Abstract
The overarching principle of three-dimensional (3D) bioprinting is the placing of cells or cell clusters in the 3D space to generate a cohesive tissue microarchitecture that comes close to in vivo characteristics. To achieve this goal, several technical solutions are available, generating considerable combinatorial bandwidth: (i) Support structures are generated first, and cells are seeded subsequently; (ii) alternatively, cells are delivered in a printing medium, so-called “bioink,” that contains them during the printing process and ensures shape fidelity of the generated structure; and (iii) a “scaffold-free” version of bioprinting, where only cells are used and the extracellular matrix is produced by the cells themselves, also recently entered a phase of accelerated development and successful applications. However, the scaffold-free approaches may still benefit from secondary incorporation of scaffolding materials, thus expanding their versatility. Reversibly, the bioink-based bioprinting could also be improved by adopting some of the principles and practices of scaffold-free biofabrication. Collectively, we anticipate that combinations of these complementary methods in a “hybrid” approach, rather than their development in separate technological niches, will largely increase their efficiency and applicability in tissue engineering.
Keywords: Tissue engineering, scaffolds, scaffold-free, bioprinting
1. Biofabrication of Tissue Analogs - The Main Approaches
Biofabrication, the three-dimensional (3D) assembly of living cells in structured systems [1], has evolved in the past decades as the biological facet of additive manufacturing [2]. Technically, biofabrication can be roughly divided into “bioprinting,” a technology akin to the classical use of ink and paper [3], and “tissue assembly” [4], where other techniques such as “tissue strands” [5], lamination of “cell sheets” [6] or cell spheroid piercing-and-stringing [7], have recently evolved. The purpose is to assemble cells into a living, functional system that serves for in vitro drug development and substance testing, or as an in vivo implantable tissue [1]. However, the boundaries between approaches are blurring, because they will be increasingly shared to create synergies with an eclectic mix of techniques for reaching specific goals. In future, the combined use of the main approaches in biofabrication will likely lead to more “hybrid” systems and subsequent expectations on appropriate instrumentation [8]. For this reason, in our overview, we will discuss the strengths and weaknesses of these approaches, according to their main features.
Moroni et al., Groll et al. [1,4] and Moldovan [9] have noticed some confusion regarding the terminology in this field. Therefore, here we need to specify again what - in our opinion - 3D Bioprinting is not: namely, it is not the mere additive manufacturing (a.k.a. 3D printing) of implantable materials which, by all intents and purposes, would be “biomaterials” [10]. The implantation of such a material aims either at total immunological inertia (sometimes also misnamed in this context as “biocompatibility),” or alternatively seeks histo-integration, and therefore the implant’s colonization by cells that migrate onto and into its structure [11]. As an example, the osteoinductive structures made of various materials can be considered [12]. This approach is common in regenerative medicine, constituting a form of “in vivo tissue engineering” with the host tissue acting as a bioreactor [13]. In this case, a tissue-repairing activity requires merely shape and mechanical function of interest; therefore, the used materials are polymeric or natural/decellularized fibrillar matrices. Hydrogels, another major medium for tissue engineering, are also occasionally injected directly in the recipients to elicit a repairing response either by themselves or often as cell carriers [14].
Many bona fide 3D biofabrication tasks primarily target the in vitro applications, and either include the spatial arrangement of living cells during the printing process or make them adhere in a directed manner on specifically preprinted structures. As a future development, pre-existing structures of living host tissues might be considered as the equivalent of preprinted structures and therefore might invite direct in vivo printing approaches onto wound grounds [15].
Whatever the approach, biofabrication is done with the understanding that besides a variety of cells, animal tissues contain various proportions of extracellular matrix (ECM) in the form of fibrils, fibrillary networks, sheaths, and hydrogels, as well as a fluid phase representing a complex mixture of molecules with metabolic or signaling functions. Correspondingly, a re-creation of tissue analogs largely recapitulates this blueprint (Figure 1).
Figure 1.
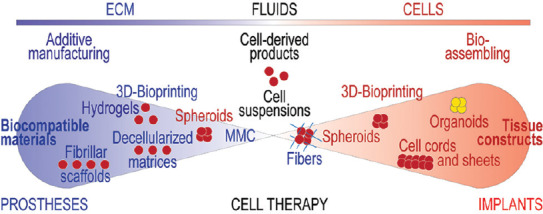
Graphic overview of the biofabrication methods.
1.1 Bioprinting
1.1.1 The Print and Populate Approach
In this approach, support structures are printed first, and cells are positioned subsequently on them in a targeted way. Often a layer-by-layer deployment method is pursued, such that lines of the hydrogel are printed first, and then covered by jetted cells [16]. The system works by printing bioinks in contact mode followed by polymerization (e.g., by ultraviolet light) of the printed lines, and subsequent jetting of cells onto printed lines in nanodroplets. One such cycle can be repeated several times resulting in a layered structure [16]. Since hydrogels are materials with an absorptive surface, they could take up the excess fluid from the jetted droplets.
The print and populate method also works with hard materials, such as polycaprolactone. In tissue engineering, another version of this method is the colonization with cells of fibrillar and/or porous scaffolds prepared by electrospinning. This is usually performed after the shape of the scaffold is pre-determined, such as layer and tube [17]. However, for cell placement, simple cell sedimentation usually does not suffice, because the superficial pores are quickly clogged, preventing their further penetration in the scaffold. To force them inside of the structure, the cells may need to be exposed to negative pressure [18], to gravitational (centrifugation) [19] or magnetic [20] forces.
