Significance
Studies have suggested a role for episodic memory in imagining future events and thinking creatively. Here, we tested the causal role played by episodic memory in future imagining and creative thinking by using fMRI-guided transcranial magnetic stimulation to disrupt neural activity in the hippocampus, a brain region involved in episodic memory. After transcranial magnetic stimulation, participants generated fewer episodic details when imagining future events and produced fewer creative ideas. fMRI analyses revealed that these behavioral reductions were linked to a reduction in hippocampal activity. Our findings have implications for brain targets and interventions to alleviate declines in memory, imagination, creativity, and other sorts of adaptive episodic functioning.
Keywords: episodic memory, creativity, imagination, TMS, fMRI
Abstract
Prior functional magnetic resonance imaging (fMRI) studies indicate that a core network of brain regions, including the hippocampus, is jointly recruited during episodic memory, episodic simulation, and divergent creative thinking. Because fMRI data are correlational, it is unknown whether activity increases in the hippocampus, and the core network more broadly, play a causal role in episodic simulation and divergent thinking. Here we employed fMRI-guided transcranial magnetic stimulation (TMS) to assess whether temporary disruption of hippocampal brain networks impairs both episodic simulation and divergent thinking. For each of two TMS sessions, continuous θ-burst stimulation (cTBS) was applied to either a control site (vertex) or to a left angular gyrus target region. The target region was identified on the basis of a participant-specific resting-state functional connectivity analysis with a hippocampal seed region previously associated with memory, simulation, and divergent thinking. Following cTBS, participants underwent fMRI and performed a simulation, divergent thinking, and nonepisodic control task. cTBS to the target region reduced the number of episodic details produced for the simulation task and reduced idea production on divergent thinking. Performance in the control task did not statistically differ as a function of cTBS site. fMRI analyses revealed a selective and simultaneous reduction in hippocampal activity during episodic simulation and divergent thinking following cTBS to the angular gyrus versus vertex but not during the nonepisodic control task. Our findings provide evidence that hippocampal-targeted TMS can specifically modulate episodic simulation and divergent thinking, and suggest that the hippocampus is critical for these cognitive functions.
Episodic memory (i.e., the ability to remember specific events from the past) (1) is a constructive process whereby the individual elements of a past event are linked together at the time of retrieval (2, 3). During the past decade numerous studies have indicated that there are neurocognitive similarities between episodic memory and episodic simulation (i.e., the ability to imagine a novel and specific future episode) (for reviews, see refs. 4, 5). These similarities have been taken as support for the constructive episodic simulation hypothesis (6, 7). According to this hypothesis, the cognitive and neural similarities reflect to a large extent the role of episodic memory in supporting simulations of the future, in that episodic retrieval processes allow for the flexible recombination of elements of past episodes to construct novel future events.
Several branches of evidence support this hypothesis. Studies employing functional magnetic resonance imaging (fMRI) demonstrate the existence of a common set of neural regions (i.e., the “core network”) engaged during episodic remembering and imagining (for review, see ref. 8). This set of regions, which largely overlaps with the default network (9), includes the medial temporal lobe (e.g., hippocampus), lateral parietal cortex (e.g., bilateral angular gyrus [AG]), medial parietal cortex, and medial prefrontal cortex (among others). Because fMRI is a correlational technique, recent studies have also employed repetitive transcranial magnetic stimulation (rTMS) to demonstrate a causal role of the core network during episodic memory retrieval and simulation. In one study (10), rTMS was applied to the left AG. Following rTMS disruption to the left AG, relative to rTMS to a control site (vertex), participants generated fewer episodic/internal details (the who, what, when, and where of an episode) (11) when remembering past and imagining novel future episodes. Critically, this effect was selective to episodic information, as performance in a nonepisodic control task did not differ as a function of TMS site. In addition, behavioral studies support the idea that remembering and imagining share common cognitive processes. One line of work used an episodic specificity induction (ESI), or brief training in recollecting specific details from a recent experience, to examine the role of episodic retrieval in related cognitive processes, such as simulating future experiences (for review, see ref. 12). After receiving the ESI, relative to a control induction, participants subsequently remember and imagine episodes in greater episodic detail but show no differences on tasks that do not draw on episodic processing (e.g., describing a picture or defining and comparing words) (13, 14).
Related studies have revealed a role for episodic retrieval in other cognitive functions that do not require episodic memory, but may still be influenced by it. One such cognitive function is divergent thinking, or the ability to generate creative ideas by combining diverse kinds of information in novel ways (15). For example, 1) episodic memories are sometimes drawn upon during divergent thinking (16–18), 2) patients with memory impairments show deficits on divergent thinking (19), 3) participant-level correlations have been observed between the amount of episodic detail in imagined future episodes and performance on divergent thinking (20), and 4) the ESI selectively boosts performance on divergent thinking (21, 22) relative to tasks that do not engage divergent thinking. Paralleling these behavioral links, several neuroimaging studies have demonstrated that core network regions, such as the hippocampus, are engaged during episodic memory, simulation, and divergent thinking (23–25). fMRI studies have also demonstrated that the hippocampus, among other core network regions, is linked to ESI-related increases in episodic detail production in imagined future events and divergent thinking (26, 27).
Taken together, these findings suggest that episodic retrieval plays a role in supporting episodic simulation and divergent thinking, but multiple caveats call this conclusion into question. First, although episodic memories are drawn upon during divergent thinking, they appear infrequently (e.g., ref. 16). Second, the data from neuropsychological studies (e.g., ref. 19) require interpretive caution because patients often exhibit deficits in both episodic and semantic memory. Third, ESI-related evidence stems from a global manipulation of episodic processing, and it is also unclear whether the hippocampus, or other brain regions involved in episodic retrieval, underlie the manipulation’s efficacy (26, 27). Fourth, given the correlational nature of fMRI, it is unknown whether any of the common neural activity observed across remembering, imagining, and divergent thinking reflects a necessary role of the common regions (e.g., ref. 23).
The aim of the present study was to provide a causal test of the role of hippocampally dependent episodic retrieval in future imagining and creative thinking by using fMRI-guided TMS in the form of continuous θ-burst stimulation (cTBS) to disrupt neural activity in the hippocampus, which has been consistently engaged during episodic memory, simulation, and divergent thinking. For each of two TMS sessions, cTBS was applied to either a control site (vertex) or to a left AG target region. Critically, the target region was identified on the basis of a participant-specific resting-state functional connectivity analysis with a hippocampal seed region previously associated with episodic memory, simulation, and divergent thinking (8, 25). Previous research has shown that TMS in the vicinity of our target region impacts activity in connected regions, including the hippocampus (28). Following application of cTBS, participants underwent fMRI and performed three tasks. In each task, participants were shown an object word and either imagined a related personal event in the next few years (episodic simulation task), generated creative and unusual object uses (the alternate uses task [AUT], or divergent thinking task), or generated associated objects and their definitions (nonepisodic control task). Following scanning, participants verbally generated their thoughts for each task cue.
Based on our prior rTMS findings (10), we predicted that cTBS to a core network region (the left AG) would produce a selective impairment in the generation of episodic details during simulation, with no deficit in the nonepisodic control task. We then tested whether cTBS would also produce a deficit in divergent thinking. Critically, we hypothesized that cTBS-related differences in the episodic detail of imagined events and the number of creative uses produced for the divergent thinking task would be linked to changes in neural activity in the hippocampus (and possibly the core network more broadly). Finally, we employed a resting-state functional connectivity analysis with the hippocampal coordinate as the seed and the TMS site as the target. This analysis assessed whether cTBS caused the left AG and the hippocampus to become less synchronized with each other, thus demonstrating that reduced coupling between these two brain regions led to reduced ability to think creatively and to imagine an episodic future event.
Results
Our primary analyses examined the influence of cTBS to the AG relative to the control site (vertex) (10, 29) on behavioral performance (episodic simulation, divergent thinking, and nonepisodic control), resting-state fMRI connectivity involving the hippocampus, and univariate fMRI effects during task performance in the hippocampus and other brain regions.
TMS Behavioral Results.
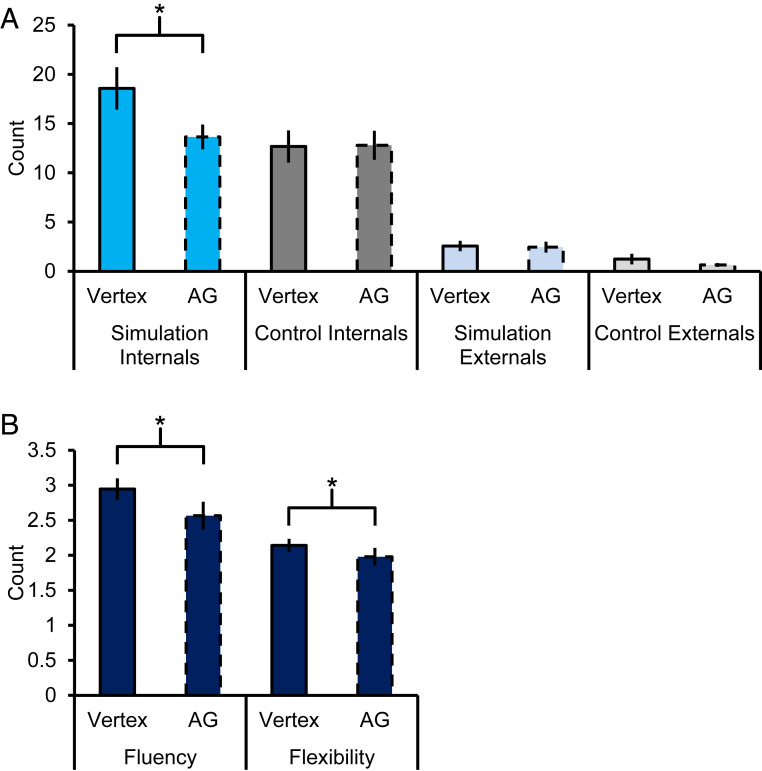
To replicate our prior findings and serve as a manipulation check for the divergent thinking analysis, we first examined impacts of cTBS on episodic simulation to assess whether the manipulation led to expected decrements in episodic detail production during episodic simulation but not the nonepisodic control task. An ANOVA with factors of cTBS site (vertex and AG), Task (episodic simulation and nonepisodic control), and Detail (internal/episodic and external/nonepisodic) conducted on the generative responses collected during the postscan interview (Fig. 1A) revealed a significant three-way interaction [F(1, 17) = 12.77, P = 0.002, partial η2 = 0.43]. The ANOVA also revealed significant main effects of cTBS site, Task, and Detail [Fs(1, 17) > 10.21, Ps < 0.005, partial η2s > 0.38], as well as a cTBS site × Detail interaction [F(1, 17) = 7.35, P = 0.02, partial η2 = 0.30]. The Task × Detail interaction was not significant [F(1, 17) = 2.12, P = 0.16].
