ABSTRACT
Background
Beverages are a source of calories and other bioactive constituents but are an understudied aspect of the diet. Different beverages have varying effects on health outcomes.
Objectives
We created the Healthy Beverage Score (HBS) to characterize participants’ beverage patterns and examined its association with chronic kidney disease (CKD) progression, incident cardiovascular disease (CVD), and all-cause mortality among individuals with CKD.
Methods
We conducted a prospective analysis of 2283 adults aged 21–74 y with a baseline estimated glomerular filtration rate of 20–70 mL · min−1 · 1.73 m−2 from the Chronic Renal Insufficiency Cohort. Diet was assessed using a 124-item FFQ at visit 1 (2003–2008). The HBS, ranging from 7 to 28 possible points, consisted of 7 components, each scored from 1 to 4 based on rank distribution by quartile, except alcohol, which was based on sex-specific cutoffs. Participants were given more points for higher consumption of low-fat milk and of coffee/tea, for moderate alcohol, and for lower consumption of 100% fruit juice, whole-fat milk, artificially sweetened beverages, and sugar-sweetened beverages. CKD progression, incident CVD, and mortality were ascertained through January 2018. We conducted multivariable Cox proportional hazards models.
Results
There were 815 cases of CKD progression, 285 cases of incident CVD, and 725 deaths over a maximum of 14 y of follow-up. Compared with participants in the lowest tertile of the HBS, participants in the highest tertile had a 25% lower likelihood of CKD progression (HR: 0.75; 95% CI: 0.63, 0.89; P-trend = 0.001) and a 17% lower likelihood of all-cause mortality (HR: 0.83; 95% CI: 0.69, 1.00; P-trend = 0.04) after adjusting for sociodemographic, clinical, and dietary factors. There was no significant trend for incident CVD.
Conclusions
Among individuals with CKD, a healthier beverage pattern was inversely associated with CKD progression and all-cause mortality. Beverage intake may be an important modifiable target in preventing adverse outcomes for individuals with CKD.
Keywords: CRIC, healthy beverages, chronic kidney disease progression, cardiovascular disease, all-cause mortality
Introduction
Dietary pattern scores are a valuable method to assess the association between diet quality and health outcomes because they represent how foods and beverages are typically consumed in a real-world setting (1). Several dietary scores have been created to assess diet quality including a priori defined scores based on dietary guidelines, randomized clinical trials, and geographic regions [e.g., Healthy Eating Index-2015 (HEI-2015), Dietary Approaches to Stop Hypertension (DASH) diet, Mediterranean diet].
Beverage intake represents ∼20% of total caloric intake, yet beverages are not well represented in dietary scores (2). Alcohol, sugar-sweetened beverages (SSBs), milk, and fruit juices are accounted for in some dietary scores, but not all (3–6). Furthermore, there are many other beverages that people consume daily such as water, coffee, tea, and artificially sweetened beverages (ASBs) that are not captured by existing dietary scores. These beverages contribute to fluid requirements, as well as contain important nutrients, bioactive constituents, and calories that may have health implications, and may interact with other nutrients in the diet.
Few studies have examined a healthy beverage pattern and health outcomes. The Healthy Beverage Index (HBI) created by Duffey and Davy (7) was designed to measure beverage quality by assigning an overall beverage score based on consumption of water, coffee and tea, low-fat milk, diet drinks, 100% fruit juice, alcohol, full-fat milk, and SSBs, total beverage energy intake, and meeting fluid requirements. The index was developed using dietary data collected in the NHANES, in which diet was assessed using 24-h recalls. A limitation of the HBI is that it cannot be used to assess beverage quality from FFQs, which often do not assess water intake and may not accurately assess total beverage energy and fluid requirements.
Given that beverages contribute nutrients and fluids that may have implications for kidney function, we aimed to explore how beverage quality is associated with adverse outcomes among people who have chronic kidney disease (CKD). Our objectives were to create a healthy beverage score that is suitable for FFQs and to examine its association with CKD progression, incident cardiovascular disease (CVD), and all-cause mortality among people with CKD.
Methods
Study population
We used data from the Chronic Renal Insufficiency Cohort (CRIC) study, which is an ongoing multicenter prospective study of people with CKD. Detailed descriptions of the study are provided elsewhere (8, 9). Briefly, 3939 men and women aged 21–74 y with an estimated glomerular filtration rate (eGFR) of 20–70 mL · min−1 · 1.73 m−2 based on the Modification of Diet in Renal Disease Study equation were recruited between 2003 and 2008 from 7 US clinical centers. Exclusion criteria for the CRIC study included inability to consent, institutionalized, pregnancy, or severe chronic conditions (8). Participants attended in-person visits each year and had telephone interviews between visits. Annual follow-up rates for visits 2, 3, 4, and 5 were 89%, 93%, 94%, and 93%, respectively. The study protocol was approved by the institutional review boards of all participating centers. All participants provided informed consent.
For our analysis, participants were excluded if they did not fill out the FFQ or were missing >60% FFQ responses (n = 1046), had extreme energy intakes [women: <500 or >3500 kcal/d; men: <700 or >4500 kcal/d (n = 50)], were missing covariates of interest (n = 533), or had incomplete responses to the beverage questions in the FFQ (n = 27). The current analysis included 2283 participants. For the analyses of incident CVD (and subtypes of CVD), the sample size was reduced to 1578 participants owing to excluding 705 participants who reported a history of CVD at baseline.
