Abstract
Purpose
Novel Coronavirus disease 2019 (COVID-19), is an acute respiratory distress syndrome (ARDS), which is emerged in Wuhan, and recently become worldwide pandemic. Strangely, ample evidences have been shown that the severity of COVID-19 infections varies widely from children (asymptomatic), adults (mild infection), as well as elderly adults (deadly critical). It has proven that COVID-19 infection in some elderly critical adults leads to a cytokine storm, which is characterized by severe systemic elevation of several pro-inflammatory cytokines. Then, a cytokine storm can induce edematous, ARDS, pneumonia, as well as multiple organ failure in aged patients. It is far from clear till now why cytokine storm induces in only COVID-19 elderly patients, and not in young patients. However, it seems that aging is associated with mild elevated levels of local and systemic pro-inflammatory cytokines, which is characterized by “inflamm-aging”. It is highly likely that “inflamm-aging” is correlated to increased risk of a cytokine storm in some critical elderly patients with COVID-19 infection.
Methods
A systematic search in the literature was performed in PubMed, Scopus, Embase, Cochrane Library, Web of Science, as well as Google Scholar pre-print database using all available MeSH terms for COVID-19, Coronavirus, SARS-CoV-2, senescent cell, cytokine storm, inflame-aging, ACE2 receptor, autophagy, and Vitamin D. Electronic database searches combined and duplicates were removed.
Results
The aim of the present review was to summarize experimental data and clinical observations that linked the pathophysiology mechanisms of “inflamm-aging”, mild-grade inflammation, and cytokine storm in some elderly adults with severe COVID-19 infection.
Keywords: ACE2 receptor, Autophagy, COVID-19, Cytokine storm, Senescent cell, Vitamin D
Introduction
The COVID-19, now named SARS-CoV2, spreading in Wuhan, China, and now spread globally rapidly [1]. It is reported that COVID-19 has the same viral genome (above 85% identity in the genome), and pathophysiology mechanisms with the SARS-CoV [2]. The COVID-19 infection affecting all age-groups, but it appears to be more severe in elderly adults [3]. It seems that very high pro-inflammatory cytokine release, which is described as cytokine storm, is a pivotal pathophysiological mechanism in elderly COVID-19 patients [4]. Aging is related to increased levels of systemic pro-inflammatory cytokines and decreased levels of systemic anti-inflammatory cytokines. Hence, a chronic condition of inflammation may be created in aged subjects, known as “inflamm-aging” [5, 6]. Ample studies have indicated elevated levels of interleukin (IL)-6, IL-1, tumor necrosis factor-α (TNF α), as well as C-reactive protein (CRP) in aged subjects [7, 8]. Although, the exact underlying mechanism of cytokine storm in elderly adults with severe COVID-19 infection is far from clear. However, it is likely that dysregulation of the cytokine homeostasis in “inflame-aging” phenomenon may play a critical role in the risk of a cytokine storm, and subsequently acute respiratory distress syndrome (ARDS) in some elderly patients with severe COVID-19 infection. It seems that “cytokine storm” phenomenon in elderly patients with severe COVID-19 infection, is associated with many age-related pathophysiologic processes, including alteration of angiotensin-converting enzyme 2 (ACE2) receptor expression [9], excess ROS production [10], alteration of autophagy [11], the inflammatory phenotype of senescent cell activity, particularly adipose tissue [12], and immune-senescence [13], as well as lack of vitamin D content [14]. Here, we are going to review and discuss all above mentioned age-related pathophysiological pathways that appear to contribute to the dysregulation of cytokine networks and possibly a cytokine storm in elderly patients with severe COVID-19 infection.
The possible pathophysiology of COVID-19 infection
It has been shown that COVID-19 infection has distinctive behavior among elderly adults (severe infection) as compared with children and young adults (none or mild infection). Indeed, COVID-19 infection can induce severe infection, including pneumonia and ARDS in some elderly adults or sick patients, and not in children or young adults [15]. What is the reason that the deadly cases of COVID-19 mainly seen in elderly patients? Here, first we are going to review and compare the possible pathophysiology mechanisms of mild infection and severe infection in young and elderly adults with COVID-19, respectively.
Normal immunologic responses in young adults with mild COVID-19 infection
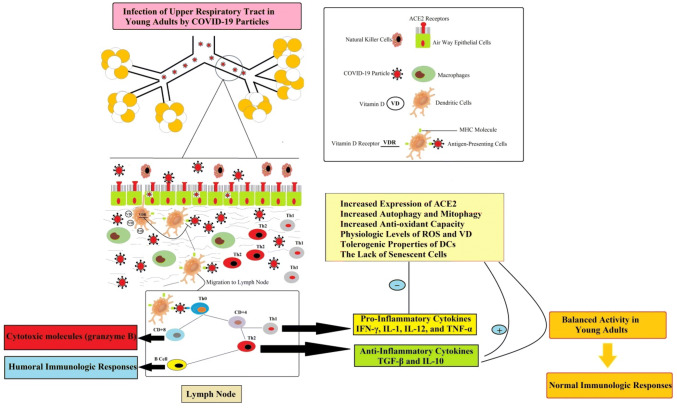
Despite increasing evidences on the immune response to pathogens, however, less is known about the exact immunologic mechanism of COVID-19 infections. As shown in Fig. 1, initiation of the immune response against invading coronavirus begins with a direct infection of the bronchi and bronchiole epithelium. First, antigen-independent innate immunity provides the first line of leukocytes defense against microorganisms. Innate immune defense involves several cell types, including leukocytes such as neutrophils, eosinophils, basophils, monocytes, macrophages, lung epithelial cells, mast cells, natural killer (NK cells) [16]. Following initial COVID-19 infection, lung-resident dendritic cells (DCs) become activated and change to antigen-presenting cells (APCs). Indeed, APCs are the first line of defense in recognizing various pathogens. In the lung, DCs resides in and below the airway epithelium, the alveolar septa, pulmonary capillaries, and airway spaces [17]. Activated APCs ingests, and processes an antigen and migrate to the lymph nodes. Then, in the lymph nodes, APC presents the antigen in the form of MHC/peptide complex to naïve circulating T helper cells (Th0), inducing the immune response [18]. Following activation of Th0 receptor by MHC/peptide complex, T helper cells become activated, proliferate and differentiated to CD4+ (T helper lymphocytes) and CD8+ (cytotoxic T lymphocytes) cells. Then, CD4+ Th lymphocytes further differentiated into Th1 and Th2, with different cytokine profiles [19]. Th1 cells drive cellular immunity and released pro-inflammatory cytokines such as IFN-γ, IL-1β, IL-12, and TNF-α [17]. It is reported that cytokine IFN-γ can inhibit viral replication and enhance antigen presentation [20]. Th2 cells activated humoral immunity and antibody production and released anti-inflammatory cytokines such as TGF-β, IL-4, IL-5, IL-9, IL-10 and IL-13 [21]. In fact, a balanced between Th1 and Th2 lymphocyte activity is observed in healthy adults with COVID-19 infection. Furthermore, CD8+ T lymphocytes cytotoxic T cells, as cytotoxic cells, secrete cytotoxic molecules such as granzyme B that kill infected epithelial cells. Indeed, CD8+ T lymphocytes and natural killer cells (NK) play a critical contribution in viral clearance [17]. Both T and B cell responses against COVID-19 observed in the systemic blood pool 1 week after the initiation of COVID-19 symptoms. The autopsy of a patient with COVID-19 identified an accumulation of mononuclear cells (likely monocytes and T cells) in the lungs, with low levels of hyperactive T cells in the peripheral blood. These findings suggested that likely T cells are attracted away from the systemic blood pool and into the infected site (lung) to control the COVID-19 infection [21]. Generally, activation of different Th cells and release of ample cytokines and chemokines recruit more innate, cell-mediated and humoral immunologic responses to control COVID-19 in adults. Additionally, it seems that the balance between pro-inflammatory and anti-inflammatory immune responses in the healthy adult can shut down immune activity at the right moment [22].
Fig. 1.
