Abstract
Sarcopenia is the loss of skeletal muscle mass with age, the precise cause of which remains unclear. Several studies have shown that sarcopenia is at least partly driven by denervation which, in turn, is related to loss of motor nerve cells. Recent data suggests degradation of the nucleocytoplasmic barrier and nuclear envelope transport process are contributors to nerve loss in a number of neurodegenerative diseases. Having recently shown that important components of the nuclear barrier are lost with advancing age, we now ask whether these emergent defects accompany increased nuclear permeability, chromatin disorganization and lower motoneuron loss in normal ageing, and if so, whether exercise attenuates these changes. Immunohistochemistry was used on young adult, old and exercised mouse tissues to examine nucleocytoplasmic transport regulatory proteins and chromatin organization. We used a nuclear permeability assay to investigate the patency of the nuclear barrier on extracts of the spinal cord from each group. We found increased permeability in nuclei isolated from spinal cords of old animals that correlated with both mislocalization of essential nuclear transport proteins and chromatin disorganization, and also found that in each case, exercise attenuated the age-associated changes. Findings suggest that the loss of nuclear barrier integrity in combination with previously described defects in nucleocytoplasmic transport may drive increased nuclear permeability and contribute to age-related motoneuron death. These events may be significant indirect drivers of skeletal muscle loss.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00155-7) contains supplementary material, which is available to authorized users.
Keywords: Ageing, Sarcopenia, Nucleocytoplasmic transport, Nuclear permeability, Motoneuron death, Neurodegeneration
Introduction
Sarcopenia is characterized by the loss of skeletal muscle mass with age (Valdez 2019), though the underlying cause of this muscle loss remains unclear. A current body of evidence suggests that the loss of muscle mass is a result of dysregulation of processes within the muscle (Bronikowski et al. 2003; Chai et al. 2011; Cruz-Jentoft et al. 2018; Deschenes et al. 2010; van Praag et al. 2005). However, many muscle properties are under direct or indirect regulation by the nervous system (Valdez 2019), so the prospect remains that muscle loss occurs as a secondary consequence of neural dysfunction (Larsson et al. 2018).
Neural dysfunction leading to weakness and loss of muscle mass is a manifestation of several neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS), and many of the cardinal features of ALS and sarcopenia are shared (Table 1). While the primary causes of neuron loss in ALS have remained obscure, a growing body of evidence suggests that defects in nucleocytoplasmic transport may contribute (Jovičić et al. 2016; Zhang et al. 2018) to both neuron loss and/or to the aberrant appearance of inclusion bodies in neuronal nuclei (Woulfe 2007; Woulfe 2008). Other research examining elderly organisms also points to progressive age-related changes in nuclear envelope (NE) and nuclear pore complex (NPC) proteins that probably contribute to cell death (D'Angelo et al. 2009; Gillon et al. 2018; Toyama et al. 2013) by permitting unrestricted transit of cellular components into and out of the cell nucleus.
Table 1.
Comparison between ALS and sarcopenia of main phenotypic features. The table identifying which phenotypical presentations of amyotrophic lateral sclerosis (ALS) are shared with sarcopenia
| Amyotrophic lateral sclerosis | Sarcopenia |
|---|---|
| Progressive muscle weakness (Kim and Taylor 2017) | ✓ (Goodpaster et al., 2006) |
| Muscle fibre atrophy (Kim and Taylor 2017) | ✓ (Barns et al., 2014) |
| Denevartion (Kim and Taylor 2017) | ✓ (Rowan et al., 2012) |
| Motoneuron loss (Kim and Taylor 2017) | ✓ (Tomlinson and Irving 1977) |
| Rostol-caudal axis of deteriotion | ✓ (Sheard & Anderson, 2012) |
| Nuclear pore complex dysfunction (Boeyenaems et al., 2016) | ✓ (D'Angelo et al. 2009) |
| Degeneration of ELLPs (Boeyenaems et al., 2016) | ✓ (Savas et al., 2012) |
| TDP43 inclusion bodies (cytoplasm) (Boeyenaems et al., 2016; Kim and Taylor 2017; Chou et al. 2018) | ? |
| SUMO1 altered activity | ? |
| C9orf72 mutation (Jovicic et al., 2015; Zhang et al., 2015) | ? |
| Alter nuclear import and export (Zhang et al., 2015; Kim and Taylor 2017) | ? |
| Nuclear permeability (D'Angelo et al. 2009) | ✓ |
Previous investigations examining nuclear permeability and altered nucleocytoplasmic transport in ALS have established that increased nuclear permeability in alpha motoneurons (MN) results in the cytoplasmic accumulation of transactivation response DNA-binding protein 43 (TDP43), a DNA- and RNA-binding protein (Chu et al. 2007; Feligioni et al. 2015). Mislocalization of TDP43, such as that which occurs in ALS, is believed to result in a toxic gain of function (normally within the cytoplasm) and/or a loss of nuclear functioning (Dewey et al. 2012; Nonaka et al. 2013). Research has also highlighted that post-translational modification of both GTPase-activating protein (RanGAP1) and regulator of chromosome condensation 1 (RCC1) by small ubiquitin-like modifier 1 (SUMO1) are important mediators of the nucleocytoplasmic transport process. Removal of SUMO1 causes reduced RCC1 mobility and the development of the premature ageing disease, Hutchinson-Gilford progeria syndrome (HGPS) (Kelley et al. 2011). RCC1 binds chromatin, and as a result, the pattern of RCC1 immunohistochemical staining is indicative of chromatin organization (Chatterjee and Paschal 2015). As a consequence, the organization of DNA can be observed (Ohtsubo et al. 1989) and RCC1 staining patterns may therefore be utilized as a proxy for DNA damage, degradation, or dysfunction within the nucleus (Espada and Esteller 2010; Larrieu et al. 2018). Investigating levels of TDP43 and SUMO1 and the distribution of RCC1 within and around MN nuclei may clarify whether nuclear permeability is altered with age, and whether a mislocalization of nuclear-associated proteins or disorganization of nuclear material occurs. This in turn may inform our understanding of processes that contribute to the MN loss that occurs with age (Gillon et al. 2018).
Since we have previously shown that MN loss with age correlates with loss of nuclear pore proteins (Nup98 and Nup93) that have essential roles in regulating nuclear permeability, we hypothesize that changes in these essential proteins may result in abnormal permeability of spinal cord neuronal nuclei with increasing age (Gillon et al. 2018; Tomlinson and Irving 1977). In addition, because regular exercise is the primary countermeasure for age-related muscle weakness, and recent data suggests that the benefits of exercise might be mediated partly by slowing the rate of MN loss (Gillon et al. 2018), we hypothesize that any changes in nuclear permeability or nuclear protein distribution will be inhibited by exercise. This study therefore explores how age and exercise are related to neuronal nuclear permeability, levels and localization of nuclear transport proteins and levels and arrangement of proteins associated with DNA organization.
Material and methods
Using immunohistochemistry (IHC), we examined age- and exercise-related changes in motoneurons from the lumbar lateral motor column (LMC) in three groups of mice (young adult, elderly, elderly exercised). Investigations of nuclear permeability, along with levels and locations of selected nuclear proteins (SUMO1, TDP43, RCC1), were performed. SUMO1 immunostaining was also investigated in neurons of the motor cortex of these three groups of mice to provide comparative data on cellular processes between upper and lower motoneurons.