Diet assessment
Diet was assessed at baseline using the National Cancer Institute's 124-item Diet History Questionnaire (DHQ), which was previously validated in another cohort (10). The DHQ asked participants to self-report the consumption frequency and portion size of foods and beverages consumed in the preceding year. We converted responses for orange or grapefruit juice, other 100% fruit juice, fruit drinks, milk, soft drinks, beer, wine, liquor, coffee, iced tea, and hot tea to fluid ounces per day. For fruit drinks and soft drinks, an additional question asked how often the drinks were diet. For milk, an additional question asked what type of milk the participant usually drank (whole, 2%, 1%, nonfat). We combined 2%, 1%, and nonfat milks into the category of low-fat milk. For coffee and tea, additional questions inquired about how frequently participants added sugar, artificial sweetener, milk, or cream. We grouped orange or grapefruit juice with other 100% fruit juice to create a fruit juice component. We created a category for ASBs, which included diet soda, diet fruit drinks, and artificially sweetened coffee and tea. We also grouped SSBs together, which included fruit drinks, regular soda, and sweetened coffee and tea. We examined correlation coefficients between beverage groups using Pearson's correlation test.
Healthy Beverage Score
We created a Healthy Beverage Score (HBS) that ranged from 7 to 28 and included 7 components (Table 1). Each component was scored 1–4 based on rank distribution by quartile, except for alcohol. Components were grouped into adequacy components, which represented beverages that were scored positively (low-fat milk, and unsweetened coffee and tea), and moderation components, which represented beverages that were scored negatively (fruit juice, whole-fat milk, ASBs, and SSBs). For alcohol, participants who never drank or were heavy drinkers (>2 drinks/d for men and >1 drink/d for women) were assigned a score of 1 and moderate drinkers (>0 and <2 drinks/d for men and >0 and <1 drink/d for women) were assigned a score of 4. This rank distribution scoring system is similar to the scoring method used for the DASH diet, which used quintiles to rank participants by each component (6). We chose these 7 components to mirror the components selected for the previously defined HBI, which was based on recommendations from the Healthy Beverage Guidance System (11).
TABLE 1.
Scoring criteria for the Healthy Beverage Score
| Component | Minimum score | Maximum score |
|---|---|---|
| Adequacy | ||
| Low-fat milk (≤2% fat milk) | 1 (Quartile 1) | 4 (Quartile 4) |
| Coffee and tea (unsweetened coffee or tea) | 1 (Quartile 1) | 4 (Quartile 4) |
| Moderation | ||
| Whole-fat milk (whole-fat milk) | 1 (Quartile 4) | 4 (Quartile 1) |
| Fruit juice (orange juice or other fruit juice) | 1 (Quartile 4) | 4 (Quartile 1) |
| Artificially sweetened beverages (diet soda, diet fruit drink, or artificiallysweetened coffee/tea) | 1 (Quartile 4) | 4 (Quartile 1) |
| Sugar-sweetened beverages (fruit drinks, regular soda, or sweetened coffee/tea) | 1 (Quartile 4) | 4 (Quartile 1) |
| Alcohol1 (beer, wine, or liquor) | 1 (Never or heavy drinker) | 4 (Moderate drinker) |
| Total | 7 | 28 |
Heavy drinker defined as >2 drinks/d for men and >1 drink/d for women. Moderate drinker defined as >0 and ≤2 drinks/d for men and >0 and ≤1 drink/d for women.
In sensitivity analyses, we created different variations of the HBS that used different score cutoffs (e.g., sex-specific median, quintiles, any compared with never), gave more weight to SSBs, separated coffee and tea into 2 components, and included vegetable juice as an additional component.
Outcomes
CKD progression was defined as ≥50% decrease in eGFR from baseline or end-stage renal disease (ESRD), which included long-term dialysis therapy or kidney transplantation. Time to eGFR halving was imputed assuming a linear decline in kidney function between annual visits (12, 13). Information on dialysis and kidney transplantation was obtained during follow-up visits and telephone interviews and confirmed by medical chart review of records from the dialysis unit or hospital. Ascertainment of ESRD was supplemented by data from the US Renal Data System.
Incident CVD was defined as a myocardial infarction (MI), congestive heart failure (CHF), or stroke event, as previously described (8, 9). Cardiovascular events were assessed using a standard Medical Event Questionnaire during all follow-up interviews. For participants who were hospitalized owing to a CVD event, their medical records were requested for verification. Two physicians adjudicated each cardiovascular event. MI was based on symptoms of angina, cardiac biomarkers, and electrographic data. CHF was based on hospital admission for new or worsening heart failure signs and symptoms and diminished cardiac output. Stroke was defined as rapid onset of a neurological deficit, headache, or other nonvascular effect, by a lesion on brain imaging for >24 h or death within 24 h.
Deaths were recorded based on reports by next of kin, death certificates, hospital records, and linkage with the Social Security Death Master File. Participant follow-up was censored at time of death, loss to follow-up, or administratively in January 2018.
