Salmonella species are bacteria that are a major source of foodborne disease through contamination of a diversity of foods, including meat, eggs, fruits, nuts, and vegetables. More than 2,600 different Salmonella enterica serovars have been identified, and only a few of them are associated with illness in humans. Despite the fact that they are genetically closely related, there is enormous variation in the virulence of different isolates of Salmonella enterica. Identification of foodborne pathogens is a lengthy process based on microbiological, biochemical, and immunological methods. Here, we worked toward new ways of integrating whole-genome sequencing (WGS) approaches into food safety practices. We used WGS to build associations between virulence and genetic diversity within 83 Salmonella isolates representing 77 different Salmonella serovars. Our work demonstrates the potential of combining a genomics approach and virulence tests to improve the diagnostics and assess risk of human illness associated with specific Salmonella isolates.
KEYWORDS: Salmonella, amoeba, epithelial cells, food safety, host cell interaction, macrophages, prediction, systemic infection, virulence, whole-genome sequencing
ABSTRACT
Salmonella comprises more than 2,600 serovars. Very few environmental and uncommon serovars have been characterized for their potential role in virulence and human infections. A complementary in vitro and in vivo systematic high-throughput analysis of virulence was used to elucidate the association between genetic and phenotypic variations across Salmonella isolates. The goal was to develop a strategy for the classification of isolates as a benchmark and predict virulence levels of isolates. Thirty-five phylogenetically distant strains of unknown virulence were selected from the Salmonella Foodborne Syst-OMICS (SalFoS) collection, representing 34 different serovars isolated from various sources. Isolates were evaluated for virulence in 4 complementary models of infection to compare virulence traits with the genomics data, including interactions with human intestinal epithelial cells, human macrophages, and amoeba. In vivo testing was conducted using the mouse model of Salmonella systemic infection. Significant correlations were identified between the different models. We identified a collection of novel hypothetical and conserved proteins associated with isolates that generate a high burden. We also showed that blind prediction of virulence of 33 additional strains based on the pan-genome was high in the mouse model of systemic infection (82% agreement) and in the human epithelial cell model (74% agreement). These complementary approaches enabled us to define virulence potential in different isolates and present a novel strategy for risk assessment of specific strains and for better monitoring and source tracking during outbreaks.
IMPORTANCE Salmonella species are bacteria that are a major source of foodborne disease through contamination of a diversity of foods, including meat, eggs, fruits, nuts, and vegetables. More than 2,600 different Salmonella enterica serovars have been identified, and only a few of them are associated with illness in humans. Despite the fact that they are genetically closely related, there is enormous variation in the virulence of different isolates of Salmonella enterica. Identification of foodborne pathogens is a lengthy process based on microbiological, biochemical, and immunological methods. Here, we worked toward new ways of integrating whole-genome sequencing (WGS) approaches into food safety practices. We used WGS to build associations between virulence and genetic diversity within 83 Salmonella isolates representing 77 different Salmonella serovars. Our work demonstrates the potential of combining a genomics approach and virulence tests to improve the diagnostics and assess risk of human illness associated with specific Salmonella isolates.
INTRODUCTION
Salmonella enterica is a facultative intracellular Gram-negative bacterium that remains one of the major sources of foodborne disease for human populations throughout the world (1). Salmonella contaminates a diversity of foods of animal origin (eggs, poultry, beef, and pork) but also fresh produce. Salmonella enterica comprises more than 2,600 different serovars classified into six subspecies, with subspecies enterica being responsible for 99% of Salmonella infections in humans (2, 3). These serovars differ in their adaptation to various hosts and the diseases they cause. Typhoidal serovars cause a systemic life-threatening disease (enteric fever) in humans. On the other hand, nontyphoidal Salmonella (NTS) serovars are capable of causing disease in a broad range of hosts (mammals, reptiles, and birds). In healthy humans, infection with NTS serovars normally develops into a localized self-limiting intestinal inflammation called salmonellosis (4, 5). When the host immunity is compromised, NTS can also cause fatal infections (6).
Although Salmonella imposes a major burden to public health, not all serovars are equally harmful and have the same levels of virulence. While a few serovars are inclined to cause severe extraintestinal systemic disease, others induce mild gastroenteritis and still others survive within the host without causing disease (7, 8). Despite the potential differences in risk to human health, all serovars isolated from food products are treated the same during food safety procedures. Benchmarking virulence combined with serotyping and its potential in foodborne disease diagnostics could lead to a reshaping of current food safety practices to recognize differences in isolate virulence and risk that could help decrease food waste while allowing public health officials to estimate the scope and impact of Salmonella outbreaks.
Given that genetic heterogeneity underlies phenotypic diversity, elucidating the genetic determinants and gene content involved in Salmonella virulence is an important step toward understanding the clinical relevance of particular serovars and their potential role in disease manifestation. It has been well established that Salmonella pathogenesis is a highly complex process which relies on a concerted effort from multiple virulence factors. More than 21 Salmonella pathogenicity islands (SPIs) containing various virulence-associated genes have been described. These functions, in addition to virulence-associated genes located outside SPIs and within virulence plasmids, can be gained and lost (9, 10). The high degree of genome, serovar, and virulence diversity within Salmonella enterica heightens the complexity of Salmonella infection, since different serovars contain different collections of SPIs in different locations (10, 11). Despite the extensive study into the genetics of Salmonella pathogenicity, many virulence-associated genes and their contributions to virulence remain unknown. Furthermore, much of the progress made to date has been based on a select group of Salmonella serovars, underrepresenting the diversity of strains associated with food products.
In humans, Salmonella enters the body following ingestion of contaminated food or water. It survives the harsh acidic conditions of the stomach and reaches the intestine where it adheres to the intestinal epithelial cells, causing a localized infection to the terminal ileum, colon, and mesenteric lymph nodes. In immunocompromised individuals, nontyphoidal serovars can cross the intestinal barrier, invade and replicate within macrophages, and then disseminate systemically (12). There are several approaches to model Salmonella virulence. Intestinal epithelial cells and macrophages have been widely used to test Salmonella virulence factors and study host-Salmonella interaction (13). The amoeba is a complementary in vitro single cell assay and a potentially useful model to study host-Salmonella interaction (14, 15). This is particularly relevant, since amoeba represent a key reservoir of Salmonella for survival in the environment and play a significant role in transmission to humans (16). The mouse is the most widely used animal model for Salmonella infection, and several models have been developed to study distinct aspects of human Salmonella infection in vivo (17).
In the present study, we report the use of a multimodel phenotyping approach and whole-genome sequencing (WGS) to assess 83 Salmonella isolates representing 77 serovars, providing a repertoire of phenotypically and genotypically defined NTS strains. Comparative genomics and 4 host models were used to determine how genomic variations between isolates may influence phenotypic traits implicated in virulence, pinpoint novel virulence markers, and predict virulence both in vitro and in vivo.
RESULTS
Identification of phylogenetically distant Salmonella isolates.
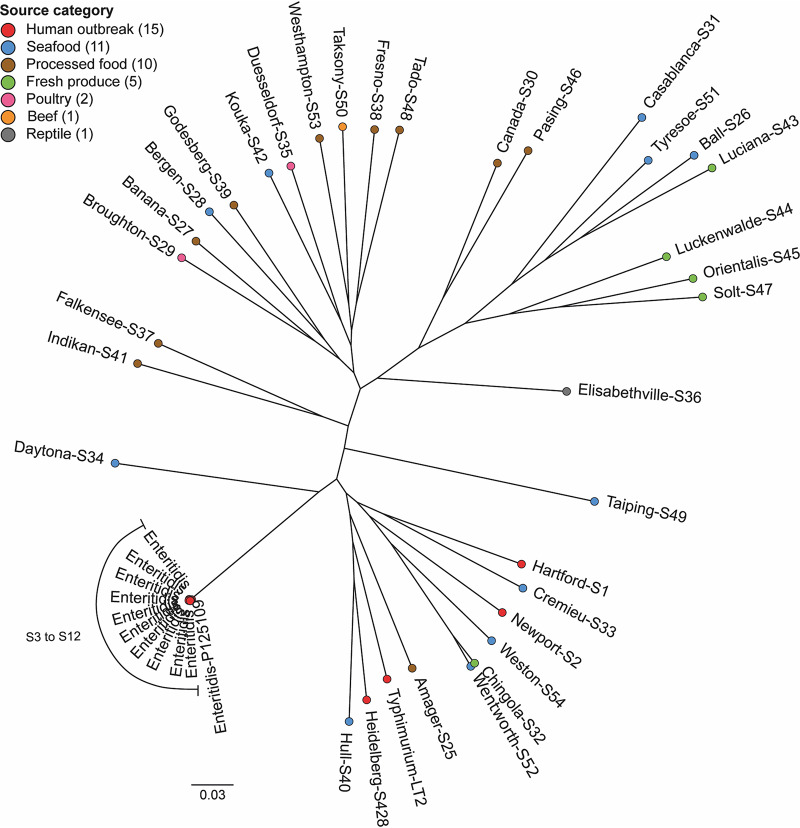
Salmonella enterica serovars have remarkable biological and genomic diversity. We first selected phylogenetically distant Salmonella serovars to evaluate their strain-specific virulence. Thirty-five isolates were selected from the SalFoS database (https://salfos.ibis.ulaval.ca), maximizing genetic diversity and range of sources, including fresh produce. The aim was to estimate whether it was possible to link genetic differences to phenotypic characteristics (Fig. 1, Table 1). In addition to these 35 isolates, 8 S. enterica serovar Enteritidis strains associated with human illness and 2 reference genomes from NCBI, S. enterica serovar Typhimurium LT2 (GenBank accession number NC_003197.2) and S. Enteritidis P125109 (GenBank accession number NC_011294.1), were included in the phylogenetic analysis. The pan-genome was evaluated using SaturnV (v1.1.0) to determine genes present in all genomes and to compute a core single nucleotide polymorphism (SNP) tree (18). The pan-genome is defined as all genes of a clade. For instance, this concept can be applied to species, genus, or even to a domain of life. The pan-genome is separated into 3 groups: core genes (present in all isolates), flexible genes (found in more than one isolate, but not in all of them), and unique genes (only found in one isolate) (19). The pan-genome of the 45 S. enterica genomes contains 3,100 core genes, 3,612 flexible genes, and 3,704 unique genes for a total of 10,461 genes. The phylogenetic tree was computed based on 168,292 core SNPs. All S. enterica serovars from human outbreaks, except for S. Enteritidis, share a more recent common ancestor and thus are more related. Of interest, 4 of the 5 S. enterica serovars isolated on fresh produce also share a recent common ancestor (S. Luciana, S. Luckenwalde, S. Orientalis, and S. Solt).
FIG 1.
Molecular phylogeny of 35 Salmonella enterica serovar isolates. A phylogenetic tree was constructed based on the whole-genome sequences of 35 Salmonella enterica serovars. Serovar font color indicates strain source category. Number of SNPs between two isolates is represented by the distance between two colored dots. The 0.03 scale is used as a unit and indicates the number of nucleotide substitutions per site.
TABLE 1.
