Abstract
The immune system protects the host from pathogenic organisms (bacteria, viruses, fungi, parasites). To deal with this array of threats, the immune system has evolved to include a myriad of specialised cell types, communicating molecules and functional responses. The immune system is always active, carrying out surveillance, but its activity is enhanced if an individual becomes infected. This heightened activity is accompanied by an increased rate of metabolism, requiring energy sources, substrates for biosynthesis and regulatory molecules, which are all ultimately derived from the diet. A number of vitamins (A, B6, B12, folate, C, D and E) and trace elements (zinc, copper, selenium, iron) have been demonstrated to have key roles in supporting the human immune system and reducing risk of infections. Other essential nutrients including other vitamins and trace elements, amino acids and fatty acids are also important. Each of the nutrients named above has roles in supporting antibacterial and antiviral defence, but zinc and selenium seem to be particularly important for the latter. It would seem prudent for individuals to consume sufficient amounts of essential nutrients to support their immune system to help them deal with pathogens should they become infected. The gut microbiota plays a role in educating and regulating the immune system. Gut dysbiosis is a feature of disease including many infectious diseases and has been described in COVID-19. Dietary approaches to achieve a healthy microbiota can also benefit the immune system. Severe infection of the respiratory epithelium can lead to acute respiratory distress syndrome (ARDS), characterised by excessive and damaging host inflammation, termed a cytokine storm. This is seen in cases of severe COVID-19. There is evidence from ARDS in other settings that the cytokine storm can be controlled by n-3 fatty acids, possibly through their metabolism to specialised pro-resolving mediators.
Keywords: malnutrition, infectious disease, microbiome, nutrient deficiencies, pulmonary disease
Introduction
The immune system exists to protect the host from noxious environmental agents especially pathogenic organisms, which may be in the form of bacteria, viruses, fungi or parasites. To deal with such an array of threats, the human immune system has evolved to include a myriad of cell types, communicating molecules and functional responses. The immune system is always active, carrying out surveillance, but its activity is enhanced if an individual becomes infected. This heightened activity is accompanied by an increased rate of metabolism, requiring energy sources, substrates for biosynthesis and regulatory molecules. These energy sources, substrates and regulatory molecules are ultimately derived from the diet. Hence an adequate supply of a wide range of nutrients is essential to support the immune system to function optimally.1 2 At the time of writing, the world is in the grip of a pandemic caused by infection with a new coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); the illness associated with infection by SARS-CoV-2 is called coronavirus disease discovered in 2019 or COVID-19.3 4 The aim of this article is to summarise the role of specific nutrients in supporting the immune system, particularly, but not exclusively, with regard to antiviral defences. The roles of nutrition in overcoming gut microbial dysbiosis and in calming a so-called ‘cytokine storm’ will also be discussed. First, some features of coronaviruses and of the immune system will be described.
Coronaviruses
Coronaviruses are a large group of single-stranded RNA viruses that are common among mammals and birds.5 6 Coronaviruses cause respiratory and, less frequently, gastrointestinal diseases.5 The respiratory symptoms caused by coronaviruses can range from common cold-like or mild influenza-like symptoms to severe pneumonia. In December 2019, a new type of coronavirus causing pneumonia and death was identified in Wuhan, China3 4; this new coronavirus is called SARS-CoV-2 because it is genetically similar to SARS-CoV which caused the 2002 outbreak of severe acute respiratory distress syndrome (ARDS). In fact, SARS-CoV-2 is the seventh known human coronavirus.7 However, SARS-CoV-2 is new to the human immune system and so there was no underlying existing natural immunity against it. This is probably why SARS-CoV-2 has spread so rapidly. SARS-CoV-2 infects respiratory epithelial cells causing the symptoms described above, and in severe cases requires ventilatory support. Older people, especially those with existing morbidities like diabetes, cardiovascular disease, respiratory disease and hypertension, are particularly susceptible to severe symptoms and mortality, as are individuals with suppressed immune systems.3 4 There is currently no treatment for infection with SARS-CoV-2 or for COVID-19. Current strategies aim to limit the spread of the virus by preventing contact between people. The search for vaccines to offer immune protection against SARS-CoV-2 and for pharmacological treatments to prevent the virus from replicating is underway. In the meantime, approaches to ensure that individuals’ immune systems are well supported should be taken. Nutrition should be at the forefront of these approaches.
The immune system
Introductory comments
The immune system becomes vital once an individual is exposed to an infectious agent. However, the nature of infectious agents varies and so different approaches are required by the immune system to deal with different types of infectious agent. These different approaches follow similar general strategies, which aim to seek out and destroy, but the precise immune mechanisms involved can differ. For example, most bacteria do not invade host cells and remain accessible to the host’s immune system; often these bacteria will be engulfed by innate phagocytic cells (typically neutrophils, monocytes, macrophages, dendritic cells), killed within intracellular phagocytic vacuoles and then digested. Remnants of the digested bacteria (antigens) can then be displayed via major histocompatibility class (MHC) II on the surface of the phagocyte. These antigens are recognised by antigen-specific CD4+ helper T lymphocytes and this triggers the acquired (also called adaptive) immune response to the bacteria, which involves the orchestrating T lymphocytes, B lymphocytes (which produce antigen-specific antibodies) and the further activation of innate immune cells. This response to extracellular bacteria is clearly targeted at killing those bacteria. Viruses (and some bacteria) invade host cells rather than remaining exclusively extracellular; this can trigger presentation of antigens via MHC I on the surface of the infected cells. Recognition of these antigens by CD8+ cytotoxic T lymphocytes results in killing of the host cell that is presenting the antigen. Natural killer cells also recognise virally infected cells and act in an analogous way to cytotoxic T lymphocytes by killing the infected cells. Thus, this response to virally infected cells is targeted at killing the host cells that harbour viruses. Killing host cells of course liberates viruses and the battle between host immune cells and virally infected cells continues.
There are four general functions of the immune system that enable effective host defence:
Creating a barrier to prevent pathogens from entering the body.
Identifying pathogens if they breech a barrier.
Eliminating pathogens.
Generating an immunological memory.
Barrier function
The barrier function of the immune system acts to prevent pathogens from entering the body from the external environment. This includes physical barriers like the skin and mucosal layers (gastrointestinal tract, respiratory tract, genitourinary tract); chemical barriers like the acid pH of the stomach; and biological barriers like the presence of commensal organisms on the skin and in the intestinal tract, secretions like IgA and antimicrobial proteins in saliva and tears, and the complement system.
Identification of pathogens
Pathogens are recognised by cells of the innate immune system, such as macrophages, monocytes and dendritic cells. This is achieved through the presence of pattern recognition receptors (PRRs) that recognise general molecular structures that are broadly shared by groups of pathogens. These structures are termed microbe-associated molecular patterns or MAMPs. When PRRs recognise MAMPs, the first line of host defensive responses is activated. PRRs include Toll-like receptors (TLRs). More than 10 functional TLRs have been identified in humans, each one detecting distinct MAMPs from bacteria, viruses, fungi and parasites. The best described of these are TLR4 which recognises the lipopolysaccharides from the cell wall of Gram-negative bacteria and TLR2 which recognises the lipoteichoic acid from the cell wall of Gram-positive bacteria. Several TLRs are expressed on the cell surface of innate immune cells because the pathogens they recognise, mainly bacteria, are extracellular. Because viruses enter host cells, it is important that there are also intracellular TLRs. Indeed, intracellular TLRs that recognise viral DNA, viral double-stranded RNA and viral single-stranded RNA exist. Among these, TLR7 and TLR8 are found in macrophages, monocytes, dendritic cells and some other cell types and are likely to be important in innate recognition of the single-stranded RNA of coronaviruses. However, proteins, including the spike glycoprotein, of the coronavirus coat are also likely to be recognised by both intracellular and extracellular PRRs.8–11
Elimination of pathogens
As mentioned earlier, extracellular bacteria can be engulfed by phagocytic cells that include macrophages and dendritic cells. After digestion of internalised bacteria, peptide fragments, termed antigens, are presented on the surface of the phagocytic cells (via MHC II) to antigen-specific CD4+ helper T lymphocytes. The activated helper T lymphocytes (specifically the T helper 1 phenotype) proliferate and produce cytokines including interleukin (IL)-2 and interferon (IFN)-γ. IFN-γ promotes antigen-specific antibody production by B lymphocytes. These antibodies coat the bacteria, neutralising them and making the process of phagocytosis more efficient.
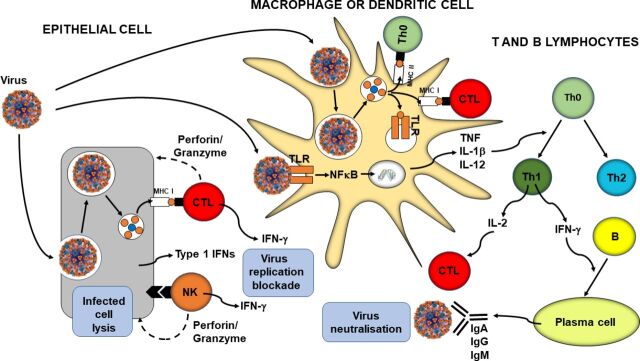
In parallel with phagocytosis, innate immune cell recognition of pathogens via PRRs triggers inflammatory signalling, activation of transcription factors like nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), inflammasome assembly, and production of classic inflammatory cytokines like tumour necrosis factor (TNF), IL-1β and IL-12. Viral infection of some cell types promotes release of type 1 IFNs (IFN-α and IFN-β) and these induce antiviral resistance, in part through activation of natural killer cells.12 13 Furthermore, as explained earlier, virally infected cells directly activate natural killer cells which act to kill the infected cell. In addition, PRR signalling induces maturation of dendritic cells which are responsible for viral antigen processing and presentation, so initiating acquired immunity. Upregulation of MHC I on virally infected cells including both respiratory epithelial cells and dendritic cells results in presentation of viral antigens to CD8+ cytotoxic T lymphocytes. This activates them to kill virally infected cells through the release of pore forming proteins like perforin. Presentation of viral antigens via MHC II and the cytokine milieu lead to the activation of CD4+ helper T lymphocytes with switching to the T helper 1 phenotype. These cells produce IL-2, which promotes cytotoxic T lymphocyte activity, and IFN-γ, which promotes differentiation of B lymphocytes to plasma cells which produce antiviral antibodies. These antibodies can bind to free viruses neutralising them. The processes involved in antiviral immunity are summarised in figure 1.
Figure 1.
Overview of antiviral immunity. The events in the figure are explained in the text. B, B lymphocyte; CTL, cytotoxic T lymphocyte; IFN, interferon; Ig, immunoglobulin; IL, interleukin; MHC, major histocompatibility class; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; NK, natural killer cell; Th, helper T lymphocyte; TLR, Toll-like receptor; TNF, tumour necrosis factor.
Immunological memory
Immunological memory refers to the ability of the immune system to quickly and specifically recognise an antigen that the body has previously encountered and initiate a corresponding immune response. There are two aspects of immunological memory. First, antibodies can persist in the circulation for many months to many years, providing protection against reinfection. Second, after the cessation of an active immune response, a small number of memory T (both CD4+ and CD8+) and B lymphocytes remain; they are in a resting state but if they encounter the same antigen that triggered their formation they are able to respond immediately and lead to rapid elimination of the source of the antigen. Memory cells have a long life (up to several decades). Immunological memory is the basis of vaccination.
Effect of ageing on the immune system
Ageing can be associated with a loss of immune competence, a process called immunosenescence.14–18 The features of immunosenescence are shown in box 1. One factor linked to immunosenescence is decreased output of immune cells from bone marrow, the site of origin of all immune cells. In addition, involution of the thymus with age decreases output of naive T lymphocytes, resulting in reduced capacity to respond to new antigens. Immunosenescence means that, compared with younger adults, older people have increased susceptibility to infections including respiratory tract infections and pneumonia and poorer responses to vaccination.14 15 19 20 The gut mucosa is the largest site of immune tissue in humans and senescence of the gut mucosal immune system has been demonstrated in murine models, with reductions in secretory IgA responses, impaired oral tolerance to new antigens and impaired mucosal dendritic cell function, as reviewed elsewhere.21 22 Immunosenescence may be one factor that predisposes older people to more severe COVID-19. Paradoxically, ageing is also linked to an increase in blood concentrations of many inflammatory mediators, a situation termed inflammageing.23 This state is considered to contribute to an increased risk of chronic conditions of ageing like cardiovascular disease, metabolic disease (diabetes, non-alcoholic fatty liver disease), neurodegeneration and some cancers23 and may predispose to mounting an excessive inflammatory response when infected. Although inflammation is part of the innate immune response and innate and acquired immunity should work in a coordinated and integrated way (see figure 1), an excessive inflammatory response can lead to impairments in acquired immunity.23
Box 1. Some key features of age-related immune decline (immunosenescence).
T lymphocytes
Decreased numbers in the circulation.
Imbalances among different phenotypes.
Decline in naive T lymphocyte production and numbers.
Accumulation of non-functional memory T lymphocytes.
Diminished antigen receptor diversity.
Impaired responsiveness.
Impaired proliferation.
Impaired production of cytokines like interleukin (IL) 2 and interferon (IFN)-γ.
B lymphocytes
Decline in naive B lymphocyte numbers.
Accumulation of non-functional memory B lymphocytes.
Impaired responsiveness.
Altered balance of immunoglobulins.
Dendritic cells
Decreased phagocytosis.
Decreased Toll-like receptor (TLR) expression.
Decreased responsiveness.
Decreased type 1 IFN production.
Neutrophils
Numbers in the circulation are preserved.
Impaired chemotaxis.
Impaired oxidative burst and bacterial killing.
Impaired phagocytosis.
Decreased TLR expression.
Decreased production of neutrophil extracellular traps.
Decreased responsiveness.
Monocytes
Altered TLR expression.
Decreased responsiveness.
Altered pattern of cytokine production.
Macrophages
Impaired phagocytosis.
Altered TLR expression.
Natural killer cells
Increased numbers in the circulation.
Imbalances among different phenotypes.
Impaired cytotoxicity.
Impaired responsiveness.