Assessment of covariates
Participants provided sociodemographic information, medical history, and medication use at baseline through self-reported questionnaires. Physical activity was summarized as metabolic equivalent tasks per week based on participants’ responses to the Multi-Ethnic Study of Atherosclerosis Typical Week Physical Activity Survey (14). Weight (kg) and height (m) were measured using standard protocols (9) and used to calculate BMI as kg/m2. eGFR was calculated using a CRIC-specific equation (12). Urinary protein excretion was measured using a 24-h urine sample and collected at each annual visit. HDL cholesterol was measured using the enzymatic colorimetric method. Diabetes mellitus was defined as a fasting plasma glucose concentration ≥126 mg/dL, a nonfasting plasma glucose concentration ≥200 mg/dL, or self-reported use of anti–diabetes mellitus medication. Systolic blood pressure was based on the mean of 3 seated measurements that were obtained by trained staff after 5 min of rest. Participants self-reported whether they had a history of CVD. HEI-2015 score ranged from 0 to 100 and consisted of 13 components (total fruits, whole fruits, total vegetables, greens and beans, whole grains, refined grains, dairy, total protein, seafood or plant protein, ratio of unsaturated to saturated fatty acids, saturated fats, sodium, and added sugars) based on recommendations from the 2015–2020 Dietary Guidelines for Americans (3). We calculated the score based on responses from the FFQs.
Statistical analysis
Participants’ baseline characteristics were summarized by tertile of HBS. We calculated the correlation coefficient between the HBS and the HEI-2015 score using Pearson's correlation. HRs for the associations of HBS tertiles with time to CKD progression, incident CVD, and all-cause mortality were calculated using Cox proportional hazards models. The assumption of proportionality was tested using Schoenfeld residuals. We did not observe substantial deviations from proportionality. For each outcome, we used 3 progressively adjusted models. Model 1 was adjusted for age, sex, race, clinical site, education, income level, baseline eGFR, urinary protein, and total energy intake; model 2 adjusted for factors in model 1 and smoking status and physical activity; and model 3 adjusted for factors in models 1 and 2 plus BMI, diabetes mellitus, history of CVD, systolic blood pressure, HDL cholesterol, angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB) use, and HEI-2015 score. We used the median value of HBS within each tertile to calculate the P-trend to test whether there was a linear relation between HBS and outcomes. In addition to the analysis according to tertile of HBS, we conducted a continuous analysis for each outcome per 1 point higher in HBS.
We examined the association between individual components of the HBS and each outcome by treating the scores as a categorical variable (1–4) and as a continuous variable. We used the same covariates as model 3 in the primary analysis and further adjusted for all other beverage components of the HBS.
We tested for interactions by age, sex, race (white compared with nonwhite), and baseline eGFR (>45 compared with ≤45 mL · min−1 · 1.73 m−2) using the likelihood ratio test. We also examined the association of HBS with each subtype of incident CVD (MI, CHF, and stroke) separately. All analyses were performed using Stata version 14.0 (StataCorp).
Results
Baseline characteristics
Compared with those who were excluded from our sample population, participants included in the final study sample were more likely to have a higher socioeconomic status, healthier lifestyle behaviors, and more favorable clinical characteristics (Supplemental Table 1). Participants who had a higher HBS, indicating a healthier beverage profile, were more likely to be older, female, white, have a higher education, higher income level, be more physically active, have diabetes, have a lower total energy intake, and have a higher diet quality score (assessed using HEI-2015) compared with participants who had a lower HBS (Table 2). In addition, those with a higher HBS were also less likely to be smokers, be hypertensive, or have a history of CVD. Kidney function (eGFR) was slightly higher and kidney damage (urinary protein) was slightly lower for those with a higher HBS. Correlation coefficients between beverage groups ranged from 0.001 (fruit juice and alcohol) to 0.70 (low-fat milk and whole-fat milk) (Supplemental Table 2). The correlation coefficient between the HBS and HEI-2015 scores was 0.09.
TABLE 2.
Baseline characteristics of CRIC participants by tertile of HBS1
| HBS | |||
|---|---|---|---|
| Characteristics | Tertile 1: 9–162 | Tertile 2: 17–182 | Tertile 3: 19–262 |
| n | 980 | 589 | 714 |
| Age, y | 57 ± 11 | 58 ± 11 | 59 ± 11 |
| Female | 45 | 48 | 52 |
| Nonwhite | 61 | 42 | 31 |
| Education ≥college or vocational/tech | 33 | 41 | 47 |
| Income ≥$50,000 | 32 | 39 | 43 |
| Current smoker | 14 | 10 | 10 |
| Physical activity, METs/wk | 200 ± 141 | 205 ± 124 | 207 ± 126 |
| BMI, kg/m2 | 32 ± 8 | 31 ± 8 | 32 ± 8 |
| Diabetes mellitus | 42 | 44 | 46 |
| History of CVD | 33 | 30 | 29 |
| Systolic BP, mm Hg | 128 ± 22 | 125 ± 20 | 124 ± 20 |
| eGFR, mL · min−1 · 1.73 m−2 | 46 ± 17 | 46 ± 17 | 48 ± 17 |
| Urinary protein, g/24 h | 1.0 ± 2.3 | 0.8 ± 1.8 | 0.8 ± 2.0 |
| HDL cholesterol, mg/dL | 48 ± 15 | 49 ± 16 | 49 ± 16 |
| ACEi or ARB use, % | 66 | 71 | 67 |
| Total energy intake, kcal/d | 1916 ± 801 | 1769 ± 718 | 1618 ± 700 |
| HEI-2015 score | 65 ± 12 | 67 ± 12 | 67 ± 12 |
Values are percentages for categorical variables and mean ± SD for continuous variables. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BP, blood pressure; CRIC, Chronic Renal Insufficiency Cohort; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HBS, Healthy Beverage Score; HEI, Healthy Eating Index; MET, metabolic equivalent task.