1.1.2 Direct Cells Printing - the Role of Bioinks
A typical commercial bioprinter offers the extrusion mode, by which a viscous medium is continuously expelled from ready-to-use cartridges through printing needles or nozzles. The extrusion can be achieved through microvalves propelling the extrudate downward, a plunger system, or in the case of very viscous materials, a screw pump [21]. The cells are delivered in a printing medium, the so-called “bioink,” that contains them during the printing process, ensures shape fidelity of the printed structure, and protects cells against shear forces [22]. Alternatively, the bioinks might be deployed by inkjet methods [23], one of which is laser-assisted droplet generation [24,25].
Technically, the bioinks represent scaffolds, which is particularly obvious after chemical, photo or enzymatic crosslinking [26]. Therefore, to avoid confusion, occasional claims (e.g., [27]) that bioink-based printing starting with more fluid solutions, or because these are further combined with fibrillar meshes, would be “scaffold-free,” should not be entertained.
1.1.3 Scaffold-in-scaffold: Integrated or Composite 3D Bioprinting
When the hydrogels alone do not attain enough strength by polymerization, another option is to print harder or stiffer scaffolds as cage-like system first and then to fill them with cell-laden bioinks on hydrogel-basis [28]. For this, the terms of “composite” [29], “integrated” [30] or even “hybrid” [31] bioprinting have been coined.
1.1.4 Advantages and Limitations of Hydrogels-based Bioprinting
Although only a few decades old, the concept of bioprinting and the instruments operated on this principle inspired from additive manufacturing have progressed impressively and already generated convincing proofs of concept. Responding to a large societal interest, a market emerged for commercialization of a variety of bioprinting materials and instruments. However, with bioink-based bioprinting, it is not trivial to satisfy simultaneously the requirement of both printability and biocompatibility [22]. Essentially, bioinks are soft biomaterials, which need to permit extrusion or jetting, yet are required to maintain a printed shape. This is achieved by employing thixotropic gels that show shear-thinning during an extrusion process and regain larger stiffness immediately after deposition or a by the post-printing chemical crosslinking process. These bioink modifications have consequences for cellular viability and proliferative and migratory behavior. Cell damage and post-printing dysfunctionality may occur for a variety of reasons, such as high shear stress, lack of growth factors or suitable ECM, or limitation of intercellular communication [9]. Despite promising results in this direction, an ideal bioink is yet to be found. Taking into consideration that there are so many different cell types in the body, all with their refined needs regarding the microenvironment, it appears that a variety of suitable bioinks still have to be developed to address the needs of different cell types. In addition, the constructs obtained by the current bioprinting techniques display a simplistic cellular architecture. For example, although pre-vascular tubes could be embedded in the structural bioink as “sacrificial” hydrogels [28], their patterns lack the fractal organization of natural microvascular networks.
In summary, this approach relies on hydrogels as primary shape generators and cell carriers. While it remains promising for large, cell-homogenous, matrix-rich tissues, representing mostly the skeletal-muscular system, it is still struggling to solve its hard to conciliate requirements, related to the hydrogels printability on one side, and cellular needs on the other. More work will need to be invested into self-organizing systems, where main (yet small) blood vessels can be printed into tissue constructs, to further allow sprouting from and to capillary systems. Cleary, the fourth dimension comes into play, namely the time-component of maturation.
2. Tissue Assembly
In this section, we describe 3D tissue engineering methods that do not make overt use of the ink and paper analogy. In most cases, the methods of this type could be called “scaffold-free,” indicating a version of biofabrication where only cells are used, and the needed matrix is produced by the cells themselves. With regard to the configuration of the cells in the initial assembly step, this approach comes in two variants: A planar and a spherical mode.
2.1 Planar Biofabrication
This mode works with cell sheets that have a preferred two-dimensional distribution. In essence, this biofabrication method is a lamination approach and hinges on the adhesion of cells to a substrate and the ECM produced by them. Thanks to a thermosensitive polymer (NiPAAM and pNIPAAM) as a support structure, a reduction of temperature below standard cultivation at 37°C will lead to a repulsion of the cell layer from the polymer, leading to the detachment of the cell culture as a contiguous cell sheet [32]. Several cell sheets can be stacked, thus resulting in a multilayered tissue [33]. The approach, originally proposed by Michael Sefton for drug delivery purposes, later developed as a tissue engineering mode [34] and was driven forward by Owaki et al. [35].
2.2 The Spheroidal Mode of Cell Assembling
2.2.1 Make and Cast
The 3D nature of tissues’ architecture is well captured by employing cell spheroids. Frequently referred to also as “microtissues” [36] they are increasingly preferred to isolated cells to improve the efficacy of cell therapy [37], or particularly as tumor models (oncospheres) [38]. When these spheroids are grown from stem cells, they are called “organoids” (or “embryoid bodies” in the case of embryonic or induced pluripotent stem cells), representing a promising new development in tissue engineering [39]. Alternatively, cell aggregates can be also prepared as cell cords, for example, for further processing into spheroids [40].