Normal immunological responses in young adults with mild COVID-19 infection. Direct infection of the bronchi and bronchioles epithelium with COVID-19 particles turn on innate and cell-mediated immune responses. COVID-19 particles convert dendritic cells to mature form, antigen-presenting cells (APCs). The APCs migrates to the lymph node and presents the antigen in the form of the MHC complex to naïve T helper cells (Th0). Th0 cells become activated, proliferate and differentiated to other cells such as CD4+ (T helper lymphocytes) and CD8+ (cytotoxic T lymphocytes) cells. In healthy adults, due to sufficient vitamin D level, VD can decrease the expression of pro-inflammatory genes in immune cells. A balanced between pro-inflammatory and anti-inflammatory activity causes shut down of the immune system at the right moment
Disrupted immunologic responses in elderly adults with severe COVID-19 infection
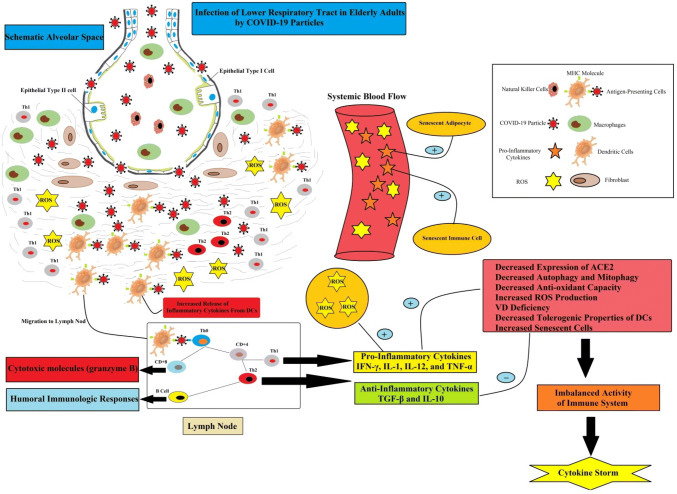
It was observed that ARDS, pneumonia and multi-organ dysfunction are the main immune-clinical symptoms of COVID-19 infection. It is well accepted that ARDS and pneumonia in deadly cases are due to severe inflammatory responses to the immune system, this so-called a cytokine storm [23]. On the other hand, severe multi-organ destruction is due to the cytokine storm rather than a direct damaging effect of the virus itself [24]. It is noteworthy when COVID-19 pathogen reached to the alveoli in elderly and weak adults, pro-inflammatory immune responses become vigorous and un-controllable active. Furthermore, impaired anti-inflammatory responses in elderly adults may correlate to increased activity of pro-inflammatory responses [25]. Hence, some elderly adults with severe COVID-19 infection cannot shut down their pro-inflammatory immune response. Several studies reported most patients with severe COVID-19 exhibit markedly increased serum levels of pro-inflammatory cytokines, including; IFN-α, IFN-γ, IL-1β, IL-6, IL-12, IL-17, IL-18, IL-33, TNF-α, G-CSF, GM-CSF, IP10, C-reactive protein (CRP), MCP1, and MIP1α [26–28]. It is necessary to mention that cytokines storm directly may lead to immune cell death, tissue damage, and respiratory shut down [25]. For example, the autopsy findings of aged subjects revealed spleen atrophy and necrosis, lymph node necrosis, hemorrhage in the kidney, hepatomegaly, and degeneration of the neurons in the central nervous system in COVID-19 patients. The number of immune cells also changed in COVID-19 infection [29, 30]. Indeed, in patients with severe COVID-19 infection, but not in patients with a mild infection, lymphopenia is a common feature, with significantly reduced numbers of CD4+ T cells, CD8+ T cells, B cells and NK cells. Furthermore, exhaustion markers, such as NKG2A receptors on NK cells and CD8+ T cells, are up-regulated in patients with COVID-19 [28, 31]. Histochemical staining showed that CD4+ T cells and CD8+ T cells were decreased in spleen and lymph nodes. In addition, in the lung with characteristic diffused alveolar damage, the major infiltrated cells were monocytes and macrophages, but very few lymphocytes [32]. Tian and colleagues in 2020 using postmortem biopsies identified alveolar damage, fibrosis of the heart and myocardial hypertrophy, and also lobular infiltration of the liver by small lymphocytes in four died cases of COVID-19 [33].
In addition to cytokine storm, viral particles of COVID-19 can also directly induce multiple organ dysfunctions. Because viral particles of COVID-19 infection were identified in the bronchial and type 2 alveolar epithelial cells, fecal, and urine samples [29, 34, 35]. Hence, it is suggested that multiple organ dysfunction in severe COVID-19 patients can also cause by a direct attack of the virus. It is far from clear whether cytokine storm, direct effects of the virus, or the synergistic effects of both, contribute to the multiple organ failures in severe COVID-19 patients [35]. Here, we are going to review the link between cytokine storm in elderly patients of COVID-19 and “inflame-aging”.
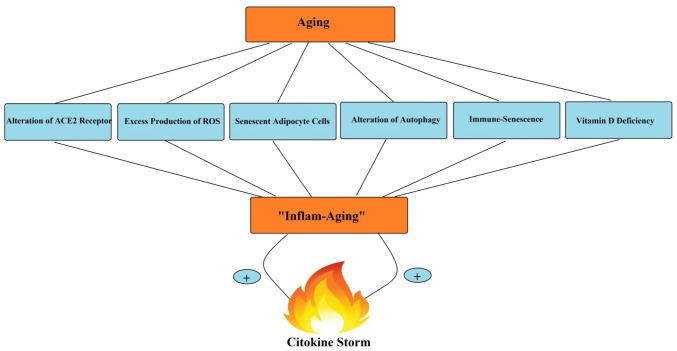
Aging is related to elevated systemic levels of pro-inflammatory cytokines, including IL-6, IL-8, TNF-α, IL-13, IFN-γ, as well as acute phase proteins. Ample studies reported a chronic mild inflammation in aging, which is described as “inflame-aging”. This phenomenon can promote age-associated disorders, including diabetes mellitus, Alzheimer's disease, atherosclerosis, etc. Accordingly, it seems that increased generation of pro-inflammatory markers and “inflame-aging” have a critical role in the process of cytokine storm in severe COVID-19 patients and enhanced mortality risk [8, 36]. As shown in Fig. 2, several factors, including alteration of ACE2 receptor expression, excess reactive oxygen species (ROS) production, senescent adipocytes activity, alteration of autophagy and mitophagy, immune- senescent, as well as vitamin D (VD) deficiency, may associate “inflame-aging” to cytokine storm in elderly patients of COVID-19.
Fig. 2.
The link between “inflame-aging” and cytokine storm in in elderly adults with severe COVID-19. Several aging-related factors may associate chronic inflammation to cytokine storm in elderly patients of COVID-19
Aging and angiotensin-converting enzyme 2 receptor (ACE2)
The renin-angiotensin system (RAS) is an important regulator of several physiologic events, including cardiovascular and blood volume, natriuresis, diabetes, chronic renal disease, and hepatic fibrosis [37, 38]. This system is composed of two different pathways, including angiotensin-converting enzyme (ACE)/angiotensin II (Ang II)/angiotensin receptor type 1 (AT1) (ACE/Ang II/AT1) pathway; and angiotensin-converting enzyme 2 (ACE2)/Ang 1–7/Mas receptor (ACE2/Ang 1–7/Mas) pathway. These two pathways have opposing effects to accommodate a coordinated response to specific triggers. The activity of ACE/Ang II/AT1 pathway related to tissue injury, inflammation and fibrosis [39]. In contrast, the activity of the ACE2/Ang 1–7/Mas pathway exerts anti-inflammatory and anti-fibrosis effects [39, 40]. ACE2 degrades Ang II, as a major substrate for ACE2, and generates Ang-(1–7) [38]. Recently, it is well accepted that ACE2 on lung epithelial cells are the entry-point receptors for COVID-19 particles [41]. It is demonstrated that the highest expression of ACE2 is in the lungs (type II alveolar epithelial cells), kidney, heart, and also vascular beds [37]. Yu and colleagues in 2018 revealed that ACE2/Ang-(1–7)/Mas pathway markedly suppressed in pancreatitis by inhibition of the p38 MAPK/NF-κB signaling pathway [38]. Fu and colleagues in 2017 transfected ACE2 plasmid in primary cultured human retinal pigment epithelium cells (hRPE) followed by stimulation with amyloid-β (Aβ). They observed that overexpression of ACE2 markedly decreased Aβ-induced inflammatory response by activating the ACE2/Ang-(1–7)/Mas pathway in hRPE [42]. In the respiratory syncytial virus, ACE2 protected against severe lung injury both in children and an experimental mouse model. In addition, in the ARDS model, ACE2 knockout mice displayed more severe symptoms of respiratory shut down compared with wild-type mice [43]. Indeed, treatment strategy using ACE2 analogs or vector containing ACE2 result in beneficial effects in diabetic nephropathy, hypertension, cardiac disease [37]. Specific inhibitors of AT1 receptors, losartan, have been shown to be effective in animal models of septic shock. Therefore, the above mentioned studies suggested that ACE2 pathway has anti-inflammatory effects. Several studies identified age-related decline of ACE2 expression [40, 44, 45]. For example, in the study of Xudong and colleagues in 2006 revealed age-related difference of ACE2 expression revealed in rat lung. They observed ACE2 expression is significantly reduced with aging. They are suggesting the more elevated ACE2 in young adults as compared to age groups may contribute to the predominance in SARS attacks in this age group [44]. Using GTEx gene expression data and analysis, Chen and colleagues in 2020 found markedly higher expression of ACE2 in Asian females compared to males. Furthermore, they found an age-dependent decline of ACE2 expression, and also a highly significant decrease in type II diabetic patients. Additionally, they established a negative correlation between ACE2 expression and COVID-19 fatality. Interestingly, in severe cases, many vital tissues, including those with little ACE2 expressed are severely damaged by COVID-19 infection [45]. These evidences may partially suggest that the increase concentration of ACE2 receptors in lung epithelial cells in children and young adults may have a protective effect on severe clinical manifestations due to COVID-19 infection. Therefore, it is highly likely that low ACE2 expression and unbalance Ang II/Ang1–7 level during aging can lead to cytokine storm and lung shut down [40, 41]. However, the genetic basis of ACE2 expression and its function in different individuals is still far from clear [46].