Range of HBS.
HBS and CKD progression, incident CVD, and all-cause mortality
There were 815 cases (36%) of CKD progression over a median follow-up of 7 y. In models 1 and 2, tertiles 2 and 3 of HBS were significantly associated with lower risk of CKD progression relative to tertile 1 (Table 3). In model 3, after also adjusting for BMI, diabetes, history of CVD, systolic blood pressure, HDL cholesterol, ACE inhibitor/ARB use, and HEI-2015 score, the association remained significant (HR for tertile 2 compared with 1: 0.80; 95% CI: 0.67, 0.95; HR for tertile 3 compared with 1: 0.75; 95% CI: 0.63, 0.89; P-trend = 0.001). For each additional 1 point higher in HBS, participants were 6% less likely to develop CKD progression (HR: 0.94; 95% CI: 0.91, 0.98).
TABLE 3.
Risk of CKD progression, incident CVD, and all-cause mortality, by tertile of HBS1
| Tertile 1 | Tertile 2 | Tertile 3 | P-trend2 | Continuous (per 1 point higher) | |
|---|---|---|---|---|---|
| CKD progression | |||||
| Cases, n | 401 | 196 | 218 | ||
| Model 1 | 1 (ref.) | 0.80 (0.68, 0.96) | 0.75 (0.63, 0.89) | 0.001 | 0.94 (0.91, 0.98) |
| Model 2 | 1 (ref.) | 0.81 (0.68, 0.96) | 0.75 (0.63, 0.89) | 0.001 | 0.94 (0.91, 0.98) |
| Model 3 | 1 (ref.) | 0.80 (0.67, 0.95) | 0.75 (0.63, 0.89) | 0.001 | 0.94 (0.91, 0.98) |
| Incident CVD (myocardial infarction, heart failure, stroke)3 | |||||
| Cases, n | 134 | 57 | 94 | ||
| Model 1 | 1 (ref.) | 0.73 (0.53, 1.01) | 1.02 (0.77, 1.34) | 0.8 | 1.01 (0.95, 1.07) |
| Model 2 | 1 (ref.) | 0.75 (0.54, 1.03) | 1.04 (0.78, 1.37) | 0.7 | 1.01 (0.95, 1.07) |
| Model 3 | 1 (ref.) | 0.71 (0.52, 0.98) | 0.95 (0.72, 1.25) | 0.8 | 0.99 (0.94, 1.05) |
| All-cause mortality | |||||
| Cases, n | 338 | 188 | 199 | ||
| Model 1 | 1 (ref.) | 0.97 (0.81, 1.16) | 0.84 (0.70, 1.01) | 0.1 | 0.97 (0.93, 1.00) |
| Model 2 | 1 (ref.) | 0.99 (0.82, 1.18) | 0.85 (0.71, 1.02) | 0.1 | 0.97 (0.93, 1.00) |
| Model 3 | 1 (ref.) | 1.01 (0.84, 1.21) | 0.83 (0.69, 1.00) | 0.04 | 0.96 (0.93, 1.00) |
Tertile HBS ranges and sample sizes were as follows: CKD progression: tertile 1, 9–16 (n = 980); tertile 2, 17–18 (n = 589); tertile 3, 19–26 (n = 714); incident CVD: tertile 1, 10–16 (n = 654); tertile 2, 17–18 (n = 414); tertile 3, 19–26 (n = 510); all-cause mortality: tertile 1, 9–16 (n = 980); tertile 2, 17–18 (n = 589); tertile 3, 19–26 (n = 714). Cox proportional hazards models were used to estimate HRs and 95% CIs. Model 1 was adjusted for age, sex, race, clinical site, education, income level, baseline estimated glomerular filtration rate, urinary protein, and total energy intake. Model 2 was further adjusted for smoking status and physical activity. Model 3 included model 2 covariates in addition to BMI, diabetes mellitus, history of CVD, systolic blood pressure, HDL cholesterol, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, and Healthy Eating Index-2015 score. CKD, chronic kidney disease; CVD, cardiovascular disease; HBS, Healthy Beverage Score; ref., reference.
Trend was tested using the median value within each tertile.
Sample size for analyses of incident CVD was 1578 participants; 705 participants with a history of CVD at baseline were excluded.
After excluding participants with a history of CVD at baseline (n = 705), there were 285 incident CVD events (18%) throughout a median follow-up time of 11 y. There were no significant associations between HBS and incident CVD in model 1 or 2. In model 3, there was a significant association between tertile 2 of HBS and incident CVD compared with tertile 1 (HR: 0.71; 95% CI: 0.52, 0.98) but not for tertile 3. There was no significant trend across tertiles for any of the models.
