Abstract
The transcription factors SRY and SOX9 and RSPO1/WNT4/β-Catenin signaling act as antagonistic pathways to drive testis and ovary development respectively, from a common gonadal primordium in mouse embryos. In this work, we took advantage of a double knockout mouse model to study gonadal development when Sox9 and Wnt4 are both mutated. We show that the XX gonad mutant for Wnt4 or for both Wnt4 and Sox9 develop as ovotestes, demonstrating that ectopic SOX9 function is not required for the partial female-to-male sex reversal caused by a Wnt4 mutation. Sox9 deletion in XY gonads leads to ovarian development accompanied by ectopic WNT/β-catenin signaling. In XY Sox9 mutant gonads, SRY-positive supporting precursors adopt a female-like identity and develop as pre-granulosa-like cells. This phenotype cannot be fully prevented by the deletion of Wnt4 or Rspo1, indicating that SOX9 is required for the early determination of the male supporting cell identity independently of repressing RSPO1/WNT4/β-Catenin signaling. However, in XY Sox9 Wnt4 double mutant gonads, pre-granulosa cells are not maintained, as they prematurely differentiate as mature granulosa cells and then trans-differentiate into Sertoli-like cells. Together, our results reveal the dynamics of the specific and independent actions of SOX9 and WNT4 during gonadal differentiation: SOX9 is essential in the testis for early specification of male-supporting cells whereas WNT4 functions in the ovary to maintain female-supporting cell identity and inhibit male-specific vascular and steroidogenic cell differentiation.
Keywords: gonad development, sex determination, SOX9, WNT signaling, ovotestis
1. Introduction
In mammals, gonadal sex determination is a highly controlled developmental process leading to the formation of a testis or ovary from a common primordium present in XY and XX organisms at embryonic stages (reviewed by [1]). Sex hormones subsequently secreted by the testis or the ovary, promote the development of secondary sexual characteristics to maintain the sexual identity and fertility [1].
The male sex determining gene Sry, located on the Y-chromosome, is expressed in mouse XY gonads from embryonic day 10.5–12.5, or E10.5–E12.5 [2,3,4,5,6]. SRY activates the expression of another high-mobility group (HMG) box-family transcription factor, SOX9, which, in turn, regulates other genes required to establish the Sertoli cell lineage that will further orchestrate testis development [7,8,9,10]. XY Sox9 mutant mice exhibit complete sex reversal and develop ovaries capable of producing oocytes that are chromosomally X or Y [11,12,13].
In the absence of Y chromosome, XX gonadal supporting cells differentiate as FOXL2-positive pre-granulosa cells and enter into mitotic arrest marked by the expression of cyclin-dependent kinase inhibitor CDKN1B/P27 [14,15]. Though FOXL2 is required to maintain granulosa cell identity in post-natal ovaries, this transcription factor is dispensable in the mouse ovary during embryonic stages [16,17]. In contrast, RSPO1/WNT4/β-Catenin signaling is required for embryonic ovarian development in both mice and human [18,19,20,21,22,23,24]. Mouse XX gonads harboring mutations in Wnt4, Rspo1, or Ctnnb1 (encoding β-Catenin) progressively develop as ovotestes, with characteristics of testes and ovaries [18,19,20,21,25]. The development of the partially sex-reversed gonads has been characterized and involves pre-granulosa cells first exiting mitotic arrest and differentiating prematurely as mature granulosa cells expressing AMH in addition to FOXL2 [25,26]. Next, mature granulosa cells loose FOXL2 expression, trans-differentiate into SOX9 and AMH positive Sertoli-like cells and organize as testis cord-like structures around birth [18,25]. In addition, RSPO1/WNT4/β-Catenin deficient XX gonads develop a testis-like coelomic vessel at E12.5 due to ectopic migration of endothelial cells from the adjacent mesonephros [18,20,21,27]. Additionally, Wnt4, Rspo1, and Ctnnb1 XX mutant gonads exhibit ectopic steroidogenic cells, which are absent in embryonic ovaries [18,19,20,21,28,29]. These cells produce testosterone and masculinize the XX genital tracts. Germ cells are depleted through apoptosis from E16.5 in Wnt4 and Ctnnb1 XX mutants [19,30,31] or by reduced proliferation from E12.5 in Rspo1 XX mutants [32].
Single-cell RNA-seq analyses of developing gonads have identified an early supporting cell precursor population with similar transcriptional profiles in XY and XX mouse embryos [33]. Differentiation of testicular Sertoli cells and ovarian granulosa cells in testes and ovaries respectively requires activation of the male or female pathway and repression of the alternate genetic cascade. Indeed, it has been demonstrated that ectopic activation of WNT/β-Catenin signaling or FOXL2 in XY gonads results in down-regulation of SOX9 and is sufficient to induce ovarian development [34,35,36]. Conversely, transgenic expression of SRY and, thus, upregulation of SOX9 or, simply, transgenic expression of SOX9, in embryonic XX supporting cells can induce testicular development [37,38,39].
Studies in double mutant mice gonads have also supported the principle of antagonistic sex determination pathways. One example involves fibroblast growth factor 9 (FGF9), which, when bound to its receptor FGFR2c, activates Sox9 expression in Sertoli cells to promote rapid expansion of the male supporting cell lineage throughout the developing testis [40,41]. Mutations in Fgf9 or Fgfr2/Fgfr2c lead to reduced SOX9 expression and partial male-to-female sex reversal [40,41,42,43,44]. In XY Fgf9 Wnt4, Fgfr2 Wnt4, and Fgfr2c Foxl2 double mutants, SOX9 expression and testicular differentiation are restored, indicating that FGF9 also functions to antagonize WNT4- and FOXL2-mediated repression of Sox9 [41,45]. The outcome of mutating Sox9 together with the female pathway components Rspo1 or Ctnnb1 has also been studied [26,46]. The gonads of both XX Sox9 Rspo1 and XX Sox9 Ctnnb1 double mutants develop as ovotestes, demonstrating that other factors besides SRY and SOX9 can drive Sertoli-like cell differentiation in Rspo1 and Ctnnb1 mutants [26,46]. In XY individuals, Sox9 Ctnnb1 double mutant embryonic gonads develop as ovotestes [26] and Sox9 Rspo1 mutant post-natal gonads develop as hypo-plastic testes [46]. These results indicate that although deletion of Rspo1 or Ctnnb1 can restore some aspects of testicular development in XY Sox9 mutant gonads, complete testis differentiation requires SOX9 function, even when the female WNT/ß-Catenin pathway is impaired.
While the gonad outcome of XY and XX Sox9 mutant mice also lacking Rspo1 or Ctnnb1 has been investigated, the gonad fate in Sox9 Wnt4 double mutants has not yet been reported. Furthermore, the sequence of events leading to the appearance of testicular characteristics in XY Sox9 Ctnnb1 and XY Sox9 Rspo1 double mutant gonads are unknown. In this study, we report the generation and analysis of double mutants for Sox9 and Wnt4 at different time points during gonadal development. We show here that similar to XX Sox9 Rspo1 and XX Sox9 Ctnnb1 mutants, SOX9 function is not required for the masculinization of XX Wnt4 mutant gonads. In addition, we demonstrate that loss of Wnt4 does not prevent the specification of XY supporting cell progenitors as pre-granulosa-like cells in XY Sox9 mutant gonads. However, in XY Sox9 Wnt4 mutants, granulosa-like cells trans-differentiate into Sertoli-like cells, leading to ovotestes development near birth.
This work highlights the specific and sequential actions of SOX9 and WNT4 during gonadal differentiation: SOX9 is essential for early specification of male supporting cells whereas WNT4 is required to maintain female supporting cell identity and to inhibit male-specific vascular and steroidogenic cell differentiation in developing ovaries.
2. Materials and Methods
2.1. Mouse Strains and Genotyping
The experiments described herein were carried out in compliance with the relevant institutional and European animal welfare laws, guidelines and policies. These procedures were approved by the French ethics committee (Comité institutionnel d’Ethique pour l’Animal de Laboratoire, number NCE/2011-2112, approved 2011/06/22). All mouse lines were kept on a mixed 129/C57Bl6/J background. Sf1-Cretg/0; Sox9flox/flox; Wnt4+/− females were crossed with Sf1-Cretg/0; Sox9flox/+; Wnt4+/− males to obtain mutant embryos at different stages. Embryos were named controls (Sf1-Cretg; Sox9flox/+; Wnt4+/−), Sox9cKO mutants (Sf1-Cretg; Sox9flox/flox; Wnt4+/−), Wnt4KO mutants (Sf1-Cretg; Sox9flox/+; Wnt4−/−), and Sox9cKO Wnt4KO double mutants (Sf1-Cretg; Sox9flox/flox; Wnt4−/−). The generation of Sf1-Cretg/0; Sox9flox/flox; Rspo1−/− mice (Sox9cKO Rspo1KO) has been previously described [46]. Genotypes of mice and embryos were determined using PCR assays on lysates from ear biopsies or tail tips as previously described [11,13,19,47]. The day when a vaginal plug was found was designated as embryonic day E0.5. E10.5–E12.5 embryos were staged by counting the number of tail somites (ts) with 8 ts corresponding to E10.5, 18 ts to E11.5 and 30 ts to E12.5 [5].