A completely different bioprinting approach is based on the notion that the cell clusters forming spheroids merge when brought into contact with each other [41]. The fusion of spheroids is based on the same principles that govern their formation in the first place: Minimization of a system’s potential energy, generated by the adhesive interactions between cells, through intercellular adhesion molecules, mainly the cadherins and connexins [42]. The cell cytoskeleton [43] and metabolism may also have indirect roles but probably are less important than direct cell-cell attachment. A good argument that spheroid fusion is relatively well understood is the ability to simulate it by computer modeling on several platforms, for example, CompuCell3D [44], initially developed around this type of applications [45]. We recently expanded a similar model to capture the role of oxygen during spheroid fusion in larger structures [46].
Spheroid generation is best achieved by placing the cells on non-binding surfaces either in large flat or small, round wells [47]. Cells sedimenting on a surface that offers no anchorage will adhere to other cells thus forming spherical clusters, a tendency that can be promoted by small inverse pyramidal depressions [48]. Depending on geometry and coating, the latter allows for homodisperse spheroid sizes, while larger non-binding wells might give rise to numerous differently sized spheroids. The spheroids so formed then have to be repositioned to induce them to fuse into 3D structures. To this end, the spheroids can be placed in molds where they form 3D structures by fusing [41]. Tissue models of cartilage [49], or cardiac patches prepared from spheroids fused by flotation on culture medium [50], as well as vascular rings [51] and tumor models [52] assembled by magnetic force, have been described. As a variant, thicker honeycomb-shaped cell toroids prepared in molds, then stacked in register, and fused in larger constructs were proposed for improved distribution of nutrients, even in the absence of a bona fide vascularization [53].
2.2.2 Pick and Place
Alternatively, spheroids can be individually manipulated by dedicated instruments (such as the “Fabion” bioprinter [54]), and placed in a pattern on support where they fuse and form hollow or mixed massive structures. Interestingly, the targeted placement of spheroids on support (which some authors address as “biopaper” [55-57]) refers back to the printing analogy.
To facilitate the formation of 3D constructs from spheroids, a method was needed to keep them in contact long enough to effect fusion, and at the same time to allow the cells to produce their own ECM [58]. The companies Organovo [59] and 3D Bioprinting Solutions [54] are performing this step on their bioprinters using “fugitive” hydrogels as supports, which are removed after the spheroid fusion process.
2.2.3 Pick and Skewer
An ingenious solution to spheroids assembling problem has been developed in Japan [60]. Essentially, this is based on using a spheroids-assembling robot, which skewers them on a rectangular array of stainless steel micro-needles (“Kenzan)” [7]. Although technologically different, this method has common goals with regular bioprinting, such as the use of a nozzle to place the cells in a layer-by-layer formed 3D pattern, as well as the computer-assisted design and operation.
Recently, several pre-clinical examples of “Kenzan” scaffold-free biofabrication have been reported: Surgically-robust small diameter vascular grafts [61], tracheal [62] and urethral [63] tubes, neural bridges [64], beating cardiac patches [65], liver buds [66], and gastric diaphragm [63]. However, the Kenzan method is not without its own limitations, as we commented previously [7]. Among the more significant ones are: (i) Inability to import anatomically-correct 3D images; (ii) dependence of this method on the cells’ propensity to make spheroids of required size (commensurate with the inter-needle distance); (iii) secretion of a matrix strong enough to keep the construct compact; and (iv) length of the constructs limited to that of the microneedles.
3. Hybrid Biofabrication
Since the inception of bioprinting, those involved in its development rightly appreciated the difficulties derived from the use of a biomaterial and contemplated alternatives (for instance,[64]). These consist of using cell spheroids as building blocks for direct assembling the 3D construct, even if the spheroids themselves may require temporary support of some sort (such as “fugitive” hydrogels, or mechanical assistance).
Thus, some of the properties of scaffold-free biofabrication could be complemented, at least in part, by including biomaterials into the “scaffold-free” constructs. For example, these biomaterials could compensate for the slower intrinsic secretion of an ECM by some cell types, when prepared as spheroids. Alternatively, “classical” bioprinting may also benefit from several principles of the scaffold-free approach. Combined, this technological inter-breeding establishes the field of “hybrid” biofabrication.
The use of sacrificial hydrogels for holding spheroids in place, until fusion and during a “post-printing maturation” phase[54,65], is a typical example of hybrid bioprinting. Combination of spheroids with hydrogels could be profitable for regular bioprinting as well if instead of single-cell suspensions pre-formed spheroids are mixed within the bioink. In practice, this solution has been used to increase the human adipose-derived stromal cells survival and promoted their ability to differentiate after bioprinting [66]. The bioprinter can be also used to prepare cells mixed in bioinks of alginate [67] or collagen [68] in droplet form, as an efficient method of cell encapsulation, for subsequent in vitro or in vivo deployment. Moreover, the addition of fibrillary materials to hydrogels has been shown to improve their mechanical properties, thus generating stronger tissue-like constructs [30,69,70].
Direct encapsulation of spheroids in polymeric cages [71], or incorporation of fibrillary matrix fragments into spheroids [72] are other examples of hybrid biofabrication, with the potential to improve spheroid-based tissue engineering. For example, polycaprolactone was prepared as a fibrillary mesh by electrospinning, then fragmented by limited hydrolysis. The resulting material was added to mesenchymal stromal cells during centrifugation-assisted spheroid preparation, becoming uniformly distributed within their volume. Consequently, the resulting spheroids were less compact, and thus better aerated and nurtured, as compared to their scaffold-free counter-parts, which positively impacted on cell survival and osteogenic differentiation [72].
