Abstract
Ovarian cancer is not a single disease and can be subdivided into at least five different histological subtypes that have different identifiable risk factors, cells of origin, molecular compositions, clinical features and treatments. Ovarian cancer is a global problem, is typically diagnosed at a late stage and has no effective screening strategy. Standard treatments for newly diagnosed cancer consist of cytoreductive surgery and platinum-based chemotherapy. In recurrent cancer, chemotherapy, anti-angiogenic agents and poly(ADP-ribose) polymerase inhibitors are used, and immunological therapies are currently being tested. High-grade serous carcinoma (HGSC) is the most commonly diagnosed form of ovarian cancer and at diagnosis is typically very responsive to platinum-based chemotherapy. However, in addition to the other histologies, HGSCs frequently relapse and become increasingly resistant to chemotherapy. Consequently, understanding the mechanisms underlying platinum resistance and finding ways to overcome them are active areas of study in ovarian cancer. Substantial progress has been made in identifying genes that are associated with a high risk of ovarian cancer (such as BRCA1 and BRCA2), as well as a precursor lesion of HGSC called serous tubal intraepithelial carcinoma, which holds promise for identifying individuals at high risk of developing the disease and for developing prevention strategies.
Although once considered a single entity, ovarian cancer can be subdivided into different histological subtypes that have different identifiable risk factors, cells of origin, molecular compositions, clinical features and treatments. These histological subtypes include epithelial cancers that account for ~90% of ovarian cancers and include serous, endometrioid, clear-cell and mucinous carcinomas (FIG. 1; TABLE 1). Of these types, high-grade serous carcinoma (HGSC) is the most commonly diagnosed. Histologically and clinically, low-grade endometrioid carcinoma and low-grade serous carcinoma (LGSC) are different compared with their high-grade counterparts; HGSC is similar to high-grade endometrioid carcinoma1–3. Other rarer histologies include small-cell carcinoma (an aggressive cancer that predominantly occurs in younger women, with a median age at diagnosis of 25 years), which has an uncertain tissue origin, and carcinosarcoma (also an aggressive cancer)4,5. Nonepithelial ovarian cancers, including germ-cell tumours and sex cord stromal tumours, which account for ~10% of ovarian cancers, are not discussed in this Primer.
Figure 1 |. Histological subtypes of ovarian cancer.
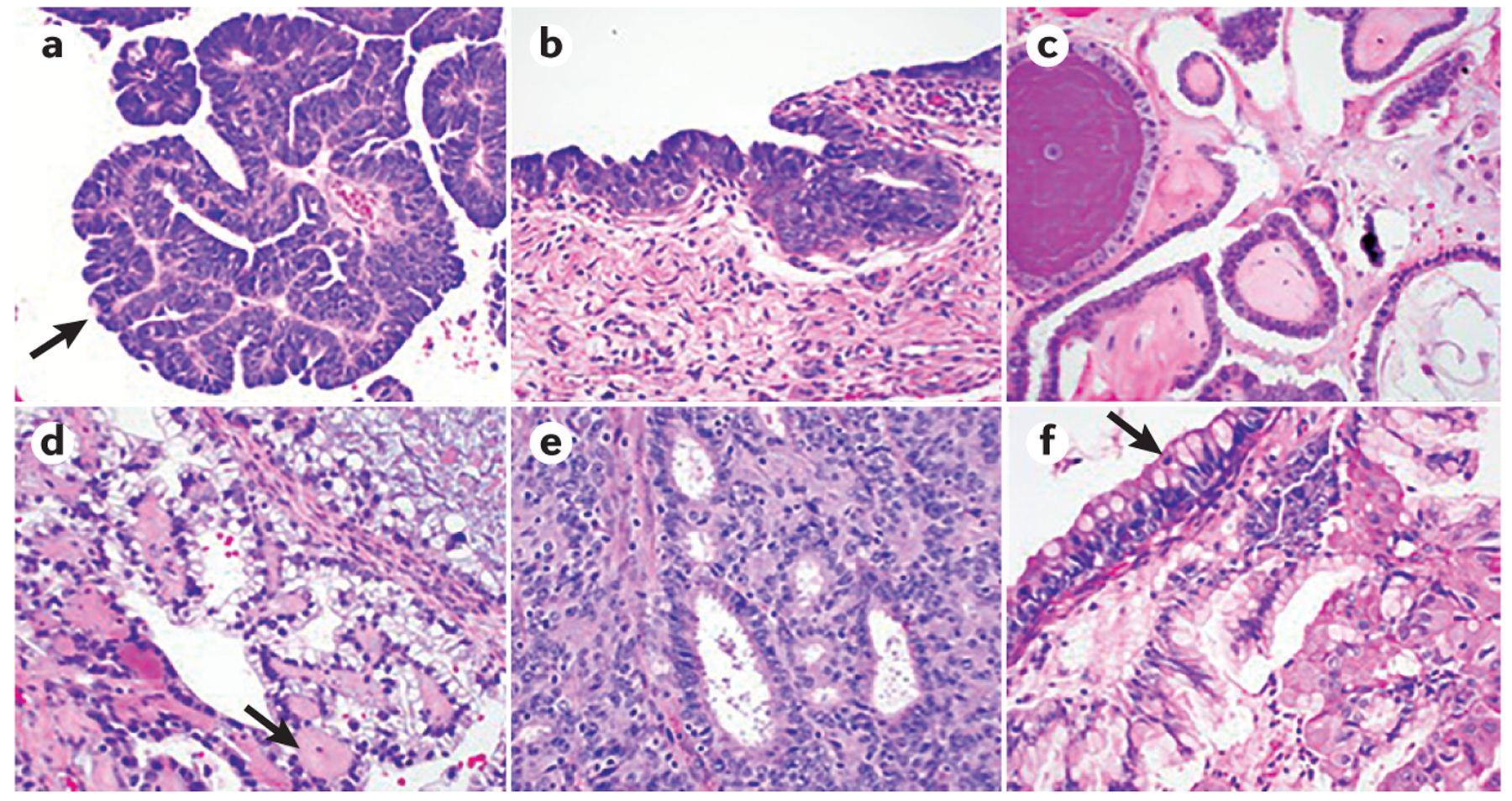
a | High-grade serous carcinoma (HGSC) is characterized by severe nuclear atypia, high nuclear-to-cytoplasmic ratio and abundant mitoses. Papillary architecture (arrow) is also often present. b | Serous tubal intraepithelial carcinoma (STIC) lesions share the same morphological features as HGSC, with severe atypia, mitoses and lack of polarity. STIC lesions are thought to be precursors for HGSC. c | Low-grade serous carcinoma (LGSC) shows papillary architecture, but only mild nuclear atypia and a lower nuclear-to-cytoplasmic ratio. d | Clear-cell carcinoma is characterized by large atypical tumour cells with frequent clearing of the cytoplasm and stromal hyalinization (arrow). e | Endometrioid adenocarcinoma is characterized by gland formation that recapitulates endometrial glands and is graded based on cellular architecture and nuclear atypia. f | Mucinous adenocarcinoma shows mucin-filled tumour cells, with frequent goblet cell forms present (arrow).
Table 1 |.
Characteristics of ovarian cancer by histology, genomic characteristics and active therapies
| Histological subtype | Clinical findings | Genetic characteristics | Treatment options |
|---|---|---|---|
| High-grade serous carcinoma and high-grade endometrioid carcinoma |
|
|
|
| Low-grade serous carcinoma |
|
|
|
| Low-grade endometrioid carcinoma |
|
|
|
| Clear-cell carcinoma |
|
|
|
| Mucinous carcinoma |
|
|
|
Some ovarian cancers originate from sites outside of the ovary; for example, many ovarian HGSCs probably originate in the fallopian tube6 and some subsets of ovarian cancer have been shown to arise from the peritoneum7. In addition, clear-cell and endometrioid carcinomas can originate from endometrial tissue located outside the uterus (endometriosis). On the basis of the new WHO classification, most of these types of ovarian cancer will now be reclassified as ‘ovarian or tubal cancers’ (REF. 8). Indeed, information about precursor sites of ovarian cancer has enabled the investigation of new primary prevention strategies, such as risk-reducing and opportunistic salpingectomy (surgical removal of the fallopian tube)6. This increased understanding of the biology underlying ovarian cancer has also translated to changes in clinical research; clinical trials are now increasingly focusing eligibility requirements on the basis of ovarian cancer histology.
Effective screening strategies for the early detection of ovarian cancer do not exist, but individuals at high risk of developing ovarian cancer, such as those with germline mutations in BRCA1 or BRCA2 (which encode proteins involved in the repair of DNA damage via homologous recombination) or other genes associated with a high risk of developing ovarian cancer can be identified. For these individuals, strategies to reduce the risk of ovarian cancer have been implemented through risk-reducing surgery, such as bilateral salpingo-oophorectomy (removal of the ovaries and the fallopian tubes). Screening strategies in women with an average risk of developing ovarian cancer have primarily focused on the biomarker CA125 (also known as mucin 16) and the use of transvaginal ultrasonography. Combinations of these screening modalities have shown success in detecting early-stage cancers, but have not yet demonstrated definitive improvements in patient mortality9,10.
The most active therapeutic agents against newly diagnosed ovarian cancer are platinum analogues (either cisplatin or carboplatin), with the addition of a taxane (either paclitaxel or docetaxel)11–15. Treatment paradigms for first-line management of newly diagnosed ovarian cancer include either primary surgical cytoreduction (to debulk tumours) followed by combination platinum-based chemotherapy or neoadjuvant chemotherapy (NACT; the administration of chemotherapy before surgery) followed by interval surgical cytoreduction and additional chemotherapy after surgery. Recurrence of cancer after initial platinum-based chemotherapy is very common for women diagnosed with advanced-stage cancer; the most difficult issue in the treatment of cancer in these women is the eventual development of platinum resistance. Advances in new therapeutics for recurrent ovarian cancer treatment include angiogenesis inhibitors, poly(ADP-ribose) polymerase (PARP) inhibitors (which block the repair of DNA damage) and immunotherapy agents. Strategies using PARP inhibitors as part of the first-line treatment, as well as combinations of these therapies for the treatment of both newly diagnosed and recurrent ovarian cancer, are underway. Overall, the treatment of ovarian cancer based on the distinct genomic make-up of the individual histological subtypes of ovarian cancer is evolving. This Primer reviews the epidemiology and known risk factors associated with epithelial ovarian cancer, in addition to tumour molecular biology, diagnostic and prevention approaches and management of both newly diagnosed and recurrent cancer. This Primer also discusses patient quality of life and concludes with the examination of the future outlook for ovarian cancer, including new prevention and screening approaches and promising new therapeutic advances.
Epidemiology
Incidence and mortality
Globally, 225,500 new cases of ovarian cancer are diagnosed each year, with 140,200 cancer-specific deaths16–18. Incidence and survival rates vary by country; Russia and the United Kingdom have the highest rates of ovarian cancer, whereas China has the lowest rates19,20. In the United States, approximately 22,280 new cases occur annually and the projected number of deaths for 2016 is 14,240 (REF. 16). Interestingly, the annual incidence of ovarian cancer reduced by 1.09% for women <65 years of age and by 0.95% for women ≥65 years of age between 1998 and 2008 (REF. 21), which might have been influenced by the changing pattern of hormonal therapy prescriptions; reduced risk of ovarian cancer coincided with the announcement of causal association between ovarian cancer and the use of hormone replacement therapy and, as such, fewer prescriptions were written21.
Over the past decade, minimal improvement in mortality has been observed17,18. The US Surveillance, Epidemiology, and End Results database reports that overall survival for all patients with ovarian cancer is 45.6%, but this varies greatly based on stage at initial diagnosis22; 5-year overall survival in patients with stage I cancer is 92.1% but is 25% for patients with stage III and stage IV cancer16,22.
Risk factors
Several factors can increase the risk of developing ovarian cancer, including genetic factors, age, postmenopausal hormonal therapy use, infertility and nulliparity.
Genetics.
A range of genetic factors are associated with an increased risk of developing ovarian cancer (TABLE 2). Germline BRCA1 and BRCA2 mutations are the most significant known genetic risk factors for ovarian cancer and either mutation is found in up to 17% of patients23,24. Moreover, mutations in BRCA increase the risk of other cancers — such as breast cancer (BRCA1 and BRCA2), pancreatic cancer (BRCA2), prostate cancer (BRCA2), melanoma (BRCA2) and, possibly, serous endometrial cancer (BRCA1) — and inheritance of these genes has been extensively studied25–27. Most subtypes of epithelial ovarian cancer are associated with germline BRCA mutations, but HGSCs are the most common25,26 and mucinous subtypes are rarely associated. Survival is improved for women with ovarian cancer carrying germline BRCA mutations compared with women who have ovarian cancer but are wild type for BRCA1 and BRCA2 (REF. 27). Germline BRCA2 mutations are associated with increased overall survival compared with germline BRCA1 mutations, probably because BRCA2 results in enhanced platinum sensitivity and thus greater killing of cancer cells than BRCA1 (REFS 27,28). Both the location of the BRCA mutation within the gene and the type of mutation might also influence the risk of developing ovarian cancer; the risk of developing breast cancer or ovarian cancer, as well as the median age at diagnosis, can vary according to the mutation type, the nucleotide position and the functional consequence of the mutation in patients with germline BRCA1 or BRCA2 mutations29. Besides BRCA1 and BRCA2, other germline mutations in genes involved in DNA repair can increase the risk of developing ovarian cancer, including genes that are part of the Fanconi anaemia–BRCA pathway, such as RAD51C, RAD51D, BRIP1, BARD1 and PALB2 (REFS 26,30–33) (TABLE 2). Inherited mutations in other genes involved in DNA repair, such as CHEK2, MRE11A, RAD50, ATM and TP53, might also increase the risk of developing ovarian cancer26,30,31.
Table 2 |.
Functions of commonly mutated inherited genes associated with increased risk of ovarian cancer*
| Gene | Protein | Protein function |
|---|---|---|
| BRCA1 | Breast cancer type 1 susceptibility protein |
|
| BRCA2 | Breast cancer type 2 susceptibility protein | |
| BARD1 | BRCA1-associated RING domain protein 1 |
|
| BRIP1 | BRCA1-interacting protein 1 (also known as Fanconi anaemia group J protein) |
|
| PALB2 | Partner and localizer of BRCA2 |
|
| RAD51C | DNA repair protein RAD51 homologue 3 |
|
| RAD51D | DNA repair protein RAD51 homologue 4 | |
| MSH2 | MutS protein homologue 2 |
|
| MLH1 | MutL protein homologue 1 | |
| MSH6 | MutS protein homologue 6 | |
| PMS2 | Mismatch repair endonuclease PMS2 |
Other inherited disorders, such as Lynch syndrome, can increase the risk of ovarian cancer. Lynch syndrome is associated with colorectal, endometrial and ovarian cancers, but can also be associated with cancers of the urinary tract, stomach, small intestine and biliary tract. The syndrome is characterized by inheritance of a germline mutation in genes of the DNA mismatch repair system — namely, MLH1, PMS2, MSH2 or MSH6, which are mutated at different frequencies34–36. Patients with Lynch syndrome-associated ovarian cancer have a mean age at presentation of 48 years (compared with a median age of ~68 years in those without Lynch syndrome), with ~50% of patients having stage I cancer. In addition, endometrioid and clear-cell carcinomas are more common in patients with Lynch syndrome than would be predicted for sporadic ovarian cancer34. Even though both the BRCA and the DNA mismatch repair pathways are involved in DNA repair, the specific mechanisms that underlie why cancers arise in specific organs associated with these inherited mutated genes are unknown.
Oral contraceptives and hormone replacement therapy.