2.2. Immunofluorescence Staining
Sections (5 µm) of samples fixed in Bouin’s solution (HT10132, Sigma-Aldrich, Munich, Germany), Antigen fix (P10016, Diapath, Martinengo, Italy) or 4% (w/v) paraformaldehyde (PFA, 15710-S, EMS, Hatfield, PA, USA) were processed for immunofluorescence staining. Paraffin was removed in xylene baths, and sections were rehydrated in 100%, 90%, 70%, 40% ethanol (EtOH) and H2O. Antigen retrieval was obtained by pressure cooking in 10 mM sodium citrate pH 6, 0.05% TWEEN® 20 (P1379, Sigma-Aldrich) for 10 min. Sections were blocked with 3% bovine serum albumin (BSA, A2153, Sigma-Aldrich), 10% inactivated normal donkey serum (NDS, D9663-10ML, Sigma-Aldrich), 0.1% TWEEN® 20 in phosphate buffered saline (PBS) for 1 h at room temperature. Primary antibodies diluted in 3% BSA, 3% NDS, 0.1% TWEEN® 20, PBS were incubated at 4 °C overnight. After three washes in 0.1% TWEEN® 20, PBS, secondary antibodies diluted in PBS were incubated for 1 h at room temperature. After three washes in 0.1% TWEEN® 20, PBS, slides were mounted with Vectashield Hardset solution containing DAPI (H-1500, Vector Laboratories, Peterborough, UK). Images were obtained on a motorized Axio Imager Z1 microscope (Zeiss, Oberkochen, Germany) coupled with an AxioCam MRm camera (Zeiss, Oberkochen, Germany) and processed with Fiji (Bethesda, MD, USA) and Adobe Photoshop (San Jose, CA, USA). Antibodies are listed in Table S1. At least three embryos of each genotype were analyzed for each marker.
2.3. RNAscope® In Situ Hybridization
Gonads were dissected, fixed in 4% (w/v) PFA (15710-S, EMS) overnight at room temperature and embedded in paraffin. Sections (5 µm) were hybridized with Wnt4 probe (Advanced Cell Diagnostics, Newark, NJ, USA). The RNAscope® assay combined with immunofluorescence was performed according to the manufacturer’s instructions. Images were obtained on a LSM 710 confocal microscope (Zeiss) and processed with Fiji and Adobe Photoshop.
2.4. RNA Extraction and Quantitative PCR Analysis
Individuals gonads without mesonephros were dissected in PBS, snap-frozen in liquid nitrogen and kept at −80 °C. Total RNAs were extracted by RNeasy Micro Kit (74004, Qiagen, Manchester, UK) and reverse transcribed by M-MLV reverse transcriptase (M170A, Promega, Madison, WI, USA). Quantitative-PCR reactions were prepared in 10 μL with LightCycler® 480 SYBR Green I Master (04887352001, Roche, Basel, Switzerland) and run in the LightCycler® 480 System (05015278001, Roche), q-PCR primers are listed in Table S2. All biological replicates of different genotypes (N = 3–5) were run in the same plate and repeated at least twice. Relative gene expression of each gonad was calculated based on a standard curve from each run and normalized to the expression of the housekeeping gene Shda. Relative normalized gene expressions in all genotypes were compared to relative normalized gene expression in XX controls. Fold change in gene expression was obtained by dividing the normalized gene expression in gonads of a given genotype by the mean of the normalized gene expression in XX control gonads. Graphs show the individual values of relative normalized gene expression compared to XX control (dots), as well as the mean fold-change (bars) ± SEM.
3. Results
3.1. Efficient Genetic Deletion of Sox9 in Sox9cKO Wnt4KO Double Mutants
To generate Sox9cKO Wnt4KO double mutants, we utilized a Wnt4 mutant line (Wnt4KO) where deletion of exon 3 leads to a WNT4 loss-of-function [19], and a conditional Sox9 mutant line, where deletion of Sox9 exons 2 and 3 occurs in cells expressing the Sf1-Cre transgene (Sox9cKO) [13,47]. Sf1-Cre is active in the somatic cells of the gonad from E10.5, and is also expressed in the adrenal cortex, the spleen, the anterior pituitary and the ventromedial hypothalamus [47]. Previous work has established that deletion of Sox9 by Sf1-Cre leads to ovarian development in XY mutant gonads, a phenotype that can be attributed to SOX9 loss-of-function in the somatic gonad independently of hypothalamus-pituitary function [13]. Quantitative PCR analysis at E12.5 and E14.5 confirmed that Sox9 was also efficiently deleted in the gonad of single and double mutant embryos generated for this study (Figure S1).
3.2. Early Phenotypic Changes in XX Wnt4KO Mutants are Independent of SOX9 Function
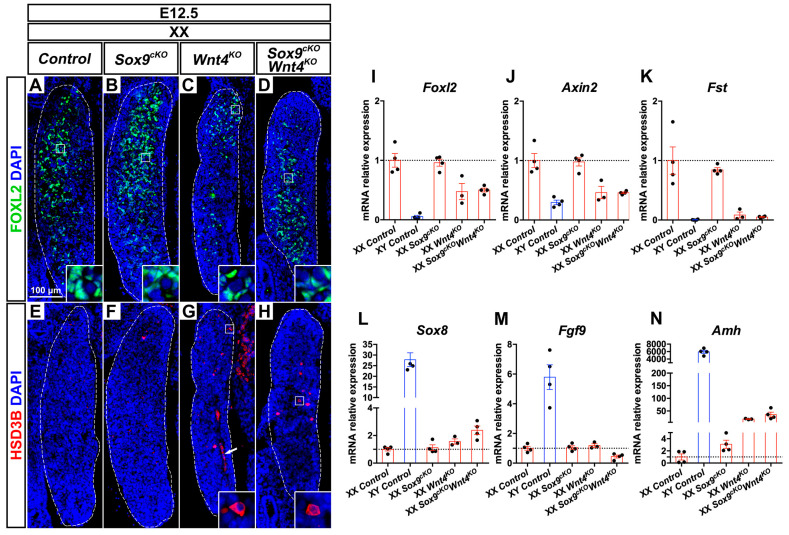
Given that Sox9 is transiently up-regulated in XX Wnt4KO mutant ovaries between E11.5 and E12.0 [40], we aimed to determine the functional contribution of SOX9 in XX Wnt4KO mutant gonads. Thus, we compared XX Sox9cKO Wnt4KO double mutant gonads at E12.5 and E14.5 with the respective single mutants and control gonads. At E12.5, XX control and XX Sox9cKO mutant gonads contain pre-granulosa cells with robust expression of the transcription factor FOXL2 (Figure 1A,B and Figure S2). In contrast, XX Wnt4KO mutants showed a marked reduction in FOXL2/Foxl2 expression at E12.5 and E14.5 (Figure 1C,I and Figure S2). This observation is consistent with reports indicating that Foxl2 expression is regulated in part by WNT/β-Catenin signaling [26,48,49]. Indeed, loss of Wnt4 in XX embryos compromised WNT/β-Catenin activity, as shown by reduced expression of Axin2 (Figure 1J and Figure S2), a downstream target of this pathway. In XX Sox9cKO Wnt4KO double mutant gonads, FOXL2/Foxl2 and Axin2 expression was similarly reduced (Figure 1D,I–J and Figure S2), indicating that down-regulation of these factors does not depend on the presence of SOX9.
Figure 1.
Early phenotypic changes in XX Wnt4 mutants are independent of SOX9 function. (A–D) Immunodetection of the pre-granulosa cell marker FOXL2 (green) in E12.5 XX gonads of Control (A), Sox9cKO (B), Wnt4KO (C) and Sox9cKO Wnt4KO (D) genotypes. (E–H) Immunodetection of the steroidogenic enzyme HSD3B (red) in E12.5 XX gonads of Control (E), Sox9cKO (F), Wnt4KO (G) and Sox9cKO Wnt4KO (H) genotypes. (I–N) RT-quantitative PCR analysis of Foxl2 (I), Axin2 (J), Follistatin (Fst) (K), Sox8 (L), Fgf9 (M) and Amh (N) expression in E12.5 gonads of XX Control, XY Control, XX Sox9cKO, XX Wnt4KO, and XX Sox9cKO Wnt4KO genotypes (N = 3–4 embryos for each genotype). Expression level in XX controls is 1. Graphs show individual values (dots), and the mean fold-change (bars) ± SEM. Nuclei labeled with DAPI are shown in blue. Magnification is the same in all panels. Scale bar = 100 μm. Gonads are outlined with broken white lines. White arrow in G indicates the coelomic vessel.
Next, we asked whether XX Sox9cKO Wnt4KO gonads contained steroidogenic cells and developed a coelomic vessel, as in XY control gonads and XX Wnt4KO mutants [19]. XX controls lacked cells expressing the steroidogenic enzyme HSD3β, and 2 out of 3 XX Sox9cKO mutant gonads exhibited only rare HSD3β positive cells (Figure 1E–F). In contrast, these cells were found in all XX Wnt4KO and XX Sox9cKO Wnt4KO mutant gonads (Figure 1G,H and Figure S2). Furthermore, XX Wnt4KO and Sox9cKO Wnt4KO mutant gonads developed a coelomic vessel (Figure 1G, arrow), similar to XY control testes [27]. In testes, coelomic vessel formation is attributed to migration of endothelial cells from the mesonephros, which in the developing ovary is inhibited by Follistatin (Fst) [27,30]. Accordingly, we found that Fst expression was severely down-regulated in XX Wnt4KO and Sox9cKO Wnt4KO mutant gonads at E12.5 (Figure 1K).
In our analyses, we did not detect up-regulation of Sox9 and Fgf9 in XX Wnt4KO mutant gonads (Figure 1M and Figure S1), as previously reported [40]. However, XX Wnt4KO and XX Sox9cKO Wnt4KO mutant gonads exhibited an enrichment for transcripts expressed in Sertoli cells, such as Sox8, Amh, and Dhh at E12.5 and E14.5, with Sox8 only being transiently expressed at E12.5 (Figure 1L,N and Figure S2).
