Abstract
NMDA receptors are ionotropic glutamate receptors that mediate excitatory neurotransmission. The diverse functions of these receptors are tuned by deploying different combinations of GluN1 and GluN2 subunits (GluN2A-D) to form either diheteromeric NMDA receptors, which contain two GluN1 and two identical GluN2 subunits, or triheteromeric NMDA receptors, which contain two GluN1 and two distinct GluN2 subunits. Here, we characterize PTC-174, a novel positive allosteric modulator (PAM) of receptors containing GluN2C or GluN2D subunits. PTC-174 potentiates maximal current amplitudes by 1.8-fold for diheteromeric GluN1/2B receptors and by >10-fold for GluN1/2C and GluN1/2D receptors. PTC-174 also potentiates responses from triheteromeric GluN1/2B/2D and GluN1/2A/2C receptors by 4.5-fold and 1.7-fold, respectively. By contrast, PTC-174 produces partial inhibition of responses from diheteromeric GluN1/2A and triheteromeric GluN1/2A/2B receptors. PTC-174 increases potencies of co-agonists glutamate and glycine by 2- to 5-fold at GluN1/2C and GluN1/2D receptors, and NMDA receptor activation facilitates allosteric modulation by PTC-174. At native NMDA receptors in GluN2D-expressing subthalamic nucleus neurons, PTC-174 increases the amplitude of responses to NMDA application and slows the decay of excitatory postsynaptic currents (EPSCs) evoked by internal capsule stimulation. Furthermore, PTC-174 increases the amplitude and slows the decay of EPSCs in hippocampal interneurons, but has not effect on the amplitudes of NMDA receptor-mediated EPSCs in hippocampal CA1 pyramidal neurons. Thus, PTC-174 provides a useful new pharmacological tool to investigate the molecular pharmacology and physiology of GluN2C- and GluN2D-containing NMDA receptors.
Keywords: NMDA receptor pharmacology, synaptic transmission, positive allosteric modulator, subthalamic nucleus, hippocampal interneurons, electrophysiology
Graphical Abstract
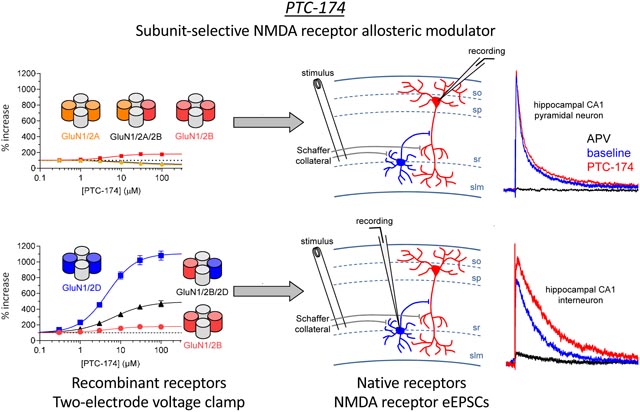
1. Introduction
NMDA receptors are glutamate-gated cation channels that mediate excitatory neurotransmission in the central nervous system (CNS) by carrying the slow component of the depolarizing postsynaptic current activated by synaptic glutamate release (Traynelis et al., 2010; Hansen et al., 2017). NMDA receptors also serve as detectors of co-incident pre- and post-synaptic activity to play a critical role in activity-dependent regulation of synaptic strength (Malenka and Bear, 2004; Citri and Malenka, 2008). Additional functions of NMDA receptors include regulation of presynaptic neurotransmitter release (Duguid and Smart, 2009) and metabotropic signaling that is independent of channel activity (Dore et al., 2016).
The diverse functions of NMDA receptors are tuned by differences in receptor subunit composition (Cull-Candy and Leszkiewicz, 2004; Paoletti et al., 2013; Glasgow et al., 2015). The majority of NMDA receptors in the CNS are composed of two GluN1 and two GluN2 subunits (Karakas and Furukawa, 2014; Lee et al., 2014). GluN1, encoded by a single gene and expressed as 8 splice variants (Durand et al., 1992; Nakanishi et al., 1992; Sugihara et al., 1992; Hollmann et al., 1993; Zukin and Bennett, 1995), harbors a binding site that must be occupied by glycine or D-serine for channel gating by glutamate binding to GluN2 (Kleckner and Dingledine, 1988; Benveniste and Mayer, 1991; Clements and Westbrook, 1991). There are 4 uniquely coded GluN2 subunits, GluN2A, GluN2B, GluN2C, and GluN2D (Ishii et al., 1993; Monyer et al., 1994; Paoletti et al., 2013). In forebrain, GluN2A- and GluN2B-containing receptors are deployed as diheteromeric GluN1/2A and GluN1/2B or as triheteromeric GluN1/2A/2B receptors at pyramidal neuron synapses and by striatal medium spiny neurons (Cull-Candy et al., 2001; Rauner and Kohr, 2011; Soares and Lee, 2013). Our understanding of the functions of GluN2A- and GluN2B-containing receptors has been facilitated by the availability of highly GluN2B-selective negative allosteric modulators (NAMs)1 (Chenard and Menniti, 1999; Layton et al., 2006). Newly described GluN2A-selective NAMs and PAMs are likely to prove equally useful in this regard (Hackos et al., 2016; Volkmann et al., 2016; Yi et al., 2016).
Compared to diheteromeric GluN1/2A and GluN1/2B receptors, recombinant diheteromeric GluN1/2C and GluN1/2D receptors have reduced channel conductance and are less sensitive to Mg2+ block (Traynelis et al., 2010; Hansen et al., 2017). Notably, the channels of these diheteromeric receptors have low probability to open in response to glutamate binding, but once open they deactivate slowly, particularly in the case of GluN1/2D. In neurons, available evidence indicates that GluN2C and GluN2D are primarily, if not exclusively, assembled with GluN2A and GluN2B, respectively, as triheteromeric GluN1/2A/2C or GluN1/2B/2D receptors. GluN2C is expressed with GluN2A in thalamic reticular neurons and cerebellar granule cells (Farrant et al., 1994; Monyer et al., 1994, Bhattacharya et al., 2018). GluN2D is most highly and widely expressed with GluN2B in early CNS development, but subsequently, GluN2D expression significantly recedes to specific neuronal populations in the adult CNS (Monyer et al., 1994; Standaert et al., 1994; Yamasaki et al., 2014). GluN2D is expressed at multiple loci within the basal ganglia circuit, including the subthalamic nucleus (STN) and globus pallidus (Standaert et al., 1994), dopaminergic neurons of the substantia nigra (Jones and Gibb, 2005; Brothwell et al., 2008), and striatal cholinergic interneurons (Zhang et al., 2014; Zhang and Chergui, 2015). In addition, GluN2D is co-expressed with GluN2B in neurons of lamina 1 in spinal cord (Hildebrand et al., 2014), cerebellar Golgi cells (Misra et al., 2000; Brickley et al., 2003), neurons in the hypothalamic supraoptic nucleus (Hagino et al., 2010), and GABAergic interneurons in the hippocampus (von Engelhardt et al., 2015; Perszyk et al., 2016; Swanger et al., 2018).
Recently, progress has been made in developing PAMs and NAMs of GluN2C- and GluN2D-containing receptors (Costa et al., 2010; Mosley et al., 2010; Mullasseril et al., 2010; Acker et al., 2011; Hansen and Traynelis, 2011; Khatri et al., 2014; Zimmerman et al., 2014; Swanger et al., 2018). In this report, we describe PTC-174 as a unique new probe for studies of GluN2C or GluN2D subunit-containing receptors. In recombinant receptors expressed in oocytes, PTC-174 is a highly efficacious PAM for GluN1/2C and GluN1/2D receptors and displays an allosteric interaction to increase co-agonist glutamate and glycine potencies. PTC-174 robustly enhances triheteromeric GluN1/2B/2D, but only weakly potentiates triheteromeric GluN1/2A/2C receptors, suggesting the compound may be a functional GluN2D-selective PAM in native tissues.
2. Materials and Methods
2.1. Reagents
APV (2-Amino-5-phosphonovalerate), 7-chlorokynurenic acid, kynurenic acid, kainate, concanavalin A, DNQX (6,7-Dinitroquinoxaline-2,3-dione), NMDA (N-methyl-D-aspartate), gabazine, and QX-314 (2-[(2,6-Dimethylphenyl)amino]-N,N,N-triethyl-2-oxoethanaminium bromide) were from Tocris (Bio-Techne Corp., Minneapolis, MN), Sigma-Aldrich (St. Louis, MO), or Hello Bio (Princeton, NJ). (+)-CIQ ((3-Chlorophenyl){6,7-dimethoxy-1-[(4-methoxyphenoxy)methyl]-3,4-dihydro-2(1H)-isoquinolinyl}methanone) was from BRANDT Labs (Atlanta GA). PTC-174 (8-(6-(tert-butyl)pyridin-3-yl)-6-oxo-3,4-dihydro-2H,6H-pyrimido[2,1-b][1,3]thiazine-7-carbonitrile) is disclosed in patent application PCT/US2017/068135 (compound 174) and was synthesized by Chinglu Pharmaceutical Research (Newington, CT).
2.2. DNA constructs
The cDNAs for rat GluN1–1a (GenBank: U08261; hereafter GluN1), rat GluN2A-D (D13211, U11419, M91563, L31611), GluA1-GluA4 (flop isoforms; X17184, M85035, M85036, M85037), GluK1 (M83561), and GluK2 (Z11548) were provided by S. Heinemann (Salk Institute), S. Nakanishi (Osaka Bioscience Institute), and P. Seeburg (University of Heidelberg).
Expression of rat triheteromeric NMDA receptors used a method previously described in detail (Hansen et al., 2014; Bhattacharya et al., 2018; Yi et al., 2019). This method involves fusing peptides (C1 and C2) containing GABAB receptor leucine zipper motifs and endoplasmic reticulum (ER) retention signals to the C-termini of GluN2 subunits. These tags cause ER retention of the expressed proteins. However, assembly of subunits containing two different tags results in coiled-coil formation between C1 and C2, which masks the ER retention signals and allows receptor trafficking to the cell surface. Triheteromeric GluN1/2A/2B expression used subunits 2AC1 and 2BC2 that contain the GluN2A C-terminal domain (CTD) with C1 and C2 peptide tags, respectively (Hansen et al., 2014). Triheteromeric GluN1/2B/2D expression used subunits 2BC1 and 2DC2 that contain the GluN2A C-terminal domain (CTD) with C1 and C2 peptide tags, respectively (Yi et al., 2019). Triheteromeric GluN1/2A/2C receptors used 2AC1 containing the C1 tag and 2CC2-NK containing the GluN2A CTD with the C2 tag as well as the N614K mutation in the channel pore, a substitution that prevents Mg2+ block of the channel (Bhattacharya et al., 2018). This additional modification to GluN2C was utilized because GluN1/2A receptors have open probabilities 30- to 50-fold higher than that of GluN1/2C. Thus, even a small proportion of GluN1/2AC1/2AC1 receptors that escape to the cell surface could confound interpretation of responses from GluN1/2A/2C receptors. Recording of the GluN1/2A C1/2C C2-NK receptors in the presence of 1 mM Mg2+ blocks any small proportion of escaped GluN1/2AC1/2AC1 receptors while leaving GluN1/2A C1/2C C2-NK unaffected (Hatton and Paoletti, 2005; Bhattacharya et al., 2018). In all cases, the contribution of currents from “escaped” receptors to the total current response was determined on the same day of the experiment with triheteromeric receptors as previously described (Hansen et al., 2014; Bhattacharya et al., 2018; Yi et al., 2019) and data were only utilized from experiments in which the “escape” current responses were <10% of the total current responses.
2.3. Two-electrode voltage-clamp electrophysiology.
Expression of NMDA receptor subtypes in Xenopus oocytes, cRNA injection, and maintenance of the oocytes were performed essentially as previously described (Hansen et al., 2013). Briefly, DNA constructs encoding the individual subunits were linearized by restriction enzymes and used as templates for in vitro cRNA transcription. Oocytes were purchased from Xenopus 1 (Dexter, MI) or Ecocyte Bio Science (Austin, TX), injected with cRNA, and used 2–4 days later. Recordings were performed at room temperature (21–23 C°) and at a holding potential of −40 mV using a two-electrode voltage-clamp amplifier (Oocyte Clamp OC-725C; Warner Instrument Corp, Hamden, CT). Oocytes were placed in a custom-made chamber and continuously perfused (bath application of approximately 5 ml/min) with extracellular recording solution containing 90 mM NaCl, 1 mM KCl, 10 mM HEPES, 0.5 mM BaCl2, and 0.01 mM EDTA (pH 7.4 with NaOH). Oocytes expressing GluN2A- or GluN2B-containing receptors were injected with 50 nl or 20 nl, respectively, of 50 mM BAPTA approximately 10–30 min before recordings to prevent activity-dependent increases in response amplitude. Oocytes expressing GluK1 and GluK2 kainate receptors were incubated in 1 mg/ml concanavalin A dissolved in extracellular recording solution for 10 minutes before recording to minimize desensitization. Test compounds, PTC-174 and (+)-CIQ, were dissolved in DMSO to make 100 mM stock solutions, and the concentration of DMSO was kept constant (< 0.1%) in all recording solutions. In these experiments, PTC-174 did not display any solubility issues at concentrations up to 100 μM (higher concentrations were not evaluated). Each test condition was replicated across a minimum of 4 oocytes prepared from 2 or more frogs. Concentration-response data was fitted to the Hill equation using Graphpad Prism (Graphpad, San Diego, CA) as previously described (Hansen et al., 2013). Where noted in the text, logEC50 values were compared for statistically significant differences (p < 0.05).
2.4. Mouse brain slice electrophysiology.
Brain slices were prepared from both male and female P12–18 C57BL/6J mice obtained from The Jackson Laboratory (Sacramento, CA). Animals were housed in specific pathogen free conditions, maintained on a 12-h light/dark cycle, with free access to food and water. All experimental procedures were carried out at the University of Montana (Missoula, MT) in accordance with the Guide for the Care and Use of Laboratory Animals (U.S. National Institutes of Health) and the Institutional Animal Care and Use Committee of the University of Montana approved all experimental protocols.