Fig. 1.
TMS-behavioral results. (A) Mean number of internal and external details for the simulation and control tasks produced following vertex cTBS and left AG cTBS. (B) Mean divergent thinking performance (measured as the total number of appropriate uses generated [fluency] and categories of appropriate uses [flexibility]) following vertex cTBS and left AG cTBS. Error bars denote mean (±1 SE). Asterisks indicate significant results (see TMS Behavioral Results for details).
To decompose the significant three-way interaction, two follow-up ANOVAs were conducted with factors cTBS site and Task: one conducted on the internal details (Fig. 1A, first four bars) and another on the external details (Fig. 1A, last four bars). The ANOVA conducted on the internal details (Fig. 1A, first four bars) revealed significant main effects of cTBS site, with more internal details produced following vertex vs. AG cTBS [F(1, 17) = 13.35, P = 0.002, partial η2 = 0.44], and Task, with more details produced for the episodic simulation vs. nonepisodic control task [F(1, 17) = 6.19, P = 0.02, partial η2 = 0.27]. Critically, the cTBS site by Task interaction was significant [F(1, 17) = 13.28, P = 0.002, partial η2 = 0.44]. Follow-up t tests revealed that, following cTBS to the AG vs. vertex, participants generated fewer internal details when imagining a future episode [t(17) = 3.90, P = 0.001, d = 0.92] with no analogous decrement in internal detail production for the nonepisodic control task (t < 1). To confirm that this decrement was selective to internal/episodic detail production, we conducted a follow-up ANOVA with factors cTBS site and Task conducted on only the external details (Fig. 1A, last four bars) and found only a main effect of Task, with more external details produced for the episodic simulation vs. nonepisodic control task [F(1, 17) = 19.78, P < 0.001, partial η2 = 0.54]; all other ANOVA results were nonsignificant [Fs(1, 17) < 1.78, Ps > 0.20]. Taken together, these findings replicate our prior findings (10) and indicate that hippocampal-targeted cTBS through the AG (vs. vertex) selectively impaired the generation of internal/episodic details during episodic simulation.
We then tested for cTBS-related effects on divergent thinking by assessing the effect of cTBS on the generative responses produced for the AUT (i.e., creative uses) in the postscan. Here, we focused on two common metrics of divergent thinking performance: fluency and flexibility (the latter being a more stringent criterion for a use) (21, 27). Following cTBS to the AG vs. vertex, participants generated significantly fewer uses (i.e., were less fluent) (Fig. 1 B, Left) [t(17) = 3.14, P = 0.006; d = 0.74], and these uses fit into significantly fewer distinct and appropriate categories (i.e., were less flexible) (Fig. 1 B, Right) [t(17) = 2.24, P = 0.04, d = 0.53] (for an analysis of other metrics of divergent thinking, see SI Appendix). We also conducted analyses on the in-scan and additional postscan ratings to examine task compliance, phenomenological characteristics, and the influence of cTBS (SI Appendix); there were no impacts of cTBS on subjective ratings.
fMRI-TMS Results.
Resting-state analyses.
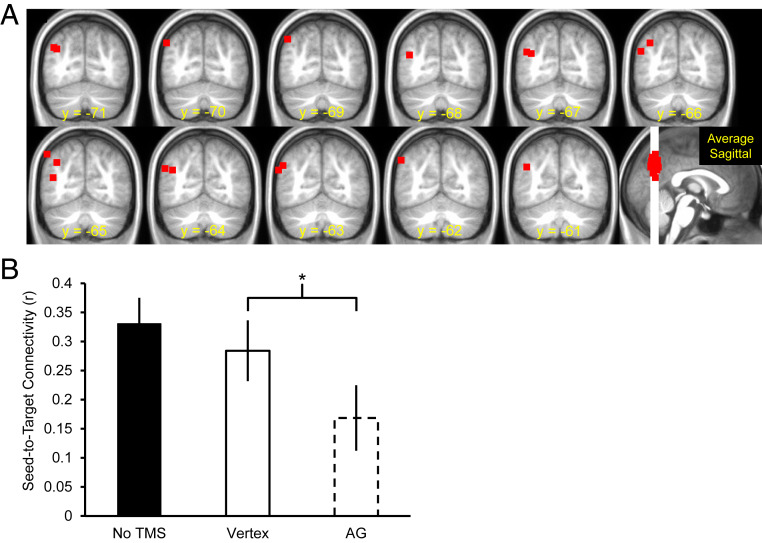
Moving from behavioral to fMRI effects of cTBS, we conducted a resting-state analysis to examine whether seed-to-target (i.e., hippocampus-to-AG) connectivity decreased after cTBS to the AG vs. vertex (Fig. 2A shows the cTBS targets across the entire sample and Fig. 2B shows mean seed-to-target connectivity as a function of the three cTBS sessions). In this analysis, we extracted the correlation value at the AG coordinate stimulated on an individual participant basis as a function of the three cTBS sessions (no-cTBS, vertex, and AG). For each session (no-cTBS, vertex cTBS, and AG cTBS), mean connectivity between the hippocampal seed and AG target was greater than 0 [ts(17) > 2.99, Ps < 0.008, ds > 0.70]. Following cTBS to the AG, there was a significant reduction in connectivity between the AG and the left anterior hippocampal seed vs. cTBS to the vertex [t(17) = 2.33, P = 0.03, d = 0.61]. Seed-to-target connectivity did not differ between the no-cTBS session and cTBS to the vertex (t < 1).
Fig. 2.
(A) cTBS target sites for each of the 18 participants overlaid on the across-participant mean T1-weighted anatomical image. Each red square denotes the cTBS target for each participant. The coronal slices are spaced every 1 mm with the most posterior (Upper Left) and anterior (Lower Right) corresponding to y = −71 and y = −61, respectively. (B) Group seed-to-target resting-state connectivity as a function of cTBS site. Error bars denote mean (±1 SE) connectivity. Asterisks indicate significant results (see fMRI-TMS Results, Resting-state analyses for details).
We also tested for the specificity of the effect of cTBS on connectivity between the hippocampal seed and AG target site. In this analysis, we examined whether cTBS to the AG relative to the vertex also changed connectivity between the hippocampal seed and two other known resting-state fMRI networks, the frontoparietal control network (FPCN) and the visual attention network (VAN; for full details, see SI Appendix). An ANOVA on the correlation values with factors Network (hippocampus-to-FPCN, hippocampus-to-VAN, hippocampus-to-AG) and cTBS site (vertex, AG) revealed main effects of Network and TMS site (Fs > 5.74, Ps > 0.03, partial η2 > 0.25), and critically, a significant Network × cTBS site interaction [F(2, 34) = 4.32, P = 0.02, partial η2 = 0.20]. Follow-up pairwise comparisons revealed that connectivity between the hippocampus and FPCN or VAN did not change as a function of cTBS site [ts(17) < 1.40, Ps > 0.18], relative to the significant change in hippocampus-to-AG connectivity reported above. The significant interaction indicates that cTBS to the AG reduced connectivity specifically between the seed (hippocampus) and target (AG), with no detectable changes between the hippocampus and other known resting-state networks (FPCN or VAN).
Univariate analyses.
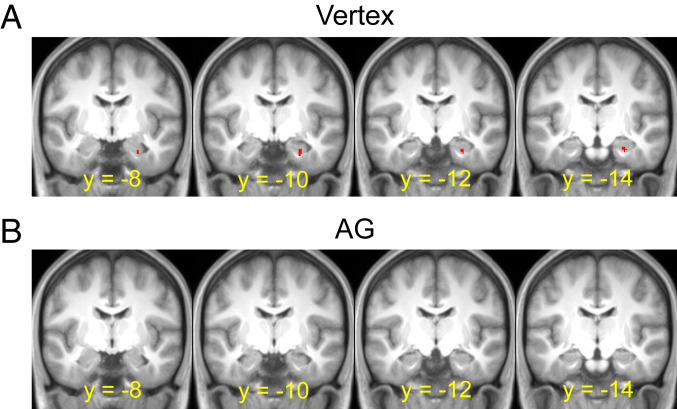
The following fMRI analyses were aimed at identifying univariate cTBS effects specific to the episodic simulation and divergent thinking tasks: a neural analog of the observed behavioral deficits in episodic simulation and divergent thinking following cTBS to the AG vs. vertex. To achieve this aim, we implemented an interaction contrast (episodic simulation + divergent thinking > nonepisodic control for the vertex > AG cTBS contrast), which identifies neural regions where task effects common to both episodic simulation and divergent thinking (i.e., episodic simulation + divergent thinking > nonepisodic control) differ as a function of cTBS site (Fig. 3). Unless otherwise noted, all results are derived from statistical parameters that survive a significance threshold of P < 0.05 corrected for multiple comparisons (see Materials and Methods).
