Abstract
Rats eating high fat chow are more sensitive to the behavioral effects of dopaminergic drugs, including methamphetamine and the dopamine D2/D3 receptor agonist quinpirole, than rats eating standard chow. However, limited work has explored possible sex differences regarding the impact of diet on drug sensitivity. It is also unknown whether eating high fat chow enhances sensitivity of rats to other dopamine (e.g., D1) receptor agonists. To explore these possibilities, male and female Sprague-Dawley rats eating standard laboratory chow (17% kcal from fat) or high fat chow (60% kcal from fat) were tested once per week for 6 weeks with dopamine D1 receptor agonist SKF 82958 (0.01–3.2 mg/kg) or methamphetamine (0.1–3.2 mg/kg) using cumulative dosing procedures. Eating high fat chow increased sensitivity of male and female rats to methamphetamine-induced locomotion; however, only female rats eating high fat chow were more sensitive to SKF 82958-induced locomotion. SKF 82958-induced eye blinking was also marginally, although not significantly, enhanced among female rats eating high fat chow, but not males. Further, although dopamine D2 receptor expression was significantly increased for SKF 82958-treated rats eating high fat chow regardless of sex, no differences were observed in dopamine D1 receptor expression. Taken together, the present study suggests that although eating high fat chow enhances sensitivity of both sexes to dopaminergic drugs, the mechanism driving this effect might be different for males versus females. These data further demonstrate the importance of studying both sexes simultaneously when investigating factors that influence drug sensitivity.
SIGNIFICANCE STATEMENT
Although it is known that diet can impact sensitivity to some dopaminergic drugs, sex differences regarding this effect are not well characterized. This report demonstrates that eating a high fat diet enhances sensitivity to methamphetamine, regardless of sex; however, sensitivity to dopamine D1 receptor agonist SKF 82958 is increased only among females eating high fat chow, but not males. This suggests that the mechanism(s) driving diet-induced changes in drug sensitivity might be different between sexes.
Introduction
In addition to causing cardiovascular and metabolic diseases, eating a diet high in fat can alter dopamine systems, the same systems that are targeted by drugs of abuse (Tomasi and Volkow, 2013). For example, mice and rats eating high fat laboratory chow are more sensitive to dopaminergic drugs than animals eating standard (low fat) laboratory chow (Baladi et al., 2012a; Collins et al., 2015; Fordahl and Jones, 2017; Naneix et al., 2017). Specifically, dopaminergic drugs produce unconditioned behavioral effects, which are increased among rats eating high fat chow (McGuire et al., 2011; Baladi et al., 2012a; Reyes, 2012). That is, dopamine D2/D3 receptor agonist–induced yawning (Baladi and France, 2009) and psychomotor stimulant–induced locomotion (McGuire et al., 2011; Baladi et al., 2015; Collins et al., 2015; Oginsky et al., 2016) are increased among male rats eating high fat chow. Some of these behavioral assays have been difficult to study in females, contributing to a relatively small number of reports exploring sex differences in the impact of diet on drug sensitivity (Collins et al., 2015; Martinez et al., 2019; Ramos et al., 2019). For example, dopamine agonist–elicited yawning is an androgen-mediated behavior (Berendsen and Nickolson, 1981) that only occurs at low frequency in females, even when they are fed a high fat diet (Serafine et al., 2014; Ramos et al., 2019). Those reports that have explored both sexes suggest that the impact of diet on sensitivity to the locomotor-stimulating effects of drugs might be greater among females than males [Baladi et al., 2012b; Collins et al., 2015; Ramos et al., 2019; although see also Martinez et al. (2019)]. Further, there are well known sex differences regarding general sensitivity to psychostimulant drugs (i.e., cocaine and methamphetamine; Camp and Robinson, 1988; Chin et al., 2001; Schindler and Carmona, 2002; Milesi-Halle et al., 2007).
Although only a few publications include both males and females in the same study (Collins et al., 2015; Ramos et al., 2019), separate experiments studying male and female rats have examined the impact of eating high fat chow on sensitivity of rats to cocaine ( Baladi et al., 2012, 2015, Serafine et al., 2014, 2016); however, less is known about how diet impacts sensitivity of female rats to methamphetamine (McGuire et al., 2011; Robinson et al., 2015). Although often grouped together as psychostimulants, several important differences between methamphetamine and cocaine necessitate the further examination of this drug of abuse in both sexes. For example, although cocaine is a monoamine transporter inhibitor, methamphetamine is a monoamine transporter substrate and can cause neurotoxicity (Howell and Kimmel, 2008). Further, methamphetamine use remains relatively high in the western/midwestern regions of the United States (Artigiani et al., 2018). Given the continued high rates of obesity in the United States (Hales et al., 2017), it is critical to understand the potential impact of eating a high fat diet on sensitivity of individuals to different drugs of abuse.
Although the mechanisms driving the effects of diet remain relatively understudied in females, high fat chow–induced enhanced sensitivity to stimulants in male rats appears to be at least partly mediated by decreases in active (i.e., membrane-bound) dopamine transporter expression (Speed et al., 2011; Cone et al., 2013). Although methamphetamine is a substrate for monoamine transporters, its rewarding, reinforcing, and locomotor-stimulating effects are due to increased endogenous activation of dopamine D1 and D2 receptors (Cabib et al., 1991; Hubner and Moreton, 1991; Caine and Koob, 1994; Bardo et al., 1999; Nazarian et al., 2004; Brennan et al., 2009). Eating a high fat diet decreases dopamine D2 receptor binding and expression [Johnson and Kenny, 2010; Tomasi and Volkow, 2013; although see also South and Huang (2008)] and increases sensitivity of male rats to the dopamine D2/D3 receptor agonist quinpirole (Baladi and France, 2009; Baladi et al., 2011; although see Ramos et al. (2019), Serafine et al. (2014) for examples with female rats]. Eating high fat chow results in the downregulation of dopamine D1 receptor gene expression (Alsio et al., 2010); however; it is not known whether eating high fat chow enhances sensitivity of rats to the behavioral effects of dopamine D1 receptor agonists (Desai et al., 2007; Graham et al., 2013; Kotani et al., 2016). There is some reason to suspect that there might be sex differences related to the effects of dopamine D1 receptor agonists, given that estradiol, a female sex hormone, has been shown to modulate dopamine D1 and D2 receptor–sensitive adenylyl cyclases (Maus et al., 1989).
To address the abovementioned gaps in knowledge, the present report examined the impact of eating high fat chow on 1) sensitivity of male and female rats to methamphetamine-induced locomotion, 2) sensitivity of male and female rats to dopamine D1 receptor agonist SKF 82958-induced eye blinking and locomotion, and 3) expression of dopamine D1 and D2 receptors.
Materials and Methods
Animals.
Male and female Sprague-Dawley rats (Envigo, Livermore, CA) weighing 20–23 g upon arrival (PND 20–21) were individually housed in Tecniplast 1284L (365 × 207 × 140 mm) individually ventilated cages in an environmentally controlled room (23 ± 3°C, 50% ± 20% relative humidity) with a 12-hour light/dark cycle (lights on at 8:00 AM). All rats had ad libitum access to food and water except when indicated. Upon arrival, all animals were habituated to the housing facilities and to the experimental procedure prior to experimental testing. All experimental procedures were conducted in accordance with the Institutional Animal Care and Use Committee, The University of Texas at El Paso, and the 2011 Guide for Care and Use of Laboratory Animals (Institute of Laboratory, Animal Resources on Life Sciences, the National Research Council, and the National Academy of Sciences).
Behavioral Observations and Timeline.
Prior to experimental testing (PND 24–26 and PND 32–33), animals were habituated to all experimental procedures. In all behavioral assays, all animals were placed into their respective testing apparatus for 30 minutes and subsequently received seven saline injections, spaced 15 minutes apart. This methodology was identical for testing procedures, except that SKF 82958 and methamphetamine dose-response curves were generated using a cumulative dosing procedure. After a 30-minute habituation period, rats were given vehicle 15 minutes before the first dose of drug, with each successive dose separated by 15 minutes. Behavioral observations began immediately after each injection. Testing procedures began on PND 39–41 with initial baseline testing, and immediately after completion of baseline testing, rats were randomly assigned to their dietary condition. Subsequent testing continued once weekly for 5 weeks, with the last session occurring on PND 74–77. Therefore, dietary manipulations and drug testing took place during the entirety of adolescence and the start of early adulthood, matching previous reports (Baladi et al., 2012, p.; Ramos et al., 2019, p.).