4. Post-printing Considerations
One of the big questions for all modes of biofabrication is what happens after the initial assembly of cells and spheroids, namely how will the cells captured in scaffolds survive, differentiate and proliferate? And how will spheroids hold together: Will there be sufficient intrinsic ECM produced to generate tissue-specific and tissue-preserving connective tissue? In fact, the post-printing ECM formation and remodeling is one of the biggest current challenges in biofabrication. ECM components are made by quite a variety of cells, but the immobilization and their firm deposition around the cells is a challenge in standard aqueous culture conditions. One way to enhance ECM accumulation is the application of macromolecular crowding (MMC). Of relevance to this discussion is the use of polydisperse additives, some akin to “particulate” hydrogels, known MMC agents [73].
A special class of polymers with hydrogel-like properties, consisting of smaller molecular weight compounds than those commonly used for tissue engineering and bioprinting (e.g. Ficol 400), are increasingly used to bring the molecular concentration of the extracellular milieu to values close to those encountered in natural tissues [74]. These molecules produce the biophysical effect of MMC and are referred to simply as “crowders.” By size, charge, and hydration shell they exclude volume and collectively generate a fractional volume occupancy [75]. This has important consequences on the codissolved materials. The effects of MMC are mediated through an increase in the chance of direct interaction between the active molecules, thus accelerating the speed of their aggregation, or increasing their stability or the rate of their processing by codissolved enzymes. Collectively, these effects lead to a denser, more structured ECM, a phenomenon studied mostly in terms of the properties of collagen I microfibrils[75-77], but also of other types of collagen, as well as laminin, fibronectin, etc. [78]. Addition of MMC to the medium used for spheroids preparation enhances matrix deposited and thus may facilitate their aggregation, shape, and stability [79]. This concept has been recently applied to bioprinting, as a means to manipulate the porosity within multilayered collagen-based hydrogels, by altering the collagen fibrillogenesis process through controlling the number of macromolecule-based bioink droplets printed on each collagen layer [80].
In summary, “hybrid biofabrication” is the cross-pollination between approaches, methods, and materials already common in this field as elsewhere in technology, which could be further expanded and exploited as a proven method to programmatically increase the “vitality” (i.e., applicability and value generation) of the resulting products.
Following the basic structure of tissues which contain cells, extracellular matrix, and fluids, the composition of biofabricated tissue analogs may vary from biomaterials-only, to cell suspensions, or cells-only constructs, with numerous intermediaries or “hybrid” situations (e.g., spheroids in hydrogels or fibers in spheroids as illustrated here). Correspondingly, these constructs can be used in a spectrum of applications ranging from inorganic biocompatible prostheses to live cell-based implants. Macromolecular crowding agents can be also added to cell preparations to bring their environment closer to the natural density. The methods of bioprinting may also come in these two versions, namely biomaterial-based or cell-based (“scaffold-free).”
Acknowledgments
We would like to acknowledge support from Indiana Institute for Medical Research (NIM) and Richard L. Roudebush VA Medical Center (NIM, LM).
References
- 1.Moroni L, Boland T, Burdick J A, et al. 2017, Biofabrication:A guide to technology and terminology. Trends Biotechnol. 36(4):384–402. doi: 10.1016/j.tibtech.2017.10.015. https://doi.org/10.1016/j.tibtech.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Atala A, Yoo J. 2015, Essentials of 3D Biofabrication and Translation. Amsterdam: Elsevier; [Google Scholar]
- 3.Mironov V. 2005, The second international workshop on bioprinting, biopatterning and bioassembly. Expert Opin Biol Ther. 5(8):1111–1115. doi: 10.1517/14712598.5.8.1111. https://doi.org/10.1517/14712598.5.8.1111. [DOI] [PubMed] [Google Scholar]
- 4.Groll J, Boland T, Blunk T, et al. 2016, Biofabrication:Reappraising the definition of an evolving field. Biofabrication. 8(1):13001. doi: 10.1088/1758-5090/8/1/013001. https://doi.org/10.1088/1758-5090/aaec52. [DOI] [PubMed] [Google Scholar]
- 5.Yu Y, Zhang Y, Ozbolat IT. 2016, Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands”as a new bioink. Sci Rep. 6:2∊. doi: 10.1038/srep28714. https://doi.org/10.1038/srep2∊. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi H, Okano T. 2015, Cell sheet-based tissue engineering for organizing anisotropic tissue constructs produced using microfabricated thermoresponsive substrates. Adv Healthc Mater. 4(16):2388–2407. doi: 10.1002/adhm.201500194. https://doi.org/10.1002/adhm.201500194. [DOI] [PubMed] [Google Scholar]