Fig. 3.
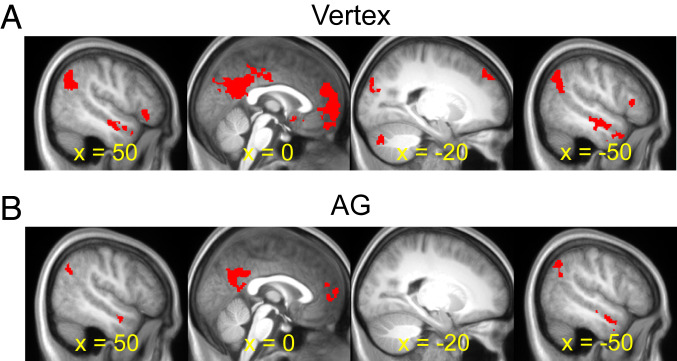
fMRI-TMS results: Hippocampus. (A) Shown in red are hippocampal regions demonstrating greater activity for the episodic simulation and divergent thinking tasks relative to the nonepisodic control task following cTBS to the vertex. (B) Hippocampal regions demonstrating greater activity for the episodic simulation and divergent thinking tasks relative to the nonepisodic control task following cTBS to the AG. Results are overlaid on the across-participant mean T1-weighted anatomical image.
Planned contrasts within the hippocampus.
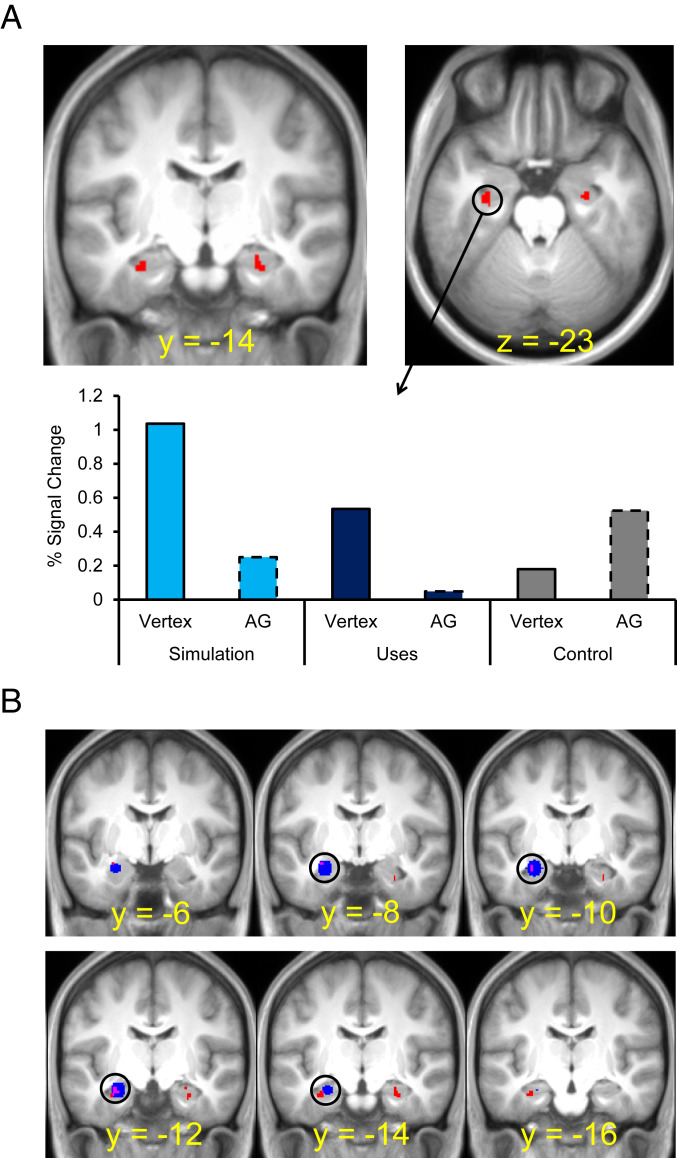
We first tested whether hippocampal cTBS effects could be identified that were common to the episodic simulation and divergent thinking tasks. Following cTBS to the vertex, common activity during episodic simulation and divergent thinking (relative to nonepisodic control) was observed in the hippocampus (peak voxel of x = 27, y = −13, z = −24, peak Z of 3.33, 26 voxels) (Fig. 3A). Following cTBS to the AG, there was a reduction of the activity observed vs. cTBS to the vertex (Fig. 3B). Critically, we then tested whether hippocampal activity showed an interaction as a function of cTBS site and task (i.e., reduced recruitment for constructing imagined events and generating creative uses following AG cTBS vs. vertex cTBS, with the opposite effect for the control task). The interaction contrast identified two hippocampal clusters, one in each hemisphere, falling primarily in the anterior hippocampus (Fig. 4A, Upper, and Table 1). To further characterize the results, we illustrate the parameter estimates for each cTBS site and task (Fig. 4 A, Lower) extracted from the left hippocampus. These estimates parallel the behavioral cTBS deficit and reveal cTBS univariate effects for episodic simulation and divergent thinking but not the nonepisodic control.
Fig. 4.
fMRI-TMS results: Hippocampus. (A) Shown in red are hippocampal regions demonstrating a cTBS site by task interaction (i.e., reduced recruitment for constructing imagined events and generating creative uses following AG cTBS compared with cTBS to the vertex with the opposite effect for the control task). The chart depicts percent signal change (extracted from the region’s peak voxel) for each task and cTBS site. Note that error bars are not plotted as a result of potential noise, and significance tests were not run on these data. (B) The fMRI-cTBS effect identified in the hippocampus (shown in red) overlapped the hippocampal seed region targeted with cTBS (shown in blue; i.e., the coordinate employed in the seed-to-voxel analysis to identify the cTBS target region in the left AG). Overlap is shown in magenta. Results are overlaid on the across-participant mean T1-weighted anatomical image.
Table 1.
Loci of fMRI-TMS effects
| MNI coordinates | Peak Z | Number of above-threshold voxels | Region | |||
| X | y | z | ||||
| Hippocampus | −31 | −17 | −22 | 3.99 | 36 | Left hippocampus |
| 27 | −13 | −22 | 3.97 | 24 | Right hippocampus | |
| Whole brain | 42 | 50 | −12 | 5.39 | 243 | Right inferior frontal gyrus |
| −27 | 7 | 17 | 5.30 | 111 | Left frontal operculum | |
| 42 | −7 | 41 | 3.65 | 228 | Right middle frontal gyrus | |
| 5 | 55 | −24 | 4.83 | 372 | Right ventromedial prefrontal cortex | |
| −12 | 51 | −16 | Left ventromedial prefrontal cortex | |||
| 13 | 0 | 24 | 4.59 | 130 | Right caudate nucleus | |
| −5 | −42 | −50 | 4.52 | 231 | Left cerebellum | |
| 5 | −39 | −38 | Right cerebellum | |||
| −21 | −78 | −33 | 4.39 | 175 | Left cerebellum | |
| 22 | 34 | −7 | 4.33 | 162 | Right orbital gyrus | |
| 56 | −22 | −19 | 4.33 | 227 | Right inferior temporal sulcus | |
| 59 | −13 | −17 | Right middle temporal gyrus | |||
| 47 | −24 | −27 | Right inferior temporal gyrus | |||
| 13 | −49 | 27 | 4.31 | 226 | Right posterior cingulate/retrosplenial cortex | |
| 44 | −53 | 25 | Right angular gyrus | |||
| −9 | −41 | 63 | 4.28 | 110 | Left paracentral lobule | |
| 3 | −30 | 64 | Right paracentral lobule | |||
| −17 | −52 | −21 | 4.27 | 177 | Left cerebellum | |
| 18 | −76 | 54 | 4.21 | 98 | Right superior parietal lobule | |
| 17 | −85 | 42 | Right superior occipital gyrus | |||
| 37 | 4 | 20 | 4.19 | 166 | Right frontal operculum | |
| −65 | −30 | −24 | 4.19 | 189 | Left inferior temporal gyrus | |
| −55 | −20 | −17 | Left middle temporal gyrus | |||
| −10 | −80 | 29 | 3.99 | 172 | Left superior occipital gyrus | |
| 13 | 46 | 15 | 3.94 | 256 | Right dorsomedial prefrontal cortex | |
| 12 | 65 | 12 | Right anterior prefrontal cortex | |||
| 10 | 44 | 39 | 3.77 | 158 | Right superior frontal gyrus | |
| −2 | −68 | 5 | 3.17 | 96 | Left extrastriate cortex | |
MNI, Montreal Neurological Institute. Coordinates for cluster subpeaks that lie in distinct cortical regions are listed directly below relevant peak cluster.
