Abstract
Pituitary adenylate cyclase activating polypeptide (PACAP; ADCYAP1) is a pleiotropic neuropeptide widely distributed in both the peripheral and central nervous systems. PACAP and its specific cognate PAC1 receptor (ADCYAP1R1) play critical roles in the homeostatic maintenance of multiple physiological and behavioral systems. Notably, maladaptations in the PACAPergic system have been associated with several psychopathologies related to fear and anxiety. PAC1 receptor transcripts are highly expressed in granule cells of the dentate gyrus (DG). Here, we examined the direct effects of PACAP on DG granule cells in brain slices using whole cell patch recordings in current clamp mode. PACAP significantly increased the intrinsic excitability of DG granule cells via PAC1 receptor activation. This increased excitability was not mediated by adenylyl cyclase/cAMP or phospholipase C/PKC activation, but instead via activation of an extracellular signal-regulated kinase (ERK) signaling pathway initiated through PAC1 receptor endocytosis/endosomal signaling. PACAP failed to increase excitability in DG granule cells pretreated with the persistent sodium current blocker riluzole, suggesting that the observed PACAP effects required this component of the inward sodium current.
Keywords: dentate gyrus, MAPK signaling, neuronal excitability, PACAP, PAC1 receptor
INTRODUCTION
Pituitary adenylate cyclase activating polypeptides (PACAP; ADCYAP1) are evolutionarily well preserved trophic and intercellular signaling molecules that are widely distributed within neural and endocrine tissues (1, 12, 19, 48). PACAP and its specific cognate PAC1 receptor (ADCYAP1R1) can play critical roles in the regulation of sensory and autonomic functioning, cognitive learning, and response to injury and stress (2, 4, 10, 11, 16, 22, 40, 43, 48). Maladaptive PACAP signaling has been associated with stress-related anxiety disorders, including posttraumatic stress disorder (20, 33).
PACAP binds with high affinity and specificity to the PAC1 receptor; PACAP and vasoactive intestinal peptide (VIP) demonstrate near equal high-affinity binding to VPAC1 (VIPR1) and VPAC2 (VIPR2) receptors. Further, there are multiple isoforms of the PAC1 receptor depending on the presence or absence of Hip and/or Hop cassettes within the third cytoplasmic loop. The PAC1 receptor can engage multiple signaling pathways upon activation and the PAC1 receptor variants may show differential potency and efficacy in stimulating Gαs/adenylyl cyclase (AC)/cAMP and Gαq/phospholipase C (PLC)/DAG/IP3 activation (39, 48). Our previous studies have shown that central activation of PACAPergic systems can produce heightened anxiety-like responses and pain hypersensitivity (28, 29, 36) and that PACAP and PAC1 receptor transcript expression is sensitive to external factors, such as chronic stress (10).
PACAP signaling has been implicated in regulation of hippocampal activity. For example, stimulation of the perforant path is enhanced in the presence of PACAP (21), and Schaeffer collateral synaptic strength is also sensitive to PACAP treatment (5, 21, 34, 35), which may be due to the regulation of AMPA (7) and/or NMDA (41) currents. However, these past studies focused mostly on CA1 or CA3 function (5, 7, 8, 21, 24, 34, 35, 38, 41, 42, 46). Recent imaging studies have suggested that PACAPergic fibers are found predominantly at the inner molecular layer (IML) that borders the dentate gyrus (DG) (6). However, there has been no examination of PACAP actions on DG granule cell function.
Earlier work from our laboratories have shown that PACAP acting via PAC1 receptors can increase excitability of autonomic neurons through modulation of various intrinsic ionic currents (26, 27, 31, 44, 45). Consequently, in the present study, we examined the effects of PACAP on DG granule cells and investigated what intracellular signaling and ionic currents might contribute to an observed PACAP enhancement of granule cell excitability.
MATERIALS AND METHODS
Animals.
Sprague-Dawley rats were obtained from Charles River Canada. All animal procedures were approved by the University of Vermont Institutional Animal Care and Use Committee and followed the National Institutes of Health’s “Guide for the Care and Use of Laboratory Animals”.
Electrophysiology.
Brains from anesthetized and decapitated rats (22–36 days old) were recovered in a slurry of sucrose-replaced artificial cerebrospinal fluid (ACSF) for coronal sectioning on a Leica VT1000 vibratome (Leica, Allendale, NJ). Younger rats were used to increase neuronal health in slice preparations. Brain sections were placed in sucrose-replaced artificial cerebrospinal fluid (32–34°C) for 30 min and then equilibrated in artificial cerebrospinal fluid (ACSF; in mM): 124 NaCl, 2.8 KCl, 2 CaCl, 1.25 NaH2PO4, 10 glucose, 0.4 sodium ascorbate, 2 sodium pyruvate, 2 MgSO4, and 26 NaHCO3. Sucrose-replaced ACSF was similar to recording-ACSF with the following exceptions (NaCl was omitted; in mM): 206 sucrose, 1 CaCl, and 1 MgCl. ACSF was adjusted to pH 7.3–7.4 with HCl; osmolarity was 310 ± 5 mOsM. Intracellular solution contained in mM: 140 potassium gluconate, 2 KCl, 3 MgCl, 10 HEPES, 5 phosphocreatine, 2 K-ATP, 0.2 Na-GTP; pH was adjusted to 7.3–7.4 with KOH.
For recording, brain sections were perfused with oxygenated ACSF (32°C) at a rate of 3–4 mL/min in a recording chamber (Warner Instruments, Hamden, CT). Electrodes were prepared from borosilicate pipettes (World Precision Instruments, Sarasota, FL) on a Sutter P-97 micropipette puller to resistances of 5–8 MΩ. Neurons were selected and identified as DG granule cells by location, and a small size using a Leica DM-LFSA microscope (Leica, Allendale, NJ) and Rolera Bolt 3000 camera (QImaging, Surrey, BC, Canada). Individual neurons were patched under visual control, and recordings were made in current-clamp mode with a Multiclamp 700B controller and MultiClamp software (Molecular Devices, San Jose, CA). Data were acquired using a Digidata 1440A interface and pClamp software (Molecular Devices, San Jose, CA). Following successful patch, neurons were allowed to equilibrate until a steady resting membrane potential was established. Baseline measurements were taken, then drugs were applied for 4–5 min, measurements were taken again, then PACAP was applied for 4–5 min, and measurements were taken a final time. Drugs were bath-applied through gravity perfusion for approximately 5 min before the second recording. PACAP was then applied for a further 5 min before the final recording. Cells were accepted if resting membrane potentials were at least −55 mV and action potential overshoot >5 mV. Access resistance was monitored continuously, and recordings were discarded if changes reached >20% or above 35 MΩ. Experiments proceeded after membrane potential stabilized and were then made from a baseline voltage of −65 mV by injecting a steady holding current. Single spike measures were obtained by injecting progressively larger 10-ms current steps until an action potential was induced. No more than two neurons were analyzed from any one animal for any one experiment.
