Abstract
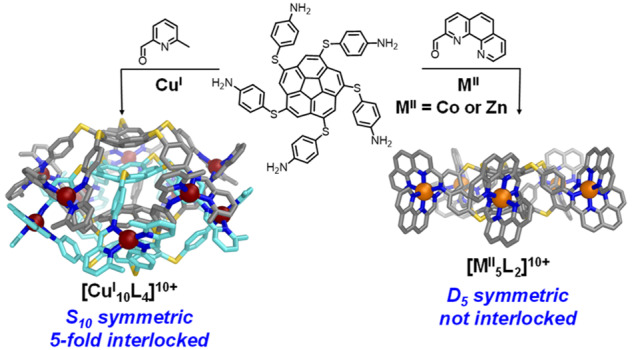
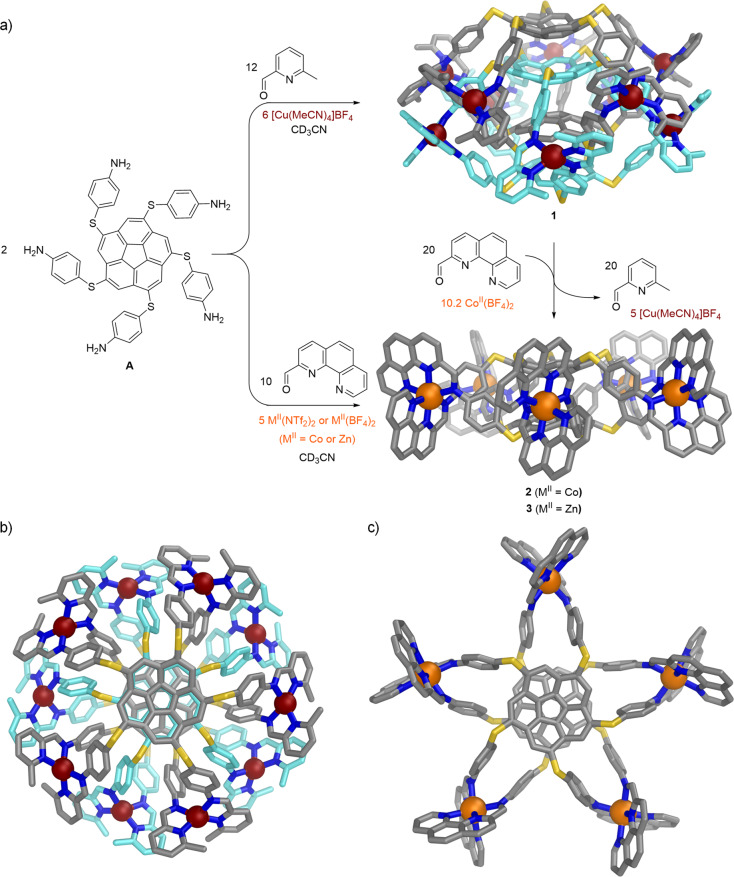
The reaction of sym-pentakis(4-aminothiophenyl)corannulene with 2-formyl-6-methylpyridine and CuI or 2-formyl-1,10-phenanthroline and MII (M = Co, Zn) yields an S10-symmetric 5-fold interlocked [2]catenane of two interpenetrating [CuI5L2]5+ cages or D5-symmetric [MII5L2]10+ cages, respectively. The new structures were characterized by X-ray crystallography, NMR spectroscopy, and mass spectrometry. Density functional theory computations point to dispersive energies on par with traditional covalent bond energies. Subcomponent exchange reactions favored formation of the [CoII5L2]10+ cage over the [CuI10L4]10+ catenane. The single cage and catenane each cocrystallized with a corannulene guest to form a bowl-in-bowl substructure.
Despite the intricate and challenging nature of interlocked molecules, knots,1 ravels,2 and catenanes3 of manifold forms have been structurally characterized, including interlocked cages of higher order.4 A rational synthetic approach for these systems is not yet available, but studies have begun to assess structure–energy relationships for these complexes.5 In favorable situations, host–guest studies6 have provided insight into structure–energy correlations of molecular aggregation phenomena more generally.7 Dispersive interactions become more important with increasing size and can rival covalent bonding energies.8
The assembly of subcomponents around metal ion templates9 yields thermodynamically stable aggregates that may have complex topologies. Judicious choice of subcomponent geometries results in high-symmetry Platonic or Archimedean polyhedra9a or even entwined higher-order topological architectures such as links and knots.10 In this context, corannulene, with 5-fold symmetry, has been the focus of dodecahedral “capsid” construction.11 Herein, pentafold-substituted corannulene subcomponents capable of generating ligands for tetrahedral CuI assembled into a surprisingly highly entangled 5-fold interlocked [2]catenane of exceedingly rare S10 symmetry.12 These interlocked [2]catenanes expand the class of interlocked cages from 3-fold13 and 4-fold14 to 5-fold structures. A related assembly binding octahedral ZnII or CoII provides a noninterlocked cognate of D5 symmetry.
Corannulene was chosen as a suitable 5-fold-symmetric scaffold11,15 because of its potential for functionalization via a variety of synthetic routes16 and its curved aromatic surface, which may enhance the guest binding properties17 of assemblies via aromatic stacking interactions.18sym-Pentakis(4-aminothiophenyl)corannulene (subcomponent A, Figure 1) was synthesized by nucleophilic aromatic substitution from sym-pentachlorocorannulene and 4-aminothiophenol in 1,2-dimethylimidazolone in the presence of sodium hydride (Scheme S1).
Figure 1.