Impaired production of cytokines.
Effect of obesity on the immune system
Obesity can be associated with a loss of immune competence,24 25 with impairments of the activity of helper T lymphocytes, cytotoxic T lymphocytes, B lymphocytes and natural killer cells,26–28 and reduced antibody and IFN-γ production.26 27 This means that, compared with healthy weight individuals, the obese have increased susceptibility to a range of bacterial, viral and fungal infections,24 29–31 and poorer responses to vaccination.26 31 The impact of obesity has been well explored in relation to influenza infection and vaccination against influenza. During the 2009 H1N1 influenza A virus pandemic, obese individuals showed delayed and weakened antiviral responses to infection and showed poorer recovery from disease compared with healthy weight individuals.26 Animal studies and case studies in humans show that obesity is associated with prolonged shedding of influenza virus, indicating an impairment in viral control and killing, and the emergence of virulent minor variants.26 Green and Beck32 note that compared with healthy weight individuals, vaccinated obese individuals have twice the risk of influenza or influenza-like illness, indicating poorer protection from vaccination in the obese. Sheridan et al 33 investigated the responses of immune cells from the blood of healthy weight, overweight and obese individuals to the influenza vaccine in vitro. Exposure of the blood immune cells to the vaccine increased the number of activated cytotoxic T lymphocytes, the number of granzyme expressing cytotoxic T lymphocytes and the number of IFN-γ producing cytotoxic T lymphocytes. However, the responses of cells from obese individuals were blunted by 40%, almost 60% and 65%, respectively. Cells from overweight individuals showed responses intermediate between those from healthy weight and obese individuals. Similar findings for the response of blood cells to the pandemic H1N1 influenza A virus were reported by Paich et al.34 Paradoxically, obesity is also linked to an increase in blood concentrations of many inflammatory mediators, a state of chronic low-grade inflammation.35 This state is considered to contribute to an increased risk of chronic conditions of ageing35 and may predispose to mounting an excessive inflammatory response when infected. Thus, obesity may be one factor that predisposes to more severe COVID-19; in support of this, a French report found that 85.7% of SARS-CoV-2 infected obese individuals required mechanical ventilation compared with 47.1% of infected healthy weight individuals.36
Nutrition, immunity and infection
Introductory comments
The immune system is functioning at all times, but cells become activated by the presence of pathogens (see The immune system). This activation results in a significant increase in the demand of the immune system for energy yielding substrates (glucose, amino acids and fatty acids). Activation of the immune response induces the production of lipid-derived mediators such as prostaglandins and leukotrienes and of many different types of protein including immunoglobulins, chemokines, cytokines, cytokine receptors, adhesion molecules and acute-phase proteins. This requires availability of the substrate fatty acids and amino acids, respectively. The immune response involves significant cellular proliferation, so increasing the number of immune cells available for defence: this requires DNA, RNA, protein and complex lipid synthesis and the ready availability of substrates to support this. The metabolic machinery involved in energy generation and biosynthesis requires many different vitamins and minerals as cofactors. Amino acids (eg, arginine) are precursors for the synthesis of polyamines, which have roles in the regulation of DNA replication and cell division. Various micronutrients (eg, iron, folate, zinc, magnesium) are also involved in nucleotide and nucleic acid synthesis. Some nutrients, such as vitamins A and D, and their metabolites are direct regulators of gene expression in immune cells and play a key role in the maturation, differentiation and responsiveness of immune cells. Creation of a pro-oxidant environment through generation of damaging reactive oxygen species is one element of innate immunity; the host needs protection against these through classic antioxidant vitamins (vitamins C and E) and the antioxidant enzymes (superoxide dismutase, catalase and glutathione peroxidase); the latter require manganese, copper, zinc, iron and selenium. Thus, the roles for nutrients in supporting the function of the immune system are many and varied and it is easy to appreciate that an adequate and balanced supply of these is essential if an appropriate immune response is to be mounted. In essence, good nutrition creates an environment in which the immune system is able to respond appropriately to challenge, irrespective of the nature of the challenge. Conversely poor nutrition creates an environment in which the immune system cannot respond well. This is amply illustrated in conditions of nutrient deficiency (either ‘real life’ or experimentally induced) which are accompanied by impairments of both innate and acquired immunity and increased susceptibility to, and severity of, infections. Both the immune impairments and the susceptibility to infection can be reversed by correcting the deficiency(ies) showing a causal relationship between availability of specific nutrients and immune defences. This is recognised by the European Food Safety Authority which permits claims of ‘maintenance of functions of the immune system’ for vitamins A, B6, B12, C, D and folate (vitamin B9) and for the trace elements zinc, iron, selenium and copper.37 There are a number of comprehensive reviews of aspects of nutrition and immunity,1 2 38–42 mainly focusing on the role of micronutrients, as well as useful single-author and multiauthor books on the topic.43–46 In addition, there are many comprehensive nutrient-specific reviews, which are cited in the relevant sections below. Some of the text of the following sections is updated from a previous publication.1
Vitamin A, immunity and infection
There are a number of reviews of the role of vitamin A and its metabolites (eg, 9-cis-retinoic acid) in immunity and in host susceptibility to infection.47–54 These reviews contain citations to the many studies of vitamin A, immunity and infection that will be summarised here. Vitamin A is important for normal differentiation of epithelial tissue and for immune cell maturation and function. Thus, vitamin A deficiency is associated with impaired barrier function, altered immune responses and increased susceptibility to a range of infections. Vitamin A-deficient mice show breakdown of the gut barrier and impaired mucus secretion (due to loss of mucus-producing goblet cells), both of which would facilitate entry of pathogens. Many aspects of innate immunity, in addition to barrier function, are modulated by vitamin A and its metabolites. Vitamin A controls neutrophil maturation and in vitamin A deficiency blood neutrophil numbers are increased, but they have impaired phagocytic function. Therefore, the ability of neutrophils to ingest and kill bacteria is impaired. Vitamin A also supports phagocytic activity and oxidative burst of macrophages, so promoting bacterial killing. Natural killer cell activity is diminished by vitamin A deficiency, which would impair antiviral defences. The impact of vitamin A on acquired immunity is less clear and may depend on the exact setting and the vitamin A metabolite involved. Vitamin A controls dendritic cell and CD4+ T lymphocyte maturation and its deficiency alters the balance between T helper 1 and T helper 2 lymphocytes. Studies in experimental model systems indicate that the vitamin A metabolite 9-cis retinoic acid enhances T helper 1 responses. Retinoic acid promotes movement (homing) of T lymphocytes to the gut-associated lymphoid tissue. Interestingly, some gut-associated immune cells are able to synthesise retinoic acid. Retinoic acid is required for CD8+ T lymphocyte survival and proliferation and for normal functioning of B lymphocytes including antibody generation. Thus, vitamin A deficiency can impair the response to vaccination, as discussed elsewhere.55 In support of this, vitamin A-deficient Indonesian children provided with vitamin A showed a higher antibody response to tetanus vaccination than seen in vitamin A-deficient children.56 Vitamin A deficiency predisposes to respiratory infections, diarrhoea and severe measles. Systematic reviews and meta-analyses of trials in children with vitamin A report reduced all-cause mortality,57 reduced incidence, morbidity and mortality from measles57 and from infant diarrhoea,57 and improved symptoms in acute pneumonia58 (table 1).
Table 1.
Summary of selected recent meta-analyses of micronutrients and respiratory infections
| Micronutrient | Authors | Sample size | Main findings | Stated conclusion in abstract |
| Vitamin A | Imdad et al 57 | 47 RCTs (1 223 856 children) | Vitamin A did not affect incidence of, or mortality from, respiratory disease; Note: vitamin A decreased all cause mortality and mortality from diarrhoea and decreased incidence of diarrhoea and measles |
Vitamin A supplementation is associated with a clinically meaningful reduction in morbidity and mortality in children. |
| Vitamin A | Hu et al 58 | 15 RCTs (3021 children) | Vitamin A did not affect mortality of children with pneumonia. Vitamin A decreased pneumonia morbidity, increased the clinical response rate, shortened clearance time of signs and shortened length of hospital stay. |
Vitamin A supplementation helps to relieve clinical symptoms and signs (of pneumonia) and shorten the length of hospital stay. |
| Vitamin C | Hemila and Louhiala65 | 3 prophylactic trials (2335 participants) two therapeutic trials (197 patients) |
All three trials found vitamin C decreased the incidence of pneumonia. One trial found vitamin C decreased severity and mortality from pneumonia; the other trial found vitamin C shortened duration of pneumonia. |
|
| Vitamin C | Hemila and Chalker66 | 29 prophylactic RCTs investigating incidence (11 306 participants) 31 prophylactic RCTs investigating duration (9745 episodes) |
Vitamin C did not affect incidence of the common cold in the general population (24 RCTs) but decreased incidence in people under heavy short-term physical stress (5 RCTs). Vitamin C shortened duration of common cold in all studies (31 RCTs), in adults (13 RCTs) and in children (10 RCTs) and decreased severity of colds. |
|
| Vitamin D | Bergman et al 86 | 11 RCTs (5660 participants) | Vitamin D decreased the risk of respiratory tract infections. | Vitamin D has a positive effect against respiratory tract infections and dosing once daily seems most effective. |
| Vitamin D | Martineau et al 87 | 25 RCTs (11 321 participants) | Vitamin D decreased the risk of acute respiratory tract infection, effects greater in those with low starting status | Vitamin D supplementation was safe and it protected against respiratory tract infection. |
| Vitamin D | Pham et al 88 | 24 studies; 14 included in meta-analysis of risk of acute respiratory tract infections and 5 in the meta-analysis of severity | Serum vitamin D was inversely associated with risk and severity of acute respiratory tract infections. | There is an inverse non-linear association between 25-hydroxyvitamin D concentration and acute respiratory tract infection. |
| Vitamin D | Zhou et al 89 | 8 observational studies (20 966 participants) | Participants with vitamin D deficiency had increased risk of community-acquired pneumonia. | (There is] an association between vitamin D deficiency and increased risk of community-acquired pneumonia. |
| Zinc, copper and iron | Mao et al 122 | 13 studies in Chinese children | Children with recurrent respiratory tract infection had lower hair levels of zinc, copper and iron. | The deficiency of zinc, copper and iron may be a contributing factor for the susceptibility of recurrent respiratory tract infection in Chinese children. |
| Zinc | Hemila123 | 7 RCTs (575 participants) | Zinc shortened duration of common cold. | |
| Zinc | Science et al 124 | 17 RCTs (2121 adults and children) | Zinc decreased duration of common cold symptoms overall and in adults but not in children. | Oral zinc formulations may shorten the duration of symptoms of the common cold. |
| Zinc | Lassi et al 125 | 6 RCTs (5193 children) | Zinc decreased incidence of pneumonia. Zinc decreased prevalence of pneumonia. |
Zinc supplementation in children is associated with a reduction in the incidence and prevalence of pneumonia. |
| Zinc | Wang and Song126 | 6 RCTs (2216 adults with severe pneumonia) | Zinc given as an adjunct therapy decreased mortality. No effect of zinc on treatment failure or antibiotic treatment. |
Zinc given as an adjunct to the treatment of severe pneumonia is effective in reducing mortality. |
RCT, randomised controlled trial.
B-group vitamins, immunity and infection
There is a recent comprehensive review of B vitamins and immunity.59 This review contains citations to the many studies of B-group vitamins and immunity that will be summarised here. B vitamins are involved in intestinal immune regulation, thus contributing to gut barrier function. Folic acid deficiency in animals causes thymus and spleen atrophy, and decreases circulating T lymphocyte numbers. Spleen lymphocyte proliferation is also reduced but the phagocytic and bactericidal capacity of neutrophils appears unchanged. In contrast, vitamin B12 deficiency decreases phagocytic and bacterial killing capacity of neutrophils, while vitamin B6 deficiency causes thymus and spleen atrophy, low blood T lymphocyte numbers and impaired lymphocyte proliferation and T lymphocyte-mediated immune responses. Vitamins B6 and B12 and folate all support the activity of natural killer cells and CD8+ cytotoxic T lymphocytes, effects which would be important in antiviral defence. Patients with vitamin B12 deficiency had low blood numbers of CD8+ T lymphocytes and low natural killer cell activity.60 In a study in healthy older humans, a vitamin B6-deficient diet for 21 days resulted in a decreased percentage and total number of circulating lymphocytes, and a decrease in T and B lymphocyte proliferation and IL-2 production.61 Repletion over 21 days using vitamin B6 at levels below those recommended did not return immune function to starting values, while repletion at the recommended intake (22.5 µg/kg body weight per day, which would be 1.575 mg/day in a 70 kg individual) did. Providing excess vitamin B6 (33.75 µg/kg body weight per day, which would be 2.362 mg/day in a 70 kg individual) for 4 days caused a further increase in lymphocyte proliferation and IL-2 production.