The use of oral contraceptives has been shown to reduce the risk of developing ovarian cancer in individuals with a germline BRCA1 mutation, as well as in those without a genetic predisposition37,38. One meta-analysis showed a lifetime reduction of 0.54% for ovarian cancer with the use of oral contraceptives for an average of 5 years38,39. Interestingly, an analysis from the Ovarian Cancer Cohort Consortium (including data from 21 studies encompassing 1.3 million women and 5,584 ovarian cancers) showed that oral contraceptive use was associated with reduction in serous, endometrioid and clear-cell carcinomas, but not mucinous carcinomas40. The relative oestrogen and progestin doses in oral contraceptives does not affect the incidence of ovarian cancer, but longer duration of oral contraceptive use is associated with reduced risk41. However, other meta-analyses have found insufficient evidence to recommend either for or against the use of oral contraceptives to prevent ovarian cancer, given their potential harm from adverse vascular events and minimal increase in other cancers (such as breast cancer) weighed against the potential for ovarian cancer risk reduction41.
Hormone replacement therapy has been shown to increase the risk of developing ovarian cancer in postmenopausal women; oestrogen-only therapy increased risk by 22% and the combined oestrogen and progesterone therapy increased risk by 10%42–44. However, a meta-analysis showed a similar increase in the risk of developing ovarian cancer, specifically, serous and endometrioid carcinomas, in menopausal women using hormone replacement therapy, regardless of whether the therapy contained oestrogen only or a combination of oestrogen and progesterone45. Others have confirmed this finding but have also shown a reduced risk of clear-cell cancer in women using hormone replacement therapy40. Interestingly, in women diagnosed with ovarian cancer and who also have severe menopausal symptoms, the use of hormone replacement therapy seems to be safe and has no effect on overall survival46. Thus, the use of hormone replacement therapy can be considered if patients are having profound menopausal symptoms46.
Reproductive factors.
Retrospective studies have identified several other factors that can influence the risk of ovarian cancer, such as parity, prior tubal ligation, salpingectomy and unilateral or bilateral oophorectomy (surgical removal of the ovary)47–49. Women who have given birth have a reduced risk of all subtypes of ovarian cancer compared with women who have not given birth, with the strongest risk reduction noted for clear-cell carcinomas. Unilateral oophorectomy is associated with a 30% reduction in the risk of ovarian cancer, which is not specific to the histological subtype. Bilateral oophorectomy is also effective in reducing the risk of ovarian cancer in women with a genetic predisposition. Interestingly, no women with a BRCA2 mutation and 1.1% with a BRCA1 mutation developed a primary peritoneal carcinoma following bilateral oophorectomy48,50. Tubal ligation and hysterectomy are also associated with a reduction in the risk of developing ovarian cancer; tubal ligation is associated with reduction in the risk of clear-cell and endometrioid carcinomas and hysterectomy is associated with reduction in the risk of clear-cell carcinoma40,47–49. In one study, reproductive risk factors, such as tubal ligation, parity of ≥2, endometriosis and younger age, were more strongly associated with the development of dominant ovarian tumours (that is, one ovarian tumour is at least twice as large as the tumour on the other ovary) than with non-dominant cancers, which are thought to arise in the fallopian tube and are mostly HGSCs51. In addition, endometriosis has been associated with endometrioid and clear-cell ovarian cancer, as well as low-grade cancers40. In women with germline BRCA mutations, tubal ligation and breastfeeding have similarly been identified as risk factors associated with a decreased risk of ovarian cancer47.
Additional factors.
Several studies have identified obesity as a possible risk factor for the development of postmenopausal ovarian cancer; one meta-analysis showed an ~13% increase in the risk of ovarian cancer in postmenopausal women with a 5 kg weight gain who did not use, or had low use of, hormone replacement therapy52. Moreover, obesity is associated with an increased risk of endometrioid and mucinous carcinomas, but not HGSCs53. However, conflicting data have been reported in other studies40. Obesity is also a risk for poor outcomes following diagnosis of ovarian cancer; women with obesity and LGSC, HGSC or endometrioid carcinoma have a worse outcome than non-obese women54. Meta-analyses have suggested a beneficial effect of regular physical activity on the risk of ovarian cancer, with a 30–60% reduction in risk in the most active women55.
Several studies have examined the association between dietary factors and the risk of developing ovarian cancer in the general population. Levels of milk consumption do not confer a significant risk of developing ovarian cancer, but one study has noted a trend that indicates an inverse association between the intake of skimmed milk and lactose in adulthood and risk of developing ovarian cancer56. Moreover, this study showed an inverse relationship between lactose intake and the risk of endometrioid carcinoma56. Studies have also assessed the association between other dietary factors, including vitamins and flavonoids and the risk of ovarian cancer. The intake of folate or vitamin A, vitamin C or vitamin E during adulthood, or intake of a specific diet (defined by dietary scores), does not alter the risk of ovarian cancer57,58. Interestingly, flavonoids and black tea might be associated with a reduced risk of ovarian cancer, but these require further study59.
Other lifestyle factors that might affect the risk of ovarian cancer include the use of talc powder (reviewed in REF. 60), medications such as NSAIDS and smoking. With respect to talc powder, results from case–control and prospective studies have been variable; one study has shown a modest increase in the risk of ovarian cancer, but other studies have shown no increase in risk with talc use61,62. Aspirin use was associated with a reduced risk of developing ovarian cancer, especially among women who took daily, low-dose aspirin, regardless of their age; the same associations were not shown for acetaminophen63. Regular aspirin use was associated with reduced risk of endometrioid and mucinous carcinomas and a significant reduction in the risk of serous carcinomas. However, no prospective trials testing aspirin for ovarian cancer risk reduction have been conducted. Non-aspirin NSAID use was associated with a trend that indicates a lower risk of ovarian cancer63, specifically, of serous carcinomas. Cigarette smoking was associated with a significantly lower risk of clear-cell carcinoma but an increased risk of mucinous carcinoma40.
Finally, data from the Nurse’s Health Study indicate that persistent depression — defined as meeting the definition of depression based on current and past questionnaires — might increase the risk of ovarian cancer compared with women who do not exhibit depressive symptoms64.
Mechanisms/pathophysiology
The Cancer Genome Atlas project, along with other projects that catalogue genetic mutations associated with cancer have produced important molecular data on the different histological subtypes of epithelial ovarian cancer65–67. These data, in turn, open the pathway to improved therapeutic, early detection and risk-reducing strategies. The recognition that ovarian cancer consists of histologically and molecularly distinct subtypes has influenced clinical trial design strategies and patient eligibility and has led to rational clinical management68,69 (TABLE 1).
Molecular alterations
The best-studied genetic alterations in ovarian cancers are those involved in DNA repair (FIG. 2). Germline or somatic mutations in homologous recombination genes have been identified in approximately one-third of ovarian carcinomas, including both serous and non-serous histologies, and subtypes that were not previously believed to have characteristics of homologous recombination deficiency (clear-cell and endometrioid carcinomas, as well as carcinosarcoma). As mentioned previously, the commonly implicated inherited genes are BRCA1, BRCA2 and BRIP1, genes that are part of the Fanconi anaemia pathway (RAD51C, RAD51D, BRIP1, PALB2 and BARD1) and genes that are involved in DNA mismatch repair (MSH2, MSH6, MLH1 and PMS2).
Figure 2 |. DNA repair mechanisms and ovarian cancer.
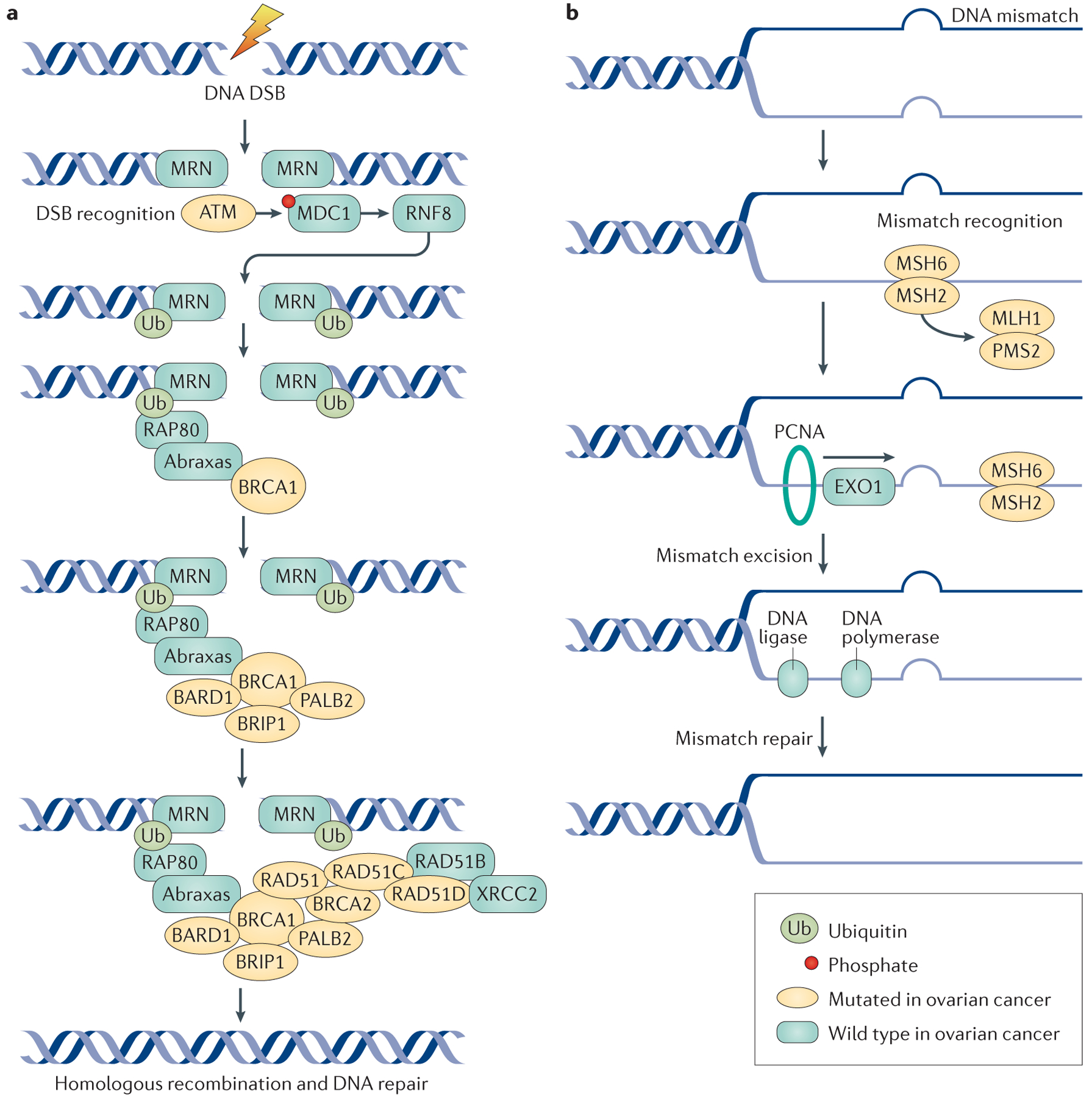
a | The double-stranded DNA break and homologous repair process begins with recognition and sensing of double-strand breaks (DSBs) by the meiotic recombination 11 homologue 1 (MRE11)–RAD50–Nijmegen breakage syndrome protein 1 (NBS1) (MRN) complex, which acts as an activation site for the serine-protein kinase ATM. ATM has a crucial role in DNA repair by coordinating homologous recombination. ATM phosphorylates the histone H2AX, which directly binds to mediator of DNA damage checkpoint protein 1 (MDC1) and NBS1 of the MRN complex, to enhance ATM binding. MDC1 phosphorylation results in a binding site for the E3 ubiquitin-protein ligase RING finger protein 8 (RNF8), which allows ubiquitin-mediated recruitment of downstream DNA damage response proteins, such as receptor-associated protein 80 (RAP80; encoded by UIMC1). RAP80 is an ubiquitin-interaction motif-containing protein that associates with the breast cancer type 1 susceptibility protein (BRCA1) complex through its interaction with Abraxas (encoded by FAM175A); Abraxas is thought to function as a central adaptor protein and contains domains required for BRCA1 interactions. The RAP80–Abraxas complex is crucial for recruiting BRCA1 to the site of DNA repair. BRCA1 and BRCA2 function as scaffolds for other proteins involved in DNA repair. BRCA1-associated RING domain protein 1 (BARD1) and BRCA1-interacting protein 1 (BRIP1; also known as Fanconi anaemia group J protein) bind directly to BRCA1; BARD1 forms a heterodimer with BRCA1, which is essential for mutual stability. BRIP1 also binds to BRCA1 and is required for S phase checkpoint activation. Partner and localizer of BRCA2 (PALB2) helps BRCA1 and BRCA2 bind at sites of DNA damage and helps load RAD51 proteins on to the BRCA proteins; the DNA repair protein XRCC2 is one of the five paralogues of RAD51. Mutations in genes involved in homologous repair lead to defective DNA repair mechanisms, the accumulation of DSBs and an increase in the risk of developing ovarian tumours. b | DNA mismatch repair is mediated by the MutS protein homologue 2 (MSH) proteins, as well as the endonuclease PMS2 and proliferating cell nuclear antigen (PCNA). DNA mismatch repair processes are aberrant in ovarian cancer due to mutations in the genes encoding MutL protein homologue 1 (MLH1), MSH2, MSH6 and PMS2. MSH2 and MSH6 form a heterodimeric complex, which initially identifies mismatched bases and initiates DNA repair. Binding of this complex to the mismatched bases enables the recruitment of MLH1 and PMS2. PCNA attaches to the sites of base mismatch and helps to recruit and tether exonuclease 1 (EXO1; a member of the RAD2 exonuclease family) to the sites of DNA damage. EXO1 excises the mismatched bases, which are then repaired by DNA polymerase and DNA ligase.
Despite genomic data showing recurrent mutations in patients with ovarian cancer, some tumours, particularly the HGSC subtype, are genetically heterogeneous65,67,70 — reflecting the underlying genomic complexity of this disease. For example, one study has demonstrated intratumour genomic heterogeneity in patients with newly diagnosed HGSC70.
HGSC.
HGSC has been extensively characterized both at the initial diagnosis of ovarian cancer and at disease recurrence after exposure to platinum-based chemotherapy.
TP53 is the most commonly mutated gene in HGSC65,67. TP53 mutations can be in-frame and frameshift insertions and deletions, as well as mis-sense or nonsense mutations71. TP53 mutations frequently occur in the region of the gene encoding the DNA-binding domain, but can also occur in regions encoding the non-DNA-binding domains. Tumours that lack TP53 mutations have signs of p53 dysfunction through a copy number gain of MDM2 or MDM4, the gene products of which are involved in the regulation and degradation of p53 (REF. 71). Genomic analyses have revealed defects in homologous recombination in ~50% of analysed HGSCs23,24. Defective homologous recombination is associated with both germline and somatic BRCA mutations, as well as alterations in other DNA repair pathway genes65 (FIG. 2). BRCA1 is crucial for DNA repair, cell cycle checkpoint control, mitosis, remodelling of chromatin and transcriptional regulation; BRCA2 is important in homologous recombination and DNA repair72. Hypermethylation of the BRCA1 promoter has also been shown in a substantial subset of HGSCs but does not influence overall survival and outcome65.
Additional recurrent molecular alterations identified in HGSC include defective Notch, phosphoinositide 3-kinase (PI3K), RAS–MEK and forkhead box protein M1 (FOXM1) signalling pathways, as well as a high level of somatic copy number alterations in the genes encoding proteins in these pathways65. Other mutated genes that play a part in the pathogenesis of HGSC and that could also serve as potential therapeutic targets for ovarian cancer include AURKA, ERBB3, CDK2, MTOR, BRD4 and MYC65,73,74. For example, one study showed that activity of the epigenetic transcription modulator, bromodomain-containing protein 4 (encoded by BRD4) is required for the proliferation and survival of HGSC cell lines73. In addition, ovarian cancer cells that are sensitive to BRD4 inhibition have a high expression of MYC, another important gene found altered in HGSC73.
