Summary:
Subtotal loss of the nose is a devastating occurrence. Traditional approaches to reconstruction have employed techniques that sequentially restore the nasal lining, support and external cover using autologous tissues. The results can be quite variable and are heavily weighted on surgical experience and expertise. We report a case of subtotal nasal reconstruction using a computer generated, 3-D printed porous polyethylene (PPE) scaffold. The patient is a 64-year-old man who presented with a sub-total nasal defect following excision of recurrent basal cell carcinoma. The missing parts comprised the distal half of the composite nose including the nasal floor and lining. The replacement nose was constructed on the patient’s right radial forearm. A computer generated PPE nasal scaffold was prelaminated with a forearm flap for lining and a free temporal fascial flap and skin graft for external cover. Following healing, nostrils were created and the nasal construct was then microsurgically transferred to the face. At 18 months post-op, the reconstructed nose has remained stable and functional with excellent aesthetic appearance. The implications for use of 3-D scaffolds for composite nasal reconstruction are enormous.
Subtotal loss of the nose is a devastating occurrence. Total nasal reconstruction is a challenging surgical procedure and even in the most skillful hands optimal results may be difficult to achieve. Experience has underscored the importance of 3 essential elements, nasal lining, support, and external cover, for achieving a successful nasal reconstruction. For total and subtotal nasal reconstruction, the amount of donor tissue required to restore these missing elements is considerable and often results in significant donor site morbidity including pain, scarring, and deformity. To address these shortcomings and to exert a more deliberate intervention for control of nasal shape and size, we sought to utilize a biocompatible 3-dimensional (3D) scaffold to serve as a support and form modulator for nasal reconstruction. Porous polyethylene (PPE) has been used extensively in facial reconstruction as onlay grafts for skeletal augmentation and as a scaffold for total ear reconstruction.1 Its advantages include excellent biocompatibility, tissue ingrowth, and adherence.2 We report our experience with a case of subtotal nasal reconstruction using a computer-generated, 3D printed PPE scaffold.
CASE REPORT
The patient is a 64-year-old man with recurrent basal cell carcinoma in a prior subtotal nasal reconstruction. His history dates back to 15 years ago when he was first diagnosed with basal cell carcinoma of his left lateral nose involving the nasal sidewall. The lesion was treated with local resection and a forehead flap at an outside hospital. He developed a recurrence 5 years later and underwent composite resection, and radiation therapy. With no evidence of recurrent disease after 1 year, the patient underwent subtotal nasal reconstruction with a radial forearm flap for adjacent cheek and vestibular lining and a second forehead flap and rib cartilage grafts for external cover and support. The patient did well until 3 years ago when he developed recurrent disease on the right side of his nose involving the ala and columella. This necessitated wide composite resection with removal of his prior nasal reconstruction (Fig. 1). He then underwent a 1-year course of Vismodegib chemotherapy. In the aftermath following his subtotal nasal resection, the patient wore an adhesive retained nasal prosthesis.
Fig. 1.

Frontal view. Subtotal nasal defect in this 64-year-old man 1 year postoperatively following excision of prior autologous nasal reconstruction due to recurrent basal cell carcinoma.