Aging and excess production of ROS
It is well accepted that ROS considered as a signaling molecule (at low concentrations), and also as a mediator of inflammation (at high concentrations) [47]. The main sources of ROS are mitochondrial respiratory chain and NADPH oxidase [48]. Garrido and colleagues in 2019 identified that the immune cells of pre-maturely aging mice presented lower values of antioxidant defenses and higher values of ROS and pro-inflammatory cytokines [10]. Hence, it is suggested that excess ROS production during aging can turn on an inflammatory machine and subsequently increased release of pro-inflammatory cytokines, including; TNF-α, IL-1β, IL-2, and IL-6, and adhesion molecules. The excess ROS production in aging can initiate the pro-inflammatory generation through activation of multiple transcription factors, including human polynucleotide phosphorylase (hPNPaseold-35), nuclear factor kappa B (NF-κB), activator protein 1 (AP-1), specificity protein 1 (Sp1), peroxisome proliferator-activated receptors (PPARs) [49, 50]. For example, it is reported that hPNPaseold-35, which is up-regulated during senescence, may promote the activation of NF-κB pathway and initiates the production of pro-inflammatory cytokines, such as IL-6 and IL-8 [51]. Furthermore, the expression of hPNPaseold-35 itself induces ROS production. This suggests that hPNPaseold-35 could be an upstream signaling molecule that increased ROS generation and subsequent pro-inflammatory cytokines during aging. In addition to hPNPaseold-35, NF-κB is also an important transcription factor that up-regulated during aging by excess ROS production. In resting states, an inhibitory protein, IkB, inactivated NF-κB in the cytoplasm. However, ROS production can phosphorylate inhibitory IkB proteins, leading to nuclear translocation of NF-κB and regulation of gene transcription. Then, activated NF-κB can initiate the secretion and release of pro-inflammatory cytokines, including TNF-a, IL-1, IL-6, IL-8, IFN-g, iNOS, COX-2 [50]. Interestingly, as the excess ROS production can increase pro-inflammatory cytokines, the pro-inflammatory cytokines can also increase ROS production [52]. For instance, it has been identified that the pro-inflammatory cytokine IL-6 can increase ROS generation by increased expression of NADPH oxidase-4 in lung cancer [53]. Additionally, it has been demonstrated that the pro-inflammatory cytokine interferon-γ and the pro-inflammatory component of the bacterial cell wall, lipopolysaccharide, can synergistically increase ROS generation in human pancreatitis by NF-κB-dependent expression of Duox2, a member of the NADPH oxidase family [54]. Hence, excess ROS production and inflammation are closely related, which are taking part in the pathogenesis of chronic inflammation and “inflame-aging” in elderly adults.
Aging and autophagy
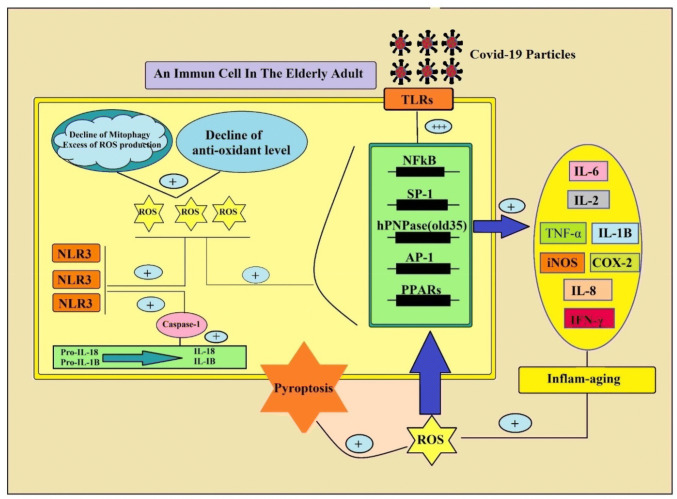
Autophagy is a conserved catabolic turnover pathway in eukaryotic cells by which cellular material delivered into the lysosomes for degradation. Autophagy process is related to the maintenance of cellular homeostasis, and its dysregulation could lead to the development of several aging-related pathophysiological diseases [11]. It has been shown that the autophagy process, decrease during aging, leads to the accumulation of damaged macromolecules and organelles. The decline of autophagy during aging can induce dysfunctional mitochondria, and subsequent increased ROS production [55]. Mitochondria are the major source of ROS. In this context, two major processes are for protection from harmful effects of ROS, including mitophagy and antioxidant capacity. In one hand, mitophagy, which is characterized by autophagic degradation of mitochondria, decreased with aging [56]. On the other hand, decreased mitophagy, together with decreased antioxidant capacity during aging can increased ROS levels in the body. The excess production of ROS in stress condition leading to memory deficits, anxiety-like behavior and increase pro-inflammatory cytokine secretion during aging [57–60]. Although, the exact underlying mechanism of how the decline in autophagy and a rise in ROS levels during aging can elevate pro-inflammatory cytokine release is far from clear. However, it is well accepted that low activity of autophagy process and high level of ROS production during aging, can activate and up-regulate Nod-like receptors (NLRs) [54]. The NLRs are a type of intracellular pattern-recognition receptors (PRRs) for pathogen recognition. They monitor both inflammation and apoptosis signaling pathways. These receptors expressed in many cell types, including immune cells (lymphocytes, macrophages, dendritic cells) and even epithelial cells [61]. It is observed that activation of cytosolic NLRs increased during aging and in many age-related diseases such as type 2 diabetes mellitus. For example, from Ebersole and colleagues study in 2017 identified that expression of NLRs increased with aging in the healthy oral mucosa [62]. Additionally, Luan and colleagues in 2018 found that NLRP3 expression increased in concanavalin A-induced hepatitis (as a model of autoimmune hepatitis) [63]. Salminen and colleagues in 2012 reported that the decrease of autophagic capacity during aging generates the inflammatory situation by means the activation of pro-inflammatory factors, in particular NLRP3 [55]. It is accepted that NLRs activity can increase expression of caspase-1, and pro-inflammatory cytokines, including IL-1β and IL-18, leading to cell death (Fig. 3). For example, Nadatani in 2016 reported that caspase-1 can induce pyroptosis, a unique form of programmed cell death, through the conversion of pro-IL1β and pro-IL18 in their active forms, which promotes further inflammation. In pyroptosis, the dying cells release their cytoplasmic pro-inflammatory contents into the extracellular fluid [64]. Similarly, Wang’s and colleagues revealed that treatment of Ac-YVAD-cmk, an inhibitor of NLRP3-caspase-1, suppressed isoflurane-induced microglial inflammatory response in aged mice [65]. This finding is a critical study for supporting that NLRP3/caspase-1 pathway is involved in the pathophysiology of chronic inflammatory disease in elderly adults (Fig. 3). Furthermore, Stranks in 2015 found that macrophages from aged mice exhibit markedly reduced autophagic flux as compared to young mice. They also reported that reduced autophagy during aging, increased macrophage populations and their phagocytosis function, decreased surface antigen expression, while the increased the inflammatory cytokine response [66]. Additionally, in animal model studies, increase autophagy by means caloric restriction and also exercise, result in down-regulation of IL-1β production and improve the aging-related pro-inflammatory profile. So, it seems that crosstalk between the decline of mitophagy pathways and elevated ROS level during aging can imbalance, immune system activity of elderly adults [67].
Fig. 3.
