Summary
Dehydrodolichyl diphosphate synthase (DHDDS) catalyzes the committed step in dolichol synthesis. Recessive mutations in DHDDS cause retinitis pigmentosa (RP59), resulting in blindness. We hypothesized that rod photoreceptor-specific ablation of Dhdds would cause retinal degeneration due to diminished dolichol-dependent protein N-glycosylation. Dhddsflx/flx mice were crossed with rod-specific Cre recombinase-expressing (Rho-iCre75) mice to generate rod-specific Dhdds knockout mice (Dhddsflx/flx iCre+). In vivo morphological and electrophysiological evaluation of Dhddsflx/flx iCre+ retinas revealed mild retinal dysfunction at postnatal (PN) 4 weeks, compared with age-matched controls; however, rapid photoreceptor degeneration ensued, resulting in almost complete loss of rods and cones by PN 6 weeks. Retina dolichol levels were markedly decreased by PN 4 weeks in Dhddsflx/flx iCre+ mice, relative to controls; despite this, N-glycosylation of retinal proteins, including opsin (the dominant rod-specific glycoprotein), persisted in Dhddsflx/flx iCre+ mice. These findings challenge the conventional mechanistic view of RP59 as a congenital disorder of glycosylation.
Subject Areas: Genetics, Cell Biology
Graphical Abstract

Highlights
-
•
Deletion of Dhdds in rod cells caused rapid retinal degeneration in mice
-
•
Retinal dolichol levels markedly decreased before onset of degeneration
-
•
Protein N-glycosylation was uncompromised despite Dhdds deletion
-
•
Degeneration also involved gliosis, microglial activation, and phagoptosis
Genetics; Cell Biology
Introduction
Retinitis pigmentosa (RP) represents a large class of inherited retinal dystrophies caused by mutations in several families of genes, leading to pigmentary retinopathy and progressive, irreversible blindness. Typically, RP is characterized by the initial loss of rod photoreceptors (PRs), deposition of pigment granules, and peripheral vision loss (Ferrari et al., 2011, Hamel, 2006). Defective asparagine-linked glycosylation (N-glycosylation) of proteins in rod cells, particularly the visual pigment rhodopsin (RHO), results in progressive, irreversible rod cell degeneration and death, with concomitant loss of vision (Murray et al., 2009, Murray et al., 2015, Kaushal et al., 1994, Fliesler et al., 1984a). Successful glycosylation of RHO is necessary for its vectorial trafficking through the inner segment (cell body) of the rod cell to the site of rod outer segment (ROS) membrane assembly at the base of the ROS. Retinal degeneration has been observed in patients harboring RHO mutations involving the N-glycosylation consensus sites, and in animal models involving comparable RHO mutations (Van Den Born et al., 1994, Zhu et al., 2004, Sullivan et al., 1993, Murray et al., 2015, Iwabe et al., 2016), as well as by tunicamycin-induced and genetic inhibition of global/RHO N-glycosylation (Fliesler and Basinger, 1985, Fliesler et al., 1985, Sabry et al., 2016, Thompson et al., 2013, Murray et al., 2015). Protein N-glycosylation involves the following steps (schematic representation, Figure 1): generation of dolichol (Dol, an important isoprenoid arising from the mevalonate pathway) and dolichyl phosphate (Dol-P, the obligate glycan carrier necessary for N-linked glycosylation, O-mannosylation, and C-mannosylation) (Burda and Aebi, 1999, Endo et al., 2003, Park et al., 2014, Cantagrel et al., 2010, Burton et al., 1979, Maeda et al., 2000, Doucey et al., 1998), generation of complex Dol-P-linked oligosaccharides (DLO) (Krasnova and Wong, 2016, Gandini et al., 2017, Behrens and Leloir, 1970), and transfer of those oligosaccharides from DLO to the N-glycosylation consensus site on the target polypeptide (Welply et al., 1983). Genetic defects affecting the glycosylation mechanism constitute a large family of syndromes termed “congenital disorders of glycosylation” (CDGs), with more than 150 causative genes (Ng and Freeze, 2018, Sparks and Krasnewich, 1993). A family of genetic diseases pertaining to Dol synthesis is classified as CDG-I (the class of CDG involving defective glycan assembly and/or their transfer in the endoplasmic reticulum [ER]) due to the requirement of DLO for N-glycosylation. Common clinical features of CDGs include failure to thrive, retarded development, protein-losing enteropathy, early-onset encephalopathy, as well as retinopathies such as RP (Sparks and Krasnewich, 1993, Thompson et al., 2013, Hamdan et al., 2017, Morava et al., 2009).
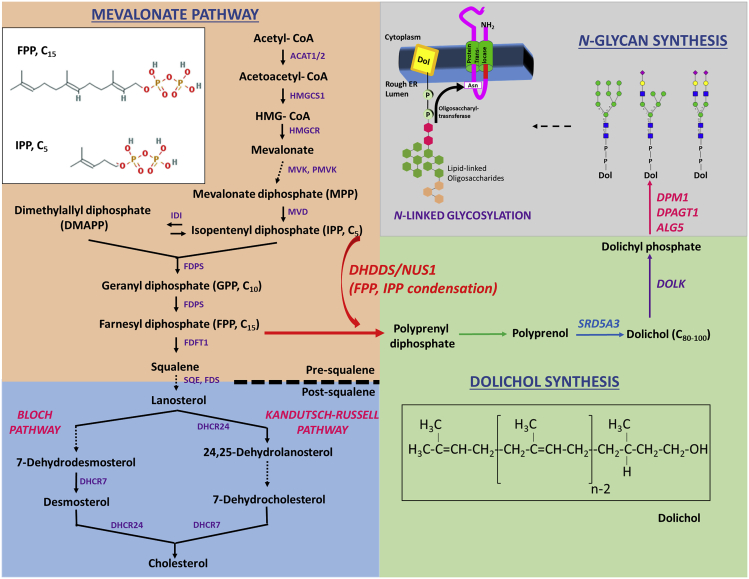
Figure 1.
Schematic Representation of the Mevalonate Pathway, Dolichol Synthesis, and the Requirement of Dolichyl Phosphate (Dol-P) for N-Linked Oligosaccharide Generation
Farnesyl diphosphate (FPP), an important pre-squalene intermediate of the mevalonate pathway, undergoes DHDDS/NUS1-catalyzed condensation with multiple isopentenyl pyrophosphate (IPP) units to generate polyprenyl pyrophosphate (diphosphate), and ultimately the obligate glycan carrier, Dol-PP. Gene products involved in synthesis of dolichol and Dol-P-saccharide/oligosaccharide intermediates, and hence implicated in CDG-1 (Congenital Disorders of Glycosylation), have been represented. The enzymatic activity provided by DHDDS and NUS1 catalyzes the commitment step of dolichol synthesis; hence mutations in either DHDDS or NUS1 are hypothesized to block protein N-glycosylation, and are classified as CDG-I. Dolichol (structure in inset), is an acyclic isoprenoid consisting of 18–21 isoprene units.
Two key intermediate metabolites in the mevalonate pathway, farnesyl pyrophosphate (FPP; also called farnesyl diphosphate) and isopentenyl pyrophosphate (IPP; also called isopentyl diphosphate), serve as precursors for squalene, cholesterol, and Dol synthesis (see Figure 1) (Grabowska et al., 1998). The committed enzymatic step in the pathway toward Dol synthesis is the catalytic condensation of FPP with multiple IPP molecules to form polyprenyl diphosphate; this reaction is catalyzed by a cis-prenyltransferase (CPT) complex composed of dehydrodolichyl diphosphate synthase (DHDDS) and Nogo-B receptor (NgBR; gene symbol NUS1) (Park et al., 2014). Polyprenyl diphosphate sequentially undergoes dephosphorylation (action of dolichyl diphosphate phosphatase 1 [DOLPP1]) (Rush et al., 2002), reduction (action of steroid Δ5 alpha-reductase 3 [SRD5A3]) (Cantagrel et al., 2010), and phosphorylation (action of dolichol kinase [DOLK]) (Shridas and Waechter, 2006) to generate Dol-P (Schenk et al., 2001). Cellular de novo synthesis and availability of Dol-P in the ER (Heesen et al., 1994, Wu et al., 2003, Ashida et al., 2006) are necessary for DLO-dependent protein glycosylation (Jakobsson et al., 1989). Genetic defects affecting Dol-P de novo synthesis at the level of DHDDS, NgBR, SRD5A3, or DOLK are classified within the CDG-1 subgroup of CDGs (Sparks and Krasnewich, 1993, Park et al., 2014).
Heretofore, animal model or in vitro studies have employed approaches such as inhibition of the Dol-P-dependent oligosaccharyltransferase (OST, e.g., using tunicamycin) (Fliesler et al., 1984a, Fliesler et al., 1984b, Fliesler et al., 1985, Fliesler and Basinger, 1985) or mutation of the glycosylation consensus sites on specific target proteins (e.g., rhodopsin; Murray et al., 2015) to evaluate the requirement of protein N-glycosylation in the retina. Herein, we addressed this requirement by genetic blockade of the rate-limiting step in dolichol synthesis, i.e., the cis-prenyltransferase (CPT) activity of DHDDS.
Recent studies involving genetic screening of families with a rare autosomal recessive form of retinitis pigmentosa (RP59) demonstrated a founder missense mutation (K42E) in the DHDDS gene (Zelinger et al., 2011, Zuchner et al., 2011). Two other rare, pathogenic DHDDS mutations (T206A, R98W), both found heterozygously with the K42E mutation, also have been reported (Wen et al., 2013, Biswas et al., 2017, Kimchi et al., 2018). In patients homozygous for the K42E mutation, the peripheral retina underwent thinning, but the cone-rich fovea is well-preserved. Functionally, in one patient examined, dark-adapted (“scotopic,” rod-driven) electroretinogrphic (ERG) responses were extinguished by 21–24 years of age, leading to a visual field limited to a small central island, consistent with rod-cone dystrophy or early-onset retinal degeneration. In an infant presenting as a compound heterozygote (W64X and p.Cys148GlufsX11), ERG responses were undetectable at 2 months of age, but the patient died at 7 months of age from multiple systemic complications due to insufficient DHHDS activity (Sabry et al., 2016), and thus is distinctly different from the K42E phenotype. The mutations in the “severe” phenotype infant led to loss of FPP/IPP-binding sites, much similar to the excision of Dhdds Exon 3 used in the current study (see Figures S1 and S2; Sabry et al., 2016). Retinal degeneration also has been observed in patients of Romani origin harboring mutations in the gene coding for the DHDDS-heterodimeric partner NgBR (Park et al., 2014). The pathological mechanisms and defective cell processes attributed to RP59, although currently hypothesized to be hypoglycosylation driven, remain to be directly tested. This is largely due to the lack of a validated vertebrate animal model of RP59 to evaluate the link between mammalian cis-prenyltransferase DHDDS activity, its requirement for protein N-glycosylation, and the establishment and preservation of normal retinal structure and function. Up until recently, only a zebrafish Dhdds knockdown model has been reported (Wen et al., 2014; Zuchner et al., 2011). However, more recently, a report of a K42E knock-in mouse model was published (Ramachandra Rao et al., 2020), but dolichol levels and cis-prenyltransferase activity were not examined.
Given this information, we hypothesized that PR-specific deletion of Dhdds would elicit a primary PR degeneration owing to the critical requirement of protein N-glycosylation for maintenance of PR structure, function, and viability. In the current study, we generated a novel murine, rod PR-specific Dhdds knockout model on a C57BL/6J background and examined the functional, morphological, and biochemical consequences to the retina. Contrary to expectation, although a profound retinal degeneration was observed, we found no evidence for defective protein N-glycosylation in this mouse model, despite confirmed ablation of Dhdds in essentially the entire population of retinal rod cells.
