(Cell 181, 1004–1015.e1–e15; May 28, 2020)
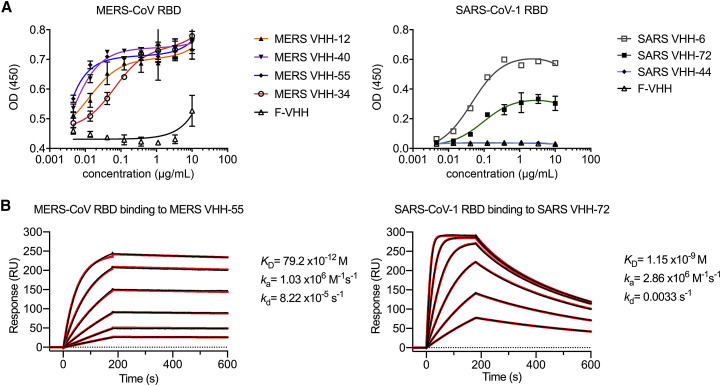
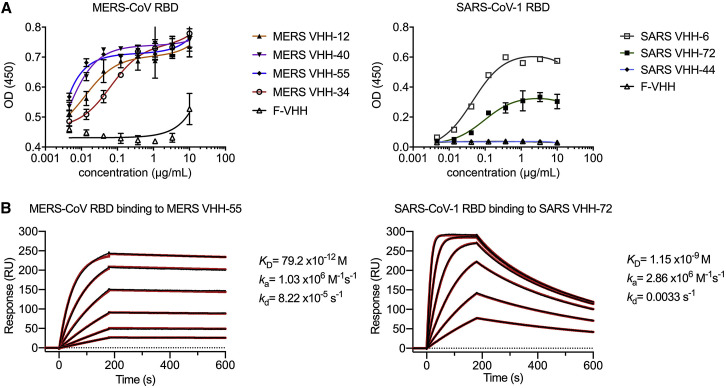
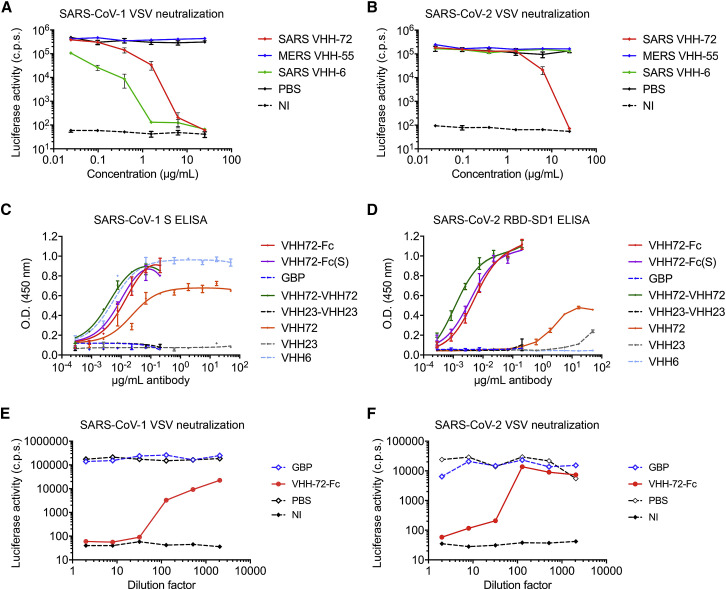
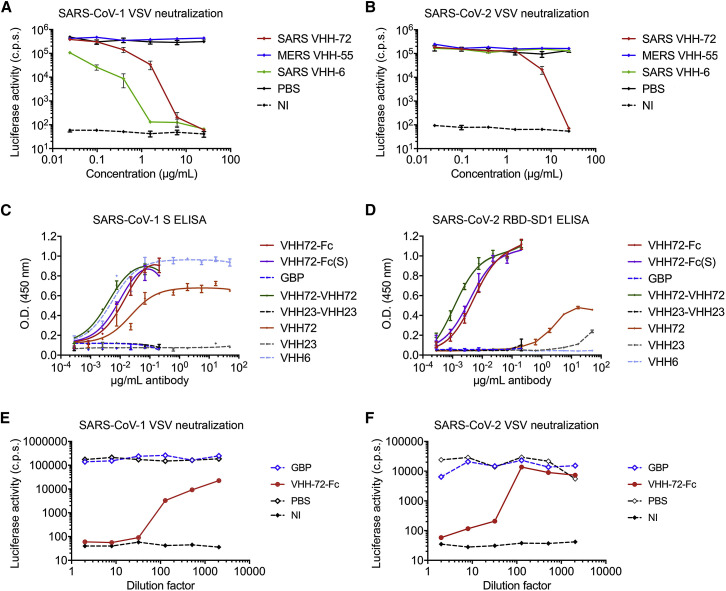
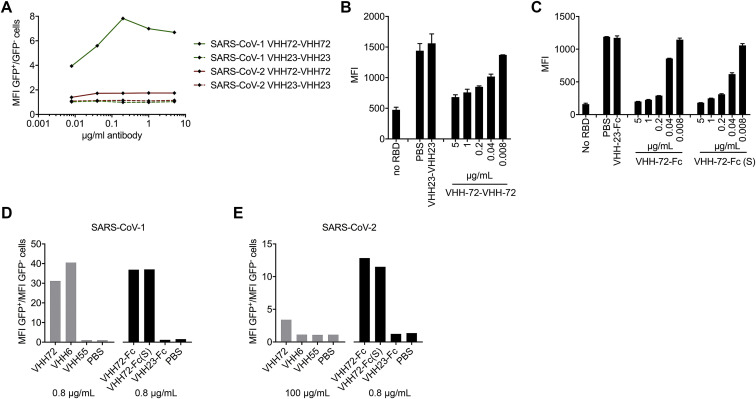
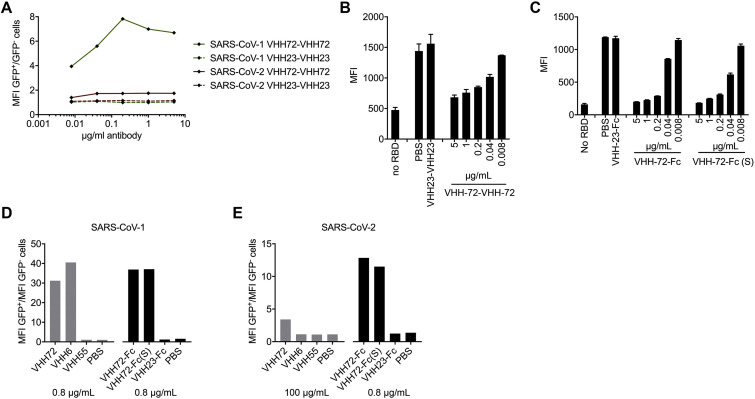
We recently discovered that two early batches of purified monovalent SARS VHH protein samples were switched during the expression in Pichia pastoris. Although this error had no effect on the biophysical or structural work, nor on the SARS-CoV-1 and −2 cross-reactivity and cross-neutralization demonstrated with the bivalent fusion constructs, and our conclusions remain unchanged, several figures (Figures 1A, 6A–6D, S1C, S3, S6D and S6E), one table (Table S1), and the corresponding text have been amended in the online and print versions to correct this. We apologize for any confusion that this error may have caused.
Figure 1.
Epitope Determination and Biophysical Characterization of MERS VHH-55 and SARS VHH-72 (corrected)
Figure 1.
Epitope Determination and Biophysical Characterization of MERS VHH-55 and SARS VHH-72 (original)
Figure 6.
SARS VHH-72 Bivalency Permits SARS-CoV-2 Pseudovirus Neutralization (corrected)
Figure 6.
