Abstract
Krüppel‐like factor 5 (KLF5) plays an oncogenic role and has diverse functions in cancer cells. However, correlation between KLF5 and clinical outcome has not been determined in patients with colorectal cancer and colorectal liver metastasis. Herein, we analyzed 65 patients with colorectal cancer who developed colorectal liver metastasis. Clinical effects were assessed through immunohistochemical analysis of primary colorectal cancer lesions and metastatic liver lesions. High expression of KLF5 in these tissues correlated with the presence of vascular invasion, elevated serum carbohydrate antigen 19‐9 levels, large diameters of metastatic liver tumors, and poor prognosis following surgery. Multivariate analyses revealed that high expression of KLF5 was an independent prognostic factor. Increased expression of KLF5 in both colorectal cancer primaries and colorectal liver metastasis was significantly associated with shorter overall survival time and time to surgical failure. Krüppel‐like factor 5 expression positively correlated with Ki‐67 and c‐Myc expression in colorectal cancer tissues. In vitro experiments with colon cancer cell lines showed that siRNA knockdown of KLF5 inhibited cell proliferation. Western blot analyses revealed that knockdown of KLF5 expression reduced cyclin D1 and c‐Myc expression. It also impaired the stem cell‐like properties of cancer cells in tumorsphere formation assays. Furthermore, anoikis assay indicated that KLF5 contributed to anoikis resistance. High KLF5 expression is associated with poor prognosis in patients with colorectal cancer and liver metastasis by promoting cell proliferation and cancer stem cell‐like properties.
Keywords: cancer stem cell, c‐Myc, colorectal cancer, colorectal liver metastasis, Krüppel‐like factor 5
High KLF5 expression in cancer cells is an independent predictor of poor prognosis in patients with primary colorectal cancer and related liver metastases. The malignant potential of cells with high Krüppel‐like factor 5 expression might be the result of high proliferative activity and cancer stem cell‐like properties, which likely occur owing to enhanced cyclin D1 and c‐Myc expression.
Abbreviations
- CA19‐9
carbohydrate antigen 19‐9
- CEA
carcinoembryonic antigen
- CRC
colorectal cancer
- CSC
cancer stem cell
- DFS
disease‐free survival
- IHC
immunohistochemistry
- KLF5
Krüppel‐like factor 5
- OS
overall survival
- TSF
time to surgical failure
1. INTRODUCTION
Colorectal cancer is one of the leading causes of mortality worldwide. 1 Distant metastasis is a major determinant of prognosis in patients with CRC. The liver is the most frequent metastatic site in CRC. Population‐based studies have shown that approximately 25%‐30% of patients diagnosed with CRC develop liver metastases during the course of their disease. 2 , 3 , 4 Therefore, an appropriate treatment strategy based on an accurate diagnosis is critical for improving clinical outcomes in patients with CRC.
Krüppel‐like factor 5 is a transcription factor that contains a triple zinc finger DNA‐binding domain. It is widely expressed in various tissues including the colon, small intestine, breast, pancreas, skeletal muscle, lung, prostate, and kidney. 5 , 6 , 7 Krüppel‐like factor 5 is reportedly essential for cell cycle regulation, apoptosis, migration, and differentiation. 8 In cancer biology, KLF5 is known to play an oncogenic role. Previous studies have reported that KLF5 is involved in angiogenesis, prevention of apoptosis, and epithelial‐mesenchymal transition, in addition to cell proliferation. 9 , 10 , 11 As for CRC, only a few in vitro studies have indicated an oncogenic role of KLF5 in colon cancer cells. 12 , 13 , 14 However, to the best of our knowledge, no studies have found a correlation between KLF5 and clinical outcome in patients with CRC and colorectal liver metastasis. Therefore, the aim of this study was to evaluate the expression of KLF5 in CRC and its correlation with patients’ clinical outcomes. Furthermore, the molecular mechanisms underlying the clinical data were explored.
2. MATERIALS AND METHODS
2.1. Patients and human tissue samples
Colorectal cancer and the corresponding liver metastasis tissues were obtained from 65 consecutive patients who underwent surgical resection for primary CRC and liver metastases at Chiba University Hospital (Chiba, Japan) between January 2006 and December 2014. Patients who underwent 2‐stage hepatectomy and primary tumor resection at other hospitals were excluded. Patients with synchronous liver metastases initially underwent primary tumor resection. As a control group, tissues were obtained from 53 consecutive patients with stage II or III CRC who did not develop distant metastases. These patients underwent surgical resection of primary CRC between 2012 and 2014. The ethics committees of Chiba University, Graduate School of Medicine (Chiba, Japan) approved the protocol of the present study (approval no. 2405). The study protocol conforms to the provisions of the Declaration of Helsinki. Written informed consent was obtained from each patient before surgery.
2.2. Indication criteria for surgical resection of metastatic tumors
Indication criteria for resection of colorectal metastases were as follows: (i) curative resection of the primary tumor is possible or has already been carried out; (ii) curative resection of metastases is possible; and (iii) preservation of physiologic function of the remaining tissue is possible (eg, 40% or more of total liver volume).
2.3. Immunohistochemistry
Formalin‐fixed paraffin‐embedded tissue samples were cut into 4‐µm‐thick slices and deparaffinized. In the IHC for KLF5 and c‐Myc, antigen retrieval was carried out by microwaving in citric acid buffer (0.01 mol/L, pH 6.0) for 25 minutes. Subsequently, endogenous peroxidase activity was blocked with hydrogen peroxide diluted to 3% in methanol for 15 minutes. Nonspecific protein was blocked with 5% BSA for 10 minutes. Following protein blocking, the slides were incubated at 4°C overnight with the following primary Abs: anti‐KLF5 polyclonal Ab (1:100 dilution, cat. no. ab24331; Abcam) and anti‐c‐Myc mAb (1:200 dilution, cat. no. MA1‐980; Thermo Fisher Scientific). In the IHC for Ki‐67, antigen retrieval was undertaken by autoclaving at 121°C for 10 minutes in citric acid buffer (0.01 mol/L, pH 9.0). Endogenous peroxidase and protein blocking were carried out in the same manner as that for KLF5 and c‐Myc. Following protein blocking, the slides were incubated at room temperature (15‐25°C) for 1 hour with the anti‐Ki‐67 primary mAb (1:75 dilution, cat. no. M7240; Dako). Counterstaining was undertaken with hematoxylin before dehydration, penetration, and mounting.
2.4. Immunohistochemical evaluation of KLF5, c‐Myc, and Ki‐67
Using an inverted microscope (BX40; Olympus), the expression levels of KLF5, c‐Myc, and Ki‐67 were evaluated independently by 2 investigators accompanied by a pathologist, all of whom were blinded to any clinical information. Percentage scores are defined as the percentage of positively stained nuclei in tumor cells relative to the total number of malignant cells in 3‐5 positive high‐power fields (×400). The evaluations were carried out after establishing interobserver consensus using samples from preliminary experiments.
2.5. Human colon cancer cell lines and culture conditions
The human colon cancer cell line DLD‐1 and the human colon cancer lymph node metastasis cell line SW620 were purchased from ATCC. The DLD‐1 cell line was cultured in RPMI‐1640 medium (Gibco) with 10% FBS (Thermo Fisher Scientific). The SW620 cell line was cultured in Leibovitz’s L‐15 medium (Gibco) with 10% FBS (Thermo Fisher Scientific).
