Abstract
Background
Although balloon-occluded retrograde transvenous obliteration (BRTO) is often selected to treat gastric varices caused by portal hypertension, data comparing BRTO and splenectomy with gastric devascularization (Sp + Dev) are limited.
Methods
From January 2009 to February 2018, 100 patients with gastric varices caused by portal hypertension who underwent Sp + Dev (n = 45) or BRTO (n = 55) were included. Overall survival (OS) and the rebleeding rate were calculated using the inverse probability of a treatment weighting-adjusted log-rank test. Independent risk factors were identified by Cox regression analysis. Changes in liver function and adverse events after the procedures were analyzed.
Results
Patients in the Sp + Dev group tended to have lower platelet counts than those in the BRTO group, but liver function did not differ between these groups. The 5-year OS rates for the Sp + Dev and BRTO groups were 73.4 and 50.0% (p = 0.005), respectively. There were no significant differences in rebleeding rates between the two groups. Multivariate analysis showed that serum albumin level ≤3.6 g/dL, prothrombin time% activity (PT%) ≤80%, and serum creatinine level ≥0.84 mg/dL were poor prognostic factors. Although the Sp + Dev group had more short-term complications after procedures, Sp + Dev tended to be more effective in improving liver function than BRTO.
Conclusions
Sp + Dev showed better OS and improvement of liver function compared with BRTO for the treatment of gastric varices caused by portal hypertension.
Electronic supplementary material
The online version of this article (10.1007/s00535-020-01693-9) contains supplementary material, which is available to authorized users.
Keywords: Splenectomy, Gastric devascularization, Balloon-occluded transvenous obliteration, Gastric varices, Portal hypertension
Introduction
Portal hypertension causes gastric variceal bleeding and hypersplenism. Variceal bleeding has high mortality and constitutes a life-threatening complication of portal hypertension [1]. Various methods have been advocated to treat portal hypertension and variceal bleeding such as splenectomy and gastric devascularization (Sp + Dev), transjugular intrahepatic portosystemic shunt, and balloon-occluded retrograde transvenous obliteration (BRTO) [2–5]. BRTO is an interventional radiological technique that is commonly used in Japan [6, 7]. BRTO has a higher rate of successful hemostasis and improves hepatic functional reserve [8]. In case of difficulties in intervention treatment, Sp + Dev has been shown to provide satisfactory results in some studies [9–11]. However, few studies have compared Sp + Dev with BRTO.
In this study, the efficacy and safety of Sp + Dev were compared to those of BRTO in patients with gastric varices caused by cirrhotic portal hypertension.
Methods
Patients and study design
Clinical data were retrospectively collected from 108 patients who had undergone either Sp + Dev or BRTO for gastric varices from January 2009 to February 2018 at a single tertiary hospital in Japan (Hiroshima University Hospital). All patients were diagnosed with gastric varices by endoscopy.
In the BRTO group, patients comprised 20 bleeding cases and 41 prophylactic cases. In the prophylactic cases, the appearance of the red color sign or F3, or rapidly growing varices with a high risk of rupture, was the indication for BRTO [12]. In bleeding cases, those with porto-systemic shunts identified by contrast-enhanced computed tomography (CT) after temporary hemostasis with balloon tamponade or endoscopically were indications for BRTO. Endoscopic findings for varices were evaluated according to the general criteria introduced by the Japanese Research Society for Portal Hypertension [13].
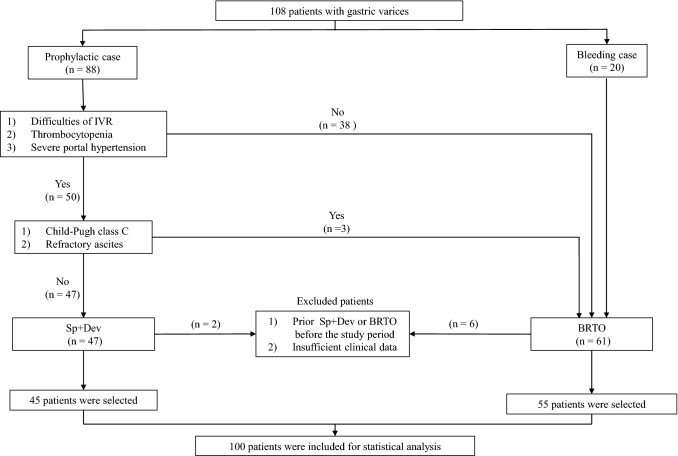
In the present study, 47 patients were treated with Sp + Dev for gastric varices caused by portal hypertension. The indicators of Sp + Dev included difficulty in interventional radiology, thrombocytopenia due to hypersplenism, and severe portal hypertension. In addition, Child–Pugh class C and refractory ascites were judged not to be operable and were an indication for BRTO. Patients who underwent Sp + Dev or BRTO for gastric varices before the study period or those with insufficient data were excluded from both groups. Ultimately, 8 patients were excluded, leaving 100 patients for statistical analysis (Fig. 1). This study adhered to the ethical principles of the Declaration of Helsinki.
Fig. 1.
Flowchart of patient population selection: 108 patients undergoing Sp + Dev or BRTO for gastric varices recruited. After excluding 8 patients, 100 patients were included for statistical analysis. Sp + Dev splenectomy and gastric devascularization, BRTO balloon-occluded retrograde transvenous obliteration, IVR interventional radiology
Surgical procedure
The procedures for Sp + Dev have been described previously [11]. In brief, an antithrombotic catheter was inserted via the jejunal vein to monitor the portal vein pressure immediately after laparotomy by a midline incision or “L” incision. A transducer was used to measure the portal vein pressure during surgery, and the catheter was removed before the abdominal operative wound was closed. Splenectomy was performed, and the ligation and division of the vessels at the splenic hilum and the spleen were removed through the incision. After splenectomy, the gastrohepatic ligament was opened and devascularization of the lesser curvature of the stomach was performed. The portal vein pressure was measured to determine the extent of devascularization to ensure the portal vein pressure did not increase by >50% from the time of laparotomy. CT was performed preoperatively and at 1-week and 6-month follow-ups after surgery or when indicated clinically. In splenectomy, aspirin was routinely used to prevent portal vein thrombosis. If a portal vein thrombus was detected, thrombolytic therapy including heparin, warfarin, and/or antithrombin III administration was initiated.