Of particular interest, the interaction effect identified in the left anterior hippocampus overlapped the hippocampal seed targeted via the seed-to-target resting-state analysis that was identified in previous metaanalyses on divergent thinking (25) and episodic memory and simulation (8) (Fig. 4B). These findings suggest that cTBS to the AG identified on an individual participant basis not only reduced functional connectivity to the hippocampus during rest (Fig. 2B), but also reduced neural activity during tasks that have been hypothesized to engage the hippocampus (i.e., episodic simulation and divergent thinking).
To further link the key cTBS-related behavioral and hippocampal results, the behavioral scores were entered as modulators of interest during the construction phase of imagined events, divergent thinking, and control tasks (for similar procedures, see ref. 26). For the behavioral index, episodic/internal details on the imagine task, fluency scores on the divergent thinking task, and internal details on the control task were used. Critically, cTBS-related reductions in hippocampal activity were observed during the construction of imagined events and divergent thinking over the control task following cTBS to the vertex vs. AG (peak voxel of x = −22, y = −10, z = −22; peak Z of 2.98; these effects were observed at an uncorrected two-tailed threshold of P < 0.005; for full details, see SI Appendix). Akin to the main univariate analysis reported above, this modulatory effect overlapped the hippocampal seed targeted via the seed-to-target resting-state analysis. The parametric modulation analysis indicates that the key cTBS-related behavioral effects (reduced episodic details and fewer creative uses following cTBS to the vertex vs. AG) modulated the key cTBS-related neural effect (i.e., reduced hippocampal activity during episodic simulation and divergent thinking following cTBS to the vertex vs. AG).
Whole-brain analyses.
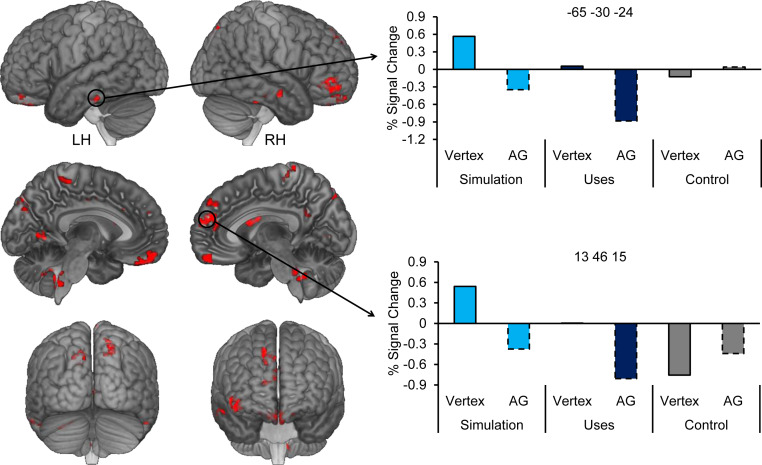
Along with impacts of cTBS on hippocampal connectivity and activity, we probed further neural differences (shown in Fig. 5) with an episodic simulation + divergent thinking > nonepisodic control contrast as a function of each cTBS site across the whole-brain. After cTBS to either site (Fig. 5 A and B, for vertex or AG, respectively), there was common recruitment during both episodic simulation and divergent thinking in core network regions (e.g., refs. 8, 25). Critically, participants exhibited significantly reduced activation in several core network regions following cTBS to AG vs. vertex for episodic simulation and divergent thinking relative to the nonepisodic control (Fig. 6), which included the ventromedial prefrontal cortex, bilateral lateral temporal cortex (i.e., right and left inferior and middle temporal gyri/sulci), and posterior cingulate/retrosplenial cortex, among others (for a full list, see Table 1). Common cTBS univariate effects were also observed in regions comprising the frontoparietal control network, such as the inferior frontal gyrus, regions previously associated with episodic simulation and divergent thinking (e.g., refs. 27, 30, 31), and thought to support cognitive control processes required for the recombination of disparate episodic details. Illustrated are the parameter estimates extracted from two representative regions, the lateral temporal cortex and medial prefrontal cortex. In each region, a cTBS effect (i.e., vertex > AG) was present for both the episodic simulation and divergent thinking tasks but not the nonepisodic control.*
Fig. 5.
fMRI-TMS results: Whole-brain. (A) Shown in red are whole-brain regions demonstrating greater activity for the episodic simulation and divergent thinking tasks relative to the nonepisodic control task following cTBS to the vertex. (B) Whole-brain regions demonstrating greater activity for the episodic simulation and divergent thinking tasks relative to the nonepisodic control task following cTBS to the AG. Results are overlaid on the across-participant mean T1-weighted anatomical image.
Fig. 6.
fMRI-TMS results: Whole-brain. Shown in red are whole-brain regions demonstrating a cTBS site by task interaction (i.e., reduced recruitment for constructing imagined events and generating creative uses following cTBS to the left AG compared with cTBS to the vertex with the opposite effect for the control task). The charts depict percent signal change extracted from peak voxels within two representative clusters within the left lateral temporal cortex (x = −65, y = −30, z = −24) and medial prefrontal cortex (x = 13, y = 46, z = 15) for each task and cTBS site. Note that error bars are not plotted as a result of potential noise, and significance tests were not run on these data. Results are projected onto a cortical surface using the skull-stripped template of MRIcroGL (see ref. 32).
Discussion
We tested the causal role played by episodic memory retrieval in future imagining and creative thinking by using cTBS to the parietal cortex (left AG) and targeting the hippocampus, two brain regions involved in episodic memory. The left AG region targeted for cTBS was identified on an individual participant basis employing a baseline (no-cTBS) resting-state functional connectivity analysis with a left anterior hippocampal seed region previously associated with episodic memory, episodic simulation, and divergent creative thinking. Replicating our prior findings, compared with cTBS to the vertex, cTBS to the AG region reduced the number of episodic details produced for the simulation task (10). Critically, cTBS to the AG relative to the vertex also reduced the number of creative uses produced for the divergent thinking task. In contrast, performance in the nonepisodic control task did not statistically differ as a function of cTBS site. Analysis of the fMRI data revealed a selective and simultaneous reduction in hippocampal activity during episodic simulation and divergent thinking following cTBS to the AG vs. vertex but not during the nonepisodic control task (i.e., a task that engages semantic retrieval and imagery but requires little divergent thinking or episodic processing). This hippocampal cTBS univariate effect overlapped the region targeted via the resting-state connectivity analysis. In addition, resting-state analyses revealed that, following cTBS to the AG vs. vertex, there was a significant reduction in functional connectivity between the left AG and hippocampus, suggesting that reduced communication between these two core network regions led to reduced ability to think creatively and to imagine an episodic future event. Taken together, our findings provide evidence that hippocampal-targeted cTBS can specifically modulate episodic simulation and divergent thinking, and suggest that the hippocampus supports a common and critical process during these cognitive functions. Further support for a brain–behavior link was identified via the parametric modulation analysis, which revealed that cTBS-related behavioral differences in episodic simulation and divergent thinking modulated cTBS-related hippocampal activity. This latter finding should be taken as preliminary, however, as it only emerged at an uncorrected statistical threshold.
The present findings have implications for our understanding of the cognitive neuroscience of creativity. The dominant view is that creativity, specifically divergent thinking is largely, if not completely, supported by semantic memory (e.g., refs. 33–35). This view has been supported by neural evidence indicating that regions associated with semantic control and retrieval are recruited during divergent thinking tasks (e.g., refs. 36–38; for review, see ref. 25). Although some evidence suggests that episodic memory and divergent thinking are supported by common cognitive and neural underpinnings, such evidence has its weaknesses (see Introduction). The current causal findings add to the small but growing body of studies indicating that the dominant view needs revision to account for the role of episodic retrieval in divergent creative thinking. Our findings are also consistent with previous literature indicating that the role of episodic retrieval in divergent thinking is best captured by quantity but not quality measures. For example, and akin to the present cTBS findings, the ESI primarily impacts fluency and flexibility metrics of divergent thinking but not creativity ratings (21, 22, 27). Taken together, the present and previous findings suggest that episodic retrieval can be considered a component process of divergent thinking, likely impacting the production of creative output, and provide support for the idea that the hippocampus, and core network more broadly, contributes to performance on generative tasks that nominally involve retrieval and reconstruction of episodic information for completion (5, 39; for related perspectives, see refs. 40–42).
There are several limitations of the present study that deserve mention. First, we failed to identify any cTBS-related univariate effects as a function of episodic simulation and divergent thinking in the region directly stimulated, the left AG. Such a finding would suggest that cTBS has no effect on underlying neural activity at the site of application, and therefore call into question our prior claim that the left AG plays a necessary role during episodic memory and simulation (10; see also, ref. 29). However, several methodological differences between the present study and our previous one (10) could explain the present null finding. First, the present study utilized a different TMS protocol (i.e., a cTBS protocol vs. the prior repetitive 1-Hz stimulation protocol), and the cTBS target was identified on an individual participant basis and varied in spatial location across participants (e.g., spanning 10 mm in the y dimension; in our prior study, the target site was identical across participants) (Fig. 2A). The relatively large spatial distribution of the cTBS target site may have limited the ability to identify a common across-participant univariate cTBS effect. Relevant to this point, the cTBS seed location (i.e., left anterior hippocampus) was held constant across participants, and was also a region where univariate cTBS effects were observed (Fig. 4B). These findings suggest that there may be a relationship between the ability to detect TMS-related task univariate effects and the variability of the target site across participants. To test this interpretation, we extracted univariate activity for each of the three tasks from the individualized target AG location as a function of the cTBS sites (both using a single 1.7-mm voxel as well as a 6-mm sphere). There were numeric decreases in univariate activity for both the simulation and divergent thinking tasks, with the reverse for the control task, following AG cTBS relative to vertex cTBS, but these differences were not significant (i.e., there was no significant reduction in univariate activity following AG cTBS relative to vertex cTBS for both the simulation and divergent thinking tasks [ts(17) < 1.08, P = 0.30]). Most important, cTBS did result in a significant reduction in seed-to-target connectivity, which provides evidence that cTBS had an effect at the sites of interest (i.e., left AG and hippocampus).
