Abstract
The bipolar mitotic spindle drives accurate chromosome segregation by capturing the kinetochore and pulling each set of sister chromatids to the opposite poles. In this review, we describe recent findings on the multiple pathways leading to bipolar spindle formation in fission yeast and discuss these results from a broader perspective. The roles of three mitotic kinesins (Kinesin-5, Kinesin-6 and Kinesin-14) in spindle assembly are depicted, and how a group of microtubule-associated proteins, sister chromatid cohesion and the kinetochore collaborate with these motors is shown. We have paid special attention to the molecular pathways that render otherwise essential Kinesin-5 to become non-essential: how cells build bipolar mitotic spindles without the need for Kinesin-5 and where the alternate forces come from are considered. We highlight the force balance for bipolar spindle assembly and explain how outward and inward forces are generated by various ways, in which the proper fine-tuning of microtubule dynamics plays a crucial role. Overall, these new pathways have illuminated the remarkable plasticity and adaptability of spindle mechanics. Kinesin molecules are regarded as prospective targets for cancer chemotherapy and many specific inhibitors have been developed. However, several hurdles have arisen against their clinical implementation. This review provides insight into possible strategies to overcome these challenges.
Keywords: bipolar mitotic spindle, fission yeast, kinesin, kinetochore, microtubule dynamics, microtubule polymerase, microtubule–associated proteins (MAPs), spindle pole body (SPB), sister chromatid cohesion
1. Introduction
1.1. Bipolar Mitotic Spindles and Kinesin Motor Proteins
The bipolar mitotic spindle is a dynamic ensemble of core microtubule polymers and a cohort of microtubule-associated proteins (MAPs). It attaches to the kinetochore on sister chromatids to align chromosomes at the spindle equator in metaphase, and pulls each pair of sister chromatids towards the opposite poles in anaphase A. During anaphase B, spindles further elongate to ensure the equal partition of each set of chromosomes, which is followed by cytokinesis [1,2]. MAPs are required for spindle assembly and coordinate the multiple events of mitosis in a spatiotemporal manner. Kinesin motors comprise one of the major families of MAPs and couple the energy of ATP hydrolysis to force generation [3]. The kinesin superfamily comprises at least 15 families, which are further structurally categorised into three groups, called N-kinesin (N-terminal), M-kinesin (middle) and C-kinesin (C-terminal), depending on the location of the motor domain within each molecule [4,5,6]. Mitotic kinesins include 10 kinesin families that are functionally designated as they are localised to the spindle microtubule and regulate structure and function of the spindle and mitotic progression [7]. This review is based upon recent work using fission yeast as a model but includes comparisons with other systems, in which evolutionary conservation and diversification are discussed.
1.2. Kinesin-5 Plays an Essential Role in Bipolar Spindle Assembly and Cell Survival
The type 5 kinesin (Kinesin-5) was originally identified in Aspergillus nidulans as one of the mitotically arrested mutants (called bimC) [8]. This kinesin belongs to the N-kinesin that moves on microtubules towards their plus ends. This motor forms homotetramers, thereby crosslinking and sliding apart antiparallel microtubules [9,10]. During early mitosis, this process generates an outward pushing force towards two duplicated spindle poles (centrosomes in animal cells and the spindle pole bodies (SPBs) in fungi), which promotes centrosome/SPB separation, thereby establishing spindle bipolarity [11,12]. In most, if not all, eukaryotes, Kinesin-5 (budding yeast Cin8 and Kip1, fission yeast Cut7, Aspergillus BimC, C. elegans BMK-1, Drosophila Klp61F, Xenopus Eg5 and human KIF11) is essential for mitosis, in which any means of its inactivation, e.g., chemical inhibition, genetic deletion or RNAi-mediated depletion, leads to the emergence of monopolar spindles, the failure of chromosome segregation and viability loss [8,13,14,15,16,17,18,19].
2. How Essential Kinesin-5 Becomes Non-Essential
Surprising findings came to light that cells can divide in the absence of Kinesin-5 function under certain conditions across a wide range of species, including human beings, frogs, flies, filamentous fungi and the budding and fission yeasts. Initial genetic studies and recent more comprehensive analysis conducted in Aspergillus nidulans and Saccharomyces cerevisiae showed that lethal mutations in Kinesin-5 are rescued by simultaneous inactivation of genes encoding Kinesin-14 (klpA and KAR3 respectively) [20,21,22,23]. This is because bipolar spindle assembly is driven by the finely tuned, antagonistic force balance exerted by opposing motors. More precisely, an outward force generated by plus end-directed Kinesin-5 is antagonised by an inward force produced by minus end-directed Kinesin-14 that belongs to the C-kinesin (budding yeast Kar3, fission yeast Pkl1 and Klp2, Aspergillus KlpA, Drosophila Ncd, Xenopus XCTK2 and human HSET/KIFC1) or Dynein [24]. Accordingly, inactivation of minus end-directed motors could neutralise the loss of Kinesin-5 activity. In other words, cells without Kinesin-5 and Kinesin-14 or Dynein are now capable of forming bipolar spindles and will continue to divide.
3. Conditions Under Which Cells Do Not Need Kinesin-5 for Survival
As aforementioned, the main means in which Kinesin-5 becomes dispensable is by simultaneous inactivation of opposing Kinesin-14 or Dynein. In order to explore the genetic network that plays a role in conferring the non-essentiality of Kinesin-5, we conducted systematic screening for suppressors against cut7 temperature sensitive (ts) mutants in fission yeast. Spontaneous survivors of cut7 mutant strains grown at the restrictive temperature (36 °C) were isolated. After standard genetic analyses and nucleotide sequencing, suppressor genes (designated skf = suppressor of kinesin five) were identified (Table 1) [25]. Suppressors can be classified into three main groups: Kinesin-14s, non-motor MAPs and tubulins.
Table 1.
List of spindle inward force generators in fission yeast.
| Gene | Synonym | Protein | Homologue | Function |
|---|---|---|---|---|
| pkl1 1 | skf1 | Kinesin-14 | HSET/KIFC1 | Minus end-directed motor |
| wdr8 | skf2 | WD40 repeats | WDRB/WRAP73 | A component of the MWP complex |
| msd1 1 | skf3 | Coiled coil | hMsd1/SSX2IP | A component of the MWP complex |
| klp2 | Kinesin-14 | HSET/KIFC1 | Minus end-directed motor | |
| nda3 | skf4 | β-tubulin | β-tubulin | Microtubule subunit |
| atb2 | skf5 | α2-tubulin | α-tubulin | Microtubule subunit |
| mal3 | skf6 | MAP | EB1 | A microtubule plus-end tracking protein |
| alp16 | GRIP repeats | GCP6 | A component of the γ-TuC | |
| alp7 | mia1 | MAP | TACC | Complex formation with Alp14 |
| alp14 | mtc1 | MAP | XMAP215/Stu2/TOG | Microtubule polymerase |
| dis1 | MAP | XMAP215/Stu2/TOG | Microtubule polymerase | |
| pka1 | Protein kinase | PKA | cAMP-dependent protein kinase |
1 Only pkl1 or msd1 deletion bypasses a complete deletion of cut7. Mutations in the remaining genes suppress only the cut7 temperature sensitive (ts) mutant, but not cut7∆. The other condition that renders cut7∆ viable is treatment with microtubule-destabilising drugs [33].
3.1. Suppression by Mutations in Kinesin-14s or Their Cofactors
In fission yeast, two Kinesin-14s, Pkl1 and Klp2, form distinct complexes with specific cofactors and are localised to different sites on the microtubule to play non-redundant roles in spindle assembly and mitotic progression [26,27,28,29]. Pkl1 forms a ternary complex with Msd1 and Wdr8 (referred to as the MWP complex) and is localised predominantly to the mitotic SPB, thereby anchoring the minus end of the spindle microtubule to the SPB [30,31,32]. During early mitosis, when the duplicated SPBs start to separate in a process driven by the Cut7-mediated outward force, the SPB-tethered MWP complex is loaded onto the spindle microtubule that nucleates from the other SPB, where this complex exerts an antagonistic inward force. Thus, the reason for suppression of cut7 mutants by pkl1∆, msd1∆ or wdr8∆ is that the two SPBs can separate because the overwhelming inward force exerted by the MWP Kinesin-14 complex disappears (Figure 1A).
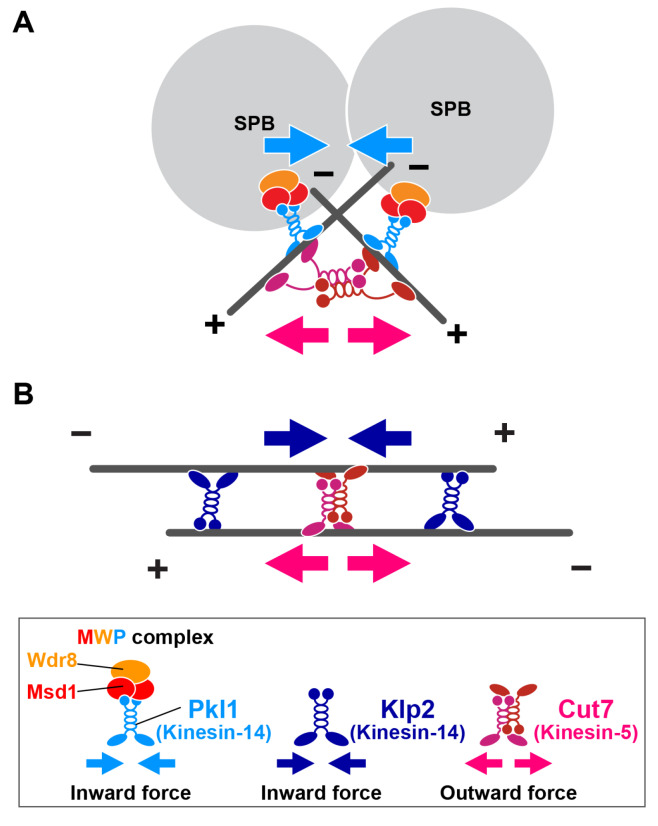
Figure 1.
