Abstract
Cellular senescence, a permanent arrest of cell proliferation, is characterized by a senescence-associated secretory phenotype (SASP), which reinforces senescence and exerts noxious effects on adjacent cells. Recent studies have suggested that transplanting small numbers of senescent cells suffices to provoke tissue inflammation. We hypothesized that senescent cells can directly augment renal injury. Primary scattered tubular-like cells (STCs) acquired from pig kidneys were irradiated by 10 Gy of cesium radiation, and 3 wk later cells were characterized for levels of senescence and SASP markers. Control or senescent STCs were then prelabeled and injected (5 × 105 cells) into the aorta of C57BL/6J mice. Four weeks later, renal oxygenation was studied in vivo using 16.4-T magnetic resonance imaging and function by plasma creatinine level. Renal markers of SASP, fibrosis, and microvascular density were evaluated ex vivo. Per flow cytometry, irradiation induced senescence in 80–99% of STCs, which showed increased gene expression of senescence and SASP markers, senescence-associated β-galactosidase staining, and cytokine levels (especially IL-6) secreted in conditioned medium. Four weeks after injection, cells were detected engrafted in the mouse kidneys with no evidence for rejection. Plasma creatinine and renal tissue hypoxia increased in senescent compared with control cells. Senescent kidneys were more fibrotic, with fewer CD31+ endothelial cells, and showed upregulation of IL-6 gene expression. Therefore, exogenously delivered senescent renal STCs directly injure healthy mouse kidneys. Additional studies are needed to determine the role of endogenous cellular senescence in the pathogenesis of kidney injury and evaluate the utility of senolytic therapy.
Keywords: kidney, microvascular injury, senescence
INTRODUCTION
Cellular senescence, a permanent arrest of cell cycle, is characterized by a senescence-associated secretory phenotype (SASP) and a prosurvival pathway (34). In turn, SASP reinforces senescence and facilitates intercellular communication with neighboring cells as well as immune surveillance (42). Senescent cells produced in vivo have been linked to a variety of pathophysiological situations, including aging (39), obesity (28), pulmonary fibrosis (16), and acute myocardial infarction (8, 36). Recent studies have shown the potent deleterious effects of senescent cells. For example, intra-peritoneal transplantation of a small number of senescent pre-adipocytes suffices to provoke significant physical dysfunction comparable to aging (39), and transplanting senescent fibroblasts into the knee joint causes an osteoarthritis-like phenotype in mice (38). Pertinently, the bystander effect of senescent cells promotes senescent cell accumulation in affected organs (9).
Cellular senescence has been implicated in renal aging and the pathogenesis of various renal diseases (32). In age-related changes such as glomerulosclerosis, interstitial fibrosis, and nephron atrophy, renal cells including tubules, glomeruli, the interstitium, and arteries can all be affected by senescence (25). Nevertheless, renal tubular cells may be particularly susceptible to senescence under ischemic or toxic injury, IgA nephropathy, and chronic kidney disease (32) and might entail tubular atrophy and interstitial fibrosis (21). However, given that the development of cellular senescence in the context of kidney disease is often accompanied by activation of additional pathomechanisms, such as inflammation and fibrosis, its specific direct effect on the kidney might be difficult to discern.
Upon renal injury, resident tubular cells undergo a phenotypic modification into reparative CD24+/CD133+ scattered tubular-like cells (STCs) that contribute to self-healing and renal recovery (30). We have previously shown that delivery of extracellular vesicles harvested from pig STCs into mice with renal artery stenosis elicited repair of the ischemic kidney (44). However, we have subsequently shown that when exposed to injurious stimuli, STCs manifest dysregulated expression of genes, including those encoding for cell cycle (2), and undergo structural and functional alterations that impair their reparative potency in vitro (1). These alterations, which are consistent with development of cellular senescence, might not only interfere with the repair potency of senescent STCs but might conceivably render them injurious for neighboring cells by virtue of SASP. Because systemic conditions may activate myriad mechanisms that induce renal injury and are difficult to distinguish, we opted to tease out the contribution of senescent cells to renal injury by injecting them in otherwise healthy animals.
We therefore tested the hypothesis that introduction of senescent cells in healthy mice would directly induce renal injury. We used 10 Gy of cesium radiation to induce STC senescence, delivered them to normal mice, and studied murine kidney function and structure.
MATERIALS AND METHODS
All animal procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee (protocol no. A1609-16).
Induction and Evaluation of STC Senescence
In healthy kidneys, STCs comprise only a little over 1% of the kidney (1). Therefore, to yield a sufficient number for delivery, we isolated them from the pig kidney, which is manifold larger than the mouse kidney and whose STCs have been well characterized. Fresh kidney tissues (4–5g, mainly the cortex) from 6-mo-old healthy female pigs were chopped with a surgical scissor under sterile conditions and digested using the Multi Tissue Dissociation Kit and gentleMACS Octo Dissociator (Miltenyi Biotec). The supernatant was carefully collected, filtered, and centrifuged. The resuspended cell pellet was incubated in medium 199 containing 3% FBS (GIBCO-BRL) at 37°C with 5% CO2. After 48 h, the culture medium was replaced to rid it of nonadherent cells. After another 24–48 h, passage 0 tubular cells became 90% confluent, and CD24/CD133 double-positive STCs were isolated using CD24 (no. 130-095-951, Miltenyi Biotec) and CD133 (no. 130-100-857, Miltenyi Biotec) MicroBead kits with a LS column on a QuadroMACS Separator.
To induce senescence, isolated renal STCs were irradiated by 10 Gy of cesium, as previously described (40), and then cultured. Irradiation is a well-accepted method to uniformly induce senescence in cells (3, 20). Control cells were cultured without irradiation. After 21 days in culture, flow cytometry was performed to identify cells undergoing senescence (4). Briefly, cells were stained with a senescence-associated β-galactosidase (SA-β-gal) staining kit (no. 9860, Cell Signaling, Boston, MA) according to the manufacturer’s protocol (18). Cells were collected and gated for single cells using the area and aspect ratio features on the bright-field image (FlowSight Imaging Flow Cytometer, Amnis, Seattle, WA). SA-β-gal-positive and SA-β-gal-negative cells were separated with the mean pixel feature of the bright-field channel and expressed as a percentage of total single cells.
