Abstract
Epidermal growth factor receptor (EGFR) expression and activation are the major causes of metastasis in cancers such as head and neck squamous cell carcinoma (HNSCC). However, the reciprocal effect of EGF‐induced COX‐2 and angiopoietin‐like 4 (ANGPTL4) on HNSCC metastasis remains unclear. In this study, we revealed that the expression of ANGPTL4 is essential for COX‐2‐derived prostaglandin E2 (PGE2)‐induced tumor cell metastasis. We showed that EGF‐induced ANGPTL4 expression was dramatically inhibited with the depletion and inactivation of COX‐2 by knockdown of COX‐2 and celecoxib treatment, respectively. Prostaglandin E2 induced ANGPTL4 expression in a time‐ and dose‐dependent manners in various HNSCC cell lines through the ERK pathway. In addition, the depletion of ANGPTL4 and MMP1 significantly impeded the PGE2‐induced transendothelial invasion ability of HNSCC cells and the binding of tumor cells to endothelial cells. The induction of molecules involved in the regulation of epithelial‐mesenchymal transition was also dependent on ANGPTL4 expression in PGE2‐treated cells. The depletion of ANGPTL4 further blocked PGE2‐primed tumor cell metastatic seeding of lungs. These results indicate that the EGF‐activated PGE2/ANGPTL4 axis enhanced HNSCC metastasis. The concurrent expression of COX‐2 and ANGPTL4 in HNSCC tumor specimens provides insight into potential therapeutic targets for the treatment of EGFR‐associated HNSCC metastasis.
Keywords: ANGPTL4, EGF, HNSCC, metastasis, PGE2
Metastasis is the major cause of cancer mortality. However, there is no significant improvement in treating advanced head and neck cancer. Our finding indicates that the monitoring levels of angiopoietin‐like 4 (ANGPTL4) are necessary for the treatment of inflammation‐ and/or growth factor‐mediated advanced or metastatic head and neck cancer. The expression of ANGPTL4, a potent angiogenetic factor in cancer, is essential for head and neck cancer metastasis caused by inflammatory response and growth factor stimulation. Therefore, the use of antiinflammatory medicines such as nonsteroidal antiinflammatory drugs and targeting ANGPTL4 could be considered as alternative approaches in treating and preventing the recurrence of head and neck cancer.
1. INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) includes cancers of the oral cavity, oropharynx, larynx, and hypopharynx and is the sixth most common malignancy worldwide. 1 Despite advances in the treatment of HNSCC, there has been no significant improvement in mortality rate over the past 40 years. 2 Metastasis is the most significant contributor to the mortality of cancer patients. For example, stage IV HNSCC long‐term survival is rare and distant metastasis is a predominant predictor of mortality. 3 The pathogenesis of cancer metastasis involves several processes, including the loss of cellular adhesion, intravasation, survival in the circulation, extravasation, and eventual colonization in the distant metastatic organs. 4 The success of HNSCC cell extravasation is regulated by matricellular proteins such as angiopoietin‐like protein 4 (ANGPTL4), which is highly associated with tumor metastasis. 5 , 6 , 7 Interestingly, upregulation of ANGPTL4 has been observed in esophageal SCC and oral tongue SCC. 8 , 9 The expression of ANGPTL4 is also essential for EGF‐induced HNSCC metastasis. 5 Hypoxic condition‐dependent induction of ANGPTL4 by prostaglandin E2 (PGE2) promotes colorectal cancer progression. 10 These results indicate that the expression of ANGPTL4 plays a role in the regulation of tumor metastasis.
A variety of biomarkers including CXC chemokine receptor 2 (CXCR2), CXCR4, interleukin (IL)‐6, IL‐8, MMPs, and cytokeratins have been reported in HNSCC. 11 The most intensive biomarker, epidermal growth factor receptor (EGFR), is overexpressed in up to 90% of HNSCC compared with normal mucosa. 12 , 13 The aberrant activation of EGFR can be achieved by overexpression of EGFR and its ligands, EGFR mutation/polymorphism and transactivation by receptor tyrosine kinases. The intensive association between EGFR expression and HNSCC led to the development of Abs directed against EGFR, such as cetuximab, panitumumab, zalutumumab, and nimotuzumab for treating HNSCC. 14 , 15 , 16 However, these anti‐EGFR inhibitors have shown limited efficacy. Chemoradiation with cetuximab in HNSCC has shown adverse events in clinical trials. 17 In addition, EGFR inhibition by erlotinib or gefitinib is overcome by epithelial‐mesenchymal transition (EMT). 18 Potential mechanisms for the failure of EGFR inhibitors in the inhibition of downstream molecules such as Ras/Raf/MAPK, PI3K/AKT/mTOR, and signal transducer and activator of transcription 3 could include the activation of EGFR‐independent signaling pathways, including hypoxia and G protein‐coupled receptors. 19 , 20 The signaling molecules work synergistically with EGFR or compensate for the loss of EGFR‐activated signaling and are likely to be targets to overcome EGFR inhibitor resistance.
Similar to EGFR, overexpression of COX‐2 is commonly found in both premalignant and malignant tissues. 21 An extensive increase in COX‐2 has been found in oral leukoplakia and seems to be correlated with high‐grade premalignant lesions. 22 In addition, evidence reveals that COX inhibitors, including nonsteroidal antiinflammatory drugs (NSAIDs), reduce the formation of a variety of tumors. 23 , 24 It is worth noting that daily aspirin can significantly prevent distant metastasis in different types of cancer. 25 This evidence suggests the possibility that the activation of COX‐2 plays a critical role in cancer progression, including HNSCC. 26 However, the cross‐talk between EGFR activation and the expression of ANGPTL4 and COX‐2 in promoting HNSCC metastasis remains unclear.
In this study, we reveal for the first time that autocrine production of EGF‐induced ANGPTL4 in tumor cells is dependent on the activation of COX‐2, resulting in HNSCC metastasis. Prostaglandin E2 directly promotes the expression of ANGPTL4 to enhance tumor‐endothelial cell interactions and lung metastasis of HNSCC cells. Inhibition of ANGPTL4 represents a new strategy for the treatment of EGFR‐ and COX‐2 proinflammatory pathway‐associated HNSCC metastasis.
2. MATERIALS AND METHODS
2.1. Cell culture
Cell lines of head and neck cancer (SCC25, KB, and FaDu) were purchased from ATCC. Head and neck cancer cells (HONE1, TU183, and SAS) and human microvascular endothelial cell line (HMEC‐1) were kindly provided by Dr Kwang‐Yu Chang (National Health Research Institutes, Taiwan) 27 and Dr Trai‐Ming Yeh (Department of Medical Laboratory Science and Biotechnology, Medical College, National Cheng Kung University), respectively. Cell culture conditions were previously described. 27 , 28
2.2. Western blot analysis
An analytical 12% SDS‐PAGE was carried out, and 30 μg protein of each was analyzed, unless stated otherwise. Western blot analysis was undertaken as previously described. 29 Antibodies against human phospho‐ERK1/2T202/Y204 (Cell Signaling Technology), COX‐2 (Lab Vision), ERK1/2 (Santa Cruz Biotechnology), and α‐tubulin (Sigma) were used as the primary Abs.