BRTO procedure
The procedures for BRTO were the same as those described previously [12]. In brief, a 5-French catheter with balloon (Seleconballoon catheter; Terumo Clinical Supply, Gifu, Japan) was inserted into the draining vein of the portal systemic shunt via the right femoral or right jugular vein under local anesthesia. During balloon occlusion of the outflow vessels, retrograde venography was performed to determine the hemodynamics of the gastric varices and collateral veins. BRTO was commonly performed using 5% ethanolamine oleate (Oldamin; Takeda Pharmaceutical, Osaka, Japan) (5% ethanolamine oleate mixed with iopamidol [EOI]) under balloon occlusion. All patients underwent gastrointestinal endoscopy and intravenous contrast-enhanced CT at approximately 1 week after BRTO.
Study end points
The primary end point in this study was overall survival (OS) of patients in the Sp + Dev group compared to that of patients in the BRTO group. We also analyzed prognostic factors of OS in the whole study population, Sp + Dev group, and BRTO group. The secondary end point was the rebleeding rate of patients. Changes in liver function and platelet counts before and after each procedure were compared and measured before each procedure, and at 6 months and 1 year after each procedure. Furthermore, we compared the rates of short-term complications and length of hospital stay. For the Sp + Dev group, surgery-related complications were also investigated. Ascites was defined as the state in which the administration of a diuretic was newly required after each procedure.
This study was conducted in accordance with the 1975 Declaration of Helsinki after receiving approval from the institutional review board of Hiroshima University (Hiroshima, Japan).
Statistical analysis
To account for differences in baseline characteristics between patients who underwent Sp + Dev and BRTO, we performed inverse probability of a treatment weighting (IPTW)-adjusted analysis. The probability (or propensity) to undergo Sp + Dev versus BRTO was estimated using a logistic regression model based on age, sex, viral hepatitis, concurrent hepatocellular carcinoma (HCC), white blood cell count, hemoglobin level, and serum levels of total bilirubin, albumin, and creatinine. Baseline characteristics were compared between the two groups before and after weighting by using the standardized differences approach, wherein significant imbalances in covariates are present if the standardized difference is ≥0.1 [14]. Propensity score weights were trimmed below and above the 1st and 99th percentiles, respectively [15].
IPTW-adjusted survivor functions for OS and the rebleeding rate were estimated using the Kaplan–Meier method, and cumulative probabilities of events were compared between the two groups using IPTW-adjusted log-rank tests. A Cox proportional hazards model was used to assess independent risk factors associated with poor OS. Statistically significant variables in the univariate analysis were entered into a multivariate Cox regression model. Changes in liver function and platelet counts were assessed using the paired t test. All statistical analyses were performed using SAS (version 9.4; SAS Institute Inc., Cary, NC, USA). All tests were two sided, and p < 0.05 was considered statistically significant.
Results
Patient characteristics and assessment of preoperative liver function
One hundred patients with gastric varices caused by liver cirrhosis and portal hypertension were divided into two groups: Sp + Dev (n = 45) and BRTO (n = 55). Baseline characteristics of the study population are described in Table 1. Before the weighting, the large standardized differences indicated that there was an imbalance between the two groups. Although patients in the Sp + Dev were younger and had a better Child–Pugh score, only prothrombin time% activity (PT%) was similar in both groups. There were no significant differences between the two groups in terms of the rate of concurrent HCC and staging and treatment method for HCC (Supplementary Table 1). The distributions of most covariates were well balanced between the groups after the IPTW adjustment except for the platelet count and ascites.
Table 1.
Baseline characteristics of the patients in the two groups
| Group variables | All (n = 100) | Before IPTW adjustment | After IPTW adjustment | ||||
|---|---|---|---|---|---|---|---|
| Sp + Dev (n = 45) | BRTO (n = 55) | Std diff | Sp + Dev (n = 45) | BRTO (n = 39) | Std diff | ||
| Age (years) | 65.1 ± 10.0 | 62.7 ± 10.7 | 67.1 ± 9.0 | 0.44 | 64.2 ± 11.0 | 64.8 ± 9.1 | 0.06 |
| Sex (male/female) | 67.0/33.0 | 71.1/28.9 | 63.6/26.4 | 0.16 | 71.7/28.3 | 73.2/26.8 | 0.03 |