HGSC has been further subdivided using data from gene expression profiling75,76. The Cancer Genome Atlas identified four subtypes of HGSC based on gene expression: differentiated, immunoreactive, mesenchymal and proliferative subtypes, which have differences in clinical outcome, although this has not been clinically useful for patient management75–77. Attempts to more-narrowly define the subgroups of HGSC have included integrated genomic analyses that incorporate multiple platforms. For example, a microRNA (miRNA)-regulated network was identified and associated with the mesenchymal subtype of HGSC and with poor clinical outcomes78. Some studies have used gene expression profiling to predict the prognosis of patients with advanced-stage HGSC, in addition to treatment resistance and response to platinum-based chemotherapy and PARP inhibitors. However, these studies relied on retrospective analyses, and prospective data from randomized trials are still needed to show usefulness of expression assays in subtyping patients79.
The level of molecular diversity of HGSC at the time of diagnosis, its evolution, change over time, the presence of few druggable driver mutations and the high rate of copy number alterations in genes of multiple signalling pathways characterize the genomic complexity of this cancer. Indeed, this molecular complexity provides insight into perhaps why the development of effective therapies for HGSC has been difficult to achieve.
Other epithelial subtypes.
The genomic landscapes of other histological subtypes of ovarian cancer have also been studied. Clear-cell carcinomas are complex at the genomic level and can have mutations in ARID1A, PIK3CA and PTEN80. BRAF and KRAS mutations are common in LGSCs81,82. In addition, LGSC mostly exhibits mutational stability such that the extent of tumour genetic evolution is low in this cancer type in each patient, but these tumours are typically more unresponsive to chemotherapy than HGSCs83.
High-grade endometrioid cancers have molecular similarities to HGSC (TABLE 1). Ovarian cancers associated with endometriosis, such as clear-cell and endometrioid carcinomas, are associated with ARID1A mutations1,84. Low-grade endometrioid carcinomas can carry loss of PTEN and mutations in PIK3CA and KRAS. Mucinous carcinomas can carry KRAS mutations85. C>T transitions in an NpCpG trinucleotide context have been shown to be the predominant mutational signature of mucinous carcinomas, indicating deamination of methylcytosines86. Approximately half of mucinous carcinomas have mutations in TP53, with other frequent mutations occurring in KRAS, BRAF, CDKN2A, RNF43, ELF3, GNAS, ERBB3 and KLF5 (REF. 86).
Hypercalcaemia-associated small-cell carcinomas are associated with somatic or germline mutations in SMARCA4 (REFS 4,87).
Precursor lesions
The distal fallopian tube has been identified as a precursor site of HGSCs in a substantial proportion of patients, owing to the presence of atypical tubal epithelial cells in women with BRCA1 or BRCA2 mutations. This site was identified with the discovery of serous tubal intraepithelial carcinoma (STIC) — an early lesion — during risk-reducing bilateral salpingo-oophorectomy in these women, with the presence of STICs in the fallopian tubes of women with advanced-stage ovarian cancer and with identification of precursors in the fallopian tube characterized by DNA damage and mutations in TP53 (REFS 6,88–94). STICs can be identified in 18–60% of cases of advanced-stage HGSCs6,88,89,91,92,94 and up to 80% of early-stage HGSCs. However, STICs are not found in all patients with HGSCs, and alternative pathways for the pathogenesis of HGSC probably exist95. One study proposed a dualistic model for HGSC pathogenesis that incorporates the variables of the patient (for example, the presence of STIC, BRCA status, patient age and morphological features of HGSC)93. The study suggested two pathways of HGSC development based on differences in STIC frequency, tumour morphology and outcome, known as classic or SET (>50% solid, pseudoendometrioid or transitional) pathways. The classic pathway involves the presence of a STIC precursor and a longer timeframe from STIC to the development of HGSC. Conversely, the SET pathway typically occurs in younger women who have a lower STIC frequency and a higher level of responsiveness to chemotherapy and PARP inhibitors. The two pathways of HGSC development might have implications for the potential ineffectiveness of risk-reducing bilateral salpingo-oophorectomy for some high-risk patients.
Immune system and tumour microenvironment
Another developing field of research in ovarian cancer pathogenesis is the role of the immune system and the tumour microenvironment. Cytotoxic T cell infiltration in ovarian cancer has been shown to correlate with improvement in overall survival in several studies96,97. For example, antitumour immune responses composed of tumour-reactive T cells and tumour-specific antibodies can be detected in peripheral blood, ovarian cancer tissue and ascites98–101. Furthermore, cytotoxic T cell infiltration in ovarian tumours correlates with improvement in overall survival, as shown by several groups96,97.
Within the many components of the tumour microenvironment, angiogenesis has a crucial role in the pathogenesis of epithelial ovarian cancer, promoting tumour growth and metastasis102. Vascular endothelial growth factor (VEGF) is one of the most potent pro-angiogenic factors identified in ovarian cancer, with other pro-angiogenic factors also identified, including fibroblast growth factor, angiopoietins, endothelins, IL-6, IL-8, macrophage chemotactic proteins and platelet-derived growth factors103,104.
Chemotherapy resistance
HGSC and other high-grade ovarian cancer histologies, for example, high-grade endometrioid carcinoma, can be further analysed on the basis of platinum sensitivity. Platinum-sensitive ovarian cancers are defined as having a platinum-free interval (PFI; the time elapsed between the last dose of platinum-based chemotherapy and evidence of cancer progression) of ≥6 months, whereas platinum-resistant cancers have a PFI of <6 months. In patients with HGSC, one study showed that inactivation of genes by disruption of transcriptional units (gene breakage) can inactivate the tumour suppressors RB1, NF1, RAD51B and PTEN, which probably contributes to increasing chemotherapy and platinum resistance67. Upregulation of ABCB1, which encodes the drug efflux pump multidrug resistance protein 1 (MDR1) leading to MDR1 overexpression, could also explain the mechanisms of platinum resistance. Moreover, germline or somatic mutations in BRCA1 or BRCA2 could lead to a favourable treatment response with improved responsiveness to chemotherapy26,65. The presence of BRCA reversion mutations (which restore the wild-type BRCA reading frame) could result in normal BRCA function and an increase in platinum resistance67,105. Amplification of the 19q12 locus, which includes CCNE1 (encoding cyclin E1, which is a cell cycle regulator), was associated with primary platinum-resistant and refractory ovarian cancers67. This leads to an abundance of cyclin E1, which subsequently activates the transcription of BRCA1 and BRCA2, increasing the levels of the BRCA proteins and leading to platinum resistance65.
Diagnosis, screening and prevention
Diagnosis
Clinical presentation.
Most women with ovarian cancer are diagnosed in later life, with a median age of diagnosis of 63 years22. Most women are symptomatic at disease presentation and have ascites (fluid in the peritoneal cavity) and gastrointestinal dysfunction (for example, constipation and/or bowel obstruction, diarrhoea, nausea, vomiting and gastrointestinal reflux). Other symptoms at initial presentation include abdominal bloating, abdominal and/or pelvic pain, fatigue and shortness of breath106. Respiratory symptoms can result from extensive intra-abdominal cancer with ascites, causing diaphragmatic pressure, pleural effusions and/or a pulmonary embolus.
Symptoms of ovarian cancer might be initially missed or attributed to other disease processes because they are general and nonspecific. Accordingly, diagnosis frequently occurs when the cancer has reached a late stage (either stage III or stage IV) because symptoms have become apparent and require intervention106, and/or the symptoms are more severe, indicative of extensive peritoneal carcinomatosis, ascites and possible cancerous involvement of the bowel. The combination of abdominal bloating, increased abdominal size and urinary symptoms has been found in 43% of patients with an eventual diagnosis of ovarian cancer, but only in 8% of patients not diagnosed with ovarian cancer107. Women presenting with severe or frequent symptoms and those of recent onset warrant further diagnostic investigation because of the association of these symptoms with ovarian masses108.
Importantly, these symptoms — and their late presentation — largely apply to those with HGSC. By contrast, histologies, such as clear-cell and small-cell carcinomas, can become symptomatic at an earlier stage. For example, hypercalcaemia can be the initial presentation of clear-cell or small-cell carcinomas. These tumour types are also associated with many of the same symptoms observed with more-advanced HGSC, such as abdominal distension, pelvic pressure and/or pain, as well as pressure of the ovarian mass on the bowel or urinary tract system. Most patients with clear-cell carcinoma present at an early stage and might present with symptoms related to pelvic pressure.
Diagnostic work-up.
In patients with indicative symptoms, diagnostic work-up includes physical examination of the patient, which consists of pelvic examination and rectovaginal examination, in addition to radiographic imaging (for example, trans vaginal ultrasonography, abdominal ultrasonography, CT (FIG. 3), MRI and/or PET). The CA125 blood test can also be used in combination with other diagnostic tests for the detection of ovarian cancer. Laparoscopic surgery with removal of the mass is recommended109 and will also give further information on the tumour histology. Results from diagnostic testing, especially trans vaginal ultrasonography, can provide information about the ovarian mass, such as size, location and level of mass complexity, which can help clinicians to determine the level of suspicion for cancer110. More-advanced cancer is associated with ascites and peritoneal carcinomatosis within the abdominal cavity; to confirm a diagnosis of ovarian cancer, a tissue biopsy must be performed.
Figure 3 |. CT scans from a patient with stage IV ovarian cancer.
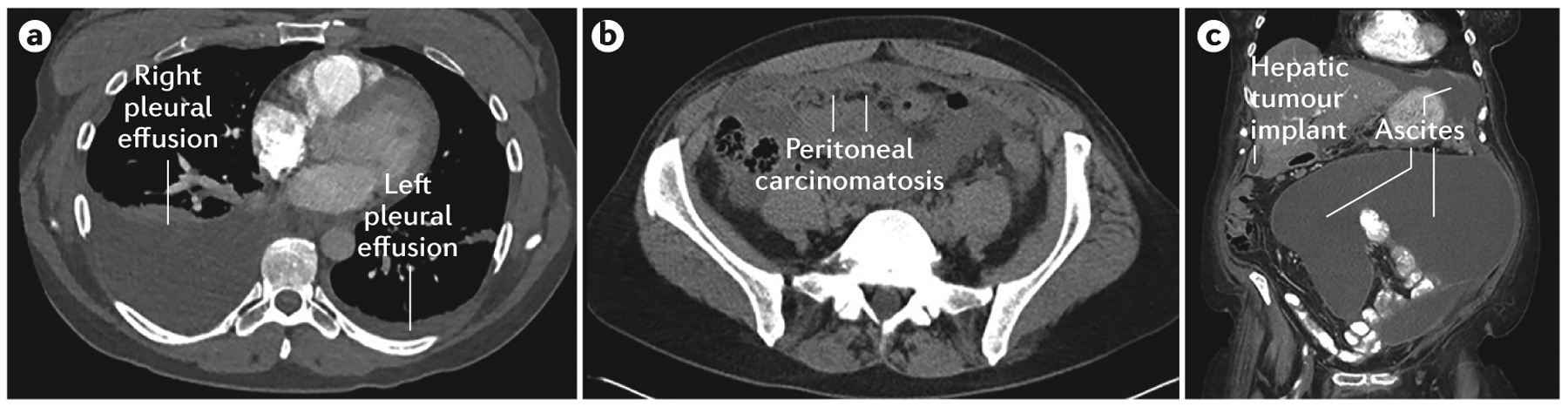
a | Right and left pleural effusions. b | Peritoneal carcinomatosis. c | Large volume ascites and a peritoneal hepatic implant.
Staging.
Pathological evaluation and tumour staging of ovarian cancer is based on surgical assessment of the cancer at initial diagnosis, including removal of lymph nodes, tissue biopsy and abdominal fluid, and uses the International Federation of Gynecology and Obstetrics (FIGO) staging system (TABLE 3). The staging system has recently changed with acceptance of the common, Müllerian-derived, multicentric origin of ovarian, fallopian tube and peritoneal cancers and that these cancers should be grouped using one system111. The latest FIGO staging system has three other notable characteristics: stage IC tumours have been subdivided based on the mechanism underlying rupture of the ovarian capsule and the presence of malignant ascites (the presence of tumour cells in ascites), stage IIC has been eliminated and stage III has a clearer definition that encompasses the size of metastases as well as the presence of metastases to the lymph nodes. Moreover, stage III was reclassified to account for differences in the clinical outcomes in patients with metastases to the lymph nodes who do not have peritoneal carcinomatosis compared with patients with peritoneal carcinomatosis112,113. In addition, stage IV has been further divided into stage IVA and stage IVB. The FIGO staging system recommends that the primary tumour site (the ovary, fallopian tube or peritoneum) and the histological grade be stated in the operative report and/or the final pathology report111.
Table 3 |.
Staging of ovarian cancer
| FIGO stage | Description | Corresponding TNM stage |
|---|---|---|
| I | Tumour confined to ovaries or fallopian tubes | T1 |
| IA | Tumour limited to one ovary (with ovarian capsule intact) or fallopian tube; no tumour on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings | T1a |
| IB | Tumour limited to both ovaries (with ovarian capsules intact) or fallopian tubes; no tumour on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings | T1b |
| IC | Tumour limited to one or both ovaries or fallopian tubes, with any of the following C substages:
|
T1c |
| II | Tumour involves one or both ovaries, or the fallopian tubes with pelvic extension below the pelvic brim or primary peritoneal cancer (Tp) | T2 |
| IIA | Extension and/or implants of tumour on uterus and/or fallopian tubes and/or ovaries | T2a |
| IIB | Extension of tumour to other pelvic intraperitoneal tissues | T2b |
| III | Tumour involves one or both ovaries, or the fallopian tubes, or primary peritoneal cancer with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes | T3 |
| IIIA | Metastasis to the retroperitoneal lymph nodes with or without microscopic peritoneal involvement beyond the pelvis | T1, T2, T3aN1 |
| IIIA1: positive retroperitoneal lymph nodes only (pathologically proven) | ||
| • IIIA1(i): metastasis up to 10 mm in greatest dimension | T3a/T3aN1 | |
| • IIIA1(ii): metastasis >10 mm in greatest dimension | ||
| IIIA2: microscopic extrapelvic (above the pelvic brim) peritoneal involvement with or without positive retroperitoneal lymph nodes | T3a/T3aN1 | |
| IIIB | Macroscopic peritoneal metastasis beyond the pelvis up to 2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes | T3b/T3bN1 |
| IIIC | Macroscopic peritoneal metastasis beyond the pelvis >2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes (includes extension of tumour to capsule of liver and spleen without parenchymal involvement of either organ) | T3c/T3cN1 |
| IV | Distant metastasis excluding peritoneal metastases | |
|
Any T, any N or M1 |
FIGO, International Federation of Gynecology and Obstetrics; TNM, TNM classification of malignant tumours. Reproduced with permission from REF. 220, Elsevier.
Surgical staging of ovarian cancer by gynaecological oncologists has been shown to be superior to that performed by non-oncological (general) surgeons, as have patient outcomes114–116. Indeed, the issue with accurate staging is pertinent; one study found that only 54% of women with ovarian cancer received correct staging as determined by a gynaecological oncologist117. When patients are operated on by non-gynaecological oncologists, such as general surgeons or general gynaecologists, the diaphragm was not visualized in 86% of cases and the omentum was not biopsied in 68% of cases117, meaning cancer was commonly missed in the diaphragm, pelvic peritoneum, peritoneal fluid and omentum.
Screening
No current screening strategy has affected the survival of patients with ovarian cancer. Creation of a successful screening strategy for ovarian cancer is challenging because this is not a common disease and includes a range of histological subtypes, each with different biological and clinical properties. For example, patients with LGSC have substantially better overall prognoses than patients with more-aggressive, high-grade cancers and might require a different screening strategy81.
