Abstract
Activins are selective regulators of FSH production by pituitary gonadotrope cells. In a gonadotrope-like cell line, LβT2, activins stimulate FSH via the activin type IIA receptor (ACVR2A) and/or bone morphogenetic protein type II receptor (BMPR2). Consistent with these observations, FSH is greatly reduced, though still present, in global Acvr2a knockout mice. In contrast, FSH production is unaltered in gonadotrope-specific Bmpr2 knockout mice. In light of these results, we questioned whether an additional type II receptor might mediate the actions of activins or related TGF-β ligands in gonadotropes. We focused on the activin type IIB receptor (ACVR2B), even though it does not mediate activin actions in LβT2 cells. Using a Cre-lox strategy, we ablated Acvr2a and/or Acvr2b in murine gonadotropes. The resulting conditional knockout (cKO) animals were compared with littermate controls. Acvr2a cKO (cKO-A) females were subfertile (~70% reduced litter size), cKO-A males were hypogonadal, and both sexes showed marked decreases in serum FSH levels compared with controls. Acvr2b cKO (cKO-B) females were subfertile (~20% reduced litter size), cKO-B males had a moderate decrease in testicular weight, but only males showed a significant decrease in serum FSH levels relative to controls. Simultaneous deletion of both Acvr2a and Acvr2b in gonadotropes led to profound hypogonadism and FSH deficiency in both sexes; females were acyclic and sterile. Collectively, these data demonstrate that ACVR2A and ACVR2B are the critical type II receptors through which activins or related TGF-β ligands induce FSH production in mice in vivo.
Keywords: ACVR2A, ACVR2B, FSH, LH, fertility, gonadotropes
The gonadotropins LH and FSH are produced by gonadotrope cells in the anterior pituitary gland, and are key regulators of gonadal function in males and females (1-4). These dimeric hormones are composed of a common alpha subunit (CGA, encoded by Cga) noncovalently linked to hormone-specific beta subunits (LHβ and FSHβ, encoded by Lhb and Fshb). CGA is produced in excess; therefore, expression of the beta subunits is rate-limiting in gonadotropin synthesis (5, 6). Beta subunit expression is regulated by hypothalamic GnRH, which binds to its receptor (GnRHR, encoded by Gnrhr) on the gonadotrope cell surface (7-9). Fshb expression is also strongly regulated by TGF-β superfamily ligands such as the activins. Activins stimulate Fshb transcription and their mechanisms of action have been extensively investigated in vitro (10-14) and in vivo (15-17).
Activins are dimeric ligands and bind type II serine-threonine kinase receptors on the cell surface of gonadotropes. Upon binding, the ligand-type II receptor complex recruits type I receptor serine-threonine kinases, which are phosphorylated and activated by the type II receptors. The type I receptors then phosphorylate intracellular SMAD proteins (mainly SMAD3 in this system), which complex with SMAD4 and accumulate in the nucleus. There, they act as transcription factors together with forkhead box L2 (FOXL2) to promote Fshb transcription (15, 17-21). Although it is clear that FSH production absolutely depends on this signaling cascade, the specific type II and type I receptors required for Fshb expression in vivo are not known. Here, we focused on the type II receptors.
Within the TGF-β family, there are 5 type II receptors: TGFBR2, AMHR2, BMPR2, ACVR2A, and ACVR2B (22). TGFBR2 and AMHR2 are not expressed in murine gonadotropes (22-24) and activins preferentially bind ACVR2A, ACVR2B, and BMPR2 (25, 26). Activin induction of Fshb promoter activity requires ACVR2A and/or BMPR2, but not ACVR2B, in LβT2 cells (a murine gonadotrope-like cell line) (25). In contrast, FSH levels are reduced in global Acvr2a knockout mice (27), but not in mice lacking BMPR2 specifically in gonadotropes (28). A role for ACVR2B in FSH production has not been investigated in vivo, in part because Acvr2b global knockout mice die shortly after birth (29). Collectively, these data suggest that ACVR2A is either the only TGF-β type II receptor required for quantitatively normal FSH production in vivo or that a second type II receptor, most likely ACVR2B, compensates in its absence. To test this hypothesis, we generated animals in which Acvr2a, Acvr2b, or both were specifically ablated in murine gonadotropes using the Cre-lox system.
Materials and Methods
Animals
The Acvr2afx/fx, Acvr2bfx/fx, and GnrhrIRES-Cre/IRES-Cre (GRIC) mice were described previously (30-32). Acvr2afx/fx males were crossed with GRIC females to generate Acvr2afx/+;GnrhrGRIC/+ progeny. Acvr2afx/+;GnrhrGRIC/+ females were then crossed to Acvr2afx/fx males to generate Acvr2afx/fx;Gnrhr+/+ (control) and Acvr2afx/fx;GnrhrGRIC/+ (conditional knockout; Acvr2a cKO [cKO-A]) animals. The same strategy was used to generate Acvr2bfx/fx;Gnrhr+/+ (control) and Acvr2bfx/fx;GnrhrGRIC/+ (Acvr2b cKO [cKO-B]) animals. Acvr2afx/fx and Acvr2bfx/fx mice were crossed to generate Acvr2afx/+;Acvr2bfx/+ animals, which were then intercrossed to generate Acvr2afx/fx;Acvr2bfx/fx mice. Acvr2afx/fx;Acvr2bfx/fx males were crossed to GRIC females to generate Acvr2afx/+;Acvr2bfx/+;GnrhrGRIC/+ females. These females were then mated to Acvr2afx/fx;Acvr2bfx/fx males to generate Acvr2afx/fx;Acvr2bfx/fx;Gnrhr+/+ (control) and Acvr2afx/fx;Acvr2bfx/fx;GnrhrGRIC/+ (double cKO; dcKO) animals. Genotyping and assessment of genomic recombination were conducted as previously described (33) (primers listed in Table 1). Animals were housed ad libitum on a 12:12 lights on/lights off cycle (lights on at 7:00, lights off at 19:00). All animal experiments were performed in accordance with institutional and federal guidelines and were approved by the McGill University and Goodman Cancer Centre Facility Animal Care Committee (Protocol 5204).
Table 1.
Genotyping Primers
| Gene | Primer Sequence |
|---|---|
| Acvr2a | |
| Forward (fx) | CCATTATGTAGAGTGCTGTCATTAGTTCAGTGCC |
| Forward (rec) | CCACTGATACCATTGTCACATGTTATCCTAATGCTAG |
| Reverse | CTAAGAGACCCAGAAGGCCCAAGGTATTC |
| Acvr2b | |
| Forward (fx) | CACTCCACTGTGTCCAGGGGCTG |
| Forward (rec) | CAGGTGGGTTATTGGAGTAGGCTGGG |
| Reverse | GATCTCTGGGGTAGCTGACAACAGCG |
| GRIC | |
| Forward | GGACATGTTCAGGGATCGCCAGGC |
| Reverse | GCATAACCAGTGAAACAGCATTGCTG |
| Rosa26 mTmG | |
| Forward (WT) | AGGGAGCTGCAGTGGAGTAG |
| Forward (mut) | TAGAGCTTGCGGAACCCTTC |
| Reverse | CTTTAAGCCTGCCCAGAAGA |
To purify gonadotropes by fluorescence-activated cell sorting (FACS), we crossed Acvr2afx/fx;Acvr2bfx/fx animals with Gt(ROSA26)ACTB-tdTomato-EGFP mice (Rosa26mTmG/mTmG, stock 007676 from Jackson Laboratories) to eventually generate Acvr2afx/fx;Acvr2bfx/fx;Rosa26mTmG/mTmG males, which were then crossed to Acvr2afx/+;Acvr2bfx/+;GnrhrGRIC/+ females to produce Acvr2afx/fx;Acvr2bfx/fx;GnrhrGRIC/+;Rosa26mTmG/+ males and females. Controls for FACS were generated by crossing Rosa26mTmG/mTmG and GRIC mice to produce Acvr2a+/+;Acvr2b+/+;GnrhrGRIC/+;Rosa26mTmG/+ males and females. FACS of gonadotropes was performed at the Flow Cytometry Core at the Montreal Clinical Research Institute. Protocols for pituitary cell dispersion and cell sorting were adapted from previous publications (16, 34). Briefly, we dispersed 3 to 4 pituitary glands per digestion per genotype per sex. Each sample was then sorted individually, and we obtained ~2.0 × 104 EGFP-positive and ~4.4 × 105 tdTomato-positive cells per sample. Male and female cells of a given genotype were then pooled together for RNA analysis.
Blood collection
Blood was collected from 8- to 10-week-old male or 9- to 10-week-old female control and cKO animals by cardiac puncture. Females were euthanized at 0700 h on estrous morning. As dcKO females were acyclic, they were collected alongside controls at 0700 hours. Blood was allowed to clot for 30 to 60 minutes at room temperature, and was then spun down at 3,000 rpm for 10 minutes to collect serum. Sera were stored at -80oC until assayed for LH and FSH.
Hormone analyses
Serum FSH was assessed with Milliplex kits (Millipore-Sigma, MPTMAG-49K [custom-made for FSH only], Oakville, Ontario, Canada) following the manufacturer’s instructions (lower detection limit: 23.7 pg/mL; dynamic range: 61.0 pg/mL to 250 000 pg/mL; limit of quantification [LOQ]: 61.0 pg/mL; intra-assay coefficient of variation [CV] < 15%). Serum LH levels were measured using an in-house sandwich ELISA as previously described (16, 35, 36) (lower detection limit: 0.117 ng/mL; dynamic range: 0.117 ng/mL to 30 ng/mL; LOQ: 0.516 ng/mL; intra-assay CV < 10%). Serum FSH and LH values that fell below each assay’s LOQ were set to the LOQ for purposes of statistical analyses and plotting of the data.