| HBV or HBC/non-B and non-C | 55.0/45.0 | 64.4/35.6 | 47.3/52.7 | 0.35 | 65.0/35.0 | 61.0/39.0 | 0.07 |
| Concurrent HCC | 44.0 | 51.1 | 38.2 | 0.26 | 54.1/45.9 | 50.6/49.4 | 0.07 |
| White blood cell counts (/mm3) | 5249 ± 7252 | 3007 ± 1043 | 7084 ± 943 | 0.61 | 3647.4 ± 1566 | 3686 ± 1295 | 0.01 |
| Hemoglobin (g/dL) | 11.0 ± 2.4 | 11.8 ± 0.34 | 10.4 ± 0.3 | 0.61 | 11.7 ± 1.8 | 11.6 ± 1.9 | 0.07 |
| Platelet counts (×104/mm3) | 9.6 ± 7.8 | 6.3 ± 1.1 | 12.3 ± 1.0 | 0.85 | 7.6 ± 4.4 | 10.3 ± 4.7 | 0.38 |
| Total bilirubin (mg/dL) | 1.4 ± 0.8 | 1.2 ± 0.1 | 1.5 ± 0.1 | 0.28 | 1.2 ± 0.6 | 1.3 ± 0.7 | 0.09 |
| Albumin (g/dL) | 3.4 ± 0.7 | 3.6 ± 0.1 | 3.3 ± 0.1 | 0.40 | 3.6 ± 0.4 | 3.6 ± 0.6 | 0.03 |
| Prothrombin time% activity (%) | 67.8 ± 15.6 | 68.5 ± 2.3 | 67.2 ± 2.1 | 0.08 | 74.8 ± 18.2 | 74.8 ± 14.9 | 0.00 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.7 ± 0.0 | 0.8 ± 0.0 | 0.29 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.06 |
| Ascites | 7.0 | 8.9 | 5.5 | 0.13 | 6.3 | 1.3 | 0.19 |
| Portosystemic encephalopathy | 9.0 | 6.7 | 10.9 | 0.15 | 4.4 | 6.1 | 0.06 |
| Child–Pugh score | 7.0 ± 1.6 | 6.6 ± 0.2 | 7.2 ± 0.2 | 0.39 | 6.4 ± 1.0 | 6.5 ± 1.6 | 0.06 |
Variables are expressed as mean ± standard deviation or %
IPTW inverse probability of a treatment weighting, Std diff. standardized difference, Sp + Dev splenectomy and gastric devascularization, BRTO balloon-occluded retrograde transvenous obliteration, HBV hepatitis B virus, HCV hepatitis C virus, HCC hepatocellular carcinoma
Supplementary Fig. 1A shows that the mean portal vein pressure before splenectomy, after splenectomy, and after Sp + Dev were 21.6 ± 4.2 mmHg, 16.6 ± 4.2 mmHg, and 19.6 ± 3.9 mmHg, respectively. The mean portal vein pressure after Sp + Dev was significantly lower than that before splenectomy (p = 0.027). In contrast, the mean hepatic vein pressure gradient (HVPG) values before and after BRTO were 12.2 ± 1.0 mmHg and 13.6 ± 1.0 mmHg, respectively (Supplementary Fig. 1B). The mean HVPG after BRTO was higher than that before BRTO, but there was no significant difference (p = 0.150).
Overall survival
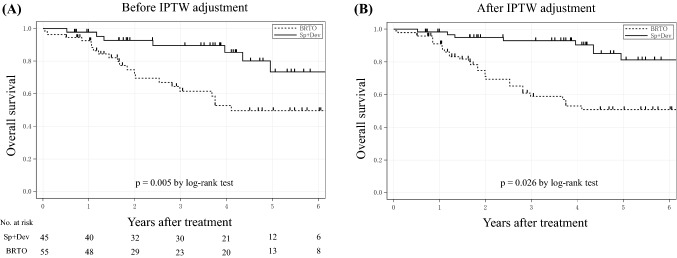
The mean follow-up period for the Sp + Dev group and BRTO group were 43.4 and 35.4 months, respectively (p = 0.332). Figure 2a shows that the OS of the Sp + Dev group was significantly longer than that of the BRTO group (p = 0.005). The 1-, 3-, and 5-year OS rates in the Sp + Dev group were 95.2, 89.7, and 73.4%, respectively, while those in the BRTO group were 92.6, 61.5, and 50.0%, respectively. Next, we used IPTW analysis to compare the OS between the two groups. Figure 2b shows that after IPTW adjustment, patients who underwent Sp + Dev had a significantly better OS than those who underwent BRTO (p = 0.026).
Fig. 2.
a Overall survival (OS) in the Sp + Dev and BRTO group in all patients. Note the significantly lower OS following Sp + Dev compared to BRTO (p = 0.005). b OS in the Sp + Dev and BRTO groups after IPTW adjustment. Note a significantly lower OS after Sp + Dev compared to the BRTO groups (p = 0.048). Sp + Dev splenectomy and gastric devascularization, BRTO balloon-occluded retrograde transvenous obliteration, IPTW inverse probability of a treatment weighting
Predictors of overall survival
Table 2 shows the results of the univariate and multivariate analyses of prognostic factors of OS in the whole study. In the univariate analysis, age ≥66 years-old, BRTO, white blood cell counts ≤3240/mm3, hemoglobin level ≤10.7 g/dL, platelet counts >6.6 × 104/mm3, serum aspartate transaminase level ≥43 IU/L, serum albumin level ≤3.6 g/dL, PT% ≤80%, and serum creatinine level ≥0.84 mg/dL were each significantly predictive of poor prognosis. In the multivariate analysis, serum albumin level ≤3.6 g/dL (hazard ratio [HR] = 4.099, 95% confidence interval [CI] 1.049–19.036; p = 0.042), PT% ≤80% (HR = 4.324, 95% CI 1.070–29.699; p = 0.039), and serum creatinine level ≥0.84 mg/dL (HR = 3.229, 95% CI 1.524–6.967; p = 0.002) emerged as independent predictors of poor survival. Furthermore, we assigned a value to these three independent factors of poor survival, which was added to obtain a total risk score ranging from zero to three. Supplementary Fig. 2 shows that the OS did not differ significantly in patients with a risk factor of zero to one between the two groups (p = 0.164); however, the OS of patients with a risk factor of two or more in the Sp + Dev group was significantly longer than that of patients in the BRTO group (p = 0.004). Regarding the cause of death, there were no significant differences except for HCC between the two groups. The Sp + Dev group had a significantly higher proportion of HCC deaths than the BRTO group (p = 0.045) (Supplementary Table 2).
Table 2.