2.6. Western blot analysis
Proteins were extracted from the above‐cultured cells with RIPA buffer (Sigma‐Aldrich). Each protein sample was lysed in buffer (Laemmli sample buffer; Bio‐Rad Laboratories) containing 5% 2‐mercaptoethanol and then incubated at 97°C for 10 minutes. After measuring the protein concentration of each sample using the Pierce BCA Protein Assay kit (Thermo Fisher Scientific), 10 µg protein was separated by electrophoresis on 7.5%‐15% XV PANTERA Gels (DRC) and transferred onto a membrane (PerkinElmer). The membranes were blocked in 5% skim milk diluted with 0.1% TBS with Tween‐20 at room temperature (15‐25°C) for 60 minutes. The membranes were then incubated at 4°C overnight with the following primary Abs: anti‐KLF5 polyclonal Ab (1:400 dilution, cat. no. ab137676; Abcam), anti‐cyclin D1 mAb (1:500 dilution, cat. no. ab16663; Abcam), anti‐c‐Myc mAb (1:1000 dilution, cat. no. ab32072; Abcam), and anti‐β‐actin mAb (1:2000 dilution, cat. no. 5125; Cell Signaling Technology). Subsequently, the membranes were incubated at room temperature (15‐25°C) for 60 minutes with anti‐rabbit IgG HRP secondary Ab (1:2000 dilution, cat. no. sc‐2301; Santa Cruz Biotechnology) in blocking buffer. The membranes were then incubated with an enhanced chemiluminescence detection reagent (Chemi‐Lumi One Ultra; Nacalai Tesque) and developed with a LAS‐4000UV mini luminescent image analyzer (Fujifilm). The intensity of each band was quantified by densitometric analysis using ImageJ software version 1.51 (NIH) and was used to calculate the relative protein level normalized to β‐actin.
2.7. Small interfering RNA transfection
The double‐stranded siRNAs used to knock down KLF5 expression were as follows: siKLF5‐1, Hs_KLF5_8 (cat. no. SI04267641; Qiagen); siKLF5‐2, Hs_KLF5_9 (cat. no. SI04281074; Qiagen); and siControl (AllStars negative control siRNA; Qiagen). These siRNAs (final concentration, 20 nmol/L) were transfected into DLD‐1 and SW620 cells using Lipofectamine RNAiMAX transfection reagent (Invitrogen; Thermo Fisher Scientific). These cells were used for subsequent assays at 24 hours post‐transfection.
2.8. Cell proliferation assay
Cell proliferation was examined using CCK‐8 (Dojindo Molecular Technologies) according to the manufacturer’s instructions. The DLD‐1 and SW620 cells transfected with siKLF5s or siControl were seeded at 1000 cells/well for DLD1 and 3000 cells/well for SW620 in 96‐well plates. At 0, 24, 48, and 72 hours, CCK‐8 (10 μL/well) solution was added to measure cell viability. After 2 hours of incubation, the absorbance of each well was measured at 450 nm.
2.9. Tumor sphere formation assay
Tumor sphere formation assays were carried out as described previously, with minor modifications. 15 Briefly, the DLD‐1 and SW620 cells transfected with siKLF5s or siControl were seeded at 15 cells/well in 96‐well ultra‐low attachment plates (Corning) with DMEM‐F12 (1:1) medium (Gibco) containing human basic fibroblast growth factor (10 ng/mL), human epidermal growth factor (20 ng/mL), and 0.4% BSA. After incubation for 10 days at 37°C, the cells were evaluated and the number of spheres with a diameter greater than 50 µm for DLD‐1 and greater than 25 µm for SW620 was counted using an inverted microscope (Axio Observer Z1; Carl Zeiss). The sphere formation rate was assessed as the percentage increase in the number of spheres on day 10 with regard to the number of spheres observed on day 1.
2.10. Anoikis resistance assay
To assess anoikis resistance, which is the resistance to apoptosis following loss of contact with the ECM, we undertook an anoikis resistance assay as described previously. 16 Briefly, DLD‐1 and SW620 cells transfected with siKLF5s or siControl were incubated at 37°C for 24 hours in a medium without growth factor under rotation. The colony formation assay was then carried out, 17 wherein 3000 cells/well suspended in a medium with 0.3% agar were seeded onto 24‐well culture plates coated with a medium containing 1% agar (bottom layer). The number of colonies was then counted at 14 days after cell seeding.
2.11. Cell invasion assay
CytoSelect 24‐well cell invasion assay kits (Cell Biolabs) utilizing basement membrane‐coated inserts were used according to the manufacturer’s protocol. In brief, cells transfected with siKLF5s or siControl were suspended in serum‐free medium. Following overnight starvation, the cells were seeded at 3.0 × 104 cells/well in the upper chamber and incubated with the medium in the lower chamber for 48 hours. The invasive cells passing through the basement membrane layer were stained, and the absorbance of each well was measured at 560 nm after extraction.
2.12. Statistical analysis
The correlations between KLF5 staining and the characteristics of patients were evaluated using the χ2‐test, Student’s t test, or Mann‐Whitney U test, as appropriate. Survival rates were calculated using Kaplan‐Meier analyses and assessed using the log‐rank test. Survival data were evaluated using univariate and multivariate Cox proportional regression analyses. When analyzing the correlation between KLF5 expression in the primary tumor and long‐term outcomes, OS and TSF were calculated from the date of primary tumor resection. When analyzing the correlation between KLF5 expression in the liver metastases and the long‐term outcome, OS and TSF were calculated from the date of initial hepatectomy. When analyzing the long‐term outcome and the difference of KLF5 expression in the primary tumor and liver metastases, OS and TSF were calculated from the date of initial hepatectomy. Correlation between KLF5 staining and Ki‐67 or c‐Myc staining was analyzed using the Pearson correlation coefficient. The in vitro experiments were undertaken at least 3 times independently, and data were analyzed using Welch’s t test and multivariate ANOVA. P values less than .05 were considered to reflect statistical significance. Values are expressed as the mean ± SEM. The above series of statistical analyses were undertaken using JMP Pro 13 software (SAS Institute).
3. RESULTS
3.1. Expression of KLF5 in primary tumor associated with poor prognosis
Krüppel‐like factor 5 protein expression was examined in primary tumors and the corresponding metastatic tumors in the liver with IHC. In 2 cases, the pathological diagnosis of liver metastases was pathological complete response. Therefore, KLF5 expression in liver metastases was examined in 63 patients. The KLF5 protein was predominantly expressed in the nucleus of cancer cells in both primary tumors and liver metastases (Figure 1). The mean KLF5‐positive rates were 67.6 ± 1.86% in primary tumors and 72.9 ± 1.93% in liver metastases (P = .0494). Based on receiver operating characteristic analyses in accordance with the 5‐year survival, the 65 patients were divided into 2 groups (cut‐off value = 76%; P = .2866; area under the curve = 0.6187).
Figure 1.
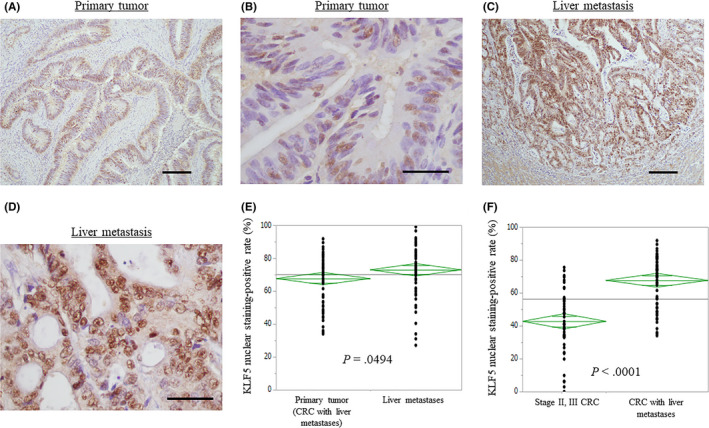
Immunohistochemistry analysis for Krüppel‐like factor 5 (KLF5) expression in colorectal cancer (CRC) and colorectal liver metastases. A, C, In both CRC (A) and its liver metastasis (C), the KLF5 protein is predominantly expressed in the nucleus of cancer cells. Scale bar = 200 μm. B, D, High magnification of A and C. Scale bar = 50 μm. E, Comparison of the KLF5 nuclear staining‐positive rates in the CRC and liver metastases. F, Comparison of the KLF5 nuclear staining‐positive rates in stage II or III CRC without metastases and CRC with liver metastases
With regard to primary tumors, high KLF5 expression (76% or more KLF5‐positive cells) was observed in 24 patients (36.9%), whereas low KLF5 expression (less than 76% KLF5‐positive cells) was observed in 41 patients (63.1%). Krüppel‐like factor 5 expression profiles in the primary tumors and clinicopathologic features are shown in Table 1. High KLF5 expression was significantly associated with synchronous liver metastasis, vascular invasion, elevated serum levels of CEA (18 ng/mL or more), elevated serum levels of CA19‐9 (50 U/mL or more), and large diameters of liver metastases (P = .0220, .0461, .0364, .0026, and .0173, respectively). No difference was noted in recurrence after hepatectomy between cancers with low and high KLF5 expression, but rehepatectomy tended to be more frequent in patients with low KLF5 expression (P = .0527). The re‐resection rate of metastatic tumors regardless of the organ was significantly higher in patients with low KLF5 expression when compared with those with high expression (P = .0279). The Kaplan‐Meier analysis showed that patients with high KLF5 expression had a significantly shorter OS time, time to liver metastasis, and TSF than did patients with low KLF5 expression (P = .0014, .0175, and .0008, respectively; Figure 2). In the univariate analysis, the primary tumor site (colon vs rectum), neoadjuvant chemotherapy before hepatectomy, molecular targeted therapy, lymph node metastasis, lymphatic invasion, number of liver metastases (3 or fewer, or 4 or more), and KLF5 expression correlated with OS. Of these, rectal cancer, large number of liver metastases, and high KLF5 expression were identified as independent prognostic factors (P = .0256, .0330, and .0137, respectively; Cox proportional hazards model; Table 2) in multivariate analyses.
