Abstract
Orexin receptor antagonists are clinically useful for treating insomnia, but thorough blockade of orexin signaling could cause narcolepsy-like symptoms. Specifically, while sleepiness is a desirable effect, an orexin antagonist could also produce cataplexy, sudden episodes of muscle weakness often triggered by strong, positive emotions. In this study, we examined the effects of dual orexin receptor antagonists (DORAs), lemborexant (E2006) and almorexant, on sleep–wake behavior and cataplexy during the dark period in wild-type (WT) mice and prepro-orexin knockout (OXKO) mice. In WT mice, lemborexant at 10 and 30 mg/kg quickly induced NREM sleep in a dose-dependent fashion. In contrast, lemborexant did not alter sleep–wake behavior in OXKO mice. Under the baseline condition, cataplexy was rare in lemborexant-treated WT mice, but when mice were given chocolate as a rewarding stimulus, lemborexant dose-dependently increased cataplexy. Almorexant produced similar results. Collectively, these results demonstrate that DORAs potently increase NREM and REM sleep in mice via blockade of orexin signaling, and higher doses can cause cataplexy when co-administered with a likely rewarding stimulus.
Keywords: orexin, dual orexin receptor antagonist, DORA, cataplexy, lemborexant, almorexant, narcolepsy
Statement of Significance.
With the rise in the prevalence of insomnia and sleep disturbances, development of effective and safe sleep aids is important. Drugs that target the orexin system have great potential to enhance sleep with relatively low risk of side effects. We found that the dual orexin receptor antagonists (DORAs), lemborexant and almorexant, potently increased NREM and REM sleep in wild-type (WT) mice but not in OXKO mice, confirming the specificity through which DORAs block orexin signaling. In addition, when WT mice had access to chocolate, both DORAs produced cataplexy. These results demonstrate that acute blockade of orexin signaling can trigger cataplexy under a likely rewarding condition. DORAs are potent hypnotics, and discussion of the risk of cataplexy should be included when prescribing DORAs.
Introduction
Sleep disturbances and insomnia are common, serious public health concerns. As many as 30% of adults in industrialized countries report some level of insomnia [1]. Difficulty initiating or maintaining sleep is associated with daytime consequences, including poor work performance, worse overall health, and reduced engagement with activities of daily life [2–6]. In addition, insufficient sleep is estimated to reduce national gross domestic products by 2%–3% [7]. Cognitive behavioral therapy is the first line of treatment for most people with insomnia, but medications are often helpful [8–10].
The discovery that narcolepsy type 1 is caused by severe loss of the orexin neurons provides new opportunities for treating insomnia. The neuropeptides orexin-A and -B (hypocretin-1 and -2) promote long periods of wake and regulate REM sleep via the orexin-1 and -2 receptors [11, 12]. Loss of the orexin-producing neurons in narcolepsy type 1 results in sleepiness and episodes of cataplexy, brief periods of muscle weakness usually triggered by positive emotional stimuli [13–17]. Similarly, prepro-orexin knockout (OXKO) mice have poor maintenance of wake and episodes of cataplexy, especially with rewarding, highly palatable food such as chocolate [18–20].
About 20% of the population take medications for insomnia [21]. Many of these medications enhance GABA signaling or block the effects of wake-promoting monoaminergic neurotransmitters (e.g. antihistamines, anticholinergics). These neurotransmitters affect many brain systems, and consequently, these medications can have troublesome side effects. Considering the more limited roles of the orexin peptides, dual orexin receptor antagonists (DORAs) are expected to improve insomnia with fewer side effects. Recent research has demonstrated the general efficacy and safety of DORAs [22–24], yet an important safety concern is whether acute, pharmacological blockade of orexin signaling by DORAs can produce cataplexy as occurs in people with narcolepsy and in animals chronically lacking orexin signaling [25–29]. A few cases of muscle weakness vaguely resembling cataplexy were reported in clinical trials of DORAs, however there has been no clear evidence of cataplexy in humans prescribed a DORA [8–10, 30, 31].
To address this question, we examined the effects of two DORAs, lemborexant and almorexant, on sleep–wake behavior in WT mice and OXKO mice. Specifically, we tested whether lemborexant and almorexant promote sleep solely by blocking orexin signaling and whether they can trigger cataplexy under baseline conditions or with chocolate.
Methods
Animals
These experiments were approved by the Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center and Harvard Medical School and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male, C57BL/6J wild-type mice (WT; n = 12) and prepro-OXKO mice on a C57BL/6J genetic background (OXKO; n = 8) were 15–18 weeks of age at time of surgery.
Surgery and polysomnography recordings
We anaesthetized mice with ketamine-xylazine (100 and 10 mg/kg, respectively, i.p.) and implanted them with a telemetry unit (DSI) for body temperature and locomotor activity acquisition and with electroencephalogram (EEG) and electromyogram (EMG) electrodes as described previously [19]. EEG signals were recorded using two ipsilateral stainless steel screws, and EMG signals were acquired by a pair of multi-stranded stainless steel wires inserted into the neck extensor muscles. The animals had ad libitum access to food and water, except on post-surgery Day 8 (see Drug administration section). All recordings were performed in a sound-attenuated chamber with a 12:12 h light/dark cycle (lights on at 07:00 and off at 19:00) and a constant temperature of about 23°C.
EEG/EMG signals were acquired using Grass Model 12 amplifiers (West Warwick, RI) and digitized at 128 Hz using a polysomnogram recording/scoring system (SleepSign software version 3.0, Kissei Comtec, Matsumoto, Japan). Mouse behavior was also video-captured simultaneously by SleepSign. The EEG/EMG signals were digitally filtered (EEG: 0.3–30 Hz, EMG: 10–64 Hz) and scored in 10 s epochs as wake, NREM, REM sleep, or cataplexy. Spectral power distribution was analyzed in 0.25 Hz bins within each behavioral state.
We scored cataplexy based on a consensus definition and our experience with OXKO mice [18–20, 32, 33]. We operationally defined cataplexy to begin with an abrupt transition from at least 40 s of active wake to atonia with prominent EEG theta (4–9 Hz) activity, and to end with an abrupt return back to active wake. Video recordings show immobility during the event, usually with a postural collapse outside the usual nest area. In prior work, we never encountered cataplexy in WT mice under regular home cage conditions, but WT mice treated with lemborexant or almorexant plus chocolate had cataplexy meeting this definition. Still, the EEG power spectra with DORA-induced cataplexy in WT mice differed slightly from that seen during typical cataplexy in OXKO mice (see Results section and Supplementary Videos S1–S3).
Drug and vehicle preparation protocol
The handling stress of gavage may reduce cataplexy in OXKO mice for several hours (unpublished observations). To avoid this stress, we administered DORAs by a voluntary eating method [34]. We suspended each drug in 0.5% methylcellulose (4000 cP solution) mixed with 14% gelatin (14%, w/v), plus a no-calorie sweetener (Splenda; 20%, w/v) and a natural flavor to form a solid jelly for oral administration. Specifically, we suspended drug in 0.525 mL methylcellulose mixed with 1.375 mL of the gelatin/splenda/flavor mixture in a well of a 24-well culture plate. After hardening, the jelly was cut into eight equal pieces and one piece (~0.24 g) was given to each mouse. Vehicle was prepared similarly. Almorexant was a gift from Novartis [35]. Lemborexant was provided by Eisai Co. Ltd.
Drug/chocolate administration and experimental design
Training for this voluntary eating method began 8 days after surgery. Mice were transferred to individual recording cages, hooked up to the recording cable, and food-deprived for 24 h. We first gave the jelly containing vehicle on Day 9, 15 min before dark onset (18:45). After Day 9, the mice were also given regular chow ad libitum, but they preferred vehicle-jelly over the chow and always finished eating the jelly within 1.5–2 min of presentation. The first sleep recordings started on Day 15, with vehicle-jelly, then three doses of lemborexant (1, 10, 30 mg/kg) were tested on the next days with 4 days washout between doses. The eight mice from the lemborexant experiments were later treated with almorexant (4 received the low dose and 4 received the high dose), and an additional cohort of four mice received the low and high dose of almorexant. In total, eight mice received low and high dose almorexant (30, 100 mg/kg) with at least 4 days of washout between doses. We administered chocolate as a rewarding stimulus as it increases cataplexy in OXKO mice [18, 32]. Specifically, we dropped one piece of milk chocolate (Hershey’s Kiss™, 4.5 g) in the cage at the dark onset (19:00). All mice were acclimated to the chocolate for three nights before the 12 h dark period EEG/EMG recordings.
Statistical analysis
All data are expressed as the mean ± SEM. Statistical analyses were performed using Prism software (versions 7.02 and 8; GraphPad Software, Inc., San Diego, CA).