Prognostic factors for overall survival identified by univariate and multivariate analyses (n = 100)
| Variables | n (%) | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| p value | HR | 95% CI | p value | ||
| Age | |||||
| <66 years | 50 (50.0) | 0.002 | 1 | 0.090 | |
| ≥66 years | 50 (50.0) | 2.026 | 0.899–4.939 | ||
| Sex | |||||
| Male | 67 (67.0) | 0.376 | |||
| Female | 33 (33.0) | ||||
| Procedure | |||||
| Sp + Dev | 45 (45.0) | 0.005 | 1 | 0.730 | |
| BRTO | 55 (55.0) | 1.253 | 0.361–4.798 | ||
| Serology of viral hepatitis | |||||
| HBV or HBC | 55 (55.0) | 0.732 | |||
| Non-B and non-C | 45 (45.0) | ||||
| Concurrent HCC | |||||
| Yes | 44 (44.0) | 0.289 | |||
| No | 56 (56.0) | ||||
| White blood cell counts | |||||
| ≥3240 (mm3) | 57 (57.0) | 1 | 0.642 | ||
| <3240 (mm3) | 43 (43.0) | 0.036 | 1.303 | 0.441–4.249 | |
| Hemoglobin | |||||
| ≥10.7 (g / dL) | 57 (57.0) | 1 | 0.945 | ||
| <10.7 (g / dL) | 43 (43.0) | 0.002 | 1.038 | 0.342–2.910 | |
| Platelet counts | |||||
| <6.6 (×104/mm3) | 33 (33.0) | 0.020 | 1 | 0.156 | |
| ≥6.6 (×104/mm3) | 67 (67.0) | 2.438 | 0.726–9.737 | ||
| Total bilirubin | |||||
| <0.8 (mg/dL) | 79 (79.0) | 0.100 | |||
| ≥0.8 (mg/dL) | 21 (21.0) | ||||
| Aspartate aminotransferase | |||||
| <43 (IU/L) | 65 (65.0) | 0.005 | 1 | 0.983 | |
| ≥43 (IU/L) | 35 (35.0) | 1.010 | 0.417–2.544 | ||
| Alanine aminotransferase | |||||
| <29 (IU/L) | 57 (57.0) | 0.091 | 1 | 0.101 | |
| ≥29 (IU/L) | 43 (43.0) | 2.382 | 0.844–6.725 | ||
| Albumin | |||||
| >3.6 (g/dL) | 37 (37.0) | 0.001 | 1 | 0.042 | |
| ≤3.6 (g/dL) | 63 (63.0) | 4.099 | 1.049–19.036 | ||
| Prothrombin time% activity | |||||
| >80 (%) | 19 (19.0) | 0.045 | 1 | 0.039 | |
| ≤80 (%) | 81 (81.0) | 4.324 | 1.070–29.699 | ||
| Creatinine | |||||
| <0.84 (mg/dL) | 64 (64.0) | 0.013 | 1 | ||
| ≥0.84 (mg/dL) | 36 (36.0) | 3.229 | 1.524–6.967 | 0.002 | |
HR hazard ratio, CI confidential interval, HCC hepatocellular carcinoma, Sp + Dev splenectomy and gastric devascularization, BRTO balloon-occluded transvenous retrograde obliteration, HBV hepatitis B virus, HCV hepatitis C virus
Rebleeding rate
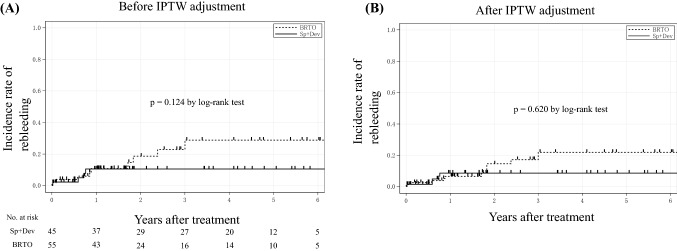
During the study period, variceal rebleeding ensued in 14 patients (Sp + Dev: n = 4, 8.9%; BRTO: n = 10, 18.2%; p = 0.183). As shown in Fig. 3a, the cumulative incidence rate of rebleeding in whole patients did not significantly differ between the two groups (p = 0.124). Figure 3b shows that the cumulative incidence rate of rebleeding after IPTW adjustment also did not significantly differ between the two groups (p = 0.620).
Fig. 3.