Altogether, by analyzing XX Sox9cKO Wnt4KO double mutant gonads, our data demonstrates that SOX9 function is not required for the reduction of WNT/β-catenin signaling and FOXL2 expression, the up-regulation of Sertoli cell markers, and the formation of ectopic vasculature and steroidogenic cells. We conclude that the establishment of early phenotypic changes in Wnt4KO XX mutants is independent of SOX9 function.
3.3. Late Phenotypic Changes in XX Wnt4KO Mutants are Independent of SOX9 Function
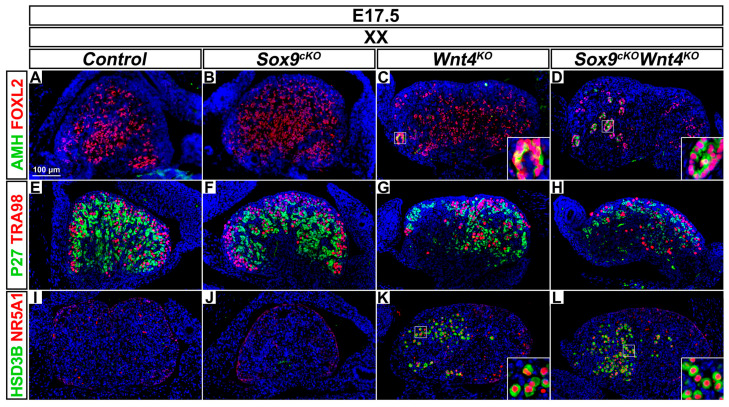
We then focused on XX Wnt4KO mutant gonads developing without Sox9 at E17.5. At this stage, a loss of fetal ovarian integrity is observed in XX Wnt4KO mutant gonads, since pre-granulosa cells differentiate prematurely [25]. Accordingly, at E17.5, FOXL2-positive pre-granulosa cells in the anterior part of XX Wnt4KO mutant gonads precociously exit their mitotic arrest, as indicated by down-regulation of P27 (CDKN1B) and prematurely differentiate as mature granulosa cells expressing AMH [25,50] (Figure 2C,G). Similarly, XX Sox9cKO Wnt4KO double mutant gonads exhibited mature granulosa cells (Figure 2D,H). These results indicate that in XX Wnt4KO mutants, SOX9 function is dispensable for premature granulosa cell differentiation, a phenotype that precedes reprogramming as Sertoli-like cells [25]. However, by using immunostaining for the Sertoli marker DMRT1 [51], we did not observe Sertoli cells forming testis cords in XX Wnt4KO mutant and XX Sox9cKO Wnt4KO double mutant gonads at E17.5 and P0.
Figure 2.
Abnormal ovarian development in XX Wnt4 mutants is independent of SOX9 function. (A–D) Immunodetection of the differentiation marker AMH (green) and the pre-granulosa cell marker FOXL2 (red) in E17.5 XX gonads of Control (A), Sox9cKO (B), Wnt4KO (C) and Sox9cKO Wnt4KO (D) genotypes. (E–H) Immunodetection of the quiescent cell marker P27 (green) and the germ cell marker TRA98 (red) in E17.5 XX gonads of Control (E), Sox9cKO (F), Wnt4KO (G) and Sox9cKO Wnt4KO (H) genotypes. (I–L) Immunodetection of the steroidogenic enzyme HSD3β (green) and the steroidogenic transcription factor NR5A1 (red) in E17.5 XX gonads of Control (I), Sox9cKO (J), Wnt4KO (K), and Sox9cKO Wnt4KO (L) genotypes. Nuclei labeled with DAPI are shown in blue. Magnification is the same in all panels. Scale bar = 100 μm.
Though the lethality of the Wnt4KO mutation after birth prevented further assessment of the role of SOX9 for the appearance of Sertoli-like cells in XX Wnt4KO mutants, additional characteristics associated with sex reversal were apparent. These include a loss of germ cells in the anterior region [30] and the ectopic presence of steroidogenic cells [19]. Here, we observed a reduction of the germ cells expressing the TRA98 antigen and an abundance of steroidogenic cells expressing HSD3β and NR5A1 in the anterior region of XX Wnt4KO and XX Sox9cKO Wnt4KO mutant gonads at E17.5 in comparison to controls and XX Sox9cKO mutants (Figure 2E–L). Thus, our analyses at E17.5 demonstrate that premature granulosa cell differentiation, germ cell loss, and ectopic steroidogenic cell formation in XX Wnt4KO mutant gonads are independent of SOX9 function.
3.4. Wnt4 Expression is Up-Regulated in XY Sox9cKO Mutant Gonads
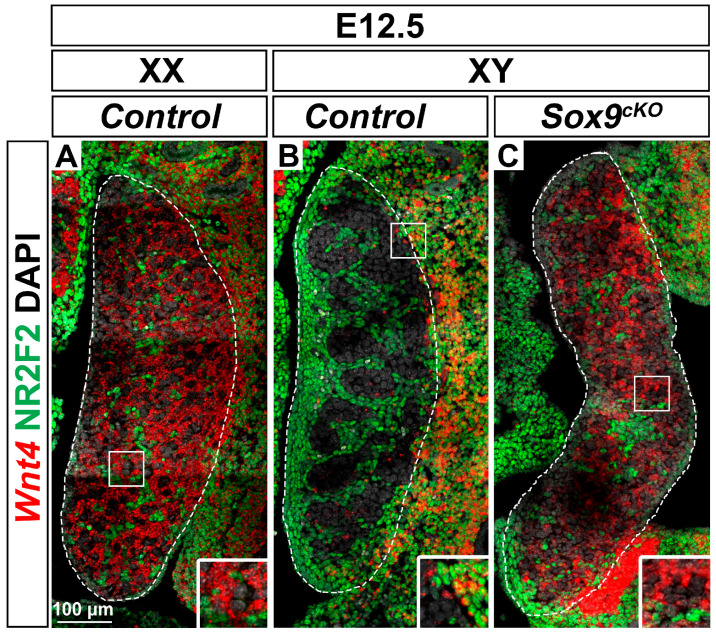
Next, we turned our attention to the role of Wnt4 in XY Sox9cKO mutant sex reversal gonads developing as ovaries [11,12,13]. At E10.5, Wnt4 is expressed in both XY and XX gonads [40] and is then down-regulated in developing testes at E11.5 [18,33,40,45,52,53]. In agreement, we detected abundant Wnt4 transcripts in E12.5 ovaries while the expression was almost absent in stage-matched testes (Figure 3A,B). In the ovary, germ cells and NR2F2 positive stromal cells exhibited low levels of Wnt4 transcripts (Figure 3A). In contrast, NR2F2 negative cells strongly expressed Wnt4 (Figure 3A) indicating that Wnt4 is predominantly expressed in pre-granulosa cells of the ovary [54,55]. These expression profiles were also observed in XY SOX9 loss-of-function gonads (Figure 3C), in agreement with the complete sex reversal reported in XY Sox9cKO mutants [11,12,13].
Figure 3.
Wnt4 expression is up-regulated in XY Sox9 mutant gonads. (A,B) RNAscope® detection of Wnt4 transcripts (red) together with immunodetection of the interstitial and stromal cell marker NR2F2 (green) in E12.5 XX Control gonad (A), XY Control gonad (B), and XY Sox9cKO gonad (C). Nuclei labeled with DAPI are shown in grey. Magnification is the same in all panels. Scale bar = 100 μm. Gonads are outlined with broken white lines.
3.5. XY Supporting Cell Precursors Adopt a Female-Like Fate in Sox9cKO Mutants Independently of WNT4
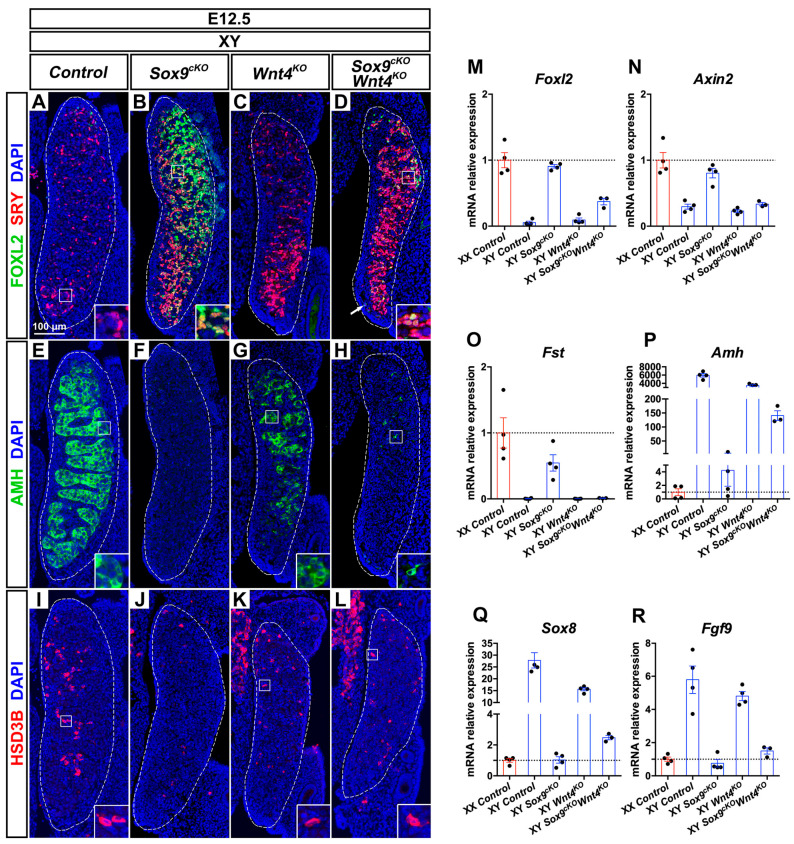
In order to investigate the contribution of WNT4 function to the XY Sox9cKO mutant phenotype, we analyzed XY single and double mutant gonads. In XY control gonads, SRY is expressed from E10.5 in pre-Sertoli cells, and is down-regulated by E12.5 upon testis differentiation (Figure 4A and Figure 5B) [6,56]. XY Wnt4KO mutant gonads following a testis fate exhibited sustained expression of SRY at E12.5 (Figure 4C). This was in agreement with delayed Sertoli cell differentiation in these mutants, which leads to transient defects in testis cords organization and size [19,57,58].