Table 1.
Clinicopathologic features of patients with colorectal cancer who have high and low Krüppel‐like factor 5 (KLF5) expression
| Feature | KLF5 expression | P value | |
|---|---|---|---|
| High (n = 24) | Low (n = 41) | ||
| Age (y) | 64 (45‐80) | 69 (42‐81) | .0888 |
| Sex, female/male | 6/18 | 15/26 | .3299 |
| Site of primary tumors, colon/rectum | 12/12 | 27/14 | .2093 |
| Site of primary tumors, right/left/rectum | 5/7/12 | 13/14/14 | .4237 |
| Time of metastasis, synchronous/metachronous | 18/6 | 19/22 | .0220 |
| Adjuvant chemotherapy, −/+ | 4/2 | 17/5 | .6031 |
| Neoadjuvant chemotherapy before hepatectomy, −/+ | 17/7 | 30/11 | .8393 |
| Adjuvant chemotherapy (post‐hepatectomy), −/+ | 5/19 | 12/29 | .4501 |
| Molecular targeted therapy, −/+ | 13/11 | 25/16 | .5914 |
| T stage, T1‐3/T4 a | 13/11 | 25/16 | .5914 |
| Lymph node metastasis, −/+ | 9/15 | 17/24 | .7525 |
| Lymphatic invasion, −/+ | 11/13 | 24/17 | .3215 |
| Vascular invasion, −/+ | 3/21 | 14/27 | .0461 |
| Differentiation (primary tumor) pap, tub/por, muc | 19/5 | 34/7 | .7078 |
| Differentiation (liver metastases) pap, tub/por, muc | 21/3 | 36/3 | .5332 |
| CEA, <18/≥18 ng/mL | 12/12 | 31/10 | .0364 |
| CA19‐9, <50/≥50 U/mL | 13/11 | 36/5 | .0026 |
| Number of liver metastases | 2.5 (1‐18) | 2 (0‐10) | .1475 |
| Liver metastasis tumor diameter (cm) | 4.63 ± 0.54 | 3.02 ± 0.34 | .0173 |
| Recurrence after hepatectomy, −/+ | 3/21 | 11/30 | .1617 |
| Intrahepatic recurrence after hepatectomy, −/+ | 12/12 | 19/22 | .7757 |
| Repeat resection (all organs) | 12/9 | 8/22 | .0279 |
| Repeat hepatectomy, −/+ | 9/3 | 9/13 | .0527 |
Bold values are significant at P < .05.
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; muc, mucinous adenocarcinoma; pap, papillary adenocarcinoma; por, poorly differentiated adenocarcinoma; tub, tubular adenocarcinoma.
Union for International Cancer Control 7th edition.
Figure 2.
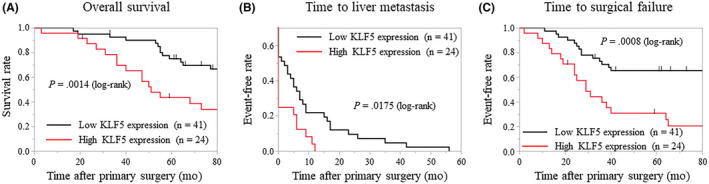
Kaplan‐Meier analysis for overall survival, time to liver metastasis, and time to surgical failure based on the Krüppel‐like factor 5 (KLF5) expression in primary tumors. A–C, The high KLF5 expression group shows significantly shorter overall survival (A), time to liver metastasis (B), and time to surgical failure (C) than the low KLF5 expression group (P = .0014, .0175, and .0008, respectively)
Table 2.
Univariate and multivariate analyses for overall survival (OS) in colorectal cancer
| Factors | n | 5‐y OS rate (%) |
Univariate P value |
Multivariate | |
|---|---|---|---|---|---|
| HR (95% CI) | P value | ||||
| Age, <65/≥65 y | 24/41 | 58.3/67.0 | .5422 | ||
| Site of primary tumors, colon/rectum | 39/26 | 76.5/44.2 | .0076 | 2.399 (1.114‐5.210) | .0259 |
| Site of primary tumors, right/left | 18/21 | 82.9/71.4 | .3941 | ||
| Time of metastasis, synchronous/metachronous | 37/28 | 58.4/70.6 | .0986 | ||
| Adjuvant chemotherapy, −/+ | 21/7 | 70.3/71.4 | .8400 | ||
| Neoadjuvant chemotherapy before hepatectomy, −/+ | 47/18 | 71.9/41.3 | .0148 | 1.462 (0.545‐3.942) | .4477 |
| Adjuvant chemotherapy (post‐hepatectomy), −/+ | 17/48 | 57.0/66.0 | .2102 | ||
| Molecular targeted therapy, −/+ | 38/27 | 70.5/53.9 | .0388 | 0.939 (0.373‐3.942) | .8938 |
| CEA, <18/≥18 ng/mL | 43/22 | 69.2/47.5 | .0886 | ||
| CA19‐9, <50/≥50 U/mL | 49/16 | 66.8/53.5 | .0900 | ||
| T stage, T1‐3/T4 a | 37/27 | 65.7/60.6 | .7814 | ||
| Lymph node metastasis, −/+ | 26/39 | 75.3/56.4 | .0164 | 2.237 (0.854‐6.586) | .1040 |
| Lymphatic invasion, −/+ | 35/30 | 73.0/41.6 | .0041 | 1.993 (0.842‐4.980) | .1179 |
| Vascular invasion, −/+ | 17/48 | 76.4/51.2 | .0899 | ||
| Differentiation (primary) pap, tub/por, muc | 53/12 | 66.8/50.0 | .3989 | ||
| Differentiation (liver) pap, tub/por, muc | 57/6 | 65.6/50.0 | .3636 | ||
| Number of liver metastases, ≤3/≥4 | 44/19 | 76.9/33.4 | .0185 | 2.403 (1.075‐5.314) | .0330 |
| Liver metastasis tumor diameter, ≤4/>4 cm | 41/22 | 70.1/52.7 | .1274 | ||
| KLF5 expression, low/high | 41/24 | 75.1/43.6 | .0027 | 2.571 (1.272‐5.283) | .0087 |
Bold values are significant at P < .05.
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; KLF5, Krüppel‐like factor 5; muc, mucinous adenocarcinoma; pap, papillary adenocarcinoma; por, poorly differentiated adenocarcinoma; tub, tubular adenocarcinoma.
Union for International Cancer Control 7th edition.