Generation of collaborative inward forces by the two Kinesin-14s, Pkl1 and Klp2. (A) When spindle bipolarity starts to be established upon onset of mitosis, spindle pole body (SPB)-tethered Pkl1 (the MWP complex) engages with the spindle microtubule that emanates from the opposite SPB. Minus end-directed motility of Pkl1 generates an inward force (blue arrows). This pulling force antagonises an outward force exerted by Cut7 (red arrows) that is also localised in the vicinity of the SPB and promotes interdigitation of the microtubules emanating from the two SPBs. In addition to inward force generation, the MWP complex plays a crucial role in anchoring the minus end of the spindle microtubule to the mitotic SPB as a barrier [32]. (B) Once bipolar spindles are formed, Klp2 and Cut7 are localised on antiparallel microtubules. These two kinesins antagonistically generate an inward force (blue arrows) and an outward force (red arrows), respectively. + and – stand for the microtubule plus and minus ends, respectively. Note that Cut7 is reportedly localised to the two other sites. One is a medial microtubule contact site. This localisation is seen when bipolar spindles are depolymerised first and then allowed to repolymerise; Cut7 promotes interpolar bundle formation [39]. The other site is the kinetochore, to which Cut7 is recruited when chromosomes are misaligned. Under this condition, Cut7 is required for chromosome gliding towards the spindle equator [40].
By contrast, Klp2 is mainly localised along spindles in a punctate manner [34]. Spindle-localising Klp2 crosslinks the antiparallel microtubule bundles, which produces an inward force by exploiting minus-end motility, and this force acts antagonistically with the Cut7-driven outward force on the spindle microtubule [33]. The reason for suppression of cut7 mutants by klp2∆ is that antiparallel microtubules can elongate in the absence of Cut7, as Klp2-driven inhibitory inward force is lost (Figure 1B). Collectively, Pkl1 acts mainly during the early stages of bipolar spindle assembly, while Klp2 plays a role in spindle elongation at later stages of mitosis. These distinct modes of the spatiotemporal regulation between Pkl1 and Klp2 underlie the collaborative actions of these two Kinesin-14s.
The deletion of either pkl1 or klp2 suppresses the temperature sensitivity caused by the cut7 mutations [27,29,35]. Intriguingly, gene deletion of pkl1, but not klp2, is capable of rescuing a complete deletion of cut7 [33,36,37,38]. Despite apparent ordinary growth, cut7∆pkl1∆ cells display mitotic delay in which cells spend a longer period of time with short spindles. This implies that in the absence of Cut7 and Pkl1, an excessive inward force is imposed during early mitosis. This inward force, at least in part, is generated by Klp2, as the slower spindle elongation rate is significantly ameliorated in the cut7∆pkl1∆klp2∆ triple mutant [33].
3.2. Suppression by Compromised Microtubule Nucleation, Polymerisation and Stability
We have found that mutations in the genes encoding tubulins and five non-motor MAPs are also capable of rescuing cut7 ts mutants. In fission yeast, tubulin molecules are encoded by nda2 (α1-tubulin), atb2 (α2-tubulin) and nda3 (β-tubulin) [41,42] (Table 1). Mutations in tubulin genes would compromise microtubule integrity. One of the five MAPs is Mal3/EB1, a conserved MAP that tracks on the microtubule plus end [43]. Its mutation leads to microtubule destabilisation and defects in kinetochore–microtubule attachment [44,45]. Alp16 is a homologue of GCP6 and a component of the microtubule-nucleator γ-tubulin complex (γ-TuC) [46,47,48]. The other three MAP-encoding suppressors are alp7 (encoding an orthologue of the transforming acidic coiled-coil protein TACC) [49,50], alp14 and dis1 (two genes encoding XMAP215/Stu2/TOG microtubule polymerases) [51,52,53,54,55,56]. Alp7 and Alp14 form a stable complex in the cell and promote microtubule polymerisation [49,54,57]. The Alp7–Alp14 complex is also required for efficient nucleation of the microtubule from the SPB through interaction with the γ-TuC [58].
Apart from its role in microtubule stabilisation, Mal3/EB1 is known to interact with Klp2, which is a prerequisite for this motor to be loaded on the spindle microtubule [34]. Thus, suppression of the cut7 ts mutation by the mal3 mutation could be ascribable to the loss of Klp2 function [25]. Overall, the common features of suppressor genes encoding tubulins and MAPs are that all these mutations lead to the destabilisation of the spindle microtubules. It is worth noting that in cut7 mutant cells, the intensities of spindle microtubules are augmented [25]. Importantly, in these mutant cells, intensities of Klp2 on the spindle microtubule are also substantially increased. Notably, in the double mutant containing cut7 ts and any of the suppressor mutations, the intensities of Klp2 are reduced. Given these observations, we propose that the rescue of cut7 mutants by these suppressors is derived from quantitative downregulation of Klp2 activity. In fact, the overproduction of Klp2 in the double mutants between cut7 and suppressor mutations restored a ts phenotype similar to a single cut7 mutant [25], corroborating the notion that the reduced localisation/activity of Klp2 is the primary, if not the sole, reason for the rescue of the cut7 mutation. In summary, suppressor analyses have uncovered that multiple factors that regulate microtubule structures are involved in several mechanisms by which Kinesin-5/Cut7 becomes dispensable, and importantly, inactivation of Kinesin-14s, Pkl1 and Klp2, is the main means for the rescue of cut7 by these suppressors.
3.3. Suppression by Microtubule-Destabilising Drugs
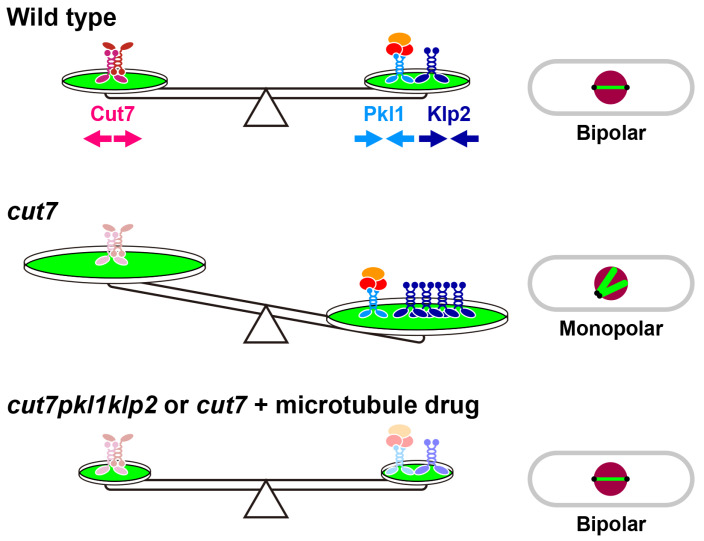
As previously mentioned, cut7 mutants exhibit increased intensities of the spindle microtubule accompanied by more Klp2 proteins on the spindle microtubule. In line with this observation, these cells display hyper-resistance against microtubule-depolymerising drugs, such as thiabendazole (TBZ) or methyl 2-benzimidazolecarbamate (MBC), and interestingly, treatment of cut7 ts mutants with TBZ or MBC rescues temperature sensitivity [25]. Under this condition, Klp2 levels are lessened as in the other suppressor mutations. Remarkably, drug treatment rescues even a complete deletion of cut7. Collectively, the impairment of microtubule stability and/or dynamics by either suppressor mutations or treatment with microtubule-destabilising drugs renders fission yeast cells viable in the absence of Kinesin-5 (Figure 2).
Figure 2.
Bipolar spindle formation requires a collaborative force balance exerted by mitotic kinesins, microtubule-associated proteins (MAPs) and microtubule dynamics. In wild type cells (top), Kinesin-5/Cut7 generates an outward force (red arrows), while Kinesin-14s/Pkl1 and Klp2 generate an opposing inward force (blue arrows). Microtubule stability and dynamics promoted by a cohort of MAPs play positive roles in Klp2 activity by enhancing its localisation to the spindle microtubule, which Kinesin-5/Cut7 opposes. In cut7 ts or cut7∆ cells (middle), Kinesin-14-mediated inward forces dominate, leading to the formation of monopolar spindles. In double mutants between cut7 and pkl1/klp2 or the cut7 mutant treated with microtubule-depolymerising drugs (bottom), loss of inward forces or the compromised microtubule dynamics, respectively, renders Cut7 dispensable for bipolar spindle assembly and therefore promotes survival.
It is worth pointing out that in cultured human cells (HeLa or U2OS), microtubule destabilisation can also effectively rescue monopolar spindle phenotypes induced by Kinesin-5/KIF11 inhibition [59,60]. This underscores the evolutionary conservation of Kinesin-5 function and its regulation from fission yeast to human beings.
3.4. Suppression through Downregulation of the cAMP/PKA Pathway
In many organisms, the extracellular environment, such as nutritional cues, regulates microtubule dynamics through intracellular signal transduction pathways. In yeasts, glucose in the media activates the cAMP/PKA pathway, by which it controls a diverse set of downstream events [61,62,63]. Deletion of the pka1 gene rescues the cut7 ts mutant [64]. Pka1 reportedly fine-tunes microtubule dynamics at least in part through downregulating the Cls1/Peg1/Stu1/CLASP MAP [65,66,67,68,69], and consistent with this notion, overproduction of Cls1/Peg1 is capable of rescuing the cut7 ts mutant as pka1∆ is [64]. Cls1/Peg1 is shown to promote microtubule bundling [70]. It is possible that enhanced bundling activity by overproduced Cls1/Peg1 would help convert the monopolar spindle in the cut7 mutation to a bipolar spindle, leading to rescue of this mutant.