(a) Synthesis of 1–3 and the conversion of 1 to 2 through subcomponent exchange. Side-on views of the crystal structures of 1 and 2 are shown. (b) View down the S10 axis of the crystal structure of 1. (c) View down the C5 axis of the crystal structure of 2. In the images of 1, the carbon atoms of the two interlocked [CuI5L2]5+ cages are colored differently, and only one of the two crystallographically unique assemblies is shown; in all cases hydrogen atoms, counterions, solvent molecules, and disorder have been omitted for clarity.
The reaction of pentaaniline A (2 equiv) with 2-formyl-6-methylpyridine (12 equiv) and tetrakis(acetonitrile)copper(I) tetrafluoroborate (CuI(MeCN)4BF4) (6 equiv)19 in CD3CN at room temperature gave product 1 (Figure 1a). The 1H NMR spectrum of 1 was well-resolved but complex, consisting of two magnetically distinct environments of equal intensity per ligand proton (Figure 2a). All of the peaks between 2.26 and 9.31 ppm displayed a single diffusion constant in the diffusion-ordered 1H NMR (DOSY) spectrum, suggesting that they belonged to a single species.20
Figure 2.
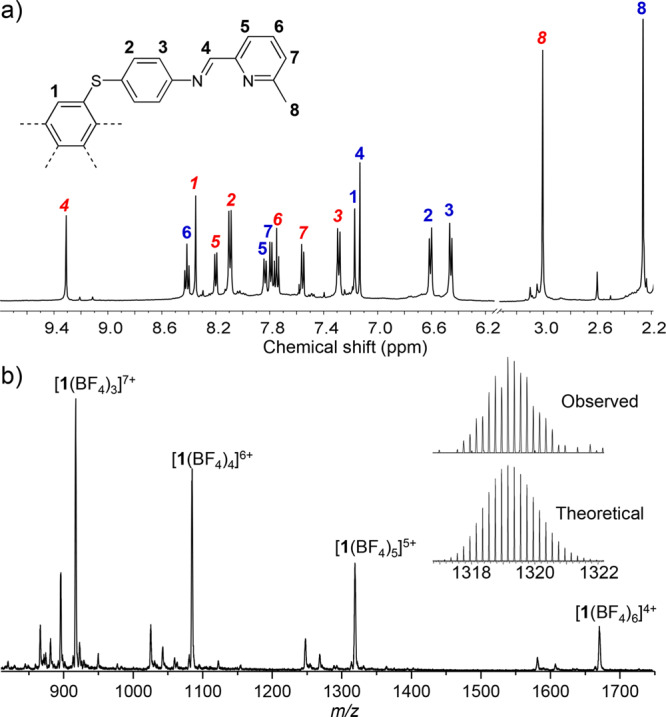
(a) 1H NMR spectra (500 MHz, CD3CN, 298 K) of 1. Peak assignments for the interior and exterior ligands are marked with bold blue and italic red labels, respectively. (b) ESI-MS spectrum of 1. The inset shows the theoretical and observed isotope patterns for the +5 peak.
Crystals of 1 were grown through vapor diffusion of diethyl ether into an acetonitrile solution, and the solid-state structure of 1 was elucidated by single-crystal X-ray diffraction using synchrotron radiation.21 The crystal structure revealed a [CuI10L4]10+ assembly consisting of a pair of 5-fold interlocked [CuI5L2]5+ cages (Figure 1). The distances of 11.3–11.9 Å between neighboring metal centers create windows of sufficient size to allow two [CuI5L2]5+ cages to interlock to form a [2]catenane. The two ligands within each [CuI5L2]5+ cage display the same handedness, resulting in idealized D5 cage symmetry. The interlocking of each cage with its enantiomer lends the complete [CuI10L4]10+ assembly S10 point-group symmetry.
The two [CuI5L2]5+ cages of the [2]catenane interlock tightly, forming two bowl-in-bowl substructures with stacked corannulenes18 separated by a distance of 3.69(1) Å. Within a single cage, the distance of 6.93(1) Å between the mean planes of the central pentagons of the corannulenes creates a cavity that matches the form of the intercalated corannulene from the other cage.
The solution NMR data for 1 in CD3CN are consistent with the solid-state structure, wherein the exo and endo ligands give rise to distinct magnetic environments (Figure 2a). The imine, corannulene, and phenylene signals for the endo ligand are shielded relative to those of the exo ligand. NOE cross-peaks are observed between NMR signals of the two ligands (Figure S8) in a manner consistent with the interlocked structure observed in the solid state. ESI-MS results are also consistent with the [CuI10L4]10+ composition (Figure 2b).