35 Salmonella strains of unknown virulence tested in 4 models of infection
| Isolate ID | Serotype | Source category | Host organism | Specific source |
|---|---|---|---|---|
| S1 | Hartford | Human | Human | |
| S2 | Newport | Human | Human | |
| S3 | Enteritidis | Human | Human | |
| S5 | Enteritidis | Human | Human | |
| S25 | Amager | Other | Animal feed | |
| S26 | Ball | Fish/shellfish | Shrimp | |
| S27 | Banana | Other | Animal feed | |
| S28 | Bergen | Fish/shellfish | Shrimp | |
| S29 | Broughton | Poultry | ||
| S30 | Canada | Other | Chocolate | |
| S31 | Casablanca | Fish/shellfish | Shrimp | |
| S32 | Chingola | Plant (fruit/veg) | Seaweed | |
| S33 | Cremieu | Fish/shellfish | Eel | |
| S34 | Daytona | Fish/shellfish | Clams | |
| S35 | Duesseldorf | Poultry | ||
| S36 | Elisabethville | Animal/other | Agamid | |
| S37 | Falkensee | Other | Rice seasoning | |
| S38 | Fresno | Other | Animal feed | |
| S39 | Godesberg | Nuts/seeds | Sesame | Halawa |
| S40 | Hull | Fish/shellfish | Conch | |
| S41 | Idikan | Nuts/seeds | Sesame | Tahini |
| S42 | Kouka | Fish/shellfish | Oysters | |
| S43 | Luciana | Plant (fruit/veg) | Cantaloupe | |
| S44 | Luckenwalde | Plant (fruit/veg) | Cocoa | Beans |
| S45 | Orientalis | Nuts/seeds | Alfalfa | Seeds |
| S46 | Pasing | Other | Chocolate | |
| S47 | Solt | Plant (fruit/veg) | Cocoa | Beans |
| S48 | Tado | Other | Animal feed | |
| S49 | Taiping | Fish/shellfish | Fish | |
| S50 | Taksony | Animal/other | Ox | |
| S51 | Tyresoe | Fish/shellfish | Shrimp | |
| S52 | Wentworth | Fish/shellfish | Cuttlefish | |
| S53 | Westhampton | Other | Animal feed | |
| S54 | Weston | Fish/shellfish | Shrimp | |
| S428 | Heidelberg | Human | Human | Feces |
Comparative virulence of 35 phylogenetically distant Salmonella isolates in four models of infection.
Phenotypic evaluation of isolate virulence was conducted using 4 models of infection: (i) human epithelial cells (bacterial adhesion, invasion, and survival), (ii) human macrophages (phagocytosis and intracellular survival at 2 and 18 h postinfection), (iii) amoeba (intracellular survival), and (iv) in vivo mouse models of systemic infection. In all cases, bacterial load was used as a marker of virulence. For each model, the reference strain S. Typhimurium SL1344 was used as a high-load control, and its isogenic mutant for the genes invA (SPI-1) and sseB (SPI-2) (here referred to as the ΔinvA ΔsseB mutant) was used as a low-load control, as this strain exhibits impaired host cell entry and intracellular survival.
For in vivo phenotyping, C57BL/6J mice were chosen because they are highly susceptible to infection and develop clinical disease within 3 to 5 days postinoculation with virulent Salmonella Typhimurium (20). We chose not to use antibiotics, since it may interfere with Salmonella growth. We then tested two routes of inoculation, oral (p.o.) and intravenous (i.v.), with the aim of identifying a robust phenotype that would provide enough statistical power to allow phenotypic discrimination among the different Salmonella isolates tested. C57BL/6J mice were inoculated with 105 (i.v.) or 107 (p.o.) CFU, and bacterial loads were measured in spleen, liver, and feces (oral infection only) 3 days postinoculation. We observed a significant difference in tissue bacterial loads between SL1344- and ΔinvA ΔsseB mutant-inoculated mice (see Fig. S1A and B in the supplemental material). The difference was greater and more significant when mice were inoculated i.v. (5-log difference for the spleen with i.v. injection compared to a 2-log difference with oral inoculation). There was no difference in fecal excretion (Fig. S1A). We then tested a subset of isolates with unknown virulence (Fig. S1C and D). The 4 isolates tested showed similar phenotypes when inoculated orally or i.v.; high bacterial loads were detected for isolates S3 and S5 and lower bacterial loads for S35 and S39. Since the i.v. inoculation route gave a more robust phenotype in terms of tissue load difference between the two control strains and a less variable phenotype, this was the route of infection that was selected for the following experiments.
Comparison of oral and systemic Salmonella enterica infection in C57BL/6J mice. Mice were either inoculated per os (A and C) or intravenously (B and D). Bacterial loads in the spleens and livers of C57BL/6J mice at day 3 after oral (A and C) and systemic (B and D) Salmonella infection. The graphs represent the CFU counts per weight of organs or feces derived from the means ± standard errors of the means (SEMs) from one experiment. Data are representative of two independent experiments. Two-way ANOVA with Sidak’s multiple-comparison test was used to assess significance. **, P ≤ 0.01; ****, P < 0.0001. Download FIG S1, PDF file, 0.1 MB (109.1KB, pdf) .
© Crown copyright 2020.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
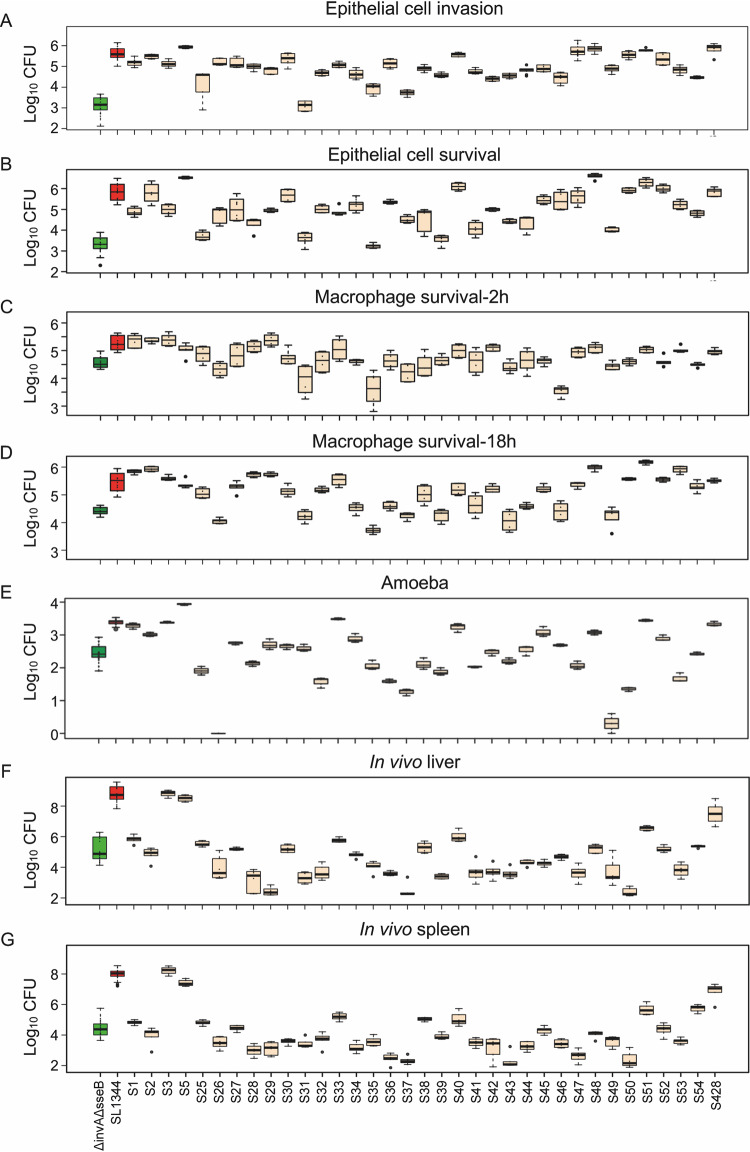
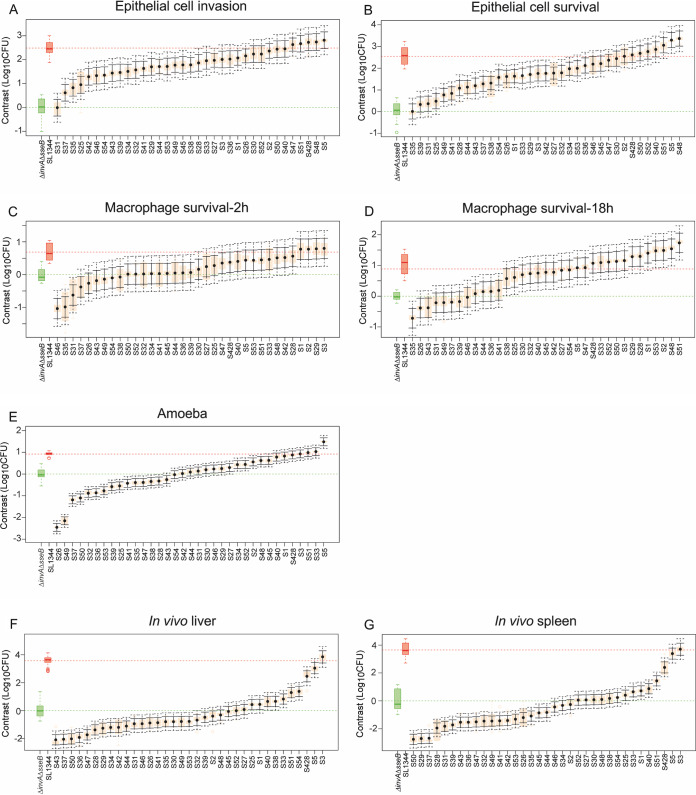
All models showed a wide distribution of phenotypes across the 35 isolates (Fig. 2). Of all phenotypic traits measured, epithelial cell adhesion and macrophage phagocytosis showed the least diversity, with most of the strains not differing significantly from the controls in terms of bacterial adhesion to epithelial cells or uptake by macrophages (see Fig. S2). Contrast analysis was used to compare the differences in bacterial burden caused by each of the 35 isolates against the low control ΔinvA ΔsseB strain within all phenotypic traits measured in each model (Fig. 3). Regarding the epithelial cell model, the majority of the isolates showed mean values that were significantly higher than those for the low control ΔinvA ΔsseB strain, for both invasion and survival (Fig. 3A and B). In macrophages, a wider distribution of CFU was observed, even if the difference between both controls was smaller. At 2 h postinfection, 3 isolates had lower bacterial load than the ΔinvA ΔsseB control, 27 isolates showed no difference compared to the ΔinvA ΔsseB strain, and 5 were higher that the ΔinvA ΔsseB control (Fig. 3C). For the bacterial loads observed 18 h postinfection, 23 isolates were higher than the ΔinvA ΔsseB isolate, 11 isolates were similar to the control, and 1 isolate was lower (Fig. 3D). The amoeba and mouse models showed the most phenotypic discrimination, including many isolates with lower bacterial burden than the ΔinvA ΔsseB control. In the amoeba model, 14 isolates caused bacterial burdens lower than the ΔinvA ΔsseB strain, 8 were similar, and 13 strains had a higher bacterial load than the ΔinvA ΔsseB strain (Fig. 3E). Similarly, in the mouse model (liver and spleen), 19 (liver) and 18 (spleen) isolates caused lower bacterial load than the ΔinvA ΔsseB strain, 9 (liver) and 10 (spleen) showed no difference compared to the ΔinvA ΔsseB strain, and 8 (liver) and 7 (spleen) isolates had a higher bacterial load (Fig. 3F and G).
FIG 2.
Phenotyping 35 phylogenetically distant Salmonella serovars in four models of infection. Bacterial load after infection was used as a marker of virulence. Infection of human epithelial cells (A and B), macrophages (C and D), amoebas (E), and in vivo mouse models of systemic disease (F and G). Human epithelial cells were used to measure Salmonella virulence in the form of adhesion, invasion, and replication within epithelial cells. Shown here are invasion (A) measured by intracellular bacterial burden (CFU) of cells 180 min postinfection and survival (B) as measured 18 h postinfection. Human macrophages were used to test strain propensity for phagocytosis and survival measured at 2 (C) and 18 h (D) postinfection. (E) Virulence in the amoeba model was measured by intracellular bacterial burden. In vivo virulence was measured by bacterial burden in mouse liver (F) and spleen (G) 3 days postinfection. Bacterial burden (CFU) in the spleen is shown here. Sample size varied across models and isolates. Either 2, 3, or 4 biological replicates with 3 technical replicates per isolate were used for epithelial cells. Either 2 or 3 biological replicates with 3 technical replicates were used for macrophages. Three biological replicates were performed per isolate in the amoeba and six biological replicates were performed per isolate in the mouse. In all cases, Log10 bacterial load was used as a marker of virulence. The distribution of data for each serovar is shown as a box plot. Low-virulence control ΔinvA ΔsseB strain is shown in green, high-virulence control SL1344 is shown in red, and test strains are in beige.