4. Outward Force Generators in the Absence of Kinesin-5
Cells without Kinesin-5 become viable if Kinesin-14 is defective (e.g., cut7∆pkl1∆) or if microtubules are destabilised (e.g., cut7∆ treated with microtubule-destabilising drugs). This finding poses the following important question: how do bipolar spindles assemble in the absence of Kinesin-5-mediated outward force? Detailed genetic and cell biological analyses have unravelled this puzzle; at least 11 gene products are capable of generating outward forces in place of Kinesin-5 (Table 2).
Table 2.
List of spindle outward force generators in fission yeast.
| Gene | Synonym | Protein | Homologue | Function |
|---|---|---|---|---|
| klp9 | Kinesin-6 | MKLP1, MKLP2 | Plus end-directed motor | |
| ase1 | MAP | PRC1 | Microtubule crosslinker | |
| cls1 | peg1 | MAP | CLASP | Microtubule stabiliser/crosslinker |
| alp7 | mia1 | MAP | TACC | Complex formation with Alp14 |
| alp14 | mtc1 | MAP | XMAP215/Stu2/TOG | Microtubule polymerase |
| dis1 | MAP | XMAP215/Stu2/TOG | Microtubule polymerase | |
| csi1 | Coiled coil | Targeting Alp7 to the mitotic SPB | ||
| csi2 | SPB localising | Targeting Csi1 to the mitotic SPB | ||
| swi6 | Chromodomain | HP1 | Heterochromatin | |
| rad21 | Kleisin | hRad21/Scc1/Mcd1 | Cohesin | |
| nuf2 | Coiled coil | Nuf2 | Kinetochore |
4.1. Outward Forces Exerted by Kinesin-6
One possibility of the survival of cut7∆pkl1∆ cells is that the other kinesin motors exert an outward force in place of Kinesin-5, thereby promoting spindle bipolarity. The fission yeast genome contains in total nine genes encoding kinesin motors. Genetic crosses indicate that only one kinesin, Klp9, becomes essential when combined with cut7∆pkl1∆; cut7∆pkl1∆klp9∆ triple mutants are inviable. Klp9 belongs to the N-kinesin Kinesin-6. Interestingly, like Cut7 it moves on the microtubule towards the plus end and forms homotetramers, thereby crosslinking antiparallel microtubules [71,72]. Previous work showed that this kinesin accumulates at the spindle midzone upon the onset of anaphase B and promotes spindle elongation during late mitosis [71], though it appears to also play additional roles during earlier stages of mitosis [73,74,75]. Detailed analysis shows that Klp9 accelerates spindle elongation only during anaphase B in both wild type and cut7∆pkl1∆ cells and that inviable cut7∆pkl1∆klp9∆ cells are in fact capable of assembling bipolar spindles. However, these spindles are shorter comparted to those in wild type or cut7∆pkl1∆ cells, and upon mitotic exit the nucleus and chromosomes are intersected by the cytokinetic actomyosin contractile ring and the septum, resulting in cell death imposed by a catastrophic “cut” (cell untimely torn) phenotype [72,76].
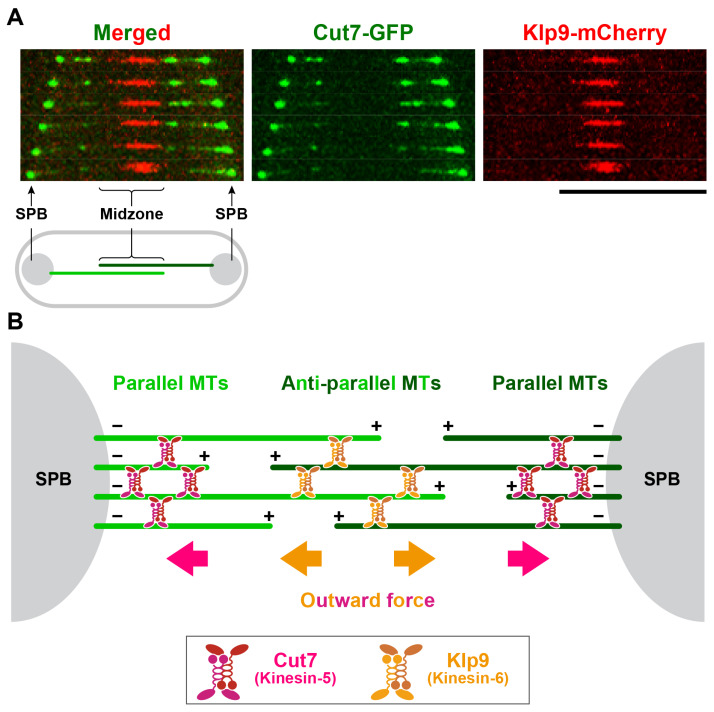
How do the two N-kinesins, Kinesin-5/Cut7 and Kinesin-6/Klp9, act in concert to drive spindle elongation in wild-type cells? Differential localisation patterns of these two kinesins on the spindle microtubule may give us a clue. While Cut7 mainly accumulates near the SPB, Klp9 is localised exclusively to the spindle midzone where antiparallel microtubules interdigitate (Figure 3A). Given these different localisations, we posit that during anaphase B, Cut7 crosslinks mainly parallel microtubules near the SPBs, while Klp9 bundles antiparallel microtubules at the spindle midzone (Figure 3B).
Figure 3.
Spatially distinct localisations between Kinesin-5/Cut7 and Kinesin-6/Klp6 on anaphase B spindles. (A) Localisation of Cut7 and Klp9 on the spindle microtubule during anaphase B. The fission yeast strain containing cut7-GFP and klp9-mCherry (expressed from individual native promoters) were observed under fluorescence microscopy and a late mitotic cell imaged. Images were obtained using a DeltaVision microscope system (DeltaVision Elite; GE Healthcare, Chicago, IL, USA) comprising a wide-field inverted epifluorescence microscope (IX71; Olympus, Tokyo, Japan) and a Plan Apochromat 60×, NA 1.42, oil immersion objective (PLAPON 60×O; Olympus Tokyo, Japan). DeltaVision image acquisition software (softWoRx 6.5.2; GE Healthcare, Chicago, IL, USA) equipped with a charge-coupled device camera (CoolSNAP HQ2; Photometrics, Tucson, AZ, USA) was used. Time-lapse live imaging was performed after the incubation of cultures at 27 °C, in which pictures were taken at 1 min intervals as 16 sections along the z-axis at 0.2 μm intervals. The sections of images acquired at each time point were compressed into a 2D projection using the DeltaVision maximum intensity algorithm. Deconvolution was applied before the 2D projection. Captured images were processed with Photoshop CS6 (version 13.0; Adobe, San Jose, CA, USA). Scale bar, 10 μm. (B) A schematic showing localisations of Cut7 and Klp9 on anaphase B spindles. Cut7 bundles parallel microtubules in the vicinity of the SPB, while Klp9 bundles antiparallel microtubules at the spindle midzone. Note that Klp9 bundles antiparallel microtubules at the spindle midzone independent of its motor activity [72]. + and – stand for the microtubule plus and minus ends, respectively.
4.2. Outward Forces Exerted by the Microtubule Crosslinker and Stabiliser
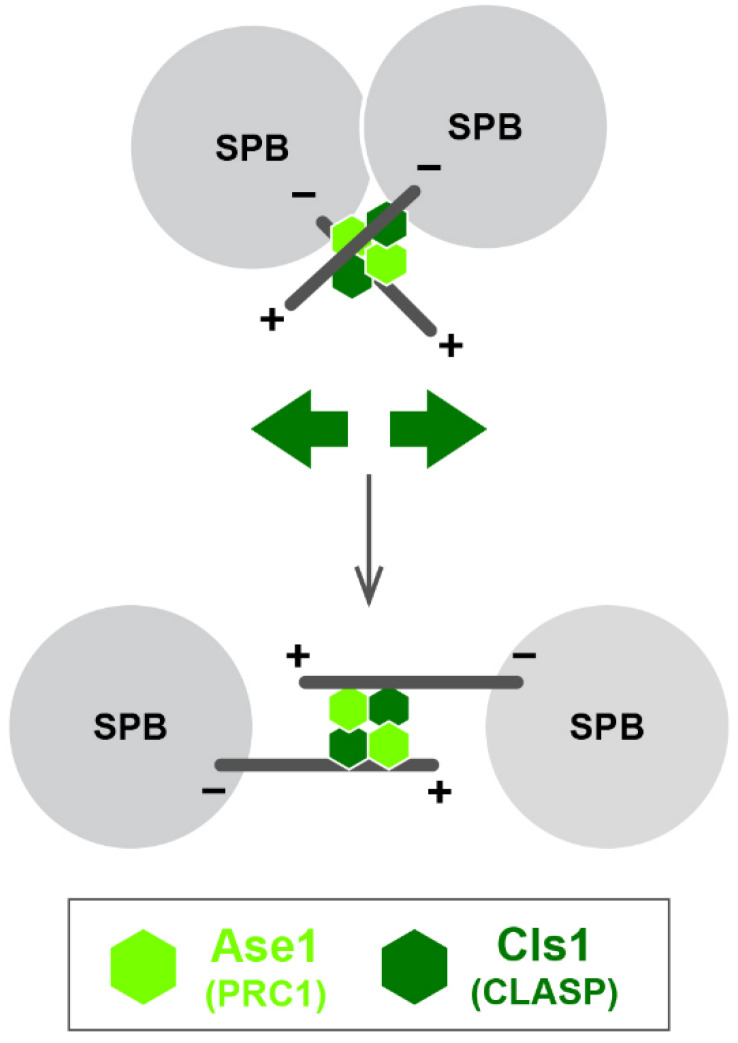
Fission yeast cells are capable of forming nearly normal bipolar spindles in the presence of only Kinesin-6 Klp9, which acts in spindle elongation later in mitosis [64]. How then could spindle bipolarity be established in the first place under this condition? It transpires that two conserved MAPs, Ase1/PRC1 [77,78,79,80] and Cls1/Peg1/Stu1/CLASP [65,66,68,69], in concert, play an indispensable role in this process. Ase1 and Cls1/Peg1 bundle and stabilise antiparallel spindle microtubules (Figure 4). Notably, theoretical modelling supports bipolar spindle assembly by these two factors; Brownian dynamics–kinetic Monte Carlo simulations show that Ase1 and Cls1/Peg1 activity are sufficient for initial bipolar spindle formation [64,68,69,81,82].