Figure 4.
Phenotypic and gene expression changes in E12.5 XY Sox9; Wnt4 mutants (A–D). Immunodetection of the pre-granulosa cell marker FOXL2 (green) and the male supporting cell marker SRY (red) in E12.5 XY gonads of Control (A), Sox9cKO (B), Wnt4KO (C), and Sox9cKO Wnt4KO (D) genotypes. (E–H) Immunodetection of the Sertoli cell marker AMH (green) in E12.5 XY gonads of Control (E), Sox9cKO (F), Wnt4KO (G), and Sox9cKO Wnt4KO (H) genotypes. (I–L) Immunodetection of the steroidogenic enzyme HSD3B (red) in E12.5 XY gonads of Control (I), Sox9cKO (J), Wnt4KO (K), and Sox9cKO Wnt4KO (L) genotypes. (M–R) RT-quantitative PCR analysis of Foxl2 (M), Axin2 (N), Follistatin (Fst) (O), Amh (P), Sox8 (Q), and Fgf9 (R) expression in E12.5 gonads of XX Control, XY Control, XY Sox9cKO, XY Wnt4KO, and XY Sox9cKO Wnt4KO genotypes (N = 3–4 embryos for each genotype). Expression level in XX controls is 1. Graphs show individual values (dots), and the mean fold-change (bars) ± SEM. Nuclei labeled with DAPI are shown in blue. Magnification is the same in all panels. Scale bar = 100 μm. Gonads are outlined with broken white lines. The white arrow in D indicates the coelomic vessel.
Figure 5.
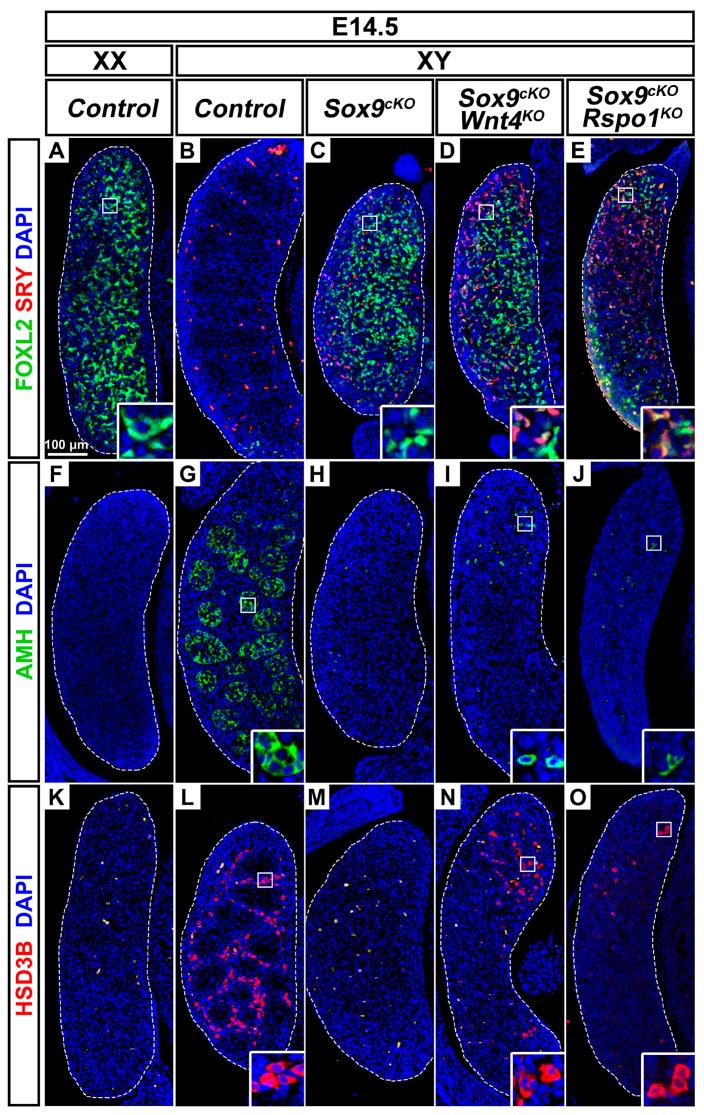
Wnt4 or Rspo1 deletion does not restore testicular development in embryonic XY gonads lacking SOX9 function. (A–E) Immunodetection of the pre-granulosa cell marker FOXL2 (green) and the male supporting cell marker SRY (red) in E14.5 gonads of XX Control (A), XY Control (B), XY Sox9cKO (C), XY Sox9cKO Wnt4KO (D), and XY Sox9cKO Rspo1KO (E) genotypes. (F–J) Immunodetection of the Sertoli cell marker AMH (green) in E14.5 gonads of XX Control (F), XY Control (G), XY Sox9cKO (H), XY Sox9cKO Wnt4KO (I), and XY Sox9cKO Rspo1KO (J) genotypes. (K–O) Immunodetection of the steroidogenic enzyme HSD3B (red) in E14.5 gonads of XX Control (K), XY Control (L), XY Sox9cKO (M), XY Sox9cKO Wnt4KO (N), and XY Sox9cKO Rspo1KO (O) genotypes. Nuclei labeled with DAPI are shown in blue. Magnification is the same in all panels. Scale bar = 100 μm. Gonads are outlined with broken white lines.
In E12.5 XY Sox9cKO mutants, SRY was also maintained at E12.5 (Figure 4B) and was down-regulated by E14.5 (Figure 5C). However, unlike XY control and XY Wnt4KO mutant gonads, SRY and FOXL2 were co-expressed in XY Sox9cKO mutants (Figure 4B and Figure 5C). This indicated that XY supporting cell precursors adopt a female-like pre-granulosa cell identity in the absence of SOX9 function. Similarly, XY Sox9cKO Wnt4KO double mutant gonads harbored cells co-expressing SRY and FOXL2 at E12.5 and E14.5 (Figure 4D and Figure 5D), though Foxl2 transcripts were reduced when compared with XY Sox9cKO mutants and XX controls (Figure 4M and Figure S3).
In our analyses, we noted that at E12.5 and E14.5, XY Sox9cKO Wnt4KO double mutants showed increased expression of male supporting markers Sox8, Dhh, and Amh, when compared with XY Sox9cKO single mutants (Figure 4P,Q and Figure S3). However, the expression level of these markers was at least 10 times lower than in XY control or Wnt4KO mutant testes. In addition, AMH protein could only be detected in rare scattered cells in E12.5 and E14.5 double mutants (Figure 4H and Figure 5I), whereas it is expressed in Sertoli cells forming testis cords in XY control or Wnt4KO mutant testes (Figure 4E,G and Figure 5G). Thus, in summary, we find that XY Sox9cKO Wnt4KO double mutant supporting cell precursors can adopt a pre-granulosa cell-like identity even in the absence of WNT4 function. Furthermore, ablation of Wnt4 cannot restore Sertoli cell development in embryonic XY gonads lacking SOX9 function.
3.6. Wnt4 Deletion Restores Coelomic Vessel and Steroidogenic Cell Development in XY Sox9cKO Mutants
XY Sox9cKO mutants exhibit high expression of the WNT/β-catenin targets Axin2 and Fst similar to XX controls (Figure 4N,O and Figure S3). In contrast, in XY double mutants, ablation of Wnt4 results in down-regulation of Axin2 and Fst to XY control levels at E12.5 and E14.5 (Figure 4N,O and Figure S3). Loss of Fst expression provides an explanation for the formation of a coelomic vessel in XY double mutants, as compared with XY Sox9cKO single mutants (Figure 4D, arrow) [30]. In addition, XY Sox9cKO Wnt4KO double mutant gonads exhibited abundant HSD3β-positive steroidogenic cells (Figure 4L and Figure 5N), unlike XY Sox9cKO single mutants where only rare HSD3β-positive cells are observed (Figure 4F). These observations indicate that Wnt4 deletion and decreased WNT/β-catenin signaling restore two testis-specific traits in XY Sox9cKO mutants, coelomic vessel formation, and steroidogenic cell development.
3.7. Pre-Granulosa-Like Cells are Specified in XY Sox9cKO Rspo1KO Double Mutants
Given that WNT4 is not involved in SRY and FOXL2 co-expression in XY Sox9cKO mutant supporting cells (Figure 4D), we asked whether RSPO1, an upstream factor of WNT4 in WNT/β-catenin signaling [18,21] would be required. Indeed, XY Sox9cKO Rspo1KO double mutant mice exhibit hypo-plastic testes at post-natal stages, indicating that Rspo1 deletion promotes testicular development in XY Sox9cKO mutants [46]. Compared with XY Sox9cKO Wnt4KO gonads at E14.5, XY Sox9cKO Rspo1KO mutant gonads exhibited fewer FOXL2-positive cells, with some co-expressing SRY (Figure 5D,E). This finding indicates that even in the absence of RSPO1 function, embryonic XY supporting cell precursors lacking SOX9 adopt a female-like identity. Like XY Sox9cKO Wnt4KO double mutants, XY Sox9cKO mutants lacking Rspo1 exhibited interspersed AMH-positive cells that were not organized in testis cords, and steroidogenic cells expressing HSD3β (Figure 5J,O).