Results
Verification of Rod Photoreceptor-Specific Dhdds Deletion
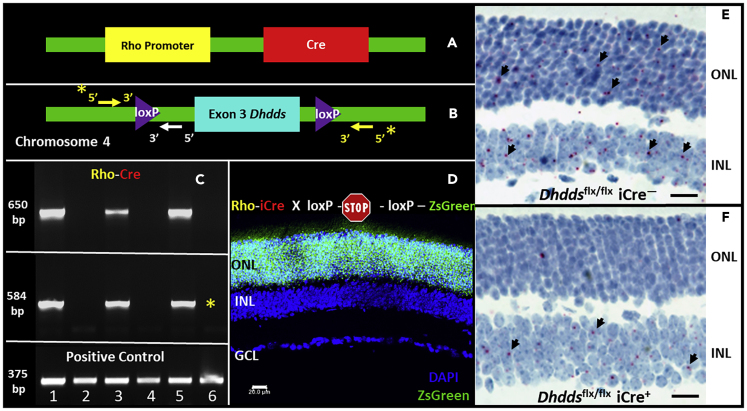
A graphical representation of the genotyping strategy utilized for the verification of Dhddsflx/flx iCre+ mice is provided in Figures S4, 2A, and 2B. Exon 3, coding for critical FPP- and IPP-binding sites, was chosen for targeted deletion (Figures S2 and S3). Initially, tail snip genotyping analysis was performed to detect floxed Dhdds allele and Rho-iCre transgenes: Dhdds allele with loxP modification and wild-type (WT) Dhdds allele yielded 393-bp and 517-bp PCR products, respectively (Figures S4A and S4C). Similarly, Rho-iCre transgene yielded a 650-bp PCR product, as shown previously (Figure S4B) (Li et al., 2005). To further validate excision of the floxed Dhdds allele in rod PRs, genomic DNA extracted from Dhddsflx/flx iCre− (lanes 2, 4, 6 in Figure 2C) and Dhddsflx/flx iCre+ retinas (lanes 1, 3, 5 in Figure 2C) (n = 3/group) (already verified by tail snip genotyping as demonstrated in Figure S4A and S4B) was subjected to PCR amplification using intronic primers designed upstream and downstream of the targeted exon (see Figures 2B and S4C). Genomic material from tail snip genotype-verified Dhddsflx/flx iCre+ retinas yielded a 650-bp PCR product for Cre recombinase, as previously reported (Li et al., 2005) (top panel, Figure 2C), as well as a 584-bp product that corresponds to the remnant intronic region upon excision of exon 3 (middle panel, Figure 2C) (see Methods for primer details, and Discussion for rationale). Dhdds WT allele (arising from other retinal cell types) would yield a predicted, larger (1.4 kbp) product (consisting of exon 3 as well as the flanking intronic regions) that is not amplified by a PCR protocol involving a short extension period of 45 s (See Methods) (Figures 2C and S4C). A non-specific, unaltered gene region (Dhcr7, 375-bp product) was PCR amplified as a positive control to verify genomic DNA quality (bottom panel, Figure 2C).
Figure 2.
Verification of Rod Photoreceptor-Specific Deletion of Dhdds
(A–E) Rod-specific ablation of Dhdds was achieved by generating a mouse model harboring homozygous loxP sites flanking Dhdds exon 3 (gene locus: murine chromosome 4, 66.47 cM] (A) and cross-breeding against a transgenic mouse line consisting of opsin promoter-driven Cre recombinase (Rho-iCre75, (B)). The cross ultimately generated the knockout line of interest: Dhddsflx/flx iCre+. Dhddsflx/flx iCre+ mice were first identified using tail snip genotyping (see also Figure S4). To verify photoreceptor-specific gene excision, whole retinas harvested from mice identified as Dhddsflx/flx iCre+ and age-matched WT controls were subjected to direct tissue genotyping (C, see also Figure S4 for primer design strategy). Retinas from Dhddsflx/flx iCre+ mice yielded short PCR product (584 bp, upper panel, (C) corresponding to the flanking intronic region only (whitearrow, B), while positive for Rho-iCre transgene (middle panel, C). Control retinal tissue, negative for Cre recombinase (upper panel, C), did not exhibit excision of exon 3 (middle panel, C). PCR reaction against a housekeeping gene (Dhcr7) demonstrates intact genomic content in all biological samples (bottom panel, C). Direct tissue genotyping targeting Cre recombinase does not provide morphological context regarding its expression or activity. Rho-iCre75 line was crossed with a ZsGreen reporter mouse: a representative confocal micrograph (D) shows ZsGreen expression exclusively in majority of cells (overwhelmingly rods) in the photoreceptor layer at PN 4 weeks (scale bar, 20 μm). In situ hybridization with a probe corresponding to exon 3 of Dhdds suggests successful ablation of Dhdds in Dhddsflx/flx iCre+ mice (F), when compared with Dhddsflx/flx iCre− mice (E). (Scale bars: 20 μm in (E and F)).
Rod PR-specific excision of Dhdds exon 3 is a function of spatiotemporal expression and activity of Cre recombinase (driven by the Rho promoter) (Li et al., 2005). Cre recombinase activity in Rho-iCre75 mice was verified using a reporter mouse strategy. Briefly, the Rho-iCre75 mouse line was crossed with a ZsGreen Ai6 reporter mouse line (harboring CAG artificial promoter-driven ZsGreen [variant of GFP]) with an upstream floxed transcriptional termination cassette (Figure 2D). Cell type/tissue-specific Cre activity, if present, relieves the ZsGreen transcription blockade by excising the upstream, loxP-modified transcription termination cassette, allowing ZsGreen expression. Retinas harvested from the first filial (F1) generation of the Rho-iCre-ZsGreen Ai6 reporter mouse crossing at PN 15 days (Figure S5) and PN 30 days (lower panel, Figure 2D) were tested for rod-specific ZsGreen expression using laser confocal fluorescence microscopy. Representative fluorescence micrographs (Figure 2D) revealed ZsGreen expression in the vast majority of rod PR nuclei by PN 15 and PN 30 days, in good agreement with the published literature (Li et al., 2005).
Retinal expression of Dhdds transcript was directly tested utilizing customized BaseScope in situ hybridization (ISH) probes (two ZZ probes) designed against a murine Dhdds transcript region corresponding to coding exon 3 (bp 361–455 of NM_026144.4). A single mRNA transcript appears as single, red chromogenic dot; bright-field images from ISH experiments in Dhddsflx/flx iCre+ and Dhddsflx/flx iCre− mice (Figures 2E and 2F, respectively) suggest PR (outer nuclear layer [ONL])-specific loss of Dhdds transcript (tested at PN 30 days), whereas the adjacent inner nuclear layer (INL) retained a comparable level of ISH label as observed in WT control retinas.
Profound and Rapid Retinal Degeneration and Dysfunction Are Observed in Dhddsflx/flx iCre+ Mice
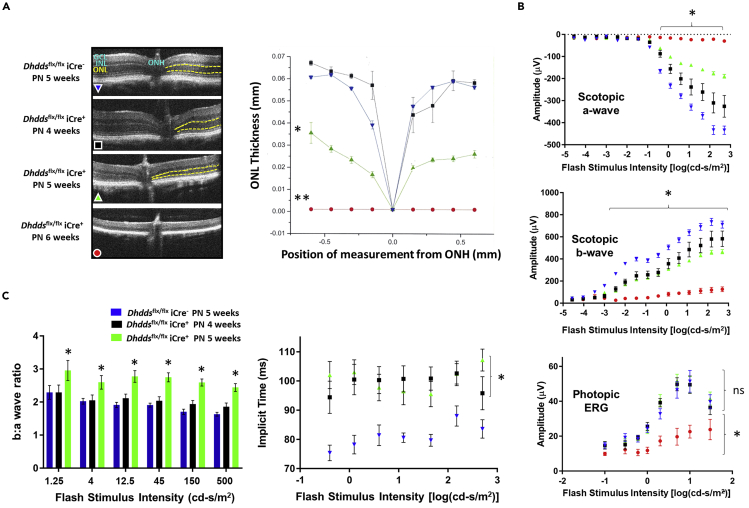
Age-matched Dhddsflx/flx iCre− and Dhddsflx/flx iCre+ mice were subjected to in vivo retinal imaging and quantitative morphometric analysis using spectral domain optical coherence tomography (SD-OCT). ONL thickness was measured (in mm) around the optic nerve head. Figure 3A (left panel) shows a set of representative SD-OCT images of retinas from PN 5-week-old controls versus PN 4-, 5-, and 6-week-old Dhddsflx/flx iCre+ mice. ONL thickness values for PN 4-week-old Dhddsflx/flx iCre+ mice were comparable to those of control mice at PN 5 weeks of age (see Figure 3A, right panel). The ONL in the SD-OCT images appears as a hyporeflective region (demarcated by yellow dotted lines, Figure 3A, left panel). The ONL in Dhddsflx/flx iCre+ mice underwent significant thinning (ca. 50% compared with age-matched controls, n = 4, p < 0.01) by PN 5 weeks and was essentially absent by PN 6 weeks of age (n = 4, p < 0.01). A graphical comparison of ONL thickness (in mm) is provided in Figure 3A (right panel). The SD-OCT data correlate well with results obtained by conventional, paraffin-embedment histological analysis (see Figures S13A and S13B).
Figure 3.
Retinal Thinning and Light Response Deficits in Dhddsflx/flx iCre+Mice
(A, left panel) Retinal thickness in Dhddsflx/flx iCre+ and age-matched controls (n = 4/age group) was measured using SD-OCT. The panel provides representative SD-OCT images (including optic nerve head [ONH]) comparing Dhddsflx/flx iCre− controls with Dhddsflx/flx iCre+ between PN 4 and 6 weeks (color code for experimental groups for all panels has been provided within the OCT panel). The ONL is demarcated by yellow dotted lines. (A, right panel) Graphical representation of the measured ONL thickness (in mm) in Dhddsflx/flx iCre+ mice and age-matched controls. Dhddsflx/flx iCre+ mice exhibit ONL thickness comparable to controls at PN 4 weeks and undergo significant thinning at PN 5 weeks (reduced by 50%) and PN 6 weeks (reduced by 80%–90%) (n = 4).
(B, top and middle panels) Scotopic ERG responses in Dhddsflx/flx iCre+ mice were markedly diminished at all time points tested, relative to age-matched Dhddsflx/flx iCre− controls (flash intensity range: 10−4 to 500 cd-s/m2). Both a- and b-wave amplitude maxima in response to flash stimuli were plotted as a function of flash intensity (top and middle panels, respectively). At very low flash intensities (10−4 cd-s/m2), pure rod response b-wave amplitudes were significantly reduced in all age groups. Both scotopic (rod-driven) a-wave and b-wave responses of PN 6-week-old Dhddsflx/flx iCre+ mice were significantly decreased. (See Figure S6 for representative scotopic ERG traces at saturating flash intensity.) (B, bottom panel) Light-adapted, photopic (cone-driven) ERG responses of Dhddsflx/flx iCre+ mice were comparable to those of controls at PN 4 and 5 weeks, despite reduced photopic responses at those time points. However, Dhddsflx/flx iCre+ photopic ERG responses were minimal at PN 6 weeks and differences were statistically significant compared with age-matched controls.
(C, left panel) Scotopic b:a wave magnitude ratios were significantly increased at all flash intensities ≥1.25 cd-s/m2 in PN 5-week-old Dhddsflx/flx iCre+ mice, when compared with age-matched controls. (C, right panel) Scotopic b-wave implicit times were significantly lengthened in both PN 4- and 5-week-old Dhddsflx/flx iCre+ mice, when compared with controls, for all flash intensities ≥0.4 cd-s/m2. (n = 12 for control; n = 9 for Dhddsflx/flx iCre+ PN 4 weeks; n = 12 for Dhddsflx/flx iCre+ PN 5 weeks; n = 7 for Dhddsflx/flx iCre+ PN 6 weeks). ∗p < 0.05, ∗∗p < 0.01; Welch's (unpaired) t test.
