Abstract
Members of the membrane spanning 4A (MS4A) gene family are clustered around 11q12–13, a region linked to allergy and asthma susceptibility. Other than the known functions of FcεRIβ (MS4A2) and CD20 (MS4A1) in mast cell and B cell signaling, respectively, functional studies for the remaining MS4A proteins are lacking. We thus explored whether MS4A4A, a mast cell expressed homologue of FcεRIβ, has related functions to FcεRIβ in FcεRI signaling. We establish in this study that MS4A4A promotes phosphorylation of PLCγ1, calcium flux and degranulation in response to IgE-mediated crosslinking of FcεRI. We previously demonstrated that MS4A4A promotes recruitment of KIT into caveolin-1-enriched microdomains and signaling through PLCγ1. Caveolin-1 itself is an important regulator of IgE-dependent store-operated Ca2+ entry (SOCE) and promotes expression of the store-operated Ca2+ channel pore-forming unit, Orai1. We thus further report that MS4A4A functions through interaction with caveolin-1 and recruitment of FcεRI and KIT into lipid rafts. In addition to proximal FcεRI signaling, we similarly show that MS4A4A regulates Orai1-mediated calcium entry downstream of calcium release from stores. Both MS4A4A and Orai1 had limited effects with compound 48/80 stimulation, demonstrating some degree of selectivity of both proteins to FcεRI receptor signaling over Mas-related G Protein coupled receptor X2 signaling. Overall, our data are consistent with the conclusion that MS4A4A performs a related function to the homologous FcεRIβ to promote PLCγ1 signaling, SOCE, and degranulation through FcεRI in human mast cells and thus represents a new target in the regulation of IgE-mediated mast cell activation.
Keywords: Mast cell, IgE receptor, MS4A, allergy, Orai1
Graphical abstract

1. Introduction
The membrane-spanning 4-subfamily A (MS4A) gene cluster are a family of at least 16 genes in humans that are related to MS4A1 (the gene encoding CD20) and are expressed primarily in immune cells (1, 2). The full-length MS4A family proteins are 4-pass transmembrane proteins that have similar topology, but low homology to tetraspanins. Therefore, MS4A proteins may have related, albeit distinct functions from tetraspanins. Although little is known about the function of MS4A proteins, recent studies have implicated the gene family in multiple signal transduction pathways for various receptors (3–6). MS4A4A (also known as MS4A4) specifically, has been identified regulator of dectin-1 signaling in macrophages that promotes NK cell-mediated resistance metastasis and found to be associated with recruitment of MS4A4A and dectin-1 into lipid rafts (3). In addition, we previously reported that MS4A4A protein functions to promote signaling and recycling of the receptor tyrosine kinase, KIT, through the PLCγ1 pathway and MS4A4A facilitated recruitment of KIT into caveolin-1-enriched lipid rafts (6). Further, the mouse homologue of MS4A4A, Ms4a4b, acts as a membrane adaptor protein for the glucocorticoid-induced tumor necrosis factor receptor-related (GITR) protein and forms complexes in lipid rafts of T cells (7). Additionally, this mouse homologue directly interacts with the store-operated Ca2+ channel pore-forming unit, Orai1 (CRACM1) (7). Collectively, these studies suggest that MS4A4A, in humans, could play an important role in lipid raft-associated signaling and complex formation of receptors and ion channels.
Contrary to the role that we and others have found for MS4A4A in promoting receptor multimeric complex formation and signaling in lipid rafts, the closely related mouse homologues of MS4A4A along with other Ms4a genes in mice, have been proposed to act directly as chemoreceptors in olfactory necklace sensory neurons where the Ms4a proteins recognize ligands including fatty acids and pheromones to trigger Ca2+ responses (8). Therefore, some MS4A proteins may recognize ligands directly to trigger signaling, or act as adaptor proteins to regulate signaling of companion receptors through multimeric complex formation in membrane microdomains. Whether ligand recognition is direct or indirect, MS4A proteins clearly have diverse functions in cells that respond to environmental cues.
We, and others, have extensively studied the closely related MS4A gene family member MS4A2, which encodes the β subunit (FcεRIβ) of the high affinity IgE receptor, FcεRI, because of the critical role it plays in FcεRI function (5, 9–15). Crosslinking FcεRI on mast cells (MCs) by IgE-bound antigens culminates in the release of proinflammatory mediators by compound exocytosis, termed degranulation. Recently, we identified expression of MS4A4A in human MCs, validated shRNA knockdown of MS4A4A, and determined that MS4A4A is associated with lipid rafts in human MCs after stimulation with the growth factor receptor KIT (6). Given the critical role of FcεRIβ in FcεRI function, and the high homology between FcεRIβ and MS4A4A, we hypothesized that in addition to the effects that we reported for MS4A4A with the KIT receptor, MS4A4A also functions in FcεRI signaling.
In this study, we tested this hypothesis and show, for the first time, that MS4A4A plays an important role in IgE-mediated MC signaling, SOCE and degranulation. Mechanistically, MS4A4A plays a role in proximal FcεRI signaling through PLCγ1 phosphorylation, which we propose is due to recruitment into lipid rafts, thereby promoting Ca2+ flux. In addition, our data support the hypothesis that MS4A4A promotes FcεRI and KIT crosstalk and synergy by promoting their interaction in lipid rafts. Further, we show that MS4A4A promotes store-operated Ca2+ entry (SOCE) through regulation of Orai1 downstream of Ca2+ release to drive MC degranulation. These mechanisms of MS4A4A function appear selective to anaphylactic, FcεRI-mediated degranulation, over anaphylactoid degranulation induced by compound 48/80 through Mas-related G Protein coupled receptor X2 (MRGPRX2) receptors. Taken together, our data sort important roles for MS4A4A in MC biology by regulating both proximal and distal IgE-mediated MC signaling pathways and thus identify a new target in the regulation of mast cell activation.
2. Materials and Methods
2.1. Cell cultures
LAD2 human MCs were cultured as described (6, 16) in StemPro-34 medium containing 13 ml supplement, penicillin (100 units/ml) and streptomycin (100 μg/ml) (all from Life Technologies, Paisley, UK), and 100 ng/ml recombinant human SCF (R&D, Abingdon, UK). Half of the medium was replaced with fresh medium and SCF every 7 d. CBMCs were cultured from de-identified, heparinized cord blood obtained from the Carolinas Cord Blood Bank at UNC Hospitals. Units with volumes or cell counts too low for use by the Cord Blood Bank for stem cell transplants were utilized and use for this research was determined to be exempt from the approval by the Biomedical Institutional Review Board at the University of North Carolina at Chapel Hill. Differentiation of mast cell progenitors was achieved as described (17). Cells were cultured for 12 weeks and mast cell purity at 12 weeks was determined to be >95% as assessed by flow cytometry and surface KIT and FcεRIα expression.
2.2. shRNA-mediated knockdown of MS4A4A and Orai1
For gene silencing of MS4A4A and ORAI1, MISSION® shRNA constructs and lentiviruses were used (5, 6) (Sigma-Aldrich, St Louis, MO). The previously validated shRNA for MS4A4A and scramble control were used as described (6). For Orai1, the following constructs were purchased and tested using the same procedures as described (5, 6). Experiments were performed after 7 days of shRNA transduction as described (5, 6).
shOrai1v1: TRCN0000439096
CGGGAGTTACTCCGAGGTGATGAGCTCGAGCTCATCACCTCGGAGTAACTCTTTTTTG
shOrai1v2: TRCN0000413611
CCGGGAGCAACGTGCACAATCTCAACTCGAGTTGAGATTGTGCACGTTGCTCTTTTTTG
shOrai1v3: TRCN0000437946
CCGGGCACCTGTTTGCGCTCATGATCTCGAGATCATGAGCGCAAACAGGTGCTTTTTTG
shOrai1v4: TRCN0000165044
(CCGGGCAACGTGCACAATCTCAACTCTCGAGAGTTGAGATTGTGCACGTTGCTTTTTTG)
shOrai1v5: TRCN0000161221
(CCGGGAAACTGTCCTCTAAGAGAATCTCGAGATTCTCTTAGAGGACAGTTTCTTTTTTG)
2.3. Quantitative real-time RT-PCR
Total RNA was isolated using an RNeasy Plus Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions, with inclusion of the QIAshredder step. For quantitative real-time RT-PCR (qPCR), first strand cDNA synthesis was conducted using a RevertAid kit (Thermo Fisher, Waltham, MA). Reactions were run on a MyiQ iCycler PCR system (Bio-Rad) with iQ5 software version 2 (Bio-Rad, Hercules, CA). Products were run on a 2% agarose gel to confirm appropriate product sizes and bands were then excised from the gel and sequenced.