FIG 3.
Distribution of contrasts in the four models of infection for all 35 phylogenetically distant Salmonella serovars. Contrast analysis was used to compare differences in bacterial burden caused by each of the 35 serovars against the low-virulence control ΔinvA ΔsseB strain (mean value shown in green) within all phenotypic traits measured in each model. Epithelial cell invasion (A) and survival (B), macrophage phagocytosis (C) and survival (D), replication in amoeba (E), and bacterial load in liver (F) and spleen (G) of mice are shown. Isolate means are shown by black dots. Orange boxes denote the ranges of values, solid black bars show the 95% confidence intervals, and dotted black bars show the confidence intervals when adjusted for multiple testing using Bonferroni’s correction. All isolates whose adjusted confidence intervals do not cross the green line were significantly different from the ΔinvA ΔsseB strain (P < 0.05). Either 2, 3, or 4 biological replicates with 3 technical replicates per isolate were used for epithelial cells. Either 2 or 3 biological replicates with 3 technical replicates were used for macrophages. Three biological replicates were performed per isolate in the amoeba and six biological replicates were performed per isolate in the mouse.
Bacterial loads and distributions of contrasts in human cells infected with 35 phylogenetically distant Salmonella serovars. Bacterial adhesion in epithelial cells (A) and bacterial phagocytosis in macrophages (C) are shown as box plots. Low- and high-virulence controls are shown in green and red, respectively. (B and D) Contrast analysis was used to compare differences in bacterial burden caused by each of the 35 serovars against the low-virulence control ΔinvA ΔsseB strain (mean value shown in green) within all phenotypic traits measured in each model. Epithelial cell adhesion (B) and macrophage phagocytosis (D). Isolate means are shown by black dots. Orange boxes denote the ranges of values, solid black bars show the 95% confidence intervals, and dotted black bars show the confidence intervals when adjusted for multiple testing using Bonferroni’s correction. All isolates whose adjusted confidence intervals do not cross the green line were significantly different from the ΔinvA ΔsseB strain (P < 0.05). Either 2, 3, or 4 biological replicates with 3 technical replicates per isolate were used for epithelial cells. Either 2 or 3 biological replicates with 3 technical replicates were used for macrophages. Download FIG S2, PDF file, 0.2 MB (223KB, pdf) .
© Crown copyright 2020.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Assessment of concordance between in vitro and in vivo models of Salmonella virulence.
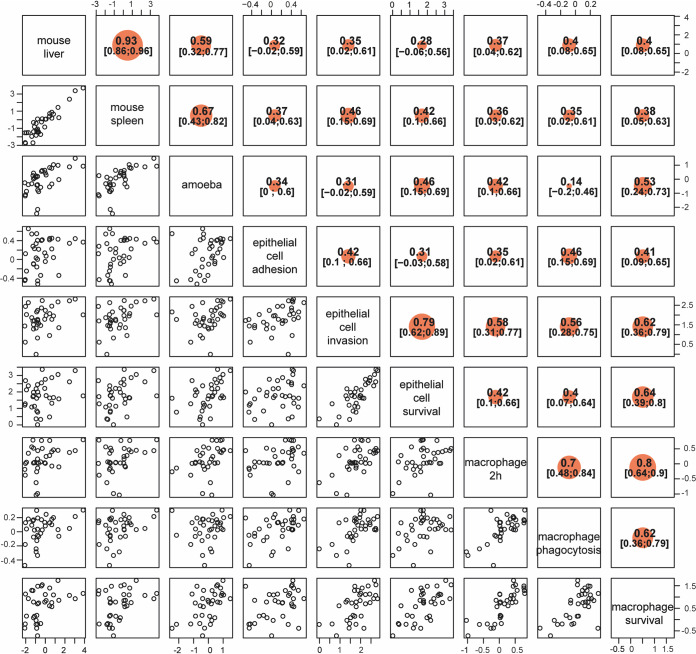
To compare the different models of Salmonella infection in the assessment of the levels of virulence for each isolate, we performed a correlation analysis between contrasts for each model (Fig. 4). All phenotypic traits from each of the models were compared against every other trait. The majority of correlations (86% [31/36]) were positive and significant (Fig. 4). As expected, comparisons that were the most significant were identified within a specific model and included bacterial burden in the mouse spleen versus mouse liver (r = 0.93; confidence interval [CI], 0.86 to 0.96), survival in macrophage 2 h p.i. versus 18 h p.i. (r = 0.8; CI, 0.64 to 0.9), and epithelial cell invasion versus epithelial cell survival (r = 0.79; CI, 0.63 to 0.89). Highly significant correlations were also identified between different models, including bacterial burden in the mouse spleen and liver versus bacterial burden in amoeba (r = 0.67 [CI, 0.43 to 0.82] and r = 0.59 [CI, 0.32 to 0.77], respectively) and epithelial cell survival and invasion versus macrophages survival (18 h p.i.) (r = 0.64 [CI, 0.39 to 0.80] and r = 0.62 [CI, 0.36 to 0.79], respectively). The amoeba and mouse models also showed significant correlations (0.42 < r < 0.53) with epithelial cell survival and macrophage survival (Fig. 4). Interestingly, the isolates (S3, S5, S33, S40, S51, and S428) with the highest bacterial burden detected in vivo in the mouse model of systemic infection were also found to be the highest isolates in the amoeba model and were significantly higher than the low-virulence control in macrophage and epithelial cell models. These isolates with high cellular and tissue burden, with the exception of S51, were all clustered in the phylogenic tree, suggesting shared determinants of virulence (Fig. 1).
FIG 4.
Correlation analysis of 35 strains across the four models of infection. Concordance between each of the four models of infection was assessed by correlation between scaled contrasts from each model. Scattergrams are shown for each of the phenotype combinations on the left of the diagonal running from the top left corner to the bottom right corner. Each point represents a Salmonella serovar. Strain positions are dictated by the level of contrast between the bacterial burden (Log10 CFUs) caused by an isolate and the bacterial burden caused by low-virulence control ΔinvA ΔsseB strain. At the top, the intraclass correlation coefficient is displayed with 95% confidence interval. Correlation coefficients and confidence intervals are shown to the right of the diagonal. Correlations are significant for all correlation coefficients whose confidence intervals do not include zero. The size of the orange dot reflects the strength of the correlation coefficient.
Comparative genomics predict Salmonella virulence phenotype.
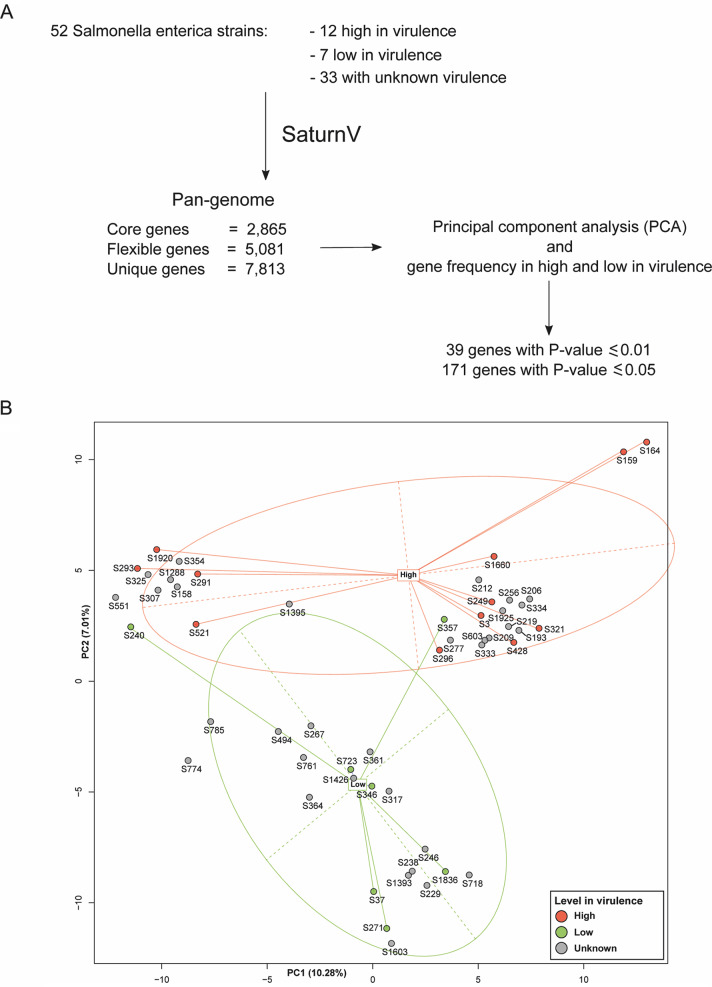
Additional isolates were chosen for comparative genomic analyses with the aim of identifying virulence-associated genes. These isolates were phenotyped in both the epithelial cell model and the mouse model. Nineteen strains showing a concordant phenotype in both models, 12 strains with a high level of associated bacteria and 7 with a reduced number of bacteria, were selected for comparative genomic analyses (Table 2). The pan-genomes of 52 S. enterica genomes were analyzed using SaturnV (v1.1.0), including 19 isolates with known levels of virulence and 33 isolates untested in the models of infection, and were used to benchmark the prediction of the number of Salmonella-associated levels as high or low. The presence/absence of flexible genes was compared by principal-component analysis (PCA) (Fig. 5A). Among the flexible genes, we identified 171 genes that were significantly (P ≤ 0.05) correlated with the predicted phenotype (see Table S1). The resulting PCA plot revealed that strains appeared to segregate based on their virulence phenotype. Isolates with high burden demonstrated a tendency to cluster, and likewise, a low-burden cluster was clearly evident. These data clearly showed a link between the level-of-virulence (high or low) phenotype in an isolate and gene content.
TABLE 2.
Salmonella isolates with high- and low-virulence phenotypes used for pan-genomic analysis
| Isolate ID | Serotype | Source category | Host organism | Specific source | Virulence |
|---|---|---|---|---|---|
| S3 | Enteritidis | Human | Human | High | |
| S51 | Tyresoe | Fish/shellfish | Shrimp | High | |
| S164 | Typhimurium | Animal other | High | ||
| S249 | Virchow | Fish/shellfish | Shrimp | High | |
| S291 | Brandenburg | Fish/shellfish | Clams | High | |
| S293 | Brandenburg | Dairy | Raw milk | High | |
| S296 | Hvittingfoss | Fish/shellfish | Shrimp | High | |
| S321 | Blockley | Poultry | Chicken | Heart | High |
| S428 | Heidelberg | Human | Human | Feces | High |
| S521 | Environmental | High | |||
| S1660 | Paratyphi B | Fish/shellfish | Shrimp | High | |
| S1920 | Montevideo | Nuts/seeds | Almond | Almond kernels, raw | High |
| S37 | Falkensee | Other | Rice seasoning | Low | |
| S240 | Montevideo | Nuts/seeds | Sesame | Sesame, halva, pistachio | Low |
| S271 | Senftenberg | Nuts/seeds | Sesame | Tahini | Low |
| S346 | Liverpool | Plant (fruit/veg) | Cantaloupe | Low | |
| S357 | London | Fish/shellfish | Shrimp | Low | |
| S723 | Enteritidis | Unknown | Low | ||
| S1836 | Kentucky | Nuts/seeds | Almond | Almond kernels, raw | Low |
FIG 5.