Expression of KLF5 was also examined in the control group patients with stage II or III CRC without any recurrence. Clinicopathologic features of these patients are shown in Table 3. The mean KLF5‐positive rate was 42.7 ± 2.6% which was significantly lower than the KLF5‐positive rates in primary tumors of patients with metastatic disease (P < .0001).
Table 3.
Clinicopathologic features of patients with colorectal cancer (CRC) with or without recurrence
| Feature | CRC without recurrence (n = 53) | CRC with liver metastases (n = 65) | P value |
|---|---|---|---|
| Age (y) | 70 (42‐85) | 67 (42‐81) | .0842 |
| Sex, female/male | 18/35 | 21/44 | .8493 |
| Site of primary tumors, colon/rectum | 39/14 | 39/26 | .1188 |
| Site of primary tumors, right/left/rectum | 10/29/14 | 18/21/26 | .0489 |
| Adjuvant chemotherapy, −/+ | 31/22 | 21/7 | .1345 |
| T stage, T1‐3/T4 a | 44/9 | 38/27 | .0033 |
| Lymph node metastasis, −/+ | 30/23 | 26/39 | .0719 |
| Lymphatic invasion, −/+ | 38/15 | 35/30 | .0456 |
| Vascular invasion, −/+ | 24/29 | 17/48 | .0299 |
| Differentiation (primary tumor) pap, tub/por, muc | 19/5 | 34/7 | .7078 |
| Differentiation (liver metastases) pap, tub/por, muc | 21/3 | 36/3 | .5332 |
| CEA, <18/≥18 ng/mL | 46/7 | 43/22 | .0080 |
| CA19‐9, <50/≥50 U/mL | 46/7 | 49/16 | .1146 |
| KLF5 expression rate (%) | 42.7 ± 2.64 | 67.6 ± 1.86 | <.0001 |
Bold values are significant at P < .05.
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; KLF5, Krüppel‐like factor 5; muc, mucinous adenocarcinoma; pap, papillary adenocarcinoma; por, poorly differentiated adenocarcinoma; tub, tubular adenocarcinoma.
Union for International Cancer Control 7th edition.
3.2. Krüppel‐like factor 5 expression in liver metastases associated with poor prognosis
Clinicopathologic features were compared between high and low levels of KLF5 expression in liver metastases. As shown in Table 4, the expression of KLF5 was significantly associated with vascular invasion of the primary tumor, elevated serum levels of CA19‐9 (≥50 U/mL), and large tumor diameter of liver metastases and the primary tumor (P = .0174, .0076, and .0133, respectively). The Kaplan‐Meier analysis showed that patients with high KLF5 expression in liver metastases had significantly shorter OS times after hepatectomy than patients with low KLF5 expression (P = .0376; Figure 3). The TSF after hepatectomy tended to be shorter in patients with high expression compared to those with low KLF5 expression in liver metastases (P = .0585; Figure 3). No significant difference in DFS after hepatectomy and time to hepatic recurrence after hepatectomy was found between patients with high and low KLF5 expression (Figure 3).
Table 4.
Clinicopathologic features of patients with colorectal liver metastasis, grouped according to Krüppel‐like factor 5 (KLF5) expression
| Feature | KLF5 expression | P value | |
|---|---|---|---|
| High (n = 30) | Low (n = 33) | ||
| Age at hepatectomy (y) | 65 (46‐81) | 70 (48‐84) | .3956 |
| Sex, female/male | 9/21 | 11/22 | .7764 |
| Site of primary tumors, colon/rectum | 19/11 | 19/14 | .6408 |
| Site of primary tumors, right/left/rectum | 10/9/11 | 8/11/14 | .7258 |
| Time of metastasis, synchronous/metachronous | 18/12 | 19/14 | .8452 |
| Adjuvant chemotherapy, −/+ | 8/4 | 11/3 | .4953 |
| Neoadjuvant chemotherapy before hepatectomy, −/+ | 25/5 | 22/11 | .1249 |
| Adjuvant chemotherapy (post‐hepatectomy), −/+ | 7/23 | 10/23 | .5327 |
| Molecular targeted therapy, −/+ | 21/9 | 17/16 | .1323 |
| Primary tumor T stage, T1‐3/T4 a | 16/14 | 21/12 | .4066 |
| Primary lymph node metastasis, −/+ | 10/20 | 15/18 | .3249 |
| Primary tumor lymphatic invasion, −/+ | 14/16 | 19/14 | .3862 |
| Primary tumor vascular invasion, −/+ | 4/26 | 13/20 | .0174 |
| Differentiation (primary tumor) pap, tub/por, muc | 24/6 | 27/6 | .8544 |
| Differentiation (liver metastases) pap, tub/por, muc | 26/4 | 31/2 | .3232 |
| CEA before hepatectomy, <18/≥18 ng/mL | 20/10 | 22/11 | 1.0000 |
| CA19‐9 before hepatectomy, <50/≥50 U/mL | 14/16 | 26/7 | .0076 |
| Number of liver metastases | 2 (1‐8) | 3 (1‐18) | .0652 |
| Liver metastasis tumor diameter (cm) | 4.48 ± 0.42 | 2.96 ± 0.42 | .0133 |
| Surgical margin, negative/positive | 19/11 | 24/9 | .4237 |
| Recurrence after hepatectomy, −/+ | 5/25 | 8/25 | .4560 |
| Intrahepatic recurrence after hepatectomy, −/+ | 14/16 | 16/17 | .8852 |
| Repeat resection (all organs) | 16/14 | 17/16 | .8852 |
| Repeat hepatectomy, −/+ | 23/7 | 25/8 | .9326 |
Bold values are significant at P < .05.
Abbreviations: CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; muc, mucinous adenocarcinoma; pap, papillary adenocarcinoma; por, poorly differentiated adenocarcinoma; tub, tubular adenocarcinoma.
Union for International Cancer Control 7th edition.
Figure 3.
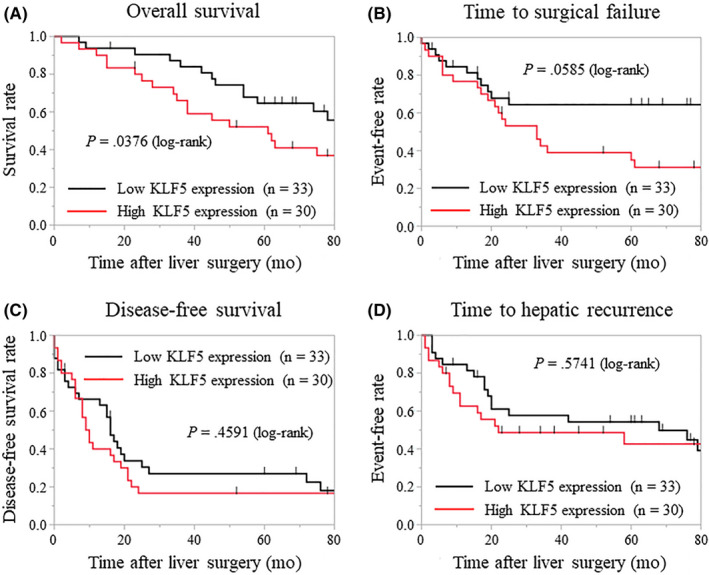
Kaplan‐Meier analysis of patients based on Krüppel‐like factor 5 (KLF5) expression in metastatic tumors. A, Patients with high KLF5 expression in the liver metastases have significantly shorter overall survival times after hepatectomy than do patients with low KLF5 expression (P = .0376). B, Time to surgical failure after hepatectomy is slightly, but not significantly, shorter in patients with high vs low KLF5 expression in liver metastases (P = .0585). C, D, No significant differences are found in disease‐free survival after hepatectomy (C) and time to hepatic recurrence after hepatectomy (D) between patients with high vs low KLF5 expression in liver metastases
3.3. Increased KLF5 expression from primary tumors to liver metastases is associated with poor prognosis
To investigate the correlation between KLF5 expression in primary tumors and liver metastases, we compared the expression rate of KLF5 in primary tumors and the corresponding liver metastases. The KLF5‐positive rate in 26 patients (41.3%) increased by at least 10%, and increased by less than 10% in 37 other patients (58.7%). The Kaplan‐Meier analysis showed that an increase of 10% or more in the KLF5‐positive rate was significantly associated with a shorter OS and TSF (P = .0075 and .0290, respectively; Figure 4). In contrast, 6 patients (9.5%) had high KLF5 expression in the primary tumor but low expression in the liver metastases. The expression pattern of KLF5 of these patients did not significantly correlate with the prognosis.
