Abstract
Background
An unexpected excess in weight gain has recently been reported in the course of dolutegravir (DTG) treatment. The aim of the present study was to investigate whether weight gain differs among different DTG-containing regimens.
Methods
Adult naïve and experienced people with HIV (PWH) initiating DTG-based antiretroviral therapy (ART) between July 2014 and December 2019 in the Surveillance Cohort Long-Term Toxicity Antiretrovirals (SCOLTA) prospective cohort were included. We used an adjusted general linear model to compare weight change among backbone groups and a Cox proportional hazard regression model to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for weight increases >10% from baseline.
Results
A total of 713 participants, 25.3% women and 91% Caucasian, were included. Of these, 195 (27.4%) started DTG as their first ART regimen, whereas 518 (72.6%) were ART-experienced. DTG was associated with abacavir/lamivudine in 326 participants, tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) in 148, boosted protease inhibitors in 60, rilpivirine in 45, lamivudine in 75, and tenofovir alafenamide (TAF)/FTC in 59. At 6 and 12 months, weight gain was highest among PWH on TDF/FTC+DTG and TAF/FTC+DTG. Baseline CD4 <200 cells/mm3 (HR, 1.84; 95% CI, 1.15 to 2.96), being ART-naïve (HR, 2.24; 95% CI, 1.24 to 4.18), and treatment with TDF/FTC+DTG (HR, 1.92; 95% CI, 1.23 to 2.98) or TAF/FTC+DTG (HR, 3.80; 95% CI, 1.75 to 8.23) were associated with weight gain >10% from baseline. Higher weight (HR, 0.97 by 1 kg; 95% CI, 0.96 to 0.99) and female gender (HR, 0.54; 95% CI, 0.33 to 0.88) were protective against weight gain.
Conclusions
Naïve PWH with lower CD4 counts and those on TAF/FTC or TDF/FTC backbones were at higher risk of weight increase in the course of DTG-based ART.
Keywords: dolutegravir, HIV metabolic complications, TAF, TDF, weight gain
The antiretroviral agents currently recommended for the treatment of HIV are all characterized by high efficacy and safety [1–3]. In particular, integrase inhibitors (INSTIs) constitute the preferred anchor drugs in modern first-line antiretroviral therapy (ART), thanks to their demonstrated durable virologic efficacy [4], and are characterized by a low rate of adverse events [5] and a favorable metabolic profile in lipids compared with protease inhibitors (PIs) and efavirenz [6–8]. INSTI, together with rilpivirine and doravirine, is the drug recommended for treating people with HIV (PWH) with high cardiovascular risk [4]. However, a new side effect has emerged in recent years, affecting the class of INSTIs in general and dolutegrvir (DTG) in particular, namely an unexpected excess in weight gain during the course of treatment [9–12]. The impact of this effect on metabolic and cardiovascular outcomes is still unclear at this time [11], sparking a heated debate between those who attribute weight gain to a return-to-health phenomenon and those who interpret it as an undesired side effect that could influence cardiovascular risk. Although the mechanisms underlying weight gain still remain unclear, the main characteristics of PWH gaining weight have been previously reported to be lower CD4 T-cell count [13–16], higher HIV-RNA [13, 15], low baseline body mass index (BMI) [14], female sex [13, 15, 16] and older age [13, 14]. Regarding the association between weight gain and specific drugs, many studies have highlighted a role of DTG that is more evident in ART-naïve PWH [11, 13, 15] and less consistent in ART-experienced PWH [14, 17, 18]. However, DTG is usually administered in combination regimens that include other antiretrovirals, whose role in weight gain has been poorly studied and is not well understood. Backbones such as abacavir/lamivudine (ABC/3TC) and tenofovir/emtricitabine (TDF/FTC) seem to have a neutral effect on weight, BMI, and lean body mass changes according to 1 previous study [19], but, on the other hand, a significant role of tenofovir alafenamide (TAF) over TDF has been reported for weight increase [13, 15, 20]. The aim of the present study was to describe the weight changes in both naïve and experienced PWH, all treated with DTG, in a large prospective cohort and explore the role of the different antiretrovirals used in combination with DTG in weight gain. Secondary aims were clarifying if weight gain is associated with changes in lipids and fasting glucose and describing incident obesity and metabolic syndrome among PWH receiving DTG-based therapies.
METHODS
We analyzed data from the Surveillance Cohort Long-Term Toxicity Antiretrovirals (SCOLTA) prospective database. The SCOLTA project is a multicenter observational study that started in 2002 and that prospectively follows PWH who start to take new antiretroviral drugs, with the aim of identifying toxicities and adverse events in a real-life setting [21]. Both ART-naïve and -experienced PWH can be included in SCOLTA if they are aged >18 years and agree to enter the study. Clinical data collected include sex, age, ethnicity, weight, height, and history of previous ART. Laboratory data include HIV-RNA, CD4 T-cell count, CD4/CD8 ratio, total cholesterol (TC), high-density lipoprotein cholesterol (HDL), triglycerides (TGs), and fasting glucose and are prospectively collected in anonymous form in a central database every 6 months.