The role of alteration of mitophagy in “inflame-aging” and subsequent cytokine storm in elderly adults. The decline of mitophagy during aging, increased ROS production. On one hand, excess ROS production can activate and up-regulate intracellular Nod-like receptor type 3 (NLR3). NLRs over-activity increase expression of caspase-1, and pro-inflammatory cytokines, including IL-1β and IL-18, leading to pyroptosis (cell death) of immune cells. In other hand, the excess ROS production can increase the pro-inflammatory molecules release through activation of multiple transcription factors, including human polynucleotide phosphorylase (hPNPaseold-35), nuclear factor kappa B (NF-κB), activator protein 1 (AP-1), specificity protein 1 (Sp1), peroxisome proliferator-activated receptors (PPARs)
Aging and senescent adipocytes
Senescent cells accumulate with aging in many animal and human tissues, leading to chronic inflammation and organ dysfunction [68]. Senescent cells have lower cell viability, as well as insufficient protection against oxidative stress [69]. Additionally, senescent cells can release pro-inflammatory cytokines, including IL-1α, IL-1β, IL-6, IL-8, IL-18, CCL-2, TNF-α, granulocyte macrophages colony-stimulating factor (GM-CSF), growth regulated oncogene (GRO), monocyte chemotactic protein (MCP)-2, MCP-3, MMP-1, MMP-3 [70, 71]. So, the immune effector is not the main source of inflammatory markers. Aging studies revealed the importance of adipose tissue inflammation in aged animals by the elevated release of interleukin 6, IL-8, IL-1β, as well as TNF-α [68–70]. Adipose tissue is a dynamic structure that plays an important contribution in modulating of metabolism and inflammation. It is highly likely that adipose tissue dysfunction (for instance obesity during aging) is associated with chronic inflammation in aged subjects [12]. Petrakis and colleagues in 2020 reported that age-related obesity leading to increased susceptibility of more serious complications of COVID-19 as compared to younger individuals. In obese COVID-19 patients, the adipose tissue interacts with the immune system and increased the lethality of the infection by fat tissue-associated cytokines (adipokines) release. Adipocytes released amyloid-A (an adipokine), which act directly on macrophages and increased generation of pro-inflammatory cytokines. The mortality rate for young adults with COVID-19 (with normal body mass index) was approximately 2%. However, the mortality rate for obese elderly adults with COVID-19 was approximately 14% [72]. In addition to obesity, Covarrubias and colleagues found that during aging senescent cells significantly accumulate in visceral white adipose tissue and inflammatory cytokines found in the supernatant from senescent cells, which are induced macrophages to proliferate and to express CD38, as a T cell activation marker [73]. Alicka and colleagues in 2020 found that adipose-derived stem cells from older groups exhibited increased gene expression of pro-inflammatory gene and miRNAs (such as IL-8, IL-1β, TNF-α, miR-203b-5p, and miR-16-5p), and apoptosis markers (such as p21, p53, caspase-3, caspase-9) [69]. Ghosh and colleagues in 2016 reported that decreased autophagy activity during aging associated with increased adipose tissue ER stress and inflammation in old adipose tissue-derived stromal vascular fraction cells (SVFs) in mice. They also revealed that accumulation of autophagy substrates LC3-II and p62 increased in old SVFs, implicated impaired autophagy activity. Furthermore, they reported that old SVFs had reduced expression of autophagy markers. They also analyzed that decreased autophagy activity in old SVFs is correlated with increased secretion of pro-inflammatory cytokines, including IL-6 and MCP-1 [74]. Therefore, the elevated release of pro-inflammatory cytokines by senescence adipocytes possibly leads to the elevated risk of the cytokine storm in COVID-19 infection in poor prognosis patients.
Aging and immune-senescence
With progressive age, the function of the innate and adaptive immune system undergoes physiological and morphological alteration throughout a lifetime, which is characterized as an immune-senescence [13, 75]. Indeed, immune-senescence is described as the progressive loss of all immune effectors in both the innate and cell-mediated immune systems with aging [76]. A normal and physiologic immunity depends on effective cross-talk between innate and adaptive immune systems, so senescent immune effectors markedly impact on the health of elderly individuals [75]. Here, we are going to briefly report age-related alteration of both innate and adaptive immune cells. Macrophages are central effector cells of the innate immune system and have many physiological functions [77]. Macrophage can produce several pro- (TNFα, IL-1, and IL-6) and anti-inflammatory (IL-10, TGFβ, acute phase proteins, and glucocorticoids) molecules, enzymes, growth factors, nitric oxide, toxic reactive radicals, metalloproteinases and inhibitors of metalloproteinases. As the clearance of pathogens proceeds, the anti-inflammatory effectors of macrophages turn off macrophage inflammatory activities [78]. Therefore, during a pathogen-induced inflammatory episode, the balance of macrophage modulating secretion present in the tissues. During aging, the generation of several macrophage-induced factors is reduced, including fibroblast growth factor, vascular endothelial growth factor, epithelial growth factor, TGFβ, toxic free radicals, and expression of nitric oxide synthase. Additionally, phagocytic and chemotactic activity of macrophages also decreased by a decline in production of macrophage-specific chemokines, including macrophage inflammatory protein (MIP)-1 and MIP-2 with advanced age. Macrophages can also display antigen-presenting activity by expressing major histocompatibility class (MHC), leading to a cross-talk between innate and cell-mediated immune system. It is reported that with progressive age, the expression of (MHC)-II, decreased in both mice and humans [79, 80]. Natural killer (NK) cells are another cytotoxic effector of the innate immune system and involved in the early, and fast, faster than T cells, defense against virus infection and other challengers [81]. The NK cells, such as macrophages, linking innate and cell-mediated immune system. NK cells regulate immune function by the generation and release of various cytokines [82]. An increase in numbers of circulating NK cells reported during aging [75]. One of the important cytokines for cytotoxic activity of NK cells is IL-2, which increases killing properties and proliferation of NK cells. In a healthy young individual, IL-2 can induce IFN secretion by NK cells, but this effect decreased in elderly [83]. Furthermore, aging can change the NK cells phenotypes, which are an increase in CD56dim cells and a decrease in CD56bright cells. In addition, aging can increase the expression of the immune-senescence marker, CD57, on NK cell populations [84]. Dendritic cells (DCs), are another component of the innate immune system and have a critical role in both immunity and tolerance. The resident DCs are immature, but the capture of pathogens converts them to mature form, known as antigen-presentation cells (APCs), through up-regulation of MHC expression. Then, mature APCs monitor Th1 and Th2 function. In contrast, immature DCs induce tolerance to self-antigens [25]. In physiological condition, DCs take up self- antigen and apoptotic cells and transport them to the lymph nodes [85]. But, during migration for the presentation of pathogens to Th0 cells, they undergo several phenotype alterations, including up-regulation of MHC class I and II molecules and down-regulation of adhesion molecules [86]. Furthermore, DCs can generate and release various cytokines, so they can modulate inflammatory responses [87]. It is reported that increased age-related pro-inflammatory cytokines can induce activation and maturation of DCs. Panda and colleagues in 2010 identified an increase of pro-inflammatory cytokines released from DCs in elderly adults [88]. Moreover, the activity of DCs in elderly adults was higher than young individuals [89]. Ageing is also accompanied by profound and consistent alterations of T-cell immunity [90]. Although, T-cell numbers do not diminish during aging, however, the T-cell pool exhibit potent age-related alterations, including poor T-cell mitogen responses, an inverted CD4+/CD8+ T-cell ratio, reduced proportion of naive cells, as well as an increased proportion of memory cells, in older animals and human [91]. Additionally, aging is related to the overproduction of pro-inflammatory cytokines by T cells, leading to immune pathology [92]. The CD8+CD28− subset of cells in the expanded memory cell population has shortened telomeres, suggesting that they have a longer reflective history [93]. In humans, almost all T cells express CD28 at birth, and the proportion of CD28+ declines by the age [81, 93]. The increase in these cells has been observed consistently and is used as a prognostic indicator of immune-senescence in older populations [94]. It is demonstrated that aging also changes the cytokine profile of Th2 cells (IL-4 and IL-10) rather than Th1 type (IL-2, IFN-g), leading to mild age-related inflammation in elderly adults. Aging can also potentially affect other Th cells pool. The ratio of Th17 cells/ Tregulatory cells increased during aging, leading to a basal inflammatory state in elderly adults [95]. Th17 cells have the pro-inflammatory phenotype, and they are in balance with Tregulatory anti-inflammatory cells. These cells are derived from a common precursor (Th0) [96]. Tregulatory cells are a subset characterized by