Scotopic ERG responses in Dhddsflx/flx iCre+ mice were diminished at all tested time points, relative to age-matched Dhddsflx/flx iCre− controls (n = 7–12/group/time point) (Figure 3B). Scotopic a- and b-waves were recorded using a customized 16-step regimen over a range of flash intensities spanning 10−5 to 500 cd-s/m2. The a-wave amplitude is a measure of PR hyperpolarization in response to photon flash stimulus. Scotopic a-wave amplitudes (at PN weeks 4, 5, and 6) were significantly lower, compared with those of age-matched controls, at all flash intensities ≥0.4 cd-s/m2. Scotopic a-wave amplitudes in Dhddsflx/flx iCre+ mice at PN 4 weeks (n = 9) were significantly diminished at all flash intensities (∼75% of the maximal control response amplitude [n = 12]). At PN 5 weeks of age (n = 12), a time point at which significant thinning of the ONL was observed, a-wave response amplitudes were significantly diminished (∼35% of control values). The a-wave ERG responses in Dhddsflx/flx iCre+ were essentially extinguished by PN 6 weeks of age (n = 7) (Figure 3B, upper panel). A similar time-dependent, significant decrease was observed in scotopic b-wave responses of Dhddsflx/flx iCre+ mice, when compared with controls (Figure 3B, middle panel). Interestingly, b-wave response amplitudes were significantly reduced in all age groups of Dhddsflx/flx iCre+ mice compared with age-matched controls (for all flash intensities ≥10−3 cd-s/m2), even at low flash intensities that may correspond to pure rod-mediated responses. Scotopic b-wave response amplitudes of Dhddsflx/flx iCre+ mice at PN 6 weeks of age (n = 7) exhibited about a 7-fold decrease compared with controls. Representative scotopic ERG traces for Dhddsflx/flx iCre+ mice and age-matched controls, at a saturating flash intensity of 500 cd-s/m2, are provided in Figure S6. Under these conditions, whereas control responses were robust at all ages tested, the Dhddsflx/flx iCre+ mice exhibited diminishing response amplitudes, being nearly extinguished by PN 6 weeks.
Scotopic b:a wave amplitude ratios were significantly increased at PN 5 weeks of age in Dhddsflx/flx iCre+ mice at all flash intensities ≥0.4 cd-s/m2, compared with age-matched controls. However, at PN 4 weeks of age, Dhddsflx/flx iCre+ mice showed increased scotopic b:a wave amplitude ratios only at flash intensities ≥150 cd-s/m2 (Figure 3C, left panel). Also, implicit time values for scotopic b-wave were significantly elevated at both PN 4 and 5 weeks of age in Dhddsflx/flx iCre+ mice (Figure 3C, right panel), compared with age-matched controls.
In contrast, cone PR-driven (photopic) ERG responses (calculated as the difference between the photopic b-wave and a-wave maxima) in Dhddsflx/flx iCre+ mice at PN 4 and 5 weeks were comparable with those of age-matched controls at all flash intensities (ranging from 10−1 to 30 cd-s/m2) (Figure 3B, bottom panel). However, photopic responses at PN 6 weeks of age were extinguished (n = 7) (i.e., equivalent to background noise levels), consistent with a rod-cone dystrophy (see Discussion).
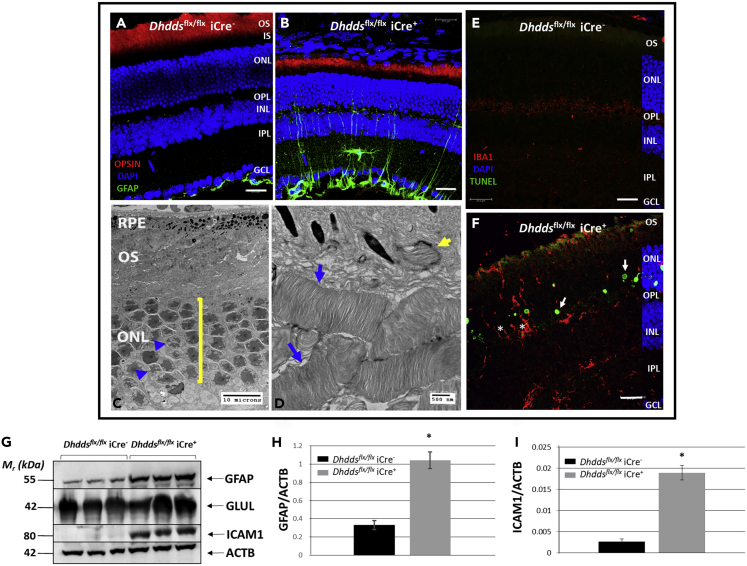
Immunohistochemical (IHC) analysis of retinas from controls and Dhddsflx/flx iCre+ mice at PN 5 weeks (a time point at which structural and functional deficits in the retina were observed) with antibodies against rod opsin and glial fibrillary acidic protein (GFAP) suggested several key degenerative features. The GFAP immunoreactivity pattern demonstrated gliosis of the degenerating Dhddsflx/flx iCre+ retina (green channel, Figure 4B). GFAP decorates only astrocytes and the Müller glial “endfeet” (i.e., the so-called inner limiting membrane) in a representative control retina (green channel, Figure 4A). Anti-opsin immunohistochemistry (red channel, Figure 4B) revealed dramatic shortening of ROS in Dhddsflx/flx iCre+ mice at PN 5 weeks, when compared with controls (red channel, Figure 4A), fully consistent with the ERG results (see earlier discussion).
Figure 4.
Morphological and Degenerative Features of the Dhddsflx/flx iCre+ Retina
(A and B) Confocal micrographs of (A) Dhddsflx/flx iCre− and (B) Dhddsflx/flx iCre+ mouse retinas at PN 5 weeks of age. Note the shortening of rod outer segments (detected using anti-opsin; red channel) in Dhddsflx/flx iCre+ retina (B) and the accompanying gliosis (detected using anti-GFAP; green channel). GFAP labeling was limited to Műller glial endfeet and astrocytes (comprising the internal limiting membrane at the vitreoretinal interface) in controls (A). Dhddsflx/flx iCre+ mice did not exhibit anti-opsin immunostaining in either the inner segments (IS) or the photoreceptor perinuclear space, suggesting unhindered opsin trafficking (scale bars, 20 μm).
(C and D) Electron micrographs of Dhddsflx/flx iCre+ outer retina at PN 5 weeks, indicating grossly shortened and poorly aligned, but otherwise ultrastructurally normal, outer segments (OS) (blue arrows, D) and functional RPE phagocytosis (yellow arrow, D). Note pyknotic nuclei (blue arrowheads, C) and thinning of ONL (6 nuclei in a row, yellow bracket, C). Scale bars: 10 μm in (C) and 500 nm in (D).
(E) Dhddsflx/flx iCre− retina did not exhibit TUNEL labeling. Iba-1 immunoreactivity of microglial cell bodies and arbors was limited to few cell bodies in PN 4- to 5-week-old Dhddsflx/flx iCre− retina (see Figure S8D) (scale bar: 20 μm).
(F) Retinal dystrophy in Dhddsflx/flx iCre+ mice was characterized by photoreceptor-specific autonomous cell death, as evidenced by TUNEL labeling (white arrows, 488-nm channel). Autonomous cell death in Dhddsflx/flx iCre+ mice was accompanied by infiltration of Iba-1-positive microglia (red channel) into the ONL and subretinal space (whitearrows. Furthermore, we observed microglial phagocytosis of TUNEL-negative photoreceptors (“phagoptosis”) leading to non-autonomous cell death (denoted by asterisks) (scale bar: 20 μm).
(G) Western blot analysis and semi-quantitative densitometry indicates significant up-regulation of GFAP (marker of gliosis) and ICAM-1 (facilitates trans-endothelial migration of leukocytes and breakdown of the blood-retinal barrier). GLUL and ACTB served as loading controls for Müller glia and total protein, respectively. (H) Quantification of GFAP levels in Dhddsflx/flx iCre− (black) vs. Dhddsflx/flx iCre+ (gray) mouse retinas on Western blots (see G), normalized to ACTB. (I) Quantification of ICAM1 levels in Dhddsflx/flx iCre− (black) vs. Dhddsflx/flx iCre+ (gray) mouse retinas on Western blots (see G), normalized to ACTB. ∗p < 0.05, ∗∗p < 0.01; Welch's (unpaired) t test.
Defective trafficking (“mislocalization”) of opsin has been observed in animal models using either tunicamycin-induced or genetic inhibition of global proteome glycosylation (Murray et al., 2015, Fliesler and Basinger, 1985, Fliesler et al., 1984a, Fliesler et al., 1985). However, in the Dhddsflx/flx iCre+ retina at an intermediate degenerative stage (PN 5 weeks), we did not observe such defects, e.g., inner segment/cytoplasmic accumulation of opsin, plasma membrane localization along the inner segment, and even to the synaptic terminal. Despite shortening of ROS, opsin trafficking was comparable to that of age-matched control (Figures 4A and 4B). We utilized an established murine model of autosomal dominate RP (adRP)—the NogRho−/- mouse, harboring mutations of the two N-terminal asparagine residues, N2 and N15—to demonstrate the trafficking fate of opsin in an animal model of true opsin hypoglycosylation. IHC revealed opsin accumulation in the inner segment, perinuclear space, as well as synapses of NogRho−/- retinas (white arrows, Figure S7). Furthermore, low- and high-magnification electron micrographs (Figures 4C and 4D, respectively) of Dhddsflx/flx iCre+ retinas also suggested thinning of the ONL (yellow bracket, Figure 4C) and disorganized ROS (blue arrows, Figure 4D), but with normal lamellar disk membrane ultrastructure (Figure 4D). Surprisingly, despite the rod-specific and essentially quantitative ablation of Dhdds, these observations are not comparable with pathological features in models of opsin glycosylation defects.
We evaluated whether the rapid thinning of the outer retina was due exclusively to rod cell death, owing to the rod-specific deletion of Dhdds. For this purpose, formaldehyde-fixed, paraffin-embedded sections of eyes from Dhddsflx/flx iCre+ and control mice (at PN 5 weeks, a time point at which the retina is in an intermediate stage of degeneration) were subjected to TUNEL analysis. Confocal fluorescence microscopy revealed TUNEL labeling (green channel) exclusively in the ONL of the Dhddsflx/flx iCre+ retina (Figure 4F; white arrows, Figures S8A–S8C); the labeling pattern was consistent with that expected for rod (not cone) PRs. Control retinas did not exhibit TUNEL-positive labeling, as expected (Figure 4E). These data are consistent with the ERG data described earlier in the discussion, suggesting a role for autonomous rod PR cell death in the observed ONL thinning.
We further observed that PR cell death was accompanied by phagocytosis of live, TUNEL-negative neurons by activated microglia, as has been reported for other models of retinal degeneration (Zhao et al., 2015). IHC analysis using antibodies to Iba-1 (a faithful marker of microglia) in Dhddsflx/flx iCre+ mice revealed infiltration of activated microglia into the ONL (asterisk, Figure 4H) as well as the subretinal space (i.e., the zone normally occupied by PR outer segments) (arrows, Figures 4H and S9A–S9C). By contrast, age-matched control retinas showed horizontally ramified microglia in the inner retinal layers only, as expected (Figures 4G and S8D). Representative high-magnification confocal microscopic images of activated microglia with phagocytic cups engulfing PR soma were observed (red arrows, Figure S8F). Activated microglia/macrophages in the subretinal space were quantified (utilizing hematoxylin and eosin [H&E] staining) as the number of nuclei in the typically acellular subretinal space (n = 3 sections of 10 μm thickness per animal, n = 8/group) (black arrows, Figures S8A and S8B). Dhddsflx/flx iCre+ mice exhibited a 4-fold increase in the number of nuclei in the subretinal space, when compared with the same region of the retina in age-matched controls (nuclei in the subretinal space were observed only in peripheral retina of WT mice). This was further observed as DAPI-positive, Iba-1-positive cells (interpreted as microglia/macrophages) in the subretinal space (white arrowheads, Figures 4F, S8E, S9A, and S9B)
Because Iba-1 immunoreactivity is indicative of an endogenous immune response, we adopted an unbiased (“shotgun”) cytokine screening approach to reveal other molecular players in the observed retinal degeneration. Surprisingly, the only two prominent targets with significant change (>2-fold increase) that emerged in Dhddsflx/flx iCre+ retinas, when compared with age-matched controls, were ICAM-1 and CXCL-10. Cytokine array data along with quantification are provided in Figure S10. Up-regulation of ICAM-1 and GFAP in Dhddsflx/flx iCre+ retinas (n = 3/group) was further validated by western blot analysis and semi-quantitative densitometry. Whole retinal ICAM-1 and GFAP levels were approximately 8- and 4-fold higher (n = 3/group), respectively, in Dhddsflx/flx iCre+ retinas compared with age-matched controls (Figures 4G–4I). Glutamine synthetase (GLUL) and β-actin (ACTB) served as loading controls for Műller glia and total retinal protein, respectively (Figure 4G).