The CA125 blood test is not an effective screening test when used alone, given that CA125 levels are only increased in 50% of stage I ovarian cancers and can also be increased in benign disorders, such as uterine fibroids, ovarian cysts and other conditions such as liver disease and infections118,119. Increased levels of CA125 are most frequently observed in HGSC, with lower levels of CA125 in other non-serous subtypes120. The combination of the CA125 blood test and radiographic imaging, such as transvaginal ultrasonography, has been evaluated for use as a screening strategy. One of the largest studies to examine this combination was the PLCO Cancer Screening trial121, which enrolled 78,216 women 55–74 years of age. Women were randomly assigned into two groups of approximately equal size, to receive either annual screening (encompassing yearly CA125 tests for 6 years and transvaginal ultrasonography for 4 years) or usual care (no yearly CA125 or transvaginal ultrasound, but could have undergone bimanual examination with ovarian palpation). Ovarian cancer was diagnosed in 212 women (5.7 per 10,000 person-years) in the screening group and in 176 women (4.7 per 10,000 person-years) in the usual care group (rate ratio: 1.21; 95% CI: 0.99–1.48), and the stage distributions of cancer were similar for the two groups (stage III and stage IV cancers comprised almost 80% of cancers in both groups). In addition, no significant reduction in overall mortality was observed with screening (3.1 deaths per 10,000 women in the screening group and 2.6 deaths per 10,000 person-years in the usual care group; mortality rate ratio of 1.18 (95% CI: 0.82–1.71)).
Although the CA125 test alone as a screening marker has been deemed ineffective, the UKCTOCS study evaluated longitudinal measurements of CA125 levels for the screening of ovarian cancer in an algorithm termed ‘risk of ovarian cancer algorithm’ (ROCA)10. In one arm of this study, ROCA was the primary screening modality, with transvaginal ultrasonography used as a secondary screening measure based on CA125 levels. ROCA interpreted longitudinal CA125 data and triaged women to normal (annual screening), intermediate (repeat CA125 testing in 3 months) and increased risk (repeat CA125 testing and transvaginal ultrasonography in 6 weeks). In the normal-risk women, annual screening used transvaginal ultrasonography as the primary test, following which patients were subdivided into three groups based on the ultrasonography results: normal (annual screening), unsatisfactory (repeat in 3 months) and abnormal (scan with a senior ultrasonographer within 6 weeks). In this study, 202,638 women were randomly assigned into one of three groups (screening based on ROCA, ultrasonography alone or no screening) and were followed-up for a median period of 11.1 years10. The proportion of women diagnosed with ovarian cancer was similar between groups (0.6–0.7%), but lower stages (stage I–IIIA) of disease were in a higher proportion of patients in the ROCA-screened group than in those who were not screened (P < 0.0001). However, there was no difference between patients in the ROCA group and those who received transvaginal ultrasonography (P = 0.57). Mortality reduction was not significant between any of the groups, thus, the ROCA test cannot currently be recommended as a screening strategy for ovarian cancer; further follow-up of this study is necessary to understand the long-term potential of this screening strategy.
Human epididymis protein 4 (HE4; also known as WFDC2) has also been tested as a potential biomarker for use in ovarian cancer screening122. A systematic review reported better sensitivity, specificity and likelihood ratios for HE4 compared with CA125, but this has not yet been analysed within a screening strategy123. The use of other novel markers for ovarian cancer screening are under investigation, including, for example, DNA analysis of uterine lavages or Pap smears for TP53 mutations124.
Prevention
Salpingectomy has gained favour as a prevention technique based on the presence of precursor lesions in the fallopian tubes of some women with ovarian cancer, as discussed above. However, no randomized prospective studies have been performed to determine the benefit or evidence of risk reduction following salpingectomy125–127.
The Society of Gynecologic Oncology guidelines and recommendations for the prevention of ovarian cancer, in addition to others, recommend that all women with invasive ovarian cancer (regardless of family history, histology or age) should undergo genetic testing and genetic counselling. The purpose of this testing is to assess women for the presence of a high-risk gene that could convey increased risk for the individual and their family members, as well as having implications for outcome and therapeutic management128,129. Moreover, the Society of Gynecologic Oncology guidelines recommend performing risk-reducing bilateral salpingo-oophorectomy in women 35–40 years of age who are at increased genetic risk (that is, the presence of germline mutations in high-risk genes) of developing ovarian cancer, as well as individualizing the age at which women undergo risk-reducing surgery128. In addition, the Society of Gynecologic Oncology guidelines mandate and recommend microscopic examination of the entire ovary and fallopian tube following risk-reducing bilateral salpingo-oophorectomy in high-risk women, to rule out early invasive cancers88–90,128.
The annual risk of ovarian cancer in individuals of specific age groups with germline BRCA mutations and intact ovaries has been estimated to help guide clinicians and patients about appropriate timing of the risk-reducing bilateral salpingo-oophorectomy130. In one study, risk-reducing salpingo-oophorectomy reduced the risk of ovarian cancer in women with BRCA mutations by 80%. The timing of risk-reducing bilateral salpingo-oophorectomy is important, as performing surgery in women <45 years of age has been associated with an increased risk of cardiovascular disease, osteoporosis and osteopenia; oestrogen replacement should be considered in these patients (if they have not had breast cancer), but the benefits and potential risks or optimal duration of oestrogen therapy have not been determined128.
Variants of unknown importance occur in BRCA1 and BRCA2, as well as other high-risk genes implicated in ovarian cancer, but the effects of these variants on ovarian tumorigenesis are currently unknown131. Variants of unknown importance represent dilemmas for women who are diagnosed with them, as these variants carry an unknown cancer risk, meaning patients and their family members cannot be accurately counselled about risk reduction and preventive surgeries.
Management
At initial diagnosis, patients are faced with the challenge of accessing appropriate medical treatment and quickly making complex decisions about their care. The choice of physician can affect outcomes116,132, as can adherence to the guidelines for the standard of care133,134; surgery performed by a gynaecological oncologist results in superior outcomes and survival than surgery performed by a non-gynaecological oncologist, such as a general surgeon.
The primary aim of treatment for ovarian cancer is to maximize cancer control and to palliate disease symptoms for as long as possible. Surgery performed by a gynaecological oncologist is the main treatment for most patients with ovarian cancer. The extent of surgery is determined by the stage of cancer and patient factors; for example, women with more-advanced cancer might undergo bilateral oophorectomy, but women with low-risk, stage I cancer (such as mucinous histologies) and young women who wish to preserve fertility might undergo unilateral oophorectomy of the affected ovary only. Surgical cytoreduction results are frequently referred to as suboptimal (that is, any focus is ≥1 cm in size (R2 resection)), optimal (that is, <1 cm residual cancer (R1 resection)) or no evidence of residual macroscopic disease (R0 resection). New studies only define optimal surgical results if macroscopic complete resection of the cancer has been achieved. Patients with macroscopic complete resection (R0) following surgery have significant improvements in outcomes, such as in overall survival and progression-free survival (PFS), compared with patients with remaining postoperative visible disease135,136. For example, in one study (GOG 182), patients with stage III or stage IV ovarian cancer with optimal cytoreduction, R1 had a worse prognosis than patients with no evidence of residual macroscopic disease (R0) following platinum-based chemotherapy. Nevertheless, patients with optimal cytoreduction have a significantly better median PFS and overall survival than patients with suboptimal cytoreduction135–137.
Newly diagnosed ovarian cancer
Primary surgery.
The primary treatment for women with newly diagnosed ovarian cancer is primary surgical cytoreduction (FIG. 4). The primary goal of surgery is to achieve macroscopic complete resection of disseminated carcinomatosis, often involving complex surgical techniques, including en bloc resection of the bowel, uterus and adnexal masses, as well as peritonectomy. In some cases, colonoscopy and/or upper endoscopy might be required to rule out the possibility of a primary gastrointestinal cancer rather than a primary ovarian cancer. Systematic pelvic and para-aortic lymph node dissection is also necessary in patients with high-risk early-stage ovarian cancer or in patients with stage II and stage IIIA disease, as nodal metastases signify a higher stage of disease, poorer prognosis and the need for different treatment strategies. Defining the best surgical approach and determining the appropriateness of surgery before the administration of chemotherapy versus NACT are crucial. If NACT is to be administered, a biopsy is needed to confirm pathology consistent with an ovarian, tubal or peritoneal primary cancer, before chemotherapy can be commenced.
Figure 4 |. Tumour burden in ovarian cancer.
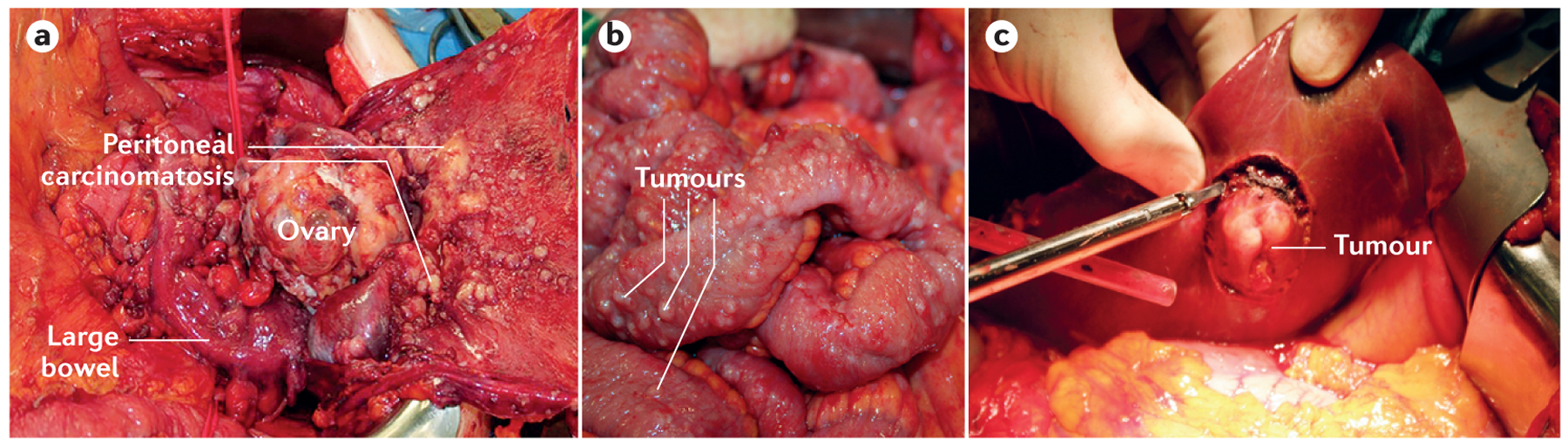
a | Surgical removal of ovarian tumours in a patient 63 years of age with bilateral advanced-stage high-grade serous carcinoma (HGSC). b | A patient with a bilateral HGSC and peritoneal carcinomatosis with involvement of intestinal surfaces. c | Surgical removal of a serosal tumour implant located on the surface of the liver.
Adjuvant chemotherapy.
Recommendations for the use of adjuvant chemotherapy using platinum-based chemotherapy for patients with early-stage ovarian cancer depend on the cancer stage, grade and histology. Many patients with grade I, stage I cancer are not treated with chemotherapy post-surgery, but those with higher grades (grade II or above) and/or specific histologies (such as HGSC and clear-cell carcinoma) undergo adjuvant systemic platinum-based chemotherapy138. Indeed, several first-line adjuvant systemic chemotherapy strategies have led to an improvement in overall survival for patients with newly diagnosed, advanced-stage ovarian cancer, including the addition of paclitaxel to platinum-based chemotherapy agents, the use of intra peritoneal cisplatin in patients with optimally cytoreduced cancer and the incorporation of dose-dense weekly paclitaxel treatment instead of administration every 3 weeks10,139–141.
Studies have examined the efficacy of different combinatorial treatments to optimize adjuvant chemotherapy, including combinations of platinum-based chemotherapy agents (cisplatin and carboplatin), taxanes (paclitaxel and docetaxel), anti-angiogenic agents (bevacizumab, nintedanib, trebananib and pazopanib) and other drugs (pegylated liposomal doxorubicin and gemcitabine) (TABLE 4).
Table 4 |.
Key clinical trials in newly diagnosed ovarian cancer
| Study | Arms | Comments | Refs |
|---|---|---|---|
| Adjuvant therapy | |||
| GOG 111 | IV cisplatin and cyclophosphamide |
|
11 |
| IV cisplatin and paclitaxel | |||
| AGO OVAR 3 | IV cisplatin and paclitaxel |
|
13 |
| IV carboplatin and paclitaxel | |||
| GOG 158 | IV cisplatin and paclitaxel |
|
14 |
| IV carboplatin and paclitaxel | |||
| GOG 182 | IV carboplatin and paclitaxel |
|
12 |
| Other platinum-based doublets | |||
| SCOTROC | IV carboplatin and paclitaxel |
|
15 |
| IV carboplatin and docetaxel | |||
| GOG 172 | IV cisplatin and paclitaxel |
|
139 |
| IP cisplatin and paclitaxel and IV paclitaxel | |||
| JGOG 3016 | IV carboplatin and paclitaxel every 21 days |
|
140, 141 |
| IV carboplatin every 21 days and weekly paclitaxel (dose-dense regimen) | |||
| GOG 252 | IP cisplatin, and IV and IP paclitaxel and bevacizumab |
|
221 |
| IP carboplatin, and IV weekly paclitaxel and bevacizumab | |||
| IV carboplatin, and IV weekly paclitaxel and bevacizumab | |||
| MITO-7 | Weekly carboplatin and weekly paclitaxel |
|
222 |
| Carboplatin and paclitaxel every 3 weeks | |||
| MITO-2 | Carboplatin and paclitaxel |
|
223 |
| Carboplatin and pegylated liposomal doxorubicin | |||
| Neoadjuvant therapy | |||
| EORTC | Upfront surgery followed by chemotherapy |
|
145 |
| NACT, interval cytoreductive surgery, followed by completion of chemotherapy | |||
| Kehoe et al. | Upfront surgery followed by chemotherapy |
|
146 |
| NACT, interval cytoreductive surgery, followed by completion of chemotherapy | |||
| Addition of anti-angiogenic agents to chemotherapy and used as maintenance therapy | |||
| GOG 218 | Carboplatin, paclitaxel and bevacizumab with bevacizumab maintenance |
|
142 |
| Carboplatin, paclitaxel and bevacizumab with placebo maintenance | |||
| Carboplatin, paclitaxel and placebo with placebo maintenance | |||
| ICON7 | Carboplatin, paclitaxel and bevacizumab with bevacizumab maintenance |
|
143 |
| Carboplatin and paclitaxel | |||
| GOG 262 | IV carboplatin and paclitaxel every 21 days with or without bevacizumab |
|
224 |
| IV carboplatin every 21 days and IV weekly paclitaxel with or without dose-dense bevacizumab | |||
| NCT00866697 | IV platinum and taxane chemotherapy followed by placebo maintenance |
|
153 |
| IV platinum and taxane chemotherapy followed by pazopanib maintenance | |||
| Addition of anti-angiogenic agents to chemotherapy and used as maintenance therapy (cont.) | |||
| AGO-OVAR 12 | Carboplatin and paclitaxel |
|
225 |
| Carboplatin and paclitaxel with nintedanib | |||
| NCT01493505 | Carboplatin and paclitaxel |
|
108 |
| Carboplatin and paclitaxel with trebananib | |||
IP intraperitoneal; IV intravenous; NACT, neoadjuvant chemotherapy; PFS progression-free survival.
In 2011, the European Medicines Agency (EMA) approved the use of bevacizumab as an addition to carboplatin and paclitaxel chemotherapy and maintenance therapy in patients with newly diagnosed, advanced-stage ovarian cancer, based on the improvement in PFS in the ICON7 and GOG 218 studies (TABLE 4). A retrospective analysis of the ICON7 study of patients with sub optimally cytoreduced stage IIIC or stage IV cancer showed an overall survival benefit with the addition of bevacizumab to a carboplatin and paclitaxel backbone, but no improvement in overall survival was observed in the intent-to-treat population of patients who were entered into either the ICON7 or the GOG 218 studies142–144. Despite being available in Europe, bevacizumab has not been approved for patients in the United States, making collaborative trial design for both newly diagnosed and recurrent ovarian cancer challenging.
NACT.