a high expression of CD25 and FOXP3, a transcriptional factor for the function and differentiation of Tregulatory cells [97]. In addition to anti-inflammatory effects of Tregulatory cells, also recognize self-antigens [98]. Furthermore, ageing is correlated with disruption of lymphocyte telomerase up-regulation [99]. It is accepted that shortened lymphocyte telomeres are associated with a variety of age-associated pathologies [100]. Furthermore, during aging naïve T-cells show multiple alterations, including the shortening of telomeres, the reduced production of IL-2 and the diminished ability to differentiate themselves into effector-cells. The loss in the number and function of the naïve T-cells, with increasing of T CD8+, CD45RO+, CD25+ clones in aged subjects [101]. CD28-cells are responsible for the production of pro-inflammatory cytokines and are resistant to apoptosis. It is proposed that they are undergoing cells to senescence, due to the shortening of telomeres and reduction of the proliferative capacity [102]. Interestingly, pro-inflammatory cytokines have been also implicated in these age-associated alterations of T-cell immune-senescence. Indeed, inflammatory conditions in elderly adults lead to alterations in T-cell immunity. For instance, age-related increased cytokine TNFα is a potent stimulator of TCD4+ cell senescence and T-cell differentiation [90]. Humoral immunity mediated by B lymphocytes has a critical role in the modulation of adaptive immunity responses. B lymphocytes produce different types of antibodies for eliminating of challengers. Additionally, B lymphocytes have an important role in the immune system through the presentation of antigens and secretion of cytokines [103]. B lymphocytes arising from hematopoietic stem cells in the bone marrow as pro-B lymphocytes. Then, they differentiate into pre-B and then B lymphocytes [104]. Ample studies identified that aging is accompanied by quantitative and qualitative alteration of B lymphocyte pool [105]. The number of B lymphocytes and the levels of serum immunoglobulin and antibody analyzed during the aging. The hematopoietic stem cells in the bone marrow from aged mice are less effective at generating both B lymphocytes as compared to young mice. The number and the size of the germinal centers of B lymphocytes also decreased with aging. Production of precursor B lymphocytes in the bone marrow and the number of pro-B and pre-B lymphocytes decreased in aged mice and human [104]. It has been shown that aged pro-B lymphocytes have an impaired ability to respond to IL-7. Also, both human naïve and IgM memory B lymphocytes impaired during aging [106]. The percentage of IgM memory B lymphocytes are not markedly decreased, however, the total numbers of B lymphocytes decreased. Additionally, the antibody level is decreased in the aged subjects. In addition, the affinity and protective ability of antibodies in aged mice decreased as compared to the young mice [75, 107]. It is also reported that the immune response to influenza in old mice has less IgG level than in young mice. Also, young mice had mostly IgG1 plasma level with high-affinity antibody. In contrast, aged mice mostly had IgM plasma cells [108]. Hence, antigen-specific antibody responses decline in old mice. The decreased function of B lymphocytes (such as antigen-specific antibody response and antibody affinity) during aging has been attributed to lack of Th function. Because the function of B lymphocytes is T-dependent [97, 109]. Taken together, immune-senescence alterations cannot properly fit cell-mediating and humeral immune response in elderly adults. The immune system appears to maintain a mild inflammatory state in elderly adult. Therefore, it is suggested that fragile and mildly over-active immune system in elderly adults cannot turn off the pro-inflammatory machine in COVID-19 infection. Clinical findings in severe patients with COVID-19 infection are in consistent with the above mention literature. Several manifestations including; lymphopenia, reduced numbers of CD4+ T cells, CD8+ T cells, B cells and natural killer (NK) cells, monocytes, eosinophils and basophils reported in severe patients with COVID-19 infection [25]. Schouten and colleagues in 2019 identified that increasing pro-inflammatory cytokines during aging also correlated with the severity of ARDS and may partially explain age-dependent difference [110].
Aging and vitamin D deficiency
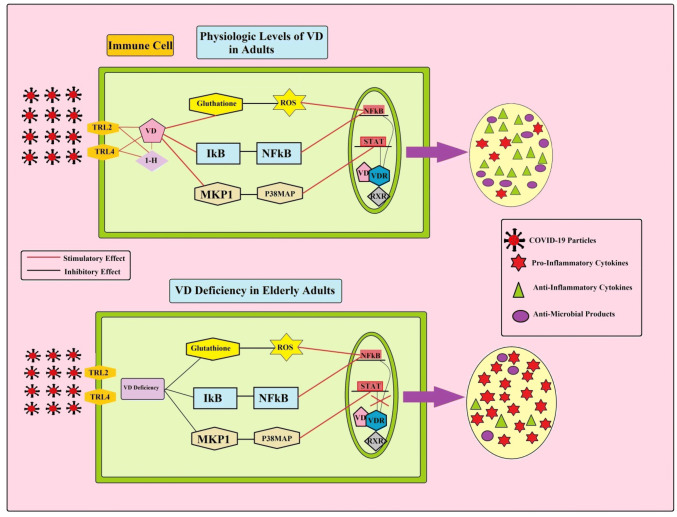
VD together with vitamin D receptor (VDR) has both classical functions (such as bone and calcium-phosphorus homeostasis), and non-classical function (such as anti-inflammatory and immune-regulatory function) [111, 112]. VDR is expressed by several types of immune cells, including monocytes, macrophages, B and T lymphocytes, as well as DCs [113]. Additionally, the α-1-hydroxilase enzyme, which converts inactive metabolite of VD (25(OH) D3) to the active form (1,25(OH)2D3), is expressed by the majority of immune cells such as macrophages [112]. Liu and colleagues in 2014 reported that the expression of VDR and α-1-hydroxylase increased in macrophages following exposure to a pathogen [114]. This finding suggested that the intracrine immune-regulatory function of VD. As shown in Fig. 4, VD can decrease the expression of pro-inflammatory genes (such as TNF-α, IL-6, monocyte chemotatic protein1 (MCP-1), and IL-12β) in immune cells through suppressing excessive ROS production, increasing intracellular glutathione levels, suppressing NF-κB and p38 MAP kinase expression. Excess ROS production can increase NF-kB expression in immune cells, leading to excess secretion of pro-inflammatory cytokines, including TNF-a, IL-1, IL-6, IL-8, IFN-g, iNOS, COX-2 [50]. Activation of p38 MAP kinase pathway can also increase IL-6 and MCP-1 generation in immune cells by stimulation of the signal transducer and activator of transcription1/5 (STAT1/5) [115]. It seems that pathogen challengers turn macrophages on by stimulation of PPRs (such as TLR2/1or NLRs). Activation of TLRs or NLR can increase intracellular VDR and α-1-hydroxylase expression. Then, a complex of VD and VDR together can decrease the pro-inflammatory cytokines, increase the autophagy activity of macrophages, and antimicrobial product generation, including cathelicidin and β-defensin in macrophages [116]. In addition, of immune regulatory effects of VD on macrophages, it can also suppress the differentiation, and migration of human DCs, and decrease the expression of MHCII on DCs, which are characterized as the tolerogenic properties [114, 117]. Tolerogenic DCs can increase the number of IL-10 producing CD4+ T-cells and Tregulatory cells. Elevated circulating CD4+ Tregulatory have of anti-inflammatory functions and also attenuated the inflammatory response of T-effector cells. These tolerogenic actions of DCs are mediated by increased expression of FOXP3 transcription factor [118]. Furthermore, VD can also make a complex with VDR on the T lymphocytes, leading to suppression of its proliferation. Hoe and colleagues in 2016 reported that VD markedly decreased pro-inflammatory cytokines (TNF-α, IFN-γ, and IL-1β, IL-8, IFN-γ) in response to bacterial ligands exposure. VD also increased the level of anti-inflammatory cytokine (IL-10) [119]. Hence, VD can modulate both innate and adaptive immune responses. Elderly adults are at risk for VD deficiency due to several factors, including decreased pre-VD production, poor skin integrity decreased dietary intake of VD, increasing adiposity, obesity, decreased renal function, as well as less time spent outdoors [120]. VD deficiency has been linked to various aging-related inflammatory diseases, including rheumatoid arthritis, asthma, inflammatory bowel disease, multiple sclerosis, cardiovascular disease, hypertension, diabetes mellitus, and cancer [121]. Additionally, there is a correlation between VD deficiency and risk of respiratory tract infection such as COVID-19 [122]. For example, Ilie and colleagues in 2020 examine the association between the mean levels of VD in 20 European countries and morbidity and mortality caused by COVID-19. Their analysis data identified negative correlations between mean levels of VD (average 56 mmol/L) in each country and the number of COVID-19 cases. In addition, a negative correlation was observed between mean levels of VD and the mortality of COVID-19 cases. They also reported that VD levels are severely low in the aging population especially in Spain, Italy and Switzerland, the most vulnerable countries in relation to COVID-19 [123]. Additionally, Ebadi and Montano-Loza in 2020 reported that VD can suppress the expression of pro-inflammatory markers, including IL-1α, IL-1β, as well as TNF-α. Therefore, VD deficiency during aging related to overexpression of Th1 cytokines. They also reported that 50% of patients with COVID-19 and about 70% mortality of COVID-19 observed in African-American population in Chicago, who are at a greater risk for VD deficiency [124]. There are reports that polymorphism of VDR gene, including polymorphisms of FokI, ApaI, and TaqI, is associated with VD deficiency and increased risk of inflammatory diseases [125]. Hence, VD and VDR pathway together have an important anti-inflammatory function in immune effectors through decreasing pro-inflammatory cytokine generation and increasing anti-inflammatory cytokines in immune cells. Furthermore, the lack of VD in aged subjects is associated with the pro-inflammatory phenotype of immune cells, leading to likely increasing the risk of elderly adults with chronic mild inflammation condition [122]. This chronic inflammatory condition likely leads to cytokine storm in elderly COVID-19 patients.