We performed a query to this prospectively collected database including participants on DTG enrolled from July 2014 until December 2019 (date of last extraction) for whom weight was registered at baseline (time of initiating DTG) and who had at least 1 follow-up. Female PWH who became pregnant during the study period were excluded. Moreover, we did not consider ART combinations that were used in ≤10 cases. Comparisons of patient demographics and baseline characteristics among different groups of backbones associated with DTG were performed using the chi-square test, analysis of variance, and the Mann-Whitney U test. Weight change from baseline was assessed using a paired t test in the univariate analysis at 6, 12, 18, and 24 months of follow-up. Overall weight change across follow-up visits (from baseline to month 24) was analyzed using a mixed model for repeated measures. We compared weight change among backbone groups, including potential confounders (differences between treatment groups or associated with baseline weight). Active hepatitis C virus (HCV) infection and statin use were updated over time. If weight was not measured at a follow-up visit, we imputed the missing value as the mean of the previous and the following visits.
Moreover, with the aim of identifying the factors associated with clinically significant weight gain, we defined as weight gainers (WGs) as those participants whose weight increased by at least 10% from baseline [15]. The associations among ART regimens, participant characteristics, and being WGs were evaluated with hazard ratios (HRs) and 95% confidence intervals (CIs) using a Cox proportional hazard regression model; time was calculated as days between starting a DTG-including regimen and the visit where the ≥10% increase was measured. Variables included in the model were age, sex, baseline weight, risk factor for HIV acquisition, baseline CD4 and Centers for Disease Control and Prevention (CDC) stage, naïve status, ART duration (set at 0 for naïve participants), statin use, HCV eradication during the first year of study, and type of DTG-including regimen.
The impact of weight gain on lipids and glucose metabolism was explored by comparing TC, HDL, TC/HDL ratio, TG, and fasting glucose changes at 6 and 12 months between participants whose weight increased by at least 10% in the first year and those whose weight did not, using a general linear model including potential confounders. For this analysis, participants with weight gain ranging from >1% to <10% in the first year of observation were excluded. Participants whose weight increased ≤1% or decreased were defined as nongainers (NGs). We also evaluated the frequency of incident obesity and metabolic syndrome. Obesity was defined by a body mass index (BMI) >30 kg/m2, while metabolic syndrome was defined by the presence of central obesity (assumed in PWH with BMI >30 kg/m2) and any 2 of the following factors: (1) TG ≥150 mg/dL or treatment for hypertriglyceridemia; (2) HDL <40 mg/dL for males or <50 mg/dL for females or specific treatment for this lipid abnormality; (3) raised blood pressure, with systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or treatment for previously diagnosed hypertension; (4) fasting glucose ≥100 mg/dL or diagnosis of type 2 diabetes [22, 23]. The study protocol of the SCOLTA group was approved by local ethical committees and conducted in accordance with the ethical principles stated in the Declaration of Helsinki. Written consent was obtained from all participants.
RESULTS
At the time of this analysis (December 2019), 987 PWH were enrolled in the SCOLTA cohort and on treatment with a DTG-containing regimen. Seven hundred sixty-six met the selection criteria for this analysis: weight available at baseline and at 6-month follow-up. Among these, 53 received a regimen used in <10 cases and were excluded.
Characteristics of the Study Population
A total of 713 participants (mean age [SD, range], 47.2 [11.6, 19–81] years) were included in the present analysis. Women represented 25.3% of the sample (n = 180, of whom 2 were transgender women), and 648 participants were Caucasian (90.9%); 22.3% were in CDC stage C (n = 159). One hundred ninety-five (27.4%) started DTG as their first ART regimen, whereas the remaining 518 (72.6%) were ART-experienced PWH who switched to DTG after a median (interquartile range [IQR]) of 9.8 (4.3–17.4) years of previous ART. DTG was associated with ABC/3TC in 326 participants (45.7%), TDF/FTC in 148 (20.8%), atazanavir or darunavir (boosted with ritonavir or cobicistat) in 60 (8.4%), rilpivirine in 45 (6.3%), 3TC in 75 (10.5%), and TAF/FTC in 59 (8.3%). Mean baseline weight (SD) was 69.2 (12.8) kg in naïve and 71.0 (13.6) kg in experienced participants. Further characterization of the study population is given in Table 1. Participants in different groups of treatment were different in terms of age, risk factor for HIV acquisition, naïve status, initial CD4 T-cell count and CD4/CD8 ratio, and active HCV infection. Among ART-experienced participants, a difference also existed in terms of detectable HIV-RNA at baseline and years of ART before DTG treatment. Among the participants, 535, 407, and 332 reached 12-, 18-, and 24-month follow-up. The median observation time (IQR) was 28 (11–39) months.
Table 1.