2.3. Reverse transcription‐PCR and real‐time quantitative RT‐PCR
Total RNA was isolated using the TRIzol RNA extraction kit (Invitrogen), and 1 μg RNA was subjected to RT‐PCR with ImProm‐II (Promega). The following primers were used: ANGPTL4 (sense, 5′‐CAAGGCTCAGAACAGCAGGA‐3′; antisense, 5′‐CCCCTGAGGCTGGATTTCAA‐3′), COX‐2 (sense, 5′‐CCCACTTCAAGGGATTTT‐3′; antisense, 5′‐CCAGACCAAAGACCTCCT‐3′), MMP1 (sense, 5′‐ATGCACAGCTTTCCTCCACT‐3′; antisense, 5′‐TTCCCAGTCACTTTCAGCCC‐3′), MMP‐2 (sense, 5′‐GCAAGTTTCCATTCCGC‐3′; antisense, 5′‐GTCGTCATCGTAGTTGGC‐3′), MMP3 (sense, 5′‐GCAAGACAGCAAGGCATAGAG‐3′; antisense, 5′‐CCGTCACCTCCAATCCAAGG‐3′), MMP9 (sense, 5′‐ACCTCGAACTTTGACAGCGACA‐3′; antisense, 5′‐GATGCCATTCACGTCGTCCTTA‐3′), Slug (sense, 5′‐GAGAGCTGCAAGAGCATGGA‐3′; antisense, 5′‐GGCAACCAGACAACCGACAT‐3′), Twist (sense, 5′‐GCCGGAGACCTAGATGTCATTG‐3′; antisense, 5′‐ AGTGGCTGATT GGCACGAC‐3′), vimentin (sense, 5′‐TGGCCGACGCCATCAACACC‐3′, antisense, 5′‐CACCTCGACGCGGGCTTTGT‐3′), N‐cadherin (sense, 5′‐GTGCCATTAGCCAAGGGAATTCAGC‐3′; antisense, 5′‐GCGTTCCTGTTCCA CTCATAGGAGG‐3′), and GAPDH (sense, 5′‐CCATCACCATCTTCCAGGAG‐3′; antisense, 5′‐CCTGCTTCACCACCTTCTTG‐3′). The PCR products were separated by 2% agarose gel electrophoresis and visualized with ethidium bromide staining. For the quantitative real‐time RT‐PCR, cDNA synthesis was carried out using the Titanium One‐Step TR‐PCR kit (Clontech) containing SYBR Green I. Real‐time fluorescence monitoring and the melting curve analysis were undertaken with LightCycler according to the manufacturer’s recommendations. The relative transcript amount of the target gene, calculated using standard curves of serial RNA dilutions, was normalized to that of GAPDH of the same RNA.
2.4. Knockdown experiments
The hairpins targeting COX‐2 (shCOX‐2) and a nontargeting hairpin (shLacZ) were obtained from the RNAi Core of the Research Center of Clinical Medicine, National Cheng Kung University Hospital in the pLKO.1 lentiviral backbone. Cells were selected in 2 μg/mL puromycin for 3 days and then expanded for 1‐2 weeks before analysis. Both shCOX‐2 #1 and shCOX‐2 #2 stable cell lines were selected from the same target sequence. Hairpin TRC clone IDs and target sequences were as follows: shLacZ/TRCN0000072223, TGTTCGCATTATCCGAACCAT; and shCOX‐2/TRCN0000045533, GCTGAATTTAACACCCTCTAT.
2.5. Transfection of cells with siRNA oligonucleotides or plasmid
Transient transfection of cells with 20 nmol/L siRNA oligonucleotides or plasmid was carried out using RNAiMAX or Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions with slight modifications. The siRNA IDs were as follows: ANGPTL4, siRNA IDs HSS181878 and HSS181879; MMP1, siRNA IDs HSS106609 and HSS106610; and negative control siRNAs, siRNA ID D‐001810‐10‐50 (Dharmacon). For use in transfection, 3.75 μL RNAiMAX or Lipofectamine 2000 was incubated with siRNA or plasmid in 1.5 mL Opti‐MEM medium (Invitrogen) for 30 minutes at room temperature. Following the removal of Opti‐MEM medium and replacement with 3 mL fresh culture medium, the cells were incubated for an additional 24 hours, unless stated otherwise.
2.6. Enzyme‐linked immunosorbent assay
Quantification of the secretory ANGPTL4 in the culture medium was achieved by a DuoSet human angiopoietin‐like 4 ELISA development kit (DY3458) according to the manufacturer’s instructions (R&D Systems). Briefly, 100 μL culture medium was collected and incubated with precoated capture Ab at room temperature in a 96‐well microplate. After washing, 100 μL detection Ab was added and incubated for another 2 hours at room temperature, and then 100 μL working dilution of streptavidin‐HRP was added to each well and incubated for 20 minutes under protection from light. After washing, 100 μL substrate solution was added and incubated for 20 minutes under protection from light. After adding 50 μL stop solution, the plate was gently tapped to ensure thorough mixing and an SpectraMax i3x Microplate Absorbance Reader (Molecular Devices) was used to immediately quantify the optical density at 450 nm.
2.7. Transendothelial invasion assay
The invasion assay was undertaken using Millicell hanging cell culture inserts (polyethylene terephthalate membranes with 8‐µm pores; Millipore). HMEC‐1 cells (1 × 105 cells per well) were plated on the upper chamber and allowed to grow to confluence, and then 10% Matrigel was loaded into the chamber. Tumor cells were treated with 50 ng/mL EGF or PGE2 in serum‐free medium and then stained with 1,1′‐dioctadecyl‐3,3,3′,3′‐tetramethyl‐indocarbocyanine perchlorate (DiI) (Invitrogen) for 30 minutes. The DiI‐stained tumor cells (2 × 105) were then loaded into the chamber, which was filled with serum‐free medium, and incubated for 2 days. Cells on the apical side of each insert were scraped off. Invasion to the basolateral side of the membrane was visualized using an immunofluorescent microscope. The number of invading cells was determined in 3 randomly chosen fields under the microscope for 3 independent experiments.
2.8. Cell adhesion assay
Briefly, tumor cells were treated with 20 μmol/L PGE2 for 3 hours, then labeled for 30 minutes at 37°C with DiI (Invitrogen) and washed twice with PBS. The medium was removed from the wells, and tumor cells (1.5 × 105 cells/mL) were added to a monolayer of HMEC‐1 cells. After incubation for 30 minutes at 37°C, the wells were gently washed twice with PBS to remove nonadherent cells. The cells were photographed and numbers were quantified under a fluorescence microscope.