Figure 4.
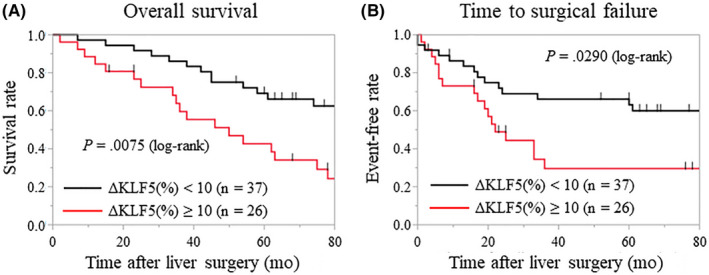
Relationship between Krüppel‐like factor 5 (KLF5) expression in primary tumors and liver metastases. A, B, Kaplan‐Meier analysis shows that patients in whom the KLF5‐positive rate increased by ≥10% (ΔKLF5 [%] ≥ 10) have significantly shorter overall survival times (A) and time to surgical failure (B) than do the patients in whom the rate increased by <10% (ΔKLF5 [%] < 10) (P = .0075 and .0290, respectively)
3.4. Krüppel‐like factor 5 expression positively correlated with Ki‐67 and c‐Myc expression in primary CRC
Immunohistochemistry for Ki‐67, a representative proliferation marker, and c‐Myc, a CSC marker, was undertaken to investigate the correlation between KLF5 expression and the expression of these markers in primary CRC. The correlations between the cancer cell nuclear staining rates of KLF5 and these markers are shown in Figure 5. Krüppel‐like factor 5 expression was positively correlated with Ki‐67 (R = 0.4435, P = .0002) and c‐Myc expression (R = 0.5590, P < .0001).
Figure 5.
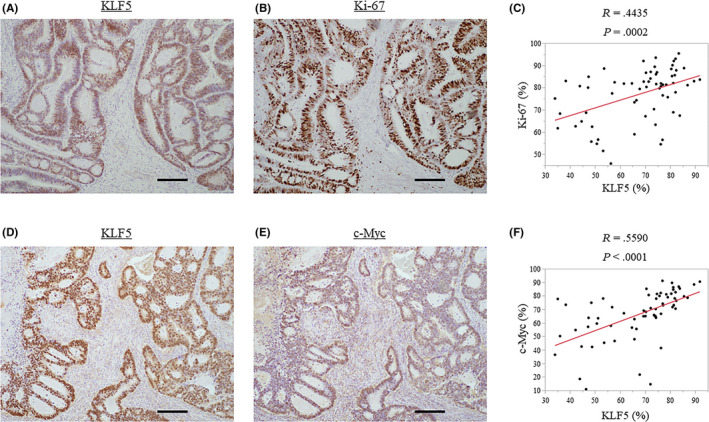
Correlations between Krüppel‐like factor 5 (KLF5) expression and Ki‐67 expression, a representative proliferation marker, and c‐Myc expression, a cancer stem cell marker, in primary colorectal cancer. A, B, Nuclear staining of KLF5 (A) and Ki‐67 (B). C, KLF5 expression positively correlates with Ki‐67 expression (R = 0.4435, P = .0002). D, E, Nuclear staining of KLF5 (D) and c‐Myc (E). F, KLF5 expression positively correlates with c‐Myc expression (R = 0.590, P < .0001). Scale bar: A, B, D, E = 200 μm
3.5. Recurrence after initial hepatectomy
Recurrences after initial hepatectomy were observed in 51 patients (78.4%). Intrahepatic recurrence was observed in 25 patients. Pulmonary metastasis was observed in 16 patients. Peritoneal dissemination was observed in 6 patients. Lymph node metastasis was observed in 8 patients (Table 5). Patients with high KLF5 expression in primary tumors tended to develop extrahepatic metastases, as well as metastases in multiple organs/sites after initial hepatectomy (P = .06 and P = .19, respectively).
Table 5.
Distribution of first recurrence site after first hepatectomy in patients with colorectal liver metastasis
| n = 61; n (%) | |
|---|---|
| Liver | 25 (41.0) |
| Lung | 16 (26.2) |
| Peritoneal dissemination | 6 (9.8) |
| Lymph nodes | 8 (13.1) |
| Others | 6 (9.8) |
Repeat resection for recurrent tumors was carried out in 16 patients but was deferred in 35 patients. The reasons for not undertaking repeat resection were multiple liver metastases in 3 patients, multiple lung metastases in 6, multiple organs/sites of metastasis in 10, peritoneal dissemination in 3, distant lymph node metastases in 4, extrahepatic and extrapulmonary metastases (bone and brain) in 5, and poor performance status in 4 patients (Table 6).
Table 6.
Contraindication to repeat resection in patients with colorectal liver metastasis
| n = 35; n (%) | |
|---|---|
| Multiple liver metastases | 3 (8.5) |
| Multiple lung metastases | 6 (17.1) |
| Multiple organ metastases | 10 (28.5) |
| Peritoneal dissemination | 3 (8.5) |
| Distant lymph node metastasis | 4 (11.4) |
| Areas that are difficult to surgically resect (eg, bone, brain) | 5 (14.2) |
| Patient factors | 4 (11.4) |
3.6. Inhibition of tumor cell proliferation through KLF5 knockdown
As the clinical data indicated that KLF5 might be oncogenic in CRC, in vitro experiments were undertaken to elucidate the molecular mechanisms by which KLF5 promotes the malignant behavior of CRC cells. The human colon cancer cell line DLD‐1 and the human colon cancer lymph node metastasis cell line SW620 were used for the experiments. To assess the effects of KLF5 knockdown on tumor cell proliferation in vitro, we undertook CCK‐8 assays following the knockdown of KLF5 using siRNAs. The CCK‐8 assays revealed that the knockdown of KLF5 significantly inhibited the proliferation of DLD‐1 and SW620 cells (Figure 6).
Figure 6.
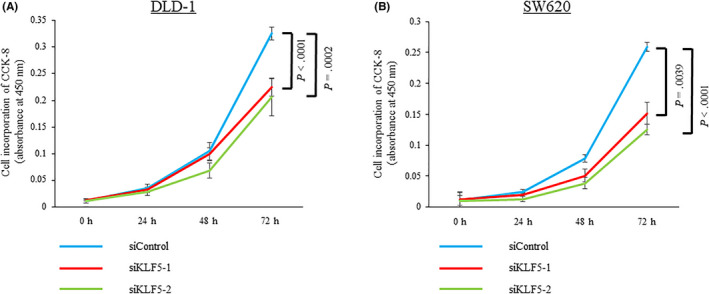
Cell proliferation assay using the CCK‐8 assay in colorectal cancer cells. A, B, Knockdown of Krüppel‐like factor 5 (KLF5) significantly inhibits the proliferation of the human colon cancer cell line DLD‐1 (A) and the human colon cancer lymph node metastasis cell line SW620 (B) cells
Subsequently, western blot analyses were undertaken to determine the effects of KLF5 knockdown on the protein expression of cyclin D1 and c‐Myc. These analyses revealed that in both colon cancer cell lines, the knockdown of KLF5 expression significantly reduced cyclin D1 and c‐Myc expression (Figure 7).
Figure 7.