Characteristics of 713 Participants in the SCOLTA Dolutegravir Cohort, According to Antiretroviral Regimen
| Total | 3TC+DTG | RPV+DTG | PI+DTG | 3TC/ABC/DTG | TDF/FTC+DTG | TAF/FTC+DTG | ||
|---|---|---|---|---|---|---|---|---|
| n = 713 | n = 75 | n = 45 | n = 60 | n = 326 | n = 148 | n = 59 | P | |
| Sex M, No. (%) | 533 (74.7) | 59 (78.7) | 26 (57.8) | 45 (75.0) | 244 (74.8) | 111 (75.0) | 48 (81.4) | .12 |
| Age, mean ± SD, y | 47.2 ± 11.6 | 50.6 ± 11.1 | 51.8 ± 10.8 | 50.4 ± 11.3 | 46.9 ± 11.5 | 45.0 ± 11.2 | 43.8 ± 12.4 | <.0001 |
| Caucasian, No. (%) | 648 (90.9) | 69 (92.0) | 43 (95.6) | 55 (91.7) | 294 (90.2) | 139 (93.9) | 48 (81.4) | .09 |
| Weight, mean ± SD, kg | 70.5 ± 13.4 | 73.3 ± 13.9 | 70.2 ± 13.3 | 70.4 ± 11.5 | 69.8 ± 13.1 | 70.6 ± 14.6 | 71.4 ± 13.0 | .46 |
| BMI (n = 608), mean ± SD, kg/m2 | 24.2 ± 4.0 | 24.5 ± 3.5 | 25.0 ± 3.8 | 24.2 ± 3.5 | 24.0 ± 4.0 | 24.0 ± 4.2 | 24.7 ± 4.3 | .61 |
| Risk factor for HIV acquisition, No. (%) | ||||||||
| Sexual | 513 (72.0) | 59 (78.7) | 33 (73.3) | 39 (65.0) | 245 (75.2) | 85 (57.4) | 52 (88.1) | |
| IVDU | 112 (15.7) | 8 (10.7) | 11 (24.2) | 14 (23.3) | 42 (12.8) | 32 (21.6) | 5 (8.5) | <.0001 |
| Other | 88 (12.3) | 8 (10.7) | 1 (2.2) | 7 (11.7) | 39 (12.0) | 31 (21.0) | 2 (3.4) | |
| CD4, mean ± SD, cells/mL | 570 ± 367 | 725 ± 422 | 699 ± 318 | 614 ± 317 | 617 ± 359 | 419 ± 330 | 364 ± 312 | <.0001 |
| CD4/CD8 (n = 616), median (IQR) | 0.64 (0.34–0.99) | 0.90 (0.57–1.23) | 0.92 (0.62–1.14) | 0.67 (0.50–0.97) | 0.68 (0.41–1.03) | 0.44 (0.17–0.74) | 0.38 (0.11–0.67) | <.0001 |
| Naïve, No. (%) | 195 (27.4) | 5 (6.7) | 1 (2.2) | 1 (1.7) | 75 (23.0) | 68 (45.9) | 45 (76.3) | <.0001 |
| CDC stage C, No. (%) | 159 (22.3) | 10 (13.3) | 9 (20.0) | 20 (33.3) | 69 (21.2) | 38 (25.7) | 13 (22.0) | .07 |
| HIV-RNA >50 copies/mL (experienced participants), No. (%) | 95 (18.3) | 6 (8.6) | 1 (2.3) | 22 (37.3) | 39 (15.5) | 24 (30.0) | 3 (21.4) | <.0001 |
| Years of ART (experienced participants), median (IQR) | 9.8 (4.3–17.4) | 10.8 (7.5–17.6) | 12.1 (6.7–18.3) | 13.5 (6.8–19.2) | 7.9 (4.0–15.5) | 9.2 (3.2–17.3) | 9.1 (4.5–19.0) | .004 |
| HCV active infection (baseline), No. (%) | 66 (9.3) | 2 (2.7) | 2 (4.4) | 7 (11.7) | 27 (8.3) | 25 (16.9) | 3 (5.1) | .004 |
| HCV eradication during follow-up (n = 66), No. (%) | 30 (45.4) | 0 (0) | 1 (50.0) | 3 (42.9) | 15 (55.6) | 10 (40.0) | 1 (33.3) | .66 |
| Statin use at baseline, No. (%) | 65 (9.1) | 11 (14.7) | 7 (15.6) | 9 (15.0) | 26 (8.0) | 9 (6.1) | 3 (5.1) | .05 |
| Statin started during the first year of observation (n = 648), No. (%) | 17 (2.6) | 2 (3.1) | 3 (7.9) | 2 (3.9) | 6 (2.0) | 3 (2.2) | 1 (1.8) | .38 |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; BMI, body mass index; CDC, Centers for Disease Control and Prevention; DTG, dolutegravir; FTC, emtricitabine; HCV, hepatitis C virus; IQR, interquartile range; IVDU, intravenous drug use; RPV, rilpivirine; PI, protease inhibitor (atazanavir, darunavir); SCOLTA, Surveillance Cohort Long-Term Toxicity Antiretrovirals; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Weight Gain in Different ART Regimens
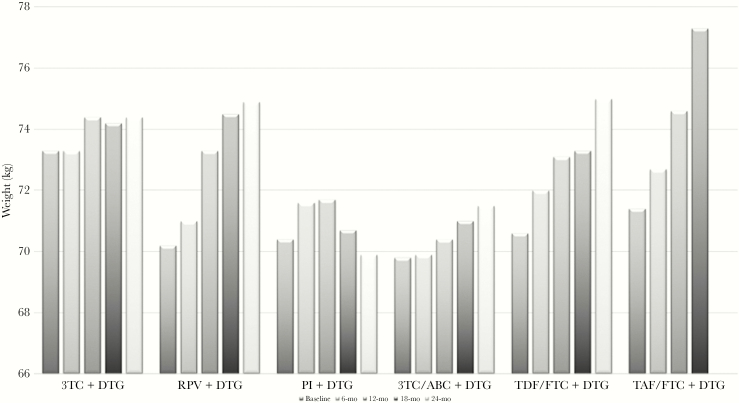
At baseline, women, participants in CDC stage C, and those with active HCV infection had significantly lower weight, whereas participants on statins were heavier than those not on statins. These findings were consistent in naïve and experienced participants, even if in some cases the probability for these comparisons was higher than the value set for significance (<.05) because of the loss of power due to subgroup analysis. The mean weights of the participants in different DTG-based regimens at baseline and during follow-up are shown in Figure 1. At 6 and 12 months, the mean weight change from baseline (± SE) was +0.6 ± 0.1 kg (P = .0005) and +1.2 ± 0.2 kg (P < .0001), respectively, in the whole study