2.9. Tumor metastasis assay in an animal model
Tumor metastasis was determined by tail vein i.v. injection of cancer cells into 4‐ to 6‐week‐old male SCID mice. Briefly, each animal was injected with 1 × 106 cells mixed with PBS, and all mice were killed up to 2 months after injection. All mice were obtained from the National Cheng Kung University Laboratory Animal Center (Tainan, Taiwan) and the National Laboratory Animal Center (Tainan, Taiwan). All animal experiments in this study were approved by the Laboratory Animal Committee of National Cheng Kung University. The H&E staining was undertaken by the Human Biobank, Research Center of Clinical Medicine, National Cheng Kung University Hospital.
3. RESULTS
3.1. Expression of ANGPTL4 is dependent on induction of COX‐2 in EGF‐treated HNSCC cells
Our previous studies revealed that EGF induces the expression of COX‐2 or ANGPTL4, resulting in the promotion of tumor metastasis. 5 , 30 However, the reciprocal regulation of ANGPTL4 and COX‐2 in HNSCC metastasis remains poorly understood. To elucidate the relationship between COX‐2 and ANGPTL4 induction in HNSCC, the sequential effect of EGF‐induced COX‐2 on ANGPTL4 expression was examined in EGF‐treated cells. As shown in Figure 1A,B, EGF induced the gene expression of COX‐2 and ANGPTL4 in a time‐dependent manner. It is worth noting that the induction of COX‐2 was earlier than that of ANGPTL4. In addition, the EGF‐induced protein expression of ANGPTL4 and COX‐2 was further confirmed using ELISA and western blotting, respectively (Figure 1C,D). To further assess the association of EGFR signaling and the expression of ANGPTL4 and COX‐2 in HNSCC patients, gene expression signatures in clinical samples were analyzed by The Cancer Genome Atlas data mining. 31 Intriguingly, concurrent expression of EGFR, COX‐2, and ANGPTL4 was observed (Figure S1). These results reveal that the induction of COX‐2 was followed by ANGPTL4 expression in EGF‐treated cells, suggesting the possibility that the expression of ANGPTL4 might be dependent on the expression of COX‐2. To assess whether COX‐2 activity was essential for EGF‐induced ANGPTL4 expression, the selective COX‐2 inhibitor celecoxib was used to suppress the activation of COX‐2. Indeed, celecoxib significantly inhibited EGF‐induced ANGPTL4 mRNA expression and protein secretion in a dose‐dependent manner (Figures 2A,B and S2A). The essential role of COX‐2 in the regulation of ANGPTL4 expression was also further confirmed by the inhibition of EGF‐induced ANGPTL4 that was observed during the depletion of COX‐2 (Figure 2C,D); however, EGF‐induced COX‐2 expression was not changed in ANGPTL4‐depleted cells (Figure 2D). These results suggested that the activation and expression of COX‐2 are essential for the expression and autocrine secretion of ANGPTL4 in EGF‐treated cells.
FIGURE 1.
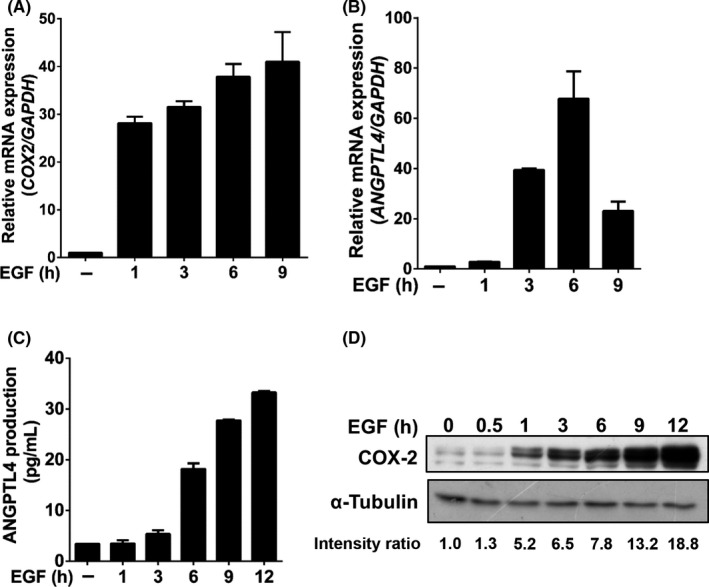
Epidermal growth factor (EGF) induces sequential expression of COX‐2 and angiopoietin‐like 4 (ANGPTL4) in head and neck squamous cell carcinoma cells. FaDu cells were treated with 50 ng/mL EGF for the indicated period of time. A, B, mRNA expression of COX‐2 and ANGPTL4 was analyzed by real‐time quantitative PCR. C, D, Expression levels of ANGPTL4 and COX‐2 protein were examined using ELISA and western blotting, respectively. Values represent the mean ± SEM of 3 replicate analyses
FIGURE 2.
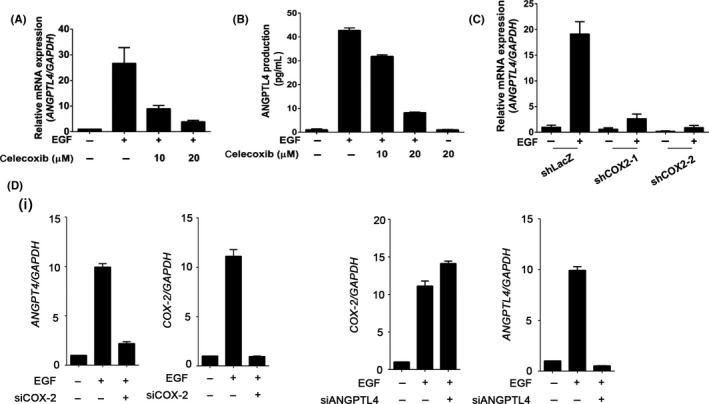
Inhibition of COX‐2 blocks epidermal growth factor (EGF)‐induced angiopoietin‐like 4 (ANGPTL4) expression and secretion. A, B, FaDu cells were treated with various concentrations of celecoxib, followed by 50 ng/mL EGF treatment for 6 h. Expression of ANGPTL4 mRNA and protein was analyzed using real‐time quantitative PCR and ELISA, respectively. C, shLacZ and shCOX‐2 (COX‐2 knockdown) cells were treated with 50 ng/mL EGF for 6 h. Expression of ANGPTL4 mRNA was analyzed using real‐time quantitative PCR. D, FaDu cells were transfected with 20 nmol/L siCOX‐2 (i), siANGPTL4 (ii), and scramble oligonucleotides by lipofection and then treated with 50 ng/mL EGF for 6 h. Real‐time quantitative PCR was carried out for analysis of mRNA levels of ANGPTL4 and COX‐2. Values represent mean ± SEM of 3 replicate analyses
3.2. Prostaglandin E2‐induced ANGPTL4 expression occurs through ERK and peroxisome proliferator activated receptor signaling pathways
To further verify whether the activation of the COX‐2 signaling pathway is essential for ANGPTL4 induction, the effect of the COX‐2 metabolite PGE2 on ANGPTL4 expression in HNSCC cell lines was investigated. Quantitative real‐time PCR revealed that PGE2 dramatically induced ANGPTL4 expression in various HNSCC cell lines (Figure 3A). The induction of ANGPTL4 expression by PGE2 was in a time‐ and dose‐dependent manner (Figures 3B,C and S2B,C). Prostaglandin E2 showed a weaker induction of ANGPTL4 expression than treatment with EGF (Figure 3D); however, the dysfunction of COX‐2 induced by either siCOX‐2 or celecoxib led to a reduction of EGF‐induced ANGPTL4 that was also reversed by cotreatment with PGE2 (Figures 3D,E and S2D). These results reveal that the expression and activation of COX‐2 play an important role in the regulation of ANGPTL4 expression. Furthermore, we confirmed that the ERK activation was also required for PGE2‐ and EGF‐induced ANGPTL4 expression by using the MEK/ERK inhibitor U0126 (Figure 4A,B). The peroxisome proliferator activated receptor (PPAR) binding sequence required for PGE2‐induced ANGPTL4 promoter activity was found in a promoter reporter assay (Figure 4C). These results reinforce the essential roles of the ERK and PPAR signaling pathways in the regulation of PGE2‐induced ANGPTL4 expression, as well as our previous studies in oleic acid‐ and EGF‐induced ANGPTL4 expression. 5 , 6
FIGURE 3.