Sagittal brain slices containing STN were obtained as described previously (Swanger et al., 2015). Transverse brain slices containing hippocampus were obtained with magic cut as described previously (Bischofberger et al., 2006). For both preparations, mice of either sex were anesthetized with isoflurane and then perfused transcardially with an ice-cold oxygenated solution containing: 3 mM KCl, 24 mM NaHCO3, 1.25 mM NaH2PO4, 10 mM glucose, 230 mM sucrose, 0.5 mM CaCl2, and 10 mM MgSO4. After decapitation, the brain was removed and immediately submerged in an oxygenated cutting solution containing: 130 mM NaCl, 3 mM KCl, 24 mM NaHCO3, 1.25 mM NaH2PO4, 10 mM glucose, 1 mM CaCl2, and 10 mM MgSO4. Hemispheres were glued onto a stage of a vibrating microtome (VT1200S, Leica Microsystems Inc., Buffalo Grove, IL). Sagittal slices (300 μm) containing STN or transverse slices (300 μm) containing ventral hippocampus of both hemispheres were cut in the ice-cold oxygenated cutting solution, then collected and incubated in cutting solution at room temperature for at least 1 hour before use.
Slices were transferred to a recording chamber continually perfused with aCSF solution containing: 130 mM NaCl, 3 mM KCl, 24 mM NaHCO3, 1.25 mM NaH2PO4, 10 mM glucose, 2 mM CaCl2, and 0.2 mM MgSO4, saturated with 95% O2 and 5% CO2, pH 7.3, with temperature maintained at 32°C (Warner Instruments, Hamden, CT). The compounds were bath-applied in the aCSF solution at a rate of 2–3 ml/min. Slices were visualized using infrared video microscopy on a SliceScope Pro 2000 (Scientifica, East Sussex, United Kingdom).
Recording electrodes were fabricated with 2–4 MΩ tip resistance using a P-1000 micropipette puller (Sutter Instruments, Novato, CA) and were filled with a solution containing: 120 mM Cs-methanesulfonate, 15 mM CsCl, 10 mM tetraethylammonium chloride, 10 mM HEPES, 8 mM NaCl, 3 mM Mg-ATP, 1.5 mM MgCl2, 10 mM QX-314, 0.3 mM Na-GTP, and 0.2 mM EGTA, pH 7.3, osmolarity 295–305 milliosmol/L. Whole-cell recordings were obtained and filtered at 4 kHz using a Multiclamp 700B amplifier (Molecular Devices, San Jose, CA) and digitized at 20 kHz (Digidata 1440A with pClamp10 software).
Whole-cell currents from STN neurons were induced by pressure applying brief pulses (20 psi; 2–20 ms) of NMDA (500 μM) plus glycine (10 μM) through a borosilicate capillary tube (~3 MΩ) using a custom-built pressure ejection system (Forman et al., 2016). DNQX (20 μM) and gabazine (10 μM) were bath-applied in the aCSF to block non-NMDA ionotropic glutamate receptors and GABAA receptors, respectively, and recordings were obtained at a holding potential of −40 mV. When stable measurements were obtained, baseline (5–10 min), treatment/vehicle (10–15 min), and APV at 400 μM (5 min) were sequentially recorded with a delivered puff of NMDA plus glycine at 30–60 s intervals.
EPSCs were stimulated by 50–500 μA current injection for 0.1 ms using a bipolar tungsten stimulating electrode (MX211EW(PC3), FHC, Bowdoin, ME) positioned near the internal capsule fibers rostral to the STN (Baufreton et al., 2009; Yamawaki et al., 2012) or positioned at Schaffer collateral for hippocampal recordings. EPSCs were stimulated every 30–60 s, and recorded at a holding potential of −40mV for STN neurons and +40 mV for hippocampal neurons. The solutions to isolate the NMDA receptor-mediated component of the EPSCs and application sequence were the same as that mentioned in the puff experiment. In some experiments, alternating puff-induced NMDA receptor currents and NMDA receptor-mediated component of the eEPSCs were recorded at an interval of 1 min from the same cell.
In all neuronal recordings, stable baseline EPSC or puff recordings were measured for 5–10 min, followed by 10–15 min recordings with 10 μM PTC-174 delivered in the bath perfusion. At the end of the recordings, 400 μM DL-APV was delivered for full inhibition of NMDA receptor-mediated responses. Stock solutions of 100 mM PTC-174 in DMSO were prepared to achieve an added DMSO concentration of 0.01% (i.e. vehicle is 0.01% DMSO) when added to the ACSF recording solution.
All electrophysiological data from mouse brain slices were analyzed with Axograph X (Axograph Scientific, Sydney, Australia). Data are presented as mean ± S.E.M. (n = number of recordings) with significance set at P<0.05 for statistical analyses (paired or unpaired t-test).
2.5. Whole-cell patch-clamp electrophysiology.
HEK293 cells were plated onto poly-D-lysine-coated (0.1 mg/ml) glass coverslips approximately 48 hours before experiments and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with GlutaMax-I and sodium pyruvate (GIBCO; Thermo Fisher Scientific) supplemented with 10% dialyzed fetal bovine serum (GIBCO; Thermo Fisher Scientific), 10 U/ml penicillin, and 10 mg/ml streptomycin (GIBCO; Thermo Fisher Scientific). Cells were transfected approximately 24 hours following plating using the calcium phosphate precipitation method with plasmid cDNAs encoding GFP, GluN1, and GluN2 subunits at a ratio of 1:1:1. The antagonists D,L-APV (200 μM) and 7-chlorokynurenic acid (200 μM) were added to the culture medium to prevent NMDA receptor-mediated cytotoxicity. Experiments were performed approximately 24 hours following transfection. Whole-cell voltage-clamp recordings (Axopatch 200B; Molecular Devices, Sunnyvale, CA) were performed at room temperature (20°C) at a holding potential of −60 mV. Recording electrodes with open-tip resistance of 2–4 MΩ were made from thin-wall glass micropipettes (TW150F-4; World Precision Instruments, Sarasota, FL) pulled using a horizontal puller (P-1000; Sutter Instruments, Novato, CA). The electrodes were filled with internal solution containing 110 mM D-gluconic acid, 110 mM CsOH, 30 mM CsCl, 5 mM HEPES, 4 mM NaCl, 0.5 mM CaCl2, 2mM MgCl2, 5 mM BAPTA, 2 mM NaATP, and 0.3 mM NaGTP (pH 7.35 with CsOH). The extracellular recording solution was composed of 150 mM NaCl, 10 mM HEPES, 3 mM KCl, 0.5 mM CaCl2, and 10 μM EDTA (pH 7.4 with NaOH). Holding potentials were not corrected for the liquid junction potential, which was which was measured to be +10.1 mV (Yi et al., 2018). Rapid (5 ms) or long pulse (2 s) solution exchange was achieved on lifted cells with a two-barrel theta-glass pipette controlled by a piezoelectric translator (MXPZT-300; Siskiyou Corporation, Grants Pass, OR) and the 10%–90% open-tip solution exchange times were 0.6–0.8 ms. Only cells with initial current responses of less than 1000 pA and series resistance of less than 10 MΩ were used for data analyses.
2.6. Data analysis.
Concentration-response data from oocytes were analyzed with GraphPad Prism (GraphPad Software, La Jolla, CA). Agonist concentration-response data for individual oocytes were fitted to the following Hill equation:
where Imax is the maximum current in response to the agonist, nH is the Hill slope, [A] is the agonist concentration, and EC50 is the agonist concentration that produces half-maximum response.
The deactivation time courses of current responses from recordings of brain slices or HEK293 cells were analyzed by Axograph X (Axograph Scientific, Sydney, Australia) and fitted using:
where τfast and τslow are the deactivation time constants for the fast and slow components, respectively, and Ifast and Islow are the current amplitudes of the fast and slow components, respectively. Weighted deactivation time constants were calculated using:
Data are presented as mean ± S.E.M and n is the cells with significance set at P<0.05 for statistical analyses.
3. Results
3.1. Potency and efficacy at diheteromeric NMDA receptors.
PTC-174 was characterized at recombinant rat GluN1/2A-2D NMDA receptor subtypes expressed in Xenopus oocytes using two-electrode voltage-clamp electrophysiology. NMDA receptor activation was induced by bath application of maximally effective concentrations of glutamate plus glycine in the presence of different concentrations of the PAM. The current response induced by maximum glutamate plus glycine was defined as 100% and potentiation was calculated relative to this value.
PTC-174 induced a concentration-dependent increase in currents mediated by diheteromeric GluN1/2C or GluN1/2D (Figure 1 and Table 1). The compound maximally increased current responses more than 10-fold, with similar potency at both receptors. PTC-174 also potentiated diheteromeric GluN1/2B receptors with similar potency as GluN1/2C or GluN1/2D, but the efficacy for potentiation of GluN1/2B was only 1.8-fold (Figure 1 and Table 1). In contrast, PTC-174 caused concentration-dependent partial inhibition of diheteromeric GluN1/2A receptors with lower potency compared to that of the PAM activities (Figure 1 and Table 1).
Figure 1. Effects of PTC-174 on recombinant NMDA receptor subtypes.
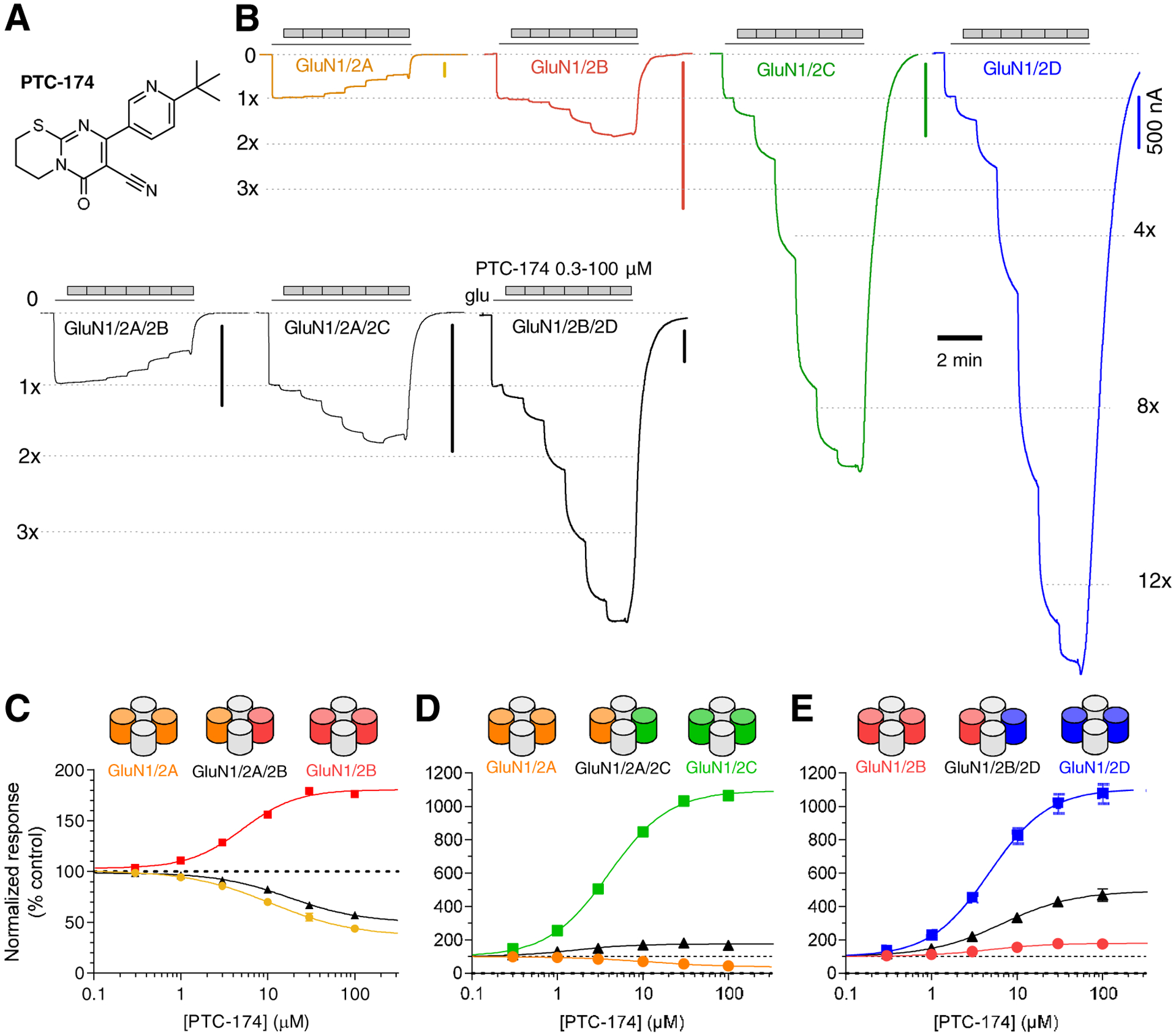
A) Chemical structure of PTC-174. B) Representative recordings of pharmacological evaluation of PTC-174 measured using two-electrode voltage-clamp electrophysiology at recombinant diheteromeric and triheteromeric NMDA receptor subtypes. The receptors were activated by maximally efficacious concentrations of glutamate plus glycine (100 μM and 50 μM, respectively) followed by increasing concentrations of PTC-174. Vertical scale bars indicate 500 nA and vertical scale bar indicates 2 min. Responses to glutamate plus glycine alone are indicated by 1x. C-E) PTC-174 concentration-response data at recombinant NMDA receptor subtypes. Responses to glutamate plus glycine alone was defined as 100% (control) and the responses to incremental additions of PTC-174 were expressed as a percentage of this value. Data are mean ± SEM from 6–16 oocytes. Note that for some data points, error bars are smaller than symbols (i.e. contained within the symbols). See Table 1 for potencies and maximal modulation.
Table 1.