- 7.Moldovan N I, Hibino N, Nakayama K. 2017, Principles of the kenzan method for robotic cell spheroid-based three-dimensional bioprinting. Tissue Eng Part B Rev. 23(3):237–244. doi: 10.1089/ten.TEB.2016.0322. https://doi.org/10.1089/ten.teb.2016.0322. [DOI] [PubMed] [Google Scholar]
- 8.Ovsianikov A, Khademhosseini A, Mironov V. 2018, The synergy of scaffold-based and scaffold-free tissue engineering strategies. Trends Biotechnol. 36(4):348–357. doi: 10.1016/j.tibtech.2018.01.005. https://doi.org/10.1016/j.tibtech.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Moldovan L. 2017, Comparison of biomaterial-dependent and independent bioprinting methods for cardiovascular medicine. Curr Opin Biomed Eng. 2:124–131. https://doi.org/10.1016/j.cobme.2017.05.009. [Google Scholar]
- 10.Zhu W, Ma X, Gou M, et al. 2016, 3D printing of functional biomaterials for tissue engineering. Curr Opin Biotechnol. 40:103–1121. doi: 10.1016/j.copbio.2016.03.014. https://doi.org/10.1016/j.copbio.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Muylaert D E, Fledderus J O, Bouten C V, et al. 2014, Combining tissue repair and tissue engineering;bioactivating implantable cell-free vascular scaffolds. Heart. 100(23):1825–1830. doi: 10.1136/heartjnl-2014-306092. https://doi.org/10.1136/heartjnl-2014-306092. [DOI] [PubMed] [Google Scholar]
- 12.Jeong C.G, Atala A. 2015, 3D printing and biofabrication for load bearing tissue engineering. Adv Exp Med Biol. 881:3–14. doi: 10.1007/978-3-319-22345-2_1. https://doi.org/10.1007/978-3-319-22345-2_1. [DOI] [PubMed] [Google Scholar]
- 13.Banu A, Tatara A M, Sutradhar A. 2018, Large animal models of an in vivo bioreactor for engineering vascularized bone. Tissue Eng Part B. 24(4):317–325. doi: 10.1089/ten.teb.2018.0005. https://doi.org/10.1089/ten.teb.2018.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho S S, Murphy K C, Binder B Y, et al. 2016, Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med. 5(6):773–781. doi: 10.5966/sctm.2015-0211. https://doi.org/10.5966/sctm.2015-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skardal A, Mack D, Kapetanovic E, et al. 2012, Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med. 1(11):792–802. doi: 10.5966/sctm.2012-0088. https://doi.org/10.5966/sctm.2012-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laternser S, Keller H, Leupin O, et al. 2018, A novel microplate 3D bioprinting platform for the engineering of muscle and tendon tissues. Slas Technol. 3:247263031≈594. doi: 10.1177/2472630318776594. https://doi.org/10.1177/247263031≈594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapoport S. 2013, Electrospinning tubular scaffolds with tissue-like mechanical properties and biomimetic surface features. Methods Mol Biol. 1001:153–165. doi: 10.1007/978-1-62703-363-3_13. https://doi.org/10.1007/978-1-62703-363-3_13. [DOI] [PubMed] [Google Scholar]
- 18.Udelsman B, Hibino N, Villalona G A, et al. 2011, Development of an operator-independent method for seeding tissue-engineered vascular grafts. Tissue Eng Part C. Methods. 17(7):731–736. doi: 10.1089/ten.tec.2010.0581. https://doi.org/10.1089/ten.tec.2010.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu W J, Xu Y D, Wang Z X, et al. 2012, New ureteral scaffold constructed with composite poly(L-lactic acid)-collagen and urothelial cells by new centrifugal seeding system. J Biomed Mater Res A. 100(7):1725–1733. doi: 10.1002/jbm.a.34134. https://doi.org/10.1002/jbm.a.34134. [DOI] [PubMed] [Google Scholar]
- 20.Thomas J, Jones D, Moldovan L, et al. 2018, Labeling of endothelial cells with magnetic microbeads by angiophagy. Biotechnol Lett. 2018 doi: 10.1007/s10529-018-2581-9. http//:doi:10.1007/s10529-018-2581-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy S V, Atala A. 2014, 3D bioprinting of tissues and organs. Nat Biotechnol. 32(8):773–785. doi: 10.1038/nbt.2958. https://doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 22.Murphy S V, Skardal A, Atala A. 2013, Evaluation of hydrogels for bio-printing applications. J Biomed Mater Res A. 101(1):272–284. doi: 10.1002/jbm.a.34326. https://doi.org/10.1002/jbm.a.34326. [DOI] [PubMed] [Google Scholar]
- 23.Boland T, Xu T, Damon B, et al. 2006, Application of inkjet printing to tissue engineering. Biotechnol J. 1(9):910–917. doi: 10.1002/biot.200600081. https://doi.org/10.1002/biot.200600081. [DOI] [PubMed] [Google Scholar]
- 24.Pirlo R K, Dean D, Knapp D R, et al. 2006, Cell deposition system based on laser guidance. Biotechnol J. 1(9):1007–1013. doi: 10.1002/biot.200600127. https://doi.org/10.1002/biot.200600127. [DOI] [PubMed] [Google Scholar]