a The incidence rate of rebleeding in the Sp + Dev and BRTO groups. No significant difference was found between the two groups (p = 0.124). b The incidence rate of rebleeding in the Sp + Dev and BRTO groups after IPTW adjustment. No significant difference was found between the two groups (p = 0.620). Sp + Dev splenectomy and gastric devascularization, BRTO balloon-occluded retrograde transvenous obliteration, IPTW inverse probability of a treatment weighting
Changes in liver function and platelet counts
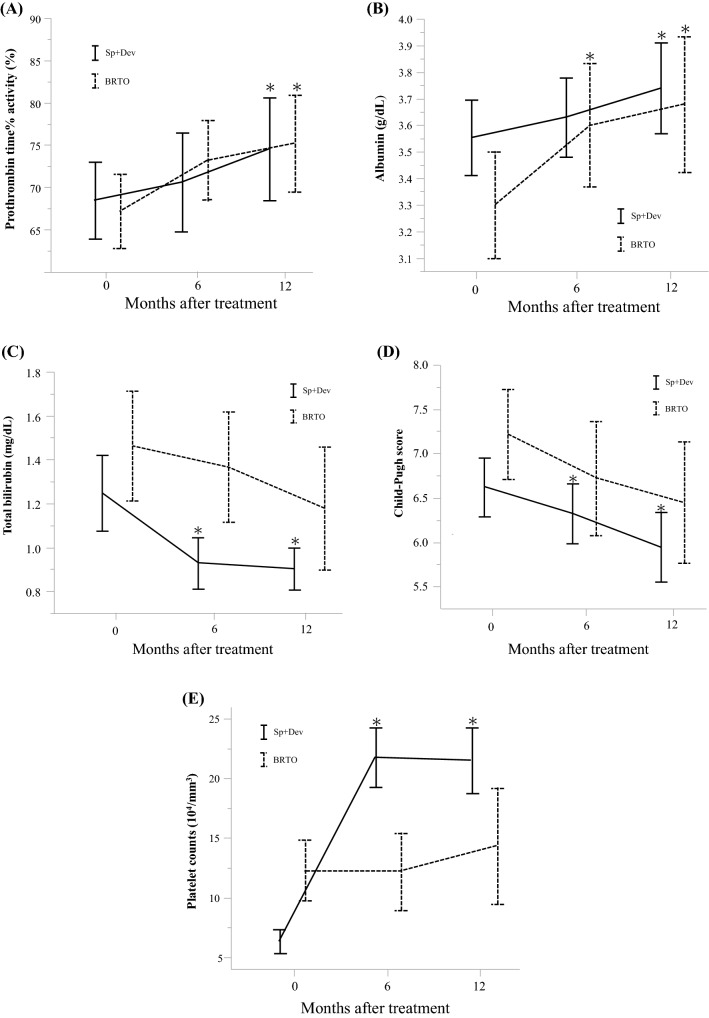
Changes in liver function before and after each procedure at 6 and 12 months are shown in Fig. 4. PT% (p = 0.011 at 12 months in the Sp + Dev group, p = 0.009 at 12 months in the BRTO group) and serum albumin level (p = 0.048 at 12 months in the Sp + Dev group, p = 0.014 at 12 months in the BRTO group) significantly increased after each procedure in both groups. However, serum total bilirubin level (p < 0.001 at 12 months), Child–Pugh score (p = 0.001 at 12 months), and platelet counts (p < 0.001 at 12 months) significantly improved after the procedure only in the Sp + Dev group. Although there was no significant difference, Child–Pugh score gradually decreased in patients who underwent BRTO (p = 0.051 at 12 months), indicating an improvement in liver function.
Fig. 4.
Dynamics of liver function after Sp + Dev (n = 45) and BRTO (n = 55) assessed by paired t test: changes in a PT%, b albumin, c total bilirubin, d Child–Pugh score, and e platelet counts differed by procedure over time. Variables expressed as the mean ± 95% confidence interval. Sp + Dev splenectomy and gastric devascularization, BRTO balloon-occluded transvenous obliteration, PT% prothrombin time% activity. *p < 0.050
Short-term outcome
Endoscopic evaluation of therapeutic effects and short-term complications after Sp + Dev and BRTO are demonstrated in Table 3. There were no significant differences between the two groups in terms of improvement of gastric varices or eradicating red color sign by endoscopy after the procedures (p = 0.188). The incidence of portal vein thrombosis (p = 0.038) and ascites (p < 0.001) was significantly higher in the Sp + Dev group than in the BRTO group. Pancreatic fistula developed in one (2.2%) patient who required drainage, and postoperative bleeding occurred in one (2.2%) patient who needed reoperation in the Sp + Dev group. One (1.8%) patient died of acute respiratory distress syndrome caused by interstitial pneumonia after BRTO. Hospital stay was significantly longer in the Sp + Dev group than in the BRTO group (17.8 versus 10.5 days, respectively; p = 0.003).
Table 3.
Post procedure outcome
| Variables | Sp + Dev (n = 45) | BRTO (n = 55) | p value |
|---|---|---|---|
| Therapeutic effect after procedure (n, %) | |||
| Improvement of endoscopic findings for gastric varices | 38(84.4) | 51(92.7) | 0.188 |
| Complications (n, %) | |||
| Ascites | 22 (48.9) | 3 (5.5) | <0.001 |
| Portal vein thrombosis | 7 (15.6) | 2 (3.6) | 0.038 |
| Splenic vein thrombosis | 1 (2.2) | 0 (0.0) | 0.267 |
| Esophageal varices bleeding | 1 (2.2) | 1 (1.8) | 0.886 |
| Infection except SSI | 5 (11.1) | 4 (7.3) | 0.505 |
| SSI | 9 (20.0) | – | – |
| Postoperative bleeding | 1 (2.2) | – | – |
| Pancreatic fistula | 1 (2.2) | – | – |
| Mortality in hospital stay | 0 (0.0) | 1 (1.8) | 0.363 |
| Hospital stay (days) | 17.8 | 10.5 | 0.003 |
Sp + Dev splenectomy and gastric devascularization, BRTO balloon-occluded transvenous obliteration, SSI surgical site infection
Discussion
In the present study, we retrospectively investigated the outcome of patients with gastric varices caused by liver cirrhosis and portal hypertension to determine the impact of treatment on clinical outcomes. We found that Sp + Dev significantly prolonged OS compared with BRTO for all patients and for those selected by IPTW method. In the multivariate analysis, low serum albumin level, low PT%, and high serum creatinine level were independent prognostic factors of OS. The rate of incidence of rebleeding did not differ between the two groups. Although liver function improved in both groups, Sp + Dev tended to be more effective in improving liver function than BRTO, and thrombocytopenia was improved in only the Sp + Dev group. Conversely, Sp + Dev demonstrated several disadvantages over BRTO, including a higher incidence of short-term complications and a longer postoperative length of hospital stay.
Although several investigators have reported that survival rates after Sp + Dev and BRTO were both favorable, few studies have compared survival outcome at a single institution. The survival rates after Sp + Dev reported at 1, 3 and 5 years ranged from 92.0 to 97.6%, 82.0 to 88.1%, and 64.0 to 76.2%, respectively [9, 16]. In contrast, the survival rates reported after BRTO at 1, 3 and 5 years ranged from 84.7 to 93.0%, 76.0 to 96.5% and 54.0 to 81.7%, respectively [17–19]. In the present study, the OS for all patients was significantly longer in the Sp + Dev group than in BRTO the group; however, this result could have been affected by a selection bias of patient. Therefore, we opted to perform IPTW analysis using the propensity score to minimize the impact of selection bias and potential confounding effects. OS, analyzed after IPTW adjustment, was also significantly longer in the Sp + Dev group than in the BRTO group. Based on the above results, the Sp + Dev procedure would be superior to BRTO under similar conditions.