A second limitation stems from the fact that the resting-state scans were acquired after the functional/task runs (see also refs. 26, 27). Because cTBS affected neural activity during the task runs, it is unclear whether the change in seed-to-target connectivity was a result of cTBS or the change in task performance/neural activity as a result of cTBS (cf. ref. 43). A final limitation stems from the lack of a cTBS effect on subjective vividness ratings. We have provided recent evidence that the hippocampus (relative to other core network regions) plays a privileged role in supporting the subjective experience of episodic content via self-rated vividness (44, 45). Although the present null finding casts doubt on the link between hippocampal activity and vividness that was based on correlational fMRI data, any null effect should be treated with caution as it can be accounted for in any number of ways (e.g., low power). These limitations should be addressed in future work.
The present study employed a cTBS protocol that was assumed to be inhibitory and therefore produce a decrement in behavioral performance. This assumption is supported by prior work showing that cTBS reduces cortical excitability (46) as well as univariate fMRI activity (47). In addition, cTBS disrupts behavioral performance in autobiographical memory tasks (29, 48). The present and earlier findings indicating that cTBS can be used to produce inhibitory behavioral effects are inconsistent with some prior findings showing that application of cTBS enhances memory performance (49, 50). One difference between the present observations and prior findings of enhancement is a difference in the control cTBS condition. Here, the control condition was cTBS to the vertex [akin to previous studies also reporting decreases in behavioral performance (29, 48)]. In the studies that have reported an increase in performance from cTBS, the control condition was either TMS to the primary somatosensory cortex (49) or sham TMS (50). An additional possibility raised by Hebscher and Voss (51) is that studies reporting behavioral increases from cTBS used complex visual memoranda with laboratory-based episodic memory paradigms, which may depend heavily on hippocampal retrieval processes (i.e., pattern completion) with relatively little necessary involvement of the AG. Thus, if stimulation were to disrupt local processing by the AG yet enhance downstream processing by the hippocampus, the disruptive effect of stimulation on episodic processing in the present study could reflect disruption of AG involvement in generating the retrieval cues that are presumably used by the hippocampus to support performance. In contrast, in previous studies finding behavioral enhancement, such cues were provided by the visual display and so beneficial effects of stimulation on the hippocampal contribution could be observed. Additional research is needed to clarify this issue.
An important point about the present pattern of results is that the effect of cTBS on neural activity was not specific to the hippocampus. As illustrated in Fig. 6, effects of cTBS on episodic simulation and divergent thinking extended to other core network regions, such as the medial prefrontal cortex, as well as noncore regions, such as the inferior frontal gyrus, regions also associated with both episodic simulation and divergent thinking. Here, we highlight the findings pertaining to the hippocampus given strong theoretical and experimental work demonstrating a link between this region and divergent thinking and episodic simulation (e.g., refs. 7, 8, 25, 39). An important avenue for future work will be to combine TMS with analytic approaches examining the direction of informational flow between regions such as effective connectivity (cf., ref. 52; see also refs. 30, 31). Finally, our analytical approach was aimed at identifying an interaction between cTBS site and task, in part to identify neural regions that tracked any cTBS behavioral effects. One drawback of this procedure is that the regions identified might be differentially sensitive to the episodic simulation and divergent thinking tasks themselves (e.g., in the hippocampus, although the average neural activity between simulation and divergent thinking was greater than in the control task following vertex cTBS, the magnitude of activity was numerically greater for simulation than divergent thinking) (Fig. 4A). This pattern of effects would suggest that the process or processes mediated by the hippocampus, although common, are engaged to a greater extent during simulation relative to divergent thinking (cf., ref. 23).
In conclusion, the present findings provide evidence indicating that episodic retrieval plays a causal role in supporting episodic simulation and divergent thinking through common hippocampal-mediated processes. It will be important for future studies to specify the precise hippocampal process or processes shared across episodic simulation and divergent thinking. In a prior study, for example, we employed multivoxel pattern analysis and demonstrated that the hippocampus supports the reinstatement of episode-specific information from memory during simulation (45). Such reinstatement may also support the generation of creative output during divergent thinking. Combining multivoxel pattern analysis with TMS may be a fruitful approach for specifying the hippocampal processes common not only to episodic simulation and divergent thinking, but also to other functions that benefit from episodic retrieval.
Materials and Methods
Participants.
Informed consent was obtained from all participants prior to participation, with protocol approval from the Institutional Review Board of Harvard University. Nineteen undergraduate and graduate students from the local community were consented. One person was excluded due to falling asleep in the scanner, leaving n = 18 in the analyzed sample (mean ± [1 SE] age of 21.2 ± 0.38 y; range of 19 to 26 y; 14 females). All participants self-reported to be native English speakers and right handed, with normal or corrected-to-normal vision. See SI Appendix for additional TMS-specific recruitment parameters. An a priori sample size of 18 was chosen based on recent fMRI-TMS within-participant studies of the left lateral parietal cortex and episodic memory (28, 53; see also refs. 10, 54), and to also provide a full counterbalanced set.
Experimental Design Overview.
A three-session within-participants design was used to assess the influence of inhibitory cTBS on hippocampal brain networks during episodic simulation and divergent thinking. Each session lasted ∼3 h and occurred on a different day (mean delay between each session was 5.5 d, with the restriction that all three sessions were done in ∼2 wk). In session 1, participants came to the laboratory for fMRI only, where they completed: 1) two task runs alternating between episodic simulation, divergent thinking, and nonepisodic control tasks; 2) a resting-state scan; and 3) an anatomic scan (in that order). On each task trial, participants viewed an object word cue, and for each cue generated a novel and specific future event (i.e., the episodic simulation task), alternate uses of the object (i.e., the divergent thinking task, AUT), or a sentence with typical associates of the object, their meanings, and definitions (i.e., the nonepisodic control task). Before scanning, participants were instructed on and practiced the three tasks. Sessions 2 and 3 involved cTBS and were similar to session 1, with the exception that an anatomic scan was not collected and, before participants entered the scanner, cTBS was applied to the control site (vertex) or the AG target. The AG target was identified on the basis of a resting-state functional connectivity analysis (i.e., a seed-to-voxel analysis) using the resting-state scan from session 1, with the left anterior hippocampus as the seed (i.e., a hippocampal coordinate previously associated with episodic memory, simulation, and divergent thinking; see below). Following fMRI data collection in each session, participants completed a postscan interview about their thoughts for each scanning cue. The order of cTBS site (vertex or AG) was counterbalanced across participants, and different object cue words were used across each session.
With the exception of the resting-state analyses, behavioral and fMRI analyses were restricted to effects between the vertex and AG cTBS sessions for two primary reasons. First, the order of cTBS session (i.e., no-cTBS, vertex, AG vs. no-cTBS, AG, vertex) was randomly assigned across participants (with half receiving vertex second and half AG second). Thus, differences associated with task familiarity (i.e., practice) between the vertex and AG cTBS sessions were controlled (relative to performance being expectedly lower in the no-cTBS session as it necessarily came first). Second, a comparison of data between the vertex and AG cTBS sessions controls for nonspecific cTBS effects (see also ref. 10). All results are collapsed across cTBS session order (i.e., vertex cTBS followed by AG cTBS, and vice versa). For all significant results (at the P < 0.05 level), we report the relevant effect sizes (partial η2 in the case of F tests, d for t tests) and in cases where P < 0.001, we report as such.
Experimental Materials and Procedure.
Main tasks.
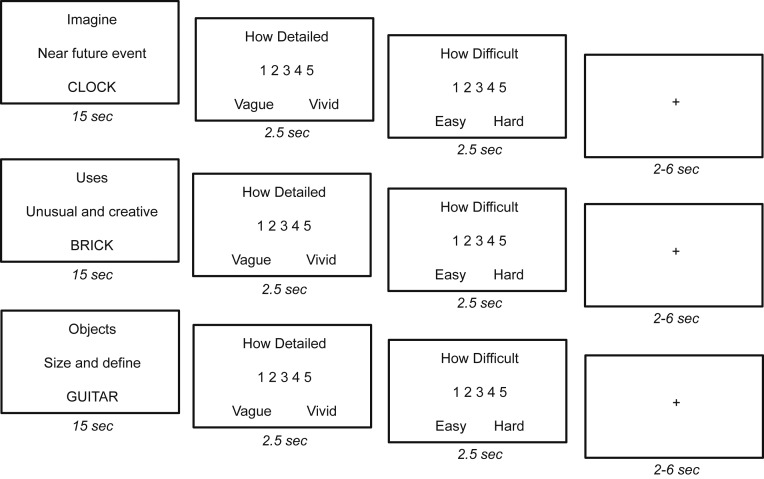
Further information on object cue words and task presentation are contained in SI Appendix. In each session, participants completed three fMRI runs. In two initial task runs, participants viewed 54 object cues and completed the main tasks (episodic simulation, divergent thinking, and nonepisodic control), and for the last run participants completed a resting-state scan. Each task run was ∼11 min and included a pseudorandom presentation of nine trials per task (for a total of 27 trials in each run) with the constraint that no more than two trials per task were presented in succession. For task trials (Fig. 7), the screen showed three lines of text for 15 s (first line: task; second line: instruction reminder; and third line: object cue word). On each trial, participants were presented with the construction–elaboration paradigm for 15 s (see below), followed by two separate ratings each presented for 2.5 s. The trial then ended with a variable fixation period (jittered at 2, 4, and 6 s). Ratings were made with a five-button response box in their left hand. Each fMRI run began and ended with a 5-s fixation period.