Over a median follow-up time of 12 y, there were 725 deaths (32%) due to any cause. The association between HBS and all-cause mortality was nonsignificant for models 1 and 2. In the fully adjusted model, participants in tertile 3 were 17% less likely to die than participants in tertile 1 (HR: 0.83; 95% CI: 0.69, 1.00; P-trend = 0.04). For each additional 1 point higher in HBS, there was a 4% reduction in all-cause mortality risk (HR: 0.96; 95% CI: 0.93, 1.00).
Individual components and CKD progression, incident CVD, and all-cause mortality
Figure 1 shows the associations of a 1-point increase in each individual component with CKD progression, incident CVD, and all-cause mortality. There were no significant independent associations for whole-fat milk, ASBs, SSBs, or alcohol and CKD progression, incident CVD, or all-cause mortality. Each additional point for low-fat milk (indicating greater consumption) was associated with lower likelihood of CKD progression, each additional point for fruit juice (indicating lower consumption) was associated with lower likelihood of incident CVD, and each additional point for coffee and tea (indicating greater consumption) was associated with lower likelihood of all-cause mortality. Supplemental Table 3 shows the HRs by category of each component's scores.
FIGURE 1.
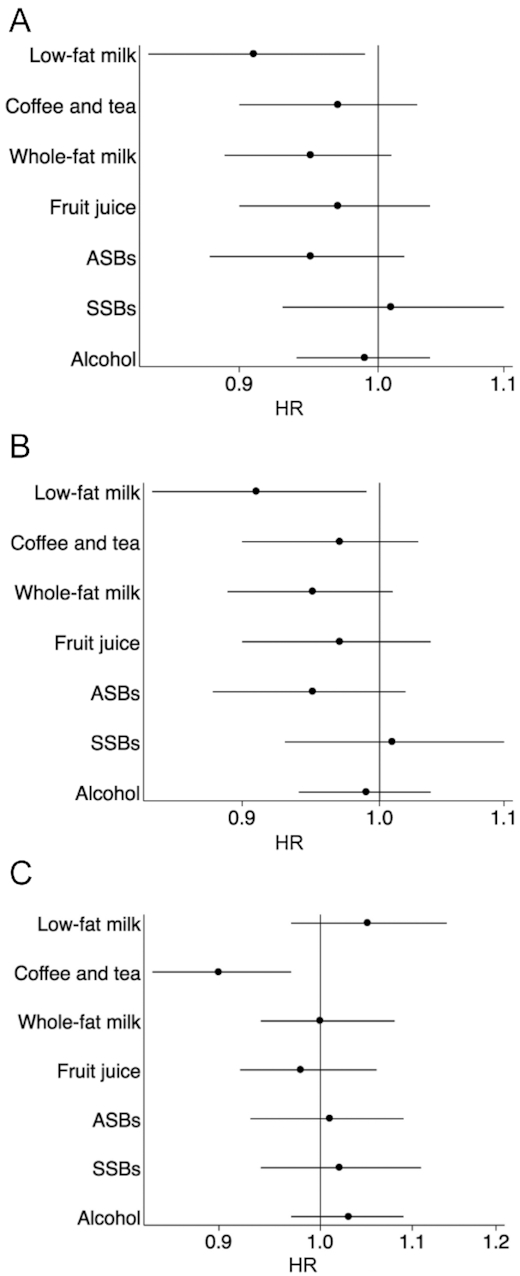
Risk of chronic kidney disease progression (A), incident CVD (B), and all-cause mortality (C) by individual components of the HBS per 1 point higher. Cox proportional hazards models were used to estimate HRs and 95% CIs. Models were adjusted for age, sex, race, clinical site, education, income level, baseline estimated glomerular filtration rate, urinary protein, total energy intake, smoking status, physical activity, BMI, diabetes mellitus, CVD, systolic blood pressure, HDL cholesterol, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use, Healthy Eating Index-2015 score, and all other components of the HBS. Alcohol was modeled as moderate drinkers compared with heavy or never drinkers. Sample size for analyses of incident CVD was 1578 participants; 705 participants with a history of CVD at baseline were excluded. ASB, artificially sweetened beverage; CVD, cardiovascular disease; HBS, Healthy Beverage Score; SSB, sugar-sweetened beverage.
When we examined the associations of different variations of the HBS with CKD progression, incident CVD, and all-cause mortality, the findings were consistent with our main results. There were no significant interactions by age, sex, race, or baseline eGFR (P-interaction > 0.05). In a sensitivity analysis according to subtype of CVD (MI, CHF, and stroke), participants in tertile 3 of the HBS relative to tertile 1 had an increased likelihood of MI in models 1 and 2 (Supplemental Table 4). However, in model 3, after adjusting for clinical and dietary variables, the association was no longer significant. For CHF, there was an inverse association with HBS comparing tertile 3 with tertile 1 but it was not significant in any of the models. For stroke, there was a direct association between HBS and stroke comparing tertile 3 with tertile 1 but it was not significant.