We thus conclude that as in XY Sox9cKO Wnt4KO mutants, ablation of Rspo1 does not completely prevent the sex reversal in embryonic XY gonads lacking SOX9 function. Together with the observations in Sox9cKO Ctnnb1cKO XY double mutants [26], our findings highlight that SOX9 function in the developing fetal testis extends beyond the inhibition of WNT/β-catenin signaling.
3.8. Pre-Granulosa-Like Cells are Not Maintained in XY Sox9cKO Wnt4KO Double Mutant Gonads
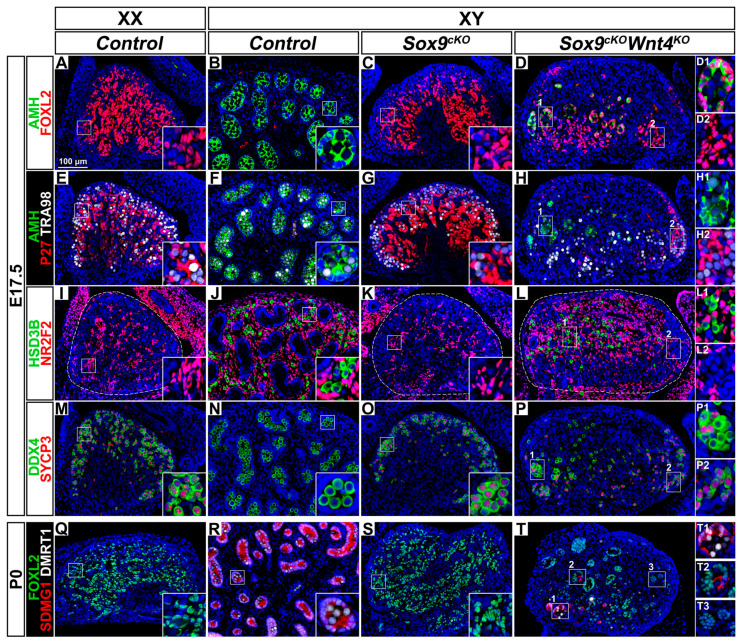
To complete our analyses in XY Sox9cKO Wnt4KO double mutant gonads, we examined gonads near birth, at E17.5. At this stage, XY Sox9cKO mutant gonads were indistinguishable from control ovaries with respect to the expression of FOXL2 and P27 in quiescent pre-granulosa cells throughout the gonad (compare Figure 6A with Figure 6C, and Figure 6E with Figure 6G) and the absence of steroidogenic cells (Figure 6I,K). Additionally, as in control fetal ovaries, germ cells in XY Sox9cKO mutant gonads initiate meiosis [11,12,13], as indicated by the expression of SYCP3, a meiotic marker (Figure 6O and Figure S4). In contrast, XY Sox9cKO Wnt4KO mutant gonads displayed a mixed, part-ovary, part-testis, gonadal fate. The more posterior regions of the double mutant gonads exhibited fetal ovarian characteristics: FOXL2 and P27 expression (Figure 6D,H), absence of steroidogenic cells (Figure 6L), and presence of meiotic germ cells marked by the co-expression of DDX4 and SYCP3 (Figure 6P). In contrast, the anterior region exhibited hallmarks of ovary-to-testis reprogramming. This includes: down-regulation of P27 and up-regulation of AMH, indicating pre-granulosa cell loss of quiescence and pre-mature differentiation (Figure 6D,H); and as in control testes, steroidogenic cells expressing HSD3β and non-meiotic germ cells (Figure 6L,P). We also noted that mature granulosa cells co-expressing FOXL2 and AMH were found in tubular structures that resembled the organization of testis cords (Figure 6D).
Figure 6.
Ovotestis development in XY Sox9; Wnt4 double mutant gonads at E17.5 and P0. (A–D) Immunodetection of the granulosa differentiation and Sertoli cell marker AMH (green) and the pre-granulosa cell marker FOXL2 (red) in E17.5 gonads of XX Control (A), XY Control (B), XY Sox9cKO (C), and XY Sox9cKO Wnt4KO (D) genotypes. (E–H) Immunodetection of AMH (green), the quiescent cell marker P27 (red) and the germ cell marker TRA98 (white) in E17.5 gonads of XX Control (E), XY Control (F), XY Sox9cKO (G), and XY Sox9cKO Wnt4KO (H) genotypes. (I–L) Immunodetection of the steroidogenic cell marker HSD3ß (green) and the interstitial and stromal cell marker NR2F2 (red) in E17.5 gonads of XX Control (I), XY Control (J), XY Sox9cKO (K), and XY Sox9cKO Wnt4KO (L) genotypes. (M–P) Immunodetection of the germ cell marker DDX4 (green) and the meiosis marker SYCP3 (red) in E17.5 gonads of XX Control (M), XY Control (N), XY Sox9cKO (O), and XY Sox9cKO Wnt4KO (P) genotypes. (Q–T) Immunodetection of the pre-granulosa cell marker FOXL2 (green), the Sertoli cell marker SDMG1 (red) and the Sertoli cell and male germ cell marker DMRT1 (white) in newborn gonads of XX Control (Q), XY Control (R), XY Sox9cKO (S), and XY Sox9cKO Wnt4KO (T) genotypes. Nuclei labeled with DAPI are shown in blue. Magnification is the same in all panels. Scale bar = 100 μm. Regions marked by numbers in panels D, H, L, P, and T are shown in the corresponding insets. Gonads are outlined with broken white lines in I, K, and L.
Our results reveal that FOXL2 expressing pre-granulosa-like cells formed during the male-to-female sex reversal caused by Sox9 mutation, fail to be maintained and prematurely differentiate in the anterior region of XY Sox9cKO Wnt4KO double mutant gonads. This observation is in agreement with the known WNT4 function in the maintenance of pre-granulosa cell identity in XX gonads [25].
We next investigated whether Sertoli cells were formed in the anterior masculinized region of XY double mutants. DMRT1 is co-expressed with SDMG1 in Sertoli cells of control testes at P0, and is also found in germ cells inside the testis cords (Figure 6R) [51,59]. In XY double mutants, cells co-expressing DMRT1 and SDMG1 were observed, indicating that Sertoli cell differentiation had occurred (Figure 6T). Some tubular structures contained both FOXL2 positive and SDMG1 positive cells, suggesting that Sertoli-like cells trans-differentiate from granulosa cells in XY double mutants (Figure 6T).
Together these results indicate that XY Sox9cKO Wnt4KO double mutant gonads develop as ovotestes near birth. In the anterior region, XY Sox9cKO Wnt4KO mutant-supporting cells cannot maintain pre-granulosa cell identity in the absence of WNT4 function, and prematurely differentiate and trans-differentiate into Sertoli-like cells. We conclude that WNT4 function in XY Sox9cKO mutant gonads is dispensable for the specification of pre-granulosa cells, but is required for their maintenance.
4. Discussion
4.1. SOX9 Function is Dispensable for Partial Sex-Reversal in XX Wnt4KO Mutants
Given that the testis-specific factor SOX9 is transiently up-regulated at E11.5 in XX Wnt4KO mutant gonads developing as ovotestes [40], it was assumed that SOX9 drives the partial sex reversal. However, we observed that in XX Sox9cKO Wnt4KO double mutant gonads, pre-granulosa cells prematurely differentiate and testis-specific traits (coelomic vessel and steroidogenic cells) appear. These phenotypes are also present in XX gonads lacking Wnt4, Rspo1, Ctnnb1, and in XX Sox9cKO mutant gonads lacking Rspo1 or Ctnnb1 [18,19,20,21,25,26,46,60]. Thus, though required for male sex determination in XY gonads [11,12,13], SOX9 is dispensable for ovotestes development in XX gonads with impaired WNT/β-Catenin signaling.
In XX WNT/β-Catenin signaling single mutants, premature granulosa cell differentiation precedes reprogramming as Sertoli-like cells in embryonic gonads near or at birth [25,26,46,60]. In XX Wnt4KO single mutant gonads specifically, a previous report shows an absence of Sertoli-like cells expressing DMRT1 at E18.5 and presence of Sertoli-like cells expressing SOX9 (albeit sporadic) at birth [25]. In the present study, we did not detect Sertoli cells expressing DMRT1 in XX Sox9cKO Wnt4KO mutants at E17.5 and P0. Note that analyses at post-natal stages in WNT4 loss-of-function mice were not possible, due to perinatal lethality [25,61]. Thus, by birth, XX Sox9cKO Wnt4KO gonads lacked Sertoli-like cells that were present in XX Sox9cKO mutant gonads additionally lacking Rspo1 or Ctnnb1 [25,26]. The absence or delay of granulosa-to-Sertoli cell reprogramming in XX Sox9cKO Wnt4KO mutant gonads is likely attributed to compensatory action of other WNT proteins to maintain pre-granulosa cells [25,26,60]. Alternatively, WNT4 function may specifically be required for aspects of Sertoli cell differentiation [57]. Altogether, our examination of XX Sox9cKO Wnt4KO mutant gonads demonstrates that both SRY and SOX9 are dispensable for the loss of fetal ovarian integrity and the masculinization of XX Wnt4KO mutant gonads.