Feeding Conditions.
All rats had free access to standard laboratory chow (3.1 kcal/g, 17% kcal from fat, Teklad 7912; Envigo) upon arrival to the facility and for the duration of habituation procedures. After the completion of baseline testing, a subset of rats were randomly assigned to eat high fat chow (5.1 kcal/g, 60% kcal from fat, Teklad 06414; Envigo) until the end of the experiment (PND 74–77). The remaining animals continued to eat standard chow for the duration of the experiment. All rats were weighed and fed daily at approximately 8:00 AM for the duration of the experiment.
Drugs.
SKF 82958 (Tocris, Minneapolis, MN), methamphetamine (Sigma Aldrich, St. Louis, MO), and urethane (Sigma Aldrich) were prepared in sterile saline (0.9% NaCl, pH 5.4). All drugs were administered in a volume of 1 ml/kg i.p.
SKF 82958- and Methamphetamine-Induced Locomotion.
When examining SKF 82958- or methamphetamine-induced locomotion, animals were placed into individual testing chambers within sound-attenuating cubicles (Med Associates Inc., Fairfax, VT). Locomotor activity counts were recorded with Activity Monitor Software (Med Associates Inc.) using the following criteria. At the start of each session, the software centered a virtual stereotypic box around each rat using horizontal and vertical infrared beams spaced 1.6 cm apart. If the rat moved outside the stereotypic box, movements were recorded via beam breaks as ambulatory counts until the rat ceased lateral movement for 0.5 seconds. Consequently, the software centered a new stereotypic box around the rat and repeated the above process until the completion of each 15-minute recording session. Any movements occurring within the stereotypic box (e.g., fine motor activity) were not included in the analysis of locomotor activity counts. The effects of eating high fat chow on locomotor activity counts induced by the dopamine D1 receptor agonist SKF 82958 or methamphetamine were assessed using a cumulative dosing procedure. Increasing doses of SKF 82958 (vehicle, 0.01, 0.032, 0.1, 0.32, 1.0, 3.2 mg/kg) or methamphetamine (vehicle, 0.1, 0.32, 1.0, 3.2 mg/kg) were delivered in 15-minute intervals, with behavioral recording occurring between injections, as mentioned above. All animals were tested once weekly, on the same day and time, for 6 weeks after assignment to dietary condition. Finally, to test for changes in the rate of sensitization between groups after weekly injections, the area under the locomotor activity counts dose-response curve (AUC) was calculated for individual rats, using GraphPad Prism (GraphPad Software, San Diego, CA). Values for AUC were then averaged across groups and analyzed for significant differences as described by Chefer and Shippenberg (2009).a
SKF 82958-Induced Eye Blinking.
When examining SKF 82958-induced eye blinking, animals were placed in custom-made restraint devices made of 6-inch length and 2-inch diameter polyvinal chloride tubing affixed to wooden platforms [based on specifications used in Desai et al. (2007) obtained via personal communication with the authors] for progressively longer sessions for 3 days, leading to a final restraint time of 2.25 hours. The head of each rat was gently placed into individual neck plates, with adjustable points to accommodate individual rat sizes, to facilitate accurate detection of eye blinks. Each session was videotaped using a digital video camera (model HF R600; Cannon, Melville, NY). Eye blinking was defined by the opening and closing of one or both eyelids for a duration of less than 1 second. Recordings were scored by observers blind to the testing conditions. The effects of eating high fat chow on SKF 82958-induced eye blinking were assessed using a cumulative dosing procedure (vehicle, 0.01, 0.032, 0.1, 0.32, 1.0, 3.2 mg/kg) delivered in 15-minute intervals, with video recording occurring as mentioned above. All animals were tested once weekly, on the same day and time, for 3 weeks after assignment to dietary condition.
Tissue Preparation.
Rats were anesthetized with urethane (1.5 g/kg) and decapitated 1 week after the final testing session. Brains were extracted, and the left and right striatum were removed using a calibrated dissection block and placed in ice-cold lysis buffer (10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1% Igepal CA) that contained protease and phosphatase inhibitors (Roche Diagnostics, Basel, Switzerland). The striatal tissue was then homogenized with a ultrasonic homogenizer. The homogenate was then centrifuged at 14,000 rpm for 5 minutes at 4°C. After centrifugation, primary protein fractions were collected and stored at −80°C until analyzed.
Western Blotting.
Striatal supernatant samples were examined via Western blot analysis following standard procedures (Speed et al., 2011). Specifically, protein concentrations (40 μg) were quantified from the cytosolic fraction using a Bio Rad Protein Assay Standard II Kit with bovine serum albumin as the standard (Bio Rad, Hercules, CA). Samples were separated on 12% SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Immobilon; Millipore) and allowed to incubate for 1 hour in blocking buffer (1% bovine serum albumin in 1× Tris-buffered saline, .25% Tween 20). After blocking, membranes were incubated with primary antibodies selective for dopamine D1 receptor (66 kDa; 1:500; Millipore Sigma) dopamine D2 receptor (50 kDa; 1:100; Santa Cruz Biotechnology), and glyceraldehyde-3-phosphate dehydrogenase (37 kDa, 1:10,000; Fitzgerald) overnight at 4°C. Membranes were then repeatedly washed three times for 10 minutes in 1× Tris-buffered saline/Tween 20 and incubated with horseradish peroxidase–conjugated secondary anti-mouse IgG (1:10,000; Sera Care) for 30 minutes at room temperature followed by washes. Subsequently, membranes were visualized using a C-DiGit Blot Scanner (LI-COR) in chemiluminescent reagents for protein intensity. All blots were normalized to total glyceraldehyde-3-phosphate dehydrogenase and quantified using ImageStudio software suite (LI-COR).
Data Analysis.
Dose-response curves were generated for locomotor activity counts for male (n = 9) and female (n = 10) rats eating standard chow that were tested with SKF 82958, male (n = 9) and female (n = 12) rats eating high fat chow that were tested with SKF 82958, male (n = 12) and female (n = 12) rats eating standard chow that were tested with methamphetamine, and male (n = 12) and female (n = 11) rats eating high fat chow that were tested with methamphetamine. Locomotion was expressed as the mean number of locomotor activity counts ± S.E.M. Three-way mixed model ANOVAs with the between-subjects variables of sex (male or female) and diet (standard or high fat) and the within-subjects variable of dose (e.g., repeated measure) were used to determine significant changes to drug-induced locomotion and eye blinking. Sidak’s multiple comparisons post hoc tests were used to examine significant main effects of diet, sex, dose, and interaction effects (between diet × sex, diet × dose, sex × dose, and diet × sex × dose). Specifically, differences between groups regarding mean locomotion or eye blinking were examined between groups of rats eating different diets (standard vs. high fat) and that were different sexes (male vs. female) at different drug doses. Because of the substantial number of comparisons for main effects and interactions effects, group means and confidence intervals are reported. All comparisons were conducted with a family-wise error rate of 0.05. SKF 82958-induced eye blinking dose-response curves were generated for male (n = 3) and female (n = 4) rats eating standard chow and male (n = 3) and female (n = 4) rats eating high fat chow. Although originally slated for five consecutive weeks, eye blinking data collection was terminated after 3 weeks of testing because of the unexpectedly large attrition rates (∼50% of animals), especially among animals eating high fat chow (70% attrition rate). To assess for differences in the rate of drug sensitization, a three-way mixed model ANOVA with the between-subjects variables of sex (male or female) and diet (standard or high fat) and the within-subjects variable of dose (e.g., repeated measure) was used to examine differences in average AUC of locomotor activity counts within and across weeks for all groups [similar to analysis used by Gerdeman et al. (2008)]. Dopamine D1 and D2 receptor intensity was measured at the conclusion of locomotor testing in a randomly selected subset of male (n = 6) and female (n = 6) rats eating standard chow and male (n = 6) and female (n = 6) rats eating high fat chow. Two-way between-subjects ANOVAs using Sidak’s multiple post hoc comparisons were used to assess for significant changes in receptor intensity. Data analysis was completed using SPSS (IBM corporation, NY) and GraphPad Prism (GraphPad Software).