Eighteen months following cessation of chemotherapy, the patient returned seeking nasal reconstruction of his subtotal nasal defect. The nasal defect comprised the distal two-thirds of his nose including the nasal floor and vestibular lining. After discussing the various options available for reconstruction, we elected to fabricate his missing nose using a computer-generated PPE scaffold laminated with autologous tissues much in the same fashion as has been described for total ear reconstruction.1 The patient’s silicone nasal prosthesis was used as a template for the reconstruction. Barium-infused medical grade silicone elastomer was cast in the patient’s existing prosthetic mould to produce a design prototype for imaging. Following processing, the silicone nose form was then imaged by cone beam computed tomography (i-CAT FLX; Imaging Sciences International LLC, Hatfield, PA) and segmented in Mimics (Materialise, Leuven, BE) to produce a virtual 3D model of the patient’s custom nose form. The resultant 3D model was exported as an *.stl file for further CADCAM processing of the PPE nasal scaffold, including offsetting to accommodate the anticipated autologous external and lining cover thickness. The final nasal scaffold model was then printed in PPE having an average thickness of 2 mm and an average pore size of 500 µm (Poriferous - Sup-or) (Fig. 2). The replacement nose was constructed on the patient’s right volar forearm. The PPE scaffold was prelaminated with a contoured radial forearm flap for lining, and the external surface was covered with a free temporal parietal fascial flap and a skin graft. Following initial healing, nostrils were created by coring out preplanned nostril apertures and lining the resulting raw surfaces with full-thickness skin grafts (Fig. 3). Two months later, the nasal construct was transferred to the face where vascular repairs were made to the facial artery and vein. The nasal lining of the construct was repaired to the existing nasal lining, and the PPE scaffold edge of the construct was suture fixated to the remnant nasal bone and cartilage framework. The external cover of the construct was repaired to the surrounding skin surfaces of the nose, face, and upper lip. Postoperative monitoring was performed using percutaneous Doppler assessment of vascular pedicle flow. In a subsequent operation, 3 months later, scalp epidermal overgrafting of the construct was performed to improve its color match to the adjacent facial skin. At 22 months postoperatively, the reconstructed nose has remained stable and functional with excellent aesthetic appearance (Fig. 4).
Fig. 2.
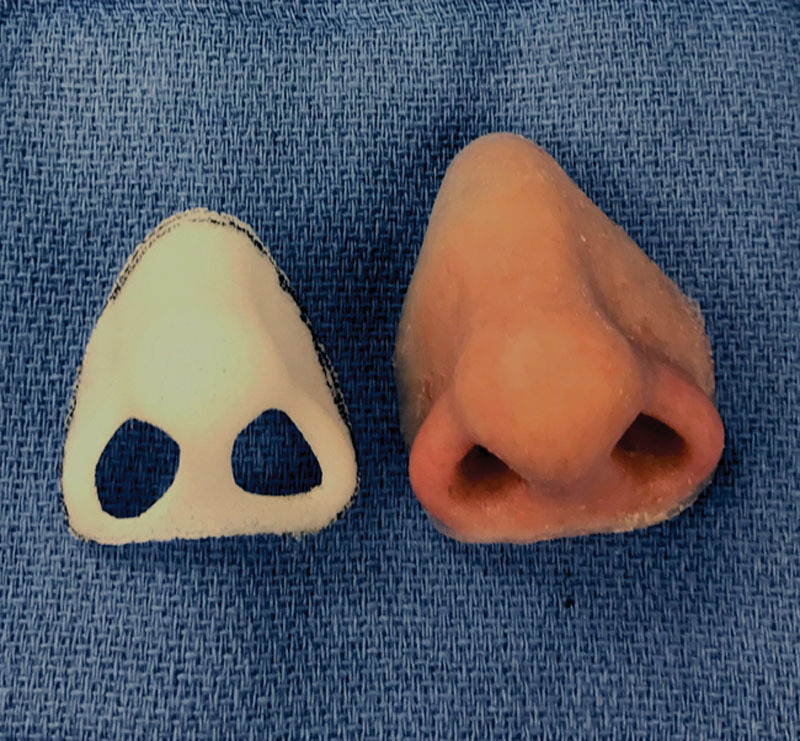
Three-dimensional printed custom PPE scaffold (left) compared to the silicone prosthesis upon which the scaffold was based.
Fig. 3.