Neuronal characteristics.
Threshold was defined as the point at which the slope of the voltage trace reached 30 mV/ms. Input resistance was estimated from 500-ms hyperpolarizing current injections of increasing intensity until reaching approximately −95 mV and was calculated from the slope of the best-fit line of current intensity injected by change in membrane potential. Excitability curves were generated from plots of action potential frequency versus progressively increasing intensity of 1-s depolarizing current steps (2, 27). An increase in slope of the excitability curve indicated that neuronal excitability was enhanced. Rheobase was determined with a 400-pA (2 pA/ms) ramped current injection and the amount of current injected to elicit the first spike was determined to be the rheobase.
Drugs.
PACAP38 (referred to as PACAP in text) and vasoactive intestinal polypeptide (VIP) were from Bachem (Torrance, CA). Pitstop2, PD98059, Dynasore, SQ22536, U73122, CNQX, AP5, picrotoxin, CGP 52432, apamin, riluzole, and phrixotoxin-1 were purchased from Abcam (Cambridge, MA). Nickel chloride and forskolin were from Sigma Aldrich (St. Louis, MO), and dendrotoxin was from Alomone Laboratories (Jerusalem, Israel). Stock peptide solutions were prepared in 0.01% BSA. All drugs were prepared in DMSO as stock solutions.
Statistics.
Statistics were done with GraphPad Prism 7 (San Diego, CA). Two-way analysis of variance (ANOVA) was performed for main effects and interactions, and Tukey’s post hoc test for group differences. One-way ANOVA and paired t tests were used for single observation metrics.
RESULTS
PACAP/PAC1R activation increases dentate gyrus granule cell excitability.
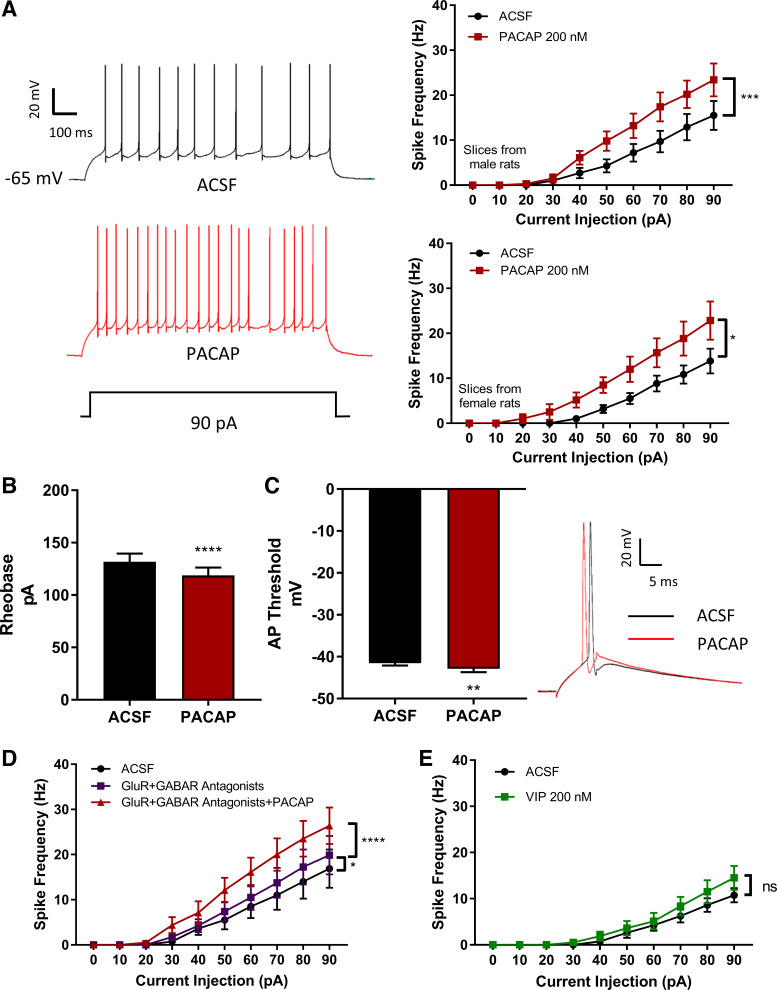
We assessed the effects of PACAP on DG granule cell excitability by quantitating action potential generation elicited by depolarizing current steps of increasing intensity. Under control current-clamp conditions, the injection of incrementally larger depolarizing currents progressively increased action potential frequency (Fig. 1A). After PACAP (200 nM) exposure for 4–5 min, the same cell reliably and significantly increased neuronal excitability in brain slices from both males (n = 10) and females (n = 6). Thus, to minimize potential variability due to hormone status, males were used for all subsequent studies. PACAP also lowered the current intensity to rheobase (n = 17) and shifted the action potential threshold (n = 12) to more negative voltages (Fig. 1, B and C), and slightly increased neuronal input resistance. Control experiments without PACAP in the perfusion demonstrated that equivalent passage of recording time did not produce changes in excitability (data not shown).
Fig. 1.
