Abstract
Lipids constitute the bulk of the dry mass of the brain and have been associated with healthy function as well as the most common pathological conditions of the brain. Demographic factors, genetics, and lifestyles are the major factors that influence lipid metabolism and are also the key components of lipid disruption in Alzheimer’s disease (AD). Additionally, the most common genetic risk factor of AD, APOE ϵ4 genotype, is involved in lipid transport and metabolism. We propose that lipids are at the center of Alzheimer’s disease pathology based on their involvement in the blood-brain barrier function, amyloid precursor protein (APP) processing, myelination, membrane remodeling, receptor signaling, inflammation, oxidation, and energy balance. Under healthy conditions, lipid homeostasis bestows a balanced cellular environment that enables the proper functioning of brain cells. However, under pathological conditions, dyshomeostasis of brain lipid composition can result in disturbed BBB, abnormal processing of APP, dysfunction in endocytosis/exocytosis/autophagocytosis, altered myelination, disturbed signaling, unbalanced energy metabolism, and enhanced inflammation. These lipid disturbances may contribute to abnormalities in brain function that are the hallmark of AD. The wide variance of lipid disturbances associated with brain function suggest that AD pathology may present as a complex interaction between several metabolic pathways that are augmented by risk factors such as age, genetics, and lifestyles. Herewith, we examine factors that influence brain lipid composition, review the association of lipids with all known facets of AD pathology, and offer pointers for potential therapies that target lipid pathways.
Keywords: amyloid precursor protein, apolipoproteins, blood-brain barrier, energy metabolism, inflammation, late-onset Alzheimer’s disease, mitochondria, myelination
Background
The Importance of Cellular Lipid Membranes
Cell membranes are composed of several lipid classes and membrane-bound proteins/receptors that interface cellular organelles, and cells with their environment. It is now recognized that these membrane lipids are important in maintaining cellular functions. Several studies show that perturbation of membrane lipids can have devastating consequences on the brain. These changes underlie Alzheimer’s disease (AD) pathology depicted in Figure 1. We will examine factors that affect lipid metabolism, describe the functions of brain lipids, and examine the consequences and contributions of lipid dyshomeostasis on AD pathology.
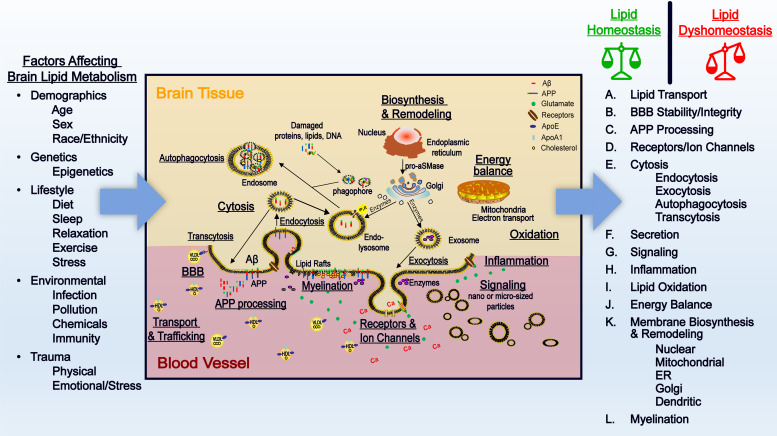
FIGURE 1.
Factorsthat affect brain lipid metabolism and the importance of lipids in healthy aging and AD. Factors that affect brain lipid metabolism – Demographic factors, genetics, lifestyle, the environment, and trauma can influence lipid metabolism in the brain. Interestingly, these factors that influence lipid metabolism are also recognized risk factors of AD. Abnormalities in lipid metabolism can contribute to dysfunctional brain networks that associate with AD pathology. Importance of lipid metabolism in brain function and AD pathology – In healthy aging, normal transport of lipids through apolipoproteins contribute to the function of the brain. Homeostatic control of the brain lipid environment is responsible for sustaining a normal BBB, providing the right environment for normal APP processing, the right composition for ion channels and receptors, cytosis, vesicle formation, and secretion, signaling, inflammation, oxidation, energy balance, and membrane biosynthesis and remodeling. Dyshomeostasis in lipid delivery into the brain and its metabolism attributes to disturbed BBB, abnormal APP processing, disturbance in cytosis, signaling, energy balance, and enhanced/sustained inflammation and oxidation. Over time, these processes lead to neuronal death that is the hallmark of AD pathology.
Brain Lipids in Healthy Aging and AD Pathology
Most of the brain is composed of lipids, which can be grouped as sphingolipids, glycerophospholipids, and cholesterol (Svennerholm et al., 1994; O’Brien and Sampson, 1965; Kishimoto et al., 1969). The brain consists of straight-chain monocarboxylic acids ranging from C12 to C26, and omega-3 (n-3) and omega-6 (n-6) fatty acids are most abundant (Kishimoto et al., 1969; Siegel, 1999). Docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are prominent polyunsaturated fatty acids (PUFA) in the brain that are derived from alpha-linolenic acid (ALA), an omega-3 fatty acid (Chappus-McCendie et al., 2019). Arachidonic acid (AA) and docosatetraenoic acid (DPA) constitute a large proportion of PUFA’s that are derived from linolenic acid (LNA), an omega 6 fatty acid (Leonard et al., 2000; Sinclair et al., 2007).
Factors That Affect Brain Lipids
Demographic Factors That Influence Brain Lipids
Brain Lipids Changes in Aging
These PUFA’s are incorporated into membrane phospholipids and therefore play a significant role in structural integrity and function of cell membranes. Lipid metabolism is changed during aging (Montanini et al., 1983; Yehuda et al., 2002; Whelan, 2008; Denis et al., 2015; Cutuli, 2017; Chappus-McCendie et al., 2019), as shown by a decline in omega-3 fatty acids and an increase in lipid peroxidation (Chen et al., 2017). Omega-3 fatty acids have antioxidant properties, and a lack of these fatty acids in one’s diet may accelerate neuronal degeneration (Yehuda et al., 2002; Janssen and Kiliaan, 2014). Susceptibility of lipids to peroxidation increases with age (Bourre, 1991; Spiteller, 2010; Denis et al., 2015; Chen et al., 2017), which supports using the level of oxidative stress as a critical determinant of neuronal health and longevity (Hulbert et al., 2006). Previous studies have suggested that DHA and EPA may protect against peroxidation and the effects of age-related brain pathology (Hasadsri et al., 2013; Chen et al., 2017). Lipids are involved in cellular signaling, energy balance, blood-brain barrier (BBB), and inflammation (Song et al., 2008; Willis et al., 2009), and such age-dependent lipidome changes that disrupt these functions may contribute to neurodegenerative diseases (Arnoldussen et al., 2016; Bos et al., 2016; Hooper et al., 2018; Luo et al., 2018; McNamara et al., 2018; Lepping et al., 2019), such as Alzheimer’s disease (AD) (Schmitt et al., 2014; Hussain et al., 2019).
Lipids and Race/Ethnicity
Race and ethnicity play a significant role in the risk of AD and related disorders. In 2014, nearly 5 million people over the age of 65 had been diagnosed with Alzheimer’s disease or related dementias (ADRD) (Matthews et al., 2019). African Americans and Hispanics had the highest prevalence of ADRD (13.8% and 12.2%, respectively), while ADRD was least common in Asian and Pacific Islanders (8.4%), followed by American Indian/Alaska Natives (9.1%), non-Hispanic whites (10.3%), and people with two or more races (11.5%) (Matthews et al., 2019). Ethnic and racial backgrounds impact many aspects of health, including diet, stress, access to medical treatment, and biological factors of disease. From past research, we can clearly see the ways in which ethnicity, race, and lipids overlap. Most clearly seen in the high incidence of both dyslipidemia, or abnormal amounts of lipids in the blood, and cardiovascular disease observed in minority populations (Frank et al., 2014), race/ethnic disparities affect the regulation of lipid metabolism. Increased concentrations of triglycerides (TG) and decreased levels of lipid carriers, such as HDL-C (high-density lipoprotein-cholesterol) in the blood of Mexican, Filipino, Indian, and Vietnamese people compared to whites may provide a possible explanation for higher risk of both ADRD and cardiovascular disease within these populations (Frank et al., 2014; Gazzola et al., 2017). HDL-C is often referred to as “good cholesterol,” has beneficial antioxidant and anti-inflammatory effects in the body, and has been observed to modulate ß-amyloid (Aß) production in the brain, a key biomarker of AD pathogenesis (Reitz, 2012; Hottman et al., 2014). Lowered levels of HDL-C have been associated with increased cognitive decline and poor cardiovascular health outcomes (Hottman et al., 2014). TG, which is increased in almost every minority population, except African Americans, has been shown to relate to central leptin- and insulin resistance in the brain and decreases in cognition (Sumner, 2009; Frank et al., 2014; Banks et al., 2018). In light of the less marked changes in lipid make-up and metabolism seen in African American populations at increased risk of ADRD, it has been suggested that African Americans are underdiagnosed with metabolic syndromes and vascular-cognitive disorders (Sumner, 2009). Furthermore, it has been observed that there is a differential expression of various molecular biomarkers of AD (phosphorylated tau and total tau) in African Americans compared to whites (Morris et al., 2019), suggesting even small, imperceptible changes in lipid distribution in this population may be sufficient to affect cognition negatively. It is important to note that despite the disproportionate impact ADRD has on minority populations, these individuals continue to be considerably underrepresented in ADRD research, contributing to large gaps in our understanding of brain lipid metabolism as it pertains to race and ethnicity (Gilmore-Bykovskyi et al., 2019).
Lipids and Sex
Sex continues to be one of the largest risk factors for developing AD. Females not only makeup two-thirds of all cases of AD diagnoses but also possess a greater lifetime risk of dementia compared to men due to longer life expectancy (Viña and Lloret, 2010; Mielke, 2018). Increased prevalence and risk of AD and other age-related disorders among females have been attributed to not only extended life expectancy but also to sudden decreases in estrogen post-menopause, among many other factors including education level and mental health status (Viña and Lloret, 2010; Mielke, 2018). Despite the many factors that may contribute to increased risk of AD in women, the contribution of sex-hormone levels and differential lipid distribution play evident roles in cognitive decline are not fully understood. Not only is fat in the form of TG distributed differently in the adipose tissue of male and females, which can be attributed in part to sex-hormone signaling, but concentrations of long-chain PUFAs (LC-PUFAs) have also been observed to be increased in women pre-menopause compared to men (Decsi and Kennedy, 2011; Lohner et al., 2013). Correspondingly, a positive association has been established between omega-3 LC-PUFA biosynthesis, i.e., the production of EPA and DHA, and circulating concentrations of estrogen and progesterone (Childs et al., 2008). Estrogen, an ovarian steroid hormone, is hypothesized to affect lipid metabolism at several points during biosynthesis, including playing a key role in lipid transport and exchange, increasing expression of metabolic enzymes, and reducing the oxidation of α-linoleic acid (ALA), the deriving fatty acid in n-3 LC-PUFA production (Childs et al., 2008; Decsi and Kennedy, 2011; Lohner et al., 2013; Palmisano et al., 2018). Estrogen has also been directly associated with inhibiting memory function impairment in premenopausal women following the surgical removal of their ovaries and loss of the ability to produce estrogen endogenously (Duka et al., 2000; Sherwin, 2012). In a study of trans-sexual subjects, those transitioning from male to female and receiving estrogen observed an increase in DHA plasma levels while those transitioning from female to male and receiving testosterone treatment experienced a marked decrease in plasma DHA (Giltay et al., 2004). The decrease in estrogen levels, as seen in post-menopausal women, has also been associated with increased TG content and lower HDL-C, both of which have been linked to cognitive decline (Derby et al., 2009; Anceline et al., 2014). This is to say, the increased prevalence and risk for AD among women can be explained in part by the abrupt decrease in estrogen production that accompanies the post-menopausal state. Not only does the lack of estrogen decrease concentrations of anti-inflammatory LC-PUFAs and HDL-C in the body, but it also increases TG levels, augmenting secretion of VLDL (very-low density lipoprotein), a lipid carrier known to induce neuroinflammation (Burgess et al., 2006; Chen et al., 2014; Nägga et al., 2018). Additionally, genetic factors, such as ApoE status, and social determinants, such as education, mental illness, and diet, interact with the post-menopausal state to amplify these detrimental effects, increasing risk of AD.