PCA-based prediction model. (A) Workflow of the strategy used to build a PCA-based pan-genome model. The genome sequences for 12 known highly virulent strains, 7 known lowly virulent strains, and 33 strains of unknown virulence were used. (B) PCA plot showing lowly virulent strains in blue, highly virulent strains in red, and strains of unknown virulence in light green. The PCA revealed clustering of genes of similar virulence. Highly virulent strains clustered at the top of the plot, and lowly virulent strains at the bottom. The position of strains of unknown virulence relative to these two groupings was used to predict their phenotype.
List of the 171 proteins that correlated (P value ≤ 0.05) with Salmonella enterica isolate virulence. Corresponding genes and positions on Salmonella Typhimurium SL1344 chromosome as well as the protein functions based on UniProt, Virulence Factor DataBase (VFDB), and BacMet databases are included. Download Table S1, XLSX file, 0.1 MB (27.2KB, xlsx) .
© Crown copyright 2020.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Given that the association between genotype and gene content was represented via the PCA plot, we hypothesized that the PCA could be used to predict the virulence of untested Salmonella isolates used as a benchmark in predicting high and low levels of Salmonella virulence without a priori knowledge of the in vitro and in vivo screening strategies described above. The pan-genomes of 33 isolates of unknown levels of virulence were included in pan-genome analysis and added to the PCA (Table 3, Fig. 5B). The positions of the 33 strains within the PCA plot were used to predict their potential virulence level, those clustering with strains with known high burden were predicted to be high in virulence while those clustering with strains with low burden were predicted to be low in virulence. As a result, 15 strains were predicted to be low and 18 were predicted to be high (Fig. 5B). This group of strains of unknown virulence was then tested in the epithelial cell and the mouse models of infection to assess this prediction and define the levels of accuracy.
TABLE 3.
Salmonella strains with virulence phenotypes predicted by PCA
| Isolate ID | Serotype | Source category | Host organism | Specific source | PCA prediction |
|---|---|---|---|---|---|
| S158 | Plant (fruit/veg) | Blueberry | High | ||
| S193 | Thompson | Plant (fruit/veg) | Spinach | High | |
| S206 | Muenchen | Plant (fruit/veg) | Cantaloupe | High | |
| S209 | Braenderup | Poultry | Egg: liquid, whole | High | |
| S212 | Stanley | Other | Spices: black pepper | High | |
| S219 | Hadar | Poultry | Chicken | Rinse | High |
| S229 | Derby | Animal/other | Pork | Muscle | Low |
| S238 | Mbandaka | Other | Pasta | Low | |
| S246 | Kentucky | Other | Animal feed meat and bone meal | Low | |
| S256 | Bovismorbificans | Animal/other | Beef | High | |
| S267 | Kiambu | Poultry | Chicken | Livers | Low |
| S277 | Uganda | Animal/other | Beef | Ground | High |
| S307 | Poona | Plant (fruit/veg) | Cantaloupe | High | |
| S317 | Ohio | Plant (fruit/veg) | Cocoa | Beans | Low |
| S325 | Bredeney | Dairy | Goat cheese | High | |
| S333 | Berta | Other | Cheese | High | |
| S334 | Kottbus | Poultry | Turkey | High | |
| S354 | Gaminara | Other | Spices: coriander | High | |
| S361 | Adelaide | Animal/other | Beef | Roast | Low |
| S364 | Cerro | Poultry | Egg: liquid frozen, albumin | Low | |
| S494 | Environmental | Low | |||
| S551 | r:e,n,x | Environmental | Multistate Salmonella in cantaloupe outbreak | High | |
| S603 | Bareilly | Environmental | Multistate Salmonella in cantaloupe outbreak | High | |
| S718 | Derby | Poultry | Turkey | Low | |
| S761 | Stanleyville | Unknown | Low | ||
| S774 | 58:d:z6 | Human | Human | Low | |
| S785 | 11:b:e,n,x | Unknown | Low | ||
| S1288 | Javiana | Plant (fruit/veg) | Soy | Soya spruce | High |
| S1393 | Tornow | Nuts/seeds | Peanut | Low | |
| S1395 | G(1):b,− | Nuts/seeds | Peanut | High | |
| S1426 | Berta | Environmental | Laurel Creek | Low | |
| S1603 | Havana | Animal/other | Porcine | Cecal content | Low |
| S1925 | Muenchen | Nuts/seeds | Almond | Almond kernel, raw nonpareil variety | High |
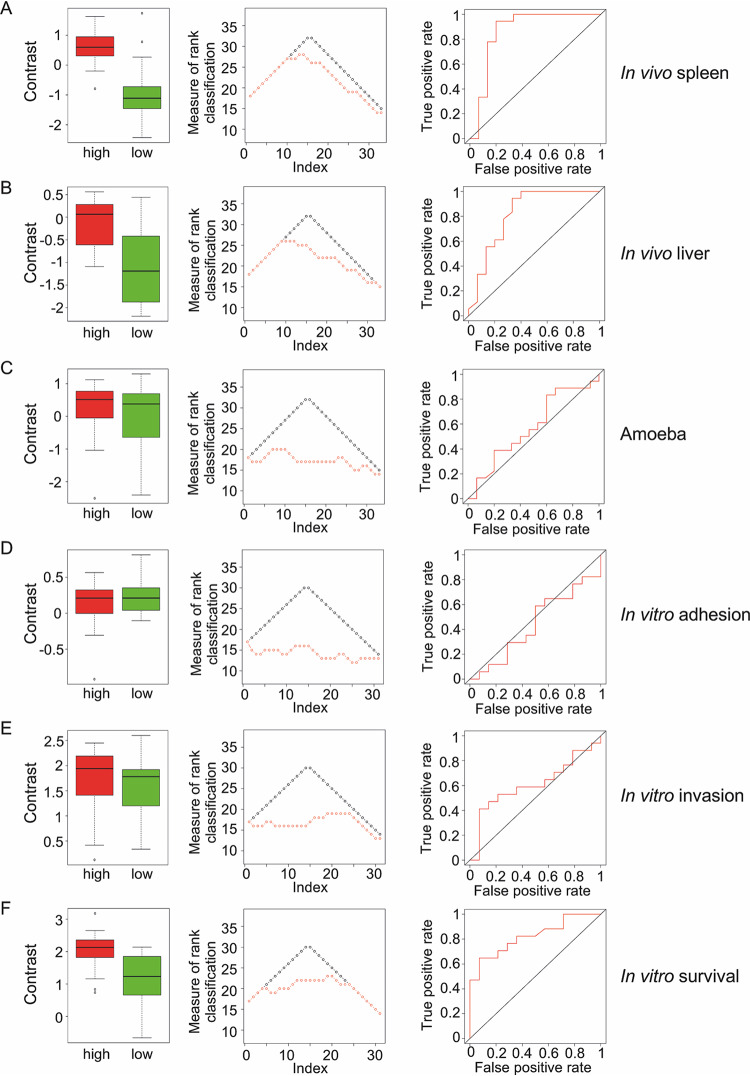
As described previously, contrast analysis was performed to classify the 33 strains based on the resulting bacterial burdens relative to the low-virulent ΔinvA ΔsseB strain for all phenotypic traits tested (see Fig. S3). The distributions of contrast values in the predicted groups (low and high) for each observed phenotypic trait are shown as boxplots (Fig. 6, left). The difference between the benchmarked predictions and expected rank was used as a measure of rank classification (Fig. 6, middle). We also used the area under the curve (AUC) of the receiver operating characteristic (ROC) as a predictive measure, with higher values showing better prediction (Fig. 6, right). Other measurements of concordance included variance explained (R2) and percentage of agreement within each group (low and high) for the observations based on contrasts (Table 4). The phenotypic traits showing significant concordance with predicted virulence were in vivo systemic infection in mice (82% of agreement for the spleen and 76% for the liver) and Salmonella survival in epithelial cells (74% agreement) (Fig. 6 and Table 4). Taken together, these data showed the predictive value of pan-genome analysis.
FIG 6.
Concordance analysis to test virulence prediction. (A to E) Distributions of contrasts in low- and high-prediction groups (left), measure of rank classification (middle) and ROC curves (right) for the different models. The distribution of contrast values in each group (predictions, low and high) was evaluated using t test and Mann-Whitney Wilcoxon test. The variance explained (R2) was calculated using ANOVA with one factor (prediction group: low or high). Percentage of agreement compared the groups (low versus high) from the prediction and the groups (low versus high) from the observations based on contrast. For the measure of rank classification, perfect prediction is shown in black and observed prediction in red.
TABLE 4.
Model comparisons for Salmonella serovar virulence prediction
| Model | t test | Wilcoxon test | R2 | % agreement | Kappa coefficient | Mean rank difference | Area under the curve |
|---|---|---|---|---|---|---|---|
| In vivo infection | |||||||
| Spleen | 1.3e−4 | 3.7e−4 | 0.45 | 0.82 | 0.63 | 2.18 | 0.87 |
| Liver | 9.6e−4 | 1.8e−3 | 0.34 | 0.76 | 0.51 | 2.85 | 0.82 |
| Epithelial cell | |||||||
| Adhesion | 0.37 | 0.6 | 0.03 | 0.55 | 0.09 | 8.55 | 0.44 |
| Invasion | 0.52 | 0.31 | 0.01 | 0.55 | 0.09 | 5.97 | 0.61 |
| Survival | 3.9e−3 | 2.6e−3 | 0.27 | 0.74 | 0.48 | 2.77 | 0.82 |
Distributions of contrasts in the four models of infection for Salmonella strains with virulence phenotypes predicted by PCA. Contrast analysis was used to compare differences in bacterial burden caused by each of the 33 serovars against the low-virulence control ΔinvA ΔsseB strains (mean value shown in green) within defined phenotypic traits measured in each model. Epithelial cell adhesion (A), invasion (B), and survival (C), replication in amoeba (D), and bacterial loads in mouse spleen (E) and liver (F). Isolate means are shown by black dots. Orange boxes denote the ranges of values, solid black bars show the 95% confidence intervals, and dotted black bars show the confidence intervals when adjusted for multiple testing using Bonferroni’s correction. All isolates whose adjusted confidence intervals do not cross the green line were significantly different from the ΔinvA ΔsseB strain (P < 0.05). Either 2, 3, or 4 biological replicates with 3 technical replicates per isolate were used for epithelial cells. Three biological replicates were performed per isolate in the amoeba. Six biological replicates were performed per isolate in the mouse. Download FIG S3, PDF file, 0.2 MB (262.8KB, pdf) .
© Crown copyright 2020.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Levels of Salmonella isolate virulence are associated with the presence or absence of new virulence genes.
