Summary
Aims:
Mouse bladder wall injection with Schistosoma haematobium eggs has been used to overcome limitations in animal models of urogenital schistosomiasis. However, the effect of the absence of cercarial infection on immune responses to eggs in this model is unknown. We hypothesized that cercarial infection would alter local bladder and systemic immune responses to eggs in this model.
Methods and results:
Mice were infected or not infected with S haematobium cercariae, and then, their bladder walls injected with S haematobium eggs or vehicle 5 weeks following cercarial infection. Three weeks later, mice were bled, sacrificed, perfused and their bladders harvested. Parasitological parameters and gross bladder pathology were not changed in egg-injected bladders by cercarial exposure. Figure S1 shows no changes in either granulomas or fibrosis. The only bladder cytokine up-regulated in egg-injected bladders by cercarial exposure (vs no exposure) was leptin. Cercarial exposure, compared to no exposure, resulted in increased serum, IL-1α, IL-13 and TGF-β in bladder egg-injected mice.
Conclusion:
Cercarial exposure altered systemic responses of several cytokines in bladder egg-injected mice, but surprisingly, only modified leptin expression in bladder tissue. This suggests that depending on the specific application, cercarial exposure may not be strictly necessary to model local immune responses in the bladder wall egg injection mouse model of urogenital schistosomiasis.
Keywords: chemokine/receptor, cytokine, Schistosoma spp., schistosomiasis
1 |. INTRODUCTION
Schistosomiasis continues to cause significant morbidity and mortality in many countries around the world; currently, over 112 million people live with urogenital schistosomiasis, and 436 million more are at risk (http://www.who.int/schistosomiasis/epidemiology/table/en/). Urogenital schistosomiasis, caused by infection with Schistosoma haematobium, the most prevalent form of human schistosomiasis, is primarily endemic in sub-Saharan Africa, resulting in over 150 000 deaths each year due to infection-induced obstructive renal failure.1 Cercariae in infested water penetrate the skin of the host and undergo maturation as they migrate through the host, ultimately arriving in the venous plexus of the bladder as adult worms. As adult pairs, schistosomes can persist as long as 40 years in human hosts, continuously laying eggs.2 The deposition of eggs into the host bladder and genital tissue causes sequelae such as granuloma formation, fibrosis, bladder dysfunction and hematuria, and urothelial changes that are believed to lead to carcinogenesis.3–5 Furthermore, female genital schistosomiasis, another potential outcome from infection with S haematobium, may be a risk factor for HIV co-infection.6–8
Despite the significant impact of urogenital schistosomiasis, relatively little is known regarding the mechanisms contributing to the pathophysiology of this disease; the majority of research efforts in schistosomiasis have been concentrated on hepatosplenic schistosomiasis caused by Schistosoma mansoni.9 This is primarily due to the lack of an experimentally tractable animal model—baboons have been useful as they can faithfully recapitulate human urogenital schistosomiasis,10 but they are expensive and controversial to use. Rodent models of urogenital schistosomiasis using mice and hamsters result in little to no involvement of the urogenital system.11,12 To bypass this limitation, viable S haematobium eggs can be injected directly into the bladder walls of mice. These eggs induce granulomatous reactions which reproduce many features of the human disease, including inflammatory cell activation and infiltration, fibrosis, egg excretion in urine and urinary dysfunction.13–15 However, this approach cannot ascertain the effect of cercarial-, schistosomular-and worm-triggered immune responses on the egg-induced granulomatous reaction. Although the majority of schistosomiasis-induced pathology is due to eggs deposited in tissue, work by others suggest that host exposure to early life cycle stages may alter subsequent liver tissue responses to eggs.16,17 We hypothesized that a mouse model combining percutaneous infection with S haematobium cercariae and bladder wall injection with S haematobium eggs would produce a unique host immune response compared to either approach alone, and would better recapitulate human urogenital schistosomiasis.
2 |. MATERIALS AND METHODS
2.1 |. Animals
Schistosoma haematobium-infected LVG hamsters were obtained from the National Institute of Allergy and Infectious Diseases Schistosomiasis Resource Center, Biomedical Research Institute (BRI) and distributed through BEI Resources, NIAID, NIH. 5-to 6-week-old female BALB/c mice were purchased from Charles River Laboratories International Inc. (Wilmington, MA). S haematobium cercariae used to infect mice were provided by BRI. We performed power calculations that showed to achieve an alpha-value of 0.80; we required at least n = 5 based on previous work with this model.14 All animal work was conducted according to relevant US and international guidelines. The animal protocols associated with the work described in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of BRI.
2.2 |. Schistosoma haematobium egg isolation
Hamsters were sacrificed 16-week post-infection to obtain eggs from livers and intestines.18 Briefly, livers and intestines were suspended in a 1.2% NaCl solution containing antibiotic-antimycotic solution (100 units penicillin, 100 μg/mL streptomycin and 0.25 μg/mL amphotericin B; Sigma-Aldrich), minced, homogenized in a Waring blender and passed through a series of stainless steel sieves with sequentially decreasing pore sizes and retained on a 45-μm sieve. Livers and intestines from uninfected hamsters were prepared in a similar fashion for control injections.