Figure 4.
Outward force generation through the spindle midzone. Short microtubules nucleating from the two SPBs are crosslinked in an antiparallel manner and stabilised by Ase1/PRC1 and Cls1/Peg1/CLASP. This provides an outward force (green arrows) towards the SPB that is sufficient to form short bipolar spindles in the absence of Kinesin-5 and Kinesin-14 [64]. + and – stand for the microtubule plus and minus ends, respectively.
Unlike in other species, C. elegans does not require Kinesin-5/BMK-1 for bipolar spindle formation [83]. During embryonic division of this organism, the spindle midzone could produce outward forces in concert with cortical pulling forces, thereby promoting chromosome segregation [18]. Interestingly, in this process, the midzone components including SPD-1/Ase1 and CLASP play vital roles in force generation, though SPD-1 seems to antagonise CLASP-mediated spindle elongation [18,84]. Taken together, the spindle midzone could produce outward forces in which the microtubule crosslinking and stabilising MAPs are key players.
4.3. Outward Forces Exerted by Microtubule Polymerases
Alp14 and Dis1 belong to a conserved MAP family of XMAP215/Stu2/TOG that catalyses microtubule polymerisation [51,52,53,54,55,56,85,86]. As aforementioned, Alp7 forms a stable complex with Alp14 and targets the Alp14 microtubule polymerase to the SPB upon mitotic onset [49,57]. Genetic analysis indicates that any of triple deletion mutants, cut7∆pkl1∆alp14∆, cut7∆pkl1∆dis1∆ or cut7∆pkl1∆alp7∆, are inviable. SPB-localising Csi1 and Csi2 are required for Alp7 localisation to the mitotic SPBs [87,88,89]. Consistent with this, deletion of either csi1 or csi2 is lethal in combination with cut7∆pkl1∆. Temperature sensitive cut7∆pkl1∆alp7 cells display monopolar spindles or very short spindles (<0.5 μm) that fail to elongate [90]. These results suggest that in cut7∆pkl1∆ cells outward forces are generated through microtubule polymerisation, in which the growing plus ends of the microtubule push the SPB, leading to separation of the SPBs (Figure 5A).
Figure 5.
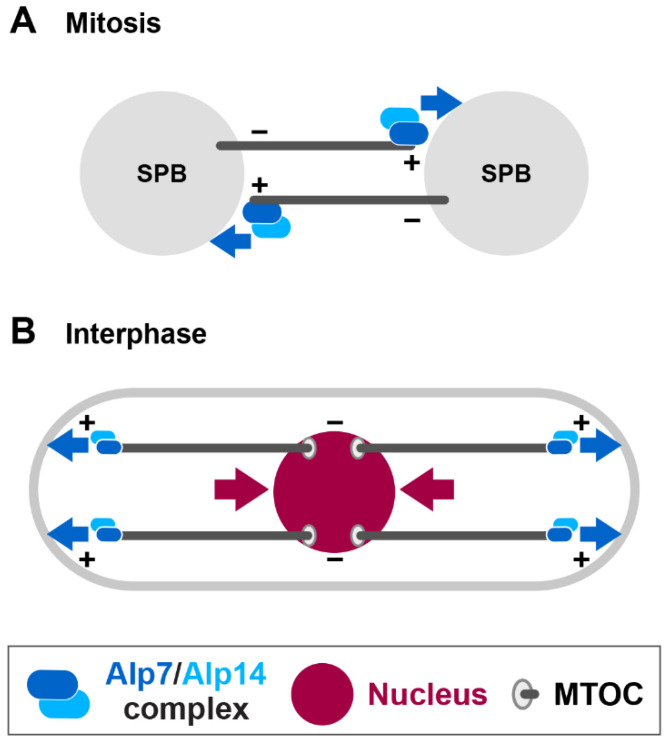
Force generation through microtubule polymerisation during interphase and mitosis. (A) During early mitosis, the Alp7/TACC-Alp14/TOG microtubule polymerase complex (blue ovals) is localised to the SPB (not shown) and the polymerising plus ends. Interaction of growing microtubule plus ends with the other SPB generates an outward force (blue arrows). This force is sufficient to separate the duplicated SPBs in the absence of Kinesin-5 and Kinesin-14, thereby promoting short bipolar spindle formation. (B) During interphase, the plus ends of the polymerising cytoplasmic microtubules reach and push the cell tip at either end (blue arrows), thereby pushing the nucleus through the opposite minus ends (deep red arrows). This allows positioning of the nucleus at the geometrical centre of the cell [91,92]. MTOC stands for microtubule organising centre, which is localised to multiple positions on the nuclear membrane during interphase [93,94]. + and – stand for the microtubule plus and minus ends, respectively.
Mutations in dis1, alp7 or alp14 rescue the cut7 ts mutation, while rather contradictorily, the same mutations become indispensable in the cut7∆pkl1∆ background; defects in the microtubule polymerisation confer both positive and negative impacts on the cut7 mutation. In a single cut7 mutation, microtubule polymerisation is negative for cell survival, while in cut7∆pkl1∆, it plays a positive role. This illuminates a remarkable mechanistic plasticity of bipolar spindle assembly; cells could generate either inward or outward forces using microtubule polymerases in a context-dependent manner.
Intriguingly, in interphase fission yeast cells the nucleus is centred by pushing forces that are generated as growing cytoplasmic microtubules hit the cell tip at each end [91,92] (Figure 5B). Hence, pushing forces generated through the polymerising microtubule plus ends play important roles in both interphase and mitosis; nuclear positioning during interphase and SPB separation/bipolar spindle assembly during mitosis. The generation of an outward pushing force by the polymerising microtubule plus end is widely observed in other systems. For instance, during embryonic divisions of animal cells the plus ends of astral microtubules physically interact with and push the cell cortex, and this force ensures proper spindle positioning [95,96].
4.4. Outward Forces Exerted by the Kinetochore and Sister Chromatid Cohesion
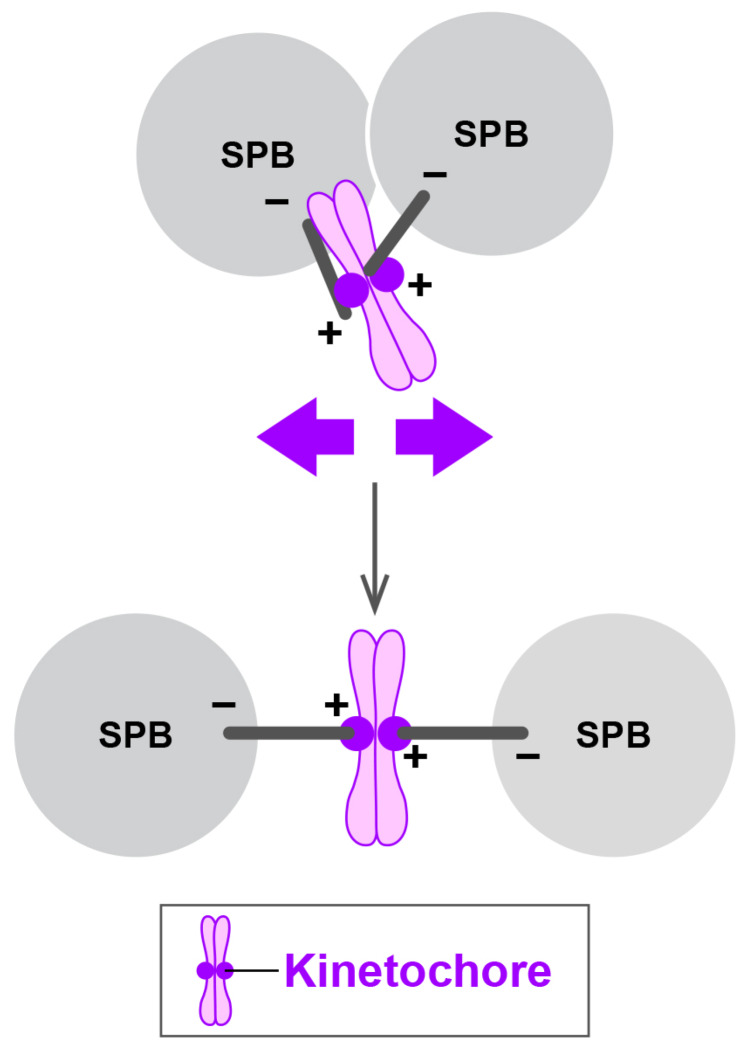
Recent work has identified a fourth class of outward force generators [97]. This force is elicited through the kinetochore, a several-MDa–sized proteinaceous structure assembled on a specialised region of the chromosome called the centromere [98]. The spindle microtubule attaches to the kinetochore for accurate sister chromatid segregation. Mutations in genes encoding the conserved kinetochore component (Nuf2) [99] or those required for centromere-mediated sister chromatid cohesion (Rad21/Scc1/Mcd1 and Swi6/HP1) [100,101,102,103] confer a severe synthetic growth defect to cut7∆pkl1∆. These triple mutant cells impair proper spindle assembly and display largely monopolar spindles. These results show that the kinetochore is captured by the plus end of the spindle microtubule, thereby producing outward forces that support bipolar spindle assembly (Figure 6) [97].
Figure 6.
Outward force generation through the kinetochore and sister chromatid cohesion. The kinetochore and sister chromatid cohesion contribute to the generation of outward forces for SPB separation in mitosis. In the absence of Kinesin-5 and Kinesin-14, outward forces (purple arrows) derived from the kinetochore and/or sister chromatid cohesion are capable of separating the SPBs, thereby promoting bipolar spindle assembly [97]. + and – stand for the microtubule plus and minus ends, respectively.