Hourly amounts of wake, NREM sleep, REM sleep, and cataplexy were averaged across animals and compared between vehicle and each dose of drug using two-way repeated measures ANOVA on factors “drug treatment” and “time,” with a post hoc Dunnett’s multiple comparison test, Tukey’s multiple comparison test or Geisser–Greenhouse’s epsilon. The summary of sleep–wake amount, total number of sleep–wake bouts and mean length of sleep–wake bouts for 6 h after the jelly/chocolate administration were compared across vehicle and drug treatment days using one-way repeated measures ANOVA with a post hoc Dunnett’s or Bonferroni’s multiple comparison test. Sleep latency was measured from the time mice finished eating the jelly (confirmed by video) to the occurrence of the first NREM sleep bout longer than 30 s. The results were compared across vehicle and drug-treatment days using one-way repeated measures ANOVA with a post hoc Dunnett’s or Bonferroni’s multiple comparison test. To compare EEG spectra during cataplexy between WT to OXKO mice, we used a two-way ANOVA followed by multiple T-tests and the two-stage linear step-up procedure of Benjamin, Krieger, and Yekutieli (GraphPad v8). For all analyses, significance was set at p < 0.05.
Results
Effects of lemborexant in WT mice
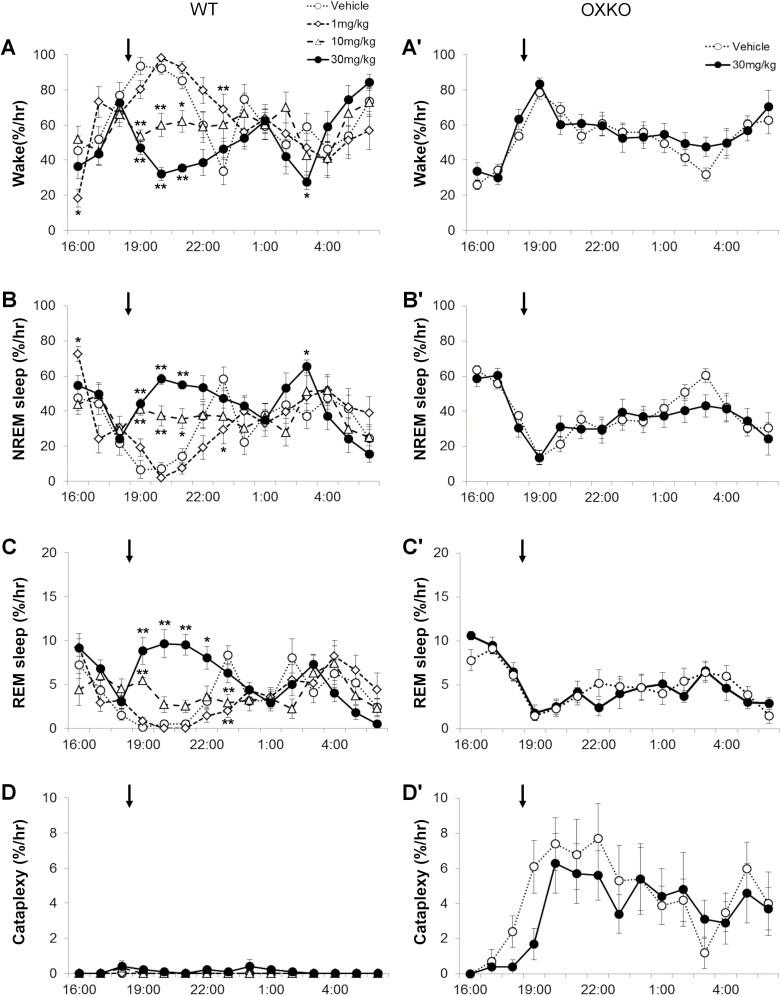
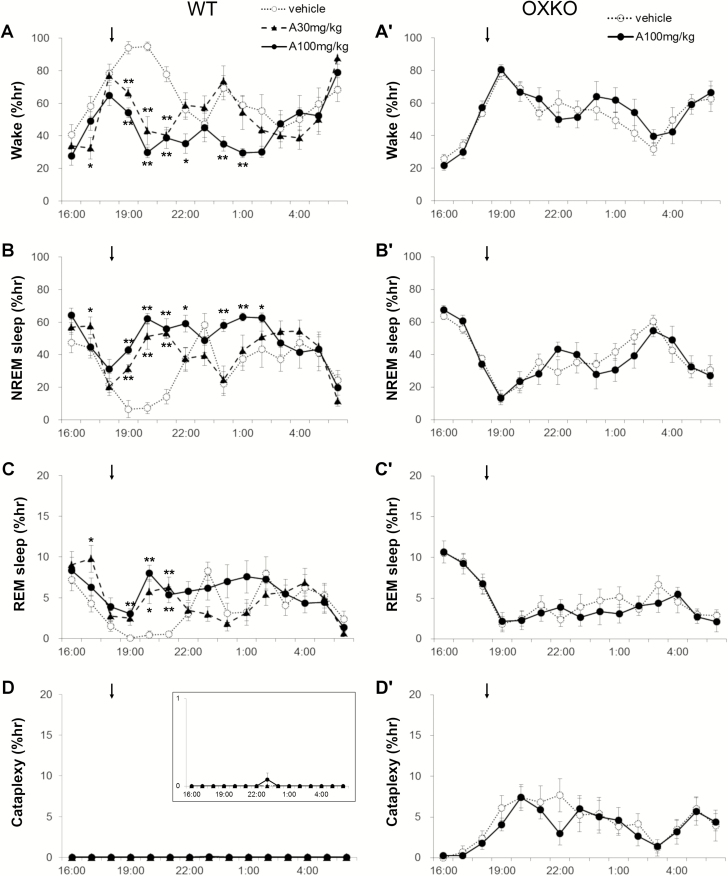
In WT mice, lemborexant dose-dependently decreased wake (interaction F(42,392) = 3.512, p < 0.0001) and increased NREM sleep (interaction F(42,392) = 3.265, p < 0.0001), and REM sleep (interaction F(42,392) = 4.324, p < 0.0001) (Figure 1, A–C). Across the 6 h after administration, lemborexant at 10 and 30 mg/kg decreased the amount of wake from 73 ± 2% (vehicle day) to 60 ± 3% (10 mg/kg) and 42 ± 3% (30 mg/kg) (Figure 2, A). NREM sleep increased from 24 ± 2% (vehicle) to 36 ± 3% (10 mg/kg) and 50 ± 3% (30 mg/kg) of the 6 h, and REM sleep increased from 2.6 ± 0.2% (vehicle) to 7.8 ± 0.8% (30 mg/kg). Lemborexant induced sleep very quickly (latency to NREM sleep was significantly reduced; F(3, 8) = 17.7, p = 0.002). WT mice were normally persistently awake at the beginning of the dark period, and they typically entered NREM sleep 130 ± 28 min after eating vehicle jelly. However, with lemborexant, the first NREM sleep occurred in 31 ± 11 (1 mg/kg vs. vehicle, p = 0.015), 10 ± 4 (10 mg/kg vs. vehicle, p = 0.008), and 5 ± 1 (30 mg/kg vs. vehicle, p = 0.007) minutes. At higher doses of lemborexant, the latency to REM sleep was also reduced (F(3, 12) = 15.4, p = 0.0006). With vehicle, mice entered REM sleep 190.3 ± 28 min after eating jelly. With lemborexant, the first REM sleep occurred in 179 ± 28 (1 mg/kg, ns), 22.9 + 7 (10 mg/kg, p < 0.001), and 31.3 ± 6.1 minutes (30 mg/kg, p = 0.002).