Results
Methamphetamine-Induced Locomotion.
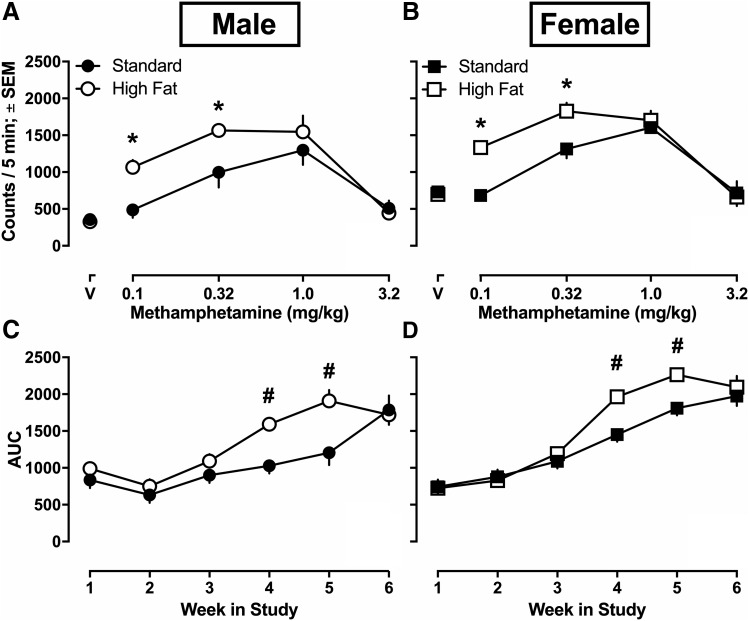
Methamphetamine dose-dependently increased and then decreased locomotion among male and female rats (Fig. 1, A and B), resulting in an inverted U-shaped dose-response curve. For each week, three-way mixed model ANOVAs were conducted to examine group differences. No significant results were revealed until week 3, during which a significant main effect of sex was revealed (P < 0.001). That is, consistent with previous reports, methamphetamine induced significantly more locomotion among females than males after 3 weeks of testing. This effect persisted for week 4 and 5, as other effects emerged. Since the effects that were revealed at week 4 and 5 were identical in terms of significance, week 5 (see Fig. 1, A and B) statistical values are reported here. The three-way mixed model ANOVA examining methamphetamine-induced locomotion dose-response curves on week 5 revealed a significant main effect of dose [F(3.083,132.6) = 71; P < 0.0001], sex [F(1,43) = 14.45; P = 0.0004], and diet [F(1,43) = 12.41; P = 0.001], as well as a significant dose × diet interaction effect [F(4,172) = 8.098; P < 0.0001]. However, no significant dose × sex [F(4,172) = 0.3439; P = 0.848], sex × diet [F(1,43) = 0.03170; P = 0.8595], or dose × sex × diet [F(4,172) = 0.1444; P = 0.9652] interactions were revealed.
Fig. 1.
Methamphetamine-induced locomotion and sensitization for male and female rats eating high fat (n = 12 male, n = 11 female) or standard chow (n = 12 male, n = 12 female). Methamphetamine-induced locomotion (e.g., ambulatory counts) for the last 5 minutes of each 15-minute recording session after 5 weeks of initial assignment to dietary conditions (A and B). Area under the weekly generated methamphetamine dose-response curves (AUC) for male and female rats eating high fat or standard chow for 1–6 weeks (C and D). Data were analyzed using a three-way (sex × diet × dose) mixed model ANOVA with Sidak’s multiple comparison post hoc tests. Data are expressed as means ± S.E.M. *P < 0.05, dietary difference within sex; #P < 0.05, weekly sex difference independent of diet.
The main effect of dose demonstrates that regardless of diet or sex, methamphetamine dose-dependently increased locomotion as compared with activity that occurred after saline injections. Specifically, locomotor activity after 0.1 mg/kg [95% bCI (801.808, 987.770)], 0.32 mg/kg [95% CI (1275.929, 1556.711)], and 1.0 mg/kg [95% CI (1361.139, 1709.638)] methamphetamine was greater than activity after saline injections [95% CI (466.321, 601.097)]. The main effect of sex demonstrates that regardless of diet or dose, methamphetamine induced significantly more locomotion among females than males. Specifically, during week 5, methamphetamine induced 265.255 more locomotor activity counts on average among females than among males [95% CI for difference (119.688, 410.823)]. The main effect of diet demonstrates that regardless of sex or dose, methamphetamine induced more locomotion among rats eating high fat chow than rats eating standard chow. Specifically, during week 5, methamphetamine induced 245.646 more locomotor activity counts on average among rats eating high fat chow than among rats eating standard chow [95% CI for difference (100.079, 391.214)]. Finally, the significant dose × diet interaction effect demonstrates that regardless of sex, methamphetamine at certain doses induced more locomotion among rats eating high fat chow than rats eating standard chow. Specifically, Sidak’s multiple comparisons revealed that rats eating high fat chow were significantly more active than rats eating standard chow after 0.1 mg/kg [standard, mean locomotor activity counts = 585.106, 95% CI (456.780, 713.432); high fat, mean locomotor activity counts = 1204.472, 95% CI (1069.883, 1339.062)] and 0.32 mg/kg [standard, mean locomotor activity counts = 1155.677, 95% CI (961.919, 1349.435); high fat, mean locomotor activity counts = 1676.963, 95% CI (1473.747, 1880.178)] methamphetamine, regardless of sex (see Fig. 1, A and B). At week 6, there was a main effect of dose (P < 0.001), but no other significant differences remained. To summarize, on average females were generally more sensitive to methamphetamine-induced locomotion than males, and rats eating high fat chow were more sensitive on average to methamphetamine-induced locomotion than rats eating standard chow, regardless of sex.
Methamphetamine-Induced Sensitization.
To analyze changes over time (e.g., across weeks) as an index of the development of sensitization to methamphetamine, AUC for methamphetamine-induced locomotion was calculated (Fig. 1, C and D). A three-way mixed model ANOVA revealed significant main effects of week [F(1.945,83.65) = 92.40; P < 0.0001], sex [F(1,43) = 12.14; P = 0.0011], and diet [F(1,43) = 14.33; P = 0.0005], as well as significant week × sex [F(5,215) = 5.229; P = 0.0001] and week × diet [F(5,215) = 6.317; P < 0.0001] interaction effects. However, no week × sex × diet interaction effect was observed [F(5,215) = 0.5674; P = 0.7250].