Expressions of senescence-related and SASP genes were studied using total RNA isolated from cells. Quantitative real-time PCR was performed using Taqman assays (ThermoFisher, Waltham, MA) for the following: cyclin-dependent kinase inhibitor 2A (CDKN2A; APYMMFE), cyclin-dependent kinase inhibitor 1A (CDKN1A; AJGJQY6), IL-1A (IL1A; Ss03391335), IL-6 (IL6; Ss03384604), chemokine (C-C motif) ligand 2 (CCL2; Ss03394377), and serpin family E member 1 (SERPINE1; Ss03392656), with TATA box-binding protein (TBP; APCE6HF) as the internal control. Analysis was done using Applied Biosystems ViiA7 Real-Time PCR systems. Fold changes of each target gene in the experimental relative to normal cells were calculated using the method (where CT is threshold cycle) (23). To evaluate SASP, levels of cytokines, IL-6 (P6000B, R&D Systems), macrophage chemoattractant protein (MCP)-1 (VS0081S-002, Kingfisher Biotech), and plasminogen activator inhibitor-1 (PAI-1; LS-F-10456, LifeSpan Bioscience), were also measured in conditioned medium by ELISA and corrected by the number of cells (11).
In Vivo Mouse Experiments: Transplantation of Senescent Cells
Eleven C57BL/6J mice (11 wk of age, Jackson Laboratory, Bar Harbor, ME) were randomly assigned to two groups. Mice were housed at 3−5/mice cage at 21–23°C on a 12:12-h light-dark cycle, with ad libitum access to municipal city water and standard chow for the duration of the study. Acute experiments were performed late morning (~11 AM) in a dedicated animal surgery suite between May and August 2018. Through a carotid cannula, either control (n = 6, 3 male and 3 female mice) or irradiated senescent (n = 5, all female mice) renal CTFR-prelabeled (far red, Invitrogen, Waltham, MA) STCs were injected (5 × 105 cells) into the aorta under 1–2% isoflurane anesthesia. No antibiotics were used after injections. Four weeks later, imaging experiments were conducted in all mice in the magnetic resonance imaging (MRI) facility starting at ~11 AM. Renal oxygenation was studied in vivo using 16.4-T MRI under 1–2% isoflurane anesthesia. Urine samples were then collected via cystocentesis, and mice euthanized in the afternoon by terminal blood sampling under isoflurane anesthesia. The kidneys were harvested, weighed, and cut into two halves to be fresh frozen or preserved in formalin.
Cell tracking.
Frozen kidney tissue embedded in OCT was sectioned at 6 μm thickness. Potential rejection of the transplanted renal STCs was evaluated by immunofluorescence staining with CD3 (Abcam, Cambridge, MA) (43). To quantify retention rate, flow cytometry was performed (1). Fresh kidney sections from control and senescent mice were diced, digested with 1 mL collagenase (2 mg/mL), and added to medium 199 (GIBCO-BRL) containing 3% FBS, and the suspension was filtered through a 100-µm cell strainer. After being centrifuged at 200 g for 10 min, cell pellets were resuspended and washed, and cells were quantified using a flow gating strategy. Single cells were gated and evaluated for the presence of prelabeled CTFR and DAPI. Acquisition was performed using an Imaging Flow Cytometer (FlowSight, Amnis, Seattle, WA) equipped with INSPIRE software. Engraftment of prelabeled cells was expressed as the percentage of events recorded in the kidneys.
Systemic measurements.
Systolic blood pressure was measured by tail cuff at baseline and 4 wk around 9 AM using the XBP1000 system (Kent Scientific, Torrington, CT) (7) located in the animal surgery suite, and blood and urine were collected before euthanasia. Plasma creatinine level and renin content were assayed using the DetectX Serum Creatinine kit (Arbor Assays, Ann Arbor, MI) and the Angiotensin-I RIA kit (ALPCO, Salem, NH), respectively. Urinary albumin was measured by the Mouse Albumin ELISA Kit (Crystal Chem, Elk Grove Village, IL), and urinary creatinine by the Creatinine Urinary Detection Kit (Arbor Assays). The urine albumin-to-creatinine ratio was calculated for each mouse.
In vivo renal function.
MRI experiments were performed to assess renal oxygenation, perfusion, and blood flow. Mice were anesthetized and maintained with 1.0–2.0% isoflurane in the supine position. ECG, respiration, and body temperature were monitored (SA Instruments, Stony Brook, NY). MRI was studied on a vertical 16.4-T animal scanner equipped with a 38-mm inner diameter birdcage coil (Bruker, Billerica, MA). Renal volume was measured from coronal images acquired using a respiration-gated three-dimensional fast imaging with steady precession sequence. Renal perfusion and blood flow were measured with arterial spin labeling with the flow-sensitive alternating inversion-recovery sequence. Renal oxygenation was assessed by blood oxygen level-dependent MRI using a respiration-gated three-dimensional multi-echo gradient echo sequence. Renal volumes were quantified from the three-dimensional fast imaging with steady precession images using ANALYZE (version 12.0, Biomedical Imaging Resource, Mayo Clinic, MN), and all other MRI images were reconstructed and analyzed offline using in-house-developed software packages in MatLab (The Mathworks, Natick, MA) (10, 15).
Histological analysis.
To estimate the degree of fibrosis, sirius red staining was performed on renal paraffin sections. After being dewaxed and rehydrated, slices were stained with sirius red in saturated picric acid for 30 min, dehydrated, and cleared through graded ethyl alcohol (15). Interstitial fibrosis was semiautomatically quantified in 10 randomly chosen fields per section using AxioVision (Carl Zeiss SMT, Oberkochen, Germany) and expressed as the percentage of sirius red-positive area to total field area. To evaluate microvascular density, renal paraffin sections were stained with anti-CD31 antibody (no. 77699, Cell Signaling). Slides were microwaved using EDTA buffer (pH 8), cooled, incubated with CD31 antibody at 1:100 dilution, and labeled with Alexa Fluoro 594. CD31+ endothelial cells were manually counted in 10 randomly chosen fields per section and expressed as the ratio to tubules. To study the spatial relationship of transplanted STCs with SASP, renal cryosections were also stained for IL-6 (ab7737, Abcam), an important SASP mediator implicated in renal injury (6). All slides were analyzed in a blinded manner.
Real-time PCR.
SASP genes were studied in frozen kidney tissue. Relative quantitative PCR was done using the following Taqman probes (ThermoFisher Scientific): Il6 (mm00446190), Ccl2 (mm00441242), Serpine1 (mm00436753), Tnfα (mm00443258), Vegf (mm00437306), and GAPDH (mm99999915) as an internal control.