Density functional theory (DFT) computations of the [CuI10L4]10+ catenane and the hypothetical [CuI5L2]5+ cage allowed an assessment of dispersion effects on their relative energetics based on the crystal structure of 1 vs those on the optimized geometry and of the effects of solvation. Neglecting dispersion effects, the energetics of the catenation process in the acetonitrile environment was calculated to be +42.0 kcal/mol (B3LYP/6-31G(d,p)//B3LYP/6-31G(d,p):acetonitrile), indicating that the complex is unbound. With fixed geometry, the energetics including dispersion in the acetonitrile environment is −212 kcal/mol (B3LYP-D3/6-31G(d,p)//B3LYP/6-31G(d,p):acetonitrile). Estimation of the effects of dispersion on the geometry carried out by single-point analysis resulted in a contribution of ∼52 kcal/mol. This gives in total ∼264 kcal/mol for the catenation process including the effects of dispersion in an acetonitrile environment. Full optimization of the complex including dispersion in the acetonitrile environment (B3LYP-D3/6-31G(d,p):acetonitrile) resulted in a complexation energy of 272.2 kcal/mol, a difference of <8 kcal/mol from the estimated value. This substantial energy contribution for the catenation process is consistent with the large surface area of the corannulene moieties giving rise to substantial van der Waals interactions. Tight packing and the large contact area between ligands within 1 play a central role in driving the selective assembly of this structure.18,32
Tridentate donor sites formed from imine condensation with 2-formyl-1,10-phenanthroline, suitable for the octahedral coordination of CoII and ZnII,22 enabled investigation of whether octahedral metal centers also give catenanes incorporating A. The reaction of A (2 equiv) with 2-formyl-1,10-phenanthroline (10 equiv) and cobalt(II) bis(trifluoromethanesulfonyl)imide (Co(NTf2)2·5H2O) (5 equiv) in CD3CN at 353 K yielded 2. The 1H NMR spectrum reveals a single resonance for each symmetry-equivalent proton, with the signals spread over the range −65.9 to +259.4 ppm as a result of the paramagnetism of CoII.23 The ESI-MS spectra are consistent with the formula [CoII5L2]10+ (Figures S14 and S17).
Crystals of 2 (cocrystallized with coronene) were obtained through vapor diffusion of diisopropyl ether into an acetonitrile solution of the BF4– salt containing excess coronene.24 Single-crystal X-ray measurements revealed non-interlocked [CoII5L2]10+ cages composed of two 5-fold-symmetric ligands of the same handedness bridging five octahedral CoII centers (Figure 1). Both enantiomers of the D5-symmetric cage are present in the unit cell, related by inversion. The hub pentagon mean planes of the two corannulene moieties sit at a distance of 3.18(1) Å from each other, closer than the 3.2–3.3 Å of graphite planes. The corannulenes in 2 are also flattened compared with those in 1, with an average bowl depth25 of 0.84(3) Å versus 0.95(3) and 0.91(1) Å for the exo and endo corannulenes in 1. Significant distortion from regular octahedral geometry around the metal centers is also observed, with angles of 81–84° between the CoIIN3 chelate planes, compared with angles of 84–90° between the CuIN2 chelate planes in 1, which displayed a more regular tetrahedral geometry.
Similarly, the reaction of A (2 equiv) with 2-formyl-1,10-phenanthroline (10 equiv) and zinc(II) bis(trifluoromethanesulfonyl)imide (Zn(NTf2)2) (5 equiv) in CD3CN at 353 K yielded [ZnII5L2]10+ assembly 3, as confirmed by ESI-MS. The 1H NMR spectrum also displays a single set of resonances for each ligand proton environment. Crystals were obtained by diffusion of diethyl ether into an acetonitrile solution of 3 containing CsCB11H12. Single-crystal X-ray analysis of 3 confirmed it to be isostructural with 2, although the weakly diffracting nature of the crystals precluded detailed analysis of the structural parameters (Figures S52 and S53).
The relative stabilities of 1 and 2 were probed via subcomponent exchange reactions.26 A mixture of Co(BF4)2·6H2O (10.2 equiv per assembly) and 2-formyl-1,10-phenanthroline (20 equiv per assembly) was added to a solution of 1 in CD3CN, and the mixture was stirred at 333 K for 24 h and then at 353 K for 72 h. 1H NMR spectra of the reaction mixture showed the disappearance of the diamagnetic signals of 1 followed by the appearance of the paramagnetically shifted signals of 2. ESI-MS analysis of the resulting mixture indicated the formation of 2 as the major product in solution, indicating its greater stability relative to 1. We infer that the formation of 2 is enthalpically favored as a consequence of the stronger coordination bonds of the tridentate ligand arms with CoII in 2 relative to the bidentate ligand arms with CuI in 1 and also entropically favored because one molecule of 1 is converted into two molecules of 2.
The potential for guests to intercalate27 within diamagnetic assemblies 1 and 3 or for guests to induce an assembly to rearrange into a suitable host28 was investigated through the addition of the prospective guests shown in Figure S33. The assemblies were initially investigated as hosts for corannulene, inspired both by the interlocked cage structure of 1 and the observation of corannulene encapsulation inside other polyaromatic hosts29 and electron-deficient macrocycles.30 Addition of corannulene (10 equiv) to CD3CN solutions of 1 or 3 led to shifts in the signals of the 1H NMR spectra of both the host and guest, consistent with fast-exchange complexation on the 1H NMR time scale (Figures S34 and S35).
The host–guest complexes of 1 and 2 with corannulene were also characterized in the solid state through single-crystal X-ray diffraction. Crystals of (corannulene)2·1 were obtained from vapor diffusion of diethyl ether into an acetonitrile solution of 1 saturated with corannulene. The structure reveals two corannulene molecules stacked on the externally facing corannulene moieties of 1 at a distance of 3.71(2) Å (Figure 3a).
Figure 3.