Lipids and Lifestyle
Diet
Dietary lipids play an integral part in physiological lipid metabolism and, consequently, in the risk of AD and cardiovascular disease. Essential fatty acids like DHA (n-3) and AA (n-6) are largely derived from the dietary consumption of their shorter-chained, slightly less-saturated counterparts ALA (n-3) and LA (n-6), respectively (Schmitz, 2008; Morris and Tangney, 2014). After consumption of these deriving fatty acids, the body is able to anabolize them, creating the LC-PUFAs that contribute to neural processes (Morris and Tangney, 2014). Early on in human existence, our diet consisted of an equal balance of n-6 to n-3 essential fatty acids, but as we have evolved, the n-3 to the n-6 ratio of dietary fatty acids has greatly shifted to one side (Simopoulos, 2006). Today, the Western diet has a ratio of about 17 to 1 n-6 to n-3 fatty acids, meaning most Americans have a lot more LA, AA, and DPA in their bodies, which are able to produce relatively large quantities of inflammatory and oxidative mediators (Simopoulos, 2006). Increased ratios of n-6 to n-3 dietary fatty acids have also been directly associated with increased cognitive decline and risk of AD (Loef and Walach, 2013; MacDonald-Wicks et al., 2019). DHA, on the other hand, an n-3 LC-PUFA usually found in fish and algae, is not largely found in the Western diet. Studies suggest, however, that DHA supplementation may work to combat neuroinflammation, oxidative stress, and cognitive decline. Fish oil supplements containing large amounts of DHA, given to older adults with varying levels of cognition, found that supplementation resulted in decreased brain atrophy and less cognitive decline compared to controls in an APOE allele-dependent manner (Daiello et al., 2015). Similarly, Morris et al. observed among subjects over the age of 65 that those who ate fish at least once a week had 60% less risk of AD than those who rarely or never ate fish (Morris et al., 2003). Dietary DHA has also been shown to improve cognition, memory, and brain development from the earliest stages of life through adulthood (Dunstan et al., 2008; McNamara et al., 2010; Muldoonm et al., 2010; Stonehouse et al., 2013; Weiser et al., 2016).
It is important to note that diet can be particularly impacted by race/ethnicity, as well as physical geography, helping to explain differences in AD risk among ethnic groups. According to a global survey of 298 studies, highest levels of DHA and EPA, another n-3 fatty acid, were observed among Japanese, Scandanavian, and indigenous populations, as well as in areas where the Westernized diet had not been fully adopted (Stark et al., 2016). Authors of this survey argue increased consumption of seafood, as dictated by culture or geographical location, greatly impact n-3 LC-PUFA levels in the bloodstream, which offer protective cognitive effects at every stage in life (Joffre et al., 2014; Stark et al., 2016; Weiser et al., 2016).
Genetical Evidence for the Importance of Lipid Metabolism in AD Pathology
Genetic Risk Factors of AD-Related to Lipid Metabolism
Genome-wide Association Studies GWAS and Transcriptome-Wide Association Studies (TWAS) associate AD pathology with several lipid genes (Shi et al., 2010; Hao et al., 2018). While the APOE4 allele carries the greatest risk for AD, other genes and gene-products commonly associated with AD pathology are linked to or interact with lipid metabolism. Several lipid genes associated with AD pathology have recently been reviewed (Tindale et al., 2017). Table 1 is the list of the major genes from GWAS that are linked with lipid metabolism (Jones et al., 2010).
TABLE 1.
Lipid metabolism-associated genes with SNP (<0.001) linked with AD from GWAS.
| Gene Symbol [Chromosome location (Mb)] (Jones et al., 2010) | The function of the gene product# | Changes and known effects on AD pathology |
| APOE [19 (50)] | As part of lipoproteins, ApoE is involved in the transport and distribution of lipids into various tissues via plasma and other interstitial fluids (Huang and Mahley, 2014) | Polymorphism of APOE is associated with age of onset (do Couto et al., 1998; Vermunt et al., 2019), cognitive and memory decline (Asada et al., 1996; El Haj et al., 2016), amyloid load (Mecca et al., 2018), cholesterol homeostasis (Leduc et al., 2010), inflammation (Tzioras et al., 2019) |
| APOC1 [19 (50)] | Involved in HDL and VLDL metabolism, inhibitor cholesteryl ester transfer protein in plasma | Gene polymorphism (Prendecki et al., 2018), oxidative stress (Prendecki et al., 2018), interaction with ApoE (Lucatelli et al., 2011), cognitive impairment (Zhou et al., 2014) |
| CLU [8 (28)] | Clusterin (ApoJ) is a component of lipoproteins associated with lipids in plasma and CSF | Polymorphism (Shuai et al., 2015; Zhu et al., 2018), interaction with PICALM (Harold et al., 2009; Kamboh et al., 2012), hippocampal function (Erk et al., 2011). |
| APOC2 [19 (50)] | A component of triglyceride (TG)-rich lipoproteins, including VLDL, HDL), and chylomicrons involved metabolism of these particles; promote VLDL1 secretion, inhibit lipoprotein lipase enzyme activity | Polymorphism associated with AD (Sun et al., 2005), decreased expression associated with increased risk (Lin et al., 2015) |
| APOC4 [19 (50)] | A lipid-binding lipoprotein thought to play a role in lipid metabolism | Decreased expression associated with increased risk (Lin et al., 2015) |
| ABCA7 [19 (1)] | Member of the ATP-binding cassette (ABC) superfamily of transporters; catalyzes the translocation of specific phospholipids from the cytoplasmic to the extracellular/lumenal leaflet of the membrane coupled with ATP hydrolysis, lipid homeostasis, binds APOA1, apolipoprotein-mediated phospholipid efflux from cells, cholesterol efflux, lipid raft organization | Polymorphism correlate with memory impairment (Chang et al., 2019), amyloid plaque burden (Zhao Q. F. et al., 2015), cognitive impairment (Chung et al., 2014; Berg et al., 2019) |
| ABCA1 [9 (107)] | A membrane of the superfamily of ATP-binding cassette (ABC) transporters with cholesterol as its substrate, it functions as a cholesterol efflux pump in the cellular lipid removal pathway | Polymorphism in AD (Chu et al., 2007; Wavrant-De Vrieze et al., 2007; Wang et al., 2013), modulates cholesterol efflux (Shibata et al., 2006; Khalil et al., 2012; Marchi et al., 2019), influences age of onset (Wollmer et al., 2003a) |
| ABCA12 [2 (216)] | A membrane of the superfamily of ATP-binding cassette (ABC) transporters involved in the transport of molecules across the cellular membrane | SNP with p < 0.001 and significantly enriched in AD (Jones et al., 2010) |
| LIPC [15 (57)] | Hepatic triglyceride lipase is a triglyceride hydrolase and ligand/bridging factor for receptor-mediated lipoprotein uptake | Gene variant might influence AD susceptibility (Xiao et al., 2012) |
| ATP8A1 [4 (42)] | ATPase Phospholipid Transporting 8A1 catalyzes ATP hydrolysis that is coupled to the transport of aminophospholipids from the outer to the inner leaflet of membranes to maintain their asymmetric distribution | SNP with p < 0.001 and significantly enriched in AD (Jones et al., 2010) |
| ATP8B4 [15 (48)] | Amninophospholipid transport across cell membranes | SNP with p < 0.001 and significantly enriched in AD (Jones et al., 2010) |
| MALL [2 (110)] | Member of the MAL proteolipid family localizes in glycolipid- and cholesterol-enriched membrane (GEM) rafts, and interacts with caveolin-1 | SNP with p < 0.001 and significantly enriched in AD (Jones et al., 2010) |
| ATP8A2 [13 (25)] | Involved in flipping phospholipids from the exoplasmic leaflet to the cytosolic leaflet of the cell membrane to generate or maintain membrane lipid asymmetry | SNP with p < 0.001 and significantly enriched in AD (Jones et al., 2010) |
| OSBPL7 [17 (43)] OSBPL9 [1 (59)] | Oxysterol-binding protein (OSBP) family, intracellular lipid receptors Oxysterol-binding protein (OSBP) family, a group of intracellular lipid receptors; cholesterol transfer protein and regulation of Golgi structure and function | Differential expression (Brown III, Theisler et al., 2004; Herold et al., 2016) |
| SCARB1 [12 (124)] | Scavenger Receptor Class B Member 1 is a plasma membrane receptor for HDL that also mediates cholesterol transfer to or from HDL | Cholesterol efflux and anti-inflammation (Khalil et al., 2012), endocytosis, transcytosis and Abeta removal (Mackic et al., 1998; Srivastava and Jain, 2002) |
| VPS4B [18 (59)] | Vacuolar Protein Sorting 4 Homolog B involved in late endosomal multivesicular bodies (MVB) pathway. Degradation of lysosomal enzymes and lipids. | SNP with p < 0.001 and significantly enriched in AD (Jones et al., 2010) |
| ABCG1 [21 (42)] | Coupled to ATP hydrolysis, catalyzes the efflux of sphingomyelin, cholesterol, and oxygenated derivatives like 7-beta-hydroxycholesterol. | Cholesterol efflux (Hirsch-Reinshagen and Wellington, 2007; Wollmer et al., 2007; Marchi et al., 2019) |
| LIPG [18 (43)] | Diverse class of lipase enzymes includes diacylglycerol lipase (DAGL) and lipoprotein lipase (LPL) and endothelial lipase (LIPG). Hydrolyzes HDL more efficiently than other lipoproteins | Polymorphism and mutation (Baum et al., 1999; Blain et al., 2006), cholesterol homeostasis (Blain and Poirier, 2004; Fidani et al., 2004), stimulation in nucleus basalis and hippocampus (Farooqui et al., 1988) |
| PCTP [17 (51)] | Phosphatidylcholine (PC) Transfer Protein; PC synthesis and metabolism, binds single PC molecule and transfers between membranes | Cholesterol transport (Knebl et al., 1994; Demeester et al., 2000) |
| SLC27A4 [9 (130)] | Family of fatty acid transport proteins; translocation of long-chain fatty acids across the plasma membrane, has acyl-CoA ligase activity for long-chain and very-long-chain fatty acids (VLCFAs) | SNP with p < 0.001 and significantly enriched in AD (Jones et al., 2010) |
| NPC1 [18 (19)] | Intracellular cholesterol transporter which is important in cholesterol removal from endosomal/lysosomal compartment | Increase expression (Kagedal et al., 2010; Maulik et al., 2015), neurocognitive deficit (Johnen et al., 2018) |
| APOA1 [11 (116)] | Apolipoprotein A-I is the major protein HDL in plasma. It promotes cholesterol efflux from tissues to the liver for excretion and is a cofactor for lecithin cholesterol acyltransferase (LCAT), an enzyme that forms cholesteryl ester | Polymorphism and decreased expression (Helbecque et al., 2008; Smach et al., 2011; Shibata et al., 2013; Lin et al., 2015) |
| APOC3 [11 (116)] | A component of triglyceride-rich VLDL, and HDL in plasma. Important in triglyceride homeostasis: promotes hepatic VLDL1 assembly and secretion, attenuates hydrolysis and clearance of triglyceride-rich lipoproteins, impairs TRL lipolysis by inhibiting lipoprotein lipase and the hepatic uptake of TRLs by remnant receptors | Polymorphism and decreased expression (Lin et al., 2015; Sun et al., 2005) |
| APOA4 [11 (116)] | Apolipoprotein A4 is a major component of HDL and chylomicrons. Important in chylomicrons and VLDL secretion and catabolism. Required for lipoprotein lipase activation by ApoC-II, a potent activator of LCAT | Decreased expression (Lin et al., 2015), enhanced susceptibility (Papassotiropoulos et al., 2005) |
| AGTR1 [3 (1490)] | Angiotensin II is a primary regulator of aldosterone secretion | Signal transduction abnormality (Pang et al., 2019) |
| SOAT1 [1 (177)] | Sterol O-Acyltransferase-1 is an acyltransferase that catalyzes the formation of fatty acid and cholesterol esters, which is important in lipoprotein assembly and dietary cholesterol absorption. It may also act as a ligase | Polymorphism (Lamsa et al., 2007), Cholesterol levels, amyloid load (Wollmer et al., 2003b) |
Genome-wide Association Studies suggest that age-related changes in brain lipid metabolism may be essential to healthy aging and longevity (Tindale et al., 2017). Identification of AD-related genes and how these interact with specific risk factors may provide the rationale for designing effective therapies.