Statistical Analysis
Statistical analysis was performed using JMP software (version 13.0, SAS Institute, Cary, NC). Preliminary power calculations, using Lamorte’s (5) for the assessment of microvascular density, indicated a needed sample size of n = 6/group with 80% power and an α level of 0.05. Analysis of both kidneys in each mouse yielded n = 12 control and n = 10 senescent cell-treated available kidneys. Normally distributed variables are expressed as means ± SD, and non-normal distributed data are expressed as medians (interquartile ranges). Parametric (Student’s t test) and nonparametric (Wilcoxon) tests were used for comparisons between groups. P values of ≤0.05 were considered statistically significant.
RESULTS
Irradiation Leads to STC Senescence
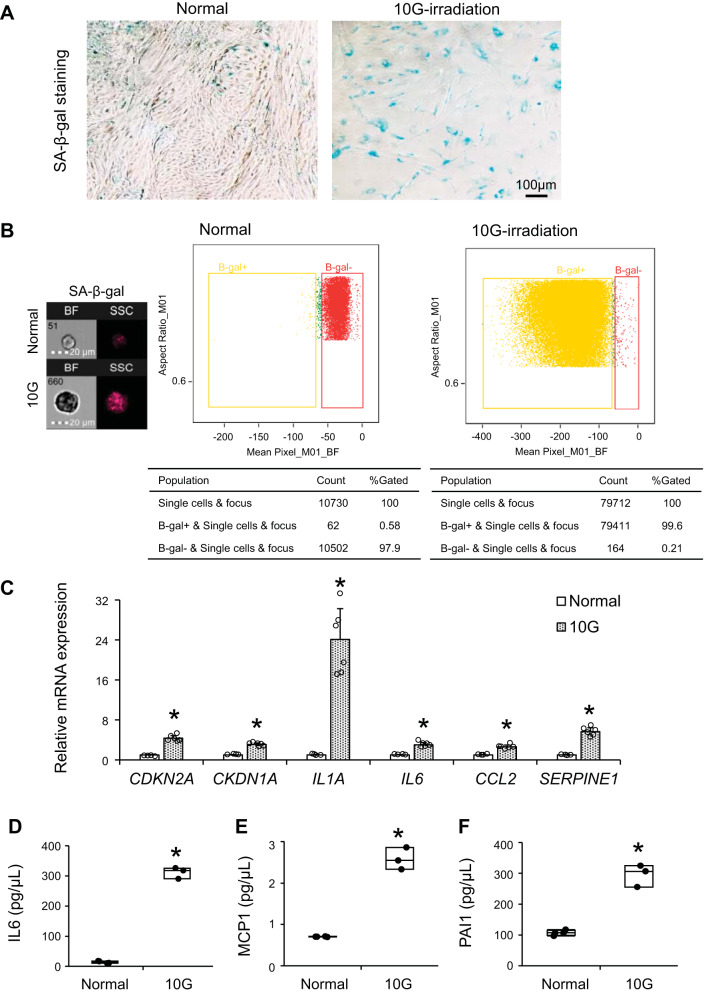
We isolated renal STCs from swine kidneys and induced cellular senescence with 10-Gy irradiation. Three weeks after irradiation, most renal STCs were morphologically large and stained positive with SA-β-gal (Fig. 1A). As per flow cytometry with SA-β-gal staining, 80–99% of renal STCs were senescent after irradiation (Fig. 1B). Irradiated cells showed increased mRNA expression characteristic of senescence (Cdkn2a and Cdkn1a) and SASP markers (Il1a, Il6, Ccl2, and Serpine1) (all P ≤ 0.0001 vs. untreated renal STCs; Fig. 1C). IL-6, MCP-1, and PAI-1 levels were elevated in conditioned medium collected from irradiated renal STCs compared with normal cells (all P ≤ 0.004; Fig. 1, D–F).
Fig. 1.
Characterization of untreated (normal) and 10-Gy irradiated senescent (10G) swine renal scattered tubular-like cells. A: representative senescence-associated β-galactosidase (SA-β-gal) staining of normal and 10G cells. B: flow cytometry analysis showing the percentage of SA-β-gal positivity in normal and 10G cells. C: gene expression of senescence and senescence-associated secretory phenotype factors quantified by RT-PCR (relative to TATA box-binding protein). Values are means ± SD; n = 4 for the normal group and n = 6 for the 10G group. CDKN2A, cyclin-dependent kinase inhibitor 2A; CDKN1A, cyclin-dependent kinase inhibitor 1A; IL1A, IL-1A; IL6, IL-6; CCL2, chemokine (C-C motif) ligand 2; SERPINE1, serpin family E member 1. D−F: levels of IL-6 (D), macrophage chemoattractant protein (MCP)-1 (E), and plasminogen activator inhibitor-1 (PAI)-1 (F) in conditioned medium (in pg/µL, corrected by the number of cells). Values are medians ± interquartile ranges; n = 4 in the normal group and n = 3 in 10G group. *P < 0.05 vs. the normal group by Student’s t tests or Wilcoxon test.