Vitamin C, immunity and infection
There are reviews of the role of vitamin C in immunity and in host susceptibility to infection.62 63 These reviews contain citations to the many studies of vitamin C, immunity and infection that will be summarised here. Vitamin C is required for collagen biosynthesis and is vital for maintaining epithelial integrity. It also has roles in several aspects of immunity, including leucocyte migration to sites of infection, phagocytosis and bacterial killing, natural killer cell activity, T lymphocyte function (especially of CD8+ cytotoxic T lymphocytes) and antibody production. Jacob et al 64 showed that a vitamin C-deficient diet in healthy young adult humans decreased mononuclear cell vitamin C content by 50% and decreased the T lymphocyte-mediated immune responses to recall antigens. Vitamin C deficiency in animal models increases susceptibility to a variety of infections.63 People deficient in vitamin C are susceptible to severe respiratory infections such as pneumonia. A meta-analysis reported a significant reduction in the risk of pneumonia with vitamin C supplementation, particularly in individuals with low dietary intakes65 (table 1). Vitamin C supplementation has also been shown to decrease the duration and severity of upper respiratory tract infections, such as the common cold, especially in people under enhanced physical stress.63 66
Vitamin D, immunity and infection
There are a number of reviews of the role of vitamin D and its metabolites in immunity and in host susceptibility to infection.67–78 These reviews contain citations to the many studies of vitamin D, immunity and infection that will be summarised here. The active form of vitamin D (1,25-dihydroxyvitamin D3) is referred to here as vitamin D. Vitamin D receptors have been identified in most immune cells and some cells of the immune system can synthesise the active form of vitamin D from its precursor, suggesting that vitamin D is likely to have important immunoregulatory properties. Vitamin D enhances epithelial integrity and induces antimicrobial peptide (eg, cathelicidin) synthesis in epithelial cells and macrophages,79 80 directly enhancing host defence. However, the effects of vitamin D on the cellular components of immunity are rather complex. Vitamin D promotes differentiation of monocytes to macrophages and increases phagocytosis, superoxide production and bacterial killing by innate immune cells. It also promotes antigen processing by dendritic cells although antigen presentation may be impaired. Vitamin D is also reported to inhibit T-cell proliferation and production of cytokines by T helper 1 lymphocytes and of antibodies by B lymphocytes, highlighting the paradoxical nature of its effects. Effects on T helper 2 responses are not clear and vitamin D seems to increase number of regulatory T lymphocytes. Vitamin D seems to have little impact on CD8+ T lymphocytes. A systematic review and meta-analysis of the influence of vitamin D status on influenza vaccination (nine studies involving 2367 individuals) found lower seroprotection rates to influenza A virus subtype H3N2 and to influenza B virus in those who were vitamin D deficient.81 Berry et al 82 described an inverse linear relationship between vitamin D levels and respiratory tract infections in a cross-sectional study of 6789 British adults. In agreement with this, data from the US Third National Health and Nutrition Examination Survey which included 18 883 adults showed an independent inverse association between serum 25(OH)-vitamin D and recent upper respiratory tract infection.83 Other studies also report that individuals with low vitamin D status have a higher risk of viral respiratory tract infections.84 Supplementation of Japanese schoolchildren with vitamin D for 4 months during winter decreased the risk of influenza by about 40%.85 Meta-analyses have concluded that vitamin D supplementation can reduce the risk of respiratory tract infections86–89 (table 1).
Vitamin E, immunity and infection
There are a number of reviews of the role of vitamin E in immunity and host susceptibility to infection.90–92 These reviews contain citations to the many studies of vitamin E, immunity and infection that will be summarised here. In laboratory animals, vitamin E deficiency decreases lymphocyte proliferation, natural killer cell activity, specific antibody production following vaccination and phagocytosis by neutrophils. Vitamin E deficiency also increases susceptibility of animals to infectious pathogens. Vitamin E supplementation of the diet of laboratory animals enhances antibody production, lymphocyte proliferation, T helper 1-type cytokine production, natural killer cell activity and macrophage phagocytosis. Vitamin E promotes interaction between dendritic cells and CD4+ T lymphocytes. There is a positive association between plasma vitamin E and cell-mediated immune responses, and a negative association has been demonstrated between plasma vitamin E and the risk of infections in healthy adults over 60 years of age.93 There appears to be particular benefit of vitamin E supplementation for the elderly.94–97 Studies by Meydani et al 94 95 demonstrated that vitamin E supplementation at high doses (one study94 used 800 mg/day and the other95 used doses of 60, 200 and 800 mg/day) enhanced T helper 1 cell-mediated immunity (lymphocyte proliferation, IL-2 production) and improved vaccination responses, including to hepatitis B virus. Supplementation of older adults with vitamin E (200 mg/day) improved neutrophil chemotaxis and phagocytosis, natural killer cell activity and mitogen-induced lymphocyte proliferation.97 Secondary analysis of data from the Alpha-Tocopherol, Beta Carotene Cancer Prevention Study identified that daily vitamin E supplements for 5 to 8 years reduced the incidence of hospital treated, community-acquired pneumonia in smokers.98 One study reported that vitamin E supplementation (200 IU/day~135 mg/day) for 1 year decreased risk of upper respiratory tract infections in the elderly,99 but another study did not see an effect of supplemental vitamin E (200 mg/day) on the incidence, duration or severity of respiratory infections in an elderly population.100
Zinc, immunity and infection
There are a number of reviews of the role of zinc in immunity and host susceptibility to infection.101–109 These reviews contain citations to the many studies of zinc, immunity and infection that will be summarised here. Of note, Read et al 110 have recently provided a very insightful evaluation of the role of zinc in antiviral immunity. Zinc inhibits the RNA polymerase required by RNA viruses, like coronaviruses, to replicate,111 suggesting that zinc may play a key role in host defence against RNA viruses. In vitro replication of influenza virus was inhibited by the zinc ionophore pyrrolidine dithiocarbamate,112 and there are indications that zinc might inhibit replication of SARS-CoVs in vitro.113 In addition, as discussed by Read et al,110 the zinc-binding metallothioneins seem to play an important role in antiviral defence.114 Zinc deficiency has a marked impact on bone marrow, decreasing the number immune precursor cells, with reduced output of naive B lymphocytes and causes thymic atrophy, reducing output of naive T lymphocytes. Therefore, zinc is important in maintaining T and B lymphocyte numbers. Zinc deficiency impairs many aspects of innate immunity, including phagocytosis, respiratory burst and natural killer cell activity. Zinc also supports the release of neutrophil extracellular traps that capture microbes.115 There are also marked effects of zinc deficiency on acquired immunity. Circulating CD4+ T lymphocyte numbers and function (eg, IL-2 and IFN-γ production) are decreased and there is a disturbance in favour of T helper 2 cells. Likewise, B lymphocyte numbers and antibody production are decreased in zinc deficiency. Zinc supports proliferation of CD8+ cytotoxic T lymphocytes, key cells in antiviral defence. Many of the in vitro immune effects of zinc are prevented by zinc chelation.116 Moderate or mild zinc deficiency or experimental zinc deficiency in humans result in decreased natural killer cell activity, T lymphocyte proliferation, IL-2 production and cell-mediated immune responses which can all be corrected by zinc repletion.117 118 In patients with zinc deficiency related to sickle cell disease, natural killer cell activity is decreased, but can be returned to normal by zinc supplementation.119 Patients with the zinc malabsorption syndrome acrodermatitis enteropathica display severe immune impairments120 and increased susceptibility to bacterial, viral and fungal infections. Zinc supplementation (30 mg/day) increased T lymphocyte proliferation in elderly care home residents in the USA, an effect mainly due to an increase in numbers of T lymphocytes.121 The wide ranging impact of zinc deficiency on immune components is an important contributor to the increased susceptibility to infections, especially lower respiratory tract infection and diarrhoea, seen in zinc deficiency. Correcting zinc deficiency lowers the likelihood of diarrhoea and of respiratory and skin infections, although some studies fail to show benefit of zinc supplementation in respiratory disease.105 Meta-analysis of studies in Chinese children showed that those with recurrent respiratory tract infection were more likely to have low hair zinc.122 Recent systematic reviews and meta-analyses of trials with zinc report shorter duration of common cold in adults,123 124 reduced incidence and prevalence of pneumonia in children125 and reduced mortality when given to adults with severe pneumonia126 (table 1).
Copper, immunity and infection
There are a number of reviews of the role of copper in immunity and host susceptibility to infection.127–129 These reviews contain citations to the many studies of copper, immunity and infection that will be summarised here. Copper itself has antimicrobial properties. Copper supports neutrophil, monocyte and macrophage function and natural killer cell activity. It promotes T lymphocyte responses such as proliferation and IL-2 production. Copper deficiency in animals impairs a range of immune functions and increases susceptibility to bacterial and parasitic challenges. Human studies show that subjects on a low copper diet have decreased lymphocyte proliferation and IL-2 production, with copper administration reversing these effects.130 Children with Menke’s syndrome, a rare congenital disease with complete absence of the circulating copper-carrying protein caeruloplasmin, show immune impairments and have increased bacterial infections, diarrhoea and pneumonia.131 Meta-analysis of studies in Chinese children showed that those with recurrent respiratory tract infection were more likely to have low hair copper.122
Selenium, immunity and infection
There are a number of reviews of the role of selenium in immunity and host susceptibility to infection.132–138 These reviews contain citations to the many studies of selenium, immunity and infection that will be summarised here. Selenium deficiency in laboratory animals adversely affects several components of both innate and acquired immunity, including T and B lymphocyte function including antibody production and increases susceptibility to infections. Lower selenium concentrations in humans have been linked with diminished natural killer cell activity and increased mycobacterial disease. Selenium deficiency was shown to permit mutations of coxsackievirus, polio virus and murine influenza virus increasing virulence.139–142 These latter observations suggest that poor selenium status could result in the emergence of more pathogenic strains of virus, thereby increasing the risks and burdens associated with viral infection. Selenium supplementation (100 to 300 µg/day depending on the study) has been shown to improve various aspects of immune function in humans,143–145 including in the elderly.146 147 Selenium supplementation (50 or 100 µg/day) in adults in the UK with low selenium status improved some aspects of their immune response to a poliovirus vaccine.148
Iron, immunity and infection
There are a number of reviews of the role of iron in immunity and host susceptibility to infection.149–159 These reviews contain citations to the many studies of iron, immunity and infection that will be summarised here. Iron deficiency induces thymus atrophy, reducing output of naive T lymphocytes, and has multiple effects on immune function in humans. The effects are wide ranging and include impairment of respiratory burst and bacterial killing, natural killer cell activity, T lymphocyte proliferation and production of T helper 1 cytokines. T lymphocyte proliferation was lower by 50% to 60% in iron-deficient than in iron-replete housebound older Canadian women.160 These observations would suggest a clear case for iron deficiency increasing susceptibility to infection. However, the relationship between iron deficiency and susceptibility to infection remains complex.150 154–158 Evidence suggests that infections caused by organisms that spend part of their life-cycle intracellularly, such as plasmodia and mycobacteria, may actually be enhanced by iron. In the tropics, in children of all ages, iron at doses above a particular threshold has been associated with increased risk of malaria and other infections, including pneumonia. Thus, iron intervention in malaria-endemic areas is not advised, particularly high doses in the young, those with compromised immunity and during the peak malaria transmission season. There are different explanations for the detrimental effects of iron administration on infections. First, iron overload causes impairment of immune function.149–159 Second, excess iron favours damaging inflammation. Third, micro-organisms require iron and providing it may favour the growth of the pathogen. Perhaps for the latter reasons several host immune mechanisms have developed for withholding iron from a pathogen.154–157 159 In a recent study giving iron (50 mg on each of 4 days a week) to iron-deficient schoolchildren in South Africa increased the risk of respiratory infections161; coadministration of n-3 fatty acids (500 mg on each of 4 days a week) mitigated the effect of iron. Meta-analysis of studies in Chinese children showed that those with recurrent respiratory tract infection were more likely to have low hair iron.122
Gut microbiota, immunity and infection
Human gut microbiota
The human body is host to a significant number of bacteria and other organisms which colonise internal and external areas, such as the skin, mouth and gut. The community of organisms in a particular location is referred to as the microbiota. The gut microbiota shows a high degree of variability among individuals,162 reflecting differing exposures to environmental factors and the influence of host phenotype such as age and ethnicity. The large intestine is the site of the greatest number and diversity of bacterial species, with recent estimates of 1011 bacteria/g of colon contents.163 The gut microbiota is strongly influenced by habitual diet.164–167 Furthermore, both ageing and the presence or absence of disease significantly influence the composition of the microbiota.168 For example, with ageing, the abundance and diversity of bifidobacteria decline,169 while bacteria including streptococci, staphylococci, enterococci and enterobacteria increase.170 Changes seen within the gut microbiota with age are environment specific, with significant differences observed between populations from different countries. Within countries there are significant differences in the microbiota of free-living older adults and those residing in residential care.171 172 An abnormal gut microbiota, termed dysbiosis, is seen in obesity and in individuals with chronic age-related conditions.166 167 173–175 It is interesting to note that Xu et al 176 comment that some Chinese patients with COVID-19 showed intestinal dysbiosis with low numbers of lactobacilli and bifidobacteria.
Gut microbiota, probiotics and the immune system
Indigenous commensal bacteria within the gastrointestinal tract are believed to play a role in host immune defence by creating a barrier against colonisation by pathogens. Disease and the use of antibiotics can disrupt this barrier, creating an environment that favours the growth of pathogenic organisms. There is now evidence that providing exogenous, live, ‘desirable’ bacteria, termed probiotics, can contribute to maintenance of the host’s gastrointestinal barrier.177 Probiotic organisms are found in fermented foods including traditionally cultured dairy products and some fermented milks; the most commonly used commercial organisms are various lactobacilli and bifidobacteria.178 These organisms are able to colonise the gut temporarily, making their regular consumption necessary. In addition to creating a physical barrier, some of the products of the metabolism of both endogenous commensal bacteria and probiotic bacteria, including lactic acid and antimicrobial proteins, can directly inhibit the growth of pathogens.179 Probiotic bacteria also compete with some pathogenic bacteria for available nutrients. In addition to these direct interactions between commensal and probiotic organisms on the one hand and pathogens on the other, commensal and probiotic organisms can interact with the host’s gut epithelium and gut-associated immune tissues.179–181 These communications with the host may occur through chemicals released from the bacteria or through direct cell-to-cell contact and it is through these interactions that probiotics are thought to be able to influence immune function, even at sites distant from the gut.181 Nevertheless, the precise nature of these interactions is not very well understood.181 A large number of studies have examined the influence of various probiotic organisms, either alone or in combination, on immune function, infection and inflammatory conditions in human subjects.182 Certain probiotic organisms appear to enhance innate immunity (particularly phagocytosis and natural killer cell activity), but they seem to have a less pronounced effect on acquired immunity.182 Studies show improved vaccination responses in individuals taking probiotics,183 184 as reviewed elsewhere.185 Recent systematic reviews and meta-analyses confirm that probiotics or prebiotics (these are usually non-digestible oligosaccharides that act as fuels for some types of bacteria enhancing their growth; many probiotics are bifidogenic) enhance the antibody response to seasonal influenza vaccination in adults.186 187 The studies with probiotics have most often used lactobacilli or bifidobacteria.