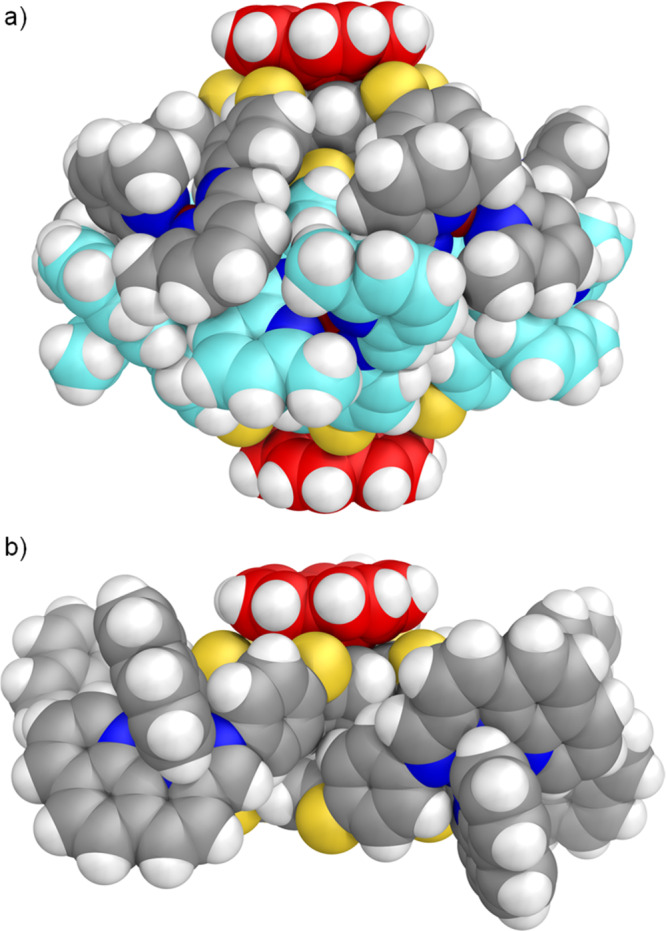
Side views of the cationic parts of the crystal structures of (a) (corannulene)2·1 and (b) corannulene·2. Counterions, solvent molecules, and disorder have been omitted for clarity, and the carbon atoms of the stacked corannulenes are colored red.
Crystals of corannulene·2 were obtained from vapor diffusion of diethyl ether into an acetonitrile solution of the triflimide salt of 2 saturated with corannulene and containing excess tetrabutylammonium perrhenate to aid crystallization. In this case only a single corannulene was observed to stack with one of the corannulene moieties of 2, with a refined occupancy of ca. 0.6 and a distance of 3.57(4) Å between the stacked rings (Figure 3b). The close packing of corannulene on the exterior of 2 in the structure of corannulene·2 contrasts with that observed in the cocrystal of 2 with coronene (Figures S46 and S47), where the cocrystallized coronene molecules intercalate between ligand arms but do not show any specific stacking interactions with the [CoII5L2]10+ cation.
The binding mode observed in the solid state is consistent with the solution NMR data, with the largest shifts in host proton signals observed for the exo-corannulene protons of 1, consistent with corannulene undergoing stacking interactions with the exterior of the cage.22 No interactions were observed between 1 or 3 and planar polycyclic aromatic hydrocarbons,31 CB11H12– anions,32 or spherical C60, despite the known tendency for fullerenes to interact with corannulenes17 and corannulene-based hosts.15a,33
In summary, a 5-fold interlocked [CuI10L4]10+ [2]catenane, representing a new structure type, has been prepared from CuI and corannulene-based subcomponent A. A DFT study revealed the dominant role of aromatic stacking interactions in driving the formation of the interlocked structure, which is a common feature13a,13d of other interlocked cage systems. This study demonstrates the power of van der Waals interactions together with coordination-driven assembly to generate new types of highly complex structures.
Acknowledgments
This work was supported by the European Research Council (695009), the U.K. Engineering and Physical Sciences Research Council (EPSRC) (EP/P027067/1), and the National Natural Science Foundation of China (21871207). We thank the EPSRC National Mass Spectrometry Centre (Swansea, U.K.) for high-resolution mass spectrometry and the Diamond Light Source (Didcot, U.K.) for synchrotron beamtime on I19 (MT15768). We thank the National Basic Research Program of China (2015CB856500), the Qian Ren Scholar Program of China, and the Synergetic Innovation Center of Chemical Science and Engineering (Tianjin) for additional support.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c03349.
Detailed descriptions of synthetic procedures, characterization of new compounds, and spectroscopic data (PDF)
X-ray data for 1·10BF4 (CCDC 1913638) (CIF)
X-ray data for (corannulene)2·1·10BF4 (CCDC 1913639) (CIF)
X-ray data for 2·10BF4 (CCDC 1913641) (CIF)
X-ray data for 2·coronene·10BF4 (CCDC 1913642) (CIF)
X-ray data for 2·10ClO4 (CCDC 1913643) (CIF)
X-ray data for (corannulene)0.6·2·4ReO4·6NTf2 (CCDC 1913646) (CIF)
X-ray data for 3·2CB11H12·8NTf2 (CCDC 1913640) (CIF)
The authors declare no competing financial interest.