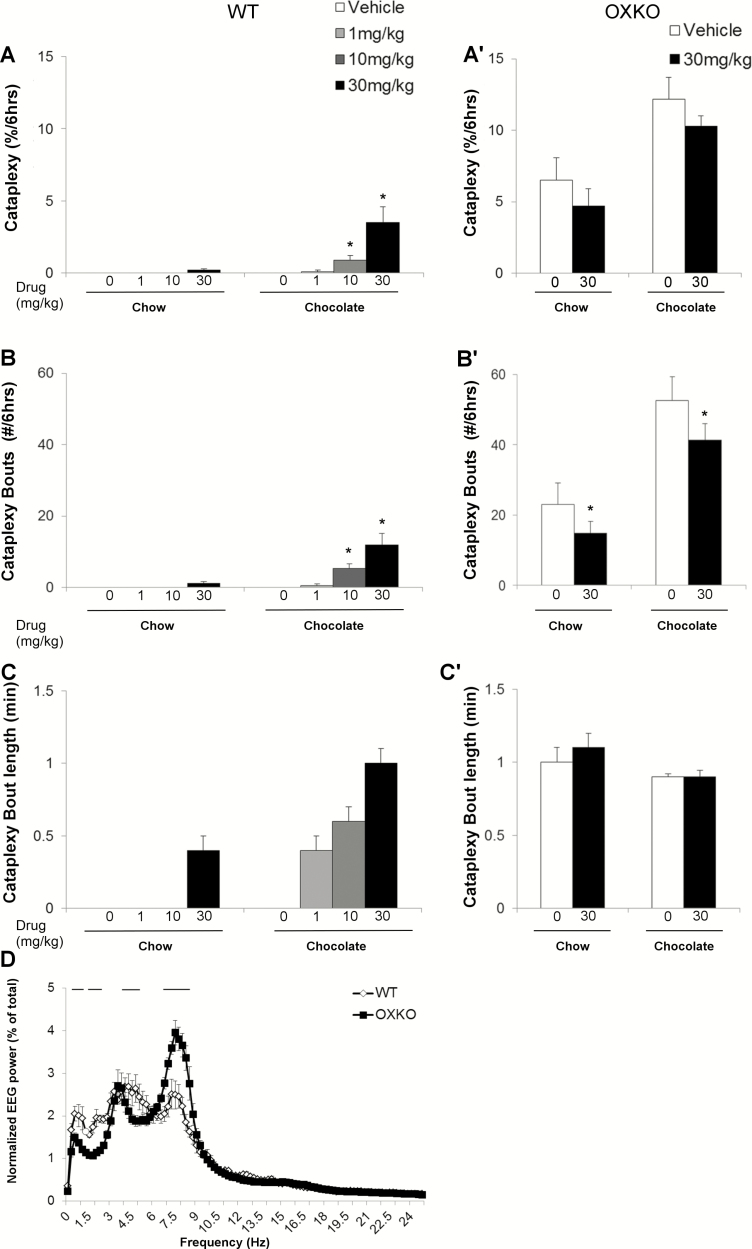
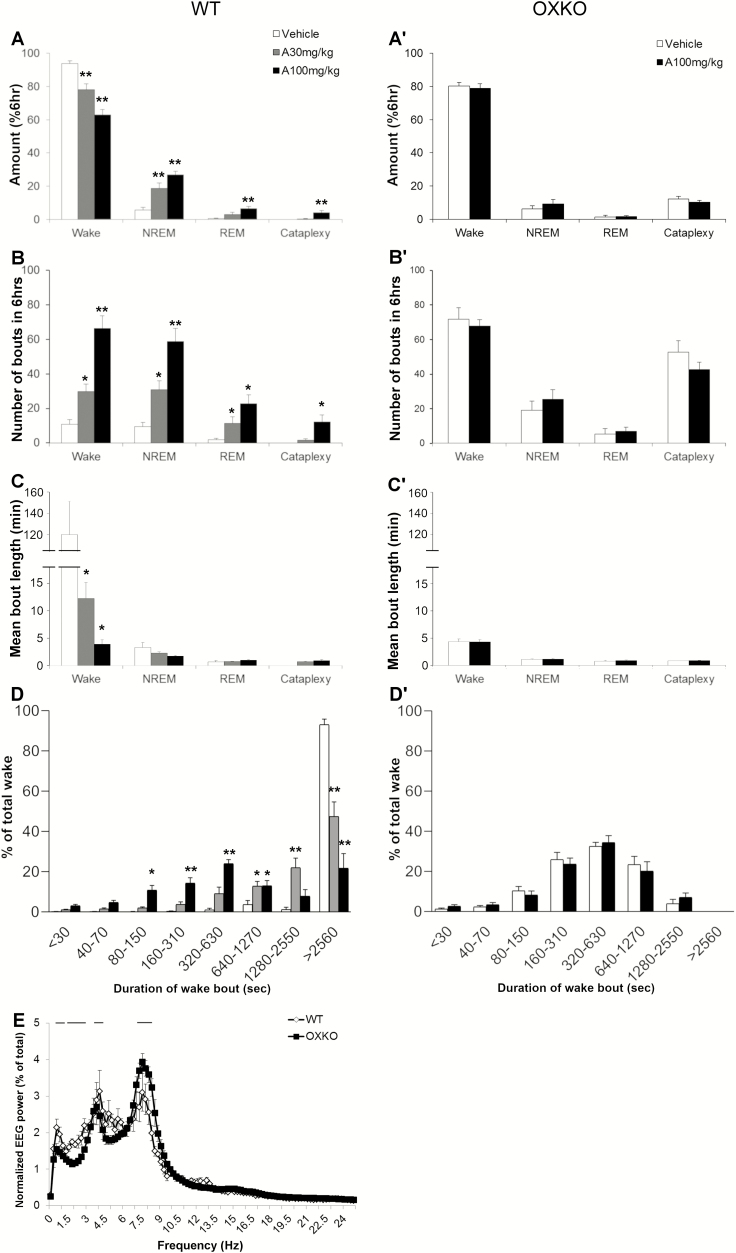
Figure 1.
Lemborexant dose-dependently increased sleep in WT mice but not in OXKO mice fed regular chow. Hourly amounts of wake, NREM sleep, REM sleep, and cataplexy in WT mice (A–D) and orexin KO (OXKO) mice (A′–D′). Arrows indicate the time of vehicle/drug presentation (at 18:45). * p < 0.05, ** p < 0.01 compared to vehicle day.
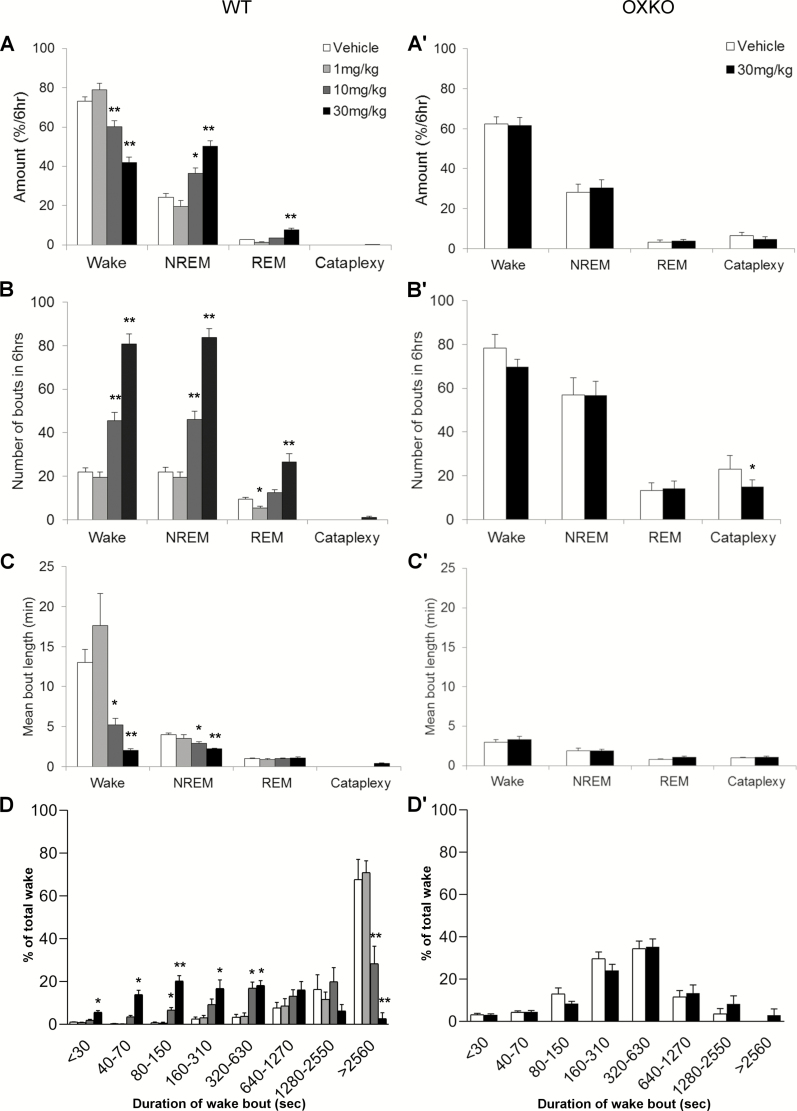
Figure 2.
Lemborexant increased transitions into NREM and REM sleep. Amounts of wake, NREM sleep, REM sleep, and cataplexy as a percentage of time in WT (A) and OXKO (A′) mice across the 6 h after lemborexant administration. Lemborexant increased the number of wake, NREM sleep, and REM sleep bouts in WT mice (B) but not in OXKO mice (B′). Lemborexant severely shortened the duration of wake bouts in WT mice (C). Time-weighted frequency histograms highlight the shift toward short wake bouts in WT mice (D). * p < 0.05, ** p < 0.01 compared to vehicle day.
Over the first 6 h, lemborexant increased transitions into NREM and REM sleep. The number of wake bouts increased from 22 ± 2 (vehicle) to 46 ± 4 (10 mg/kg) and 81 ± 5 (30 mg/kg), and the mean length of wake bouts decreased from 13.0 ± 1.6 min (vehicle) to 5.2 ± 0.8 (10 mg/kg) and 2.0 ± 0.2 min (30 mg/kg) (Figure 2, B and C). Time-weighted frequency histograms indicated a shift to much shorter wake bouts (Figure 2, D; F(3,28) = 3.8, p = 0.02).
REM sleep bouts also increased from 9 ± 1 (vehicle) to 27 ± 4 (30 mg/kg, p = 0.02) in proportion to the increase of NREM sleep, though the mean duration of REM sleep bouts was unchanged (Figure 2, B and C). REM sleep ratio (REM sleep as percentage of total sleep) was reduced after low (6.5 + 0.77%, p = 0.0057) and moderate (8.6 + 0.45%, p = 0.02) doses of lemborexant compared to vehicle (9.7 + 0.438%; F(3,7) = 12.5, p = 0.0043).
In these WT mice receiving regular chow, lemborexant did not significantly promote cataplexy. Four mice (out of 8) had cataplexy, based on our scoring rules (see Methods section), at the highest dose of 30 mg/kg. On average, these mice had only one bout of cataplexy and spent only 0.2 ± 0.2% of time in cataplexy across the 6 h (Figure 2, C) and 0.3 ± 0.2% of wake was spent in cataplexy (calculated as (amount of cataplexy/(amount of wake + amount of cataplexy))*100). This slight increase in cataplexy was not statistically significant. Cataplexy was too sparse to analyze the EEG power distribution.