Fig. 4.
The role of vitamin D content on pro-inflammatory cytokine release in young and elderly adults with COVID-19 infection. Following exposure to COVID-19 particles in young adults, sufficient vitamin D content increasing intracellular glutathione levels, suppressing excessive ROS production, suppressing NF-κB (nuclear factor kappa b) and p38 MAP kinase expression. In contrast, vitamin D deficiency in elderly adults leads to over-activity of p38 MAP kinase/ STAT (signal transducers and activator of transcription) and ROS/ NF-Κb pathways in the immune cells. So, elderly adults with severe COVID-19 infection cannot turn off their pro-inflammatory immune machine
Conclusion
In summary, it seems that young adults have balanced between pro-inflammatory and anti-inflammatory cytokine networks (Fig. 1). Therefore, their balanced immune system can limit the progression of COVID-19 infection. However, elderly patients do not have the same balanced immune response as young adults. As shown Fig. 5, with advancing age, the immune system appears to maintain a condition of mild inflammation. So, the activation of the body with pathogens, such as COVID-19 infection can exaggeratedly increase the amplitude of the immune response, which is known as a cytokine storm. As mentioned above, alteration of ACE2 receptor expression, oxidative stress, adipose tissue- and immune-senescent cell activity, lack of VD content, as well as decrease of autophagy and mitophagy may contribute to high amplitude of the immune response to external challengers in elderly adults. This high amplitude of the immune response in elderly adults can favor induction of the cytokine storm and death in severe and critical cases of COVID-19 infection. Nevertheless, the COVID-19 infection is not deadly in all elderly patients, because the aging process is dependent on several markers, including genes, lifestyles, individual variety of immune responses to challengers.
Fig. 5.
Disrupted immunological responses in elderly adults with severe COVID-19 infection. Several factors can un-controllable turn on the inflammatory machine in elderly adults with COVID-19, including (1) decreased ACE2 expression in alveolar cells, (2) decreased mitophagy and subsequently excess ROS production, (3) accumulation of senescent adipocyte cells in visceral white adipose tissue, (4), increased number of senescent immune cells, as well as (5) vitamin D deficiency. Hence, elderly adults with severe COVID-19 infection cannot shut down their pro-inflammatory immune response
Acknowledgements
This study was supported by Neuroscience Sciences Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sun D, Li H, Lu XX, Xiao H, Ren J, Zhang FR, Liu ZS. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center’s observational study. World J Pediatr. 2020;19:1–9. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Meng J, Zhu Z, Zhang Z, Wang J, Sheng J. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–327. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molloy EJ, Bearer CF. COVID-19 in children and altered inflammatory responses. Pediatr Res. 2020;In Press. [DOI] [PubMed]
- 4.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020;In Press. [DOI] [PMC free article] [PubMed]
- 5.Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, Morishita R. Source of chronic inflammation in aging. Front Cardiovasc Med. 2018;5:12. doi: 10.3389/fcvm.2018.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koelman L, Pivovarova-Ramich O, Pfeiffer AF, Grune T, Aleksandrova K. Cytokines for evaluation of chronic inflammatory status in ageing research: reliability and phenotypic characterisation. Immun Ageing. 2019;16(1):11. doi: 10.1186/s12979-019-0151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoon HE, Kim EN, Kim MY, Lim JH, Jang I, Ban TH, Shin SJ, Park CW, Chang YS, Choi BS. Age-associated changes in the vascular renin-angiotensin system in mice. Oxid Med Cell Longev. 2016;5:1–14. doi: 10.1155/2016/6731093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garrido A, Cruces J, Ceprián N, Vara E, de la Fuente M. Oxidative-inflammatory stress in immune cells from adult mice with premature aging. Int J Mol Sci. 2019;20(3):769. doi: 10.3390/ijms20030769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbosa MC, Grosso RA, Fader CM. Hallmarks of aging: an autophagic perspective. Front Endocrinol. 2019;9:790. doi: 10.3389/fendo.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology. 2017;32(1):9–19. doi: 10.1152/physiol.00012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuentes E, Fuentes M, Alarcon M, Palomo I. Immune system dysfunction in the elderly. An Acad Bras Ciênc. 2017;89(1):285–299. doi: 10.1590/0001-3765201720160487. [DOI] [PubMed] [Google Scholar]
- 14.Meehan M, Penckofer S. The role of vitamin D in the aging adult. J Aging Gerontol. 2014;2(2):60–71. doi: 10.12974/2309-6128.2014.02.02.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16(1):69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robb CT, Regan KH, Dorward DA, Rossi AG. Key mechanisms governing resolution of lung inflammation. Semin Immunopathol. 2016;38:425–448. doi: 10.1007/s00281-016-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moldoveanu B, Otmishi P, Jani P, Walker J, Sarmiento X, Guardiola J, Saad M, Yu J. Inflammatory mechanisms in the lung. J Inflamm Res. 2009;2:1. [PMC free article] [PubMed] [Google Scholar]
- 18.Kambayashi T, Laufer TM. Atypical MHC class II-expressing antigen-presenting cells: can anything replace a dendritic cell? Nat Rev Immunol. 2014;14(11):719–730. doi: 10.1038/nri3754. [DOI] [PubMed] [Google Scholar]
- 19.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+ T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:1–12. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Channappanavar R, Fehr AR, Zheng J, Wohlford-Lenane C, Abrahante JE, Mack M, Sompallae R, McCray PB, Meyerholz DK, Perlman S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Investig. 2019;129(9):3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grødeland G, Fossum E, Bogen B. Polarizing T and B cell responses by APC-targeted subunit vaccines. Front Immunol. 2015;6:367. doi: 10.3389/fimmu.2015.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. 2020;10(2):102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LF. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;28:1–2. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schett G, Sticherling M, Neurath MF. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020;20:271–272. doi: 10.1038/s41577-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, Wang Q, Miao H. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):1–3. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, Su X, Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;In Press. [DOI] [PMC free article] [PubMed]
- 30.Park MD. Macrophages: a Trojan horse in COVID-19? Nat Rev Immunol. 2020;20:351–351. doi: 10.1038/s41577-020-0317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, Xu Y, Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zeng X. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin Immunol. 2020;214:18393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;e20462:1–8. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–4. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia S, Zhang X, Zheng S, Khanabdali R, Kalionis B, Wu J, Wan W, Tai X. An update on inflamm-aging: mechanisms, prevention, and treatment. J Immunol Res. 2016;2016:1–12. doi: 10.1155/2016/8426874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tikellis C, Thomas MC. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012;2012:1–12. doi: 10.1155/2012/256294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu X, Cui L, Hou F, Liu X, Wang Y, Wen Y, Chi C, Li C, Liu R, Yin C. Angiotensin-converting enzyme 2-angiotensin (1–7)-Mas axis prevents pancreatic acinar cell inflammatory response via inhibition of the p38 mitogen-activated protein kinase/nuclear factor-κB pathway. Int J Mol Med. 2018;41(1):409–420. doi: 10.3892/ijmm.2017.3252. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues Prestes TR, Rocha NP, Miranda AS, Teixeira AL, Simoes-e-Silva AC. The anti-inflammatory potential of ACE2/angiotensin-(1–7)/mas receptor axis: evidence from basic and clinical research. Curr Drug Targets. 2017;18(11):1301–1313. doi: 10.2174/1389450117666160727142401. [DOI] [PubMed] [Google Scholar]