population; the same figures were +1.2 ± 0.3 kg (P < .0001) and +2.4 ± 0.4 kg (P < .0001) in naïve and +0.4 ± 0.2 kg (P = .02) and +0.9 ± 0.2 kg (P < .0001) in experienced participants. Weight over time was analyzed including age, sex, risk factor for HIV acquisition, CD4 at baseline, naïve status, CDC stage C, updated statin use, and DTG-including ART. We found that weight significantly increased and that naïve status (P = .002) and type of ART regimen (P = .033) affected weight gain over time. Table 2 shows the mean change from baseline by ART regimen: at 6-month follow-up, weight increased significantly in participants on RPV+DTG, PI+DTG, TDF/FTC+DTG, and TAF/FTC+DTG. After adjusting for age, sex, baseline weight and CD4, naïve status, CDC stage, statin use (use at enrollment and 1-year updated information), and active HCV infection (at enrollment and 1-year updated information), weight increase was confirmed to be significant at 6-month follow-up; further increases at 12-month follow-up were only significant for TDF/FTC+DTG and TAF/FTC+DTG. In the same model, using 3TC/ABC+DTG as the reference, weight change was significantly higher in the TDF/FTC+DTG (P = .004) and PI+DTG groups (P = .02) at 6-month follow-up. However, in subsequent follow-up, no difference was observed.
Figure 1.
Weight by regimen. Mean weight (kg) at baseline and 6-month, 12-month, 18-month, and 24-month follow-up in people treated with different dolutegravir-containing regimens. Dolutegravir was associated with abacavir/lamivudine in 326 participants, tenofovir disoproxil fumarate/emtricitabine in 148, boosted protease inhibitors in 60, rilpivirine in 45, lamivudine in 75, and tenofovir alafenamide/emtricitabine in 59. Abbreviations: 3TC, lamivudine; ABC, abacavir; DTG, dolutegravir; FTC, emtricitabine; PI, protease inhibitor; RPV, rilpivirine; TDF, tenofovir disoproxil fumarate; TAF, tenofovir alafenamide.
Table 2.
Weight Change According to DTG-Containing Regimen
| Weight | 3TC + DTG | RPV + DTG | PI + DTG | 3TC/ABC/DTG | TDF/FTC + DTG | TAF/FTC + DTG | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Mean | No. | Mean | No. | Mean | No. | Mean | No. | Mean | No. | Mean | |
| T1-T0 | 75 | –0.1 ± 0.4 | 45 | 0.8 ± 0.3a | 60 | 1.2 ± 0.3a | 326 | 0.1 ± 0.2 | 148 | 1.5 ± 0.3a | 59 | 1.3 ± 0.5a |
| T2-T1 | 44 | 1.0 ± 0.4a | 34 | 0.6 ± 0.6 | 51 | 0.1 ± 0.3 | 250 | 0.1 ± 0.2 | 137 | 1.0 ± 0.4a | 14 | 1.6 ± 0.4a |
| T3-T2 | 28 | –0.7 ± 0.2a | 30 | 0.4 ± 0.3 | 40 | –0.2 ± 0.3 | 191 | –0.3 ± 0.2 | 115 | 0.3 ± 0.5 | 3 | –1.0 ± 0.6 |
| T4-T3 | 24 | –0.3 ± 1.0 | 27 | –0.2 ± 0.4 | 36 | 0.3 ± 0.5 | 150 | 0.5 ± 0.3 | 92 | 0.5 ± 0.3 | 1 | – |
T0 = baseline; T1 = 6 months; T2 = 12 months; T3 = 18 months; T4 = 24 months.
Abbreviations: 3TC, lamivudine; ABC, abacavir; DTG, dolutegravir; FTC, emtricitabine; RPV, rilpivirine; PI, protease inhibitor (atazanavir, darunavir); TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
a P < .05 for change from baseline (T1-T0) or previous follow-up visit (T2-T1, T3-T2, T4-T3).
Factors Associated With Weight Gain
According to our definition, 75 participants (10.5%) became WGs during the whole observation period. In multivariate Cox regression (Table 3), higher baseline weight and female sex represented factors that were protective against becoming a WG. Baseline CD4 <200 cells/mm3 and being ART-naïve were associated with higher weight change from baseline. Eradication of HCV infection was suggested as a risk factor, although of borderline significance. With regard to regimens, we used the most used regimen, 3TC/ABC+DTG, as the reference category: HRs were higher for all the remaining regimens, but only significantly so for TDF/FTC+DTG and TAF/FTC+DTG (Table 3). The analysis of factors associated with weight gain in patients who were ART-naïve or -experienced is available in the Supplementary Data.
Table 3.