Probiotic bacteria and gastrointestinal infections
A number of studies in children report lower incidence and duration of diarrhoea with certain probiotics. Recent systematic reviews and meta-analyses report that Lactobacillus paracasei CBA L74 reduces the risk of diarrhoea,188 that Lactobacillus acidophilus LB reduces duration of diarrhoea,188 that probiotics and synbiotics (combinations of probiotics and prebiotics) reduce durations of diarrhoea and hospitalisation and hasten recovery,189 that Lactobacillus rhamnosus GG reduces duration of diarrhoea,190 that Lactobacillus reuteri DSM 17938 reduces durations of diarrhoea and hospitalisation and increases early cure rate,191 192 and that Bacillus clausii reduces durations of diarrhoea and hospitalisation.193 In adults, there is now good evidence that probiotics protect against antibiotic-associated diarrhoea.194–198 Recent systematic reviews and meta-analyses report that probiotics reduce the risk of antibiotic-associated diarrhoea in adults aged 18 to 64 years but not in older adults (>65 years),199 that probiotics reduce the risk of Clostridium difficile-associated diarrhoea,200 that probiotics reduce the incidence and duration of antibiotic-associated diarrhoea and C. difficile-associated diarrhoea, with lactobacilli especially Lactobacillus casei being most effective,201 that L. rhamnosus GG may be most effective at treating antibiotic-associated diarrhoea,202 and that L. casei may be most effective at treating C. difficile-associated diarrhoea.202 What is evident from this research is that, although probiotics are effective in preventing and treating diarrhoea in both children and adults, there are considerable differences in the effects of different probiotic species and strains and the effects observed with one type of probiotic cannot be extrapolated to another. The recently released Handbook of COVID-19 Prevention and Treatment 203 comments that ‘some COVID-19 patients have gastrointestinal symptoms (such as abdominal pain and diarrhoea) due to direct viral infection of the intestinal mucosa or anti-viral and anti-infective drugs’. The handbook goes on to say that the dysbiosis seen in these patients176 ‘may lead to bacterial translocation and secondary infection, so it is important to maintain the balance of intestinal microecology [i.e. microbiota] by microecological modulator and nutritional support’ and that ‘microecologics [probiotics?] can reduce bacterial translocation and secondary infection … inhibit intestinal harmful bacteria, reduce toxin production and reduce infection caused by gut microflora dysbiosis … improve the gastrointestinal symptoms of patients … improve faecal character and defaecation frequency, and reduce diarrhoea by inhibiting intestinal mucosal atrophy … antibiotics can be adjusted timely and probiotics can be prescribed … these can reduce the chances of intestinal bacterial translocation and gut-derived infection’. These statements seem to be based on the existing literature rather than evidence from patients with COVID-19, and there is no clear description that such interventions have been successfully (or even unsuccessfully) performed in these patients. Indeed, Gao et al 204 state that ‘there is no direct clinical evidence that the modulation of gut microbiota plays the (sic) therapeutic role in the treatment of COVID-19’. Nevertheless, the observations suggest a potential for probiotic administration to have clinical relevance in patients with COVID-19.
Probiotic bacteria and respiratory infections
The gut microbiota seems to be protective against respiratory infection, as its depletion or absence in mice leads to impaired immune responses and worsens outcomes following bacterial or viral respiratory infection.205 206 These observations suggest a gut–lung axis of some importance in maintaining respiratory fitness during infection. There are a number of studies of probiotics in human respiratory disease, mainly in children, and mainly using different lactobacilli and bifidobacteria. Many of these studies find benefits of probiotics in terms of reduced incidence or severity of respiratory tract infections. These studies have been subject to a number of systematic reviews and meta-analyses over recent years207–215; the findings of these are summarised in table 2. Taken together, these findings provide some evidence that probiotics, in particular some lactobacilli and bifidobacteria, reduce the incidence, and improve the outcomes, of respiratory infections in humans. Thus, the observation that some Chinese patients with COVID-19 showed intestinal dysbiosis with low numbers of lactobacilli and bifidobacterial176 is important, but whether this dysbiosis is a predisposing factor to COVID-19 in those patients is not known. However, the totality of the evidence demonstrating that lactobacilli and bifidobacteria may improve immune function, enhance the response to seasonal influenza vaccination (which mimics a viral infection), reduce the incidence of respiratory infections, including those caused by viruses, and improve outcomes in those with respiratory infections would favour the use of these organisms as a strategy to reduce the risk and severity of viral respiratory infections.
Table 2.
Summary of selected systematic reviews and meta-analyses reporting on probiotics and respiratory infections
| Authors | Population | Included trials | Probiotic | Outcome | Effect |
| Vouloumanou et al 207 | Children and adults | 14 RCTs (3580 participants) | Any (mainly lactobacilli and bifidobacteria) | RTI | 4/10 RCTs reported probiotics reduced incidence of RTI 5/6 RCTs reported probiotics reduced severity of symptoms of RTI 3/9 RCTS reported probiotics shortened duration of RTI |
| Liu et al 208 | Critically ill adults | 12 RCTs (1546 patients) | Any | Nosocomial pneumonia | OR of nosocomial pneumonia with probiotics 0.75 (95% CI 0.57 to 0.97) No effect of probiotics on in hospital mortality, intensive care unit mortality, duration of hospital stay, duration of intensive care unit stay |
| Liu et al 209 | Children | 4 RCTs (1805 children) | Lactobacillus rhamnosus GG | RTI | RR of URTI with probiotics 0.62 (95% CI 0.50 to 0.78) RR for antibiotic treatment for URTI with probiotics 0.80 (95% CI 0.71 to 0.91) No effect of probiotics on LRTI or overall respiratory infections |
| King et al 210 | Children and adults | 20 RCTs (>4141 participants) | Any | RTI | SMD days of illness per person with probiotics −0.31 (95% CI −0.41 to −0.11); WMD days of illness with probiotics −0.77 (95% CI −1.50 to −0.04); SMD days of absence with probiotics −0.17 (95% CI −0.31 to −0.03) |
| Hao et al 211 | Children and adults | 13 RCTs; 12 RCTS in meta-analysis (3750 participants) | Any | URTI | OR of one URTI with probiotics 0.53 (95% CI 0.37 to 0.76) OR of at least three URTIs with probiotics 0.53 (95% CI 0.36 to 0.80) Mean duration of episode of URTI with probiotics −1.89 days (95% CI −2.03 to −1.75) OR for antibiotic prescription for URTI with probiotics 0.65 (95% CI 0.45 to 0.94) |
| Ozen et al 212 | Children | 14 RCTs | Any (mainly lactobacilli and bifidobacteria) | URTI | At least one beneficial effect of probiotics was observed in most of the RCTs |
| Araujo et al 213 | Children | 11 RCTs (2417 children) | Any | RTI | Several RCTs reports fewer new episodes, decreased duration of episodes and less severe symptoms |
| Wang et al 214 | Children | 23 RCTs (6269 children) | Any | RTI | RR of one RTI with probiotics 0.80 (95% CI 0.82 to 0.96); Days of RTI per child with probiotics −0.16 (95% CI −0.29 to 0.02) Days absent with probiotics −0.94 (95% CI −1.72 to −0.15) |
| Laursen and Hojsak215 | Children | 15 RCTs; 12 RCTs in meta-analysis (4527 children) | Any | RTI |
L. rhamnosus GG reduced duration of RTI −0.78 days (95% CI −1.46 to −0.090) Meta-analysis for other probiotics is not possible |
LRTI, lower respiratory tract infection; RCT, randomised controlled trial; RR, relative risk; RTI, respiratory tract infection; SMD, standardised mean difference; URTI, upper respiratory tract infection; WMD, weighted mean difference.
Nutritional intervention to control a cytokine storm
Coronaviruses cause respiratory disease and can lead to substantial lung damage.3–7 In trying to deal with this damage, cells of the immune system infiltrate the lungs initiating a significant inflammatory reaction. This can cause small blood vessels in the lung to leak fluid and fill up the alveoli, which makes it difficult for oxygen to enter the bloodstream for delivery to the body’s organs. This is when a patient will need ventilatory support. In the course of the battle between the host immune system and coronaviruses, excessive stimulation of the inflammatory response can occur. This is manifested as substantial production of reactive oxygen species, inflammatory eicosanoids and inflammatory chemokines and cytokines such as TNF-α, IL-1β and IL-6. This pro-oxidative, proinflammatory state is referred to as a ‘cytokine storm’; this is because this response of the innate immune system becomes damaging to host tissue and actually contributes to lung injury and respiratory failure. This condition is ARDS. The link between coronavirus infection, a cytokine storm and ARDS is elegantly described elsewhere.216 Mortality from ARDS is typically high. Patients with advanced COVID-19 are described to have markedly elevated inflammatory markers in their bloodstream,217 218 and ferritin, C reactive protein and IL-6 levels were significantly higher in non-survivors than survivors reports from Wuhan, China.219 220 As well as being directly damaging to host tissue, the excessive inflammatory response (ie, the cytokine storm) suppresses the acquired immune response; for example, numbers of CD4+ and CD8+ T lymphocytes are reduced,217 218 and the ability of CD4+ T lymphocytes to produce IFN-γ is impaired.217 This impairment of acquired immunity means that the individual’s ability to deal with the virus is seriously hindered.
Because of its pro-oxidant, proinflammatory state, ARDS may be amenable to treatment with nutrients that target oxidative stress and inflammation. The former would include classic antioxidants like vitamin C221 222 and vitamin E and also trace elements223 224 to support the activity of antioxidant enzymes, while the latter would include the bioactive n-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). EPA and DHA have anti-inflammatory properties acting to decrease the production of inflammatory eicosanoids produced from arachidonic acid, to inhibit NFκB signalling and to reduce production of inflammatory cytokines, as reviewed elsewhere.225–227 EPA is metabolised to weakly inflammatory eicosanoids,228 and both EPA and DHA are metabolised to metabolites known as specialised pro-resolving mediators (SPMs) which are known to resolve (turn off) ongoing inflammatory processes.229–231 Using an injury model with the isolated rabbit lung, EPA was shown to decrease arachidonic acid-derived inflammatory eicosanoids, arterial pressure and vascular leakage.232 Perfusion of isolated rabbit lungs with a fish oil-containing lipid emulsion markedly attenuated the vascular inflammatory reaction.233 Animal models of lung injury have shown that fish oil attenuates pulmonary accumulation of neutrophils,234 reduces lung permeability,235 reduces pulmonary oedema236 and attenuates cardiopulmonary dysfunction.237 The pro-resolving effects of EPA- and DHA-derived SPMs seem to be highly relevant to the effect of n-3 fatty acids in lung injury: Hecker et al 238 reported that the beneficial effects of fish oil in a murine model of acute lung injury were abrogated in mice lacking ChemR23, a receptor for some SPMs. Hence, the effects of n-3 fatty acids in lung injury might be due to their conversion to SPMs. In accordance with this, a number of studies of individual SPMs in various animal models of lung injury report reduced lung inflammation, increased bacterial killing and less or resolved lung injury.239–250 The active SPMs described in these animal studies include resolvin D1, aspirin-triggered resolvin D1, resolvin D3, aspirin-triggered resolvin D3, all produced from DHA, and resolvin E1, produced from EPA.
A number of trials of n-3 fatty acids in patients with ARDS have been performed. The earliest trials251–253 used the same enteral formulation which provided a high dose of EPA and DHA along with antioxidants and γ-linolenic acid. All three of these trials reported favourable effects on multiple inflammatory, respiratory and clinical outcomes, and a meta-analysis of these trials found significant improvements in ventilator-free days, new organ failures, length of stay in the intensive care unit and mortality.254 A more recent Cochrane meta-analysis255 pooled data from 10 randomised controlled trials of n-3 fatty acids in patients with ARDS. Six of these trials used the n-3 fatty acid, antioxidant and γ-linolenic acid formulation, while three used other enteral formulas dominated by n-3 fatty acids and one used parenteral n-3 fatty acids. The findings of the meta-analysis are summarised in table 3. The meta-analysis concluded that administration of n-3 fatty acids usually in combination with other bioactive nutrients led to ‘reductions in duration of mechanical ventilation and intensive care unit length of stay, along with improved oxygenation’. Putting all these observations together, it seems that patients with ARDS can be treated favourably with n-3 fatty acids, perhaps in combination with antioxidants, which act to reduce inflammation and the cytokine storm most likely through their conversion to SPMs, although EPA and DHA do have anti-inflammatory effects in their own right.
Table 3.
Summary of the findings of the meta-analysis of Dushianthan et al 255 of the effects of n-3 fatty acid-rich formulas in patients with ARDS
| Outcome | Effect | 95% CI | P value |
| PaO2/FiO2 at day 4 (mean difference, mm Hg) | 38.88 | 10.75 to 67.02 | 0.0068 |
| PaO2/FiO2 at day 8 (mean difference, mm Hg) | 23.44 | 1.73 to 45.15 | 0.034 |
| Ventilator days (mean difference, days) | −2.24 | −3.77 to −0.71 | 0.0042 |
| New organ failure (relative risk) | 0.45 | 0.32 to 0.63 | <0.00001 |
| Length of intensive care unit stay (mean difference, days) | −3.09 | −5.19 to −0.99 | 0.004 |
| 28-day mortality (relative risk) | 0.64 | 0.49 to 0.84 | 0.0015 |
| All-cause mortality (relative risk) | 0.79 | 0.59 to 1.07 | Not given |
ARDS, acute respiratory distress syndrome; FiO2, fractional inspired oxygen; PaO2, arterial oxygen tension (or pressure).