4.2. SRY Regulation in XY Sox9cKO Wnt4KO Double Mutants
Given that XY Sox9cKO mutant gonads develop as ovaries expressing Wnt4 [11,12,13], we analyzed XY Sox9cKO Wnt4KO double mutants to determine the contribution of WNT4 function in the XY sex reversal ovary.
We have observed that SRY expression is maintained in E12.5 XY Sox9cKO and Sox9cKO Wnt4KO mutants, indicating that without the downstream activation of SOX9, SRY cannot antagonize granulosa cell formation to initiate Sertoli cell differentiation. SRY expression is down-regulated in control testes around E12.5 [6,56]. We found that SRY was widely detected in XY Sox9cKO mutants at E12.5 and was further maintained in E14.5 Sox9cKO Wnt4KO and Sox9cKO Rspo1KO mutants. It has been previously suggested that SOX9 might be involved in Sry down-regulation in the XY developing gonad [11]. However, additional factors are at play as demonstrated by the correct down-regulation of SRY expression at the poles of B6-XYPOS ovotestes devoid of SOX9 expression [62]. Interestingly, SRY expression is rescued in XY Cbx2 Wnt4 mutant gonads [63], indicating that WNT4 function, possibly through the activation of β-catenin signaling, directly or indirectly contributes to a negative regulation of Sry.
4.3. Pre-Granulosa Cells are Specified in Sox9cKO Mutants Without WNT4 Function
We show here that in the absence of WNT4 function, SRY-positive cells in XY Sox9cKO Wnt4KO mutant gonads still adopt a pre-granulosa cell-like fate at E12.5, though Foxl2 is expressed at reduced levels compared to XY Sox9cKO single mutants. Thus, WNT4 function is dispensable for early differentiation of XY Sox9cKO mutant supporting cells as pre-granulosa-like cells.
In XX gonads, granulosa cell specification and ovarian development require the WNT/β-Catenin and FOXL2 pathways acting in parallel. Ectopic up-regulation of β-Catenin signaling or FOXL2 in XY gonads has been shown to be sufficient to trigger granulosa cell differentiation [34,36]. In addition, the two pathways can compensate for each other as evidenced by (i) pre-granulosa cell specification before reprogramming as Sertoli-like cells in XX gonads lacking Rspo1, Wnt4, Ctnnb1, or Foxl2, [16,17,18,19,20,21,25,26] and (ii) enhancement of this phenotype in XX Rspo1 or Wnt4 mutants also carrying a Foxl2 mutation [48,49]. In XY Sox9cKO Wnt4KO mutant gonads, FOXL2 expression and reduced levels of WNT/β-Catenin signaling are sufficient for pre-granulosa-like cell specification from XY supporting cell precursors thus preventing embryonic testicular development. In contrast, mutations in Wnt4 can rescue Sox9 expression and testis development in XY embryos lacking FGF9 or its receptor FGFR2, indicating that WNT4 function antagonizes testis differentiation downstream of FGF9/FGFR2 signaling [45]. In Fgf9 Wnt4 and Fgfr2 Wnt4 double mutant gonads, the maintenance of SOX9 expression leads to the activation/reinforcement of a testis-differentiating pathway independent of FGF9/FGFR2 signaling [45]. This likely involves prostaglandin D2 signaling given that this pathway can activate Sox9 expression independently of FGF signaling to promote Sertoli cell specification [64].
Altogether, our results further highlight that Sox9 is indispensable to promote Sertoli cell specification at the time of sex determination.
4.4. RSPO1/WNT/β-Catenin Mutations Cannot Restore Sertoli Cell Specification in Sox9cKO Mutants
The pre-granulosa-like cells observed at E14.5 both in XY Sox9cKO Wnt4KO mutant gonads (this study) and in XY Sox9cKO Ctnnb1cKO mutant gonads [26], prompted us to examine early XY Sox9cKO Rspo1KO mutant gonad development. We previously reported post-natal hypo-plastic testis development with rare FOXL2 expressing follicles in XY Sox9cKO Rspo1KO double mutants [46]. This raised the question of the early events leading to the hypo-plastic testis. We show here that at E14.5, XY Sox9cKO Rspo1KO gonads contain SRY-positive cells also expressing FOXL2, indicating transient pre-granulosa cell-like specification. We presume that after initial pre-granulosa-like cell specification, these cells trans-differentiate as Sertoli-like cells, as in XX Rspo1KO single mutant gonads [25,46]. We conclude that SOX9 function is required for the early choice that drives XY Sertoli cell specification from a bipotential supporting cell precursor and that this function extends beyond the inhibition of RSPO1/WNT/β-Catenin signaling.
FOXL2-positive cells observed in XY Sox9cKO mutant gonads also harboring mutations in the WNT/β-Catenin pathway suggests that β-Catenin signaling is not required for Foxl2 expression in these gonads. This may be explained by the loss of SOX9, which represses Foxl2 expression in XY supporting cells either directly, or through the action of its target FGF9 [10,41]. Interestingly, ChIP-seq experiments have identified FOXL2 binding peaks in a region containing an enhancer involved in Sox9 repression in granulosa cells [36]. Thus, FOXL2 and SOX9 might repress each other expression to ensure the appropriate differentiation of bipotential supporting precursors along the male or female pathway.
4.5. Vasculature Formation and Steroidogenic Cell Development Do Not Require SOX9 Function
In studying early gonad development in XY double mutants, it was apparent that inactivation of Wnt4 in XY Sox9cKO mutant gonads restored a subset of testis-specific traits, including coelomic vessel formation and steroidogenic cell differentiation. The coelomic vessel forms in developing testes through the migration of endothelial cells of mesonephric origin [27]. This requires stimulation by Activin B, a homodimer with monomeric subunits (Inhbb) expressed by Sertoli and granulosa cells [65]. In developing ovaries, this process is inhibited by FST, an antagonist of Activin, a down-stream factor of the RSPO1/WNT/β-Catenin and FOXL2 pathways [18,20,21,30,31,36,66]. Reduced Fst expression in the absence of Wnt4 in XY Sox9cKO Wnt4KO double mutants is the most likely cause of coelomic vessel formation in these gonads.
With respect to steroidogenic cell differentiation in control developing testes, Hedgehog signaling (Desert Hedgehog, Dhh) is required for fetal Leydig cells differentiation from undifferentiated interstitial precursors [67]. Dhh expression is up-regulated in XY Sox9cKO Wnt4KO double mutants compared to XY Sox9cKO mutants, suggesting that Hedgehog signaling might be involved in steroidogenic cell formation in these gonads.
Altogether our observations indicate that at least two male specific processes, coelomic vasculature and fetal steroidogenic cell formation, do not depend on SOX9 itself but require the inhibition of WNT signaling in XY gonads. In XX gonads lacking WNT4 function (XX Wnt4KO and XX Sox9cKO Wnt4KO mutants), we also observed down-regulation of Fst and up-regulation of Dhh expression, when compared with control and XX Sox9cKO mutant ovaries, providing an explanation for the formation of male-like coelomic vessel and steroidogenic cells in these XX mutant gonads.
4.6. Premature Granulosa Cell Differentiation and Sertoli Trans-Differentiation in XY Sox9cKO Wnt4KO Double Mutants
In XY Sox9cKO Wnt4KO double mutant gonads, a fraction of pre-granulosa cells exit mitotic arrest at E17.5 and begin to express AMH, indicating precocious granulosa cell differentiation. We conclude that the maintenance of pre-granulosa cell identity in XY Sox9cKO mutant ovaries requires WNT4 function. Premature granulosa cell differentiation is a common feature of XX gonads lacking Rspo1, Wnt4 or Ctnnb1 [25,26]. This process is independent of SOX9 function as shown here in XX Sox9cKO Wnt4KO double mutants, but also in XX Sox9cKO Rspo1KO and XX Sox9cKO Ctnnb1cKO double mutant gonads [26,46]. Currently, it is unknown whether premature granulosa cell differentiation is a cell autonomous process triggered by the decrease of β-Catenin signaling or linked to other testis-like phenotypes such as coelomic vasculature and steroidogenic cells.
It is noteworthy that premature granulosa cell differentiation in XY double mutant gonads occurs predominantly in the anterior region of the gonad. This is similar to the situation observed in XX Sox9cKO Wnt4KO, XX Sox9cKO Rspo1KO, XX Sox9cKO Ctnnb1cKO double mutants and XX Wnt4KO, XX Rspo1KO and XX Ctnnb1cKO single mutants [25,26,46]. Thus, it appears that premature granulosa cell differentiation in XX and XY ovotestes proceeds along an anterior-to-posterior axis that recapitulates the gradient of differentiation observed in normal ovarian development [68].
We observed testis cord-like structures with Sertoli-like cells co-expressing DMRT1 and SDMG1 in newborn XY Sox9cKO Wnt4KO mutant gonads. These structures contained granulosa cells expressing FOXL2, suggesting that the Sertoli-like cells originate from reprogramming of granulosa cells. We did not observe Sertoli-like cells expressing DMRT1 in XX double mutants, indicating that the Y chromosome, and possibly some target genes of SRY [26], favor Sertoli cell development in XY Sox9cKO Wnt4KO ovotestes.
Altogether, we report that Sertoli-like cells in XY Sox9cKO Wnt4KO mutant gonads arise from a two-step process: differentiation of XY pre-granulosa cells due to loss of SOX9 function, then premature granulosa cell differentiation followed by Sertoli cell reprogramming, due to loss of WNT4 function.