Cox Regression Analysis for Weight Gain >10%
| Hazard Ratio | 95% CI | P | ||
|---|---|---|---|---|
| Baseline age (by 5 y) | 0.97 | 0.89 | 1.06 | .48 |
| Gender F vs M | ||||
| M | 1.00 | |||
| F | 0.54 | 0.33 | 0.88 | .01 |
| Baseline weight (by 1 kg) | 0.97 | 0.96 | 0.99 | .002 |
| Risk factor for HIV acquisition | ||||
| Sexual | 1.00 | |||
| IVDU | 1.23 | 0.68 | 2.24 | .49 |
| Other | 0.94 | 0.56 | 1.61 | .84 |
| Baseline CD4, cells/mm3 | ||||
| >500 | 1.00 | |||
| 200–500 | 0.98 | 0.64 | 1.52 | .94 |
| <200 | 1.84 | 1.15 | 2.96 | .01 |
| CDC stage | ||||
| A–B | 1.00 | |||
| C | 1.40 | 0.94 | 2.10 | .10 |
| Status | ||||
| ART-experienced | 1.00 | |||
| ART-naïve | 2.24 | 1.20 | 4.18 | .01 |
| Years of previous ART (by 1 y) | 0.99 | 0.96 | 1.02 | .59 |
| Statin use | ||||
| Never | 1.00 | |||
| Since baseline | 1.18 | 0.55 | 2.55 | .67 |
| Started after enrollment | 1.37 | 0.49 | 3.82 | .55 |
| Baseline active HCV | ||||
| No | 1.00 | |||
| Active HCV, untreated | 1.23 | 0.58 | 2.61 | .60 |
| Active HCV, eradicated | 1.84 | 0.92 | 3.69 | .08 |
| ART regimen | ||||
| 3TC/ABC/DTG | 1.00 | |||
| 3TC + DTG | 1.72 | 0.82 | 3.61 | .15 |
| RPV + DTG | 1.37 | 0.60 | 3.12 | .45 |
| PI + DTG | 1.33 | 0.68 | 2.57 | .40 |
| TDF/FTC + DTG | 1.92 | 1.23 | 2.98 | .004 |
| TAF/FTC + DTG | 3.80 | 1.75 | 8.23 | .0007 |
The model equation included age, sex, baseline weight, risk factor for HIV acquisition, baseline CD4 and CDC stage, naïve status, ART duration (set at 0 for naïve participants), statin use, HCV eradication during the first year of study, DTG-including regimen. The model for naïve status estimate did not include ART duration.
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; CDC, Centers for Disease Control and Prevention; CI, confidence interval; DTG, dolutegravir; FTC, emtricitabine; HCV, hepatitis C virus; IVDU, intravenous drug use; RPV, rilpivirine; PI, protease inhibitor (atazanavir, darunavir); TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Impact of Weight Gain on Lipids and Blood Glucose
To explore the possible effect of weight gain on blood lipids and glucose, we compared TC, HDL, TG, and fasting glucose between 12-month WG and NG participants. One hundred five (14.7%) participants with intermediate weight gain were excluded from this analysis. Thus, we compared 49 WGs vs 526 NGs. The trends of TC, HDL, TG, and fasting glycemia during the study period are shown in Table 4. Controlling for age, sex, statin use, and regimen, all these values were not significantly different by weight gain, except for HDL variation, which showed a higher increase in WG participants (P = .04).
Table 4.
Blood Lipids and Glucose Changes in 548 Nongainers and 49 Weight Gainers
| NGs | WGs | P a | |
|---|---|---|---|
| Total cholesterol, mean ± SD, mg/dL | BL, .04 | ||
| Baseline | 186 ± 46 | 172 ± 49 | .22 |
| At 6 mo | 186 ± 45 | 176 ± 52 | |
| At 12 mo | 184 ± 42 | 181 ± 46 | |
| HDL cholesterol, mean ± SD, mg/dL | BL, .008 | ||
| Baseline | 47 ± 17 | 40 ± 13 | .046 |
| At 6 mo | 48 ± 16 | 41 ± 13 | |
| At 12 mo | 49 ± 15 | 47 ± 15 | |
| TC/HDL, median (IQR) | BL, .10 | ||
| Baseline | 4.1 (3.2–5.1) | 4.3 (3.8–5.3) | .18 |
| At 6 mo | 3.9 (3.1–4.9) | 4.3 (3.3–5.5) | |
| At 12 mo | 3.9 (3.1–4.7) | 4.0 (3.2–5.0) | |
| Triglycerides, median (IQR), mg/dL | BL, .75 | ||
| Baseline | 117 (87–172) | 108 (86–180) | .63 |
| At 6 mo | 112 (80–162) | 119 (88–173) | |
| At 12 mo | 107 (79–159) | 110 (73–174) | |
| Triglycerides/HDL | BL, .16 | ||
| Baseline | 2.7 (1.6–4.7) | 3.0 (2.0–6.1) | .15 |
| At 6 mo | 2.5 (1.5–4.1) | 2.9 (1.9–5.1) | |
| At 12 mo | 2.3 (1.5–4.0) | 2.7 (1.4–4.8) | |
| Blood glucose, mean ± SD, mg/dL | BL, .64 | ||
| Baseline | 91 ± 27 | 90 ± 16 | .49 |
| At 6 mo | 92 ± 23 | 92 ± 16 | |
| At 12 mo | 93 ± 22 | 93 ± 15 |
Abbreviations: BL, baseline; HDL, high-density lipoprotein; IQR, interquartile range; NGs, nongainers; TC, total cholesterol; WGs, weight gainers.
aTest for repeated measures, adjusted for age, sex, baseline statin use, regimen type.
