Abstract
Background/objective
Marked to abundant crystalluria may cause significant morbidity due to acute renal injury. Intravenous acyclovir administration may result in a pathologic crystalluria, especially in cases with increased renal concentration of the drug. It is important that clinical laboratory staff recognize and communicate the presence of abundant crystalluria to clinical staff to avoid irreversible kidney injury.
Methods
We report a case of crystalluria in a patient treated empirically with intravenous acyclovir for possible viral meningitis.
Results
Opaque “milky” urine was submitted for urine analysis which showed abundant long needle-shaped brightly birefringent crystals under polarized light microscopy and was diagnosed as acyclovir crystalluria.
Conclusions
Any case of moderate to abundant crystalluria should be reported in a timely manner to the clinical staff to facilitate treatment modification to reduce the risk of acute kidney injury. Laboratory staff should be aware and recognize acyclovir treatment as a possible cause of pathologic crystalluria.
Key words: acyclovir, crystals, crystalluria, acute kidney injury, adverse drug reaction
INTRODUCTION
Rapid identification of abundant crystals in urine is a clinically important skill for laboratory staff because prompt treatment actions can avoid acute kidney injury and crystalluria may be the first clinical indication of a serious adverse drug reaction (1). Drug-induced crystalluria and acute kidney injury can be reduced by discontinuation of the drug, enhanced drug clearance by patient hydration, urine alkalization, or dialysis (in some cases). Detection of trace amounts of common urine crystals such as calcium oxalate, struvite (triple phosphate) and uric acid are often transient observations with little clinical sequelae. Identification and reporting moderate abundance of urine crystals are useful clinical correlates that may support diagnoses. The most common agents causing crystalluria are sulfonamide antibiotics, ethylene glycol, high dose ascorbic acid, methotrexate and antiviral protease inhibitors (2). It is important to distinguish the rare observation of milky white urine with a pathologic crystalluria from the common milder events.
Pathologic crystalluria is usually associated with administration of pharmaceuticals with limited water solubility to patients at risk with intravascular volume depletion or pre-existing kidney or liver disease, who are predisposed to high urine concentrations of drug and drug metabolites (2-4). This scenario is particularly common among patients that receive intravenous medications and are not able to access drinking water to maintain their hydration status such as pediatric patients, patients verbally impaired and patients that experience intravascular volume contraction due to acute fluid loss (e.g. diarrhea, vomiting).
Acyclovir is a common antiviral drug used to treat herpes simplex infection or varicella zoster and is administered orally, as a cream, or intra-venously (5). As a purine derivative, it has limited water solubility and 60-90% is excreted unchanged in the urine. It is known to cause nausea and diarrhea and should be used with caution in patients with poor liver or kidney function. Three recent studies reported the incidence of acute kidney injury among patients administered parenteral acyclovir to be 5.1-10.5%, 13%, and 17.5% (6, 7, 8). It is critical for laboratories to identify and report detection of acyclovir crystalluria to alert clinicians, so they can make immediate therapeutic changes to avoid irreversible acute kidney injury (9, 10). We report a recent case to highlight this importance.
CLINICAL-DIAGNOSTIC CASE
A 53-year-old man living in a long-term care facility presented to the emergency department with fever (39.4° C) and decreasing level of consciousness (Glasgow coma score = 8). This gentleman had a history of multiple cerebral infarctions and an intraventricular arteriovenous malformation (AVM) with baseline left sided deficits and epilepsy and was verbally impaired. The patient had chronic Hepatitis C infection.
The patient was taking 250 mg phenytoin daily for epilepsy. Phenytoin toxicity, new cerebral infarction, and meningitis were differential diagnoses in this case. He was treated empirically for meningitis with 3.375 g of piperacillin-tazobactam IV, 1 g of vancomycin IV, 500 mg azithromycin IV, and 500 mg of acyclovir IV in the emergency department. A lumbar puncture was performed and Varicella Zoster Virus (VZV) and Herpes Simplex Virus (HSV) PCR was assessed. The non-contrast CT of the brain was consistent with previous imaging; no acute infarctions were identified.
On admission, the patient had normal electrolytes (Table 1), elevated urea and normal creatinine. As the patient became better hydrated during this admission, urea, hemoglobin and creatinine concentrations fell (Table 1).
Table 1.