The main effect of week demonstrates that all rats, regardless of sex or diet, developed sensitization to the locomotor-stimulating effects of methamphetamine after repeated testing during weeks 3–5. Specifically, the AUC for methamphetamine-induced locomotion increased significantly during weeks 3–5: week 3 [mean area = 1068.46, 95% CI (977.21, 1159.71)], 4 [mean area = 1509.58, 95% CI (1416.73, 1602.43)], and 5 [mean area = 1796.58, 95% CI (1666.65, 1926.50)], see Fig. 1, C and D. The main effect of sex demonstrates that, regardless of diet and week, AUC of methamphetamine-induced locomotor activity was greater among female rats as compared with males. Specifically, AUC for female rats was increased on average by 214.232 as compared with male rats [95% CI of difference (90.24, 338.23)]. The main effect of diet demonstrates that, regardless of week or sex, the AUC of methamphetamine-induced locomotor activity was greater among rats eating high fat chow as compared with rats eating standard chow. Specifically, AUC for rats eating high fat chow was increased on average by 232.755 compared with standard chow [95% CI of difference (108.76, 356.75)]. The significant interaction effects were explored using Sidak’s multiple comparisons. The significant week × sex effect demonstrates that, regardless of diet, at certain weeks methamphetamine induced greater locomotion among females than among males. Specifically, Sidak’s multiple comparisons revealed that the AUC was greater among female rats during week 4 [female, mean AUC = 1708.966, 95% CI (1576.206, 1841.726); male, mean AUC = 1310.192, 95% CI (1180.350, 1440.033)] and week 5 [female, mean AUC = 2036.266, 95% CI (1850.700, 2222.232); male, mean AUC = 1556.683, 95% CI (1375.001, 1738.366)]. By week 6, this effect was no longer apparent, suggesting that after several weeks of testing, sensitization occurred at the same magnitude eventually in both male and female rats. The significant week × diet interaction effect demonstrates that regardless of sex, at certain weeks methamphetamine induced greater locomotor activity among rats eating high fat chow than rats eating standard chow. Specifically, Sidak’s multiple comparisons revealed that the AUC was greater for rats eating high fat chow at week 4 [standard, mean AUC = 1240.608, 95% CI (1110.767, 1370.450); high fat, mean AUC = 1778.549, 95% CI (1645.789, 1911.309)] and week 5 [standard, mean AUC = 1506.808, 95% CI (1325.767, 1688.491); high fat, mean AUC = 2086.341, 95% CI (1900.575, 2272.107)] as compared with rats eating standard chow. To summarize, methamphetamine-induced sensitization was larger among females than males, as evidenced by greater AUC initially (e.g., during weeks 4 to 5), but this effect dissipated with repeated testing. That is, by week 6, males and females developed comparable sensitization to methamphetamine. Methamphetamine-induced sensitization was also greater among rats eating high fat chow than rats eating standard chow, regardless of sex, as demonstrated by greater AUC at weeks 4 and 5, but this effect dissipated with repeated testing. That is, by week 6, rats eating high fat or standard chow developed comparable sensitization to methamphetamine.
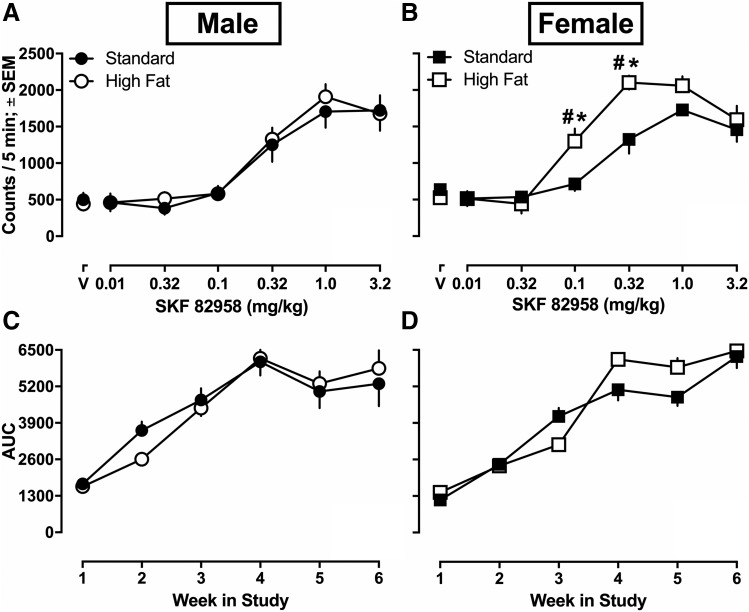
SKF 82958-Induced Locomotion.
SKF 82958 dose-dependently increased locomotor activity among male and female rats (Fig. 2, A and B). For each week of locomotor activity, three-way mixed model ANOVAs were conducted to examine group differences. No significant results were revealed until week 4. Since the effects that were revealed at week 4 and 5 were identical in terms of significance, week 5 (see Fig. 2, A and B) statistical values are reported here. The three-way mixed model ANOVA examining SKF 82958-induced locomotion dose-response curves on week 5 revealed a significant main effect of dose [F(3.912,140.8.0) = 115.0; P < 0.0001], as well as a significant dose × diet interaction effect [F(6,216) = 2.876; P = 0.0102], dose × sex interaction effect [F(6,216) = 3.731; P = 0.0015], and a significant dose × sex × diet interaction effect [F(6,216) = 2.329; P = 0.0336]. However, no significant effects of sex [F(1,36) = 2.306; P = 0.1376], diet [F(1,36) = 2.328; P = 0.1358], or sex × diet interaction effect [F(1,36) = 1.1214; P = 0.298] were observed.
Fig. 2.
SKF 82958-induced locomotion and sensitization for male and female rats eating high fat (n = 9 male, n = 10 female) or standard chow (n = 9 male, n = 12 female). SKF 82958-induced locomotion (e.g., ambulatory counts) for the last 5 minutes of each 15-minute recording session after 5 weeks of initial assignment to dietary conditions (A and B). Area under the weekly generated SKF 82958 dose-response curves (AUC) for male and female rats eating high fat or standard chow for 1–6 weeks (C and D). Three female rats eating high fat chow were lost due to attrition: two during week 4 and one during week 5 (data not included in analysis). Data were analyzed using a three-way (sex × diet × dose) mixed model ANOVA with Sidak’s multiple comparison post hoc tests. Data are expressed as means ± S.E.M. *P < 0.05, dietary difference within sex; #P < 0.05, female rats eating high fat chow had significantly increased locomotion compared with all other groups.
The main effect of dose demonstrates that regardless of diet or sex, SKF 82958 dose-dependently increased locomotion as compared with activity that occurred after saline injections. Specifically, Sidak’s multiple comparisons revealed that locomotion after 0.1 mg/kg [95% CI (676.459, 917.737)], 0.32 mg/kg [95% CI (1319.386, 1682.897)], 1.0 mg/kg [95% CI (1698.832, 2001.015)], and 3.2 mg/kg [95% CI (1413.482, 1812.493)] was greater than activity after saline injections [95% CI (442.680, 609.011)]. The significant dose × sex interaction demonstrates that regardless of diet, SKF 82958 induced significantly more locomotion among female rats at certain doses. Specifically, Sidak’s multiple comparisons revealed greater locomotion among females after 0.1 mg/kg [female, mean locomotor activity counts = 1007.725, 95% CI (845.484, 1169.966); male, mean locomotor activity counts = 586.444, 95% CI (407.823, 765.066)] and 0.32 mg/kg SKF 82958 [standard, mean locomotor activity counts = 1155.677, 95% CI (961.919, 1349.435); high fat, mean locomotor activity counts = 1676.963, 95% CI (1473.747, 1880.178)] as compared with males. The significant dose × diet interaction demonstrates that regardless of sex, SKF 82958 induced more locomotion among rats eating high fat chow than rats eating standard chow. Specifically, Sidak’s multiple comparisons revealed that SKF 82958 induced more locomotion in rats eating high fat chow after 0.1 mg/kg [standard, mean locomotor activity counts = 653.097, 95% CI (586.012, 720.182); high fat, mean locomotor activity counts = 941.072, 95% CI (766.974, 1115.171)] and 0.32 mg/kg [standard, mean locomotor activity counts = 1206.917, 95% CI (992.022, 1421.812); high fat, mean locomotor activity counts = 1715.367, 95% CI (1453.097, 1977.636)] SKF 82958. Finally, the significant dose × sex × diet interaction demonstrates that SKF 82958 at certain doses induced more locomotion among female rats eating high fat chow as compared with other groups. Specifically, Sidak’s multiple comparisons demonstrated greater SKF 82958-induced locomotion among female rats eating high fat chow [mean locomotor activity counts = 1300.700, 95% CI (1061.055, 1540.345)] compared with females eating standard chow [mean locomotor activity counts = 714.750, 95% CI (495.985, 933.515)], male rats eating high fat chow [mean locomotor activity counts = 581.444, 95% CI (328.836, 834.053)], and male rats eating standard chow [mean locomotor activity counts = 591.444, 95% CI (338.836, 844.053)] after 0.1 mg/kg SKF 82958. Similar differences were observed after 0.32 mg/kg SKF 82958, after which female rats eating high fat chow had significantly increased locomotion compared with all other groups (all P < 0.05). At week 6, there was a main effect of dose (P < 0.001), but no other significant differences remained. Three female rats eating high fat chow were lost due to attrition: two during week 4 of testing and one during week 5 (data were excluded for all analyses). To summarize, females were generally more sensitive to SKF 82958 locomotion than males. Some toxicity associated with repeated injections of SKF 82958 was also observed and only impacted female rats. Further, female rats eating high fat chow (but not males) were more sensitive to SKF 82958-induced locomotion than female rats eating standard chow.