Senescent STCs Induce Renal Fibrosis and Microvascular Loss
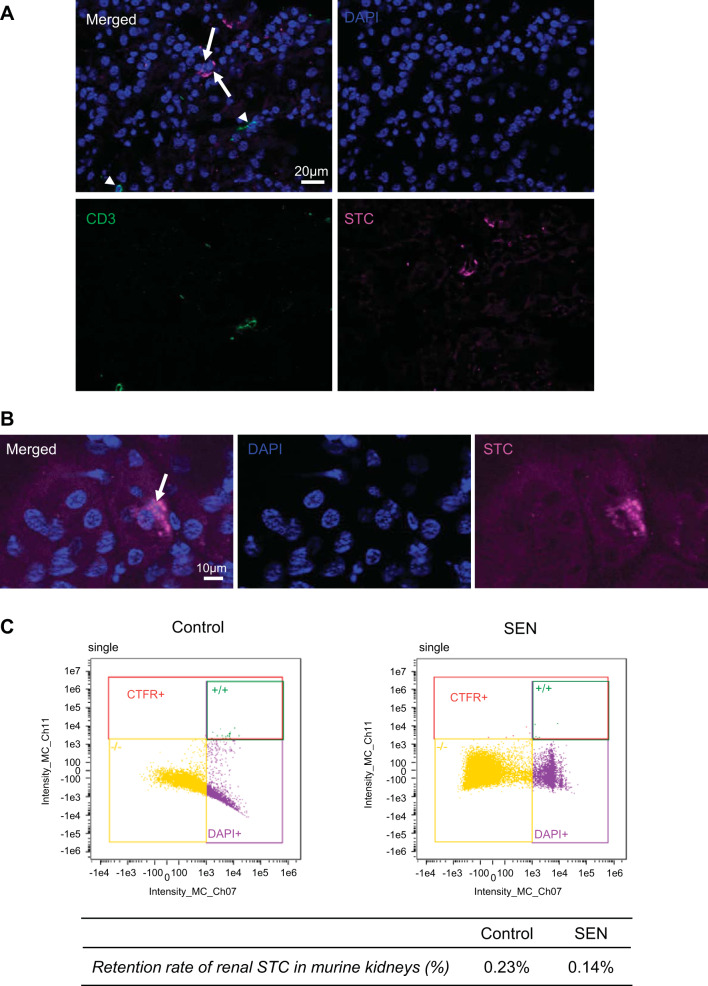
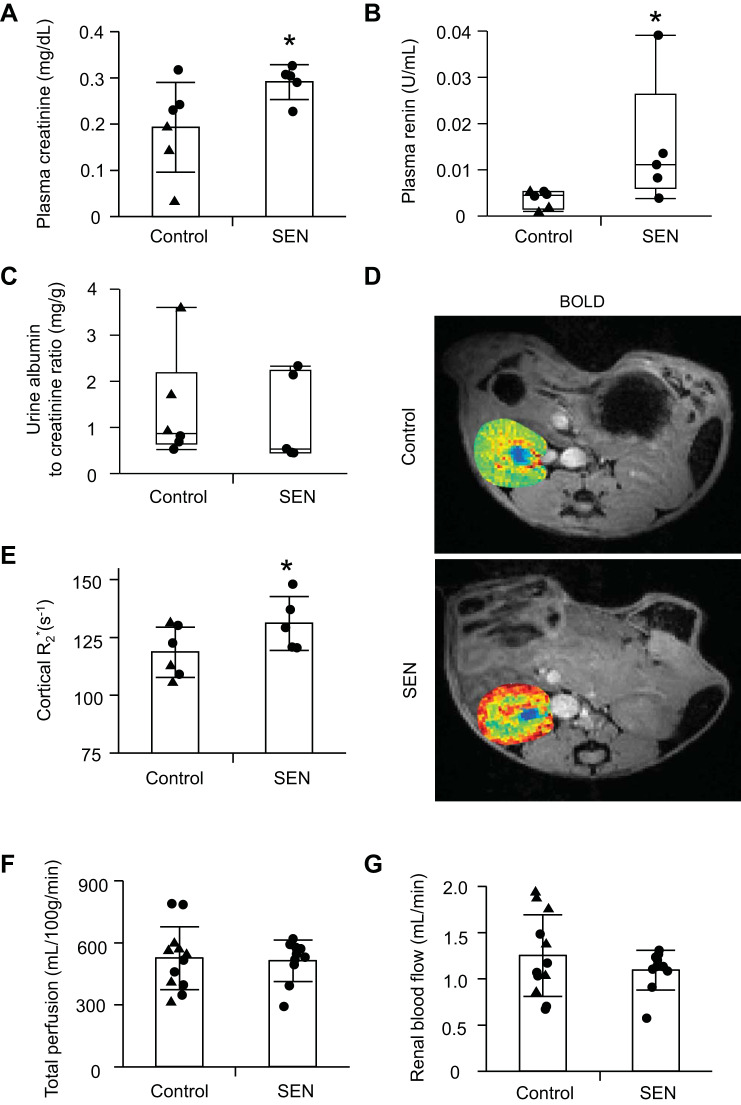
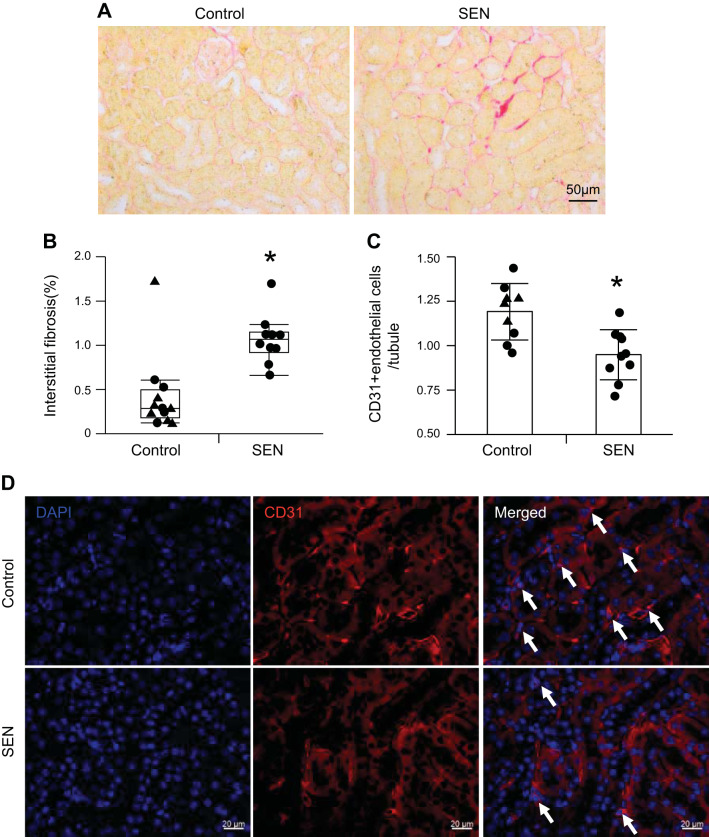
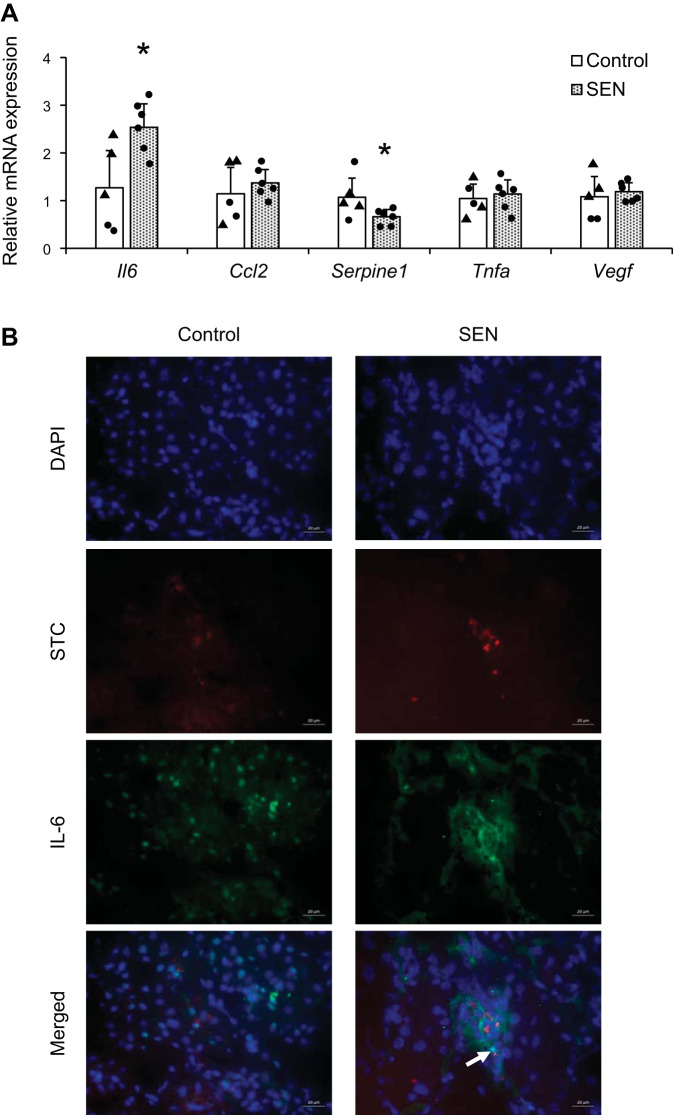
Control or senescent STCs were transplanted into young normal mice. By four weeks after injection there was no significant change in systolic blood pressure in either the control or senescent group (P = 0.11 and P = 0.19, respectively), and body weight, systolic blood pressure, and kidney weights showed no significant difference between the groups (P = 0.22, P = 0.84, and P = 0.14, respectively; Table 1). Four weeks after injection, cells were detected engrafted in the kidneys, especially in tubules, and no colocalization with CD3+ T cells argued against rejection (Fig. 2, A and B). Retention rates of renal STCs per flow cytometry analysis were 0.23% STCs in control kidneys and 0.14% in senescent STC-treated kidneys (Fig. 2C). Plasma creatinine and renin levels were significantly elevated in the senescent group compared with the control group (P = 0.03 and P = 0.04, respectively; Fig. 3, A and B), whereas urine albumin-to-creatinine ratios were comparable between them (P = 0.5; Fig. 3C). Renal cortical hypoxia increased in the senescent group compared with the control group (P = 0.05; Fig. 3, D and E), while renal perfusion and blood flow were not significantly different (P = 0.41 and P = 0.16, respectively; Fig. 3, F and G). Senescent kidneys were more fibrotic by sirius red staining than control kidneys (P = 0.007; Fig. 4, A and B), and CD31-stained renal sections showed fewer CD31+ capillary endothelial cells per tubule (P = 0.003; Fig. 4, C and D). Senescent kidneys showed upregulation of Il6 (P = 0.01) and downregulation of Serpine1 gene expression (P = 0.04), whereas gene expression of Ccl2, Tnfa, and Vegf remained unchanged (P = 0.4, P = 0.6, and P = 0.6, respectively; Fig. 5A). We observed colocalization of senescent STCs with IL-6 in senescent kidneys but not in control kidneys (Fig. 5B), linking senescent STCs to the release of SASP with potential harmful effects.
Table 1.
Characteristics
| Control Cell-Injected Mice | Senescent Cell-Injected Mice | P Value | |
|---|---|---|---|
| Number of mice/group | 6 | 5 | |
| Change in body weight (4 wk – baseline), g | −1.9 (−2.3 to approximately −1.6) | −0.2 (−2.3 to approximately −0.1) | 0.22 |
| Change in systolic blood pressure (4 wk − baseline), mmHg | 19.3 ± 16.8 | 16.6 ± 22.5 | 0.84 |
| Kidney weight, mg | 202.9 ± 17.5 | 190.2 ± 15.4 | 0.14 |