Pituitary adenylate cyclase-activating polypeptide (PACAP) increases dentate gyrus granule cell excitability. A: DG granule cell patch recordings were performed as described in methods. Left: representative trace demonstrating PACAP effects on spike generation in response to depolarizing current. Right, top: from male rats, 1-s current pulses of increasing intensity were injected into patched neurons for action potential measurements before and after PACAP (200 nM) application. Effect of PACAP: F (1,9) = 34.9, P = 0.0002; effect of current: F (9,81) = 30.25, P < 0.0001, and PACAP × current interaction: F (9, 81) = 13.34; P < 0.0001; n = 10 cells. Right, bottom: from the same protocol in female rat preparations: effect of PACAP: F (1,5) = 7.882, P = 0.0377; effect of current: F (9,45) = 33.15; P < 0.0001; and a significant PACAP × current interaction: F (9,45) = 6.715, P < 0.0001. B: in PACAP, dentate gyrus (DG) granule cells required less injected current to initiate an action potential. Two-tailed paired t-test showed smaller rheobase (t = 6.058, df = 16, ****P < 0.0001). C: in PACAP, left panel: action potential threshold was shifted to more negative voltages (two-tailed paired t test, t = 3.219, df = 11; **P = 0.0082). Right: traces showing shift of action potential initiation in PACAP. D: PACAP-induced granule cell excitability was not due to release of transmitters from glutamatergic or GABAergic terminals. Treatment of granule cells with an inhibitor cocktail (purple trace) to block AMPA receptors (CNQX, 10 µM) NMDA receptors (AP5, 50 µM), GABA-A receptors (picrotoxin, 10 µM), and GABA-B receptors (CGP52432, 1 µM), did not abrogate PACAP-induced increase in excitability (red trace). Effect of drug: F (2,14) = 64.61, P < 0.0001, effect of current: F (9, 63) = 23.23, P < 0.0001, and a significant drug × current interaction: [F (18, 126) = 16.53, P < 0.0001]. Tukey’s post hoc test revealed significant column differences among groups: artificial cerebrospinal fluid (ACSF) vs. antagonists (P = 0.0162), vehicle control vs. antagonists + PACAP (P < 0.0001), and antagonists vs. antagonists + PACAP (P < 0.0001). E: PACAP-induced granule cell excitability was not mediated by VPAC receptors. Exposing DG granule cells to vasoactive intestinal peptide (VIP; 200 nM, green trace) did not increase excitability. Effect of VIP: F (1,7) = 4.443; P = 0.0730, effect of current: F (9,63) = 28.87; P < 0.0001, and VIP × current interaction: F (9,63) = 4.073; P = 0.0004. Data were analyzed with two-way ANOVA with both current and drug effects as repeated measures. ns, not significantly different. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n = 7–17 cells per group. Values are means ± SE.
To ensure that the PACAP-induced increase in granule cell excitability was a direct effect on granule cells and not mediated by presynaptic effects of PACAP releasing glutamate and GABA from afferent inputs, parallel experiments were performed in the presence of glutamate and GABA receptor antagonists (n = 8). Pretreatment with a cocktail of glutamatergic and GABAergic receptor antagonists had no apparent effects on the PACAP-induced increase in excitability (Fig. 1D), supporting a direct PACAP action on DG granule cells.
Although PACAP is 100- to 1,000-fold more potent than VIP in binding and activating the PAC1 receptor, PACAP and VIP bind with similar high affinity at VPAC1 and VPAC2 receptors (13). In addition to PAC1 receptor transcript expression, DG granule cells also express VPAC1 transcript; VPAC2 transcript appeared minimal (23). However, 200 nM VIP did not recapitulate the effects of PACAP on granule cell excitability (n = 8; Fig. 1E). Hence, the PACAP-mediated responses were attributed to PAC1 receptor activation.
PACAP-mediated increase in granule cell excitability are dependent on MEK/ERK mechanisms.
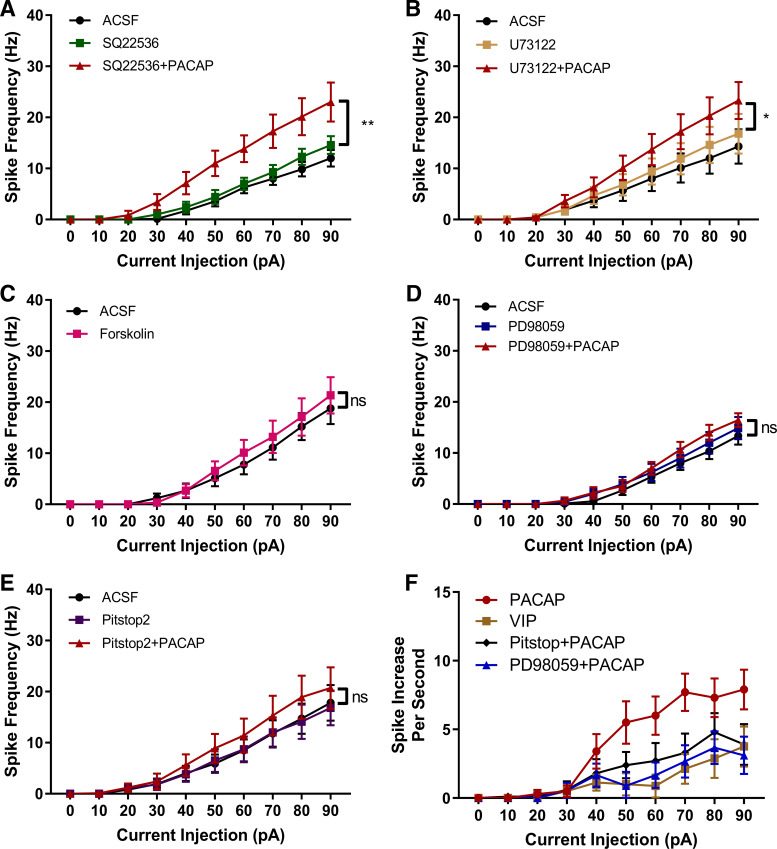
The PAC1 receptor is unique in its abilities to dually engage Gαs/AC/cAMP and Gαq/PLC/IP3/DAG signaling pathways (39). Both cAMP and PLC signaling pathways can lead to MEK/ERK activation. In addition, PAC1 receptor internalization/endosomal signaling can recruit and sustain long-term activation of MEK/ERK (25). All of these intracellular signaling pathways can potentially contribute to the modulation of ionic conductances that determine neuronal excitability (26, 31). To determine the signaling pathways facilitating the PACAP-induced increase in DG granule cell excitability, we examined whether pretreatment of the preparations with well-established selective signaling pathway inhibitors could delineate the mechanisms underlying the PACAP-mediated responses. For these experiments, recordings were obtained from granule cells to establish baseline activity; one of the signaling inhibitors was then bath-applied to assess potential actions of the inhibitors alone before subsequent application of inhibitor with PACAP. The results are reported and analyzed as within-subjects comparisons. If in the presence of a specific signaling pathway inhibitor, PACAP still produced a significant increase in excitability, we concluded that activation of this pathway did not play a primary role in the PACAP enhancement of excitability.