NACT consisting of carboplatin and paclitaxel for three cycles is then followed by interval (that is, between rounds of chemotherapy) surgical cytoreduction and additional chemotherapy post-surgery for a total of six cycles of chemotherapy. NACT is a possible treatment alternative to upfront surgical cytoreduction for ovarian cancer, especially for patients who are too ill for initial surgery or if the cancer burden is too extensive to allow macroscopic complete resection. Two trials have demonstrated comparable outcomes for first-line surgery with adjuvant chemotherapy compared with NACT followed by surgery and postoperative chemotherapy, with less morbidity and mortality but similar outcomes in PFS and overall survival in the group that received NACT145,146. Data from the first study indicated that NACT followed by interval cytoreductive surgery is not inferior to primary cytoreductive surgery followed by chemotherapy and no significant difference in PFS (12 months) or overall survival (29–30 months) was found between the groups145. The second study (CHORUS) in patients with advanced stage III or stage IV cancer randomly assigned to either primary cytoreductive surgery followed by chemotherapy (consisting of either carboplatin and paclitaxel or carboplatin alone) or NACT (three cycles) followed by cytoreductive surgery and three more cycles of chemotherapy (carboplatin and paclitaxel or carboplatin alone) showed a non-significant difference in overall survival between the group that received NACT (24.1 months) and those that received upfront surgery (22.6 months; hazard ratio (HR): 0.87; 95% CI: 0.72–1.05)146. In addition, PFS was similar for both groups: 12 months in the NACT group compared with 10.7 months for the primary surgery group (HR: 0.91; 95% CI: 0.76–1.09). However, the number of postoperative deaths was lower in the NACT group than in the upfront surgery group146.
Some medical centres are testing the use of surgical algorithms with diagnostic laparoscopy to determine tumour resectability and to identify patients who are appropriate for first-line cytoreductive surgery versus those suitable for NACT; however, no validated preoperative instrument has currently been established147. Controversy persists over the identification of the most appropriate candidates for NACT and whether NACT induces upfront platinum resistance. Accordingly, a general consensus regarding the equivalence of NACT followed by surgery and upfront surgery followed by adjuvant chemotherapy is lacking148. In addition, some groups have argued that the overall survival and PFS outcomes used in the aforementioned randomized trials of NACT versus upfront surgical cytoreduction145,146 are inferior to other trials and that inferior complete resection rates were observed in the primary surgery control group, particularly in the CHORUS study146,149.
Maintenance therapy following NACT.
Aims of maintenance therapy are to prolong a clinically meaningful survival end point, such as PFS, and to also preserve the quality of life of the patient. The use of maintenance therapy following platinum-based chemotherapy has been investigated and reviewed150,151. Monthly paclitaxel treatment (for a duration of either 3 months or 12 months) has been assessed in patients with ovarian cancer following completion of NACT152; no benefit in overall survival was observed with paclitaxel treatment for 12 months compared with treatment for 3 months, but PFS was longer in the 12-month versus 3-month groups. However, owing to the risk of developing adverse effects with continuation of monthly paclitaxel for 12 months (for example, alopecia and peripheral neuropathy), the use of paclitaxel for maintenance therapy after platinum-based chemotherapy is not commonly used; currently, the standard of care following completion of platinum-based chemotherapy is observation alone138.
Pazopanib has also been studied for use in maintenance therapy, resulting in an increase in PFS but no improvement in overall survival153. Pazopanib is also associated with a significant toxicity profile, such as fatigue, gastrointestinal toxicities (such as nausea and/or diarrhoea), hypertension and myelosuppression153. Bevacizumab is approved in Europe as maintenance therapy following initial platinum with taxane and bevacizumab chemotherapy based on GOG 218 and ICON7 results.
Recurrent disease
Monitoring for recurrence.
>80% of patients with advanced-stage ovarian cancer will experience recurrence of their primary cancer. Recurrent ovarian cancer is generally incurable, but rare exceptions to this exist, such as patients with isolated metastatic cancer in whom the disease can be fully resected after secondary cytoreductive surgery or treatment with localized radiotherapy.
Many patients with recurrent ovarian cancer are asymptomatic at the time of their relapse and, as such, recurrent ovarian cancer is most frequently detected by increased levels of CA125; the sensitivity and specificity of this test for recurrence detection range from ~60% to 94% and ~91% to 100%, respectively154,155. CA125 levels are monitored following completion of the initial treatment, but guidelines regarding the frequency of CA125 and clinical monitoring of patients with ovarian cancer change with different guidelines154,155. The Society of Gynecologic Oncology recommends a review of clinical symptoms and a physical examination of patients following the initial treatment for ovarian cancer every 3 months with an optional CA125 test and radiographic imaging (CT, PET or MRI) in patients with suspected recurrence (such as those with an increased level of CA125, findings on clinical examination and/or suspicious symptoms)155. Conversely, the National Comprehensive Cancer Network guidelines recommend follow-up visits every 2–4 months for 2 years after treatment, including the measurement of CA125 levels; radiographic imaging should be done if recurrence of ovarian cancer is suspected138.
The limitations of disease detection and the role of CA125 should be discussed with all patients who have completed therapy. Sufficient clinical information should be available to make a definitive diagnosis of cancer recurrence, including increased levels of CA125, radiographic evidence of cancer, physical examination evidence, symptoms related to the disease burden and/or a positive biopsy. Increased levels of CA125 in the absence of other clinical indicators are generally not a reason to initiate treatment, unless the patient is enrolling into a clinical trial. Some patients might not have increased levels of CA125 at either initial diagnosis or with recurrence of ovarian cancer, which makes the CA125 test less useful when used for recurrent cancer. In these patients, alternative biomarkers, such as HE4, and/or the use of interval radiographic imaging might be of use for monitoring of recurrent cancer, but this needs further evaluation. Although used, measurement of CA125 levels for the early detection of recurrence has not been shown to improve outcomes in patients with recurrent disease. In one study, no improvement in patient survival was observed following early treatment of recurrent ovarian cancer (diagnosed on the basis of increased levels of CA125 in the absence of clinical symptoms) compared with delayed treatment (until the manifestation of clinical symptoms of disease progression)156. This trial has been criticized because of the long period of time needed to accrue patients (almost 10 years), the lack of predefined subsequent therapies and the lack of access to newer treatments (such as bevacizumab) and other drugs through clinical trials, or to the potential use of secondary cytoreductive surgery157.
Treatment options
Following definitive diagnosis of recurrent ovarian cancer, several factors should be considered before deciding appropriate treatment options, including the level of disease burden (such as symptomatic versus asymptomatic cancer and the location of metastases), the presence of complications from previous therapies (such as peripheral neuropathy, pancytopaenias and/or drug hypersensitivity reactions), the availability of clinical trials, the degree of platinum sensitivity, end-organ function, performance status of the patient and, also, wishes and goals of the patient. Treatment of recurrent ovarian cancer has been made more complex with oncologists factoring in tumour histology and underlying BRCA status, given the recent US FDA and EMA approvals of the PARP inhibitor olaparib. Secondary surgical cytoreduction can be considered for patients with a long PFI with recurrent cancer that is limited and isolated (for example, cancer in one location such as the spleen or an isolated lymph node), although meta-analyses did not demonstrate any benefit of this surgery158. One randomized trial (GOG 213) investigating the efficacy of secondary surgical cytoreduction for the treatment of platinum-sensitive recurrent ovarian cancer is underway159. The German AGO Study Group has demonstrated a potential survival benefit only in patients with no postoperative residual cancer following secondary cytoreductive surgery160. This study also established a preoperative clinical score to predict the target population with the best outcomes following secondary cytoreductive surgery, including the amount of ascites (<500 ml) and the result of primary surgery (macroscopically free). On the basis of these findings, a prospective study (DESKTOP-Trial III) was conducted to compare the overall survival of patients with platinum-sensitive recurrent ovarian cancer undergoing cytoreductive surgery followed by platinum-based chemotherapy, with patients receiving chemotherapy alone, the results of which are expected in 2017 (REF. 161).
Recurrent ovarian cancer is classified as platinum-sensitive or platinum-resistant. However, the Institute of Medicine called for an improved classification system for recurrent ovarian cancer, as the current classification does not reflect the effect of BRCA status on treatment responses and the varied responses to treatment in women with platinum-resistant cancer162. In addition, some groups have called for diminishing the importance of the PFI as this definition is flawed, with no universally accepted objective definition and, instead, incorporating key disease parameters, such as molecular signature (such as BRCA mutation), immunological features and tumour histology163.
Platinum-sensitive disease.
For patients with platinum-sensitive recurrent ovarian cancer, the standard of care is re-use of a platinum-based regimen138. However, re-use of platinum-based chemotherapy is associated with the development of potentially life-threatening platinum drug allergies164. Response rates using platinum doublets in patients with platinum-sensitive recurrent cancer are ~50%138,165–167, although the length of the PFI decreases with subsequent platinum use168. Various combinations of therapies are being investigated for the treatment of platinum-sensitive ovarian cancer (TABLE 5), including paclitaxel and carboplatin165, carboplatin and pegylated liposomal doxorubicin166 and carboplatin and gemcitabine167. Use of a platinum-based combination has been shown to improve outcomes compared with the use of single-agent platinum in patients with platinum-sensitive recurrent ovarian cancer165.
Table 5 |.
Key trials for the treatment of recurrent ovarian cancer
| Study | Arms | Comments | Refs |
|---|---|---|---|
| Platinum-sensitive disease | |||
| ICON4/AGO-OVAR-2.2 | Carboplatin |
|
165 |
| Carboplatin and paclitaxel | |||
| OCEANS | Carboplatin, gemcitabine and bevacizumab with bevacizumab maintenance therapy |
|
226 |
| Carboplatin and gemcitabine | |||
| ICON6 | Platinum-based chemotherapy and cediranib with cediranib maintenance therapy |
|
177 |
| Platinum-based chemotherapy | |||
| NOVA | Platinum-based chemotherapy for platinum-sensitive recurrence followed by maintenance niraparib |
|
227 |
| Platinum-based chemotherapy for platinum-sensitive recurrence, followed by maintenance with placebo drug | |||
| NCT00113607 | Pegylated liposomal doxorubicin and trabectedin |
|
228 |
| Pegylated liposomal doxorubicin | |||
| NCT00753545 | Platinum-based chemotherapy |
|
229 |
| Platinum-based chemotherapy with olaparib maintenance therapy | |||
| NCT01081951 | Carboplatin and paclitaxel |
|
230 |
| Carboplatin and paclitaxel with olaparib maintenance therapy | |||
| NCT01116648 | Olaparib |
|
231 |
| Olaparib and cediranib | |||
| Platinum-resistant disease | |||
| AURELIA | Paclitaxel with or without bevacizumab |
|
171, 172 |
| Pegylated liposomal doxorubicin with or without bevacizumab | |||
| Topotecan with or without bevacizumab | |||
PFS, progression-free survival.
Approved therapies for the treatment of patients with platinum-sensitive recurrent ovarian cancer in Europe include bevacizumab (in combination with carboplatin and gemcitabine) and trabectedin (an agent that binds to DNA, resulting in cell cycle arrest and apoptosis)167. Carboplatin and gemcitabine are approved for use in the United States. Trabectedin was not ultimately approved for use in the United States owing to toxicity concerns; adverse effects of this agent include bone marrow suppression, fatigue and gastrointestinal complications (such as nausea, vomiting and diarrhoea), in addition to increased levels of liver enzymes.
Olaparib has been approved by the EMA as a maintenance therapy for platinum-sensitive ovarian cancer, after response and completion of platinum-based chemotherapy in patients with either a germline or a tumour BRCA mutation. However, accelerated approval for olaparib as a maintenance therapy in patients with germline BRCA mutations was rejected by the FDA Oncologic Drugs Advisory Committee, owing to a lack of evidence supporting improvements in overall survival; the final results of a confirmatory phase III study (SOLO2) will probably factor into future FDA decisions169,170. Nonetheless, the FDA has granted accelerated approval to olaparib as a single agent for use in patients with germline BRCA mutations who have received at least three prior lines of chemotherapy, regardless of platinum sensitivity170. The combination of olaparib and cediranib showed an improvement in PFS compared with olaparib alone as treatment for platinum-sensitive recurrent ovarian cancer (TABLE 5), and two phase III trials are ongoing for both platinum-resistant and platinum-sensitive recurrent disease.
Platinum-resistant disease.
For patients with platinum-resistant cancer, bevacizumab with weekly paclit axel, pegylated liposomal doxorubicin or topotecan treatment in the first platinum-resistant setting was approved by both the FDA and the EMA, following the results of the AURELIA trial171,172. Although promising, care should be taken when using bevacizumab in patients with ovarian cancer, owing to the risk of severe adverse effects, such as gastrointestinal perforation173, hypertension, proteinuria and fistula development. Other single agents available to treat platinum-resistant ovarian cancer include gemcitabine, etoposide and vinorelbine138, which have response rates of up to 10–15% and median PFS of approximately 3–4 months. Anti-angiogenic agents that have been studied in recurrent ovarian cancer include nintedanib, trebananib, sunitinib, cabozantinib and cediranib174,175. Notably, cediranib has single-agent activity in both platinum-resistant and platinum-sensitive recurrent ovarian cancer176, can increase PFS when combined with platinum-based chemotherapy and can also be used as maintenance therapy in patients with platinum-sensitive recurrent cancer177. Furthermore, cediranib is being tested in combination with olaparib in two actively accruing phase III studies: GY004 and GY005 (REFS 178,179).
Ultimately, treatment of recurrent ovarian cancer should be tailored to the patient to prevent worsening of pre-existing adverse effects, such as myelosuppression and neuropathy, as well as respecting the wishes of the patient and the avoidance of other adverse effects, such as alopecia and gastrointestinal complications.
Quality of life
The diagnosis of any life-threatening disease, coupled with the acute and long-term adverse effects of treatment, can be associated with reductions in quality-of-life domains, including physical, functional, emotional, sexual, social and occupational well-being. Moreover, the large number of medical decisions required in a short period of several days to weeks following the initial diagnosis of ovarian cancer can add to the emotional stress felt by patients. The responses to these issues vary; for example, some patients might re-evaluate their attitudes to relationships, work and day-to-day life following a diagnosis of ovarian cancer180.
Although current treatment advances give more women with ovarian cancer the prospect of living longer, minimizing and/or ameliorating the adverse effects associated with treatments are crucial if quality, as well as length, of life is to be improved. Improvements in PFS or overall survival in trials might excite clinical scientists but be of less value to patients experiencing treatment-related adverse effects; because of this, many phase III studies have incorporated standardized, validated measures of quality of life (commonly referred to as patient-reported outcome (PRO) end points) into studies181,182. PROs are important as there are increasing doubts raised about the validity of data regarding adverse events collected during clinical trials; several studies have shown that the symptoms of disease and adverse effects of treatment are often under-recognized, under-reported and consequently undertreated183. Indeed, patients report adverse effects (such as fatigue, nausea, vomiting, constipation, alopecia, appetite loss and pain) earlier, more frequently and of greater severity than clinicians and nurses using Common Terminology Criteria for Adverse Events grading or proxy raters183. Quantification of quality-of-life issues faced by women with ovarian cancer requires well-constructed, reliable PRO measures that need to be essential components of phase III studies. Both the FDA and the EMA have clear guidelines on PRO instruments that are acceptable for conducting health technology assessments, defined as outcomes reported by patients, without the intervention of a third party and that have been constructed using appropriate psychometric methodology184. One key issue is that the PRO measures should be defined upfront and during trial development, with patients involved in their production. PRO measures used for ovarian cancer include generic, tumour-specific, treatment-specific or symptom-specific measures185–187 and involve face-to-face interview schedules188, questionnaires on quality of life186,187,189–191, satisfaction scales and patient preference approaches192. For example, a PRO might include a series of questions related to the severity of various symptoms, such as lack of energy, pain, discomfort, sexual dysfunction, feeling ill, insomnia, sweating, bowel control and constipation, as used in the GY004 trial.
Thorough monitoring using validated instruments in clinical trials is needed to compile a database of the trajectory and severity of issues, such as adverse effects of treatment as well as emotional distress, permitting better evaluation of the benefits and harms of therapies, but also to establish the case for more research to develop therapies to reduce the adverse effects. The traditional end points of clinical trials (such as PFS and overall survival) need to be integrated with PROs to improve quality as well as length of life.
Outlook
Now is a very exciting and promising time for ovarian cancer research, yet challenges remain in early detection, the identification of women who are at higher risk of developing ovarian cancer, overcoming platinum resistance and resistance to other treatments, in addition to developing rationale and effective immunotherapeutic strategies.