2.3 |. Schistosoma haematobium cercarial exposure and egg injection
To test our hypothesis, we used four groups of BALB/c mice (Figure 1): mice received bladder wall injection with vehicle alone (uninfected hamster liver and intestine extract [NN]); bladder wall injection with 3000 freshly harvested S haematobium eggs (NI); percutaneous infection with 150 S haematobium cercariae via tail immersion and bladder wall injection with vehicle (IN); or both percutaneous infection and egg bladder wall injection (II). Mice were infected at week 0 with 150 S haematobium cercariae. At week 5, roughly the time when cercariae develop into worms,19 mice received either bladder wall injections with eggs or vehicle as previously described.14,15 Briefly, mice were anaesthetized with isoflurane followed by subcutaneous injection with buprenorphine (0.05 mg/kg) and line block with bupivacaine (1 mg/kg) for analgesia. A midline lower abdominal incision was made, and the bladder was exteriorized. Freshly harvested S haematobium eggs (3000 eggs suspended in 50 μL of phosphate-buffered saline) or vehicle was injected submucosally into the anterior aspect of the bladder dome.14 Abdominal incisions were then closed with 4–0 Vicryl sutures and treated with topical antibiotic ointment. Previous work shows that peak host immune responses occur at 3-week post–egg deposition.14,20 Therefore, mice were sacrificed at week 8 to collect bladder specimens for cytokine analysis. Sera were collected 1 day prior to sacrifice for cytokine analysis.
FIGURE 1.
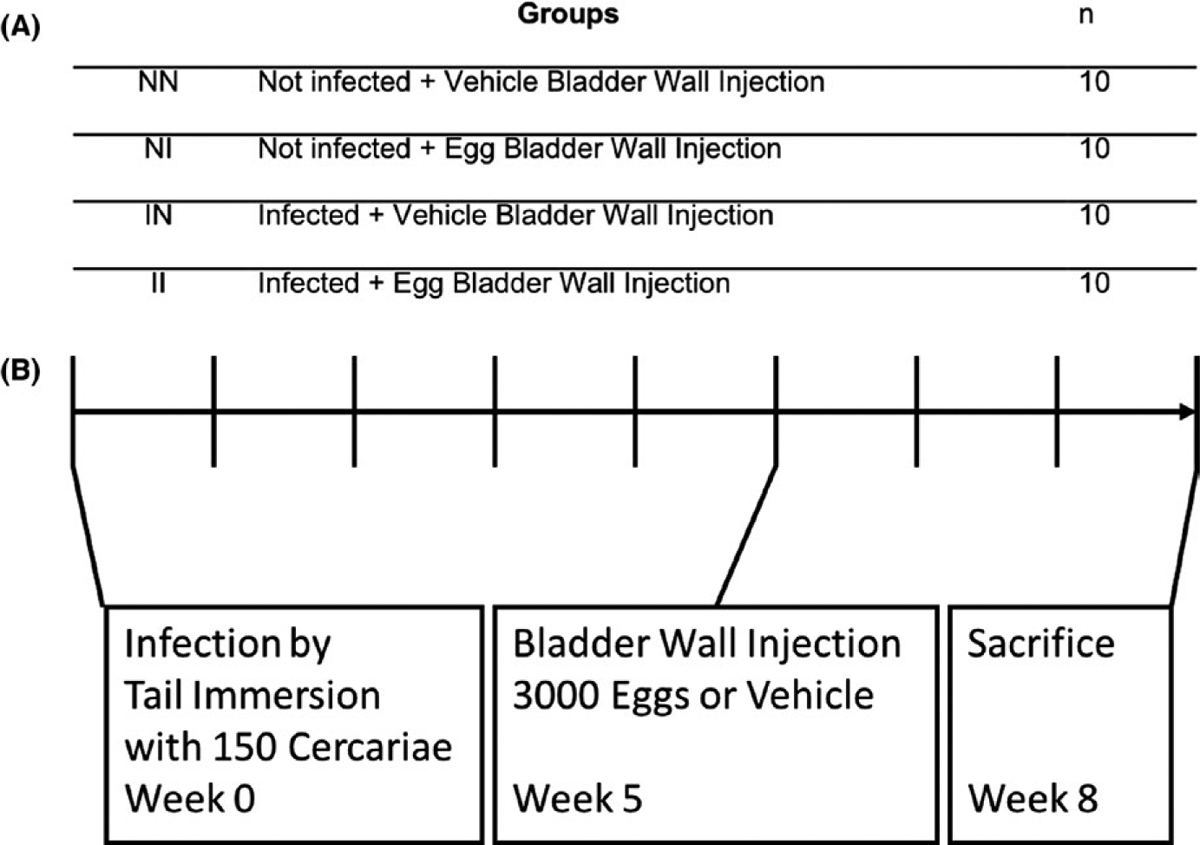
Experimental design. A, Mice were divided into four groups and were exposed or not exposed to Schistosoma haematobium cercariae. Their bladder walls injected with S haematobium eggs or vehicle 5 wk following infection. B, Timeline for mouse exposure to cercaria and bladder wall injections
2.4 |. Cytokine analysis
Bladders were excised and suspended in Tissue Extraction Reagent I (Invitrogen, Camarillo, CA) supplemented with protease inhibitor cocktail at a ratio of 10 mL of reagent to 1 g of tissue. Bladder tissues were then homogenized using a bead beater and centrifuged at 10 000 × g for 5 minutes to pellet tissue debris. The supernatants were collected and frozen at −80°C. The first five bladder extract and serum samples for each group were assayed using a mouse 38-plex cytokine kit (Affymetrix, Santa Clara, CA). The second five were assayed using an expanded mouse 39-plex kit (Affymetrix). The two kits were identical except for the addition of a leptin assay in the second kit. Assays were performed in the Human Immune Monitoring Center at Stanford University following manufacturer’s instructions. Analysed proteins are listed in Table 1.