We initially tested the potential role of AC/cAMP signaling. Pretreatment with the AC-inhibitor SQ22536 (20 µM) (3) alone had no effect on granule cell excitability. Further, SQ22536 did not eliminate the PACAP-induced increase in granule cell excitability, suggesting that this pathway was not critical for the PACAP action (n = 7; Fig. 2A). Similarly, pretreatment with the PLC inhibitor U73122 (20 µM) (3) did not affect granule cell excitability alone, and subsequent PACAP treatment still stimulated a significant increase in excitability (n = 9; Fig. 2B). Thus, given exposure to U73122 did not eliminate the ability of PACAP to enhance excitability, PLC activation is apparently not exclusively required for this effect.
Fig. 2.
Pituitary adenylate cyclase activating polypeptide (PACAP)-induced granule cell excitability can be attenuated by MEK and endocytosis inhibitors. A: PACAP-induced increase in excitability remained in slice preparations treated with the adenylyl cyclase inhibitor SQ22536 (20 µM). Effect of drug: F (2,12) = 13.85; P = 0.0009; current: F (9,54) = 50.25; P < 0.0001; and drug × current interaction F (18,108) = 13.63; P < 0.0001. Tukey’s post hoc test exhibited column differences between ACSF condition and SQ22536 + PACAP (P = 0.0010), and between SQ22536 and SQ22536 + PACAP condition (P = 0.0040). B: PACAP-enhanced excitability remained in slice preparations treated with the PLC inhibitor U73122 (20 µM). Effect of drug: F (9,81) = 23.67; P = 0.0101; current: F (9,81) = 23.67; P = <0.0001; and drug × current interaction: F (18,162) = 10.07; P = 0.0001. Tukey’s post hoc multiple-comparisons test exhibited column differences between artificial cerebrospinal fluid (ACSF) and U73122 + PACAP (P = 0.0010), and between U73122 and U73122 + PACAP (P = 0.0133). C: direct adenylyl cyclase activation with forskolin (5 µM) did significantly increase dentate gyrus (DG) granule cell excitability. Effect of forskolin: F (1,8) = 3.145, P = 0.1141, current [F (9, 72) = 28.83, P < 0.0001], and forskolin × current interaction F (1,8) = 3.145; P = 0.005. D: PACAP-induced increase in excitability was essentially eliminated by treatment with the MEK/ERK inhibitor PD98059 (20 µM). Effect of drug F (2,16) = 1.881; P = 0.1846), effect of current F (9,72) = 65.49; P < 0.0001, and current × drug interaction [F (18,144) = 1.784, P = 0.0323]. Tukey’s post hoc test did not show significant group effects. E: PACAP-induced increase in excitability was essentially eliminated by exposure to Pitstop2 (15 µM), an inhibitor of clathrin-mediated endocytosis. Effect of drug F (2,18) = 3.856; P = 0.0404 effect of current F (9,81) = 23.14; P < 0.0001), and drug × current interaction F (18,162) = 3.642; P < 0.0001. Tukey’s post hoc multiple-comparisons test exhibited no significant column differences. F: normalized excitability curves demonstrate that the MEK/ERK inhibitor PD98059 and the clathrin inhibitor Pitstop2 reduced the PACAP-induced increase in excitability by the same extent. For the normalized PACAP excitability curve, the number of action potentials generated by the current steps in control was subtracted from the number of action potentials generated by similar current steps in PACAP. For the inhibitor-normalized excitability curves, the number of action potentials generated by the current steps with only inhibitor present was subtracted from the number of action potentials generated by similar current steps in the presence of inhibitor plus PACAP. The normalized excitability curve for vasoactive intestinal peptide (VIP) is included as a control excitability curve. Data were analyzed with two-way ANOVA with drug and current as repeated measures. ns, not significantly different. *P < 0.05, **P < 0.01; n = 6–10 cells per group. Values are means ± SE.
The inability of the AC inhibitor to block the PACAP-mediated increase in neuronal excitability was unanticipated as PACAP can potently increase cAMP production 10- to 20-fold (25) to impact neuronal excitability in other neuronal populations (27, 45). Corroborating these results, direct activation of AC with forskolin (5 µM) did not mimic the effects of PACAP on excitability (n = 9; Fig. 2C), reinforcing the conclusion that AC signaling mechanisms apparently do not contribute significantly to the PACAP modulation of DG granule cell excitability.
In addition to plasma membrane delimited AC/cAMP or PLC/DAG/IP3 mechanisms, MEK/ERK signaling can be initiated through cytosolic endosomal signaling following PAC1 receptor internalization (25). Unlike the previous experiments with AC and PLC inhibitors, the MEK inhibitor PD98059 (20 µM) essentially eliminated the PACAP-induced increase in excitability (n = 9; Fig. 2D). To test whether PAC1 receptor internalization is potentially a key step in the PACAP-induced MEK/ERK activation, subsequent experiments tested whether treatment with Pitstop2 to inhibit clathrin-mediated endocytosis (49) also would significantly blunt the PACAP effect. From Fig. 2E, it is evident that Pitstop2 virtually eliminated the PACAP enhancement of excitability (n = 10), like that seen with PD98059 pretreatment.
Figure 2F demonstrates that PD98059 and Pitstop2 treatments produced a comparable suppression of the PACAP effect. For this comparison, excitability curves generated with PACAP alone or with PACAP in the presence of PD98059 or Pitstop2 were normalized. For PACAP alone, the number of action potentials generated by the current steps in control was subtracted from the number of action potentials generated by similar current steps in PACAP. For the inhibitor experiments, the number of action potentials generated by the current steps with only inhibitor present was subtracted from the number of action potentials generated by similar current steps in the presence of inhibitor plus PACAP. The normalized excitability curve for VIP also is included to represent a normalized control excitability curve, given that VIP did not significantly affect excitability. The normalized excitability curves are shown in Fig. 2F. Together, with past work in other cell types, these data suggest that the effects of PACAP rely on MEK/ERK activation, likely driven by receptor endocytosis.
PACAP-mediated increase in granule cell excitability requires the presence of a persistent sodium current.