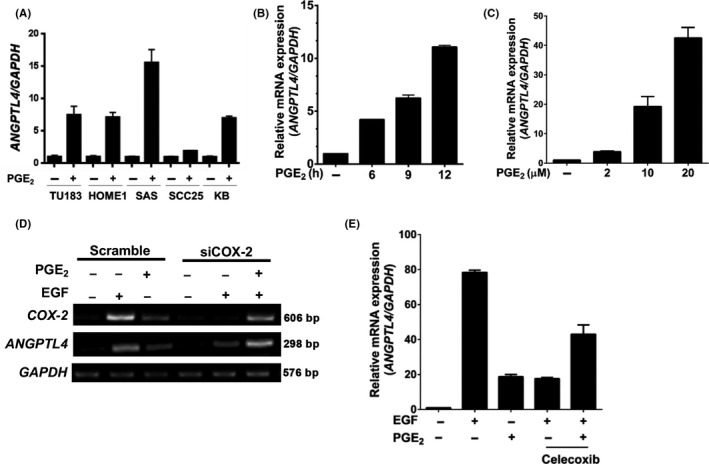
Cyclooxygenase‐2‐derived prostaglandin E2 (PGE2) significantly induces angiopoietin‐like 4 (ANGPTL4) expression. A‐C, Cell lines (A) and FaDu cells (B, C) were treated with 20 μmol/L PGE2 for 6 h or the indicated period of time, or with various concentrations as indicated. Expression of ANGPTL4 was analyzed using real‐time quantitative PCR. D, E, Cells were transfected with 20 nmol/L COX‐2 siRNA (siCOX‐2) and scramble oligonucleotides by lipofection and then treated with 20 μmol/L PGE2 and 50 ng/mL epidermal growth factor (EGF) or 20 μmol/L celecoxib for 6 h. mRNA levels of COX‐2, ANGPTL4, and GAPDH were analyzed by RT‐PCR and examined by 2% agarose gel electrophoresis or real‐time quantitative PCR. Values represent mean ± SEM of 3 replicate analyses
FIGURE 4.
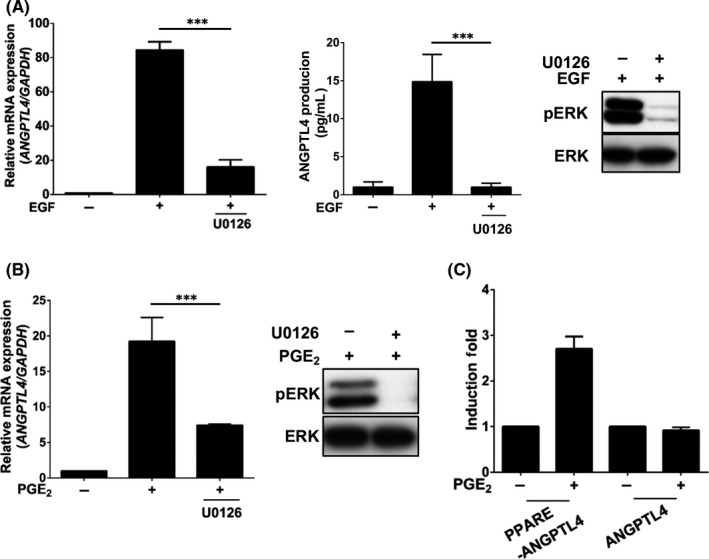
Epidermal growth factor (EGF)‐ and prostaglandin E2 (PGE2)‐induced angiopoietin‐like 4 (ANGPTL4) expression occurs through the ERK pathway. A, B, Cells were treated with 10 μmol/L U0126 and 50 ng/mL EGF (A) or 20 μmol/L PGE2 (B) for 6 h. Expression of ANGPTL4 mRNA and protein was analyzed by real‐time quantitative PCR and ELISA, respectively. Activation of ERK in cells treated with 50 ng/mL EGF or 20 μmol/L PGE2 for 1 h was analyzed by western blotting using anti‐phospho‐(p)ERK1/2 (Thr202/Tyr204) Abs. C, Cells were transfected with 0.5 μg of PGL3 vector containing the ANGPTL4 promoter with an intronic PPAR element (PPARE) by lipofection and then treated with 20 μmol/L PGE2 for 12 h. Luciferase activities were determined and normalized. Values represent mean ± SEM of 3 replicate analyses. ***P < .001
3.3. Prostagladin E2‐induced ANGPTL4 enhances HNSCC cell metastasis
Overexpression of ANGPTL4 has been identified in various cancers. 32 To test whether ANGPTL4 expression is required for PGE2‐ and EGF‐induced tumor cell invasion, ANGPTL4 siRNA was used in transendothelial invasion assays. As shown in Figure 5A, the depletion of ANGPTL4 inhibited EGF‐ and PGE2‐induced tumor cell transendothelial invasion. In addition, COX‐2 siRNA also inhibited EGF‐triggered tumor cell transendothelial invasion (Figure 5A). Our previous studies showed that the expression of MMP1 is essential for EGF‐induced HNSCC metastasis. 5 Next, we further identified whether MMP1 was required for PGE2‐induced cancer cell invasion. As shown in Figure 5A, siMMP1 significantly inhibited PGE2‐induced tumor cell invasion. The infiltration of tumor cells into distant locations initially relies on the attachment to endothelial cells. Therefore, we further investigated the effect of EGF‐ and PGE2‐induced ANGPTL4 expression on the interaction between tumor and endothelial cells. As shown in Figure 5B, the EGF‐ and PGE2‐induced tumor‐endothelial cell interaction was significantly inhibited in the ANGPTL4‐knockdown cells. The depletion of COX‐2 also blocked EGF‐enhanced tumor‐endothelial cell interaction (Figure 5B). These results indicated that PGE2‐induced autocrine production of ANGPTL4 stimulated the binding of tumor cells to endothelial cells. The EGF/PGE2/ANGPTL4/MMP1 axis was required for HNSCC invasion.
FIGURE 5.