Animals
Data were derived from five young adult (6 months), five elderly (24 months) and five elderly exercised (24 months) female C57Bl/6j mice. The C57Bl/6j strain was chosen as it is a commonly used model for investigating age-related neuromuscular deterioration (losses in muscle mass and force-generating capacity, deterioration of the neuromuscular junction) (Ballak et al. 2014; Bhattacharya et al. 2019; Chai et al. 2011; Luff 1998; Valdez et al. 2010; Vinel et al. 2018). The mean life span of the C57Bl/6j strain is 26.7 months (Ballak et al. 2014); young adult and elderly mice in this study were approximately equivalent to 19 and 76 human years respectively using the life span equivalence equation (Dutta and Sengupta 2016). Animals were kept under a 12-h light/dark regimen with environment enrichment toys, standard mouse chow and water available ad libitum. The animal tissue utilized for immunohistochemistry was from the same cohort of mice as those from previously published work (Gillon et al. 2018), while permeability assays were conducted on tissues from a separate cohort of animals. Prior approval for all experiments was obtained from the University of Otago Animal Ethics Committee.
Exercised animals
Elderly exercised animals (exercised) were given access to a monitored running wheel for a period of 4 months, starting at 20 months of age. Animals were housed in large smooth-walled and topped cages that prevented climbing, allowing any benefits seen in exercised animals to be associated with running only. Distance covered, time spent running and average running speeds were collected weekly for each animal over the exercise period.
Euthanasia and tissue extraction
Animals were deeply anaesthetized by intraperitoneal injection of sodium pentobarbital and perfused with heparinized phosphate-buffered saline (PBS), followed by perfusion with 1% paraformaldehyde in warmed phosphate buffer via a peristaltic minipump. For fresh tissue extraction, animals were euthanized via an overdose of pentobarbital. Following both perfusion and euthanasia, the spinal cords and brains were carefully removed and placed in Tissue-Tek® OCT embedding compound (Sakura, Alphen aan den Rijn, The Netherlands) pre-chilled to 4 °C to optimize snap freezing by partial immersion in L-isopentane chilled to − 170 °C with liquid nitrogen. Specimens were stored at − 80 °C until required for subsequent examination.
Tissue processing
A Leica CM1850 cryostat (Leica, Wetzlar, Germany) was used to cut frozen tissue sections. All sections were mounted on APES (Sigma-Aldrich no. A3648) coated microscope slides and left to dry at room temperature for 30 min.
Nuclear isolation
Fresh spinal cords were homogenized in a micro-centrifuge tube using a potter homogenizer (roughly 15–20 strokes) with 15 mL homogenisation solution, containing 0.25% Triton-X100 per 1 g of tissue. The homogenate was then centrifuged at 850rcf, at 4 °C, for 10mins. The supernatant was discarded and the pellet was re-suspended in the same volume of homogenisation solution without Triton-X100. The re-suspended material was centrifuged again at 1000rcf for 10mins. The resuspension protocol was repeated twice more, with the re-suspended material being centrifuged at 5000rcf (15mins) and finally 10,000rcf (30 mins), respectively. The samples were kept on ice between centrifuge protocols to maintain the viability of the nuclei (Løvtrup-Rein and McEwen 1966).
Frozen sectioning for immunohistochemistry
Sixteen micrometre transverse frozen sections of the lumbar enlargement were cut and left to dry at room temperature for 30 min. Serial sections of spinal cord were cut and picked up on numbered microscope slides so they could be subsequently assigned for immunohistochemistry using one of three primary antibodies. Sixteen micrometre sections were also taken of precentral gyrus of the frontal lobe (primary motor cortex) in the coronal plane, to isolate the layer five neurons. The sections were left to dry at room temperature for 30 min and stored in a − 80 °C freezer until use.
Fluorescent dextrans and immunohistochemistry
Nuclear permeability assay
Isolated neuron nuclei were suspended in a warmed glycerol gelatin-dextran cocktail (GG1-10 mL, Sigma-Aldrich Missouri, USA). The gelatin cocktail was formed by mixing 200 μL of two fluorescent dextran solutions; a green 70 kDa fluorophore (Fluorescein isothiocyanate-dextran (FITC), FD70S, Sigma-Aldrich, St-Louis, MI) and a red 30 kDa fluorophore (Tetramethylrhodamine isothiocyanate-dextran, 73766 Sigma-Aldrich, St-Louis, MI) which were both made into a stock solution of 1 mg of dextran per mL gelatin with addition of a 1 in 500 dilution of DAPI to identify nuclei. The gelatin cocktail was kept warm while the nuclear pellet was added; the mixture was then put on a warmed microscope slide, coverslipped and left to cool. Dextran size choice was based on previous literature and an understanding of normal nuclear permeability (D'Angelo et al. 2009). As the nucleus allows passive movement of molecules smaller than 40 kDa, the 30 kDa dextran acts as a positive control that should always be able to enter. By contrast, under normal conditions, the 70 kDa dextran should be excluded from the nucleus, and so the admission of the 70 kDa probe indicates an increase in nuclear permeability.
The two sizes of fluorescent dextran were produced by limited hydrolysis and fractionation to ensure the size of the coupled dextrans and that no uncoupled fluorophore was present within the samples.
Spinal cord proteins
Immunohistochemical protocols were conducted on transverse serial sections of the spinal cord. All slides were rinsed in Tris-buffered saline (1× TBS) for 45 min prior to processing. Slides were incubated in primary antibodies to detect SUMO1 (rabbit polyclonal at 1:500 dilution, Abcam®, Cambridge, UK, AB30258), TDP43 (rabbit polyclonal at 1:100, NOVNB110-55376, Novus-Biologicals, Colorado, USA), at room temperature for 4 h. For RCC1, slides were incubated in primary antibodies (rabbit polyclonal at 1:100, Novus-biological, NBP1-85638, Novus-Biologicals, Colorado, USA) across serial transverse sections on separate slides. Slides immunostained with RCC1 primary antibody underwent a heat-induced epitope retrieval step in citrate-buffered saline (Shi et al. 2001) prior to primary antibody incubation.
All spinal cord sections were washed in several changes of TBS and subsequently incubated with the appropriate AlexaFluor488 conjugated anti-species secondary antibody (1:500 dilution; Life Technologies, Carlsbad, CA) in the dark at 4 °C overnight. Slides were washed with TBS and coverslipped with glycerol mounting medium.
Motor cortex protein
Identification of the layer five MNs of primary motor cortex was possible by the use of chicken ovalbumin upstream promotor transcription factor-interacting protein 2 (CTIP2) immunohistochemical staining (1:50 dilution; Abcam®, Cambridge, UK, AB18465). CTIP2 is a zinc finger transcription factor that has been shown to label the nuclei of cortical spinal projection neurons in layers 5A and 5B of the mature mouse cortex (Tantirigama et al. 2016), allowing us to identify these neurons and to investigate SUMO1 within their nuclei. Slides stained with CTIP2 primary antibody underwent a heat-induced epitope retrieval step in sodium citrate-buffered saline (Shi et al. 2001) prior to primary antibody incubation at 4 °C overnight, and a secondary antibody incubation for 3 h at room temperature.
Microscopy and image processing
Nuclear permeability
Isolated MN nuclei were imaged at × 600 magnification under a Zeiss LSM710 upright confocal microscope with LSM710 scanning head and photomultiplier (Carl Zeiss, Oberkochen, Germany). The use of the laser scanning confocal microscope allowed for thin optical sectioning through a mid-nuclear plane, allowing determination of whether the fluorescent dextrans were located in, on, or outside each nucleus.