Effects of lemborexant plus chocolate in WT mice
Chocolate substantially increases cataplexy in OXKO mice [18, 20], so we next examined whether chocolate could increase cataplexy in WT mice treated with lemborexant.
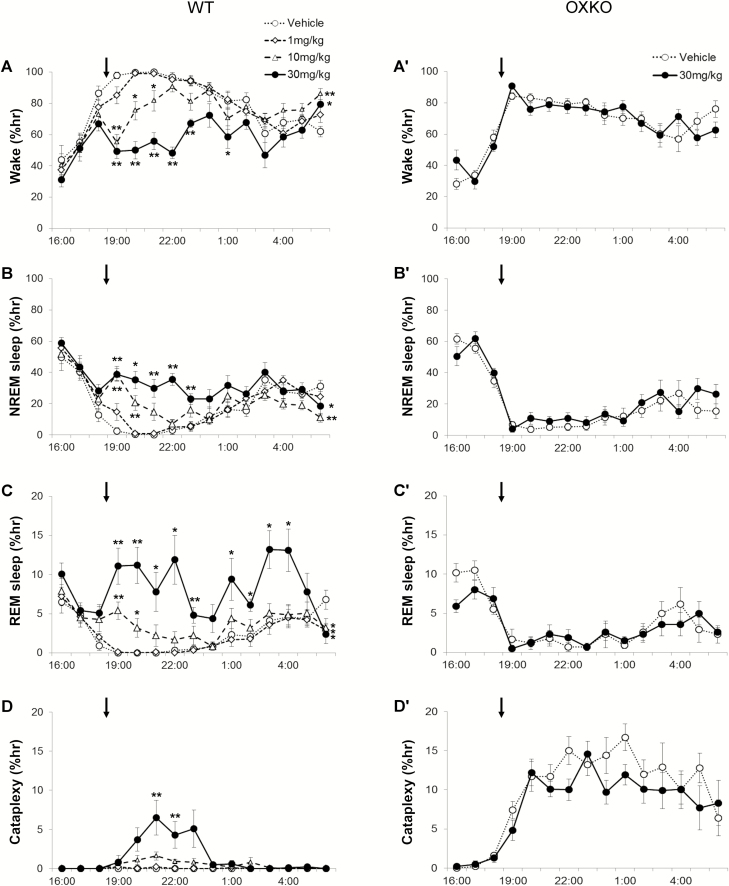
Administration of chocolate at dark onset increased wake to 96 ± 1.6% across the first 6 h in WT mice given vehicle (Figures 3 and 4). Lemborexant dose-dependently decreased wake (interaction F(42,392) = 4.385, p < 0.0001) and increased NREM sleep (interaction F(42,392) = 3.499, p < 0.0001) and REM sleep (interaction F(42,392) = 3.005, p < 0.0001) even in the presence of chocolate (Figures 3 and 4).
Figure 3.
In the presence of chocolate, lemborexant dose-dependently increased sleep and cataplexy in WT mice. Mice with access to chocolate had high levels of wake that are reduced by lemborexant in WT mice (A), but not OXKO mice (A′). Lemborexant increased NREM sleep, REM sleep, and cataplexy in WT mice (B–D) but not in OXKO mice (B′–D′). Arrows indicate the time of vehicle/drug jelly (at 18:45) and chocolate (at 19:00) presentation. * p < 0.05, ** p < 0.01 compared to vehicle day.
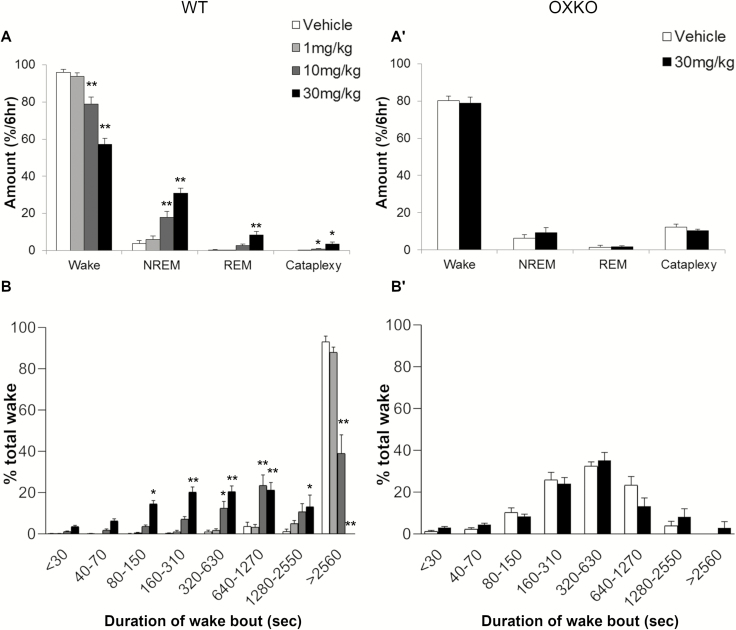
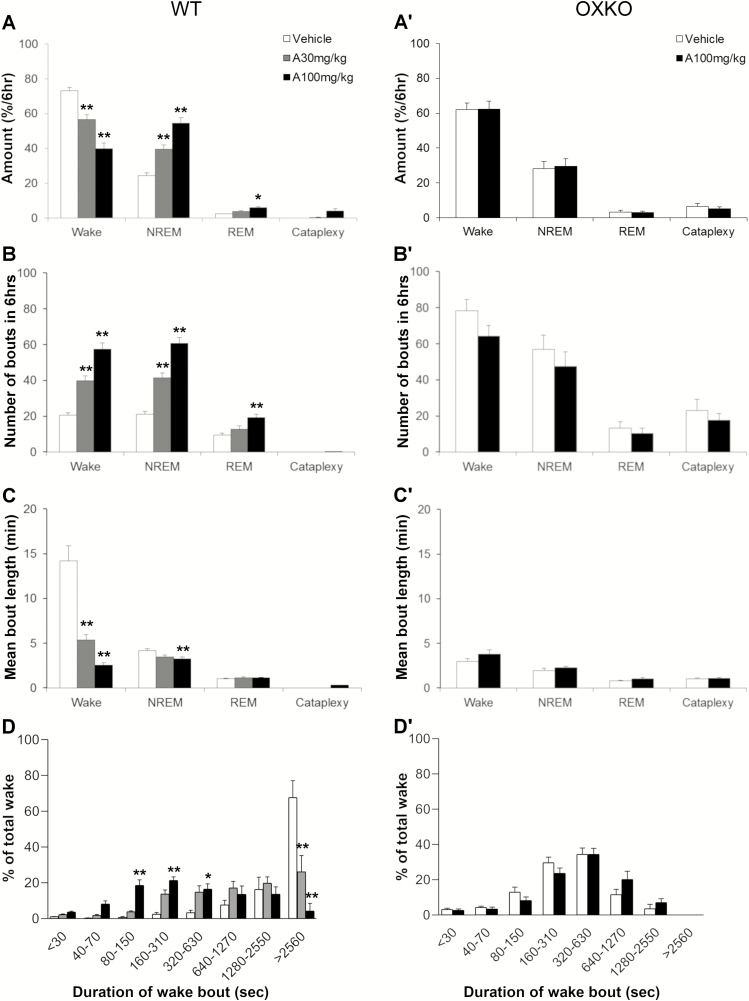
Figure 4.
Lemborexant increased NREM sleep, REM sleep, and cataplexy and shortens wake bouts in the presence of chocolate. Average state amounts in WT mice (A) and OXKO mice (A′) in the 6 h after vehicle or lemborexant administration. Time-weighted wake bout histogram for WT (B) and OXKO mice (B′). * p < 0.05, ** p < 0.01 compared to vehicle day.
Lemborexant shortened the latency to NREM sleep in the presence of chocolate. WT mice were normally persistently awake at the beginning of the dark period, and they typically started sleeping 219 ± 37 min after eating vehicle jelly. However, with lemborexant, the first NREM sleep occurred in 57 ± 35 (1 mg/kg vs. vehicle, ns), 24 ± 2 (10 mg/kg vs. vehicle, p = 0.005) and 17 ± 2 (30 mg/kg vs. vehicle, p = 0.005) minutes. At higher doses of lemborexant, the latency to REM sleep was also reduced. With vehicle, mice entered REM sleep 267 ± 11 min after eating jelly. With lemborexant, the first REM sleep occurred in 140 ± 50 (1 mg/kg, ns), 39 ± 3 (10 mg/kg, p < 0.001), and 27 ± 4 (30 mg/kg, p < 0.001) minutes after eating jelly.