It is noteworthy that a similar result was reported in human cells, in which stable kinetochore–microtubule attachment plays a crucial role in centrosome separation [104] and becomes essential for maintenance of the bipolar spindle in the absence of Kinesin-5 [105]. Taking all these findings together, the balance between inward and outward forces generated by opposing motor proteins, multiple MAPs, the kinetochore and sister chromatid cohesion underlies mechanisms by which bipolar spindles are formed and maintained.
4.5. Outward Force Generation by Kinesin-12 in Human Cells
In fission yeast, Kinesin-6/Klp9 collaborates with Kinesin-5/Cut7 to generate an outward force. However, Klp9 acts in spindle elongation only during late mitosis irrespective of the presence or absence of Cut7, and furthermore, Klp9 cannot be substituted for Cut7 [64,90]. By contrast, in human cells a similar role appears to be executed by the N-kinesin Kinesin-12/KIF15/HKLP2, which does not exist in yeasts. It has been shown that Kinesin-12 functions redundantly with Kinesin-5 to promote spindle bipolarity [59,106,107,108], and curiously the overproduction of Kinesin-12 can drive bipolar spindle assembly even when Kinesin-5 activity is fully inhibited. This indicates that Kinesin-12 has the potential to execute all essential functions of Kinesin-5. Therefore, functions of fission yeast Kinesin-6/Klp9 and human Kinesin-12/KIF15/HKLP2 appear similar but are mechanistically different.
5. Force Generation in Human Prophase Cells
Human cells undergo centrosome separation through two temporally distinct pathways, the prophase pathway and the prometaphase pathway [109,110,111]. By contrast, yeasts have adopted only the prometaphase pathway. Recent analysis has uncovered key players acting in the prophase pathway and their individual roles [110]. Duplicated centrosomes in human cells remain closely linked during interphase, in a process called centrosome cohesion. Centrosome cohesion is maintained through dual mechanisms. The first mechanism depends upon a structural linker composed of two proteins, Rootletin and C-NAP1. This linker physically joins the two centrosomes in a side-by-side configuration. The second mechanism involves the Kinesin-14/KIFC3-mediated inward force. KIFC3 forms homotetramers and interconnects a special centrosome-associated microtubule network, thereby producing pulling forces towards the centrosomes. Upon mitotic entry, KIFC3 is inactivated by the NEK2 protein kinase, which also promotes the dissolution of the linker [110]. Antagonising outward forces are produced by Kineisn-5/KIF11 in both prophase and prometaphase pathways.
We contemplate that the differences between human beings and yeasts, in which human cells have developed more complex regulatory mechanisms, stem from different modes of mitosis; an open mitosis in higher eukaryotes vs. a closed mitosis in yeasts. As the nuclear membrane disassembles upon mitotic onset, human cells have acquired an additional regulatory process (the prophase pathway), which ensures the temporal order of bipolar spindle assembly to be synchronised with mitotic onset. Implementation of dual, redundant pathways might be also beneficial for the robustness of the system, disrupting one pathway would not result in a catastrophic impact on spindle formation and therefore the fidelity of chromosome segregation.
6. Force Generation in the Acentrosomal Cells
In higher eukaryotes and plants, the bipolar spindle is formed independent of the centrosome through the pathway referred to as the acentrosomal pathway [112,113,114]. Historically, the acentrosomal pathway is extensively characterised in vitro using extracts prepared from Xenopus oocytes, where the Ran GTPase acts as a master regulator [115,116]. This pathway is functional in vivo in several cell types which are naturally devoid of centrosomes (e.g., vertebrate oocytes and plants) or animal somatic cells which are experimentally (chemically, genetically or physically) manipulated to eliminate their centrosomes [117,118,119]. Interestingly, in this acentrosomal pathway, both in vitro and in vivo, Kinesin-5 also plays a major role in the formation of antiparallel microtubule bundles and the generation of an outward force. However, in contrast with the centrosome-dependent pathway, Kinesin-14 and Dynein appear to act collaboratively, rather than antagonistically, with Kinesin-5. It is reported that in the Xenopus oocyte and human acentrosomal cells, Kinesin-5 crosslinks the antiparallel microtubules while Kinesin-14 and/or Dynein are required for spindle pole focusing [118,120], two processes needed to establish spindle bipolarity. Therefore, the importance of the force balance for bipolar spindle formation and its underlying mechanism have not been addressed explicitly in the acentrosomal cells and await further investigation.
7. Towards Cancer Therapeutics
Kinesin-5 is required for successive cell division. Furthermore, in actively growing cancer cell lines, its activity is tightly, and sometimes causally, linked to tumour progression and malignancy [121]. Accordingly, this kinesin has been deemed to be an attractive target of cancer chemotherapeutics. Indeed, several Kinesin-5 inhibitors were developed and their clinical trials conducted [122]. However, drug-resistant cell lines often emerged which hampered the clinical usage of these inhibitors [123,124,125]. To tackle this conundrum, a comprehensive understanding of in vivo Kinesin-5 functions and regulations, in addition to structural information on the interaction between Kinesin-5 and specific inhibitors [122,126], are necessary. As Kinesin-12 is essential for the survival of HeLa cells that become resistant to Kinein-5 inhibitors, the development of specific Kinesin-12 inhibitors would be important [106,108,125]. Given that destabilisation and/or the reduced dynamics of the microtubule rescues the lethality derived from Kinesin-5 inactivation [25,59], the combined treatment of Kinesin-5 inhibitors and microtubule stabilising reagents would be worth consideration. In this context, treatment with Paclitaxel (Taxol), a microtubule-stabilising drug which on its own is widely used for chemotherapy (though the underlying mechanism of its anti-cancer activity remains to be resolved) [127,128,129] or suppressing Kinesin-13 microtubule depolymerases [60], might provide a more effective treatment for cancer therapeutics.
Acknowledgments
We thank all the laboratory members, past and present, and collaborators for their contributions to our work described in this review. We are grateful to Risa Mori for critical reading of the manuscript.
Abbreviations
| γ-TuC | γ-tubulin complex |
| MBC | methyl 2-benzimidazolecarbamate |
| MAPs | microtubule-associated proteins |
| SPB | spindle pole body |
| TBZ | thiabendazole |
| ts | temperature-sensitive |
Author Contributions
T.T. wrote the manuscript with input from M.Y. and Y.T. M.Y. and Y.T. performed experiments and analysed the data with T.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Japan Society for the Promotion of Science (JSPS) [KAKENHI Scientific Research (A) (16H02503 to T.T.) and Scientific Research (C) (19K05813 to M.Y.)].
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Mitchison T.J., Salmon E.D. Mitosis: A history of division. Nat. Cell Biol. 2001;3:E17–E21. doi: 10.1038/35050656. [DOI] [PubMed] [Google Scholar]
- 2.Woodruff J.B., Wueseke O., Hyman A.A. Pericentriolar material structure and dynamics. Philos. Trans. R. Soc. Lond B Biol. Sci. 2014;369:20130459. doi: 10.1098/rstb.2013.0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vale R.D., Reese T.S., Sheetz M.P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell. 1985;42:39–50. doi: 10.1016/S0092-8674(85)80099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirokawa N., Noda Y., Tanaka Y., Niwa S. Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 5.Wickstead B., Gull K., Richards T.A. Patterns of kinesin evolution reveal a complex ancestral eukaryote with a multifunctional cytoskeleton. BMC Evol. Biol. 2010;10:110. doi: 10.1186/1471-2148-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrence C.J., Dawe R.K., Christie K.R., Cleveland D.W., Dawson S.C., Endow S.A., Goldstein L.S., Goodson H.V., Hirokawa N., Howard J., et al. A standardized kinesin nomenclature. J. Cell Biol. 2004;167:19–22. doi: 10.1083/jcb.200408113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yount A.L., Zong H., Walczak C.E. Regulatory mechanisms that control mitotic kinesins. Exp. Cell Res. 2015;334:70–77. doi: 10.1016/j.yexcr.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enos A.P., Morris N.R. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell. 1990;60:1019–1027. doi: 10.1016/0092-8674(90)90350-N. [DOI] [PubMed] [Google Scholar]
- 9.Kashina A.S., Baskin R.J., Cole D.G., Wedaman K.P., Saxton W.M., Scholey J.M. A bipolar kinesin. Nature. 1996;379:270–272. doi: 10.1038/379270a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapitein L.C., Peterman E.J., Kwok B.H., Kim J.H., Kapoor T.M., Schmidt C.F. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435:114–118. doi: 10.1038/nature03503. [DOI] [PubMed] [Google Scholar]
- 11.Hagan I., Yanagida M. Kinesin-related cut7 protein associates with mitotic and meiotic spindles in fission yeast. Nature. 1992;356:74–76. doi: 10.1038/356074a0. [DOI] [PubMed] [Google Scholar]