SKF 82958-Induced Sensitization.
To analyze changes over time (e.g., across weeks) as an index of the development of sensitization to SKF 82958, AUC for SKF 82958-induced locomotion was calculated (Fig. 2). A three-way mixed model ANOVA revealed significant main effects of week [F(1.720,61.91) = 124.00; P < 0.0001], as well as a significant week × sex interaction effect [F(5,180) = 3.892; P = 0.0023] and a significant week × diet interaction effect [F(5,180) = 3.047; P = 0.0115]. However, no significant main effects of sex [F(1,36) = 1.828; P = 0.1848] or diet [F(1, 36) = 0.2319; P = 0.6330] and no week × diet × sex interaction effects [F(5, 180) = 1.195; P = 0.313] were revealed.
The significant main effect of week demonstrates that regardless of sex and diet, rats developed sensitization to the locomotor-stimulating effects of SKF 82958 after repeated testing during weeks 1–4. Specifically, the AUC for SKF 82958-induced locomotion increased weekly, from week 1 [mean area = 1486.618, 95% CI (1384.434, 1588.803)], week 2 [mean area = 2758.811, 95% CI (2556.518, 2961.104)], week 3 [mean area = 4101.808, 95% CI (3800.026, 4403.591)], and week 4 [mean area = 5881.296, 95% CI (5499.353, 6263.239)]. There were no continued increases in AUC at weeks 5 and 6, suggesting a ceiling effect regarding locomotor sensitization after week 4.
Although significant week × sex and week × diet interactions were revealed with the three-way ANOVA, Sidak’s post hoc multiple comparisons did not reveal any specific significant differences within the same week (all P > 0.05), suggesting that the rate of SKF 82958-induced sensitization was the same for all groups as measured by AUC (although see Fig. 2, A and B for differences among females for the week 5 SKF 82958 dose-response curve). To summarize, no differences in AUC were observed between males and females or between rats eating different diets; however, all rats (regardless of sex and diet) developed sensitization to SKF 82958 after repeated testing (as indicated by increased AUC in all groups during weeks 1–4).
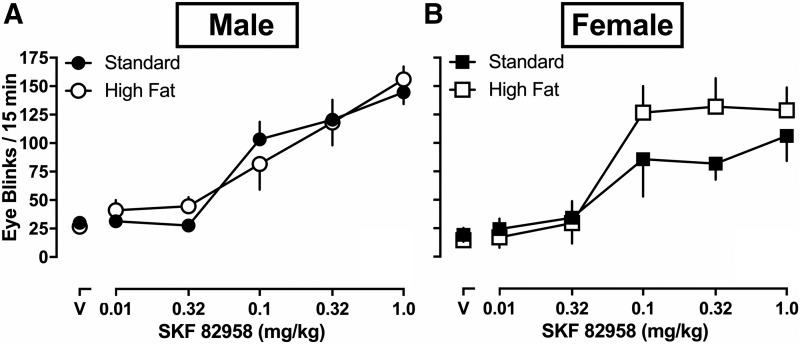
SKF 82958-Induced Eye Blinking.
SKF 82958 dose-dependently increased eye blinking among male and female rats. For each week of eye blinking activity, mixed model ANOVAs were conducted to examine group differences. The mixed model ANOVAs for week 1 and 2 revealed significant main effects of dose (all P’s < 0.5). The mixed model ANOVA for week 3 (Fig. 3) revealed a significant main effect of dose [F(2.422,24.22) = 49.07; P < 0.0001] but no significant main effects of diet [F(1,10) = 0.6580; P = 0.4362] or sex [F(1,10) = 0.9019; P = 0.3647], and no significant dose × diet [F(5,50) = 0.5301; P = 0.7524], diet × sex [F(5,50) = 1.176; P = 0.3342], sex × diet interaction [F(1,10) = 0.4290; P = 0.5273], or dose × sex × diet interaction effects [F(5,50) = 1.618; P = 0.1724] were observed. Specifically, SKF 82958 dose-dependently increased eye blinking on average among rats (Fig. 3) after 0.1 mg/kg [95% CI (66.709, 132.041)], 0.32 mg/kg [95% CI (78.469, 147.739)], and 1.0 mg/kg [95% CI (99.599, 168.234)] SKF 82958 as compared with eye blinking after saline injections [95% CI (466.321, 601.097)].
Fig. 3.
SKF 82958-induced eye blinking for male and female rats eating high fat (n = 3 male, n = 4 female) or standard chow (n = 3 male, n = 4 female) for the full 15-minute recording session after 3 weeks of initial assignment to dietary conditions (A and B). Although underpowered, data were analyzed using a three-way (sex × diet × dose) mixed model ANOVA. After 3 weeks of testing, treatment was discontinued because of apparent toxic effects of a combination of SKF 82958 and restraint testing. Attrition included 50% male rats eating standard chow, 50% male rats eating high fat chow, 50% female rats eating standard chow, and 60% female rats eating high fat chow. Data are expressed as means ± S.E.M.
During the experiment, the toxic effects of repeated SKF 82958 testing in combination with restraint became apparent, and treatment was discontinued after week 3. Attrition included three of six male rats eating standard chow, three of six male rats eating high fat chow, three of six female rats eating standard chow, and four of eight female rats eating high fat chow (data were excluded for all analyses). To summarize, repeated testing with SKF 82958 combined with eye blink restraints revealed toxic effects that precluded the ability to achieve statistical power to detect significant effects in the present report. Toxicity was observed in all groups for both sexes and both dietary conditions.
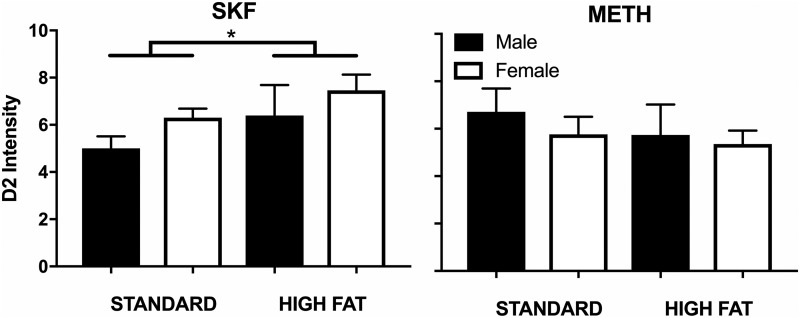
Dopamine D1 and D2 Receptor Expression.
Striatal dopamine receptor expression was examined using two-way between-subjects ANOVAs. The two-way between-subjects ANOVA examining striatal dopamine D2 receptor expression among rats that had been treated with SKF 82958 revealed a significant main effect of diet [F(1,20) = 7.15; P = 0.0146] but no main effect of sex [F(1,20) = 2.89; P = 0.1042] or a diet × sex interaction effect [F(1,20) = 0.02; P = 0.8959]. The significant main effect of diet demonstrates that regardless of sex, dopamine D2 receptor expression was significantly increased among rats eating high fat chow that were tested with SKF 82958 compared with rats eating standard chow (Fig. 4). In contrast, the two-way between-subjects ANOVA examining striatal dopamine D2 receptor expression among rats that had been treated with methamphetamine revealed no significant effects (all P > 0.05).
Fig. 4.
Dopamine D2 receptor intensity for male and female rats eating high fat (n = 6 male, n = 6 female) or standard chow (n = 6 male, n = 6 female) after 7 weeks of initial assignment to dietary conditions. Data were analyzed using an ordinary two-way (diet × sex) ANOVA with Sidak’s multiple comparison post hoc tests. Rats treated with SKF 82958 and eating high fat chow had significantly increased dopamine D2 receptor expression compared with rats eating standard chow regardless of sex (left). No changes in dopamine D2 receptor expression were observed in rats treated with methamphetamine. Data are expressed as means + S.E.M. *P < 0.05, compared with rats eating standard chow.