2.4. Mediator release assays
LAD2 cells were cultured for 6 d after transduction of shRNA, and then sensitized with 100 ng/mL biotinylated human myeloma IgE and incubated overnight (16 h). Cells were subsequently activated by streptavidin (SA) (Sigma Aldrich), thapsigargin or compound 48/80 for 30 min. Degranulation was assayed by β-hexosaminidase release, and PGD2 was measured as described (18). CBMCs were sensitized the same as LAD2 cells, but were activated by IgE crosslinking with anti-IgE.
2.5. Flow cytometry
Surface receptor expression was assessed using flow cytometry as described (5, 19).
2.6. Calcium signaling assay
Changes in cytosolic Ca2+ levels were determined following loading of the cells with Fura-2 AM ester (Molecular Probes, Eugene, OR), as described (5, 20). LAD2 cells were transduced with shRNA as described above, and calcium signaling assays were conducted on Day 7. For IgE-mediated calcium mobilization, cells were sensitized with 100 ng/mL biotinylated human myeloma IgE on Day 6 and incubated overnight (16 h). Fluorescence was measured at two excitation wavelengths (340 and 380 nm) and an emission wavelength of 510 nm using a Biotek Synergy Neo2 multimode plate reader.
2.7. SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis
MC activation was performed with a dose-response of SA as for degranulation assays and immunoblotting was carried out as described (5, 6).
2.8. Cholesterol depletion and repletion
Experiments using cholesterol depletion and repletion were carried out as reported (21). In brief, LAD2 cells were split into 3 treatment conditions with all incubations carried out at 37°C. Untreated cells were incubated in BSA-containing buffered saline solution (BSA/BSS: 20 mM Hepes, pH 7.4, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, 1 mg/ml BSA) as used previously (21). Cholesterol depleted cells were incubated with 10 μM methyl-β-cyclodextrin (MβCD) (Sigma) in BSA/BSS for 1 hour. Additionally, LAD2 cells were subjected to cholesterol depletion with 10 μM MβCD in BSA/BSS for 1 hour followed by washing and cholesterol repletion (0.4 μM cholesterol in BSA/BSS incubation for 2 hours). After all incubations concluded, cells were washed and resuspended in BSA/BSS and then challenged against increasing doses of SA for β-hexosaminidase assays. All conditions were carried out concurrently and in parallel.
2.9. Confocal microscopy
Confocal microscopy on LAD2 cells was carried out within the Biological Imaging Section of the Research Technologies Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health, on a Leica TCS SP8 confocal microscope.as described (6).
3. Results
3.1. MS4A4A functions in FcεRI-mediated degranulation
We hypothesized that MS4A4A has a related function to its homologue, FcεRIβ, in FcεRI function. Therefore, we employed shMS4A4A and studied degranulation in response to IgE and FcεRI crosslinking. Transduction of LAD2 MCs with shMS4A4A resulted in a significant reduction in MS4A4A protein expression (Supplemental Fig. 1) and IgE-dependent MC degranulation in response to streptavidin (SA) alone (Fig. 1A) or SA plus SCF co-stimulation (Fig. 1B). Given the effects of shMS4A4A on FcεRI-induced degranulation, we examined whether shMS4A4A treatment resulted in a downregulation of surface FcεRI expression. There was no significant reduction in surface FcεRI with shMS4A4A treatment (Fig. 1C), but as we have reported, surface KIT expression was markedly reduced (6). These data are consistent with the conclusion that MS4A4A is involved in FcεRI signaling rather than regulating expression of FcεRI.
Figure 1. MS4A4A functions in FcεRI-mediated degranulation.
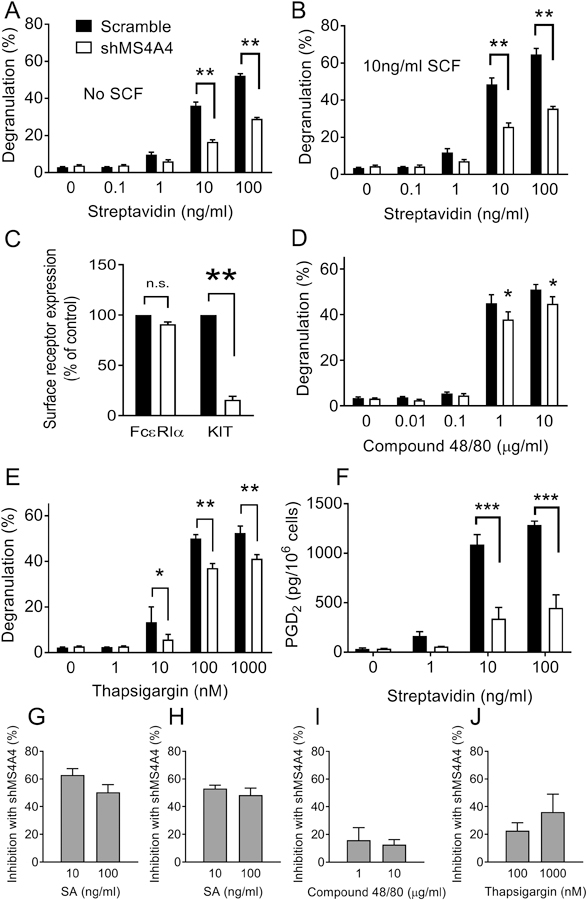
Transduction with shMS4A4A reduces release of β-hexosaminidase from LAD2 cells stimulated with (A) SA (n=11) and (B) SA and 10 ng/ml SCF co-stimulation (n=5). (C) Flow-cytometric analysis of surface FcεRIα and KIT in LAD2 cells after transduction with either scramble or shMS4A4A (n=3). (D) Compound 48/80-induced release of β-hexosaminidase from LAD2 cells was not significantly inhibited by shMS4A4A (n=3). (E) Transduction with shMS4A4A reduces release of β-hexosaminidase from LAD2 cells stimulated with thapsigargin (n=6). (F) PGD2 release from LAD2 cells was inhibited by shMS4A4A (n=3). (G-J) Inhibition of degranulation with shMS4A4A for each stimulus was calculated as the percent inhibition at the top two concentrations. Data are the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, n.s. = not significant. ANOVA with Sidak’s posttest.
Because MCs are activated by IgE-dependent and IgE-independent stimuli, we next examined the effects of shMS4A4A in response to compound 48/80, which activates MCs via the G protein-coupled MRGPRX2 receptors (22). There was a small inhibition of compound 48/80-induced degranulation with shMS4A4A (Fig. 1D), but this inhibition was not as marked as with IgE crosslinking (XL) with SA, suggesting a degree of specificity of MS4A4A function to FcεRI signaling over MRGPRX2 receptors. As an additional control, we used thapsigargin, because thapsigargin induces degranulation (23) by depleting the endoplasmic reticular pool of Ca2+ and subsequently triggering Ca2+ influx via SOCE (24, 25). This effectively bypasses both FcεRI and PLCγ1 to induce degranulation. We found that shMS4A4A had a significant inhibitor y effect on thapsigargin-induced degranulation (Fig. 1E). Because thapsigargin stimulation bypasses surface receptors, this suggests that MS4A4A may play a role in MC degranulation downstream of Ca2+ release from stores.
We next tested PGD2 production, which is Ca2+-dependent, but secretory granule independent, and found that PGD2 production in response to FcεRI stimulation was significantly inhibited in shMS4A4A treated MCs (Fig. 1F). This suggests that the defect in degranulation was not due to a secretory granule defect in trafficking. Calculation of the inhibition of degranulation with each stimuli demonstrated that SA stimulation was inhibited by ~50–60% (Fig 1G), SA + SCF degranulation was inhibited by >50% (Fig 1H), compound 48/80 degranulation was inhibited by <20% (Fig 1I) and thapsigargin degranulation was inhibited by <40% (Fig 1J). Taken together, these data indicate that MS4A4A promotes MC activation and degranulation preferentially, but not solely, through FcεRI and that the inhibition of secretion was not a result of reduced FcεRI expression.
3.2. MS4A4A promotes FcεRI-dependent PLCγ1 signaling
We have shown that MS4A4A actively participates in trafficking and signaling of the growth factor receptor KIT (6). MS4A4A promotes KIT recruitment into caveolin-1 enriched lipid raft domains, altering endocytosis and recycling of the receptor, and facilitating PLCγ1 signaling in response to ligand (6). Because PLCγ1 and Ca2+ influx are critical drivers of MC degranulation (20, 26), we predicted that inhibition of MC degranulation with shMS4A4A was through reduced PLCγ1 signaling. IgE-dependent activation of MCs triggered a dose-dependent phosphorylation of PLCγ1 Y783, which was significantly inhibited by shMS4A4A (Fig. 2A & B). Phosphorylation of AKT S473, was not inhibited (Fig. 2A & C), but phosphorylation of AKT was only weakly induced by stimulation and interference from SCF used to maintain human MCs in culture could interfere with basal AKT signaling. The downstream MAPK, ERK T202-Y204 was strongly induced by FcεRI stimulation and markedly reduced with shMS4A4A (Fig. 2A & D). In summary, these data indicate that the PLCγ1 pathway, rather than the PI3K/AKT pathway is responsible for the inhibition of MC degranulation by MS4A4A silencing.