Protein N-glycosylation Is Active in the Dhddsflx/flx iCre+ Retina
We investigated the glycosylation status of proteins in PN 5-week-old Dhddsflx/flx iCre+ retinas compared with retinas from age-matched Dhddsflx/flx iCre− mice (which served as controls, instead of WT mice). This was achieved by adopting a dual-lectin staining strategy that exhibits differential binding of lectins to rod versus cone PRs: Concanavalin-A (Con-A, a lectin with specific affinity for non-reducing α-D-mannosyl and α-D-glucosyl residues and which binds to the interphotoreceptor rod matrix sheath) and peanut agglutinin (PNA, a lectin that preferentially binds to galactosyl-β(1,3)-N-acetylgalactosamine, a constituent of the interphotoreceptor cone matrix sheath) (Varner et al., 1987, Hageman and Johnson, 1986). If Dhdds ablation inhibited N-glycosylation as hypothesized, we expected to observe a lack of Con-A binding to rod PRs. Surprisingly, Con-A labeling of the rod-rich outer retinal layer of Dhddsflx/flx iCre+ mice at PN 5 weeks was robust (including ROS, ONL, and outer plexiform layer [OPL]), comparable to age-matched controls (green channel, Figures 5A and 5B). PNA staining (far-red channel, pseudocolored magenta) suggested that cone PR density and distribution in rod-Dhdds-null mice was not altered at PN 5 weeks (Figures 5A–5D). PNGase-F-treated sections subsequently subjected to dual lectin staining were utilized as true “negative” controls (Figures 5C and 5D), as PNGase-F treatment should remove all N-linked glycans and, hence, abolish Con-A binding. Similar to Con-A labeling, wheat germ agglutinin (which detects N-acetyl-D-glucosamine residues) also exhibited robust binding to Dhddsflx/flx iCre+ retinas, comparable to that of controls (Figure S11).
Figure 5.
Protein N-Glycosylation is Functional in the Dhddsflx/flx iCre+ Retina
(A–F) The glycosylation status of Dhddsflx/flx iCre+ retinas (PN 5 weeks) was evaluated and compared with that of controls using a dual-lectin cytochemical staining strategy (Concanavalin-A [Con-A] in green channel, and cone PR-specific peanut agglutinin [PNA] in far-red [pseudocolored magenta] channel). Confocal fluorescence micrographs demonstrate robust Con-A labeling in Dhddsflx/flx iCre+ retina (B) comparable to that in controls (A), suggesting normal protein N-glycosylation in Dhddsflx/flx iCre+ mouse retinas. This was validated by testing the PNGase-F sensitivity of Con-A binding. Both control and Dhddsflx/flx iCre+ retinas (C and D, respectively) were sensitive to PNGase-F activity and exhibited markedly reduced Con-A binding. Furthermore, cone density and distribution (as indicated by PNA binding) was comparable in both control and Dhddsflx/flx iCre+ retinas. PNA labeling was unaffected by PNGase-F treatment, because PNGase-F does not cleave the cognate disaccharide Gal-β(1,3)-GalNAc to which PNA binds. Scale bars: 20 μm in (A–D). Retinal protein extracts from Dhddsflx/flx iCre+ and control mice (n = 3/group, at PN 5 weeks) were subjected to PNGase-F treatment, and western blot analysis was performed, probing the blot with antibodies to opsin (RHO) and LAMP2 (E and F, respectively). Untreated protein extracts served as negative controls for PNGase-F treatment. Opsin and LAMP2 proteins from Dhddsflx/flx iCre+ retinas exhibited PNGase-F sensitivity, and a subsequent Mr mobility shift (from ∼37 to ∼35 kDa, and from ∼120 to ∼40 kDa, respectively) as a consequence of cleaving their N-linked glycans, relative to the lanes containing untreated retinal extracts. This same behavior was observed when retinal extracts from control mice were subjected to PNGase-F treatment. Hence, RHO and LAMP2 were N-glycosylated in Dhddsflx/flx iCre+ mice. Note the decreased levels of opsin in lanes corresponding to Dhddsflx/flx iCre+, when compared with controls.
We further verified protein N-glycosylation in Dhddsflx/flx iCre+ retinas by examining PNGase-F sensitivity of opsin (the most abundant rod PR-specific glycoprotein) and LAMP2 (lysosome-associated membrane glycoprotein). Opsin and LAMP2 western blot analysis of PNGase F-treated versus untreated retinal protein extracts from Dhddsflx/flx iCre+ and control mice at PN 5 weeks was consistent with the lectin cytochemical staining results described earlier in the article. Both opsin and LAMP2 from Dhddsflx/flx iCre+ retinas (Figures 5E and 5F) were sensitive to PNGase-F treatment, and exhibited mobility shifts relative to the untreated specimens (biological triplicates), as expected. These findings were comparable to those obtained with PNGase-F-treated versus untreated control retinas (Figures 5E and 5F).
Retinal Dolichol Levels Are Markedly Reduced in Dhddsflx/flx iCre+ Mice
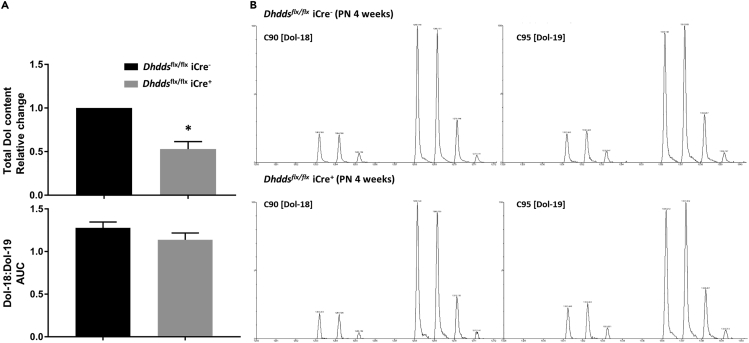
Age-dependent reduction of PR number in Dhddsflx/flx iCre+ retinas (Figures 3A and 3B) presents a challenge in delineating the expected decrease in total dolichol content due to ablation of Dhdds, as opposed to the decrease in the PR population (cell mass). Therefore, we utilized retinas from PN 4-week-old Dhddsflx/flx iCre− and Dhddsflx/flx iCre+ mice to measure whole retinal dolichol content—a time point at which no significant attrition in PR mass was observed (Figures 3A and 3B). For mass spectrometry quantification of dolichol, five retinas were pooled per sample to obtain sufficient tissue mass (n = 3 for Dhddsflx/flx iCre− mice, n = 4 for Dhddsflx/flx iCre+ mice). Liquid chromatography-mass spectrometry (LC-MS) analysis was performed to quantify the two dominant dolichol species—C90 (Dol-18) and C95 (Dol-19) (denoting dolichol species with 18 or 19 isoprene units and containing 90 or 95 carbon atoms, respectively); the sum of C90 plus C95 species was taken to represent the total retinal dolichol content. As shown in Figure 6 (top panel, Figure 6A), retinas from Dhddsflx/flx iCre+ mice exhibited about a 50% reduction (p < 0.01) in total dolichol content, relative to Dhddsflx/flx iCre− (control) retinas. Despite this, the distribution of dolichol species remained unaltered: no significant difference was observed in the ratio of Dol-18 to Dol-19 (bottom panel, Figure 6A). Representative mass spectra of C90 and C95 dolichols from Dhddsflx/flx iCre+ retinas are shown in Figure 6B.
Figure 6.
Dolichol Content Is Markedly Decreased in Dhddsflx/flx iCre+ Retinas, Relative to Age-Matched Controls
(A) LC-MS analysis of dolichols extracted from Dhddsflx/flx iCre+ and control retinas demonstrated ∼50% decrease in the total dolichol content of Dhddsflx/flx iCre+ retinas (n = 4), when compared with controls (n = 3) (upper panel, A). However, there was no selectivity as a function of dolichol chain lengths: the Dol-18:Dol-19 ratio [C90:C95] in Dhddsflx/flx iCre+ retinas was comparable to that of age-matched controls (lower panel, A).
(B) Mass spectra of Dol-18 and Dol-19 species from Dhddsflx/flx iCre− (control) and Dhddsflx/flx iCre+ retinas. Note that signals of ammoniated [M + NH4]+ and sodiated [M + Na]+ forms of respective Dol isotopologues are clearly visible. Each sample analyzed was prepared by pooling five retinas from the respective genotype group. ∗p < 0.05; Welch's (unpaired) t test.
Discussion
Several in vivo biochemical, pharmacological, and genetic studies (Fliesler et al., 1984a, Fliesler et al., 1985, Kean, 1980, Fliesler and Basinger, 1985, Murray et al., 2015) have demonstrated retinal de novo synthesis of dolichol and the requirement of dolichol-dependent N-glycosylation in maintaining the normal structure and function of the retina, particularly as regards the intracellular trafficking and incorporation of opsin, the predominant PR-specific glycoprotein, into ROS membranes. Recent whole-exome sequencing studies have revealed the RP59-causative missense mutations in the gene coding for DHDDS, which catalyzes the committed step of the mevalonate pathway directed toward dolichol synthesis (Zelinger et al., 2011, Zuchner et al., 2011). The present study is the first to employ a genetic approach, using conditional deletion of DHDDS in retinal rod PRs, to study the effects of altered PR dolichol homeostasis, as a tractable step toward understanding the pathological mechanisms underlying RP59. The following discussion provides broad insights into the challenges in modeling a complex metabolic disorder pertaining to dolichol homeostasis and compares the findings of this study (i.e., rapid structural and functional degeneration of PRs and markedly decreased dolichol content, but without any obvious impact on protein N-glycosylation), using a murine model, with the human RP59 disease.
Important Considerations in Generation and Verification of Rod PR-Specific Dhdds Knockout Mice
Multiple rod PR-specific, Cre recombinase-expressing mouse lines (driven by rod PR-specific Nrl,/LMOP/RHO promoters) may be utilized for the targeted excision of the loxP-modified gene of interest selectively in rod cells (Brightman et al., 2016, Le et al., 2006, Li et al., 2005). We set out to compare the spatiotemporal expression/activity of Cre recombinase in two Cre lines (LMOP-Cre and Rho-iCre75), utilizing the ZsGreen reporter strategy (Figures 2D,S3, and S5). The Rho-iCre75 mouse line exhibited Cre recombinase activity in almost all terminally differentiated rod PRs (Figure S5 and 2D), in good agreement with the original report describing the Rho-iCre75 mouse line (Li et al., 2005), whereas the LMOP-Cre line exhibited mosaic Cre-recombinase activity (ZsGreen reporter expression in ∼30%–40% of rod PRs only at PN 90 days) (Figure S12). Therefore, we utilized the Rho-iCre75 line to generate the Dhddsflx/flx iCre+ model, which successfully induced Dhdds deletion in terminally differentiated rod PRs. Importantly, Cre expression in the Rho-iCre75 line commences at around PN day 7; at that stage of retinal development, rod cell fate has been determined, but initiation of ROS assembly has just barely commenced and full maturation of rods has yet to be achieved.