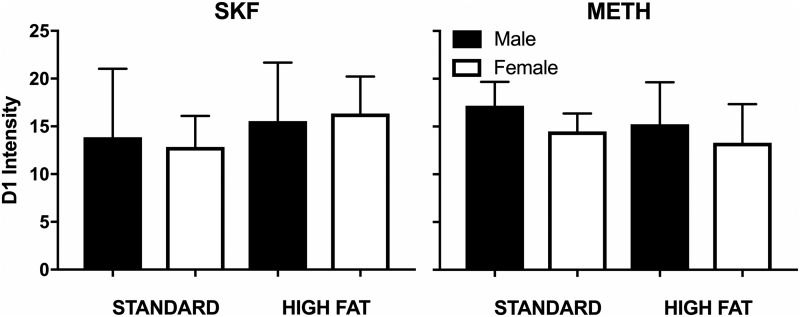
The two-way between-subjects ANOVAs examining striatal dopamine D1 receptor expression among rats that had been treated with SKF 82958 or methamphetamine revealed no significant effects (all P > 0.05; Fig. 5). To summarize, dopamine D2 receptor expression was increased among rats eating high fat chow that were tested with SKF 82958 (but not methamphetamine), and no differences were observed for dopamine D1 receptor expression.
Fig. 5.
Dopamine D1 receptor intensity for male and female rats eating high fat (n = 6 male, n = 6 female) or standard chow (n = 6 male, n = 6 female) after 7 weeks of initial assignment to dietary conditions. Data were analyzed using an ordinary two-way (diet × sex) ANOVA. Rats treated with SKF 82958 or methamphetamine did not have significantly different dopamine D1 receptor expression regardless of diet or sex. Data are expressed as means + S.E.M.
Discussion
Eating a high fat diet increases risk of cardiovascular disease, metabolic syndrome, and obesity (U.S. Department of Agriculture and U.S. Department of Health and Human Services, 2010). Eating high fat laboratory chow enhances sensitivity of rats to the behavioral effects of drugs that act directly and indirectly on dopamine systems (McGuire et al., 2011; Baladi et al., 2012a; Reyes, 2012; Collins et al., 2015). However, the majority of this work either has not considered potential sex differences or has focused on one sex exclusively. The present report evaluated the effects of eating high fat chow on sensitivity of male and female rats to the behavioral effects of methamphetamine and SKF 82958. Consistent with the literature, female rats were more sensitive to the locomotor-stimulating effects of methamphetamine and SKF 82958 as compared with male rats initially; however, after repeated injections, all rats developed sensitization, washing out these sex differences by week 4 of testing. As in previous reports with males (McGuire et al., 2011), eating high fat chow enhanced sensitivity of rats to methamphetamine-induced locomotion (Fig. 1B). Rats in all groups, regardless of sex or diet, developed sensitization to methamphetamine as indicated by increased AUC throughout weekly testing (Fig. 1, C and D). Female rats eating high fat chow were also more sensitive to the locomotor-stimulating effects of SKF 82958 (Fig. 2B). In contrast, male rats eating high fat chow tested weekly with SKF 82958 were not more sensitive than standard chow controls at any time point (Fig. 2A). Males and females developed sensitization to the locomotor-stimulating effects of SKF 82958 (Fig. 2, C and D); however, eating high fat chow did not enhance the rate of sensitization for either sex (Fig. 2D). SKF 82958 significantly increased eye blinking in all rats regardless of sex or diet; however, unexpectedly high attrition rates (∼50% of rats) contributed to inadequate statistical power to detect significant effects. Despite this, it does appear as though female rats were more sensitive than males to the toxic effects of SKF 82958 repeated testing in combination with restraint, given the larger rate of attrition among females as compared with males. Taken together, the SKF 82958 experiments suggest that enhanced sensitivity of females to methamphetamine might be largely due to changes involving dopamine D1 receptors, given that females eating high fat chow are also more sensitive to SFK 82958-induced locomotion. In contrast, diet did not impact sensitivity of males to SKF 82958, suggesting that high fat diet–induced enhanced sensitivity to methamphetamine might not be related to changes at the level of the dopamine D1 receptor among males.
This hypothesis is supported by previous work demonstrating that, in the absence of dietary manipulation, there are already sex differences with regard to dopamine D1 receptors. For example, dopamine D1 receptor agonists differentially impact cocaine-induced conditioned place preference in male and female rats (Nazarian et al., 2004). Further, in cell culture, the female sex hormone estradiol suppresses dopamine D2 receptor–mediated inhibition of adenylyl cyclase activity and enhances dopamine D1 receptor–mediated activation of adenylyl cyclase (Maus et al., 1989; Becker and Hu, 2008). Although fewer reports have examined dopamine receptors directly in studies using both sexes and high fat diets, female sex hormones (i.e., estradiol) are increased in rats eating high fat chow (Hilakivi-Clarke et al., 1996) and decreased in rats eating a low fat chow (Rosenthal et al., 1985; Heber et al., 1991), suggesting a potential interaction between diet and sex hormones that might be underlying some of the sex differences reported here.
Although increases in dopamine D1 receptor gene expression in rats eating a high fat/high sugar diet have been described previously (Alsio et al., 2010), in the present report, dopamine D1 receptor expression was not altered by diet regardless of sex (Fig. 5). Therefore, the high fat chow–induced enhancements in SKF 82958-induced locomotion in female rats demonstrated here (Fig. 2) are likely due to factors other than general receptor expression changes. In fact, repeated psychomotor stimulant administration itself can enhance cellular and behavioral activity in the absence of changes to receptor density (Kleven et al., 1990; Peris et al., 1990; Henry and White, 1991). For example, repeated cocaine administration increases the cellular effects of dopamine D1 receptor agonist SKF 38393 without altering dopamine D1 receptor density (Henry and White, 1991). Similarly, supersensitivity to dopamine has also been reported in the absence of dopamine receptor density changes in studies examining nigral denervation as a result of intracerebral 6-hydroxydopamine infusions (Leslie and Bennett, 1987; Ariano, 1988). These instances of supersensitivity are likely partly mediated by enhancements in dopamine D1 receptor downstream transduction mechanisms (Henry and White, 1991; Terwilliger et al., 1991). Taken together, these previous studies suggest that although dopamine D1 receptor expression was not changed by diet in the present report, some other aspect of dopamine D1 receptor function is likely altered specifically among female rats eating high fat chow, resulting in enhanced sensitivity to methamphetamine and SKF 82958.
Although dopamine D1 receptor–specific changes might underlie the high fat chow–induced changes in drug sensitivity among females, enhanced sensitivity of males eating high fat chow to methamphetamine might be predominantly driven by changes at the level of the dopamine transporter (South and Huang, 2008; Speed et al., 2011; Cone et al., 2013) dopamine D2 receptor (South and Huang, 2008; Baladi et al., 2012a) and/or dopamine D3 receptor (Heidbreder et al., 2005; Baladi et al., 2011). For example, male rats eating high fat chow have decreased dopamine clearance rate as well as decreased dopamine transporter expression (Speed et al., 2011). Further, those observed decreases are posited to be mediated by high fat chow–induced decreases in an insulin signaling central kinase (phosphorylated protein kinase B [pAkt ]; see Speed et al., 2011). Interestingly, decreases in pAkt are not observed in female rats eating high fat chow (Ramos et al., 2019), supporting the hypothesis that high fat chow–induced changes in drug sensitivity are differentially mediated in males versus females. Additionally, previous reports have demonstrated increased behavioral sensitivity to the dopamine D2/D3 receptor agonist quinpirole (Baladi et al., 2011) as well as increased dopamine D2 receptor binding density (South and Huang, 2008) among male rats eating high fat chow as compared with male rats eating standard chow. Although drug-naïve rats eating different diets were not examined in the present report, dopamine D2 receptor expression was only increased among male and female rats eating high fat chow that were tested with SKF 82958 (Fig. 4). However, it is possible that the behavioral changes in drug sensitivity among male rats [e.g., enhanced sensitivity to methamphetamine (McGuire et al., 2011; Speed et al., 2011) and quinpirole (Baladi and France, 2009; Ramos et al., 2019)] also occur in the absence of changes to dopamine D2 receptor expression (see Fig. 4). For example, at least one other report found no differences in dopamine D2/D3 receptor availability in rats eating high fat chow (van de Giessen et al., 2012). One potential factor that might underlie the discordant results in the literature regarding receptor expression changes is body weight itself. For example, it has been well demonstrated that eating a high fat diet produces changes to drug sensitivity, similar to those demonstrated in this report, even in the absence of changes to body weight (Baladi et al., 2011; Hernandez-Casner et al., 2019; Ramos et al., 2019). In contrast, many of the reports demonstrating changes in dopamine receptor expression also describe increases in body weight among animals eating a high fat diet (South and Huang, 2008; Johnson and Kenny, 2010; Konner et al., 2011). Further, changes to dopamine D2 receptor binding has also been demonstrated among obese human patients (Wang et al., 2001; de Weijer et al., 2011; Tomasi and Volkow, 2013). Although the rats in the present experiments were not definable as obese by many recognized definitions, we have previously demonstrated that body fat composition can be altered even in the absence of changes to overall body weight (Hernandez-Casner et al., 2019). Future research will explore the possible factors involved in weight gain versus body fat distribution changes that might be occurring concurrent to changes in dopamine receptor expression and sensitivity.