Figure 2. MS4A4A promotes FcεRI-dependent PLCγ1 signaling.
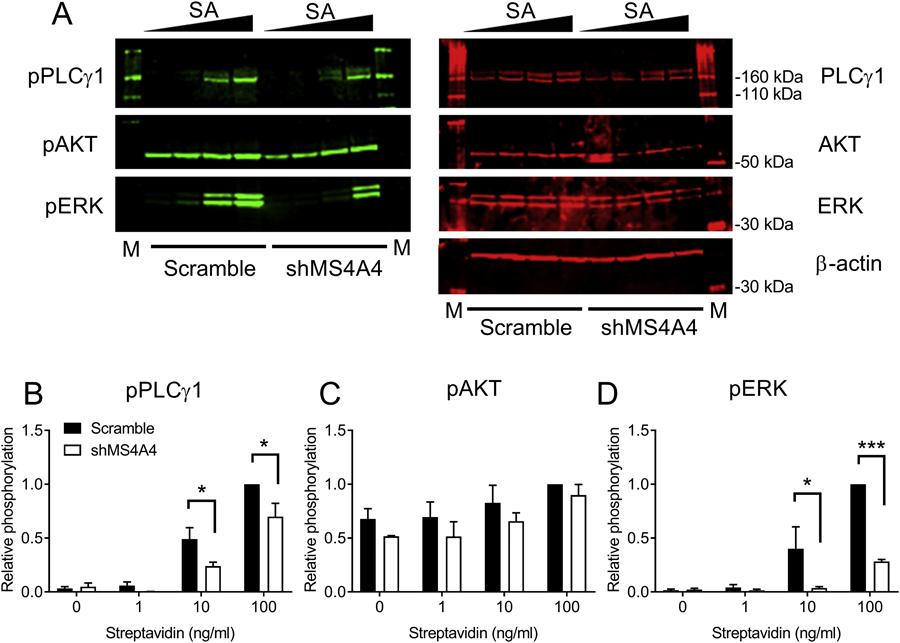
(A) Immunoblots from LAD2 cells transduced with either scramble or shMS4A4A lentiviruses. (B-D) Quantification of PLCγ1 (B), AKT (C), and ERK (D) phosphorylation for scramble or shMS4A4A-transduced LAD2 cells calculated as relative phosphorylation after correction against total protein and normalized to maximal phosphorylation for each phosphoprotein. Data are the mean ± SEM from three independent experiments. *p<0.05, ***p<0.001, ANOVA with Sidak’s posttest.
3.3. MS4A4A promotes FcεRI-dependent and KIT-mediated Ca2+ conductance promoting synergy in Ca2+ responses
Activation of PLCγ1 in MCs leads to the production of inositol triphosphate (IP3) that triggers the release of Ca2+ from intracellular stores by binding to the IP3 receptor on the ER membrane (26). Ca2+ release from stores is critical for influx of extracellular Ca2+ through SOCE. Therefore, we examined the effects of shMS4A4A on Ca2+ conductance using Fura2 ratiometric assays. Addition of medium alone did not induce a Ca2+ response and no difference was observed between scramble control and shMS4A4A (Fig. 3A). Addition of SCF induced a small but significant increase in intracellular Ca2+ in scramble control, but not in shMS4A4A-treated MCs (Fig. 3B). Stimulation of MCs with SA to crosslink IgE bound to FcεRI resulted in a robust Ca2+ influx, which was significantly reduced with shMS4A4A treatment and more evident at the higher SA dose (Fig. 3C & D). Compound 48/80 stimulation resulted in a trend for reduced Ca2+ responses with shMS4A4A treatment, only significant at late time-points (Fig. 3E). There was no significant difference with thapsigargin stimulation (Fig. 3F). Taken together, these data are in agreement with the degranulation studies and further suggest that MS4A4A plays an important role in FcεRI and SCF global Ca2+ flux, but has limited effects on compound 48/80 and thapsgargin stimulation.
Figure 3. MS4A4A promotes FcεRI-dependent and KIT-mediated Ca2+ conductance promoting synergy in Ca2+ responses.
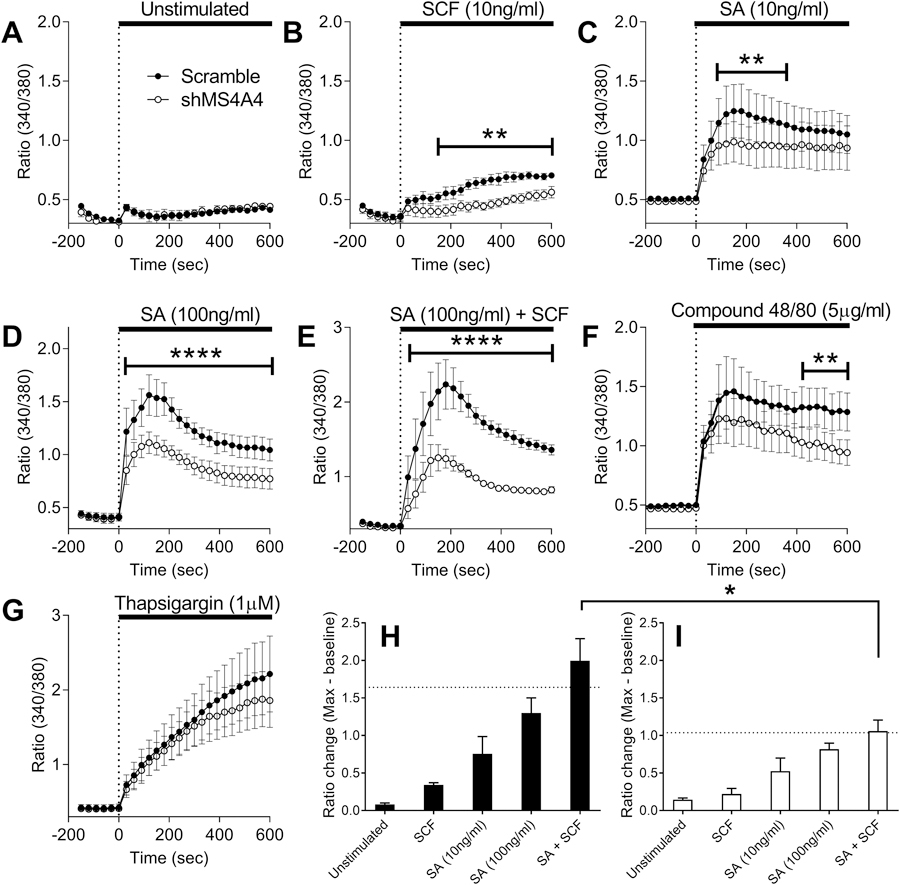
(A-G) Ratiometric calcium signaling compare calcium conductance between scramble control and shMS4A4A following incubations of various stimuli. (A) medium alone, (B) SCF, (C) streptavidin 10 ng/ml, (D) streptavidin 100 ng/ml, (E) compound 48/80 5 μg/ml, (F) thapsigargin 1 μM, (G) streptavidin 100 ng/ml + SCF 10ng/ml. (H) Comparison in scramble control treated cells of fluorescence ratio pre-stimulation to maximum ratio post-stimulation; dashed line indicates additive fluorescence between SCF and streptavidin 100 ng/ml. (I) Comparison of fluorescence ratio in shMS4A4A treated cells. Data are the mean ± SEM from three to seven independent experiments. *p<0.05, **p<0.01, ****p<0.0001. ANOVA with Sidak’s posttest.
An important crosstalk interaction exists between two key receptors in MCs, FcεRI and KIT, the receptor tyrosine kinase for stem cell factor (SCF) (26–28). SCF is a growth and survival factor for MCs (29, 30) and synergistically enhances antigen-induced degranulation (26, 31). Given the effects of shMS4A4A on FcεRI (Fig. 2) and KIT (6) signaling through PLCγ1, we postulated that MS4A4A functions to promote synergy between KIT and FcεRI, because the PLCγ1 pathway is an important convergent of FcεRI and KIT signaling (32). The effects of shMS4A4A on Ca2+ conductance were more evident when the cells were co-stimulated with IgE crosslinking and SCF (Fig. 3G). Calculating the change in fluorescence ratio pre-stimulation to maximum ratio post-stimulation demonstrated that co-stimulation of FcεRI and KIT in scramble control cells resulted in a synergistic effect with fluorescence above the additive level (Fig. 3H). Conversely, in shMS4A4A treated MCs, co-stimulation of FcεRI and KIT resulted in an additive increase in Ca2+ flux (Fig. 3I). These data are consistent with the conclusion that MS4A4A also functions to promote synergy between these two canonical MC receptors.