Steps to take to support the immune system through good nutrition
The foregoing discussion highlights that a number of vitamins (A, B6, B12, folate, C, D and E) and trace elements (zinc, copper, selenium, iron) are vital for supporting immune function. Other essential nutrients including other vitamins and trace elements, amino acids and fatty acids are also important in this regard. The understanding of the importance of these nutrients in immunity and in ensuring the host is better able to cope with pathogen exposure comes from states of deficiency (either experimental or ‘real world’) and their reversal. Thus, it is clear that situations of frank essential nutrient deficiency impair immune function and increase susceptibility to infections and that these two outcomes can both be prevented or reversed by treating the deficiency(ies). This may be through diet or in some cases may require supplementation or some other form of therapeutic administration, depending on the nutrient, the extent of the deficiency and the setting. Moving away from frank deficiency, there will be individuals in all populations who have ‘suboptimal’ intakes and status of one or more essential nutrients. It is not entirely clear the extent to which immune function in those individuals will be compromised. However, it seems likely that individuals with suboptimal intakes of a range of essential nutrients are likely to show suboptimal immune responses; this probably contributes to the variation in immune outcomes that is seen in the general population.256 257 In the interests of assuring the best possible immune response if an individual becomes infected, it would seem prudent to consume sufficient amounts of essential nutrients, although in most cases these amounts are not explicitly defined. Table 4 lists good dietary sources of key nutrients that support the immune system. This listing conveys that the best diet to support the immune system is one with a diverse and varied intake of vegetables, fruits, berries, nuts, seeds, grains and pulses along with some meats, eggs, dairy products and oily fish. This diet is consistent with those regarded as generally healthy258 and is consistent with current dietary guidelines.259 Such a diet would preclude too much processed and ‘junk’ food and excessive amounts of saturated fat and sugar. A randomised controlled trial of >5 servings of fruits and vegetables per day compared with <2 servings per day in older people (age 65 to 85 years) reported a better response to pneumococcal vaccination in the group consuming the higher amount of fruits and vegetables, although the response to tetanus vaccination was not different between the two groups.260 Human trials suggest that the intakes of some micronutrients needed to optimally support the immune system are likely to be in excess of intakes that can easily be achieved through diet alone. This is the case for vitamins C, D and E and zinc and selenium. There may be a role for immune-targeted supplements to achieve the intakes of these nutrients necessary to fully support the immune system. In addition to considering the ‘direct’ effects of nutrition on the immune system, many plant foods, fibre and fermented foods play a role in creating and maintaining a healthy gut microbiota167 175 that will also help to support the immune system.179–181
Table 4.
Important dietary sources of nutrients that support the immune system
| Nutrient | Good dietary sources |
| Vitamin A (or equivalents) | Milk and cheese, eggs, liver, oily fish, fortified cereals, dark orange or green vegetables (eg, carrots, sweet potatoes, pumpkin, squash, kale, spinach, broccoli), orange fruits (eg, apricots, peaches, papaya, mango, cantaloupe melon), tomato juice |
| Vitamin B6 | Fish, poultry, meat, eggs, whole grain cereals, fortified cereals, many vegetables (especially green leafy) and fruits, soya beans, tofu, yeast extract |
| Vitamin B12 | Fish, meat, some shellfish, milk and cheese, eggs, fortified breakfast cereals, yeast extract |
| Folate | Broccoli, brussels sprouts, green leafy vegetables (spinach, kale, cabbage), peas, chick peas, fortified cereals |
| Vitamin C | Oranges and orange juice, red and green peppers, strawberries, blackcurrants, kiwi, broccoli, brussels sprouts, potatoes |
| Vitamin D | Oily fish, liver, eggs, fortified foods (spreads and some breakfast cereals) |
| Vitamin E | Many vegetable oils, nuts and seeds, wheat germ (in cereals) |
| Zinc | Shellfish, meat, cheese, some grains and seeds, cereals, seeded or wholegrain breads |
| Selenium | Fish, shellfish, meat, eggs, some nuts especially brazil nuts |
| Iron | Meat, liver, beans, nuts, dried fruit (eg, apricots), wholegrains (eg, brown rice), fortified cereals, most dark green leafy vegetables (spinach, kale) |
| Copper | Shellfish, nuts, liver, some vegetables |
| Essential amino acids | Meat, poultry, fish, eggs, milk and cheese, soya, nuts and seeds, pulses |
| Essential fatty acids | Many seeds, nuts and vegetable oils |
| Long chain omega-3 fatty acids (EPA and DHA) | Oily fish |
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Summary and conclusions
The immune system protects the host from pathogenic organisms (bacteria, viruses, fungi, parasites). To deal with such an array of threats, the human immune system has evolved to include a myriad of specialised cell types, communicating molecules and functional responses. The immune system is always active, carrying out surveillance, but its activity is enhanced if an individual becomes infected. This heightened activity is accompanied by an increased rate of metabolism, requiring energy sources, substrates for biosynthesis and regulatory molecules, which are all ultimately derived from the diet. Through experimental research and studies of people with deficiencies, a number of vitamins (A, B6, B12, folate, C, D and E) and trace elements (zinc, copper, selenium, iron) have been demonstrated to have key roles in supporting the human immune system and reducing risk of infections. Other essential nutrients including other vitamins and trace elements, amino acids and fatty acids are also important in this regard. All of nutrients named above have roles in supporting antibacterial and antiviral defences but zinc and selenium seem to be particularly important for the latter. It would seem prudent for individuals to consume sufficient amounts of essential nutrients to support their immune system to help them to deal with pathogens should they become infected. Consumption of a diet of diverse and varied plant-based and animal-based foods that is consistent with current healthy eating guidelines would be best to support the immune system. However, human trials suggest that the intakes of some micronutrients (vitamins C, D and E and zinc and selenium) needed to optimally support the immune system are in excess of intakes that can easily be achieved through diet alone and in this case supplementation might be considered. The gut microbiota plays a role in educating and regulating the immune system and gut dysbiosis is a feature of disease including many infectious diseases. Therefore, dietary approaches to achieve a healthy microbiota can also benefit the immune system. There is evidence that probiotic bacteria, particularly some lactobacilli and bifidobacteria, can modify the microbiota, modulate the immune response and protect against infections, including of the respiratory tract. Many plant foods, fibre and fermented foods play a role in creating and maintaining a healthy gut microbiota and so will also help to support the immune system. Thus, specific nutrients and the foods that provide them can play a role in supporting the immune system in order that the host can better defend against bacteria and viruses if infected. Therefore, having a healthy diet could be an important factor, but one of many, in determining outcome in individuals should they become infected with coronavirus. However, it is important to note that there are no published nutrition studies in the context of SARS-CoV-2 or COVID-19. Chinese researchers have noted dysbiosis in patients with severe COVID-19 and have recommended treatment with probiotics, but it is not clear whether this was done and, if it was, whether it was successful in improving clinical outcome. Severe infection of the respiratory epithelium can lead to ARDS, characterised by excessive and damaging host inflammation, termed a cytokine storm. This is seen in cases of severe COVID-19. There is evidence from ARDS in other settings that the cytokine storm can be controlled by the n-3 fatty acids EPA and DHA, possibly through their metabolism to SPMs. This therapeutic approach has not been attempted in severe COVID-19 and warrants investigation.
Acknowledgments
PCC is supported by the National Institute for Health Research (NIHR) through the NIHR Southampton Biomedical Research Centre.
Footnotes
Contributors: The author was solely responsible for all aspects of preparation of the manuscript.
Funding: The author has not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PCC has research funding from Bayer Consumer Care; acts as an advisor/consultant to BASF AS, DSM, Cargill, Smartfish, Nutrileads, Bayer Consumer Care and Pfizer (now GSK) Consumer Healthcare; has received reimbursement for travel and/or speaking from Danone, Fresenius Kabi, Baxter Healthcare, B Braun Melsungen, Pfizer (now GSK) Consumer Healthcare, Abbott, Smartfish, Biogredia and the California Walnut Commission; and is President and member of the Board of Directors of the European Branch of the International Life Sciences Institute.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Calder PC. Feeding the immune system. Proc. Nutr. Soc. 2013;72:299–309. 10.1017/S0029665113001286 [DOI] [PubMed] [Google Scholar]
- 2. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune System–Working in harmony to reduce the risk of infection. Nutrients 2020;12:E236 10.3390/nu12010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leung C. Clinical features of deaths in the novel coronavirus epidemic in China. Rev Med Virol 2020;30:e2103. 10.1002/rmv.2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu D, Wu T, Liu Q, et al. The SARS-CoV-2 outbreak: what we know. Int J Infect Dis 2020;94:44–8. 10.1016/j.ijid.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv. Virus Res 2011;81:85–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 2016;24:490–502. 10.1016/j.tim.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y, Liu Q, Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 2020;92:418–23. 10.1002/jmv.25681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dosch SF, Mahajan SD, Collins AR. Sars coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res 2009;142:19–27. 10.1016/j.virusres.2009.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hu W, Yen Y-T, Singh S, et al. Sars-Cov regulates immune function-related gene expression in human monocytic cells. Viral Immunol 2012;25:277–88. 10.1089/vim.2011.0099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Liu L. The membrane protein of severe acute respiratory syndrome coronavirus functions as a novel cytosolic pathogen-associated molecular pattern to promote beta interferon induction via a toll-like-receptor-related TRAF3-independent mechanism. mBio 2016;7:e01872–15. 10.1128/mBio.01872-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Qahtani AA, Lyroni K, Aznaourova M, et al. Middle east respiratory syndrome corona virus spike glycoprotein suppresses macrophage responses via DPP4-mediated induction of IRAK-M and PPARγ. Oncotarget 2017;8:9053–66. 10.18632/oncotarget.14754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in Detente. Science 2006;312:879–82. 10.1126/science.1125676 [DOI] [PubMed] [Google Scholar]
- 13. Sallard E, Lescure FX, Yazdanpanah Y, et al. C-20-15 discovery French Steering Committee (2020) type 1 interferons as a potential treatment against COVID-19. Antiviral Res;178:104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pawelec G, Larbi A, Derhovanessian E. Senescence of the human immune system. J Comp Pathol 2010;142:S39–44. 10.1016/j.jcpa.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 15. Pera A, Campos C, López N, et al. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas 2015;82:50–5. 10.1016/j.maturitas.2015.05.004 [DOI] [PubMed] [Google Scholar]
- 16. Agarwal S, Busse PJ. Innate and adaptive immunosenescence. Ann Allergy Asthma Immunol 2010;104:183–90. 10.1016/j.anai.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 17. Montgomery RR, Shaw AC. Paradoxical changes in innate immunity in aging: recent progress and new directions. J Leukoc Biol 2015;98:937–43. 10.1189/jlb.5MR0315-104R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ventura MT, Casciaro M, Gangemi S, et al. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy 2017;15:21 10.1186/s12948-017-0077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fulop T, Pawelec G, Castle S, et al. Immunosenescence and vaccination in nursing home residents. Clin Infect Dis 2009;48:443–8. 10.1086/596475 [DOI] [PubMed] [Google Scholar]
- 20. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine 2006;24:1159–69. 10.1016/j.vaccine.2005.08.105 [DOI] [PubMed] [Google Scholar]
- 21. Fujihashi K, Kiyono H. Mucosal immunosenescence: new developments and vaccines to control infectious diseases. Trends Immunol 2009;30:334–43. 10.1016/j.it.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 22. Ogra PL. Ageing and its possible impact on mucosal immune responses. Ageing Res Rev 2010;9:101–6. 10.1016/j.arr.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 23. Calder PC, Bosco N, Bourdet-Sicard R, et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev 2017;40:95–119. 10.1016/j.arr.2017.09.001 [DOI] [PubMed] [Google Scholar]
- 24. Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc 2012;71:298–306. 10.1017/S0029665112000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andersen CJ, Murphy KE, Fernandez ML. Impact of obesity and metabolic syndrome on immunity. Adv Nutr 2016;7:66–75. 10.3945/an.115.010207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Honce R, Schultz-Cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol 2019;10:1071 10.3389/fimmu.2019.01071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frasca D, Diaz A, Romero M, et al. Ageing and obesity similarly impair antibody responses. Clin Exp Immunol 2017;187:64–70. 10.1111/cei.12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O’Shea D, Hogan AE. Dysregulation of natural killer cells in obesity. Cancers 2019;11:E573 10.3390/cancers11040573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huttunen R, Syrjänen J. Obesity and the risk and outcome of infection. Int J Obes 2013;37:333–40. 10.1038/ijo.2012.62 [DOI] [PubMed] [Google Scholar]