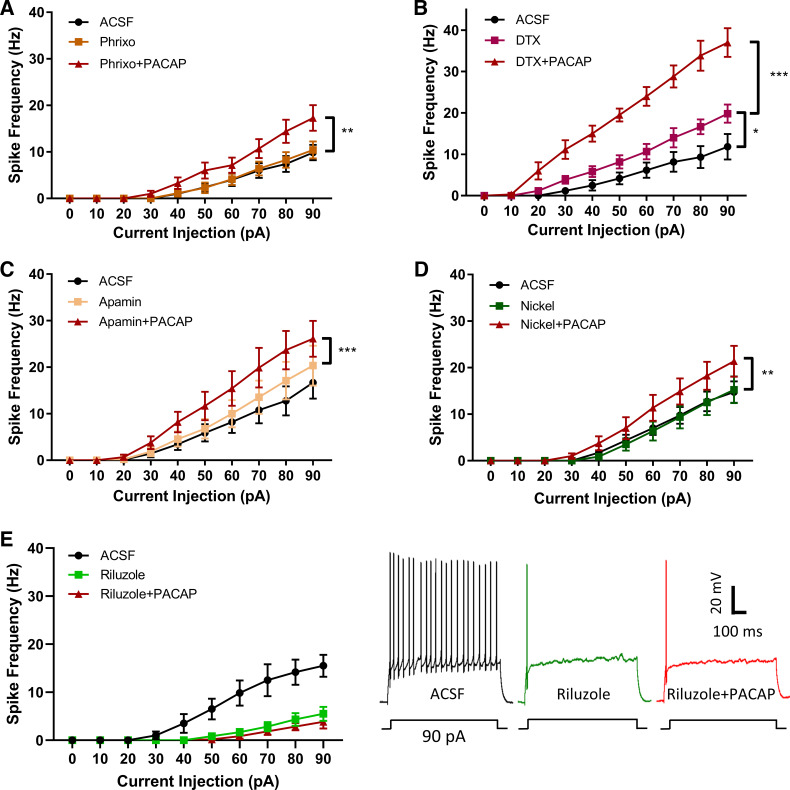
We next sought to determine the ionic currents modulated by PACAP-mediated MEK/ERK signaling to increase granule cell excitability. PACAP/PAC1 receptor signaling has been shown to modulate several ionic currents, flowing through sodium, potassium, and calcium channels (26, 44). Hence, we evaluated the effects of several ion channel inhibitors on PACAP-induced DG excitability. As PACAP activity in mixed hippocampal neuronal cultures has been shown to diminish voltage-gated Kv4.2 channel surface expression (9) and resultant A-type current to facilitate excitability (17), we initially tested whether PACAP-mediated downregulation of Kv4.2 channels may contribute to DG granule cell excitability. In the slice preparation, exposure to the Kv4.2/4.3 inhibitor phrixotoxin-1 (50 nM) alone had no effect on granule cell excitability, and PACAP significantly increased excitability with phrixotoxin-1 pretreatment (n = 7; Fig. 3A), suggesting Kv4.2/4.3 does not contribute to the PACAP/PAC1-mediated effect under the conditions of our experiments. Other voltage-gated potassium channels containing α-subunits Kv1.1, Kv1.2, and Kv1.6, contribute to outwardly rectifying currents and can be blocked by dendrotoxin. Unlike phrixotoxin-1, dendrotoxin alone significantly increased granule cell excitability, suggesting that members of the Kv1 channel family regulate DG granule cell action potential generation. In the presence of dendrotoxin, the addition of PACAP significantly augmented granule cell excitability beyond levels observed with the inhibitor alone (n = 6; Fig. 3B).
Fig. 3.
The pituitary adenylate cyclase activating polypeptide (PACAP)-induced granule cell excitability is eliminated by treatment with the persistent sodium current inhibitor riluzole. A: inhibition of Kv 4.2/Kv4.3 with phrixotoxin-1 (phrixo; 50 nM) alone did not enhance cell excitability and did not block PACAP effects on excitability. Effect of drug: F (2,12) = 12.39; P = 0.0012; current: F (9,54) = 31.99; P < 0.0001, and drug × current interaction: F (18,108) = 11.78; P < 0.0001. Tukey’s post hoc tests showed significant column differences between ACSF and phrixo + PACAP (P = 0.0021), and between phrixo and phrixo + PACAP (P = 0.0036). B: inhibition of Kv1.1, Kv1.2, and Kv1.6 with dendrotoxin (DTX; 50 nM) alone increased granule cell excitability, suggesting that these channels contributed to inherent neuronal activities; the addition of PACAP amplified neuronal excitability beyond levels of DTX alone. Effect of drug: F (2,10) = 79.29; P < 0.0001; current: F (9,45) = 67.53; P < 0.0001; and drug × current interaction: F (18,90) = 39.98; P < 0.0001. Tukey’s post hoc multiple-comparison test exhibited column differences between artificial cerebrospinal fluid (ACSF) and DTX (P = 0.0171), between ACSF and DTX +P ACAP (P < 0.0001), and between DTX and DTX+PACAP (P < 0.0001). C: treatment with apamin (200 nM) to block SK channels did not increase excitability and did not eliminate a PACAP-induced increase in excitability. Effect of drug: F (2,16) = 25.27; P < 0.0001; effect of current: F (9, 72) = 24.14; P < 0.0001; and drug × current injection interaction: F (18,144) = 13.91; P < 0.0001. Tukey’s post hoc test exhibited column differences between ACSF and apamin + PACAP (P < 0.0001), and between apamin and apamin + PACAP (P = 0.0005). D: DG granule cell treatment with nickel chloride (500 µM) did not affect cell excitability alone and did not block PACAP-mediated excitability. Effect of drug: F (2,14) = 10.31; P = 0.0018; effect of current F (9,63) = 32.16; P < 0.0001; and drug × current interaction: F (18,126) = 7.677; P < 0.0001. Tukey’s post hoc tests showed significant column differences between ACSF and nickel + PACAP (P = 0.0057) and between nickel and nickel + PACAP (P = 0.0029). E: persistent sodium current is required for DG granule cell tonic firing, and its presence is required for the PACAP-induced enhancement of cell excitability. Exposure to the persistent sodium current inhibitor riluzole (10 µM) effectively eliminated tonic firing induced by depolarizing current steps and subsequent exposure of PACAP did not restore the tonic firing pattern. Effect of drug: F (2,10) = 7.563; P = 0.0100; effect of current: F (9,45) = 79.06; and drug × current interaction F (18,90) = 7.836; P < 0.0001. Tukey’s post hoc tests exhibited significant column differences between ACSF and Riluzole (P = 0.0249) and between ACSF and Riluzole+PACAP (P = 0.0135), but no significant difference between Riluzole and Riluzole+PACAP Groups. Data were analyzed with two-way ANOVA with drug and current as repeated measures. *P < 0.05, **P < 0.01, ***P < 0.001; n = 6–10 cells per group. Values are means ± SE.