Modulation of recombinant NMDA receptor subtypes by PTC-174.
| EC50 (μM) | Maximal modulation (% control) | nH | n | |
|---|---|---|---|---|
| GluN1/2A | 11.3 ± 1.6 | 39 ± 4 | −1.1 | 7 |
| GluN1/2B | 5.0 ± 0.3 | 180 ± 4 | 1.3 | 16 |
| GluN1/2C | 4.1 ± 0.2 | 1095± 29 | 1.2 | 12 |
| GluN1/2D | 5.7 ± 0.4 | 1265 ± 67 | 1.2 | 16 |
| GluN1/2AC1/2BC2 | 19.7 ± 1.5 | 47 ± 2 | −1.0 | 8 |
| GluN1/2BC1/2DC2 | 6.9 ± 0.5 | 447 ± 25 | 1.0 | 10 |
| GluN1/2CC1/2CC2-nk | 5.0 ± 0.5 | 961 ± 39 | 1.4 | 6 |
| GluN1/2CC1/2CC2-nk (Mg2+) | 4.5 ± 0.2 | 902 ± 18 | 1.2 | 8 |
| GluN1/2AC1/2CC2-nk (Mg2+) | 1.7 ± 0.1 | 169 ± 3 | 1.2 | 12 |
Recombinant diheteromeric and triheteromeric NMDA receptor subtypes were expressed in Xenopus oocytes and concentration-response data for PTC-174 were measured using two-electrode voltage-clamp electrophysiology. Triheteromeric NMDA receptors are expressed as described in Materials and Methods. 2CC2-NK indicates a GluN2C subunit containing the GluN2A CTD with the C2 tag, as well as the N614K mutation in the channel pore to prevent Mg2+ block of the channel. Concentration-response data generated in the presence of 1 mM Mg2+ are indicated by (Mg2+). The receptors were activated with maximally efficacious concentrations of glutamate plus glycine (100 μM and 50 μM, respectively) in the absence and presence of increasing, sequentially added concentrations of PTC-174. The negative Hill slope (nH) for GluN1/2A and GluN1/2AC1/2BC2 indicates inhibition of glutamate plus glycine responses by PTC-174. Maximal modulation is determined as percent of control in the same recording, which is the response to glutamate plus glycine in the absence of PTC-174. Data are presented as mean ± SEM, and n is the number of oocytes used to generate the data.
3.2. Potency and efficacy at triheteromeric NMDA receptors.
GluN2D is co-expressed with GluN2B in neurons of several brain regions (Standaert et al., 1994; Jones and Gibb, 2005; Brothwell et al., 2008; Zhang et al., 2014; Zhang and Chergui, 2015). Thus, we examined the effect of PTC-174 on triheteromeric GluN1/2B/2D receptors expressed as previously described (Yi et al., 2019). PTC-174 potentiated the response of GluN1/2B/2D receptors with potency similar to that at diheteromeric GluN1/2D and GluN1/2B. Maximal potentiation was 4.5-fold, which is intermediate between that for GluN1/2D (13-fold) and GluN1/2B (1.8-fold) (Figure 1 and Table 1).
GluN2C is co-expressed with GluN2A in cerebellum and in thalamic reticular neurons (Farrant et al., 1994; Monyer et al., 1994, Bhattacharya et al., 2018). Thus, we also examined the activity of PTC-174 on triheteromeric GluN1/2A/2C receptors. For this analysis, GluN2CC2-NK was co-expressed with GluN2AC1. GluN2CC2-NK harbors the N614K mutation in the channel pore that prevents Mg2+ block of the channel (Bhattacharya et al., 2018). Thus, experiments were conducted in the presence of 1 mM Mg2+ to block any small proportion of currents from GluN1/2AC1/2AC1 that escaped the ER (see section 2.2). In control experiments, the N614K mutation had little effect on the potency and efficacy of PTC-174 (Table 1). The PAM potency of PTC-174 at GluN1/2C (4.1 ± 0.2 μM) and GluN1/2CC1/2CC2-NK (4.5 ± 0.2 μM) receptors was not statistically different (unpaired t-test, t (18) = 1.3558, p = 0.19). PAM efficacy of PTC-174 at GluN1/2CC1/2CC2-NK (902 ± 18% of the response to glutamate and glycine alone) was slightly, albeit statistically significant, lower than at GluN1/2C (1095 ± 29%, unpaired t-test, t(18) = 4.9918, p = 0.001). However, when GluN2CC2-NK was co-expressed with GluN2AC1, the PAM efficacy of PTC-174 was reduced to 1.7-fold (Figure 1 and Table 1).
GluN2A and GluN2B are co-expressed in pyramidal neurons in the hippocampus and cortex of the adult rodent brain (Luo et al., 1997; Gray et al., 2011; Rauner and Köhr, 2011; Sheng et al., 1994; Yi et al., 2019). PTC-174 produced partial inhibition of triheteromeric GluN1/2A/2B receptors expressed as previously described (Yi et al., 2019) with a potency and maximal inhibition similar to the inhibition of diheteromeric GluN1/2A receptors (Figure 1 and Table 1). In summary, these data demonstrate that PTC-174 potentiate triheteromeric GluN1/2B/2D by 4.5-fold and GluN1/2A/2C receptors by 1.7-fold, whereas PTC-174 produce partial inhibition of triheteromeric GluN1/2A/2B receptors.
3.3. Effects on agonist potencies.
All PTC-174 concentration-response data in Figure 1 were generated in the presence of maximally effective concentrations of glutamate and glycine, indicating a mechanism at the level of channel activity once agonist binding has triggered channel opening. We also examined another potential PAM mechanism, namely that of increasing the potency of agonists for activating receptors. In these experiments, the effect of PTC-174 was compared to (+)-CIQ, the prototypical GluN2C/D-selective PAM (Mullasseril et al., 2010). Thus, we evaluated activation of GluN1/2D receptors by sequentially increasing concentrations of glutamate in the presence of a fixed maximal concentration of glycine (50 μM) and in the absence or presence of PTC-174 (10 μM) or (+)-CIQ (10 μM). The maximal fitted glutamate-induced currents in the absence or presence of PAM were defined as 100 % and the remainder of the responses expressed as a percentage of this maximum. We found that 10 μM PTC-174 increased glutamate potency at diheteromeric GluN1/2D by 2.5-fold (Figure 2A and Table 2). This difference between the EC50 values was statistically significant (unpaired t test, p < 0.0001, t(10) = 13.9). In contrast, 10 μM (+)-CIQ had no effect on glutamate potency (unpaired t test, t(10) = 0.66, p = 0.52, Figure 2C and Table 2). In similar experiments, oocytes incubated in the absence or presence of 10 μM PTC-174 or 10 μM (+)-CIQ were exposed sequentially to increasing concentrations of glycine in the presence of a fixed maximal concentration of glutamate (100 μM). As for glutamate potency, PTC-174 increased glycine potency at diheteromeric GluN1/2D receptors by 4.8 -fold (unpaired t test, t(9) = 22.96, p < 0.0001), whereas (+)-CIQ had no significant effect on glycine potency (unpaired t test, t(12) = 0.3576, p = 0.73, Figure 2B,2D).
Figure 2. Effects of PTC-174 and CIQ on glutamate and glycine potencies.
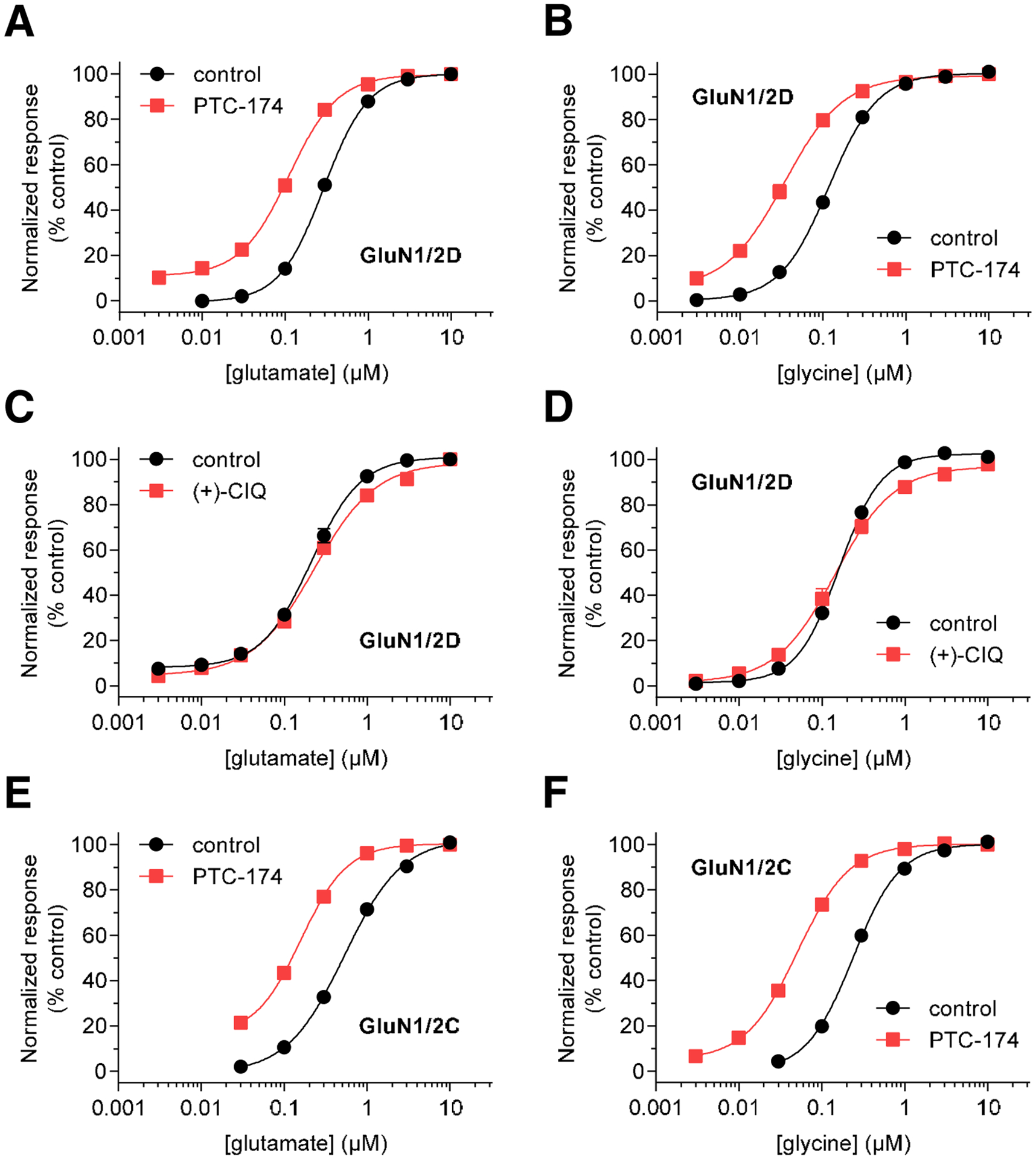
A-D) Glutamate (A,C) and glycine (B,D) concentration-response data in the presence and absence of 10 μM PTC-174 (A,B) or 10 μM (+)-CIQ (C,D) were measured using two-electrode voltage-clamp electrophysiology at recombinant GluN1/2D receptors. Responses to maximal glutamate plus glycine alone was defined as 100% (control). E,F) Glutamate (E) and glycine (F) concentration-response data in the presence and absence of 10 μM PTC-174 were measured using two-electrode voltage-clamp electrophysiology at recombinant GluN1/2C receptors. Data are mean ± SEM from 5–8 oocytes. See Table 2 for EC50 values.
Table 2.
Effects of PTC-174 and (+)-CIQ on agonist potencies at NMDA receptor subtypes.
| glutamate EC50 (μM) | nH | n | glycine EC50 (μM) | nH | n | ||
|---|---|---|---|---|---|---|---|
| GluN1/2C | control | 0.52 ± 0.05 | 1.3 | 6 | 0.24 ± 0.03 | 1.6 | 6 |
| + 10 μM PTC-174 | 0.19 ± 0.02* | 1.8 | 6 | 0.05 ± 0.00* | 1.4 | 6 | |
| GluN1/2D | control | 0.30 ± 0.01 | 1.7 | 6 | 0.12 ± 0.01 | 1.5 | 6 |
| + 10 μM PTC-174 | 0.11 ± 0.01* | 1.5 | 6 | 0.03 ± 0.01* | 1.2 | 5 | |
| GluN1/2D | control | 0.21 ± 0.02 | 1.5 | 6 | 0.16 ± 0.01 | 1.8 | 8 |
| + 10 μM (+)-CIQ | 0.23 ± 0.01 | 1.2 | 6 | 0.15 ± 0.02 | 1.2 | 6 | |
Recombinant GluN1/2C and GluN1/2D NMDA receptor subtypes were expressed in Xenopus oocytes and concentration-response data for glutamate and glycine were measured using two-electrode voltage-clamp electrophysiology. Glutamate EC50 values were generated by activating the receptors with increasing concentrations of glutamate in the continuous presence of 50 μM glycine in the absence and presence of 10 μM PTC-174 or 10 μM (+)-CIQ. Glycine EC50 values were generated by activating the receptors with increasing concentrations of glycine in the continuous presence of 100 μM glutamate in the absence and presence of 10 μM PTC-174 or 10 μM (+)-CIQ. Data are presented as mean ± SEM, nH is the Hill slope, and n is the number of oocytes used to generate the data. Statistical tests were performed using logEC50 values and
indicates significantly different from control on the same receptor for the same agonist (P < 0.05; unpaired t-test).
At GluN1/2C receptors (Figure 2E, F), 10 μM PTC-174 also significantly increased glutamate potency by 3.3-fold (unpaired t test, t(10) = 5.975, p < 0.0001) and significantly increased glycine potency by 4.7-fold (unpaired t test, t(10) = 39.34, p < 0.0001). These effects at GluN1/2C receptors are very similar to those at GluN1/2D receptors, consistent with a conserved mechanism of action at these two receptor subtypes.