- 40.Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Inflammasomes in cancer: a double-edged sword. Protein cell. 2014;5(1):12–20. doi: 10.1007/s13238-013-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cristiani L, Mancino E, Matera L, Nenna R, Pierangeli A, Scagnolari C, Midulla F. Will children reveal their secret? The coronavirus dilemma. Eur Respir J. 2020;55:2000749. doi: 10.1183/13993003.00749-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fu X, Lin R, Qiu Y, Yu P, Lei B. Overexpression of angiotensin-converting enzyme 2 ameliorates amyloid β-induced inflammatory response in human primary retinal pigment epithelium. Investig Ophthalmol Vis Sci. 2017;58(7):3018–3028. doi: 10.1167/iovs.17-21546. [DOI] [PubMed] [Google Scholar]
- 43.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xudong X, Junzhu C, Xingxiang W, Furong Z, Yanrong L. Age-and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78(19):2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Jiang Q, Xia X, Liu K, Yu Z, Tao W, Gong W, Han JD. Individual variation of the SARS-CoV2 receptor ACE2 gene expression and regulation. Preprints. 2020;2020030191. [DOI] [PMC free article] [PubMed]
- 46.Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X, Wen F, Huang X, Ning G, Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6(1):1–4. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kozieł R, Pircher H, Kratochwil M, Lener B, Hermann M, Dencher NA, Jansen-Dürr P. Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. Biochem J. 2013;452(2):231–239. doi: 10.1042/BJ20121778. [DOI] [PubMed] [Google Scholar]
- 49.Lavrovsky Y, Chatterjee B, Clark RA, Roy AK. Role of redox-regulated transcription factors in inflammation, aging and age-related diseases. Exp Gerontol. 2000;35(5):521–532. doi: 10.1016/S0531-5565(00)00118-2. [DOI] [PubMed] [Google Scholar]
- 50.Sarkar D, Fisher PB. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236(1):13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Sarkar D, Lebedeva IV, Emdad L, Kang DC, Baldwin AS, Jr, Fisher PB. Human polynucleotide phosphorylase (hPNPaseold-35): a potential link between aging and inflammation. Cancer Res. 2004;64(20):7473–7478. doi: 10.1158/0008-5472.CAN-04-1772. [DOI] [PubMed] [Google Scholar]
- 52.Biswas SK. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid Med Cell Longev. 2016;2016:1–9. doi: 10.1155/2016/5698931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Lan T, Zhang C, Zeng C, Hou J, Yang Z, Zhang M, Liu J, Liu B. Reciprocal activation between IL-6/STAT3 and NOX4/Akt signalings promotes proliferation and survival of non-small cell lung cancer cells. Oncotarget. 2015;6(2):1031. doi: 10.18632/oncotarget.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y, Lu J, Antony S, Juhasz A, Liu H, Jiang G, Meitzler JL, Hollingshead M, Haines DC, Butcher D, Roy K. Activation of TLR4 is required for the synergistic induction of dual oxidase 2 and dual oxidase A2 by IFN-γ and lipopolysaccharide in human pancreatic cancer cell lines. J Immunol. 2013;190(4):1859–1872. doi: 10.4049/jimmunol.1201725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging. 2012;4(3):166. doi: 10.18632/aging.100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen G, Kroemer G, Kepp O. Mitophagy: an emerging role in aging and age-associated diseases. Front Cell Dev Biol. 2020;8:200. doi: 10.3389/fcell.2020.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghobadi N, Sahraei H, Meftahi GH, Bananej M, Salehi S. Effect of estradiol replacement in ovariectomized NMRI micein response to acute and chronic stress. J Appl Pharm Sci. 2016;6(11):176–184. doi: 10.7324/JAPS.2016.601128. [DOI] [Google Scholar]
- 58.Faraji N, Shiravi A, Bahari Z, Shirvani H, Meftahi GH. Basolateral amygdala α1-adrenergic receptor suppression attenuates stress-induced anxiety-like behavior and spine morphology impairment on hippocampal CA1 pyramidal neurons. Neurochem J. 2020;14:77–89. doi: 10.1134/S1819712420010079. [DOI] [Google Scholar]
- 59.Vernucci E, Tomino C, Molinari F, Limongi D, Aventaggiato M, Sansone L, Tafani M, Russo MA. Mitophagy and oxidative stress in cancer and aging: focus on sirtuins and nanomaterials. Oxid Med Cell Longev. 2019;2019:1–19. doi: 10.1155/2019/6387357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bahari Z, Meftahi GH, Meftahi MA. Dopamine effects on stress-induced working memory deficits. Behav Pharmacol. 2018;29(7):584–591. doi: 10.1097/FBP.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 61.Heim VJ, Stafford CA, Nachbur U. NOD signaling and cell death. Front Cell Dev Biol. 2019;7:1–15. doi: 10.3389/fcell.2019.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ebersole JL, Kirakodu S, Novak MJ, Exposto CR, Stromberg AJ, Shen S, Orraca L, Gonzalez-Martinez J, Gonzalez OA. Effects of aging in the expression of NOD-like receptors and inflammasome-related genes in oral mucosa. Mol Oral Microbiol. 2016;31(1):18–32. doi: 10.1111/omi.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luan J, Zhang X, Wang S, Li Y, Fan J, Chen W, Zai W, Wang S, Wang Y, Chen M, Meng G. NOD-like receptor protein 3 inflammasome-dependent IL-1β accelerated ConA-induced hepatitis. Front Immunol. 2018;9:758. doi: 10.3389/fimmu.2018.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nadatani Y, Huo X, Zhang X, Yu C, Cheng E, Zhang Q, Dunbar KB, Theiss A, Pham TH, Wang DH, Watanabe T. NOD-like receptor protein 3 inflammasome priming and activation in Barrett’s epithelial cells. Cell Mol Gastroenterol Hepatol. 2016;2(4):439–453. doi: 10.1016/j.jcmgh.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Fu H, Nanayakkara G, Li Y, Shao Y, Johnson C, Cheng J, Yang WY, Yang F, Lavallee M, Xu Y. Novel extracellular and nuclear caspase-1 and inflammasomes propagate inflammation and regulate gene expression: a comprehensive database mining study. J Hematol Oncol. 2016;9(1):122. doi: 10.1186/s13045-016-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stranks AJ, Hansen AL, Panse I, Mortensen M, Ferguson DJ, Puleston DJ, Shenderov K, Watson AS, Veldhoen M, Phadwal K, Cerundolo V. Autophagy controls acquisition of aging features in macrophages. J Innate Immun. 2015;7(4):375–391. doi: 10.1159/000370112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saxena M, Yeretssian G. NOD-like receptors: master regulators of inflammation and cancer. Front Immunol. 2014;5:327. doi: 10.3389/fimmu.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davalos AR, Coppe JP, Campisi J, Desprez PY. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010;29(2):273–283. doi: 10.1007/s10555-010-9220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alicka M, Kornicka-Garbowska K, Kucharczyk K, Kępska M, Rӧcken M, Marycz K. Age-dependent impairment of adipose-derived stem cells isolated from horses. Stem Cell Res Ther. 2020;11(1):1–20. doi: 10.1186/s13287-019-1512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16(5):238–246. doi: 10.1016/j.molmed.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stojanović SD, Fiedler J, Bauersachs J, Thum T, Sedding DG. Senescence-induced inflammation: an important player and key therapeutic target in atherosclerosis. Eur Heart J. 2020;1–14. [DOI] [PMC free article] [PubMed]
- 72.Petrakis D, Margină D, Tsarouhas K, Tekos F, Stan M, Nikitovic D, Kouretas D, Spandidos DA, Tsatsakis A. Obesity - a risk factor for increased COVID-19 prevalence, severity and lethality (Review) Mol Med Rep. 2020;22(1):9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Covarrubias AJ, Kale A, Perrone R, Lopez-Dominguez JA, Pisco AO, Kasler HG, Schmidt MS, Wiley CD, Iyer SS, Basisty N, Wu Q. Aging-related inflammation driven by cellular senescence enhances NAD consumption via activation of CD38+ pro-inflammatory macrophages. bioRxiv. 2019:609438.