The onset of age related disease can be accelerated with suppression of anti-aging genes, such as Sirtuin 1 (SIRT1). SIRT1 is a histone deacetylase involved with gene expression related to metabolic activity (Grabowska et al., 2017). SIRT1 interacts with lipid metabolism regulation and hepatic oxidative stress and inflammation (Ding et al., 2017). It also regulates circadian rhythms in the liver and brain, maintaining the body’s regulation of glucogenesis, fatty acid beta-oxidation, and cholesterol biosynthesis (Bellet et al., 2016). Its involvement in metabolism explains its effects on energy metabolism, neurogenesis, glucose and cholesterol metabolism, and amyloidosis. Sirt 1 also contributes to neuron apoptosis and survival. Downregulation of this anti-aging gene may lead to acceleration of neurodegenerative disease. Nutritional interventions, such as a reduction in overconsumption of carbohydrates, are recommended because they may be associated with preventing cell senescence and maintaining anti-aging gene activity (Martins et al., 2017). SIRT1 expression promotes APP processing on a non-amyloidogenic pathway and clearance of tau from the brain (Herskovits and Guarente, 2014). SIRT1’s deacetylase activity increases the activity of lysosome-related genes, facilitating Aβ degradation (Li et al., 2018). SIRT1 is a potential therapeutic target for AD because of its involvement in many amyloid beta and cholesterol pathways.
Contribution of Lipids to AD Pathology
Although the brain has a very high concentration of long-chain omega-3 and omega-6 fatty acids, there is no conclusive explanation for how these fatty acids participate in various signaling cascades and in AD (Torres et al., 2014; Mohaibes et al., 2017). However, lipodomic studies related to AD pathology have demonstrated a decrease in DHA levels within the brain, predominantly in the hippocampus (Belkouch et al., 2016). Damage to the hippocampus is associated with impaired learning and memory abilities, a symptom of AD onset (Sarrafpour et al., 2019). With growing evidence that AD is associated with dysregulation of fatty acid metabolism, fatty acid levels may be potential biomarkers of this disease (Fonteh et al., 2014; Wong et al., 2017). In addition to omega fatty acids, the levels of several lipids change with AD pathology (Table 2).
TABLE 2.
Summary of lipids that change in AD.
| Lipids | Changes observed in AD |
| Fatty acids | |
| Omega-3 fatty acids (#DHA, EPA, DPA, ALA) | DHA decreased in brains, circulation, and CSF of AD individuals (Fonteh et al., 2014, 2020; de Wilde et al., 2017; Snowden et al., 2017; Hosseini et al., 2020). EPA decreased in brain and circulation of AD individuals (Hosseini et al., 2020). DPA increased in livers of AD (Dyall, 2015). ALA increased in plasma and peripheral tissues (Cherubini et al., 2007). |
| Omega-6 fatty acids (#AA, LA) | AA increased in brains, erythrocytes, and CSF of AD individuals (Thomas et al., 2016; Goozee et al., 2017; Fonteh et al., 2020). LA decreased in AD brain and plasma (Snowden et al., 2017; Cunnane et al., 2012). |
| Saturated fatty acids (#PA, SA, C15:0) | Increased in the CSF and brains of AD individuals (Fonteh et al., 2014). Odd chain saturated fatty acids derived from microbiome or measures of dairy consumption decreased in CSF of AD (Fonteh et al., 2014; Nasaruddin et al., 2016). |
| Eicosanoids | Pro-inflammatory eicosanoid pathways are upregulated in AD individuals, while anti-inflammatory eicosanoids are decreased (Biringer, 2019). Prostaglandin and thromboxane B2 increased in AD brains (Iwamoto et al., 1989). Pro-resolvin mediators, such as lipoxins, are reduced in AD brains (Wang et al., 2015b). |
| Endocannabinoids | Decreased levels of endocannabinoids and receptors in AD brains (Bedse et al., 2015). |
| Glycerolipids | |
| Triglycerides | Total TG lipid levels decreased in the serum of individuals with probable AD (Lepara et al., 2009). Polyunsaturated TG decreased in AD brains (Bernath et al., 2019). |
| Glycerophospholipids | |
| Phosphatidylcholine (PC) Phosphatidylethanolamine (PE) Phosphatidylserine (PS) | Total PC lipids decreased in AD brains (Wood, 2012). PC species decreased in CSF of AD individuals #PC32:0, PC34p:0/34e:1, PC34:1, PC34:0, PC36:1, PC38a:5 (PC-EPA), PC36:0/38p:6, 38a:6 (PC-DHA) (Fonteh et al., 2013). PC species decreased in plasma of AD individuals. PC36:5 (PC-EPA), PC38:6 (PC-DHA), PC40:6 (PC-DHA) (Whiley et al., 2014). PC species increased in the prefrontal cortex of AD individuals. PC38:6 (PC-DHA), PC40:6 (PC-DHA) (Igarashi et al., 2011). Total PE lipids decreased in the hippocampus of AD individuals (Prasad et al., 1998). PE species decreased in the hippocampus of AD individuals. PE-SA, PE-OA, PE-AA, PE-DHA (Guan et al., 1999). A decrease in PE plasmalogen in AD (Farooqui and Horrocks, 1998). Total PS lipids decreased in the occipital lobe and inferior parietal lobule of AD brains (Farooqui and Horrocks, 1998). |
| Sphingolipids | |
| Sphingomyelin (SM) Ceramides (CM) Sulfatides Gangliosides | Total SM lipids lower in CSF of AD individuals (Fonteh et al., 2015). SM species decreased in the CSF of AD individuals #SM18/14:0, SM18/16:0, SM18/16:1, SM18/17:0 SM species (SM18/18:0, SM18/18:1) increased in the CSF of prodromal AD individuals (Kosicek et al., 2012). Total CM lipids increased in AD brains (Filippov et al., 2012). CM species increased in AD brains and plasma CM16:0 (PA), CM18:0 (SA), CM20:0, CM24:0, CM24:1 (Kim et al., 2017). Total sulfatide levels significantly lower in AD brains in both gray and white matter. The compositional distribution of sulfatide subtypes is unaltered (Han et al., 2002). Ganglioside lipid levels reduced in the temporal lobe of AD brains (Molander-Melin et al., 2005). |
| Sterols | |
| Cholesterol Oxysterols Hormones | Cholesterol decreased, and oxysterol/cholesterol precursors increased in MCI and sporadic AD brains (Hascalovici et al., 2009). Total oxidized cholesterol increased in AD brains (Heverin et al., 2004). Oxidized cholesterol species decreased in AD brains, 24S-hydroxycholesterol (Heverin et al., 2004). Oxidized cholesterol species increased in AD brains, 27-hydroxycholesterol (Heverin et al., 2004). Lower estrogen increases the risk of AD (Ratnakumar et al., 2019; Uddin et al., 2020). Increased basal cortisol levels in the plasma of demented individuals (Csernansky et al., 2006). Association of cortisol with Aβ deposition (Toledo et al., 2012) and with hypometabolism (Wirth et al., 2019). |
#DHA, Docosahexaenoic acid (C22:6, n-3); EPA, Eicosapentaenoic acid (C20:5, n-3); DPA, Docosapentaenoic acid (C22:5, n-3); ALA, Alpha-linolenic acid (C18:3, n-3); AA, Arachidonic acid (C20:4, n-6); LA, Linoleic acid (C18:2, n-6); PA, Palmitic acid (C16:0); SA, Stearic acid (C18:0); OA, Oleic Acid (C18:1, n-9); PC, phosphatidylcholine; PCxxa:#, PC specie with xx carbon number and acyl-linked fatty acid at the sn-1 position containing # (number) double bonds; PCxxp:#, PC specie with xx carbon number and alk-1-enyl (plasmalogen)-linked fatty acid at the sn-1 position containing # (number) double bonds PCxxe:# PC specie with xx carbon number and alkyl (ether)-linked fatty acid at the sn-1 position containing # (number) double bond; PE, phosphatidylethanolamine; PS, phosphatidylserine; SM, sphingomyelin; CM, ceramide; TG, triglyceride.
Lipid Transport: Apolipoproteins
Brain Lipoproteins and Their Function
Lipoproteins are molecules with a hydrophobic lipid core composed of cholesterol, esters, and triglycerides and a hydrophilic exterior of phospholipids, apolipoproteins, and free cholesterol (Alaupovic, 1996; Hoofnagle and Heinecke, 2009; Braun and Hantke, 2019). Lipoproteins assist with the transport of lipids and amphipathic compounds throughout the body (Feingold and Grunfeld, 2000). However, circulating plasma lipoproteins differ from those within the CNS because only high-density lipoproteins (HDL) can cross the blood-brain barrier (Balazs et al., 2004). The most abundant apolipoproteins, apolipoprotein E (ApoE), and apolipoprotein J (ApoJ) are synthesized by astrocytes and serve as enzyme cofactors and receptor ligands on HDL (Pitas et al., 1987; Feingold and Grunfeld, 2000; Ito et al., 2014).
Apolipoproteins are greatly involved in metabolism, serving as both activators and inhibitors of metabolic enzymes, ligands for lipoprotein receptors, and providing structural support (Feingold and Grunfeld, 2000; Bolanos-Garcia and Miguel, 2003; Filou et al., 2016). They also regulate lipid transport by controlling interactions with receptors, enzymes, and lipid-transport proteins (Bolanos-Garcia and Miguel, 2003; Ramasamy, 2014). Apolipoproteins have receptor binding domains containing low-density lipoprotein (LDL) receptors that direct lipid and substrate delivery to specific brain cells (Clavey et al., 1995; Dehouck et al., 1997; Herz, 2001). Their amphipathic-helices facilitate lipid-binding and lipid transport (Clavey et al., 1995; Prevost and Kocher, 1999; Elliott et al., 2010). LDL receptors also facilitate the clearance of amyloid peptides through the BBB (Shibata et al., 2000).
Contribution of Lipoproteins to AD Pathology
Brain lipoproteins with ApoE are responsible for phospholipid and cholesterol transport (Growdon and Hyman, 2014; Wong et al., 2019). ApoE is mainly expressed in astrocytes and microglia and appears as three major isoforms, ApoE2, ApoE3, and ApoE4, of which ApoE4 is the strongest genetic risk factor for AD (Stone et al., 1997; Ito et al., 2005; Vance and Hayashi, 2010; Chung et al., 2016; Liu et al., 2017; Montoliu-Gaya et al., 2018; Tulloch et al., 2018). ApoE4 demonstrates a lower affinity for lipids than ApoE2 and ApoE3, limiting CNS transport of lipids needed for neuronal remodeling and repair (Bradley and Gianturco, 1986; Barbagallo et al., 1998; Li et al., 2002; Frieden et al., 2017). Furthermore, levels of ApoE LDL receptors directly correlate with Aβ clearance, and promoting the expression of these receptors are potential therapeutic targets for AD treatment (Zhao et al., 2018). ApoJ, also known as clusterin, is expressed in astrocytes, neurons, and ependymal cells (Nuutinen et al., 2005, 2007). This neuroprotectant initiates a defense response to neuronal damage and clears Aβ across the BBB via LDLR-2 (Merino-Zamorano et al., 2016; Nelson et al., 2017; Zandl-Lang et al., 2018). ApoJ’s role in Aβ accumulation and toxicity is still undetermined because variability under different contexts and environments confound results (Foster et al., 2019).