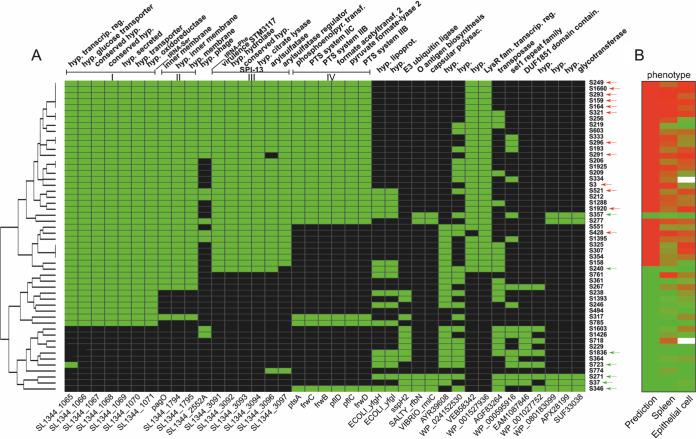
We analyzed the genetic diversity and population structure of Salmonella isolates used in the PCA analysis. We performed hierarchical clustering for the 39 most significant genes (P ≤ 0.01) present or absent in the 19 Salmonella isolates with known virulence and visualized the 33 isolates with predicted levels of virulence (Fig. 7). Of major interest in Fig. 7 was the identification of 4 major clusters, including SPI-13 and a collection of 21 hypothetical and conserved hypothetical proteins which may play a significant role in virulence. The low-virulence phenotype appeared to be conferred by the absence of several clusters of genes encoding proteins for metabolic functions or virulence factors. Cluster I is delimited by the serine tRNA and encodes a putative sialic acid transport system, a member of a sodium solute symporter family. Sialic acid is involved in several biological process and can be used as a nutrient source. This system was shown to be upregulated under anaerobic conditions (21) and to be a reversible carrier, a propriety of sialic acid transporters associated with bacteria that colonize humans (22). Cluster II encodes 3 putative membrane proteins, including pagO, a PhoP-activated gene (23). In S. Typhimurium, this cluster was found to be upregulated when grown under an SPI-2-inducing condition (21) and in both macrophages and HeLa cells (24). These 3 genes were also found to be regulated by RcsB (25), a two-component system involved in virulence, cell envelope stress, antimicrobial resistance, and oxidative and acidic stress (26–28). Cluster III encodes the first 6 genes of SPI-13 (2 operons), which were shown to be involved in systemic infection of mice (29). Open reading frame (ORF) STM3117 is involved in methylglyoxal detoxification and is known to promote the replication of Salmonella Typhimurium inside host macrophages (30) and epithelial cells (31). The first 4 genes were upregulated in macrophages but not in HeLa cells (24). Cluster IV encodes a putative sugar-transporting phosphotransferase system (PTS) that maybe involved in anaerobic carbon metabolism (32). This cluster was upregulated during anaerobic growth (21). In Klebsiella, the frw system was involved in virulence in mice (33). Three hypothetical and uncharacterized proteins, including a putative regulator (WP_001527936), were associated with the isolates with the highest bacterial burden.
FIG 7.
Distribution of virulence genes in Salmonella isolates used in the PCA-based prediction model. (A) Heat map showing the distribution of 39 genes correlating with the levels of virulence. Column labels indicate gene names (bottom) and functions (top). The gene names at the bottom refer either to the genes in Salmonella Typhimurium SL1344 or, when no annotation was available, to a generic name generated by Prokka (linked to Table S1 in the supplemental material). The lines above mark contiguous genes on the S. Typhimurium SL1344 chromosome and show four clusters (I to IV). Green cells indicate that the gene is present, whereas black cells indicate its absence from a given isolate. The tree on the left shows isolates ordered based on hierarchical clustering. The identifiers (IDs) of the isolates are shown on the right. Red arrows indicate high-virulence isolates, and green arrows indicate low-virulence isolates that were used for PCA analysis. hyp, hypothetical; transcrip, transcriptional; reg, regulator; phosphoenolpyr transf, phosphoenolpyruvate transferase; acetyltransf, acetyltransferase; lipoprot, lipoprotein; polysac, polysaccharide; fam, family. (B) Heat map showing the distribution of predicted phenotype and phenotype in vivo (spleen) and in vitro (epithelial cell survival). Isolates were ranked according to the distribution of contrasts. Red cells, high virulence; green cells, low virulence. Isolates S324 and S718 (empty cells) were gentamicin-resistant and not tested in vitro.
Within the isolates used to build the PCA, there were 2 low-virulence isolates (S357 and S240) that segregated with the cluster with high virulence (Fig. 5B and Fig. 7). When we compared the pan-genome of S357 to those of the 12 selected strains with high bacterial loads, we detected an indel (deletion of a T) at the 6th nucleotide position in ssaK resulting in a frameshift and giving a truncated protein (see Fig. S4A). This indel affecting the function of SsaK may have a major impact on the level of virulence of S357, because SsaK is known to be an essential component of the SPI-2 secretion system apparatus of S. Typhimurium (34). SsaK was described as a cytoplasmic protein interacting with SsaN forming the C ring complex in the SPI-2 type 3 secretion system (T3SS), an essential part of a 13-gene transcriptional unit of 10,161 nucleotides from ssaK to ssaU (35–37). Moreover, mutants in the ssaK-ssaU operon were shown to invade fewer epithelial cells by reducing SPI-1 secretion (34).
Genomic alterations in isolates S357 London and S240 Montevideo. (A) Graphical representation of the indel in the ssaK gene of S357 causing a frameshift and resulting in a premature stop codon. The slash bars represent truncated regions for better visualization of the key regions. The putative ssaK ORF in the S357 genome begins 102 nucleotides (nt) downstream of the premature stop codon and encodes a putative 537-amino-acid protein instead of the expected 675-amino-acid SsaK protein in SL1344. (B) Genomic deletion of 75.2 kbp in the S240 isolate was identified between hycE (start position on SL1344, 3,017,328 bp) and truD genes (end position on SL1344, 3,092,532 bp) and includes the SPI-1 T3SS essential for Salmonella virulence. (C) invA deletion impaired the virulence of Salmonella Typhimurium in vivo as shown by reduced bacterial recovery in spleen and liver and low competitive index (CI). C57BL/6J mice were coinfected with a mixed inoculum containing equal numbers of SL1344 and invA-deficient SL1344 cells. CIs were calculated by dividing the number of cells for the ΔinvA strain (kanamycin resistant) by that for SL1344 (streptomycin resistant). Download FIG S4, PDF file, 0.2 MB (235.9KB, pdf) .
© Crown copyright 2020.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
We also noted the S240 isolate clustered with the high-virulence group, contradicting the in vitro and in vivo analysis showing that S240 has a low-virulence phenotype. The low virulence of S240 is presumably caused by a deletion (between the 3′ end of hycE and the 5′ end of truD) of 73 genes located within a 75.2-kb region encompassing SPI-1 and including invA (Fig. S4B). Consistent with this deletion reported previously by others, the loss of invA diminished the virulence of SL1344 in vivo (Fig. S4C).
It is possible that strains S240 and S357 have evolved by genome degradation to adapt to their host (nut and shrimp) and lost their virulence potential in the mammalian host. Gene decay was linked with Salmonella host specialization (38).
For all the isolates with predicted levels of virulence, only 3 of 33 isolates had in vivo and/or in vitro phenotypes that did not match the prediction: S219 was predicted high but presented low virulence in vivo and in vitro, and isolates and S718 and S761 were predicted as low virulence but were high. In S219, there were nine altered genes (yebT, phsA, yegD, queG, basR, bcsQ, nifJ, osmX, and yadI), 4 with IS6 insertions, 4 with premature stop codons, and 1 missing. The high number of IS6 insertions in isolate S219 may render this strain of serovar Hadar less virulent. Strain S718 belongs to serovar Derby, and we have not identified why this isolate was virulent. For S761, phylogenetic analysis of the SalFoS strains indicated that this isolate of serovar Stanleyville shares a recent common ancestor with several S. Typhi and S. Paratyphi A strains (data not shown).
One major observation from these detailed combined in vitro, in vivo, and genomics-based analyses is the identification of several novel genes and clusters associated with virulence.
DISCUSSION
The systematic approach of high-quality WGS coupled with comparative genomics analysis is a powerful strategy to determine the phylogenetic relationships between Salmonella enterica isolates (39, 40). Combining in vitro and in vivo systematic analysis to define isolates as high and low in virulence with the presence and absence of genes clearly showed a significant correlation for most isolates. In addition, WGS-derived phylogenetic analysis is an invaluable tool in epidemiological investigations and has enabled fast and accurate identification of the sources of foodborne disease outbreaks (41–43). Other studies have used WGS to characterize Salmonella serovars for antimicrobial resistance and virulence genes using collections of human, animal, and environmental isolates (44–46). However, virulence prediction was mainly based on already-known virulence genes, and only one phenotypic model was tested.
Here, we hypothesized that the levels of virulence, as measured by the Salmonella burden in cells and tissues using different models of infection, may be predicted as high or low when combining WGS, phylogeny, and pan-genome studies for the presence and absence of genes in association with virulence. As additional supporting evidence, we benchmarked a collection of 33 isolates with no a priori knowledge of their levels of virulence, and which remarkably supported the potential of this approach. We entertain the possibility that this is a first step toward new ways of identifying other genes implicated in virulence, as we identified here a collection of 20 hypothetical and conserved hypothetical proteins. Additional studies will be essential to clearly define the role of these unknown genes in virulence. As most of these genes are involved in metabolism, they may represent genes important for nutrient acquisition within the host. We propose that gaining a clear understanding of the genetic determinants causing virulence in different in vitro and in vivo models of infection will enable pathogenic potential to be predicted by WGS analysis. We have used WGS to build associations between phenotypic and genetic diversity within 83 isolates representing 77 different Salmonella enterica serovars. Salmonella isolates were selected to maximize genetic diversity, and accordingly, we observed a wide range of phenotypes, with the numbers of bacteria spanning beyond the ranges of the high- and low-virulence controls. Interestingly, the magnitudes of the differences between the controls varied between the different models tested. Even if the difference between the wild type (WT) and the ΔinvA ΔsseB strain was small in the macrophage and amoeba models, the distribution of phenotypes was broad in these models. The correlation between the different models of infection (epithelial cell, macrophage, amoeba, and mouse models) in assessing virulence was highly significant. The isolates with the highest burden were found to be high in all models. The isolates that did not replicate well either in epithelial cells, in macrophages, or in amoeba were the least virulent in the mouse model. Some serovars replicated at high levels in epithelial cells and macrophages but showed low virulence in the amoeba and/or mouse models, highlighting the fact that a high propensity for adhesion to, invasion of, or survival in epithelial cells does not guarantee that a strain is pathogenic at the level of the whole organism. On the other hand, Salmonella needs to be able to replicate in epithelial cells and/or macrophages to cause disease, showing the importance of studying the biodiversity of Salmonella isolates in various models of infection.
Our analysis showed that the virulence of clinical isolates of different serovars (Hartford, Enteritidis, Heidelberg, Newport, Bareilly, and r:e,n,x) was consistently high in all Salmonella infection models (in vitro and in vivo), highlighting the validity of using modeling in cells and animals to identify highly virulent isolates from different serovars. Other highly virulent serovars were isolated from fish/shellfish (Tyresoe, Cremieu, and Hull), from meat and animal products (Derby, Uganda, and Berta), and from plant/fruits/vegetables/nuts (Javiana, Stanley, and Muenchen). Salmonella isolates within one serovar did not necessarily present the same degree of virulence as observed in the present study for the serovars Derby, Enteritidis, and Montevideo, stressing the potentially high genomic variability not only between serovars but also within one individual serovar. Hence, this shows that WGS coupled to other genomics approaches has an excellent potential in studying virulence in foodborne disease outbreaks.
Analysis of genetic relatedness among highly and poorly virulent Salmonella serovars was based on pan-genome analysis where the pool of flexible genes was used to identify gene frequency for high- and low-virulence serovars. The predictive value of the PCA-based model was high in the model of epithelial cell infection (74% concordance) and in the systemic infection mouse model (82% concordance). It is clear that a gene-based frequency approach to determine virulence has some limitations. In fact, there were 3 serovars (S219, S718, and S761) that did not fit the prediction, contradicting the in vitro and in vivo phenotypes. False negatives are a major concern, as they pose the greatest public health risk.
Genetic variability between serovars occurs for a variety of reasons, such as point mutations and single nucleotide insertions or deletions; additionally, there is a role of unknown genes in virulence. Further refinement may be improved by using a larger number of serovars with known phenotypes to predict virulence together with an approach that weighs in nucleotide variants and kmers. These studies will certainly assist in integrating WGS approaches into food safety practices and prediction of outbreaks.
MATERIALS AND METHODS
Salmonella strains.