- 25.Xiong R, Zhang Z, Chai W, et al. 2015, Freeform drop-on-demand laser printing of 3D alginate and cellular constructs. Biofabrication. 7(4):45011. doi: 10.1088/1758-5090/7/4/045011. https://doi.org/10.1088/1758-5090/7/4/045011. [DOI] [PubMed] [Google Scholar]
- 26.Hospodiuk M, Dey M, Sosnoski D, et al. 2017, The bioink:A comprehensive review on bioprintable materials. Biotechnol Adv. 35(2):217–239. doi: 10.1016/j.biotechadv.2016.12.006. https://doi.org/10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Pourchet L J, Thepot A, Albouy M, et al. 2017, Human skin 3D bioprinting using scaffold-free approach. Adv Healthc Mater. 6(4):345. doi: 10.1002/adhm.201601101. https://doi.org/10.1002/adhm.201601101. [DOI] [PubMed] [Google Scholar]
- 28.Kolesky D B, Homan K A, Skylar-Scott A M, et al. 2016, Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. 113(12):3179–3184. doi: 10.1073/pnas.1521342113. https://doi.org/10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S J, Lim G J, Lee J W, et al. 2006 In vitro evaluation of a poly(lactide-co-glycolide)–collagen composite scaffold for bone regeneration. Biomaterials. 27(18):3466–3472. doi: 10.1016/j.biomaterials.2006.01.059. https://doi.org/10.1016/j.biomaterials.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 30.Kang H W, Lee S J, Ko I K, et al. 2016, A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 34(3):312–319. doi: 10.1038/nbt.3413. https://doi.org/10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 31.Xu T, Binder K W, Albanna M Z, et al. 2013, Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 5(1):15001. doi: 10.1088/1758-5082/5/1/015001. https://doi.org/10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 32.Kikuchi T, Shimizu T, Wada M, et al. 2014, Automatic fabrication of 3-dimensional tissues using cell sheet manipulator technique. Biomaterials. 35(8):2428–2435. doi: 10.1016/j.biomaterials.2013.12.014. https://doi.org/10.1016/j.biomaterials.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Kim K, Utoh R, Ohashi K, et al. 2015, Fabrication of functional 3D hepatic tissues with polarized hepatocytes by stacking endothelial cell sheets in vitro. J Tissue Eng Regen Med. 2015:124–126. doi: 10.1002/term.2102. [DOI] [PubMed] [Google Scholar]
- 34.Leung B M, Sefton M V. 2010, A modular approach to cardiac tissue engineering. Tissue Eng Part A. 16(10):3207–3218. doi: 10.1089/ten.tea.2009.0746. https://doi.org/10.1089/ten.tea.2009.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owaki T, Shimizu T, Yamato M, et al. 2014, Cell sheet engineering for regenerative medicine:Current challenges and strategies. Biotechnol J. 9(7):904–914. doi: 10.1002/biot.201300432. https://doi.org/10.1002/biot.201300432. [DOI] [PubMed] [Google Scholar]
- 36.Gartner Z J, Bertozzi C R. 2017, Programmed assembly of 3-dimensional microtissues with defined cellular connectivity. Proc Natl Acad Sci U S A. 106(12):4606–4610. doi: 10.1073/pnas.0900717106. https://doi.org/10.1073/pnas.0900717106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhang S H, Cho S W, La W G, et al. 2011, Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 32(11):2734–2747. doi: 10.1016/j.biomaterials.2010.12.035. https://doi.org/10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 38.Haji-Karim M, Carlsson J. 1978, Proliferation and viability in cellular spheroids of human origin. Cancer Res. 38(5):1457–1464. [PubMed] [Google Scholar]
- 39.Takebe T, Enomura M, Yoshizawa E, et al. 2015, Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell. 16(5):556–565. doi: 10.1016/j.stem.2015.03.004. https://doi.org/10.1016/j.stem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Shafiee A. 2015, Post-deposition bioink self-assembly:A quantitative study. Biofabrication. 7(4):45005. doi: 10.1088/1758-5090/7/4/045005. https://doi.org/10.1088/1758-y5090/7/4/045005. [DOI] [PubMed] [Google Scholar]
- 41.Mironov V, Visconti R P, Kasyanov V, et al. 2009, Organ printing:Tissue spheroids as building blocks. Biomaterials. 30(12):2164–2174. doi: 10.1016/j.biomaterials.2008.12.084. https://doi.org/10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bao B, Jiang J, Yanase T, et al. 2011, Connexon-mediated cell adhesion drives microtissue self-assembly. FASEB J. 25(1):255–264. doi: 10.1096/fj.10-155291. https://doi.org/10.1096/fj.10-155291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dean D M, Morgan J R. 2008, Cytoskeletal-mediated tension modulates the directed self-assembly of microtissues. Tissue Eng Part A. 14(12):1989–1997. doi: 10.1089/ten.tea.2007.0320. https://doi.org/10.1089/ten.tea.2007.0320. [DOI] [PubMed] [Google Scholar]
- 44.Thomas G L, Nagy-Mehez V, Mombach A. 2014, Dynamics of cell aggregates fusion:Experiments and simulations. Physica. 395:247–254. https://doi.org/10.1016/j.physa.2013.10.037. [Google Scholar]
- 45.Izaguirre J A, Chaturvedi R, Huang C, et al. 2004, Compucell, a multi-model framework for simulation of morphogenesis. Bioinformatics. 20(7):1129–1137. doi: 10.1093/bioinformatics/bth050. https://doi.org/10.1093/bioinformatics/bth050. [DOI] [PubMed] [Google Scholar]