Lipids and the Blood-Brain Barrier
The Blood-Brain Barrier
The blood-brain barrier (BBB) is a semipermeable membrane that carefully regulates the exchange of solutes between blood and brain to maintain CNS homeostasis and block entry of toxins and pathogens into the CNS (Bradbury, 1984; Abbott et al., 2010; Betsholtz, 2014; Daneman and Prat, 2015; Ferreira, 2019; Moura et al., 2019). The integrity of the BBB is largely dependent on its tight junctions (Brown and Davis, 2002; Castro Dias et al., 2019), adherens junction proteins, and ability to control the vesicular movement of macromolecules through transcytosis and pinocytosis (Dehouck et al., 1997; Baldo et al., 2014). The BBB permits free diffusion of gases, such as oxygen and carbon dioxide, but small solutes such as lipophilic molecules and ions enter through receptor-mediated transcytosis or via channels (Fishman et al., 1987; Zlokovic, 2008; Preston et al., 2014; Andreone et al., 2017; Villasenor et al., 2017; Ayloo and Gu, 2019). The BBB is critical in linking multiple major organ systems, and any dysfunction in the lipid bilayer’s ability to act as a barrier may lead to neuronal degeneration (Zhao Z. et al., 2015; Halliday et al., 2016; Muszynski et al., 2017; Nation et al., 2019).
Importance of Lipids in BBB Function
In addition to composing the BBB lipid bilayer, lipids, including phospholipids, sphingolipids, and cholesterol, also compose the plasma membrane of vesicles involved with receptor-mediated transcytosis within the CNS (Kramer et al., 2002; Dodelet-Devillers et al., 2009; Campbell et al., 2014; Andreone et al., 2017). The formation and function of vesicles required to transport essential macromolecules across the BBB may be affected by the plasma membrane lipid composition (Lingwood et al., 2009; Lingwood and Simons, 2010; Kaiser et al., 2011). In particular, DHA disrupts the membrane domains necessary to form such transport vesicles and therefore contributes to BBB integrity and suppression of transcytosis (Ouellet et al., 2009; Freund Levi et al., 2014; Pan et al., 2015, 2016; Belayev et al., 2018). There is also recent evidence that the membrane transport protein, Mfsd2a, controls lipid exchange and plays a key role in the transport of DHA into the brain, though this pathway is largely undetermined (Segi-Nishida, 2014; Zhao and Zlokovic, 2014; Keaney and Campbell, 2015; Andreone et al., 2017). Loss of Msfd2a transport function resulted in decreased DHA transport and increased activity levels of transcytosis within CNS endothelial cells (Andreone et al., 2017). A leaky barrier increases the brain’s susceptibility to toxins and pathogens and homeostasis disruption, and ultimately, neuronal dysfunction (Abbott, 2000; Hutchinson, 2010; Ikeshima-Kataoka and Yasui, 2016; Block, 2019).
The Contribution of the BBB to AD Pathology
Loss of BBB function may contribute to neurodegenerative diseases, including AD (Banks, 1999; Gilgun-Sherki et al., 2001; Zlokovic, 2008; Carvey et al., 2009; Karamanos et al., 2014; Sweeney et al., 2018; Katt et al., 2019). According to multiple independent studies, BBB breakdown in AD is demonstrated by decreased integrity of BBB tight junctions, pericyte and endothelial degeneration, RBC extravasation, and brain capillary leakages (Zlokovic, 2008; Carvey et al., 2009; de Vries et al., 2012; Nelson et al., 2016; Sweeney et al., 2018). A buildup of blood proteins and macromolecules due to barrier leakiness may damage vasculature and brain parenchyma, which induces neuronal degeneration. Studies have also indicated that AD pathology includes reduced expression of glucose transporters in the BBB (Kalaria and Harik, 1989; Harik and Kalaria, 1991; Guo et al., 2005; Agrawal et al., 2017; Block, 2019). This may exacerbate AD cerebrovascular degeneration and cognitive function, considering that the brain requires a continuous supply of glucose and utilizes the most glucose of the major organs (Benton et al., 1996; Dienel et al., 1997; Benton, 2001; Gong et al., 2006). The BBB contains a wide variety of structural components to regulate the brain’s health and function, but a loss of function in any such component may lead to dyshomeostasis and a rapid cascade of dysfunctions in other structures within the brain.
Lipids Contribute to Amyloid Precursor Protein Processing
Amyloid Precursor Protein Processing
Amyloid precursor protein (APP) is a type I transmembrane protein that is cleaved into amyloid β-peptide (Aβ) by β- and γ-secretases (Nunan and Small, 2000; Hartmann, 2012). APP is synthesized in the endoplasmic reticulum and is found in the highest concentrations in neuron’s trans-Golgi-network, suggesting that APP is associated with secretory pathways (Palacios et al., 1992; Stephens and Austen, 1996; Kitazume et al., 2001; Tam et al., 2014; Toh et al., 2017; Liu et al., 2019). There are two accepted proteolytic pathways for APP processing − non-amyloidogenic and amyloidogenic (Ishiura, 1991; Kojima and Omori, 1992; Sisodia, 1992; Roberts et al., 1994; Mills and Reiner, 1999; Soriano et al., 2001; Irizarry et al., 2004; Song et al., 2004; Chow et al., 2010; Wang et al., 2010; Tomita and Wong, 2011). The non-amyloidogenic pathway involves cleavage of APP by α-secretase at the plasma membrane, releasing soluble APPα (sAPPα) fragments into the extracellular environment, and normalizes AG genes and memory (Volmar et al., 2017). The amyloidogenic pathway involves cleavage of APP by β-secretase in early endosomes, releasing sAPPβ fragments in the endosomal lumen, and increasing susceptibility to Aβ plaques that are relevant to AD pathology (Estus et al., 1992; Golde et al., 1992; Saftig et al., 1996; Ehehalt et al., 2003; Andrew et al., 2016; Grimm et al., 2016).
The Role of Lipids in APP Processing
The β-site APP-cleaving enzyme 1 (BACE-1) is the major β-secretase that targets endosomes with APP in transit to endocytosis sites on the plasma membrane (Shimokawa et al., 1993; O’Brien and Wong, 2011; Chun et al., 2015; Audagnotto et al., 2018). Both APP and BACE-1 are associated with lipid rafts, which are membrane domains enriched with cholesterol, sphingolipids, and gangliosides that are crucial to vesicle trafficking and intracellular transport (Ehehalt et al., 2003; Yoon et al., 2007; Marquer et al., 2011; Bhattacharyya et al., 2013). Recent studies have proposed that BACE-1 in cholesterol depleted environments displayed inhibited β-secretase activity, suggesting that cholesterol and lipid composition of the intracellular environment may be a large determinant of whether BACE-1 can access APP endosomes (Dash and Moore, 1993; Cheng et al., 2014; Mukadam et al., 2018). However, other studies suggest that both homeostasis of lipid composition and oxidation state of lipids, including DHA, are critical to APP processing (Grimm et al., 2012; Bhattacharyya et al., 2013; Figure 2). Under conditions with high concentrations of oxidized lipids, levels of sAPPα fragments decreased while sAPPβ levels increased (Grimm et al., 2016). A novel mechanism of proteolytic activity regulation of secretases involves a separating lipid boundary with their substrates, APP (Kaether and Haass, 2004). Lipid mediators of inflammation also interact with APP processing at the level of O-GlcNAcylation (Sastre et al., 2008; Jean-Louis et al., 2018). Thus, oxidized or inflammatory lipids may shift APP processing from the non-amyloidogenic to an amyloidogenic pathway (Figure 2).
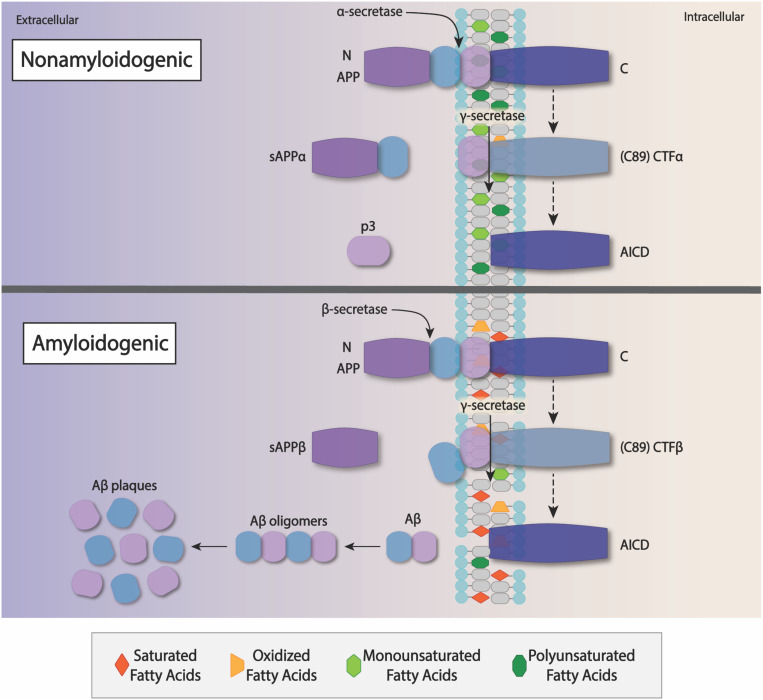
FIGURE 2.
The importance of Lipids on APP processing – APP is a transmembrane protein that is cleaved by several proteases: α-secretase, β-secretases, and γ-secretases. Non-amyloidogenic processing of APP− In a cell with a membrane containing normal or high amounts of unsaturated fatty acids, especially DHA, preference is given to cleavage by α-secretase In this case, a well-structured membrane holds onto an intact APP as it is cleaved by the α-secretase and subsequently the α-secretase releasing the secreted ectodomain sAPPα, along with a small protein fragment, p3, and APP intracellular C-terminal domain (AICD) peptide in the extracellular space. sAPPα and p3 do not form neurotoxic fibrils and plaques, and so this process is referred to as non-amyloidogenic APP processing. Amyloidogenic processing of APP – In contrast, PUFA enriched structure of healthy neurons, the presence of saturated and oxidized fatty acids results in the disruption of the cell membrane structure, and this favors β-secretase activation. APP is cleaved at its’ N-terminus by β-secretase, releasing a soluble ectodomain sAPPβ into the extracellular space. γ-secretase subsequently cleaves the cell-associated C-terminus releasing and Aβ peptides of varying lengths into the extracellular space. Insoluble Aβ fibrils aggregate as oligomers that ultimately clump to form plaques within the brain. These plaques contribute to oxidative stress, neuroinflammation, and eventually decreased brain function.
The Intersection of Lipids, APP Processing, and AD Pathology
The Aβ fragments of APP is the major component of AD amyloid plaques, and such dysregulation of APP trafficking and processing are relevant to understanding AD pathology (Caporaso et al., 1994; Thinakaran and Koo, 2008; Zhang et al., 2011; Tan and Gleeson, 2019; Yuksel and Tacal, 2019). Intracellular Aβ accumulation in neurons of patients with AD and metabolic analysis of brain function indicate a possible dysfunction in Aβ transport exiting the brain (O’Brien and Wong, 2011; Yuksel and Tacal, 2019). Lipids rafts play important roles in APP trafficking (Yoon et al., 2007; Yang et al., 2013). Moreover, palmitoylation dictates how APP is processed (Bhattacharyya et al., 2013). Trans fatty acids influence amylogenic APP processing, while the level of fatty acid unsaturation determines the activity of secretases (Yang et al., 2011; Grimm et al., 2012). Future research relating to changes in brain lipid composition in pre-symptomatic AD may provide a link with early disease onset, dysregulation of lipid metabolism, and APP processing.
The Intersection of Lipid Rafts, APP Processing, and AD Pathology
Lipid rafts are dynamic clusters of membrane lipids that interact with protein complexes to promote intracellular signal transduction (Mesa-Herrera et al., 2019). Normal aging is associated with gradual reductions in cholesterol and polyunsaturated fatty acids (PUFAs) in lipid rafts. With age-related changes lipid rafts composition, alterations in intracellular communication may be associated with age-associated reductions in synaptic plasticity. In neurodegenerative diseases, the composition of lipid rafts changes more rapidly, most notably in n-3 and n-6 PUFAs (Li et al., 2018). Lipid raft aging appears to be exacerbated in Alzheimer’s Disease, which may serve as the underlying contribution to disrupted signal transduction, increased APP processing, and rapid formation of AB aggregates (Grassi et al., 2019). Normal APP signal transduction involves cleaving APP into AB into the extracellular environment. However, if APP interacts with ApoE and tau on a lipid raft with an atypical lipid composition, signal transduction may be disrupted, promoting the formation of AB aggregates. Other alterations include reductions in unsaturation of FA in AD patients, as compared to controls (Kao et al., 2020). Lipid raft aging also appears to exhibit gender differences, such that women had more severe changes in lipid raft composition as compared to men. This may serve as supportive evidence for the finding that postmenopausal women are more likely to progress from MCI to AD than age-matchd men (Herrera). Considering that lipid raft function is sensitive to aging, further characterization of composition changes in lipid rafts within the brain may be useful as a biomarker of neurodegenerative stages.