The LOQ for each assay was defined as the lowest concentration of analyte that could be reliably quantified. As described elsewhere (37), the LOQ was calculated by adding 2 times the standard deviation of blank matrix absorbance values to the mean absorbance value of the blanks (based on 6 independent experiments). In other words, LOQ = A + 2σ, where A is the mean absorbance value of the blank matrix (i.e., no analyte present), and σ is the standard deviation of absorbance values using blank matrix. The obtained absorbance value (from A + 2σ) was then interpolated on a standard curve.
Organ collection
Pituitary glands, testes, and seminal vesicles were dissected from 8- to 10-week-old control and cKO males. Control and cKO females were sacrificed at 9 to 10 weeks of age at 0700 hours on the morning of estrus (confirmed by assessing vaginal cytology), at the approximate time of the secondary FSH surge, to collect pituitary glands and ovaries. Acyclic dcKO females were collected along with control females at 0700 hours. Pituitary glands were snap frozen in liquid nitrogen and stored at -80oC. All other organs were weighed on an analytical scale.
One ovary per female was fixed in 10% formalin (HT501128, Millipore-Sigma) overnight at room temperature, and then stored in 70% ethanol. Fixed ovaries were sent for embedding, sectioning, and hematoxylin/eosin staining at the McGill Centre for Bone and Periodontal Research. Follicle counting (done by H.S. and blinded to genotype) was done on fully cut-through ovaries using an inverted microscope. For each female, the other ovary was snap frozen in liquid nitrogen and stored at -80oC.
One testis per male was immersed in Bouin’s fixative solution (1120-16, Ricca Chemical Company, Pocomoke City, MD, USA) overnight at room temperature, followed by an overnight incubation in 100% ethanol. Finally, the testis was left in 70% ethanol and processed like the ovaries at the McGill Centre for Bone and Periodontal Research. For each male, the second testis was homogenized in a solution containing 10% dimethyl sulfoxide and 0.9% NaCl. Sperm were then counted in a 0.1% trypan blue solution on a hemocytometer. The data represent the average of 2 counts performed by 2 independent observers (G.S. and L.O.; both were blinded to genotype).
Assessment of female puberty onset, estrous cyclicity, and fertility
Vaginal opening was monitored daily following weaning (postnatal day 21). At 6 weeks of age, females were swabbed daily for 3 weeks to assess estrous cyclicity. Vaginal cytology was assessed using 0.1% methylene blue, following previously established guidelines (38).
At 9 weeks of age, females were mated with wild-type, age-matched C57BL/6 males (Charles River, Senneville, Quebec, Canada) for 3 to 4 months. Breeding cages were monitored daily to record the number of pups produced. Pups were euthanized 2 weeks after birth.
Primary pituitary cultures
Pituitaries from 8- to 10-week-old male and female mice (Acvr2afx/fx, Acvr2bfx/fx, or Acvr2afx/fx;Acvr2bfx/fx) were extracted and dispersed as previously described (28, 39, 40). On day 1, cells pooled from both sexes were seeded in 48-well plates (250 000-400 000 cells/well). At the time of plating, cells were treated with adenoviruses expressing green fluorescent protein (Ad-GFP) or Cre-IRES-GFP (Ad-Cre) (Baylor College of Medicine Vector Development Laboratory, Houston, Texas, USA) at a multiplicity of infection of 600 in medium 199 (M199; 31100-035, Invitrogen, Burlington, Ontario, Canada) supplemented with 10% fetal bovine serum (10438026, ThermoFisher Scientific, Burlington, Ontario, Canada). Approximately 24 hours later, on day 2, the medium was changed to fresh culture medium (10% fetal bovine serum) without viruses. On day 4, RNA was harvested as described in the following section.
Reverse transcription and quantitative-PCR
Pituitaries and ovaries were homogenized in TRIzol reagent (15596018, ThermoFisher Scientific), and total RNA was extracted following the manufacturer’s guidelines. For FACS samples and primary pituitary cultures, total RNA and DNA were extracted using Total RNA Mini Kits (Geneaid, RB300, New Taipei City, Taiwan).
Reverse transcription was performed as previously described (41) using Moloney murine leukemia virus reverse transcriptase (0000172807, Promega, Madison, WI, USA) and random hexamers (0000184865, Promega). Quantitative PCR (qPCR) runs were conducted on a Corbett Rotorgene 600 instrument (Corbett Life Science) using EvaGreen qPCR Mastermix (ABMMmix-S-XL; Diamed, Mississauga, Ontario, Canada) and the primers listed in Table 2. Expression levels of genes of interest were determined using the 2-ΔΔCt method (41) and ribosomal protein L19 (Rpl19) for normalization (unless otherwise specified). All primers were validated for efficiency and specificity (primers listed in Table 2).
Table 2.
qPCR Primers
| Gene | Primer Sequence |
|---|---|
| Actb | |
| Forward | TGGCGCTTTTGACTCAGGAT |
| Reverse | GGGATGTTTGCTCCAACCAA |
| Acvr2a | |
| Forward | AAGATGGCCTACCCTCCTGT |
| Reverse | ACCAAATCTTCCCCTTGCTT |
| Acvr2b | |
| Forward | GGCTGCGTTTGGAAAGCTC |
| Reverse | GCAACAAGTTTTCGTGCTTCA |
| Bmpr2 | |
| Forward | GAATGTTGACAGGAGACCGGA |
| Reverse | TTATCCAGGTCAAGGGAGGGC |
| Cga | |
| Forward | TCCCTCAAAAAGTCCAQGAGC |
| Reverse | GAAGAGAATGAAGAATATGCAG |
| Fshb | |
| Forward | GTGCGGGCTACTGCTACACT |
| Reverse | CAGGCAATCTTACGGTCTCG |
| Gnrhr | |
| Forward | TTCGCTACCTCCTTTGTCGT |
| Reverse | CACGGGTTTAGGAAAGCAAA |
| Lhb | |
| Forward | ACTGTGCCGGCCTGTCAACG |
| Reverse | AGCAGCCGGCAGTACTCGGA |
| Rpl19 | |
| Forward | CGGGAATCCAAGAAGATTGA |
| Reverse | TTCAGCTTGTGGATGTGCTC |
Superovulation
Superovulation was performed in juvenile females (23-30 days of age). Control and dcKO animals were IP injected with 5 IU equine chorionic gonadotropin (HOR-272-a, East Brunswick, NJ, USA) between 1600 and 1700 hours. Two days later, females were IP injected with 5 IU of human chorionic gonadotropin (C1063, Millipore-Sigma) between 1700 and 1800 hours. On the next morning, between 0700 and 0730 hours, mice were euthanized, and cumulus-oocyte complexes (COCs) were harvested from the ampullae of the oviduct. COCs were enzymatically digested with 0.5 mg/mL hyaluronidase (H3884, Millipore-Sigma) for 20 minutes at 37°C. The number of oocytes was counted (by G.S., who was blinded to genotype) using an inverted microscope.
Gonadectomy
Males and females were gonadectomized at 7 to 8 weeks of age in compliance with standard operating procedures 206 and 207 of the McGill University and Goodman Cancer Centre Facility Animal Care Committee. Briefly, for males, an incision was made at the midline of the scrotum at the level of the skin, and then the tunica. Each testis was pulled out with tweezers, and the tissue was cauterized at the level of the fat pad such that entire testes and epididymides could be removed. The wound was then closed with sutures and veterinary glue. For females, an incision was made at the midline of the mid-dorsum of the animal. A small incision was made in the muscle above the ovary on each side, and the ovary pulled out with forceps by the surrounding fat pad. The oviduct was then cauterized so that the entire ovary could be removed. All incisions were closed by sutures. In the case of sham-operated animals, all the procedures were the same, except that there was no cauterization. Animals were left to recover for 2 weeks following surgery, at which point they were euthanized to collect serum and pituitary glands. All analyses from gonadectomized animals were conducted as described previously, except that control females were collected at random estrous cycle stages.
Statistical analysis
All data were analyzed on GraphPad Prism 8 using Student t tests, unless otherwise specified. In situations where 2-way ANOVA was used, analyses were corrected for multiple comparisons using the Holm-Sidak method (all P values were adjusted for multiple comparisons). Results were considered statistically significant when P < 0.05.
Results
Generation of Acvr2a and Acvr2b conditional knockout mice
To investigate the relative roles of ACVR2A and ACVR2B in gonadotropes, we generated gonadotrope-specific Acvr2a and Acvr2b conditional knockout (cKO-A and cKO-B) mice. First, we examined the specificity of Acvr2a and Acvr2b gene recombination in the conditional KO animals. To that end, we collected several tissues from cKO-A or cKO-B males and females and extracted genomic DNA. We confirmed by PCR that recombination occurred specifically in the pituitary glands of males and females, and in testes and epididymides of males (Figs. S1A-B in ref. (42)). The Cre-driver line used here (GRIC) is active in the male germline in addition to gonadotropes (16, 30, 43, 44). Therefore, the recombination in testes and epididymides was expected. It is for this reason that the GRIC allele is always introduced via the female parent.
We then generated animals in which both Acvr2a and Acvr2b were ablated in gonadotropes (dcKOs). We detected little to no Acvr2a and Acvr2b mRNA in purified gonadotropes of dcKOs relative to control mice (~93%-94% reduction) (GFP+ cells in Figs. S1C-D in ref. (42)). In contrast, expression of both receptors was intact in non-gonadotropes from dcKO mice (Tomato + cells in Figs. S1C-D in ref. (42)). Bmpr2 mRNA expression was intact in both cell populations in dcKOs (Fig. S1E in ref. (42)). Finally, we confirmed by PCR that Cre-mediated recombination of the floxed alleles occurred exclusively in the purified gonadotrope population (Fig. S1F in ref. (42)).