Over the 6 h, lemborexant increased transitions into NREM and REM sleep. The number of wake bouts increased from 6.5 ± 1.7 (vehicle) to 38.5 ± 5.3 (10 mg/kg, p = 0.008) and 74.6 ± 5.1 (30 mg/kg, p ≤ 0.0001), and the mean length of wake bouts decreased from 98.1 ± 38.4 min (vehicle) to 9.1 ± 2 (10 mg/kg) and 2.9 ± 0.3 min (30 mg/kg). Time-weighted frequency analysis demonstrated a shift toward shorter wake bouts (F(3,28) = 6.97, p = 0.0012; multiple comparisons vehicle vs. lemborexant 30 mg/kg; Figure 4, B). This interruption of long wake bouts resulted from more frequent transitions into NREM sleep, from 6.0 ± 1.8 (vehicle) to 34 ± 5.6 (10 mg/kg, p = 0.013) and 66.6 ± 4.4 (30 mg/kg, p ≤ 0.0001) transitions in the 6 h, and the length of NREM sleep bouts slightly decreased. REM sleep bouts also increased from 1.1 ± 0.5 (vehicle) to 11.1 ± 3.8 (10 mg/kg, ns) and 28.5 ± 5.2 (30 mg/kg, p = 0.008). Compared to vehicle (3.8 ± 1.6%; F(3,7) = 21.7, p < 0.0001), the REM sleep ratio increased after the moderate (12 ± 2.2, p = 0.0098) and high (22 ± 3.6, p = 0.0022) dose of lemborexant.
The combination of lemborexant plus chocolate increased cataplexy in WT mice, but chocolate alone did not induce cataplexy. The amount of cataplexy (interaction F(42,392) = 3.691, p < 0.0001) (Figure 3) and the percent of wake in cataplexy (interaction F(42,392) = 4.174, p < 0.0001) were significantly increased. In the lemborexant plus chocolate condition, cataplexy occurred in 3 out of 8 WT mice at 1 mg/kg, in 7 mice at 10 mg/kg, and in all 8 mice at 30 mg/kg. At 1 mg/kg, however, the total amount (0.1 ± 0.04% of time) and number (0.6 ± 0.4 bouts) of cataplexy bouts across 6 h were not statistically significant compared to vehicle plus chocolate (Figures 3–5). At 10 and 30 mg/kg, the total amount of cataplexy (0.9 ± 0.3% and 3.5 ± 1.1% of time, respectively) and the number of cataplexy bouts (5 and 12 bouts, respectively) were both increased significantly. The amount of cataplexy as a percent of wake increased from 0% of wake (vehicle) to 1.1 ± 0.3% (10 mg/kg) and 5.2 ± 1.6% (30 mg/kg) of wake.
In most respects, this lemborexant-induced cataplexy was very similar to cataplexy in OXKO mice, with transitions from active wake to sudden atonia and regular EEG theta activity throughout the episode (Supplementary Video S3). However, cataplexy in WT mice treated with lemborexant included more transitions into brief periods of wake with muscle activity, not typically observed in OXKO mice, suggesting partial cataplexy due to less persistent muscle atonia. In addition, high amplitude theta bursts were infrequent [36], and the amplitude of EEG theta activity during cataplexy in the WT mice was lower than in OXKO mice (30 mg/kg, interaction F(99,1386) = 6.702, p < 0.0001) (Figure 5, D).
Figure 5.
Characterization of lemborexant-induced cataplexy with chocolate. Lemborexant dose-dependently increased time in cataplexy in the presence of chocolate in WT mice (A) but not in OXKO mice (A′) in the 6 h after vehicle (dose 0 mg/kg) or lemborexant. Lemborexant increased the number of cataplexy bouts in WT (B) but not in OXKO mice (B′). * p < 0.05, ** p < 0.01 compared to the respective vehicle day. Lemborexant did not alter the duration of cataplexy bouts (C and C′). (D) EEG theta activity was lower with lemborexant-induced cataplexy in WT mice than in OXKO mice. – p < 0.05.
Effects of lemborexant in OXKO mice
Lemborexant did not alter sleep–wake behavior in OXKO mice (Figures 1 and 2). These mice had no change in amount, bout number or bout length of wake, NREM sleep and REM sleep when treated with the highest dose of lemborexant (30 mg/kg). There was no significant change in the latency to NREM or REM sleep. There was no difference in the time-weighted frequency distribution of wake bouts.
Effects of lemborexant plus chocolate in OXKO mice
In OXKO mice treated with chocolate, lemborexant (30 mg/kg) did not substantially affect sleep–wake behavior (Figures 3–5), except for a slight reduction in the number of cataplexy bouts.
Effects of almorexant in WT mice
In WT mice, almorexant dose-dependently decreased wake (interaction F(28,350) = 3.35, p < 0.0001) and increased NREM sleep (interaction F(28,350) = 3.29, p < 0.0001) and REM sleep (interaction F(28,350) = 2.4, p < 0.0001) (Figures 6 and 7). Across the 6 h after administration, almorexant at 30 and 100 mg/kg decreased the amount of wake from 73 ± 2% (vehicle day) to 56.5 ± 2.8% (30 mg/kg) and 40 ± 3.3% (100 mg/kg) (Figure 7). NREM sleep increased from 24 ± 2% (vehicle) to 40 ± 3% (30 mg/kg) and 54.4 ± 3.8% (100 mg/kg) of time, and REM sleep increased from 2.4 ± 0.2% (vehicle) to 3.8 ± 0.5% (30 mg/kg) and 5.9 ± 0.6% (100mg/kg) of time. Almorexant induced NREM sleep and REM sleep very quickly (F(2,7) = 15.17, p = 0.0068; F(2,7) = 36.33, p = 0.0002, respectively). Time to first NREM sleep bout was 129.3 + 21.3 min after eating vehicle jelly. However, with almorexant, the first NREM sleep occurred in 31.5 ± 4 (30 mg/kg, p = 0.0066) and 29.6 ± 3.6 (100 mg/kg, p = 0.0058 vs. vehicle) min. Time to first REM sleep was also reduced from 185.3 ± 18.4 min after eating vehicle jelly, to 64 + 5.4 min (30 mg/kg, p = 0.0011 vs. vehicle) and 53.1 ± 4 min (100 mg/kg, p < 0.0001 vs. vehicle).
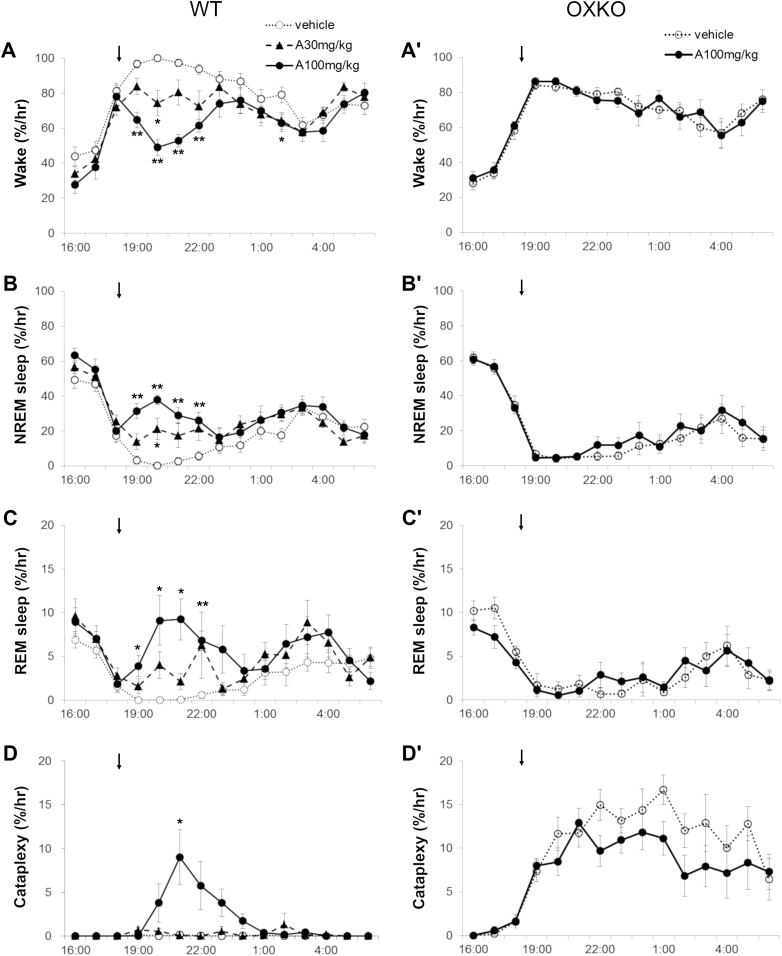
Figure 6.
Almorexant dose-dependently increased NREM and REM sleep in WT mice but not in OXKO mice with normal chow. Hourly amounts of wake, NREM sleep, REM sleep, and cataplexy in WT (A–D) and OXKO mice (A′–D′). Arrows indicate the time of vehicle/drug jelly presentation (at 18:45). One WT mouse had one cataplexy bout at hour 24 highlighted in inset with expanded scale (D). * p < 0.05, ** p < 0.01 compared to vehicle day.
Figure 7.
Almorexant increased transitions into NREM and REM sleep and shortened wake bouts. In mice fed regular chow, almorexant reduced wake and increased NREM and REM sleep, but did not increase cataplexy in the 6 h after dosing (A and B). Almorexant increased the number of wake and NREM sleep bouts (B), and severely shortened the duration of wake bouts (C and D). Almorexant had no effects in OXKO mice (A′–D′). * p < 0.05, ** p < 0.01 compared to vehicle day.