Tail snip genotyping does not provide direct evidence for exon excision in the target tissue/cell type. Owing to the lack of availability of reliable antibodies against murine DHDDS, it is challenging to directly verify DHDDS protein levels, or cell type-specific knockout of the target. Therefore, we verified the establishment of this model by multiple approaches: (1) indirect tail snip genotype analysis (Figure S4), (2) direct retinal genotyping (testing whole tissue genomic DNA extract for Dhdds excision, Figure 2C), (3) the ZsGreen reporter mouse approach to detect Cre-recombinase activity (Figure 2D), and (4) ISH (Figures 2E and 2F) to obtain histology-level verification of cell type-specific Dhdds ablation. PCR assay involving whole retinal genetic material utilized a short extension period to only select for the 584-bp intronic PCR product (upon exon excision). One would predict that using a long extension period would amplify the long, non-excised Dhdds allele arising from other retinal cell types and may not distinguish heterozygous from homozygous knockouts. Therefore, retinal genotyping, when preceded by preliminary tail snip analysis (to confirm loxP homozygosity), and the reporter mouse strategy may provide better qualitative evidence for target excision. ISH using an RNA-based technology (BaseScope; ACD Bio) to probe control and Dhddsflx/flx iCre+ retinas (Figures 2E and 2F) provided important morphological and physiological context regarding Dhdds expression in the retina, as well as direct verification of successful Dhdds excision in a cell type-specific manner. Importantly, ISH labeling of the adjacent INL provides an on-section internal positive control for validating Dhdds mRNA detection by the BaseScope ZZ probes.
DHDDS belongs to the superfamily of CPT enzymes involved in the synthesis of long-chain polyisoprenoid products upon cis-condensation of FPP and IPP (yielding ≥ C55 long-chain products). Structural elucidation of E. coli undecaprenyl pyrophosphate synthase (UPPS) has revealed critical amino acids involved in hydrogen bonding and hydropathic interactions with FPP and IPP (Guo et al., 2005). Multiple sequence analysis using CLUSTALW2 algorithm suggests that these critical amino acids are well conserved in murine DHDDS CPT and other mammalian CPT enzymes (compare Figures S1 and S2) (Larkin et al., 2007). Targeted ablation of coding exon 3 of Dhdds is predicted to render the enzyme fully inactive due to the loss of the amino acids required for hydropathic interaction with FPP and hydrogen bonding with the phosphate groups of IPP (Figures S1 and S2). Furthermore, compensatory mechanisms for dolichol synthesis can be ruled out due to the absence of DHDDS homologs.
Whole-exome sequencing of patients with RP59 revealed point mutations in the DHDDS gene, the predominant mutation being K42E. The K42E mutation renders the human DHDDS gene product hypoactive, unlike the mouse rod-specific Dhdds exon 3 knockout (with expected total loss of function of DHDDS activity) (Figure S2). Another reported pathogenic DHDDS mutation is W64X (phenylalanine to STOP codon), which resulted in early postnatal lethality (Sabry et al., 2016). This clinically “severe” mutation generates a fully dysfunctional gene product comparable to the loss of the DHDDS catalytic activity in the Dhddsflx/flx iCre+ mouse model. The Dhddsflx/flx iCre+ mouse model differs from the human RP59 disease in at least two respects: (1) the RP59-causing DHDDS mutations (see Figure S3) globally affect all tissues and cell types, including the retina, whereas our mouse knockout model employs targeted Dhdds ablation only in rod PRs and (2) the predominant RP59 point mutation, K42E, is only hypomorphic, whereas our knockout model is a total loss-of-function insult, although cell type specific. Despite these differences, the findings in this study suggest that conditional deletion of Dhdds in rod PRs is sufficient to elicit a profound and rapid retinal degeneration. Furthermore, the Dhddsflx/flx iCre+ mouse line serves as a reliable biochemical model (owing to the presumed complete loss of DHDDS activity) to investigate the rod PR requirement for the dolichol pathway (Figure S2).
Structural, Functional, and Pathological Consequences of Rod Photoreceptor-Specific Dhdds Deletion
The current study was aimed at investigating the PR-specific disease mechanism(s) of RP59. In vivo retinal imaging (SD-OCT analysis, Figure 3A) suggested that ONL thickness in Dhddsflx/flx iCre+ mice was comparable to that of age-matched controls at PN 4 weeks. Surprisingly, the unaltered structural integrity at PN 4 weeks in Dhddsflx/flx iCre+ retinas did not correspond to full functional integrity (see Figures 3B, and S6): scotopic ERG responses (a- and b-waves) at all flash intensities (10−4 to 500 cd-s/m2) were significantly lower compared with those of age-matched (PN 4 weeks) controls. Dhddsflx/flx iCre+ retinas exhibited both profound structural and functional deficits at PN 5 and 6 weeks. ONL thickness was significantly reduced at PN 5 weeks (by ∼50% [p < 0.05], compared with age-matched WT C57BL/6J controls, Figure 3B). Low-magnification images of H&E-stained sections of eyes from PN 5-week-old Dhddsflx/flx iCre+ mice showed PR thinning (Figures S13B and S13D) compared with age-matched controls (Figures S13A and S13C). The rapid PR loss observed in Dhddsflx/flx iCre+ mice can be attributed to the following two degenerative processes: (1) autonomous rod PR-specific cell death, as observed by TUNEL labeling, and (2) phagocytosis of live PRs by activated, Iba-1-positive microglia (Figures 4F, S8E, S8F, and S9), leading to non-autonomous loss of PRs. The exact details of rod PR-autonomous defective cell processes leading to cell death upon Dhdds deletion remain to be elucidated. The Dhddsflx/flx iCre+ mice also exhibited activated microglia in the subretinal space, i.e., between the outer neural retina and the underlying retinal pigment epithelium (Figures 4F, S8E, S8F, and S9), which is normally “virtual” space filled with PR outer segments and associate extracellular matrix constituents. By contrast, the microglial population in retinas of young, age-matched control mice was notably sparse, and restricted to just the inner retinal layers (nner plexiform layer [IPL], ganglion cell layer [GCL]), as judged by Iba-1 immunostaining (Figures 4E and S8D). Although previous investigators have made similar observations regarding the distribution of microglia in the normal C57BL/6 mouse retina (Xu et al., 2007, Kaneko et al., 2008, Chen and Xu, 2015), often the Iba-1-positive cells were more numerous than what we observed in the present study. The reasons for these differences are not fully apparent, but could be due to age-dependent population differences (e.g., prior studies tended to use older mice than those employed here), or possibly to differences in the type of embedment media used (i.e., optimal cutting temperature embedment medium [O.C.T.] frozen sections versus paraffin sections) (Atiskova et al., 2019, Noailles et al., 2019). Proteomic and/or transcriptomic approaches offer potentially fruitful avenues to investigate the downstream cellular and molecular processes that are altered upon Dhdds-dependent inhibition of dolichol synthesis. Pharmacological inhibition of microglial activity in the retina also may provide a therapeutic avenue for preventing the rapid retinal degeneration observed in the Dhddsflx/flx iCre+ mouse model. Our cytokine array analysis (and correlative western blot analysis) revealed >8-fold up-regulation of ICAM-1 levels in Dhddsflx/flx iCre+ retinas compared with age-matched controls; such has been implicated in leukocyte adhesion, as well as breakdown in the blood-retinal barrier (Williams and Luscinskas, 2011, Devine et al., 1996, Mesri et al., 1994). The presumed involvement of ICAM-1 in microglial recruitment and in the observed retinal degeneration in this animal model also remains to be investigated further. Another key degenerative feature of the Dhddsflx/flx iCre+ retina is gliosis, as evidenced by significant up-regulation of GFAP relative to that of the Müller glial marker glutamine synthetase (Figures 4B and 4G–4I).
The SD-OCT results (i.e., ONL thinning) demonstrated rapid retinal degeneration in Dhddsflx/flx iCre+ mice; however, whereas most of that loss reasonably could be attributed to rod cell degeneration and demise (given that rods account for ∼97% of the PRs in the mouse retina; Carter-Dawson and Lavail, 1979), the involvement of cone degeneration and demise cannot be discounted. Cone function was directly assessed utilizing photopic ERG analysis at all tested time points (PN 4, 5, and 6 weeks) (Figure 3B, bottom panel). Electrophysiological responses of cone PRs were unaltered and robust until massive rod dropout was observed at PN 6 weeks (Figure 3B, bottom panel), suggesting a rapid rod-cone dystrophy. The rod-cone dystrophy observed in this animal model, although consistent with the rod-cone dystrophy observed in patients with RP59 (Kimchi et al., 2018), should be interpreted with caution. The rod-cone dystrophy observed in the Dhddsflx/flx iCre+ mouse model is “by design,” i.e., rod PR primary cell death is attributable to the rod-targeted excision of Dhdds, whereas cone PRs exhibit secondary dysfunction (i.e., a “bystander effect”; Ripps, 2002, Cusato et al., 2006, Ma et al., 2018), which is independent of Dhdds activity. Cone-specific PNA staining, as expected, did not reflect any significant cone PR dropout even at PN 5 weeks (Figures 5C and 5D). The density and distribution of PNA-positive structures in Dhddsflx/flx iCre+ mouse retinas appeared qualitatively comparable to that of age-matched controls, in agreement with the photopic ERG responses observed at PN 5 weeks (Figure 4C). PNA binds to O-linked glycans (specifically, the disaccharide Gal-β(1–3)-GalNAc), and therefore its binding to cone PRs is unaltered by PNGase-F treatment. We further documented cone arrestin distribution in PN 5-week-old Dhddsflx/flx iCre+ retinas, compared with age-matched controls. We observed cone arrestin-positive structures both in the inner/outer segment region and in the OPL (PR synapses) (Figures S13C and S13D).
Furthermore, we observed age-dependent increase in the scotopic b:a wave ratio (Figure 3C, left panel), in accordance with severe reductions in scotopic a-wave amplitudes, in relation to the reduction in b-wave amplitude. A similar increase in b:a wave ratio has been described in patients with PMM2-CDG (Thompson et al., 2012). However, we observe a significant decrease in both scotopic b-wave and a-wave amplitudes in the Dhddsflx/flx iCre+ mice. Given that the Dhdds excision occurs exclusively in rod PRs, we interpret the increase in b:a wave ratio to be due to a significant and specific decrease in the a-wave component of the ERG. In addition, implicit times for Dhddsflx/flx iCre+ mice were significantly longer compared with those of controls (Figure 3C, right panel). This finding along with the ERG amplitude analysis at all flash intensities clearly suggest less robust and more sluggish scotopic response in Dhddsflx/flx iCre+ mice compared with age-matched controls. The increased b:a wave ratio in the conditional Dhddsflx/flx iCre+ mouse model may not be directly suggestive of similar trends in patients carrying systemic DHDDS point mutations; however, a:b wave ratios have not been characterized, to date, in the DHDDS-CDG patient population.
In summary, the key features of this novel Dhddsflx/flx iCre+ model include progressive ROS shortening, gliosis, PR cell death, and microglial activation, microglial migration to ONL and subretinal space, and phagoptosis of entire PR cells, leading to rapid rod-cone dystrophy by PN 5–6 weeks (Figures 2, 3, and 4). The findings in this model (characterized by presumed total loss of DHDDS CPT activity) may be important in ultimately understanding the underlying mechanisms involved in RP59 (caused by homozygous point mutations with mildly hindered CPT activity). Parallel efforts to this work in our laboratories currently involve generation of DHDDS dual point mutant knock-in mice harboring both K42E and T206A mutations, to create a potentially more representative model of RP59 (unpublished studies). Surprisingly, the K42E homozygous knock-in model (even up to PN 1 year of age) does not replicate the structural or functional deficit features observed either in patients with RP59 or in the Dhddsflx/flx iCre+ model reported here, with the exception of marked gliosis (Ramachandra Rao et al., 2020).