Lipids and Cellular Remodeling
Role of Lipid Remodeling in Synaptogenesis
Lipid bodies (LBs) are spherical lipid-rich organelles associated with lipid storage, metabolism, cell signaling, and inflammation (Schmitz and Muller, 1991; Melo et al., 2011). At regulated levels, LBs maintain lipid homeostasis and cellular function, but in response to brain inflammation and increased neuronal oxidative stress, these LBs grow in size and accumulate within microglial cells (Tremblay et al., 2016; Hu et al., 2017). Though the pathway is still largely undiscovered, LBs in microglia appear to communicate with organelles such as the mitochondria, which control cell-death mechanisms (Tyurina et al., 2014). When exposed to lipopolysaccharides, LBs contact to mitochondria was disrupted, but DHA treatment reduced such effects. DHA may be a key factor in preserving mitochondrial health and regulation of microglial activity (Tremblay et al., 2016; Maysinger et al., 2018). When regulated in rodent models of AD, microglia slows the accumulation of Aβ plaques, but a proliferation of microglia activity may result in brain inflammation and degradation of neuronal synapses (Lim et al., 2000; Stahl et al., 2006; McClean et al., 2015). Microglial dysfunction has been implicated as a contributor to AD pathogenesis (Hansen et al., 2018). Microglia cells in the brain contribute to the reorganization of neuronal circuits by phagocytosing dead neurons and their dendritic spines and axon terminals. These immune cells contribute to neural plasticity (Wu and Zhuo, 2008; Yates, 2014; Yang et al., 2019), which refers to the brain’s ability to maintain, modify, and strengthen these synapses in order to permit neuronal communication (Tremblay et al., 2011).
Importance of Lipid Remodeling/Synaptogenesis in AD Pathology
Synaptogenesis is the formation of nerve synapses involving the reorganization of cell structural components (Aoki et al., 2003; Kelsch et al., 2010). Several studies suggest that presynaptic and postsynaptic development is initiated by signaling pathways involving cholesterol (Mauch et al., 2001; Fester et al., 2009). Changes in fatty acid content occur prior to synaptogenesis in cones (Martin and Bazan, 1992). Studies have shown that neurons deprived of lipid rafts underwent a cascade of effects inhibiting synaptic growth and development (Bazan, 2005; Welberg, 2014; Mochel, 2018). Depletion of lipid rafts decreased dendritic density and increased the synapse, disrupting neuronal communication (Martin, 2000; Hering et al., 2003; Sebastiao et al., 2013; Wang, 2014). The transport protein, apolipoprotein E (apoE), monitors cholesterol transport from glial cells to neurons, and impaired ApoE is implicated in deficits in synaptic plasticity and cognitive function (Periyasamy et al., 2017). Of the three isoforms of ApoE, ApoE4 is a prevalent risk factor that is synergistic with obesity and age for AD (Butler, 1994; Riedel et al., 2016; Jones and Rebeck, 2018; O’Donoghue et al., 2018; Glorioso et al., 2019). ApoE4 binds fewer lipids and is most likely involved in changes in cholesterol flux and metabolism (de Chaves and Narayanaswami, 2008; van den Kommer et al., 2012; Mahley, 2016; Nunes et al., 2018), accounting for altered synaptogenesis and neural plasticity.
Lipids and Myelination
The Importance of Myelination
Action potentials propagate along axons through rapid saltatory conduction. Synthesized by oligodendrocytes in the CNS and Schwann glial cells in the PNS, myelin membranes act as electrical insulators, permitting higher nerve conduction velocities and greater neuronal communication efficiency (Almeida and Lyons, 2014; Almeida and Lyons, 2017). Without myelin, axons would require more energy to depolarize its membrane (Stassart et al., 2018). Myelin is composed of several lipids and protein layers that wrap around most of the axon, except at nodes of Ranvier, which are regions highly concentrated with sodium ion channels (Finean and Robertson, 1958; Davison, 1972; Burgisser et al., 1986; Wender et al., 1988; Ando et al., 2003; Schmitt et al., 2015; Montani and Suter, 2018). Myelination of axons is a dynamic process through development and adulthood, and this process, in addition to myelin sheath modification and myelin repair, contributes to synaptic remodeling and neural plasticity (Zatorre et al., 2012).
The Role of Lipids in Myelination
The myelin membrane consists of myelin-specific proteins and high-level synthesis of lipids representative of all major classes, such as cholesterol, glycosphingolipids, glycerophospholipids, and galactolipids (Chrast et al., 2011). Lipids comprise approximately 80% of myelin’s dry weight, accounting for glia’s high demand for fatty acids, which are fundamental building blocks of its lipid structure (Dimas et al., 2019). Myelin accounts for a majority of the white matter in the brain, which is consistent with reported reduced myelin density associated with AD white matter changes in the brain (Nasrabady et al., 2018).
Brain Myelination and AD Pathology
Reduced number and activity of oligodendrocytes and precursor cells can damage myelin integrity, contributing to AD pathology’s characteristic neuronal loss (Bartzokis, 2011). Oligodendrocytes support and regulate neurons, but they are primarily responsible for myelin production (Simons and Nave, 2015). Myelinating oligodendrocytes are sensitive to lipid peroxidation because oxidative stress inhibits expression of genes that promote oligodendrocyte differentiation (French et al., 2009). This implies that disruption of myelin synthesis may be a central feature of AD pathology, and can be exploited for therapy (Desai et al., 2010). Dysfunction in these processes may be linked to white matter abnormalities and cognitive impairment associated with AD due to damaged signal conductivity and synchronicity needed for information processing between neurons (Ihara et al., 2010; Alexander, 2017; Nasrabady et al., 2018). The causal relationship between myelination and AD has not been elucidated, but white matter changes arising from myelination dysfunctions have been described in AD brains (Kohama et al., 2012). Additional evidence for the contribution of myelin breakdown on AD pathology comes from studies showing that the rate and severity of myelin breakdown in healthy seniors are associated with APOE status, a major risk factor of AD (Bartzokis et al., 2006).
Lipids and Receptor-Mediated Signaling
Neuronal Receptor Signaling Pathways
Neurons communicate via electrochemical signals and neurotransmitters across gaps called synapses associated with several integrated networks (Mayer, 1993; Laughlin and Sejnowski, 2003; Salinas, 2009; Hahn et al., 2019). The presynaptic neuron releases neurotransmitters through exocytosis, and those chemicals bind to the postsynaptic neuron’s neurotransmitter receptors to alter postsynaptic neuronal activity (Kennedy, 2013). One class of neurotransmitter receptors, called ligand-gated ion channel receptors, opens an ion pore through the membrane upon ligand binding. Ions cannot travel through the hydrophobic lipid membrane and, therefore, can only pass through channels controlled by these receptors. Ions entering the ligand-gated channel can initiate excitatory or inhibitory signals, but both rapidly influence neuronal function (Cantor, 2018). Another class of neurotransmitter receptors, G-protein-coupled receptors (GPCRs), bind to the ligand and initiate an intracellular mechanism in which its G-proteins alter cAMP levels to stimulate or inhibit the neuron, and may involve lipid agonists (Hansen, 2015). Unlike ligand-gated ion channel receptors, GPCRs are slower but longer-lasting in affecting neuronal activity (Lovinger, 2008).
Role of Lipids in Neuronal Signaling
While cascades of protein kinases and phosphatases have been largely studied, there is an increasing interest in lipid-based pathways involving lipid kinases and phosphatases. Lipids are versatile in signal transduction pathways and act as hormones, ligands, substrates, and mediators (Eyster, 2007; Piomelli et al., 2007; Piomelli, 2012). Sphingolipids and cholesterol comprise lipid rafts, which are regions in the plasma membrane that organize signaling molecules, amplify intracellular signaling cascades, and regulate both neurotransmission and membrane protein trafficking (Levental and Veatch, 2016). Additionally, lipids are integral to GPCR signaling cascades. Following GPCR binding, phospholipase C (PLC) cleaves the polar phosphate head of phospholipids and forms diacylglycerol (DAG), a lipid second messenger (Black et al., 2016). Fatty acids (FAs), especially those belonging to the omega-3 and omega-6 classes, act as ligands for membrane receptors in a variety of pathways (Mobraten et al., 2013). The wide diversity of lipids and their structures contributes to AD, and their multiple roles in signal transduction may influence AD pathology.
Signaling Lipids Contribute to AD Pathology
Endocannabinoid signaling is responsible for inhibition and excitation in modulating synaptic strength, implicating its possible role in AD and associated inflammatory pathology (Skaper and Di Marzo, 2012). Although the mechanism has not been elucidated yet, free radicals and oxidative stress increase GPCR cannabinoid 2 receptors (CB2) expression in AD microglial cells, increasing neuroinflammation (Paloczi et al., 2018). Inflammation protects the brain against neurotoxins, but excessive inflammation may contribute to neurodegeneration. Another study suggested that monoacylglycerol lipase (MAGL) produces neuroinflammatory prostaglandins through the hydrolysis of endocannabinoids (Piro et al., 2012). Inhibiting MAGL activity is a potential AD therapeutic target because it is reported to prevent neuroinflammation, neurodegeneration, and impaired synaptic plasticity (Chen et al., 2012). Dysregulation in neuronal signaling cascades may contribute to increased susceptibility to neuronal dysfunction and are, therefore, important in studying its effects and relation to AD.
Lipids and Inflammation
The Importance of Inflammation
Inflammation is a defense mechanism initiated by the immune system in response to pathogens, injured cells, infections, and other toxic stimuli. A signaling cascade results in leukocyte migration to damaged sites, in which released cytokines recruit other immune cells to heal injured tissue (Robinson et al., 2018). Specifically, within the CNS, activation of microglia and its associated cytokine production are primarily responsible for the inflammatory responses (Frank et al., 2007; Ghosh et al., 2012; Zhu et al., 2019). However, unregulated inflammation, excessive cytokine production, and failure to resolve inflammatory responses all contribute to chronic neuroinflammation, a biomarker of many neurodegenerative diseases, including AD (Wang et al., 2015a).
Lipids and Inflammation
Several studies implicate the role of lipids and lipid metabolism in inflammatory responses (Janciauskiene and Wright, 1998; Kang and Rivest, 2012; Zhang et al., 2018; Ntambi, 2019). Eicosanoids are a class of lipid mediators inflammation produced by innate immune cells that contribute to acute inflammation, resulting in pain, loss of function, heat, and swelling (Higgs et al., 1984; Williams and Higgs, 1988; Hedqvist et al., 1991; Umamaheswaran et al., 2018). Following the elimination of toxic stimuli, innate immune cells cease the production of eicosanoids and begin production of specialized pro-resolving lipid mediators (SPMs) to resolve inflammation (Serhan, 2010; Chandrasekharan and Sharma-Walia, 2015; Chiurchiu et al., 2018; Maclean et al., 2018). Synthesized from omega-3 fatty acids, docosahexaenoic acid (DHA), and eicosapentaenoic acid (EPA), SPMs resolve inflammation by inhibiting polymorphonuclear leukocytes (PMN) and lowering vascular permeability This process may be impaired in AD (Whittington et al., 2017).