Supplementary Material
References
- Fielden S. D. P.; Leigh D. A.; Woltering S. L. Molecular Knots. Angew. Chem., Int. Ed. 2017, 56, 11166. 10.1002/anie.201702531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.; Clegg J. K.; Lindoy L. F.; MacQuart R. B.; Meehan G. V. Metallosupramolecular Self-Assembly of a Universal 3-Ravel. Nat. Commun. 2011, 2, 205. 10.1038/ncomms1208. [DOI] [PubMed] [Google Scholar]
- Ng A. W. H.; Yee C.-C.; Au-Yeung H. Y. Radial Hetero[5]catenanes: Peripheral Isomer Sequences of the Interlocked Macrocycles. Angew. Chem., Int. Ed. 2019, 58, 17375. 10.1002/anie.201908576. [DOI] [PubMed] [Google Scholar]
- a Zhu R.; Ding J.; Jin L.; Pang H. Interpenetrated structures appeared in supramolecular cages, MOFs, COFs. Coord. Chem. Rev. 2019, 389, 119. 10.1016/j.ccr.2019.03.002. [DOI] [Google Scholar]; b Frank M.; Johnstone M. D.; Clever G. H. Interpenetrated Cage Structures. Chem. - Eur. J. 2016, 22, 14104. 10.1002/chem.201601752. [DOI] [PubMed] [Google Scholar]; c Zhang G.; Presly O.; White F.; Oppel I. M.; Mastalerz M. A Shape-Persistent Quadruply Interlocked Giant Cage Catenane with Two Distinct Pores in the Solid State. Angew. Chem., Int. Ed. 2014, 53, 5126. 10.1002/anie.201400285. [DOI] [PubMed] [Google Scholar]
- a Cougnon F. B. L.; Au-Yeung H. Y.; Pantoş G. D.; Sanders J. K. M. Exploring the Formation Pathways of Donor–Acceptor Catenanes in Aqueous Dynamic Combinatorial Libraries. J. Am. Chem. Soc. 2011, 133, 3198. 10.1021/ja111407m. [DOI] [PubMed] [Google Scholar]; b Hasell T.; Wu X.; Jones J. T. A.; Bacsa J.; Steiner A.; Mitra T.; Trewin A.; Adams D. J.; Cooper A. I. Triply interlocked covalent organic cages. Nat. Chem. 2010, 2, 750. 10.1038/nchem.739. [DOI] [PubMed] [Google Scholar]; c Zhu K.; Baggi G.; Loeb S. J. Ring-through-ring molecular shuttling in a saturated [3]rotaxane. Nat. Chem. 2018, 10, 625. 10.1038/s41557-018-0040-9. [DOI] [PubMed] [Google Scholar]
- a Liu W.; Johnson A.; Smith B. D. Guest Back-Folding: A Molecular Design Strategy That Produces a Deep-Red Fluorescent Host/Guest Pair with Picomolar Affinity in Water. J. Am. Chem. Soc. 2018, 140, 3361. 10.1021/jacs.7b12991. [DOI] [PubMed] [Google Scholar]; b Gropp C.; Fischer S.; Husch T.; Trapp N.; Carreira E. M.; Diederich F. Molecular Recognition and Cocrystallization of Methylated and Halogenated Fragments of Danicalipin A by Enantiopure Alleno-Acetylenic Cage Receptors. J. Am. Chem. Soc. 2020, 142, 4749. 10.1021/jacs.9b13217. [DOI] [PubMed] [Google Scholar]; c Löffler S.; Wuttke A.; Zhang B.; Holstein J. J.; Mata R. A.; Clever G. H. Influence of size, shape, heteroatom content and dispersive contributions on guest binding in a coordination cage. Chem. Commun. 2017, 53, 11933. 10.1039/C7CC04855F. [DOI] [PubMed] [Google Scholar]
- a Turega S.; Cullen W.; Whitehead M.; Hunter C. A.; Ward M. D. Mapping the internal recognition surface of an octanuclear coordination cage using guest libraries. J. Am. Chem. Soc. 2014, 136, 8475. 10.1021/ja504269m. [DOI] [PubMed] [Google Scholar]; b Davis A. V.; Fiedler D.; Seeber G.; Zahl A.; Van Eldik R.; Raymond K. N. Guest Exchange Dynamics in an M4L6 Tetrahedral Host. J. Am. Chem. Soc. 2006, 128, 1324. 10.1021/ja056556+. [DOI] [PubMed] [Google Scholar]; c Jiang W.; Ajami D.; Rebek J. Alkane Lengths Determine Encapsulation Rates and Equilibria. J. Am. Chem. Soc. 2012, 134, 8070. 10.1021/ja302669y. [DOI] [PubMed] [Google Scholar]; d Qiao B.; Sengupta A.; Liu Y.; McDonald K. P.; Pink M.; Anderson J. R.; Raghavachari K.; Flood A. H. Electrostatic and Allosteric Cooperativity in Ion-Pair Binding: A Quantitative and Coupled Experiment–Theory Study with Aryl–Triazole–Ether Macrocycles. J. Am. Chem. Soc. 2015, 137, 9746. 10.1021/jacs.5b05839. [DOI] [PubMed] [Google Scholar]
- Toyota S.; Woods C. R.; Benaglia M.; Haldimann R.; Wärnmark K.; Hardcastle K.; Siegel J. S. Tetranuclear Copper(I)-Biphenanthroline Gridwork: Violation of the Principle of Maximal Donor Coordination Caused by Intercalation and CH-to-N Forces. Angew. Chem., Int. Ed. 2001, 40, 751.. [DOI] [PubMed] [Google Scholar]