Discussion
In our study of 2283 participants with CKD at baseline, we created a novel index to assess overall beverage quality and found that higher scores were inversely associated with CKD progression and all-cause mortality, but not incident CVD. Lifestyle and clinical factors did not substantially change the associations between HBS and the 3 outcomes. Low-fat milk was inversely associated with CKD progression, fruit juice was directly associated with incident CVD, and unsweetened coffee and tea was inversely associated with all-cause mortality. The association between the HBS and CKD progression was stronger than any of the independent associations for the individual components, suggesting a potential cumulative effect of beverages on CKD progression. The correlation between the HBS and the HEI-2015 score was very low, suggesting that diet quality scores such as HEI-2015 do not adequately assess beverage quality, an important dimension of total diet.
To our knowledge, this is the first study to examine the association between overall beverage quality and health outcomes longitudinally and the second a priori index created to assess overall beverage quality. Previously, Duffey and Davy (7) created the HBI in NHANES and found that the score was associated in a cross-sectional analysis with cardiometabolic outcomes (e.g., hypertension, fasting insulin, fasting glucose, and cholesterol). The HBI was created based on 24-h recalls to assess diet. Therefore, the HBI was not easily transferrable to a longitudinal cohort in which FFQs were used to assess diet. For example, water was not assessed in the FFQ used in the CRIC Study, and total beverage energy and fluid requirements could not be accurately estimated because the FFQ was based on average intake over the previous year rather than intake for a single day. Thus, we attempted to create a beverage score that included the beverage components in the HBI, with the exception of water. Whereas the HBI scored components based on percentage of fluid requirements, the HBS scored participants based on rank distribution by quartiles. Whereas optimal consumption of alcohol was moderate consumption in the HBS, none to moderate alcohol consumption was scored the highest in the HBI. Furthermore, the HBS weighted all of the components equally, whereas the HBI assigned SSBs 3 times the weight of the other beverage components.
We found that low-fat milk was inversely associated with CKD progression. To our knowledge, this is the first study to examine the association between low-fat milk and CKD progression. However, a previous study found that in a population of 14,882 generally healthy participants, low-fat dairy was inversely associated with incident CKD (15). Dairy peptides (casein and whey proteins) and nutrients (e.g., calcium, potassium, and magnesium) in milk may have a protective renal effect through lowering blood pressure and acting as ACE inhibitors to hinder the renin–angiotensin system (16, 17). Furthermore, low-fat milk was inversely associated with CKD progression but whole-fat milk was not, suggesting that high fat content may counter the beneficial constituents in milk. Future studies should investigate further the role of low-fat milk in CKD progression.
Higher consumption of fruit juice was directly associated with incident CVD in our study. Evidence is mixed regarding the health effects of fruit juices considering they provide important nutrients but are also high in sugar, contribute calories, and have a high glycemic index (18–20). For example, 100% fruit juice has been found to be associated with long-term weight gain and type 2 diabetes but evidence is inconsistent for CVD (21–23). A pooled analysis of the Nurses’ Health Study and Health Professionals’ Follow-Up Study found a null association between citrus fruit juice and incident CVD (24). In the Danish Diet, Cancer, and Health cohort study, fruit juice was associated with acute coronary syndrome among women but not men (25). These studies were conducted in healthy populations; therefore, more evidence is needed for fruit juice consumption among people with CKD, who may have other comorbidities such as obesity or diabetes. Although the FFQ in our study specified that fruit juice was 100% fruit juice, it is possible that participants reported juices that were not 100% fruit juice.
We found the coffee and tea component to be inversely associated with all-cause mortality, but not CKD progression. Coffee consumption has consistently been shown to be associated with lower risk of all-cause mortality (26, 27) and possibly with incident CKD (28); however, there is less evidence on the association with CKD progression. We expected that coffee consumption may help lower risk of CKD progression by improving glycemia and therefore lowering risk of diabetic nephropathy or by protecting the glomerular endothelium from oxidative stress and inflammation (29, 30). However, these health-promoting attributes of coffee may not have the same effects after the onset of CKD. In a prospective study of a general population from the Multiethnic Cohort, participants who drank ≥1 cup of coffee per day had a significantly lower risk of death due to kidney disease than people who never drank coffee (27). In the Singapore Chinese Health Study, a prospective cohort of 63,257 adults, higher coffee consumption was inversely associated with ESRD, but tea was not associated with ESRD (31). Evidence is limited on the association of coffee and tea with CKD progression among people with CKD.