Additional experiments tested whether pretreatment with apamin (200 nM), to block small-conductance potassium channels (17), or nickel (500 µM) to block low-voltage activated calcium channels (18), impacted the excitability caused by PACAP. Neither apamin (n = 9) nor nickel (n = 8) appeared to have any effect on their own and did not prevent the effects of PACAP on neuronal excitability (Fig. 3, C and D).
Persistent sodium currents can regulate neuronal excitability through their ability not to inactivate over prolonged depolarizations (47), and the responses can be modulated by channel phosphorylation (37). Accordingly, the effects of blocking persistent sodium currents with riluzole (10 µM) on the firing properties of DG granule cells were examined. Under control conditions, the granule cells demonstrated a regular repetitive firing pattern, which was greatly attenuated following 4–5 min of riluzole treatment (n = 6; Fig. 3E), indicating a riluzole-sensitive current, most likely a persistent sodium current, is required for tonic firing produced by long depolarizing steps in DG granule cells. It was also noted that even though repetitive firing was blunted in riluzole, the properties of the initial action potentials were comparable before and during drug exposure. There were no difference in threshold F (2,10) = 0.14, P = 0.8669 or half-width F (2, 10) = 0.07, P = 0.9270. There was a small ~4-mV decrease in amplitude observed over time; however, the peak response was still robust following PACAP application, averaging +34.73 mV. Following the riluzole pretreatment, PACAP did not restore or increase the regular firing pattern induced by depolarizing current steps.
DISCUSSION
There are three key observations derived from this study. First, PACAP through activation of PAC1 receptors significantly increases excitability of DG granule cells. Second, the enhancement of neuronal excitability by PACAP requires activation of MEK/ERK signaling, which is recruited via PAC1 internalization and endosomal signaling. Last, the PACAP enhancement of DG granule cell excitability is eliminated by riluzole, indicating a role of the persistent sodium current in the PACAP effect.
In situ hybridization studies have clearly demonstrated that PACAP and PAC1 receptors are expressed in DG hilar and granule cells, respectively (12, 15, 19). Although the PACAPergic synapses were not identified, it was suggested they were at the mossy fiber-CA3 synapse (30). By contrast, recent studies using PACAP-EGFP mice have suggested that PACAP terminals were preferentially restricted to the IML (6). In unpublished data with PACAP-Ires-Cre mice, we confirmed these more recent results (May V and Aktar M, unpublished observations). Results reported here demonstrate that rat DG granule cells are activated by PACAP, consistent with a similar PAC1 receptor localization between species (14, 50).
Earlier results from our laboratories have shown that a PACAP/PAC1 interaction can engage multiple plasma membrane delimited and intracellular signaling cascades to coordinate the modulation of several ionic currents to increase excitability of parasympathetic postganglionic cardiac neurons (26, 27, 31). The results of the current studies suggest that PACAP modulation of DG granule cell excitability did not appear to require plasma membrane delimited AC or PLC signaling, but was essentially eliminated upon inhibition of MEK/ERK activation. GPCRs, including PAC1 receptors, can undergo β-arrestin-mediated internalization for long-term MEK/ERK signaling from endosomal scaffolds. Treatment with Pitstop2 to block PAC1 receptor endocytosis essentially eliminated the PACAP enhancement of granule cell excitability. Our results suggest recruitment of MEK/ERK through β-arrestin-mediated internalization of the PAC1 receptor, and endosomal signaling is the predominant mechanism initiating the PACAP-initiated MEK/ERK activation that contributes to the modulation of DG granule cells by PACAP. The importance of PAC1 internalization/endosomal signaling leading to recruitment of MEK/ERK activation is common to PACAP actions in other neuronal systems (26, 27, 29). Accordingly, inhibition of MEK/ERK and PAC1 receptor endocytosis disrupts the effects of PACAP on both cardiac ganglion neurons and DG granule cells and on behaviors associated with hyperalgesia (26, 27, 29). Increasingly, the roles of endosomal activities to sustain signaling at targeted intracellular sites have been appreciated in various neural systems (32).
The PACAP/PAC1 receptor-induced ERK activation could potentially modulate the properties of multiple voltage-gated ion currents by alteration of their voltage dependence of activation/inactivation or current density. We used well-established inhibitors to systematically inhibit specific ionic conductances potentially mediating the PACAP-induced increase in excitability. Our results suggested that the PACAP effects on DG granule cells were unlikely due to modulation of apamin-, dendrotoxin-, nickel-, or phrixotoxin-sensitive ionic currents. These observations indicated that the slow AHP, A-current, or T-type calcium currents likely did not contribute significantly to the PACAP-mediated modulation of excitability in the DG granule cells. With the concentration of nickel used, it is suggested that other voltage-dependent calcium channels also are not involved in the PACAP modulation of DG granule cells. Previously, Taylor et al. (41) demonstrated that inhibition of the late slow component of the AHP increased excitability of CA1 pyramidal cells, an effect mediated by PKA and p38 MAPK signaling. There was no consistent evidence in our recordings of a late-frequency adaptation (nonadapting interspike interval) during repetitive spiking elicited by the 1-s depolarizing current injections. Furthermore, AC/cAMP/PKA manipulation failed to modulate excitability. Thus, this current either is not significantly expressed in the granule cells or is not a target of PACAP/PAC1-induced intracellular signaling.
PACAP failed to increase excitability in DG granule cells pretreated with the persistent sodium current blocker riluzole (Fig. 3E), suggesting that the observed PACAP effects required this component of the inward sodium current. Riluzole effectively reduced the repetitive spiking of DG granule cells, strongly suggesting that the persistent sodium current is required for repetitive firing induced by depolarizing steps in these cells. However, in the presence of riluzole, an action potential was still generated at the beginning of a suprathreshold depolarization and its properties were similar to those determined for untreated controls, indicating that the amplitude of the fast inactivating inward sodium current was not significantly affected by the drug at the concentrations used. We suggest two interpretations of these results. First, a PACAP enhancement of the persistent sodium current component of the inward sodium current mediated by MEK/ERK signaling might be responsible for the enhanced excitability in DG granule cells. Alternatively, PACAP through MEK/ERK signaling could also modulate inactivation/inactivation properties of the fast component of the inward sodium current promoting increased action potential firing. In either case, this modulation by ERK signaling requires the presence of the persistent sodium current.