Salmonella strains used in this study were selected from SalFoS (The Salmonella Foodborne Sys-OMICS) database, a repository of thousands of Salmonella isolates from environmental, plant, animal, food or clinical sources (https://salfos.ibis.ulaval.ca/). Salmonella strains used in this study are listed in Tables 1 to 3. Salmonella serovars rarely implicated in foodborne disease in Canada were selected and compared to clinical and foodborne isolates of Salmonella Enteritidis, Salmonella Heidelberg, and Salmonella Typhimurium, all of which are among the top ten serovars that commonly cause salmonellosis in Canada. The rationale for including isolates from outside the top 70 salmonellosis-causing serovars was based on the hypothesis that since these isolates do not cause many salmonellosis cases, they were likely to be less virulent. The high-virulence control was Salmonella Typhimurium SL1344, and the isogenic ΔinvA ΔsseB mutant was used as the low-virulence control. All the bacteria used in this study had similar growth curves to that of the high-virulence control in LB.
In vitro human cell studies.
(i) Interactions with human epithelial intestinal cells. A high-throughput gentamicin protection assay was adapted for 96-well plates to quantify Salmonella interactions with human intestinal epithelial cells (47). INT-407 cells (ATCC CCL-6) were grown in minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 25 mM HEPES (Wisent). Bacteria were grown overnight under static conditions (low aeration) in LB-NaCl (300 mM) to induce SPI-1 and were added at a multiplicity of infection of 20:1 in triplicates. After 90 min, infected cells were washed with phosphate-buffered saline (PBS), and fresh medium supplemented with 50 μg/ml gentamicin sulfate was added to kill the extracellular bacteria. Cells were lysed with PBS-deoxycholate (DOC) 0.1% at 90 min (adhesion), 180 min (invasion), and 18 h (survival) postinfection. Serial dilutions were performed for enumeration of viable colony counts (CFU/ml). Sample size varied across isolates, ranging from 2 to 4 biological replicates, with 3 technical replicates per biological replicate.
(ii) Interactions with human macrophages. THP-1 cells (ATCC TIB-202) were maintained in RPMI 1640 supplemented with 10% heat-inactivated FBS, 1 mM sodium pyruvate, and 1% MEM nonessential amino acids (Wisent). The monocyte cells were differentiated into macrophages by addition of 10−7 M phorbol 12-myristate 13 acetate (PMA) (Sigma) for 48 h before the infection. Following an overnight growth in LB broth, the strains were added in triplicates at a multiplicity of infection of 10:1. After 30 min, infected cells were washed with PBS, treated with gentamicin (50 μg/ml), and lysed with PBS-DOC 0.1% at 30 min (phagocytosis), 2 h, and 18 h (survival) postinfection; then, serial dilutions were performed for enumeration of viable colony counts (CFU/ml). Either 2 or 3 biological replicates were performed per isolate, with 3 technical replicates per biological replicate. Control SL1344 and ΔinvA ΔsseB strains each had a total of 6 biological replicates per phenotypic trait.
Amoeba studies.
(i) Amoebic cells and culture conditions. The strain of Acanthamoeba used in this study was A. rhysodes (ATCC 50368). It was obtained as an axenic strain and maintained as adherent cells in an axenic culture medium, peptone-yeast extract-glucose (PYG) broth, in 25-cm3 tissue cultures flasks incubated at 30°C until near confluence was reached. Amoeba suspensions were examined by bright-field microscopy before use, and the number of cells was determined by counting with a Neubauer chamber.
(ii) Preparation of bacterial inocula. Salmonella strains were grown in LB-NaCl (300 mM) at 37°C until late-logarithmic growth phase to promote induction of SPI-1. The bacteria were then washed twice and diluted in PBS to a concentration of 100 bacteria to 1 amoeba.
(iii) Intracellular bacterial growth in A. rhysodes. A. rhysodes was grown in 10 ml of PYG broth. The tissue culture flask containing near confluent A. rhysodes was gently shaken, and the PYG medium containing nonadherent amoeba was removed. Adherent amoebae were removed by agitating the empty flask and resuspending with fresh PYG broth. The suspension was seeded into a 12-well plate (105 cells/well). Amoeba were then incubated for 24 h at 30°C to permit adherence to the wells. The number of amoebae per well was calculated an additional time prior to infection. To prepare Salmonella inocula, bacteria were diluted in PBS to give a multiplicity of infection of 100. Following inoculation, the 12-well plate was centrifuged for 5 min at 500 × g to promote contact between bacteria and amoebae. The plate was then incubated at 30°C for 60 min. After incubation, the medium was removed, wells were washed with PBS, and fresh PYG broth supplemented with 50 μg/ml gentamicin sulfate was added to kill the extracellular bacteria. After incubation for 30 min at 30°C, the wells were washed twice with PBS and lysed in 0.5% sodium deoxycholate. CFU enumeration was then determined by serially diluting the samples and plating them on LB agar. Three biological replicates were performed per isolate. Control SL1344 and ΔinvA ΔsseB strains were replicated a total of 51 times.
Mouse studies.
(i) Animals. All animal experiments were performed under guidelines specified by the Canadian Council for Animal Care. The animal use protocol was approved by the McGill University Facility Animal Care Committee (protocol no. 7760). C57BL/6J mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and then bred in-house.
(ii) In vivo Salmonella infection. For intravenous (i.v.) infection, bacteria were grown shaking at 37°C in tryptic soy broth (TSB) to an optical density at 660 nm (OD660) of between 0.1 and 0.2, cooled to 4°C, and plated overnight on tryptic soy agar. The day after, the infectious dose was adjusted to 500 CFU/μl with sterile saline, and 200 μl was injected into each of the caudal veins of 7- to 13-week-old mice of both sexes. For oral infections, bacteria were grown overnight in 3 ml of TSB shaking at 37°C. Mice were infected by oral gavage with 200 μl of a 10-fold dilution of the overnight culture containing 2 × 107 to 3 × 107 CFU of Salmonella spp. The infectious doses were verified by the plating of serial dilutions on Trypticase soy agar (48, 49). Mice infected orally were first deprived of food for 4 h before infection with Salmonella enterica. Spleens, livers, and feces were collected day 3 postinfection and homogenized in saline, and CFUs were determined by plating serial dilutions on either Trypticase soy agar (spleen and liver) or MacConkey (feces) plates. A sample size of 6 mice was used per isolate, 3 female and 3 male mice, with the exception of S3 (4 mice), S5 (5 mice), S32 (5 mice), S428 (5 mice), and S334 (4 mice). Three mice were tested per control for each experiment. Across all experiments, SL1344 was replicated 57 times and the ΔinvA ΔsseB strain was replicated 48 times.
Statistical analyses.
(i) Comparing virulence levels. The bacterial burden, or colony counts, found in each of the 35 serovars was compared to that of the low-virulence control by analysis of variance (ANOVA). Models were fit separately for each of the four models:
(a) Infection of human epithelial cells. The data consist of CFU counts for 35 serovars and the low-virulence control, under 3 conditions (bacterial adhesion, invasion, and survival), and for several epithelial cells per serovar. There were 2 to 3 replicates per combination, leading to an unbalanced study design containing 840 measurements. We fit the following model that contains a random effect accounting for dependence between results from the same cell (equation 1):
| (1) |
where yijkl is the CFU counts (in log10 units) measured in the ith cell from serovar j under condition k, replicate l; Sj is the serovar j, Ck is the condition k; bi is the random term associated with the cell i. Finally, eijkl represents the normally distributed error term of the statistical model. This statistical model is equivalent to a two-way ANOVA for serovar and condition, with the additional random term for cells. Contrasts compared serovars to the low-virulence control strain.
(b) Human macrophages. The data consist of 731 measures of CFU counts across the serovars and three conditions (uptake, survival 2 h pos infection, and survival at 18 h postinfection). As for the epithelial cells, an ANOVA model was fit to log CFU counts in this unbalanced design, including terms for serovar, condition, and their interaction, and again including a random term to account for intracell correlations. The model is similar to that in equation 1.
(c) Amoeba model. The data consist of 147 measures of CFU counts. The bacterial burden was modeled as a function of serovar with a one-way ANOVA analysis as follows (equation 2):
| (2) |
where yij is the CFU counts (in log10 units) measured in the ith cell from serovar Sj; eij represents the normally distributed error term of the statistical model.
(d) Mouse model. The data consist of 514 CFU counts. We modeled the bacterial burden as a function of the serovar and the organ (called the condition: liver and spleen) as well as their interaction. A random term was added to account for the intramouse correlation, as each mouse provided two observations, one from each organ. The model (equation 3) is as follows:
| (3) |
where yijk is the CFU counts (in log10) measured in the ith mouse from serovar Sj under condition k; Ck is the condition or organ k; bi is the random term associated with mouse i. Finally, eijk represents the normally distributed error term of the statistical model. This statistical model is equivalent to a two-way ANOVA with a random effect for mouse.
For each of the four models, type III tests of the hypothesis were computed to evaluate the significance of the serovar-condition interaction (except for experiment 3, where the effect of the serovar was tested directly). Contrasts were constructed to evaluate the difference in log10 CFU counts between each of the 35 serovars and the low-virulence control for each condition—i.e., for each phenotypic trait—yielding 70 comparisons all together. Test results and confidence intervals obtained for each contrast were adjusted for multiple comparisons using Bonferroni’s correction. Analyses were performed using R software. The nlme package was used for random effect models and the multcomp package was used for multiple comparisons.
(ii) Concordance analysis. Pairwise Pearson’s correlation coefficients were calculated between the estimated contrast values, resulting from the ANOVAs, for each condition (phenotypic trait) and each model.
(iii) Performance of predictions on new strains. To assess the accuracy of the predictions of virulence obtained from the PCA analysis (high versus low virulence), 33 new strains with unknown virulence were examined. First, we repeated the analysis similar to that described above (“Comparing virulence levels”) for log CFU counts for each of the 33 new strains compared to low-virulence controls for each phenotypic trait and for three of the four infection models (mouse, amoeba, and epithelial). Contrasts were obtained as described above. Then, the resulting contrasts were compared to predictions from the PCA analysis. A prediction would be defined as perfect if the largest contrast value among the group of strains predicted as low virulence is smaller than the smallest contrast value among the group of strains predicted as high virulence. Performance of the predictions was assessed in five different ways.
Boxplots show the distributions of estimated contrasts in each predicted group. The distributions were compared with t tests and Mann-Whitney tests. Larger differences imply better predictions.
One-way ANOVA models were fit predicting the contrasts as a function of the predicted group. Larger coefficients of determination () mean a better prediction.
Contrast values were dichotomized into low versus high while matching the numbers of strains in the predicted low and high groups. Accuracy was calculated as the percentage of strains given the same label and also using a Kappa coefficient for concordance between two binary variables. Larger Kappa values imply better agreement.
For measures of rank classification, strains were ranked by their contrast values. For each rank, we counted the number of strains in the predicted low-virulence group among all strains with lower rank, and correspondingly, we counted the number of strains predicted to be in the high-virulence group among all strains with higher rank. These counts were plotted together with counts expected under perfect predictions, and we averaged the absolute value of the difference between these counts. A lower value means a better prediction.
The receiver operating characteristic (ROC) curves and areas under the curves (AUCs) were calculated. A higher AUC means a better prediction. The P value associated with the AUC corresponds to the test of the null hypothesis H0: AUC = 0.5 corresponding to a random prediction.
Genomic analyses.
(i) DNA sequencing. Genomic DNA was extracted from overnight LB broth cultures at 37°C using the E-Z 96 tissue DNA kit (Omega Bio-Tek, Norcross, GA, USA). Around 500 ng of genomic DNA was mechanically fragmented during 40 s by a Covaris M220 (Covaris, Woburn, MA, USA) using the default settings. Libraries were synthesized using an NEBNext Ultra II DNA library prep kit for Illumina (New England BioLabs, Ipswich, MA, USA) according to manufacturer’s instructions and were sequenced by an Illumina MiSeq 300-bp paired-end run at the Plateforme d’Analyses Génomiques of the Institut de Biologie Intégrative et des Systèmes (Laval University, QC, Canada).