Fig. 7.
Experimental design. In each task participants were shown an object word for 15 s. For the episodic simulation task (Top), participants were asked to silently imagine a novel future experience related to the object. For the divergent thinking task (Middle), participants were asked to generate creative and unusual uses for the object. For the control task (Bottom), participants were asked to generate two associated objects, put them in a sentence by their relative size, and then generate definitions related to the objects. After each trial, participants rated the level of detail and difficulty on a 5-point scale. The trial ended with a variable fixation period.
For simulation trials (Fig. 7, Top), participants were instructed to silently imagine a novel and specific future episode from a first-person perspective related to the cue. Each event had to occur in the next few years, be specific in time and place over the course of a few minutes to an hour (i.e., an episodic event), and be as detailed as possible. With respect to construction–elaboration, participants pressed their thumb when the future event had come to mind (i.e., been constructed), following which they filled in all of the details and elaborated on the event until the first rating appeared. These details included—but were not limited to—the people, actions, and emotions of the event. At the end of each simulation trial, participants first rated the level of vividness or amount of subjectively rated detail associated with the event they had generated on a 5-point scale (“least vivid with no or few details” to “very vivid and highly detailed”), followed by the level of difficulty on a 5-point scale (“very easy” to “very difficult”).
For divergent thinking trials (Fig. 7, Middle), participants were instructed to silently generate as many unusual and creative uses related to the cue during its 15-s presentation. Participants were told to be both creative and to generate as many uses as possible given past research indicating that type of instruction can impact divergent thinking (e.g., ref. 55). With respect to construction–elaboration, participants pressed their thumb when they had generated their first creative use (i.e., constructed it), following which they were to generate as many additional uses as possible until the first rating appeared. At the end of each divergent thinking trial, participants first rated the level of vividness or amount of subjectively rated detail associated with the uses they had generated on a 5-point scale (“least vivid with no or few details” to “very vivid and highly detailed”), followed by the level of difficulty on a 5-point scale (i.e., “very easy” to “very difficult”).
For nonepisodic control trials (Fig. 7, Bottom), participants were first instructed to silently generate two associated objects related to the cue word, and then to put all three in a sentence sorting the objects by their relative physical size. With respect to construction–elaboration, participants pressed their thumb when they had generated the size sentence, following which they generated meanings and definitions for each object in as much detail as possible. Participants were instructed to focus on meaning and definition details, which included but were not limited to typical attributes, functions, and characteristics. They were also told to generate details as if they were coming from a dictionary or encyclopedia rather than related to themselves or their lives. At the end of each control trial, participants first rated the level of detail associated with the meanings and definitions they had generated on a 5-point scale (“least vivid with no or few details” to “very vivid and highly detailed”), followed by the level of difficulty (“very easy” to “very difficult”). Here, the nonepisodic control task was chosen as a comparison to both simulation and divergent thinking as it requires the search, retrieval, and integration of information related to an object cue, but did not involve the generation of a coherent episodic event or divergent thinking (see analogous control tasks in prior studies as a comparison to both episodic memory/simulation and divergent thinking) (8, 10, 21, 26, 27, 44, 56).
Resting state.
After the two main task runs, participants completed a resting-state scan for 7 min and 8 s (for similar procedures, see refs. 26, 27). During this scan, participants were shown a white central fixation cross on a black screen and were instructed to keep their eyes open for the scan.
Postscan interview.
Immediately after scanning, participants completed a postscan interview where they viewed each object cue from the scanner and were instructed to verbally generate whatever they had thought about for each cue (for similar postscan procedures, see refs. 14, 26, 27, 56). They were specifically instructed to not add anything they had not thought about. Each trial was self-paced, and participants hit the space bar when they had finished speaking. Following each trial, participants made additional ratings regarding their responses (more details are contained in SI Appendix).
Participants’ verbal responses were audio-recorded and transcribed for analysis. For the episodic simulation task, each future event was scored in accordance with the Autobiographical Interview (11). For the nonepisodic control task, meanings and definitions were also segmented into internal and external details (14, 26). Finally, for the divergent thinking task, we computed standard measures related to the quantity and quality of the uses (15, 20, 21, 27, 57). More details on scoring criteria are included in SI Appendix. All scoring was conducted by two raters who were blind to cTBS session (i.e., no-cTBS, vertex, or AG). We confirmed interrater reliability with a separate and third rater who scored a random selection of ∼25 responses randomly sampled from each task and cTBS session and obtained high interrater reliability (Cronbach’s α > 0.90 across the divergent thinking measures, and internal and external details for the episodic simulation and control task).
fMRI Acquisition and Analysis.
Univariate analysis.
Anatomic and functional images were acquired on a 3-Tesla Siemens scanner with a 32-channel head coil, and handled with standard preprocessing steps in Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, London, United Kingdom). SI Appendix contains additional information on scanning and preprocessing parameters. Univariate analysis was conducted using a two-stage mixed effects general linear model (GLM). In the first stage, neural activity associated with the construction and elaboration periods were modeled separately for each simulation, divergent thinking, and control trial using the canonical hemodynamic response function in SPM12 (for similar univariate analyses, see refs. 26, 56). The construction period was modeled with a delta/stick function 2 s after cue onset, and the elaboration period with a delta/stick function 2 s after participants made a button response (mean [±1 SE] construction time of 5.09 ± 0.39 s across tasks and cTBS conditions).† The associated blood-oxygen level-dependent (BOLD) response was modeled by convolving the boxcar functions with a canonical hemodynamic response function to yield regressors in a GLM that modeled the BOLD response for each event type. Each first-level model comprised 12 events modeling neural activity as a function of task (episodic simulation, divergent thinking, and nonepisodic control), cTBS site (vertex and AG), and trial phase (construction and elaboration). Two additional events of no-interest included trials without a response and the rating period. Six regressors modeling movement-related variance were also included in the first-level model (three for rigid-body translation and three for rotation). An autoregressive model of order 1 was used to correct for nonsphericity of the error covariance (58). The data across the two cTBS sessions were concatenated. Temporal smoothing was conducted before estimation of the parameter estimates using the default high-pass filter of 128 s. Note that, although we modeled the elaboration period of each trial, here we focus on construction-related neural activity as this portion of the trial has been associated with retrieval processes hypothesized to be shared across episodic simulation and divergent thinking (20, 26, 27, 39, 44, 45, 52).
In the second stage, parameter estimates for the six events of interest (i.e., construction-related activity for each of the three tasks and two cTBS sites) and for each participant were entered into a repeated-measures ANOVA with participants modeled as a random effect. To evaluate effects within the hippocampus, a mask was created by manually tracing the hippocampus using the across-participant mean normalized anatomical image based on standard anatomical landmarks (59; for similar approaches, see refs. 45, 60). An individual voxel two-tailed threshold of P < 0.005 was used for targeted a priori contrasts aimed at identifying cTBS effects (see below) within the hippocampus (44, 60–64). Correction for multiple comparisons (to P < 0.05) was affected by imposition of a cluster extent threshold (65, 66) of 16 voxels within the hippocampal mask. The threshold was estimated using a Monte Carlo simulation of 10,000 iterations with a full-width half-maximum (FWHM) of 7.65 mm estimated using the residual mean-square image of the participant-specific first-level models (i.e., the maximum FWHM across all images). For whole-brain analyses, an individual voxel two-tailed threshold of P < 0.005 was used, corrected to P < 0.05 by imposition of a cluster extent of 94 voxels (estimated using the same procedure as just described). All contrasts were conducted using the error term derived from the parent ANOVA.
Our aim was to assess whether the hippocampus in particular would demonstrate a common cTBS effect for both the episodic simulation and divergent thinking tasks. To formally test for the presence of such an effect, the vertex > AG contrast for the episodic simulation + divergent thinking > nonepisodic control was analyzed (i.e., the interaction contrast; for similar procedures, see refs. 26, 27). This interaction identifies regions exhibiting significant reductions in activity following cTBS to the AG compared with cTBS to the vertex during episodic simulation and divergent thinking, over the nonepisodic control task.
Resting-state analysis.
