ (2018). Associations between depression, anxious arousal and manifestations of psychological inflexibility. Journal of Behavior Therapy and Experimental Psychiatry, 21, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, & Donchin E (1983). A new method for off-line removal of ocular artifact. Clinical Neurophysiology, 55(4), 468–484. 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grunewald M, Döhnert M, Brandeis D, Klein AM, Klitzing K von, Matuschek T, & Stadelmann S (2018). Attenuated LPP to emotional face stimuli associated with parent- and self-reported depression in children and adolescents. Journal of Abnormal Child Psychology, 1–10. 10.1007/s10802-018-0429-3. [DOI] [PubMed] [Google Scholar]
- Hajcak G, & Dennis TA (2009). Brain potentials during affective picture processing in children. Biological Psychology, 80(3), 333–338. 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, & Olvet DM (2010). Event-related potentials, emotion, and emotion regulation: An integrative review. Developmental Neuropsychology, 35(2), 129–155. 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hankin BL (2015). Depression from childhood through adolescence: Risk mechanisms across multiple systems and levels of analysis. Current Opinion in Psychology, 4, 13–20. 10.1016/j.copsyc.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Klein DN, Durbin CE, & Olino TM (2006). Positive emotionality at age 3 predicts cognitive styles in 7-year-old children. Development and Psychopathology, 18(02), 409–423. [DOI] [PubMed] [Google Scholar]
- Hua M, Han ZR, Chen S, Yang M, Zhou R, & Hu S (2014). Late positive potential (LPP) modulation during affective picture processing in preschoolers. Biological Psychology, 101, 77–81. 10.1016/j.biopsycho.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Jaworska N, Blier P, Fusee W, & Knott V (2012). The temporal electrocortical profile of emotive facial processing in depressed males and females and healthy controls. Journal of Affective Disorders, 136(3), 1072–1081. 10.1016/j.jad.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, & Gotlib IH (2007). Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology, 116(1), 80–85. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stewart JE, & Quitkin FM (2000). Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: Differences between depressed patients and healthy adults in P3 amplitude and asymmetry. International Journal of Psychophysiology, 36(3), 211–236. 10.1016/S0167-8760(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE, Abraham KS, Alschuler DM, Alvarenga JE, Skipper J, Warner V, Bruder GE, & Weissman MM (2017). Motivated attention and family risk for depression: Neuronal generator patterns at scalp elicited by lateralized aversive pictures reveal blunted emotional responsivity. NeuroImage: Clinical, 14, 692–707. 10.1016/j.nicl.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel EM, Dougherty LR, Kujawa A, Hajcak G, Carlson GA, & Klein DN (2016). Longitudinal associations between preschool disruptive mood dysregulation disorder symptoms and neural reactivity to monetary reward during preadolescence. Journal of Child and Adolescent Psychopharmacology, 26(2), 131–137. 10.1089/cap.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel EM, Kujawa A, Goldstein B, Hajcak G, Bufferd SJ, Dyson M, & Klein DN (2017). Behavioral observations of positive and negative valence systems in early childhood predict physiological measures of emotional processing three years later. Journal of Affective Disorders, 216, 70–77. 10.1016/j.jad.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, & Burkhouse KL (2017). Vulnerability to depression in youth: Advances from affective neuroscience. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(1), 28–37. 10.1016/j.bpsc.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, & Klein DN (2012a). Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry and Allied Disciplines, 53(2), 207–215. 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, & Hajcak G (2012b). Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience, 2(4), 458–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, & Proudfit GH (2013). Two-year stability of the late positive potential across middle childhood and adolescence. Biological psychology, 94(2), 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, & Phan KL (2015). Enhanced neural reactivity to threatening faces in anxious youth: Evidence from event-related potentials. Journal of Abnormal Child Psychology, 43(8), 1493–1501. 10.1007/s10802-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (1995). International affective picture system. Center for Research in Psychophysiology: University of Florida. [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (1997). Motivated attention: Affect, activation, and action In Attention and orienting: Sensory and motivational processes (1st ed., pp. 97–135). Mawah NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, & Hamm AO (1993). Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology, 30(3), 261–273. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, & Phillips ML (2004). Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry, 55(6), 578–587. [DOI] [PubMed] [Google Scholar]