With the fields of genomics yielding more genetic information about ovarian cancer, in addition to the genotype of patients and with costs of sequencing decreasing, understanding the pathophysiology of ovarian cancer and rationale design of therapeutics are poised to move forward. In fact, the National Comprehensive Cancer Network genetics guidelines as well as several European organizations have recommended universal germline BRCA mutation screening for all women diagnosed with ovarian cancer. Screening women with ovarian cancer will enable the identification of family members at high risk and the risk of the patient developing other types of cancer, thus allowing the performance of risk-reducing surgeries for both patients and affected family members. Moreover, the extent of genetic testing, including panel testing that includes genes other than BRCA1 and BRCA2, continues to evolve and will contribute to our understanding of the genetics underlying the formation of ovarian cancer and its biology. Improved understanding of the genomics of different histological subtypes of ovarian cancer will be an important target over the upcoming years, to facilitate the understanding of the risk factors associated with this disease, as well as the development of prevention and therapeutic strategies.
Early detection efforts are promising, with ROCA testing demonstrating increased detection of early ovarian cancers compared with no testing. However, results of the UKCTOCS study did not show an overall survival advantage to using the ROCA testing, thus no screening test exists at this time. In addition, further research elucidating the role of variants of unknown importance in both BRCA genes and in other associated genes (such as BRIP1 and RAD51) in the risk of developing ovarian cancer is crucial for the appropriate recommendation of risk-reducing surgeries.
Other risk-reducing efforts, including surgical techniques such as bilateral salpingectomy, not directed at the high-risk population but more at a general risk population, are ongoing. Understanding the pathogenesis of the various types of ovarian cancer, such as the precursor STIC lesions for HGSC, is crucial for the appropriate use of surgical interventions for the prevention of ovarian cancer. Establishing uniform criteria for the definition of the site of origin of HGSCs, based on specific pathology findings, is being called for by consensus statements193.
Emerging therapies
Promising future therapies for ovarian cancer include PARP inhibitors and antibody–drug conjugates. PARP inhibitors, initially olaparib, have shown single-agent response rates of up to 30% in recurrent ovarian cancer, with the greatest activity in cancers with BRCA mutations and in platinum-sensitive disease194–196. Other PARP inhibitors (such as niraparib, rucaparib and veliparib) that have single-agent activity in ovarian cancer are in phase III studies of, for example, use as maintenance therapy in patients with platinum-sensitive recurrent ovarian cancer following a response to treatment with platinum-based chemotherapy197,198 (TABLE 6). Rucaparib was recently given breakthrough status (to accelerate the development and review of the drug) by the FDA based on results from the ARIEL2 trial199. Veliparib has been added to the NACT armamentarium with carboplatin and paclitaxel for newly diagnosed advanced ovarian cancer in a phase III study, in addition to testing as a maintenance therapy200.
Table 6 |.
Key ongoing or completed clinical trials of biologic agents for the treatment of ovarian cancer
| Example | Target | Comments | Refs |
|---|---|---|---|
| Anti-angiogenics | |||
| Bevacizumab | VEGF | Single-agent activity in both platinum-resistant and platinum-sensitive cancer | 173–176, 178,179 |
| Cediranib | VEGFR1, VEGFR2 and VEGFR3 | ||
| PARP inhibitors | |||
| Olaparib | PARP | Olaparib has been approved by the European Medicines Agency for the treatment of platinum-sensitive recurrent cancer as a maintenance therapy and by the US FDA for recurrent ovarian cancer in women with germline BRCA mutations and who have received at least three prior lines of chemotherapy | 170,178, 179, 194–199, 229,230,232–236 |
| Rucaparib | PARP | Single-agent activity in BRCA-mutated and wild-type ovarian cancer | |
| Veliparib | |||
| Niraparib | |||
| Immunotherapies | |||
| Nivolumab | Programmed cell death protein 1 | 15% response rate and 50% disease control rate | 237 |
| Pembrolizumab | Programmed cell death protein 1 | 11.5% response rate and 23.1% of patients had stable disease | 238 |
| Avelumab | Programmed cell death 1 ligand 1 | 10.7% risk reduction and 54.7% disease control rate; two patients with a clear-cell histology showed evidence of an objective response rate | 239 |
| Ipilimumab | Cytotoxic T lymphocyte 4 | Results pending | 240,241 |
| Antibody-drug conjugate | |||
| IMGN853 | Folate receptor-a | Promising preliminary data | 209 |
| Combination | |||
| Olaparib and cediranib | PARP, and VEGFR1, VEGFR2 and VEGFR3 | Two phase III studies are ongoing in both platinum-resistant and platinum-sensitive recurrent ovarian cancer | 178,179 |
| Pembrolizumab and niraparib | Various | Results pending | 206 |
| BKM120 or BYL719 and olaparib | Various | Maximum tolerated dose achieved for BKM120 and olaparib; anticancer activity noted for this combination | 205 |
PARP, poly(ADP-ribose) polymerase; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Acknowledging that the effectiveness of single-agent biologic therapies has reached a therapeutic plateau, one promising approach has been the development of combinations of biologic agents (anti-angiogenics, PARP inhibitors and immunotherapy agents)201–204. Such a strategy would target multiple cancer-promoting pathways or mechanisms and might be effective particularly in HGSC owing to its genomic complexity. Other histological subtypes such as clear-cell carcinoma that can harbour deficiencies in homologous recombination, such as mutations in ARID1A and PIK3CA, might also be clinically responsive. Furthermore, biologic combinations have the advantage of including agents that have non-overlapping adverse effects that might potentially reduce treatment-related toxicities.
Combining PARP inhibitors with targeted therapies against the PI3K pathway is being investigated, based on preclinical evidence from patient-derived xenograft models. Combinations of PARP inhibitors and, for example, cyclin-dependent kinase (CDK) inhibitors, immunotherapy agents and heat shock protein 90 (HSP90) inhibitors205,206, are also being assessed. Combining PARP inhibitors with chemotherapy has already proved challenging owing to overlapping myelosuppression associated with both therapies.
Study of immunotherapy strategies for the treatment of recurrent ovarian cancer is underway207, with several immune checkpoint inhibitors tested in recurrent disease (TABLE 6). At this time, many questions remain about the optimal strategies for the use of immunotherapies for the treatment of either newly diagnosed or recurrent ovarian cancer, but several studies are planned and are underway. Combinations of either chemotherapy and immunotherapy or two immunotherapy agents are under investigation. For example, nivolumab and ipilimumab (which has shown efficacy in melanoma) compared with nivolumab alone is being investigated, results of which are pending208. More research is needed to understand the selection of optimal immunotherapy through the use of biomarkers, the effect of the tumour microenvironment on cancer growth and determining the best and most effective therapeutic agents and combinations.
Antibody–drug conjugates have shown single-agent activity. One antibody–drug conjugate, IMGN853, targets the folate receptor-α and is linked to a highly potent maytansinoid that targets microtubules and suppresses microtubule dynamic instability, inducing cell cycle arrest and cell death209. IMGN853 has demonstrated impressive single-agent activity in patients with platinum-resistant recurrent ovarian cancer.
One other strategy for the therapeutic management of ovarian cancer is replacing mutated TP53 using gene therapy, as well as inhibition of MDM2 (the ligase that regulates p53 levels through small molecules). However, TP53 gene therapy using adenoviral vectors has been met with limited success partly owing to toxicity related to the approaches used210,211. Other emerging therapies targeting tumours carrying mutant TP53 include COTI-2, which is thought to induce a ‘wild-type-like’ conformational change in mutant p53 and is currently in clinical trials212.
Importance of tumour histology.
With the improved understanding that ovarian cancer is composed of several histologically and molecularly distinct subtypes, certain classes of therapeutics have histology-specific mechanisms of action, such as PARP inhibitors for the treatment of HGSC and MEK inhibitors for the treatment of LGSC. Activity of the MEK inhibitor selumetinib in LGSC has been demonstrated213; clinical trials comparing the use of MEK inhibitors to chemotherapy for the treatment of recurrent LGSC are underway, including a phase III study of binimetinib (also known as MEK162) compared with the physician’s choice of chemotherapy agent (the MILO study)214. However, the MILO study was terminated because of futility, based on a planned interim analysis showing that the HR for PFS crossed a predefined futility boundary. One other study assessing MEK inhibition for the management of LGSC is investigating trametinib (also known as GSK 1120212) in patients with recurrent or progressive LGSC215, but this study has been suspended owing to problems with the drug supply.
Drug approvals.
One challenge for the development of new therapies in ovarian cancer is the approval mechanisms of the FDA and the EMA. Demonstrating an improvement in overall survival is a requirement for regulatory approval but is difficult to demonstrate in ovarian cancer; explanations for this are not fully understood but possibly include the use of active study agents after disease progression, which dilutes the effect of the active agent on overall survival but not on PFS216,217. In addition, the lack of subclassification of histological subtypes for clinical trial eligibility might have diluted the efficacy of some therapeutic agents, such as bevacizumab, and also because no biomarker currently exists to select patients to receive this therapy. Several groups are calling for the achievement of significant improvement in PFS, coupled with PRO measures demonstrating the benefit of treatment, as a reason for drug approval; the approval of bevacizumab and olaparib was owing to improvement in PFS, quality of life, duration of response or response rate; however, approvals based on PROs are rare218,219. The time frame between subsequent therapies (that is, the second PFS) or time between paracentesis or thoracentesis procedures could also be important measurements of patient benefit from a specific therapy. PROs should be a vital component of any phase III study, particularly those testing agents for potential regulatory approval for the treatment of ovarian cancer.
Acknowledgements
U.A.M. has received research support from the Ovarian Cancer Research Foundation, the Breast Cancer Research Foundation and the US Department of Defense. A.S. has received research support from the US National Institute of Health (CA109298, P50 CA083639 and P50 CA098258).
Footnotes
Competing interests
U.A.M. has served as a consultant for AstraZeneca, ImmunoGen, Pfizer, Genentech and Merck. All other authors declare no competing interests.
References
- 1.Oswald AJ & Gourley C Low-grade epithelial ovarian cancer: a number of distinct clinical entities? Curr. Opin. Oncol 27, 412–419 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Groen RS, Gershenson DM & Fader AN Updates and emerging therapies for rare epithelial ovarian cancers: one size no longer fits all. Gynecol. Oncol 136, 373–383 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Mangili G et al. Unraveling the two entities of endometrioid ovarian cancer: a single center clinical experience. Gynecol. Oncol 126, 403–407 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Callegaro-Filho D et al. Small cell carcinoma of the ovary-hypercalcemic type (SCCOHT): a review of 47 cases. Gynecol. Oncol 140, 53–57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witkowski L et al. The influence of clinical and genetic factors on patient outcome in small cell carcinoma of the ovary, hypercalcemic type. Gynecol. Oncol 141, 454–460 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kindelberger DW et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am. J. Surg. Pathol 31, 161–169 (2007). [DOI] [PubMed] [Google Scholar]; This paper associates STICs that arise in the distal fallopian tube with eventual development of HGSCs of the ovary and fallopian tube.
- 7.Pentheroudakis G & Pavlidis N Serous papillary peritoneal carcinoma: unknown primary tumour, ovarian cancer counterpart or a distinct entity? A systematic review. Crit. Rev. Oncol. Hematol 75, 27–42 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Kurman RJ, Carcangiu ML, Herrinton CS & Young RH (eds) WHO Classification of Tumours Female Reproductive Organs (IARC, 2014). [Google Scholar]
- 9.Menon U et al. Risk algorithm using serial biomarker measurements doubles the number of screen-detected cancers compared with a single-threshold rule in the United Kingdom Collaborative Trial of Ovarian Cancer Screening. J. Clin. Oncol 33, 2062–2071 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs IJ et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 387, 945–956 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGuire WP et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N. Engl. J. Med 334, 1–6 (1996). [DOI] [PubMed] [Google Scholar]; This trial was the first to demonstrate a PFS and overall survival benefit of the addition of paclitaxel to platinum therapy, thereby helping to establish the standard of care of a platinum plus taxane chemotherapy regimen for newly diagnosed ovarian cancer.
- 12.Bookman MA et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III Trial of the Gynecologic Cancer Intergroup. J. Clin. Oncol 27, 1419–1425 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.du Bois A et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl Cancer Inst 95, 1320–1329 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Ozols RF et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J. Clin. Oncol 21, 3194–3200 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Vasey PA et al. Phase III randomized trial of docetaxel–carboplatin versus paclitaxel–carboplatin as first-line chemotherapy for ovarian carcinoma. J. Natl Cancer Inst 96, 1682–1691 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD & Jemal A Cancer statistics, 2016. CA Cancer J. Clin 66, 7–30 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Jemal A et al. Global cancer statistics. CA Cancer J. Clin 61, 69–90 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Sant M et al. Survival of women with cancers of breast and genital organs in Europe 1999–2007: results of the EUROCARE-5 study. Eur. J. Cancer 51, 2191–2205 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Lowe KA et al. An international assessment of ovarian cancer incidence and mortality. Gynecol. Oncol 130, 107–114 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Sung P-L, Chang Y-H, Chao K-C & Chuang C-M Global distribution pattern of histological subtypes of epithelial ovarian cancer: a database analysis and systematic review. Gynecol. Oncol 133, 147–154 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Yang HP et al. Ovarian cancer incidence trends in relation to changing patterns of menopausal hormone therapy use in the United States. J. Clin. Oncol 31, 2146–2151 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute. SEER stat fact sheets: ovarian cancer. SEER http://seer.cancer.gov/statfacts/html/ovary.html (2016). [Google Scholar]
- 23.Zhang S et al. Frequencies of BRCA1 and BRCA2 mutations among 1,342 unselected patients with invasive ovarian cancer. Gynecol. Oncol 121, 353–357 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Alsop K et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol 30, 2654–2663 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castilla LH et al. Mutations in the BRCA1 gene in families with early-onset breast and ovarian cancer. Nat. Genet 8, 387–391 (1994). [DOI] [PubMed] [Google Scholar]
- 26.Pennington KP & Swisher EM Hereditary ovarian cancer: beyond the usual suspects. Gynecol. Oncol 124, 347–353 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Bolton KL et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 307, 382–390 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; The presence of germline BRCA mutations improves the survival of women with ovarian cancer compared with women with sporadic ovarian cancer; BRCA2 mutations are associated with a better outcome than BRCA1 mutations.
- 28.Liu G et al. Differing clinical impact of BRCA1 and BRCA2 mutations in serous ovarian cancer. Pharmacogenomics 13, 1523–1535 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rebbeck TR et al. Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA 313, 1347–1361 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh T et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc. Natl Acad. Sci. USA 108, 18032–18037 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norquist BM et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol 2, 482–490 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reviews the known and established inherited germline mutations that are associated with an increased risk of developing ovarian cancer.