- 12.Sawin K.E., LeGuellec K., Philippe M., Mitchison T.J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- 13.Blangy A., Lane H.A., d’Herin P., Harper M., Kress M., Nigg E.A. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995;83:1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 14.Hagan I., Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature. 1990;347:563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- 15.Heck M.M., Pereira A., Pesavento P., Yannoni Y., Spradling A.C., Goldstein L.S. The kinesin-like protein KLP61F is essential for mitosis in Drosophila. J. Cell Biol. 1993;123:665–679. doi: 10.1083/jcb.123.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Guellec R., Paris J., Couturier A., Roghi C., Philippe M. Cloning by differential screening of a Xenopus cDNA that encodes a kinesin-related protein. Mol. Cell Biol. 1991;11:3395–3398. doi: 10.1128/MCB.11.6.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapoor T.M., Mayer T.U., Coughlin M.L., Mitchison T.J. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 2000;150:975–988. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nahaboo W., Zouak M., Askjaer P., Delattre M. Chromatids segregate without centrosomes during Caenorhabditis elegans mitosis in a Ran- and CLASP-dependent manner. Mol. Biol. Cell. 2015;26:2020–2029. doi: 10.1091/mbc.E14-12-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mann B.J., Wadsworth P. Kinesin-5 regulation and function in mitosis. Trends Cell Biol. 2019;29:66–79. doi: 10.1016/j.tcb.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 20.O’Connell M.J., Meluh P.B., Rose M.D., Morris N.R. Suppression of the bimC4 mitotic spindle defect by deletion of klpA, a gene encoding a KAR3-related kinesin-like protein in Aspergillus nidulans. J. Cell Biol. 1993;120:153–162. doi: 10.1083/jcb.120.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders W.S., Hoyt M.A. Kinesin-related proteins required for structural integrity of the mitotic spindle. Cell. 1992;70:451–458. doi: 10.1016/0092-8674(92)90169-D. [DOI] [PubMed] [Google Scholar]
- 22.Hoyt M.A., He L., Totis L., Saunders W.S. Loss of function of Saccharomyces cerevisiae kinesin-related CIN8 and KIP1 is suppressed by KAR3 motor domain mutations. Genetics. 1993;135:35–44. doi: 10.1093/genetics/135.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B., Li K., Jin M., Qiu R., Liu B., Oakley B.R., Xiang X. The Aspergillus nidulans bimC4 mutation provides an excellent tool for identification of kinesin-14 inhibitors. Fungal Genet. Biol. 2015;82:51–55. doi: 10.1016/j.fgb.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.She Z.Y., Yang W.X. Molecular mechanisms of kinesin-14 motors in spindle assembly and chromosome segregation. J. Cell Sci. 2017;130:2097–2110. doi: 10.1242/jcs.200261. [DOI] [PubMed] [Google Scholar]
- 25.Yukawa M., Yamada Y., Toda T. Suppressor analysis uncovers that MAPs and microtubule dynamics balance with the Cut7/Kinesin-5 motor for mitotic spindle assembly in Schizosaccharomyces pombe. G3 (Bethesda) 2019;9:269–280. doi: 10.1534/g3.118.200896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braun M., Drummond D.R., Cross R.A., McAinsh A.D. The kinesin-14 Klp2 organizes microtubules into parallel bundles by an ATP-dependent sorting mechanism. Nat. Cell Biol. 2009;11:724–730. doi: 10.1038/ncb1878. [DOI] [PubMed] [Google Scholar]
- 27.Pidoux A.L., LeDizet M., Cande W.Z. Fission yeast pkl1 is a kinesin-related protein involved in mitotic spindle function. Mol. Biol. Cell. 1996;7:1639–1655. doi: 10.1091/mbc.7.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furuta K., Edamatsu M., Maeda Y., Toyoshima Y.Y. Diffusion and directed movement: In vitro motile properties of fission yeast kinesin-14 Pkl1. J. Biol. Chem. 2008;283:36465–36473. doi: 10.1074/jbc.M803730200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Troxell C.L., Sweezy M.A., West R.R., Reed K.D., Carson B.D., Pidoux A.L., Cande W.Z., McIntosh J.R. pkl1+ and klp2+: Two kinesins of the Kar3 subfamily in fission yeast perform different functions in both mitosis and meiosis. Mol. Biol. Cell. 2001;12:3476–3488. doi: 10.1091/mbc.12.11.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikebe C., Konishi M., Hirata D., Matsusaka T., Toda T. Systematic localization study on novel proteins encoded by meiotically up-regulated ORFs in fission yeast. Biosci. Biotechnol. Biochem. 2011;75:2364–2370. doi: 10.1271/bbb.110558. [DOI] [PubMed] [Google Scholar]
- 31.Toya M., Sato M., Haselmann U., Asakawa K., Brunner D., Antony C., Toda T. γ-Tubulin complex-mediated anchoring of spindle microtubules to spindle-pole bodies requires Msd1 in fission yeast. Nat. Cell Biol. 2007;9:646–653. doi: 10.1038/ncb1593. [DOI] [PubMed] [Google Scholar]
- 32.Yukawa M., Ikebe C., Toda T. The Msd1-Wdr8-Pkl1 complex anchors microtubule minus ends to fission yeast spindle pole bodies. J. Cell Biol. 2015;209:549–562. doi: 10.1083/jcb.201412111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yukawa M., Yamada Y., Yamauchi T., Toda T. Two spatially distinct kinesin-14 proteins, Pkl1 and Klp2, generate collaborative inward forces against kinesin-5 Cut7 in S. pombe. J. Cell Sci. 2018;131:jcs.210740. doi: 10.1242/jcs.210740. [DOI] [PubMed] [Google Scholar]
- 34.Mana-Capelli S., McLean J.R., Chen C.T., Gould K.L., McCollum D. The kinesin-14 Klp2 is negatively regulated by the SIN for proper spindle elongation and telophase nuclear positioning. Mol. Biol. Cell. 2012;23:4592–4600. doi: 10.1091/mbc.e12-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez A.S., Batac J., Killilea A.N., Filopei J., Simeonov D.R., Lin I., Paluh J.L. Protein complexes at the microtubule organizing center regulate bipolar spindle assembly. Cell Cycle. 2008;7:1246–1253. doi: 10.4161/cc.7.9.5808. [DOI] [PubMed] [Google Scholar]
- 36.Syrovatkina V., Tran P.T. Loss of kinesin-14 results in aneuploidy via kinesin-5-dependent microtubule protrusions leading to chromosome cut. Nat. Commun. 2015;6:7322. doi: 10.1038/ncomms8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olmsted Z.T., Colliver A.G., Riehlman T.D., Paluh J.L. Kinesin-14 and kinesin-5 antagonistically regulate microtubule nucleation by γ-TuRC in yeast and human cells. Nat. Commun. 2014;5:5339. doi: 10.1038/ncomms6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeda A., Saitoh S., Ohkura H., Sawin K.E., Goshima G. Identification of 15 new bypassable essential genes of fission yeast. Cell Struct. Funct. 2019;44:113–119. doi: 10.1247/csf.19025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winters L., Ban I., Prelogovic M., Kalinina I., Pavin N., Tolic I.M. Pivoting of microtubules driven by minus-end-directed motors leads to spindle assembly. BMC Biol. 2019;17:42. doi: 10.1186/s12915-019-0656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akera T., Goto Y., Sato M., Yamamoto M., Watanabe Y. Mad1 promotes chromosome congression by anchoring a kinesin motor to the kinetochore. Nat. Cell Biol. 2015;17:1124–1133. doi: 10.1038/ncb3219. [DOI] [PubMed] [Google Scholar]
- 41.Toda T., Adachi Y., Hiraoka Y., Yanagida M. Identification of the pleiotropic cell division cycle gene NDA2 as one of two different α-tubulin genes in Schizosaccharomyces pombe. Cell. 1984;37:233–242. doi: 10.1016/0092-8674(84)90319-2. [DOI] [PubMed] [Google Scholar]
- 42.Hiraoka Y., Toda T., Yanagida M. The NDA3 gene of fission yeast encodes β-tubulin: A cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984;39:349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- 43.Carvalho P., Tirnauer J.S., Pellman D. Surfing on microtubule ends. Trends Cell Biol. 2003;13:229–237. doi: 10.1016/S0962-8924(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 44.Beinhauer J.D., Hagan I.M., Hegemann J.H., Fleig U. Mal3, the fission yeast homologue of the human APC-interacting protein EB-1 is required for microtubule integrity and the maintenance of cell form. J. Cell Biol. 1997;139:717–728. doi: 10.1083/jcb.139.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asakawa K., Toya M., Sato M., Kanai M., Kume K., Goshima T., Garcia M.A., Hirata D., Toda T. Mal3, the fission yeast EB1 homologue, cooperates with Bub1 spindle checkpoint to prevent monopolar attachment. EMBO Rep. 2005;6:1194–1200. doi: 10.1038/sj.embor.7400540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita A., Vardy L., Garcia M.A., Toda T. A fourth component of the fission yeast γ-tubulin complex, Alp16, is required for cytoplasmic microtubule integrity and becomes indispensable when γ-tubulin function is compromised. Mol. Biol. Cell. 2002;13:2360–2373. doi: 10.1091/mbc.02-01-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anders A., Lourenco P.C., Sawin K.E. Noncore components of the fission yeast γ-tubulin complex. Mol. Biol. Cell. 2006;17:5075–5093. doi: 10.1091/mbc.e05-11-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masuda H., Toda T. Synergistic role of fission yeast Alp16GCP6 and Mzt1MOZART1 in γ-tubulin complex recruitment to mitotic spindle pole bodies and spindle assembly. Mol. Biol. Cell. 2016;27:1753–1763. doi: 10.1091/mbc.e15-08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato M., Vardy L., Angel Garcia M., Koonrugsa N., Toda T. Interdependency of fission yeast Alp14/TOG and coiled coil protein Alp7 in microtubule localization and bipolar spindle formation. Mol. Biol. Cell. 2004;15:1609–1622. doi: 10.1091/mbc.e03-11-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peset I., Vernos I. The TACC proteins: TACC-ling microtubule dynamics and centrosome function. Trends Cell Biol. 2008;18:379–388. doi: 10.1016/j.tcb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Nabeshima K., Kurooka H., Takeuchi M., Kinoshita K., Nakaseko Y., Yanagida M. p93dis1, which is required for sister chromatid separation, is a novel microtubule and spindle pole body-associating protein phosphorylated at the Cdc2 target sites. Genes Dev. 1995;9:1572–1585. doi: 10.1101/gad.9.13.1572. [DOI] [PubMed] [Google Scholar]