It is well known that the Child–Pugh classification and model for end-stage liver disease score are commonly used as predictors of survival in patients with liver cirrhosis [20, 21]. The present study showed that low PT%, low serum albumin level and high serum creatinine level were independent prognostic factors of OS. Gastric varices were also a result of the pathophysiology of liver cirrhosis and portal hypertension; therefore, our results appear to be consistent with previous reports.
Patients with major shunts such as gastric varices have decreased portal blood flow, resulting in a gradually decreased hepatic functional reservoir. A decreased portal blood flow can be recovered by the occlusion of shunts in BRTO, resulting in improvement of liver function [18, 22]. Although the mechanism of improvement of liver function following Sp + Dev interventions has yet to be fully elucidated, several studies have reported that splenectomy ameliorates liver function [23, 24]. With regard to hemodynamics in liver cirrhosis, excessive splenic artery blood flowing into spleen causes portal hypertension and a splenohepatic arterial steal syndrome, which leads to the development of a decrease in the hepatic artery blood flow [25]. The portal blood flow decreases after ligation of the splenic artery, thereby decompressing the portal vein pressure. Although decreasing the portal vein pressure and increasing hepatic artery blood flow may be associated with improved liver function, no relationship was found between the changes in portal vein pressure by Sp + Dev and BRTO and liver function and OS. These results suggest that increasing hepatic blood flow may have a greater effect on improvement of liver function than on reduction of portal vein pressure. Furthermore, various reports have demonstrated the mechanism by which splenectomy improves liver function, including reducing levels of transforming growth factor-beta, which is a hepatocyte growth inhibitor derived from spleen; impairment of overloading the capacity of the liver to metabolize bilirubin; and promoting liver regeneration by preserving the secretion of tumor necrosis factor-alpha [26–28]. Other studies have shown similar evidence in the improvement of liver function after BRTO and splenectomy, but few reports have described the transition of liver function following Sp + Dev. Although liver function in patients who underwent Sp + Dev and BRTO gradually improved over 12 months in the present study, serum total bilirubin level and Child–Pugh score improved significantly only in those who underwent Sp + Dev. This suggests that Sp + Dev is more effective in improving liver function than BRTO. Furthermore, our study demonstrated that Sp + Dev is particularly effective in patients with risk factors.
In addition, the presence of thrombocytopenia has been shown to be a prognostic factor in cirrhotic patients according to its association with significant mortality [29, 30]. Thrombocytopenia that is associated with liver cirrhosis causes bleeding tendency, difficulties of induction of interferon for hepatitis C virus (HCV), and difficulties with interventional radiology for HCC. Yamamoto et al. reported that they could perform the treatment of HCC safely for patients who received splenectomy, and this contributed to prolonged OS in patients who underwent splenectomy compared with those who did not undergo splenectomy [31]. Although partial splenic embolization is another effective modality that is less invasive in patients with hypersplenism, some investigators have reported disadvantages such as high recurrence rates of thrombocytopenia and the possibility of infectious complications [32–34]. The Sp + Dev group showed greater improvement of liver function and thrombocytopenia according to splenectomy and increased blood flow into the liver. These factors could be associated with later treatments including direct-acting antivirals for HCV, interventional radiology for HCC and procedures including surgery or biopsy for other diseases due to bleeding tendency. Therefore, patients in the Sp + Dev group had a better prognosis than those in the BRTO group.
In the present study, patients in the Sp + Dev group had longer hospital stays and higher rates of complications, including portal vein thrombosis and ascites, than those in the BRTO group. In the treatment of gastric varices, the BRTO group was likely to have included many cases of poor liver function than the Sp + Dev group, because the invasiveness of the procedures differs. The cumulative rebleeding rates were similar between the two groups. Essentially, BRTO is often first choice when attempting to eradicate varices because it is less invasive. Sp + Dev is performed for patients with BRTO-resistant varices associated with enlarged inflow routes, high-grade portal hypertension, and difficulties in coagulation. However, the surgical procedure has become easy and safe to perform with the recent introduction of a vessel-sealing system and auto-suture devices [23, 35–37]. Together with a trend toward less invasive treatment for portal hypertension, the Sp + Dev approach for gastric varices with liver cirrhosis is a reasonable treatment option for operable patients.
The limitations of the study include its retrospective nature and that it was performed at a single institution, which may have led to potential bias. However, even after IPTW adjustment, selection bias may not have been avoided completely. Further investigations with a larger number of patients may be necessary.