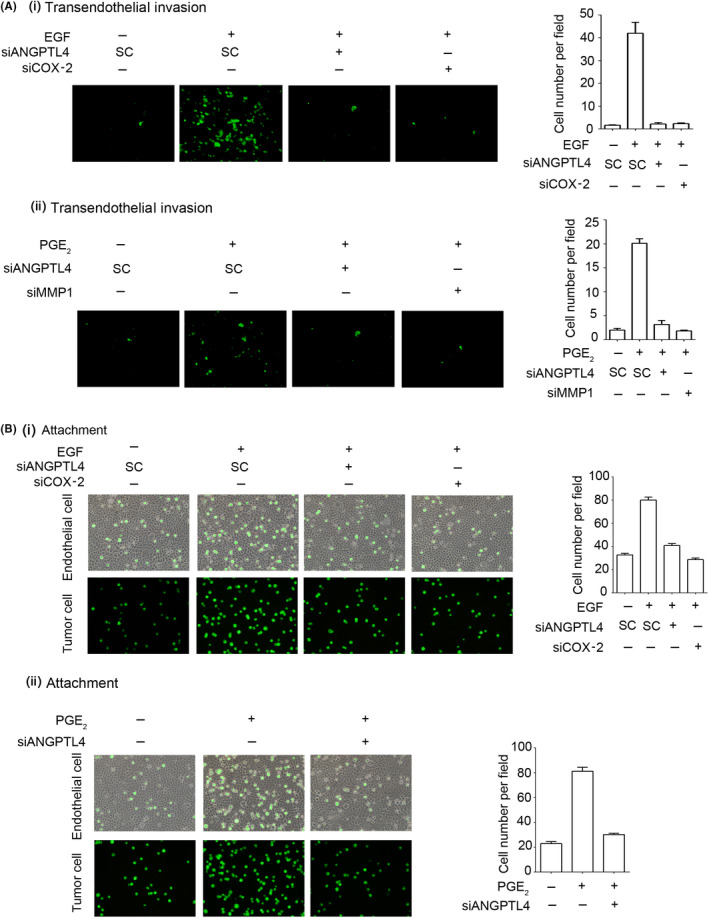
Depletion of angiopoietin‐like 4 (ANGPTL4) inhibits epidermal growth factor (EGF)‐ and prostaglandin E2 (PGE2)‐induced invasion of tumor cells and interaction with endothelial cells. A, FaDu cells were transfected with 20 nmol/L ANGPTL4, COX‐2, or MMP1 siRNA oligonucleotides (siANGPTL4, siCOX‐2, and siMMP1, respectively) and scrambled control oligonucleotides (SC) by lipofection. Endothelial cells were grown to form a monolayer on the bottom of a thick layer of ECM proteins to mimic intravasation in transendothelial invasion assays. After 50 ng/mL EGF (i) and 20 μmol/L PGE2 (ii) treatment for 6 h and stained with 1,1′‐dioctadecyl‐3,3,3′,3′‐tetramethyl‐indocarbocyanine perchlorate, cells were loaded in the upper chamber of filter inserts. After incubation for 48 h, noninvasive cells were removed. Invasive images were examined using a microscope (left panel). Original magnification, ×200. Invasive cells were counted (right panel). Values represent mean ± SEM of 3 determinations. B, Tumor cells were further cultured with endothelial cells for 3 h. Attachment of tumor cells was examined using a microscope (left panel). Number of attached cells was counted using 3 randomly chosen fields under the microscope from 3 independent experiments (right panel). Values represent mean ± SEM of 3 replicate analyses
3.4. Angiopoietin‐like 4 is essential for EMT marker expression and PGE2‐primed cancer metastasis
Based on the EGF/PGE2/ANGPTL4/MMP1 axis being essential for PGE2‐induced cancer cell invasion, we further studied whether the expression of EMT markers is involved in ANGPTL4‐regulated cell metastasis. As shown in Figure 6, the expression levels of fibronectin, Slug, Snail, Twist, vimentin, and N‐cadherin were increased in PGE2‐treated cells. In addition, the induction of EMT markers was significantly inhibited in ANGPTL4‐depleted cells. In contrast, the induction and inhibition of Snail, Twist, and fibronectin, and E‐cadherin by EGF, respectively, were reversed in ANGPTL4‐depleted cells (Figure S3). Our previous studies have shown that MMP expression is regulated by ANGPTL4 levels and is associated with cancer metastasis. 5 , 33 To clarify whether ANGPTL4 was essential for PGE2‐induced MMP expression, the effects of ANGPTL4 depletion on MMP gene expression were examined. Quantitative real‐time PCR showed that PGE2‐induced expression of MMPs was inhibited in ANGPTL4‐depleted cells (Figures 6 and S4). In addition, ANGPTL4 siRNA attenuated EGF‐induced expressions of MMP1 and MMP9 (Figure S3), consistent with our previous studies. 5 Because PGE2‐induced ANGPTL4 expression promoted expression of EMT markers and MMPs, resulting in tumor cell invasion in in vitro studies, we further examined whether ANGPTL4 was essential for PGE2‐enhanced metastasis in vivo. Parental and siANGPTL4 cells were pretreated with PGE2 for 3 hours and then injected into the tail veins of mice for 60 days. Hematoxylin and eosin staining showed that the lungs of mice receiving the PGE2‐pretreated parental tumor cells contained more and larger micrometastatic colonies than those receiving the PGE2‐pretreated siANGPTL4 cells (Figure 7). These results suggest that the induction of ANGPTL4 is essential for PGE2‐primed HNSCC metastasis as well as the requirement of ANGPTL4 for EGF‐primed tumor metastasis. 5
FIGURE 6.
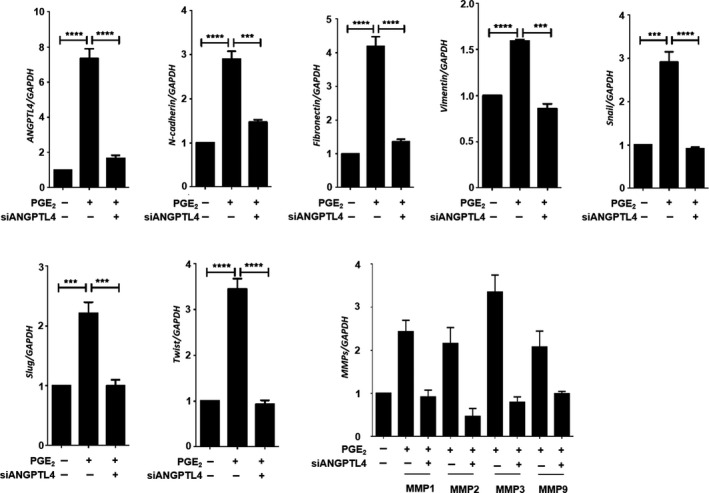
Depletion of angiopoietin‐like 4 (ANGPTL4) inhibits prostaglandin E2 (PGE2)‐induced expression of epithelial‐mesenchymal transition markers. FaDu cells were transfected with 20 nmol/L ANGPTL4 siRNA oligonucleotides (siANGPTL4) by lipofection and then treated with 20 μmol/L PGE2. mRNA expression of ANGPTL4, N‐cadherin, fibronectin, Snail, Slug, Twist, vimentin, and MMPs was analyzed by real‐time quantitative PCR. Values represent mean ± SEM of 3 replicate analyses. *** P < .001, ****P < .0001
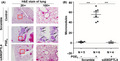
FIGURE 7.