To determine if binding of PTC-174 affects the requirement for simultaneous glutamate and glycine binding for NMDA receptor activation, we evaluated responses to glutamate alone and glycine alone in the presence of PTC-174 at GluN1/2C receptors using two-electrode voltage-clamp electrophysiology (Figure 3). Application of glutamate only + PTC-174 or glycine only + PTC-174 resulted in negligible current responses of 1.5% ± 0.1% and 4.0% ± 0.9%, respectively, relative to the response activated by glutamate + glycine + PTC-174. This indicates that PTC-174 has negligible, if any, intrinsic ability to activate NMDA receptors and that the requirement of simultaneous glutamate and glycine co-agonist binding for receptor activation is unaffected by PTC-174. The small responses to glutamate + PTC-174 and glycine + PTC-174 are presumed to result from low levels of contaminating glycine and glutamate in the recording solution. The reduced efficacy of the competitive glutamate site antagonist APV (400 μM) for inhibition of responses to glutamate + glycine in the presence of PTC-174 is consistent with increased glutamate potency in the presence of PTC-174 (Figure 3).
Figure 3. Effects of PTC-174 on the requirement for co-agonists glutamate and glycine for NMDA receptor activation.
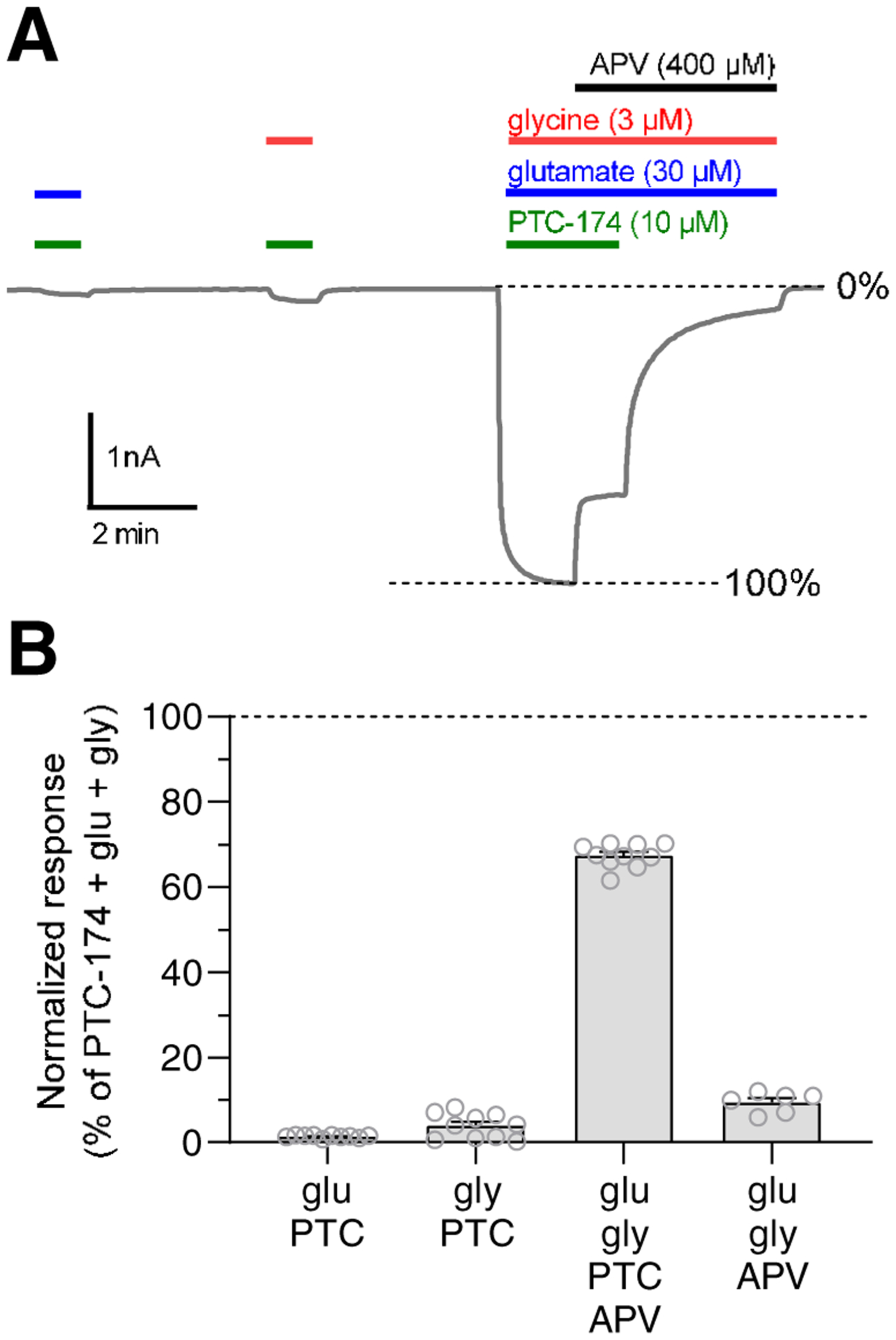
A) Representative two-electrode voltage-clamp recording shows responses to glutamate (blue) alone and glycine (green) alone, as well as glutamate + glycine, in the presence of PTC-174 (red) at recombinant rat GluN1/2C receptors. Inhibition of responses by the competitive glutamate antagonist, APV, is also shown (black). B) The bar graph summarizes responses shown in A at recombinant rat GluN1/2C receptors. Responses are normalized to the response to glutamate + glycine + PTC-174. Data are mean ± SEM from 10 oocytes. Open circles are responses from individual oocytes.
3.4. Selectivity of PTC-174 for NMDA receptors over AMPA and kainate receptors.
To evaluate the selectivity for modulation of NMDA receptors, we determined the effects of PTC-174 on responses from recombinant AMPA and kainate receptor (GluK1 and GluK2) subtypes using two-electrode voltage-clamp electrophysiology. PTC-174 (10 μM) did not affect current responses from AMPA (GluA1–4) or kainate (GluK1–2) receptors activated by 30 μM kainate or 100 μM glutamate, resepectively (Figure 4). In addition, we determined the effects of PTC-174 (10 μM) on responses from diheteromeric NMDA receptor subtypes activated by 10 μM glutamate in the presence of a low concentration of glycine (1 μM). As expected, PTC-174 produced robust ~10-fold potentiation of responses from GluN1/2C and GluN1/2D receptors and more modest 1.8-fold potentiation of responses from GluN1/2B receptors (Figure 4), consistent with the potency and efficacy of PTC-174 at these diheteromeric NMDA receptor subtypes (Figure 1 and Table 1). However, PTC-174 also potentiated responses from GluN1/2A by 1.6-fold (Figure 4), suggesting that PTC-174 enhances agonist potencies for GluN1/2A receptors similar to at GluN1/2C and GluN1/2D receptors (Figure 2 and Table 2). This potentiation of GluN1/2A by 10 μM PTC-174 is consistent with a mixed effect caused by some inhibition due to the relatively low antagonist potency of PTC-174 (IC50 11.3 μM; Table 1) and increased activation due to enhancement of glycine potency (EC50 is 1.1 μM at GluN1/2A in the absence of PTC-174; Yi et al. 2016). In summary, these results demonstrate that allosteric modulation by 10 μM PTC-174 is selective for NMDA receptors over AMPA and kainate receptors.
Figure 4. Selectivity of PTC-174 for NMDA receptors over AMPA and kainate receptors.
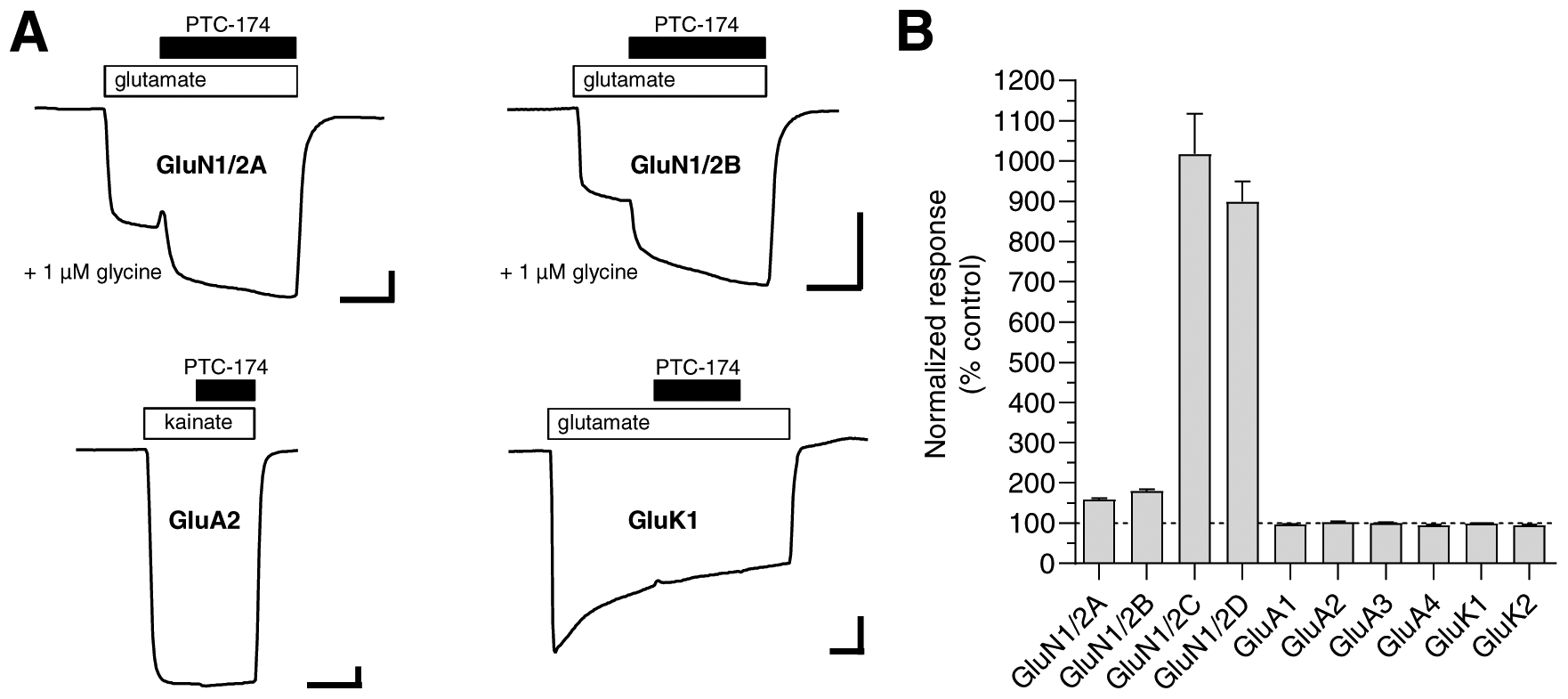
A) Representative two-electrode voltage-clamp recordings of current responses from recombinant glutamate receptors. Current responses were activated by 10 μM glutamate in the continuous presence of 1 μM glycine (NMDA receptors; GluN1/2A-D), 30 μM kainate (AMPA receptors; GluA1–4), or 100 μM glutamate (kainate receptors; GluK1–2) in the absence and presence of 10 μM PTC-174 as indicated above the recordings. Oocytes expressing kainate receptors were incubated with concanavalin A prior to the recordings in order to minimize desensitization (see Materials and Methods). Scale bars are 30 seconds (horizontal) and 100 nA (vertical). B) Summary of modulation by 10 μM PTC-174 of current responses from recombinant GluN1/2 NMDA receptors, GluA1–4 AMPA receptors, and GluK1–2 kainate receptors. Data are normalized to the control response in the absence of PTC-174 and are mean ± SEM of 5–8 oocytes.
3.5. Effects of PTC-174 on native NMDA receptors expressed by STN neurons.
The GluN2D subunit is expressed by neurons of the STN (Monyer et al., 1994; Standaert et al., 1994; Swanger et al., 2015), and (+)-CIQ has been shown to potentiate NMDA receptor-mediated responses of STN neurons (Swanger et al., 2015). Therefore, we examined the effect of PTC-174 on native NMDA receptor-mediated responses in STN neurons in mouse brain slices using whole-cell patch clamp electrophysiology. For these experiments, slices were superfused with aCSF containing 0.2 mM Mg2+ as well as 20 μM DNQX to block AMPA receptors and 10 μM gabazine to block GABAA receptors. First, STN neurons were voltage clamped at −40 mV and stimulated with NMDA plus glycine applied in the immediate vicinity of the patched cell using a picospritzer (Figure 5A). Under these experimental conditions, pulses of NMDA evoked current responses with peak amplitudes of 40–100 pA. These currents were eliminated by inclusion of the NMDA receptor antagonist APV in the superfusion solution (Figure 5B). When 10 μM PTC-174 was included in the superfusion solution, the NMDA-activated peak current responses were strongly potentiated (80.76 ± 8.02 vs 119.10 ± 9.76 pA for baseline vs PTC-174, paired t test, t(11) = 6.582 p <0.0001, Figure 5B, C). Next, we examined the effect of PTC-174 on amplitude and duration of EPSCs evoked in STN neurons by stimulation of the putative cortical input via the internal capsule. PTC-174 (10 μM) had no effect on EPSCs amplitudes (paired t test, t(8) = 1.198, p = 0.2635) (Figure 5D,E, Table 3), but did increase the weighted EPSC decay time constant by prolonging the slow component of the EPSC decay (111.0 ± 7.1 vs 166.7 ± 21.3 ms for baseline and PTC-174, paired t test, t(8) = 3.158, p = 0.0134, Figure 5F; Table 3).
Figure 5. Effects of PTC-174 on NMDA receptor responses in mouse STN neurons.