- 30. Dobner J, Kaser S. Body mass index and the risk of infection - from underweight to obesity. Clinical Microbiology and Infection 2018;24:24–8. 10.1016/j.cmi.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 31. Frasca D, Blomberg BB. The impact of obesity and metabolic syndrome on vaccination success. Interdiscip. Top. Gerontol. Geriatr 2020;43:86–97. [DOI] [PubMed] [Google Scholar]
- 32. Green WD, Beck MA. Obesity impairs the adaptive immune response to influenza virus. Ann Am Thorac Soc 2017;14:S406–9. 10.1513/AnnalsATS.201706-447AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes 2012;36:1072–7. 10.1038/ijo.2011.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paich HA, Sheridan PA, Handy J, et al. Overweight and obese adult humans have a defective cellular immune response to pandemic H1N1 influenza A virus. Obesity 2013;21:2377–86. 10.1002/oby.20383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Calder PC, Ahluwalia N, Brouns F, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr 2011;106:S5–78. 10.1017/S0007114511005460 [DOI] [PubMed] [Google Scholar]
- 36. Simonnet A, Chetboun M, Poissy J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. In Press 2020. 10.1002/oby.22831. [Epub ahead of print: 09 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Guidance on the scientific requirements for health claims related to the immune system, the gastrointestinal tract and defence against pathogenic microorganisms. Efsa J 2016;14:4369. [Google Scholar]
- 38. Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab 2007;51:301–23. 10.1159/000107673 [DOI] [PubMed] [Google Scholar]
- 39. Maggini S, Wintergerst ES, Beveridge S, et al. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr 2007;98:S29–35. 10.1017/S0007114507832971 [DOI] [PubMed] [Google Scholar]
- 40. Maggini S, Pierre A, Calder P. Immune function and micronutrient requirements change over the life course. Nutrients 2018;10:1531 10.3390/nu10101531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alpert PT. The role of vitamins and minerals on the immune system. Home Health Care Manag Pract 2017;29:199–202. 10.1177/1084822317713300 [DOI] [Google Scholar]
- 42. Wu D, Lewis ED, Pae M, et al. Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol 2019;9:3160 10.3389/fimmu.2018.03160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calder PC, Field CJ, Gill HA. Nutrition and immune function (EDS). Wallingford: CAB International, 2002. [Google Scholar]
- 44. Shetty P. Nutrition immunity infection. Wallingford: CAB International, 2010. [Google Scholar]
- 45. Calder PC, Yaqoob P. Diet, immunity and inflammation (EDS). Cambridge: Woodhead Publishing, 2013. [Google Scholar]
- 46. Calder PC, Kulkarni A. Nutrition, immunity and infection (EDS). Boca Raton: CRC Press, 2018. [Google Scholar]
- 47. Villamor E, Fawzi WW. Effects of vitamin A supplementation on immune responses and correlation with clinical outcomes. Clin Microbiol Rev 2005;18:446–64. 10.1128/CMR.18.3.446-464.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ross AC. Vitamin A and retinoic acid in T cell–related immunity. Am J Clin Nutr 2012;96:1166S–72. 10.3945/ajcn.112.034637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Raverdeau M, Mills KHG. Modulation of T cell and innate immune responses by retinoic acid. J Immunol 2014;192:2953–8. 10.4049/jimmunol.1303245 [DOI] [PubMed] [Google Scholar]
- 50. Brown CC, Noelle RJ. Seeing through the dark: new insights into the immune regulatory functions of vitamin A. Eur J Immunol 2015;45:1287–95. 10.1002/eji.201344398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Larange A, Cheroutre H. Retinoic acid and retinoic acid receptors as pleiotropic modulators of the immune system. Annu Rev Immunol 2016;34:369–94. 10.1146/annurev-immunol-041015-055427 [DOI] [PubMed] [Google Scholar]
- 52. Erkelens MN, Mebius RE. Retinoic acid and immune homeostasis: a balancing act. Trends Immunol 2017;38:168–80. 10.1016/j.it.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 53. Huang Z, Liu Y, Qi G, et al. Role of vitamin A in the immune system. JCM 2018;7:258 10.3390/jcm7090258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oliveira LdeM, Teixeira FME, Sato MN. Impact of retinoic acid on immune cells and inflammatory diseases. Mediators Inflamm 2018;2018:1–17. 10.1155/2018/3067126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ross AC. Vitamin A deficiency and retinoid repletion regulate the antibody response to bacterial antigens and the maintenance of natural killer cells. Clin Immunol Immunopathol 1996;80:S63–72. 10.1006/clin.1996.0143 [DOI] [PubMed] [Google Scholar]
- 56. Semba RD, Scott AL, et al. , Muhilal . Depressed immune response to tetanus in children with vitamin A deficiency. J Nutr 1992;122:101–7. 10.1093/jn/122.1.101 [DOI] [PubMed] [Google Scholar]
- 57. Imdad A, Mayo-Wilson E, Herzer K, et al. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database Syst Rev 2017;3:CD008524. 10.1002/14651858.CD008524.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu N, QB L, Zou SY. Effect of vitamin A as an adjuvant therapy for pneumonia in children: a meta analysis. Zhongguo Dang Dai Er. Ke. Za Zhi 2018;20:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yoshii K, Hosomi K, Sawane K, et al. Metabolism of dietary and microbial vitamin B family in the regulation of host immunity. Front. Nutr. 2019;6:48 10.3389/fnut.2019.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tamura J, Kubota K, Murakami H, et al. Immunomodulation by vitamin B12: augmentation of CD8+ T lymphocytes and natural killer (NK) cell activity in vitamin B12-deficient patients by methyl-B12 treatment. Clin Exp Immunol 1999;116:28–32. 10.1046/j.1365-2249.1999.00870.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Meydani SN, Ribaya-Mercado JD, Russell RM, et al. Vitamin B − 6 deficiency impairs interleukin 2 production and lymphocyte proliferation in elderly adults. Am J Clin Nutr 1991;53:1275–80. 10.1093/ajcn/53.5.1275 [DOI] [PubMed] [Google Scholar]
- 62. Carr A, Maggini S. Vitamin C and immune function. Nutrients 2017;9:1211 10.3390/nu9111211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hemilä H. Vitamin C and infections. Nutrients 2017;9:339 10.3390/nu9040339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jacob RA, Kelley DS, Pianalto FS, et al. Immunocompetence and oxidant defense during ascorbate depletion of healthy men. Am J Clin Nutr 1991;54:1302S–9. 10.1093/ajcn/54.6.1302s [DOI] [PubMed] [Google Scholar]
- 65. Hemilä H, Louhiala P. Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev 2013:CD005532. 10.1002/14651858.CD005532.pub3 [DOI] [PubMed] [Google Scholar]
- 66. Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev 2013:CD000980. 10.1002/14651858.CD000980.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ooi JH, Chen J, Cantorna MT. Vitamin D regulation of immune function in the gut: why do T cells have vitamin D receptors? Mol Aspects Med 2012;33:77–82. 10.1016/j.mam.2011.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hewison M. An update on vitamin D and human immunity. Clin Endocrinol 2012;76:315–25. 10.1111/j.1365-2265.2011.04261.x [DOI] [PubMed] [Google Scholar]
- 69. Di Rosa M, Malaguarnera M, Nicoletti F, et al. Vitamin D3: a helpful immuno-modulator. Immunology 2011;134:123–39. 10.1111/j.1365-2567.2011.03482.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Van Belle TL, Gysemans C, Mathieu C. Vitamin D in autoimmune, infectious and allergic diseases: a vital player? Best Pract Res Clin Endocrinol Metab 2011;25:617–32. 10.1016/j.beem.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 71. Hewison M. Vitamin D and immune function: an overview. Proc Nutr Soc 2012;71:50–61. 10.1017/S0029665111001650 [DOI] [PubMed] [Google Scholar]
- 72. Sassi F, Tamone C, D’Amelio P. Vitamin D: nutrient, hormone, and immunomodulator. Nutrients 2018;10:1656 10.3390/nu10111656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cantorna MT, Snyder L, Lin Y-D, et al. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015;7:3011–21. 10.3390/nu7043011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Medrano M, Carrillo-Cruz E, Montero I, et al. Vitamin D: effect on haematopoiesis and immune system and clinical applications. Int J Mol Sci 2018;19:2663 10.3390/ijms19092663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gois P, Ferreira D, Olenski S, et al. Vitamin D and infectious diseases: simple bystander or contributing factor? Nutrients 2017;9:651 10.3390/nu9070651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chirumbolo S, Bjørklund G, Sboarina A, et al. The role of vitamin D in the immune system as a pro-survival molecule. Clin Ther 2017;39:894–916. 10.1016/j.clinthera.2017.03.021 [DOI] [PubMed] [Google Scholar]
- 77. Aranow C. Vitamin D and the immune system. J Investig Med 2011;59:881–6. 10.2310/JIM.0b013e31821b8755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Prietl B, Treiber G, Pieber T, et al. Vitamin D and immune function. Nutrients 2013;5:2502–21. 10.3390/nu5072502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol 2009;4:1151–65. 10.2217/fmb.09.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang T-T, Nestel FP, Bourdeau V, et al. Cutting Edge: 1,25-Dihydroxyvitamin D 3 Is a Direct Inducer of Antimicrobial Peptide Gene Expression. J Immunol 2004;173:2909–12. 10.4049/jimmunol.173.5.2909 [DOI] [PubMed] [Google Scholar]
- 81. Lee M-D, Lin C-H, Lei W-T, et al. Does vitamin D deficiency affect the immunogenic responses to influenza vaccination? A systematic review and meta-analysis. Nutrients 2018;10:409 10.3390/nu10040409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Berry DJ, Hesketh K, Power C, et al. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr 2011;106:1433–40. 10.1017/S0007114511001991 [DOI] [PubMed] [Google Scholar]
- 83. Ginde AA, Mansbach JM, Camargo CA. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third National Health and Nutrition Examination Survey. Arch Intern Med 2009;169:384–90. 10.1001/archinternmed.2008.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sabetta JR, DePetrillo P, Cipriani RJ, et al. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One 2010;5:e11088 10.1371/journal.pone.0011088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Urashima M, Segawa T, Okazaki M, et al. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 2010;91:1255–60. 10.3945/ajcn.2009.29094 [DOI] [PubMed] [Google Scholar]
- 86. Bergman P, Lindh Åsa U., Björkhem-Bergman L, et al. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2013;8:e65835 10.1371/journal.pone.0065835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017;356:i6583 10.1136/bmj.i6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pham H, Rahman A, Majidi A, et al. Acute respiratory tract infection and 25-hydroxyvitamin D concentration: a systematic review and meta-analysis. Int J Environ Res Public Health 2019;16:3020 10.3390/ijerph16173020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhou YF, Luo BA, Qin LL. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine 2019;98:17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Meydani SN, Han SN, Wu D. Vitamin E and immune response in the aged: molecular mechanisms and clinical implications. Immunol Rev 2005;205:269–84. 10.1111/j.0105-2896.2005.00274.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wu D, Meydani SN. Age-Associated changes in immune function: impact of vitamin E intervention and the underlying mechanisms. Endocr Metab Immune Disord Drug Targets 2014;14:283–9. 10.2174/1871530314666140922143950 [DOI] [PubMed] [Google Scholar]
- 92. Lee G, Han S. The role of vitamin E in immunity. Nutrients 2018;10:614 10.3390/nu10111614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chavance M, Herbeth B, Fournier C, et al. Vitamin status, immunity and infections in an elderly population. Eur J Clin Nutr 1989;43:827–35. [PubMed] [Google Scholar]
- 94. Meydani SN, Barklund MP, Liu S, et al. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr 1990;52:557–63. 10.1093/ajcn/52.3.557 [DOI] [PubMed] [Google Scholar]
- 95. Meydani SN, et al. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. JAMA 1997;277:1380–6. 10.1001/jama.1997.03540410058031 [DOI] [PubMed] [Google Scholar]
- 96. Pallast EG, Schouten EG, de Waart FG, et al. Effect of 50- and 100-mg vitamin E supplements on cellular immune function in noninstitutionalized elderly persons. Am J Clin Nutr 1999;69:1273–81. 10.1093/ajcn/69.6.1273 [DOI] [PubMed] [Google Scholar]
- 97. De la Fuente M, Hernanz A, Guayerbas N, et al. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic Res 2008;42:272–80. 10.1080/10715760801898838 [DOI] [PubMed] [Google Scholar]
- 98. Hemilä H. Vitamin E administration may decrease the incidence of pneumonia in elderly males. Clin Interv Aging 2016;11:1379–85. 10.2147/CIA.S114515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Meydani SN, Leka LS, Fine BC, et al. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA 2004;292:828–36. 10.1001/jama.292.7.828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Graat JM, Schouten EG, Kok FJ. Effect of daily vitamin E and multivitamin-mineral supplementation on acute respiratory tract infections in elderly persons: a randomized controlled trial. JAMA 2002;288:715–21. 10.1001/jama.288.6.715 [DOI] [PubMed] [Google Scholar]
- 101. Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med 2008;14:353–7. 10.2119/2008-00033.Prasad [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Walker CF, Black RE. Zinc and the risk for infectious disease. Annu Rev Nutr 2004;24:255–75. 10.1146/annurev.nutr.23.011702.073054 [DOI] [PubMed] [Google Scholar]
- 103. Wessels I, Maywald M, Rink L, et al. Zinc as a gatekeeper of immune function. Nutrients 2017;9:1286 10.3390/nu9121286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Maywald M, Wessels I, Rink L. Zinc signals and immunity. Int J Mol Sci 2017;18:2222 10.3390/ijms18102222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Gammoh NZ, Rink L. Zinc in infection and inflammation. Nutrients 2017;9:624. 10.3390/nu9060624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Hojyo S, Fukada T. Roles of zinc signaling in the immune system. J Immunol Res 2016;2016:1–21. 10.1155/2016/6762343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Maares M, Haase H. Zinc and immunity: an essential interrelation. Arch Biochem Biophys 2016;611:58–65. 10.1016/j.abb.2016.03.022 [DOI] [PubMed] [Google Scholar]