Nuclear proteins
Sections subject to immunohistochemical processing using antibodies against TDP43, SUMO1 and RCC1 were illuminated using a CoolLED fluorescence illuminator on an Olympus BX-50 (Olympus® Corporation, Tokyo, Japan) compound widefield fluorescence microscope and digitally photographed with a Spot-RT™ Slider (SPOT Imaging Solutions: Diagnostic Instruments, Inc., Sterling Heights, USA) cooled digital microscope camera. Illumination and exposure conditions were carefully controlled for both spinal cord and brain measurements to eliminate specimen-to-specimen variation in these parameters and to ensure that variation between grey level values between specimens was not due to variability in imaging conditions. It is important to note that although brain samples were from the same animals as spinal cord samples, the fluorescent imaging parameters for SUMO1 were different as the samples were run at different time points, by two separate investigators. Image region of interest (nucleus) greyscale data was acquired using FoveaPro 4.0 (Reindeer Graphics, Ashville, USA) and Image J (National Institutes of Health, Bethesda, USA). Co-staining with 4′,6-diamidino-2-phenylindole (DAPI) was used to verify the presence of motoneuron nuclei during the imaging of each fluorescently labelled protein, while negative controls ensured the absence of staining due to non-specific binding of fluorescent secondary antibody. Motoneurons for each section were identified based on their size and location within the lateral column of the ventral horn of the spinal cord. Presence and levels of TDP43 were assessed within the nucleus and cytoplasm of spinal MN, while SUMO1 was assessed within the nucleus of both spinal and cortical MN. For RCC1, the distribution of nuclear staining was assessed by qualitative assessment of the presence or absence of condensed chromatin to allow analysis of chromatin organization.
We have recently validated the accuracy and reliability of our immunohistochemical measures of relative protein levels on tissue sections (Brady and Sheard 2015); these methods are an extension and adaptation of previously validated principles (Matkowskyj et al. 2003) establishing that both chromogen- and fluorescence-based immunohistochemical techniques can be made reliably quantitative (by comparison with e.g. mass spectrometry, Toki et al. 2017) by understanding and controlling processing and imaging variables. In our adaptation, we dissolve known amounts of purified protein of interest at a variety of concentrations in agarose gel. We then freeze, section and immunohistochemically process the sections of protein gel on the slides along with the tissue sections. When processed and imaged, the known-concentration protein gel provides an accurate reference enabling reliable relative quantification of protein as detected on identically processed, photographed and analyzed tissue sections.
Statistical analyses
All data was organized and analyzed in Microsoft Excel (Microsoft Corporation, Redmond, WA), Prism 7 (GraphPad Software Inc., La Jolla, CA) and R statistical data analysis packages (Davies 2016). Significance was p ≤ 0.05 for all analyses.
Nuclear permeability analysis
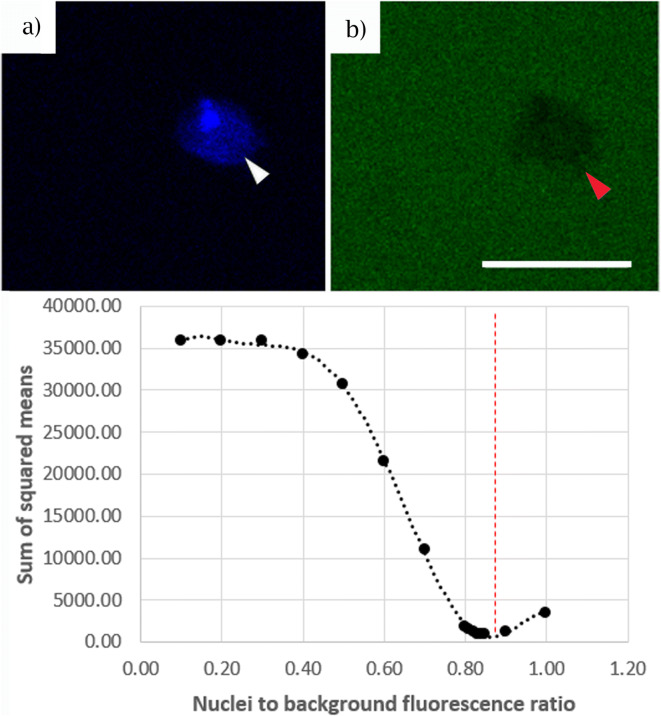
Images were analyzed in Fiji (Image J; National Health, Bethesda, MD) image processing software. The images were processed both visually (observed, qualitative measurements—permeable/not-permeable) and using empirical measurement of the relative grey values. DAPI staining was used to identify isolated nuclei to allow creation of a mask (Fig. 1a) to measure the nuclear fluorescence intensity for the two dextrans (30 kDa and 70 kDa) as well as the background fluorescence intensity. Measurement of both intranuclear and background staining allowed a ratio of the fluorescence of the DAPI masked area to background to be calculated (Fig. 1b/c).
Fig. 1.
Sum of squared means calculation for nuclear permeability. DAPI staining of isolated nuclei ((a) white arrow) was used as a mask to both empirically measure subsequent levels of fluorescent 30 kDa dextran and 70 kDa dextran ((b) red arrow) relative to the background, and to compare this to the observed measurements of permeable and impermeable nuclei. The sum of squared means calculation was used to determine at which point the observed and empirical measurements intersect and determines the cut-off ratio for intranuclear to background ratio (0.84), which was then used conservatively as 0.9 in the current investigation
Collection of both qualitative and empirical measurements allowed calculation of a sum of squares value. This allowed determination of the closest approximation of the number of nuclei that were permeable to dextran, with the aim of aligning what was observed visually with empirical measurement. As shown in Fig. 3, the sum of squares values calculation compared the data collected from subjective judgements (permeable vs. impermeable) and compared it with empirical measurements (fluorescence of DAPI-stained area vs. background). In doing so, the sum of squares values calculation allowed us to determine where the two different measurement processes converged and provided a foreground to background ratio to use as a cut-off point for permeable vs non-permeable. The resulting value was 0.84, so we used the slightly more conservative value of 0.9 as the threshold for separating permeable nuclei from impermeable.
Fig. 3.
Nuclear permeability assay. The pattern of fluorescence for nuclear marker DAPI (a), 70 kDa dextran (b) and 30 kDa dextran (c). The images presented in a–c are typical of the majority of isolated nuclei imaged, featuring a DAPI positive nucleus, with fluorescent 30 kDa dextran localized within the nucleus (b) and no fluorescent 70 kDa dextran present within the nucleus (c). These results show that spinal cord nuclei are typically permeable to 30 kDa but not to 70 kDa probes. Frames d–f illustrate the uncommon observation of a nucleus that was permeable to both 30 kDa and 70 kDa dextran (scale bar 25 μm). Young, elderly and exercised permeability comparison of subjective measures of nuclear permeability were made in young, elderly and elderly exercised isolated nuclei. The fraction of DAPI positive nuclei which are visually deemed to be permeable to 30 kDa and 70 kDa fluorescent dextrans in young, elderly and elderly exercised animals. f More than 98% of DAPI positive nuclei were permeable to 30 kDa in all groups; however, less than 10% of nuclei were permeable to 70 kDa in young, 40% in elderly and 30% in exercised
Statistical analysis of spinal cord and motor cortex protein levels
The comparison of structural and transport proteins between young adult, elderly and exercised mice involved measurement of 50 motoneuron nuclei per animal and five animals per group in spinal cord measurements, while measurements of the cortex were from n = 4 per group and a minimum of 20 measurements per animal. The statistical comparison was conducted using a linear mixed model in order to analyze correlation between measurements from multiple mice without violating the assumption of independence (Davies 2016). Statistical comparison of permeability between young adult, elderly and exercised mice was conducted using an unpaired t test.