Taken together with previous research, the present report suggests that the mechanism(s) by which high fat diets enhance sensitivity of rats to dopaminergic drugs might be distinct between males and females. These data highlight the importance of examining both sexes in experiments related to both dietary manipulations, as well as drug sensitivity, since behavioral as well as receptor expression changes do not always generalize across sex. Future research will further examine the role of sex hormones in modulating these sex differences. Additionally, future research should explore how these differences in drug sensitivity as a function of diet and/or sex might contribute to differences in abuse vulnerability among males and females using animal models of substance use disorder.
Acknowledgments
The authors would like to acknowledge Jorge Gonzalez for his technical assistance in building the eye blinking restraint devices.
Abbreviations
- PND
postnatal day
- AUC
area under the curveb
- CI
confidence interval
Authorship Contributions
Participated in research design: Ramos, Serafine.
Conducted experiments: Ramos, Hardin, Grant, Flores-Robles, Gonzalez, Cruz, Martinez, Beltran, Serafine.
Performed data analysis: Ramos, Grant, Serafine.
Wrote or contributed to the writing of the manuscript: Ramos, Hardin, Grant, Flores-Robles, Gonzalez, Cruz, Martinez, Beltran, Serafine.
Footnotes
The authors declare no conflict of interest. The present study was supported by the National Institute on Drug Abuse [R25DA033613] and the National Institute of General Medical Sciences [R25GM069621] of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Alsiö J, Olszewski PK, Norbäck AH, Gunnarsson ZEA, Levine AS, Pickering C, Schiöth HB. (2010) Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience 171:779–787 DOI: 10.1016/j.neuroscience.2010.09.046. [DOI] [PubMed] [Google Scholar]
- Ariano MA. (1988) Striatal D1 dopamine receptor distribution following chemical lesion of the nigrostriatal pathway. Brain Res 443:204–214 DOI: 10.1016/0006-8993(88)91614-9. [DOI] [PubMed] [Google Scholar]
- Artigiani EE, Hsu MH, McCandlish D, Wish ED. (2018) Methamphetamine a Regional Drug Crisis, National Drug Early Warning System, College Park, MD. [Google Scholar]
- Baladi MG, Daws LC, France CP. (2012b) You are what you eat: influence of type and amount of food consumed on central dopamine systems and the behavioral effects of direct- and indirect-acting dopamine receptor agonists. Neuropharmacology 63 (1):76–86, doi: 10.1016/j.neuropharm.2012.02.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. (2009) High fat diet and food restriction differentially modify the behavioral effects of quinpirole and raclopride in rats. Eur J Pharmacol 610:55–60 DOI: 10.1016/j.ejphar.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Horton RE, Owens WA, Daws LC, France CP. (2015) Eating high fat chow decreases dopamine clearance in adolescent and adult male rats but selectively enhances the locomotor stimulating effects of cocaine in adolescents. Int J Neuropsychopharmacol 18:pyv024 DOI: 10.1093/ijnp/pyv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Koek W, Aumann M, Velasco F, France CP. (2012) Eating high fat chow enhances the locomotor-stimulating effects of cocaine in adolescent and adult female rats. Psychopharmacology (Berl) 222:447–457 DOI: 10.1007/s00213-012-2663-7. [DOI] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. (2011) Influence of body weight and type of chow on the sensitivity of rats to the behavioral effects of the direct-acting dopamine-receptor agonist quinpirole. Psychopharmacology (Berl) 217:573–585 DOI: 10.1007/s00213-011-2320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Bevins RA. (1999) Locomotion and conditioned place preference produced by acute intravenous amphetamine: role of dopamine receptors and individual differences in amphetamine self-administration. Psychopharmacology (Berl) 143:39–46 DOI: 10.1007/s002130050917. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. (2008) Sex differences in drug abuse. Front Neuroendocrinol 29:36–47 DOI: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen HHG, Nickolson VJ. (1981) Androgenic influences on apomorphine-induced yawning in rats. Behav Neural Biol 33:123–128 DOI: 10.1016/s0163-1047(81)92306-2. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Carati C, Lea RA, Fitzmaurice PS, Schenk S. (2009) Effect of D1-like and D2-like receptor antagonists on methamphetamine and 3,4-methylenedioxymethamphetamine self-administration in rats. Behav Pharmacol 20:688–694 DOI: 10.1097/fbp.0b013e328333a28d. [DOI] [PubMed] [Google Scholar]
- Cabib S, Castellano C, Cestari V, Filibeck U, Puglisi-Allegra S. (1991) D1 and D2 receptor antagonists differently affect cocaine-induced locomotor hyperactivity in the mouse. Psychopharmacology (Berl) 105:335–339 DOI: 10.1007/bf02244427. [DOI] [PubMed] [Google Scholar]
- Caine SB, Koob GF. (1994) Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther 270:209–218. [PubMed] [Google Scholar]
- Camp DM, Robinson TE. (1988) Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav Brain Res 30:55–68 DOI: 10.1016/0166-4328(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Shippenberg TS. (2009) Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology 34:887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Fletcher H, Perrotti LI, Jenab S, Quiñones-Jenab V. (2001) Sex differences in cocaine-induced behavioral sensitization. Cell Mol Biol (Noisey-le-grand) 47: 1089–1095. [PubMed] [Google Scholar]
- Collins GT, Chen Y, Tschumi C, Rush EL, Mensah A, Koek W, France CP. (2015) Effects of consuming a diet high in fat and/or sugar on the locomotor effects of acute and repeated cocaine in male and female C57BL/6J mice. Exp Clin Psychopharmacol 23:228–237 DOI: 10.1037/pha0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JJ, Chartoff EH, Potter DN, Ebner SR, Roitman MF. (2013) Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS One 8:e58251 DOI: 10.1371/journal.pone.0058251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Neumeyer JL, Bergman J, Paronis CA. (2007) Pharmacological characterization of the effects of dopamine D(1) agonists on eye blinking in rats. Behav Pharmacol 18:745–754 DOI: 10.1097/fbp.0b013e3282f14ee6. [DOI] [PubMed] [Google Scholar]
- de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, van de Laar A, Fliers E, Serlie MJ, Booij J. (2011) Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res 1:37 DOI: 10.1186/2191-219x-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordahl SC, Jones SR. (2017) High-fat-diet-induced deficits in dopamine terminal function are reversed by restoring insulin signaling. ACS Chem Neurosci 8:290–299 DOI: 10.1021/acschemneuro.6b00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Schechter JB, French ED. (2008) Context-specific reversal of cocaine sensitization by the CB1 cannabinoid receptor antagonist rimonabant. Neuropsychopharmacology 33:2747–2759 DOI: 10.1038/sj.npp.1301648. [DOI] [PubMed] [Google Scholar]
- Graham DL, Amos-Kroohs RM, Braun AA, Grace CE, Schaefer TL, Skelton MR, Williams MT, Vorhees CV. (2013) Neonatal +-methamphetamine exposure in rats alters adult locomotor responses to dopamine D1 and D2 agonists and to a glutamate NMDA receptor antagonist, but not to serotonin agonists. Int J Neuropsychopharmacol 16:377–391 DOI: 10.1017/S1461145712000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, Ogden CL. (2017) Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief 1–8 [PubMed]
- Heber D, Ashley JM, Leaf DA, Barnard RJ. (1991) Reduction of serum estradiol in postmenopausal women given free access to low-fat high-carbohydrate diet. Nutrition 7:137–139; discussion 139–140. [PubMed] [Google Scholar]
- Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, Ashby CR., Jr (2005) The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Brain Res Rev 49:77–105 DOI: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DJ, White FJ. (1991) Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther 258:882–890. [PubMed] [Google Scholar]
- Hernandez-Casner C, Woloshchuk CJ, Poisson C, Hussain S, Ramos J, Serafine KM. (2019) Dietary supplementation with fish oil reverses high fat diet-induced enhanced sensitivity to the behavioral effects of quinpirole. Behav Pharmacol 30:370–375 DOI: 10.1097/fbp.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Cho E, Onojafe I. (1996) High-fat diet induces aggressive behavior in male mice and rats. Life Sci 58:1653–1660 DOI: 10.1016/0024-3205(96)00140-3. [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. (2008) Monoamine transporters and psychostimulant addiction. Biochem Pharmacol 75:196–217 DOI: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Hubner CB, Moreton JE. (1991) Effects of selective D1 and D2 dopamine antagonists on cocaine self-administration in the rat. Psychopharmacology (Berl) 105:151–156 DOI: 10.1007/bf02244301. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. (2010) Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats [published correction appears in Nat Neurosci (2010) 13:1033]. Nat Neurosci 13:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Perry BD, Woolverton WL, Seiden LS. (1990) Effects of repeated injections of cocaine on D1 and D2 dopamine receptors in rat brain. Brain Res 532:265–270. [DOI] [PubMed] [Google Scholar]
- Könner AC, Hess S, Tovar S, Mesaros A, Sánchez-Lasheras C, Evers N, Verhagen LA, Brönneke HS, Kleinridders A, Hampel B, et al. (2011) Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell Metab 13:720–728. [DOI] [PubMed] [Google Scholar]
- Kotani M, Kiyoshi A, Murai T, Nakako T, Matsumoto K, Matsumoto A, Ikejiri M, Ogi Y, Ikeda K. (2016) The dopamine D1 receptor agonist SKF-82958 effectively increases eye blinking count in common marmosets. Behav Brain Res 300:25–30 DOI: 10.1016/j.bbr.2015.11.028. [DOI] [PubMed] [Google Scholar]
- Leslie CA, Bennett JP., Jr (1987) Striatal D1- and D2-dopamine receptor sites are separately detectable in vivo. Brain Res 415:90–97 DOI: 10.1016/0006-8993(87)90271-x. [DOI] [PubMed] [Google Scholar]
- Martinez LA, Lees ME, Ruskin DN, Masino SA. (2019) A ketogenic diet diminishes behavioral responses to cocaine in young adult male and female rats. Neuropharmacology 149:27–34 DOI: 10.1016/j.neuropharm.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus M, Bertrand P, Drouva S, Rasolonjanahary R, Kordon C, Glowinski J, Premont J, Enjalbert A. (1989) Differential modulation of D1 and D2 dopamine-sensitive adenylate cyclases by 17 beta-estradiol in cultured striatal neurons and anterior pituitary cells. J Neurochem 52:410–418 DOI: 10.1111/j.1471-4159.1989.tb09136.x. [DOI] [PubMed] [Google Scholar]
- McGuire BA, Baladi MG, France CP. (2011) Eating high-fat chow enhances sensitization to the effects of methamphetamine on locomotion in rats. Eur J Pharmacol 658:156–159 DOI: 10.1016/j.ejphar.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. (2007) Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav 86:140–149 DOI: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naneix F, Tantot F, Glangetas C, Kaufling J, Janthakhin Y, Boitard C, De Smedt-Peyrusse V, Pape JR, Vancassel S, Trifilieff P, et al. (2017) Impact of early consumption of high-fat diet on the mesolimbic dopaminergic system. eNeuro 4 DOI: 10.1523/eneuro.0120-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian A, Russo SJ, Festa ED, Kraish M, Quinones-Jenab V. (2004) The role of D1 and D2 receptors in the cocaine conditioned place preference of male and female rats. Brain Res Bull 63:295–299 DOI: 10.1016/j.brainresbull.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Oginsky MF, Goforth PB, Nobile CW, Lopez-Santiago LF, Ferrario CR. (2016) Eating ‘junk-food’ produces rapid and long-lasting increases in NAc CP-AMPA receptors: implications for enhanced cue-induced motivation and food addiction. Neuropsychopharmacology 41:2977–2986 DOI: 10.1038/npp.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, Boyson SJ, Cass WA, Curella P, Dwoskin LP, Larson G, Lin LH, Yasuda RP, Zahniser NR. (1990) Persistence of neurochemical changes in dopamine systems after repeated cocaine administration. J Pharmacol Exp Ther 253:38–44. [PubMed] [Google Scholar]
- Ramos J, Hernandez-Casner C, Cruz B, Serafine KM. (2019) Sex differences in high fat diet-induced impairments to striatal Akt signaling and enhanced sensitivity to the behavioral effects of dopamine D2/D3 receptor agonist quinpirole. Physiol Behav 203:25–32 DOI: 10.1016/j.physbeh.2017.11.014. [DOI] [PubMed] [Google Scholar]
- Reyes TM. (2012) High-fat diet alters the dopamine and opioid systems: effects across development. Int J Obes Suppl 2 (Suppl 2):S25–S28 DOI: 10.1038/ijosup.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJF, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, Berridge KC, Ferrario CR. (2015) Individual differences in cue-induced motivation and striatal systems in rats susceptible to diet-induced obesity. Neuropsychopharmacology 40:2113–2123 DOI: 10.1038/npp.2015.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal MB, Barnard RJ, Rose DP, Inkeles S, Hall J, Pritikin N. (1985) Effects of a high-complex-carbohydrate, low-fat, low-cholesterol diet on levels of serum lipids and estradiol. Am J Med 78:23–27 DOI: 10.1016/0002-9343(85)90456-5. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Carmona GN. (2002) Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol Biochem Behav 72:857–863 DOI: 10.1016/s0091-3057(02)00770-0. [DOI] [PubMed] [Google Scholar]
- Serafine KM, Bentley TA, Grenier AE, France CP. (2014) Eating high fat chow and the behavioral effects of direct-acting and indirect-acting dopamine receptor agonists in female rats. Behav Pharmacol 25:287–295 DOI: 10.1097/fbp.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafine KM, Labay C, France CP. (2016) Dietary supplementation with fish oil prevents high fat diet-induced enhancement of sensitivity to the locomotor stimulating effects of cocaine in adolescent female rats. Drug Alcohol Depend 165:45–52 DOI: 10.1016/j.drugalcdep.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South T, Huang XF. (2008) High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem Res 33:598–605 DOI: 10.1007/s11064-007-9483-x. [DOI] [PubMed] [Google Scholar]
- Speed N, Saunders C, Davis AR, Owens WA, Matthies HJG, Saadat S, Kennedy JP, Vaughan RA, Neve RL, Lindsley CW, et al. (2011) Impaired striatal Akt signaling disrupts dopamine homeostasis and increases feeding. PLoS One 6:e25169 DOI: 10.1371/journal.pone.0025169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. (1991) A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res 548:100–110 DOI: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. (2013) Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol Biol 48:1–19 DOI: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services (2010) Dietary Guidelines for Americans, 7th ed, U.S. Government Printing Office, Washington, DC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Giessen E, la Fleur SE, de Bruin K, van den Brink W, Booij J. (2012) Free-choice and no-choice high-fat diets affect striatal dopamine D2/3 receptor availability, caloric intake, and adiposity. Obesity (Silver Spring) 20:1738–1740 DOI: 10.1038/oby.2012.17. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. (2001) Brain dopamine and obesity. Lancet 357:354–357 DOI: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]