- 46.Sego T J, Kasacheuski U, Hauersperger D, et al. 2017, A heuristic computational model of basic cellular processes and oxygenation during spheroid-dependent biofabrication. Biofabrication. 9(2):24104. doi: 10.1088/1758-5090/aa6ed4. https://doi.org/10.1088/1758-5090/aa6ed4. [DOI] [PubMed] [Google Scholar]
- 47.Gobaa S, Hoehnel S, Roccio M, et al. 2011, Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods. 8(11):949–955. doi: 10.1038/nmeth.1732. https://doi.org/10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 48.Ungrin M D, Joshi C, Nica A, et al. 2008, Reproducible, ultra high-throughput formation of multicellular organization from single cell suspension-derived human embryonic stem cell aggregates. PLoS One. 3(2):e1565. doi: 10.1371/journal.pone.0001565. https://doi.org/10.1371/journal.pone.000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murata D, Tokunaga S, Tamura T, et al. 2015, A preliminary study of osteochondral regeneration using a scaffold-free three-dimensional construct of porcine adipose tissue-derived mesenchymal stem cells. J Orthop Surg Res. 10:35. doi: 10.1186/s13018-015-0173-0. https://doi.org/10.1186/s13018-015-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noguchi R, Nakayama K, Itoh M, et al. 2016 Development of a three-dimensional pre-vascularized scaffold-free contractile cardiac patch for treating heart disease. J Heart Lung Transplant. 35(1):137–145. doi: 10.1016/j.healun.2015.06.001. https://doi.org/10.1016/j.healun.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 51.Tseng H, Gage J A, Shen T, et al. 2016, A high-throughput in vitro ring assay for vasoactivity using magnetic 3D bioprinting. Sci Rep. 6:30640. doi: 10.1038/srep30640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaganathan H, Gage J, Leonard F, et al. 2014, Three-dimensional in vitro co-culture model of breast tumor using magnetic levitation. Sci Rep. 4:6468. doi: 10.1038/srep06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dean D M, Sasagawa T, Shimizu T, et al. 2007, Rods, tori, and honeycombs:The directed self-assembly of microtissues with prescribed microscale geometries. FASEB J. 21(14):4005–4012. doi: 10.1096/fj.07-8710com. https://doi.org/10.1096/fj.07-8710com. [DOI] [PubMed] [Google Scholar]
- 54.Bulanova E A, Koudan E V, Degosserie J, et al. 2017, Bioprinting of a functional vascularized mouse thyroid gland construct. Biofabrication. 9(3):34105. doi: 10.1088/1758-5090/aa7fdd. https://doi.org/10.1088/1758-5090/aa7fdd. [DOI] [PubMed] [Google Scholar]
- 55.Jakab K, Damon B, Neagu A, et al. 2006, Three-dimensional tissue constructs built by bioprinting. Biorheology. 43(3-4):509–513. [PubMed] [Google Scholar]
- 56.Lee W. 2012, Cellular hydrogel biopaper for patterned 3D cell culture and modular tissue reconstruction. Adv Healthc Mater. 1(5):635–639. doi: 10.1002/adhm.201200158. https://doi.org/10.1002/adhm.201290023. [DOI] [PubMed] [Google Scholar]
- 57.Hakam M S, Imani R, Abolfathi N, et al. 2016, Evaluation of fibrin-gelatin hydrogel as biopaper for application in skin bioprinting:An in-vitro study. Biomed Mater Eng. 27(6):669–682. doi: 10.3233/BME-161617. https://doi.org/10.3233/BME-161617. [DOI] [PubMed] [Google Scholar]
- 58.Olsen T R, Mattix B, Casco M, et al. 2015, Manipulation of cellular spheroid composition and the effects on vascular tissue fusion. Acta Biomater. 13:188–198. doi: 10.1016/j.actbio.2014.11.024. https://doi.org/10.1016/j.actbio.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen D G, Funk J, Robbins J B, et al. 2016, Bioprinted 3D primary liver tissues allow assessment of organ-level response to clinical drug induced toxicity in vitro. PLoS One. 11(7):e0158674. doi: 10.1371/journal.pone.0158674. https://doi.org/10.1371/journal.pone.0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimoto T. 2012, Building of HD MACs using cell processing robot for cartilage regeneration. J Robot Mechatr. 24(2):347–353. https://doi.org/10.20965/jrm.2012.p0347. [Google Scholar]
- 61.Itoh M, Nakayama K, Noguchi R, et al. 2015, Scaffold-free tubular tissues created by a bio-3D printer undergo remodeling and endothelialization when implanted in rat aortae. PLoS One. 10(9):e0136681. doi: 10.1371/journal.pone.0136681. https://doi.org/10.1371/journal.pone.0136681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Machino R, Taniguchi D, Takeoka Y, et al. 2015, Scaffold-free trachea tissue engineering using bioprinting. Am J Respir Crit Care Med. 191:A5343. [Google Scholar]
- 63.Zhang X Y, Yanagi Y, Takeoka Y, et al. 2018, Regeneration of diaphragm with bio-3D cellular patch. Biomaterials. 167:1–14. doi: 10.1016/j.biomaterials.2018.03.012. https://doi.org/10.1016/j.biomaterials.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 64.Mironov V, Kasyanov V, Drake C, et al. 2008, Organ printing:Promises and challenges. Regen Med. 3(1):93–103. doi: 10.2217/17460751.3.1.93. https://doi.org/10.2217/17460751.3.1.93. [DOI] [PubMed] [Google Scholar]