Serum Chemistry and Hemoglobin Results Days 1-4
| Test | Day 1 | Day 2 | Day 3 | Day 4 | Reference Interval |
|---|---|---|---|---|---|
| Sodium | 147 | 148 | 151 | 150 | 135-146 mmol/L |
| Potassium | 3.8 | 3.5 | 3.2 | 3.0 | 3.5-5.1 mmol/L |
| Chloride | 103 | 109 | 114 | 117 | 100-110 mmol/L |
| Total CO2 | 30 | 26 | 29 | 26 | 22-31 mmol/L |
| Urea | 12.8 | 16.7 | 11.1 | 7.1 | 3.7-7.0 mmol/L |
| Creatinine | 58 | 81 | 49 | 44 | 60-104 μmol/L |
| Hemoglobin | 168 | 150 | 136 | 130 | 135-180 g/L |
The unusually low creatinine level may be attributed to the chronic hepatitis C infection. With continued fluids, antipyretics, and antibiotic/antiviral treatment, the patient’s fever settled over the next few hours and his level of consciousness improved to baseline.
On admission day 3, the catheter derived urine specimen grossly appeared pale, cloudy, and milky (Table 2).
Table 2.
Day 3 Automated Urinalysis and manual Urine Microscopy
| Collection type | Catheter | WBCs Urine | Present |
| Appearance Urine | Cloudy | RBCs Urine | Present |
| Color Urine | Cloudy | Crystals Urine | - |
| Specific Gravity | - | ||
| pH Urine | 6.5 | Needle-shaped crystals | |
| Leukocyte Esterase crystals | 6.5 | Presumptive identification: Acyclovir | |
| Nitrites Urine | Negative | ||
| Protein Urine | 0.25 g/L (+) | ||
| Glucose Urine | Normal | ||
| Glucose Urine | Negative | ||
| Hemoglobin Urine | 250 (++++) | ||
Automated urinalysis (Roche Diagnostics Canada, Laval, Quebec, Canada; u6500 analyzer) showed presence of leukocyte esterase, protein and blood. The specimen was too turbid for determination of specific gravity. The specimen was flagged for manual microscopy, which showed very abundant needle-shaped crystals. These were initially reported as “needle-like crystals, unidentified” and the biochemist was called to review the specimen. The crystal morphology resembled previous cases of acyclovir crystalluria encountered (Figure 1 and Figure 2), and the report was modified to reflect this. The on-call pharmacist as well as the attending physician caring for the patient were notified and informed of the abundant crystals present in the urine and the risk of renal injury. At this time the viral PCR results had been reported as negative for VZV and HSV, and the patient’s acyclovir was discontinued. Evidence of renal insufficiency was not present at follow up based on serum creatinine levels.
Figure 1.

Acyclovir crystalluria: Light microscopy of a wet mount of urine sediment showed abundant sharp needle-shaped transparent crystals (400 × magnification)
Figure 2.
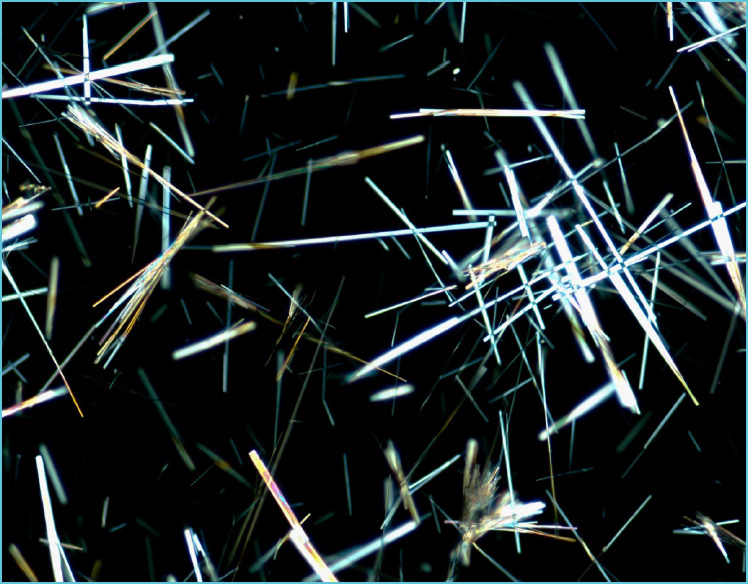
Acyclovir crystalluria: Polarized light microscopy of a wet mount of urine sediment. With crossed polarization filters the needle shaped crystals are very bright and birefringent (400 × magnification)
DISCUSSION
This case highlights several important points. Patients presenting to the emergency department with vague non-specific symptoms may have a broad differential diagnosis which may be treated with initial empiric pharmaceutical therapies while awaiting confirmatory laboratory results. Volume status and renal function can significantly impact the adverse effects of these pharmaceutical agents. In this case, a transient period of hemo-concentration likely secondary to dehydration may have contributed to a markedly elevated concentration of acyclovir in the urine.
Acyclovir crystalluria is a rare laboratory observation due to extensive clinical utilization warnings from the drug manufacturers and pharmacists which encourage maintaining patient hydration, monitoring renal function, and dose adjustments for patients with known renal impairment (11). Substitution with alternative antiviral agents (e.g. valacyclovir or famciclovir) that are also effective against HSV or VSV and have lower risk of crystalluria to reduce the risk for acute kidney injury is another common strategy (12).