Results
Running data
Daily averages for exercise parameters for each animal were recorded (Fig. 2). Exercised elderly animals ran between 216 and 805 total kilometres at an average of 4.26 km ± 1.74 per day. These distances are similar to running distances previously recorded for elderly mice (Fig. 2) (Bronikowski et al. 2003; Gillon et al. 2018; Lightfoot et al. 2004; McMahon et al. 2014; Valdez et al. 2010; van Praag et al. 2005).
Fig. 2.

Average daily distance covered by elderly mice (Distance, km/day) in the current study (red symbol) compared to running distances previouslypublished by other authors. Data adapted from Bronikowski et al. 2003; Gillon et al. 2018; Lightfoot et al. 2004; McMahon et al. 2014; Valdez et al. 2010;van Praag et al. 2005
Permeability of nuclei
Young adult, elderly and elderly exercised comparison
Spinal cords were extracted from young adult, elderly and elderly exercised animals and homogenized. Nuclei identified by DAPI staining (Fig. 3) had permeability to fluorescent 30 kDa and 70 kDa dextrans measured. Both subjective and empirical methods were used to assess the permeability of isolated nuclei. Note that tissue homogenisation and nuclear extraction isolates nuclei from all cells in the spinal cord and does not allow the cellular origin of the nuclei to be established, unlike immunohistochemistry which permits analysis of specific cells. Virtually all nuclei (Fig. 3a) were permeable to the 30 kDa dextran (Fig. 3b) but most remained impermeable to the 70 kDa dextran (Fig. 3c, f) as shown by the absence of FITC (green) fluorescence in the optical section through the equator of the nucleus.
Subjective visual investigation of the age-related changes in nuclear permeability is presented below. All nuclei from elderly animals admitted the 30 kDa probe, but by contrast with young adult animals, nuclei that admitted the 70 kDa dextran were also common (Fig. 4). The proportion of nuclei permeable to 70 kDa dextran from elderly exercised animals was somewhat lower than that for sedentary older animals, but the difference failed to reach statistical significance (Fig. 4).
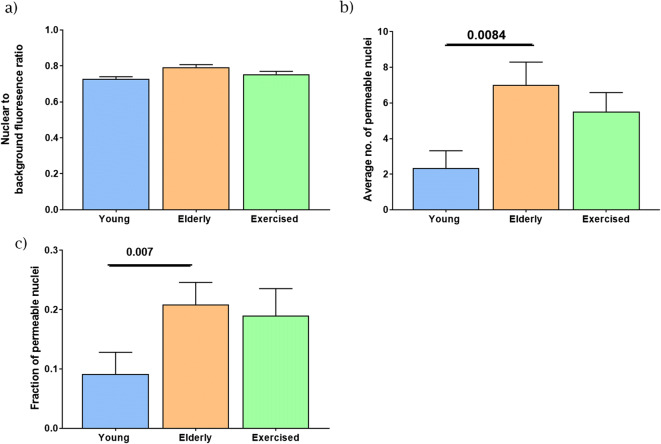
Fig. 4.
Young, elderly and exercised permeability comparison. Empirical measures of nuclear permeability were made in young, elderly and elderly exercised isolated nuclei. Empirical: The average ratio of nuclear to background fluorescence for the 70 kDa fluorescent dextran (a), the average number of permeable nuclei present per animal in young adult, elderly and elderly exercised groups (b) and the fraction of permeable nuclei (c). a No significant difference in nuclear to background ratio was present across all three groups. b Significant increases in the fraction of permeable nuclei were evident in elderly animals, an increase also evident in exercised animals. c Comparison of the average number of permeable nuclei per group showed that an average of two out of 25 nuclei investigated per young animal were permeable, while elderly and exercised animals had seven and five permeable nuclei per 25 nuclei measured, respectively. Data expressed as mean ± SEM, n = 6 animals per group, 25 nuclei per animal
Nuclear transport protein levels and localization
Spinal cord motoneurons
Relative levels of the nuclear signalling proteins TDP43 (nuclear, cytoplasmic) and SUMO1 (nuclear) were measured in lumbar motoneurons in all three groups of mice (Fig. 5). Nuclear TDP43 levels were significantly reduced in the older animals, and exercise did not impact on the decline (Fig. 5d). No differences in cytoplasmic TDP43 were evident across the three groups (Fig. 5d). Nuclear SUMO1 immunofluorescence was significantly higher in nuclei of neurons in the elderly than in the young adult animals, while exercise prevented much of this increase since neurons from old exercised animals had nuclear SUMO1 levels similar to young adult animals (Fig. 5h).
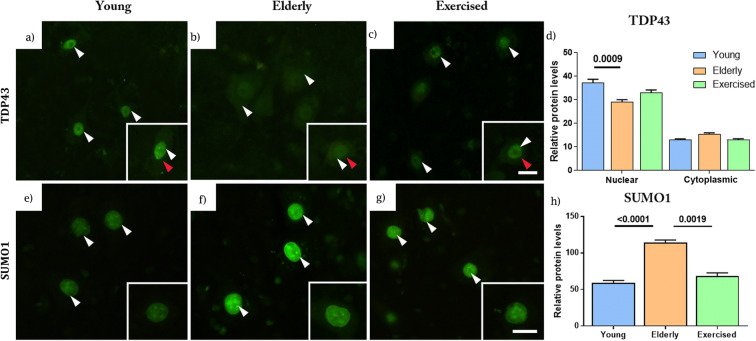
Fig. 5.
Nuclear signalling protein levels in the spinal cord. Young (a), elderly (b) and elderly exercised (c) animal immunostaining for nuclear signalling proteins, Tar DNA-binding protein 43 (TDP43) with nuclear and cytoplasmic staining identified by white and red arrows, respectively. Nuclear (red arrow) but not cytoplasmic (white arrow) TDP43 staining showed age-related declines (d). Young (e), elderly (f) and elderly exercised (g) animal immunostaining for nuclear signalling protein small ubiquitin-related modifier 1 (SUMO1). In each case, higher magnification images are in the insets. Sedentary ageing accompanied a significant decline in immunostaining of nuclear TDP43 compared with young but not exercised animals. In sedentary animals, there was a significant increase in immunostaining of SUMO1 (h) compared with both young and exercised animals. Data expressed as mean ± SEM, young n = 5, elderly n = 5 and exercised n = 5, 50 nuclei per animal (scale 20 μm)
Motor cortex
Relative levels of nuclear SUMO1 were measured in neurons of the motor cortex of each of the three groups of mice (Fig. 6). The SUMO1 immunofluorescence showed the same trend as that evident in MNs of the spinal cord. That is, SUMO1 levels increased with sedentary ageing, but the increase was prevented by 4 months of wheel running (Fig. 6h).
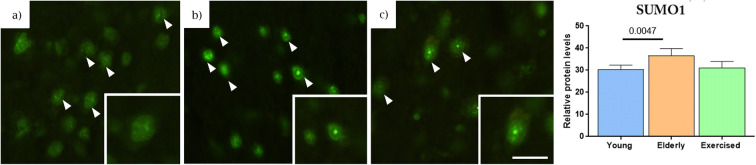
Fig. 6.