TABLE 1.
Assayed cytokines (Luminex platform)
| Mouse 38-plex |
|---|
| Eotaxin |
| G-CSF |
| GM-CSF |
| GRO-alpha/KC (murine) |
| IFN-α |
| IFN-gamma |
| IL-10 |
| IL-12p70 |
| IL-13 |
| IL-15R |
| IL-15 |
| IL-17A |
| IL-18 |
| IL-1α |
| IL-1β |
| IL-2 |
| IL-22 |
| IL-23 |
| IL-27 |
| IL-28 |
| IL-3 |
| IL-31 |
| IL-4 |
| IL-5 |
| IL-6 |
| IL-9 |
| IP-10 |
| LIF |
| ENA-78/LIX (murine) |
| M-CSF |
| MCP-1 |
| MCP-3 |
| MIP-1α |
| MIP-1β |
| MIP-2 (murine) |
| RANTES |
| TGF-β |
| TNF-α (active) |
| VEGF-A |
2.5 |. Statistical analysis
Groups were compared using unpaired Student’s t test or two-way analysis of variance followed by Bonferroni’s test for multiple comparisons. Data were expressed as mean ± standard deviation. P < 0.05 was considered statistically significant. The figures included report descriptive statistics for visual comparison as well as significant statistics where applicable.
For principal component analysis (PCA), datasets were loaded into R, and the prcomp() function was used to perform PCAs. The first two dimensions were then used to generate PCAs. Charts were created using ggplot2.
3 |. RESULTS
3.1 |. Cercarial infection did not change parasitological parameters
Adult worms were perfused from the hepatic portal system and manually removed from the mesenteric veins to estimate worm burden. Consistent with prior literature, no worms were seen in the pelvic venous system.21 To quantify tissue egg burden, liver and intestine samples were collected from each animal, digested in 4% KOH, washed and resuspended in 1.2% NaCl.18 Egg bladder wall injection did not alter worm burdens in cercaria-exposed mice compared to vehicle bladder wall-injected, cercaria-infected mice (Figure 2), consistent with prior literature.22 No liver egg burden was observed on gross examination.
FIGURE 2.
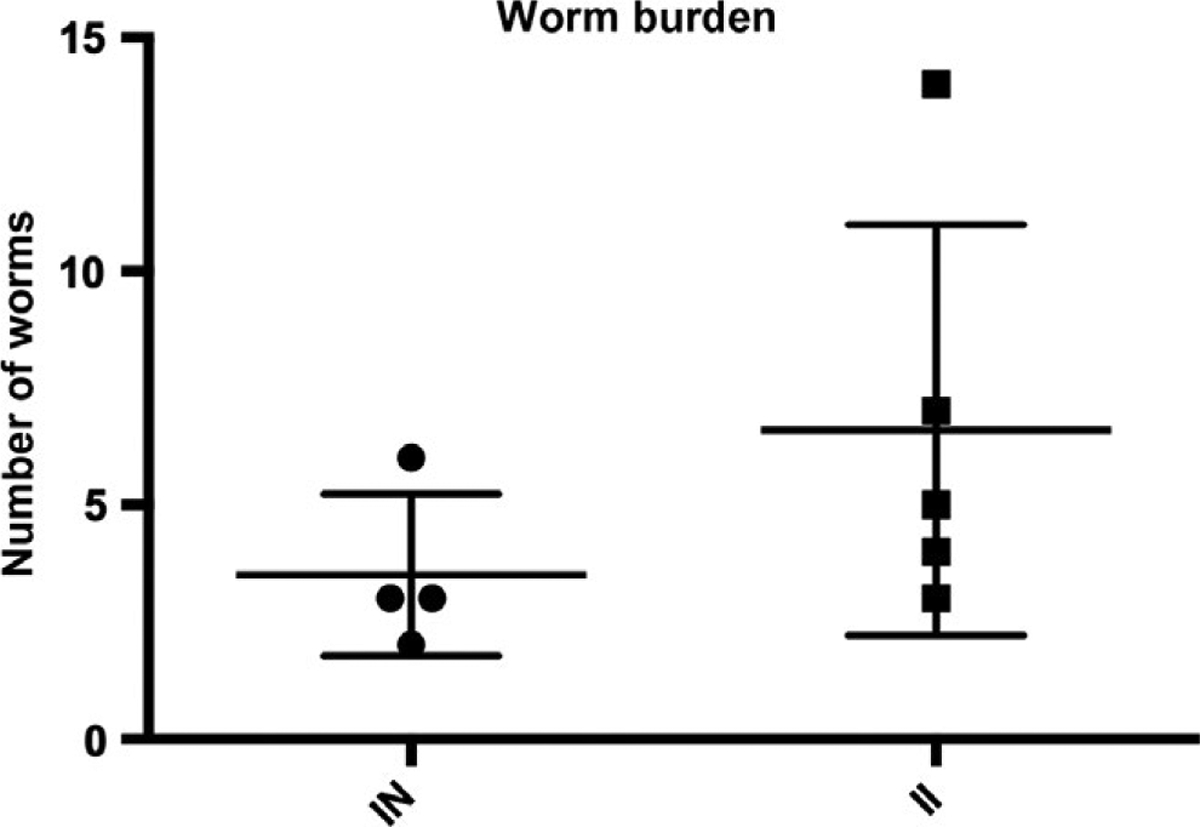
Worm burdens in cercaria-exposed, vehicle bladder wall-injected (“IN”) vs cercaria-exposed, egg bladder wall-injected (“II”) mice. Mice underwent perfusion 8-wk post-cercarial exposure. All worms identified were in the mesenteric veins
Egg deposition in the bladder causes granuloma formation, which would increase bladder wet weight. To determine the effects of prior cercarial exposure, bladder wet weights of the mice were recorded. The bladders of egg-injected mice (“NI” and “II”, Figure 3) were significantly heavier than those of vehicle bladder wall-injected mice (“NN” and “IN” [P < 0.0001]). We observed no significant differences in bladder wet weights in the vehicle bladder wall-injected groups with or without cercarial infection, and no significant differences between egg bladder wall-injected mice with or without cercarial infection, suggesting that granuloma mass was not significantly affected by adult worms (Figure 3).