FSH production is impaired in male and female Acvr2a cKO mice
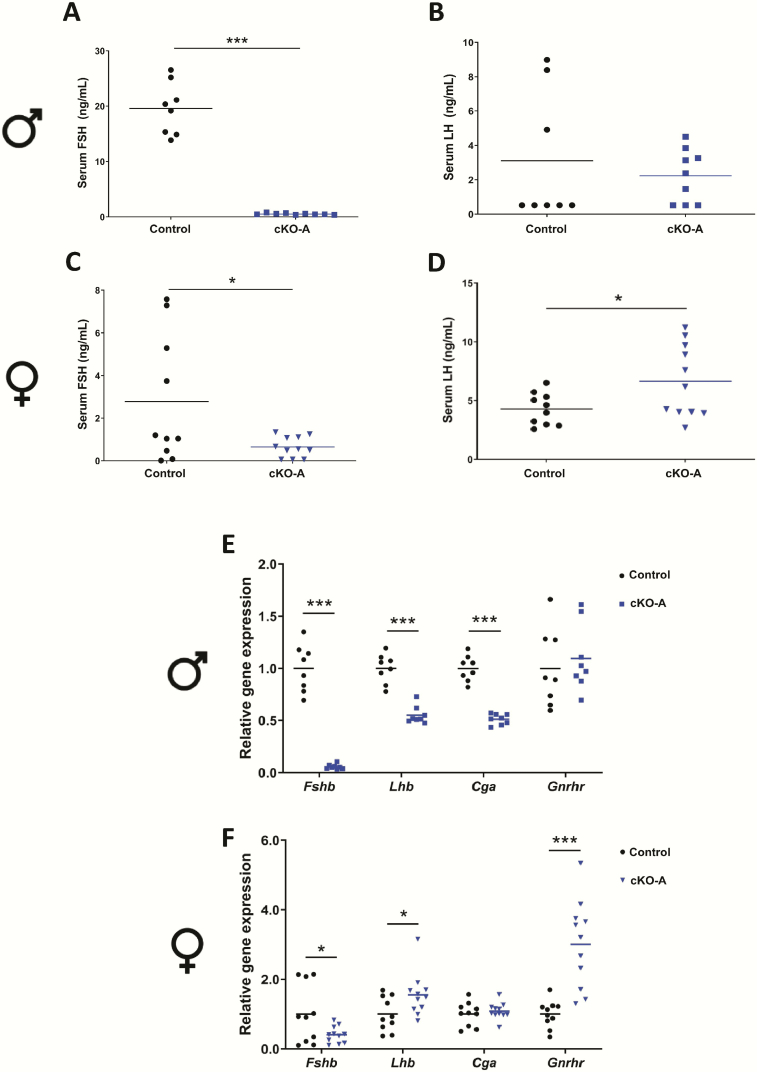
Serum FSH levels were significantly reduced in male and female Acvr2a cKO (cKO-A) animals compared to controls (Figs. 1A and 1C). The average serum FSH levels were 19.6 ng/mL (±4.7 ng/mL) and 0.52 ng/mL (±0.15 ng/mL) in control and cKO-A males, respectively. In females, they were 2.78 ng/mL (±2.97 ng/mL) in control and 0.65 ng/mL (±0.48 ng/mL) in cKO-A mice. Serum LH levels were unaffected in cKO-A males (Fig. 1B) but elevated in cKO-A females (Fig. 1D). Pituitary analyses revealed decreased Fshb, Lhb, and Cga mRNA expression in cKO-A relative to control males (Fig. 1E). Gnrhr mRNA levels were normal in cKO-A males. Fshb expression was decreased in cKO-A females, whereas their Lhb and Gnrhr levels were significantly elevated (Fig. 1F). Cga expression was unaltered in female cKO-A animals relative to controls. The variability in serum FSH and pituitary Fshb levels in control females (for this and the following models) likely stemmed from the fact that some females had completed the secondary FSH surge by 0700 hours on the morning of estrus, whereas others had not.
Figure 1.
Acvr2a expression in gonadotropes is required for quantitatively normal FSH production and Fshb expression in both male and female mice. Serum FSH levels in control and Acvr2a cKO (cKO-A) (A) males and (C) females. Serum LH levels in control and cKO-A (B) males and (D) females. Pituitary Fshb, Lhb, Cga, and Gnrhr mRNA levels assessed by RT-qPCR in (E) male and (F) female control and cKO-A mice. Rpl19 was used as the housekeeping gene. t-tests were used for statistical analyses, *P < 0.05, ***P < 0.001.
In primary pituitary cultures from Acvr2afx/fx animals, Fshb mRNA expression levels were reduced by ~60% in cells transduced with a Cre-expressing adenovirus (Ad-Cre), relative to cells transduced with a GFP-expressing adenovirus (Ad-GFP; Fig. S2A in ref. (42)). Acvr2a mRNA levels were reduced by ~85% in Ad-Cre relative to Ad-GFP cells (Fig. S2A in ref. (42)).
Acvr2a cKO mice are hypogonadal
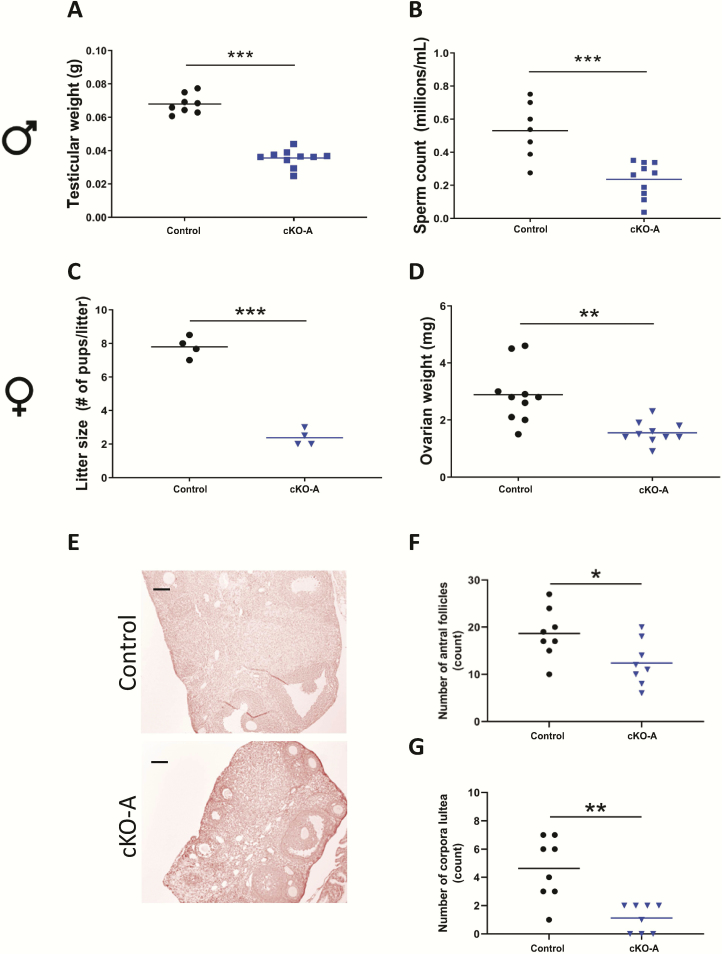
cKO-A males exhibited decreased testicular weights compared with controls (Fig. 2A), whereas their seminal vesicles weights were unaffected (Fig. S3A in ref. (42)). The hypogonadism was associated with a decrease in testicular sperm counts (Fig. 2B), with no structural abnormalities in the testes (Fig. S3B in ref. (42)).
Figure 2.
Acvr2a cKO animals are hypogonadal. (A) Testicular weights and (B) testicular sperm counts in 8- to 10-week-old control and cKO-A males. (C) Average litter sizes from control and cKO-A females paired with wild-type males for 3 to 4 months. Ovarian (D) weights, (E) histology, (F) antral follicle counts, and (G) corpora lutea numbers in 9- to 10-week-old control and cKO-A females. Scale bar: 100 μm. t-tests were used for statistical analyses, *P < 0.05, **P < 0.01, ***P < 0.001.
Female cKO-A mice did not display impairments in puberty onset (measured by vaginal opening, Fig. S3C in ref. (42)) or estrous cyclicity (Fig. S3D in ref. (42)). When paired to wild-type males, however, cKO-A females had significantly smaller litters compared with controls (Fig. 2C), despite producing litters at normal intervals (data not shown). cKO-A females had smaller ovaries than controls (Fig. 2D-E), which was associated with a reduced number of antral follicles (Fig. 2F) and corpora lutea (Fig. 2G).