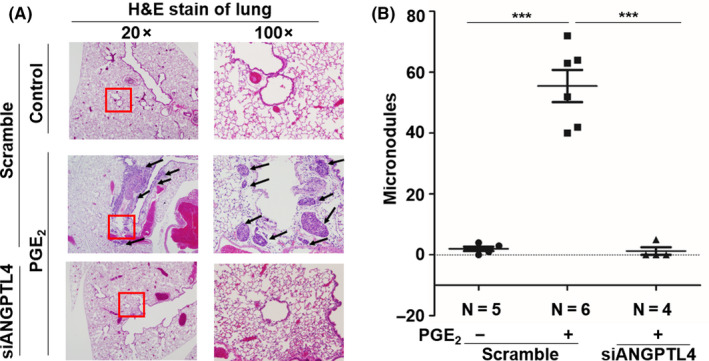
Angiopoietin‐like 4 (ANGPTL4) mediates prostaglandin E2 (PGE2) priming for tumor dissemination to lungs. FaDu cells were transfected with 20 nmol/L ANGPTL4 siRNA oligonucleotides (siANGPTL4) by lipofection for 24 h and then treated with 20 μmol/L PGE2 for 3 h. Lung colonization analysis was carried out by injecting 2 × 105 cells into a lateral tail vein of mice. Nodules were examined and photographed at 2 mo. Arrows indicate metastatic nodules. Images of tumors (A) and numbers of nodules (B) were examined using H&E staining and counted under a microscope, respectively. The 100x was enlarged from the red box in 20x. Values represent mean ± SEM of indicated number (N) of mice. ***P < .001
4. DISCUSSION
Head and neck squamous cell carcinoma progression is associated with EGFR and/or the proinflammatory pathway, which are targeted by using inhibitors of EGFR and COX‐2, such as cetuximab and celecoxib, respectively. 34 , 35 Unfortunately, the combination of cetuximab and celecoxib is likely limited in cancer therapy due to anticancer drug resistance and ultimately lack of effect on metastatic tumors. The understanding of cross‐talk between EGFR‐ and COX‐2‐associated HNSCC metastasis can provide better approaches to treat tumors. In this study, for the first time, we provide evidence that the activation of EGFR signaling promotes the upregulation of COX‐2, followed by the induction of ANGPTL4, resulting in the increase of HNSCC metastasis. However, the production of PGE2 either from EGF‐stimulated tumors or surrounding cells, such as tumor‐associated macrophages and fibroblasts, has been found to contribute to tumor cell metastasis. 30 Intriguingly, we found that ANGPTL4 was essential for fibronectin expression and HNSCC metastasis in PGE2‐treated cells. These results were consistent with our previous study that showed that the expression of ANGPTL4 and fibronectin is also required for EGF‐ and PGE2‐primed HNSCC metastasis, respectively. 5 , 30 The studies reveal that the ANGPTL4/fibronectin pathway plays a role in growth factor‐ and inflammation‐associated tumor metastasis. Therefore, the blocking of proinflammatory factors, such as PGE2‐regulated metastasis by targeting ANGPTL4, provides new insight into treating inflammation and growth factor‐initiated tumor metastasis.
The modest effect of the COX‐2 inhibitor celecoxib against advanced cancers has been determined from a metaanalysis of clinical trials and there is no significant effect on the 1‐year survival rate. 36 Although COX‐2 inhibition is not sufficient to suppress tumor progression, the risk of developing certain cancers, including HNSCC and breast, prostate, and pancreatic cancers, is dramatically reduced, 37 , 38 , 39 , 40 suggesting that selective COX‐2 inhibitors have strong potential for the chemoprevention of cancers. Indeed, our studies revealed that the depletion of ANGPTL4 reduced PGE2‐primed HNSCC metastasis, suggesting that the inhibition of the inflammatory response, such as the COX‐2 signaling pathway, is a new approach to reduce the risk of tumor recurrence by preventing cancer metastasis. In addition, previous studies indicated that COX‐2 is involved in immunity‐regulated tumor progression. For example, COX‐2 inhibitors also suppress tumor immune evasion by inhibiting M2 macrophages and T regulatory cells. 41 , 42 Cyclooxygenase‐2 in tumor‐associated macrophages (TAMs) promotes breast cancer metastasis through the induction of MMP9 and the promotion of EMT in tumor cells. 43 In addition, cancer‐associated fibroblasts (CAFs) are major sources of COX‐2/PGE2 in the tumor microenvironment. 44 These results suggest that the regulation of EMT by PGE2 produced from TAMs, CAFs, or tumors, could further promote tumor metastasis. Considering sources of PGE2 and their wide effect on inflammation‐associated tumors, inhibition of the inflammatory response by using NSAIDs or selective COX‐2 inhibitors is necessary for the treatment of cancer. Elevated expression of ANGPTL4 also enhances pulmonary tissue leakiness and intensified inflammation‐induced lung damage during influenza infection. 45 These results further suggest that ANGPTL4 might play a role in the regulation of the immune response. Chronic inflammation is highly associated with the risk of developing cancer. 46 Therefore, whether PGE2‐induced ANGPTL4 regulates chronic inflammation‐associated tumor progression and immunotherapeutic effects should be further investigated.
In this study, it is worth noting that PGE2‐induced EMT markers, including Snail, Slug, Twist, and fibronectin, and MMPs were reduced with the depletion of ANGPTL4 in HNSCC. These results were consistent with the finding that ANGPTL4‐regulated EMT participated in various tumor metastases. 5 , 47 In addition to the regulation of EMT, ANGPTL4 enhances vascular permeability through the integrin pathway to promote tumor metastasis. 7 Our study further provides evidence to suggest that ANGPTL4 also confers metastasis to tumor cells through the enhancement of PGE2‐regulated interactions between tumor cells and endothelial cells. These results reveal that the PGE2/ANGPTL4 axis regulates tumor cell migration and invasion through the promotion of tumor‐endothelial cell interactions. In addition, previous reports indicated that COX‐2 enhances β1 integrin expression to promote non‐small‐cell lung cancer cell invasion. 48 In our previous studies, we have shown that EGF‐induced ANGPTL4 activates the integrin pathway to promote HNSCC metastasis. 5 Together with previous reports and results from this study showing that PGE2‐induced ANGPTL4 promotes HNSCC cell metastasis, we propose that PGE2/ANGPTL4 might regulate tumor cell metastasis through the integrin pathway.