Across the 6 h, almorexant increased transitions into NREM sleep. The number of wake bouts increased from 20.5 ± 1.6 (vehicle) to 40 ± 2.6 (30 mg/kg, p = 0.001) and 57.5 ± 3.4 (100 mg/kg, p < 0.0001), and the mean length of wake bouts decreased from 14.2 ± 1.7 min (vehicle) to 5.2 + 0.8 (30 mg/kg, p = 0.001) and 2.0 ± 0.2 min (100 mg/kg, p = 0.001) (Figure 7). Time-weighted frequency analysis indicated a shift to short duration wake bouts (Figure 7; F(2,21) = 3.25, p = 0.05; multiple comparisons vehicle vs. almorexant 100 mg/kg). This poor maintenance of long wake bouts resulted from more frequent transitions into NREM sleep, from 22 ± 2 (vehicle) to 41.5 ± 2.7 (30 mg/kg, p = 0.0006) and 60.6 ± 3.2 (100 mg/kg; p < 0.0001) transitions in the 6 h, and the length of NREM sleep bouts slightly decreased (4.2 ± 0.2 to 3.3 ± 0.2 at 100 mg/kg, p = 0.004). REM sleep bouts also increased from 9.6 ± 0.9 (vehicle) to 12.6 ± 1.9 (30 mg/kg, ns) and 19 ± 2 (100 mg/kg, p = 0.002), though the mean bout length was unchanged (Figure 7). REM sleep ratio did not differ across treatments.
In WT mice receiving regular chow, almorexant did not significantly cause cataplexy. One mouse (out of 8) had cataplexy at the highest dose of 100 mg/kg. On average, this mouse spent only 0.1% of time in the cataplexy across the 6 h after administration, without statistical significance compared to the vehicle day (Figure 7). These events were too sparse to assess the EEG power distribution of the EEG.
Effects of almorexant plus chocolate in WT mice
In WT mice given chocolate, almorexant dose-dependently decreased wake (interaction F(28,350) = 3.53, p < 0.0001) and increased NREM sleep (interaction F(28,350) = 2.5, p < 0.0001) and REM sleep (interaction F(28,350) = 2.62, p < 0.0001) (Figure 8). Specifically, almorexant dose-dependently decreased wake from 94 ± 1.6% (vehicle) to 78 ± 3.5% (30 mg/kg) and 63 ± 3% (100 mg/kg) and increased NREM sleep from 5.8 ± 1.4% (vehicle) to 18.7 ± 3% (30 mg/kg) and 26.7 ± 2.3% (100 mg/kg) and REM sleep from 0.5 ± 0.2% (vehicle) to 3 ± 1.2% (30 mg/kg) and 6.4 ± 1.6% (100 mg/kg) (Figure 9).
Figure 8.
In the presence of chocolate, almorexant dose-dependently increased sleep and cataplexy in WT mice. Hourly amounts of wake, NREM sleep, REM sleep, and cataplexy in WT mice (A–D) and OXKO mice (A′–D′). Arrows indicate the time of vehicle/drug jelly (at 18:45) and chocolate (at 19:00) presentation. * p < 0.05, ** p < 0.01 compared to vehicle day.
Figure 9.
Almorexant increased transitions into sleep and shortened wake bouts in the presence of chocolate. Average amount of wake, NREM sleep, REM sleep, and cataplexy in WT (A) or cataplexy in OXKO (A′) in the 6 h after vehicle or almorexant administration. Number of wake, NREM sleep, REM sleep, and cataplexy bouts in WT mice (B) and cataplexy bouts in OXKO mice (B′) for 6 h after almorexant administration. Mean duration of wake, NREM sleep, REM sleep, and cataplexy bouts in WT mice (C) and OXKO mice (C′). * p < 0.05, ** p < 0.01 compared to vehicle day. Time-weighted frequency histograms show that almorexant shortens wake bouts in WT mice (D) but not in OXKO mice (D′). (E) Peak theta frequency during almorexant-induced cataplexy is slightly lower in WT mice than in OXKO mice, – p < 0.05.
Almorexant shortened the latency to sleep even in the presence of chocolate. With vehicle, WT mice were persistently awake at the beginning of the dark period, and they typically started sleeping 219 ± 37 min after eating vehicle jelly. However, with almorexant, the first NREM sleep occurred in 51 ± 9 (30 mg/kg vs. vehicle, ns) and 36 ± 4 (100 mg/kg vs. vehicle, p = 0.005) minutes. At higher doses of almorexant, the latency to REM sleep was also reduced. With vehicle, mice entered REM sleep 267 ± 11 min after eating jelly. With almorexant, the first REM sleep occurred in 120 ± 34 (30 mg/kg, ns) and 60 ± 8 (100 mg/kg, p < 0.001) minutes after eating jelly.
Over the 6 h, almorexant increased transitions into sleep. The number of wake bouts increased from 10.8 ± 2.7 (vehicle) to 29.8 ± 4.4 (30 mg/kg) and 66.3 ± 7.4 (100 mg/kg), and the mean length of wake bouts decreased from 120.2 ± 31 min (vehicle) to 12.3 ± 2.9 (30 mg/kg) and 4.0 ± 0.8 min (100 mg/kg) (Figure 9). Time-weighted frequency analysis demonstrated a shift toward shorter wake bouts (F(2,21) = 5.82, p = 0.0098). This inability to maintain long wake bouts resulted from more frequent transitions into NREM sleep, from 9.3 ± 2.7 (vehicle) to 30.9 ± 5.2 (30 mg/kg) and 59 ± 7.7 (100 mg/kg) transitions over 6 h, but no change in the length of NREM sleep bouts. REM sleep bouts also increased from 2 ± 0.75 (vehicle) to 11.4 ± 3.8 (30 mg/kg) and 22.6 ± 5.3 (100 mg/kg) with no change in mean bout length (Figure 9). Compared to vehicle (0.038 ± 0.016; F(2,7) = 0.07), almorexant increased the REM sleep ratio at the high dose (0.19 ± 0.04, p = 0.015).
The combination of almorexant plus chocolate significantly increased cataplexy (interaction F(28,350) = 6.08, p < 0.0001) and the percent of wake in cataplexy (interaction F(28,350) = 5.545, p < 0.0001) (Figure 8). Cataplexy occurred in 4 out of 8 WT mice at 30 mg/kg, and in all 8 mice at 100 mg/kg. Compared to vehicle (0.0 ± 0, F(2,7) = 7.55, p = 0.028), the amount of cataplexy increased with the high dose of almorexant to 4 ± 1.4% (p = 0.037) of time. The amount of cataplexy as a percent of wake was significantly increased to 6 ± 1.8% (p = 0.022) compared to vehicle (0.0 ± 0, F(2,7) = 9.73, p = 0.016). Almorexant significantly increased the number of cataplexy bouts (F(2,7) = 7.03, p = 0.03, vehicle (0.0 ± 0 bouts) vs. 100 mg/kg (12 ± 4 bouts), p = 0.025). In most respects, this almorexant-induced cataplexy was very similar to cataplexy in OXKO mice, with transitions from active wake to sudden atonia and regular EEG-theta activity throughout the episode, however the amplitude of theta activity was mildly reduced (Figure 9).
Additionally, this almorexant-induced cataplexy was occasionally interrupted by brief periods of wake with normal muscle tone.
Effects of almorexant in OXKO mice
Almorexant did not alter sleep/wake behavior in OXKO mice (Figures 6 and 7). These mice had no change in amount, bout number and bout length of wake, NREM sleep and REM sleep, even when treated with the highest dose of almorexant (100 mg/kg). There was no significant change in the latency to NREM or REM sleep. There was no difference between the time-weighted frequency distribution of wake bouts. There was no difference in REM sleep ratio across treatment. Almorexant 100 mg/kg did not alter the number of cataplexy bouts (17.6 ± 3.8) compared to vehicle (23 ± 6).
Effects of almorexant plus chocolate in OXKO mice
In OXKO mice, almorexant (100 mg/kg) did not affect sleep–wake behavior or chocolate-induced cataplexy (Figures 8 and 9).
Discussion
We examined the effects of the DORAs, lemborexant and almorexant on sleep–wake behavior and their potential for cataplexy. We confirmed that both DORAs potently increase NREM and REM sleep in WT mice, yet they are highly selective, with essentially no effects in OXKO mice. Cataplexy is rare in WT mice treated with lemborexant or almorexant under the regular chow condition, but with the addition of chocolate, a rewarding stimulus, both DORAs produce a moderate amount of cataplexy. These results highlight the potent sleep-promoting effects of these medications mediated by antagonism of orexin signaling, and they also demonstrate that acute blockade of orexin signaling can trigger cataplexy, especially in the presence of a likely motivating stimulus.
Sleep-promoting effects
The orexin neuropeptides help promote long periods of wake, and the most striking behavioral effect with both lemborexant and almorexant is the substantial shortening of the duration of wake bouts. As in mice lacking orexins, the mean duration of wake bouts was only about 2 min or 15% of the normal duration of wake in the dark period with high dose DORAs. Additionally, both DORAs shifted the time weighted frequency histogram to shorter wake bouts.
Moderate and high doses of lemborexant and almorexant potently increased NREM and REM sleep for several hours in WT mice, and shortened the latency to NREM sleep. The shorter latency to REM sleep most likely contributed to the increased REM sleep ratio under the chocolate conditions with higher doses of the DORAs. These results are similar to prior reports on these DORAs in rodents and similar to the effects of another DORA, suvorexant [27, 35, 37–39].