FIGURE 3.
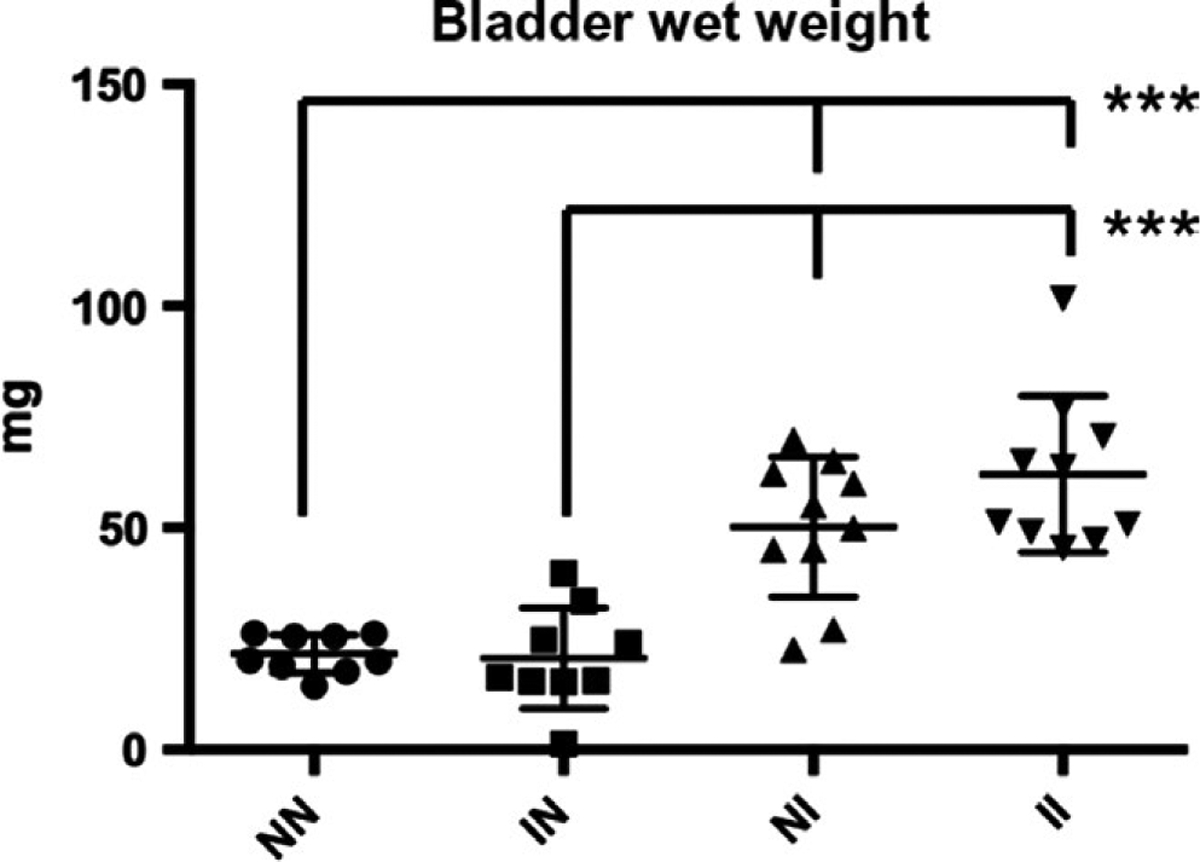
Bladder Wet Weights. No significant differences were observed between bladder wall-injected mice with vs without cercarial exposure. No significant differences were observed between egg-injected mice with vs without cercarial exposure. Mice that received egg injections (NI and II) exhibited significantly greater bladder wet weights compared to mice that received vehicle (NN and IN) bladder wall injections (P < 0.001)***P < 0.005
3.2 |. Cercarial infection combined with egg bladder wall injection induces local and systemic immune responses distinct from bladder wall injection with eggs alone
Analysis of cytokines in bladder tissue homogenates and sera revealed significant differences between egg-injected groups with or without cercarial challenge (Figure 4 and Supporting Information). Cercarial infection combined with egg bladder wall injection resulted in higher bladder leptin levels compared to those of mice that received egg bladder wall injections without cercarial exposure (“II” vs “NI”, Figure 4A). Exposure to cercaria followed by egg bladder wall injection led to increased serum concentrations of IL-1α, IL-13 and TGF-β vs those of mice that underwent egg bladder wall injection without cercarial infection (“II” vs “NI”, Figure 4B). In several cases, the combined model (“II”) produced a significant increase in cytokines and chemokines compared to uninfected, vehicle bladder wall injection-negative controls (“NN”), whereas egg bladder wall injections alone (“NI”) did not. For instance, bladder homogenate levels were increased for eotaxin, IFN-γ, IL-4, IL-13, LIF, M-CSF, RANTES, leptin, IL-1α, IL-13 and TGF-β. In the sera, the combined model (“II”) induced elevated serum.