There are several limitations of our study. Our results are generalizable only to individuals with CKD stages 2–4 and we cannot draw conclusions related to beverage quality among people with renal failure. ESRD patients may have different nutrient and fluid restrictions that should be considered when assessing beverage quality and intake. Diet was self-reported using FFQs, which may result in measurement error. However, measurement error would likely make our estimates conservative in comparison with the true associations. Many participants (n = 702) did not respond or completely respond to the FFQ at baseline, which may be an indicator of a less healthy lifestyle. Participants excluded from our study sample had lower socioeconomic status and poorer baseline health markers than participants included. Therefore, our estimates may underestimate the true associations. The FFQ did not assess intake of water, which is a frequently consumed beverage. Because water was not assessed, we also could not calculate total fluid intake, which is an important factor in assessing beverage consumption. Future questionnaires to assess dietary and beverage habits should measure water intake. Another limitation was that we did not incorporate daily fluid requirements into the HBS. Because fluid requirements requires total volume of beverages consumed in a day and our FFQ assessed diet in the past year, we felt that our data may not be appropriate for this metric. Therefore, we did not include beverage energy intake or fluid requirements in the HBS. Owing to many participants having CVD at baseline, our sample size was much smaller for analyses for incident CVD (n = 1578). Therefore, the power for these analyses was most likely too low to detect significant associations. We found that tertile 2 of the HBS was significantly inversely associated with incident CVD; however, the P-trend was not significant. Furthermore, our results from analyses of subtypes of CVD suggest that participants in tertile 3 of the HBS may have opposite associations for MI and stroke compared with participants in tertile 2. However, the sample sizes for these analyses were too small to interpret these findings. Lastly, there may be residual confounding due to incomplete adjustment of covariates.
Despite the limitations, there were many strengths of our study. This was one of the first studies that created an a priori score for beverage patterns suitable for studies using FFQs to assess diet and was the first study to longitudinally assess the association between beverage quality and health outcomes. The follow-up time was long with a maximum of 14 y. Our study included white, black, and Hispanic men and women with CKD from 7 sites in the United States, allowing for broad generalizability.
Our findings have several important implications. Identifying beverages with a protective association with CKD progression could help inform future US Dietary Guidelines and clinical guidelines for people with CKD. In a clinical setting, clinicians may recommend people with chronic illnesses consume more of certain beverages (e.g., coffee, low-fat milk) or avoid certain beverage types (e.g., fruit juice). Furthermore, the HBS may be applied to other cohorts with FFQ data to assess beverage quality and health outcomes in other populations of healthy individuals and those with other common comorbid conditions (CVD, diabetes). Overall, our study highlights the importance of beverages as a critical contributor to the overall diet and to health.
In conclusion, we created a novel index that assessed beverage quality. Our findings suggest that an overall healthy beverage pattern is inversely associated with CKD progression and all-cause mortality, and the health implications of individual beverage components may be cumulative. This index may be a valuable method of assessment that can be applied in other cohorts to assess beverage quality and health outcomes. These findings may aid in identifying optimal beverage patterns to prevent adverse health outcomes among the general population and among those with chronic illnesses.
Supplementary Material
ACKNOWLEDGEMENTS
The CRIC Study investigators include Harold I Feldman (University of Pennsylvania), Alan S Go (Kaiser Permanente Division of Research), James P Lash (University of Illinois), Panduranga S Rao (University of Michigan), Mahboob Rahman (Case Western Reserve University), and Raymond R Townsend (University of Pennsylvania). The authors’ responsibilities were as follows—EAH and CMR: designed the research plan, analyzed the data, and had primary responsibility for the final content; EAH: wrote the paper; and all authors: read and approved the final manuscript.
Notes
Supported by NIH/National Heart, Lung, and Blood Institute (NHLBI) training grant T32 HL007024 (to EAH), in part by NHLBI grant 1K24 HL148181 (to DCC), in part by National Institute of General Medical Sciences grant P20GM109036 (to KTM), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) mentored research scientist development award K01 DK107782 (to CMR), and NHLBI grant R21 HL143089 (to CMR). Funding for the Chronic Renal Insufficiency Cohort study was obtained under a cooperative agreement from the NIDDK, grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902. In addition, this work was supported in part by a Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award (CTSA), NIH/National Center for Advancing Translational Sciences (NCATS) grant UL1TR000003, Johns Hopkins University grant UL1 TR-000424, University of Maryland grant GCRC M01 RR-16500, the Clinical and Translational Science Collaborative of Cleveland, grant UL1TR000439 from the NCATS component of the NIH and NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research grant UL1TR000433, University of Illinois at Chicago CTSA grant UL1RR029879, Tulane Center of Biomedical Research Excellence for Clinical and Translational Research in Cardiometabolic Diseases grant P20 GM109036, and Kaiser Permanente NIH/National Center for Research Resources University of California San Francisco-Clinical & Translational Science Institute grant UL1 RR-024131. Some of the data reported here were supplied by the US Renal Data System.
Author disclosures: The authors report no conflicts of interest.
The funders had no role in the study design; collection, analysis, and interpretation of these data; writing the report; and the decision to submit the report for publication.
Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ASB, artificially sweetened beverage; CHF, congestive heart failure; CKD, chronic kidney disease; CRIC, Chronic Renal Insufficiency Cohort; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DHQ, Diet History Questionnaire; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HBI, Healthy Beverage Index; HBS, Healthy Beverage Score; HEI-2015, Healthy Eating Index-2015; MI, myocardial infarction; SSB, sugar-sweetened beverage.
Contributor Information
Emily A Hu, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Cheryl A M Anderson, Department of Family Medicine and Public Health, University of California San Diego School of Medicine, San Diego, CA, USA.
Deidra C Crews, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Katherine T Mills, Department of Medicine, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA.
Jiang He, Department of Medicine, School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA.
Haochang Shou, Department of Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Jonathon J Taliercio, Department of Nephrology and Hypertension, Cleveland Clinic, Cleveland, OH, USA.