In general, EGF activates the EGFR signaling pathway to induce COX‐2 expression and promotes tumor metastasis. 30 In addition to EGF, we provided evidence revealing that the COX‐2 metabolite PGE2 triggered tumor metastasis through the induction of ANGPTL4. However, the effect of PGE2 on tumor metastasis might be mediated through the activation of EGFR signaling due to the reciprocal effect between EGFR signaling and COX‐2, which has been found to regulate tumor metastasis. For example, PGE2 triggers transactivation and phosphorylation of EGFR to promote colorectal cancer cell migration and invasion. 49 In addition, both EGFR and COX‐2 inhibitors, such as erlotinib and celecoxib, respectively, have been used as single agents for chemoprevention in HNSCC. 50 , 51 Preclinical and clinical studies have revealed that targeting the EGFR and COX‐2 pathways can synergistically inhibit HNSCC growth. 52 , 53 , 54 Our study also found a strong correlation between the expression of EGFR, COX‐2, and ANGPTL4 in HNSCC tumor specimens. Therefore, it is interesting to further investigate whether EGFR inhibitors initiate a direct effect on proinflammatory cytokines in PGE2‐regulated HNSCC metastasis. In summary, we conclude that targeting ANGPTL4 provides new insight into the treatment of growth factor/inflammation‐associated HNSCC metastasis.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Supporting information
Fig S1‐S4
ACKNOWLEDGMENTS
This work was supported by the Ministry of Science and Technology of Taiwan (grants NSC 102‐2628‐B‐006‐011‐MY3, MOST 105‐2320‐B‐006‐022‐MY3, and 105‐2320‐B‐038‐064‐MY3).
Chiang K‐H, Shieh J‐M, Shen C‐J, et al. Epidermal growth factor‐induced COX‐2 regulates metastasis of head and neck squamous cell carcinoma through upregulation of angiopoietin‐like 4. Cancer Sci. 2020;111:2004–2015. 10.1111/cas.14400
Kuo‐Hwa Chiang and Jiunn‐Min Shieh contributed equally to this work.
Contributor Information
Wen‐Chang Chang, Email: wcchang@tmu.edu.tw.
Ben‐Kuen Chen, Email: bkchen58@mail.ncku.edu.tw.
REFERENCES
- 1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893‐2917. [DOI] [PubMed] [Google Scholar]
- 2. Matta A, Ralhan R. Overview of current and future biologically based targeted therapies in head and neck squamous cell carcinoma. Head Neck Oncol. 2009;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hauswald H, Simon C, Hecht S, Debus J, Lindel K. Long‐term outcome and patterns of failure in patients with advanced head and neck cancer. Radiat Oncol. 2011;6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453‐458. [DOI] [PubMed] [Google Scholar]
- 5. Liao Y‐H, Chiang K‐H, Shieh J‐M, et al. Epidermal growth factor‐induced ANGPTL4 enhances anoikis resistance and tumour metastasis in head and neck squamous cell carcinoma. Oncogene. 2017;36:2228‐2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen CJ, Chan SH, Lee CT, Huang WC, Tsai JP, Chen BK. Oleic acid‐induced ANGPTL4 enhances head and neck squamous cell carcinoma anoikis resistance and metastasis via up‐regulation of fibronectin. Cancer Lett. 2017;386:110‐122. [DOI] [PubMed] [Google Scholar]
- 7. Huang R‐L, Teo Z, Chong HC, et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE‐cadherin and claudin‐5 clusters. Blood. 2011;118:3990‐4002. [DOI] [PubMed] [Google Scholar]
- 8. Shibata K, Nakayama T, Hirakawa H, Hidaka S, Nagayasu T. Clinicopathological significance of angiopoietin‐like protein 4 expression in oesophageal squamous cell carcinoma. J Clin Pathol. 2010;63:1054‐1058. [DOI] [PubMed] [Google Scholar]
- 9. Wang Z, Han B, Zhang Z, Pan J, Xia H. Expression of angiopoietin‐like 4 and tenascin C but not cathepsin C mRNA predicts prognosis of oral tongue squamous cell carcinoma. Biomarkers. 2010;15:39‐46. [DOI] [PubMed] [Google Scholar]
- 10. Kim SH, Park YY, Kim SW, Lee JS, Wang D, DuBois RN. ANGPTL4 induction by prostaglandin E2 under hypoxic conditions promotes colorectal cancer progression. Cancer Res. 2011;71:7010‐7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dahiya K, Dhankhar R. Updated overview of current biomarkers in head and neck carcinoma. World J Methodol. 2016;6:77‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666‐2672. [DOI] [PubMed] [Google Scholar]
- 13. Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579‐3584. [PubMed] [Google Scholar]
- 14. Vermorken JB, Mesia R, Rivera F, et al. Platinum‐based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116‐1127. [DOI] [PubMed] [Google Scholar]
- 15. Mesía R, Henke M, Fortin A, et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous‐cell carcinoma of the head and neck (CONCERT‐1): a randomised, controlled, open‐label phase 2 trial. Lancet Oncol. 2015;16:208‐220. [DOI] [PubMed] [Google Scholar]
- 16. Zibelman M, Mehra R. Overview of current treatment options and investigational targeted therapies for locally advanced squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2016;39:396‐406. [DOI] [PubMed] [Google Scholar]
- 17. Pfister DG, Su YB, Kraus DH, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined‐modality paradigm. J Clin Oncol. 2006;24:1072‐1078. [DOI] [PubMed] [Google Scholar]
- 18. Frederick BA, Helfrich BA, Coldren CD, et al. Epithelial to mesenchymal transition predicts gefitinib resistance in cell lines of head and neck squamous cell carcinoma and non‐small cell lung carcinoma. Mol Cancer Ther. 2007;6:1683‐1691. [DOI] [PubMed] [Google Scholar]
- 19. Morgillo F, Bareschino MA, Bianco R, Tortora G, Ciardiello F. Primary and acquired resistance to anti‐EGFR targeted drugs in cancer therapy. Differentiation. 2007;75:788‐799. [DOI] [PubMed] [Google Scholar]
- 20. Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007;19:2013‐2023. [DOI] [PubMed] [Google Scholar]
- 21. Dannenberg AJ, Altorki NK, Boyle JO, et al. Cyclo‐oxygenase 2: a pharmacological target for the prevention of cancer. Lancet Oncol. 2001;2:544‐551. [DOI] [PubMed] [Google Scholar]
- 22. Banerjee AG, Gopalakrishnan VK, Bhattacharya I, Vishwanatha JK. Deregulated cyclooxygenase‐2 expression in oral premalignant tissues. Mol Cancer Ther. 2002;1:1265‐1271. [PubMed] [Google Scholar]
- 23. Backlund MG, Mann JR, Dubois RN. Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E2. Oncology. 2005;69(Suppl 1):28‐32. [DOI] [PubMed] [Google Scholar]
- 24. Williams CS, Watson AJ, Sheng H, Helou R, Shao J, DuBois RN. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045‐6051. [PubMed] [Google Scholar]