- 32.Prakash R, Zhang Y, Feng W & Jasin M Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb. Perspect. Biol 7, a016600 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suwaki N, Klare K & Tarsounas M RAD51 paralogs: roles in DNA damage signalling, recombinational repair and tumorigenesis. Semin. Cell Dev. Biol 22, 898–905 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Ketabi Z et al. Ovarian cancer linked to lynch syndrome typically presents as early-onset, non-serous epithelial tumors. Gynecol. Oncol 121, 462–465 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Engel C et al. Risks of less common cancers in proven mutation carriers with lynch syndrome. J. Clin. Oncol 30, 4409–4415 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Crispens MA Endometrial and ovarian cancer in lynch syndrome. Clin. Colon Rectal Surg 25, 97–102 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moorman PG et al. Oral contraceptives and risk of ovarian cancer and breast cancer among high-risk women: a systematic review and meta-analysis. J. Clin. Oncol 31, 4188–4198 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Bassuk SS & Manson JE Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann. Epidemiol 25, 193–200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Havrilesky LJ et al. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Obstet. Gynecol 122, 139–147 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Wentzensen N et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. J. Clin. Oncol June 20 2016. [epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Havrilesky LJ et al. Oral contraceptive use for the primary prevention of ovarian cancer. Evid. Rep. Technol. Assess. (Full Rep.) 212, 1–514 (2013). [PMC free article] [PubMed] [Google Scholar]
- 42.Pearce CL, Chung K, Pike MC & Wu AH Increased ovarian cancer risk associated with menopausal estrogen therapy is reduced by adding a progestin. Cancer 115, 531–539 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mørch LS, Løkkegaard E, Andreasen AH, Krüger-Kjaer S & Lidegaard O Hormone therapy and ovarian cancer. JAMA 302, 298–305 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Hildebrand JS et al. Postmenopausal hormone use and incident ovarian cancer: associations differ by regimen. Int. J. Cancer 127, 2928–2935 (2010). [DOI] [PubMed] [Google Scholar]
- 45.Collaborative Group On Epidemiological Studies Of Ovarian Cancer et al. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet 385, 1835–1842 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eeles RA et al. Adjuvant hormone therapy may improve survival in epithelial ovarian cancer: results of the AHT randomized trial. J. Clin. Oncol 33, 4138–4144 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Friebel TM, Domchek SM & Rebbeck TR Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J. Natl Cancer Inst 106, dju091 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rice MS, Hankinson SE & Tworoger SS Tubal ligation, hysterectomy, unilateral oophorectomy, and risk of ovarian cancer in the Nurses’ Health Studies. Fertil. Steril 102, 192–198.e3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaitskell K et al. Tubal ligation and ovarian cancer risk in a large cohort: substantial variation by histological type. Int. J. Cancer 138, 1076–1084 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Domchek SM et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. JAMA 304, 967–975 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kotsopoulos J et al. Ovarian cancer risk factors by tumor dominance, a surrogate for cell of origin. Int. J. Cancer 133, 730–739 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keum N et al. Adult weight gain and adiposity-related cancers: a dose–response meta-analysis of prospective observational studies. J. Natl. Cancer Inst 107, djv088 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Olsen CM et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr. Relat. Cancer 20, 251–262 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nagle CM et al. Obesity and survival among women with ovarian cancer: results from the Ovarian Cancer Association Consortium. Br. J. Cancer 113, 817–826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cannioto RA & Moysich KB Epithelial ovarian cancer and recreational physical activity: a review of the epidemiological literature and implications for exercise prescription. Gynecol. Oncol 137, 559–573 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merritt MA, Poole EM, Hankinson SE, Willett WC & Tworoger SS Dairy food and nutrient intake in different life periods in relation to risk of ovarian cancer. Cancer Causes Control 25, 795–808 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koushik A et al. Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control 26, 1315–1327 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xie J et al. A prospective cohort study of dietary indices and incidence of epithelial ovarian cancer. J. Ovarian Res 7, 112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cassidy A, Huang T, Rice MS, Rimm EB & Tworoger SS Intake of dietary flavonoids and risk of epithelial ovarian cancer. Am. J. Clin. Nutr 100, 1344–1351 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Narod SA Talc and ovarian cancer. Gynecol. Oncol 141, 410–412 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Terry KL et al. Genital powder use and risk of ovarian cancer: a pooled analysis of 8,525 cases and 9,859 controls. Cancer Prev. Res. (Phila) 6, 811–821 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Houghton SC et al. Perineal powder risk ovarian Cancer. J. Natl Cancer Inst 106, dju208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trabert B et al. Aspirin, nonaspirin nonsteroidal anti-inflammatory drug, acetaminophen risk invasive epithelial ovarian cancer: pooled analysis in the Ovarian Cancer Association Consortium. J. Natl Cancer Inst 106, djt431 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang T et al. Depression and risk of epithelial ovarian cancer: results from two large prospective cohort studies. Gynecol. Oncol 139, 481–486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bell D et al. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper analyses the molecular composition of HGSC, which is a cancer of genomic instability, DNA repair defects and copy number alterations.
- 66.Berns EMJJ & Bowtell DD The changing view of high-grade serous ovarian cancer. Cancer Res 72, 2701–2704 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Patch A-M et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 521, 489–494 (2015). [DOI] [PubMed] [Google Scholar]; This paper demonstrates that many genetic abnormalities contribute to platinum and overall chemotherapy insensitivity, including cyclin E1 amplification, MDR1 overexpression and BRCA reversion mutations.
- 68.Liu J & Matulonis UA New strategies in ovarian cancer: translating the molecular complexity of ovarian cancer into treatment advances. Clin. Cancer Res 20, 5150–5156 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Galic V, Coleman RL & Herzog TJ Unmet needs in ovarian cancer: dividing histologic subtypes to exploit novel targets and pathways. Curr. Cancer Drug Targets 13, 698–707 (2013). [DOI] [PubMed] [Google Scholar]
- 70.Bashashati A et al. Distinct evolutionary trajectories of primary high-grade serous ovarian cancers revealed through spatial mutational profiling. J. Pathol 231, 21–34 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed AA et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol 221, 49–56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Donovan PJ & Livingston DM BRCA1 and BRCA2: breast/ovarian cancer susceptibility gene products and participants in DNA double-strand break repair. Carcinogenesis 31, 961–967 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Baratta MG et al. An in-tumor genetic screen reveals that the BET bromodomain protein, BRD4, is a potential therapeutic target in ovarian carcinoma. Proc. Natl Acad. Sci. USA 112, 232–237 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reyes-González JM et al. Targeting c-MYC in platinum-resistant ovarian cancer. Mol. Cancer Ther 14, 2260–2269 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tothill RW et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin. Cancer Res 14, 5198–5208 (2008). [DOI] [PubMed] [Google Scholar]
- 76.Bentink S et al. Angiogenic mRNA and microRNA gene expression signature predicts a novel subtype of serous ovarian cancer. PLoS ONE 7, e30269 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verhaak RGW et al. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J. Clin. Invest 123, 517–525 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang D et al. Integrated analyses identify a master microRNA regulatory network for the mesenchymal subtype in serous ovarian cancer. Cancer Cell 23, 186–199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Etemadmoghadam D et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin. Cancer Res 15, 1417–1427 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tan DSP et al. Genomic analysis reveals the molecular heterogeneity of ovarian clear cell carcinomas. Clin. Cancer Res 17, 1521–1534 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Della Pepa C et al. Low grade serous ovarian carcinoma: from the molecular characterization to the best therapeutic strategy. Cancer Treat. Rev 41, 136–143 (2015). [DOI] [PubMed] [Google Scholar]
- 82.Romero I, Sun CC, Wong KK, Bast RC & Gershenson DM Low-grade serous carcinoma: new concepts and emerging therapies. Gynecol. Oncol 130, 660–666 (2013). [DOI] [PubMed] [Google Scholar]
- 83.Tone AA et al. Intratumoral heterogeneity in a minority of ovarian low-grade serous carcinomas. BMC Cancer 14, 982 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wiegand KC et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med 363, 1532–1543 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown J & Frumovitz M Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr. Oncol. Rep 16, 389 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ryland GL et al. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med 7, 87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jelinic P et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat. Genet 46, 424–426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kurman RJ & Shih I-M The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am. J. Surg. Pathol 34, 433–443 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Medeiros F et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am. J. Surg. Pathol 30, 230–236 (2006). [DOI] [PubMed] [Google Scholar]
- 90.Carlson JW et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J. Clin. Oncol 26, 4160–4165 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perets R et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell 24, 751–765 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee Y et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J. Pathol 211, 26–35 (2007). [DOI] [PubMed] [Google Scholar]
- 93.Howitt BE et al. Evidence for a dualistic model of high-grade serous carcinoma: BRCA mutation status, histology, and tubal intraepithelial carcinoma. Am. J. Surg. Pathol 39, 287–293 (2015). [DOI] [PubMed] [Google Scholar]
- 94.Crum CP et al. Through the glass darkly: intraepithelial neoplasia, top-down differentiation, and the road to ovarian cancer. J. Pathol 231, 402–412 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Powell CB et al. Long term follow up of BRCA1 and BRCA2 mutation carriers with unsuspected neoplasia identified at risk reducing salpingo-oophorectomy. Gynecol. Oncol 129, 364–371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang L et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med 348, 203–213 (2003). [DOI] [PubMed] [Google Scholar]
- 97.Hwang W-T, Adams SF, Tahirovic E, Hagemann IS & Coukos G Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol. Oncol 124, 192–198 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Musrap N & Diamandis EP Revisiting the complexity of the ovarian cancer microenvironment — clinical implications for treatment strategies. Mol. Cancer Res 10, 1254–1264 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Zsiros E, Tanyi J, Balint K & Kandalaft LE Immunotherapy for ovarian cancer: recent advances and perspectives. Curr. Opin. Oncol 26, 492–500 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Schlienger K et al. TRANCE- and CD40 ligand-matured dendritic cells reveal MHC class I-restricted T cells specific for autologous tumor in late-stage ovarian cancer patients. Clin. Cancer Res 9, 1517–1527 (2003). [PubMed] [Google Scholar]
- 101.Santin AD et al. In vitro induction of tumor-specific human lymphocyte antigen class I-restricted CD8+ cytotoxic T lymphocytes by ovarian tumor antigen-pulsed autologous dendritic cells from patients with advanced ovarian cancer. Am. J. Obstet. Gynecol 183, 601–609 (2000). [DOI] [PubMed] [Google Scholar]
- 102.Yang X, Shen F, Hu W, Coleman RL & Sood AK New ways to successfully target tumor vasculature in ovarian cancer. Curr. Opin. Obstet. Gynecol 27, 58–65 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bottsford-Miller JN, Coleman RL & Sood AK Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J. Clin. Oncol 30, 4026–4034 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lu C et al. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res 67, 1757–1768 (2007). [DOI] [PubMed] [Google Scholar]
- 105.Sakai W et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature 451, 1116–1120 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goff BA, Mandel L, Muntz HG & Melancon CH Ovarian carcinoma diagnosis. Cancer 89, 2068–2075 (2000). [DOI] [PubMed] [Google Scholar]
- 107.Goff BA, Mandel LS, Melancon CH & Muntz HG Frequency of symptoms of ovarian cancer in women presenting to primary care clinics. JAMA 291, 2705–2712 (2004). [DOI] [PubMed] [Google Scholar]
- 108.US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01493505 (2014).
- 109.Demir RH & Marchand GJ Adnexal masses suspected to be benign treated with laparoscopy. JSLS 16, 71–84 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sokalska A et al. Diagnostic accuracy of transvaginal ultrasound examination for assigning a specific diagnosis to adnexal masses. Ultrasound Obstet. Gynecol 34, 462–470 (2009). [DOI] [PubMed] [Google Scholar]
- 111.Prat J & FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynecol. Obstet 124, 1–5 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Ferrandina G, Legge F, Petrillo M, Salutari V & Scambia G Ovarian cancer patients with ‘node-positive-only’ stage IIIC disease have a more favorable outcome than stage IIIA/B. Gynecol. Oncol 107, 154–156 (2007). [DOI] [PubMed] [Google Scholar]
- 113.Baek S-J et al. Stage IIIC epithelial ovarian cancer classified solely by lymph node metastasis has a more favorable prognosis than other types of stage IIIC epithelial ovarian cancer. J. Gynecol. Oncol 19, 223–228 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Piver MS, Barlow JJ & Lele SB Incidence of subclinical metastasis in stage I and II ovarian carcinoma. Obstet. Gynecol 52, 100–104 (1978). [PubMed] [Google Scholar]
- 115.Piver MS Optimal surgical therapy in stage I and II ovarian malignancies. Int. J. Radiat. Oncol 8, 247–249 (1982). [DOI] [PubMed] [Google Scholar]
- 116.Mayer AR et al. Ovarian cancer staging: does it require a gynecologic oncologist? Gynecol. Oncol 47, 223–227 (1992). [DOI] [PubMed] [Google Scholar]
- 117.McGowan L, Lesher LP, Norris HJ & Barnett M Misstaging of ovarian cancer. Obstet. Gynecol 65, 568–572 (1985). [PubMed] [Google Scholar]
- 118.Kobayashi H et al. A randomized study of screening for ovarian cancer: a multicenter study in Japan. Int. J. Gynecol. Cancer 18, 414–420 (2008). [DOI] [PubMed] [Google Scholar]
- 119.Jacobs IJ et al. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet 353, 1207–1210 (1999). [DOI] [PubMed] [Google Scholar]
- 120.Tian C et al. CA-125 change after chemotherapy in prediction of treatment outcome among advanced mucinous and clear cell epithelial ovarian cancers: a Gynecologic Oncology Group study. Cancer 115, 1395–1403 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Buys SS et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 305, 2295–2303 (2011). [DOI] [PubMed] [Google Scholar]
- 122.Hellström I et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res 63, 3695–3700 (2003). [PubMed] [Google Scholar]
- 123.Wu L et al. Diagnostic value of serum human epididymis protein 4 (HE4) in ovarian carcinoma: a systematic review and meta-analysis. Int. J. Gynecol. Cancer 22, 1106–1112 (2012). [DOI] [PubMed] [Google Scholar]
- 124.Maritschnegg E et al. Lavage of the uterine cavity for molecular detection of mullerian duct carcinomas: a proof-of-concept study. J. Clin. Oncol 33, 4293–4300 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Falconer H, Yin L, Grönberg H & Altman D Ovarian cancer risk after salpingectomy: a nationwide population-based study. J. Natl. Cancer Inst 107, dju410 (2015). [DOI] [PubMed] [Google Scholar]
- 126.McAlpine JN et al. Opportunistic salpingectomy: uptake, risks, and complications of a regional initiative for ovarian cancer prevention. Am. J. Obstet. Gynecol 210, 471.e1–471.e11 (2014). [DOI] [PubMed] [Google Scholar]
- 127.Kwon JS et al. Costs and benefits of opportunistic salpingectomy as an ovarian cancer prevention strategy. Obstet. Gynecol 125, 338–345 (2015). [DOI] [PubMed] [Google Scholar]
- 128.Walker JL et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer 121, 2108–2120 (2015). [DOI] [PubMed] [Google Scholar]
- 129.National Comprehensive Cancer Network. Clinical practice guidelines in oncology (NCCN guidelines). Genetic/familial high-risk assessment: breast and ovarian. NCCN https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf (2016). [Google Scholar]
- 130.Finch APM et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J. Clin. Oncol 32, 1547–1553 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Eccles DM et al. BRCA1 and BRCA2 genetic testing-pitfalls and recommendations for managing variants of uncertain clinical significance. Ann. Oncol 26, 2057–2065 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chan JK et al. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet. Gynecol 109, 1342–1350 (2007). [DOI] [PubMed] [Google Scholar]
- 133.Cliby WA et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol. Oncol 136, 11–17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bristow RE, Chang J, Ziogas A & Anton-Culver H Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet. Gynecol 121, 1226–1234 (2013). [DOI] [PubMed] [Google Scholar]
- 135.Chang S-J, Hodeib M, Chang J & Bristow RE Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol. Oncol 130, 493–498 (2013). [DOI] [PubMed] [Google Scholar]
- 136.Horowitz NS et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: an analysis of GOG 182. J. Clin. Oncol 33, 937–943 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chang S-J, Bristow RE & Ryu H-S Impact of complete cytoreduction leaving no gross residual disease associated with radical cytoreductive surgical procedures on survival in advanced ovarian cancer. Ann. Surg. Oncol 19, 4059–4067 (2012). [DOI] [PubMed] [Google Scholar]
- 138.National Comprehensive Cancer Netwwork. Clinical practice guidelines in oncology (NCCN guidelines). Ovarian cancer including fallopian tube cancer and primary peritoneal cancer. NCCN https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (2016). [Google Scholar]
- 139.Armstrong DK et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N. Engl. J. Med 354, 34–43 (2009). [DOI] [PubMed] [Google Scholar]
- 140.Katsumata N et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet 374, 1331–1338 (2009). [DOI] [PubMed] [Google Scholar]; Weekly paclitaxel is superior to paclitaxel every 3 weeks when combined with carboplatin for newly diagnosed ovarian cancer.