The increase in transitions between wake and NREM sleep in the current study of WT mice is similar to the fragmentation of wake reported by Black et al. when adjusted for the different amount of time reported [23]. The frequent transitions are similar to those of OXKO mice, indicating that both DORAs potently block orexin signaling, resulting in poor maintenance of wake. We dosed mice at dark onset, the beginning of their active phase, but effects may have been different if given at light onset when the sedating effects of a DORA would not conflict with the circadian waking drive. A similar phenomena occurs in people treated with suvorexant who spend about 60% less time in long (>2 min) wake bouts [40]. This inability to maintain long wake bouts results in more transitions into sleep, but it does not reduce the frequency of short awakenings. Thus, we propose that DORAs may be especially helpful for people with sleep maintenance insomnia with prolonged periods of wake, but they may be less helpful for those prone to brief awakenings triggered by factors such as pain, sleep apnea, and environmental noise. In addition, as orexin tone is highest in the active period [41–43], DORAs may help initiate sleep in people with jet lag or circadian phase delay who are aiming to sleep during their habitual wake phase.
To determine if the effect of these DORAs promote sleep solely by blocking orexin signaling, we tested the highest doses of lemborexant and almorexant in OXKO mice. Neither DORA changed the amount of wake, NREM sleep, or REM sleep in these mice under chow or chocolate conditions. This aligns well with prior research showing no effect of almorexant in mice lacking both orexin receptors, and no effect of lemborexant in mice lacking the orexin neurons [35, 38]. Collectively, these results strongly indicate that these DORAs promote sleep solely through blockade of orexin signaling.
Cataplexy-promoting effects of DORAs
Prior preclinical and clinical reports on orexin receptor antagonists have emphasized the lack of cataplexy with these medications [8–10, 28, 30, 31, 44–46]. Daily administration of suvorexant to dogs for 4–7 days resulted in transient limb buckling and a prone posture when the dogs were presented with canned food, a stimulus that strongly increases cataplexy in dogs with OX2R mutations [45]. However, this collapsing was probably related to sedation as a high dose DORA had no effect in a formal canine food-elicited cataplexy test [46]. Under baseline conditions, almorexant, suvorexant, and lemborexant were reported to produce no cataplexy in mice [27, 35, 38]. In line with this, we found that under the chow condition, cataplexy was quite rare, even with high doses of lemborexant and almorexant. However, in people and mice, cataplexy occurs during periods of high arousal and is often triggered by rewarding stimuli. Thus, it is expected that cataplexy will generally be rare with DORAs as any tendency toward cataplexy would be masked by acute sedation and reduced activity in brain reward pathways.
In contrast, cataplexy was common in WT mice treated with a DORA plus chocolate. Both lemborexant and almorexant dose-dependently increased cataplexy in mice with access to chocolate. This cataplexy was not simply due to chocolate as WT mice with access to chocolate had no cataplexy when treated with vehicle.
This cataplexy in WT mice with chocolate plus DORA was quite similar to that seen in OXKO mice. WT mice were active just prior to transitions into cataplexy, and the EEG during cataplexy was dominated by theta activity (4–9 Hz). However, DORA-induced cataplexy in the WT mice was more fragmented, with occasional, brief increases in muscle tone and movement with sudden returns to typical cataplexy. In addition, the amplitude of theta activity during DORA-induced cataplexy was slightly lower than seen in OXKO mice. As previously reported, OXKO mice often have very high amplitude bursts of theta activity during cataplexy [36, 47]. These bursts were common in OXKO mice, but rare in DORA-treated WT mice. Why DORA-induced cataplexy shows these small differences is unknown, but more severe cataplexy may require a greater loss of orexin signaling, chronic loss of orexin signaling, or changes in other neural systems [48, 49].
Cataplexy clearly requires a severe loss of orexin signaling. Mice completely lacking orexins have moderate amounts of cataplexy whereas mice with partial loss of the orexin neurons have less [23, 50]. Black et al. reported an increase in cataplexy after administration of almorexant in orexin ataxin-3 mice with a partial loss of the orexin neurons [23]. In contrast, we did not observe any increase in cataplexy in OXKO mice with high doses of either DORA, most likely because they already lack all orexin signaling. Similarly, Mang et al. saw no increase in cataplexy with almorexant (100 mg/kg) in mice lacking both orexin receptors [35]. The findings of Black et al. may be due to low, residual orexin tone in the orexin ataxin-3 mice which can be further reduced by a DORA versus no orexin tone in mice fully lacking orexin signaling [23].
We propose that the stimulating and likely rewarding aspects of chocolate permit expression of cataplexy in WT mice treated with DORAs. Mice with ad lib access consume about 1.9–3.3 g chocolate across the dark period, and this amount of chocolate clearly increases wake [18, 20]. We found that vehicle-treated mice with access to chocolate were awake for 96% of the first 6 h of the dark period, compared with 73% with regular chow. Most likely, this stimulant effect is due to caffeine, theobromine, and other wake-promoting compounds in chocolate. However, arousal alone is not a sufficient explanation as the increase in cataplexy is much larger than the increase in time awake in OXKO mice with access to chocolate [18, 20, 51]. Additionally, caffeine and paraxanthine suppressed cataplexy in orexin/ataxin-3 transgenic narcoleptic mice [52]. Most likely, the rewarding aspects of chocolate also contribute to cataplexy [53, 54]. Cataplexy in people with narcolepsy is often triggered by positive stimuli such as laughter or pleasant surprise [17], and cataplexy in mice is increased by other rewards such as tasty food (e.g. Froot Loops®, peanuts) and running wheels [32, 55]. Black et al. observed rare cataplexy-like episodes in WT mice that received 100 or 300 mg/kg almorexant [23]; importantly, these events occurred immediately after wheel running, suggesting the running may have triggered the event. Mang et al. [35] did not observe cataplexy in WT mice treated with almorexant (up to 300 mg/kg) plus a running wheel, Froot Loops® and novel objects, but mice were exposed to these stimuli acutely and novel objects may induce anxiety. For example, we find that mice take many days to acclimate to running wheels, and they avoid chocolate when first exposed and require 2–3 days acclimation [20, 55]. We administered the drugs by a voluntary eating method, and reducing stress may have enabled expression of cataplexy. Overall, we propose that cataplexy requires severe loss of orexin signaling plus high arousal, and is further increased by rewarding stimuli.
More broadly, these results demonstrate that in WT mice, acute blockade of orexin signaling is capable of producing cataplexy, especially in conjunction with chocolate. This is an important observation as researchers have speculated that chronic changes in other neurochemical systems such as histamine and melanin-concentrating hormone may contribute to cataplexy [20, 48, 49, 56]. These results now show that acute loss of orexin signaling is sufficient to produce cataplexy, at least when a likely rewarding stimulus such as chocolate is present. Still, orexin deficiency may not be the sole explanation for cataplexy as the amount of cataplexy with DORAs plus chocolate in WT mice was still much less than seen in OXKO mice, suggesting that additional neurochemical systems may contribute in typical narcolepsy with chronically low orexin tone.
Limitations
We administered DORAs during the dark phase as this is the period when OXKO mice have cataplexy. This helps demonstrate the sleep- and cataplexy-promoting aspects of DORAs, but if they were administered during the light phase, the mice may have had longer sleep bouts and less cataplexy. Additionally, we administered the DORAs in ascending order, and although we allowed ample time for drug wash out, randomized dosing may have produced slightly different results. Furthermore, we cannot yet explain the small differences between DORA-induced cataplexy and that seen in OXKO mice. For example, the brief interruptions of cataplexy in DORA-treated WT mice may have suppressed the typical very high theta power seen during typical cataplexy.
Conclusions
We examined the effects on sleep/wake behavior of the DORAs lemborexant and almorexant in WT mice and OXKO mice. Both compounds potently increased NREM and REM sleep in WT mice but not in OXKO mice, confirming that they promote sleep solely through blockade of orexin signaling. In addition, both DORAs produced cataplexy in WT mice with access to chocolate, demonstrating that acute blockade of orexin signaling can trigger cataplexy under arousing and likely rewarding conditions.
These results also demonstrate that when a stimulus such as chocolate is present, acute loss of orexin signaling is sufficient to trigger cataplexy, yet other basic questions remain. Can this occur with other rewards? Might cataplexy occur with high doses of a selective OX1R or OX2R antagonist [57]? If the cataplexy is due to remaining awake even when orexin receptor blockade is intense, then might modafinil, caffeine or another wake-promoting medication promote cataplexy if co-administered with a high dose DORA?
Is cataplexy a concern for people with insomnia taking a DORA? In general, we believe that cataplexy should be quite rare as typical clinical doses of DORAs do not produce severe blockade of orexin signaling, and if a patient were to take a high dose, severe sleepiness would likely surmount any tendency towards cataplexy. Still, in clinical trials of suvorexant, about 1% of participants had hypnagogic hallucinations or sleep paralysis, indicating the potential for mixed, REM sleep-like states [40, 58]. Further, people with insomnia sometimes find themselves awake despite taking a medication, and high arousal in combination with a high dose of a DORA might be sufficient to trigger cataplexy, especially if coupled with a positive emotional stimulus at night such as sex or social interactions that could trigger cataplexy. We suggest that in clinic, the potential for cataplexy be mentioned when discussing potential side effects of a DORA.