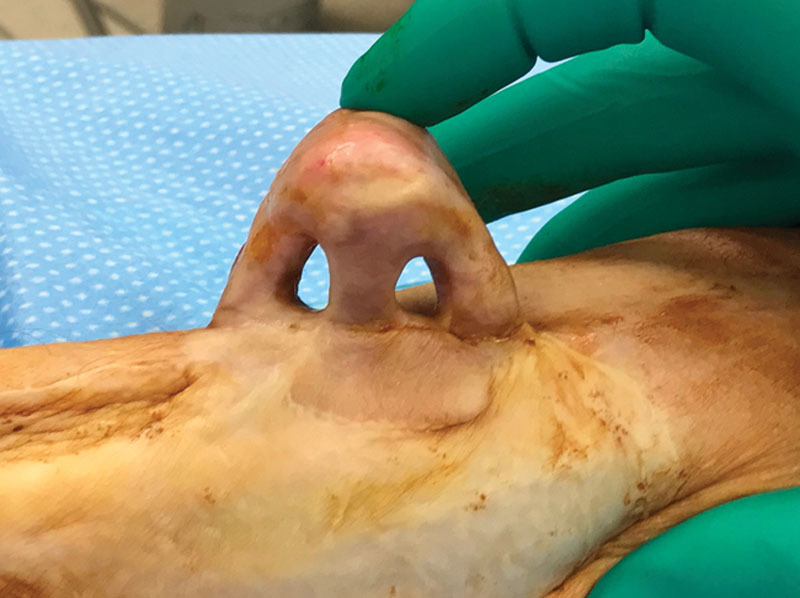
The composite flap construct is shown on the donor forearm before transfer. Nostril apertures were created following the initial prelamination procedure.
Fig. 4.
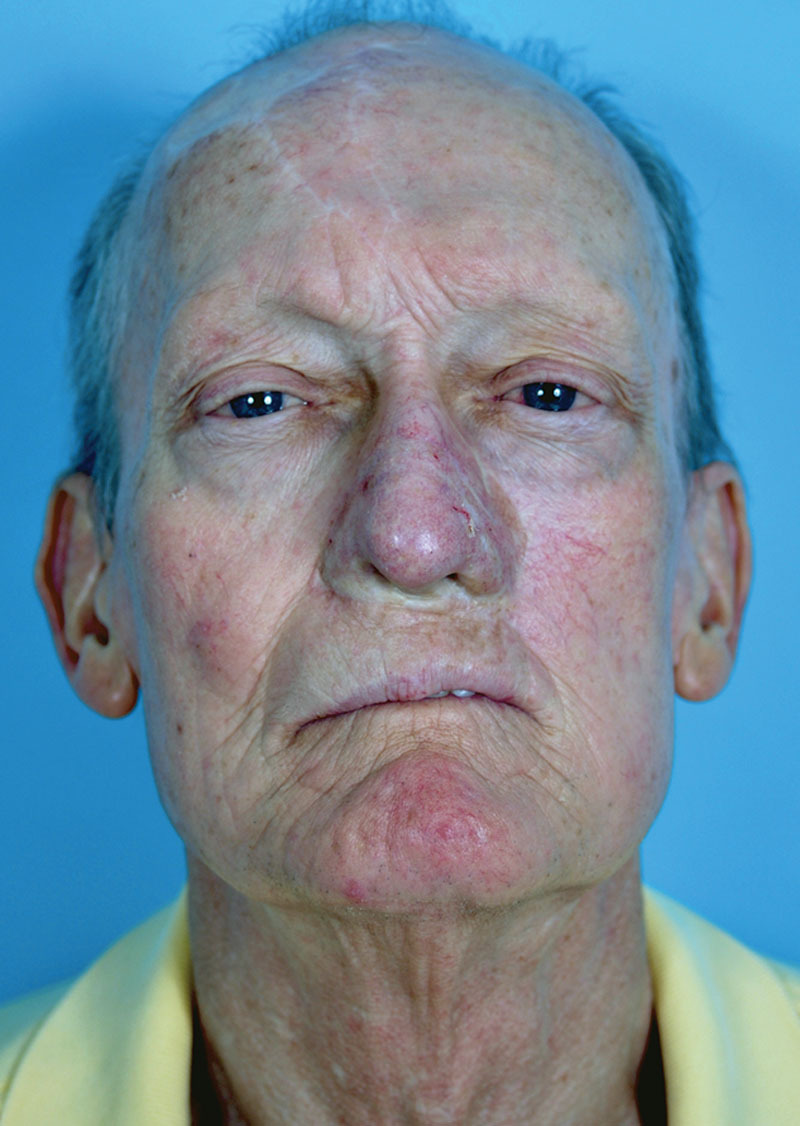
Frontal view. The patient is shown 22 months following completion of the reconstruction. Three months following microsurgical transfer, epidermal overgrafting was performed to improve color match of the construct to the adjacent facial skin.