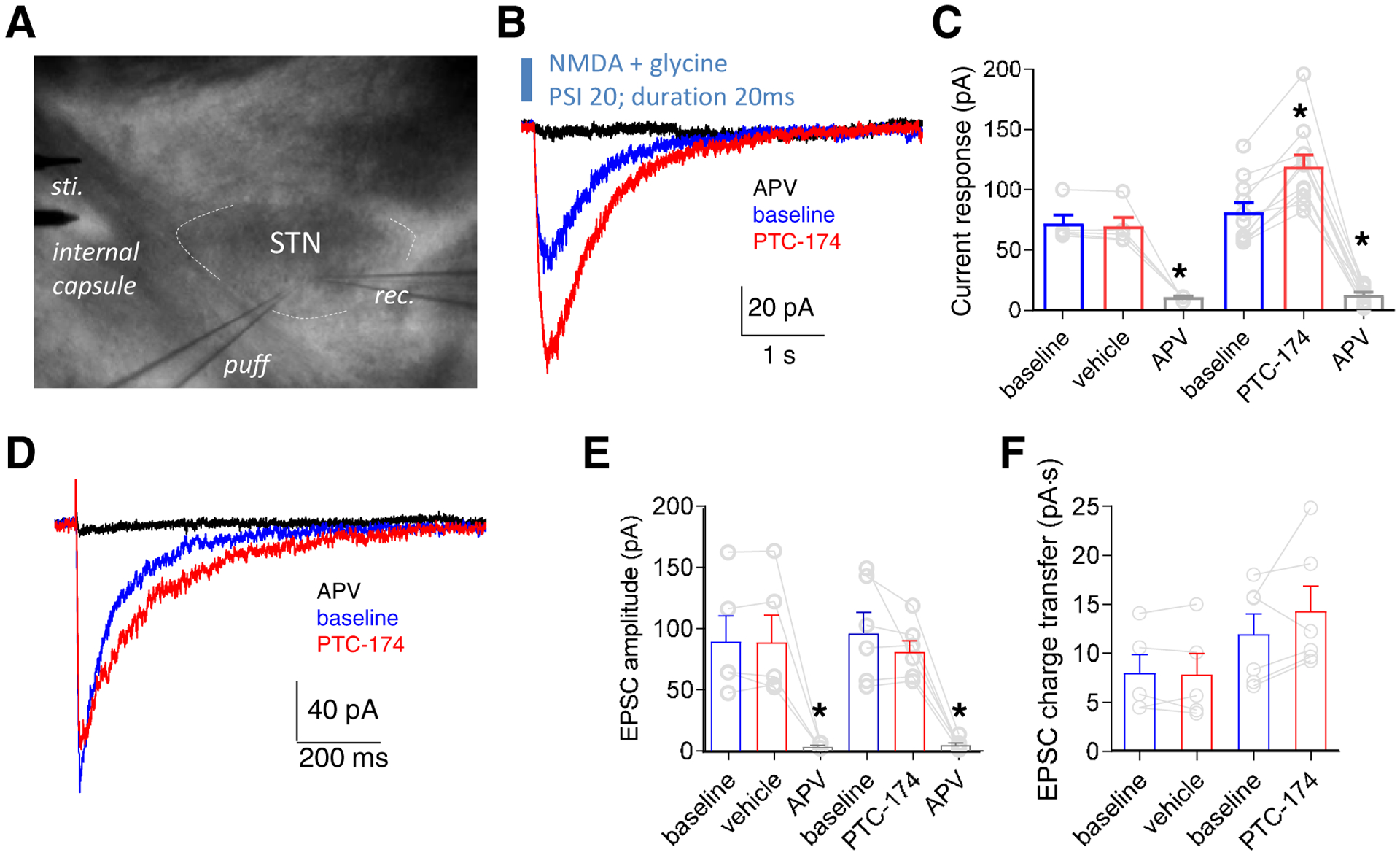
A) Sagittal brain slices that included the STN were prepared from P12–18 mice and superfused with the AMPA receptor antagonist DNQX and the GABAA receptor antagonist gabazine to isolate NMDA receptor-mediated currents. STN neurons were identified visually and pressure-induced puff electrode and bipolar stimulation electrode were positioned to activate extrasynaptic and synaptic NMDA receptors, respectively. B) STN neurons were voltage clamped at −40 mV and stimulated by brief applications of 0.5 mM NMDA plus 10 μM glycine via a pressure-induced puff electrode and the absence (baseline) and presence of bath-applied 10 μM PTC-174. Superfusion with the NMDA receptor antagonist APV (400 μM) completely blocked the response to NMDA. C) Summary of effects on peak responses by vehicle and 10 μM PTC-174 treatments for extrasynaptic NMDA receptor-mediated currents. * indicates significantly different from baseline (P < 0.05; paired t-test). D) Representative excitatory postsynaptic currents (EPSCs) from one STN neuron in response to electrode stimulation in the absence (baseline) and presence of bath-applied 10 μM PTC-174 (holding potential at −40 mV). Superfusion with the NMDA receptor antagonist APV (400 μM) completely blocked the NMDA receptor-mediated EPSCs. E) Summary of peak amplitudes for NMDA receptor-mediated EPSCs. * indicates significantly different from baseline (P < 0.05; paired t-test). F) Summary of charge transfer during NMDA receptor-mediated EPSCs. See Table 3 for summary of all the data.
Table 3.
Effects of PTC-174 on NMDA receptor-mediated EPSCs in distinct neuronal populations.
| τfast (ms) | τslow (ms) | % fast | τw (ms) | Peak amplitude (pA) |
Charge transfer (pA·S) | n | ||
|---|---|---|---|---|---|---|---|---|
| STN neurons | baseline | 36.0 ± 5.7 | 170.9 ± 18.5 | 60.3 ± 6.0 | 87.7 ± 4.7 | 97.6 ± 26.1 | 8.8 ± 2.2 | 4 |
| + vehicle | 38.5 ± 5.5 | 250.4 ± 42.3 | 67.6 ± 5.7 | 100.1 ± 5.1 | 100.2 ± 26.0 | 8.8 ± 2.4 | 4 | |
| STN neurons | baseline | 49.6 ± 5.5 | 226.2 ± 21.8 | 62.8 ± 4.2 | 111.0 ± 7.1 | 107.6 ± 12.8 | 12.9 ± 1.4 | 9 |
| + PTC-174 | 69.3 ± 10.1 | 397.7 ± 131.5 | 58.3 ± 5.0 | 166.7 ± 2.1* | 94.3 ± 11.8 | 15.9 ± 2.4 | 9 | |
| Hippocampal interneurons | baseline | 113.7 ± 27.2 | 321.4 ± 36.0 | 60.3 ± 12.7 | 191.6 ± 6.6 | 132.6 ± 20.1 | 26.9 ± 3.8 | 5 |
| + PTC-174 | 147.3 ± 46.5 | 522.4 ± 154.9 | 45.3 ± 14.5 | 292.0 ± 41.8* | 156.6 ± 23.3* | 49.8 ± 8.3* | 5 | |
| Hippocampal pyramidal neurons | baseline | 56.0 ± 3.3 | 382.5 ± 38.8 | 70.8 ± 2.5 | 150.1 ± 10.0 | 183.5 ± 27.8 | 29.1 ± 6.1 | 5 |
| + PTC-174 | 64.3 ± 9.3 | 416.1 ± 36.9 | 64.5 ± 5.3 | 187.3 ± 20.0* | 176.4 ± 24.0 | 35.7 ± 8.7 | 5 |
Sagittal mouse brain slices that included the STN were prepared from P12–18 mice and NMDA receptor-mediated EPSCs (holding potential at −40 mV) were evoked by electrical stimulation of the internal capsule before (baseline) or during superfusion with vehicle (0.01% DMSO) or 10 μM PTC-174. Transverse hippocampal brain slices were prepared from P12–18 mice and hippocampal CA1 interneurons in stratum radiatum/lacunosum moleculare and CA1 pyramidal neurons were identified visually for recordings of NMDA receptor-mediated EPSCs (holding potential at +40 mV) evoked by stimulation of the Schaffer collaterals. EPSC decay time courses were analyzed as stated in Materials and Methods. Charge transfer was measured as the area under the current response of the NMDA receptor-mediated EPSC. Data are presented as mean ± SEM, and n is the number of cells used to generate the data.
indicates significantly different from baseline (P < 0.05; paired t-test). For the deactivation time course, only τW values were compared.
3.6. Effects of PTC-174 on native NMDA receptors expressed by hippocampal interneurons.
GluN2D-containing NMDA receptors are expressed by several classes of interneurons in hippocampus (Monyer et al., 1994; von Engelhardt et al., 2015; Perszyk et al., 2016; Swanger et al., 2018; Yi et al., 2019). In contrast, hippocampal pyramidal neurons express GluN2A and GluN2B subunits, but little or no GluN2C or GluN2D (Gray et al., 2011; Rauner and Köhr, 2011; Perszyk et al., 2016; Swanger 2018; Yi et al., 2019). We evaluated the effects of PTC-174 on NMDA receptor-mediated responses from interneurons in the CA1 region of hippocampus in mouse brain slices (Figure 6A). Superfusion with 10 μM PTC-174 increased the amplitude of interneuron EPSCs induced by Schaffer collateral stimulation from 133 ± 20 to 157 ± 23 pA (paired t test, t(4) = 3.871, p = 0.018, Figure 6B–C, Table 3) and significantly slowed the weighted EPSC decay time constant from 191.6 ± 6.6 to 292.0 ± 41.8 ms (paired t test, t(4) = 2.778, p = 0.049, Figure 6B, Table 3). The increased amplitude and slower decay of the interneuron EPSCs in the presence of PTC-174 resulted in a significant increase in charge transfer during the NMDA receptor-mediated EPSCs (paired t test, t(4) = 3.731, p = 0.02, Figure 6D, Table 3).
Figure 6. Effects of PTC-174 on EPSCs in mouse hippocampal CA1 interneurons.
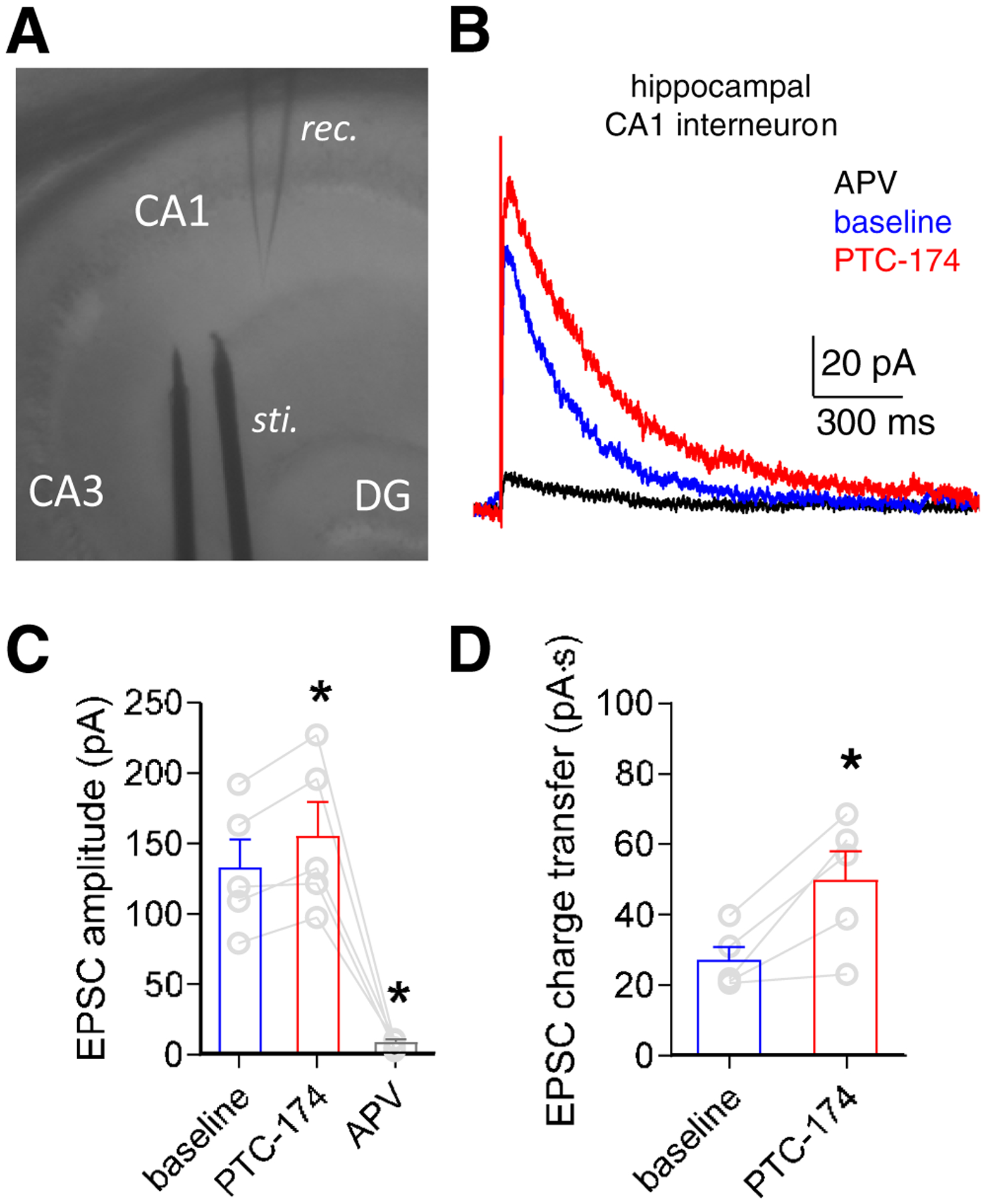
A) Transverse hippocampal brain slices were prepared from P12–18 mice and superfused with the AMPA receptor antagonist DNQX and the GABAA receptor antagonist gabazine to isolate NMDA receptor-mediated currents. Hippocampal interneurons were identified visually in the n stratum radiatum/lacunosum moleculare layers of the CA1 field and a bipolar stimulation electrode was positioned to evoke EPSCs by stimulation of the Schaffer collaterals. B) Representative excitatory postsynaptic currents (EPSCs) from one hippocampal interneuron in response to electrode stimulation in the absence (baseline) and presence of bath-applied 10 μM PTC-174 (holding potential at +40 mV). Superfusion with the NMDA receptor antagonist APV (400 μM) completely blocked the NMDA receptor-mediated EPSCs. E) Summary of peak amplitudes for NMDA receptor-mediated EPSCs. * indicates significantly different from baseline (P < 0.05; paired t-test). F) Summary of charge transfer during NMDA receptor-mediated EPSCs. * indicates significantly different from baseline (P < 0.05; paired t-test). See Table 3 for summary of all the data.