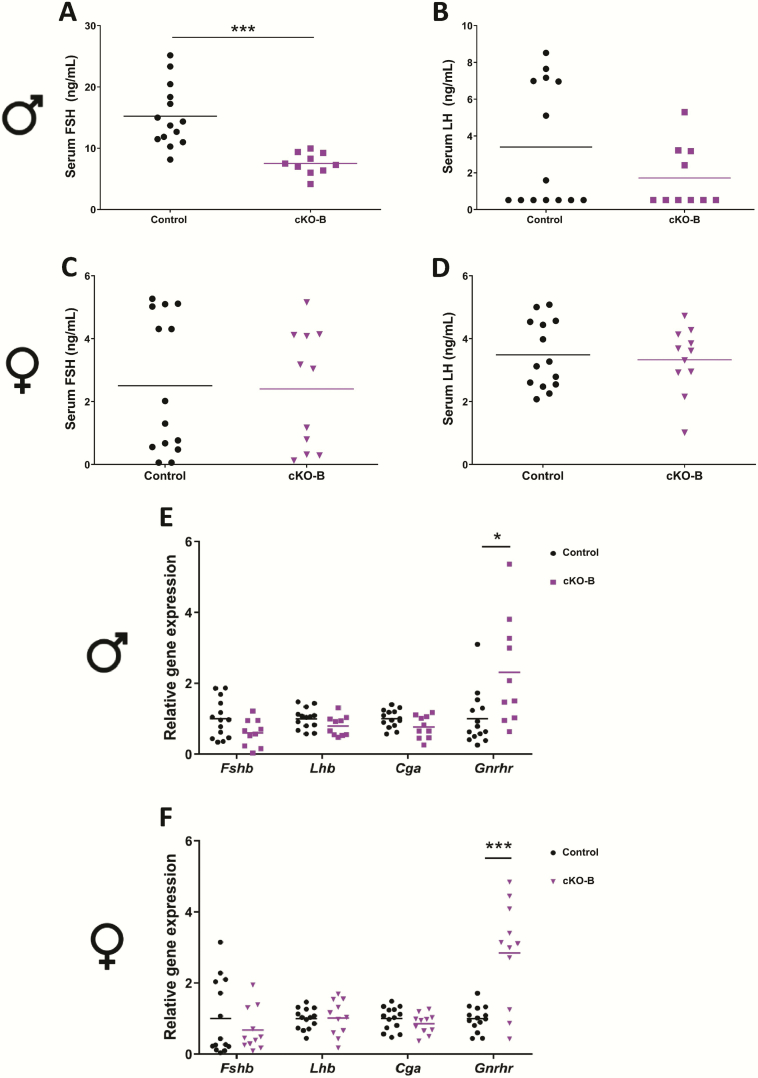
Male, but not female, Acvr2b cKO mice show a mild impairment in FSH production
Acvr2b cKO (cKO-B) males showed a measurable impairment in FSH production relative to controls (Fig. 3A; from 15.2 ng/mL (±5.06 ng/mL) in controls to 7.52 ng/mL (±1.77 ng/mL) in cKO-Bs), with serum LH unaffected (Fig. 3B). In contrast, FSH and LH levels did not differ between cKO-B and control females (Figs. 3C-D). Mean serum FSH levels were 2.51 ng/mL (±2.18 ng/mL) and 2.40 ng/mL (±1.88 ng/mL) in control and cKO-B females, respectively. In the pituitary glands, there were no significant changes in gene expression in Fshb, Lhb, or Cga in males (Fig. 3E) or females (Fig. 3F). However, Gnrhr mRNA levels were statistically significantly increased in both male and female cKO-B animals relative to controls (Figs. 3E-F).
Figure 3.
Acvr2b expression in gonadotropes is required for quantitatively normal FSH production in male, but not female, mice. Serum FSH levels in control and Acvr2b cKO (cKO-B) (A) males and (C) females. Serum LH levels in control and cKO-B (B) males and (D) females. Pituitary Fshb, Lhb, Cga, and Gnrhr mRNA levels assessed by RT-qPCR in (E) male and (F) female control and cKO-B mice. Rpl19 was used as the housekeeping gene. t-tests were used for statistical analyses, *P < 0.05, ***P < 0.001.
In primary pituitary cultures from Acvr2bfx/fx animals, Fshb mRNA expression levels were not statistically significantly different between Ad-GFP and Ad-Cre-transduced cells (Fig. S2B in ref. (42)). However, Acvr2b mRNA levels were reduced by ~85% in Ad-Cre transduced cells, relative to controls (Fig. S2B in ref. (42)).
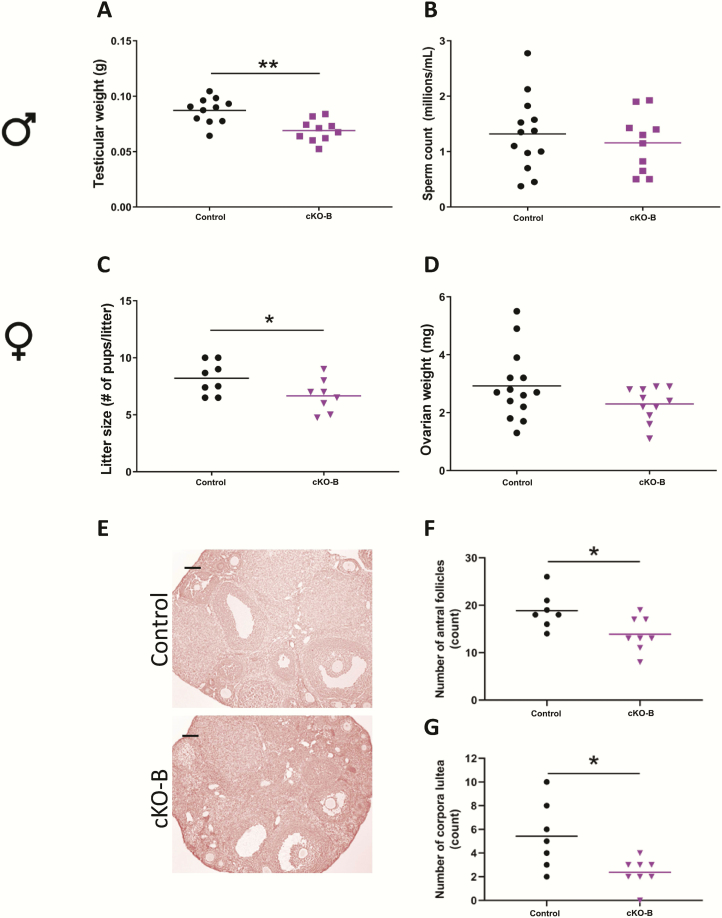
Acvr2b cKOs show milder reproductive phenotypes than Acvr2a cKOs
Gonadotrope-specific Acvr2b deletion in males led to a decrease in testicular weight (Fig. 4A) with no change in seminal vesicle weight (Fig. S4A in ref. (42)). However, there were no detectable changes in testicular sperm production or testis morphology between genotypes (Fig. 4B and S4B in ref. (42)).
Figure 4.
Acvr2b cKO males are hypogonadal and females display impaired ovarian function. (A) Testicular weights and (B) testicular sperm counts in 8- to 10-week-old control and cKO-B males. (C) Average litter sizes from control and cKO-B females paired with wild-type males for 3 to 4 months. Ovarian (D) weights, (E) histology, (F) antral follicle counts, and (G) corpora lutea numbers in 9- to 10-week-old control and cKO-B females. Scale bar: 100 μm. t-tests were used for statistical analyses, *P < 0.05, **P < 0.01.
cKO-B females had normal puberty onset (Fig. S4C in ref. (42)) and estrous cyclicity (Fig. S4D in ref. (42)) relative to controls; however, they produced modestly smaller litters relative to controls when paired to wild-type males (Fig. 4C). Although cKO-B females had no measurable changes in ovarian weights (Fig. 4D) or ovarian morphology (Fig. 4E), their ovaries contained fewer antral follicles (Fig. 4F) and corpora lutea than controls (Fig. 4G).
Pituitary FSH production is abolished in dcKO mice
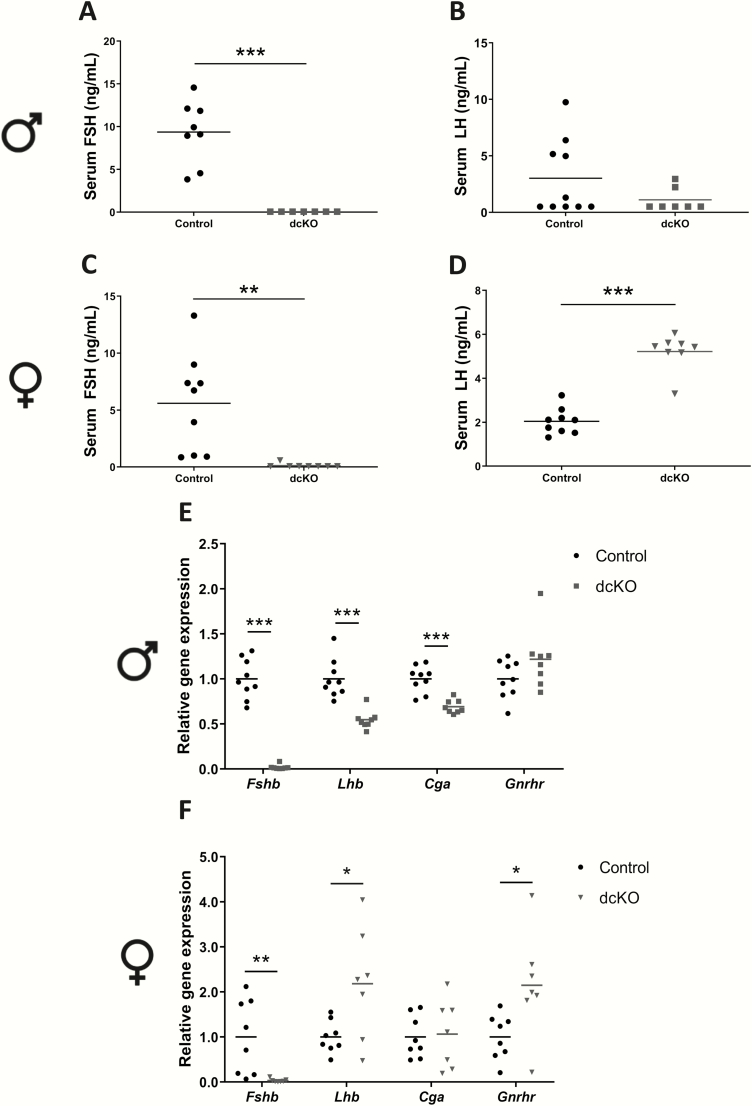
Serum FSH was below detection levels in Acvr2a and Acvr2b dcKO males (Fig. 5A), whereas their serum LH levels were normal (Fig. 5B). dcKO females similarly had low to undetectable serum FSH (Fig. 5C), but also exhibited elevated serum LH levels (Fig. 5D) compared with controls. FSH, but not LH, protein levels were also profoundly decreased in pituitaries of dcKO males and females, as assessed by immunofluorescence (Figs. S5A-B in ref. (42, 45-49)). dcKO males showed decreased pituitary Fshb, Lhb, and Cga mRNA levels (Fig. 5E), whereas expression of Fshb was decreased and Lhb increased in dcKO females (Fig. 5F). Gnrhr expression was normal in dcKO males (Fig. 5E), whereas Gnrhr was elevated and Cga was normal in dcKO females (Fig. 5F).