- a Ronson T. K.; Zarra S.; Black S. P.; Nitschke J. R. Metal-organic container molecules through subcomponent self-assembly. Chem. Commun. 2013, 49, 2476. 10.1039/c2cc36363a. [DOI] [PubMed] [Google Scholar]; b Zhang D.; Ronson T. K.; Nitschke J. R. Functional Capsules via Subcomponent Self-Assembly. Acc. Chem. Res. 2018, 51, 2423. 10.1021/acs.accounts.8b00303. [DOI] [PubMed] [Google Scholar]; c Luo D.; Wang X.-Z.; Yang C.; Zhou X.-P.; Li D. Self-Assembly of Chiral Metal–Organic Tetartoid. J. Am. Chem. Soc. 2018, 140, 118. 10.1021/jacs.7b11285. [DOI] [PubMed] [Google Scholar]; d Li X.; Wu J.; He C.; Zhang R.; Duan C. Multicomponent self-assembly of a pentanuclear Ir–Zn heterometal–organic polyhedron for carbon dioxide fixation and sulfite sequestration. Chem. Commun. 2016, 52, 5104. 10.1039/C6CC00064A. [DOI] [PubMed] [Google Scholar]
- a Bilbeisi R. A.; Prakasam T.; Lusi M.; El Khoury R.; Platas-Iglesias C.; Charbonnière L. J.; Olsen J.-C.; Elhabiri M.; Trabolsi A. [C–H···anion] interactions mediate the templation and anion binding properties of topologically non-trivial metal–organic structures in aqueous solutions. Chem. Sci. 2016, 7, 2524. 10.1039/C5SC04246A. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wood C. S.; Ronson T. K.; Belenguer A. M.; Holstein J. J.; Nitschke J. R. Two-stage directed self-assembly of a cyclic [3]catenane. Nat. Chem. 2015, 7, 354. 10.1038/nchem.2205. [DOI] [PubMed] [Google Scholar]; c Ayme J.-F.; Beves J. E.; Leigh D. A.; McBurney R. T.; Rissanen K.; Schultz D. A synthetic molecular pentafoil knot. Nat. Chem. 2012, 4, 15. 10.1038/nchem.1193. [DOI] [PubMed] [Google Scholar]; d Chichak K. S.; Cantrill S. J.; Pease A. R.; Chiu S.-H.; Cave G. W. V.; Atwood J. L.; Stoddart J. F. Molecular Borromean Rings. Science 2004, 304, 1308. 10.1126/science.1096914. [DOI] [PubMed] [Google Scholar]
- Chen Y.-S.; Solel E.; Huang Y.-F.; Wang C.-L.; Tu T.-H.; Keinan E.; Chan Y.-T. Chemical mimicry of viral capsid self-assembly via corannulene-based pentatopic tectons. Nat. Commun. 2019, 10, 3443. 10.1038/s41467-019-11457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John R. P.; Lee K.; Kim B. J.; Suh B. J.; Rhee H.; Lah M. S. Modulation of the Ring Size and Nuclearity of Metallamacrocycles via the Steric Effect of Ligands: Preparation and Characterization of 18-Membered Hexanuclear, 24-Membered Octanuclear, and 30-Membered Decanuclear Manganese Metalladiazamacrocycles with α- and β-Branched N-Acylsalicylhydrazides. Inorg. Chem. 2005, 44, 7109. 10.1021/ic050891h. [DOI] [PubMed] [Google Scholar]
- a Fujita M.; Fujita N.; Ogura K.; Yamaguchi K. Spontaneous assembly of ten components into two interlocked, identical coordination cages. Nature 1999, 400, 52. 10.1038/21861. [DOI] [Google Scholar]; b Westcott A.; Fisher J.; Harding L. P.; Rizkallah P.; Hardie M. J. Self-assembly of a 3-D triply interlocked chiral [2]catenane. J. Am. Chem. Soc. 2008, 130, 2950. 10.1021/ja8002149. [DOI] [PubMed] [Google Scholar]; c Henkelis J. J.; Ronson T. K.; Harding L. P.; Hardie M. J. M3L2 metallo-cryptophanes: [2]catenane and simple cages. Chem. Commun. 2011, 47, 6560. 10.1039/c1cc10806a. [DOI] [PubMed] [Google Scholar]; d Mishra A.; Dubey A.; Min J. W.; Kim H.; Stang P. J.; Chi K.-W. Molecular self-assembly of arene-Ru based interlocked catenane metalla-cages. Chem. Commun. 2014, 50, 7542. 10.1039/C4CC01991A. [DOI] [PubMed] [Google Scholar]; e Yamauchi Y.; Yoshizawa M.; Fujita M. Engineering Stacks of Aromatic Rings by the Interpenetration of Self-Assembled Coordination Cages. J. Am. Chem. Soc. 2008, 130, 5832. 10.1021/ja077783+. [DOI] [PubMed] [Google Scholar]; f Yang L.; Jing X.; An B.; He C.; Yang Y.; Duan C. Binding of anions in triply interlocked coordination catenanes and dynamic allostery for dehalogenation reactions. Chem. Sci. 2018, 9, 1050. 10.1039/C7SC04070A. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Li Y.; Mullen K. M.; Claridge T. D. W.; Costa P. J.; Felix V.; Beer P. D. Sulfate anion templated synthesis of a triply interlocked capsule. Chem. Commun. 2009, 7134. 10.1039/b915548a. [DOI] [PubMed] [Google Scholar]; h Wang Q.; Yu C.; Long H.; Du Y.; Jin Y.; Zhang W. Solution-Phase Dynamic Assembly of Permanently Interlocked Aryleneethynylene Cages through Alkyne Metathesis. Angew. Chem., Int. Ed. 2015, 54, 7550. 10.1002/anie.201501679. [DOI] [PubMed] [Google Scholar]; i Li H.; Zhang H.; Lammer A. D.; Wang M.; Li X.; Lynch V. M.; Sessler J. L. Quantitative self-assembly of a purely organic three-dimensional catenane in water. Nat. Chem. 2015, 7, 1003. 10.1038/nchem.2392. [DOI] [PubMed] [Google Scholar]