- 108. Subramanian Vignesh K, Deepe GS. Immunological orchestration of zinc homeostasis: the battle between host mechanisms and pathogen defenses. Arch Biochem Biophys 2016;611:66–78. 10.1016/j.abb.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bonaventura P, Benedetti G, Albarède F, et al. Zinc and its role in immunity and inflammation. Autoimmun Rev 2015;14:277–85. 10.1016/j.autrev.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 110. Read SA, Obeid S, Ahlenstiel C, et al. The role of zinc in antiviral immunity. Adv Nutr 2019;10:696–710. 10.1093/advances/nmz013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kaushik N, Subramani C, Anang S, et al. Zinc salts block hepatitis E virus replication by inhibiting the activity of viral RNA-dependent RNA polymerase. J. Virol 2017;91i:e00754–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Uchide N, Ohyama K, Bessho T, et al. Effect of antioxidants on apoptosis induced by influenza virus infection: inhibition of viral gene replication and transcription with pyrrolidine dithiocarbamate. Antiviral Res 2002;56:207–17. 10.1016/S0166-3542(02)00109-2 [DOI] [PubMed] [Google Scholar]
- 113. te Velthuis AJW, van den Worm SHE, Sims AC, et al. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog 2010;6:e1001176 10.1371/journal.ppat.1001176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Subramanian Vignesh K, Deepe Jr. G. Metallothioneins: emerging modulators in immunity and infection. Int J Mol Sci 2017;18:2197 10.3390/ijms18102197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hasan R, Rink L, Haase H. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun 2013;19:253–64. 10.1177/1753425912458815 [DOI] [PubMed] [Google Scholar]
- 116. Hasan R, Rink L, Haase H. Chelation of free Zn²⁺ impairs chemotaxis, phagocytosis, oxidative burst, degranulation, and cytokine production by neutrophil granulocytes. Biol Trace Elem Res 2016;171:79–88. 10.1007/s12011-015-0515-0 [DOI] [PubMed] [Google Scholar]
- 117. Kahmann L, Uciechowski P, Warmuth S, et al. Zinc supplementation in the elderly reduces spontaneous inflammatory cytokine release and restores T cell functions. Rejuvenation Res 2008;11:227–37. 10.1089/rej.2007.0613 [DOI] [PubMed] [Google Scholar]
- 118. Beck FW, Prasad AS, Kaplan J, et al. Changes in cytokine production and T cell subpopulations in experimentally induced zinc-deficient humans. Am J Physiol 1997;272:E1002–7. 10.1152/ajpendo.1997.272.6.E1002 [DOI] [PubMed] [Google Scholar]
- 119. Tapazoglou E, Prasad AS, Hill G, et al. Decreased natural killer cell activity in patients with zinc deficiency with sickle cell disease. J Lab Clin Med 1985;105:19–22. [PubMed] [Google Scholar]
- 120. Sandström B, et al. Acrodermatitis enteropathica, zinc metabolism, copper status, and immune function. Arch Pediatr Adolesc Med 1994;148:980–5. 10.1001/archpedi.1994.02170090094017 [DOI] [PubMed] [Google Scholar]
- 121. Barnett JB, Dao MC, Hamer DH, et al. Effect of zinc supplementation on serum zinc concentration and T cell proliferation in nursing home elderly: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2016;103:942–51. 10.3945/ajcn.115.115188 [DOI] [PubMed] [Google Scholar]
- 122. Mao S, Zhang A, Huang S. Meta-Analysis of Zn, Cu and Fe in the hair of Chinese children with recurrent respiratory tract infection. Scand J Clin Lab Invest 2014;74:561–7. 10.3109/00365513.2014.921323 [DOI] [PubMed] [Google Scholar]
- 123. Hemilä H. Zinc lozenges and the common cold: a meta-analysis comparing zinc acetate and zinc gluconate, and the role of zinc dosage. JRSM Open 2017;8:205427041769429. 10.1177/2054270417694291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Science M, Johnstone J, Roth DE, et al. Zinc for the treatment of the common cold: a systematic review and meta-analysis of randomized controlled trials. Can Med Assoc J 2012;184:E551–61. 10.1503/cmaj.111990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lassi ZS, Moin A, Bhutta ZA. Zinc supplementation for the prevention of pneumonia in children aged 2 months to 59 months. Cochrane Database Syst Rev 2016;12:CD005978. 10.1002/14651858.CD005978.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang L, Song Y. Efficacy of zinc given as an adjunct to the treatment of severe pneumonia: a meta-analysis of randomized, double-blind and placebo-controlled trials. Clin Respir J 2018;12:857–64. 10.1111/crj.12646 [DOI] [PubMed] [Google Scholar]
- 127. Percival SS. Copper and immunity. Am J Clin Nutr 1998;67:1064S–8. 10.1093/ajcn/67.5.1064S [DOI] [PubMed] [Google Scholar]
- 128. Li C, Li Y, Ding C. The role of copper homeostasis at the host-pathogen axis: from bacteria to fungi. Int J Mol Sci 2019;20:175 10.3390/ijms20010175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Besold AN, Culbertson EM, Culotta VC. The yin and yang of copper during infection. J Biol Inorg Chem 2016;21:137–44. 10.1007/s00775-016-1335-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Hopkins RG, Failla ML. Copper deficiency reduces interleukin-2 (IL-2) production and IL-2 mRNA in human T-lymphocytes. J Nutr 1997;127:257–62. 10.1093/jn/127.2.257 [DOI] [PubMed] [Google Scholar]
- 131. Vyas D, Chandra RK. Thymic factor activity, lymphocyte stimulation response and antibody producing cells in copper deficiency. Nutr Res 1983;3:343–9. 10.1016/S0271-5317(83)80084-0 [DOI] [Google Scholar]
- 132. McKenzie RC, S. Rafferty T, Beckett GJ. Selenium: an essential element for immune function. Immunol Today 1998;19:342–5. 10.1016/S0167-5699(98)01294-8 [DOI] [PubMed] [Google Scholar]
- 133. Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 2012;16:705–43. 10.1089/ars.2011.4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Arthur JR, McKenzie RC, Beckett GJ. Selenium in the immune system. J Nutr 2003;133:1457S–9. 10.1093/jn/133.5.1457S [DOI] [PubMed] [Google Scholar]
- 135. Avery J, Hoffmann P. Selenium, selenoproteins, and immunity. Nutrients 2018;10:1203 10.3390/nu10091203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Huang Z, Rose AH, Hoffmann PR. The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 2012;16:705–43. 10.1089/ars.2011.4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hoffmann PR, Berry MJ. The influence of selenium on immune responses. Mol Nutr Food Res 2008;52:1273–80. 10.1002/mnfr.200700330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Guillin OM, Vindry C, Ohlmann T, et al. Selenium, selenoproteins and viral infection. Nutrients 2019;11:2101 10.3390/nu11092101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Beck MA, Levander OA. Host nutritional status and its effect on a viral pathogen. J Infect Dis 2000;182:S93–6. 10.1086/315918 [DOI] [PubMed] [Google Scholar]
- 140. Beck M, Handy J, Levander O. Host nutritional status: the neglected virulence factor. Trends Microbiol 2004;12:417–23. 10.1016/j.tim.2004.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Beck MA, Nelson HK, Shi Q, et al. Selenium deficiency increases the pathology of an influenza virus infection. Faseb J 2001;15:1481–3. 10.1096/fj.00-0721fje [DOI] [PubMed] [Google Scholar]
- 142. Nelson HK, Shi Q, Van Dael P, et al. Host nutritional selenium status as a driving force for influenza virus mutations. FASEB j. 2001;15:1727–38. 10.1096/fj.01-0108com [DOI] [PubMed] [Google Scholar]
- 143. Roy M, Kiremidjian-Schumacher L, Wishe HI, et al. Supplementation with selenium and human immune cell functions. I. Effect on lymphocyte proliferation and interleukin 2 receptor expression. Biol Trace Elem Res 1994;41:103–14. 10.1007/BF02917221 [DOI] [PubMed] [Google Scholar]
- 144. Hawkes WC, Kelley DS, Taylor PC. The effects of dietary selenium on the immune system in healthy men. Biol Trace Elem Res 2001;81:189–213. 10.1385/BTER:81:3:189 [DOI] [PubMed] [Google Scholar]
- 145. Kiremidjian-Schumacher L, Roy M, Wishe HI, et al. Supplementation with selenium and human immune cell functions. II. Effect on cytotoxic lymphocytes and natural killer cells. Biol Trace Elem Res 1994;41:115–27. 10.1007/BF02917222 [DOI] [PubMed] [Google Scholar]
- 146. Peretz A, Nève J, Desmedt J, et al. Lymphocyte response is enhanced by supplementation of elderly subjects with selenium-enriched yeast. Am J Clin Nutr 1991;53:1323–8. 10.1093/ajcn/53.5.1323 [DOI] [PubMed] [Google Scholar]
- 147. Roy M, Kiremidjian-Schumacher L, Wishe HI, et al. Supplementation with selenium restores age-related decline in immune cell function. Exp Biol Med 1995;209:369–75. 10.3181/00379727-209-43909 [DOI] [PubMed] [Google Scholar]
- 148. Broome CS, McArdle F, Kyle JAM, et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr 2004;80:154–62. 10.1093/ajcn/80.1.154 [DOI] [PubMed] [Google Scholar]
- 149. Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest 2002;32 Suppl 1:70–8. 10.1046/j.1365-2362.2002.0320s1070.x [DOI] [PubMed] [Google Scholar]
- 150. Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr 2001;131:616S–35. 10.1093/jn/131.2.616S [DOI] [PubMed] [Google Scholar]
- 151. Schaible UE, Kaufmann SHE. Iron and microbial infection. Nat Rev Microbiol 2004;2:946–53. 10.1038/nrmicro1046 [DOI] [PubMed] [Google Scholar]
- 152. Cherayil BJ. Iron and immunity: immunological consequences of iron deficiency and overload. Arch Immunol Ther Exp 2010;58:407–15. 10.1007/s00005-010-0095-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Kumar V, Choudhry VP. Iron deficiency and infection. Indian J Pediatr 2010;77:789–93. 10.1007/s12098-010-0120-3 [DOI] [PubMed] [Google Scholar]
- 154. Ganz T, Nemeth E. Iron homeostasis in host defence and inflammation. Nat Rev Immunol 2015;15:500–10. 10.1038/nri3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Ward RJ, Crichton RR, Taylor DL, et al. Iron and the immune system. J Neural Transm 2011;118:315–28. 10.1007/s00702-010-0479-3 [DOI] [PubMed] [Google Scholar]
- 156. Nairz M, Dichtl S, Schroll A, et al. Iron and innate antimicrobial immunity-Depriving the pathogen, defending the host. J Trace Elem Med Biol 2018;48:118–33. 10.1016/j.jtemb.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 157. Ganz T. Iron and infection. Int J Hematol 2018;107:7–15. 10.1007/s12185-017-2366-2 [DOI] [PubMed] [Google Scholar]
- 158. Nairz M, Theurl I, Swirski FK, et al. “Pumping iron”—how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflugers Arch - Eur J Physiol 2017;469:397–418. 10.1007/s00424-017-1944-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Drakesmith H, Prentice AM. Hepcidin and the Iron-Infection axis. Science 2012;338:768–72. 10.1126/science.1224577 [DOI] [PubMed] [Google Scholar]
- 160. Ahluwalia N, Sun J, Krause D, et al. Immune function is impaired in iron-deficient, homebound, older women. Am J Clin Nutr 2004;79:516–21. 10.1093/ajcn/79.3.516 [DOI] [PubMed] [Google Scholar]
- 161. Malan L, Baumgartner J, Calder PC, et al. n–3 long-chain PUFAs reduce respiratory morbidity caused by iron supplementation in iron-deficient South African schoolchildren: a randomized, double-blind, placebo-controlled intervention. Am J Clin Nutr 2015;101:668–79. 10.3945/ajcn.113.081208 [DOI] [PubMed] [Google Scholar]
- 162. Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature 2012;486:207–14. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016;14:1002533 10.1371/journal.pbio.1002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011;334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Scott KP, Gratz SW, Sheridan PO, et al. The influence of diet on the gut microbiota. Pharmacol Res 2013;69:52–60. 10.1016/j.phrs.2012.10.020 [DOI] [PubMed] [Google Scholar]
- 166. Willson K, Situ C. Systematic review on effects of diet on gut microbiota in relation to metabolic syndromes. J Clin Nutr Metab 2017;1:2. [Google Scholar]
- 167. Singh RK, Chang H-W, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017;15:73 10.1186/s12967-017-1175-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 2001;48:198–205. 10.1136/gut.48.2.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Arboleya S, Watkins C, Stanton C, et al. Gut bifidobacteria populations in human health and aging. Front Microbiol 2016;7:1204 10.3389/fmicb.2016.01204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Biagi E, Candela M, Turroni S, et al. Ageing and gut microbes: perspectives for health maintenance and longevity. Pharmacol Res 2013;69:11–20. 10.1016/j.phrs.2012.10.005 [DOI] [PubMed] [Google Scholar]
- 171. Claesson MJ, Jeffery IB, Conde S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178–84. 10.1038/nature11319 [DOI] [PubMed] [Google Scholar]
- 172. OToole PW, Jeffery IB. Gut microbiota and aging. Science 2015;350:1214–5. 10.1126/science.aac8469 [DOI] [PubMed] [Google Scholar]
- 173. Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science 2018;362:776–80. 10.1126/science.aau5812 [DOI] [PubMed] [Google Scholar]
- 174. Valdes AM, Walter J, Segal E, et al. Role of the gut microbiota in nutrition and health. BMJ 2018;361:k2179 10.1136/bmj.k2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol 2019;16:35–56. 10.1038/s41575-018-0061-2 [DOI] [PubMed] [Google Scholar]
- 176. Xu K, Cai H, Shen Y, et al. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020;49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bron PA, Kleerebezem M, Brummer R-J, et al. Can probiotics modulate human disease by impacting intestinal barrier function? Br J Nutr 2017;117:93–107. 10.1017/S0007114516004037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hill C, Guarner F, Reid G, et al. The International scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014;11:506–14. 10.1038/nrgastro.2014.66 [DOI] [PubMed] [Google Scholar]
- 179. Thomas CM, Versalovic J. Probiotics-host communication: modulation of signaling pathways in the intestine. Gut Microbes 2010;1:148–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol 2013;6:39–51. 10.1177/1756283X12459294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Ahern PP, Maloy KJ. Understanding immune–microbiota interactions in the intestine. Immunology 2020;159:4–14. 10.1111/imm.13150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Lomax A, Calder P. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr. Pharmaceut. Design 2009;15:1428–518. 10.2174/138161209788168155 [DOI] [PubMed] [Google Scholar]
- 183. Boge T, Rémigy M, Vaudaine S, et al. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine 2009;27:5677–84. 10.1016/j.vaccine.2009.06.094 [DOI] [PubMed] [Google Scholar]
- 184. Rizzardini G, Eskesen D, Calder PC, et al. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br J Nutr 2012;107:876–84. 10.1017/S000711451100420X [DOI] [PubMed] [Google Scholar]
- 185. Maidens C, Childs C, Przemska A, et al. Modulation of vaccine response by concomitant probiotic administration. Br J Clin Pharmacol 2013;75:663–70. 10.1111/j.1365-2125.2012.04404.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Lei W-T, Shih P-C, Liu S-J, et al. Effect of probiotics and prebiotics on immune response to influenza vaccination in adults: a systematic review and meta-analysis of randomized controlled trials. Nutrients 2017;9:1175 10.3390/nu9111175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Yeh T-L, Shih P-C, Liu S-J, et al. The influence of prebiotic or probiotic supplementation on antibody titers after influenza vaccination: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther 2018;12:217–30. 10.2147/DDDT.S155110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Malagón-Rojas JN, Mantziari A, Salminen S, et al. Postbiotics for preventing and treating common infectious diseases in children: a systematic review. Nutrients 2020;12:389 10.3390/nu12020389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Yang B, Lu P, Li M-X, et al. A meta-analysis of the effects of probiotics and synbiotics in children with acute diarrhea. Medicine 2019;98:e16618. 10.1097/MD.0000000000016618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Li Y-T, Xu H, Ye J-Z, et al. Efficacy of Lactobacillus rhamnosus GG in treatment of acute pediatric diarrhea: A systematic review with meta-analysis. World J Gastroenterol 2019;25:4999–5016. 10.3748/wjg.v25.i33.4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Urbańska M, Gieruszczak-Białek D, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment Pharmacol Ther 2016;43:1025–34. 10.1111/apt.13590 [DOI] [PubMed] [Google Scholar]