Inflammatory Lipids and AD Pathology
A disproportionate level of inflammation can disrupt the balance between eicosanoids and SPMs, overwhelming the brain’s ability to return to a non-inflammatory state. This suggests the brain’s dependence on SPMs and its omega-3 precursors, DHA, and EPA (Serhan et al., 2018). AD pathology includes decreased DHA levels (Fonteh et al., 2014; Yassine et al., 2017), which may account for heightened brain inflammation that leads to declining cognitive health. Moreover, many studies have reported alterations to the eicosanoid pathway in AD (Biringer, 2019), further heightening research interest in the balance between eicosanoids and SPMs (Serhan et al., 2015). AD is also associated with elevated microglia-induced neuroinflammation, increases in proinflammatory cytokines, and upregulated expression of phagocytic receptors in white matter microglia (Zheng et al., 2016). One receptor, CD36, promotes both pro-inflammatory and oxidative pathways upon binding to ligands, including lipids and Aβ (Doens et al., 2017). Overexpression may lead to dysregulated inflammation and increased oxidative stress, a biomarker of the inflammatory response, and AD (Park et al., 2014; Koizumi et al., 2016). White matter is critical to neuronal connectivity and processing speed, and such white matter inflammation may result in neurodegeneration and, therefore, the cognitive decline (Raj et al., 2017). Further studies aim to determine if inflammation contributes to the onset of AD or exacerbates already-existing neuropathology (Heppner et al., 2015).
Lipids and Oxidative Stress
Oxidative Stress
Oxidative stress is defined as a disruption in homeostasis between antioxidants and oxidants, and more specifically, an accumulation of reactive oxidative species (ROS) and reactive nitrogen species (RNS) (Apak et al., 2016; Hameister et al., 2020). ROS belongs to a family of compounds containing partially reduced oxygen species, such as O2– and HO-, that are generated primarily by the electron transport chain during aerobic respiration (Zhao et al., 2019). ROS are involved in many redox-dependent processes, including cell signaling, homeostasis, immune system responses, energy metabolism, and tissue remodeling. However, an excess of ROS or impaired control of the balance between antioxidants and oxidants leads to oxidative stress, which is implicated in the progression of neurodegenerative diseases (Cheignon et al., 2017). Because the brain consumes approximately 25% of the body’s glucose, its high energy consumption increases neurons’ susceptibility to oxidative stress and overproduction of ROS (Wezyk et al., 2018).
Membrane Lipids Are Damaged During Oxidative Stress
Excess ROS can lead to increased lipid peroxidation within the brain, altering membrane permeability and activity of membrane receptors and their associated enzymes (Birben et al., 2012). Lipid peroxidation produces reactive aldehydes, including malondialdehyde (MDA) and 4-hydroxynonenal (HNE), that modify and bind to proteins involved in metabolism, antioxidant defense systems, and axonal growth. By modifying Tau protein, 4-HNE can indirectly lead to an increase in neurofibrillary tangles, which is consistent with proteomic reports of increased 4-HNE in AD hippocampal tissue and neurofibrillary tangles (Cheignon et al., 2018). Moreover, low-density lipid lipoprotein receptor-related protein (LRP1) is involved in Aβ peptide removal. LRP1 is oxidized by Aβ, inhibiting its ability to clear Aβ and therefore leading to Aβ accumulation in the brain (Shinohara et al., 2017). LRP1 is another protein that is covalently modified by 4-HNE, further supporting that unrestrained lipid peroxidation produces excess reactive products that initiate a cascade of dysregulations within pathways necessary to neuronal health (Butterfield et al., 2002). Oxidant/antioxidant imbalance forms blood-based biomarkers that can be used for early, non-invasive diagnosis (Wojsiat et al., 2018), or for AD therapies (Yatin et al., 2000; Sultana et al., 2004).
Oxidative Stress and AD Pathology
Many trials seek to assess different antioxidant therapeutic approaches to alleviate oxidative stress, a key biomarker of AD. CoQ10, creatine, idebenone, latrepirdine, triterpenoids, omega-3 PUFAs, vitamin E, and vitamin C are just a few antioxidants that are extensively studied in their treatment of neurodegenerative diseases (Yatin et al., 2000; Kumar and Singh, 2015).
Lipids and Immune Response
The Immune System
The immune system, which is divided into the innate and adaptive immune system, is critical to defending the body against infectious and toxic stimuli (Simon et al., 2015). The innate immune system utilizes cytokine production and modulation to mount a quick but sufficient response to pathogens, including viruses, bacteria, and parasites. The innate immune system is also responsible for activating the adaptive immune system, which is slower due to the lengthy production of specific antibodies to the foreign antigen (Iwasaki and Medzhitov, 2015). Studies in the past 20 years have refuted the notion of the brain as being “immunologically privileged” in relying largely on innate immune system mechanisms within the CNS. While it was thought that the CNS and immune system were separate due to the blood-brain barrier, the detection of lymphatic vessels connecting T-cells in lymph nodes to cerebrospinal fluid (CSF) in the meninges provided evidence for the brain’s semi-dependence on the adaptive immune system (Louveau et al., 2015). Neuroimmune processes are activated by vagal nerve signaling, immune signals, and complement proteins, resulting in increased activity of microglia and astrocytes (Tchessalova et al., 2018).
Lipids and Immunity
Studies reported increased levels of platelets and vascular lesions in AD patients outside of the brain, contributing to cerebral amyloid angiopathy, a biomarker of AD that shows increased amyloid protein in the brain arteries (Kniewallner et al., 2015). Although platelets combat vascular injury, they are also involved in APP processing, and transitively, the formation of Aβ plaques (Evin et al., 2003; Evin and Li, 2012). The balance of omega-3 and omega-6 PUFAs may affect platelet levels, as membrane essential fatty acids (EFAs), primarily DHA and EPA, form prostaglandins PGE1, PGE2, and PGE3, all of which participate in a variety of immunological and signaling pathways in the brain (Chang et al., 2009). PGE1 has anti-inflammatory properties, and conversely, PGE2 strongly promotes inflammation by acting on different receptors (Iyu et al., 2011). PGE3 is responsible for regulating PGE2’s inflammatory effects by competing with its formation from precursor EFAs (Chang et al., 2009). Imbalances in the omega-6 to omega-3 PUFA ratios disrupt the formation of PGE3, which minimizes the regulation of PGE2 induced inflammation. Moreover, this imbalance of PUFAs is associated with changes in neuronal brain composition that, in combination with drug therapies, can reduce the risks and slow the progression of AD (Giulietti et al., 2016).
Immunity and AD Pathology
An impaired BBB is implicated with the onset of AD, which may increase the BBB’s permeability to pathogens and immune cells (Veerhuis et al., 2011). Levels of cytotoxic and helper T-cells are upregulated in brain parenchyma of AD patients (Oberstein et al., 2018). Helper T cells and pro-inflammatory cytokines target neurofibrillary tangles and plaques composed of Aβ and Tau and activate microglia at these sites, further exacerbating neuroinflammation (Gold et al., 2014; Martinez-Frailes et al., 2019). One class of cytokines, called chemokines, stimulates leukocyte migration from blood to tissues. CCL5 is a chemokine that is amplified in response to reactive oxygen species and oxidative stress within the brain’s endothelial cells, promoting even more T cell migration across the leaky BBB. These inflammatory mediators are elevated in the CSF and blood and are possible biomarkers for detecting AD and its progression (Mietelska-Porowska and Wojda, 2017).
Lipids and Energy Regulation
Sources of Brain Energy
Although the human brain comprises only 2% of the body weight, it consumes approximately 20% of glucose, demonstrating its disproportionately high energy demand (Mergenthaler et al., 2013). The majority of the energy utilized by the brain is dedicated to returning neurons to their resting states after depolarization, and the remaining 20−25% of energy is allocated toward synthesizing vesicles and neurotransmitters (Harris et al., 2012). The brain relies on a constant flow of glucose and oxygen, which are delivered through the blood. However, during fasting periods, when glucose levels are decreased, the liver can supply ketone bodies to support metabolism within the brain (Patel et al., 1975; Hawkins and Biebuyck, 1979; Nehlig, 2004). These delivered ketone bodies are primarily utilized by astrocytes, and upon arrival, ketolysis of the ketone bodies generates acetyl CoA, an important substrate for the tricarboxylic acid (TCA) cycle and therefore, ATP production. Although the brain has a large ATP requirement, it does not utilize these ketone bodies or fatty acids as a significant source of energy like in other organs, such as the liver. It is hypothesized that evolution selected against this pathway because it produces ROS and therefore, contributes to oxidative stress that contributes to neurodegeneration (Schonfeld and Reiser, 2017).
Role of Brain Energy Regulation in AD Pathology
Transport and utilization of glucose within the brain are tightly regulated, but mitochondrial dysfunction and decreased expression of glucose transporters (GLUT) are potential contributors to AD (Yin et al., 2016). Highly concentrated in the BBB, GLUT1 transports glucose across the endothelium and into astrocytes, whereas GLUT3 is predominantly found in axons and dendrites, underscoring its role in neuronal glucose transport and distribution (Vannucci et al., 1998). Reduced GLUTs expression at the BBB and within neurons is associated with AD, which may explain overall decreased glucose metabolism in AD pathology (Yin et al., 2016).
Mitochondrial Dysfunction and AD Pathology
Mitochondria are organelles central to brain energy processes, and altering glucose availability or dysregulating oxidative phosphorylation can have direct effects on neuronal function and cognitive health (Picard and McEwen, 2014; Anderson, 2018). Recent reports have hypothesized that Aβ may initiate mitochondrial dysfunction, and one theory proposes that Aβ raises cytosolic calcium levels, inhibiting oxidative phosphorylation and, therefore, ATP production (Cardoso et al., 2001; Eckert et al., 2010; Spuch et al., 2012; Kaminsky et al., 2015; Brewer et al., 2020). Moreover, mitochondria delivery to needed brain regions is dependent on tau, a protein related to microtubules (Quintanilla et al., 2012; Amadoro et al., 2014). Mitochondria are observed to be differentially localized in AD brains, suggesting that mitochondria trafficking is affected (Nicholls and Budd, 2000; Duchen, 2012; Devine and Kittler, 2018; Son and Han, 2018; Rangaraju et al., 2019), and provides further support for mitochondrial-based contributors to neurodegeneration.
Potential AD Therapies Targeting Lipid Metabolism
Dietary Modification Studies
With the realization that lipids are altered in AD pathology, several studies have identified specific lipids that may be used as dietary supplements to alleviate AD symptoms (Table 3). The major lipids include omega-3 fatty acids (DHA, EPA), choline-containing lipids, cholesterol, and lipids with antioxidant properties (CoQ10, Vitamin K).
TABLE 3.