3.4. MS4A4A promotes KIT and FcεRI colocalization after co-stimulation of receptors
We have established that MS4A4A co-immunoprecipitates with caveolin-1 in MCs (6). We also showed that a proportion of KIT is recruited into lipid rafts after stimulation with SCF and that this recruitment is promoted by expression of MS4A4A (6). Phosphorylation of PLCγ1 in response to SCF is weak and could represent a small proportion of KIT that are recruited into lipid rafts that signal through the PLCγ1 pathway (6). Therefore, we tested whether MS4A4A promotes colocalization of FcεRI and KIT following activation of both receptors. IgE crosslinking alone in MCs did not induce colocalization of FcεRI and KIT (Fig. 4A&C). However, co-stimulation of MCs with IgE crosslinking and SCF induced colocalization of both receptors at or near the plasma membrane (Fig. 4B&C), suggesting that both receptors have the potential to be recruited into the same membrane microdomains. Colocalization between KIT and FcεRI was significantly reduced with shMS4A4A (Fig. 4C). Therefore, we propose that MS4A4A promotes KIT and FcεRI crosstalk by recruiting both receptors into lipid rafts.
Figure 4. MS4A4A promotes KIT and FcεRI colocalization after co-stimulation of receptors and MS4A4A likely functions in lipid rafts.
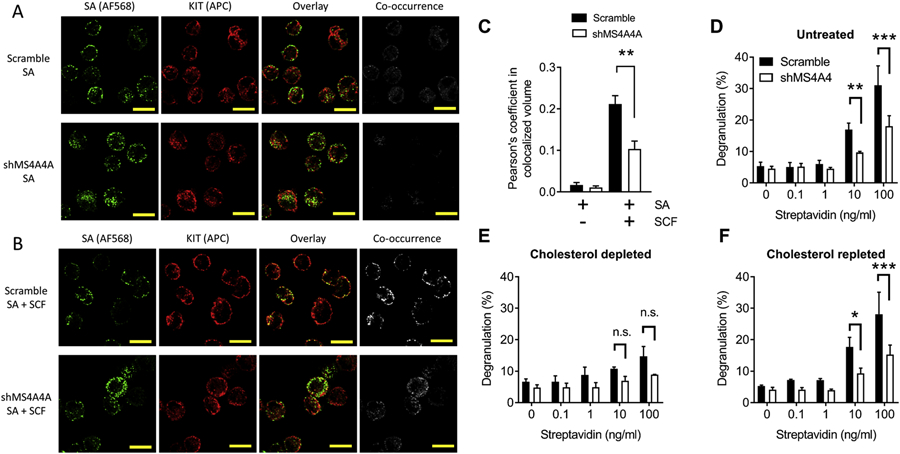
(A-B) Confocal microscopy of human LAD2 MCs show effect of MS4A4A on colocalization of crosslinked FcεRI (green; AF568) and KIT (red; Allophycocyanin) when MCs were stimulated by biotinylated IgE plus AF568 streptavidin (A) compared with stimulation by biotinylated IgE plus AF568 streptavidin in the presence of SCF (B). Top panels show representative images of scramble control treated cells and bottom panels show representative images of shMS4A4A treated cells. (C) Quantification of colocalization assessed by Pearson’s coefficient in colocalized volume above threshold of each stack of high power images. (D) Knockdown of MS4A4A results in reduced degranulation. (E) Depletion of cholesterol with MβCD reduces degranulation and the difference between scramble control and shMS4A4A is no longer significant. (F) Depletion of cholesterol, followed by repletion of cholesterol restores the phenotype to that of untreated cells. Data from D-F are the mean ± SEM from three independent experiments. *p<0.05, **p<0.01, ***p<0.001, ANOVA with Sidak’s posttest.
3.5. Inhibition of degranulation with shMS4A4A is reduced with depletion of cholesterol
As further evidence of lipid raft involvement in MS4A4A function and FcεRI signaling, we chose to utilize depletion and repletion of cholesterol experiments. Cholesterol is a critical component of lipid rafts and depletion of cholesterol disrupts binding of lipid raft-associated proteins (21, 33). To deplete cholesterol, we used methyl-β-cyclodextrin (MβCD) as previously used for MCs (21). MβCD is water soluble and rapidly depletes cholesterol from the plasma membrane in living cells without incorporating itself or alternative lipid products into the membrane (34). Using this approach, we established that in mock treated cells, there was a significant inhibition of degranulation by shMS4A4A (Fig. 4D). Cholesterol depletion with MβCD resulted in marked inhibition of degranulation in response to IgE crosslinking, with reduced differences in degranulation between scramble control and shMS4A4A, which no longer reached significance (Fig. 4E). Although MβCD preferentially depletes cholesterol, it can result in depletion of other lipids from the plasma membrane. Therefore, we also examined cholesterol repletion, after depletion, to confirm that the effects of MβCD were due to cholesterol. With repletion of cholesterol, a complete restoration of phenotype was achieved (Fig. 4F). Overall, these data suggest that MS4A4A participates in proximal FcεRI signaling, most likely in lipid raft microdomains.
3.6. MS4A4A functions in SOCE
Having established that MS4A4A functions in proximal FcεRI signaling by promoting recruitment into lipid rafts and signaling through PLCγ1, we next asked whether MS4A4A was also functioning in distal FcεRI signaling. Knockdown of MS4A4A has an inhibitory effect on degranulation in response to thapsigargin (Fig. 1E), but has limited effects on global Ca2+ particularly during early time-points (Fig. 3F). Thapsigargin strongly induces SOCE, which is downstream of Ca2+ release from stores, and not measurable with global Ca2+ flux assays. We have shown that MS4A4A and caveolin-1 coimmunoprecipitate (6). In addition to the roles of caveolin-1 in caveolae, lipid rafts and endocytosis, caveolin-1 also regulates SOCE and expression of Orai1 (35), where caveolin-1 and lipid rafts are critical for Orai1-STIM1 interaction (36). Since SOCE in human MCs is primarily driven by Orai1 (37), we predicted that the effects of shMS4A4A on degranulation of MCs with thapsigargin stimulation was due to Orai1 regulation by MS4A4A and caveolin-1 interactions. To examine SOCE, we stimulated MCs with either SA, compound 48/80 or thapsigargin in the absence of extracellular Ca2+ followed by addition of Ca2+ (Fig. 5A–C). Using this approach, we established that SOCE in response to SA and SCF co-stimulation (Fig. 5A) and thapsigargin stimulation (Fig. 5B) were both significantly reduced with shMS4A4A, but SOCE in response to compound 48/80 was not (Fig. 5C). To confirm that the effects of MS4A4A on Ca2+ influx were indeed due to SOCE, we added the Ca2+-release activated Ca2+ channel (CRAC) blocker YM-58483 to the cells and examined SOCE. With the addition of this CRAC inhibitor, there was a marked reduction in SOCE and the differences between scramble and shMS4A4A were diminished (Fig. 5A–C). SOCE with compound 48/80 was inhibited with YM-58483, but not with shMS4A4 suggesting that while ion channels that carry Ca2+ were involved in SOCE with compound 48/80 stimulation, MS4A4A was not playing a role. Taken together, these data support the conclusion that MS4A4A functions in Ca2+ conductance and SOCE from SA and thapsigargin, but has limited impact on compound 48/80.
Figure 5. MS4A4A functions in SOCE.