Figure 5.
Acvr2a/Acvr2b dcKO males and females are FSH-deficient. Serum FSH levels in control and dcKO (A) males and (C) females. Serum LH levels in control and dcKO (B) males and (D) females. Pituitary Fshb, Lhb, Cga, and Gnrhr mRNA levels assessed by RT-qPCR in (E) male and (F) female control and dcKO mice. Rpl19 was used as the housekeeping gene. t-tests were used for statistical analyses, *P < 0.05, **P < 0.01, ***P < 0.001.
In primary pituitary cultures from Acvr2afx/fx;Acvr2bfx/fx animals, Fshb mRNA expression levels were reduced by ~70% in cells transduced with Ad-Cre, relative to cells transduced with ad-GFP (Fig. S2C in ref. (42)). In Ad-Cre transduced cells, Acvr2a and Acvr2b mRNA levels were reduced by ~90% and 80%, respectively (Fig. S2C in ref. (42)).
Acvr2a and Acvr2b dcKO mice are hypogonadal and females are sterile
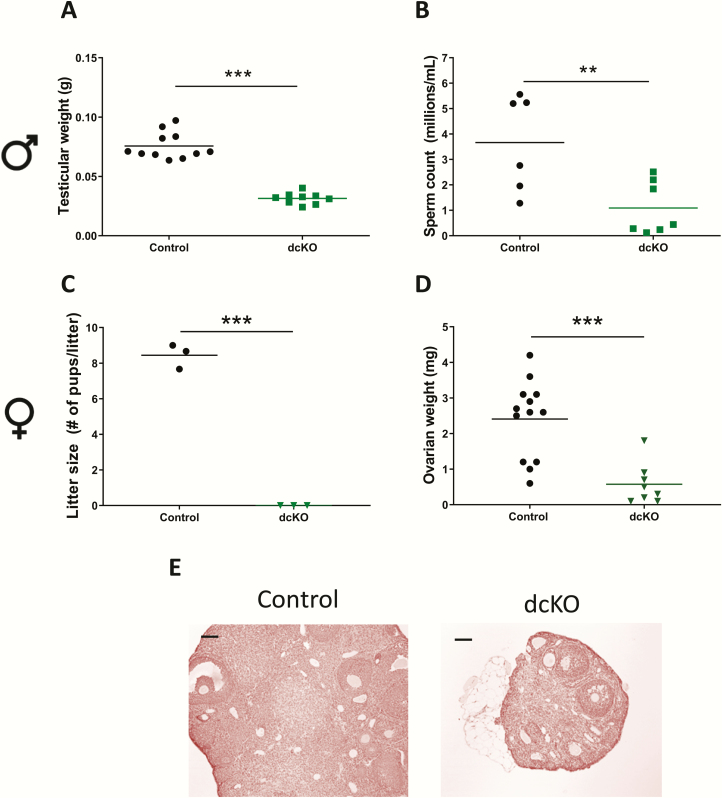
Consistent with the hormone data, testicular (Figs. 6A and S6A in ref. (42)) but not seminal vesicle weights (Figs. S6A-B in ref. (42)) were significantly reduced in dcKO males relative to controls. Testicular sperm production in these animals was also impaired (Fig. 6B) without morphological changes in testicular structure (Fig. S6C in ref. (42)).
Figure 6.
dcKO animals are profoundly hypogonadal. (A) Testicular weights and (B) testicular sperm counts in 8- to 10-week-old control and dcKO males. (C) Average litter sizes from control and dcKO females paired with wild-type males for 3 months. Ovarian (D) weights and (E) histology in 9- to 10-week-old control and dcKO females. Scale bar: 100 μm. t-tests were used for statistical analyses, *P < 0.05, **P < 0.01.
dcKO females were sterile, hypogonadal, and had thread-like uteri (Figs. 6C, 6D and S6D in ref. (42)). There was no delay in vaginal opening in dcKO females (Fig. S6E in ref. (42)), but they did not exhibit estrous cycles (Fig. S6F in ref. (42)). Consistent with their sterility, dcKO females had no late antral follicles or corpora lutea in their ovaries (Fig. 6E). Expression of Cyp19a1 (encoding aromatase), an FSH target gene, was significantly decreased in ovaries of dcKOs relative to controls (Fig. S6G in ref. (42)). To examine ovarian integrity in these animals, we superovulated control and dcKO females. Both genotypes were able to ovulate in response to exogenous gonadotropins, although dcKO females ovulated fewer oocytes on average compared with controls (Fig. S6H in ref. (42)).
To assess potential Acvr2a and Acvr2b gene dosage effects, we measured gonadal weights in males and females with varying degrees of allelic recombination of Acvr2a and/or Acvr2b (Figs. S7A-B in ref. (42)). Of note, the loss of a single Acvr2a allele did not decrease testicular weights relative to controls (Fig. S7A in ref. (42); see Acvr2afx/fx;Acvr2b+/+;Gnrhr+/+ compared with Acvr2afx/+;Acvr2b+/+;GnrhrGRIC/+); however, in the context of ACVR2B-deficient gonadotropes, the loss of a single Acvr2a allele led to a decrease in gonadal weights (see Acvr2afx/fx;Acvr2bfx/fx;Gnrhr+/+ compared with Acvr2afx/+;Acvr2bfx/fx;GnrhrGRIC/+). Finally, though Acvr2afx/+;Acvr2b+/+;GnrhrGRIC/+ females were not investigated, Acvr2afx/+;Acvr2bfx/fx;GnrhrGRIC/+ females did not appear to have a significant decrease in ovarian weights (Fig. S7B in ref. (42)).
Sex differences in LH levels in dcKO mice are dependent on gonadal and extragonadal factors
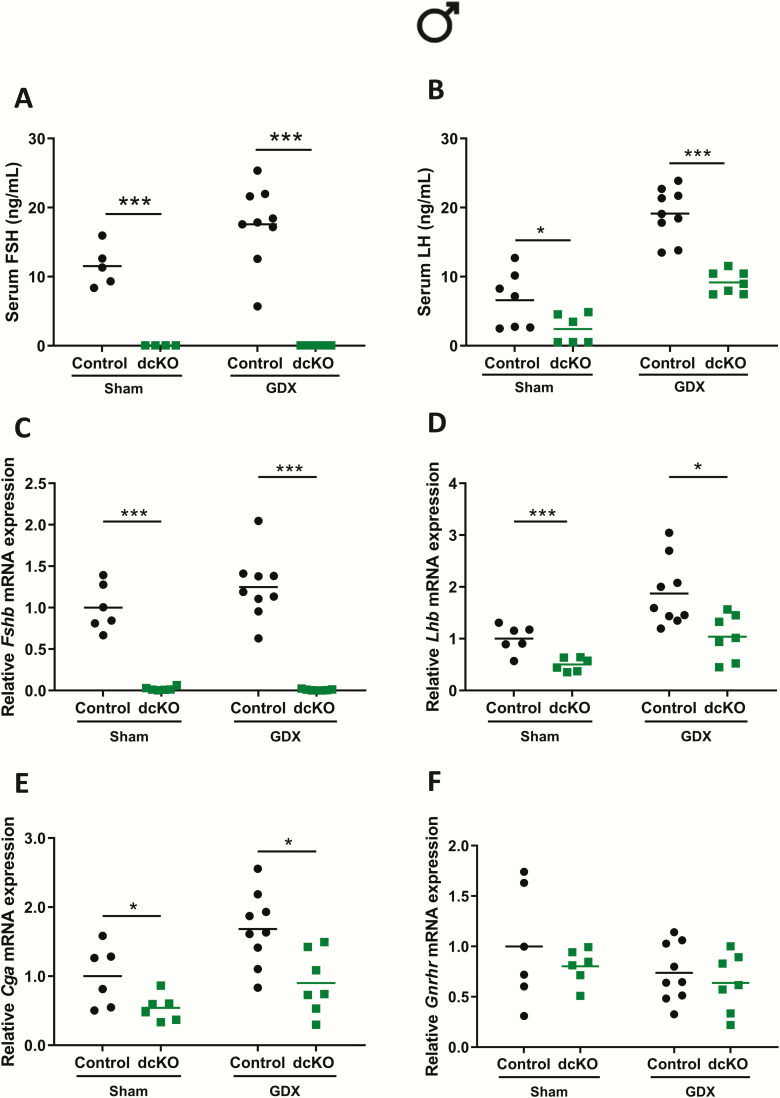
LH levels were elevated in dcKO females (Fig. 5D), but not males (Fig. 5B). This could have stemmed from the loss of estrogen negative feedback in females (Fig. S6G in ref. (42)) or might reflect a sex difference in LH regulation via ACVR2A and ACVR2B at the pituitary level. To begin to address these possibilities, we compared sham-operated or gonadectomized control and dcKO mice. In males, castration led to a modest increase in serum FSH levels in control animals, whereas FSH remained undetectable in both sham-operated and castrated dcKOs (Fig. 7A). Both genotypes displayed increases in serum LH following castration, though levels were lower in dcKO males relative to controls (Fig. 7B). These results were paralleled by the pattern of pituitary mRNA expression. Fshb in control, but not dcKO, males modestly increased postcastration (Fig. 7C). Lhb and Cga expression increased in males of both genotypes postcastration (Figs. 7D-E), though levels were lower in dcKOs relative to controls. Gnrhr mRNA levels were not significantly different between groups (Fig. 7F).