Data are expressed as medians (interquartile ranges) or means ± SD. Analysis of body weight was by a Wilcoxon test; analysis of kidney weight and systolic blood pressure was by two-tailed Student’s t tests.
Fig. 2.
Transplanted control and senescent (SEN) renal scattered tubular-like cells (STCs) in the murine kidneys. A: representative immunofluorescence of prelabeled transplanted renal STCs (far red, arrow) with CD3 staining (green, arrowhead) in the kidney. DAPI is shown in blue. B: representative images showing a STC (arrow) to be incorporated into a renal tubule. C: flow cytometry analysis showing the distribution of transplanted cells 4 wk after injection, as a fraction of cellular content in control and SEN kidneys.
Fig. 3.
Renal function in control and senescent (SEN) cell-transplanted mice. A: plasma creatinine level. B: plasma renin content. C: urine albumin-to-creatinine ratio. D and E: hypoxia by blood oxygen level-dependent MRI (brighter red represents greater hypoxia, unit of the color bar is s−1). n = 6 in the control group and n = 5 in the SEN group. F and G: renal perfusion and blood flow by MRI. Values are means ± SD or medians ± interquartile ranges; n = 12 control kidneys and n = 10 SEN kidneys. ▲, male; ●, female. *P < 0.05 vs. the control group by Student’s t tests or Wilcoxon test.
Fig. 4.
Renal injury in control and senescent (SEN) cell-transplanted mice. A and B: sirius red staining and quantification of interstitial fibrosis. C and D: CD31 immunofluorescence staining and quantification of CD31+ endothelial cells. Values are means ± SD or medians ± interquartile ranges; n = 12 control kidneys and n = 10 SEN kidneys. ▲, male; ●, female. *P < 0.05 vs. the control group by Student’s t tests or Wilcoxon test.
Fig. 5.
Expression of senescence-associated secretory phenotype factors in murine kidneys after transplantation of control or senescent (SEN) renal scattered tubular-like cells (STCs). A: gene expression of senescence-associated secretory phenotype factors quantified by RT-PCR (relative to GAPDH). Values are means ± SD; n = 5 in the control group and n = 6 in the SEN group. ▲, male; ●, female. *P < 0.05 vs. the control group by Student’s t tests. B: representative immunofluorescence of prelabeled transplanted renal STCs (red) colocalizing (yellow) with IL-6 staining (green, arrow) in the kidney. DAPI is shown in blue. Il6, interleukin-6; Ccl2, chemokine (C-C motif) ligand 2; Serpine1, serpin family E member 1; Tnfa, tumor necrosis factor-α; Vegf, vascular endothelial growth factor.