FIGURE 4.
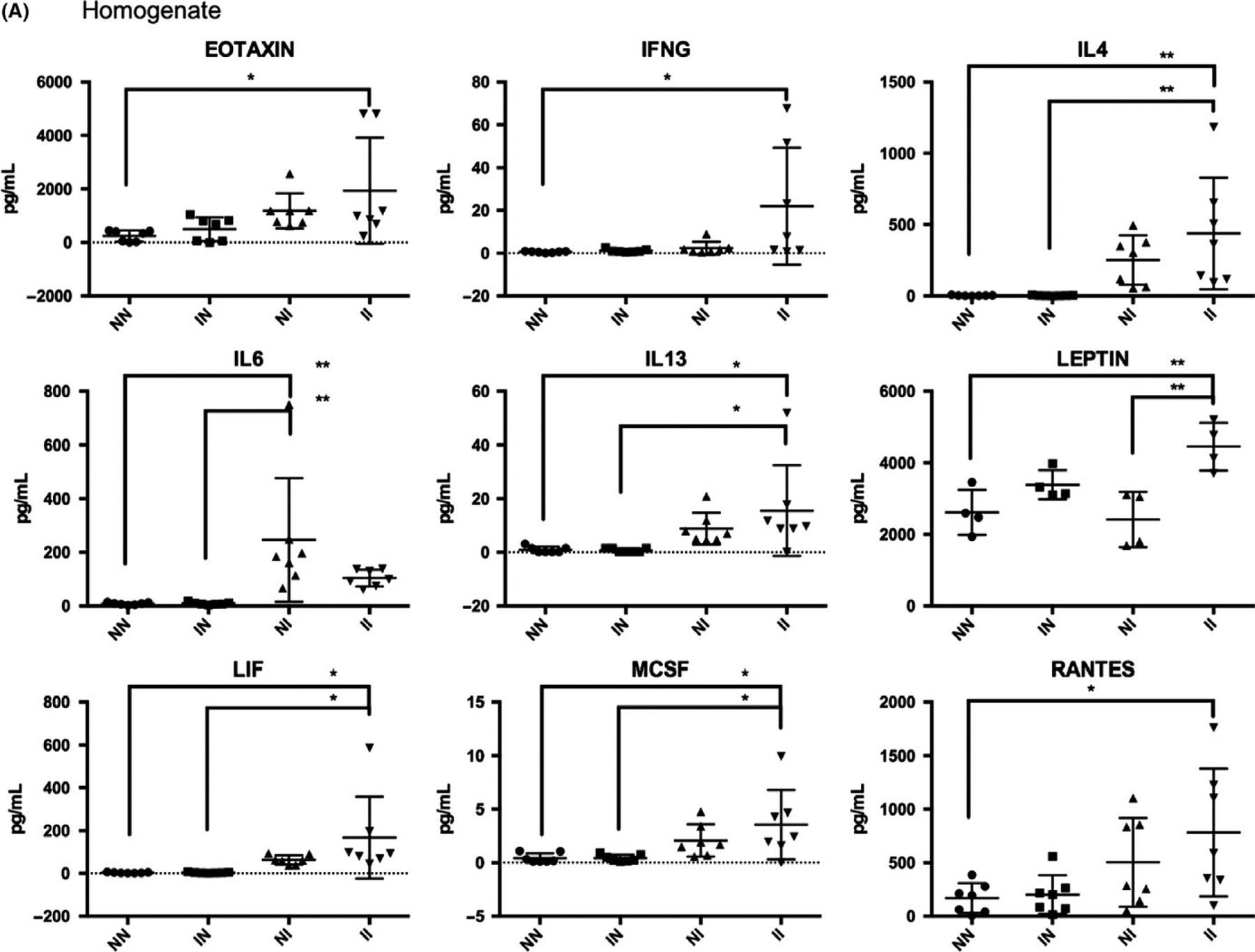
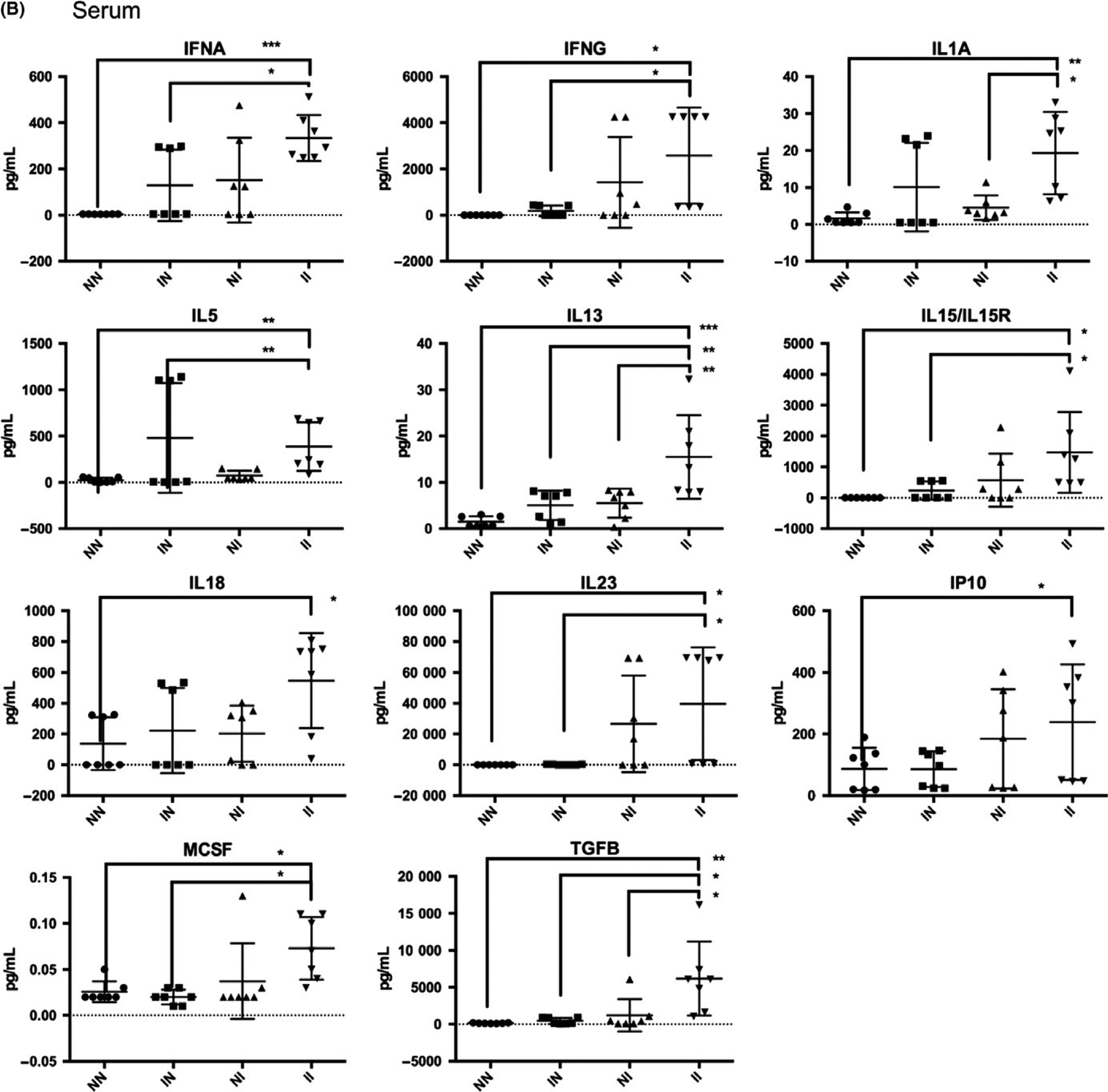
Cytokine analysis of bladder homogenates (A) and sera (B) from mice undergoing vehicle bladder wall injection alone (“NN”), cercarial infection and vehicle bladder wall injection (“IN”), egg bladder wall injection alone (“NI”), and both cercarial infection and egg bladder wall injection (“II”). *P < 0.05, **P < 0.01, ***P < 0.001
IFN-α, IFN-γ, IL1A, IL-5, IL-13, IL-15, IL-18, IL-23, IP10, M-CSF and TGF-β relative to uninfected, vehicle bladder wall injection-negative controls (“NN”), whereas egg bladder wall injections alone (“NI”) did not.
Principal component analysis demonstrated good clustering of sera samples by treatment group (Figure S3). For bladder samples, the majority of II (Infected Egg Injection) mice clustered together. Similarly, most of the NN samples were tightly clustered. Conversely, the NI samples exhibited more heterogeneity. Consistent with observations that serum cytokines exhibited more pronounced responses than bladder cytokines, PCA of bladder samples demonstrated less inter-group variation (Figure S4).
4 |. DISCUSSION
The complex interactions between hosts and schistosomes necessitate the use of live animal models for schistosomal research.23 Although nonhuman primate models have been invaluable to progress in schistosome research, they are associated with prohibitively high housing and care costs and sensitive ethical considerations, especially when large biological replicates are required.23–27 Rodent models have several advantages, including ease of access and low cost, permitting large sample sizes and ease of replication. Past rodent models of urogenital schistosomiasis have failed to reproduce key aspects of the human disease, most notably urinary tract involvement.11,12 Here, we have described a mouse model that bypasses the limitations of previous methods by direct injection of viable eggs following percutaneous cercarial infection.