Madhumita J Mohanty, Department of Medicine, Wayne State University, Detroit, MI, USA.
Zeenat Bhat, Department of Medicine, Wayne State University, Detroit, MI, USA.
Josef Coresh, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
Lawrence J Appel, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Casey M Rebholz, Email: crebhol1@jhu.edu, Department of Epidemiology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, USA.
CRIC Study Investigators:
Harold I Feldman, Alan S Go, James P Lash, Panduranga S Rao, Mahboob Rahman, and Raymond R Townsend
References
- 1. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 2. US Department of Health and Human Services (USDHHS), USDA. 2015–2020 Dietary Guidelines for Americans. [Internet] 8th ed Washington (DC): USDHHS and USDA; 2015; [cited 2019 Sep 3]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 3. Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet. 2018;118(9):1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82(1):163–73. [DOI] [PubMed] [Google Scholar]
- 6. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168(7):713–20. [DOI] [PubMed] [Google Scholar]
- 7. Duffey KJ, Davy BM. The Healthy Beverage Index is associated with reduced cardiometabolic risk in US adults: a preliminary analysis. J Acad Nutr Diet. 2015;115(10):1682–9.e2. [DOI] [PubMed] [Google Scholar]
- 8. Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL et al.. The Chronic Renal Insufficiency Cohort (CRIC) study: design and methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–53. [DOI] [PubMed] [Google Scholar]
- 9. Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J et al.. Chronic Renal Insufficiency Cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4(8):1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, McIntosh A, Rosenfeld S. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089–99. [DOI] [PubMed] [Google Scholar]
- 11. Popkin BM, Armstrong LE, Bray GM, Caballero B, Frei B, Willett WC. A new proposed guidance system for beverage consumption in the United States. Am J Clin Nutr. 2006;83(3):529–42. [DOI] [PubMed] [Google Scholar]
- 12. Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P et al.. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2012;60(2):250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, Hsu CY, Fink JC, He J, Lash JP et al.. Association of kidney disease outcomes with risk factors for CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2014;63(2):236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169(4):444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rebholz CM, Crews DC, Grams ME, Steffen LM, Levey AS, Miller ER 3rd, Appel LJ, Coresh J. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis. 2016;68(6):853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kris-Etherton PM, Grieger JA, Hilpert KF, West SG. Milk products, dietary patterns and blood pressure management. J Am Coll Nutr. 2009;28(Suppl 1):103S–19S. [DOI] [PubMed] [Google Scholar]
- 17. FitzGerald RJ, Murray BA, Walsh DJ. Hypotensive peptides from milk proteins. J Nutr. 2004;134(4):980S–8S. [DOI] [PubMed] [Google Scholar]
- 18. Walker RW, Dumke KA, Goran MI. Fructose content in popular beverages made with and without high-fructose corn syrup. Nutrition. 2014;30(7–8):928–35. [DOI] [PubMed] [Google Scholar]
- 19. Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care. 2008;31(12):2281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Auerbach BJ, Dibey S, Vallila-Buchman P, Kratz M, Krieger J. Review of 100% fruit juice and chronic health conditions: implications for sugar-sweetened beverage policy. Adv Nutr. 2018;9(2):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes. 2013;37(10):1378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008;31(7):1311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, Sun Q. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hung HC, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, Colditz GA, Rosner B, Spiegelman D, Willett WC. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96(21):1577–84. [DOI] [PubMed] [Google Scholar]
- 25. Hansen L, Dragsted LO, Olsen A, Christensen J, Tjønneland A, Schmidt EB, Overvad K. Fruit and vegetable intake and risk of acute coronary syndrome. Br J Nutr. 2010;104(2):248–55. [DOI] [PubMed] [Google Scholar]
- 26. Gunter MJ, Murphy N, Cross AJ, Dossus L, Dartois L, Fagherazzi G, Kaaks R, Kuhn T, Boeing H, Aleksandrova K et al.. Coffee drinking and mortality in 10 European countries: a multinational cohort study. Ann Intern Med. 2017;167(4):236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park SY, Freedman ND, Haiman CA, Le Marchand L, Wilkens LR, Setiawan VW. Association of coffee consumption with total and cause-specific mortality among nonwhite populations. Ann Intern Med. 2017;167(4):228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu EA, Lazo M, Selvin E, Hamilton JP, Grams ME, Steffen LM, Coresh J, Rebholz CM. Coffee consumption and liver-related hospitalizations and deaths in the ARIC study. Eur J Clin Nutr. 2019;73(8):1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim BH, Park YS, Noh HM, Sung JS, Lee JK. Association between coffee consumption and renal impairment in Korean women with and without diabetes: analysis of the fourth Korea National Health and Nutrition Examination Survey in 2008. Korean J Fam Med. 2013;34(4):265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Butt MS, Sultan MT. Coffee and its consumption: benefits and risks. Crit Rev Food Sci Nutr. 2011;51(4):363–73. [DOI] [PubMed] [Google Scholar]
- 31. Lew QJ, Jafar TH, Jin A, Yuan JM, Koh WP. Consumption of coffee but not of other caffeine-containing beverages reduces the risk of end-stage renal disease in the Singapore Chinese Health Study. J Nutr. 2018;148(8):1315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