(ii) Core SNP phylogeny. Core genes of the distant Salmonella isolates were determined by SaturnV (v1.1.0) (https://github.com/ejfresch/saturnV) (18) with ≥50% identity between proteins and ≥85% alignment coverage. To avoid bias in the phylogenetic analysis caused by duplicated genes (paralogs), single-copy core genes were retained and aligned using MUSCLE (v3.8.31) (50). Uninformative SNPs were removed by BMGE (v1.12) (51), and the phylogenetic tree was computed by FastTree (v.2.1.9) (52).
(iii) Pan-genome analysis. Pan-genome analysis of 52 Salmonella strains, of which 12 were highly virulent, 7 were lowly virulent, and 33 strains had an unknown virulence phenotype, was performed using SaturnV (v1.1.0) with ≥90% identity between proteins and ≥85% alignment covering their length to be considered ortholog proteins.
(iv) Principal-component analysis. The table of ortholog proteins was converted to a binary table using a perl script and imported into R. Ade4 package was used to perform the principal-component analysis (PCA) [> data_pca <- dudi.pca(table.binary.tsv, scale = F, scannf = FALSE, nf = 5)]. The plot was created using the two first principal components, and the strains were colored based on their virulence levels (red, high virulence; green, low virulence; gray, unknown virulence phenotype) {> plot(data_pca$li[,1:2], col=color_t_condition, pch =19)}.
Data availability.
All genomic sequences are publicly available through NCBI (BioProject accession PRJNA634019).
ACKNOWLEDGMENTS
This work was supported by Genome Canada and Genome Quebec (Syst-OMICS project grant number 8505).
We thank Lei Zhu and Patricia D’Arcy for mouse breeding and screening and Line Larivière for technical assistance.
We declare no competing interests.
REFERENCES
- 1.WHO. 2018. Salmonella (non-typhoidal). https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal). Accessed December 2019.
- 2.Achtman M, Wain J, Weill FX, Nair S, Zhou Z, Sangal V, Krauland MG, Hale JL, Harbottle H, Uesbeck A, Dougan G, Harrison LH, Brisse S, S. Enterica MLST Study Group. 2012. Multilocus sequence typing as a replacement for serotyping in Salmonella enterica. PLoS Pathog 8:e1002776. doi: 10.1371/journal.ppat.1002776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popoff MY, LeMinor L. 1997. Antigenic formulas of the Salmonella serovars, 7th revision. WHO Collaborating Centre for Reference and Research on Salmonella. Institut Pasteur, Paris, France. [Google Scholar]
- 4.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM, International Collaboration on Enteric Disease ‘Burden of Illness’ Studies. 2010. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 5.Thomas MK, Murray R, Flockhart L, Pintar K, Pollari F, Fazil A, Nesbitt A, Marshall B. 2013. Estimates of the burden of foodborne illness in Canada for 30 specified pathogens and unspecified agents, circa 2006. Foodborne Pathog Dis 10:639–648. doi: 10.1089/fpd.2012.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bustamante J, Boisson-Dupuis S, Abel L, Casanova JL. 2014. Mendelian susceptibility to mycobacterial disease: genetic, immunological, and clinical features of inborn errors of IFN-gamma immunity. Semin Immunol 26:454–470. doi: 10.1016/j.smim.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perron GG, Quessy S, Letellier A, Bell G. 2007. Genotypic diversity and antimicrobial resistance in asymptomatic Salmonella enterica serotype Typhimurium DT104. Infect Genet Evol 7:223–228. doi: 10.1016/j.meegid.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Suez J, Porwollik S, Dagan A, Marzel A, Schorr YI, Desai PT, Agmon V, McClelland M, Rahav G, Gal-Mor O. 2013. Virulence gene profiling and pathogenicity characterization of non-typhoidal Salmonella accounted for invasive disease in humans. PLoS One 8:e58449. doi: 10.1371/journal.pone.0058449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulig PA. 1990. Virulence plasmids of Salmonella typhimurium and other salmonellae. Microb Pathog 8:3–11. doi: 10.1016/0882-4010(90)90003-9. [DOI] [PubMed] [Google Scholar]
- 10.Sabbagh SC, Forest CG, Lepage C, Leclerc JM, Daigle F. 2010. So similar, yet so different: uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol Lett 305:1–13. doi: 10.1111/j.1574-6968.2010.01904.x. [DOI] [PubMed] [Google Scholar]
- 11.Betancor L, Yim L, Fookes M, Martinez A, Thomson NR, Ivens A, Peters S, Bryant C, Algorta G, Kariuki S, Schelotto F, Maskell D, Dougan G, Chabalgoity JA. 2009. Genomic and phenotypic variation in epidemic-spanning Salmonella enterica serovar Enteritidis isolates. BMC Microbiol 9:237. doi: 10.1186/1471-2180-9-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon MA, Banda HT, Gondwe M, Gordon SB, Boeree MJ, Walsh AL, Corkill JE, Hart CA, Gilks CF, Molyneux ME. 2002. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 16:1633–1641. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 13.Marcus SL, Brumell JH, Pfeifer CG, Finlay BB. 2000. Salmonella pathogenicity islands: big virulence in small packages. Microbes Infect 2:145–156. doi: 10.1016/s1286-4579(00)00273-2. [DOI] [PubMed] [Google Scholar]
- 14.Riquelme S, Varas M, Valenzuela C, Velozo P, Chahin N, Aguilera P, Sabag A, Labra B, Alvarez SA, Chavez FP, Santiviago CA. 2016. Relevant genes linked to virulence are required for Salmonella Typhimurium to survive intracellularly in the social amoeba Dictyostelium discoideum. Front Microbiol 7:1305. doi: 10.3389/fmicb.2016.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tezcan-Merdol D, Ljungstrom M, Winiecka-Krusnell J, Linder E, Engstrand L, Rhen M. 2004. Uptake and replication of Salmonella enterica in Acanthamoeba rhysodes. Appl Environ Microbiol 70:3706–3714. doi: 10.1128/AEM.70.6.3706-3714.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douesnard-Malo F, Daigle F. 2011. Increased persistence of Salmonella enterica serovar Typhi in the presence of Acanthamoeba castellanii. Appl Environ Microbiol 77:7640–7646. doi: 10.1128/AEM.00699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsolis RM, Xavier MN, Santos RL, Baumler AJ. 2011. How to become a top model: impact of animal experimentation on human Salmonella disease research. Infect Immun 79:1806–1814. doi: 10.1128/IAI.01369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freschi L, Bertelli C, Jeukens J, Moore MP, Kukavica-Ibrulj I, Emond-Rheault JG, Hamel J, Fothergill JL, Tucker NP, McClean S, Klockgether J, de Soyza A, Brinkman FSL, Levesque RC, Winstanley C. 2018. Genomic characterisation of an international Pseudomonas aeruginosa reference panel indicates that the two major groups draw upon distinct mobile gene pools. FEMS Microbiol Lett 365:fny120. doi: 10.1093/femsle/fny120. [DOI] [PubMed] [Google Scholar]
- 19.Tettelin H, Masignani V, Cieslewicz MJ, Donati C, Medini D, Ward NL, Angiuoli SV, Crabtree J, Jones AL, Durkin AS, Deboy RT, Davidsen TM, Mora M, Scarselli M, Margarit y Ros I, Peterson JD, Hauser CR, Sundaram JP, Nelson WC, Madupu R, Brinkac LM, Dodson RJ, Rosovitz MJ, Sullivan SA, Daugherty SC, Haft DH, Selengut J, Gwinn ML, Zhou L, Zafar N, Khouri H, Radune D, Dimitrov G, Watkins K, O'Connor KJB, Smith S, Utterback TR, White O, Rubens CE, Grandi G, Madoff LC, Kasper DL, Telford JL, Wessels MR, Rappuoli R, Fraser CM. 2005. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome. Proc Natl Acad Sci U S A 102:13950–13955. doi: 10.1073/pnas.0506758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidal S, Tremblay ML, Govoni G, Gauthier S, Sebastiani G, Malo D, Skamene E, Olivier M, Jothy S, Gros P. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp Med 182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroger C, Colgan A, Srikumar S, Handler K, Sivasankaran SK, Hammarlof DL, Canals R, Grissom JE, Conway T, Hokamp K, Hinton JC. 2013. An infection-relevant transcriptomic compendium for Salmonella enterica serovar Typhimurium. Cell Host Microbe 14:683–695. doi: 10.1016/j.chom.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Severi E, Hosie AH, Hawkhead JA, Thomas GH. 2010. Characterization of a novel sialic acid transporter of the sodium solute symporter (SSS) family and in vivo comparison with known bacterial sialic acid transporters. FEMS Microbiol Lett 304:47–54. doi: 10.1111/j.1574-6968.2009.01881.x. [DOI] [PubMed] [Google Scholar]
- 23.Gunn JS, Belden WJ, Miller SI. 1998. Identification of PhoP-PhoQ activated genes within a duplicated region of the Salmonella typhimurium chromosome. Microb Pathog 25:77–90. doi: 10.1006/mpat.1998.0217. [DOI] [PubMed] [Google Scholar]
- 24.Hautefort I, Thompson A, Eriksson-Ygberg S, Parker ML, Lucchini S, Danino V, Bongaerts RJ, Ahmad N, Rhen M, Hinton JC. 2008. During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell Microbiol 10:958–984. doi: 10.1111/j.1462-5822.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariscotti JF, Garcia-del Portillo F. 2009. Genome expression analyses revealing the modulation of the Salmonella Rcs regulon by the attenuator IgaA. J Bacteriol 191:1855–1867. doi: 10.1128/JB.01604-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blatter EE, Abriola DP, Pietruszko R. 1992. Aldehyde dehydrogenase. Covalent intermediate in aldehyde dehydrogenation and ester hydrolysis. Biochem J 282:353–360. doi: 10.1042/bj2820353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farris C, Sanowar S, Bader MW, Pfuetzner R, Miller SI. 2010. Antimicrobial peptides activate the Rcs regulon through the outer membrane lipoprotein RcsF. J Bacteriol 192:4894–4903. doi: 10.1128/JB.00505-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pescaretti M. d L M, Farizano JV, Morero R, Delgado MA. 2013. A novel insight on signal transduction mechanism of RcsCDB system in Salmonella enterica serovar Typhimurium. PLoS One 8:e72527. doi: 10.1371/journal.pone.0072527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haneda T, Ishii Y, Danbara H, Okada N. 2009. Genome-wide identification of novel genomic islands that contribute to Salmonella virulence in mouse systemic infection. FEMS Microbiol Lett 297:241–249. doi: 10.1111/j.1574-6968.2009.01686.x. [DOI] [PubMed] [Google Scholar]
- 30.Shi L, Adkins JN, Coleman JR, Schepmoes AA, Dohnkova A, Mottaz HM, Norbeck AD, Purvine SO, Manes NP, Smallwood HS, Wang H, Forbes J, Gros P, Uzzau S, Rodland KD, Heffron F, Smith RD, Squier TC. 2006. Proteomic analysis of Salmonella enterica serovar typhimurium isolated from RAW 264.7 macrophages: identification of a novel protein that contributes to the replication of serovar Typhimurium inside macrophages. J Biol Chem 281:29131–29140. doi: 10.1074/jbc.M604640200. [DOI] [PubMed] [Google Scholar]
- 31.Chakraborty S, Chaudhuri D, Balakrishnan A, Chakravortty D. 2014. Salmonella methylglyoxal detoxification by STM3117-encoded lactoylglutathione lyase affects virulence in coordination with Salmonella pathogenicity island 2 and phagosomal acidification. Microbiology 160:1999–2017. doi: 10.1099/mic.0.078998-0. [DOI] [PubMed] [Google Scholar]
- 32.Reizer J, Reizer A, Saier MH Jr.. 1995. Novel phosphotransferase system genes revealed by bacterial genome analysis–a gene cluster encoding a unique enzyme I and the proteins of a fructose-like permease system. Microbiology 141:961–971. doi: 10.1099/13500872-141-4-961. [DOI] [PubMed] [Google Scholar]