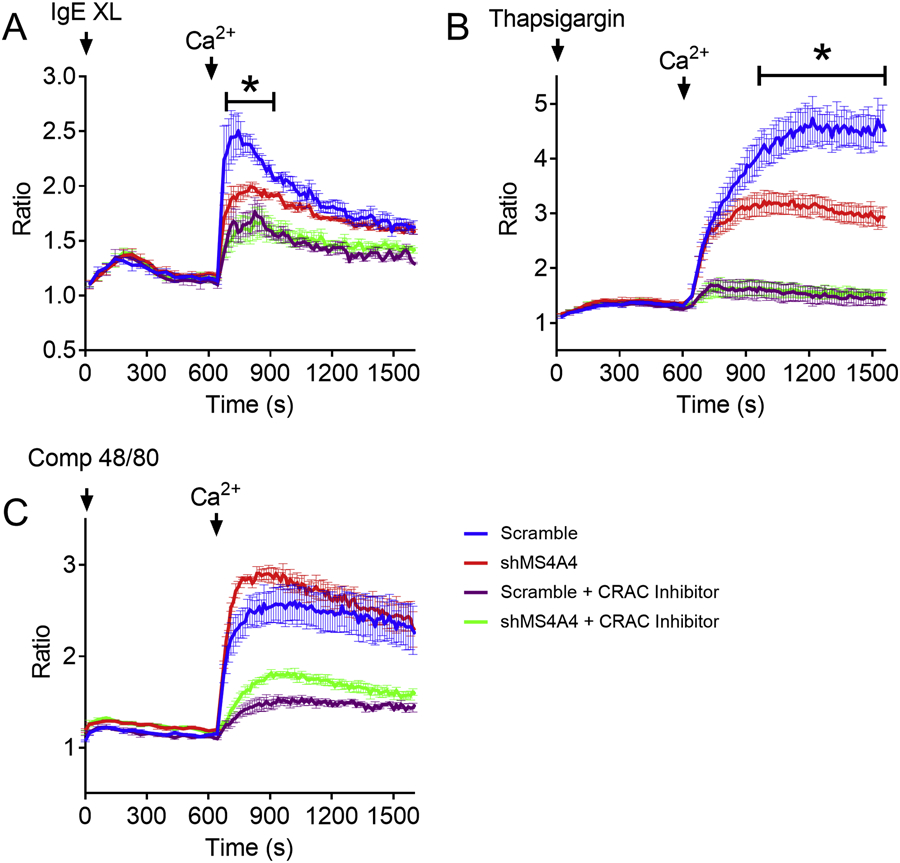
Store-operated calcium entry measured by ratiometric Ca2+ assays in the absence of Ca2+, followed by addition of 2mM Ca2+. The first peak before the addition of Ca2+ is release from stores and the peak after Ca2+ is SOCE. (A) Streptavidin and SCF, (B) thapsigargin, and (C) Compound 48/80. Arrows indicate the introduction of stimulus and Ca2+. Data from are the mean ± SEM from three independent experiments. ***p<0.001, ****p<0.0001, ANOVA with Sidak’s posttest.
3.7. MS4A4A function in SOCE is through regulation of Orai1
Because of the reported role of caveolin-1 on promoting Orai1 function discussed above (35, 36), we predicted that MS4A4A was affecting degranulation and SOCE by regulating function of Orai1. We therefore examined the effects of MS4A4A knockdown with additional knockdown of Orai1. If MS4A4A was affecting Orai1 function, we would expect to see a similar phenotype if either protein was knocked down, and no additional effect of double knockdown. We tested five shRNA constructs for Orai1 and chose shOrai1v2, the most efficient shRNA construct (Fig. 6A). None of the knockdowns showed evidence of cytotoxicity over the course of the experiments, which was measured by trypan blue counts (not shown). shMS4A4A resulted in a significant reduction in MC degranulation in response to IgE crosslinking (Fig. 6B). shOrai1 resulted in greater reduction in degranulation than shMS4A4A, but double knockdown with shMS4A4A and shOrai1 was comparable to shOrai1 alone (Fig. 6B). Stimulation with compound 48/80 was considerably less affected by shMS4A4A and shOrai1 (Fig. 6C), suggesting that not only was MS4A4A less involved in compound 48/80 degranulation compared to IgE crosslinking, but that Orai1 was also playing less of a role in this pathway. In addition, thapsigargin-induced degranulation was inhibited by shMS4A4A and shOrail, but again there was no additional effect of shMS4A4A and shOrai1 double knockdown (Fig. 6D). Taken together, these data are consistent with the conclusion that MS4A4A and Orai1 are functioning in a common pathway, and that SOCE with MRGPRX2 GPCR activation in MCs is less dependent on both MS4A4A and Orail.
Figure 6. MS4A4A function in SOCE is through regulation of Orai1.
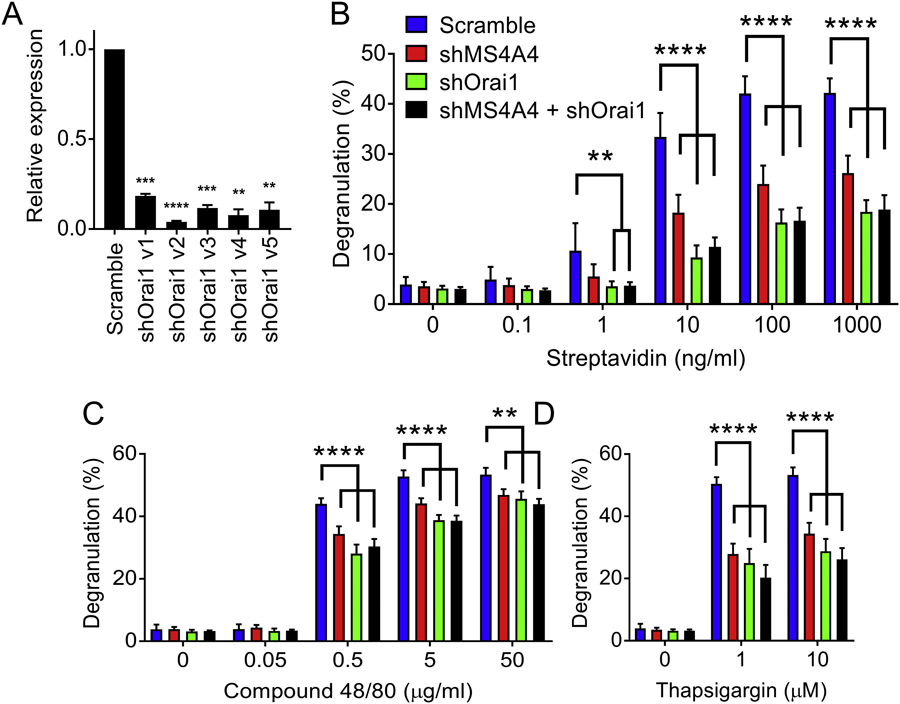
(A) qRT-PCR for Orai1 mRNA in LAD2 cells using five shRNA constructs targeting Orai1. (B-D) Comparison of β-hexosaminidase release from LAD2 cells transduced with scramble, shMS4A4A, shOrai1 (v2, the most effective construct) or shMS4A4A plus shOrai1 (v2). Stimulants of degranulation included biotinylated IgE with streptavidin (B), compound 48/80 (C), and thapsigargin (D). Data are the mean ± SEM from three (A) or five (B-D) independent experiments. **p<0.01, ***p<0.001, ****p<0.0001, ANOVA with Sidak’s posttest.
3.8. MS4A4A is expressed in primary human cord blood-derived MCs and its function is conserved
We next sought to confirm that MS4A4A expression and function was conserved in primary human MCs. We employed human cord blood-derived MCs (HCBDMCs) (17). HCBDMCs demonstrated low degranulation capacity in response to IgE crosslinking (XL) in the absence of IL-4 (Fig. 7A). However, with 7 days of 100 ng/ml IL-4 in culture, HCBDMCs demonstrated more robust degranulation in response to IgE XL (Fig. 7A). Ionomycin was used as a positive control, and this was unaffected by IL-4 (Fig 7B). The low degranulation response appeared to be due to low FcεRIα expression on the surface of the HCBDMCs, which was upregulated with IL-4 priming (Fig. 7C & D). We next examined surface MS4A4A expression, as described (3) and found that surface MS4A4A was present on HCBDMCs, but expression was lower than in LAD2 cells (Fig. 7E). After we had reported the expression of MS4A4A by human MCs (6), MS4A4A was reported to be a macrophage tetraspan that acts as a marker of alternatively activated M2 macrophages (3, 38). Because IL-4 is a key regulator of the M2 macrophage phenotype, IL-4 has been examined and shown to induce or upregulate surface expression of MS4A4A in macrophages (3, 38). Therefore, we expected surface MS4A4A expression to be upregulated in MCs after IL-4 priming. However, we did not find any change in surface MS4A4A expression with IL-4 addition in either HCBDMCs or LAD2 cells (Fig. 7E), despite surface FcεRIα expression increasing in HCBMCs (Fig. 7D). These findings suggest that while MS4A4A expression modulates FcεRI function, MS4A4A does not appear to regulate surface FcεRI expression, which is in agreement with the data showing that knockdown of MS4A4A does not affect surface FcεRIα expression in LAD2 cells (Fig. 1C).
Figure 7. MS4A4 is expressed in primary cord blood-derived MCs and function is conserved.