We were able to reproduce fundamental immunological aspects of human urogenital schistosomiasis, such as a collective type 2 inflammatory response (IL-4 [bladder], IL-5 [sera], IL-13 [bladder and sera]) in the bladders and sera of egg-injected, cercaria-exposed animals compared to vehicle injected, cercaria unexposed animals (“II” vs “NN”, Figure 4 and Supporting Information). The level of eotaxin, an eosinophil chemotactic protein, was significantly increased in bladder tissue homogenate of the combined model (“II”) compared to the vehicle injected, cercaria unexposed animals (“NN”). This finding is consistent with reports that eosinophiluria is a hallmark of human urogenital schistosomiasis.28 Another interesting observation was the increased level of Th1 (IFN-γ [bladder and sera], IL-1α [bladder], and IL-18 and IP-10 [sera]) and serum Th17 cytokines (IL-23) (Figure 4). Th0 and Th1 responses were not observed in the sera of mice receiving only egg bladder wall injections (“NI”), which may be attributed to the lack of exposure to cercariae, schistosomula and/or adult worms, thus highlighting an important advantage to the combined model for understanding systemic immune responses to S haematobium.29,30 Several key differences in serum cytokine levels were detected when comparing egg-injected mice with or without cercarial exposure (“NI” vs “II”, Figure 4B): the proinflammatory cytokine IL-1α, the type 2 cytokine IL-13 and the regulatory/pro-fibrotic cytokine TGF-β. This suggests that cercarial exposure triggers additional systemic immune responses not induced by bladder eggs alone. In contrast, the only bladder cytokine showing significantly different levels in egg bladder wall-injected, cercarial unexposed (“NI”) vs egg bladder wall-injected, cercarial exposed (“II”) mice was leptin. This cytokine has been implicated in metabolism and wasting and has been noted to be inversely correlated with antibodies against S haematobium in infected boys.31 In addition, leptin downmodulates the proliferation of Foxp3+CD4+CD25+ Treg cells32 and induces the release of IFN-γ and TNF-α, shifting the immune response towards a Th1 response.33 Although the immunomodulatory role of leptin in urogenital schistosomiasis has not been fully explored, parasite-induced leptin manipulation has been implicated in host behavioural changes that enhance helminth transmission.34
Though a range of cytokines were reviewed, not every cytokine and chemokine were surveyed. To address this limitation, RNA-Seq-based studies of the bladder are planned to explore the immune response to schistosome eggs with more nuance. In addition, the study described herein discerns solely cytokines and chemokines as metrics for local and systemic immune responses; however, based on the lack of changes in key cytokines, we opted not to characterize circulating or infiltrating leucocyte phenotypes via FACS analysis.
In conclusion, we have found that bladder wall injection with S haematobium eggs alone may overall be sufficient to study immune responses localized to the bladder. However, our findings suggest that cercarial exposure should be added to our egg bladder wall injection model to better understand systemic immune responses induced by S haematobium cercariae, which precede egg deposition in the parasite life cycle. This study only examined the effects of a single cercarial infection, and so we acknowledge that a trickle cercarial infection may result in different and interesting findings worthy of further study. Furthermore, the biological significance of leptin in urogenital schistosomiasis remains to be determined and should continue to be researched. Future work could include RNA-Seq analysis of the serum and bladder at different time points post-infection to tease out the subtle contributions of cercaria and eggs to urogenital schistosomiasis on the host transcriptional level. Our findings suggest that cercarial exposure should be added to our egg bladder wall injection model to better understand systemic immune responses induced by S haematobium.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Stirewalt Endowed Directorship (MHH), Biomedical Research Institute (www.afbr-bri.org), R01DK113504 (MHH) and R56AI119168 (MHH). We thank the team at the Stanford Human Immune Monitoring Core for processing Luminex samples.
Funding information
National Institute of Allergy and Infectious Diseases, Grant/Award Number: AI119168; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: DK113504; Margaret A. Stirewalt Endowment; Biomedical Research Institute
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Van Der Werf MJ, De Vlas SJ, Brooker S, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. [DOI] [PubMed] [Google Scholar]
- 2.Chabasse D, Bertrand G, Leroux JP, Gauthey N, Hocquet P. Developmental bilharziasis caused by Schistosoma mansoni discovered 37 years after infestation. Bull Soc Pathol Exot Filiales. 1985;78(5):643–647. [PubMed] [Google Scholar]
- 3.Wilkins HA, Goll P, Marshall TFDC, Moore P. The significance of proteinuria and haematuria in Schistosoma haematobium infection. Trans R Soc Trop Med Hyg. 1979;73(1):74–80. [DOI] [PubMed] [Google Scholar]
- 4.von Lichtenberg F. Schistosomiasis as a worldwide problem: pathology. J Toxicol Environ Health. 1975;1(2):175–184. [DOI] [PubMed] [Google Scholar]
- 5.Mayer DA, Fried B. The role of helminth infections in carcinogenesis. Adv Parasitol. 2007;65:239–296. [DOI] [PubMed] [Google Scholar]
- 6.Downs JA, Mguta C, Kaatano GM, et al. Urogenital schistosomiasis in women of reproductive age in Tanzania’s Lake Victoria region. Am J Trop Med Hyg. 2011;84(3):364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leutscher PDC, Ramarokoto C-E, Hoffmann S, et al. Coexistence of urogenital schistosomiasis and sexually transmitted infection in women and men living in an area where Schistosoma haematobium is endemic. Clin Infect Dis. 2008;47(6):775–782. [DOI] [PubMed] [Google Scholar]