DISCUSSION
This study shows that engraftment of senescent renal STCs directly engenders renal inflammation, fibrosis, and microvascular loss, despite their low retention in the kidney. This further activated the renin-angiotensin system and hampered renal function and cortical oxygenation, possibly related to upregulated renal IL6 gene expression. These observations suggest that exposure to a small number of senescent cells can directly induce renal injury, consistent with a potential role for cellular senescence in the pathogenesis of kidney injury.
We have previously shown that various insults, including metabolic burden and renal artery stenosis, give rise to cellular senescence in renal tubules (17, 18). In swine chronic renal ischemic injury, gene ontology analysis of STCs has shown that genes encoding for the cell cycle and cytoskeleton are downregulated (2), consistent with the development of cellular senescence. Accordingly, decreased chromosome condensin complex subunits, such as structural maintenance of chromosomes 2 and 4 (SMC2 and SMC4, respectively), has a close relationship with cellular senescence (26). The cytoskeleton has emerged as a modulator of cellular and organismal aging (19), and loss of centrosomal structures hinders cell cycle progression (14).
Here, we studied the direct effect of senescent STCs on normal renal cells via transplantation of exogenous cells, in turn underscoring the clinically important role of endogenous senescent STCs in the injured kidney. Our findings showed that exogenous senescent STCs increased renal fibrosis and microvascular rarefaction, which may be linked to upregulated renal expression of IL-6. Indeed, the most prominent SASP factor detected in radiation-induced senescent swine renal STCs was IL-6, which was secreted in 50-fold higher levels compared with conditioned medium of normal cells. After delivery of senescent cells to mice in vivo, renal Il6 gene expression was also amplified. Furthermore, we observed senescent STCs colocalizing with IL-6 staining, implying potential detrimental effects on neighboring cells. IL-6 trans-signaling has been implicated in the development and progression of renal interstitial fibrosis (6). IL-6 can trigger fibroblasts to produce extracellular matrix proteins, and tubular epithelial cells to transit to mesenchymal phenotype, leading to peritubular microvascular rarefaction via increased distance between the capillaries and tubules (22). Moreover, IL-6 may be related to the increase in plasma renin content after delivery of senescent cells. Prenatal IL-6 exposure provokes activation of the renal and circulating renin-angiotensin system in female rats (31), whereas inhibition of the renin-angiotensin system blunts proinflammatory and profibrotic pathways, including IL-6 (35). Additional studies are needed to determine if inhibition of IL-6 could be a potential strategy targeting renal senescence.
In our model, transplantation of senescent cells increased plasma creatinine in normal mice but did not change albuminuria, which often involves structural changes in podocyte foot process and glomerular endothelial injury (37). Our results indicate that transplanted renal STCs may engraft adjacent to, or possibly incorporate into, tubules and provoke the ripple effect into surrounding tubules and the interstitium. In addition, renal perfusion and blood flow were not different between the control and senescent groups, whereas cortical oxygenation was decreased in the senescent group, although this did not reached statistical difference levels, and capillary number was decreased. This indicates that the presence of a small number of senescent tubular cells in the kidney elicits microvascular injury.
Interestingly, while Serpine1 gene expression was increased in senescent cells in vitro, it was unexpectedly lower in kidneys transplanted with senescent cells. Notably, PAI-1 plays a protective role in glomerulonephritis (13), and its levels fall with aging (24), so that its downregulation might reflect the disease process. Additionally, considering the low retention rate of transplanted senescent cells, the effect of original net tissue gene expression in renal cells might have been dominant. Although transplanted senescent cells can secrete PAI-1, it is mainly produced by endothelial cells (33). The decreased number of endothelial cells in kidneys with transplanted senescent cells might have therefore accounted for their lower expression of PAI-1.
In this study, swine renal STCs were engrafted in the murine kidneys with no evidence for rejection, albeit at a retention rate of <0.3%. Interestingly, we found that the percentage of engrafted senescent cells among dissociated kidney cells was lower than that of untreated cells. Senescent stem cells have shown downregulation of genes related to focal adhesion, organization, and cytoskeleton turnover, leading to impaired migratory and homing ability (12). Our group has previously shown that mesenchymal stem cell retention 48 h after injection was <1% in the healthy murine kidney (43). Nevertheless, despite this low retention, our observations demonstrate that the presence of a small number of senescent cells suffice to exert deleterious effects on normal neighboring tissue. Moreover, the antiapoptotic attribute of senescent cells may extend their survival in the kidney, thereby potentiating their injurious capacity (38).
We acknowledge some limitations in our study. We studied normal kidneys from relatively young mice, which might have decreased homing of xenotransplanted cells and were likely more resilient to the effect of senescent cells compared with injured kidneys affected by concomitant aging or disease. We used xenogeneic STCs, because pig kidneys readily yield a sufficient number for delivery and their STCs have been well characterized (1). Yet, we did not observe rejection. The control group included both male and female mice, whereas the senescent group included only female mice, because of mouse availability from the vendor at that time. While we did not observe sex-based differences within our control group in renal function, fibrosis, or microvascular density, our study was likely underpowered to detect sex differences because of small sample size, increasing the risk of type II error or false negative (29). Furthermore, given that female mice have a lower propensity for kidney damage and development of senescence than male mice (41), detecting significant effects of senescent STCs in female mice underscores their impact. Murine renal arteries do not lend themselves to intrarenal injections; thus, we used systemic injections, leading to lower engraftment. Additionally, we did not investigate retention and potential injurious effects of transplanted STCs in other organs, which was beyond the scope of this study. However, while we cannot exclude the possibility that senescent STCs lodged in other organs induced renal injury remotely via systemic inflammation, this effect would have likely been small compared with their direct effect within the kidney. We also did not measure renal function before injections; future longitudinal studies might be informative. Finally, the unaltered body weight implied overall well-being.
In conclusion, exposure to a small number of exogenous senescent STCs induces damage to neighboring cells in the murine kidney, eliciting inflammation, fibrosis, and microvascular loss. SASP factors, particularly IL-6, might play an important role in this process. These findings imply that the development of senescence in endogenous tubular cells, or possibly other renal cell types, might contribute to the pathomechanism of kidney disease. Plausibly, senescent cells clearance may be an effective strategy in preventing renal injury. These findings support novel intervention strategies and targeted senolytic therapy in renal senescence.