- 141.Katsumata N et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol 14, 1020–1026 (2013). [DOI] [PubMed] [Google Scholar]
- 142.Burger RA et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med 365, 2473–2483 (2011). [DOI] [PubMed] [Google Scholar]; This is the first phase III study to test a biologic agent, bevacizumab, combined with carboplatin and paclitaxel chemotherapy for newly diagnosed ovarian cancer.
- 143.Perren TJ et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med 365, 2484–2496 (2011). [DOI] [PubMed] [Google Scholar]
- 144.Oza AM et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol 16, 928–936 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vergote I et al. Neoadjuvant chemotherapy or primary surgery in stage IIIc or IV ovarian cancer. N. Engl. J. Med 363, 943–953 (2010). [DOI] [PubMed] [Google Scholar]; Overall survival and PFS are similar for upfront cytoreductive surgery followed by platinum-based and taxane-based chemotherapy versus NACT followed by interval cytoreductive surgery followed by completion chemotherapy; complications of surgery are less with NACT than with upfront cytoreductive surgery.
- 146.Kehoe S et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 386, 249–257 (2015). [DOI] [PubMed] [Google Scholar]
- 147.Gómez-Hidalgo NR et al. Predictors of optimal cytoreduction in patients with newly diagnosed advanced-stage epithelial ovarian cancer: time to incorporate laparoscopic assessment into the standard of care. Gynecol. Oncol 137, 553–558 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.du Bois A et al. Neoadjuvant chemotherapy cannot be regarded as adequate routine therapy strategy of advanced ovarian cancer. Int. J. Gynecol. Cancer 22, 182–185 (2012). [DOI] [PubMed] [Google Scholar]
- 149.Mahner S et al. Neoadjuvant chemotherapy in ovarian cancer revisited. Ann. Oncol 27 (Suppl. 1), i30–i32 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Markman M Maintenance chemotherapy in the management of epithelial ovarian cancer. Cancer Metastasis Rev 34, 11–17 (2015). [DOI] [PubMed] [Google Scholar]
- 151.Mei L et al. Maintenance chemotherapy for ovarian cancer. Cochrane Database Syst. Rev 6, CD007414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Markman M et al. Phase III randomized trial of 12 versus 3 months of maintenance paclitaxel in patients with advanced ovarian cancer after complete response to platinum and paclitaxel-based chemotherapy: a Southwest Oncology Group and Gynecologic Oncology Group Trial. J. Clin. Oncol 21, 2460–2465 (2003). [DOI] [PubMed] [Google Scholar]
- 153.du Bois A et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J. Clin. Oncol 32, 3374–3382 (2014). [DOI] [PubMed] [Google Scholar]
- 154.Rustin GJ, Marples M, Nelstrop AE, Mahmoudi M & Meyer T Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J. Clin. Oncol 19, 4054–4057 (2001). [DOI] [PubMed] [Google Scholar]
- 155.Salani R et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am. J. Obstet. Gynecol 204, 466–478 (2011). [DOI] [PubMed] [Google Scholar]
- 156.Rustin GJS et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet 376, 1155–1163 (2010). [DOI] [PubMed] [Google Scholar]
- 157.Morris RT & Monk BJ Ovarian cancer: relevant therapy, not timing, is paramount. Lancet 376, 1120–1122 (2010). [DOI] [PubMed] [Google Scholar]
- 158.Al Rawahi T et al. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst. Rev 2, CD008765 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT00565851 (2007).
- 160.Harter P et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann. Surg. Oncol 13, 1702–1710 (2006). [DOI] [PubMed] [Google Scholar]
- 161.US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01166737 (2010).
- 162.The National Academies of Sciences, Engineering and Medicine. Ovarian cancers: evolving paradigms in research and care. National Academies http://www.nationalacademies.org/hmd/Reports/2016/state-of-ovarian-cancer.aspx (2016). [PubMed] [Google Scholar]
- 163.Alvarez RD et al. Moving beyond the platinum sensitive/resistant paradigm for patients with recurrent ovarian cancer. Gynecol. Oncol 141, 405–409 (2016). [DOI] [PubMed] [Google Scholar]
- 164.Castells MC et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J. Allergy Clin. Immunol 122, 574–580 (2008). [DOI] [PubMed] [Google Scholar]
- 165.Parkin D et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet 361, 2099–2106 (2003). [DOI] [PubMed] [Google Scholar]
- 166.Pujade-Lauraine E et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum-sensitive ovarian cancer in late relapse. J. Clin. Oncol 28, 3323–3329 (2010). [DOI] [PubMed] [Google Scholar]
- 167.Pfisterer J et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J. Clin. Oncol 24, 4699–4707 (2006). [DOI] [PubMed] [Google Scholar]
- 168.Markman M et al. Duration of response to second-line, platinum-based chemotherapy for ovarian cancer: implications for patient management and clinical trial design. J. Clin. Oncol 22, 3120–3125 (2004). [DOI] [PubMed] [Google Scholar]
- 169.US Food and Drug Administration. Oncologic drugs advisory committee meeting announcement. FDA http://www.fda.gov/AdvisoryCommittees/Calendar/ucm394126.htm (2014). [Google Scholar]
- 170.US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01874353 (2013).
- 171.Pujade-Lauraine E et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J. Clin. Oncol 32, 1302–1308 (2014). [DOI] [PubMed] [Google Scholar]
- 172.Poveda AM et al. Bevacizumab combined with weekly paclitaxel, pegylated liposomal doxorubicin, or topotecan in platinum-resistant recurrent ovarian cancer: analysis by chemotherapy cohort of the randomized phase III AURELIA trial. J. Clin. Oncol 33, 3836–3838 (2015). [DOI] [PubMed] [Google Scholar]
- 173.Cannistra SA et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J. Clin. Oncol 25, 5180–5186 (2007). [DOI] [PubMed] [Google Scholar]
- 174.Gadducci A, Lanfredini N & Sergiampietri C Antiangiogenic agents in gynecological cancer: state of art and perspectives of clinical research. Crit. Rev. Oncol. Hematol 96, 113–128 (2015). [DOI] [PubMed] [Google Scholar]
- 175.Jackson AL, Eisenhauer EL & Herzog TJ Emerging therapies: angiogenesis inhibitors for ovarian cancer. Expert Opin. Emerg. Drugs 20, 331–346 (2015). [DOI] [PubMed] [Google Scholar]
- 176.Matulonis UA et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J. Clin. Oncol 27, 5601–5606 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ledermann JA et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 387, 1066–1074 (2016). [DOI] [PubMed] [Google Scholar]
- 178.US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02446600 (2015).
- 179.US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02502266 (2015).
- 180.Park CL, Edmondson D, Fenster JR & Blank TO Meaning making and psychological adjustment following cancer: the mediating roles of growth, life meaning, and restored just-world beliefs. J. Consult. Clin. Psychol 76, 863–875 (2008). [DOI] [PubMed] [Google Scholar]
- 181.Stockler MR et al. Patient-reported outcome results from the open-label phase III AURELIA trial evaluating bevacizumab-containing therapy for platinum-resistant ovarian cancer. J. Clin. Oncol 32, 1309–1316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Monk BJ et al. Patient reported outcomes of a randomized, placebo-controlled trial of bevacizumab in the front-line treatment of ovarian cancer: a Gynecologic Oncology Group study. Gynecol. Oncol 128, 573–578 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Basch E The missing voice of patients in drug-safety reporting. N. Engl. J. Med 362, 865–869 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.US Food and Drug Administration. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. FDA http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Donovan KA et al. Recommended patient-reported core set of symptoms and quality-of-life domains to measure in ovarian cancer treatment trials. J. Natl Cancer Inst 106, dju128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wenzel L et al. Validation of FACT/GOG-AD subscale for ovarian cancer-related abdominal discomfort: a Gynecologic Oncology Group study. Gynecol. Oncol 110, 60–64 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Calhoun EA et al. Psychometric evaluation of the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (Fact/GOG-Ntx) questionnaire for patients receiving systemic chemotherapy. Int. J. Gynecol. Cancer 13, 741–748 (2003). [DOI] [PubMed] [Google Scholar]
- 188.Jenkins V et al. Patients’ and oncologists’ views on the treatment and care of advanced ovarian cancer in the U. K.: results from the ADVOCATE study. Br. J. Cancer 108, 2264–2271 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Jensen SE, Kaiser K, Lacson L, Schink J & Cella D Content validity of the NCCN-FACT Ovarian Symptom Index-18 (NFOSI-18). Gynecol. Oncol 136, 317–322 (2015). [DOI] [PubMed] [Google Scholar]
- 190.Cella DF et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J. Clin. Oncol 11, 570–579 (1993). [DOI] [PubMed] [Google Scholar]
- 191.Basen-Engquist K et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. J. Clin. Oncol 19, 1809–1817 (2001). [DOI] [PubMed] [Google Scholar]
- 192.Havrilesky LJ et al. Patient preferences in advanced or recurrent ovarian cancer. Cancer 120, 3651–3659 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Singh N et al. Primary site assignment in tuboovarian high-grade serous carcinoma: consensus statement on unifying practice worldwide. Gynecol. Oncol 141, 195–198 (2016). [DOI] [PubMed] [Google Scholar]
- 194.Audeh MW et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376, 245–251 (2010). [DOI] [PubMed] [Google Scholar]
- 195.Gelmon KA et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 12, 852–861 (2011). [DOI] [PubMed] [Google Scholar]
- 196.Matulonis UA et al. Olaparib monotherapy in patients with advanced relapsed ovarian cancer and a germline BRCA1/2 mutation: a multi-study analysis of response rates and safety. Ann. Oncol 27, 1013–1019 (2016). [DOI] [PubMed] [Google Scholar]
- 197.Sandhu SK et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol 14, 882–892 (2013). [DOI] [PubMed] [Google Scholar]
- 198.Coleman RL et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation. Gynecol. Oncol 137, 386–391 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.McNeish IA et al. Results of ARIEL2: a phase 2 trial to prospectively identify ovarian cancer patients likely to respond to rucaparib using tumor genetic analysis. ASCO Meeting Abstr 33, 5508 (2015). [Google Scholar]
- 200.US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02470585 (2015).
- 201.Glickman MS & Sawyers CL Converting cancer therapies into cures: lessons from infectious diseases. Cell 148, 1089–1098 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hanahan D & Weinberg RA Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 203.Yap TA, Omlin A & de Bono JS Development of therapeutic combinations targeting major cancer signaling pathways. J. Clin. Oncol 31, 1592–1605 (2013). [DOI] [PubMed] [Google Scholar]
- 204.Paller CJ et al. Design of phase I combination trials: recommendations of the Clinical Trial Design Task Force of the NCI Investigational Drug Steering Committee. Clin. Cancer Res 20, 4210–4217 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Matulonis U et al. Phase I study of oral BKM120 and oral olaparib for high-grade serous ovarian cancer (HGSC) or triple-negative breast cancer (TNBC). ASCO Meeting Abstr 32, 2510 (2014). [Google Scholar]
- 206.US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02657889 (2016).
- 207.Coukos G, Tanyi J & Kandalaft LE Opportunities in immunotherapy of ovarian cancer. Ann. Oncol 27, i11–i15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT02498600 (2015).
- 209.Moore KN et al. Preliminary single agent activity of IMGN853, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in platinum-resistant epithelial ovarian cancer (EOC) patients (pts): phase I trial. ASCO Meeting Abstr 33, 5518 (2015). [Google Scholar]
- 210.Vazquez A, Bond EE, Levine AJ & Bond GL The genetics of the p53 pathway, apoptosis and cancer therapy. Nat. Rev. Drug Discov 7, 979–987 (2008). [DOI] [PubMed] [Google Scholar]
- 211.Cheok CF, Verma CS, Baselga J & Lane DP Translating p53 into the clinic. Nat. Rev. Clin. Oncol 8, 25–37 (2011). [DOI] [PubMed] [Google Scholar]
- 212.Liu Y et al. TP53 loss creates therapeutic vulnerability in colorectal cancer. Nature 520, 697–701 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Farley J et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol 14, 134–140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01849874 (2013).
- 215.US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT02101788 (2014).
- 216.Herzog TJ et al. Ovarian cancer clinical trial endpoints: Society of Gynecologic Oncology white paper. Gynecol. Oncol 132, 8–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Matulonis UA, Oza AM, Ho TW & Ledermann JA Intermediate clinical endpoints: a bridge between progression-free survival and overall survival in ovarian cancer trials. Cancer 121, 1737–1746 (2015). [DOI] [PubMed] [Google Scholar]
- 218.Herzog TJ et al. SGO guidance document for clinical trial designs in ovarian cancer: a changing paradigm. Gynecol. Oncol 135, 3–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gnanasakthy A et al. Patient-reported outcomes labeling for products approved by the Office of Hematology and Oncology Products of the US Food and Drug Administration (2010–2014). J. Clin. Oncol 34, 1928–1934 (2016). [DOI] [PubMed] [Google Scholar]
- 220.Mutch DG & Prat J 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol. Oncol 133, 401–404 (2014). [DOI] [PubMed] [Google Scholar]; This reference, along with reference 111, is the current staging system for ovarian cancer.
- 221.Walker J et al. A phase III trial of bevacizumab with IV versus IP chemotherapy for ovarian, fallopian tube, and peritoneal carcinoma: an NRG oncology study. Gynecol. Oncol 141 (Suppl. 1), 208 (2016). [Google Scholar]
- 222.Pignata S et al. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 15, 396–405 (2014). [DOI] [PubMed] [Google Scholar]
- 223.Pignata S et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: the MITO-2 randomized phase III trial. J. Clin. Oncol 29, 3628–3635 (2011). [DOI] [PubMed] [Google Scholar]
- 224.Chan JK et al. Weekly versus every-3-week paclitaxel and carboplatin for ovarian cancer. N. Engl. J. Med 374, 738–748 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.du Bois A et al. Standard first-line chemotherapy with or without nintedanib for advanced ovarian cancer (AGO-OVAR 12): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 17, 78–89 (2016). [DOI] [PubMed] [Google Scholar]
- 226.Aghajanian C et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol 30, 2039–2045 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Tesaro. Tesaro’s niraparib significantly improved progression-free survival for patients with ovarian cancer in both cohorts of the phase 3 NOVA trial. Tesaro http://ir.tesarobio.com/releasedetail.cfm?ReleaseID=977524 (2016). [Google Scholar]
- 228.Monk BJ et al. Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J. Clin. Oncol 28, 3107–3114 (2010). [DOI] [PubMed] [Google Scholar]
- 229.Ledermann J et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med 366, 1382–1392 (2012). [DOI] [PubMed] [Google Scholar]; Olaparib (an oral PARP inhibitor) is effective as a maintenance therapy after platinum therapy response in patients with platinum-sensitive HGSC compared with placebo.
- 230.Oza AM et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol 16, 87–97 (2015). [DOI] [PubMed] [Google Scholar]
- 231.Liu JF et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol 15, 1207–1214 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liu JF, Konstantinopoulos PA & Matulonis UA PARP inhibitors in ovarian cancer: current status and future promise. Gynecol. Oncol 133, 362–369 (2014). [DOI] [PubMed] [Google Scholar]
- 233.Scott CL, Swisher EM & Kaufmann SH Poly(ADP-ribose) polymerase inhibitors: recent advances and future development. J. Clin. Oncol 33, 1397–1406 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ledermann J et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol 15, 852–861 (2014). [DOI] [PubMed] [Google Scholar]
- 235.Kaufman B et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol 33, 244–250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.US National Library of Medicine. ClinicalTrials.gov http://www.clinicaltrials.gov/ct2/show/NCT01844986 (2013).
- 237.Hamanishi J et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J. Clin. Oncol 33, 4015–4022 (2015). [DOI] [PubMed] [Google Scholar]
- 238.Varga A et al. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: interim results from a phase Ib study. ASCO Meeting Abstr 33, 5510 (2015). [Google Scholar]
- 239.Disis ML et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with previously treated, recurrent or refractory ovarian cancer: a phase Ib, open-label expansion trial. ASCO Meeting Abstr 33, 5509 (2015). [Google Scholar]
- 240.Hodi FS et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc. Natl Acad. Sci. USA 105, 3005–3010 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01611558 (2012).