Obesity and Metabolic Syndrome
Out of 608 participants with available baseline BMI, 46 (7.6%) were obese and 186 (30.6%) were overweight. Among overweight participants, 15 (8.1%) crossed the threshold for the obesity definition, and 37 (19.9%) lost enough weight to become normal weight.
At baseline, among 464 (65.1%) participants with complete data for metabolic syndrome evaluation, 26 (5.6%) had metabolic syndrome. Out of 438 without baseline metabolic syndrome, 10 developed it during follow-up, resulting in a rate of 9.9 cases per 1000 person-years (95% CI, 5.0 to 17.7).
DISCUSSION
In this work, we described the weight changes in one of the largest cohorts of DTG-treated PWH prospectively followed up in a real-life context. Similar to previous data, we confirmed that PWH gained weight during DTG treatment, especially if naïve to ART [11, 14, 17]. We also found that people treated with DTG in association with TAF/FTC or TDF/FTC were more prone to experience the weight increase. A possible role of TAF has been previously observed in ART-naïve and -experienced PWH [13, 15, 20, 24] and was confirmed in our work in a real-life context, where TAF was mainly used to treat ART-naïve participants and, despite low numbers, seemed to be linked to nearly 4-fold higher risk of weight gain compared with ABC/3TC + DTG. The reasons underlying this observation remain unclear, despite a possible effect on lipid metabolism having been previously observed for TAF that might in some way also influence fat accumulation [25, 26]. It is also possible that the inverse correlation, namely the accumulation of fat during TAF treatment, could be one of the reasons for the elevation of lipid levels that has been observed in the course of TAF treatment. In fact, in the general population, a correlation between visceral fat and higher levels of TG and TC exists, as omental and mesenteric fat play an important role in the hepatic lipid metabolism, contributing to hypertriglyceridemia and low HDL concentrations [27]. However, due to the recent introduction of TAF in clinical practice, observational data are still scarce [24], and more studies will be needed to identify the potential metabolic pathway by which TAF could interfere with fat accumulation or blood lipid metabolism. Moreover, we did not observe different effects on lipids between WGs and NGs, or participants treated with TDF, a drug considered to have a neutral effect on lipids [28], who experienced nearly double the risk of weight gain compared with those on ABC/3TC in SCOLTA. The effect of TDF/FTC on weight has been studied in the past, but the results were not univocal. No effect was found in previous studies in naïve [15, 19] or experienced PWH [18], but in a recently published pooled analysis of 8 randomized controlled clinical trials of treatment-naïve PWH initiating ART between 2003 and 2015, Sax et al. showed that PWH treated with TDF gained more weight compared with those treated with AZT (2.07 kg; 95% CI, 1.84 to 2.30; vs 0.39 kg; 95% CI, –0.57 to 1.34) [15]. Then, according to our results, tenofovir could influence weight gain in both its prodrug forms, TDF and TAF, but on the contrary, previous studies have found that PWH on ABC/3TC gained more weight than those on tenofovir [9, 29], whereas in other studies both ABC/3TC and TDF/FTC had a neutral effect on weight, BMI, and lean body mass changes [15, 19]. The discordant results of the abovementioned studies might reside in different patient populations and unmeasured confounding factors; dietary, psychological, social, and economic factors can play a role. Moreover, it is possible that not only the single drugs, but also their association could increase the risk of gaining weight, although with an unknown mechanism, or simply enhancing the effect of DTG in certain combinations.
Last, but not least, HIV infection itself and the virus activity may also play a role in weight changes. In our study, ART-naïve PWH and those with the lowest CD4 T-cell counts were at higher risk of gaining weight, supporting the hypothesis that weight gain might be explained, at least in part, as a “return to health” phenomenon, the result of successfully suppressing viral replication, controlling inflammation, and normalizing resting energy expenditure [30]. Of note, in healthy HIV-uninfected males, weight did not change with use of either TDF/FTC or cabotegravir [31, 32].
We also found higher risk of being a WG for male PWH. This is in contrast with literature data collected in PWH [9, 13, 15], where black females especially were at higher risk. However, in our study, participants were mainly Caucasian, and women were little represented. The difference in country of origin and ethnicity might indeed play a role in the evaluation of weight gain and gender differences, as in developed countries more men than women are overweight and obese, whereas in developing countries overweight and obesity are more prevalent in women [33]. Moreover, in the general population living in developed countries, overweight and obesity are expected to peak in men at about 55 years of age, different from women, in whom the peak is expected at a slightly older age, closer to 60 years [33]. PWH enrolled in the present study, mainly male and about 50 years old, might be more representative of the general population of developed countries than PWH enrolled in previous studies; our findings may be similar to the recently presented data from the Swiss cohort, where male PWH were shown to be at greater risk of weight gain than women [34].
Finally, we found that the incidence of MS was about 10 cases per 1000 person-years. A higher prevalence of metabolic syndrome in PWH than in the general population has been reported in the past [35], with an estimated incidence in developed countries ranging between 8 and 14 cases per 100 person-years [36–38]. Although the follow-up was limited in our study, we did not find an excess incidence of metabolic syndrome. Moreover, blood levels of TC, TC/TG, and fasting glucose did not change differently in WGs and NGs in our study, suggesting that the metabolic impact of weight gain was low in our cohort.