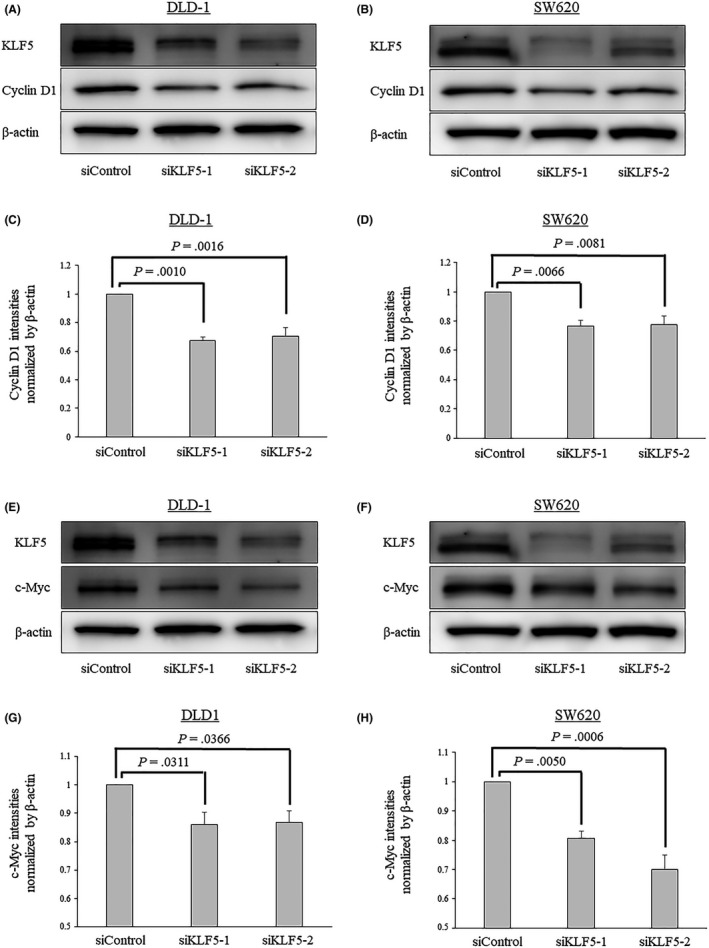
Effects of Krüppel‐like factor 5 (KLF5) knockdown evaluated using western blot analyses in the human colon cancer cell line DLD‐1 and the human colon cancer lymph node metastasis cell line SW620 cells. A, B, Knockdown of KLF5 expression significantly reduces the expression of cyclin D1 expression in both colorectal cancer (CRC) cell lines. C, D, Comparative analyses of cyclin D1 expression between CRC cells treated with siControl and siKLF5s. The band intensities were normalized to that of β‐actin. E, F, Knockdown of KLF5 expression significantly reduces the expression of c‐Myc expression in both CRC cell lines. G, H, Comparative analyses of c‐Myc expression between CRC cells treated with siControl and siKLF5s. Experiments were carried out at least 3 times. Error bars represent SEM
3.7. Knockdown of KLF5 expression impairs the CSC‐like properties and anoikis resistance in colon cancer cells
To evaluate the effects of KLF5 expression on the properties of CSCs, the self‐renewal capacity of colon cancer cells was investigated in vitro with the tumorsphere formation assay. This assay showed that KLF5 knockdown significantly reduced the number of sphere‐forming cells (Figure 8). These results indicate that KLF5 might be involved in maintaining the CSC‐like properties in colon cancer cells. Furthermore, the effects of KLF5 on anoikis resistance in colon cancer cells were investigated because anoikis resistance is a major characteristic of CSCs that promotes metastasis. 18 In the anoikis assay, KLF5 knockdown resulted in a significant reduction in the number and size of colonies (Figure 8). These results indicate that KLF5 might play a role in the maintenance of anoikis resistance in colon cancer cells.
Figure 8.
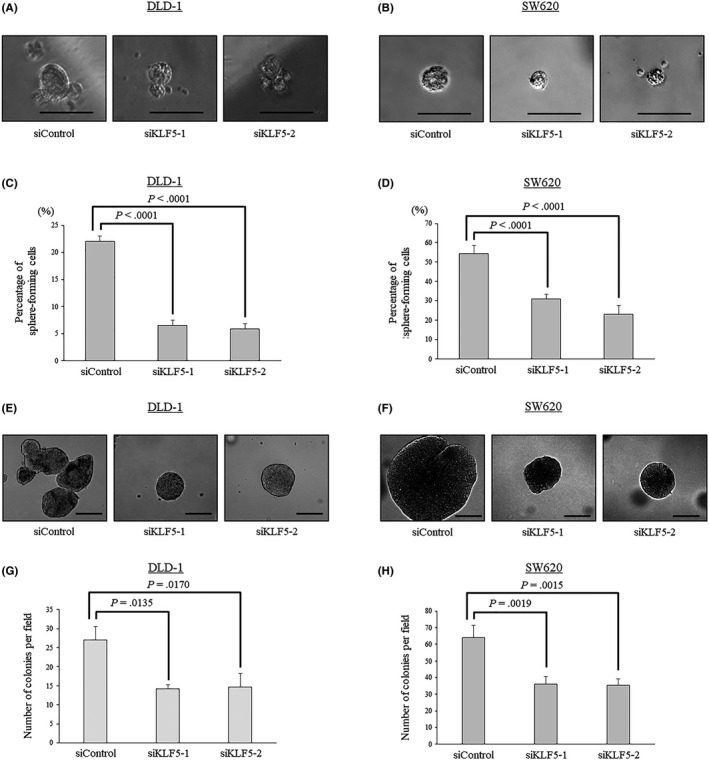
Krüppel‐like factor 5 (KLF5) expression maintains the properties of cancer stem cells and anoikis resistance in colon cancer cells. A, B, Representative findings of sphere formation in DLD‐1 (A) and SW620 (B) cells treated with siControl and siKLF5s. C, D, Percentages of sphere‐forming cells are evaluated in DLD‐1 (C) and SW620 (D) cells treated with siControl and siKLF5s. E, F, Representative findings of colony formation in DLD‐1 (E) and SW620 (F) cells treated with siControl and siKLF5s. G, H, The number of colonies is significantly reduced after KLF5 knockdown in DLD‐1 (G) and SW620 (H) cells. Experiments were carried out at least 3 times. Error bars represent SEM. Scale bar: A, B = 50 μm, E, F = 200 μm
3.8. Knockdown of KLF5 expression did not alter cell invasiveness
As cell invasiveness is one of the important properties in the metastatic cascade of cancer, the effect of KLF5 on cell invasiveness was assessed. The cell invasion assays revealed that the knockdown of KLF5 did not alter the invasiveness of DLD‐1 or SW620 cells (Figure 9).
Figure 9.
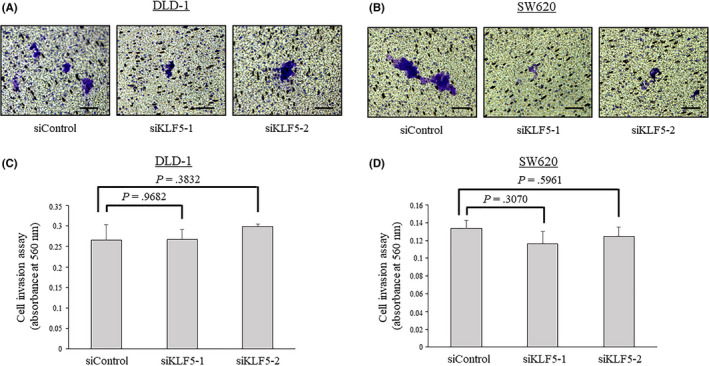
Cell invasion assay in colorectal cancer cells. Knockdown of Krüppel‐like factor 5 (KLF5) did not alter the cell invasiveness of human colon cancer cell line DLD‐1 cells (A, C) or human colon cancer lymph node metastasis cell line SW620 (B, D) cells
4. DISCUSSION
To the best of our knowledge, the present study is the first to reveal the roles that KLF5 plays in clinical outcomes in patients with CRC and liver metastasis. Our results showed that high KLF5 expression in both primary CRC tumors and their liver metastases was significantly associated with a poor prognosis. Furthermore, high KLF5 expression was significantly associated with several clinicopathologic factors that are known to be associated with the malignant behavior of CRC. Our in vitro experiments indicated that KLF5 promoted cell proliferation through cyclin D1. Moreover, our data suggested that KLF5 was associated with the CSC‐like properties in colon cancer cells. These factors might contribute to the early and unresectable recurrence of CRC, ultimately leading to shorter survival times in patients with high KLF5 expression.