Nuclear signalling protein levels in brain tissue. Young (a), elderly (b) and exercised (c) animal immunostaining for SUMO1 (white arrows). In each case, high magnification images are in the insets. Sedentary animals showed a significant increase in immunostaining of SUMO1 (h), compared with young but not exercised animals. Data expressed as mean ± SEM, young n = 5, elderly n = 5 and exercised n = 4, 20 nuclei per animal (scale 20 μm)
Chromatin organization
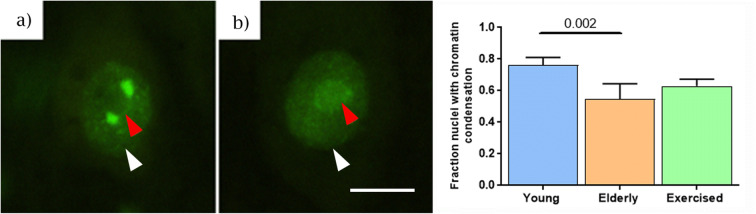
RCC1 immunostaining localized within the nucleus (Fig. 7). Although there was no significant change in relative brightness of RCC1 nuclear staining between young adult (Fig. 7a) and elderly (Fig. 7b) animals (red arrow), a change in the distribution of the stained region was evident, reflective of an altered chromatin distribution (white arrow). Qualitative assessment revealed a significant increase in the reduced chromatin condensation in elderly MNs (Fig. 7). Investigation in exercised animals showed no significant change compared with elderly animals.
Fig. 7.
Qualitative analysis of chromatin-bound RCC1 immunostaining. Assessment of changes in the RCC1 staining between young and elderly animals. There is a significant reduction in RCC1 staining at the chromatin condensations of motoneurons in elderly animals compared with young, with no significant effect of exercise in elderly animals. Data expressed as mean ± SEM, young n = 5, elderly n = 5, elderly exercised n = 5, 50 nuclei per animal
Discussion
This investigation of the effects of age and exercise on spinal cord MN nuclei has three main findings. First, there was an age-related increase in the permeability of nuclei isolated from cells of the spinal cord. Second, ageing featured a change in the levels of nuclear signalling proteins TDP43 and SUMO1 as well as an altered chromatin distribution. Finally, wheel-running exercise from 20 to 24 months reduced the increase in nuclear permeability, as well as the changes to nuclear signalling proteins and chromatin disorganization. These findings suggest that age is associated with increased nuclear permeability and altered chromatin distribution that may contribute in the MN loss previously described in old age. Exercise appears to attenuate these age-related changes in MNs, suggesting that the therapeutic benefit of exercise in sarcopenia may derive at least in part from positive effects in the central nervous system.
Age-related changes in neuronal nuclei
Nuclear permeability
The nuclear envelope provides a diffusion barrier allowing the separation of DNA transcription from mRNA translation. The loss of this barrier can lead to the free exchange of nuclear and cytosolic contents leading to the accumulation of cytosolic proteins in the nucleus and nucleic acids in the cytosol (Lusk and King 2017). Movement of DNA or RNA to the cytosol can activate pattern recognition receptors that elicit an immune response, while cytoplasmic DNAases could enter the nucleus and drive DNA disorganization and damage (Lusk and King 2017). These manifestations may be evident in the current investigation as elderly animals had both significant increases in nuclear permeability and altered distribution of chromatin.
Chromatin disorganization
The appearance of altered chromatin distribution within the nucleus correlated with increased nuclear permeability. Chromatin is normally organized through interactions with the surrounding nuclear envelope. The organization and regional segmentation of the chromatin is driven through reversible histone modifications such as methylation, ATP-dependent remodelling of the nucleosome core, and modifications to histone tails (Espada and Esteller 2010). Disruption or disorganization of this system may result in altered gene transcription and DNA damage (Espada and Esteller 2010; Larrieu et al. 2018). Several conditions, including both normal and early onset ageing, are associated with chromatin disorganization due to loss of heterochromatin anchorage and altered histone modifications (Espada and Esteller 2010; Larrieu et al. 2018). In Hutchinson-Gilford progeria syndrome, the resultant chromatin disorganization can make the DNA susceptible to corruption, and this damage can result in downstream production of mutant and aberrant proteins which ultimately trigger cell death (Madabhushi et al. 2014). RCC1 binds to chromatin (Chatterjee and Paschal 2015), so in this investigation, the pattern of RCC1 immunoreactivity within the nucleus provides a means of detecting overt changes to chromatin distribution. Elderly animal RCC1 staining was more diffuse, which is clear evidence of reduced condensation and is coincident with increased nuclear permeability. While we have no direct causal link between loss of chromatin condensation, increased nuclear permeability and neuron death, other studies have shown that the loss of chromatin stability results in the accumulation of DNA damage and activation of cell death pathways (Warren and Shanahan 2011).
Nuclear signalling proteins
The potential for age-related activation of cell death pathways is further supported by the increased nuclear localisation of SUMO1 in MNs of elderly animals (Fig. 6). SUMO1 is a free-exchange substance (able to traverse the nuclear envelope) that plays important roles in the post-translational modification of several nucleocytoplasmic transport substances including RCC1 and RanGAP1 (Kelley et al. 2011). SUMO1 is involved in multiple cellular pathways of which one is nucleocytoplasmic transport; however, another interaction of particular interest to the current investigation is SUMO1 binding to promyelocytic leukaemia protein (PML) promoting the formation of nuclear PML bodies (Sternsdorf et al. 1997). The PML bodies function as protein scaffolds for correct targeting of multiple proteins including SUMO1 and RanGAP1 within their nuclear domain, as SUMO1 knockout models resulted in reduced PML body formation (Evdokimov et al. 2008; Hofmann and Will 2003). PML body formation has been associated with apoptotic pathway activation, as PML knockout models show defective apoptosis, while PML also co-localizes alongside SUMO1 with the tumour suppressor p53, a key regulator of cellular apoptosis (Hofmann and Will 2003) that facilitates the transcription of pro-apoptotic stress-induced pathways resulting in cell death (Müller et al. 2001). SUMOylation of p53 has been shown to stimulate its transcriptional and pro-apoptotic properties, while this activity can be enhanced through co-factor activation from TP53INP1 in response to oxidative stress (Müller et al. 2001; Peuget et al. 2014). For p53 to exert its transcriptional activity on its target apoptotic genes in response to oxidative stress, it requires interaction with SUMOylated TP53INP1 (Peuget et al. 2014). It therefore appears that SUMO1 acts as an oxidative stress sensor for p53 activation of pro-apoptotic pathways. Thus, in the current investigation, the age-associated increase in nuclear SUMO1 in both upper and lower MNs may promote both PML body formation and activation of apoptotic pathways resulting in cell death in response to increased oxidative stress, and is an indication of increased nuclear permeability resulting in the potential activation of cell death cascades.
One of the well-documented features of ALS MNs is the appearance of cytoplasmic TDP43 aggregates, thought to have both toxic gain and loss of function effects (Dewey et al. 2012). Recently, the mislocalization of TDP-43 to the cytoplasm has been thought to be due in part to altered nuclear permeability (Chou et al. 2018). We investigated the changes evident in both cytoplasmic and nuclear TDP43 levels within elderly MNs, finding significant age-related reductions in nuclear TDP43 levels accompanied by a non-significant increase in cytoplasmic TDP43. Such altered distribution of TDP43 from its nuclear localization to a cytoplasmic one is similar to what occurs in ALS. The presentation of reduced nuclear levels of TDP43 and trending increase in cytoplasmic levels further supports our hypothesis that disrupted regulation and increased permeability of the nuclear envelope permits inappropriate movement of proteins across the nuclear envelope, with this leading to loss of lower MNs in elderly animals.