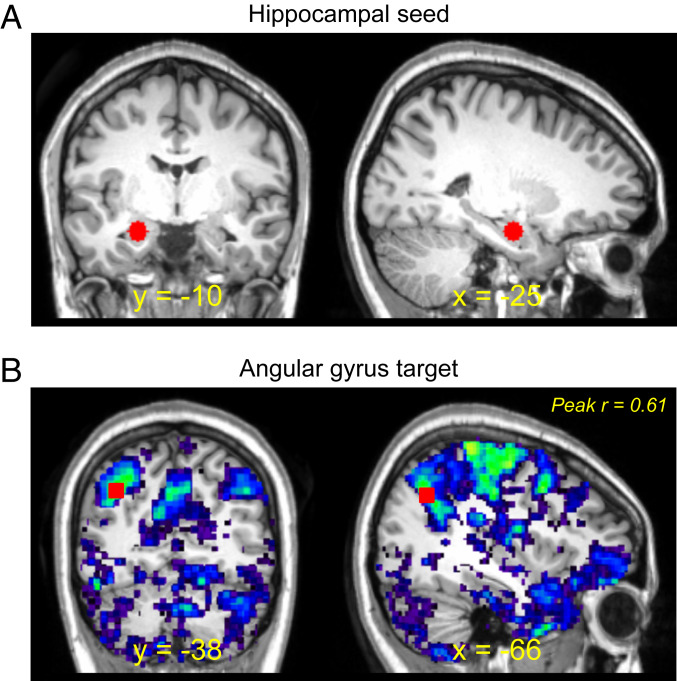
Resting-state images were acquired on the 3-Tesla Siemens scanner with a 32-channel head coil, and preprocessed according to standard guidelines in FSL 4.1.7 (FMRIB) and SPM12 (see details in SI Appendix). A seed-to-voxel connectivity analysis was carried out on an individual participant basis (67) to identify the left AG cTBS target functionally coupled to the hippocampal coordinate of interest. A 6-mm sphere was centered at the left hippocampal coordinate of x = −25, y = −10, z = −19 (Fig. 8A). This coordinate was selected on the basis of a prior metaanalysis demonstrating that the left hippocampus is associated with divergent relative to convergent thinking (25). Of importance, this coordinate overlapped the hippocampal cluster reported in a relevant metaanalysis to be jointly recruited during episodic memory and simulation (see figure 2 and table 3 in ref. 8). These findings support the assumption that the chosen hippocampal seed is associated with the three cognitive processes of interest. To create whole-brain correlation images for each individual participant and cTBS session, the averaged time series across all voxels comprising a seed region-of-interest (ROI) was used as the variable of interest with the time series corresponding to each voxel across the brain via Pearson’s correlation; all statistical analyses of correlation data were performed on Fisher’s z-transformations. Our resting-state analysis was based on two of our prior fMRI studies (26, 27). These two studies not only employed the same resting-state data acquisition protocols, but also the same resting-state analysis pipeline. Before conducting the present study, we ran pilot seed-to-target connectivity analyses on individual participant resting-state data from our prior studies to confirm that a 6-mm sphere was sufficient to identify left AG target sites on an individual participant basis. These pilot analyses determined that a 6-mm sphere together with the resting-state scanning protocol was sufficient, and therefore we adopted the identical procedures.
Fig. 8.
Representative-participant cTBS target identification. (A) On an individual participant basis, a seed-to-voxel analysis was conducted using the resting-state data from the no-cTBS session (i.e., session 1). A 6-mm sphere (shown in red) in the left anterior hippocampus was centered on a peak voxel previously associated with divergent thinking, episodic simulation, and episodic memory (x = −25, y = −10, z = −19; see fMRI Aquisition and Analysis, Resting-state analysis for details). (B) Whole-brain correlation images were created by using the averaged time series across all voxels comprising the seed and the time series corresponding to each voxel across the brain with Pearson’s correlation. The AG cTBS target (shown in red) was selected as the peak coordinate in the left AG demonstrating the greatest resting-state connectivity (i.e., Fisher-transformed correlation, r) and that fell within the left Brodmann area 39 (i.e., the left AG). Resting-state results are overlaid onto a representative participant anatomic image.
fMRI-TMS procedure.
Following similar procedures employed in prior fMRI-TMS studies targeting hippocampal networks through the AG (e.g., refs. 28, 49, 50, 53), the cTBS target was selected on an individual participant basis as the left AG coordinate with the strongest connectivity to the left hippocampal seed (i.e., highest correlation) (Fig. 8B). The coordinate had to fall within the left Brodmann area 39 (i.e., the left AG). Fig. 2A illustrates the cTBS targets across the entire sample (mean AG cTBS coordinate [±1 SE] of x = −45.6 ± 1.45, y = −65.9 ± 0.70, z = 33.1 ± 1.85). As in our prior study (10), the vertex was chosen as the control cTBS site. The vertex was identified on each participant’s anatomic image as the location at which the central sulci in each hemisphere met (for other studies employing the same control site, see refs. 29, 48, 68, 69).
A MagPro X100 Magnetic Stimulator equipped with a Cool-B65 A/P 75-mm coil was used to apply cTBS (46). The Brainsight (Rogue Research) neuronavigation system was used to apply the cTBS and allow real-time tracking of the TMS coil relative to physical head and linked anatomic image. Further details on the adopted cTBS approach and Brainsight neuronavigation system on a participant-to-participant basis are contained in SI Appendix. Following application of cTBS, participants were placed into the fMRI scanner. The functional and resting-state scan began ∼5 to 7 min and ∼22 to 25 min following cTBS offset, respectively. fMRI data acquisition was completed within 60 min (i.e., the assumed duration of the effects of cTBS) (46). Postscan data collection began ∼60 min following the cTBS offset. Although prior studies indicate that the effects of the current cTBS protocol dissipate within 60 min (46), no study has directly examined the timecourse of cTBS over the parietal cortex. We note that an analysis of additional postscan data revealed null effects of TMS (e.g., as reported in SI Appendix, Table S2, there were no TMS effects in rated plausibility of event, self-rated creativity, and so forth). These null effects suggest that the cTBS effects had dissipated. Importantly, we did replicate our original TMS study where participants verbally generated fewer episodic details during future imagining (with no deficit in a nonepisodic control task) directly following rTMS to the left AG relative to the vertex.
Data Availability.
Data and materials are available upon reasonable request.
Supplementary Material
Acknowledgments
We thank Ethan Harris for assistance with behavioral transcriptions; Zulkayda Mamat for assistance with scoring; Stephanie McMains for assistance in the resting-state analysis; Mark Eldaif for transcranial magnetic stimulation consultation; and Aleea Devitt for insightful comments. This research was supported by National Institute of Mental Health Grant R01MH60941 (to D.L.S.) and National Institute on Aging Grant F32AG059341 (to K.P.M.).
Footnotes
The authors declare no competing interest.
*Note that the interaction contrast does not ensure that the magnitude of the cTBS effects as a function of the two tasks of interests (episodic simulation and divergent thinking) are statistically equivalent in magnitude. The interaction contrast only indicates that the parameter estimates associated with neural activity following cTBS to the vertex for both episodic simulation and divergent thinking are numerically greater than the respective task conditions following cTBS to the AG (and the opposite for the non-episodic control). To ensure a statistically common cTBS effect, we employed the interaction contrast restricted to only the episodic simulation and divergent thinking as a function of cTBS site as an exclusive mask. That is, we statistically removed all voxels at the lenient threshold of P < 0.05 where the magnitude of the cTBS effect for episodic simulation differed from divergent thinking (and vice versa). Critically, the same peak clusters were identified both at the whole-brain level and in the hippocampus. The analysis confirms the reported commonality of the cTBS effect across the two tasks.
†An ANOVA with factors Task (simulation, divergent thinking, and control) by cTBS site (vertex and AG) on the construction times revealed solely a main effect of Task [F(2, 34) = 10.21, P < 0.001, partial η2 =0.38] with construction times for the control (5.65 ± 0.47 s) greater than both simulation (4.69 ± 0.40 s) and divergent thinking [5.01 ± 0.34 s; ts(17) > 2.44, Ps < 0.03, ds > 0.56]. Of most importance, the Task × cTBS site interaction was not significant (F < 1).
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003535117/-/DCSupplemental.