- 52.Garcia M.A., Vardy L., Koonrugsa N., Toda T. Fission yeast ch-TOG/XMAP215 homologue Alp14 connects mitotic spindles with the kinetochore and is a component of the Mad2-dependent spindle checkpoint. EMBO J. 2001;20:3389–3401. doi: 10.1093/emboj/20.13.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuo Y., Maurer S.P., Yukawa M., Zakian S., Singleton M.R., Surrey T., Toda T. An unconventional interaction between Dis1/TOG and Mal3/EB1 in fission yeast promotes the fidelity of chromosome segregation. J. Cell Sci. 2016;129:4592–4606. doi: 10.1242/jcs.197533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Bassam J., Kim H., Flor-Parra I., Lal N., Velji H., Chang F. Fission yeast Alp14 is a dose dependent plus end tracking microtubule polymerase. Mol. Biol. Cell. 2012;23:2878–2890. doi: 10.1091/mbc.e12-03-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hussmann F., Drummond D.R., Peet D.R., Martin D.S., Cross R.A. Alp7/TACC-Alp14/TOG generates long-lived, fast-growing MTs by an unconventional mechanism. Sci. Rep. 2016;6:20653. doi: 10.1038/srep20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winey M., Bloom K. Mitotic spindle form and function. Genetics. 2012;190:1197–1224. doi: 10.1534/genetics.111.128710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sato M., Toda T. Alp7/TACC is a crucial target in Ran-GTPase-dependent spindle formation in fission yeast. Nature. 2007;447:334–337. doi: 10.1038/nature05773. [DOI] [PubMed] [Google Scholar]
- 58.Flor-Parra I., Iglesias-Romero A.B., Chang F. The XMAP215 ortholog Alp14 promotes microtubule nucleation in fission yeast. Curr. Biol. 2018;28:1681–1691. doi: 10.1016/j.cub.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Florian S., Mayer T.U. Modulated microtubule dynamics enable Hklp2/Kif15 to assemble bipolar spindles. Cell Cycle. 2011;10:3533–3544. doi: 10.4161/cc.10.20.17817. [DOI] [PubMed] [Google Scholar]
- 60.Kollu S., Bakhoum S.F., Compton D.A. Interplay of microtubule dynamics and sliding during bipolar spindle formation in mammalian cells. Curr. Biol. 2009;19:2108–2113. doi: 10.1016/j.cub.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta D.R., Paul S.K., Oowatari Y., Matsuo Y., Kawamukai M. Multistep regulation of protein kinase A in its localization, phosphorylation and binding with a regulatory subunit in fission yeast. Curr. Genet. 2011;57:353–365. doi: 10.1007/s00294-011-0354-2. [DOI] [PubMed] [Google Scholar]
- 62.Hoffman C.S. Glucose sensing via the protein kinase A pathway in Schizosaccharomyces pombe. Biochem. Soc. Trans. 2005;33:257–260. doi: 10.1042/BST0330257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hanyu Y., Imai K.K., Kawasaki Y., Nakamura T., Nakaseko Y., Nagao K., Kokubu A., Ebe M., Fujisawa A., Hayashi T., et al. Schizosaccharomyces pombe cell division cycle under limited glucose requires Ssp1 kinase, the putative CaMKK, and Sds23, a PP2A-related phosphatase inhibitor. Genes Cells. 2009;14:539–554. doi: 10.1111/j.1365-2443.2009.01290.x. [DOI] [PubMed] [Google Scholar]
- 64.Rincon S.A., Lamson A., Blackwell R., Syrovatkina V., Fraisier V., Paoletti A., Betterton M.D., Tran P.T. Kinesin-5-independent mitotic spindle assembly requires the antiparallel microtubule crosslinker Ase1 in fission yeast. Nat. Commun. 2017;8:15286. doi: 10.1038/ncomms15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grallert A., Beuter C., Craven R.A., Bagley S., Wilks D., Fleig U., Hagan I.M. S. pombe CLASP needs dynein, not EB1 or CLIP170, to induce microtubule instability and slows polymerization rates at cell tips in a dynein-dependent manner. Genes Dev. 2006;20:2421–2436. doi: 10.1101/gad.381306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bratman S.V., Chang F. Stabilization of overlapping microtubules by fission yeast CLASP. Dev. Cell. 2007;13:812–827. doi: 10.1016/j.devcel.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelkar M., Martin S.G. PKA antagonizes CLASP-dependent microtubule stabilization to re-localize Pom1 and buffer cell size upon glucose limitation. Nat. Commun. 2015;6:8445. doi: 10.1038/ncomms9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin H., You L., Pasqualone D., Kopski K.M., Huffaker T.C. Stu1p is physically associated with beta-tubulin and is required for structural integrity of the mitotic spindle. Mol. Biol. Cell. 2002;13:1881–1892. doi: 10.1091/mbc.01-09-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pasqualone D., Huffaker T.C. STU1, a suppressor of a β-tubulin mutation, encodes a novel and essential component of the yeast mitotic spindle. J. Cell Biol. 1994;127:1973–1984. doi: 10.1083/jcb.127.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ebina H., Ji L., Sato M. CLASP promotes microtubule bundling in metaphase spindle independently of Ase1/PRC1 in fission yeast. Biol. Open. 2019;8:bio045716. doi: 10.1242/bio.045716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu C., Ward J.J., Loiodice I., Velve-Casquillas G., Nedelec F.J., Tran P.T. Phospho-regulated interaction between kinesin-6 Klp9p and microtubule bundler Ase1p promotes spindle elongation. Dev. Cell. 2009;17:257–267. doi: 10.1016/j.devcel.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yukawa M., Okazaki M., Teratani Y., Furuta K., Toda T. Kinesin-6 Klp9 plays motor-dependent and -independent roles in collaboration with Kinesin-5 Cut7 and the microtubule crosslinker Ase1 in fission yeast. Sci. Rep. 2019;9:7336. doi: 10.1038/s41598-019-43774-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi S.H., McCollum D. A role for metaphase spindle elongation forces in correction of merotelic kinetochore attachments. Curr. Biol. 2012;22:225–230. doi: 10.1016/j.cub.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meadows J.C., Lancaster T.C., Buttrick G.J., Sochaj A.M., Messin L.J., Del Mar Mora-Santos M., Hardwick K.G., Millar J.B. Identification of a Sgo2-dependent but Mad2-independent pathway controlling anaphase onset in fission yeast. Cell Rep. 2017;18:1422–1433. doi: 10.1016/j.celrep.2017.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kruger L.K., Sanchez J.L., Paoletti A., Tran P.T. Kinesin-6 regulates cell-size-dependent spindle elongation velocity to keep mitosis duration constant in fission yeast. eLife. 2019;8:e42182. doi: 10.7554/eLife.42182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yanagida M. Fission yeast cut mutations revisited: Control of anaphase. Trends Cell Biol. 1998;8:144–149. doi: 10.1016/S0962-8924(98)01236-7. [DOI] [PubMed] [Google Scholar]
- 77.Loiodice I., Staub J., Setty T.G., Nguyen N.P., Paoletti A., Tran P.T. Ase1p organizes antiparallel microtubule arrays during interphase and mitosis in fission yeast. Mol. Biol. Cell. 2005;16:1756–1768. doi: 10.1091/mbc.e04-10-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamashita A., Sato M., Fujita A., Yamamoto M., Toda T. The roles of fission yeast ase1 in mitotic cell division, meiotic nuclear oscillation, and cytokinesis checkpoint signaling. Mol. Biol. Cell. 2005;16:1378–1395. doi: 10.1091/mbc.e04-10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pellman D., Bagget M., Tu Y.H., Fink G.R., Tu H. Two microtubule-associated proteins required for anaphase spindle movement in Saccharomyces cerevisiae. J. Cell Biol. 1995;130:1373–1385. doi: 10.1083/jcb.130.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schuyler S.C., Liu J.Y., Pellman D. The molecular function of Ase1p: Evidence for a MAP-dependent midzone-specific spindle matrix. J. Cell Biol. 2003;160:517–528. doi: 10.1083/jcb.200210021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blackwell R., Edelmaier C., Sweezy-Schindler O., Lamson A., Gergely Z.R., O’Toole E., Crapo A., Hough L.E., McIntosh J.R., Glaser M.A., et al. Physical determinants of bipolar mitotic spindle assembly and stability in fission yeast. Sci. Adv. 2017;3:e1601603. doi: 10.1126/sciadv.1601603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edelmaier C., Lamson A.R., Gergely Z.R., Ansari S., Blackwell R., McIntosh J.R., Glaser M.A., Betterton M.D. Mechanisms of chromosome biorientation and bipolar spindle assembly analyzed by computational modeling. eLife. 2020;9:e48787. doi: 10.7554/eLife.48787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bishop J.D., Han Z., Schumacher J.M. The Caenorhabditis elegans Aurora B kinase AIR-2 phosphorylates and is required for the localization of a BimC kinesin to meiotic and mitotic spindles. Mol. Biol. Cell. 2005;16:742–756. doi: 10.1091/mbc.e04-08-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saunders A.M., Powers J., Strome S., Saxton W.M. Kinesin-5 acts as a brake in anaphase spindle elongation. Curr. Biol. 2007;17:R453–R454. doi: 10.1016/j.cub.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yukawa M., Kawakami T., Pinder C., Toda T. Two XMAP215/TOG microtubule polymerases, Alp14 and Dis1, play non-exchangeable, distinct roles in microtubule organisation in fission yeast. Int. J. Mol. Sci. 2019;20:5108. doi: 10.3390/ijms20205108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Al-Bassam J., Chang F. Regulation of microtubule dynamics by TOG-domain proteins XMAP215/Dis1 and CLASP. Trends Cell Biol. 2011;21:604–614. doi: 10.1016/j.tcb.