This study has several potential limitations. First, owing to the observational nature of the study, PWH were not randomly assigned to drug therapies, and confounding bias could have occurred, based on clinicians’ decisions to prescribe different drug associations in PWH with different baseline characteristics. In addition, dietary habits and personal choices or sociopsychological conditions influencing weight loss or gain were not recorded. Finally, our ability to detect incident obesity and metabolic syndrome might be limited by the lack of complete data to define these events in part of the study population and by the relatively short follow-up.
In conclusion, in our study, naïve PWH and those with lower CD4 counts were at higher risk of weight gain, and TAF/FTC and TDF/FTC were the drugs that in combination with DTG had the highest impact on weight. The real clinical implications of weight gain on cardiovascular risk still need to be defined, whereas weight gain in people who are not overweight should be considered a favorable effect, as it has a demonstrated benefit in terms of mortality [39]. Moreover, weight gain seemed to have no impact on fasting glucose or cholesterol levels in our cohort, where, despite weight gain, we did not find a high impact on incident obesity and MS. The fact that studies with differing designs and populations have found different risk factors for weight gain in the literature highlights how treatment should always be tailored to the individual patient, without falling to the temptation of generalizing the study results to all PWH.
Acknowledgments
We want to acknowledge all the members of Coordinamento Italiano Studio Allergie e Infezione da HIV (CISAI). Coordinators: Paolo Bonfanti (Lecco), Antonio Di Biagio (Genova). Data Management: Elena Ricci (Milano). Participating centers: E. Sarchi, G. Chichino, C. Bolla (Alessandria); C. Bellacosa, G. Angarano (Bari); L. Calza (Bologna); B. Menzaghi, M. Farinazzo (Busto Arsizio); G. Angioni (Cagliari); M. Gussio, B. M. Celesia (Catania); K. Falasca (Chieti); A. Mastroianni, G. Guadagnino (Cosenza); F. Vichi, E. Salomoni (Firenze); C. Martinelli (Firenze); A. Di Biagio, L. Nicolini (Genova); G. Cenderello (Genova); P. Bonfanti, C. Molteni (Lecco); G. F. Pellicanò, G. Nunnari (Messina); L. Valsecchi, L. Cordier, S. Parisini, G. Rizzardini (Milano); S. Rusconi, F. Conti (Milano); A. Bandera, L. Taramasso, A. Gori (Milano); D. Motta, M. Puoti (Milano); N. Squillace, G. M. Migliorino (Monza); P. Maggi, S. Martini (Napoli); A. Cascio, M. Trizzino (Palermo); R. Gulminetti (Pavia); G. V. De Socio, M. Nofri, D. Francisci (Perugia); D. Cibelli, G. Parruti (Pescara); C. Dentone (Sanremo); G. Madeddu, M. S. Mameli (Sassari); G. Orofino, M. Guastavigna (Torino).
Financial support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Potential conflicts of interest. None of the authors reported any conflict of interest relevant to the present work. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Antinori A, Di Biagio A, Marcotullio S, et al. Evidence-based renewal of the Italian guidelines for the use of antiretroviral agents and the diagnostic-clinical management of HIV-1 infected persons. New Microbiol 2018; 41:247–55. [PubMed] [Google Scholar]
- 2. EACS guidelines Available at: http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html. Accessed 24 September 2019.
- 3. What’s new in the guidelines? Adult and adolescent ARV Available at: https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/37/whats-new-in-the-guidelines-. Accessed 24 September 2019.
- 4. DHHS Panel on Antiretroviral Guidelines for Adults, and Adolescents – a Working Group of the Office of AIDS Research, Advisory Council (OARAC). Guidelines for the use of antiretroviral agents in adults and adolescents with HIV Available at: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf#page=1&zoom=auto,-14,792. Accessed 1 March 2020.
- 5. Lepik KJ, Yip B, Ulloa AC, et al. Adverse drug reactions to integrase strand transfer inhibitors. AIDS 2018; 32:903–12. [DOI] [PubMed] [Google Scholar]
- 6. Taramasso L, Tatarelli P, Ricci E, et al. ; CISAI Study Group Improvement of lipid profile after switching from efavirenz or ritonavir-boosted protease inhibitors to rilpivirine or once-daily integrase inhibitors: results from a large observational cohort study (SCOLTA). BMC Infect Dis 2018; 18:357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gatell JM, Assoumou L, Moyle G, et al. Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS 2017; 31:2503–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bagella P, Squillace N, Ricci E, et al. Lipid profile improvement in virologically suppressed HIV-1-infected patients switched to dolutegravir/abacavir/lamivudine: data from the SCOLTA project. Infect Drug Resist 2019; 12:1385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menard A, Meddeb L, Tissot-Dupont H, et al. Dolutegravir and weight gain: an unexpected bothering side effect? AIDS 2017; 31:1499–500. [DOI] [PubMed] [Google Scholar]
- 10. Norwood J, Turner M, Bofill C, et al. Brief report: weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr 2017; 76:527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020;. 70:1267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Waters L, Assoumou L, Rusconi S, et al. Switch to dolutegravir (DTG) from a boosted protease inhibitor (PI/r) associated with significant weight gain over 48 weeks in NEAT-022, a randomised 96-week trial. Poster presented at: HIV Drug Therapy; October 28–31, 2018, Glasgow P102.