- 8.Kjetland EF, Ndhlovu PD, Gomo E, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20(4):593–600. [DOI] [PubMed] [Google Scholar]
- 9.Rollinson D. A wake up call for urinary schistosomiasis: reconciling research effort with public health importance. Parasitology. 2009;136:1593–1610. [DOI] [PubMed] [Google Scholar]
- 10.Jordan P, Von Lichtenberg F, Goatly KD. Experimental schistosomiasis in primates in Tanzania. Preliminary observations on the susceptibility of the baboon Papio anubis to Schistosoma haematobium and Schistosoma mansoni. Bull World Health Organ. 1967;37(3):393–403. [PMC free article] [PubMed] [Google Scholar]
- 11.Loker ES. A comparative study of the life-histories of mammalian schistosomes. Parasitology. 1983;87(2):343–369. [DOI] [PubMed] [Google Scholar]
- 12.Rheinberg CE, Moné H, Caffrey CR, Imbert-Establet D, Jourdane J, Ruppel A. Schistosoma haematobium, S. intercalatum, S. japonicum, S. mansoni and S. rodhaini in mice: relationship between patterns of lung migration by schistosomula and perfusion recovery of adult worms. Parasitol Res. 1998;84(4):338–342. [DOI] [PubMed] [Google Scholar]
- 13.Honeycutt J, Hammam O, Hsieh MH. Schistosoma haematobium egg-induced bladder urothelial abnormalities dependent on p53 are modulated by host sex. Exp Parasitol. 2015;158:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu CL, Odegaard JI, Herbert DR, Hsieh MH. A novel mouse model of Schistosoma haematobium egg-induced immunopathology. PLoS Pathog. 2012;8(3):e1002605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu CL, Apelo CA, Torres B, Thai KH, Hsieh MH. Mouse bladder wall injection. J Vis Exp. 2011;53:e2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheever AW. Differential regulation of granuloma size and hepatic fibrosis in schistosome infections. Mem Inst Oswaldo Cruz. 1997;92(5):689–692. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs W, Bogers J, Deelder A, Wéry M, Van Marck E. Adult Schistosoma mansoni worms positively modulate soluble egg antigen-induced inflammatory hepatic granuloma formation in vivo. Stereological analysis and immunophenotyping of extracellular matrix proteins, adhesion molecules, and chemokines. Am J Pathol. 1997;150(6):2033–2045. [PMC free article] [PubMed] [Google Scholar]
- 18.Tucker MS, Karunaratne LB, Lewis FA, Freitas TC, Liang YS. Schistosomiasis. Curr Protoc Immunol. 2013;103:1–58. [DOI] [PubMed] [Google Scholar]
- 19.Botros SS, Hammam OA, El-Lakkany NM, El-Din SHS, Ebeid FA. Schistosoma haematobium (Egyptian strain): rate of development and effect of praziquantel treatment. J Parasitol. 2008;94(2): 386–394. [DOI] [PubMed] [Google Scholar]
- 20.Ray D, Nelson TA, Fu CL, et al. Transcriptional profiling of the bladder in urogenital schistosomiasis reveals pathways of inflammatory fibrosis and urothelial compromise. PLoS Negl Trop Dis. 2012;6:e1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vuong PN, Bayssade-dufour C, Albaret L, Farhati K. Histopathological observations in new and classic models of experimental Schistosoma haematobiurn infections. Trop Med Int Health. 1996;1(3):348–358. [DOI] [PubMed] [Google Scholar]
- 22.Farag HF, Awadalla HN, Girgis RS, Michael AI. Schistosoma haematobium infection in the white mouse–histopathological and histo-chemical studies. Angew Parasitol. 1980;21(1):20–26. [PubMed] [Google Scholar]
- 23.Farah IO, Kariuki TM, King CL, Hau J. An overview of animal models in experimental schistosomiasis and refinements in the use of non-human primates. Lab Anim. 2001;35:205–212. [DOI] [PubMed] [Google Scholar]
- 24.Fenwick A. Baboons as reservoir hosts of Schistosoma mansoni. Trans R Soc Trop Med Hyg. 1969;63(5):557–567. [DOI] [PubMed] [Google Scholar]
- 25.Farah IO, Nyindo M, Suleman MA, et al. Schistosoma mansoni: development and modulation of the granuloma after single or multiple exposures in the baboon (Papio cynocephalus anubis). Exp Parasitol. 1997;86(2):93–101. [DOI] [PubMed] [Google Scholar]
- 26.Mola PW, Farah IO, Kariuki TM, Nyindo M, Blanton RE, King CL. Cytokine control of the granulomatous response in Schistosoma mansoni- infected baboons: role of exposure and treatment. Infect Immun. 1999;67(12):6565–6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips KA, Bales KL, Capitanio JP, et al. Why primate models matter. Am J Primatol. 2014;76:801–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eltoum IA, Ghalib HW, Sualaiman S, Kordofani A, Mustafa MD, Homeida M. Significance of eosinophiluria in urinary schistosomiasis. A study using Hansel’s stain and electron microscopy. Am J Clin Pathol. 1989;92(3):329–338. [DOI] [PubMed] [Google Scholar]
- 29.Chikunguwo SM, Quinn JJ, Harn DA, Stadecker MJ. The cell-mediated response to schistosomal antigens at the clonal level. III. Identification of soluble egg antigens recognized by cloned specific granulomagenic murine CD4+ Th1-type lymphocytes. J Immunol. 1993;150(4):1413–1421. [PubMed] [Google Scholar]
- 30.Vella AT, Pearce EJ. CD4+ Th2 response induced by Schistosoma mansoni eggs develops rapidly, through an early, transient, Th0-like stage. J Immunol. 1992;148(7):2283–2290. [PubMed] [Google Scholar]
- 31.Hermann E, Gaayeb L, Sow PS, et al. Sex-dependent interactions between leptin, wasting and humoral immunity in two ethnic communities of school-aged children differentially exposed to Schistosoma haematobium. Trans R Soc Trop Med Hyg. 2017;111(10):448–456. [DOI] [PubMed] [Google Scholar]
- 32.De Rosa V, Procaccini C, Calì G, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. [DOI] [PubMed] [Google Scholar]
- 33.Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394: 897–901. [DOI] [PubMed] [Google Scholar]
- 34.Lõhmus M, Moalem S, Björklund M. Leptin, a tool of parasites? Biol Lett. 2012;8:849–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