DISCUSSION
This is the first clinical report of a subtotal nasal reconstruction performed using a computer-generated, 3D printed PPE scaffold in a human. The implications for use of 3D scaffold materials for composite nasal reconstruction are enormous owing to the improved precision in rendering size and form in these complex reconstructions. Unlike traditional total and subtotal nasal reconstructions that use separate pieces of bone and cartilage to stabilize and render form to the nasal construct through a series of operative stages, a 3D alloplast scaffold combines all of these elements into 1 unit in 1 setting with no donor site. The nasal scaffold becomes the primary determinate of the construct size, shape, and contour. The beauty of this lies in its simplicity in providing a stable, accurate, predictable nasal form. That the scaffold can be computer generated to any desirable dimension introduces a heretofore unprecedented latitude of options for the reconstructive effort.
PPE implants have been utilized extensively in facial skeletal reconstruction as onlay implants and as scaffold material for total ear reconstruction.1,3 Prior applications using PPE implants in nasal reconstruction have focused on basic sheet or single strut fabrications, and these applications have shown excellent fibrovascular ingrowth of the explanted nasal implants.2,4 Although PPE has demonstrated efficacy in this construct setting, as a scaffold material it remains imperfect. An ideal scaffold should be biocompatible (preferably autologous), inert, provide for surface cellular adhesion/integration with minimal foreign body reaction, possess sufficient strength and rigidity to provide support but have a modicum of flexibility to dampen the daily environmental traumas and lessen the tendency for soft tissue shear, inflammation, erosion, and exposure.5 An ideal scaffold would exhibit biomechanical confluence with its viscoelastic soft tissue environment, and if exposed, would promote cellular migration and secondary healing.6
Numerous alloplastic materials have been used for support in nasal reconstruction including silicone, titanium, cartilage allograft, with each having its unique advantages and disadvantages.3–5 Three-dimensional printing of implants for nasal reconstruction is relatively new and has found early clinical application in the fabrication of nasal models and airway splints.7–9 The use of 3D printing to create custom nasal implants in aesthetic and reconstructive nasal surgery is an evolving technology.10–12 Recent success has been demonstrated in the fabrication of patient-specific silicone polymer buttons for treatment of nasal septal perforations.13 Experimental studies have demonstrated the feasibility of using 3D printing technology to create anatomic alloplastic facsimiles of native supporting cartilage for nasal reconstruction.14,15 In a tissue engineering study in a porcine model, 3D printed nasal and ear polycaprolactone scaffolds were seeded with chondrogenic growth factors in a hyaluronic acid/collagen hydrogel. Cartilage regeneration within the scaffolds was observed, demonstrating the feasibility of employing this technology to engineer composite, alloplastic/biologic scaffolds approximating the biomechanical properties of native cartilage while retaining a defined shape and stability over time.16
PPE has many attributes that satisfy the requisites of an ideal scaffold. It is biocompatible, provides for soft tissue integration, has a modicum of flexibility while providing for stable support, and lends well to 3D rendering. Shortcomings of PPE relate to the susceptibility of its integrated soft tissue envelope to shear trauma and healing problems if the implant becomes exposed. It can also fracture if subjected to significant trauma. Although most of these issues can be dealt with using thoughtful construct design, scaffold placement, and judicious wound care intervention, optimal application of this new technology will require the development of improved scaffolds that further approximate the ideal.