GRANTS
This work was partly supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-120292, DK-104273, DK-122734, and DK-102325.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). Dr. Lerman receives grant funding from Novo Nordisk, and is an advisor to Weijian Technologies and AstraZeneca.
AUTHOR CONTRIBUTIONS
S.R.K., K.J., X.-J.C., X.Y.Z., and L.O.L. conceived and designed research; S.R.K., K.J., C.M.F., H.T., X.-J.C., and X.Y.Z. performed experiments; S.R.K., K.J., C.M.F., and X.-J.C. analyzed data; S.R.K., T.T., and L.O.L. interpreted results of experiments; S.R.K., K.J., C.M.F., and X.-J.C. prepared figures; S.R.K., K.J., and L.O.L. drafted manuscript; S.R.K., K.J., C.M.F., H.T., X.-J.C., X.Y.Z., L.J.H., T.T., J.L.K., and L.O.L. edited and revised manuscript; S.R.K., K.J., C.M.F., H.T., X.-J.C., X.Y.Z., L.J.H., T.T., J.L.K., and L.O.L. approved final version of manuscript.
REFERENCES
- 1.Nargesi AA, Zhu XY, Conley SM, Woollard JR, Saadiq IM, Lerman LO, Eirin A. Renovascular disease induces mitochondrial damage in swine scattered tubular cells. Am J Physiol Renal Physiol 317: F1142–F1153, 2019. doi: 10.1152/ajprenal.00276.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghajani Nargesi A, Zhu XY, Liu Y, Tang H, Jordan KL, Lerman LO, Eirin A. Renal artery stenosis alters gene expression in swine scattered tubular-like cells. Int J Mol Sci 20: 5069, 2019. doi: 10.3390/ijms20205069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aratani S, Tagawa M, Nagasaka S, Sakai Y, Shimizu A, Tsuruoka S. Radiation-induced premature cellular senescence involved in glomerular diseases in rats. Sci Rep 8: 16812, 2018. doi: 10.1038/s41598-018-34893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biran A, Zada L, Abou Karam P, Vadai E, Roitman L, Ovadya Y, Porat Z, Krizhanovsky V. Quantitative identification of senescent cells in aging and disease. Aging Cell 16: 661–671, 2017. doi: 10.1111/acel.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol Pharmacother 4: 303–306, 2013. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Yuan H, Cao W, Wang T, Chen W, Yu H, Fu Y, Jiang B, Zhou H, Guo H, Zhao X. Blocking interleukin-6 trans-signaling protects against renal fibrosis by suppressing STAT3 activation. Theranostics 9: 3980–3991, 2019. doi: 10.7150/thno.32352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, Lerman LO, Platt JL, Romero JC, Textor SC, Nath KA, Grande JP. Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol 297: F1055–F1068, 2009. doi: 10.1152/ajprenal.90439.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui S, Xue L, Yang F, Dai S, Han Z, Liu K, Liu B, Yuan Q, Cui Z, Zhang Y, Xu F, Chen Y. Postinfarction hearts are protected by premature senescent cardiomyocytes via GATA 4-dependent CCN 1 secretion. J Am Heart Assoc 7: e009111, 2018. doi: 10.1161/JAHA.118.009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva PFL, Ogrodnik M, Kucheryavenko O, Glibert J, Miwa S, Cameron K, Ishaq A, Saretzki G, Nagaraja-Grellscheid S, Nelson G, von Zglinicki T. The bystander effect contributes to the accumulation of senescent cells in vivo. Aging Cell 18: e12848, 2019. doi: 10.1111/acel.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebrahimi B, Crane JA, Knudsen BE, Macura SI, Grande JP, Lerman LO. Evolution of cardiac and renal impairment detected by high-field cardiovascular magnetic resonance in mice with renal artery stenosis. J Cardiovasc Magn Reson 15: 98, 2013. doi: 10.1186/1532-429X-15-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eirin A, Zhu XY, Ebrahimi B, Krier JD, Riester SM, van Wijnen AJ, Lerman A, Lerman LO. Intrarenal delivery of mesenchymal stem cells and endothelial progenitor cells attenuates hypertensive cardiomyopathy in experimental renovascular hypertension. Cell Transplant 24: 2041–2053, 2015. doi: 10.3727/096368914X685582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geißler S, Textor M, Kühnisch J, Könnig D, Klein O, Ode A, Pfitzner T, Adjaye J, Kasper G, Duda GN. Functional comparison of chronological and in vitro aging: differential role of the cytoskeleton and mitochondria in mesenchymal stromal cells. PLoS One 7: e52700, 2012. doi: 10.1371/journal.pone.0052700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertig A, Berrou J, Allory Y, Breton L, Commo F, Costa De Beauregard MA, Carmeliet P, Rondeau E. Type 1 plasminogen activator inhibitor deficiency aggravates the course of experimental glomerulonephritis through overactivation of transforming growth factor beta. FASEB J 17: 1–24, 2003. doi: 10.1096/fj.03-0084fje. [DOI] [PubMed] [Google Scholar]
- 14.Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science 291: 1547–1550, 2001. doi: 10.1126/science.1056866. [DOI] [PubMed] [Google Scholar]
- 15.Jiang K, Ferguson CM, Ebrahimi B, Tang H, Kline TL, Burningham TA, Mishra PK, Grande JP, Macura SI, Lerman LO. Noninvasive assessment of renal fibrosis with magnetization transfer mr imaging: validation and evaluation in murine renal artery stenosis. Radiology 283: 77–86, 2017. doi: 10.1148/radiol.2016160566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, Prata L, Masternak MM, Kritchevsky SB, Musi N, Kirkland JL. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 40: 554–563, 2019. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SR, Eirin A, Zhang X, Lerman A, Lerman LO. Mitochondrial protection partly mitigates kidney cellular senescence in swine atherosclerotic renal artery stenosis. Cell Physiol Biochem 52: 617–632, 2019. doi: 10.33594/000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SR, Jiang K, Ogrodnik M, Chen X, Zhu XY, Lohmeier H, Ahmed L, Tang H, Tchkonia T, Hickson LJ, Kirkland JL, Lerman LO. Increased renal cellular senescence in murine high-fat diet: effect of the senolytic drug quercetin. Transl Res 213: 112–123, 2019. doi: 10.1016/j.trsl.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kounakis K, Tavernarakis N. The cytoskeleton as a modulator of aging and neurodegeneration. Adv Exp Med Biol 1178: 227–245, 2019. doi: 10.1007/978-3-030-25650-0_12. [DOI] [PubMed] [Google Scholar]