- 33.Lin D, Fan J, Wang J, Liu L, Xu L, Li F, Yang J, Li B. 2018. The fructose-specific phosphotransferase system of Klebsiella pneumoniae is regulated by global regulator CRP and linked to virulence and growth. Infect Immun 86:e00340-18. doi: 10.1128/IAI.00340-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hensel M, Shea JE, Raupach B, Monack D, Falkow S, Gleeson C, Kubo T, Holden DW. 1997. Functional analysis of ssaJ and the ssaK/U operon, 13 genes encoding components of the type III secretion apparatus of Salmonella pathogenicity island 2. Mol Microbiol 24:155–167. doi: 10.1046/j.1365-2958.1997.3271699.x. [DOI] [PubMed] [Google Scholar]
- 35.Geddes K, Worley M, Niemann G, Heffron F. 2005. Identification of new secreted effectors in Salmonella enterica serovar Typhimurium. Infect Immun 73:6260–6271. doi: 10.1128/IAI.73.10.6260-6271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niemann GS, Brown RN, Gustin JK, Stufkens A, Shaikh-Kidwai AS, Li J, McDermott JE, Brewer HM, Schepmoes A, Smith RD, Adkins JN, Heffron F. 2011. Discovery of novel secreted virulence factors from Salmonella enterica serovar Typhimurium by proteomic analysis of culture supernatants. Infect Immun 79:33–43. doi: 10.1128/IAI.00771-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida Y, Miki T, Ono S, Haneda T, Ito M, Okada N. 2014. Functional characterization of the type III secretion ATPase SsaN encoded by Salmonella pathogenicity island 2. PLoS One 9:e94347. doi: 10.1371/journal.pone.0094347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langridge GC, Fookes M, Connor TR, Feltwell T, Feasey N, Parsons BN, Seth-Smith HM, Barquist L, Stedman A, Humphrey T, Wigley P, Peters SE, Maskell DJ, Corander J, Chabalgoity JA, Barrow P, Parkhill J, Dougan G, Thomson NR. 2015. Patterns of genome evolution that have accompanied host adaptation in Salmonella. Proc Natl Acad Sci U S A 112:863–868. doi: 10.1073/pnas.1416707112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeukens J, Kukavica-Ibrulj I, Emond-Rheault JG, Freschi L, Levesque RC. 2017. Comparative genomics of a drug-resistant Pseudomonas aeruginosa panel and the challenges of antimicrobial resistance prediction from genomes. FEMS Microbiol Lett 364:fnx161. doi: 10.1093/femsle/fnx161. [DOI] [PubMed] [Google Scholar]
- 40.Painset A, Bjorkman JT, Kiil K, Guillier L, Mariet JF, Felix B, Amar C, Rotariu O, Roussel S, Perez-Reche F, Brisse S, Moura A, Lecuit M, Forbes K, Strachan N, Grant K, Moller-Nielsen E, Dallman TJ. 2019. LiSEQ - whole-genome sequencing of a cross-sectional survey of Listeria monocytogenes in ready-to-eat foods and human clinical cases in Europe. Microb Genom 5:e000257. doi: 10.1099/mgen.0.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dallman T, Inns T, Jombart T, Ashton P, Loman N, Chatt C, Messelhaeusser U, Rabsch W, Simon S, Nikisins S, Bernard H, Le Hello S, Jourdan da-Silva N, Kornschober C, Mossong J, Hawkey P, de Pinna E, Grant K, Cleary P. 2016. Phylogenetic structure of European Salmonella Enteritidis outbreak correlates with national and international egg distribution network. Microb Genom 2:e000070. doi: 10.1099/mgen.0.000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inns T, Ashton PM, Herrera-Leon S, Lighthill J, Foulkes S, Jombart T, Rehman Y, Fox A, Dallman T, DE Pinna E, Browning L, Coia JE, Edeghere O, Vivancos R. 2017. Prospective use of whole genome sequencing (WGS) detected a multi-country outbreak of Salmonella Enteritidis. Epidemiol Infect 145:289–298. doi: 10.1017/S0950268816001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phillips A, Sotomayor C, Wang Q, Holmes N, Furlong C, Ward K, Howard P, Octavia S, Lan R, Sintchenko V. 2016. Whole genome sequencing of Salmonella Typhimurium illuminates distinct outbreaks caused by an endemic multi-locus variable number tandem repeat analysis type in Australia, 2014. BMC Microbiol 16:211. doi: 10.1186/s12866-016-0831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuijpers AFA, Bonacic Marinovic AA, Wijnands LM, Delfgou-van Asch EHM, van Hoek A, Franz E, Pielaat A. 2018. Phenotypic prediction: linking in vitro virulence to the genomics of 59 Salmonella enterica strains. Front Microbiol 9:3182. doi: 10.3389/fmicb.2018.03182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monte DF, Lincopan N, Berman H, Cerdeira L, Keelara S, Thakur S, Fedorka-Cray PJ, Landgraf M. 2019. Genomic features of high-priority Salmonella enterica serovars circulating in the food production chain, Brazil, 2000–2016. Sci Rep 9:11058. doi: 10.1038/s41598-019-45838-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pornsukarom S, van Vliet AHM, Thakur S. 2018. Whole genome sequencing analysis of multiple Salmonella serovars provides insights into phylogenetic relatedness, antimicrobial resistance, and virulence markers across humans, food animals and agriculture environmental sources. BMC Genomics 19:801. doi: 10.1186/s12864-018-5137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu J, Pugh R, Laughlin RC, Andrews-Polymenis H, McClelland M, Baumler AJ, Adams LG. 2014. High-throughput assay to phenotype Salmonella enterica Typhimurium association, invasion, and replication in macrophages. J Vis Exp 11:e51759. doi: 10.3791/51759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy MF, Riendeau N, Bedard C, Helie P, Min-Oo G, Turcotte K, Gros P, Canonne-Hergaux F, Malo D. 2007. Pyruvate kinase deficiency confers susceptibility to Salmonella typhimurium infection in mice. J Exp Med 204:2949–2961. doi: 10.1084/jem.20062606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuki KE, Marei H, Fiskin E, Eva MM, Gopal AA, Schwartzentruber JA, Majewski J, Cellier M, Mandl JN, Vidal SM, Malo D, Dikic I. 2019. CYRI/FAM49B negatively regulates RAC1-driven cytoskeletal remodelling and protects against bacterial infection. Nat Microbiol 4:1516–1531. doi: 10.1038/s41564-019-0484-8. [DOI] [PubMed] [Google Scholar]
- 50.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Criscuolo A, Gribaldo S. 2010. BMGE (Block Mapping and Gathering with Entropy): a new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol Biol 10:210. doi: 10.1186/1471-2148-10-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price MN, Dehal PS, Arkin AP. 2010. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One 5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of oral and systemic Salmonella enterica infection in C57BL/6J mice. Mice were either inoculated per os (A and C) or intravenously (B and D). Bacterial loads in the spleens and livers of C57BL/6J mice at day 3 after oral (A and C) and systemic (B and D) Salmonella infection. The graphs represent the CFU counts per weight of organs or feces derived from the means ± standard errors of the means (SEMs) from one experiment. Data are representative of two independent experiments. Two-way ANOVA with Sidak’s multiple-comparison test was used to assess significance. **, P ≤ 0.01; ****, P < 0.0001. Download FIG S1, PDF file, 0.1 MB (109.1KB, pdf) .
© Crown copyright 2020.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bacterial loads and distributions of contrasts in human cells infected with 35 phylogenetically distant Salmonella serovars. Bacterial adhesion in epithelial cells (A) and bacterial phagocytosis in macrophages (C) are shown as box plots. Low- and high-virulence controls are shown in green and red, respectively. (B and D) Contrast analysis was used to compare differences in bacterial burden caused by each of the 35 serovars against the low-virulence control ΔinvA ΔsseB strain (mean value shown in green) within all phenotypic traits measured in each model. Epithelial cell adhesion (B) and macrophage phagocytosis (D). Isolate means are shown by black dots. Orange boxes denote the ranges of values, solid black bars show the 95% confidence intervals, and dotted black bars show the confidence intervals when adjusted for multiple testing using Bonferroni’s correction. All isolates whose adjusted confidence intervals do not cross the green line were significantly different from the ΔinvA ΔsseB strain (P < 0.05). Either 2, 3, or 4 biological replicates with 3 technical replicates per isolate were used for epithelial cells. Either 2 or 3 biological replicates with 3 technical replicates were used for macrophages. Download FIG S2, PDF file, 0.2 MB (223KB, pdf) .
© Crown copyright 2020.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
List of the 171 proteins that correlated (P value ≤ 0.05) with Salmonella enterica isolate virulence. Corresponding genes and positions on Salmonella Typhimurium SL1344 chromosome as well as the protein functions based on UniProt, Virulence Factor DataBase (VFDB), and BacMet databases are included. Download Table S1, XLSX file, 0.1 MB (27.2KB, xlsx) .
© Crown copyright 2020.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Distributions of contrasts in the four models of infection for Salmonella strains with virulence phenotypes predicted by PCA. Contrast analysis was used to compare differences in bacterial burden caused by each of the 33 serovars against the low-virulence control ΔinvA ΔsseB strains (mean value shown in green) within defined phenotypic traits measured in each model. Epithelial cell adhesion (A), invasion (B), and survival (C), replication in amoeba (D), and bacterial loads in mouse spleen (E) and liver (F). Isolate means are shown by black dots. Orange boxes denote the ranges of values, solid black bars show the 95% confidence intervals, and dotted black bars show the confidence intervals when adjusted for multiple testing using Bonferroni’s correction. All isolates whose adjusted confidence intervals do not cross the green line were significantly different from the ΔinvA ΔsseB strain (P < 0.05). Either 2, 3, or 4 biological replicates with 3 technical replicates per isolate were used for epithelial cells. Three biological replicates were performed per isolate in the amoeba. Six biological replicates were performed per isolate in the mouse. Download FIG S3, PDF file, 0.2 MB (262.8KB, pdf) .
© Crown copyright 2020.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Genomic alterations in isolates S357 London and S240 Montevideo. (A) Graphical representation of the indel in the ssaK gene of S357 causing a frameshift and resulting in a premature stop codon. The slash bars represent truncated regions for better visualization of the key regions. The putative ssaK ORF in the S357 genome begins 102 nucleotides (nt) downstream of the premature stop codon and encodes a putative 537-amino-acid protein instead of the expected 675-amino-acid SsaK protein in SL1344. (B) Genomic deletion of 75.2 kbp in the S240 isolate was identified between hycE (start position on SL1344, 3,017,328 bp) and truD genes (end position on SL1344, 3,092,532 bp) and includes the SPI-1 T3SS essential for Salmonella virulence. (C) invA deletion impaired the virulence of Salmonella Typhimurium in vivo as shown by reduced bacterial recovery in spleen and liver and low competitive index (CI). C57BL/6J mice were coinfected with a mixed inoculum containing equal numbers of SL1344 and invA-deficient SL1344 cells. CIs were calculated by dividing the number of cells for the ΔinvA strain (kanamycin resistant) by that for SL1344 (streptomycin resistant). Download FIG S4, PDF file, 0.2 MB (235.9KB, pdf) .
© Crown copyright 2020.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
All genomic sequences are publicly available through NCBI (BioProject accession PRJNA634019).