References
- 1.Tulving E., Episodic memory: From mind to brain. Annu. Rev. Psychol. 53, 1–25 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Bartlett F. C., Remembering, (Cambridge University Press, Cambridge, England, 1932). [Google Scholar]
- 3.Schacter D. L., Norman K. A., Koutstaal W., The cognitive neuroscience of constructive memory. Annu. Rev. Psychol. 49, 289–318 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Schacter D. L., Benoit R. G., Szpunar K. K., Episodic future thinking: Mechanisms and functions. Curr. Opin. Behav. Sci. 17, 41–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schacter D. L. et al., The future of memory: Remembering, imagining, and the brain. Neuron 76, 677–694 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schacter D. L., Addis D. R., The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 773–786 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schacter D. L., Addis D. R., “Memory and imagination: Perspectives on constructive episodic simulation” in The Cambridge Handbook of Imagination, Abraham A., Ed. (Cambridge University Press, Cambridge, England, 2020), pp. 111–131. [Google Scholar]
- 8.Benoit R. G., Schacter D. L., Specifying the core network supporting episodic simulation and episodic memory by activation likelihood estimation. Neuropsychologia 75, 450–457 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raichle M. E., The brain’s default mode network. Annu. Rev. Neurosci. 38, 433–447 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Thakral P. P., Madore K. P., Schacter D. L., A role for the left angular gyrus in episodic simulation and memory. J. Neurosci. 37, 8142–8149 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine B., Svoboda E., Hay J. F., Winocur G., Moscovitch M., Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychol. Aging 17, 677–689 (2002). [PubMed] [Google Scholar]
- 12.Schacter D. L., Madore K. P., Remembering the past and imagining the future: Identifying and enhancing the contribution of episodic memory. Mem. Stud. 9, 245–255 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madore K. P., Gaesser B., Schacter D. L., Constructive episodic simulation: Dissociable effects of a specificity induction on remembering, imagining, and describing in young and older adults. J. Exp. Psychol. Learn. Mem. Cogn. 40, 609–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madore K. P., Schacter D. L., Remembering the past and imagining the future: Selective effects of an episodic specificity induction on detail generation. Q J Exp Psychol (Hove) 69, 285–298 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guilford J. P., The Nature of Human Intelligence, (McGraw Hill, New York, New York, 1967). [Google Scholar]
- 16.Gilhooly K. J., Fioratou E., Anthony S. H., Wynn V., Divergent thinking: Strategies and executive involvement in generating novel uses for familiar objects. Br. J. Psychol. 98, 611–625 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Runco M. A., Acar S. A., Do tests of divergent thinking have an experiential bias? Psychol. Aesthet. Creat. Arts 4, 144–148 (2010). [Google Scholar]
- 18.Storm B. C., Patel T. N., Forgetting as a consequence and enabler of creative thinking. J. Exp. Psychol. Learn. Mem. Cogn. 40, 1594–1609 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Duff M. C., Kurczek J., Rubin R., Cohen N. J., Tranel D., Hippocampal amnesia disrupts creative thinking. Hippocampus 23, 1143–1149 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Addis D. R., Pan L., Musicaro R., Schacter D. L., Divergent thinking and constructing episodic simulations. Memory 24, 89–97 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madore K. P., Addis D. R., Schacter D. L., Creativity and memory: Effects of an episodic-specificity induction on divergent thinking. Psychol. Sci. 26, 1461–1468 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madore K. P., Jing H. G., Schacter D. L., Divergent creative thinking in young and older adults: Extending the effects of an episodic specificity induction. Mem. Cognit. 44, 974–988 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaty R. E., Thakral P. P., Madore K. P., Benedek M., Schacter D. L., Core network contributions to remembering the past, imagining the future, and thinking creatively. J. Cogn. Neurosci. 30, 1939–1951 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellamil M., Dobson C., Beeman M., Christoff K., Evaluative and generative modes of thought during the creative process. Neuroimage 59, 1783–1794 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Wu X. et al., A meta-analysis of neuroimaging studies on divergent thinking using activation likelihood estimation. Hum. Brain Mapp. 36, 2703–2718 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madore K. P., Szpunar K. K., Addis D. R., Schacter D. L., Episodic specificity induction impacts activity in a core brain network during construction of imagined future experiences. Proc. Natl. Acad. Sci. U.S.A. 113, 10696–10701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madore K. P., Thakral P. P., Beaty R. E., Addis D. R., Schacter D. L., Neural mechanisms of episodic retrieval support divergent creative thinking. Cereb. Cortex 29, 150–166 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J. X. et al., Targeted enhancement of cortical-hippocampal brain networks and associative memory. Science 345, 1054–1057 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnici H. M., Cheke L. G., Green D. A. E., FitzGerald T. H. M. B., Simons J. S., Specifying a causal role for angular gyrus in autobiographical memory. J. Neurosci. 38, 10438–10443 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spreng R. N., Stevens W. D., Chamberlain J. P., Gilmore A. W., Schacter D. L., Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage 53, 303–317 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerlach K. D., Spreng R. N., Madore K. P., Schacter D. L., Future planning: Default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Soc. Cogn. Affect. Neurosci. 9, 1942–1951 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rorden C., Karnath H. O., Bonilha L., Improving lesion-symptom mapping. J. Cogn. Neurosci. 19, 1081–1088 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Welch L., Recombination of ideas in creative thinking. J. Appl. Psychol. 30, 638–643 (1946). [DOI] [PubMed] [Google Scholar]
- 34.Mednick S. A., The associative basis of the creative process. Psychol. Rev. 69, 220–232 (1962). [DOI] [PubMed] [Google Scholar]
- 35.Smith S. M., Ward T. B., Finke R. A., Eds., The Creative Cognition Approach, (Oxford University Press, New York, 1995). [Google Scholar]
- 36.Fink A. et al., Training of verbal creativity modulates brain activity in regions associated with language- and memory-related demands. Hum. Brain Mapp. 36, 4104–4115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun J. et al., Training your brain to be more creative: Brain functional and structural changes induced by divergent thinking training. Hum. Brain Mapp. 37, 3375–3387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beaty R. E. et al., Default network contributions to episodic and semantic processing during divergent creative thinking: A representational similarity analysis. Neuroimage 209, 116499 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moscovitch M., Cabeza R., Winocur G., Nadel L., Episodic memory and beyond: The hippocampus and neocortex in transformation. Annu. Rev. Psychol. 67, 105–134 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romero K., Moscovitch M., Episodic memory and event construction in aging and amnesia. J. Mem. Lang. 67, 270–284 (2012). [Google Scholar]
- 41.Rubin R. D., Watson P. D., Duff M. C., Cohen N. J., The role of the hippocampus in flexible cognition and social behavior. Front. Hum. Neurosci. 8, 742 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richmond L. L., Zacks J. M., Constructing experience: Event models from perception to action. Trends Cogn. Sci. 21, 962–980 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grigg O., Grady C. L., Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS One 5, e13311 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thakral P. P., Madore K. P., Schacter D. L., The core episodic simulation network dissociates as a function of subjective experience and objective content. Neuropsychologia 136, 107263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thakral P. P., Madore K. P., Addis D. R., Schacter D. L., Reinstatement of event details during episodic simulation in the hippocampus. Cereb. Cortex 30, 2321–2337, 10.1093/cercor/bhz242 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y. Z., Edwards M. J., Rounis E., Bhatia K. P., Rothwell J. C., Theta burst stimulation of the human motor cortex. Neuron 45, 201–206 (2005). [DOI] [PubMed] [Google Scholar]
- 47.Hubl D. et al., Time course of blood oxygenation level-dependent signal response after theta burst transcranial magnetic stimulation of the frontal eye field. Neuroscience 151, 921–928 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Hebscher M., Meltzer J. A., Gilboa A., A causal role for the precuneus in network-wide theta and gamma oscillatory activity during complex memory retrieval. eLife 8, e43114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tambini A., Nee D. E., D’Esposito M., Hippocampal-targeted theta-burst stimulation enhances associative memory formation. J. Cogn. Neurosci. 30, 1452–1472 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hermiller M. S., VanHaerents S., Raij T., Voss J. L., Frequency-specific noninvasive modulation of memory retrieval and its relationship with hippocampal network connectivity. Hippocampus 29, 595–609 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hebscher M., Voss J. L., Testing network properties of episodic memory using non-invasive brain stimulation. Curr. Opin. Behav. Sci. 32, 35–42 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell K. L., Madore K. P., Benoit R. G., Thakral P. P., Schacter D. L., Increased hippocampus to ventromedial prefrontal connectivity during the construction of episodic future events. Hippocampus 28, 76–80 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nilakantan A. S., Bridge D. J., Gagnon E. P., VanHaerents S. A., Voss J. L., Stimulation of the posterior cortical-hippocampal network enhances precision of memory recollection. Curr. Biol. 27, 465–470 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sestieri C., Capotosto P., Tosoni A., Luca Romani G., Corbetta M., Interference with episodic memory retrieval following transcranial stimulation of the inferior but not the superior parietal lobule. Neuropsychologia 51, 900–906 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Nusbaum E. C., Silvia P. J., Beaty R. E., Ready, set, create: What instructing people to “be creative” reveals about the meaning and mechanisms of divergent thinking. Psychol. Aesthet. Creat. Arts 8, 423–432 (2014). [Google Scholar]
- 56.Addis D. R., Wong A. T., Schacter D. L., Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45, 1363–1377 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guilford J. P., Christensen P. R., Merrifield P. R., Wilson R. C., Alternate Uses Manual, (Mind Garden, Menlo Park, California, 1960). [Google Scholar]
- 58.Friston K. J. et al., Classical and Bayesian inference in neuroimaging: Applications. Neuroimage 16, 484–512 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Frisoni G. B. et al.; EADC-ADNI Working Group on The Harmonized Protocol for Manual Hippocampal Volumetry and for the Alzheimer’s Disease Neuroimaging Initiative , The EADC-ADNI harmonized protocol for manual hippocampal segmentation on magnetic resonance: Evidence of validity. Alzheimers Dement. 11, 111–125 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thakral P. P., Yu S. S., Rugg M. D., The hippocampus is sensitive to the mismatch in novelty between items and their contexts. Brain Res. 1602, 144–152 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson J. D., Muftuler L. T., Rugg M. D., Multiple repetitions reveal functionally and anatomically distinct patterns of hippocampal activity during continuous recognition memory. Hippocampus 18, 975–980 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson J. D., Suzuki M., Rugg M. D., Recollection, familiarity, and content-sensitivity in lateral parietal cortex: A high-resolution fMRI study. Front. Hum. Neurosci. 7, 219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki M., Johnson J. D., Rugg M. D., Decrements in hippocampal activity with item repetition during continuous recognition: An fMRI study. J. Cogn. Neurosci. 23, 1522–1532 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki M., Johnson J. D., Rugg M. D., Recollection-related hippocampal activity during continuous recognition: A high-resolution fMRI study. Hippocampus 21, 575–583 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slotnick S. D., Moo L. R., Segal J. B., Hart J. Jr., Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res. Cogn. Brain Res. 17, 75–82 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Slotnick S. D., Cluster success: fMRI inferences for spatial extent have acceptable false-positive rates. Cogn. Neurosci. 8, 150–155 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Van Dijk K. R. et al., Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J. Neurophysiol. 103, 297–321 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yazar Y., Bergström Z. M., Simons J. S., Continuous theta burst stimulation of angular gyrus reduces subjective recollection. PLoS One 9, e110414 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ryals A. J., Rogers L. M., Gross E. Z., Polnaszek K. L., Voss J. L., Associative recognition memory awareness improved by theta-burst stimulation of frontopolar cortex. Cereb. Cortex 26, 1200–1210 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and materials are available upon reasonable request.