Figure 7.
Gonadectomized dcKO males do not produce FSH and display impaired LH production. (A) Serum FSH and (B) LH in sham-operated (Sham) or castrated/gonadectomized (GDX) control and dcKO males. (C-F) Pituitary Fshb, Lhb, Cga, and Gnrhr mRNA levels assessed by RT-qPCR in male Sham or GDX control and dcKO mice. Rpl19 was used for normalization in all RT-qPCR experiments. Two-way ANOVAs, followed by Holm-Sidak multiple comparison post-hoc tests, were used for statistical analyses, *P < 0.05, ***P < 0.001.
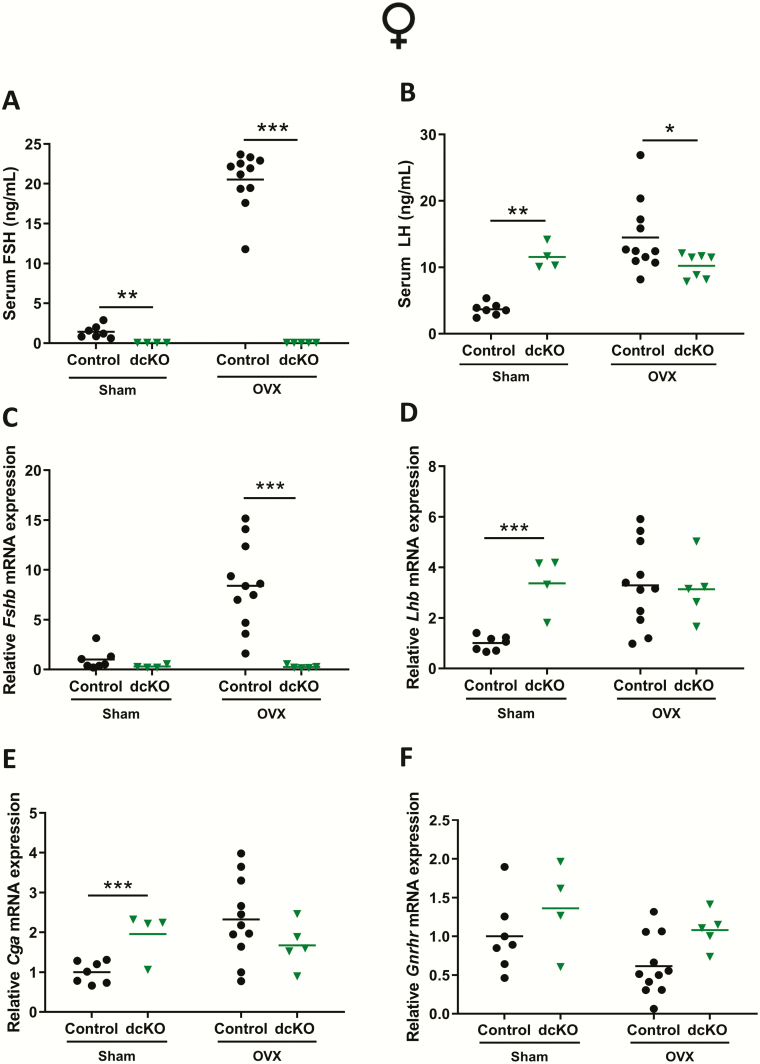
In females, serum FSH increased markedly postovariectomy (OVX) in control mice, but remained undetectable in intact and OVX dcKOs (Fig. 8A). In sham-operated females (collected at random estrous cycle stages, as opposed to morning of estrus in Fig. 5), serum LH levels were increased in dcKO relative to control mice (Fig. 8B). Post-OVX, LH levels increased in control but not dcKO females (Fig. 8B). Pituitary gene expression matched the serum hormone levels, with Fshb and Lhb increasing only in control females following OVX (Fig. 8C-D). In dcKO females, Fshb and Lhb expression remained low and high, respectively (Fig. 8C-D). Importantly, there were no differences in Lhb mRNA levels between OVX controls and dcKOs (Fig. 8D). Cga expression patterns closely matched those of Lhb (Fig. 8E), while Gnrhr expression was not significantly different between groups (Fig. 8F).
Figure 8.
FSH, but not LH production is impaired in gonadectomized dcKO females. (A) Serum FSH and (B) LH in sham-operated (Sham) or ovariectomized (OVX) control and dcKO females. (C-F) Pituitary Fshb, Lhb, Cga, and Gnrhr mRNA levels assessed by RT-qPCR in female Sham or OVX control and dcKO mice. Rpl19 was used for normalization in all RT-qPCR experiments. Two-way ANOVAs, followed by Holm-Sidak multiple comparison post-hoc tests, were used for statistical analyses, *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
We generated conditional Acvr2a and Acvr2b knockout mice to investigate the relative role of these TGF-β family type II receptors in gonadotrope function in vivo. Our data suggest that FSH production is dependent on ACVR2A and, to a lesser extent, ACVR2B. Given the high recombination efficiency in the context of the dcKO mice, it is unlikely that the residual reproductive function and FSH production observed in the single Acvr2a or Acvr2b cKO animals stemmed from preservation of Acvr2a or Acvr2b expression.
ACVR2A and ACVR2B are required for FSH production in murine gonadotropes
ACVR2A had already been suggested to be a necessary type II receptor for FSH production in vivo, based on the phenotype of Acvr2a global knockout mice (27). By specifically ablating Acvr2a in gonadotropes, we both confirmed and extended these earlier observations. One important difference between the 2 studies is the extent to which FSH was reduced. Although we observed a reduction of ~98% and ~80% in serum FSH levels in Acvr2a cKO males and females, respectively, Acvr2a global KO animals (of both sexes) showed a ~60% reduction in FSH (27). Interstudy variation may stem from differences in the genetic backgrounds of the mice and/or from the hormone assays used. Indeed, there were reportedly no sex differences in serum FSH levels in either global Acvr2a KOs or their wild-type counterparts (27), even though male mice are well known to have higher FSH levels than females (17, 50, 51), as we show here. Moreover, the loss of ACVR2A outside of gonadotropes in the global KOs, such as in the gonads, may have contributed to the milder decrease in serum FSH levels observed in these animals relative to Acvr2a cKO mice.
Despite differences between the 2 studies, FSH was still detected in circulation in both the global and gonadotrope-specific Acvr2a knockout models, raising the possibility that another type II receptor may compensate for the absence of ACVR2A. Given that Amhr2 and Tgfbr2 are not expressed in gonadotropes (22-24), and that gonadotrope-specific Bmpr2 cKO animals produce FSH at normal levels (28), ACVR2B was the only other candidate type II receptor remaining that might mediate TGF-β signaling in gonadotropes.
Gonadotrope-specific Acvr2b cKO mice showed milder forms of the phenotypes observed in Acvr2a cKO mice. This was most notable in males, which had detectable decreases in testicular weights and serum FSH levels. Acvr2b cKO females produced smaller litters and had impaired folliculogenesis relative to controls, though their FSH production was apparently normal. It is possible that we missed alterations in serum FSH and Fshb mRNA expression in these mice because of intrinsic biological variability. Regardless, ACVR2B’s role in gonadotropes of both males and females was made evident by the complete loss of FSH production in dcKO animals, and the sterility observed in dcKO females. The data conclusively demonstrate that FSH production in mice depends on ACVR2A and ACVR2B in gonadotropes.
Sex differences
Interestingly, ablation of Acvr2a alone or in combination with Acvr2b produced sex-specific effects. First, Acvr2a cKO and dcKO males, but not females, had decreased pituitary Lhb and Cga mRNA levels. We attempted to elucidate the basis for these sex differences. Castrated dcKO males continued to express reduced levels of Lhb and Cga relative to controls, indicating that androgens or other testicular factors likely do not explain the sex differences in Lhb and Cga expression. Moreover, there did not appear to be a “protective” factor from the ovary because Lhb and Cga mRNA levels were not reduced in ovariectomized dcKO relative to control females. It may be that TGF-β signaling regulates Lhb and Cga expression (5, 52-54) in vivo either alone or in combination with GnRH (55-57). However, it remains unclear why this signaling and regulation might be male-specific.