Surgical resection is the gold standard of treatment for colorectal liver metastases. A number of studies have reported the utility of curative resection in improving the prognosis of patients with colorectal liver metastasis. 19 , 20 , 21 Recent evidence has also shown that repeat resection of distant metastases, including liver and lung metastases, could be useful. 22 , 23 Repeat hepatectomy for successive hepatic recurrences has been shown to yield a survival outcome that is comparable to that of the initial hepatectomy. 24 These findings suggest that recurrence itself might not always reflect a patient’s prognosis accurately. Whether or not the recurrence can be resected should be considered when discussing the clinical outcome of metastatic CRC. Therefore, a traditional indicator, DFS, does not necessarily reflect OS. The TSF, a newly reported end‐point, is defined as the interval from the initial surgery to the first unresectable recurrence or death. 25 Oba et al 25 reported that TSF is much more strongly correlated with OS than DFS in patients with colorectal liver metastasis. Our data indicated that repeat resection for distant metastasis was less frequent in patients with high expression compared to low KLF5 expression in primary tumors. The TSF of patients with high KLF5 expression was significantly shorter than that of patients with low KLF5 expression. Given these findings, we hypothesized that KLF5 promotes cancer cell proliferation and CSC‐like properties in CRC because these factors could lead to multiple and rapidly growing metastases that cannot be surgically resected. To validate this hypothesis, we undertook in vitro experiments using 2 cell lines. Cell proliferation was significantly inhibited by KLF5 knockdown through cyclin D1. This finding is in line with those of previous studies that reported pro‐proliferative activities of KLF5. 26 , 27 , 28 Moreover, these in vitro experiments support our clinical findings, such as the significant positive correlations between high KLF5 expression and large numbers and diameters of tumors.
A novel finding of this study is that the KLF5‐c‐Myc axis might play a pivotal role in promoting the CSC‐like properties of CRC cells. We found that knockdown of KLF5 reduced c‐Myc expression in vitro. c‐Myc is one of the well‐known oncoproteins of several cancers. Dysregulation of c‐Myc is reportedly associated with aggressive tumor behavior and poor clinical outcomes. 29 , 30 , 31 With regard to the role of c‐Myc in CSCs, Wang et al 31 found that high c‐Myc expression is required for proliferation, growth, survival, and tumorigenesis in glioma CSCs. Therefore, our data indicate that KLF5 might similarly regulate the stemness of CRC cells by modulating c‐Myc expression. This finding was supported by IHC for c‐Myc in primary tumors, in which a significant positive correlation was identified between KLF5 expression and c‐Myc expression. This result bridges the gap between our in vitro data and clinical data by indicating the oncogenic role of the KLF5‐c‐Myc axis in CRC.
Regarding the mechanisms underlying KLF5 modulation of c‐Myc and cyclin D1 expression, several studies have reported that KLF5 transactivates promoters of c‐Myc and cyclin D1 using luciferase reporter assays. Guo et al 32 found that KLF5 knockdown significantly reduced MYC expression and that KLF5 directly binds to 2 different sites of the MYC promoter in HaCaT epidermal epithelial cells. Furthermore, Nandan et al showed that KLF5 knockdown reduced cell proliferation and colony formation and that KLF5 was able to stimulate cyclin D1 promoter activity. 33 , 34 Given those findings, we speculate that KLF5 plays a role in the behavior of CRC and its liver metastases through binding to the promoters of c‐Myc and cyclin D1. How these mechanisms manifest in CRC cells needs to be investigated in future experiments.
Notably, we did not find a significant correlation between TSF and KLF5 expression in liver metastases. Because the mean rate of KLF5 expression in liver metastases was high compared with that of the primary tumor, identification of a significant difference might be difficult given the small sample size of the present study. The number of liver metastases among patients with high KLF5 expression in the primary tumor was greater than that in patients with low KLF5 expression. However, a contrasting result was observed when comparing KLF5 expression in liver metastases, although it was not significant. The difference might be explained by the number of liver metastases reflecting the KLF5 expression in the primary tumor but not in the liver metastases. In fact, the number of recurrent tumors after initial hepatectomy tended to be high in patients with high KLF5 expression in liver metastases.
Interestingly, KLF5 expression in liver metastases was elevated compared with that in the corresponding primary tumor. Moreover, the OS of patients in whom the KLF5‐positive rate increased by 10% or more in metastatic tumors relative to primary tumors was significantly worse than that of other patients. As shown in the present study, colon cancer cells with high KLF5 expression might exhibit CSC‐like properties, at least in part, by modulating c‐Myc expression. Additionally, previous studies indicated that KLF5 is involved in EMT. 35 , 36 Therefore, cells with high KLF5 expression might acquire more aggressive metastatic properties during the course of cancer progression than other cells. Generally, the distribution of cancer cells in solid tumors is heterogeneous. 37 , 38 Subsequently, cells with aggressive behavior could develop distant metastases, thus explaining the high KLF5 expression rate in metastatic tumors compared with that in primary tumors. Once the cells colonize distant organs, cells with higher KLF5 expression might develop larger and higher numbers of tumors and/or accelerate other distant metastases that lead to shorter TSF and OS times.
Given these data, several clinical implications can be proposed. Our data indicate that aggressive tumor behavior, such as rapid and multiple recurrences, are presumed in patients with high KLF5 expression and an increase of more than 10% in KLF5 expression in the liver metastases. Therefore, intensive follow‐up examination using tumor markers, computed tomography scans, and MRI should be carried out for these patients. However, once multiple organ/site metastases have developed, surgical resection is not an option. Thus, it is more important to reduce the risk of recurrence. Because perioperative chemotherapy might be an option to reduce the risk, more effective drug regimens need to be explored, and their administration to patients with high KLF5 expression should be of high priority.
Herein, we focused on the roles KLF5 plays in the proliferation and CSC‐like properties of cancer cells using in vitro experiments. However, KLF5 might have other roles in the malignant behavior of cancer cells because our clinical data showed that high KLF5 expression was associated with vascular invasion, high CEA levels, and high CA19‐9 levels. Previous studies have reported that KLF5 promotes cell adhesion, migration, and invasion in various types of cancer cells. 39 , 40 Hence, KLF5 expression might affect these cell properties in CRC, which could lead to tumor progression and accelerate metastatic activity to distant organs. These factors need to be examined in future experiments.
The present study has several limitations. First, all data were collected retrospectively; therefore, patients’ background data are not homogeneous. Several prognostic factors were not randomized for the analyses of long‐term outcome. Second, we only examined the effects of KLF5 with loss‐of‐function experiments. Further investigation with gain‐of‐function experiments and in vivo experiments are warranted to verify our data and elucidate the roles of KLF5 in CRC progression more accurately.
In conclusion, high KLF5 expression in cancer cells is an independent predictor of poor prognosis in patients with primary CRC and corresponding liver metastases. The malignant potential of cells with high KLF5 expression might be the result of high proliferative activity and CSC‐like properties, which likely occur owing to enhanced cyclin D1 and c‐Myc expression. These findings provide a rationale for the development of a novel therapeutic approach that targets KLF5 in the CRC progression and metastatic cascade. Further studies are necessary to explore the precise mechanism(s) by which KLF5 accelerates the malignant potential of CRC cells.
DISCLOSURE
The authors have no conflict of interest to declare.
ACKNOWLEDGMENTS
This research was supported by the Japan Society for the Promotion of Science KAKENHI (Grant No. JP16K10564 to N. Sakai).