Ageing features declines in key nuclear gating proteins that correlate with increases in nuclear permeability. The increased nuclear permeability in turn correlates with altered distribution of nuclear transport proteins and upregulation of SUMO1, both of which may trigger activation of cell death pathways in ageing MNs. These changes correlate, and are temporally coincident with previously described the loss of MNs (Gillon et al. 2018; Tomlinson and Irving 1977), suggesting that changes at the MN nucleus may be a primary driver of MN neurodegeneration and thereby a secondary driver of the muscle fibre denervation, cardinal features of sarcopenia. These findings also highlight several parallels shared between sarcopenia and other neurodegenerative conditions including ALS, Parkinson’s disease, frontotemporal lobe dementia and Alzheimer’s disease. Each of these neurodegenerative diseases has individually been associated with altered nucleocytoplasmic transport, impaired nuclear permeability, mislocalization of protein aggregates and neuron death (Kim and Taylor 2017; Li and Lagier-Tourenne 2018; Woulfe 2008). Although the underpinning manifestation of sarcopenia and these neurodegenerative conditions may differ, our results highlight the potential for a common pathophysiological mechanisms.
Effects of exercise
Nuclear permeability
Although the current investigation showed that exercise did not significantly reduce the age-related increase in nuclear permeability (Fig. 4), exercise did appear to slow the rate at which permeability increased. A similar inhibitory effect was present when assessing lower MN number with age, which showed that endurance exercise from 20 to 24 months slowed or inhibited MN loss (Gillon et al. 2018).
The difficulty with assessing the effect of exercise on nuclear permeability is that the point at which nuclei are becoming “more” permeable, or permeable to the point of pathology, is unknown. Based on our previous (Gillon et al. 2018) and current research, it seems likely that this process is happening over a prolonged period and accelerates into older age. Counts of human MNs suggest that cell loss is well underway by age 60 (Tomlinson and Irving 1977). By the use of lifespan equivalence data, we speculate that similar neuronal loss in mice has begun by about 18 months, so if elevated nuclear permeability is causally related to MN loss, it is likely also to be in progress by 18 months. Understanding how prior to 18 months (beginning of MN loss) increases in nuclear permeability are evident compared with “normal” homeostatic levels will significantly alter our interpretation of how beneficial exercise is at inhibiting nuclear permeability. As the animals in the current investigation were given access to running wheels at 20 months, it is likely that some MN nuclei will have become permeable and resulted in MN loss by that time, while other MN nuclei are likely to have initiated the events that ultimately lead to their loss. Based on data from apoptotic cell death which shows that once “cell” permeability is initiated, the cell is committed cell to die, we suggest that increased nuclear permeability will also trigger cell death (Kim et al. 2003). Investigations in models of ALS have shown similar results, in which neurons with impaired nucleocytoplasmic transport feature increased nuclear permeability correlated with neurodegenerative progression (Kim and Taylor 2017; Li and Lagier-Tourenne 2018). If increased nuclear permeability is a potentially irreversible process, cells with increased nuclear permeability prior to the beginning of the exercise protocol may not be “rescued” by exercise, and so ongoing cell loss after initiation of exercise may mask some of the potential benefits of aerobic exercise. This also suggests that earlier implementation of an exercise regimen is likely to be more beneficial in regard to prevention of MN loss.
Nuclear signalling proteins and chromatin disorganization
Similar to the effects of exercise on lower MN number previously described (Gillon et al. 2018) and on nuclear permeability in the current investigation, exercise in elderly animals inhibited the TDP43 and SUMO1 mislocalization and chromatin disorganization that occurs with age. Along with our previously published data showing that exercise slows the loss of essential NPC proteins and the loss of MNs (Gillon et al. 2018), the current results show that the age-related loss of nuclear pore proteins that leads to increased nuclear permeability and mislocalization of TDP43/SUMO is also slowed or inhibited by regular exercise in late life.
How does exercise slow or prevent this cascade of disruption to the nuclear pore that appears to be a feature of sedentary ageing? Ageing is associated with increased oxidative stress due to increased reactive oxygen species (ROS) production and reduced antioxidant defence due to reduced protein synthesis (Sallam and Laher 2016). The increased ROS and oxidative stress results in macromolecule damage and initiation of inflammatory pathways resulting in the activation of pro-inflammatory mediators and inflammatory pathways that have deleterious effects on ageing cells (Sallam and Laher 2016). Although multiple studies have shown that exercise increases the levels of ROS present (Davies et al. 1982; Higuchi et al. 1985; Poulsen et al. 1996), recent literature has suggested that exercise-induced increases in oxidative stress are actually a necessary part of cellular adaptation, including activation of cell signalling pathways and optimal force production in skeletal muscle, which suggests that the benefits associated with exercise are more likely due to increases in antioxidants (Powers and Jackson 2008). Exercise-induced increases in antioxidant enzymes including superoxide dismutase (SOD) and glutathione peroxidase are well documented to show activity-dependent responses to bouts of endurance exercise (Higuchi et al. 1985): (Elosua et al. 2003). It has also been shown to reduce pro-inflammatory markers such as IL-6 and TNF-α through reduction of adipose body fat, one of the main points of release of pro-inflammatory markers (Esposito et al. 2006). Exercise therefore appears to have roles in both reducing release of pro-inflammatory markers and stimulating upregulation of the antioxidant defence.
As well as pro-inflammatory and antioxidant effects, exercise has long been associated with inducing activity-dependent release of substances (e.g. neurotrophins) considered to be important to the survival and/or maintenance of the MN (Funakoshi et al. 1995; Vinel et al. 2018). Interestingly, research has shown that the antioxidant defence is upregulated in response to neurotrophic signalling (Mattson et al. 1995), suggesting a potential mechanism linking exercise to antioxidant defence within the neuromuscular system. We propose that increased nuclear permeability arising from reduction of key nucleocytoplasmic transport proteins (Nup93, Nup98) (Gillon et al. 2018) is caused by oxidative stress that modifies these key nuclear components. Exercise inhibits this Nup modification (Gillon et al. 2018), potentially as a result of activity-dependent release of neurotrophins from the muscle and their retrograde transport back to the MN cell body. Similar roles for neurotrophins are proposed in rat hippocampal cells undergoing glutamate-induced excitotoxicity (Mattson et al. 1995), where the hippocampal cells featured calcium-induced peroxide accumulation that was attenuated by the induction of the antioxidant defence by brain-derived neurotrophic factor (Mattson et al. 1995). Furthermore, reduced levels of neurotrophins within the neuron cell body have been associated with increased free radical production and induction of apoptotic pathways (Miller et al. 2002). Given the impact of exercise on nuclear permeability in the current study, we suggest the beneficial effects may be a result of both activity-dependent release of antioxidant species, and further antioxidant release in response to neurotrophin signalling from the muscle.