3.7. Effects of PTC-174 on native NMDA receptors expressed by hippocampal CA1 pyramidal neurons.
We evaluated the effects of PTC-174 on NMDA receptor-mediated responses from pyramidal neurons in the CA1 region of hippocampus in mouse brain slices (Figure 7A), which express GluN2A and GluN2B subunits (Gray et al., 2011; Rauner and Köhr, 2011; Yi et al., 2019). Superfusion with 10 μM PTC-174 had no effect on the amplitude of EPSCs induced by Schaffer collateral stimulation (paired t test, t(4) = 1.434, p = 0.225, Figure 7B–C, Table 3), but significantly slowed the weighted EPSC decay time constant from 150.1 ± 10.0 to 187.3 ± 20.0 ms (paired t test, t(4) = 3.145, p = 0.035, Figure 7B, Table 3). This slower EPSC decay in the presence of PTC-174, which was not accompanied by increased EPSC amplitude, did not result in increased charge transfer during the NMDA receptor-mediated EPSCs (paired t test, t(4) = 2.195, p = 0.093, Figure 7D, Table 3).
Figure 7. Effects of PTC-174 on EPSCs in mouse hippocampal CA1 pyramidal neurons.
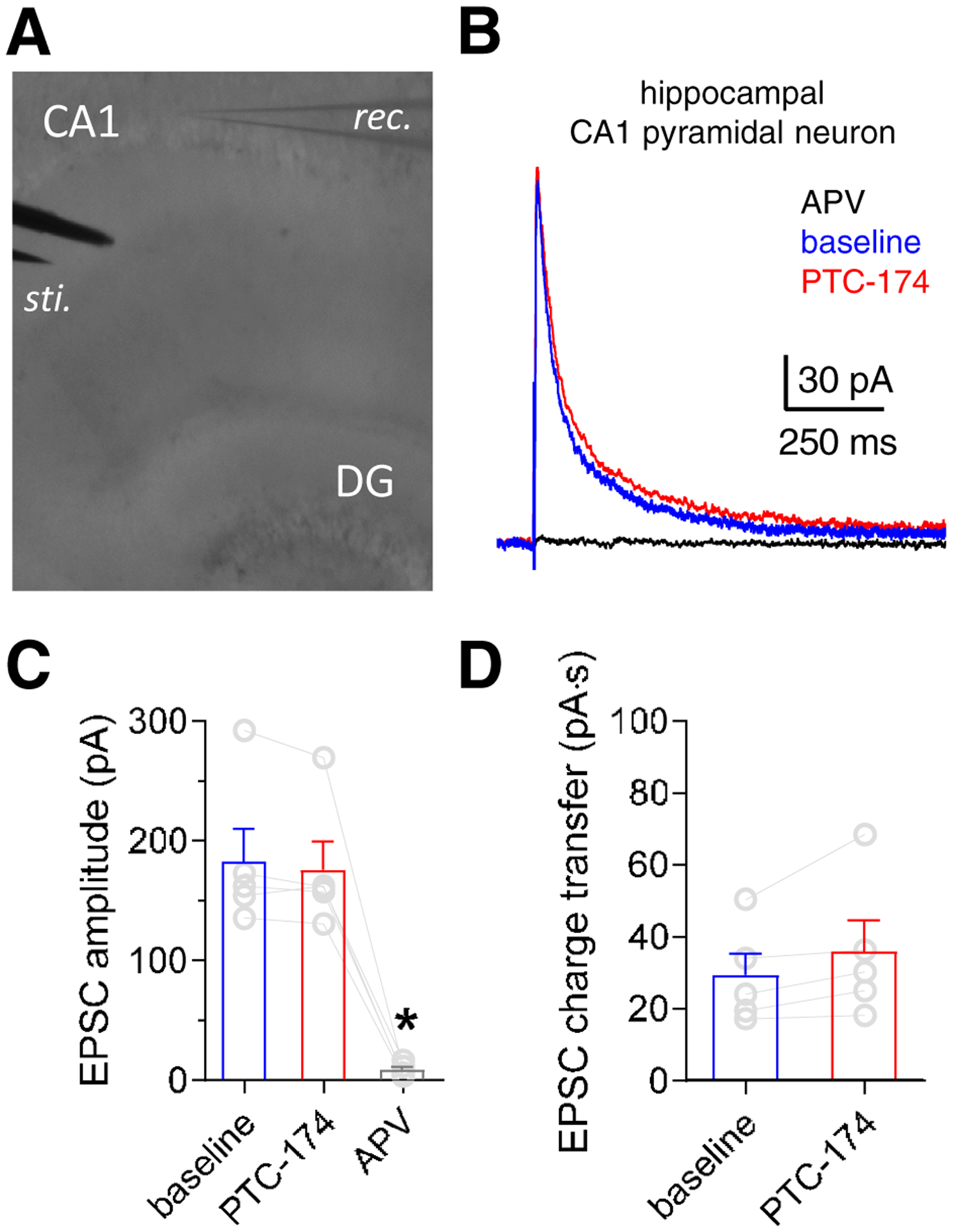
A) Hippocampal CA1 pyramidal neurons were identified visually, and a bipolar stimulation electrode was positioned to evoke EPSCs by stimulation of the Schaffer collaterals. B) Representative excitatory postsynaptic currents (EPSCs) from one hippocampal pyramidal neuron in response to electrode stimulation in the absence (baseline) and presence of bath-applied 10 μM PTC-174 (holding potential at +40 mV). Superfusion with the NMDA receptor antagonist APV (400 μM) completely blocked the NMDA receptor-mediated EPSCs. E) Summary of effects on peak EPSC amplitudes at different conditions for NMDA receptor-mediated EPSCs. * indicates significantly different from baseline (P < 0.05; paired t-test). F) Summary of charge transfer during NMDA receptor-mediated EPSCs. See Table 3 for summary of all the data.
3.8. Evaluation of use-dependence of modulation by PTC-174.
The pronounced increase in agonist potencies in the presence of PTC-174 (Figure 2 and Table 2) raises the possibility that agonist binding or receptor activation may reciprocally increase PTC-174 potency. Such agonist-dependence or use-dependence could account for the lack of PTC-174 effects on peak amplitudes of NMDA receptor-mediated EPSCs in STN neurons and hippocampal CA1 pyramidal neurons if glutamate is not present for sufficient time to enable PTC-174 modulation in these synapses. To evaluate this possibility, we measured synaptic-like responses from recombinant GluN1/2C and GluN1/2D receptors expressed in HEK293 cells using fast-application whole-cell patch-clamp electrophysiology. The responses were activated by brief 3–5 ms applications of 1 mM glutamate in the continuous presence of 10 μM glycine and in the absence and presence of 10 μM PTC-174 (Figure 8A). In these conditions, PTC-174 enhanced peak responses of both GluN1/2C and GluN1/2D by 3.7-fold and 5.3-fold, respectively (Figure 8B). The weighted deactivation time constant for GluN1/2C significantly increased from 299 ± 8 ms to 1850 ± 50 ms in the presence of PTC-174 (paired t test, t(6) = 31.88, p < 0.001, Figure 8C). Similarly, the weighted deactivation time constant for GluN1/2D significantly increased from 4050 ± 600 ms to 13350 ± 1740 ms in the presence of PTC-174 (paired t test, t(4) = 4.3, p = 0.013, Figure 8C). These results demonstrate that the continuous presence of 10 μM PTC-174 results in pronounced modulation of NMDA receptors activated by synaptic-like exposures to glutamate, even in the absence of ambient glutamate binding. Thus, the lack of PTC-174 modulation of NMDA receptor-mediated EPSC amplitudes in STN neurons and hippocampal CA1 pyramidal neurons is unlikely to be a result of a combination of low or no ambient glutamate levels and insufficient time for PTC-174 during synaptic glutamate release.
Figure 8. Effects of PTC-174 on the time course of responses from GluN1/2C and GluN1/2D receptors.
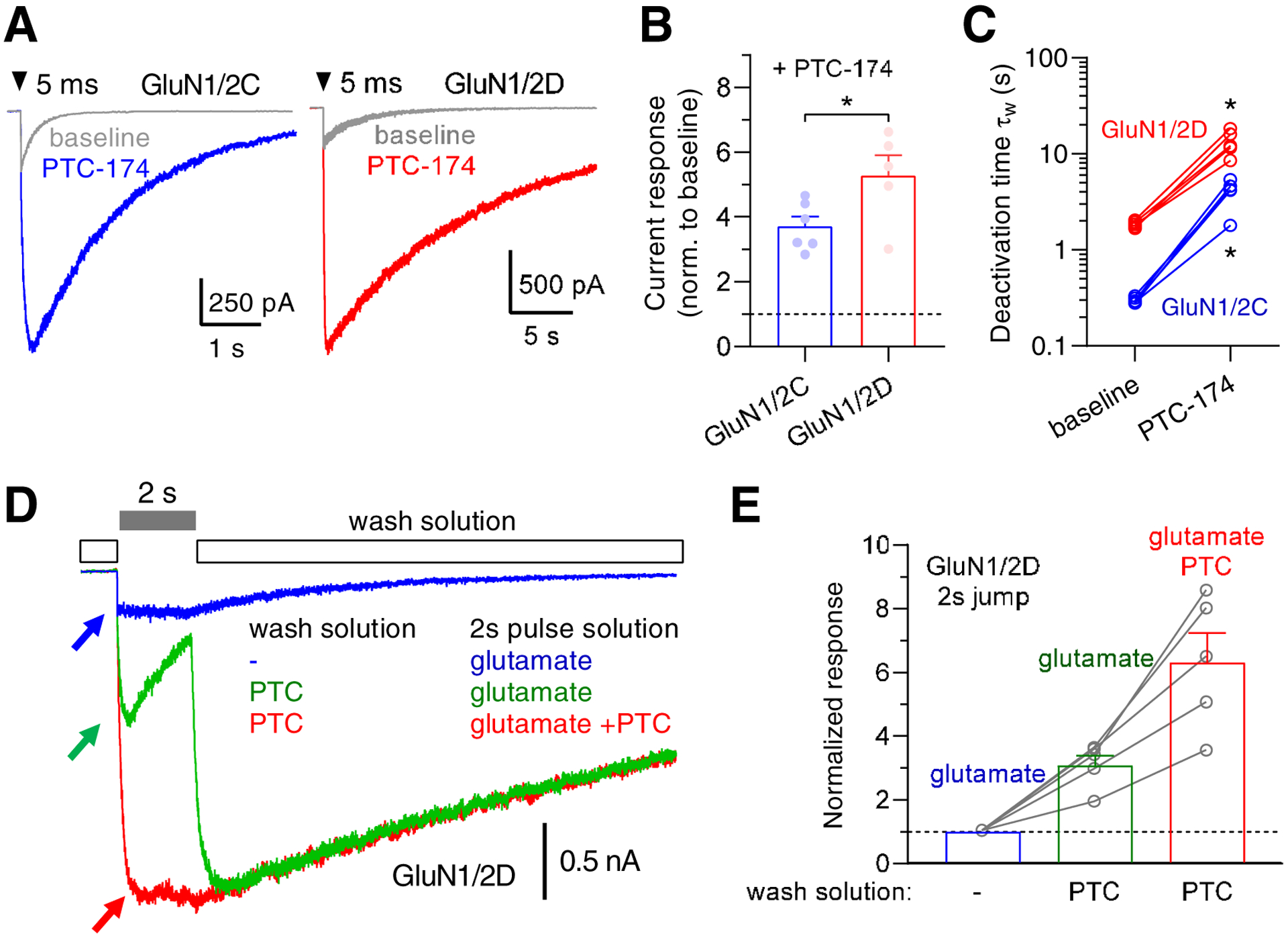
A) Representative whole-cell patch-clamp recordings of responses from recombinant GluN1/2C and GluN1/2D receptors expressed in HEK293 cells and activated by fast-application of 1 mM glutamate in the continuous presence of 10 μM glycine in the absence (baseline) or presence of 10 μM PTC-174. B) Summary of peak amplitudes of responses to brief application of 1 mM glutamate in the presence of 10 μM PTC-174 normalized to responses in the absence of PTC-174 (baseline). * indicates significant difference between PTC-174 potentiation of GluN1/2C and GluN1/2D (P < 0.05; t-test). C) Summary of weighted deactivation time constants in the absence (baseline) and presence of 10 μM PTC-174. * indicates significantly different from baseline (P < 0.05; paired t-test). D). Representative recordings of current responses from GluN1/2D receptors measured using whole-cell patch-clamp electrophysiology. The responses were activated in the continuous presence of 10 μM glycine using different ligands in the wash solution and during the 2 second pulse to activate responses. Blue recording shows a response resulting from changing the solution from no ligand (−), except glycine, to 1 mM glutamate. Green recording shows a response resulting from changing the solution from 10 μM PTC-174 (PTC) to 1 mM glutamate without PTC-174. Red recording shows a response resulting from changing the solution from 10 μM PTC-174 (PTC) to 1 mM glutamate plus 10 μM PTC-174. Arrows indicate the peak amplitude of the different responses. E) Summary of current responses activated by 2-s pulse solutions from different wash solutions. The responses are normalized to the response to 1 mM glutamate in the complete absence of PTC-174.
To explore the potential use-dependence of PTC-174 modulation, we measured responses from GluN1/2D receptors activated by long 2 second applications of 1 mM glutamate in the continuous presence of 10 μM glycine, but in the absence or presence of 10 μM PTC-174 before or during glutamate exposure (Figure 8D). When PTC-174 was present before, but not during glutamate exposure, the response was potentiated by 3.1-fold relative to the response in the complete absence of PTC-174 (Figure 8E). However, when PTC-174 was present before and during glutamate exposure, the response was potentiated by 6.3-fold (Figure 8E). These results suggest that PTC-174 can bind GluN1/2D receptors in the absence of glutamate binding (or receptor activation), but that glutamate binding (or receptor activation) facilitates binding of PTC-174 and that maximal potentiation by PTC-174 requires the continuous presence of PTC-174 during prolonged NMDA receptor activation.