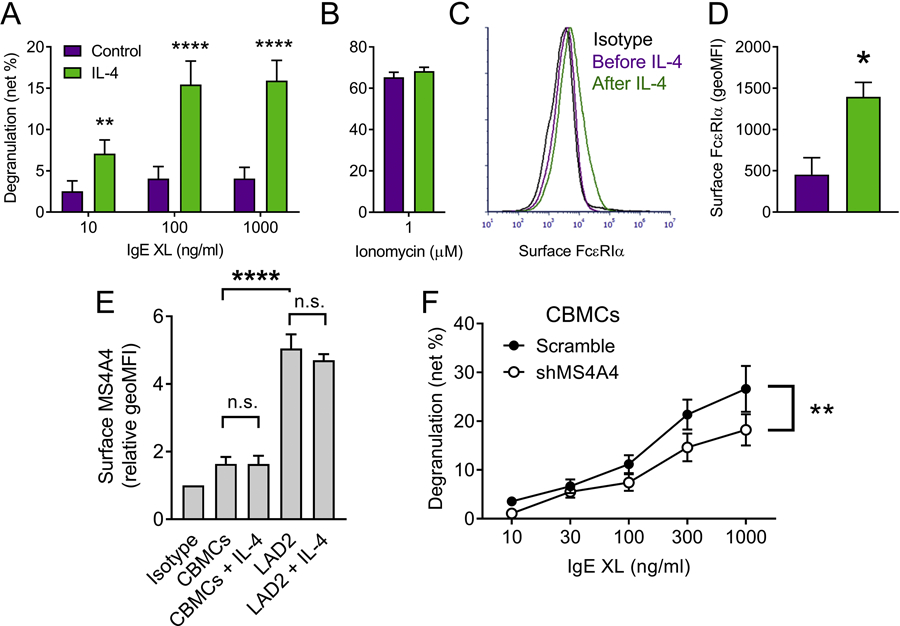
(A) CBMCs degranulate when primed with IL-4. Dose response of IgE crosslinking (XL) with anti-IgE in the absence and presence of IL-4. (B) Ionomycin degranulation was unaffected by IL-4. (C) Histograms of flow cytometry analysis of FcεRIα surface expression. (D) combined data from multiple different donors shows an increase in surface FcεRIα expression with IL-4 stimulation. (E) CBMCs and LAD2 MCs stained for surface MS4A4A expression with and without IL-4 stimulation. (F) Degranulation of CBMCs with increasing concentrations of anti-IgE. Data are the mean ± SEM from four (A-E) or five (F) independent experiments. *p<0.05, **p<0.01, ****p<0.0001, n.s. = not significant. ANOVA with Sidak’s posttest was used for (A), t-test for (D), and ANOVA with or without post-test for (E & F, respectively).
Finally, having established expression of MS4A4A in HCBDMCs we next confirmed that shMS4A4A also resulted in reduced degranulation of HCBDMCs (Fig. 7F). The effects of shMS4A4A on degranulation in HCBDMCs were significant, but less marked than in LAD2 cells (compare Fig. 7D to Fig. 1A). We propose that this was due to the lower expression of MS4A4A in HCBDMCs compared to LAD2 cells (Fig. 7E) and that higher expression of MS4A4A results in greater degranulation in response to IgE XL.
4. Discussion
The function of the human MS4A4A gene is unknown and this is the first study to identify that the four transmembrane protein product, MS4A4A (also named MS4A4), functions in MC degranulation and signaling through FcεRI. We have further established that MS4A4A functions to promote human MC degranulation through FcεRI crosslinking by two distinct mechanisms. Firstly, MS4A4A promotes proximal FcεRI signaling through the PLCγ1 pathway, which is likely due to promoting lipid raft association and interaction of the complex with adaptor proteins and signaling molecules. Secondly, MS4A4A promotes Orai1-mediated SOCE downstream of Ca2+ release from ER stores.
MS4A4A is a homologue of FcεRIβ and thus we postulated that it could have related functions. We have shown that MS4A4A actively participates in trafficking and signaling of the growth factor receptor KIT (6). MS4A4A promotes KIT recruitment into caveolin-1 enriched lipid raft domains facilitating PLCγ1 signaling in response to ligand, altering endocytosis and recycling of the receptor (6). The result is that MS4A4A regulates KIT signaling pathways to alter MC phenotype. We now show that MC degranulation in response to antigen is significantly reduced with shMS4A4A and we propose that this effect is due to recruitment of crosslinked FcεRI into lipid rafts at the plasma membrane. We examined signaling in response to antigen and found that PLCγ1 signaling was significantly reduced and this correlated with reduction in Ca2+ influx. Importantly, the effects of MS4A4A knockdown on Ca2+ were more evident when the cells were co-stimulated with antigen and SCF (the ligand for KIT) and act synergistically to promote MC functions (31). Since MS4A4A knockdown reduced PLCγ1 signaling for both receptors, we predict that MS4A4A plays a role in recruitment of both receptors into lipid rafts. Indeed, FcεRI and KIT colocalized to membrane domains only when co-stimulated and knockdown of MS4A4A reduced this colocalization, suggesting that MS4A4A function in lipid raft association of receptors is not limited to a single receptor and could have relevance in several immune cells that express MS4A4A.
In support of our hypothesis that MS4A4A functions to recruit receptors into lipid rafts in a promiscuous manner, recent studies have identified MS4A4A expression in alternatively activated macrophages (3, 38), which differ considerably from MCs in receptor expression. MS4A4A expression was identified in peripheral blood, which was principally in monocytes, and on the surface of M2, but not M1 macrophages (38). The precise role that MS4A4A plays in monocytes and M2 macrophages is not yet established, but it was shown that MS4A4A promotes dectin-1 signaling and both MS4A4A and dectin-1 were recruited into lipid rafts after zymosan engagement where they were shown to interact (3). These observations, taken together with our findings, lead us to propose that MS4A4A functions in signaling through multiple receptors by promoting complex formation within lipid rafts. The mechanisms underlying this function of MS4A4A are yet to be elucidated, but we have shown that MS4A4A traffics through caveolae and interacts with caveolin-1 (6), suggesting that this interaction could be critical. While it seems that signaling through several receptors are affected by MS4A4A, we show that MS4A4A provides some selectivity between receptors. For FcεRI signaling, phosphorylation of FcεRIγ ITAM occurs mainly by the Src family kinase, Lyn kinase, which is recruited to the non-canonical ITAM of FcεRIβ (39). Upon activation by crosslinking, FcεRI is rapidly recruited into lipid rafts enriched in glycosphingolipids, cholesterol, and glycosylphosphatidylinositol-anchored proteins, where it is phosphorylated by Lyn kinase (40, 41). The recruitment of FcεRI complexes into lipid rafts likely plays an important role in downstream signaling events. Downstream signaling through FcεRI is critically dependent on the linker protein, LAT (42), which is resident in lipid rafts and crucial for PLCγ1 phosphorylation (43). Bone marrow-derived MCs from LAT deficient mice exhibit intact proximal phosphorylation of FcεRI subunits and Syk in response to FcεRI aggregation, but phosphorylation of PLCγ1 and Ca2+ influx were markedly reduced (42).
It has been demonstrated that while LAT is critical for complete FcεRI signal transduction, Ca2+ influx and degranulation (42) in lipid rafts (43), LAT phosphorylation and downstream signaling can still occur independently from Lyn kinase translocation into lipid rafts (41). Lyn is critical for proximal FcεRI signaling, but these data suggest that signaling through LAT within lipid rafts can occur without Lyn translocation to lipid rafts. Indeed, FcεRI can be phosphorylated outside of lipid rafts (44), and thus signaling through FcεRI and LAT can occur without FcεRI or Lyn kinase translocation to lipid rafts. However, lipid raft translocation may amplify signaling, which could be evidenced by the reduced PLCγ1 phosphorylation we observed with shMS4A4A. We propose that by facilitating FcεRI recruitment into lipid rafts, MS4A4A promotes FcεRI signal transduction through PLCγ1 to promote distal signaling and Ca2+ mobilization. However, we show in the present study that knockdown of MS4A4A reduces IgE-dependent LAD2 cell degranulation by around 50%, and while PLCγ1 signaling is reduced, it still occurs, and is thus unlikely to be the only mechanism for the inhibitory effect of shMS4A4A on distal FcεRI signaling.