Second, we observed that Acvr2a cKO and dcKO females, but not males, had elevated Lhb mRNA expression and serum LH levels relative to controls. We previously reported similar observations in other FSH-deficient models and argued that this may reflect endocrine effects (15, 16). Although we did not measure estradiol levels here because of concerns about the reliability of estradiol assays in mice (15, 16, 58), Cyp19a1 mRNA levels were decreased in Acvr2a/Acvr2b dcKO ovaries relative to controls. As a consequence, estradiol production was likely impaired in these mice. This is supported by their hypoplastic uteri, a biological indicator of hypoestrogenemia. The loss of estradiol negative feedback in Acvr2a/Acvr2b dcKO females could therefore explain their increased LH. This idea is substantiated by the equivalency of LH levels in ovariectomized dcKO and control females. In males, testosterone is the principal testicular steroid that negatively regulates GnRH and LH secretion. Testosterone production is FSH independent, helping to explain why elevated LH levels were not observed in dcKO males. Indeed, their LH levels were reduced, likely due to reductions in Lhb and Cga expression.
Are activins the TGF-β ligands driving FSH synthesis in vivo?
Our observations raise questions regarding the necessity for activins in FSH production in vivo. In the gonadotrope-like cell line, LβT2, activins act via ACVR2A and BMPR2, but not ACVR2B, to regulate Fshb transcription (25). However, mice lacking Bmpr2 expression in gonadotropes produce FSH normally (28) and the data reported here clearly indicate that the TGF-β ligand regulating FSH in vivo acts via ACVR2A and ACVR2B. Therefore, activins either use different receptors in the cell line than in bona fide gonadotropes or a non-activin TGF-β ligand may be the principal driver of FSH production in vivo. Elevated, rather than reduced, FSH levels in activin B-deficient mice (59), as well as other unpublished observations from our laboratory, are consistent with the latter possibility.
Study limitations
It is important to note some potential limitations of the study. First, Cre activity in the GRIC mice starts at embryonic day 12.75 (30). Therefore, it is possible that the phenotypes observed in the different conditional knockout strains stemmed from defects in gonadotrope development. However, we also observed robust decreases in Fshb expression following Acvr2a, but not Acvr2b, knockdown in pituitary cultures from adult mice (Fig. S2 in ref. (42)). The loss of both Acvr2a and Acvr2b led to an even more pronounced decrease in Fshb mRNA levels. These data indicate that: (1) the endogenous pituitary TGF-β ligand(s) that regulate(s) Fshb synthesis use(s) ACVR2A and ACVR2B, with preference for ACVR2A, and (2) the effects observed in single cKO and dcKO animals in vivo are likely not developmental in nature. Indeed, intact LH production in these mice suggests that gonadotrope development was likely unimpaired.
Second, we assessed the extent of recombination in the double knockout model (Fig. S1 in ref. (42)), but not in either of the individual receptor knockout lines. Although there is no reason to suspect that recombination efficiency would be lower in the latter lines (especially as there are fewer floxed alleles), it would have been valuable to assess the expression of one receptor subtype in the absence of the other. For example, it is possible that Acvr2a may have been upregulated in Acvr2b cKO gonadotropes, which could have contributed to the relatively modest FSH phenotype in these mice. Finally, deleting the type II receptors would also be predicted to affect inhibin as well as activin action (60-62). However, impairments in inhibin activity should manifest as increased FSH production (40, 63). In all 3 models presented here, FSH production was either modestly or profoundly reduced. Therefore, the reported phenotypes are not likely explained by alterations in inhibin action or any such changes are, at least, superseded by the impairments in activin-like signaling.
In summary, TGF-β signaling via ACVR2A and ACVR2B in gonadotropes is required for FSH production in mice. Whether the same receptors mediate activin or activin-like regulation of FSH synthesis in other mammals, including humans, is not yet clear. However, it should be noted that an ACVR2A-Fc fusion protein dose-dependently inhibits FSH levels in postmenopausal women (64). Similarly, an ACVR2A neutralizing antibody suppresses FSH levels in the same clinical population (65). Therefore, common TGF-β ligands and type II receptors may regulate FSH production in mice and humans.
Acknowledgments
The authors thank Julie Lord (Flow Cytometry Core, Montreal Clinical Research Institute) for her help with fluorescence-activated cell sorting.
Funding: This work was supported by the Canadian Institutes of Health Research (operating/project grants MOP-123447, MOP-133394, and PJT-162343 to DJB, and Doctoral Research Award 152308 to GS), the Natural Sciences and Engineering Research Council of Canada (2015–05178 to DJB and Doctoral fellowship to EB), Fonds de Recherche du Québec—Santé (fellowship number 31338 to GS), a Samuel Solomon Fellowship in Reproductive Endocrinology (to GS), and a Ferring Postdoctoral Fellowship in Reproductive Health (to LO).
Author Contributions: G.S. and D.J.B. were responsible for the experimental design, data analyses, and manuscript preparation. G.S. conducted most of the experiments. L.O. and X.Z. helped with tissue collection, mouse colony management, and analyses. H.S. was responsible for the follicle counts. Y.W. conducted the FSH assays. E.B. performed the immunofluorescence. U.B. and S.J.L. generated and provided the mouse strains. All authors approved the final version of the manuscript.
Glossary
Abbreviations
- Ad-Cre
adenovirus expressing Cre-IRES-GFP
- Ad-GFP
adenovirus expressing green fluorescent protein
- cKO
conditional knockout
- COC
cumulus-oocyte complex
- CV
coefficient of variation
- dcKO
double conditional knockout
- FACS
fluorescence-activated cell sorting
- FOXL2
forkhead box L2
- LOQ
limit of quantification
- OVX
postovariectomy
- qPCR
quantitative PCR
- TGF-β
transforming growth factor-β
Additional Information
Disclosure Summary: The authors declare that they have no conflicts of interest.
Data Availability. The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Kumar TR, Wang Y, Lu N, Matzuk MM. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet. 1997;15(2):201-204. [DOI] [PubMed] [Google Scholar]
- 2. Ma X, Dong Y, Matzuk MM, Kumar TR. Targeted disruption of luteinizing hormone beta-subunit leads to hypogonadism, defects in gonadal steroidogenesis, and infertility. Proc Natl Acad Sci U S A. 2004;101(49):17294-17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tapanainen JS, Aittomäki K, Min J, Vaskivuo T, Huhtaniemi IT. Men homozygous for an inactivating mutation of the follicle-stimulating hormone (FSH) receptor gene present variable suppression of spermatogenesis and fertility. Nat Genet. 1997;15(2):205-206. [DOI] [PubMed] [Google Scholar]
- 4. Lofrano-Porto A, Barra GB, Giacomini LA, et al. Luteinizing hormone beta mutation and hypogonadism in men and women. N Engl J Med. 2007;357(9):897-904. [DOI] [PubMed] [Google Scholar]
- 5. Attardi B, Miklos J. Rapid stimulatory effect of activin-A on messenger RNA encoding the follicle-stimulating hormone beta-subunit in rat pituitary cell cultures. Mol Endocrinol. 1990;4(5):721-726. [DOI] [PubMed] [Google Scholar]
- 6. Weiss J, Guendner MJ, Halvorson LM, Jameson JL. Transcriptional activation of the follicle-stimulating hormone beta-subunit gene by activin. Endocrinology. 1995;136(5):1885-1891. [DOI] [PubMed] [Google Scholar]
- 7. Dalkin AC, Burger LL, Aylor KW, et al. Regulation of gonadotropin subunit gene transcription by gonadotropin-releasing hormone: measurement of primary transcript ribonucleic acids by quantitative reverse transcription-polymerase chain reaction assays. Endocrinology. 2001;142(1):139-146. [DOI] [PubMed] [Google Scholar]
- 8. Miller WL, Shafiee-Kermani F, Strahl BD, Huang HJ. The nature of FSH induction by GnRH. Trends Endocrinol Metab. 2002;13(6):257-263. [DOI] [PubMed] [Google Scholar]
- 9. Fortin J, Lamba P, Wang Y, Bernard DJ. Conservation of mechanisms mediating gonadotrophin-releasing hormone 1 stimulation of human luteinizing hormone beta subunit transcription. Mol Hum Reprod. 2009;15(2):77-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernard DJ, Lee KB, Santos MM. Activin B can signal through both ALK4 and ALK7 in gonadotrope cells. Reprod Biol Endocrinol. 2006;4:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamba P, Santos MM, Philips DP, Bernard DJ. Acute regulation of murine follicle-stimulating hormone beta subunit transcription by activin A. J Mol Endocrinol. 2006;36(1):201-220. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Bernard DJ. Activin A induction of murine and ovine follicle-stimulating hormone β transcription is SMAD-dependent and TAK1 (MAP3K7)/p38 MAPK-independent in gonadotrope-like cells. Cell Signal. 2012;24(8):1632-1640. [DOI] [PubMed] [Google Scholar]
- 13. Dupont J, McNeilly J, Vaiman A, Canepa S, Combarnous Y, Taragnat C. Activin signaling pathways in ovine pituitary and LbetaT2 gonadotrope cells. Biol Reprod. 2003;68(5):1877–1887. [DOI] [PubMed] [Google Scholar]
- 14. Suszko MI, Balkin DM, Chen Y, Woodruff TK. Smad3 mediates activin-induced transcription of follicle-stimulating hormone beta-subunit gene. Mol Endocrinol. 2005;19(7):1849-1858. [DOI] [PubMed] [Google Scholar]