Lipid diets and their effects on AD.
| Lipid diet interventions | Effects on AD |
| Algal DHA#; 2 g daily for 18 months | Supplementation with DHA compared with placebo did not slow the rate of cognitive and functional decline in patients with mild to moderate Alzheimer’s disease (Quinn et al., 2010). |
| Consumption of fish once or more per week | In adults above the age of 65, participants who consumed fish once or more per week had 60% less risk of developing Alzheimer’s compared to participants who rarely or never ate fish (Morris et al., 2003). |
| Omega-3 PUFA; 600 mg EPA and 625 mg DHA daily for 4 months | In adults with mild cognitive impairment and probable AD, omega-3 supplementation had negligible effects on cognition or mood (Phillips et al., 2015). |
| EPA-DHA for 26 weeks; stratified into high-dose (180 mg EPA-DHA daily) and low-dose (400 mg daily) | In cognitively healthy adults over 65 years old, there were no significant differential changes in any of the cognitive domains for either low-dose or high-dose fish oil supplementation compared with placebo (van de Rest et al., 2008). |
| Study participants are postmenopausal women (60−84 years); 1g DHA, 160 mg EPA, 240 mg Ginkgo biloba, 60 mg PS, 20 mg per day for 6 months | In a randomized, double-blind study, a high dose of omega-3 nutrients has cognition and mobility benefits to older women (Strike et al., 2016). |
| DHA-EPA; 1.7 g DHA and 0.6 g EPA daily for 6 months (OmegAD Study) | Omega-3 fatty acids did not delay the rate of cognitive decline, nor did it have marked effects on neuropsychiatric symptoms except for possible positive effects on depressive symptoms in non-APOE4 carriers and agitation symptoms in APOE4 carriers (Freund-Levi et al., 2006; Freund-Levi et al., 2008). Plasma levels of AA decreased while DHA and EPA levels increased at 6 months. Specialized pro-resolving mediators (SPMs) do not change in the omega-3 group but a decrease in the placebo group. SPM changes associate with cognitive changes in AD (Lopez et al., 2011). |
| Omega-3 PUFAs; 1.8 g daily for 24 weeks | The omega-3 supplementation treatment group showed significant improvement in the Alzheimer’s Disease Assessment Scale compared to the placebo group in participants with mild cognitive impairment. However, there was no significant improvement in Alzheimer’s disease study participants (Chiu et al., 2008). |
| Supplementation with omega-3 fatty acids alone or omega-3 plus alpha-lipoic acid; 675 mg DHA and 975 mg EPA or 675 mg DHA and 975 mg EPA plus 600 mg lipoic acid daily for 12 months | Combining omega-3 fatty acids with lipoic acid slowed both cognitive and functional decline in mild to moderately impaired AD participants over 12 months compared to placebo (Shinto et al., 2014). |
| 3 DHA exposure variables used in separate analyses; plasma DHA, dietary DHA, and consumption of cold-water fish | Plasma and dietary DHA were associated with a decreased risk of dementia and AD (Lopez et al., 2011). |
| Arachidonic acid and DHA supplementation;240 mg of AA and DHA daily for 90 days | Participants with mild cognitive impairment showed a significant improvement in the immediate memory and attention score compared to placebo, but there was no significant improvement in participants with AD (Kotani et al., 2006). |
| Docosahexaenoic acid-concentrated fish oil supplementation; 430 mg of DHA and 150 mg of EPA daily for 12 months | In participants with mild cognitive impairment, supplementation resulted in a significant improvement in short-term memory, working memory, immediate verbal memory, and delayed recall capability (Lee et al., 2013). |
| Fortasyn Connect supplementation; 125 mL once-a-day drink containing Fortasyn Connect for 24 months (LipiDiDiet Trial) | In individuals with prodromal AD, Fortasyn Connect supplementation had no significant effect on neuropsychological test battery results (Soininen et al., 2017). |
| FINGER Study − Dietary intervention using a diet with 10−20% of daily energy (E%) from proteins, 25−35% from fat (less than 10E% from SAFA, 10−20% from MUFA, 5−10% from PUFA (including 2,5−3 g/day n-3 fatty acids); 45−55% from carbohydrates (less than 10% refined sugar); 25−35 g/day dietary fiber; less than 5 g/day salt; and less than 5 E% from alcohol for 2 years | In adults over 60 years old, there was a significant beneficial intervention effect on overall cognitive performance, including memory, executive function, and psychomotor speed (Rosenberg et al., 2020). |
#DHA, Docosahexaenoic acid (C22:6, n-3); EPA, Eicosapentaenoic acid (C20:5, n-3); MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SAFA, saturated fatty acids.
Several dietary intervention studies using DHA have yielded mixed effects on AD symptoms. A likely reason for these mixed results is that different disease severity, different formulations, and variable endpoint and time of interventions were studied (Fonteh, 2018). Recent studies indicate that the best form of DHA delivery into the brain is through the Msf2a LPC receptors (Sugasini et al., 2019). A better understanding of the right formulation and optimal concentrations of DHA probably supplemented at the prodromal phase of AD will likely yield beneficial outcomes.
Modification of Lipid Metabolism
Metabolism of lipids can be altered to prevent depletion of their levels in the AD by targeting pathways that transport or catabolize these lipids in the brain.
Lipid Transport Into the Brain
Several lipoproteins and their receptor complexes are the major form by which lipids bypass the BBB to be delivered into the brain. Several of these lipoprotein genes are linked to AD pathology (Table 1). Some lipoproteins have protective effects, while others have AD enhancing properties. For example, HDL has been shown to be protective by improving Aβ clearance, delaying Aβ fibrillization, suppressing vascular inflammation, and inducing endothelial nitric oxide production (Button et al., 2019).
Cholesterol Metabolism
Since cholesterol metabolism altered at several stages of AD, modulation of its metabolism may have beneficial effects on disease progression. Modification of cholesterol homeostasis can be influenced during its consumption, at the level of its biosynthesis, and during its transport into the brain. The use of statin to alter cholesterol biosynthesis is proposed to be insightful in AD pathophysiology and therapy (Wolozin et al., 2004; Hoglund et al., 2005; Biondi, 2007; Evans et al., 2009). Gene therapy targeting cholesterol 24-hydroxylase reduces the amyloid pathology before or after the onset of amyloid plaques in mouse AD models (Hudry et al., 2010). Studies in mouse models show that blocking the conversion of cholesterol to cholesterol esters has beneficial effects on AD (Shibuya et al., 2015). The relationship between hypercholesterolemia, cholesterol-lowering therapies, and the role of oxysterols in AD pathology have led to the proposition that cholesterol metabolites are valuable targets for alternative AD treatments or prevention (Loera-Valencia et al., 2019). Neuroinflammatory pathways mediated by toll-like receptor 4 (TLR4)-mediated signaling can aggravate AD symptoms. In a rodent AD model, treatment with an anti-inflammatory steroid (atorvastatin) regulates this inflammatory process and results in the amelioration of cognitive deficits (Wang et al., 2018).
Lipolytic Enzymes
The activity or expression of several lipolytic enzymes are altered in AD. Phospholipase A2 (PLA2) is associated with amyloid plaques, and reduction of its activity and expression ameliorates AD. Plasmalogen selective PLA2 is also altered in AD. Our studies show an increase in PLA2 activity of CSF of AD participants accompanied by an increase in lysophosphatidylcholine (LPC). LPC is known to disrupt the BBB, and changes in PLA2 are associated with inflammation. The association of PLA2 with AD pathology suggests that inhibitors of PLA2 activity or expression may be an effective means of preventing AD. Ong et al. (2015) reviewed the importance of several natural and synthetic PLA2 inhibitors on the treatment of neurological disorders. Since PLA2 isoforms may have divergent effects on membrane remodeling and function, there is a need for isoform-specific inhibitors in order to avoid toxicity encountered with non-selective inhibitors. In addition to PLA2, phospholipase D (PLD) and phospholipase C (PLC) expression and activities are associated with AD pathology. These lipases that are linked with neurite growth and signaling, respectively, offer other avenues for exploring AD treatments.
Lipid Oxidation Inhibitors
There is convincing evidence for the importance of oxidative stress on AD pathology (Sun et al., 2001; Bassett and Montine, 2003; Bacchetti et al., 2015). The most important brain fatty acid, DHA, is a polyunsaturated fatty that is easily susceptible to oxidative damage. While HDL is protective against oxidative damage, VLDL is easily oxidized. Oxidatively damaged lipids contribute to AD pathology by their highly neurotoxic properties (Bassett et al., 1999). Approaches that reduce oxidation are expected to reduce AD progression. These include the use of natural antioxidants, carnosine, lipoic acid, Ginkgo biloba flavonoids, soybean isoflavones, vitamin K, homocysteine, curcumin (Rutten et al., 2002; Vina et al., 2004; Frank and Gupta, 2005; Mancuso et al., 2007; Zhao, 2009; Cankurtaran et al., 2013). A limitation of natural antioxidant is the lack of demonstration of efficacy. Given that oxidative stress destroys mitochondrial function, an objective measure of any antioxidant can be their ability to restore mitochondrial function (Kumar and Singh, 2015; Kwon et al., 2016; Yu et al., 2016). The role of endogenous lipids in oxidative stress can be exploited when there is an uncontrolled formation of ROS and RNS or when the antioxidants contribute to disease pathology (Leuti et al., 2019). Also, the source of ROS determines the effects on cellular physiology and manipulation of the ubiquinone redox state is proposed to be a viable approach of delaying aging and therapy (Scialo et al., 2016; Wojsiat et al., 2018).
Concluding Remarks
Biochemical, physiological, and genetic analyses show that lipid metabolism interphases with all the major facets of AD pathology (Figure 1). In normal aging, lipid metabolic homeostasis ensures that the basic functions of the brain are met. In AD, there is dyshomeostasis of lipid metabolism, and this results in abnormal functions of the brain that characterize disease progression. This underscores the need for detailed analyses of brain lipid homeostasis in order to unravel more comprehensive mechanisms, specific biomarkers, and novel therapies of AD.
Author Contributions
AF contributed to the conceptualization and study design, supervised the data, and carried out the project administration and funding acquisition. HC, VS, and AF contributed to writing of the original draft and manuscript preparation. HC and AF contributed to the manuscript review and editing. VS and AF prepared the figures and illustrations.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank HMRI and Dr. M. Harrington for providing support and a scholarly environment for the summer student program. Dr. H. Yassine provided mentorship to VS.
Footnotes
Funding. AF was supported by funds from the L. K. Whittier Foundation and HMRI. VS is supported by R01 AG054434 and R01 AG055770 to Dr. H. Yassine.