In addition to proximal FcεRI signaling, we also demonstrate that MS4A4A functions in distal FcεRI signaling downstream of Ca2+ release from stores to promote SOCE through Orai1. MS4A1 and MS4A12 have been proposed to act as distinct Ca2+ channels because ectopic expression induces a Ca2+ current post-transfection (45, 46). We found that MS4A4A promotes Ca2+ conductance and SOCE in human MCs suggesting that MS4A4A could function as a Ca2+ channel. However, SOCE in human MCs is primarily driven by the Ca2+ channel pore-forming unit, Orai1 (37). Thus, we examined MC degranulation with shRNA knockdown of MS4A4A and/or Orai1 and found that the phenotype with shMS4A4A closely resembled shOrai1 and knockdown of both Orai1 and MS4A4A had no additive effect. It is possible that MS4A4A forms a Ca2+ channel that is dependent upon Orai1. However, we propose that it is more likely that MS4A4A is not acting as a Ca2+ channel itself, but rather regulating Orai1 function. Caveolin-1 functions in SOCE by promoting Orai1 interaction with STIM1 and by regulating of expression of Orai1 (35, 36). Caveolin-1 also channels Ca2+ microdomain signaling through distinct pathways to activate transcription factors where caveolin-1 and lipid rafts are critical for Orai1-STIM1 interactions (36). We propose that MS4A4A, most likely in the ER and juxtanuclear regions of the cells, functions in this pathway to regulate Orai1-mediated SOCE.
Strong supporting evidence in our study that MS4A4A functions in an Orai1 mediated SOCE pathway was the finding that human MC degranulation was less dependent upon either Orai1 or MS4A4A when the cells were activated by compound 48/80 when compared to IgE crosslinking. SOCE was not reduced by shMS4A4A with compound 48/80 treated cells, but blocking CRAC channels with compound YM-58483 did inhibit SOCE in both control and shMS4A4A treated cells. It has been established that Orai1 is the primary CRAC channel responsible for SOCE in human MCs with IgE-dependent degranulation (37), but this has been less characterized with MRGPRX2 in human MCs. Although blocking SOCE with compound 48/80 suggests CRAC channel involvement and YM-58483 potently blocks Orai1, it also blocks other ion channels, such as the non-selective cation TRPC channels (47). Indeed, compound 48/80-induced Ca2+ influx in rat MCs is primarily driven by non-selective cation channels (48–51). We found that degranulation in response to compound 48/80 was significantly less sensitive to Orai1 knockdown than either IgE-crosslinking or thapsigargin stimulation. Our findings with MS4A4A knockdown also followed this same pattern. These observations, taken together, suggest that the primary ion channels that carry SOCE in IgE-mediated (anaphylactic) and MRGPRX2-mediated (anaphylactoid) human MC degranulation differ, with Orai1 driving IgE-mediated SOCE and another ion channel, perhaps a TRP channel, mediating MRGPRX2 SOCE.
5. Summary
In summary, we show that MS4A4A promotes MC degranulation by facilitating the PLCγ1 signaling pathway most likely within lipid rafts, as well as SOCE through Orai1. Further study of the mechanisms and functions of MS4A4A are warranted to establish whether MS4A4A contributes to the membrane and organelle trafficking during degranulation in MCs and establish the role MS4A4A plays in allergic and inflammatory diseases. The MS4A family are clustered around chromosome 11q12-q13 (1, 2), a region previously linked to allergy and asthma susceptibility (52–54). Given the role of FcεRIβ in MCs, FcεRIβ is considered a candidate for the linkage of these genetic loci with allergy. However, given the high homology between FcεRIβ other MS4A cluster proteins, other members of the MS4A cluster, in particular MS4A4A, could well contribute to the development, and pathophysiology of allergy, and may represent a novel therapeutic target.
Supplementary Material
Highlights.
Human mast cells express MS4A4A on the cell surface.
MS4A4A functions in a related manner to its homologue, the β subunit of the high affinity IgE receptor (the MS4A2 gene).
MS4A4A functions in proximal IgE receptor signaling in a lipid raft-dependent manner to promote degranulation.
MS4A4A also functions in distal IgE receptor signaling by promoting store-operated calcium entry through Orai1 in human mast cells.
8. Funding
Research reported in this publication was supported by the National Institutes of Health (NIH), National Institute of Allergy and Infectious Diseases (NIAID), Award Number R01AI143985, and National Institute of Environmental Health Science (NIEHS), Award Number R01ES025198. From the Department of Molecular Biomedical Sciences, College of Veterinary Medicine, NC State University start-up funds, and NC TraCS Institute CTSA grant UL1TR002489. D.D.M. was supported by the Division of Intramural Research, NIAID, NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflicts of interest related to the reported research.
9 References
- 1.Liang Y, and Tedder TF 2001. Identification of a CD20-, FcepsilonRIbeta-, and HTm4-related gene family: sixteen new MS4A family members expressed in human and mouse. Genomics 72: 119–127. [DOI] [PubMed] [Google Scholar]
- 2.Liang Y, Buckley TR, Tu L, Langdon SD, and Tedder TF 2001. Structural organization of the human MS4A gene cluster on Chromosome 11q12. Immunogenetics 53: 357–368. [DOI] [PubMed] [Google Scholar]
- 3.Mattiola I, Tomay F, De Pizzol M, Silva-Gomes R, Savino B, Gulic T, Doni A, Lonardi S, Astrid Boutet M, Nerviani A, Carriero R, Molgora M, Stravalaci M, Morone D, Shalova IN, Lee Y, Biswas SK, Mantovani G, Sironi M, Pitzalis C, Vermi W, Bottazzi B, Mantovani A, and Locati M 2019. The macrophage tetraspan MS4A4A enhances dectin-1-dependent NK cell-mediated resistance to metastasis. Nat Immunol 20: 1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruse G, Kaur D, Leyland M, and Bradding P 2010. A novel FcεRIβ-chain truncation regulates human mast cell proliferation and survival. FASEB J 24: 4047–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cruse G, Beaven MA, Ashmole I, Bradding P, Gilfillan AM, and Metcalfe DD 2013. A truncated splice-variant of the FcεRIβ receptor subunit is critical for microtubule formation and degranulation in mast cells. Immunity 38: 906–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruse G, Beaven MA, Music SC, Bradding P, Gilfillan AM, and Metcalfe DD 2015. The CD20 homologue MS4A4 directs trafficking of KIT toward clathrin-independent endocytosis pathways and thus regulates receptor signaling and recycling. Molecular biology of the cell 26: 1711–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howie D, Nolan KF, Daley S, Butterfield E, Adams E, Garcia-Rueda H, Thompson C, Saunders NJ, Cobbold SP, Tone Y, Tone M, and Waldmann H 2009. MS4A4B is a GITR-associated membrane adapter, expressed by regulatory T cells, which modulates T cell activation. J Immunol 183: 4197–4204. [DOI] [PubMed] [Google Scholar]
- 8.Greer PL, Bear DM, Lassance JM, Bloom ML, Tsukahara T, Pashkovski SL, Masuda FK, Nowlan AC, Kirchner R, Hoekstra HE, and Datta SR 2016. A Family of non-GPCR Chemosensors Defines an Alternative Logic for Mammalian Olfaction. Cell 165: 1734–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruse G, Kaur D, Leyland M, and Bradding P 2010. A novel FcεRIβ-chain truncation regulates human mast cell proliferation and survival. The FASEB Journal 24: 4047–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruse G, Yin Y, Fukuyama T, Desai A, Arthur GK, Bäumer W, Beaven MA, and Metcalfe DD 2016. Exon skipping of FcεRIβ eliminates expression of the high-affinity IgE receptor in mast cells with therapeutic potential for allergy. Proc Natl Acad Sci U S A 113: 14115–14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dombrowicz D, Lin S, Flamand V, Brini AT, Koller BH, and Kinet J-P 1998. Allergy-associated FcRβ is a molecular amplifier of IgE-and IgG-mediated in vivo responses. Immunity 8: 517–529. [DOI] [PubMed] [Google Scholar]
- 12.Dombrowicz D, Brini AT, Flamand V, Hicks E, Snouwaert JN, Kinet JP, and Koller BH 1996. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol 157: 1645–1651. [PubMed] [Google Scholar]
- 13.Alber G, Miller L, Jelsema CL, Varin-Blank N, and Metzger H 1991. Structure-function relationships in the mast cell high affinity receptor for IgE. Role of the cytoplasmic domains and of the beta subunit. J Biol Chem 266: 22613–22620. [PubMed] [Google Scholar]
- 14.Donnadieu E, Jouvin M-H, and Kinet J-P 2000. A second amplifier function for the allergy-associated FcεRI-β subunit. Immunity 12: 515–523. [DOI] [PubMed] [Google Scholar]