Supplementary Material
Acknowledgments
The OXKO mice were a gift from Takeshi Sakurai and Masashi Yanagisawa (University of Tsukuba).
Funding
This research was supported by a grant from Eisai Co, Ltd. and National Institutes of Health (grant NS106032).
Conflict of interest statement: T.E.S. has consulted with Eisai, Merck, and Idorsia (makers of DORAs).
References
- 1. Ellis JG, et al. . The natural history of insomnia: focus on prevalence and incidence of acute insomnia. J Psychiatr Res. 2012;46(10):1278–1285. [DOI] [PubMed] [Google Scholar]
- 2. Diagnostic and Statistical Manual of Mental Disorders. Vol. 5 Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 3. Leigh JP. Employee and job attributes as predictors of absenteeism in a national sample of workers: the importance of health and dangerous working conditions. Soc Sci Med. 1991;33(2):127–137. [DOI] [PubMed] [Google Scholar]
- 4. Spira AP, et al. . Association between insomnia symptoms and functional status in U.S. older adults. J Gerontol B Psychol Sci Soc Sci. 2014;69(Suppl 1):S35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DiBonaventura M, et al. . The association between insomnia and insomnia treatment side effects on health status, work productivity, and healthcare resource use. PLoS One. 2015;10(10):e0137117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hafner M, et al. . Why sleep matters-the economic costs of insufficient sleep: a cross-country comparative analysis. Rand Health Q. 2017;6(4):11. [PMC free article] [PubMed] [Google Scholar]
- 8. Black SW, et al. . Challenges in the development of therapeutics for narcolepsy. Prog Neurobiol. 2017;152:89–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murphy P, et al. . Lemborexant, A dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a Bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roth T, et al. . Dual orexin receptor antagonist, almorexant, in elderly patients with primary insomnia: a randomized, controlled study. Sleep. 2017;40(2). doi:10.1093/sleep/zsw034 [DOI] [PubMed] [Google Scholar]
- 11. España RA, et al. . Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106(4):699–715. [DOI] [PubMed] [Google Scholar]
- 12. Adamantidis AR, et al. . Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Lecea L, et al. . The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95(1):322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakurai T, et al. . Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(5): 573–585. [DOI] [PubMed] [Google Scholar]
- 15. Li SB, et al. . Optical probing of orexin/hypocretin receptor antagonists. Sleep. 2018;41(10). doi:10.1093/sleep/zsy141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Crocker A, et al. . Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65(8):1184–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Overeem S, et al. . The clinical features of cataplexy: a questionnaire study in narcolepsy patients with and without hypocretin-1 deficiency. Sleep Med. 2011;12(1):12–18. [DOI] [PubMed] [Google Scholar]
- 18. Burgess CR, et al. . Amygdala lesions reduce cataplexy in orexin knock-out mice. J Neurosci. 2013;33(23):9734–9742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mochizuki T, et al. . Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24(28):6291–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oishi Y, et al. . Role of the medial prefrontal cortex in cataplexy. J Neurosci. 2013;33(23):9743–9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pillai V, et al. . Prevalence and predictors of prescription sleep aid use among individuals with DSM-5 insomnia: the role of hyperarousal. Sleep. 2016;39(4):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Betschart C, et al. . Identification of a novel series of orexin receptor antagonists with a distinct effect on sleep architecture for the treatment of insomnia. J Med Chem. 2013;56(19):7590–7607. [DOI] [PubMed] [Google Scholar]
- 23. Black SW, et al. . Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy. Sleep. 2013;36(3):325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roundtree HM, et al. . Orexin receptor antagonism improves sleep and reduces seizures in kcna1-null mice. Sleep. 2016;39(2):357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chemelli RM, et al. . Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98(4):437–451. [DOI] [PubMed] [Google Scholar]
- 26. Lin L, et al. . The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98(3):365–376. [DOI] [PubMed] [Google Scholar]
- 27. Brisbare-Roch C, et al. . Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13(2):150–155. [DOI] [PubMed] [Google Scholar]
- 28. Morairty SR, et al. . Dual hypocretin receptor antagonism is more effective for sleep promotion than antagonism of either receptor alone. PLoS One. 2012;7(7):e39131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Fabio R, et al. . Discovery process and pharmacological characterization of a novel dual orexin 1 and orexin 2 receptor antagonist useful for treatment of sleep disorders. Bioorg Med Chem Lett. 2011;21(18):5562–5567. [DOI] [PubMed] [Google Scholar]
- 30. Hoever P, et al. . Orexin receptor antagonism, a new sleep-promoting paradigm: an ascending single-dose study with almorexant. Clin Pharmacol Ther. 2010;87(5):593–600. [DOI] [PubMed] [Google Scholar]
- 31. Vermeeren A, et al. . On-the-road driving performance the morning after bedtime administration of lemborexant in healthy adult and elderly volunteers. Sleep. 2019;42(4). doi:10.1093/sleep/zsy260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clark EL, et al. . Feeding-elicited cataplexy in orexin knockout mice. Neuroscience. 2009;161(4):970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scammell TE, et al. . A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32(1):111–116. [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang L. Voluntary oral administration of drugs in mice. Protocol (Version 1).. 2011. doi:10.1038/protex.2011.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mang GM, et al. . The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep. 2012;35(12):1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vassalli A, et al. . Electroencephalogram paroxysmal θ characterizes cataplexy in mice and children. Brain. 2013;136(Pt 5):1592–1608. [DOI] [PubMed] [Google Scholar]
- 37. Cao M, et al. . Hypocretin and its emerging role as a target for treatment of sleep disorders. Curr Neurol Neurosci Rep. 2011;11(2):227–234. [DOI] [PubMed] [Google Scholar]
- 38. Beuckmann CT, et al. . Preclinical in vivo characterization of lemborexant (E2006), a novel dual orexin receptor antagonist for sleep/wake regulation. Sleep. 2019;42(6). doi:10.1093/sleep/zsz076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Winrow CJ, et al. . Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25(1–2):52–61. [DOI] [PubMed] [Google Scholar]
- 40. Svetnik V, et al. . Insight into reduction of wakefulness by suvorexant in patients with Insomnia: analysis of wake bouts. Sleep. 2018;41(1). doi:10.1093/sleep/zsx178 [DOI] [PubMed] [Google Scholar]
- 41. Gotter AL, et al. . The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold. BMC Neurosci. 2013;14:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeitzer JM, et al. . Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci. 2003;23(8):3555–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fujiki N, et al. . Changes in CSF hypocretin-1 (orexin A) levels in rats across 24 hours and in response to food deprivation. Neuroreport. 2001;12(5):993–997. [DOI] [PubMed] [Google Scholar]
- 44. Brisbare-Roch C, et al. . Transient orexin receptor blockade induces sleep without cataplexy in rats. In: Paper presented at: WorldSleep072007; Cairns.
- 45. Foutz AS, et al. . Monoaminergic mechanisms and experimental cataplexy. Ann Neurol. 1981;10(4):369–376. [DOI] [PubMed] [Google Scholar]
- 46. Tannenbaum PL, et al. . Orexin receptor antagonist-induced sleep does not impair the ability to wake in response to emotionally salient acoustic stimuli in dogs. Front Behav Neurosci. 2014;8:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bastianini S, et al. . High-amplitude theta wave bursts during REM sleep and cataplexy in hypocretin-deficient narcoleptic mice. J Sleep Res. 2012;21(2):185–188. [DOI] [PubMed] [Google Scholar]
- 48. Valko PO, et al. . Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol. 2013;74(6):794–804. [DOI] [PubMed] [Google Scholar]
- 49. John J, et al. . Greatly increased numbers of histamine cells in human narcolepsy with cataplexy. Ann Neurol. 2013;74(6):786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tabuchi S, et al. . Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci. 2014;34(19):6495–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mahoney CE, et al. . GABAergic neurons of the central amygdala promote cataplexy. J Neurosci. 2017;37(15):3995–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Okuro M, et al. . Effects of paraxanthine and caffeine on sleep, locomotor activity, and body temperature in orexin/ataxin-3 transgenic narcoleptic mice. Sleep. 2010;33(7):930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hsu CT, et al. . Palatable meal anticipation in mice. PLoS One. 2010;5(9). doi: 10.1371/journal.pone.0012903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Martín-García E, et al. . New operant model of reinstatement of food-seeking behavior in mice. Psychopharmacology (Berl). 2011;215(1):49–70. [DOI] [PubMed] [Google Scholar]
- 55. España RA, et al. . Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep. 2007;30(11):1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Naganuma F, et al. . Melanin-concentrating hormone neurons contribute to dysregulation of rapid eye movement sleep in narcolepsy. Neurobiol Dis. 2018;120:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gotter AL, et al. . Orexin 2 receptor antagonism is sufficient to promote NREM and REM sleep from mouse to man. Sci Rep. 2016;6:27147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Norman JL, et al. . Novel class of medications, orexin receptor antagonists, in the treatment of insomnia—critical appraisal of suvorexant. Nat Sci Sleep. 2016;8:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.