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng F., Li T., Jin D.Y., Syrovatkina V., Scheffler K., Tran P.T., Fu C. Csi1p recruits alp7p/TACC to the spindle pole bodies for bipolar spindle formation. Mol. Biol. Cell. 2014;25:2750–2760. doi: 10.1091/mbc.e14-03-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Costa J., Fu C., Khare V.M., Tran P.T. csi2p modulates microtubule dynamics and organizes the bipolar spindle for chromosome segregation. Mol. Biol. Cell. 2014;25:3900–3908. doi: 10.1091/mbc.e14-09-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hou H., Zhou Z., Wang Y., Wang J., Kallgren S.P., Kurchuk T., Miller E.A., Chang F., Jia S. Csi1 links centromeres to the nuclear envelope for centromere clustering. J. Cell Biol. 2012;199:735–744. doi: 10.1083/jcb.201208001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yukawa M., Kawakami T., Okazaki M., Kume K., Tang N.H., Toda T. A microtubule polymerase cooperates with the kinesin-6 motor and a microtubule cross-linker to promote bipolar spindle assembly in the absence of kinesin-5 and kinesin-14 in fission yeast. Mol. Biol. Cell. 2017;28:3647–3659. doi: 10.1091/mbc.e17-08-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tran P.T., Marsh L., Doye V., Inoue S., Chang F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J. Cell Biol. 2001;153:397–412. doi: 10.1083/jcb.153.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Daga R.R., Yonetani A., Chang F. Asymmetric microtubule pushing forces in nuclear centering. Curr. Biol. 2006;16:1544–1550. doi: 10.1016/j.cub.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 93.Bao X.X., Spanos C., Kojidani T., Lynch E.M., Rappsilber J., Hiraoka Y., Haraguchi T., Sawin K.E. Exportin Crm1 is repurposed as a docking protein to generate microtubule organizing centers at the nuclear pore. eLife. 2018;7:e33465. doi: 10.7554/eLife.33465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu W., Zheng F., Wang Y., Fu C. Alp7-Mto1 and Alp14 synergize to promote interphase microtubule regrowth from the nuclear envelope. J. Mol. Cell Biol. 2019;11:944–955. doi: 10.1093/jmcb/mjz038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu H.Y., Nazockdast E., Shelley M.J., Needleman D.J. Forces positioning the mitotic spindle: Theories, and now experiments. Bioessays. 2017;39:1600212. doi: 10.1002/bies.201600212. [DOI] [PubMed] [Google Scholar]
- 96.Garzon-Coral C., Fantana H.A., Howard J. A force-generating machinery maintains the spindle at the cell center during mitosis. Science. 2016;352:1124–1127. doi: 10.1126/science.aad9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shirasugi Y., Sato M. Kinetochore-mediated outward force promotes spindle pole separation in fission yeast. Mol. Biol. Cell. 2019;30:2802–2813. doi: 10.1091/mbc.E19-07-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hara M., Fukagawa T. Dynamics of kinetochore structure and its regulations during mitotic progression. Cell Mol. Life Sci. 2020;77:s00018-020-03472-4. doi: 10.1007/s00018-020-03472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nabetani A., Koujin T., Tsutsumi C., Haraguchi T., Hiraoka Y. A conserved protein, Nuf2, is implicated in connecting the centromere to the spindle during chromosome segregation: A link between the kinetochore function and the spindle checkpoint. Chromosoma. 2001;110:322–334. doi: 10.1007/s004120100153. [DOI] [PubMed] [Google Scholar]
- 100.Bernard P., Maure J.F., Partridge J.F., Genier S., Javerzat J.P., Allshire R.C. Requirement of heterochromatin for cohesion at centromeres. Science. 2001;294:2539–2542. doi: 10.1126/science.1064027. [DOI] [PubMed] [Google Scholar]
- 101.Nonaka N., Kitajima T., Yokobayashi S., Xiao G., Yamamoto M., Grewal S.I., Watanabe Y. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 2002;4:89–93. doi: 10.1038/ncb739. [DOI] [PubMed] [Google Scholar]
- 102.Michaelis C., Ciosk R., Nasmyth K. Cohesins: Chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/S0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- 103.Tatebayashi K., Kato J., Ikeda H. Isolation of a Schizosaccharomyces pombe rad21ts mutant that is aberrant in chromosome segregation, microtubule function, DNA repair and sensitive to hydroxyurea: Possible involvement of Rad21 in ubiquitin-mediated proteolysis. Genetics. 1998;148:49–57. doi: 10.1093/genetics/148.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Toso A., Winter J.R., Garrod A.J., Amaro A.C., Meraldi P., McAinsh A.D. Kinetochore-generated pushing forces separate centrosomes during bipolar spindle assembly. J. Cell Biol. 2009;184:365–372. doi: 10.1083/jcb.200809055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gayek A.S., Ohi R. Kinetochore-microtubule stability governs the metaphase requirement for Eg5. Mol. Biol. Cell. 2014;25:2051–2060. doi: 10.1091/mbc.e14-03-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tanenbaum M.E., Macurek L., Janssen A., Geers E.F., Alvarez-Fernandez M., Medema R.H. Kif15 cooperates with eg5 to promote bipolar spindle assembly. Curr. Biol. 2009;19:1703–1711. doi: 10.1016/j.cub.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 107.Vanneste D., Takagi M., Imamoto N., Vernos I. The role of Hklp2 in the stabilization and maintenance of spindle bipolarity. Curr. Biol. 2009;19:1712–1717. doi: 10.1016/j.cub.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 108.Sturgill E.G., Norris S.R., Guo Y., Ohi R. Kinesin-5 inhibitor resistance is driven by kinesin-12. J. Cell Biol. 2016;213:213–227. doi: 10.1083/jcb.201507036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaseda K., McAinsh A.D., Cross R.A. Dual pathway spindle assembly increases both the speed and the fidelity of mitosis. Biol. Open. 2012;1:12–18. doi: 10.1242/bio.2011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hata S., Pastor Peidro A., Panic M., Liu P., Atorino E., Funaya C., Jakle U., Pereira G., Schiebel E. The balance between KIFC3 and EG5 tetrameric kinesins controls the onset of mitotic spindle assembly. Nat. Cell Biol. 2019;21:1138–1151. doi: 10.1038/s41556-019-0382-6. [DOI] [PubMed] [Google Scholar]
- 111.Tanenbaum M.E., Medema R.H. Mechanisms of centrosome separation and bipolar spindle assembly. Dev. Cell. 2010;19:797–806. doi: 10.1016/j.devcel.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 112.Hashimoto T. A ring for all: γ-tubulin-containing nucleation complexes in acentrosomal plant microtubule arrays. Curr. Opin. Plant. Biol. 2013;16:698–703. doi: 10.1016/j.pbi.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 113.Meunier S., Vernos I. Acentrosomal microtubule assembly in mitosis: The where, when, and now. Trends Cell Biol. 2016;26:80–87. doi: 10.1016/j.tcb.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 114.Takeda Y., Kuroki K., Chinen T., Kitagawa D. Centrosomal and non-centrosomal functions emerged through eliminating centrosomes. Cell Struct Funct. 2020;45:csf.20007. doi: 10.1247/csf.20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gruss O.J. Animal female meiosis: The challenges of eliminating centrosomes. Cells. 2018;7:73. doi: 10.3390/cells7070073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karsenti E., Vernos I. The mitotic spindle: A self-made machine. Science. 2001;294:543–547. doi: 10.1126/science.1063488. [DOI] [PubMed] [Google Scholar]
- 117.Khodjakov A., Cole R.W., Oakley B.R., Rieder C.L. Centrosome-independent mitotic spindle formation in vertebrates. Curr. Biol. 2000;10:59–67. doi: 10.1016/S0960-9822(99)00276-6. [DOI] [PubMed] [Google Scholar]
- 118.Chinen T., Yamamoto S., Takeda Y., Watanabe K., Kuroki K., Hashimoto K., Takao D., Kitagawa D. NuMA assemblies organize microtubule asters to establish spindle bipolarity in acentrosomal human cells. EMBO J. 2020;39:e102378. doi: 10.15252/embj.2019102378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Basto R., Lau J., Vinogradova T., Gardiol A., Woods C.G., Khodjakov A., Raff J.W. Flies without centrioles. Cell. 2006;125:1375–1386. doi: 10.1016/j.cell.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 120.Walczak C.E., Vernos I., Mitchison T.J., Karsenti E., Heald R. A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr. Biol. 1998;8:903–913. doi: 10.1016/S0960-9822(07)00370-3. [DOI] [PubMed] [Google Scholar]
- 121.Shu S., Iimori M., Wakasa T., Ando K., Saeki H., Oda Y., Oki E., Maehara Y. The balance of forces generated by kinesins controls spindle polarity and chromosomal heterogeneity in tetraploid cells. J. Cell Sci. 2019;132:jcs.231530. doi: 10.1242/jcs.231530. [DOI] [PubMed] [Google Scholar]
- 122.El-Nassan H.B. Advances in the discovery of kinesin spindle protein (Eg5) inhibitors as antitumor agents. Eur. J. Med. Chem. 2013;62:614–631. doi: 10.1016/j.ejmech.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 123.Wacker S.A., Houghtaling B.R., Elemento O., Kapoor T.M. Using transcriptome sequencing to identify mechanisms of drug action and resistance. Nat. Chem. Biol. 2012;8:235–237. doi: 10.1038/nchembio.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ma H.T., Erdal S., Huang S., Poon R.Y. Synergism between inhibitors of Aurora A and KIF11 overcomes KIF15-dependent drug resistance. Mol. Oncol. 2014;8:1404–1418. doi: 10.1016/j.molonc.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dumas M.E., Sturgill E.G., Ohi R. Resistance is not futile: Surviving Eg5 inhibition. Cell Cycle. 2016;15:2845–2847. doi: 10.1080/15384101.2016.1204864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pena A., Sweeney A., Cook A.D., Topf M., Moores C.A. Structure of microtubule-trapped human kinesin-5 and its mechanism of inhibition revealed using cryoelectron microscopy. Structure. 2020;28:1–8. doi: 10.1016/j.str.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huszar D., Theoclitou M.E., Skolnik J., Herbst R. Kinesin motor proteins as targets for cancer therapy. Cancer Metastasis Rev. 2009;28:197–208. doi: 10.1007/s10555-009-9185-8. [DOI] [PubMed] [Google Scholar]
- 128.Mitchison T.J. The proliferation rate paradox in antimitotic chemotherapy. Mol. Biol. Cell. 2012;23:1–6. doi: 10.1091/mbc.e10-04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mitchison T.J., Pineda J., Shi J., Florian S. Is inflammatory micronucleation the key to a successful anti-mitotic cancer drug? Open Biol. 2017;7:170182. doi: 10.1098/rsob.170182. [DOI] [PMC free article] [PubMed] [Google Scholar]