5. Conclusions
This work demonstrates that SOX9 and WNT/β-Catenin signaling act at different levels in the mutual antagonistic network that drives supporting cell differentiation and gonadal development. SOX9 function is essential for the initial decision in the supporting cell lineage to drive Sertoli cell differentiation. WNT/β-Catenin signaling co-operates with FOXL2 for granulosa cell specification, inhibits male vascular and steroidogenic development and is essential to maintain pre-granulosa cells in an undifferentiated quiescent state during ovarian development.
Acknowledgments
We acknowledge the help from members of the Experimental Histpathology Platform, the PRISM Imaging Platform and the Animal house at iBV (Institut de Biologie Valrose, Université Côte d’Azur, CNRS, Inserm, iBV, France). We are grateful to Ian Adams and Dagmar Wilhelm for sharing reagents, to members of the A. Schedl and M.C. Chaboissier groups for helpful discussions and to Eric Pailhoux for critical reading of the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/9/5/1103/s1, Figure S1: Sox9 expression in control and mutant gonads at E12.5 and E14.5; Figure S2: Phenotype of E14.5 XX control and mutant gonads; Figure S3: Quantitative PCR analysis of gene expression in XY control and mutant gonads at E14.5; Figure S4: Germ cell development in XY control and mutant gonads at E14.5; Table S1: Antibodies for immunodetection; Table S2: Quantitative-PCR primers.
Author Contributions
Conceptualization: A.P.-G. and M.-C.C.; formal analysis: F.T.; investigation: F.T, A.A., N.R., and A.P.-G.; writing—original draft preparation: F.T. and A.P.-G.; writing—review and editing: A.P.-G., N.R., and M.-C.C.; visualization: F.T. and A.P.-G.; supervision: A.P.-G.; funding acquisition: M.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Agence Nationale de la Recherche: ANR-19-CE14-0022-01, SexDiff (to M.C.C.), and ANR-11-LABX-0028-01 9 (to N.R.), and by a scholarship from the China Scholarship Council (to F.T.).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- 1.Nef S., Stévant I., Greenfield A. Current Topics in Developmental Biology. Academic Press; Cambridge, MA, USA: 2019. Characterizing the bipotential mammalian gonad; pp. 167–194. [DOI] [PubMed] [Google Scholar]
- 2.Koopman P., Gubbay J., Vivian N., Goodfellow P., Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 3.Sinclair A.H., Berta P., Palmer M.S., Hawkins J.R., Griffiths B.L., Smith M.J., Foster J.W., Frischauf A.M., Lovell-Badge R., Goodfellow P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 4.Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 5.Hacker A., Capel B., Goodfellow P., Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- 6.Bullejos M., Koopman P. Spatially Dynamic Expression of Sry in Mouse. Dev. Dyn. 2001;205:201–205. doi: 10.1002/dvdy.1134. [DOI] [PubMed] [Google Scholar]
- 7.Gonen N., Futtner C.R., Wood S., Alexandra Garcia-Moreno S., Salamone I.M., Samson S.C., Sekido R., Poulat F., Maatouk D.M., Lovell-Badge R. Sex reversal following deletion of a single distal enhancer of Sox9. Science. 2018;360:1469–1471. doi: 10.1126/science.aas9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekido R., Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- 9.Rahmoun M., Lavery R., Laurent-Chaballier S., Bellora N., Philip G.K., Rossitto M., Symon A., Pailhoux E., Cammas F., Chung J., et al. In mammalian foetal testes, SOX9 regulates expression of its target genes by binding to genomic regions with conserved signatures. Nucleic Acids Res. 2017;45:7191–7211. doi: 10.1093/nar/gkx328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y., Zheng M., Lau Y.F.C. The sex-determining factors SRY and SOX9 regulate similar target genes and promote testis cord formation during testicular differentiation. Cell Rep. 2014;8:723–733. doi: 10.1016/j.celrep.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 11.Chaboissier M.-C. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- 12.Barrionuevo F., Bagheri-Fam S., Klattig J., Kist R., Taketo M.M., Englert C., Scherer G. Homozygous Inactivation of Sox9 Causes Complete XY Sex Reversal in Mice1. Biol. Reprod. 2005;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- 13.Lavery R., Lardenois A., Ranc-Jianmotamedi F., Pauper E., Gregoire E.P., Vigier C., Moreilhon C., Primig M., Chaboissier M.C. XY Sox9 embryonic loss-of-function mouse mutants show complete sex reversal and produce partially fertile XY oocytes. Dev. Biol. 2011;354:111–122. doi: 10.1016/j.ydbio.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Nef S., Schaad O., Stallings N.R., Cederroth C.R., Pitetti J.L., Schaer G., Malki S., Dubois-Dauphin M., Boizet-Bonhoure B., Descombes P., et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev. Biol. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Gustin S.E., Hogg K., Stringer J.M., Rastetter R.H., Pelosi E., Miles D.C., Sinclair A.H., Wilhelm D., Western P.S. WNT/β-catenin and p27/FOXL2 differentially regulate supporting cell proliferation in the developing ovary. Dev. Biol. 2016;412:250–260. doi: 10.1016/j.ydbio.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Ottolenghi C., Omari S., Garcia-Ortiz J.E., Uda M., Crisponi L., Forabosco A., Pilia G., Schlessinger D. Foxl2 is required for commitment to ovary differentiation. Hum. Mol. Genet. 2005;14:2053–2062. doi: 10.1093/hmg/ddi210. [DOI] [PubMed] [Google Scholar]
- 17.Uhlenhaut N.H., Jakob S., Anlag K., Eisenberger T., Sekido R., Kress J., Treier A.C., Klugmann C., Klasen C., Holter N.I., et al. Somatic Sex Reprogramming of Adult Ovaries to Testes by FOXL2 Ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Chassot A.A., Ranc F., Gregoire E.P., Roepers-Gajadien H.L., Taketo M.M., Camerino G., de Rooij D.G., Schedl A., Chaboissier M.C. Activation of β-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum. Mol. Genet. 2008;17:1264–1277. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- 19.Vainio S., Heikkilä M., Kispert A., Chin N., McMahon A.P. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- 20.Liu C.F., Bingham N., Parker K., Yao H.H.C. Sex-specific roles of β-catenin in mouse gonadal development. Hum. Mol. Genet. 2009;18:405–417. doi: 10.1093/hmg/ddn362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomizuka K., Horikoshi K., Kitada R., Sugawara Y., Iba Y., Kojima A., Yoshitome A., Yamawaki K., Amagai M., Inoue A., et al. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum. Mol. Genet. 2008;17:1278–1291. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- 22.Parma P., Radi O., Vidal V., Chaboissier M.C., Dellambra E., Valentini S., Guerra L., Schedl A., Camerino G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat. Genet. 2006;38:1304–1309. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- 23.Mandel H., Shemer R., Borochowitz Z.U., Okopnik M., Knopf C., Indelman M., Drugan A., Tiosano D., Gershoni-Baruch R., Choder M., et al. SERKAL Syndrome: An Autosomal-Recessive Disorder Caused by a Loss-of-Function Mutation in WNT4. Am. J. Hum. Genet. 2008;82:39–47. doi: 10.1016/j.ajhg.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biason-Lauber A., Konrad D., Navratil F., Schoenle E.J. A WNT4 mutation associated with Müllerian-Duct regression and virilization in a 46,XX woman. N. Engl. J. Med. 2004;351:792–798. doi: 10.1056/NEJMoa040533. [DOI] [PubMed] [Google Scholar]
- 25.Maatouk D.M., Mork L., Chassot A.A., Chaboissier M.C., Capel B. Disruption of mitotic arrest precedes precocious differentiation and transdifferentiation of pregranulosa cells in the perinatal Wnt4 mutant ovary. Dev. Biol. 2013;383:295–306. doi: 10.1016/j.ydbio.2013.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicol B., Yao H.H.-C. Gonadal Identity in the Absence of Pro-Testis Factor SOX9 and Pro-Ovary Factor Beta-Catenin in Mice. Biol. Reprod. 2015;93:1–12. doi: 10.1095/biolreprod.115.131276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeays-Ward K. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- 28.Heikkilä M., Peltoketo H., Leppäluoto J., Ilves M., Vuolteenaho O., Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- 29.Heikkilä M., Prunskaite R., Naillat F., Itäranta P., Vuoristo J., Leppäluoto J., Peltoketo H., Vainio S. The partial female-to-male sex reversal in Wnt-4 deficient females involves induced expression of testosterone biosynthetic genes and testosterone production and depends on androgen action. Endocrinology. 2005;146:4016–4023. doi: 10.1210/en.2005-0463. [DOI] [PubMed] [Google Scholar]
- 30.Yao H.H.C., Matzuk M.M., Jorgez C.J., Menke D.B., Page D.C., Swain A., Capel B. Follistatin operates downstream of Wnt4 in mammalian ovary organogenesis. Dev. Dyn. 2004;230:210–215. doi: 10.1002/dvdy.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu C.F., Parker K., Humphrey Y. WNT4/β-catenin pathway maintains female germ cell survival by inhibiting activin βB in the mouse fetal ovary. PLoS ONE. 2010;5:e10382. doi: 10.1371/journal.pone.0010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chassot A.A., Gregoire E.P., Lavery R., Taketo M.M., de Rooij D.G., Adams I.R., Chaboissier M.C. RSPO1/β-Catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS ONE. 2011;6:e25641. doi: 10.1371/journal.pone.0025641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stévant I., Kühne F., Greenfield A., Chaboissier M.C., Dermitzakis E.T., Nef S. Dissecting Cell Lineage Specification and Sex Fate Determination in Gonadal Somatic Cells Using Single-Cell Transcriptomics. Cell Rep. 2019;26:3272–3283. doi: 10.1016/j.celrep.2019.02.069. [DOI] [PubMed] [Google Scholar]