Biochemical Features of the Degenerating Dhddsflx/flx iCre+ Retina
We originally predicted that rod-specific Dhdds ablation would cause defective protein N-glycosylation in rods because of the biochemical requirement of protein glycosylation for Dol-P (see Figure 1) (Denecke and Kranz, 2009, Buczkowska et al., 2015, Kean, 1977, Kean, 1980, Kean, 1999, Behrens and Leloir, 1970). We first verified excision of Dhdds exon 3 in terminally differentiated rod PRs (Figures S5 and Figure 2) and utilized this model to analyze dolichol content and N-glycosylation status. However, contrary to our initial expectations, the observed rapid PR degeneration was not accompanied by a glycosylation defect (Figures 5 and S11). In vitro and in vivo studies involving deletion of genes encoding enzymes involved in dolichol synthesis have utilized different model systems, different genes of interest, various gene editing techniques, and different time points during cell growth/tissue differentiation. A study utilizing global ablation of Dhdds at the one-cell embryo stage in zebrafish, which has a cone-rich retina (unlike mouse), indicated defective protein glycosylation in the PRs, as evidenced by lack of cone PR-specific PNA staining (Zuchner et al., 2011). Mouse embryonic/conditional ablation of NUS1, the gene encoding NgBR, the dimeric partner of the gene product of Dhdds, causes embryonic lethality before E6.5, as well as loss of N-glycosylation (Park et al., 2014, Park et al., 2016). However, curiously, biological samples (blood, urine, and fibroblasts) from patients with RP59 have been reported to exhibit a shift toward shorter dolichol chain length (increased Dol-18:Dol-19 mole ratios), without any appreciable hypoglycosylation defects (Wen et al., 2013, Lam et al., 2014). Furthermore, DHDDS knockdown experiments in fully confluent cell culture models did not lead to an observable glycosylation defect (Sabry et al., 2016). One possible explanation for the divergent observations across various models may involve elevated dolichol synthesis during tissue development and the unusual biological stability (long half-life) of dolichol, particularly in neural tissue. The rate of dolichol synthesis and accumulation is highest during cell growth and tissue development (Sakakihara and Volpe, 1984, Wong and Lennarz, 1982, Carson and Lennarz, 1981, Volpe et al., 1987, Adair and Cafmeyer, 1987, Doyle and Kandutsch, 1988, Larsson and Wejde, 1992). Furthermore, dolichols are biologically very stable lipids with long half-lives, and the only known forms of dolichols are the free alcohol, phosphorylated derivatives, and esters of carboxylic acids (Chojnacki and Dallner, 1988, Parentini et al., 2005). Oxidative catabolism of dolichol, although postulated, has not yet been fully documented in vivo (Swiezewska and Danikiewicz, 2005). It should be noted that the control and Dhddsflx/flx iCre+ mice utilized in this study were on a C57BL/6J background, and this study did not directly test the potential effects of other modifier mutations on the severity of the phenotype. However, we posit that the Dhdds K42E point knock-in mouse model may provide a better avenue to investigate the effects of genetic background on the RP59 phenotype (Westphal et al., 2002, Slijkerman et al., 2015, Ramachandra Rao et al., 2020). LC-MS analysis of whole retinal dolichol content suggests ∼50% reduction upon RhoiCre-mediated deletion of Dhdds in rod PRs (see Figures 2 and 6). Retinal dolichol content in our Dhddsflx/flx iCre+ model was significantly reduced, whereas the Dol-18/Dol-19 ratio was unaltered, unlike observations as mentioned earlier regarding tissues and fluids obtained from patients with RP59 (Wen et al., 2013) (see Figure 6). Reduction in whole retinal dolichol levels can be reasonably attributed to PR-specific loss of Dhdds. However, this finding may not account for any residual dolichol in PRs that may have been synthesized before Rho-iCre-mediated deletion of Dhdds had taken place in rod PRs (Figures 2 and S5). Future investigations into the role of mammalian DHDDS and its heterodimeric partner, NgBR, during PR genesis and retinal development may more fully explain the “hypoglycosylation-free” phenotype observed in the Dhddsflx/flx iCre+ mouse model described in this study (see Figures 5 and S10), as well as provide a deeper understanding of dolichol synthesis and homeostasis in retinal PRs. In addition, studying the effects of PR-specific Dhdds deletion on other important glycan modifications of proteins, such as O-mannosylation, may provide additional clues regarding the underlying mechanism driving RP59 pathology.
Inhibition of N-linked glycosylation perturbs vectorial trafficking of opsin and other ROS-destined proteins in PR cells and consequently compromises the inability to form PR outer segments (Fliesler et al., 1984a, Fliesler et al., 1985, Fliesler and Basinger, 1985, Ulshafer et al., 1986, Defoe et al., 1986, St Jules et al., 1990). However, surprisingly, although we observed shortening of ROS, we did not observe mislocalization of opsin, e.g., aberrant accumulation in the inner segment or in the perinuclear space of the ONL (see Figures 5A and 5B). In fact, we observed successful outer segment formation (see Figures 4C and 4D). Thus, our observations (see Figures 5 and 6) would argue against defective N-glycosylation upon Rho-iCre-mediated Dhdds excision, where Rho-iCre recombinase is expressed (starting at PN 7 days) after cell fate commitment to rod PR terminal differentiation (Li et al., 2005). However, the PR-specific ablation of Dhdds is sufficient to elicit primary degeneration of PRs, suggesting the requirement of continued endogenous dolichol synthesis in terminally differentiated rod PRs.
Limitations of the Study
RP59 is an autosomal recessive, non-syndromic form of RP, caused by mutations in DHDDS (Zelinger et al., 2011, Zuchner et al., 2011). Hence, although all cells require dolichol derivatives to carry out protein N-glycosylation, only the retina seems to be negatively impacted by such mutations in this disease. The present study was aimed at neither addressing the question of why RP59 is non-syndromic nor creating a faithful animal model that replicates the human disease phenotype. The immediate goal of the present study was to create a tractable in vivo model that would allow a further understanding of the requirement for dolichol homeostasis and DHDDS activity in retinal PR cells specifically. This targeted approach was taken because (1) global deletion of Dhdds likely would be embryonic lethal, and (2) PRs represent the predominant cell type affected in the RP59 retinal degeneration. Such targeted gene ablation in rods is best achieved by using the Dhddsflx/flx iCre+ model as employed here. Also, the synthesis of dolichol and its derivatives requires the activity of two enzymes— DHDDS and Nogo-B receptor (NgBR)—functioning together as a heterodimeric complex (Park et al., 2014, Park et al., 2016). In this study, we have modeled the effect of PR-specific deletion of the gene that encodes only one of the two heterodimeric partners. In addition, this cell type-specific targeted gene ablation occurs only in rod PRs and is dependent on the onset of opsin gene expression (the promoter driving Cre expression), which starts at PN day 4–7 (after the onset of expression of the transcription factor Nrl, which determines rod cell fate). This scenario is significantly different from what occurs in human patients with RP59: first, RP59 involves DHDDS point mutations, rather than gene ablation; second, those mutations are found in every cell type throughout the retina (and the entire body), rather than being restricted just to rod cells; third, the global expression of mutant DHDDS in humans is initiated in utero during early embryogenesis, rather than commencing after birth.
The lack of an observable defect in protein N-glycosylation in the retina in the Dhddsflx/flx iCre+ model may be attributed to at least two factors: (1) the other heterodimeric partner, NgBR (gene: Nus1), required for dolichol synthesis (which, presumably, remains active in the absence of Dhdds) may be able to at least partly take over the role of Dhdds (i.e., enzyme activity is impaired, but not completely lost) and/or (2) the dolichol synthesized in PR cells (or their precursors) before onset of Cre-recombinase expression may persist and be sufficient to support protein N-glycosylation even after Dhdds has been ablated in the rod cells. The lack of suitable reagents or methods to directly study the distribution and turnover rates of dolichol and its derivatives at the single cell and subcellular levels also presents a further impediment to obtaining a more complete understanding of exactly how PR-specific ablation of Dhdds alters this aspect of isoprenoid homeostasis in those cells.
Resource Availability
Lead Contact
Dr. Steven J. Fliesler, VA Western NY Healthcare System, Buffalo, NY 14215 U.S.A.; Email: fliesler@buffalo.edu.
Materials Availability
All novel reagents and genetically modified animals, as described herein, are available to qualified researchers per the requirements stipulated by the N.I.H., Department of Health and Human Services, USA. All associated costs must be the responsibility of the requesting investigator or institution, and are subject to review.
Data and Code Availability
Not applicable.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Supported, in part, by U.S.P.H.S. (NIH) grants 1 R01 EY029341 and a UAB Vision Science Research Center Pilot grant (S.J.P. and S.J.F.), 1UL1 TR001412 (to SUNY-University at Buffalo [S.J.F.]), P30 EY003039 (S.J.P.), EY020545 (X.M.), the National Science Centre of Poland grant no. UMO-2018/29/B/NZ3/01033 (E.S.), and by facilities and resources provided by VA Western New York Healthcare System (S.J.F.). S.J.F. is the recipient of a Research Career Scientist (RCS) Award from the Department of Veterans Affairs, BLR&D Service. S.R.R. was the recipient of two Fight for Sight Summer Student Fellowships (2013 and 2016), a Knights Templar Eye Foundation Career-Starter grant award, and a Fight For Sight Postdoctoral Fellowship. We gratefully acknowledge the technical assistance of Rebecca Benz, Cheryl Dann, and Kristie Kilby in the course of this study. We thank Drs. Muna Naash and Muayyad-Al-Ubaidi (University of Houston, Houston, TX, USA) for the generous gift of eyes from the NOGRho−/− genetic murine RP model, Aimee Stablewski (Gene Targeting and Transgenic Shared Facility, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA) for the generation of Dhddsflx/fflx mice, and Dr. Jack M. Sullivan (SUNY, University at Buffalo, and VA Western NY Healthcare System, Buffalo, NY, USA) for helpful discussions and use of his ERG system during the course of this project. Finally, we thank Dr. Tirthankar Sinha (University of Houston, Houston, TX, USA) for his assistance with graphic arts. Portions of this work have been presented in preliminary form at the Annual Meeting of the Association for Research in Vision and Ophthalmology (ARVO 2017). The opinions expressed herein do not reflect those of the Department of Veteran Affairs, the National Institutes of Health, or the US Government.
Author Contributions
S.R.R., S.J.F., and S.J.P. designed the study; D.A.S. and S.J.P. identified initial Dhddsflx/flx founders and verified FRT excision; S.R.R., L.A.S., M.C.B., F.W., A.O., L.S., G.S., and W.D. collected the data; S.J.F., S.J.P., S.R.R., E.S., X.M. interpreted the data; S.R.R. wrote the initial manuscript draft and generated the figures; S.J.F. and S.J.P. were primary editors of the manuscript. All persons listed as coauthors reviewed and contributed to editing of drafts of the manuscript contents and approved the final version before submission for publication.
Declaration of Interests
The authors declare no competing interests.
Published: June 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101198.