- 13. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 14. Taramasso L, Ricci E, Menzaghi B, et al. Weight gain: a possible side effect of all antiretrovirals. Open Forum Infect Dis 2017; 4:ofx239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bakal DR, Coelho LE, Luz PM, et al. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother 2018; 73:2177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burns JE, Stirrup OT, Dunn D, et al. No overall change in the rate of weight gain after switching to an integrase-inhibitor in virologically suppressed adults with HIV. AIDS 2020; 34:109–14. [DOI] [PubMed] [Google Scholar]
- 18. Lake JE, Wu K, Bares SH, et al. Risk Factors for Weight Gain Following Switch to Integrase Inhibitor-Based Antiretroviral Therapy [published online ahead of print, 2020 Feb 26]. Clin Infect Dis. 2020;ciaa177. doi: 10.1093/cid/ciaa177 [DOI] [PMC free article] [PubMed]
- 19. Erlandson KM, Kitch D, Tierney C, et al. Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. AIDS 2013; 27:2069–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taramasso L, Berruti M, Briano F, Di Biagio A. The switch from tenofovir disoproxil fumarate to tenofovir alafenamide determines weight gain in patients on rilpivirine-based regimen. AIDS 2020; 34:877–81. [DOI] [PubMed] [Google Scholar]
- 21. Bonfanti P, Martinelli C, Ricci E, et al. ; CISAI Group (Italian Coordinators for the Study of Allergies HIV Infection) An Italian approach to postmarketing monitoring: preliminary results from the SCOLTA (Surveillance Cohort Long-Term Toxicity Antiretrovirals) project on the safety of lopinavir/ritonavir. J Acquir Immune Defic Syndr 2005; 39:317–20. [DOI] [PubMed] [Google Scholar]
- 22. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006; 23:469–80. [DOI] [PubMed] [Google Scholar]
- 23. International Diabetes Federation (IDF). The IDF consensus worldwide definition of the metabolic syndrome Available at: https://www.idf.org/e-library/consensus-statements/60-idfconsensus-worldwide-definitionof-the-metabolicsyndrome.html. Accessed 1 March 2020.
- 24. Gomez M, Seybold U, Roider J, et al. A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)- to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015–2017. Infection 2019; 47:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hagins D, Orkin C, Daar ES, et al. Switching to coformulated rilpivirine (RPV), emtricitabine (FTC) and tenofovir alafenamide from either RPV, FTC and tenofovir disoproxil fumarate (TDF) or efavirenz, FTC and TDF: 96-week results from two randomized clinical trials. HIV Med 2018; 19:724–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taramasso L, Di Biagio A, Riccardi N, et al. Lipid profile changings after switching from rilpivirine/tenofovir disoproxil fumarate/emtricitabine to rilpivirine/tenofovir alafenamide/emtricitabine: different effects in patients with or without baseline hypercholesterolemia. PLoS One 2019; 14:e0223181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ebbert J, Jensen M. Fat depots, free fatty acids, and dyslipidemia. Nutrients 2013; 5:498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Milinkovic A, Berger F, Arenas-Pinto A, Mauss S. Reversible effect on lipids by switching from tenofovir disoproxil fumarate to tenofovir alafenamide and back. AIDS 2019; 33:2387–91. [DOI] [PubMed] [Google Scholar]
- 29. Martin A, Bloch M, Amin J, et al. Simplification of antiretroviral therapy with tenofovir‐emtricitabine or abacavir‐lamivudine: a randomized, 96‐week trial. Clin Infect Dis 2009; 49:1591–601. [DOI] [PubMed] [Google Scholar]
- 30. Tate T, Willig AL, Willig JH, et al. HIV infection and obesity: where did all the wasting go? Antivir Ther 2012; 17:1281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grant RM, Lama JR, Anderson PL, et al. ; iPrEx Study Team Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363:2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Landovitz RJ, Zangeneh SZ, Chau G, et al. Cabotegravir is not associated with weight gain in HIV-uninfected individuals in HPTN 077. Clin Infect Dis 2020; 70:319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mugglin C, Calmy A, Guntard H, et al. ; Swiss HIV Cohort Study Changes in weight after switching to dolutegravir containing antiretroviral therapy in the Swiss HIV cohort study 2019. Available at: http://resourcelibrary.eacs.cyim.com/mediatheque/media.aspx?mediaId=78040&channel=28172. Accessed 1 March 2020.
- 35. Bonfanti P, Giannattasio C, Ricci E, et al. HIV and metabolic syndrome: a comparison with the general population. J Acquir Immune Defic Syndr 2007; 45:426–31. [DOI] [PubMed] [Google Scholar]
- 36. Palacios R, Santos J, González M, et al. Incidence and prevalence of the metabolic syndrome in a cohort of naive HIV-infected patients: prospective analysis at 48 weeks of highly active antiretroviral therapy. Int J STD AIDS 2007; 18:184–7. [DOI] [PubMed] [Google Scholar]
- 37. Freitas P, Carvalho D, Souto S, et al. Impact of lipodystrophy on the prevalence and components of metabolic syndrome in HIV-infected patients. BMC Infect Dis 2011; 11:246–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jacobson DL, Tang AM, Spiegelman D, et al. Incidence of metabolic syndrome in a cohort of HIV-infected adults and prevalence relative to the US population (National Health and Nutrition Examination Survey). J Acquir Immune Defic Syndr 2006; 43:458–66. [DOI] [PubMed] [Google Scholar]
- 39. Yuh B, Tate J, Butt AA, et al. Weight change after antiretroviral therapy and mortality. Clin Infect Dis 2015; 60:1852–9. [DOI] [PMC free article] [PubMed] [Google Scholar]