4. Discussion
The most abundant and well-studied NMDA receptors are those containing GluN2A and GluN2B subunits at pyramidal neuron synapses in the hippocampus and cortex. The GluN2B-selective NAM, ifenprodil (Williams, 1993), has been instrumental in elucidating the coordination of GluN2A- and GluN2B-containing NMDA receptors in plasticity and the ifenprodil analog, CP-101,606, has demonstrated the therapeutic potential of subunit-selective targeting of NMDA receptors (Sang et al., 2003; Nutt et al., 2008; Preskorn et al., 2008). In other-than-pyramidal neurons, GluN2A and GluN2B are often expressed in combination with GluN2C and GluN2D subunits, respectively. Recently, new pharmacological tools have become available that selectively modulate the activities of GluN2C- and GluN2D-containing NMDA receptors (Feng et al., 2004; Costa et al., 2010; Mosley et al., 2010; Mullasseril et al., 2010; Hansen and Traynelis, 2011; Monaghan et al., 2012; Acker et al., 2013; Santangelo Freel et al., 2013; Khatri et al., 2014; Swanger et al., 2018). Here, we describe one such new compound, PTC-174, which displays unprecedented efficacy as a PAM of GluN2C- and GluN2D-containing NMDA receptors.
PTC-174 produces greater than 10-fold increases in currents from recombinant diheteromeric GluN1/2C and GluN1/2D receptors expressed in oocytes and stimulated with maximal concentrations of glutamate plus glycine. The channels formed by these subunit combinations have low probabilities of entering the open state following agonist binding (Dravid et al., 2008; Yuan et al., 2009; Vance et al., 2012). Thus, the efficacy of PTC-174 can be accounted for by an increase in channel open probability. PTC-174 binding also has an allosteric effect to increase both glutamate and glycine potencies at GluN1/2D and GluN1/2C receptors. Reciprocally, binding of PTC-174 is increased by the activation of NMDA receptors following glutamate and glycine binding.
Available data suggest that GluN2C and GluN2D are expressed in neurons primarily as triheteromeric NMDA receptors in combination with GluN2A or GluN2B, respectively. Recently, techniques have been developed to express triheteromeric NMDA receptors in heterologous expression systems (Hansen et al., 2014; Bhattacharya et al., 2018; Yi et al., 2019), thereby enabling investigation of the effect of PTC-174 on recombinant GluN1/2A/2B, GluN1/2A/2C, and GluN1/2B/2D receptors. PTC-174 increases current responses from diheteromeric GluN1/2B receptors by 1.8-fold, whereas PTC-174 produces a robust 4.5-fold potentiation of triheteromeric GluN1/2B/2D receptors, which is intermediate in magnitude compared to GluN1/2B and GluN1/2D. In contrast, PTC-174 induces a partial inhibition of diheteromeric GluN1/2A and triheteromeric GluN1/2A/2B receptors and produces a modest 1.7-fold potentiation of currents for triheteromeric GluN1/2A/2C receptors. These data suggest that the GluN2A subunit attenuates PTC-174 PAM activity when co-assembled with GluN2B and GluN2C.
The prototype GluN2C/D-selective PAM, (+)-CIQ has been used to study GluN2D-containing receptors in hippocampal interneurons and STN neurons in slice preparations (Mullasseril et al., 2010; Swanger et al., 2015; Perszyk et al., 2016). We investigated the effects of PTC-174 in similar preparations to evaluate the pharmacology of PTC-174 at native receptors. Immunohistochemical analysis indicates that parvalbumin-positive and somatostatin-positive interneurons in the murine hippocampus express GluN2D (von Engelhardt et al., 2015), but not GluN2C (Alsaad et al. 2019; Karavanova et al., 2007). More recently, Perszyk et al (2016) reported that the majority of hippocampal interneurons express mRNA for GluN2D, but not GluN2C. We investigated the effects of PTC-174 on the activities of rat hippocampal interneurons located in stratum radiatum/lacunosum moleculare layers of the CA1 field. We observed that PTC-174 increased the amplitude and slowed the decay of the NMDA receptor-mediated component of EPSCs recorded from these interneurons following stimulation of Schaffer collaterals. The effects of PTC-174 on the amplitude of EPSCs from hippocampal interneurons are consistent with those previously reported for (+)-CIQ in similar experiments (Perszyk et al., 2016). By contrast, (+)-CIQ did not affect the EPSC decay time in hippocampal interneurons (Perszyk et al., 2016), whereas PTC-174 prolonged the EPSC decay time in these GluN2D-expressing neurons. Furthermore, (+)-CIQ has also been shown have no effect on NMDA receptor-mediated EPSCs in hippocampal CA1 pyramidal neurons (Perszyk et al., 2016), whereas PTC-174 also prolonged the EPSC decay time in these neurons, which lack expression of GluN2C and GluN2D subunits (Yi et al., 2019). These results suggest that these GluN2D-selective PAMs, (+)-CIQ and PTC-174, may have distinct effects on excitation/inhibition balance in this hippocampal circuit.
GluN2D is also highly expressed with GluN2B in STN neurons (Standaert et al., 1994; Swanger et al., 2015) to mediate signaling of the excitatory input from cortical regions via the hyperdirect pathway (Magill et al., 2004). In mouse brain slices, we observed that superfusion with PTC-174 caused a robust increase in the amplitude of whole cell currents recorded from STN neurons in response to a brief pulse of NMDA applied with a picospritzer. When EPSCs were evoked by stimulation of the excitatory input, PTC-174 slowed the EPSC decay time with no effect on amplitude. Thus, the effect of PTC-174 on EPSCs in STN neurons appeared to differ from that observed in hippocampal interneurons, where both an increase in amplitude and slowed decay were observed. To explore this difference, we assessed the use-dependence of PTC-174 and demonstrated that maximal potentiation by PTC-174 requires the continuous presence of PTC-174 during prolonged NMDA receptor activation, suggesting that modulation by PTC-174 is facilitated by glutamate binding and/or activation of the NMDA receptor. However, synaptic-like responses activated in the absence and continuous presence of PTC-174 revealed robust potentiation of glutamate responses in these conditions relevant to synaptic transmission. Thus, it is possible that the subunit composition of synaptic and extrasynaptic NMDA receptors is different for STN neurons investigated in our study.
We noted several differences between PTC-174 and (+)-CIQ compounds. (+)-CIQ is nearly as efficacious at recombinant GluN1/2A/2C as at GluN1/2C (Bhattacharya et al., 2018), which contrasts with PTC-174 that is only weakly efficacious for potentiation of recombinant GluN1/2A/2C compared to GluN1/2C. Given that GluN1/2A/2C receptors are the predominant, if not exclusive, native configuration of GluN2C-containing NMDA receptors (Bhattacharya et al., 2018), this difference implies that PTC-174 may display functional selectivity for GluN2D-containing NMDA receptors in native systems, whereas (+)-CIQ may have broader effects that reflect potentiation of both GluN2D-containing receptors as well as triheteromeric GluN1/2A/2C receptors. Another potentially significant functional difference is that PTC-174 increases potencies for both glutamate and glycine at GluN2C and GluN2D receptors, whereas (+)-CIQ has no effect on agonist potencies. Synaptic glutamate rapidly pulses to levels that far exceed those needed to saturate the NMDA receptor glutamate binding site. However, extrasynaptic glutamate levels are much lower and may vary in the range of glutamate binding site affinities (Herman and Jahr, 2007). Thus, PTC-174 may have unique effects on extrasynaptic GluN2D-containing NMDA receptors signaling in response to changes in extrasynaptic glutamate concentration to modulate this aspect of glutamatergic signaling. It will be of interest in future studies to investigate whether the functional differences between PTC-174 and (+)-CIQ reflect differences in molecular mechanism of action that differentially impact synaptic activities.
5. Conclusions
Clinical studies have revealed that functional differences among NMDA receptor modulators, even within a specific pharmacological class, can yield different outcomes. Whereas the channel blocker ketamine is both psychotomimetic and antidepressant (Berman et al., 2000) (Niciu et al., 2014), the channel blockers memantine and lanicemine do not have these effects (Zarate et al., 2006; Sanacora et al., 2017). On the other hand, the GluN2B-selective NAM CP-101,606 has antidepressant and psychotomimetic effects similar to ketamine (Preskorn et al., 2008), despite the distinct subunit selectivity and mode of action (Nagy et al., 2015). Interpreting the complex actions of NMDA receptor modulators at the systems level in terms of differences in molecular mechanisms enables a better understanding of the systems neurobiology and a path for development and refinement of compounds that are more effective and safe therapeutics. PTC-174 has drug-like properties and pharmacokinetics, and readily achieves CNS exposure after systemic administration. Preliminary studies on the behavioral effects of PTC-174 in rodents are presented in a separate manuscript. This work with PTC-174 and other GluN2C/D modulators promises to yield insight into the physiology and therapeutic potential of targeting these classes of NMDA receptors.
Highlights.
PTC-174 potentiates diheteromeric GluN1/2C, and GluN1/2D receptors by >10-fold.
PTC-174 also potentiates triheteromeric GluN1/2B/2D and GluN1/2A/2C receptors.
PTC-174 is a partial antagonist of GluN1/2A and GluN1/2A/2B receptors.
Agonist potencies of glutamate and glycine increase in the presence of PTC-174.
PTC-174 modulates native GluN2D-containing NMDA receptors in distinct neuron types.
Acknowledgements
We thank Kollol Pal (Mnemosyne Pharmaceuticals) for grant preparation, Jinming Xiong (Chinglu Pharmaceutical Research) for contributing to compound synthesis, and Robert Boswick, Robert Dougherty, David Gurley, Julian Wooltorton, and Zina Itkin (Jubilant Discovery Services) for screening support.
Funding
This work was funded by SBIR grants from the National Institutes of Health (1R43MH098467-01 and 5R43MH098467-02) awarded to Chinglu Pharmaceutical Research LLC, Yuelian Xu Principal Investigator, as well as funding to Kasper B. Hansen from the National Institutes of Health (P20GM103546 and R01NS097536). Additional funding and resources to support this work were from Mnemosyne Pharmaceuticals, Inc., Luc Therapeutics, Inc. and Cadent Therapeutics, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations- aCSF, artificial cerebrospinal fluid; EPSC, excitatory postsynaptic current; NAM, negative allosteric modulator; PAM, positive allosteric modulator; STN, subthalamic nucleus.
Declaration of Conflicting Interests
Volkmann, Xu, Fanger, and Menniti are co-inventors on patent applications that claim PTC-174. The intellectual property rights to PTC-174 are assigned to Cadent Pharmaceuticals and Volkmann and Menniti own stock in this company. Hansen is the recipient of a small research contract from Janssen Research & Development. All other authors have no commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Acker TM, Khatri A, Vance KM, Slabber C, Bacsa J, Snyder JP, Traynelis SF and Liotta DC (2013) Structure-activity relationships and pharmacophore model of a noncompetitive pyrazoline containing class of GluN2C/GluN2D selective antagonists. J Med Chem 56:6434–6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acker TM, Yuan H, Hansen KB, Vance KM, Ogden KK, Jensen HS, Burger PB, Mullasseril P, Snyder JP, Liotta DC and Traynelis SF (2011) Mechanism for noncompetitive inhibition by novel GluN2C/D N-methyl-D-aspartate receptor subunit-selective modulators. Mol Pharmacol 80:782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaad HA, DeKorver NW, Mao Z, Dravid SM, Arikkath J and Monaghan DT (2019) In the Telencephalon, GluN2C NMDA Receptor Subunit mRNA is Predominately Expressed in Glial Cells and GluN2D mRNA in Interneurons. Neurochem Res 44:61–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufreton J, Kirkham E, Atherton JF, Menard A, Magill PJ, Bolam JP and Bevan MD (2009) Sparse but selective and potent synaptic transmission from the globus pallidus to the subthalamic nucleus. J Neurophysiol 102:532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste M and Mayer ML (1991) Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophysical J 59:560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS and Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Khatri A, Swanger SA, DiRaddo JO, Yi F, Hansen KB, Yuan H and Traynelis SF (2018) Triheteromeric GluN1/GluN2A/GluN2C NMDARs with unique single-channel properties are the dominant receptor population in cerebellar granule cells. Neuron 99:315–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Li L, Geiger JR and Jonas P (2006) Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat Protoc 1:2075–2081. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Misra C, Mok MH, Mishina M and Cull-Candy SG (2003) NR2B and NR2D subunits coassemble in cerebellar Golgi cells to form a distinct NMDA receptor subtype restricted to extrasynaptic sites. J Neurosci 23:4958–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothwell S, Barber J, Monaghan D, Jane D, Gibb A and Jones S (2008) NR2B-and NR2D-containing synaptic NMDA receptors in developing rat substantia nigra pars compacta dopaminergic neurones. J Physiol 586:739–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenard BL and Menniti FS (1999) Antagonists selective for NMDA receptors containing the NR2B subunit. Curr Pharm Des 5:381–404. [PubMed] [Google Scholar]
- Citri A and Malenka RC (2008) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacol 33:18–41. [DOI] [PubMed] [Google Scholar]
- Clements J and Westbrook G (1991) Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron 7:605–613. [DOI] [PubMed] [Google Scholar]
- Costa BM, Irvine MW, Fang G, Eaves RJ, Mayo-Martin MB, Skifter DA, Jane DE and Monaghan DT (2010) A novel family of negative and positive allosteric modulators of NMDA receptors. J Pharmacol Exp Ther 335:614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Brickley S and Farrant M (2001) NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol 11:327–335. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG and Leszkiewicz DN (2004) Role of distinct NMDA receptor subtypes at central synapses. Science STKE 16:1–9. [DOI] [PubMed] [Google Scholar]
- Dore K, Aow J and Malinow R (2016) The emergence of NMDA receptor metabotropic function: Insights from imaging. Front Synaptic Neurosci 8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid SM, Prakash A and Traynelis SF (2008) Activation of recombinant NR1/NR2C NMDA receptors. J Physiol 586:4425–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid IC and Smart TG (2009) Presynaptic NMDA receptors, Chapter 14 in Biology of the NMDA Receptor (Van Dongen AM ed), Boca Raton (FL). [PubMed] [Google Scholar]