After IPTW adjustment, we found that Sp + Dev significantly prolonged OS compared to BRTO in patients with gastric varices caused by portal hypertension. Besides, the incidence rate of rebleeding did not differ between the Sp + Dev and BRTO groups, both for the entire study population and after IPTW adjustment. BRTO could reasonably be attempted for gastric varices because it is less invasive than surgery; however, Sp + Dev is a useful treatment option that leads to improvements in liver function and thrombocytopenia in operable patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1Supplementary Figure 1. (A) Dynamics of portal vein pressure before and after splenectomy and after Sp+Dev as assessed by paired-t test. The mean portal vein pressure after Sp+Dev was significantly lower than that before splenectomy (p = 0.027). (B) Dynamics of hepatic vein pressure gradient before and after BRTO as assessed by paired-t test. The mean HVPG after BRTO was higher than that before BRTO, but there was no significant difference (p = 0.150). Sp, splenectomy; Sp+Dev, splenectomy and gastric devascularization; HVPG, hepatic vein pressure gradient; BRTO, balloon-occluded transvenous obliteration. * p < 0.050. Supplementary Figure 2. One point was assigned to each of the following independent risk factors; serum albumin level ≥ 3.6 mg/dL, PT% ≤ 80% and serum creatinine level ≥ 0.84 mg/dL. (A) Overall survival in patients with zero to one risk factors in the Sp+Dev group and BRTO groups. No significant difference was found between the two groups. (B) Overall survival in patients with two or more risk factor in the Sp+Dev group and BRTO groups. Note there was a significantly higher rate after Sp+Dev procedures compared to BRTO (p = 0.004). Sp+Dev, splenectomy and gastric devascularization; BRTO, balloon-occluded retrograde transvenous obliteration; PT%, prothrombin time% activity. (PPTX 62 kb)
Acknowledgements
This work was supported in part by the JSPS KAKENHI [Grant Numbers JP17K10669], AMED [Grant Numbers JP18fk0210007, 19fk0310109h0003, and 19fk0210020h0003], and the Takeda Science Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
KO: contributed to the study design, collected samples, and drafted the manuscript. MO: contributed to the study design, conducted analysis and interpretation of the data, and assisted in drafting and revising the manuscript. NH: collected samples, contributed to the study design, and assisted in drafting and revising the manuscript. TK: contributed to the study design and assisted in drafting and revising the manuscript. EM: collected samples, contributed to the study design, and assisted in drafting and revising the manuscript. HA: contributed to the study design and assisted in drafting and revising the manuscript. YB: contributed to the study design and assisted in drafting and revising the manuscript. RK: conducted analysis of the data and assisted in drafting and revising the manuscript. KA: contributed to the study design and assisted in drafting and revising the manuscript. KC: contributed to the study design and assisted in drafting and revising the manuscript. HO: contributed to the study design and assisted in drafting and revising the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest concerning this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grace ND, Groszmann RJ, Garcia-Tsao G, et al. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology. 1998;28:868–880. doi: 10.1002/hep.510280339. [DOI] [PubMed] [Google Scholar]
- 2.Wolff M, Hirner A. Current state of portosystemic shunt surgery. Langenbecks Arch Surg. 2003;388:141–149. doi: 10.1007/s00423-003-0367-5. [DOI] [PubMed] [Google Scholar]
- 3.Wu X, Ding W, Cao J, et al. Favorable clinical outcome using a covered stent following transjugular intrahepatic portosystemic shunt in patients with portal hypertension. J Hepatobilary Pancreat Sci. 2010;17:701–708. doi: 10.1007/s00534-010-0270-8. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto N, Akahoshi T, Yoshida D, et al. The efficacy of balloon-occluded retrograde transvenous obliteration on small intestinal variceal bleeding. Surgery. 2010;148:145–150. doi: 10.1016/j.surg.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 5.Hassab MA. Gastroesophageal decongestion and splenectomy in the treatment of esophageal varices in bilharzial cirrhosis: further studies with a report on 355 operations. Surgery. 1967;61:169–176. [PubMed] [Google Scholar]
- 6.Kanagawa H, Mima S, Kouyama H, et al. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996;11:51–58. doi: 10.1111/j.1440-1746.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 7.Chikamori F, Shibuya S, Takase Y, et al. Transjugular retrograde obliteration for gastric varices. Abdom Imaging. 1996;21:299–303. doi: 10.1007/s002619900068. [DOI] [PubMed] [Google Scholar]
- 8.Hirota S, Kobayashi K, Maeda H, et al. Balloon-occluded retrograde transvenous obliteration for portal hypertension. Radiat Med. 2006;24:315–320. doi: 10.1007/s11604-006-2407-x. [DOI] [PubMed] [Google Scholar]
- 9.Tomikawa M, Hashizume M, Saku M, et al. Effectiveness of gastric devascularization and splenectomy for patients with gastric varices. J Am Coll Surg. 2000;191:498–503. doi: 10.1016/S1072-7515(00)00735-3. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Li Y, Ma J, et al. A modified Hassab's operation for portal hypertension: experience with 562 cases. J Surg Res. 2013;185:463–468. doi: 10.1016/j.jss.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 11.Tashiro H, Ide K, Amano H, et al. Surgical treatment for portosystemic encephalopathy in patients with liver cirrhosis: occlusion of portosystemic shunt in combination with splenectomy. Hepatol Res. 2013;43:249–254. doi: 10.1111/j.1872-034X.2012.01059.x. [DOI] [PubMed] [Google Scholar]
- 12.Naeshiro N, Aikata H, Kakizawa H, et al. Long-term outcome of patients with gastric varices treated by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 2014;29:1035–1042. doi: 10.1111/jgh.12508. [DOI] [PubMed] [Google Scholar]
- 13.Tajiri T, Yoshida H, Obara K, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition) Dig Endosc. 2010;22:1–9. doi: 10.1111/j.1443-1661.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- 14.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawanaka H, Akahoshi T, Nagao Y, et al. Customization of laparoscopic gastric devascularization and splenectomy for gastric varices based on CT vascular anatomy. Surgical Endosc. 2018;32:114–126. doi: 10.1007/s00464-017-5646-2. [DOI] [PubMed] [Google Scholar]