Supplemental Information
References
- Adair W.L., Jr., Cafmeyer N. Cell-cycle dependence of dolichyl phosphate biosynthesis. Arch. Biochem. Biophys. 1987;258:491–497. doi: 10.1016/0003-9861(87)90370-5. [DOI] [PubMed] [Google Scholar]
- Ashida H., Maeda Y., Kinoshita T. DPM1, the catalytic subunit of dolichol-phosphate mannose synthase, is tethered to and stabilized on the endoplasmic reticulum membrane by DPM3. J. Biol. Chem. 2006;281:896–904. doi: 10.1074/jbc.M511311200. [DOI] [PubMed] [Google Scholar]
- Atiskova Y., Bartsch S., Danyukova T., Becker E., Hagel C., Storch S., Bartsch U. Mice deficient in the lysosomal enzyme palmitoyl-protein thioesterase 1 (PPT1) display a complex retinal phenotype. Sci. Rep. 2019;9:14185. doi: 10.1038/s41598-019-50726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens N.H., Leloir L.F. Dolichol monophosphate glucose: an intermediate in glucose transfer in liver. Proc. Natl. Acad. Sci. U S A. 1970;66:153–159. doi: 10.1073/pnas.66.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas P., Duncan J.L., Maranhao B., Kozak I., Branham K., Gabriel L., Lin J.H., Barteselli G., Navani M., Suk J. Genetic analysis of 10 pedigrees with inherited retinal degeneration by exome sequencing and phenotype-genotype association. Physiol. Genomics. 2017;49:216–229. doi: 10.1152/physiolgenomics.00096.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman D.S., Razafsky D., Potter C., Hodzic D., Chen S. Nrl-Cre transgenic mouse mediates loxP recombination in developing rod photoreceptors. Genesis. 2016;54:129–135. doi: 10.1002/dvg.22918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buczkowska A., Swiezewska E., Lefeber D.J. Genetic defects in dolichol metabolism. J. Inherit. Metab. Dis. 2015;38:157–169. doi: 10.1007/s10545-014-9760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda P., Aebi M. The dolichol pathway of N-linked glycosylation. Biochim. Biophys. Acta. 1999;1426:239–257. doi: 10.1016/s0304-4165(98)00127-5. [DOI] [PubMed] [Google Scholar]
- Burton W.A., Scher M.G., Waechter C.J. Enzymatic phosphorylation of dolichol in central nervous tissue. J. Biol. Chem. 1979;254:7129–7136. [PubMed] [Google Scholar]
- Cantagrel V., Lefeber D.J., Ng B.G., Guan Z., Silhavy J.L., Bielas S.L., Lehle L., Hombauer H., Adamowicz M., Swiezewska E. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142:203–217. doi: 10.1016/j.cell.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D.D., Lennarz W.J. Relationship of dolichol synthesis to glycoprotein synthesis during embryonic development. J. Biol. Chem. 1981;256:4679–4686. [PubMed] [Google Scholar]
- Carter-Dawson L.D., Lavail M.M. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol. 1979;188:245–262. doi: 10.1002/cne.901880204. [DOI] [PubMed] [Google Scholar]
- Chen M., Xu H. Parainflammation, chronic inflammation, and age-related macular degeneration. J. Leukoc. Biol. 2015;98:713–725. doi: 10.1189/jlb.3RI0615-239R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chojnacki T., Dallner G. The biological role of dolichol. Biochem. J. 1988;251:1–9. doi: 10.1042/bj2510001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusato K., Ripps H., Zakevicius J., Spray D.C. Gap junctions remain open during cytochrome c-induced cell death: relationship of conductance to 'bystander' cell killing. Cell Death Differ. 2006;13:1707–1714. doi: 10.1038/sj.cdd.4401876. [DOI] [PubMed] [Google Scholar]
- Defoe D.M., Besharse J.C., Fliesler S.J. Tunicamycin-induced dysgenesis of retinal rod outer segment membranes. II. Quantitative freeze-fracture analysis. Invest. Ophthalmol. Vis. Sci. 1986;27:1595–1601. [PubMed] [Google Scholar]
- Denecke J., Kranz C. Hypoglycosylation due to dolichol metabolism defects. Biochim. Biophys. Acta. 2009;1792:888–895. doi: 10.1016/j.bbadis.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Devine L., Lightman S.L., Greenwood J. Role of LFA-1, ICAM-1, VLA-4 and VCAM-1 in lymphocyte migration across retinal pigment epithelial monolayers in vitro. Immunology. 1996;88:456–462. doi: 10.1046/j.1365-2567.1996.d01-666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucey M.A., Hess D., Cacan R., Hofsteenge J. Protein C-mannosylation is enzyme-catalysed and uses dolichyl-phosphate-mannose as a precursor. Mol. Biol. Cell. 1998;9:291–300. doi: 10.1091/mbc.9.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle J.W., Kandutsch A.A. Requirement for mevalonate in cycling cells: quantitative and temporal aspects. J. Cell Physiol. 1988;137:133–140. doi: 10.1002/jcp.1041370116. [DOI] [PubMed] [Google Scholar]
- Endo S., Zhang Y.W., Takahashi S., Koyama T. Identification of human dehydrodolichyl diphosphate synthase gene. Biochim. Biophys. Acta. 2003;1625:291–295. doi: 10.1016/s0167-4781(02)00628-0. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Di Iorio E., Barbaro V., Ponzin D., Sorrentino F.S., Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr. Genomics. 2011;12:238–249. doi: 10.2174/138920211795860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler S.J., Basinger S.F. Tunicamycin blocks the incorporation of opsin into retinal rod outer segment membranes. Proc. Natl. Acad. Sci. U S A. 1985;82:1116–1120. doi: 10.1073/pnas.82.4.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliesler S.J., Rapp L.M., Hollyfield J.G. Photoreceptor-specific degeneration caused by tunicamycin. Nature. 1984;311:575–577. doi: 10.1038/311575a0. [DOI] [PubMed] [Google Scholar]
- Fliesler S.J., Tabor G.A., Hollyfield J.G. Glycoprotein synthesis in the human retina: localization of the lipid intermediate pathway. Exp. Eye Res. 1984;39:153–173. doi: 10.1016/0014-4835(84)90005-8. [DOI] [PubMed] [Google Scholar]
- Fliesler S.J., Rayborn M.E., Hollyfield J.G. Membrane morphogenesis in retinal rod outer segments: inhibition by tunicamycin. J. Cell Biol. 1985;100:574–587. doi: 10.1083/jcb.100.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini R., Reichenbach T., Tan T.C., Divne C. Structural basis for dolichylphosphate mannose biosynthesis. Nat. Commun. 2017;8:120. doi: 10.1038/s41467-017-00187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska D., Karst F., Szkopinska A. Effect of squalene synthase gene disruption on synthesis of polyprenols in Saccharomyces cerevisiae. FEBS Lett. 1998;434:406–408. doi: 10.1016/s0014-5793(98)01019-9. [DOI] [PubMed] [Google Scholar]
- Guo R.T., Ko T.P., Chen A.P., Kuo C.J., Wang A.H., Liang P.H. Crystal structures of undecaprenyl pyrophosphate synthase in complex with magnesium, isopentenyl pyrophosphate, and farnesyl thiopyrophosphate: roles of the metal ion and conserved residues in catalysis. J. Biol. Chem. 2005;280:20762–20774. doi: 10.1074/jbc.M502121200. [DOI] [PubMed] [Google Scholar]
- Hageman G.S., Johnson L.V. Biochemical characterization of the major peanut-agglutinin-binding glycoproteins in vertebrate retinae. J. Comp. Neurol. 1986;249:499–510. doi: 10.1002/cne.902490406. 482-483. [DOI] [PubMed] [Google Scholar]
- Hamdan F.F., Myers C.T., Cossette P., Lemay P., Spiegelman D., Laporte A.D., Nassif C., Diallo O., Monlong J., Cadieux-Dion M. High rate of recurrent de novo mutations in developmental and epileptic encephalopathies. Am. J. Hum. Genet. 2017;101:664–685. doi: 10.1016/j.ajhg.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel C. Retinitis pigmentosa. Orphanet J. Rare Dis. 2006;1:40. doi: 10.1186/1750-1172-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesen S., Lehle L., Weissmann A., Aebi M. Isolation of the ALG5 locus encoding the UDP-glucose:dolichyl-phosphate glucosyltransferase from Saccharomyces cerevisiae. Eur. J. Biochem. 1994;224:71–79. doi: 10.1111/j.1432-1033.1994.tb19996.x. [DOI] [PubMed] [Google Scholar]
- Iwabe S., Ying G.S., Aguirre G.D., Beltran W.A. Assessment of visual function and retinal structure following acute light exposure in the light sensitive T4R rhodopsin mutant dog. Exp. Eye Res. 2016;146:341–353. doi: 10.1016/j.exer.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson A., Swiezewska E., Chojnacki T., Dallner G. Uptake and modification of dietary polyprenols and dolichols in rat liver. FEBS Lett. 1989;255:32–36. doi: 10.1016/0014-5793(89)81055-5. [DOI] [PubMed] [Google Scholar]
- Kaneko H., Nishiguchi K.M., Nakamura M., Kachi S., Terasaki H. Characteristics of bone marrow-derived microglia in the normal and injured retina. Invest. Ophthalmol. Vis. Sci. 2008;49:4162–4168. doi: 10.1167/iovs.08-1738. [DOI] [PubMed] [Google Scholar]
- Kaushal S., Ridge K.D., Khorana H.G. Structure and function in rhodopsin: the role of asparagine-linked glycosylation. Proc. Natl. Acad. Sci. U S A. 1994;91:4024–4028. doi: 10.1073/pnas.91.9.4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean E.L. The biosynthesis of mannolipids and mannose-containing complex glycans by the retina. J. Supramol Struct. 1977;7:381–395. doi: 10.1002/jss.400070310. [DOI] [PubMed] [Google Scholar]
- Kean E.L. The lipid intermediate pathway in the retina for the activation of carbohydrates involved in the glycosylation of rhodopsin. Neurochem. Int. 1980;1C:59–68. doi: 10.1016/0197-0186(80)90050-9. [DOI] [PubMed] [Google Scholar]
- Kean E.L. The dolichol pathway in the retina and its involvement in the glycosylation of rhodopsin. Biochim. Biophys. Acta. 1999;1473:272–285. doi: 10.1016/s0304-4165(99)00198-1. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Khateb S., Wen R., Guan Z., Obolensky A., Beryozkin A., Kurtzman S., Blumenfeld A., Pras E., Jacobson S.G. Nonsyndromic retinitis pigmentosa in the Ashkenazi Jewish population: genetic and clinical aspects. Ophthalmology. 2018;125:725–734. doi: 10.1016/j.ophtha.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Krasnova L., Wong C.H. Understanding the chemistry and biology of glycosylation with glycan synthesis. Annu. Rev. Biochem. 2016;85:599–630. doi: 10.1146/annurev-biochem-060614-034420. [DOI] [PubMed] [Google Scholar]
- Lam B.L., Zuchner S.L., Dallman J., Wen R., Alfonso E.C., Vance J.M., Pericak-Vance M.A. Mutation K42E in dehydrodolichol diphosphate synthase (DHDDS) causes recessive retinitis pigmentosa. Adv. Exp. Med. Biol. 2014;801:165–170. doi: 10.1007/978-1-4614-3209-8_21. [DOI] [PubMed] [Google Scholar]
- Larkin M.A., Blackshields G., Brown N.P., Chenna R., Mcgettigan P.A., Mcwilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Larsson O., Wejde J. Dolichol delays G1-arrest for one cell cycle in human fibroblasts subjected to depletion of serum or mevalonate. J. Cell Sci. 1992;103(Pt 4):1065–1072. doi: 10.1242/jcs.103.4.1065. [DOI] [PubMed] [Google Scholar]
- Le Y.Z., Zheng L., Zheng W., Ash J.D., Agbaga M.P., Zhu M., Anderson R.E. Mouse opsin promoter-directed Cre recombinase expression in transgenic mice. Mol. Vis. 2006;12:389–398. [PubMed] [Google Scholar]
- Li S., Chen D., Sauve Y., Mccandless J., Chen Y.J., Chen C.K. Rhodopsin-iCre transgenic mouse line for Cre-mediated rod-specific gene targeting. Genesis. 2005;41:73–80. doi: 10.1002/gene.20097. [DOI] [PubMed] [Google Scholar]