- 34.Maatouk D.M., Dinapoli L., Alvers A., Parker K.L., Taketo M.M., Capel B. Stabilization of β-catenin in XY gonads causes male-to-female sex-reversal. Hum. Mol. Genet. 2008;17:2949–2955. doi: 10.1093/hmg/ddn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris A., Siggers P., Corrochano S., Warr N., Sagar D., Grimes D.T., Suzuki M., Burdine R.D., Cong F., Koo B.-K., et al. ZNRF3 functions in mammalian sex determination by inhibiting canonical WNT signaling. Proc. Natl. Acad. Sci. USA. 2018;115:5474–5479. doi: 10.1073/pnas.1801223115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicol B., Grimm S.A., Gruzdev A., Scott G.J., Ray M.K., Yao H.H.C. Genome-wide identification of FOXL2 binding and characterization of FOXL2 feminizing action in the fetal gonads. Hum. Mol. Genet. 2018;27:4273–4287. doi: 10.1093/hmg/ddy312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vidal V.P.I., Chaboissier M.C., De Rooij D.G., Schedl A. Sox9 induces testis development in XX transgenic mice. Nat. Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- 38.Gregoire E.P., Lavery R., Chassot A.A., Akiyama H., Treier M., Behringer R.R., Chaboissier M.C. Transient development of ovotestes in XX Sox9 transgenic mice. Dev. Biol. 2011;349:65–77. doi: 10.1016/j.ydbio.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiramatsu R., Matoba S., Kanai-Azuma M., Tsunekawa N., Katoh-Fukui Y., Kurohmaru M., Morohashi K.I., Wilhelm D., Koopman P., Kanai Y. A critical time window of Sry action in gonadal sex determination in mice. Development. 2009;136:129–138. doi: 10.1242/dev.029587. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y., Kobayashi A., Sekido R., DiNapoli L., Brennan J., Chaboissier M.C., Poulat F., Behringer R.R., Lovell-Badge R., Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:1000–1009. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagheri-Fam S., Bird A.D., Zhao L., Ryan J.M., Yong M., Wilhelm D., Koopman P., Eswarakumar V.P., Harley V.R. Testis determination requires a specific FGFR2 isoform to repress FOXL2. Endocrinology. 2017;158:3832–3843. doi: 10.1210/en.2017-00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bagheri-Fam S., Sim H., Bernard P., Jayakody I., Taketo M.M., Scherer G., Harley V.R. Loss of Fgfr2 leads to partial XY sex reversal. Dev. Biol. 2008;314:71–83. doi: 10.1016/j.ydbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Colvin J.S., Green R.P., Schmahl J., Capel B., Ornitz D.M. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell. 2001;104:875–889. doi: 10.1016/S0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- 44.Kim Y., Bingham N., Sekido R., Parker K.L., Lovell-Badge R., Capel B. Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc. Natl. Acad. Sci. USA. 2007;104:16558–16563. doi: 10.1073/pnas.0702581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jameson S.A., Lin Y.T., Capel B. Testis development requires the repression of Wnt4 by Fgf signaling. Dev. Biol. 2012;370:24–32. doi: 10.1016/j.ydbio.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lavery R., Chassot A.A., Pauper E., Gregoire E.P., Klopfenstein M., de Rooij D.G., Mark M., Schedl A., Ghyselinck N.B., Chaboissier M.C. Testicular Differentiation Occurs in Absence of R-spondin1 and Sox9 in Mouse Sex Reversals. PLoS Genet. 2012;8:e1003170. doi: 10.1371/journal.pgen.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bingham N.C., Verma-Kurvari S., Parada L.F., Parker K.L. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. doi: 10.1002/dvg.20231. [DOI] [PubMed] [Google Scholar]
- 48.Ottolenghi C., Pelosi E., Tran J., Colombino M., Douglass E., Nedorezov T., Cao A., Forabosco A., Schlessinger D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum. Mol. Genet. 2007;16:2795–2804. doi: 10.1093/hmg/ddm235. [DOI] [PubMed] [Google Scholar]
- 49.Auguste A., Chassot A.A., Grégoire E.P., Renault L., Pannetier M., Treier M., Pailhoux E., Chaboissier M.C. Loss of R-spondin1 and Foxl2 amplifies female-to-male sex reversal in XX mice. Sex. Dev. 2012;5:304–317. doi: 10.1159/000334517. [DOI] [PubMed] [Google Scholar]
- 50.Durlinger A.L.L., Gruijters M.J.G., Kramer P., Karels B., Ingraham H.A., Nachtigal M.W., Uilenbroek J.T.J., Grootegoed J.A., Themmen A.P.N. Anti-Müllerian Hormone Inhibits Initiation of Primordial Follicle Growth in the Mouse Ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 51.Lei N., Hornbaker K.I., Rice D.A., Karpova T., Agbor V.A., Heckert L.L. Sex-Specific Differences in Mouse DMRT1 Expression Are Both Cell Type- and Stage-Dependent During Gonad Development1. Biol. Reprod. 2007;77:466–475. doi: 10.1095/biolreprod.106.058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munger S.C., Natarajan A., Looger L.L., Ohler U., Capel B. Fine Time Course Expression Analysis Identifies Cascades of Activation and Repression and Maps a Putative Regulator of Mammalian Sex Determination. PLoS Genet. 2013;9:e1003630. doi: 10.1371/journal.pgen.1003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao L., Wang C., Lehman M.L., He M., An J., Svingen T., Spiller C.M., Ng E.T., Nelson C.C., Koopman P. Transcriptomic analysis of mRNA expression and alternative splicing during mouse sex determination. Mol. Cell. Endocrinol. 2018;478:84–96. doi: 10.1016/j.mce.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Chen H., Palmer J.S., Thiagarajan R.D., Dinger M.E., Lesieur E., Chiu H., Schulz A., Spiller C., Grimmond S.M., Little M.H., et al. Identification of novel markers of mouse fetal ovary development. PLoS ONE. 2012;7:e41683. doi: 10.1371/journal.pone.0041683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maatouk D.M., Mork L., Hinson A., Kobayashi A., McMahon A.P., Capel B. Germ Cells Are Not Required to Establish the Female Pathway in Mouse Fetal Gonads. PLoS ONE. 2012;7:e47238. doi: 10.1371/journal.pone.0047238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilhelm D., Martinson F., Bradford S., Wilson M.J., Combes A.N., Beverdam A., Bowles J., Mizusaki H., Koopman P. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev. Biol. 2005;287:111–124. doi: 10.1016/j.ydbio.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 57.Jeays-Ward K., Dandonneau M., Swain A. Wnt4 is required for proper male as well as female sexual development. Dev. Biol. 2004;276:431–440. doi: 10.1016/j.ydbio.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 58.Chassot A.-A., Bradford S.T., Auguste A., Gregoire E.P., Pailhoux E., de Rooij D.G., Schedl A., Chaboissier M.-C. WNT4 and RSPO1 together are required for cell proliferation in the early mouse gonad. Development. 2012;139:4461–4472. doi: 10.1242/dev.078972. [DOI] [PubMed] [Google Scholar]
- 59.Best D., Sahlender D.A., Walther N., Peden A.A., Adams I.R. Sdmg1 is a conserved transmembrane protein associated with germ cell sex determination and germline-soma interactions in mice. Development. 2008;135:1415–1425. doi: 10.1242/dev.019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chassot A.A., Gregoire E.P., Magliano M., Lavery R., Chaboissier M.C. Genetics of ovarian differentiation: Rspo1, a major player. Sex. Dev. 2008;2:219–227. doi: 10.1159/000152038. [DOI] [PubMed] [Google Scholar]
- 61.Stark K., Vainio S., Vassileva G., McMahon A.P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 62.Wilhelm D., Washburn L.L., Truong V., Fellous M., Eicher E.M., Koopman P. Antagonism of the testis- and ovary-determining pathways during ovotestis development in mice. Mech. Dev. 2009;126:324–336. doi: 10.1016/j.mod.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garcia-Moreno S.A., Lin Y.-T., Futtner C.R., Salamone I.M., Capel B., Maatouk D.M. CBX2 is required to stabilize the testis pathway by repressing Wnt signaling. PLoS Genet. 2019;15:e1007895. doi: 10.1371/journal.pgen.1007895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moniot B., Declosmenil F., Barrionuevo F., Scherer G., Aritake K., Malki S., Marzi L., Cohen-Solal A., Georg I., Klattig J., et al. The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in Sertoli cells during male sexual differentiation. Development. 2009;136:1813–1821. doi: 10.1242/dev.032631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao H.H.-C., Aardema J., Holthusen K. Sexually Dimorphic Regulation of Inhibin Beta B in Establishing Gonadal Vasculature in Mice1. Biol. Reprod. 2006;74:978–983. doi: 10.1095/biolreprod.105.050286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kashimada K., Pelosi E., Chen H., Schlessinger D., Wilhelm D., Koopman P. FOXL2 and BMP2 act cooperatively to regulate follistatin gene expression during ovarian development. Endocrinology. 2011;152:272–280. doi: 10.1210/en.2010-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao H.H.C., Whoriskey W., Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harikae K., Miura K., Shinomura M., Matoba S., Hiramatsu R., Tsunekawa N., Kanai-Azuma M., Kurohmaru M., Morohashi K.-I., Kanai Y. Heterogeneity in sexual bipotentiality and plasticity of granulosa cells in developing mouse ovaries. J. Cell Sci. 2013;126:2834–2844. doi: 10.1242/jcs.122663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.