Laboratory technologists often do not have access to patient charts or medication records. However, timely reporting of moderate to abundant crystalluria by the laboratory to clinical staff, even if the crystals have not been specifically identified, is important to raise concern for the risk of acute renal injury. Crystalluria secondary to pharmaceutical products may not have distinct morphology. Laboratory procedures including assessment of the reaction of the crystals to the addition of water, saline, acid, and base, as well as the use of a polarizer for microscopic examination may be useful. Direct communication with the clinical team and a review of the patient chart including prescribed pharmaceutical agents by the biochemist is extremely useful in elucidating the etiology of urinary crystals.
TAKE HOME MESSAGES/LEARNING POINTS
Laboratory staff should recognize that milky white urine with abundant crystalluria is a clinically critical observation that needs to be immediately reported to medical staff, even if the initial report indicates an abundant crystalluria with an unidentified crystal.
Acyclovir crystalluria is often observed following intravenous administration of the drug.
Acyclovir crystals do not have a unique morphology. However, long needle shaped crystals, with bright birefringence under polarized light microscopy that readily dissolve when diluted by water, saline, acid or base in the urine of patients receiving acyclovir IV is most likely to acyclovir crystalluria.
Physicians and pharmacists treating patients with acyclovir crystalluria may consider alternative antiviral therapies to reduce the risk of acute kidney injury.
Footnotes
Author disclosures & contributions
A. R. Andrews: Reviewed the clinical case, conducted microscopic photography and literature review, wrote and reviewed the manuscript.
D. Yu: Directed photography, wrote and reviewed the manuscript.
A. W. Lyon: Reviewed the clinical case, wrote and reviewed the manuscript.
REFERENCES
- 1.Cavanaugh C, Perazella MA. Urine Sediment Examination in the Diagnosis and Management of Kidney Disease: Core Curriculum 2019. Am J Kidney Dis. 2019. February; 73(2): 258-272. [DOI] [PubMed] [Google Scholar]
- 2.Daudon M, Frochot V. Crystalluria. Clin Chem Lab Med 2015; 53(Suppl): S1479-S1487. [DOI] [PubMed] [Google Scholar]
- 3.Daudon M, Frochot V, Bazin D, Jungers P. Drug-Induced Kidney Stones and Crystalline Nephropathy: Pathophysiology, Prevention and Treatment. Drugs. 2018; 78: 163-201. [DOI] [PubMed] [Google Scholar]
- 4.Becker GJ, Garigali G, Fogazzi GB. Advances in Urine Microscopy. Am J Kidney Dis. 2016; 67:954-964. [DOI] [PubMed] [Google Scholar]
- 5.King DH. History, pharmacokinetics, and pharmacology of acyclovir. J Am Acad Dermatol. 1988; 18:176-179. [DOI] [PubMed] [Google Scholar]
- 6.Ryan L, Heed A, Foster J, Valappil M, Schmid ML, Duncan CJA. Acute kidney injury (AKI) associated with intravenous aciclovir in adults: Incidence and risk factors in clinical practice. Int J Infect Dis. 2018. September; 74:97-99. [DOI] [PubMed] [Google Scholar]
- 7.Richelsen RKB, Jensen SB, Nielsen H. Incidence and predictors of intravenous acyclovir-induced nephrotoxicity. Eur J Clin Microbiol Infect Dis. 2018; 37: 1965-1971. [DOI] [PubMed] [Google Scholar]
- 8.Lee EJ, Jang HN, Cho HS, Bae E, Lee TW, Chang SH, Park DJ. The incidence, risk factors, and clinical outcomes of acute kidney injury (staged using the RIFLE classification) associated with intravenous acyclovir administration. Ren Fail. 2018; 40: 688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinot M, Klein A, Demesmay K, Groza M, Mohseni-Zadeh M, Tebacher-Alt M, Fafi-Kremer S. Acute renal failure related to high doses of acyclovir (15 mg/kg/8 h) during treatment of varicella zoster virus encephalitis. Antivir Ther. 2019; [DOI] [PubMed] [Google Scholar]
- 10.Lyon AW, Mansoor A, Trotter MJ. Urinary Gems, Acyclovir Crystalluria, Arch Path Lab Med. 2002; 126: 753-754. [DOI] [PubMed] [Google Scholar]
- 11.Zovirax [Product Monograph]. Mississauga, Ontario: GlaxoSmithKline Inc; 2016. [Google Scholar]
- 12.De SK, Hart JCL, Breuer J. Herpes simplex virus and varicella zoster virus: recent advances in therapy. Curr Opin Infec Dis. 2015; 28(6), 589-595. [DOI] [PubMed] [Google Scholar]