- a Fukuda M.; Sekiya R.; Kuroda R. A Quadruply Stranded Metallohelicate and Its Spontaneous Dimerization into an Interlocked Metallohelicate. Angew. Chem. 2008, 120, 718. 10.1002/ange.200703162. [DOI] [PubMed] [Google Scholar]; b Han M.; Engelhard D. M.; Clever G. H. Self-assembled coordination cages based on banana-shaped ligands. Chem. Soc. Rev. 2014, 43, 1848. 10.1039/C3CS60473J. [DOI] [PubMed] [Google Scholar]
- a Sun W.; Wang Y.; Ma L.; Zheng L.; Fang W.; Chen X.; Jiang H. Self-Assembled Carcerand-like Cage with a Thermoregulated Selective Binding Preference for Purification of High-Purity C60 and C70. J. Org. Chem. 2018, 83, 14667. 10.1021/acs.joc.8b02674. [DOI] [PubMed] [Google Scholar]; b Huang F.; Ma L.; Che Y.; Jiang H.; Chen X.; Wang Y. Corannulene-Based Coordination Cage with Helical Bias. J. Org. Chem. 2018, 83, 733. 10.1021/acs.joc.7b02709. [DOI] [PubMed] [Google Scholar]
- a Pappo D.; Mejuch T.; Reany O.; Solel E.; Gurram M.; Keinan E. Diverse Functionalization of Corannulene: Easy Access to Pentagonal Superstructure. Org. Lett. 2009, 11, 1063. 10.1021/ol8028127. [DOI] [PubMed] [Google Scholar]; b Nestoros E.; Stuparu M. C. Corannulene: a molecular bowl of carbon with multifaceted properties and diverse applications. Chem. Commun. 2018, 54, 6503. 10.1039/C8CC02179A. [DOI] [PubMed] [Google Scholar]; c Steinauer A.; Butterfield A. M.; Linden A.; Molina-Ontario A.; Buck D. C.; Cotta R. W.; Echegoyen L.; Baldridge K. K.; Siegel J. S. Tunable Photochemical/Redox Properties of (Phenylthio)ncorannulenes: Application to a Photovoltaic Device. J. Braz. Chem. Soc. 2016, 27, 1866. 10.5935/0103-5053.20160072. [DOI] [Google Scholar]
- Dawe L. N.; AlHujran T. A.; Tran H.-A.; Mercer J. I.; Jackson E. A.; Scott L. T.; Georghiou P. E. Corannulene and its penta-tert-butyl derivative co-crystallize 1:1 with pristine C60-fullerene. Chem. Commun. 2012, 48, 5563. 10.1039/c2cc30652b. [DOI] [PubMed] [Google Scholar]
- a Dubceac C.; Sevryugina Y.; Kuvychko I. V.; Boltalina O. V.; Strauss S. H.; Petrukhina M. A. Self-Assembly of Aligned Hybrid One-Dimensional Stacks from Two Complementary π-Bowls. Cryst. Growth Des. 2018, 18, 307. 10.1021/acs.cgd.7b01258. [DOI] [Google Scholar]; b Haupt A.; Lentz D. Tuning the Electron Affinity and Stacking Properties of Corannulene by Introduction of Fluorinated Thioethers. Chem. - Asian J. 2018, 13, 3022. 10.1002/asia.201801311. [DOI] [PubMed] [Google Scholar]
- Incomplete self-assembly was observed in the absence of excess 2-formyl-6-methylpyridine and CuI(MeCN)4BF4.
- Peaks for a second, minor species accounting for less than 10% of the signal intensity were also evident in the 1H NMR spectrum of 1. Those peaks were not intense enough to allow for full NMR characterization. They may correspond to an isomer of 1 with a different stacking pattern having a symmetry too low to be consistent with a single [CuI5L2]5+ cage of the type that constitute 1 or to a high-symmetry assembly of such cages.
- Allan D.; Nowell H.; Barnett S.; Warren M.; Wilcox A.; Christensen J.; Saunders L.; Peach A.; Hooper M.; Zaja L.; Patel S.; Cahill L.; Marshall R.; Trimnell S.; Foster A.; Bates T.; Lay S.; Williams M.; Hathaway P.; Winter G.; Gerstel M.; Wooley R. A Novel Dual Air-Bearing Fixed-χ Diffractometer for Small-Molecule Single-Crystal X-ray Diffraction on Beamline I19 at Diamond Light Source. Crystals 2017, 7, 336. 10.3390/cryst7110336. [DOI] [Google Scholar]
- Rizzuto F. J.; Wu W.-Y.; Ronson T. K.; Nitschke J. R. Peripheral Templation Generates an MII6L4 Guest-Binding Capsule. Angew. Chem., Int. Ed. 2016, 55, 7958. 10.1002/anie.201602135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. R.; Manck L. E.; Tidmarsh I. S.; Stephenson A.; Taylor B. F.; Blaikie E. J.; Griend D. A. V.; Ward M. D. Structures, host-guest chemistry and mechanism of stepwise self-assembly of M4L6 tetrahedral cage complexes. Dalton Trans. 2011, 40, 12132. 10.1039/c1dt10781j. [DOI] [PubMed] [Google Scholar]
- X-ray-quality crystals of the pure BF4– and ClO4– salts of 2 in the absence of coronene were also obtained. However, those data sets showed lower resolution and/or more disorder in the main cation compared with that of the coronene cocrystal that is discussed here in detail. Details of those other data sets can be found in the Supporting Information.