The PPE scaffold in this case provided an excellent framework upon which to integrate a soft tissue lining and external cover. The composition and pore size of the PPE scaffold utilized in our construct is very similar to other common facial implants fabricated commercially that accommodate soft tissue adherence and ingrowth (eg, Medpor I Stryker). There has been no evidence to date of breakdown of the external cover or the coaptation sites of the construct to the facial skin and nasal lining. Obviously, the success of this technique for use in nasal reconstruction must be measured from the perspective of time. Historically, ear constructs using a PPE scaffold and fascia/skin graft cover have performed quite well with very few complications despite the relatively exposed position on the head.1 Like the ear, the nose is also prone to surface trauma. Importantly, the nasal lining presents an added level of complexity owing to the relatively moist environment and increased surface bacteria to which it is exposed. The long-term effects in this setting are not known. Experience with traditional techniques in total and subtotal nasal reconstruction where epidermal lining flaps have been utilized for nasal lining has shown no untoward sequelae as a result of chronic exposure to a moist internal nasal environment.17–19
In a “worse-case” scenario where the scaffold might become exposed, this could potentially lead to infection of the construct, its possible extrusion, and loss of the reconstruction. In reviewing the current literature on PPE implant use in the nose, this has not been the general experience, as most PPE exposures have been successfully treated with local wound care, minimal debridement, and soft tissue closure.5
For this case, we utilized a computer-generated 3D PPE scaffold as the basis for the nasal reconstruction. Unlike previously reported ear reconstructions that utilize a PPE scaffold covered with a fascial flap and skin graft, we chose to cover 1 side of the construct (the lining side) with a well-vascularized radial forearm flap. The rationale for this approach was to line the hidden interior of the construct with stable soft tissue not requiring a modulus of motion to dampen shear forces, such as provided by a free fascial flap, because of the reduced exposure to surface trauma compared to the exterior. As well, the physical coverage of the convoluted surfaces of both lining and external cover of the construct was simplified by using 2 separate flaps. The external cover of the construct was provided by a free fascial flap and a skin graft, much in the same fashion as has been described for ear reconstruction.1 The fascia imparts a unique characteristic to the external cover of a PPE scaffold in that it allows for slight motion of the skin graft cover in relation to the underlying framework. This serves to dampen the effect of surface trauma by buffering shear forces, thereby stifling the tendency for skin and soft tissue injury. The thin fascia and skin graft external cover also provided excellent contour resolution of the delicate contours of the underlying scaffold, thereby improving the aesthetic appearance of the reconstruction—which approximates the normal appearance of a nose. A much thicker flap would likely not have provided such refinement without subsequent thinning or debulking procedures.
Unlike total ear reconstructions that utilize generic size PPE scaffold components, our PPE nasal scaffold was computer modeled after the patient’s nasal prosthesis imparting a unique dimension and contour to the construct to comport with the patient’s facial topography and aesthetic. The accuracy in which the PPE scaffold can be 3D printed to mirror the precise, desired anatomy of the nose represents a new pathway for achieving “normal” in these complex reconstructions.
Future Considerations
Although this case has demonstrated the feasibility of constructing a subtotal nasal defect using a PPE scaffold, this approach to management will necessitate further simplification and refinement for general application in the reconstructive arena. With improvements in this technique, it is feasible that the number of operative steps and morbidity traditionally associated with total and subtotal nasal reconstruction could be reduced. This might also represent a quantum shift in our approach to management of these challenging problems and potentially provide an improved means for achieving consistent aesthetic and functional results.
We have used PPE as a scaffold but other materials, such as noted earlier, having better biocompatibility and characteristics that approximate the ideal scaffold are desirable. Similarly, 3D printing technology holds much promise for engineering a soft tissue covering and perhaps a soft tissue lining that actually approximates the form and function of native lining tissues.
CONCLUSIONS
We report a case of subtotal nasal reconstruction using a computer-generated, 3D PPE scaffold. The construct demonstrates excellent function and aesthetics 22 months postoperatively. This approach to management has great potential for application in total and subtotal nasal reconstruction and represents a harbinger of future reconstructive approaches to management.