Conclusion
The current investigation shows that the previously described loss of long-lived nuclear pore proteins from the nuclei of old mouse lower MNs is associated with increased nuclear permeability, mislocalization of nuclear signalling proteins and altered chromatin organization. Further, we show that regular late life wheel-running exercise prevents or slows these changes. Taken together, our recent findings suggest that loss of the integrity of the nuclear envelope in late life results in increased nuclear permeability with consequential dysregulation of important cell survival and regulatory processes leading to cell death. Our data also suggests that the muscle-protecting benefits of exercise in the elderly may derive partly from inhibition of these changes at the nuclear envelope, which extends the lifespan of MNs and slows the progression of motor nerve terminal withdrawal, muscle denervation atrophy and consequential weakness arising from premature loss of MNs.
Electronic supplementary material
Permeability controls. A series of control experiments was conducted to validate the technique used in the current investigation (Fig. S1). First, both brain tissue homogenate and clear microspheres (impermeable) were incubated in 70 kDa dextran, and the infiltration measured. Second, nuclear fraction isolated from spinal cord homogenate was incubated in large fluorescent microspheres (0.24 μm, much larger than the nuclear pore) to determine whether nuclei retained any barrier. (PNG 222 kb)
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ashley Gillon, Email: ashgillon@gmail.com.
Philip Sheard, Email: phil.sheard@otago.ac.nz.
References
- Ballak SB, Degens H, de Haan A, Jaspers RT. Aging related changes in determinants of muscle force generating capacity: a comparison of muscle aging in men and male rodents. Ageing Res Rev. 2014;14:43–55. doi: 10.1016/j.arr.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Barns M, Gondro C, Tellam RL, Radley-Crabb HG, Grounds MD, Shavlakadze T (2014) Molecular analyses provide insight into mechanisms underlying sarcopenia and myofibre denervation in old skeletal muscles of mice The International Journal of Biochemistry & Cell Biology 53:174-185 [DOI] [PubMed]
- Bhattacharya A, et al. Neuromuscular function during aging is protected in baicalein-treated C57BL/6 mice. FASEB J. 2019;33:651.652–651.652. [Google Scholar]
- Boeynaems S, Bogaert E, Van Damme P, Van Den Bosch L (2016) Inside out: the role of nucleocytoplasmic transport in ALS and FTLD ActaNeuropathologica 132:159-173 [DOI] [PMC free article] [PubMed]
- Brady J, Sheard P (2015) Implementation of internal intensity controls for semi-quantitative immunohistochemistry. In: New Zealand Microscopy Society, Dunedin
- Bronikowski AM, et al. Lifelong voluntary exercise in the mouse prevents age-related alterations in gene expression in the heart. Physiol Genomics. 2003;12:129. doi: 10.1152/physiolgenomics.00082.2002. [DOI] [PubMed] [Google Scholar]
- Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS One. 2011;6:e28090. doi: 10.1371/journal.pone.0028090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Paschal BM. Disruption of the Ran system by cysteine oxidation of the nucleotide exchange factor RCC1. Mol Cell Biol. 2015;35:566–581. doi: 10.1128/MCB.01133-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C-C, Zhang Y, Umoh ME, Vaughan SW, Lorenzini I, Liu F, Sayegh M, Donlin-Asp PG, Chen YH, Duong DM, Seyfried NT, Powers MA, Kukar T, Hales CM, Gearing M, Cairns NJ, Boylan KB, Dickson DW, Rademakers R, Zhang YJ, Petrucelli L, Sattler R, Zarnescu DC, Glass JD, Rossoll W. TDP-43 pathology disrupts nuclear pore complexes and nucleocytoplasmic transport in ALS/FTD. Nat Neurosci. 2018;21:228–239. doi: 10.1038/s41593-017-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT, Plowey ED, Wang Y, Patel V, Jordan-Sciutto KL. Location, location, location: altered transcription factor trafficking in neurodegeneration. J Neuropathol Exp Neurol. 2007;66:873–883. doi: 10.1097/nen.0b013e318156a3d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ et al. (2018) European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2, Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing, 48: 16–31
- D'Angelo MA, Raices M, Panowski SH, Hetzer MW. Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell. 2009;136:284–295. doi: 10.1016/j.cell.2008.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TM (2016) The book of R: a first course in programming and statistics. Network Security, Vol 9. William Pollock
- Davies KJA, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey CM, Cenik B, Sephton CF, Johnson BA, Herz J, Yu G. TDP-43 aggregation in neurodegeneration: are stress granules the key? Brain Res. 2012;1462:16–25. doi: 10.1016/j.brainres.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152:244–248. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Elosua R, Molina L, Fito M, Arquer A, Sanchez-Quesada JL, Covas MI, Ordoñez-Llanos J, Marrugat J. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis. 2003;167:327–334. doi: 10.1016/s0021-9150(03)00018-2. [DOI] [PubMed] [Google Scholar]
- Espada J, Esteller M. DNA methylation and the functional organization of the nuclear compartment. Semin Cell Dev Biol. 2010;21:238–246. doi: 10.1016/j.semcdb.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Esposito K, Giugliano G, Scuderi N, Giugliano D. Role of adipokines in the obesity–inflammation relationship: the effect of fat removal. Plast Reconstr Surg. 2006;118:1048–1057. doi: 10.1097/01.prs.0000232281.49432.ce. [DOI] [PubMed] [Google Scholar]
- Evdokimov E, Sharma P, Lockett SJ, Lualdi M, Kuehn MR. Loss of SUMO1 in mice affects RanGAP1 localization and formation of PML nuclear bodies, but is not lethal as it can be compensated by SUMO2 or SUMO3. J Cell Sci. 2008;121:4106. doi: 10.1242/jcs.038570. [DOI] [PubMed] [Google Scholar]
- Feligioni M, Marcelli S, Knock E, Nadeem U, Arancio O, Fraser PE (2015) SUMO modulation of protein aggregation and degradation vol 2. vol 4. AIMs Molecular Science 2(4): 382-410
- Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Gillon A, Nielsen K, Steel C, Cornwall J, Sheard P. Exercise attenuates age-associated changes in motoneuron number, nucleocytoplasmic transport proteins and neuromuscular health. GeroScience. 2018;40:177–192. doi: 10.1007/s11357-018-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH et al. (2006) The Loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 61:1059-1064 [DOI] [PubMed]
- Higuchi M, L-j C, Chen M, Holloszy JO (1985) Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol 40:281–286 [DOI] [PubMed]
- Hofmann TG, Will H. Body language: the function of PML nuclear bodies in apoptosis regulation. Cell Death Differ. 2003;10:1290. doi: 10.1038/sj.cdd.4401313. [DOI] [PubMed] [Google Scholar]
- Jovicic A et al. (2015) Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS Nature Neuroscience 18:1226-1229 [DOI] [PMC free article] [PubMed]
- Jovičić A, Paul JW, Gitler AD. Nuclear transport dysfunction: a common theme in amyotrophic lateral sclerosis and frontotemporal dementia. J Neurochem. 2016;138:134–144. doi: 10.1111/jnc.13642. [DOI] [PubMed] [Google Scholar]