- 192. Patro-Gołąb B, Szajewska H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for treating acute gastroenteritis in children. An update. Nutrients 2019;11:2762 10.3390/nu11112762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ianiro G, Rizzatti G, Plomer M, et al. Bacillus clausii for the treatment of acute diarrhea in children: a systematic review and meta-analysis of randomized controlled trials. Nutrients 2018;10:1074 10.3390/nu10081074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Hickson M, D'Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007;335:80–3. 10.1136/bmj.39231.599815.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. McFarland LV. Meta-Analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. Am J Gastroenterol 2006;101:812–22. 10.1111/j.1572-0241.2006.00465.x [DOI] [PubMed] [Google Scholar]
- 196. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev 2010:CD003048. 10.1002/14651858.CD003048.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea. JAMA 2012;307:1959–69. [DOI] [PubMed] [Google Scholar]
- 198. Calder P, Hall V. Understanding gut-immune interactions in management of acute infectious diarrhoea. Nurs Older People 2012;24:29–39. 10.7748/nop2012.11.24.9.29.c9367 [DOI] [PubMed] [Google Scholar]
- 199. Jafarnejad S, Shab-Bidar S, Speakman JR, et al. Probiotics Reduce the Risk of Antibiotic-Associated Diarrhea in Adults (18–64 Years) but Not the Elderly (>65 Years). Nutr Clin Pract 2016;31:502–13. 10.1177/0884533616639399 [DOI] [PubMed] [Google Scholar]
- 200. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Int J Gen Med 2016;9:27–37. 10.2147/IJGM.S98280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Ma Y, Yang JY, Peng X, et al. Which probiotic has the best effect on preventing Clostridium difficile-associated diarrhea? A systematic review and network meta-analysis. J Dig Dis 2020;21:69–80. 10.1111/1751-2980.12839 [DOI] [PubMed] [Google Scholar]
- 202. Cai J, Zhao C, Du Y, et al. Comparative efficacy and tolerability of probiotics for antibiotic-associated diarrhea: systematic review with network meta-analysis. United European Gastroenterol J 2018;6:169–80. 10.1177/2050640617736987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Llang T. Handbook of COVID-19 prevention and treatment, 2020. Available: https://covid19.alnap.org/help-library/handbook-of-covid-19-prevention-and-treatment
- 204. Gao QY, Chen YX, Fang JY. 2019 novel coronavirus infection and gastrointestinal tract. J Dig Dis 2020;21:125–6. 10.1111/1751-2980.12851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via NOD-like receptor ligands. Infect Immun 2014;82:4596–606. 10.1128/IAI.02212-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zhang N, He Q-S. Commensal microbiome promotes resistance to local and systemic infections. Chin Med J 2015;128:2250–5. 10.4103/0366-6999.162502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Vouloumanou EK, Makris GC, Karageorgopoulos DE, et al. Probiotics for the prevention of respiratory tract infections: a systematic review. Int J Antimicrob Agents 2009;34:197.e1–197.e10. 10.1016/j.ijantimicag.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 208. Liu K-xiong, Zhu Y-gang, Zhang J, et al. Probiotics' effects on the incidence of nosocomial pneumonia in critically ill patients: a systematic review and meta-analysis. Crit Care 2012;16:R109. 10.1186/cc11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Liu S, Hu P, Du X, et al. Lactobacillus rhamnosus GG supplementation for preventing respiratory infections in children: a meta-analysis of randomized, placebo-controlled trials. Indian Pediatr 2013;50:377–81. 10.1007/s13312-013-0123-z [DOI] [PubMed] [Google Scholar]
- 210. King S, Glanville J, Sanders ME, et al. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr 2014;112:41–54. 10.1017/S0007114514000075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Hao Q, Lu Z, Dong BR, et al. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev 2011:CD006895. 10.1002/14651858.CD006895.pub2 [DOI] [PubMed] [Google Scholar]
- 212. Ozen M, Kocabas Sandal G, Dinleyici EC. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther 2015;15:9–20. 10.1517/14712598.2015.980233 [DOI] [PubMed] [Google Scholar]
- 213. Araujo GVde, Oliveira Junior MHde, Peixoto DM, et al. Probiotics for the treatment of upper and lower respiratory-tract infections in children: systematic review based on randomized clinical trials. J Pediatr 2015;91:413–27. 10.1016/j.jped.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 214. Wang Y, Li X, Ge T, et al. Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine 2016;95:e4509. 10.1097/MD.0000000000004509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Laursen RP, Hojsak I. Probiotics for respiratory tract infections in children attending day care centers—a systematic review. Eur J Pediatr 2018;177:979–94. 10.1007/s00431-018-3167-1 [DOI] [PubMed] [Google Scholar]
- 216. Pedersen SF, Ho Y-C. SARS-CoV-2: a storm is raging. J Clin Invest 2020;130:2202–5. 10.1172/JCI137647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130:2620–9. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). medRxiv 2020;20024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ruan Q, Yang K, Wang W, et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:846–8. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Fowler AA, Kim C, Lepler L, et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. world J. Crit. Care Med 2017;6:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Boretti A, Banik BK. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition 2020:100190. 10.1016/j.phanu.2020.100190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Boudreault F, Pinilla-Vera M, Englert JA, et al. Zinc deficiency primes the lung for ventilator-induced injury. JCI Insight 2017;2:86507 10.1172/jci.insight.86507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Mahmoodpoor A, Hamishehkar H, Shadvar K, et al. The effect of intravenous selenium on oxidative stress in critically ill patients with acute respiratory distress syndrome. Immunol Invest 2019;48:147–59. 10.1080/08820139.2018.1496098 [DOI] [PubMed] [Google Scholar]
- 225. Calder PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 2013;75:645–62. 10.1111/j.1365-2125.2012.04374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Calder PC. Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim Biophys Acta 2015;1851:469–84. 10.1016/j.bbalip.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 227. Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans 2017;45:1105–15. 10.1042/BST20160474 [DOI] [PubMed] [Google Scholar]
- 228. Wada M, DeLong CJ, Hong YH, et al. Enzymes and receptors of prostaglandin pathways with arachidonic acid-derived versus eicosapentaenoic acid-derived substrates and products. J Biol Chem 2007;282:22254–66. 10.1074/jbc.M703169200 [DOI] [PubMed] [Google Scholar]
- 229. Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin Immunol 2015;27:200–15. 10.1016/j.smim.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Chiang N, Serhan CN. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med 2017;58:114–29. 10.1016/j.mam.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 2018;128:2657–69. 10.1172/JCI97943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Grimminger F, Wahn H, Kramer HJ, et al. Differential influence of arachidonic vs. eicosapentaenoic acid on experimental pulmonary hypertension. Am J Physiol Heart Circ Physiol 1995;268:H2252–9. 10.1152/ajpheart.1995.268.6.H2252 [DOI] [PubMed] [Google Scholar]
- 233. Breil I, Koch T, Heller A, et al. Alteration of n-3 fatty acid composition in lung tissue after short-term infusion of fish oil emulsion attenuates inflammatory vascular reaction. Crit Care Med 1996;24:1893–902. 10.1097/00003246-199611000-00021 [DOI] [PubMed] [Google Scholar]
- 234. Mancuso P, Whelan J, DeMichele SJ, et al. Effects of eicosapentaenoic and gamma-linolenic acid on lung permeability and alveolar macrophage eicosanoid synthesis in endotoxic rats. Crit Care Med 1997;25:523–32. 10.1097/00003246-199703000-00024 [DOI] [PubMed] [Google Scholar]
- 235. Mancuso P, Whelan J, DeMichele SJ, et al. Dietary fish oil and fish and borage oil suppress intrapulmonary proinflammatory eicosanoid biosynthesis and attenuate pulmonary neutrophil accumulation in endotoxic rats. Crit Care Med 1997;25:1198–206. 10.1097/00003246-199707000-00023 [DOI] [PubMed] [Google Scholar]
- 236. Sane S, Baba M, Kusano C, et al. Eicosapentaenoic acid reduces pulmonary edema in endotoxemic rats. J Surg Res 2000;93:21–7. 10.1006/jsre.2000.5960 [DOI] [PubMed] [Google Scholar]
- 237. Murray MJ, Kumar M, Gregory TJ, et al. Select dietary fatty acids attenuate cardiopulmonary dysfunction during acute lung injury in pigs. Am J Physiol Heart Circ Physiol 1995;269:H2090–9. 10.1152/ajpheart.1995.269.6.H2090 [DOI] [PubMed] [Google Scholar]
- 238. Hecker M, Linder T, Ott J, et al. Immunomodulation by lipid emulsions in pulmonary inflammation: a randomized controlled trial. Crit Care 2015;19:226 10.1186/s13054-015-0933-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Seki H, Fukunaga K, Arita M, et al. The anti-inflammatory and proresolving mediator resolvin E1 protects mice from bacterial pneumonia and acute lung injury. J Immunol 2010;184:836–43. 10.4049/jimmunol.0901809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Wang B, Gong X, Wan J-yuan, et al. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm Pharmacol Ther 2011;24:434–41. 10.1016/j.pupt.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 241. El Kebir D, Gjorstrup P, Filep JG. Resolvin E1 promotes phagocytosis-induced neutrophil apoptosis and accelerates resolution of pulmonary inflammation. Proc Natl Acad Sci U S A 2012;109:14983–8. 10.1073/pnas.1206641109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Liao Z, Dong J, Wu W, et al. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARγ/NF-κB pathway. Respir Res 2012;13:110. 10.1186/1465-9921-13-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Eickmeier O, Seki H, Haworth O, et al. Aspirin-Triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol 2013;6:256–66. 10.1038/mi.2012.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Yaxin W, Shanglong Y, Huaqing S, et al. Resolvin D1 attenuates lipopolysaccharide induced acute lung injury through CXCL-12/CXCR4 pathway. J Surg Res 2014;188:213–21. 10.1016/j.jss.2013.11.1107 [DOI] [PubMed] [Google Scholar]
- 245. Wang L, Yuan R, Yao C, et al. Effects of resolvin D1 on inflammatory responses and oxidative stress of lipopolysaccharide-induced acute lung injury in mice. Chin Med J 2014;127:803–9. [PubMed] [Google Scholar]
- 246. Cox R, Phillips O, Fukumoto J, et al. Enhanced resolution of hyperoxic acute lung injury as a result of aspirin triggered resolvin D1 treatment. Am J Respir Cell Mol Biol 2015;53:422–35. 10.1165/rcmb.2014-0339OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Liu Y, Zhou D, Long F-W, et al. Resolvin D1 protects against inflammation in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 2016;310:G303–9. 10.1152/ajpgi.00355.2014 [DOI] [PubMed] [Google Scholar]
- 248. Colby JK, Abdulnour R-EE, Sham HP, et al. Resolvin D3 and aspirin-triggered resolvin D3 are protective for injured epithelia. Am J Pathol 2016;186:1801–13. 10.1016/j.ajpath.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Zhang H-W, Wang Q, Mei H-X, et al. RvD1 ameliorates LPS-induced acute lung injury via the suppression of neutrophil infiltration by reducing CXCL2 expression and release from resident alveolar macrophages. Int Immunopharmacol 2019;76:105877 10.1016/j.intimp.2019.105877 [DOI] [PubMed] [Google Scholar]
- 250. Sekheri M, El Kebir D, Edner N, et al. 15-Epi-LXA 4and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc Natl Acad Sci U S A 2020;117:7971–80. 10.1073/pnas.1920193117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Gadek JE, DeMichele SJ, Karlstad MD, et al. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Crit Care Med 1999;27:1409–20. 10.1097/00003246-199908000-00001 [DOI] [PubMed] [Google Scholar]
- 252. Singer P, Theilla M, Fisher H, et al. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med 2006;34:1033–8. 10.1097/01.CCM.0000206111.23629.0A [DOI] [PubMed] [Google Scholar]
- 253. Pontes-Arruda A, Aragão AMA, Albuquerque JD. Effects of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in mechanically ventilated patients with severe sepsis and septic shock. Crit Care Med 2006;34:2325–33. 10.1097/01.CCM.0000234033.65657.B6 [DOI] [PubMed] [Google Scholar]
- 254. Pontes-Arruda A, DeMichele S, Seth A, et al. The use of an inflammation-modulating diet in patients with acute lung injury or acute respiratory distress syndrome: a meta-analysis of outcome data. JPEN J Parenter Enteral Nutr 2008;32:596–605. 10.1177/0148607108324203 [DOI] [PubMed] [Google Scholar]
- 255. Dushianthan A, Cusack R, Burgess VA, et al. Immunonutrition for acute respiratory distress syndrome (ARDS) in adults. Cochrane Database Syst Rev 2019;1:CD012041. 10.1002/14651858.CD012041.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Cummings JH, Antoine J-M, Azpiroz F, et al. PASSCLAIM1?Gut health and immunity. Eur J Nutr 2004;43:ii118–73. 10.1007/s00394-004-1205-4 [DOI] [PubMed] [Google Scholar]
- 257. Calder PC, Kew S. The immune system: a target for functional foods? Br J Nutr 2002;88:S165–76. 10.1079/BJN2002682 [DOI] [PubMed] [Google Scholar]
- 258. Cena H, Calder PC. Defining a healthy diet: evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020;12:334 10.3390/nu12020334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. US Department of health and human services and U.S. department of agriculture. 2015–2020 dietary guidelines for Americans. 8th edition, 2015. Available: http://health.gov/dietaryguidelines/2015/guidelines/
- 260. Gibson A, Edgar JD, Neville CE, et al. Effect of fruit and vegetable consumption on immune function in older people: a randomized controlled trial. Am J Clin Nutr 2012;96:1429–36. 10.3945/ajcn.112.039057 [DOI] [PubMed] [Google Scholar]