- Levinson AR, Speed BC, & Hajcak G (2018). Neural response to pleasant pictures moderates prospective relationship between stress and depressive symptoms in adolescent girls. Journal of Clinical Child & Adolescent Psychology, 48, 1–13. 10.1080/15374416.2018.1426004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, & Belden AC (2008). Clinical characteristics of bipolar vs. unipolar depression in preschool children: An empirical investigation. The Journal of Clinical Psychiatry, 69(12), 1960–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Hessler MJ, Brown KM, & Hildebrand T (2002). Preschool major depressive disorder: Preliminary validation for developmentally modified DSM-IV criteria. Journal of the American Academy of Child & Adolescent Psychiatry, 41(8), 928–937. 10.1097/00004583-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown KM, Hessler MJ, Wallis JM, & Spitznagel E (2003). The clinical picture of depression in preschool children. Journal of the American Academy of Child & Adolescent Psychiatry, 42(3), 340–348. 10.1097/00004583-200303000-00015. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Koenig-McNaught AL, Brown K, & Spitznagel E (2004). The preschool feelings checklist: A brief and sensitive screening measure for depression in young children. Journal of the American Academy of Child & Adolescent Psychiatry, 43(6), 708–717. 10.1097/01.chi.0000121066.29744.08. [DOI] [PubMed] [Google Scholar]
- Luby JL, Belden AC, Pautsch J, Si X, & Spitznagel E (2009a). The clinical significance of preschool depression: Impairment in functioning and clinical markers of the disorder. Journal of Affective Disorders, 112(1), 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Si X, Belden AC, Tandon M, & Spitznagel E (2009b). Preschool depression: Homotypic continuity and course over 24 months. Archives of General Psychiatry, 66(8), 897–905. 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Gaffrey MS, Tillman R, April LM, & Belden AC (2014). Trajectories of preschool disorders to full DSM depression at school age and early adolescence: Continuity of preschool depression. The American Journal of Psychiatry, 171(7), 768–776. 10.1176/appi.ajp.2014.13091198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Belden AC, Jackson JJ, Lessov-Schlaggar CN, Harms MP, Tillman R, Botteron K, Whalen D, & Barch DM (2016). Early childhood depression and alterations in the trajectory of gray matter maturation in middle childhood and early adolescence. JAMA Psychiatry, 73(1), 31–38. 10.1001/jamapsychiatry.2015.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Barch DM, Whalen D, Tillman R, & Freedland KE (2018). A randomized controlled trial of parent-child psychotherapy targeting emotion development for early childhood depression. American Journal of Psychiatry, 175(11), 1102–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Repovs G, Belden AC, Gaffrey MS, Botteron KN, Luby JL, & Barch DM (2011). Functional connectivity of the amygdala in early-childhood-onset depression. Journal of the American Academy of Child & Adolescent Psychiatry, 50(10), 1027–1041.e3. 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Kotov R, & Hajcak G (2016). Diagnostic and symptom-based predictors of emotional processing in generalized anxiety disorder and major depressive disorder: An event-related potential study. Cognitive Therapy and Research, 40(3), 275–289. 10.1007/s10608-015-9717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardaga S, & Iakimova G (2014). Neurocognitive processing of emotion facial expressions in individuals with self-reported depressive symptoms: The role of personality and anxiety. Neurophysiologie Clinique/Clinical Neurophysiology, 44(5), 447–455. 10.1016/j.neucli.2014.08.007. [DOI] [PubMed] [Google Scholar]
- McCabe C (2018). Linking anhedonia symptoms with behavioural and neural reward responses in adolescent depression. Current Opinion in Behavioral Sciences, 22, 143–151. 10.1016/j.cobeha.2018.07.001. [DOI] [Google Scholar]
- Nelson TD, Nelson JM, Kidwell KM, James TD, & Espy KA (2015a). Preschool sleep problems and differential associations with specific aspects of executive control in early elementary school. Developmental Neuropsychology, 40(3), 167–180. 10.1080/87565641.2015.1020946. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Perlman G, Hajcak G, Klein DN, & Kotov R (2015b). Familial risk for distress and fear disorders and emotional reactivity in adolescence: An event-related potential investigation. Psychological Medicine, 45(12), 2545–2556. 10.1017/S0033291715000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Luby JL, Luking KR, Belden AC, & Barch DM (2014). Brain–behavior relationships in the experience and regulation of negative emotion in healthy children: Implications for risk for childhood depression. Development and Psychopathology, 26- (special issue 4pt2), 1289–1303. 