- 25. Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591‐1601. [DOI] [PubMed] [Google Scholar]
- 26. Abrahao AC, Castilho RM, Squarize CH, Molinolo AA, dos Santos‐Pinto D, Gutkind JS. A role for COX2‐derived PGE2 and PGE2‐receptor subtypes in head and neck squamous carcinoma cell proliferation. Oral Oncol. 2010;46:880‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang KY, Tsai SY, Wu CM, Yen CJ, Chuang BF, Chang JY. Novel phosphoinositide 3‐kinase/mTOR dual inhibitor, NVP‐BGT226, displays potent growth‐inhibitory activity against human head and neck cancer cells in vitro and in vivo. Clin Cancer Res. 2011;17:7116‐7126. [DOI] [PubMed] [Google Scholar]
- 28. Yeh T‐M, Liu S‐H, Lin K‐C, et al. Dengue virus enhances thrombomodulin and ICAM‐1 expression through the macrophage migration inhibitory factor induction of the MAPK and PI3K signaling pathways. PLoS ONE. 2013;8:e55018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang KY, Shen MR, Lee MY, et al. Epidermal growth factor‐activated aryl hydrocarbon receptor nuclear translocator/HIF‐1{beta} signal pathway up‐regulates cyclooxygenase‐2 gene expression associated with squamous cell carcinoma. J Biol Chem. 2009;284:9908‐9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsu J‐Y, Chang K‐Y, Chen S‐H, et al. Epidermal growth factor‐induced cyclooxygenase‐2 enhances head and neck squamous cell carcinoma metastasis through fibronectin up‐regulation. Oncotarget. 2015;6:1723‐1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98‐W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tan MJ, Teo Z, Sng MK, Zhu P, Tan NS. Emerging roles of angiopoietin‐like 4 in human cancer. Mol Cancer Res. 2012;10:677‐688. [DOI] [PubMed] [Google Scholar]
- 33. Akishima‐Fukasawa Y, Ishikawa Y, Akasaka Y, et al. Histopathological predictors of regional lymph node metastasis at the invasive front in early colorectal cancer. Histopathology. 2011;59:470‐481. [DOI] [PubMed] [Google Scholar]
- 34. Mehra R, Cohen RB, Burtness BA. The role of cetuximab for the treatment of squamous cell carcinoma of the head and neck. Clin Adv Hematol Oncol. 2008;6:742‐750. [PMC free article] [PubMed] [Google Scholar]
- 35. Saba NF, Hurwitz SJ, Kono SA, et al. Chemoprevention of head and neck cancer with celecoxib and erlotinib: results of a phase ib and pharmacokinetic study. Cancer Prev Res (Phila). 2014;7:283‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen J, Shen P, Zhang XC, Zhao MD, Zhang XG, Yang L. Efficacy and safety profile of celecoxib for treating advanced cancers: a meta‐analysis of 11 randomized clinical trials. Clin Ther. 2014;36:1253‐1263. [DOI] [PubMed] [Google Scholar]
- 37. Ahmadi N, Goldman R, Seillier‐Moiseiwitsch F, Noone AM, Kosti O, Davidson BJ. Decreased risk of squamous cell carcinoma of the head and neck in users of nonsteroidal anti‐inflammatory drugs. Int J Otolaryngol. 2010;2010:424161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Howe LR, Subbaramaiah K, Patel J, et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER‐2)/neu‐induced breast cancer. Cancer Res. 2002;62:5405‐5407. [PubMed] [Google Scholar]
- 39. Dandekar DS, Lopez M, Carey RI, Lokeshwar BL. Cyclooxygenase‐2 inhibitor celecoxib augments chemotherapeutic drug‐induced apoptosis by enhancing activation of caspase‐3 and ‐9 in prostate cancer cells. Int J Cancer. 2005;115:484‐492. [DOI] [PubMed] [Google Scholar]
- 40. Arjona‐Sánchez Á, Ruiz‐Rabelo J, Perea MD, et al. Effects of capecitabine and celecoxib in experimental pancreatic cancer. Pancreatology. 2010;10:641‐647. [DOI] [PubMed] [Google Scholar]
- 41. Palmowski MJ, Gileadi U, Salio M, et al. Role of immunoproteasomes in cross‐presentation. J Immunol. 2006;177:983‐990. [DOI] [PubMed] [Google Scholar]
- 42. Dubey P, Shrivastava R, Tripathi C, et al. Cyclooxygenase‐2 inhibition attenuates hypoxic cancer cells induced m2‐polarization of macrophages. Cell Mol Biol (Noisy‐le‐grand). 2014;60:10‐15. [PubMed] [Google Scholar]
- 43. Gan LU, Qiu Z, Huang J, et al. Cyclooxygenase‐2 in tumor‐associated macrophages promotes metastatic potential of breast cancer cells through Akt pathway. Int J Biol Sci. 2016;12:1533‐1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Su CW, Zhang Y, Zhu YT. Stromal COX‐2 signaling are correlated with colorectal cancer: a review. Crit Rev Oncol Hematol. 2016;107:33‐38. [DOI] [PubMed] [Google Scholar]
- 45. Li L, Chong H, Ng S, et al. Angiopoietin‐like 4 increases pulmonary tissue leakiness and damage during influenza pneumonia. Cell Rep. 2015;10:654‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Multhoff G, Molls M, Radons J. Chronic inflammation in cancer development. Front Immunol. 2011;2:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Teo Z, Sng MK, Chan JSK, et al. Elevation of adenylate energy charge by angiopoietin‐like 4 enhances epithelial‐mesenchymal transition by inducing 14‐3‐3gamma expression. Oncogene. 2017;36:6408‐6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pan J, Yang Q, Shao J, et al. Cyclooxygenase‐2 induced beta1‐integrin expression in NSCLC and promoted cell invasion via the EP1/MAPK/E2F‐1/FoxC2 signal pathway. Sci Rep. 2016;6:33823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278:35451‐35457. [DOI] [PubMed] [Google Scholar]
- 50. Steinbach G, Lynch PM, Phillips RKS, et al. The effect of celecoxib, a cyclooxygenase‐2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946‐1952. [DOI] [PubMed] [Google Scholar]
- 51. Rosenthal EL, Chung TK, Carroll WR, Clemons L, Desmond R, Nabell L. Assessment of erlotinib as adjuvant chemoprevention in high‐risk head and neck cancer patients. Ann Surg Oncol. 2014;21:4263‐4269. [DOI] [PubMed] [Google Scholar]
- 52. Chen Z, Zhang X, Li M, et al. Simultaneously targeting epidermal growth factor receptor tyrosine kinase and cyclooxygenase‐2, an efficient approach to inhibition of squamous cell carcinoma of the head and neck. Clin Cancer Res. 2004;10:5930‐5939. [DOI] [PubMed] [Google Scholar]
- 53. Zhang X, Chen ZG, Choe MS, et al. Tumor growth inhibition by simultaneously blocking epidermal growth factor receptor and cyclooxygenase‐2 in a xenograft model. Clin Cancer Res. 2005;11:6261‐6269. [DOI] [PubMed] [Google Scholar]
- 54. Choe MS, Zhang X, Shin HJ, Shin DM, Chen ZG. Interaction between epidermal growth factor receptor‐ and cyclooxygenase 2‐mediated pathways and its implications for the chemoprevention of head and neck cancer. Mol Cancer Ther. 2005;4:1448‐1455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S4