- 15. Fortin J, Boehm U, Deng CX, Treier M, Bernard DJ. Follicle-stimulating hormone synthesis and fertility depend on SMAD4 and FOXL2. Faseb J. 2014;28(8):3396-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Schang G, Boehm U, Deng CX, Graff J, Bernard DJ. SMAD3 regulates follicle-stimulating hormone synthesis by pituitary gonadotrope cells in vivo. J Biol Chem. 2017;292(6):2301-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Y, Schang G, Wang Y, et al. Conditional deletion of FOXL2 and SMAD4 in gonadotropes of adult mice causes isolated FSH deficiency. Endocrinology. 2018;159(7):2641-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ. A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone beta subunit transcription. Mol Endocrinol. 2009;23(7):1001-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lamba P, Wang Y, Tran S, et al. Activin A regulates porcine follicle-stimulating hormone beta-subunit transcription via cooperative actions of SMADs and FOXL2. Endocrinology. 2010;151(11):5456-5467. [DOI] [PubMed] [Google Scholar]
- 20. Tran S, Lamba P, Wang Y, Bernard DJ. SMADs and FOXL2 synergistically regulate murine FSHbeta transcription via a conserved proximal promoter element. Mol Endocrinol. 2011;25(7):1170-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tran S, Zhou X, Lafleur C, et al. Impaired fertility and FSH synthesis in gonadotrope-specific Foxl2 knockout mice. Mol Endocrinol. 2013;27(3):407-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gore AJ, Philips DP, Miller WL, Bernard DJ. Differential regulation of follicle stimulating hormone by activin A and TGFB1 in murine gonadotropes. Reprod Biol Endocrinol. 2005;3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheung LYM, George AS, McGee SR, et al. Single-Cell RNA sequencing reveals novel markers of male pituitary stem cells and hormone-producing cell types. Endocrinology. 2018;159(12):3910-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayran A, Sochodolsky K, Khetchoumian K, et al. Pioneer and nonpioneer factor cooperation drives lineage specific chromatin opening. Nat Commun. 2019;10(1):3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rejon CA, Hancock MA, Li YN, Thompson TB, Hébert TE, Bernard DJ. Activins bind and signal via bone morphogenetic protein receptor type II (BMPR2) in immortalized gonadotrope-like cells. Cell Signal. 2013;25(12):2717-2726. [DOI] [PubMed] [Google Scholar]
- 26. Thompson TB, Woodruff TK, Jardetzky TS. Structures of an ActRIIB:activin A complex reveal a novel binding mode for TGF-beta ligand:receptor interactions. Embo J. 2003;22(7):1555-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matzuk MM, Kumar TR, Bradley A. Different phenotypes for mice deficient in either activins or activin receptor type II. Nature. 1995;374(6520):356-360. [DOI] [PubMed] [Google Scholar]
- 28. Ongaro L, Zhou X, Cui Y, Boehm U, Bernard DJ. Gonadotrope-specific deletion of the BMP type 2 receptor does not affect reproductive physiology in mice. Biol Reprod. 2020;102(3):639-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oh SP, Li E. The signaling pathway mediated by the type IIB activin receptor controls axial patterning and lateral asymmetry in the mouse. Genes Dev. 1997;11(14):1812-1826. [DOI] [PubMed] [Google Scholar]
- 30. Wen S, Schwarz JR, Niculescu D, et al. Functional characterization of genetically labeled gonadotropes. Endocrinology. 2008;149(6):2701-2711. [DOI] [PubMed] [Google Scholar]
- 31. Lee SJ, Huynh TV, Lee YS, et al. Role of satellite cells versus myofibers in muscle hypertrophy induced by inhibition of the myostatin/activin signaling pathway. Proc Natl Acad Sci U S A. 2012;109(35):E2353-E2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goh BC, Singhal V, Herrera AJ, et al. Activin receptor type 2A (ACVR2A) functions directly in osteoblasts as a negative regulator of bone mass. J Biol Chem. 2017;292(33):13809-13822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhou X, Wang Y, Ongaro L, et al. Normal gonadotropin production and fertility in gonadotrope-specific Bmpr1a knockout mice. J Endocrinol. 2016;229(3):331-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ho CC, Zhou X, Mishina Y, Bernard DJ. Mechanisms of bone morphogenetic protein 2 (BMP2) stimulated inhibitor of DNA binding 3 (Id3) transcription. Mol Cell Endocrinol. 2011;332(1-2):242-252. [DOI] [PubMed] [Google Scholar]
- 35. Czieselsky K, Prescott M, Porteous R, et al. Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology. 2016;157(12):4794-4802. [DOI] [PubMed] [Google Scholar]
- 36. Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939-4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riepsamen AH, Chan K, Lien S, et al. Serum concentrations of oocyte-secreted factors BMP15 and GDF9 during IVF and in women with reproductive pathologies. Endocrinology. 2019;160(10):2298-2313. [DOI] [PubMed] [Google Scholar]
- 38. Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schang G, Toufaily C, Bernard DJ. HDAC inhibitors impair Fshb subunit expression in murine gonadotrope cells. J Mol Endocrinol. 2019;62(2):67-78. [DOI] [PubMed] [Google Scholar]
- 40. Li Y, Fortin J, Ongaro L, et al. Betaglycan (TGFBR3) functions as an Inhibin A, but not Inhibin B, coreceptor in pituitary gonadotrope cells in mice. Endocrinology. 2018;159(12):4077-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402-408. [DOI] [PubMed] [Google Scholar]
- 42. Schang G, Ongaro L, Schultz H, et al. Murine FSH production depends on the activin type II receptors ACVR2A and ACVR2B (supplementary figures). Figshare Digital Repository 2020. Deposited March 22, 2020. 10.6084/m9.figshare.11923617.v1. [DOI]
- 43. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci U S A. 2010;107(37):16372-16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toufaily C, Schang G, Zhou X, et al. Impaired LH surge amplitude in gonadotrope-specific progesterone receptor knockout mice. J Endocrinol. 2020;244(1):111-122. [DOI] [PubMed] [Google Scholar]
- 45. Schang G, Ongaro L, Schultz H, et al. Murine FSH production depends on the activin type II receptors ACVR2A and ACVR2B (supplementary materials and methods) Figshare Digital Repository 2020. Deposited March 22nd 2020. 10.6084/m9.figshare.11923623.v2. [DOI]
- 46.RRID:AB_141607. https://scicrunch.org/resolver/AB_141607.
- 47.RRID:AB_141637. https://scicrunch.org/resolver/AB_141637.
- 48.RRID:AB_2338476. https://scicrunch.org/resolver/AB_2338476.
- 49.RRID:AB_2665514. https://scicrunch.org/resolver/AB_2665514.
- 50. Michael SD, Kaplan SB, Macmillan BT. Peripheral plasma concentrations of LH, FSH, prolactin and GH from birth to puberty in male and female mice. J Reprod Fertil. 1980;59(1):217-222. [DOI] [PubMed] [Google Scholar]
- 51. Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM. The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology. 2000;141(5):1795-1803. [DOI] [PubMed] [Google Scholar]
- 52. Coss D, Thackray VG, Deng CX, Mellon PL. Activin regulates luteinizing hormone beta-subunit gene expression through Smad-binding and homeobox elements. Mol Endocrinol. 2005;19(10):2610-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stouffer RL, Woodruff TK, Dahl KD, Hess DL, Mather JP, Molskness TA. Human recombinant activin-A alters pituitary luteinizing hormone and follicle-stimulating hormone secretion, follicular development, and steroidogenesis, during the menstrual cycle in rhesus monkeys. J Clin Endocrinol Metab. 1993;77(1):241-248. [DOI] [PubMed] [Google Scholar]
- 54. Blumenfeld Z, Ritter M. Inhibin, activin, and follistatin in human fetal pituitary and gonadal physiology. Ann N Y Acad Sci. 2001;943:34-48. [DOI] [PubMed] [Google Scholar]
- 55. Fortin J, Bernard DJ. SMAD3 and EGR1 physically and functionally interact in promoter-specific fashion. Cell Signal. 2010;22(6):936-943. [DOI] [PubMed] [Google Scholar]
- 56. McLachlan RI, Dahl KD, Bremner WJ, et al. Recombinant human activin-A stimulates basal FSH and GnRH-stimulated FSH and LH release in the adult male macaque, Macaca fascicularis. Endocrinology. 1989;125(5):2787-2789. [DOI] [PubMed] [Google Scholar]
- 57. Burger LL, Dalkin AC, Aylor KW, Haisenleder DJ, Marshall JC. GnRH pulse frequency modulation of gonadotropin subunit gene transcription in normal gonadotropes-assessment by primary transcript assay provides evidence for roles of GnRH and follistatin. Endocrinology. 2002;143(9):3243-3249. [DOI] [PubMed] [Google Scholar]
- 58. Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152(11):4443-4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vassalli A, Matzuk MM, Gardner HA, Lee KF, Jaenisch R. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev. 1994;8(4):414-427. [DOI] [PubMed] [Google Scholar]
- 60. Chapman SC, Bernard DJ, Jelen J, Woodruff TK. Properties of inhibin binding to betaglycan, InhBP/p120 and the activin type II receptors. Mol Cell Endocrinol. 2002;196(1-2):79-93. [DOI] [PubMed] [Google Scholar]
- 61. de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 2004;15(1):1-11. [DOI] [PubMed] [Google Scholar]
- 62. Bernard DJ, Chapman SC, Woodruff TK. Mechanisms of inhibin signal transduction. Recent Prog Horm Res. 2001;56: 417-450. [DOI] [PubMed] [Google Scholar]
- 63. Rivier C, Vale W. Immunoneutralization of endogenous inhibin modifies hormone secretion and ovulation rate in the rat. Endocrinology. 1989;125(1):152-157. [DOI] [PubMed] [Google Scholar]
- 64. Ruckle J, Jacobs M, Kramer W, et al. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res. 2009;24(4):744-752. [DOI] [PubMed] [Google Scholar]
- 65. Garito T, Zakaria M, Papanicolaou DA, et al. Effects of bimagrumab, an activin receptor type II inhibitor, on pituitary neurohormonal axes. Clin Endocrinol (Oxf). 2018;88(6): 908-919. [DOI] [PubMed] [Google Scholar]