- 15.On M, Billingsley JM, Jouvin M-H, and Kinet J-P 2004. Molecular dissection of the FcRβ signaling amplifier. Journal of Biological Chemistry 279: 45782–45790. [DOI] [PubMed] [Google Scholar]
- 16.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, and Metcalfe DD 2003. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res 27: 677–682. [DOI] [PubMed] [Google Scholar]
- 17.Hua X, Chason KD, Patel JY, Naselsky WC, and Tilley SL 2011. IL-4 amplifies the pro-inflammatory effect of adenosine in human mast cells by changing expression levels of adenosine receptors. PLoS One 6: e24947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuehn HS, Radinger M, and Gilfillan AM 2010. Measuring mast cell mediator release. Curr Protoc Immunol Chapter 7: Unit7.38. [DOI] [PMC free article] [PubMed]
- 19.Arthur GK, and Cruse G 2018. Exon Skipping of FcεRIβ for Allergic Diseases. Methods Mol Biol 1828: 503–518. [DOI] [PubMed] [Google Scholar]
- 20.Tkaczyk C, Beaven MA, Brachman SM, Metcalfe DD, and Gilfillan AM 2003. The phospholipase C gamma 1-dependent pathway of Fc epsilon RI-mediated mast cell activation is regulated independently of phosphatidylinositol 3-kinase. J Biol Chem 278: 48474–48484. [DOI] [PubMed] [Google Scholar]
- 21.Sheets ED, Holowka D, and Baird B 1999. Critical role for cholesterol in Lyn-mediated tyrosine phosphorylation of FcepsilonRI and their association with detergent-resistant membranes. J Cell Biol 145: 877–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatemoto K, Nozaki Y, Tsuda R, Konno S, Tomura K, Furuno M, Ogasawara H, Edamura K, Takagi H, Iwamura H, Noguchi M, and Naito T 2006. Immunoglobulin E-independent activation of mast cell is mediated by Mrg receptors. Biochem Biophys Res Commun 349: 1322–1328. [DOI] [PubMed] [Google Scholar]
- 23.Ali H, Christensen SB, Foreman JC, Pearce FL, Piotrowski W, and Thastrup O 1985. The ability of thapsigargin and thapsigargicin to activate cells involved in the inflammatory response. Br J Pharmacol 85: 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, and Dawson AP 1990. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A 87: 2466–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lytton J, Westlin M, and Hanley MR 1991. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem 266: 17067–17071. [PubMed] [Google Scholar]
- 26.Gilfillan AM, and Tkaczyk C 2006. Integrated signalling pathways for mast-cell activation. Nature reviews. Immunology 6: 218–230. [DOI] [PubMed] [Google Scholar]
- 27.Cruse G, Metcalfe DD, and Olivera A 2014. Functional deregulation of KIT: link to mast cell proliferative diseases and other neoplasms. Immunology and allergy clinics of North America 34: 219–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennartsson J, and Ronnstrand L 2012. Stem cell factor receptor/c-Kit: from basic science to clinical implications. Physiological reviews 92: 1619–1649. [DOI] [PubMed] [Google Scholar]
- 29.Jensen BM, Metcalfe DD, and Gilfillan AM 2007. Targeting kit activation: a potential therapeutic approach in the treatment of allergic inflammation. Inflammation & allergy drug targets 6: 57–62. [DOI] [PubMed] [Google Scholar]
- 30.Okayama Y, and Kawakami T 2006. Development, migration, and survival of mast cells. Immunologic research 34: 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bischoff SC, and Dahinden CA 1992. c-kit ligand: a unique potentiator of mediator release by human lung mast cells. J Exp Med 175: 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, and Beaven MA 2004. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood 104: 2410–2417. [DOI] [PubMed] [Google Scholar]
- 33.Kabouridis PS, Janzen J, Magee AL, and Ley SC 2000. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur J Immunol 30: 954–963. [DOI] [PubMed] [Google Scholar]
- 34.Mahammad S, and Parmryd I 2015. Cholesterol depletion using methyl-β-cyclodextrin. Methods Mol Biol 1232: 91–102. [DOI] [PubMed] [Google Scholar]
- 35.Sathish V, Abcejo AJ, Thompson MA, Sieck GC, Prakash YS, and Pabelick CM 2012. Caveolin-1 regulation of store-operated Ca(2+) influx in human airway smooth muscle. Eur Respir J 40: 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeh YC, and Parekh AB 2015. Distinct structural domains of caveolin-1 independently regulate Ca2+ release-activated Ca2+ channels and Ca2+ microdomain-dependent gene expression. Mol Cell Biol 35: 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wajdner HE, Farrington J, Barnard C, Peachell PT, Schnackenberg CG, Marino JP, Xu X, Affleck K, Begg M, and Seward EP 2017. Orai and TRPC channel characterization in Fc. Physiol Rep 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanyal R, Polyak MJ, Zuccolo J, Puri M, Deng L, Roberts L, Zuba A, Storek J, Luider JM, Sundberg EM, Mansoor A, Baigorri E, Chu MP, Belch AR, Pilarski LM, and Deans JP 2017. MS4A4A: a novel cell surface marker for M2 macrophages and plasma cells. Immunol Cell Biol 95: 611–619. [DOI] [PubMed] [Google Scholar]
- 39.Kimura T, Kihara H, Bhattacharyya S, Sakamoto H, Appella E, and Siraganian RP 1996. Downstream signaling molecules bind to different phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) peptides of the high affinity IgE receptor. J Biol Chem 271: 27962–27968. [DOI] [PubMed] [Google Scholar]
- 40.Field KA, Holowka D, and Baird B 1995. Fc epsilon RI-mediated recruitment of p53/56lyn to detergent-resistant membrane domains accompanies cellular signaling. Proc Natl Acad Sci U S A 92: 9201–9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovárová M, Tolar P, Arudchandran R, Dráberová L, Rivera J, and Dráber P 2001. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fcepsilon receptor I aggregation. Mol Cell Biol 21: 8318–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saitoh S, Arudchandran R, Manetz TS, Zhang W, Sommers CL, Love PE, Rivera J, and Samelson LE 2000. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity 12: 525–535. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Trible RP, and Samelson LE 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 9: 239–246. [DOI] [PubMed] [Google Scholar]
- 44.Surviladze Z, Dráberová L, Kovárová M, Boubelík M, and Dráber P 2001. Differential sensitivity to acute cholesterol lowering of activation mediated via the high-affinity IgE receptor and Thy-1 glycoprotein. Eur J Immunol 31: 1–10. [DOI] [PubMed] [Google Scholar]
- 45.Bubien JK, Zhou LJ, Bell PD, Frizzell RA, and Tedder TF 1993. Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J Cell Biol 121: 1121–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koslowski M, Sahin U, Dhaene K, Huber C, and Tureci O 2008. MS4A12 is a colon-selective store-operated calcium channel promoting malignant cell processes. Cancer research 68: 3458–3466. [DOI] [PubMed] [Google Scholar]
- 47.Liu S, Hasegawa H, Takemasa E, Suzuki Y, Oka K, Kiyoi T, Takeda H, Ogasawara T, Sawasaki T, Yasukawa M, and Maeyama K 2017. Efficiency and Safety of CRAC Inhibitors in Human Rheumatoid Arthritis Xenograft Models. J Immunol 199: 1584–1595. [DOI] [PubMed] [Google Scholar]
- 48.Fasolato C, Hoth M, Matthews G, and Penner R 1993. Ca2+ and Mn2+ influx through receptor-mediated activation of nonspecific cation channels in mast cells. Proc Natl Acad Sci U S A 90: 3068–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Penner R, Matthews G, and Neher E 1988. Regulation of calcium influx by second messengers in rat mast cells. Nature 334: 499–504. [DOI] [PubMed] [Google Scholar]
- 50.Kuno M, and Kimura M 1992. Noise of secretagogue-induced inward currents dependent on extracellular calcium in rat mast cells. J Membr Biol 128: 53–61. [DOI] [PubMed] [Google Scholar]
- 51.Freichel M, Almering J, and Tsvilovskyy V 2012. The Role of TRP Proteins in Mast Cells. Front Immunol 3: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandford AJ, Shirakawa T, Moffatt MF, Daniels SE, Ra C, Faux JA, Young RP, Nakamura Y, Lathrop GM, Cookson WO, and et al. 1993. Localisation of atopy and beta subunit of high-affinity IgE receptor (Fc epsilon RI) on chromosome 11q. Lancet 341: 332–334. [DOI] [PubMed] [Google Scholar]
- 53.Stafford AN, Rider SH, Hopkin JM, Cookson WO, and Monaco AP 1994. A 2.8 Mb YAC contig in 11q12-q13 localizes candidate genes for atopy: Fc epsilon RI beta and CD20. Human molecular genetics 3: 779–785. [DOI] [PubMed] [Google Scholar]
- 54.Lympany P, Welsh KI, Cochrane GM, Kemeny DM, and Lee TH 1992. Genetic analysis of the linkage between chromosome 11q and atopy. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology 22: 1085–1092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