- Kelley JB, et al. The defective nuclear lamina in Hutchinson-gilford progeria syndrome disrupts the nucleocytoplasmic Ran gradient and inhibits nuclear localization of Ubc9. Mol Cell Biol. 2011;31:3378–3395. doi: 10.1128/MCB.05087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Taylor JP. Lost in transportation: nucleocytoplasmic transport defects in als and other neurodegenerative diseases. Neuron. 2017;96:285–297. doi: 10.1016/j.neuron.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-S, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- Larrieu D, Viré E, Robson S, Breusegem SY, Kouzarides T, Jackson SP (2018) Inhibition of the acetyltransferase NAT10 normalizes progeric and aging cells by rebalancing the Transportin-1 nuclear import pathway. Sci Signal eaar5401 11(537) [DOI] [PMC free article] [PubMed]
- Larsson L, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. 2018;99:427–511. doi: 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lagier-Tourenne C. Nuclear pores: the gate to neurodegeneration. Nat Neurosci. 2018;21:156–158. doi: 10.1038/s41593-017-0066-0. [DOI] [PubMed] [Google Scholar]
- Lightfoot JT, Turner MJ, Daves M, Vordermark A, Kleeberger SR. Genetic influence on daily wheel running activity level. Physiol Genomics. 2004;19:270–276. doi: 10.1152/physiolgenomics.00125.2004. [DOI] [PubMed] [Google Scholar]
- Løvtrup-Rein H, McEwen BS. Isolation and fractionation of rat brain nuclei. J Cell Biol. 1966;30:405–415. doi: 10.1083/jcb.30.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann N Y Acad Sci. 1998;854:92–101. doi: 10.1111/j.1749-6632.1998.tb09895.x. [DOI] [PubMed] [Google Scholar]
- Lusk CP, King MC. The nucleus: keeping it together by keeping it apart. Curr Opin Cell Biol. 2017;44:44–50. doi: 10.1016/j.ceb.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madabhushi R, Pan L, Tsai LH. DNA damage and its links to neurodegeneration. Neuron. 2014;83:266–282. doi: 10.1016/j.neuron.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkowskyj KA, Cox R, Jensen RT, Benya RV. Quantitative immunohistochemistry by measuring cumulative signal strength accurately measures receptor number. J Histochem Cytochem. 2003;51:205–214. doi: 10.1177/002215540305100209. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- McMahon CD, et al. Lifelong exercise and locally produced insulin-like growth factor-1 (IGF-1) have a modest influence on reducing age-related muscle wasting in mice. Scand J Med Sci Sports. 2014;24:e423–e435. doi: 10.1111/sms.12200. [DOI] [PubMed] [Google Scholar]
- Miller JM, Miller AL, Yamagata T, Bredberg G, Altschuler RA. Protection and regrowth of the auditory nerve after deafness: neurotrophins, antioxidants and depolarization are effective in vivo. Audiol Neurotol. 2002;7:175–179. doi: 10.1159/000058306. [DOI] [PubMed] [Google Scholar]
- Müller S, Hoege C, Pyrowolakis G, Jentsch S. Sumo, ubiquitins mysterious cousin. Nat Rev Mol Cell Biol. 2001;2:202. doi: 10.1038/35056591. [DOI] [PubMed] [Google Scholar]
- Nonaka T, et al. Prion-like properties of pathological TDP-43 aggregates from diseased brains. Cell Rep. 2013;4:124–134. doi: 10.1016/j.celrep.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M, Okazaki H, Nishimoto T. The RCC1 protein, a regulator for the onset of chromosome condensation locates in the nucleus and binds to DNA. J Cell Biol. 1989;109:1389. doi: 10.1083/jcb.109.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuget S, Bonacci T, Soubeyran P, Iovanna J, Dusetti NJ. Oxidative stress-induced p53 activity is enhanced by a redox-sensitive TP53INP1 SUMOylation. Cell Death Differ. 2014;21:1107. doi: 10.1038/cdd.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen HE, Loft S, Vistisen K. Extreme exercise and oxidative DNA modification. J Sports Sci. 1996;14:343–346. doi: 10.1080/02640419608727720. [DOI] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT (2012) Denervation causes fiber atrophy and myosin heavy chainco-expression in senescent skeletal muscle PLoS One 7:e29082 [DOI] [PMC free article] [PubMed]
- Sallam N, Laher I. Exercise modulates oxidative stress and inflammation in aging and cardiovascular diseases. Oxidative Med Cell Longev. 2016;2016:32. doi: 10.1155/2016/7239639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas JN, Toyama BH, Xu T, Yates JR, Hetzer MW (2012) Extremely long-lived nuclear pore proteins in the rat brain Science 335:942-942 [DOI] [PMC free article] [PubMed]
- Sheard PW, Anderson RD (2012) Age-related loss of muscle fibres is highly variable amongst mouse skeletal muscles Biogerontology 13:157-167 [DOI] [PubMed]
- Shi S-R, Cote RJ, Taylor CR. Antigen retrieval techniques: current perspectives. J Histochem Cytochem. 2001;49:931–937. doi: 10.1177/002215540104900801. [DOI] [PubMed] [Google Scholar]
- Sternsdorf T, Jensen K, Will H. Evidence for covalent modification of the nuclear dot–associated proteins PML and Sp100 by PIC1/SUMO-1. J Cell Biol. 1997;139:1621. doi: 10.1083/jcb.139.7.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantirigama MLS, Oswald MJ, Clare AJ, Wicky HE, Day RC, Hughes SM, Empson RM. Fezf2 expression in layer 5 projection neurons of mature mouse motor cortex. J Comp Neurol. 2016;524:829–845. doi: 10.1002/cne.23875. [DOI] [PubMed] [Google Scholar]
- Toki MI, Cecchi F, Hembrough T, Syrigos KN, Rimm DL. Proof of the quantitative potential of immunofluorescence by mass spectrometry. Lab Investig. 2017;97:329–334. doi: 10.1038/labinvest.2016.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34:213–219. doi: 10.1016/0022-510x(77)90069-7. [DOI] [PubMed] [Google Scholar]
- Toyama BH, Savas JN, Park SK, Harris MS, Ingolia NT, Yates Iii JR, Hetzer MW. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G. Effects of disease-afflicted and aging neurons on the musculoskeletal system. Bone. 2019;122:31–37. doi: 10.1016/j.bone.2019.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Kang H, Clemenson GD, Gage F, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinel C, et al. The exerkine apelin reverses age-associated sarcopenia. Nat Med. 2018;24:1360–1371. doi: 10.1038/s41591-018-0131-6. [DOI] [PubMed] [Google Scholar]
- Warren DT, Shanahan CM. Defective DNA-damage repair induced by nuclear lamina dysfunction is a key mediator of smooth muscle cell aging. Biochem Soc Trans. 2011;39:1780–1785. doi: 10.1042/BST20110703. [DOI] [PubMed] [Google Scholar]
- Woulfe J. Abnormalities of the nucleus and nuclear inclusions in neurodegenerative disease: a work in progress. Neuropathol Appl Neurobiol. 2007;33:2–42. doi: 10.1111/j.1365-2990.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- Zhang K et al. (2015) The C9orf72 repeat expansion disrupts nucleocytoplasmic transport Nature 525:56-61 [DOI] [PMC free article] [PubMed]
- Woulfe J. Nuclear bodies in neurodegenerative disease. Biochim Biophys Acta (BBA) - Mol Cell Res. 2008;1783:2195–2206. doi: 10.1016/j.bbamcr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Zhang K, et al. Stress granule assembly disrupts nucleocytoplasmic transport. Cell. 2018;173:958–971.e917. doi: 10.1016/j.cell.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Permeability controls. A series of control experiments was conducted to validate the technique used in the current investigation (Fig. S1). First, both brain tissue homogenate and clear microspheres (impermeable) were incubated in 70 kDa dextran, and the infiltration measured. Second, nuclear fraction isolated from spinal cord homogenate was incubated in large fluorescent microspheres (0.24 μm, much larger than the nuclear pore) to determine whether nuclei retained any barrier. (PNG 222 kb)