- Durand GM, Gregor P, Zheng X, Bennett M, Uhl GR and Zukin RS (1992) Cloning of an apparent splice variant of the rat N-methyl-D-aspartate receptor NMDAR1 with altered sensitivity to polyamines and activators of protein kinase C. Proc Nat Acad Sci USA 89:9359–9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Feldmeyer D, Takahashi T and Cull-Candy SG (1994) NMDA-receptor channel diversity in the developing cerebellum. Nature 368:335–339. [DOI] [PubMed] [Google Scholar]
- Feng B, Tse HW, Skifter DA, Morley R, Jane DE and Monaghan DT (2004) Structure-activity analysis of a novel NR2C/NR2D-preferring NMDA receptor antagonist: 1-(phenanthrene-2-carbonyl) piperazine-2,3-dicarboxylic acid. Br J Pharmacol 141:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman CJ, Tomes H, Mbobo B, Baden T and Raimondo JV (2017) Openspritzer: an open hardware pressure ejection system for reliably delivering picolitre volumes. Sci Reports 7: 2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow NG, Siegler Retchless B and Johnson JW (2015) Molecular bases of NMDA receptor subtype-dependent properties. J Physiol 593:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray John A, Shi Y, Usui H, During Matthew J, Sakimura K and Nicoll Roger A (2011) Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: Single-cell NMDA receptor subunit deletion in vivo. Neuron 71:1085–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackos DH, Lupardus PJ, Grand T, Chen Y, Wang TM, Reynen P, Gustafson A, Wallweber HJ, Volgraf M, Sellers BD, Schwarz JB, Paoletti P, Sheng M, Zhou Q and Hanson JE (2016) Positive allosteric modulators of GluN2A-containing NMDARs with distinct modes of action and impacts on circuit function. Neuron 89:983–999. [DOI] [PubMed] [Google Scholar]
- Hagino Y, Kasai S, Han W, Yamamoto H, Nabeshima T, Mishina M and Ikeda K (2010) Essential role of NMDA receptor channel ε4 subunit (GluN2D) in the effects of phencyclidine, but not methamphetamine. PloS one 5:e13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Mullasseril P, Dawit S, Kurtkaya NL, Yuan H, Vance KM, Orr AG, Kvist T, Ogden KK, Le P, Vellano KM, Lewis I, Kurtkaya S, Du Y, Qui M, Murphy TJ, Snyder JP, Bräuner-Osborne H and Traynelis SF (2010) Implementation of a fluorescence-based screening assay identifies histamine H3 receptor antagonists clobenpropit and iodophenpropit as subunit-selective N-methyl-D-aspartate receptor antagonists. J Pharmacol Exp Ther 333:650–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Ogden KK, Yuan H and Traynelis SF (2014) Distinct functional and pharmacological properties of triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron 81:1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Tajima N, Risgaard R, Perszyk RE, Jørgensen L, Vance KM, Ogden KK, Clausen RP, Furukawa H and Traynelis SF (2013) Structural determinants of agonist efficacy at the glutamate binding site of N-methyl-D-aspartate receptors. Mol Pharmacol 84:114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB and Traynelis SF (2011) Structural and mechanistic determinants of a novel site for noncompetitive inhibition of GluN2D-containing NMDA receptors. J Neurosci 31:3650–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Yi F, Perszyk RE, Menniti FS and Traynelis SF (2017) NMDA receptors in the central nervous system, Methods Mol Biol 1677:1–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton CJ and Paoletti P (2005) Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron 46:261–274. [DOI] [PubMed] [Google Scholar]
- Herman MA and Jahr CE (2007) Extracellular glutamate concentration in hippocampal slice. J Neurosci 27:9736–9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand ME, Pitcher GM, Harding EK, Li H, Beggs S and Salter MW (2014) GluN2B and GluN2D NMDARs dominate synaptic responses in the adult spinal cord. Sci Rep 4:4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Boulter J, Maron C, Beasley L, Sullivan J, Pecht G and Heinemann S (1993) Zinc potentiates agonist-lnduced currents at certain splice variants of the NMDA receptor. Neuron 10:943–954. [DOI] [PubMed] [Google Scholar]
- Ishii T, Moriyoshi K, Sugihara H, Sakurada K, Kadotani H, Yokoi M, Akazawa C, Shigemoto R, Mizuno N, Masu M and Nakanishi S (1993) Molecular characterization of the family of the N-methyl-D-aspartate receptor subunits. J Biol Chem 268:2836–2843. [PubMed] [Google Scholar]
- Jones S and Gibb AJ (2005) Functional NR2B-and NR2D-containing NMDA receptor channels in rat substantia nigra dopaminergic neurones. J Physiol 569:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E and Furukawa H (2014) Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 344:992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavanova I, Vasudevan K, Cheng J and Buonanno A (2007) Novel regional and developmental NMDA receptor expression patterns uncovered in NR2C subunit-beta-galactosidase knock-in mice. Mol Cell Neurosci 34:468–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri A, Burger PB, Swanger SA, Hansen KB, Zimmerman S, Karakas E, Liotta DC, Furukawa H, Snyder JP and Traynelis SF (2014) Structural determinants and mechanism of action of a GluN2C-selective NMDA receptor positive allosteric modulator. Mol Pharmacol 86:548–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner NW and Dingledine R (1988) Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science 241:835–837. [DOI] [PubMed] [Google Scholar]
- Layton ME, Kelly MJ 3rd and Rodzinak KJ (2006) Recent advances in the development of NR2B subtype-selective NMDA receptor antagonists. Curr Top Med Chem 6:697–709. [DOI] [PubMed] [Google Scholar]
- Lee CH, Lu W, Michel JC, Goehring A, Du J, Song X and Gouaux E (2014) NMDA receptor structures reveal subunit arrangement and pore architecture. Nature 511:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JH, Wang YH, Yasuda RP, Dunah AW and Wolfe BB (1997) The majority of N-methyl-D-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B). Mol Pharmacol 51:79–86. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Sharott A, Bevan MD, Brown P and Bolam JP (2004) Synchronous unit activity and local field potentials evoked in the subthalamic nucleus by cortical stimulation. J Neurophysiol 92:700–714. [DOI] [PubMed] [Google Scholar]
- Malenka RC and Bear MF (2004) LTP and LTD: an embarrassment of riches. Neuron 44:5–21. [DOI] [PubMed] [Google Scholar]
- Misra C, Brickley SG, Farrant M and Cull-Candy SG (2000) Identification of subunits contributing to synaptic and extrasynaptic NMDA receptors in Golgi cells of the rat cerebellum. J Physiol 524 Pt 1:147–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan DT, Irvine MW, Costa BM, Fang G and Jane DE (2012) Pharmacological modulation of NMDA receptor activity and the advent of negative and positive allosteric modulators. Neurochem Int 61:581–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B and Seeburg PH (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12:529–540. [DOI] [PubMed] [Google Scholar]
- Mosley CA, Acker TM, Hansen KB, Mullasseril P, Andersen KT, Le P, Vellano KM, Bräuner-Osborne H, Liotta DC and Traynelis SF (2010) Quinazolin-4-one derivatives: A novel class of noncompetitive NR2C/D subunit-selective N-methyl-D-aspartate receptor antagonists. J Med Chem 53:5476–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullasseril P, Hansen KB, Vance KM, Ogden KK, Yuan H, Kurtkaya NL, Santangelo R, Orr AG, Le P and Vellano KM (2010) A subunit-selective potentiator of NR2C-and NR2D-containing NMDA receptors. Nat Comm 1:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy D, Stoiljkovic M, Menniti FS and Hajos M (2015) Differential effects of an NR2B NAM and ketamine on synaptic potentiation and gamma synchrony: Relevance to rapid-onset antidepressant efficacy. Neuropsychopharmacol 41:1486–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N, Axel R and Shneider NA (1992) Alternative splicing generates functionally distinct N-methyl-D-aspartate receptors. Proc Natl Acad Sci U S A 89:8552–8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CA Jr. and Charney DS (2014) Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu Rev Pharmacol Toxicol 54:119–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, Gunzler SA, Kirchhoff T, Hogarth P, Weaver JL, Krams M, Jamerson B, Menniti FS and Landen JW (2008) Effects of a NR2B selective NMDA glutamate antagonist, CP-101,606, on dyskinesia and Parkinsonism. Movement Dis 23:1860–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C and Zhou Q (2013) NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14:383–400. [DOI] [PubMed] [Google Scholar]
- Perszyk RE, DiRaddo JO, Strong KL, Low CM, Ogden KK, Khatri A, Vargish GA, Pelkey KA, Tricoire L, Liotta DC, Smith Y, McBain CJ and Traynelis SF (2016) GluN2D-containing N-methyl-D-aspartate receptors mediate synaptic transmission in hippocampal interneurons and regulate interneuron activity. Mol Pharmacol 90:689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M and Landen JW (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28:631–637. [DOI] [PubMed] [Google Scholar]
- Rauner C and Köhr G (2011) Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem 286:7558–7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Johnson MR, Khan A, Atkinson SD, Riesenberg RR, Schronen JP, Burke MA, Zajecka JM, Barra L, Su HL, Posener JA, Bui KH, Quirk MC, Piser TM, Mathew SJ and Pathak S (2017) Adjunctive lanicemine (AZD6765) in patients with major depressive disorder and history of inadequate response to antidepressants: A randomized, placebo-controlled study. Neuropsychopharmacol 42:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang CN, Weaver JJ, Jinga L, Wouden J and Saltarelli MD (2003) The NR2B subunit-selective NMDA receptor antagonist CP-101,606 reduces pain intensity in patients with central and peripheral neuropathic pain, in Society for Neuroscience p 814–819., New Orleans, LA. [Google Scholar]
- Santangelo Freel RM, Ogden KK, Strong KL, Khatri A, Chepiga KM, Jensen HS, Traynelis SF and Liotta DC (2013) Synthesis and structure activity relationship of tetrahydroisoquinoline-based potentiators of GluN2C and GluN2D containing N-methyl-D-aspartate receptors. J Med Chem 56:5351–5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN and Jan LY (1994) Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 368:144–147. [DOI] [PubMed] [Google Scholar]
- Soares C and Lee KF (2013) A prominent role for triheteromeric GluN1/GluN2A/GluN2B NMDARs at central synapses. J Neurosci 33:14975–14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert DG, Testa CM, Young AB and Penney JB Jr., (1994) Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J Comp Neurol 343:1–16. [DOI] [PubMed] [Google Scholar]
- Sugihara H, Moriyoshi K, Ishii T, Masu M and Nakanishi S (1992) Structures and properties of seven isoforms of the NMDA receptor generated by alternative splicing. Biochem Biophys Res Comm 185:826–832. [DOI] [PubMed] [Google Scholar]
- Swanger SA, Vance KM, Acker TM, Zimmerman SS, DiRaddo JO, Myers SJ, Bundgaard C, Mosley CA, Summer SL, Menaldino DS, Jensen HS, Liotta DC and Traynelis SF (2018) A novel negative allosteric modulator selective for GluN2C/2D-containing NMDA receptors inhibits synaptic transmission in hippocampal interneurons. ACS Chem Neurosci 9:306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanger SA, Vance KM, Pare JF, Sotty F, Fog K, Smith Y and Traynelis SF (2015) NMDA receptors containing the GluN2D subunit control neuronal function in the subthalamic nucleus. J Neurosci 35:15971–15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ and Dingledine R (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62:405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance KM, Hansen KB and Traynelis SF (2012) GluN1 splice variant control of GluN1/GluN2D NMDA receptors. J Physiol 590:3857–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann RA, Fanger CM, Anderson DR, Sirivolu VR, Paschetto K, Gordon E, Virginio C, Gleyzes M, Buisson B, Steidl E, Mierau SB, Fagiolini M and Menniti FS (2016) MPX-004 and MPX-007: New pharmacological tools to study the physiology of nmda receptors containing the GluN2A subunit. PLoS One 11:e0148129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt J, Bocklisch C, Tonges L, Herb A, Mishina M and Monyer H (2015) GluN2D-containing NMDA receptors-mediate synaptic currents in hippocampal interneurons and pyramidal cells in juvenile mice. Front Cell Neurosci 9:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K (1993) Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor - Selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol 44:851–859. [PubMed] [Google Scholar]
- Yamasaki M, Okada R, Takasaki C, Toki S, Fukaya M, Natsume R, Sakimura K, Mishina M, Shirakawa T and Watanabe M (2014) Opposing role of NMDA receptor GluN2B and GluN2D in somatosensory development and maturation. J Neurosci 34:11534–11548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N, Magill P, Woodhall G, Hall S and Stanford I (2012) Frequency selectivity and dopamine-dependence of plasticity at glutamatergic synapses in the subthalamic nucleus. Neurosci 203:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Bhattacharya S, Thompson CM, Traynelis SF and Hansen KB (2019) Functional and pharmacological properties of triheteromeric GluN1/2B/2D NMDA receptors. J Physiol. Manuscript in press. doi: 10.1113/JP278168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Mou TC, Dorsett KN, Volkmann RA, Menniti FS, Sprang SR and Hansen KB (2016) Structural basis for negative allosteric modulation of GluN2A-containing NMDA receptors. Neuron 91:1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Zachariassen LG, Dorsett KN and Hansen KB (2018) Properties of Triheteromeric N-Methyl-d-Aspartate Receptors Containing Two Distinct GluN1 Isoforms. Mol Pharmacol 93:453–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, Hansen KB, Vance KM, Ogden KK and Traynelis SF (2009) Control of NMDA receptor function by the NR2 subunit amino-terminal domain. J Neurosci 29:12045–12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, Manji HK and Charney DS (2006) A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry 163:153–155. [DOI] [PubMed] [Google Scholar]
- Zhang X and Chergui K (2015) Dopamine depletion of the striatum causes a cell-type specific reorganization of GluN2B-and GluN2D-containing NMDA receptors. Neuropharmacol 92:108–115. [DOI] [PubMed] [Google Scholar]
- Zhang X, Feng ZJ and Chergui K (2014) GluN2D-containing NMDA receptors inhibit neurotransmission in the mouse striatum through a cholinergic mechanism: implication for Parkinson’s disease. J Neurochem 129:581–590. [DOI] [PubMed] [Google Scholar]
- Zimmerman SS, Khatri A, Garnier-Amblard EC, Mullasseril P, Kurtkaya NL, Gyoneva S, Hansen KB, Traynelis SF and Liotta DC (2014) Design, synthesis, and structure-activity relationship of a novel series of GluN2C-selective potentiators. J Med Chem 57:2334–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukin RS and Bennett MV (1995) Alternatively spliced isoforms of the NMDARI receptor subunit. Trends Neurosci 18:306–313. [DOI] [PubMed] [Google Scholar]