Footnotes
Published online 11 December 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Reinisch J, Tahiri Y. Polyethylene ear reconstruction: a state-of-the-art surgical journey. Plast Reconstr Surg. 2018;141:461–470. [DOI] [PubMed] [Google Scholar]
- 2.Choi SY, Shin HI, Kwon TY, et al. Histopathological and scanning electron microscopy findings of retrieved porous polyethylene implants. Int J Oral Maxillofac Surg. 2017;46:582–585. [DOI] [PubMed] [Google Scholar]
- 3.Genther DJ, Papel ID. Surgical nasal implants: indications and risks. Facial Plast Surg. 2016;32:488–499. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y, Fan F, Kang N, et al. Tissue engineering of human nasal alar cartilage precisely by using three-dimensional printing. Plast Reconstr Surg. 2015;135:451–458. [DOI] [PubMed] [Google Scholar]
- 5.Niechajev I. Porous polyethylene implants for nasal reconstruction: clinical and histologic studies. Aesthetic Plast Surg. 1999;23:395–402. [DOI] [PubMed] [Google Scholar]
- 6.Walton RL, Brown RE. Tissue engineering of biomaterials for composite reconstruction: an experimental model. Ann Plast Surg. 1993;30:105–110. [DOI] [PubMed] [Google Scholar]
- 7.Khan G, Choi YS, Park ES, et al. The application of three-dimensional simulation program and three-dimensional printing in secondary rhinoplasty. J Craniofac Surg. 2018;29:e774–e777. [DOI] [PubMed] [Google Scholar]
- 8.Jung JW, Ha DH, Kim BY, et al. Nasal reconstruction using a customized three-dimensional-printed stent for congenital arhinia: three-year follow-up. Laryngoscope. 2019;129:582–585. [DOI] [PubMed] [Google Scholar]
- 9.Yen CI, Zelken JA, Chang CS, et al. Computer-aided design and three-dimensional printing improves symmetry in heminasal reconstruction outcomes. J Plast Reconstr Aesthet Surg. 2019;72:1198–1206. [DOI] [PubMed] [Google Scholar]
- 10.Ghai S, Sharma Y, Jain N, et al. Use of 3-D printing technologies in craniomaxillofacial surgery: a review. Oral Maxillofac Surg. 2018;22:249–259. [DOI] [PubMed] [Google Scholar]
- 11.Kim YS, Shin YS, Park DY, et al. The application of three-dimensional printing in animal model of augmentation rhinoplasty. Ann Biomed Eng. 2015;43:2153–2162. [DOI] [PubMed] [Google Scholar]
- 12.Bauermeister AJ, Zuriarrain A, Newman MI. Three-dimensional printing in plastic and reconstructive surgery: a systematic review. Ann Plast Surg. 2016;77:569–576. [DOI] [PubMed] [Google Scholar]
- 13.Stokken JK, Pallanch JF. The emerging role of 3-dimensional printing in rhinology. Otolaryngol Clin North Am. 2017;50:583–588. [DOI] [PubMed] [Google Scholar]
- 14.Park SH, Yun BG, Won JY, et al. New application of three-dimensional printing biomaterial in nasal reconstruction. Laryngoscope. 2017;127:1036–1043. [DOI] [PubMed] [Google Scholar]
- 15.Visscher DO, van Eijnatten M, Liberton NPTJ, et al. MRI and additive manufacturing of nasal alar constructs for patient-specific reconstruction. Sci Rep. 2017;7:10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zopf DA, Mitsak AG, Flanagan CL, et al. Computer aided-designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction. Otolaryngol Head Neck Surg. 2015;152:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walton RL, Burget GC, Beahm EK. Microsurgical reconstruction of the nasal lining. Plast Reconstr Surg. 2005;115:1813–1829. [DOI] [PubMed] [Google Scholar]
- 18.Burget GC, Walton RL. Optimal use of microvascular free flaps, cartilage grafts, and a paramedian forehead flap for aesthetic reconstruction of the nose and adjacent facial units. Plast Reconstr Surg. 2007;120:1171–1207. [DOI] [PubMed] [Google Scholar]
- 19.Menick FJ. Nasal reconstruction. Plast Reconstr Surg. 2010;125:138e–150e. [DOI] [PubMed] [Google Scholar]