SARS VHH-72 Bivalency Permits SARS-CoV-2 Pseudovirus Neutralization (original)
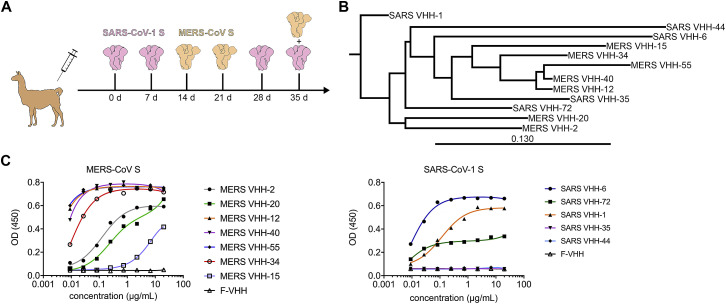
Figure S1.
CoV VHH Immunization and Panning (corrected)
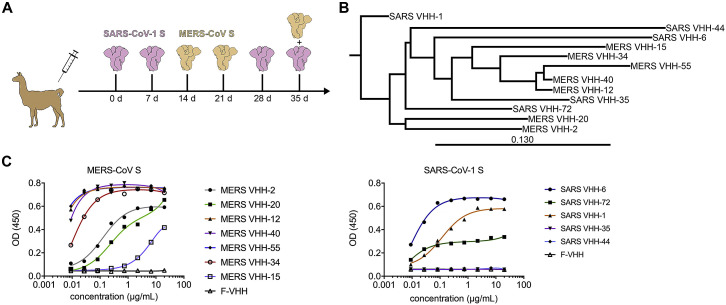
Figure S1.
CoV VHH Immunization and Panning (original)
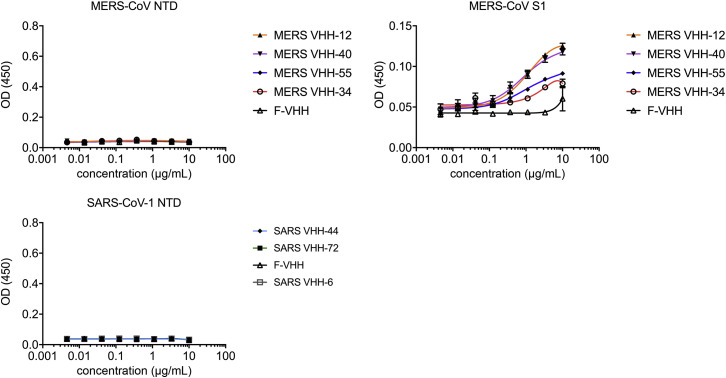
Figure S3.
Lack of Binding of MERS-CoV and SARS-CoV-Directed VHHs to Non-RBD Epitopes (corrected)
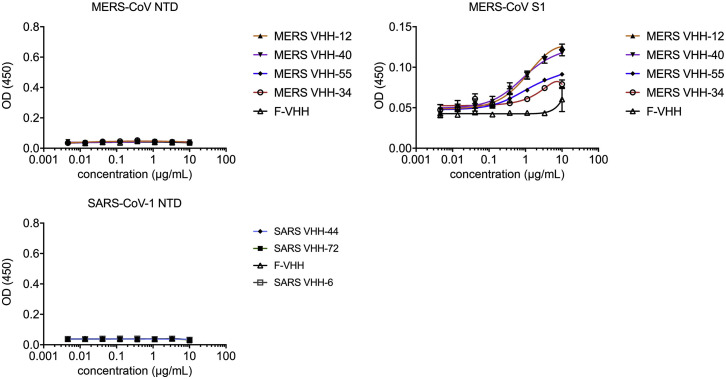
Figure S3.
Lack of Binding of MERS-CoV and SARS-CoV-Directed VHHs to Non-RBD Epitopes (original)
Figure S6.
Engineering a Functional Bivalent VHH Construct (corrected)
Figure S6.
Engineering a Functional Bivalent VHH Construct (original)