10.1017/S0954579414001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaccio D, Luking KR, Anokhin AP, Hayden EP, Peng C-Z, Gotlib IH, et al. (2015). Revising the BIS/BAS scale to study development: Measurement invariance and normative effects of age and sex from childhood through adulthood. Psychological Assessment, 28(4), 429–442. 10.1037/pas0000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, & Gotlib IH (2005). Emotion context insensitivity in major depressive disorder. Journal of Abnormal Psychology, 114(4), 627–639. 10.1037/0021-843X.114.4.627 [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Tiffany I, & Lang PJ (2003). Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology, 37(2), 257–261. 10.1111/1469-8986.3720257. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, & Junghöfer M (2006). Emotion and attention: Event-related brain potential studies. In Anders S, Ende G, Junghofer M, Kissler J, & Wildgruber D (Eds.), Progress in Brain Research (pp. 31–51). 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Shestyuk AY, & Deldin PJ (2010). Automatic and strategic representation of the self in major depression: Trait and state abnormalities. The American Journal of Psychiatry, 167(5), 536–544. 10.1176/appi.ajp.2009.06091444. [DOI] [PubMed] [Google Scholar]
- Solomon B, DeCicco JM, & Dennis TA (2012). Emotional picture processing in children: An ERP study. Developmental Cognitive Neuroscience, 2(1), 110–119. 10.1016/j.dcn.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed BC, Nelson BD, Auerbach RP, Klein DN, & Hajcak G (2016). Depression risk and electrocortical reactivity during self-referential emotional processing in 8 to 14 year-old girls. Journal of Abnormal Psychology, 125(5), 607–619. 10.1037/abn0000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, MacNamara A, Kennedy AE, Hajcak G, Phan KL, & Klumpp H (2017). Brain-behavioral adaptability predicts response to cognitive behavioral therapy for emotional disorders: A person-centered event-related potential study. Neuropsychologia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schöning S, ... & Arolt V (2010). Automatic mood-congruent amygdala responses to masked facial expressions in major depression. Biological Psychiatry, 67(2), 155–160. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Botteron KN, Luby JL, Belden AC, Gaffrey MS, Babb CM, Nishino T, Miller MI, Ratnanather JT, & Barch DM (2012). Structural-functional correlations between hippocampal volume and cortico-limbic emotional responses in depressed children. Cognitive, Affective, & Behavioral Neuroscience, 13(1), 135–151. 10.3758/s13415-012-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Henrichs J, Donkers F, & Van den Bergh B (2015). Effects of prenatal exposure to maternal anxiety and mindfulness on the child’s affective processing. Altered Neurocognitive Functioning in Infants and Young Children Prenatally Exposed to Maternal Anxiety and Mindfulness, 81. [Google Scholar]
- Webb CA, Auerbach RP, Bondy E, Stanton CH, Foti D, & Pizzagalli DA (2017). Abnormal neural responses to feedback in depressed adolescents. Journal of Abnormal Psychology, 126(1), 19–31. 10.1037/abn0000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, & Hajcak G (2011). The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience, 23(10), 2994–3007. 10.1162/jocn.2011.21630. [DOI] [PubMed] [Google Scholar]
- Weinberg A, & Sandre A (2018). Distinct associations between low positive affect, panic, and neural responses to reward and threat during late stages of affective picture processing. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(1), 59–68. 10.1016/j.bpsc.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Venables NC, Proudfit GH, & Patrick CJ (2015). Heritability of the neural response to emotional pictures: Evidence from ERPs in an adult twin sample. Social Cognitive and Affective Neuroscience, 10(3), 424–434. 10.1093/scan/nsu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Perlman G, Kotov R, & Hajcak G (2016). Depression and reduced neural response to emotional images: Distinction from anxiety, and importance of symptom dimensions and age of onset. Journal of Abnormal Psychology, 125(1), 26–39. 10.1037/abn0000118. [DOI] [PubMed] [Google Scholar]
- Weinberg A, May AM, Klonsky ED, Kotov R, & Hajcak G (2017). Decreased neural response to threat differentiates patients who have attempted suicide from nonattempters with current ideation. Clinical Psychological Science, 5(6), 952–963. 10.1177/2167702617718193. [DOI] [Google Scholar]
- Whalen DJ, Luby JL, Tilman R, Mike A, Barch D, & Belden AC (2016). Latent class profiles of depressive symptoms from early to middle childhood: Predictors, outcomes, and gender effects. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 57(7), 794–804. 10.1111/jcpp.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen DJ, Sylvester CM, & Luby JL (2017). Depression and anxiety in preschoolers: A review of the past 7 years. Child and Adolescent Psychiatric Clinics, 26(3), 503–522. 10.1016/j.chc.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.