In conclusion, our results show that PACAP markedly enhances excitability of DG granule cells, an effect requiring MEK/ERK activation. We further conclude that a PACAP-induced PAC1 internalization and recruitment of endosomal signaling leads to the activation of the ERK signaling modulating ionic conductances underlying the enhanced excitability by PACAP.
GRANTS
This work was supported in part by NIH National Institute of Mental Health Grant MH097988 to S.H. and V.M.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.C.J., R.L.P., V.M., and S.E.H. conceived and designed research; G.C.J. performed experiments; G.C.J. analyzed data; G.C.J., R.L.P., V.M., and S.E.H. interpreted results of experiments; G.C.J. prepared figures; G.C.J., R.L.P., V.M., and S.E.H. drafted manuscript; G.C.J., R.L.P., V.M., and S.E.H. edited and revised manuscript; G.C.J., R.L.P., V.M., and S.E.H. approved final version of manuscript.
REFERENCES
- 1.Arimura A. Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol 48: 301–331, 1998. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- 2.Braas KM, May V, Harakall SA, Hardwick JC, Parsons RL. Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci 18: 9766–9779, 1998. doi: 10.1523/JNEUROSCI.18-23-09766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braas KM, May V. Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem 274: 27702–27710, 1999. doi: 10.1074/jbc.274.39.27702. [DOI] [PubMed] [Google Scholar]
- 4.Cho J-H, Zushida K, Shumyatsky GP, Carlezon WA Jr, Meloni EG, Bolshakov VY. Pituitary adenylate cyclase-activating polypeptide induces postsynaptically expressed potentiation in the intra-amygdala circuit. J Neurosci 32: 14165–14177, 2012. doi: 10.1523/JNEUROSCI.1402-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciranna L, Cavallaro S. Opposing effects by pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide on hippocampal synaptic transmission. Exp Neurol 184: 778–784, 2003. doi: 10.1016/S0014-4886(03)00300-5. [DOI] [PubMed] [Google Scholar]
- 6.Condro MC, Matynia A, Foster NN, Ago Y, Rajbhandari AK, Van C, Jayaram B, Parikh S, Diep AL, Nguyen E, May V, Dong HW, Waschek JA. High-resolution characterization of a PACAP-EGFP transgenic mouse model for mapping PACAP-expressing neurons. J Comp Neurol 524: 3827–3848, 2016. doi: 10.1002/cne.24035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa L, Santangelo F, Li Volsi G, Ciranna L. Modulation of AMPA receptor-mediated ion current by pituitary adenylate cyclase-activating polypeptide (PACAP) in CA1 pyramidal neurons from rat hippocampus. Hippocampus 19: 99–109, 2009. doi: 10.1002/hipo.20488. [DOI] [PubMed] [Google Scholar]
- 8.Gardoni F, Saraceno C, Malinverno M, Marcello E, Verpelli C, Sala C, Di Luca M. The neuropeptide PACAP38 induces dendritic spine remodeling through ADAM10-N-cadherin signaling pathway. J Cell Sci 125: 1401–1406, 2012. doi: 10.1242/jcs.097576. [DOI] [PubMed] [Google Scholar]
- 9.Gupte RP, Kadunganattil S, Shepherd AJ, Merrill R, Planer W, Bruchas MR, Strack S, Mohapatra DP. Convergent phosphomodulation of the major neuronal dendritic potassium channel Kv4.2 by pituitary adenylate cyclase-activating polypeptide. Neuropharmacology 101: 291–308, 2016. doi: 10.1016/j.neuropharm.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology 34: 833–843, 2009. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammack SE, May V. Pituitary adenylate cyclase activating polypeptide in stress-related disorders: data convergence from animal and human studies. Biol Psychiatry 78: 167–177, 2015. doi: 10.1016/j.biopsych.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol 453: 389–417, 2002. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- 13.Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol 166: 4–17, 2012. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto H, Ishihara T, Shigemoto R, Mori K, Nagata S. Molecular cloning and tissue distribution of a receptor for pituitary adenylate cyclase-activating polypeptide. Neuron 11: 333–342, 1993. doi: 10.1016/0896-6273(93)90188-W. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, Matsuda T, Mizuno N, Nagata S, Baba A. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol 371: 567–577, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 16.Hill J, Chan S-A, Kuri B, Smith C. Pituitary adenylate cyclase-activating peptide (PACAP) recruits low voltage-activated T-type calcium influx under acute sympathetic stimulation in mouse adrenal chromaffin cells. J Biol Chem 286: 42459–42469, 2011. doi: 10.1074/jbc.M111.289389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer, 2001. [Google Scholar]
- 18.Iftinca MC, Zamponi GW. Regulation of neuronal T-type calcium channels. Trends Pharmacol Sci 30: 32–40, 2009. doi: 10.1016/j.tips.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Jaworski DM, Proctor MD. Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Brain Res Dev Brain Res 120: 27–39, 2000. doi: 10.1016/S0165-3806(99)00192-3. [DOI] [PubMed] [Google Scholar]
- 20.Jovanovic T, Norrholm SD, Davis J, Mercer KB, Almli L, Nelson A, Cross D, Smith A, Ressler KJ, Bradley B. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol Psychiatry 18: 742–743, 2013. doi: 10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo T, Tominaga T, Ichikawa M, Iijima T. Differential alteration of hippocampal synaptic strength induced by pituitary adenylate cyclase activating polypeptide-38 (PACAP-38). Neurosci Lett 221: 189–192, 1997. doi: 10.1016/S0304-3940(96)13323-1. [DOI] [PubMed] [Google Scholar]
- 22.Legradi G, Das M, Giunta B, Hirani K, Mitchell EA, Diamond DM. Microinfusion of pituitary adenylate cyclase-activating polypeptide into the central nucleus of amygdala of the rat produces a shift from an active to passive mode of coping in the shock-probe fear/defensive burying test. Neural Plast 2007: 79102, 2007. doi: 10.1155/2007/79102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176, 2007. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 24.Macdonald DS, Weerapura M, Beazely MA, Martin L, Czerwinski W, Roder JC, Orser BA, MacDonald JF. Modulation of NMDA receptors by pituitary adenylate cyclase-activating peptide in CA1 neurons requires G alpha q, protein kinase C, and activation of Src. J Neurosci 25: 11374–11384, 2005. doi: 10.1523/JNEUROSCI.3871-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.May V, Buttolph TR, Girard BM, Clason TA, Parsons RL. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. Am J Physiol Cell Physiol 306: C1068–C1079, 2014. doi: 10.1152/ajpcell.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.May V, Parsons RL. G protein-coupled receptor endosomal signaling and regulation of neuronal excitability and stress responses: signaling options and lessons from the PAC1 receptor. J Cell Physiol 232: 698–706, 2017. doi: 10.1002/jcp.25615. [DOI] [PubMed] [Google Scholar]