- 20.Li M, You L, Xue J, Lu Y. Ionizing radiation-induced cellular senescence in normal, non-transformed cells and the involved DNA damage response: a mini review. Front Pharmacol 9: 522, 2018. doi: 10.3389/fphar.2018.00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Yang JR, He YN, Cai GY, Zhang JG, Lin LR, Zhan J, Zhang JH, Xiao HS. Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin A (IgA) nephropathy. Transl Res 159: 454–463, 2012. doi: 10.1016/j.trsl.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Liu M, Ning X, Li R, Yang Z, Yang X, Sun S, Qian Q. Signalling pathways involved in hypoxia-induced renal fibrosis. J Cell Mol Med 21: 1248–1259, 2017. doi: 10.1111/jcmm.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.McBane RD II, Hardison RM, Sobel BE; BARI 2D Study Group . Comparison of plasminogen activator inhibitor-1, tissue type plasminogen activator antigen, fibrinogen, and D-dimer levels in various age decades in patients with type 2 diabetes mellitus and stable coronary artery disease (from the BARI 2D trial). Am J Cardiol 105: 17–24, 2010. doi: 10.1016/j.amjcard.2009.08.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF. Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int 65: 510–520, 2004. doi: 10.1111/j.1523-1755.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 26.Meng Q, Gao J, Zhu H, He H, Lu Z, Hong M, Zhou H. The proteomic study of serially passaged human skin fibroblast cells uncovers down-regulation of the chromosome condensin complex proteins involved in replicative senescence. Biochem Biophys Res Commun 505: 1112–1120, 2018. doi: 10.1016/j.bbrc.2018.10.065. [DOI] [PubMed] [Google Scholar]
- 28.Ogrodnik M, Zhu Y, Langhi LGP, Tchkonia T, Krüger P, Fielder E, Victorelli S, Ruswhandi RA, Giorgadze N, Pirtskhalava T, Podgorni O, Enikolopov G, Johnson KO, Xu M, Inman C, Palmer AK, Schafer M, Weigl M, Ikeno Y, Burns TC, Passos JF, von Zglinicki T, Kirkland JL, Jurk D. Obesity-induced cellular senescence drives anxiety and impairs neurogenesis. Cell Metab 29: 1061–1077.e8, 2019. [Erratum in Cell Metab 29: 1233, 2019.] doi: 10.1016/j.cmet.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rich-Edwards JW, Kaiser UB, Chen GL, Manson JE, Goldstein JM. Sex and gender differences research design for basic, clinical, and population studies: essentials for investigators. Endocr Rev 39: 424–439, 2018. doi: 10.1210/er.2017-00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romagnani P, Remuzzi G. CD133+ renal stem cells always co-express CD24 in adult human kidney tissue. Stem Cell Res (Amst) 12: 828–829, 2014. doi: 10.1016/j.scr.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Samuelsson AM, Alexanderson C, Mölne J, Haraldsson B, Hansell P, Holmäng A. Prenatal exposure to interleukin-6 results in hypertension and alterations in the renin-angiotensin system of the rat. J Physiol 575: 855–867, 2006. doi: 10.1113/jphysiol.2006.111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sturmlechner I, Durik M, Sieben CJ, Baker DJ, van Deursen JM. Cellular senescence in renal ageing and disease. Nat Rev Nephrol 13: 77–89, 2017. doi: 10.1038/nrneph.2016.183. [DOI] [PubMed] [Google Scholar]
- 33.Svenningsen P, Hinrichs GR, Zachar R, Ydegaard R, Jensen BL. Physiology and pathophysiology of the plasminogen system in the kidney. Pflugers Arch 469: 1415–1423, 2017. doi: 10.1007/s00424-017-2014-y. [DOI] [PubMed] [Google Scholar]
- 34.Tchkonia T, Kirkland JL. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA 320: 1319–1320, 2018. doi: 10.1001/jama.2018.12440. [DOI] [PubMed] [Google Scholar]
- 35.Vaziri ND, Xu ZG, Shahkarami A, Huang KT, Rodríguez-Iturbe B, Natarajan R. Role of AT-1 receptor in regulation of vascular MCP-1, IL-6, PAI-1, MAP kinase, and matrix expressions in obesity. Kidney Int 68: 2787–2793, 2005. doi: 10.1111/j.1523-1755.2005.00750.x. [DOI] [PubMed] [Google Scholar]
- 36.Walaszczyk A, Dookun E, Redgrave R, Tual-Chalot S, Victorelli S, Spyridopoulos I, Owens A, Arthur HM, Passos JF, Richardson GD. Pharmacological clearance of senescent cells improves survival and recovery in aged mice following acute myocardial infarction. Aging Cell 18: e12945, 2019. doi: 10.1111/acel.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weil EJ, Lemley KV, Mason CC, Yee B, Jones LI, Blouch K, Lovato T, Richardson M, Myers BD, Nelson RG. Podocyte detachment and reduced glomerular capillary endothelial fenestration promote kidney disease in type 2 diabetic nephropathy. Kidney Int 82: 1010–1017, 2012. doi: 10.1038/ki.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson KO, Lowe V, Tchkonia T, Westendorf JJ, Kirkland JL. Transplanted senescent cells induce an osteoarthritis-like condition in mice. J Gerontol A Biol Sci Med Sci 72: 780–785, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nat Med 24: 1246–1256, 2018. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci USA 112: E6301–E6310, 2015. doi: 10.1073/pnas.1515386112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yousefzadeh MJ, Zhao J, Bukata C, Wade EA, McGowan SJ, Angelini LA, Bank MP, Gurkar AU, McGuckian CA, Calubag MF, Kato JI, Burd CE, Robbins PD, Niedernhofer LJ. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19: e13094, 2020. doi: 10.1111/acel.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 17: 324–328, 2014. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 43.Zou X, Jiang K, Puranik AS, Jordan KL, Tang H, Zhu X, Lerman LO. Targeting murine mesenchymal stem cells to kidney injury molecule-1 improves their therapeutic efficacy in chronic ischemic kidney injury. Stem Cells Transl Med 7: 394–403, 2018. doi: 10.1002/sctm.17-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou X, Kwon SH, Jiang K, Ferguson CM, Puranik AS, Zhu X, Lerman LO. Renal scattered tubular-like cells confer protective effects in the stenotic murine kidney mediated by release of extracellular vesicles. Sci Rep 8: 1263, 2018. doi: 10.1038/s41598-018-19750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]