- 17.Ninoi T, Nishida N, Kaminou T, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol. 2005;184:1340–1346. doi: 10.2214/ajr.184.4.01841340. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Kim SU, Kim MD, et al. Comparison of treatment outcomes between balloon-occluded retrograde transvenous obliteration and transjugular intrahepatic portosystemic shunt for gastric variceal bleeding hemostasis. J Gastroenterol Hepatol. 2017;32:1487–1494. doi: 10.1111/jgh.13729. [DOI] [PubMed] [Google Scholar]
- 19.Akahoshi T, Hashizume M, Tomikawa M, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding and risky gastric varices: a 10-year experience. J Gastroenterol Hepatol. 2008;23:1702–1709. doi: 10.1111/j.1440-1746.2008.05549.x. [DOI] [PubMed] [Google Scholar]
- 20.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 21.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Gimm G, Chang Y, Kim HC, et al. Balloon-occluded retrograde transvenous obliteration versus transjugular intrahepatic portosystemic shunt for the management of gastric variceal bleeding. Gut Liver. 2018;11:173–188. doi: 10.5009/gnl17515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto J, Nagai M, Smith B, et al. Hand-assisted laparoscopic splenectomy and devascularization of the upper stomach in the management of gastric varices. World J Surg. 2006;30:1520–1525. doi: 10.1007/s00268-005-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ushitora Y, Tashiro H, Takahashi S, et al. Splenectomy in chronic hepatic disorders: portal vein thrombosis and improvement of liver function. Dig Surg. 2011;28:9–14. doi: 10.1159/000321886. [DOI] [PubMed] [Google Scholar]
- 25.Quintini C, Hirose K, Hashimoto K, et al. "Splenic artery steal syndrome" is a misnomer: the cause is portal hyperperfusion, not arterial siphon. Liver Transpl. 2008;14:374–379. doi: 10.1002/lt.21386. [DOI] [PubMed] [Google Scholar]
- 26.Murata K, Shiraki K, Sugimoto K, et al. Splenectomy enhances liver regeneration through tumor necrosis factor (TNF)-alpha following dimethylnitrosamine-induced cirrhotic rat model. Hepatogastroenterology. 2001;48:1022–1027. [PubMed] [Google Scholar]
- 27.Sugawara Y, Yamamoto J, Shimada K, et al. Splenectomy in patients with hepatocellular carcinoma and hypersplenism. J Am Coll Surg. 2000;190:446–450. doi: 10.1016/S1072-7515(99)00294-X. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Sakata R, Ueno T, et al. Inhibition of transforming growth factor beta prevents progression of liver fibrosis and enhances hepatocyte regeneration in dimethylnitrosamine-treated rats. Hepatology. 2000;32:247–255. doi: 10.1053/jhep.2000.9109. [DOI] [PubMed] [Google Scholar]
- 29.Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48:1000–1007. doi: 10.1016/j.jhep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Peck-Radosavljevic M. Thrombocytopenia in liver disease. Can J Gastroenterol. 2000;14(Suppl D):60d–66d. doi: 10.1155/2000/617428. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto N, Okano K, Oshima M, et al. Laparoscopic splenectomy for patients with liver cirrhosis: improvement of liver function in patients with Child–Pugh class B. Surgery. 2015;158:1538–1544. doi: 10.1016/j.surg.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Cai M, Huang W, Lin C, et al. Partial splenic embolization for thrombocytopenia in liver cirrhosis: predictive factors for platelet increment and risk factors for major complications. Eur Radiol. 2016;26:370–380. doi: 10.1007/s00330-015-3839-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhu K, Meng X, Qian J, et al. Partial splenic embolization for hypersplenism in cirrhosis: a long-term outcome in 62 patients. Dig Liver Dis. 2009;41:411–416. doi: 10.1016/j.dld.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Noguchi H, Hirai K, Aoki Y, et al. Changes in platelet kinetics after a partial splenic arterial embolization in cirrhotic patients with hypersplenism. Hepatology. 1995;22:1682–1688. doi: 10.1002/hep.1840220611. [DOI] [PubMed] [Google Scholar]
- 35.Anegawa G, Kawanaka H, Uehara H, et al. Effect of laparoscopic splenectomy on portal hypertensive gastropathy in cirrhotic patients with portal hypertension. J Gastroenterol Hepatol. 2009;24:1554–1558. doi: 10.1111/j.1440-1746.2009.05906.x. [DOI] [PubMed] [Google Scholar]
- 36.Kawanaka H, Akahoshi T, Kinjo N, et al. Laparoscopic splenectomy with technical standardization and selection criteria for standard or hand-assisted approach in 390 patients with liver cirrhosis and portal hypertension. J Am Coll Surg. 2015;221:354–366. doi: 10.1016/j.jamcollsurg.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 37.Zheng S, Sun P, Liu X, et al. Efficacy and safety of laparoscopic splenectomy and esophagogastric devascularization for portal hypertension: a single-center experience. Medicine. 2018;97:e13703. doi: 10.1097/MD.0000000000013703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1Supplementary Figure 1. (A) Dynamics of portal vein pressure before and after splenectomy and after Sp+Dev as assessed by paired-t test. The mean portal vein pressure after Sp+Dev was significantly lower than that before splenectomy (p = 0.027). (B) Dynamics of hepatic vein pressure gradient before and after BRTO as assessed by paired-t test. The mean HVPG after BRTO was higher than that before BRTO, but there was no significant difference (p = 0.150). Sp, splenectomy; Sp+Dev, splenectomy and gastric devascularization; HVPG, hepatic vein pressure gradient; BRTO, balloon-occluded transvenous obliteration. * p < 0.050. Supplementary Figure 2. One point was assigned to each of the following independent risk factors; serum albumin level ≥ 3.6 mg/dL, PT% ≤ 80% and serum creatinine level ≥ 0.84 mg/dL. (A) Overall survival in patients with zero to one risk factors in the Sp+Dev group and BRTO groups. No significant difference was found between the two groups. (B) Overall survival in patients with two or more risk factor in the Sp+Dev group and BRTO groups. Note there was a significantly higher rate after Sp+Dev procedures compared to BRTO (p = 0.004). Sp+Dev, splenectomy and gastric devascularization; BRTO, balloon-occluded retrograde transvenous obliteration; PT%, prothrombin time% activity. (PPTX 62 kb)