- Seiders T. J.; Baldridge K. K.; Grube G. H.; Siegel J. S. Structure/Energy Correlation of Bowl Depth and Inversion Barrier in Corannulene Derivatives: Combined Experimental and Quantum Mechanical Analysis. J. Am. Chem. Soc. 2001, 123, 517. 10.1021/ja0019981. [DOI] [PubMed] [Google Scholar]
- a Miller T. F.; Holloway L. R.; Nye P. P.; Lyon Y.; Beran G. J. O.; Harman W. H.; Julian R. R.; Hooley R. J. Small Structural Variations Have Large Effects on the Assembly Properties and Spin State of Room Temperature High Spin Fe(II) Iminopyridine Cages. Inorg. Chem. 2018, 57, 13386. 10.1021/acs.inorgchem.8b01973. [DOI] [PubMed] [Google Scholar]; b Struch N.; Topić F.; Schnakenburg G.; Rissanen K.; Lützen A. Electron-Deficient Pyridylimines: Versatile Building Blocks for Functional Metallosupramolecular Chemistry. Inorg. Chem. 2018, 57, 241. 10.1021/acs.inorgchem.7b02412. [DOI] [PubMed] [Google Scholar]
- Freye S.; Michel R.; Stalke D.; Pawliczek M.; Frauendorf H.; Clever G. H. Template Control over Dimerization and Guest Selectivity of Interpenetrated Coordination Cages. J. Am. Chem. Soc. 2013, 135, 8476. 10.1021/ja403184a. [DOI] [PubMed] [Google Scholar]
- a Custelcean R.; Bonnesen P. V.; Duncan N. C.; Zhang X.; Watson L. A.; Van Berkel G.; Parson W. B.; Hay B. P. Urea-Functionalized M4L6 Cage Receptors: Anion-Templated Self-Assembly and Selective Guest Exchange in Aqueous Solutions. J. Am. Chem. Soc. 2012, 134, 8525. 10.1021/ja300677w. [DOI] [PubMed] [Google Scholar]; b Bai X.; Jia C.; Zhao Y.; Yang D.; Wang S.-C.; Li A.; Chan Y.-T.; Wang Y.-Y.; Yang X.-J.; Wu B. Peripheral Templation-Modulated Interconversion between an A4L6 Tetrahedral Anion Cage and A2L3 Triple Helicate with Guest Capture/Release. Angew. Chem., Int. Ed. 2018, 57, 1851. 10.1002/anie.201712080. [DOI] [PubMed] [Google Scholar]; c Kaifer A. E.; Yi S.; Brega V.; Captain B. Sulfate-templated self-assembly of new M4L6 tetrahedral metal organic cages. Chem. Commun. 2012, 48, 10295. 10.1039/c2cc35095e. [DOI] [PubMed] [Google Scholar]; d Nakamura T.; Ube H.; Miyake R.; Shionoya M. A C60-Templated Tetrameric Porphyrin Barrel Complex via Zinc-Mediated Self-Assembly Utilizing Labile Capping Ligands. J. Am. Chem. Soc. 2013, 135, 18790. 10.1021/ja4110446. [DOI] [PubMed] [Google Scholar]
- a Kishi N.; Li Z.; Sei Y.; Akita M.; Yoza K.; Siegel J. S.; Yoshizawa M. Wide-Ranging Host Capability of a PdII-Linked M2L4 Molecular Capsule with an Anthracene Shell. Chem. - Eur. J. 2013, 19, 6313. 10.1002/chem.201204010. [DOI] [PubMed] [Google Scholar]; b Schmidt B. M.; Osuga T.; Sawada T.; Hoshino M.; Fujita M. Compressed Corannulene in a Molecular Cage. Angew. Chem., Int. Ed. 2016, 55, 1561. 10.1002/anie.201509963. [DOI] [PubMed] [Google Scholar]; c Fan Q.-J.; Lin Y.-J.; Hahn F. E.; Jin G.-X. Host–guest capability of a three-dimensional heterometallic macrocycle. Dalton Trans. 2018, 47, 2240. 10.1039/C7DT04453D. [DOI] [PubMed] [Google Scholar]
- Juríček M.; Strutt N. L.; Barnes J. C.; Butterfield A. M.; Dale E. J.; Baldridge K. K.; Stoddart J. F.; Siegel J. S. Induced-fit catalysis of corannulene bowl-to-bowl inversion. Nat. Chem. 2014, 6, 222. 10.1038/nchem.1842. [DOI] [PubMed] [Google Scholar]
- In all cases the signals for the hosts appeared at the same or similar chemical shifts (±0.05 ppm) as in the absence of the prospective guest, and the signals for the prospective guest were identical to those in the absence of host, indicating no interaction or the presence of weak nonspecific π-stacking interactions at high guest concentrations.
- Although the crystal structure of 3 showed CB11H12– anions intercalated between the corannulene bowls of neighboring [ZnII5L2]10+ cages to form 1D stacks (Figure S55), no significant 1H NMR chemical shift changes were observed in solution upon addition of CsCB11H12 to CD3CN solutions of 1 or 3.
- a Yang D.-C.; Li M.; Chen C.-F. A bis-corannulene based molecular tweezer with highly sensitive and selective complexation of C70 over C60. Chem. Commun. 2017, 53, 9336. 10.1039/C7CC03519E. [DOI] [PubMed] [Google Scholar]; b Álvarez C. M.; García-Escudero L. A.; García-Rodríguez R.; Martín-Álvarez J. M.; Miguel D.; Rayón V. M. Enhanced association for C70 over C60 with a metal complex with corannulene derivate ligands. Dalton Trans. 2014, 43, 15693. 10.1039/C4DT02078B. [DOI] [PubMed] [Google Scholar]; c Abeyratne Kuragama P. L.; Fronczek F. R.; Sygula A. Bis-corannulene Receptors for Fullerenes Based on Klärner’s Tethers: Reaching the Affinity Limits. Org. Lett. 2015, 17, 5292. 10.1021/acs.orglett.5b02666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.