- 74.Ghosh AK, Mau T, O'Brien M, Garg S, Yung R. Impaired autophagy activity is linked to elevated ER-stress and inflammation in aging adipose tissue. Aging. 2016;8(10):2525. doi: 10.18632/aging.101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ponnappan S, Ponnappan U. Aging and immune function: molecular mechanisms to interventions. Antioxid Redox Sign. 2011;14(8):1551–1585. doi: 10.1089/ars.2010.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, Ligotti ME, Zareian N, Accardi G. The immunosenescence and its hallmarks: how to oppose ageing strategically? A review of potential options for therapeutic intervention. Front Immunol. 2019;10:1–19. doi: 10.3389/fimmu.2019.02247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rahat MA, Coffelt SB, Granot Z, Muthana M, Amedei A. Macrophages and neutrophils: regulation of the inflammatory microenvironment in autoimmunity and cancer. Mediat Inflamm. 2016;2016:1–3. doi: 10.1155/2016/5894347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Linehan E, Fitzgerald D. Ageing and the immune system: focus on macrophages. Eur J Microbiol Immunol. 2015;5(1):14–24. doi: 10.1556/EuJMI-D-14-00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27(5):717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 81.Rosenberg J, Huang J. CD8+ T cells and NK cells: parallel and complementary soldiers of immunotherapy. Curr Opin Chem Eng. 2018;19:9–20. doi: 10.1016/j.coche.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Camous X, Pera A, Solana R, Larbi A. NK cells in healthy aging and age-associated diseases. BioMed Res Int. 2012;2012:1–8. doi: 10.1155/2012/195956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Przemska-Kosicka A, Childs CE, Maidens C, Dong H, Todd S, Gosney MA, Tuohy KM, Yaqoob P. Age-related changes in the natural killer cell response to seasonal influenza vaccination are not influenced by a synbiotic: a randomised controlled trial. Front Immunol. 2018;9:591. doi: 10.3389/fimmu.2018.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, Caminschi I, Shortman K, Henson PM, Jakubzick CV. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell–associated antigen. J Exp Med. 2011;208(9):1789–1797. doi: 10.1084/jem.20110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saiz ML, Rocha-Perugini V, Sánchez-Madrid F. Tetraspanins as organizers of antigen-presenting cell function. Front Immunol. 2018;9:1074. doi: 10.3389/fimmu.2018.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jensen SS, Gad M. Differential induction of inflammatory cytokines by dendritic cells treated with novel TLR-agonist and cytokine based cocktails: targeting dendritic cells in autoimmunity. J Inflamm. 2010;7(1):37. doi: 10.1186/1476-9255-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Panda A, Qian F, Mohanty S, Van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, Allore HG. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184(5):2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agrawal A, Tay J, Ton S, Agrawal S, Gupta S. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J Immunol. 2009;182(2):1138–1145. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Macaulay R, Akbar AN, Henson SM. The role of the T cell in age-related inflammation. Age. 2013;35(3):563–572. doi: 10.1007/s11357-012-9381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bektas A, Schurman SH, Sen R, Ferrucci L. Human T cell immunosenescence and inflammation in aging. J Leukocyte Biol. 2017;102(4):977–988. doi: 10.1189/jlb.3RI0716-335R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weng NP, Akbar AN, Goronzy J. CD28− T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30(7):306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zanni F, Vescovini R, Biasini C, Fagnoni F, Zanlari L, Telera A, Di Pede P, Passeri G, Pedrazzoni M, Passeri M, Franceschi C. Marked increase with age of type 1 cytokines within memory and effector/cytotoxic CD8+ T cells in humans: a contribution to understand the relationship between inflammation and immunosenescence. Exp Gerontol. 2003;38(9):981–987. doi: 10.1016/S0531-5565(03)00160-8. [DOI] [PubMed] [Google Scholar]
- 95.Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol. 2013;48:1379–1386. doi: 10.1016/j.exger.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 96.Caza T, Landas S. Functional and phenotypic plasticity of CD4(+) T cell subsets. BioMed Res Int. 2015;2015:521957. doi: 10.1155/2015/521957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ventura MT, Casciaro M, Gangemi S, Buquicchio R. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15(1):21. doi: 10.1186/s12948-017-0077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X, Zheng Y. Regulatory T cell identity: formation and maintenance. Trends Immunol. 2015;36:344–353. doi: 10.1016/j.it.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van de Berg PJ, Griffiths SJ, Yong SL, Macaulay R, Bemelman FJ, Jackson S, Henson SM, ten Berge IJ, Akbar AN, van Lier RA. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J Immunol. 2010;184(7):3417–3423. doi: 10.4049/jimmunol.0903442. [DOI] [PubMed] [Google Scholar]
- 100.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schwaiger S, Wolf AM, Robatscher P, Jenewein B, Grubeck-Loebenstein B. IL-4-producing CD8+ T cells with a CD62L ++(bright) phenotype accumulate in a subgroup of older adults and are associated with the maintenance of intact humoral immunity in old age. J Immunol. 2003;170:613–619. doi: 10.4049/jimmunol.170.1.613. [DOI] [PubMed] [Google Scholar]
- 102.Sansoni P, Vescovini R, Fagnoni F, Biasini C, Zanni F, Zanlari L, Telera A, Lucchini G, Passeri G, Monti D, Franceschi C, Passeri M. The immune system in extreme longevity. Exp Gerontol. 2008;43:61–65. doi: 10.1016/j.exger.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 103.Ma S, Wang C, Hao Y. B cell dysfunction associated with aging and autoimmune diseases. Front Immunol. 2019;10:318. doi: 10.3389/fimmu.2019.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holodick NE, Rothstein TL. B cells in the aging immune system: time to consider B-1 cells. Ann N Y Acad Sci. 2015;1362(1):176. doi: 10.1111/nyas.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alves AS, Bueno V. Immunosenescence: participation of T lymphocytes and myeloid-derived suppressor cells in aging-related immune response changes. Einstein. 2019;17(2):1–5. doi: 10.31744/einstein_journal/2019RB4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Frasca D, Blomberg BB. Effects of aging on B cell function. Curr Opin Immunol. 2009;21(4):425–430. doi: 10.1016/j.coi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holodick NE, Vizconde T, Hopkins TJ, Rothstein TL. Age-related decline in natural IgM function: diversification and selection of the B-1a cell pool with age. J Immunol. 2016;196(10):4348–4357. doi: 10.4049/jimmunol.1600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Han S, Yang K, Ozen Z, Peng W, Marinova E, Kelsoe G, Zheng B. Enhanced differentiation of splenic plasma cells but diminished long-lived high-affinity bone marrow plasma cells in aged mice. J Immunol. 2003;170:1267–1273. doi: 10.4049/jimmunol.170.3.1267. [DOI] [PubMed] [Google Scholar]
- 109.Cancro MP, Hao Y, Scholz JL, Riley RL, Frasca D, Dunn- Walters DK, Blomberg BB. B cells and aging: molecules and mechanisms. Trends Immunol. 2009;30:313–318. doi: 10.1016/j.it.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schouten LR, van Kaam AH, Kohse F, Veltkamp F, Bos LD, de Beer FM, van Hooijdonk RT, Horn J, Straat M, Witteveen E, Glas GJ. Age-dependent differences in pulmonary host responses in ARDS: a prospective observational cohort study. Ann Intensive Care. 2019;9(1):55. doi: 10.1186/s13613-019-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chirumbolo S, Bjørklund G, Sboarina A, Vella A. The role of vitamin D in the immune system as a pro-survival molecule. Clin Ther. 2017;39(5):894–916. doi: 10.1016/j.clinthera.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 112.Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients. 2018;10(11):1656. doi: 10.3390/nu10111656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Joshi S, Pantalena LC, Liu XK, Gaffen SL, Liu H, Rohowsky-Kochan C, Ichiyama K, Yoshimura A, Steinman L, Christakos S, Youssef S. 1, 25-Dihydroxyvitamin D3 ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31(17):3653–3669. doi: 10.1128/MCB.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu CY, Zhang ZH, Yang HF, Xu H, Cheng FF, Xu JZ. Effect of vitamin D3 on maturation and antigen-presenting function of dendritic cells treated with Mycobacterium tuberculosis. Asian Pac J Trop Med. 2016;9(1):54–57. doi: 10.1016/j.apjtm.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 115.Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PloS One. 2015;10(11):1–12. doi: 10.1371/journal.pone.0141770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wöbke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Front Physiol. 2014;5:244. doi: 10.3389/fphys.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barragan M, Good M, Kolls JK. Regulation of dendritic cell function by vitamin D. Nutrients. 2015;7(9):8127–8151. doi: 10.3390/nu7095383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoe E, Nathanielsz J, Toh ZQ, Spry L, Marimla R, Balloch A, Mulholland K, Licciardi PV. Anti-inflammatory effects of vitamin D on human immune cells in the context of bacterial infection. Nutrients. 2016;8(12):806. doi: 10.3390/nu8120806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Elizondo-Montemayor L, Castillo EC, Rodríguez-López C, Villarreal-Calderón JR, Gómez-Carmona M, Tenorio-Martínez S, Nieblas B, García-Rivas G. Seasonal variation in vitamin D in association with age, inflammatory cytokines, anthropometric parameters, and lifestyle factors in older adults. Mediat Inflamm. 2017;2017:1–14. doi: 10.1155/2017/5719461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aslam MM, John P, Bhatti A, Jahangir S, Kamboh MI. Vitamin D as a principal factor in mediating rheumatoid arthritis-derived immune response. BioMed Res Int. 2019;2019:1–12. doi: 10.1155/2019/3494937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sişmanlar T, Aslan AT, Gülbahar Ö, Özkan S. The effect of vitamin D on lower respiratory tract infections in children. Turk Pediatr Ars. 2016;51(2):94. doi: 10.5152/TurkPediatriArs.2016.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;1:1–4. doi: 10.1007/s40520-020-01570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ebadi M, Montano-Loza AJ. Perspective: improving vitamin D status in the management of COVID-19. Eur J Clin Nutr. 2020;1:1–4. doi: 10.1038/s41430-020-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zaki M, Kamal S, Basha WA, Youness E, Ezzat W, El-Bassyouni H, Amr K. Association of vitamin D receptor gene polymorphism (VDR) with vitamin D deficiency, metabolic and inflammatory markers in Egyptian obese women. Gen Dis. 2017;4(3):176–182. doi: 10.1016/j.gendis.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]