- 27.Merriam LA, Baran CN, Girard BM, Hardwick JC, May V, Parsons RL. Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. J Neurosci 33: 4614–4622, 2013. doi: 10.1523/JNEUROSCI.4999-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miles OW, Thrailkill EA, Linden AK, May V, Bouton ME, Hammack SE. Pituitary adenylate cyclase-activating peptide in the bed nucleus of the stria terminalis mediates stress-induced reinstatement of cocaine seeking in rats. Neuropsychopharmacology 43: 978–986, 2018. doi: 10.1038/npp.2017.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Missig G, Mei L, Vizzard MA, Braas KM, Waschek JA, Ressler KJ, Hammack SE, May V. Parabrachial pituitary adenylate cyclase-activating polypeptide activation of amygdala endosomal extracellular signal-regulated kinase signaling regulates the emotional component of pain. Biol Psychiatry 81: 671–682, 2017. doi: 10.1016/j.biopsych.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otto C, Zuschratter W, Gass P, Schütz G. Presynaptic localization of the PACAP-type I-receptor in hippocampal and cerebellar mossy fibres. Brain Res Mol Brain Res 66: 163–174, 1999. doi: 10.1016/S0169-328X(99)00010-8. [DOI] [PubMed] [Google Scholar]
- 31.Parsons RL, May V. PACAP-induced PAC1 receptor internalization and recruitment of endosomal signaling regulate cardiac neuron excitability. J Mol Neurosci 68: 340–347, 2019. doi: 10.1007/s12031-018-1127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson YK, Luttrell LM. The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol Rev 69: 256–297, 2017. doi: 10.1124/pr.116.013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 470: 492–497, 2011. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberto M, Brunelli M. PACAP-38 enhances excitatory synaptic transmission in the rat hippocampal CA1 region. Learn Mem 7: 303–311, 2000. doi: 10.1101/lm.34200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberto M, Scuri R, Brunelli M. Differential effects of PACAP-38 on synaptic responses in rat hippocampal CA1 region. Learn Mem 8: 265–271, 2001. doi: 10.1101/lm.40501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roman CW, Lezak KR, Hartsock MJ, Falls WA, Braas KM, Howard AB, Hammack SE, May V. PAC1 receptor antagonism in the bed nucleus of the stria terminalis (BNST) attenuates the endocrine and behavioral consequences of chronic stress. Psychoneuroendocrinology 47: 151–165, 2014. doi: 10.1016/j.psyneuen.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheuer T. Regulation of sodium channel activity by phosphorylation. Semin Cell Dev Biol 22: 160–165, 2011. doi: 10.1016/j.semcdb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmidt SD, Myskiw JC, Furini CR, Schmidt BE, Cavalcante LE, Izquierdo I. PACAP modulates the consolidation and extinction of the contextual fear conditioning through NMDA receptors. Neurobiol Learn Mem 118: 120–124, 2015. doi: 10.1016/j.nlm.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Spongier D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburgt PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature 365: 170–175, 1993. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 40.Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, Eiden LE. PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice. Endocrinology 154: 330–339, 2013. doi: 10.1210/en.2012-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor RD, Madsen MG, Krause M, Sampedro-Castañeda M, Stocker M, Pedarzani P. Pituitary adenylate cyclase-activating polypeptide (PACAP) inhibits the slow afterhyperpolarizing current sIAHP in CA1 pyramidal neurons by activating multiple signaling pathways. Hippocampus 24: 32–43, 2014. doi: 10.1002/hipo.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toda AM, Huganir RL. Regulation of AMPA receptor phosphorylation by the neuropeptide PACAP38. Proc Natl Acad Sci USA 112: 6712–6717, 2015. doi: 10.1073/pnas.1507229112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tompkins JD, Ardell JL, Hoover DB, Parsons RL. Neurally released pituitary adenylate cyclase-activating polypeptide enhances guinea pig intrinsic cardiac neurone excitability. J Physiol 582: 87–93, 2007. doi: 10.1113/jphysiol.2007.134965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tompkins JD, Clason TA, Hardwick JC, Girard BM, Merriam LA, May V, Parsons RL. Activation of MEK/ERK signaling contributes to the PACAP-induced increase in guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol 311: C643–C651, 2016. doi: 10.1152/ajpcell.00164.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tompkins JD, Merriam LA, Girard BM, May V, Parsons RL. Nickel suppresses the PACAP-induced increase in guinea pig cardiac neuron excitability. Am J Physiol Cell Physiol 308: C857–C866, 2015. doi: 10.1152/ajpcell.00403.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J 279: 12–19, 2012. doi: 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- 47.Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. Eur J Neurosci 12: 3567–3574, 2000. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- 48.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BKC, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev 61: 283–357, 2009. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 49.von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Kräusslich HG, Shupliakov O, Robinson PJ, McCluskey A, Haucke V. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 146: 471–484, 2011. doi: 10.1016/j.cell.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 50.Zhou CJ, Kikuyama S, Shibanuma M, Hirabayashi T, Nakajo S, Arimura A, Shioda S. Cellular distribution of the splice variants of the receptor for pituitary adenylate cyclase-activating polypeptide (PAC(1)-R) in the rat brain by in situ RT-PCR. Brain Res Mol Brain Res 75: 150–158, 2000. doi: 10.1016/S0169-328X(99)00300-9. [DOI] [PubMed] [Google Scholar]