Takagi Y, Sakai N, Yoshitomi H, et al. High expression of Krüppel‐like factor 5 is associated with poor prognosis in patients with colorectal cancer. Cancer Sci. 2020;111:2078–2092. 10.1111/cas.14411
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier A‐M. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006;244:254‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer‐Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten‐year population‐based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases – a population‐based study on incidence, management and survival. BMC Cancer. 2018;18:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi H, Zhang Z, Wang X, Liu S, Teng CT. Isolation and characterization of a gene encoding human Kruppel‐like factor 5 (IKLF): binding to the CAAT/GT box of the mouse lactoferrin gene promoter. Nucleic Acids Res. 1999;27:4807‐4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sur I, Undén AB, Toftgård R. Human Krüppel‐like factor5/KLF5: synergy with NF‐κB/Rel factors and expression in human skin and hair follicles. Eur J Cell Biol. 2002;81:323‐334. [DOI] [PubMed] [Google Scholar]
- 7. Chen C, Bhalala HV, Qiao H, Dong J‐T. A possible tumor suppressor role of the KLF5 transcription factor in human breast cancer. Oncogene. 2002;21:6567‐6572. [DOI] [PubMed] [Google Scholar]
- 8. Dong J‐T, Chen C. Essential role of KLF5 transcription factor in cell proliferation and differentiation and its implications for human diseases. Cell Mol Life Sci. 2009;66:2691‐2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao Y, Wu K, Chen Y, et al. Beyond proliferation: KLF5 promotes angiogenesis of bladder cancer through directly regulating VEGFA transcription. Oncotarget. 2015;6:43791‐43805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tong D, Czerwenka K, Heinze G, et al. Expression of KLF5 is a prognostic factor for disease‐free survival and overall survival in patients with breast cancer. Clin Cancer Res. 2006;12:2442‐2448. [DOI] [PubMed] [Google Scholar]
- 11. David C, Huang Y‐H, Chen MO, et al. TGF‐β tumor suppression through a lethal EMT. Cell. 2016;164:1015‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bialkowska AB, Liu Y, Nandan MO, Yang VW. A colon cancer‐derived mutant of Krüppel‐like factor 5 (KLF5) is resistant to degradation by glycogen synthase kinase 3beta (GSK3beta) and the E3 ubiquitin ligase F‐box and WD repeat domain‐containing 7alpha (FBW7alpha). J Biol Chem. 2014;289:5997‐6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shin J, Carr A, Corner GA, et al. The intestinal epithelial cell differentiation marker intestinal ALPi is selectively induced by HDACi in colon cancer cells in a KLF5‐dependent manner. J Biol Chem. 2014;289:25306‐25316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wei X, Ye J, Shang Y, et al. Ascl2 activation by YAP1/KLF5 ensures the self‐renewability of colon cancer progenitor cells. Oncotarget. 2017;8:109301‐109318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neri F, Dettori D, Incarnato D, et al. TET1 is a tumour suppressor that inhibits colon cancer growth by derepressing inhibitors of the WNT pathway. Oncogene. 2015;34:4168‐4176. [DOI] [PubMed] [Google Scholar]
- 16. Yoneura N, Takano S, Yoshitomi H, et al. Expression of annexin II and stromal tenascin C promotes epithelial to mesenchymal transition and correlates with distant metastasis in pancreatic cancer. Int J Mol Med. 2018;42:821‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takano S, Reichert M, Bakir B, et al. Prrx1 isoform switching regulates pancreatic cancer invasion and metastatic colonization. Genes Dev. 2016;30:233‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sugiura K, Mishima T, Takano S, et al. The expression of Yes‐associated protein (YAP) maintains putative cancer stemness and is associated with poor prognosis in intrahepatic cholangiocarcinoma. Am J Pathol. 2019;189:1863‐1877. [DOI] [PubMed] [Google Scholar]
- 19. Simmonds P, Primrose J, Colquitt J, Garden O, Poston G, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long‐term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125‐135. [DOI] [PubMed] [Google Scholar]
- 21. Takahashi M, Hasegawa K, Oba M, et al. Repeat resection leads to long‐term survival: analysis of 10‐year follow‐up of patients with colorectal liver metastases. Am J Surg. 2015;210:904‐910. [DOI] [PubMed] [Google Scholar]
- 22. Pulitanò C, Bodingbauer M, Aldrighetti L, et al. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi‐institutional analysis. Ann Surg Oncol. 2011;18:1380‐1388. [DOI] [PubMed] [Google Scholar]
- 23. Leung U, Gönen M, Allen PJ, et al. Colorectal cancer liver metastases and concurrent extrahepatic disease treated with resection. Ann Surg. 2017;265:158‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oba M, Hasegawa K, Shindoh J, et al. Survival benefit of repeat resection of successive recurrences after the initial hepatic resection for colorectal liver metastases. Surgery. 2016;159:632‐640. [DOI] [PubMed] [Google Scholar]
- 25. Oba M, Hasegawa K, Matsuyama Y, et al. Discrepancy between recurrence‐free survival and overall survival in patients with resectable colorectal liver metastases: a potential surrogate endpoint for time to surgical failure. Ann Surg Oncol. 2014;21:1817‐1824. [DOI] [PubMed] [Google Scholar]
- 26. Sogawa K, Imataka H, Yamasaki Y, Kusume H, Abe H, Fujii‐Kuriyama Y. cDNA cloning and transcriptional properties of a novel GC box‐binding protein, BTEB2. Nucleic Acids Res. 1993;21:1527‐1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chanchevalap S, Nandan MO, Merlin D, Yang VW. All‐trans retinoic acid inhibits proliferation of intestinal epithelial cells by inhibiting expression of the gene encoding Kruppel‐like factor 5. FEBS Lett. 2004;578:99‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghaleb AM, Nandan MO, Chanchevalap S, Dalton WB, Hisamuddin IM, Yang VW. Krüppel‐like factors 4 and 5: the yin and yang regulators of cellular proliferation. Cell Res. 2005;15:92‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vita M, Henriksson M. The Myc oncoprotein as a therapeutic target for human cancer. Semin Cancer Biol. 2006;16:318‐330. [DOI] [PubMed] [Google Scholar]
- 30. Wang H, Mannava S, Grachtchouk V, et al. c‐Myc depletion inhibits proliferation of human tumor cells at various stages of the cell cycle. Oncogene. 2008;27:1905‐1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang J, Wang H, Li Z, et al. c‐Myc is required for maintenance of glioma cancer stem cells. PLoS ONE. 2008;3:e3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo P, Dong XY, Zhao K, et al. Opposing effects of KLF5 on the transcription of MYC in epithelial proliferation in the context of transforming growth factor beta. J Biol Chem. 2009;284:28243‐28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nandan MO, Yoon HS, Zhao W, et al. Kruppel‐like factor 5 mediates the transforming activity of oncogenic H‐Ras. Oncogene. 2004;23:3404‐3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nandan MO, Chanchevalap S, Dalton WB, et al. Kruppel‐like factor 5 promotes mitosis by activating the cyclin B1/Cdc2 complex during oncogenic Ras‐mediated transformation. FEBS Lett. 2005;579:4757‐4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jia X, Shi L, Wang X, et al. KLF5 regulated lncRNA RP1 promotes the growth and metastasis of breast cancer via repressing p27kip1 translation. Cell Death Dis. 2019;10:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. An T, Dong T, Zhou H, et al. The transcription factor Krüppel‐like factor 5 promotes cell growth and metastasis via activating PI3K/AKT/Snail signaling in hepatocellular carcinoma. Biochem Biophys Res Commun. 2019;508:159‐168. [DOI] [PubMed] [Google Scholar]
- 37. Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jia L, Zhou Z, Liang H, et al. KLF5 promotes breast cancer proliferation, migration and invasion in part by upregulating the transcription of TNFAIP2. Oncogene. 2016;35:2040‐2051. [DOI] [PubMed] [Google Scholar]
- 40. Ma Y, Wang Q, Liu F, et al. KLF5 promotes the tumorigenesis and metastatic potential of thyroid cancer cells through the NF‐kappaB signaling pathway. Oncol Rep. 2018;40:2608‐2618. [DOI] [PMC free article] [PubMed] [Google Scholar]