- Ma Y., Han X., De Castro R.B., Zhang P., Zhang K., Hu Z., Qin L. Analysis of the bystander effect in cone photoreceptors via a guided neural network platform. Sci. Adv. 2018;4:eaas9274. doi: 10.1126/sciadv.aas9274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y., Tanaka S., Hino J., Kangawa K., Kinoshita T. Human dolichol-phosphate-mannose synthase consists of three subunits, DPM1, DPM2 and DPM3. EMBO J. 2000;19:2475–2482. doi: 10.1093/emboj/19.11.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesri M., Liversidge J., Forrester J.V. ICAM-1/LFA-1 interactions in T-lymphocyte activation and adhesion to cells of the blood-retina barrier in the rat. Immunology. 1994;83:52–57. [PMC free article] [PubMed] [Google Scholar]
- Morava E., Wosik H.N., Sykut-Cegielska J., Adamowicz M., Guillard M., Wevers R.A., Lefeber D.J., Cruysberg J.R. Ophthalmological abnormalities in children with congenital disorders of glycosylation type I. Br. J. Ophthalmol. 2009;93:350–354. doi: 10.1136/bjo.2008.145359. [DOI] [PubMed] [Google Scholar]
- Murray A.R., Fliesler S.J., Al-Ubaidi M.R. Rhodopsin: the functional significance of asn-linked glycosylation and other post-translational modifications. Ophthalmic Genet. 2009;30:109–120. doi: 10.1080/13816810902962405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.R., Vuong L., Brobst D., Fliesler S.J., Peachey N.S., Gorbatyuk M.S., Naash M.I., Al-Ubaidi M.R. Glycosylation of rhodopsin is necessary for its stability and incorporation into photoreceptor outer segment discs. Hum. Mol. Genet. 2015;24:2709–2723. doi: 10.1093/hmg/ddv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng B.G., Freeze H.H. Perspectives on glycosylation and its congenital disorders. Trends Genet. 2018;34:466–476. doi: 10.1016/j.tig.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noailles A., Kutsyr O., Maneu V., Ortuno-Lizaran I., Campello L., de Juan E., Gomez-Vicente V., Cuenca N., Lax P. The absence of toll-Like receptor 4 mildly affects the structure and function in the adult mouse retina. Front Cell Neurosci. 2019;13:59. doi: 10.3389/fncel.2019.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parentini I., Cavallini G., Donati A., Gori Z., Bergamini E. Accumulation of dolichol in older tissues satisfies the proposed criteria to be qualified a biomarker of aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2005;60:39–43. doi: 10.1093/gerona/60.1.39. [DOI] [PubMed] [Google Scholar]
- Park E.J., Grabinska K.A., Guan Z., Sessa W.C. NgBR is essential for endothelial cell glycosylation and vascular development. EMBO Rep. 2016;17:167–177. doi: 10.15252/embr.201540789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E.J., Grabinska K.A., Guan Z., Stranecky V., Hartmannova H., Hodanova K., Baresova V., Sovova J., Jozsef L., Ondruskova N. Mutation of Nogo-B receptor, a subunit of cis-prenyltransferase, causes a congenital disorder of glycosylation. Cell Metab. 2014;20:448–457. doi: 10.1016/j.cmet.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra Rao S., Fliesler S.J., Kotla P., Nguyen M.N., Pittler S.J. Lack of overt retinal degeneration in a K42E Dhdds knock-in mouse model of RP59. Cells. 2020;9:896. doi: 10.3390/cells9040896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H. Cell death in retinitis pigmentosa: gap junctions and the 'bystander' effect. Exp. Eye Res. 2002;74:327–336. doi: 10.1006/exer.2002.1155. [DOI] [PubMed] [Google Scholar]
- Rush J.S., Cho S.K., Jiang S., Hofmann S.L., Waechter C.J. Identification and characterization of a cDNA encoding a dolichyl pyrophosphate phosphatase located in the endoplasmic reticulum of mammalian cells. J. Biol. Chem. 2002;277:45226–45234. doi: 10.1074/jbc.M207076200. [DOI] [PubMed] [Google Scholar]
- Sabry S., Vuillaumier-Barrot S., Mintet E., Fasseu M., Valayannopoulos V., Heron D., Dorison N., Mignot C., Seta N., Chantret I. A case of fatal Type I congenital disorders of glycosylation (CDG I) associated with low dehydrodolichol diphosphate synthase (DHDDS) activity. Orphanet J. Rare Dis. 2016;11:84. doi: 10.1186/s13023-016-0468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakihara Y., Volpe J.J. Dolichol deposition in developing mammalian brain: content of free and fatty-acylated dolichol and proportion of specific isoprenologues. Brain Res. 1984;316:255–262. doi: 10.1016/0165-3806(84)90310-9. [DOI] [PubMed] [Google Scholar]
- Schenk B., Fernandez F., Waechter C.J. The ins(ide) and out(side) of dolichyl phosphate biosynthesis and recycling in the endoplasmic reticulum. Glycobiology. 2001;11:61R–70R. doi: 10.1093/glycob/11.5.61r. [DOI] [PubMed] [Google Scholar]
- Shridas P., Waechter C.J. Human dolichol kinase, a polytopic endoplasmic reticulum membrane protein with a cytoplasmically oriented CTP-binding site. J. Biol. Chem. 2006;281:31696–31704. doi: 10.1074/jbc.M604087200. [DOI] [PubMed] [Google Scholar]
- Slijkerman R.W., Song F., Astuti G.D., Huynen M.A., Van Wijk E., Stieger K., Collin R.W. The pros and cons of vertebrate animal models for functional and therapeutic research on inherited retinal dystrophies. Prog. Retin. Eye Res. 2015;48:137–159. doi: 10.1016/j.preteyeres.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Sparks S.E., Krasnewich D.M. Congenital disorders of N-linked glycosylation and multiple pathway overview. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. GeneReviews((R)) University of Washington; 1993. [PubMed] [Google Scholar]
- St Jules R.S., Smith S.B., O'brien P.J. The localization and timing of post-translational modifications of rat rhodopsin. Exp. Eye Res. 1990;51:427–434. doi: 10.1016/0014-4835(90)90155-n. [DOI] [PubMed] [Google Scholar]
- Sullivan L.J., Makris G.S., Dickinson P., Mulhall L.E., Forrest S., Cotton R.G., Loughnan M.S. A new codon 15 rhodopsin gene mutation in autosomal dominant retinitis pigmentosa is associated with sectorial disease. Arch. Ophthalmol. 1993;111:1512–1517. doi: 10.1001/archopht.1993.01090110078029. [DOI] [PubMed] [Google Scholar]
- Swiezewska E., Danikiewicz W. Polyisoprenoids: structure, biosynthesis and function. Prog. Lipid Res. 2005;44:235–258. doi: 10.1016/j.plipres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Thompson D.A., Lyons R.J., Liasis A., Russell-Eggitt I., Jagle H., Grunewald S. Retinal on-pathway deficit in congenital disorder of glycosylation due to phosphomannomutase deficiency. Arch. Ophthalmol. 2012;130:712–719. doi: 10.1001/archophthalmol.2012.130. [DOI] [PubMed] [Google Scholar]
- Thompson D.A., Lyons R.J., Russell-Eggitt I., Liasis A., Jagle H., Grunewald S. Retinal characteristics of the congenital disorder of glycosylation PMM2-CDG. J. Inherit. Metab. Dis. 2013;36:1039–1047. doi: 10.1007/s10545-013-9594-2. [DOI] [PubMed] [Google Scholar]
- Ulshafer R.J., Allen C.B., Fliesler S.J. Tunicamycin-induced dysgenesis of retinal rod outer segment membranes. I. A scanning electron microscopy study. Invest. Ophthalmol. Vis. Sci. 1986;27:1587–1594. [PubMed] [Google Scholar]
- Van Den Born L.I., Van Schooneveld M.J., de Jong L.A., Riemslag F.C., de Jong P.T., Gal A., Bleeker-Wagemakers E.M. Thr4Lys rhodopsin mutation is associated with autosomal dominant retinitis pigmentosa of the cone-rod type in a small Dutch family. Ophthalmic Genet. 1994;15:51–60. doi: 10.3109/13816819409098864. [DOI] [PubMed] [Google Scholar]
- Varner H.H., Rayborn M.E., Osterfeld A.M., Hollyfield J.G. Localization of proteoglycan within the extracellular matrix sheath of cone photoreceptors. Exp. Eye Res. 1987;44:633–642. doi: 10.1016/s0014-4835(87)80135-5. [DOI] [PubMed] [Google Scholar]
- Volpe J.J., Sakakihara Y., Rust R.S. Dolichol kinase and the regulation of dolichyl phosphate levels in developing brain. Brain Res. 1987;428:193–200. doi: 10.1016/0165-3806(87)90117-9. [DOI] [PubMed] [Google Scholar]
- Welply J.K., Shenbagamurthi P., Lennarz W.J., Naider F. Substrate recognition by oligosaccharyltransferase. Studies on glycosylation of modified Asn-X-Thr/Ser tripeptides. J. Biol. Chem. 1983;258:11856–11863. [PubMed] [Google Scholar]
- Wen R., Dallman J.E., Li Y., Züchner S.L., Vance J.M., Peričak-Vance M.A., Lam B.L. Knock-down DHDDS expression Induces photoreceptor degeneration in zebrafish. In: Ash J., Grimm C., Hollyfield J.G., Anderson R.E., LaVail M.M., Bowes Rickman C., editors. Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology. Vol. 801. Springer; 2014. pp. 543–550. [DOI] [PubMed] [Google Scholar]
- Wen R., Lam B.L., Guan Z. Aberrant dolichol chain lengths as biomarkers for retinitis pigmentosa caused by impaired dolichol biosynthesis. J. Lipid Res. 2013;54:3516–3522. doi: 10.1194/jlr.M043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal V., Kjaergaard S., Schollen E., Martens K., Grunewald S., Schwartz M., Matthijs G., Freeze H.H. A frequent mild mutation in ALG6 may exacerbate the clinical severity of patients with congenital disorder of glycosylation Ia (CDG-Ia) caused by phosphomannomutase deficiency. Hum. Mol. Genet. 2002;11:599–604. doi: 10.1093/hmg/11.5.599. [DOI] [PubMed] [Google Scholar]
- Williams M.R., Luscinskas F.W. Leukocyte rolling and adhesion via ICAM-1 signals to endothelial permeability. Focus on "Leukocyte rolling and adhesion both contribute to regulation of microvascular permeability to albumin via ligation of ICAM-1". Am. J. Physiol. Cell Physiol. 2011;301:C777–C779. doi: 10.1152/ajpcell.00250.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T.K., Lennarz W.J. Biosynthesis of dolichol and cholesterol during embryonic development of the chicken. Biochim. Biophys. Acta. 1982;710:32–38. doi: 10.1016/0005-2760(82)90186-2. [DOI] [PubMed] [Google Scholar]
- Wu X., Rush J.S., Karaoglu D., Krasnewich D., Lubinsky M.S., Waechter C.J., Gilmore R., Freeze H.H. Deficiency of UDP-GlcNAc:dolichol phosphate N-Acetylglucosamine-1 phosphate transferase (DPAGT1) causes a novel congenital disorder of glycosylation type Ij. Hum. Mutat. 2003;22:144–150. doi: 10.1002/humu.10239. [DOI] [PubMed] [Google Scholar]
- Xu H., Chen M., Mayer E.J., Forrester J.V., Dick A.D. Turnover of resident retinal microglia in the normal adult mouse. Glia. 2007;55:1189–1198. doi: 10.1002/glia.20535. [DOI] [PubMed] [Google Scholar]
- Zelinger L., Banin E., Obolensky A., Mizrahi-Meissonnier L., Beryozkin A., Bandah-Rozenfeld D., Frenkel S., Ben-Yosef T., Merin S., Schwartz S.B. A missense mutation in DHDDS, encoding dehydrodolichyl diphosphate synthase, is associated with autosomal-recessive retinitis pigmentosa in Ashkenazi Jews. Am. J. Hum. Genet. 2011;88:207–215. doi: 10.1016/j.ajhg.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Zabel M.K., Wang X., Ma W., Shah P., Fariss R.N., Qian H., Parkhurst C.N., Gan W.B., Wong W.T. Microglial phagocytosis of living photoreceptors contributes to inherited retinal degeneration. EMBO Mol. Med. 2015;7:1179–1197. doi: 10.15252/emmm.201505298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Jang G.F., Jastrzebska B., Filipek S., Pearce-Kelling S.E., Aguirre G.D., Stenkamp R.E., Acland G.M., Palczewski K. A naturally occurring mutation of the opsin gene (T4R) in dogs affects glycosylation and stability of the G protein-coupled receptor. J. Biol. Chem. 2004;279:53828–53839. doi: 10.1074/jbc.M408472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S., Dallman J., Wen R., Beecham G., Naj A., Farooq A., Kohli M.A., Whitehead P.L., Hulme W., Konidari I. Whole-exome sequencing links a variant in DHDDS to retinitis pigmentosa. Am. J. Hum. Genet. 2011;88:201–206. doi: 10.1016/j.ajhg.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.