- 65.Collin de H A, Takeishi K, Guzman-Lepe J, et al. 2016, Liver-regenerative transplantation:Regrow and reset. Am J Transplant. 16(6):1688–1696. doi: 10.1111/ajt.13678. https://doi.org/10.1111/ajt.13678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yipeng J, Yongde X, Yuanyi W, et al. 2017, Microtissues enhance smooth muscle differentiation and cell viability of hADSCs for three dimensional bioprinting. Front Physiol. 8:534. doi: 10.3389/fphys.2017.00534. https://doi.org/10.3389/fphys.2017.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams S K, Touroo J S, Church K H, et al. 2013, Encapsulation of adipose stromal vascular fraction cells in alginate hydrogel spheroids using a direct-write three-dimensional printing system. Biores Open Access. 2(6):448–454. doi: 10.1089/biores.2013.0046. https://doi.org/10.1089/biores.2013.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gettler B C, Zakhari J S, Gandhi P S, et al. 2017, Formation of adipose stromal vascular fraction cell-laden spheroids using a three-dimensional bioprinter and superhydrophobic surfaces. Tissue Eng Part C. 23(9):516–524. doi: 10.1089/ten.TEC.2017.0056. https://doi.org/10.1089/ten.tec.2017.0056. [DOI] [PubMed] [Google Scholar]
- 69.Izadifar Z, Chang T, Kulyk W, et al. 2016, Analyzing biological performance of 3D-printed, cell-impregnated hybrid constructs for cartilage tissue engineering. Tissue Eng Part C. Methods. 22(3):173–188. doi: 10.1089/ten.TEC.2015.0307. https://doi.org/10.1089/ten.tec.2015.0307. [DOI] [PubMed] [Google Scholar]
- 70.Castilho M, Feyen D, Flandes-Iparraguirre M, et al. 2017, Melt electrospinning writing of poly-hydroxymethylglycolide-co-epsilon-caprolactone-based scaffolds for cardiac tissue engineering. Adv Healthc Mater. 2017:6–18. doi: 10.1002/adhm.201700311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Danilevicius P, Rezende R A, Pereira F D, et al. 2015, Burr-like, laser-made 3D microscaffolds for tissue spheroid encagement. Biointerphases. 10(2):21011. doi: 10.1116/1.4922646. https://doi.org/10.1116/1.4922646. [DOI] [PubMed] [Google Scholar]
- 72.Ahmad T, Lee J, Shin Y M, et al. 2017, Hybrid-spheroids incorporating ECM like engineered fragmented fibers potentiate stem cell function by improved cell/cell and cell/ECM interactions. Acta Biomater. 64:161–175. doi: 10.1016/j.actbio.2017.10.022. https://doi.org/10.1016/j.actbio.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 73.Lareu R R, Arsianti I, Subramhanya H K, et al. 2007 In vitro enhancement of collagen matrix formation and crosslinking for applications in tissue engineering:A preliminary study. Tissue Eng. 13(2):385–391. doi: 10.1089/ten.2006.0224. https://doi.org/10.1089/ten.2006.0224. [DOI] [PubMed] [Google Scholar]
- 74.Satyam A, Kumar P, Fan X, et al. 2014 Macromolecular crowding meets tissue engineering by self-assembly:A paradigm shift in regenerative medicine. Adv Mater. 26(19):3024–3034. doi: 10.1002/adma.201304428. https://doi.org/10.1002/adma.201304428. [DOI] [PubMed] [Google Scholar]
- 75.Chen C, Loe F, Blocki A, et al. 2011, Applying macromolecular crowding to enhance extracellular matrix deposition and its remodeling in vitro for tissue engineering and cell-based therapies. Adv Drug Deliv Rev. 63(4-5):277–290. doi: 10.1016/j.addr.2011.03.003. https://doi.org/10.1016/j.addr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 76.Dewavrin J Y, Hamzavi N, Shim V P, et al. 2014, Tuning the architecture of three-dimensional collagen hydrogels by physiological macromolecular crowding. Acta Biomater. 10(10):4351–4359. doi: 10.1016/j.actbio.2014.06.006. https://doi.org/10.1016/j.actbio.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 77.Rashid R, Lim N S, Chee S M, et al. 2014, Novel use for polyvinylpyrrolidone as a macromolecular crowder for enhanced extracellular matrix deposition and cell proliferation. Tissue Eng Part C. Methods. 20(12):994–1002. doi: 10.1089/ten.tec.2013.0733. https://doi.org/10.1089/ten.tec.2013.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Magno V, Friedrichs J, Weber H M, et al. 2017 Macromolecular crowding for tailoring tissue.derived fibrillated matrices. Acta Biomater. 55:109–119. doi: 10.1016/j.actbio.2017.04.018. https://doi.org/10.1016/j.actbio.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 79.Leung B M, Lesher-Perez S C, Matsuoka T, et al. 2015, Media additives to promote spheroid circularity and compactness in hanging drop platform. Biomater Sci. 3(2):336–344. doi: 10.1039/c4bm00319e. https://doi.org/10.1039/C4BM00319E. [DOI] [PubMed] [Google Scholar]
- 80.Ng W L, Goh M H, Yeong W Y, et al. 2018, Applying macromolecular crowding to 3D bioprinting:Fabrication of 3D hierarchical porous collagen-based hydrogel constructs. Biomater Sci. 6(3):562–574. doi: 10.1039/c7bm01015j. https://doi.org/10.1039/C7BM01015J. [DOI] [PubMed] [Google Scholar]