References
- Abbott N. J. (2000). Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol. 20 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott N. J., Patabendige A. A., Dolman D. E., Yusof S. R., Begley D. J. (2010). Structure and function of the blood-brain barrier. Neurobiol. Dis. 37 13–25. [DOI] [PubMed] [Google Scholar]
- Agrawal M., Ajazuddin, Tripathi D. K., Saraf S., Saraf S., Antimisiaris S. G., et al. (2017). Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J. Control. Release 260 61–77. 10.1016/j.jconrel.2017.05.019 [DOI] [PubMed] [Google Scholar]
- Alaupovic P. (1996). Significance of apolipoproteins for structure, function, and classification of plasma lipoproteins. Methods Enzymol. 263 32–60. 10.1016/s0076-6879(96)63004-3 [DOI] [PubMed] [Google Scholar]
- Alexander G. E. (2017). An Emerging role for imaging white matter in the preclinical risk for Alzheimer disease: linking beta-amyloid to myelin. JAMA Neurol. 74 17–19. [DOI] [PubMed] [Google Scholar]
- Almeida R. G., Lyons D. A. (2014). On the resemblance of synapse formation and CNS myelination. Neuroscience 276 98–108. 10.1016/j.neuroscience.2013.08.062 [DOI] [PubMed] [Google Scholar]
- Almeida R. G., Lyons D. A. (2017). On myelinated axon plasticity and neuronal circuit formation and function. J. Neurosci. 37 10023–10034. 10.1523/jneurosci.3185-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadoro G., Corsetti V., Florenzano F., Atlante A., Ciotti M. T., Mongiardi M. P., et al. (2014). AD-linked, toxic NH2 human tau affects the quality control of mitochondria in neurons. Neurobiol. Dis. 62 489–507. 10.1016/j.nbd.2013.10.018 [DOI] [PubMed] [Google Scholar]
- Anceline M.-L., Ripoche E., Dupuy A.-M., Samieri C., Rouaud O., Berr C., et al. (2014). Gender-specific associations between lipids and cognitive decline in the elderly. Eur. Neuropsychopharmacol. 24 1056–1066. 10.1016/j.euroneuro.2014.02.003 [DOI] [PubMed] [Google Scholar]
- Anderson G. (2018). Linking the biological underpinnings of depression: role of mitochondria interactions with melatonin, inflammation, sirtuins, tryptophan catabolites, DNA repair and oxidative and nitrosative stress, with consequences for classification and cognition. Prog. Neuropsychopharmacol. Biol. Psychiatry 80 255–266. 10.1016/j.pnpbp.2017.04.022 [DOI] [PubMed] [Google Scholar]
- Ando S., Tanaka Y., Toyoda Y., Kon K. (2003). Turnover of myelin lipids in aging brain. Neurochem. Res. 28 5–13. [DOI] [PubMed] [Google Scholar]
- Andreone B. J., Chow B. W., Tata A., Lacoste B., Ben-Zvi A., Bullock K., et al. (2017). Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron 94 581.e5–594.e5. 10.1016/j.neuron.2017.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew R. J., Kellett K. A., Thinakaran G., Hooper N. M. (2016). A greek tragedy: the growing complexity of alzheimer amyloid precursor protein proteolysis. J. Biol. Chem. 291 19235–19244. 10.1074/jbc.r116.746032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki C., Fujisawa S., Mahadomrongkul V., Shah P. J., Nader K., Erisir A. (2003). NMDA receptor blockade in intact adult cortex increases trafficking of NR2A subunits into spines, postsynaptic densities, and axon terminals. Brain Res. 963 139–149. 10.1016/s0006-8993(02)03962-8 [DOI] [PubMed] [Google Scholar]
- Apak R., Ozyurek M., Guclu K., Capanoglu E. (2016). Antioxidant activity/capacity measurement. 3. reactive oxygen and nitrogen species (ROS/RNS) scavenging assays, oxidative stress biomarkers, and chromatographic/chemometric assays. J. Agric. Food Chem. 64 1046–1070. 10.1021/acs.jafc.5b04744 [DOI] [PubMed] [Google Scholar]
- Arnoldussen I. A., Zerbi V., Wiesmann M., Noordman R. H., Bolijn S., Mutsaers M. P., et al. (2016). Early intake of long-chain polyunsaturated fatty acids preserves brain structure and function in diet-induced obesity. J. Nutr. Biochem. 30 177–188. 10.1016/j.jnutbio.2015.12.011 [DOI] [PubMed] [Google Scholar]
- Asada T., Kariya T., Yamagata Z., Kinoshita T., Asaka A. (1996). ApoE epsilon 4 allele and cognitive decline in patients with Alzheimer’s disease. Neurology 47:603. 10.1212/wnl.47.2.603 [DOI] [PubMed] [Google Scholar]
- Audagnotto M., Kengo Lorkowski A., Dal Peraro M. (2018). Recruitment of the amyloid precursor protein by gamma-secretase at the synaptic plasma membrane. Biochem. Biophys. Res. Commun. 498 334–341. 10.1016/j.bbrc.2017.10.164 [DOI] [PubMed] [Google Scholar]
- Ayloo S., Gu C. (2019). Transcytosis at the blood-brain barrier. Curr. Opin. Neurobiol. 57 32–38. 10.1016/0006-8993(87)90236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetti T., Vignini A., Giulietti A., Nanetti L., Provinciali L., Luzzi S., et al. (2015). Higher levels of oxidized low density lipoproteins in Alzheimer’s disease patients: roles for platelet activating factor acetyl hydrolase and paraoxonase-1. J. Alzheimers Dis. 46 179–186. 10.3233/JAD-143096 [DOI] [PubMed] [Google Scholar]
- Balazs Z., Panzenboeck U., Hammer A., Sovic A., Quehenberger O., Malle E., et al. (2004). Uptake and transport of high-density lipoprotein (HDL) and HDL-associated alpha-tocopherol by an in vitro blood-brain barrier model. J. Neurochem. 89 939–950. 10.1111/j.1471-4159.2004.02373.x [DOI] [PubMed] [Google Scholar]
- Baldo G., Giugliani R., Matte U. (2014). Lysosomal enzymes may cross the blood-brain-barrier by pinocytosis: implications for enzyme replacement therapy. Med. Hypotheses 82 478–480. 10.1016/j.mehy.2014.01.029 [DOI] [PubMed] [Google Scholar]
- Banks W. A. (1999). Physiology and pathology of the blood-brain barrier: implications for microbial pathogenesis, drug delivery and neurodegenerative disorders. J. Neurovirol. 5 538–555. 10.3109/13550289909021284 [DOI] [PubMed] [Google Scholar]
- Banks W. A., Farr S., Salameh T. S., Niehoff M. L., Rhea E. M., Morley J. E., et al. (2018). Triglycerides cross the blood–brain barrier and induce central leptin and insulin receptor resistance. Int. J. Obes. 42 391–397. 10.1038/ijo.2017.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo C. M., Levine G. A., Blanche P. J., Ishida B. Y., Krauss R. M. (1998). Influence of apoE content on receptor binding of large, bouyant LDL in subjects with different LDL subclass phenotypes. Arterioscler. Thromb. Vasc. Biol. 18 466–472. 10.1161/01.atv.18.3.466 [DOI] [PubMed] [Google Scholar]
- Bartzokis G. (2011). Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol. Aging 32 1341–1371. 10.1016/j.neurobiolaging.2009.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G., Lu P. H., Geschwind D. H., Edwards N., Mintz J., Cummings J. L. (2006). Apolipoprotein E genotype and age-related myelin breakdown in healthy individuals: implications for cognitive decline and dementia. Arch. Gen. Psychiatry 63 63–72. [DOI] [PubMed] [Google Scholar]
- Bassett C. N., Montine T. J. (2003). Lipoproteins and lipid peroxidation in Alzheimer’s disease. J. Nutr. Health Aging 7 24–29. [PubMed] [Google Scholar]
- Bassett C. N., Neely M. D., Sidell K. R., Markesbery W. R., Swift L. L., Montine T. J. (1999). Cerebrospinal fluid lipoproteins are more vulnerable to oxidation in Alzheimer’s disease and are neurotoxic when oxidized ex vivo. Lipids 34 1273–1280. 10.1007/s11745-999-0478-1 [DOI] [PubMed] [Google Scholar]
- Baum L., Chen L., Masliah E., Chan Y. S., Ng H. K., Pang C. P. (1999). Lipoprotein lipase mutations and Alzheimer’s disease. Am. J. Med. Genet. 88 136–139. [DOI] [PubMed] [Google Scholar]
- Bazan N. G. (2005). Synaptic signaling by lipids in the life and death of neurons. Mol. Neurobiol. 31 219–230. 10.1385/mn:31:1-3:219 [DOI] [PubMed] [Google Scholar]
- Bedse G., Romano A., Lavecchia A. M., Cassano T., Gaetani S. (2015). The role of endocannabinoid signaling in the molecular mechanisms of neurodegeneration in Alzheimer’s disease. J. Alzheimers Dis. 43 1115–1136. 10.3233/jad-141635 [DOI] [PubMed] [Google Scholar]
- Belayev L., Hong S. H., Menghani H., Marcell S. J., Obenaus A., Freitas R. S., et al. (2018). Docosanoids promote neurogenesis and angiogenesis, blood-brain barrier integrity, penumbra protection, and neurobehavioral recovery after experimental ischemic stroke. Mol. Neurobiol. 55 7090–7106. 10.1007/s12035-018-1136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkouch M., Hachem M., Elgot A., Lo Van A., Picq M., Guichardant M., et al. (2016). The pleiotropic effects of Omega-3 docosahexaenoic acid on the hallmarks of Alzheimer’s disease. J. Nutr. Biochem. 38 1–11. 10.1016/j.jnutbio.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Bellet M. M., Masri S., Astarita G., Sassone-Corsi P., Della Fazia M. A., Servillo G. (2016). Histone deacetylase SIRT1 controls proliferation, circadian rhythm, and lipid metabolism during liver regeneration in mice. J. Biol. Chem. 291 23318–23329. 10.1074/jbc.m116.737114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton D. (2001). The impact of the supply of glucose to the brain on mood and memory. Nutr. Rev. 59 S20–S21. [DOI] [PubMed] [Google Scholar]
- Benton D., Parker P. Y., Donohoe R. T. (1996). The supply of glucose to the brain and cognitive functioning. J. Biosoc. Sci. 28 463–479. 10.1017/s0021932000022537 [DOI] [PubMed] [Google Scholar]
- Berg C. N., Sinha N., Gluck M. A. (2019). The effects of APOE and ABCA7 on cognitive function and Alzheimer’s disease risk in african americans: a focused mini review. Front. Hum. Neurosci. 13:387. 10.3389/fnhum.2019.00387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernath M. M., Bhattacharyya S., Nho K., Barupal D. K., Fiehn O., Baillie R., et al. (2019). Serum triglycerides in Alzheimer’s disease: relation to neuroimaging and CSF biomarkers. bioRxiv [Preprint]. 10.1101/441394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C. (2014). Physiology: double function at the blood-brain barrier. Nature 509 432–433. 10.1038/nature13339 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya R., Barren C., Kovacs D. M. (2013). Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts. J. Neurosci. 33 11169–11183. 10.1523/jneurosci.4704-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi E. (2007). Statin-like drugs for the treatment of brain cholesterol loss in Alzheimer’s disease. Curr. Drug. Saf. 2 173–176. 10.2174/157488607781668927 [DOI] [PubMed] [Google Scholar]
- Birben E., Sahiner U. M., Sackesen C., Erzurum S., Kalayci O. (2012). Oxidative stress and antioxidant defense. World Allergy Organ. J. 5 9–19. 10.1097/WOX.0b013e3182439613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biringer R. G. (2019). The role of eicosanoids in Alzheimer’s disease. Int. J. Environ. Res. Public Health 16:2560. 10.3390/ijerph16142560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black J. B., Premont R. T., Daaka Y. (2016). Feedback regulation of G protein-coupled receptor signaling by GRKs and arrestins. Semin. Cell Dev. Biol. 50 95–104. 10.1016/j.semcdb.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain J. F., Poirier J. (2004). Cholesterol homeostasis and the pathophysiology of Alzheimer’s disease. Expert. Rev. Neurother. 4 823–829. 10.1586/14737175.4.5.823 [DOI] [PubMed] [Google Scholar]
- Blain J. F., Aumont N., Theroux L., Dea D., Poirier J. (2006). A polymorphism in lipoprotein lipase affects the severity of Alzheimer’s disease pathophysiology. Eur. J. Neurosci. 24 1245–1251. 10.1111/j.1460-9568.2006.05007.x [DOI] [PubMed] [Google Scholar]
- Block J. (2019). Alzheimer’s disease might depend on enabling pathogens which do not necessarily cross the blood-brain barrier. Med. Hypotheses. 125 129–136. 10.1016/j.mehy.2019.02.044 [DOI] [PubMed] [Google Scholar]
- Bolanos-Garcia V. M., Miguel R. N. (2003). On the structure and function of apolipoproteins: more than a family of lipid-binding proteins. Prog. Biophys. Mol. Biol. 83 47–68. 10.1016/s0079-6107(03)00028-2 [DOI] [PubMed] [Google Scholar]
- Bos D. J., van Montfort S. J., Oranje B., Durston S., Smeets P. A. (2016). Effects of Omega-3 polyunsaturated fatty acids on human brain morphology and function: what is the evidence? Eur. Neuropsychopharmacol. 26 546–561. 10.1016/j.euroneuro.2015.12.031 [DOI] [PubMed] [Google Scholar]
- Bourre J. M. (1991). [Vitamin E: protection of membrane polyunsaturated fatty acids against radical peroxidation in the course of cerebral aging, particularly in cerebral capillaries and microvessels]. Bull. Acad. Natl. Med. 175 1305–1317. [PubMed] [Google Scholar]
- Bradbury M. W. (1984). The structure and function of the blood-brain barrier. Fed. Proc. 43 186–190. [PubMed] [Google Scholar]
- Bradley W. A., Gianturco S. H. (1986). ApoE is necessary and sufficient for the binding of large triglyceride-rich lipoproteins to the LDL receptor; apoB is unnecessary. J. Lipid. Res. 27 40–48. [PubMed] [Google Scholar]
- Braun V., Hantke K. (2019). Lipoproteins: structure, function, biosynthesis. Subcell. Biochem. 92 39–77. 10.1007/978-3-030-18768-2_3 [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Herrera R. A., Philipp S., Sosna J., Reyes-Ruiz J. M., Glabe C. G. (2020). Age-related intraneuronal aggregation of amyloid-beta in endosomes, mitochondria, autophagosomes, and lysosomes. J. Alzheimers Dis. 73 229–246. 10.3233/jad-190835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J., III, Theisler C., Silberman S., Magnuson D., Gottardi-Littell N., Lee J. M., et al. (2004). Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J. Biol. Chem. 279 34674–34681. 10.1074/jbc.m402324200 [DOI] [PubMed] [Google Scholar]
- Brown R. C., Davis T. P. (2002). Calcium modulation of adherens and tight junction function: a potential mechanism for blood-brain barrier disruption after stroke. Stroke 33 1706–1711. 10.1161/01.str.0000016405.06729.83 [DOI] [PubMed] [Google Scholar]
- Burgess B. L., McIsaac S., Naus K. E., Chan J. Y., Tansley G. H., Yang J., et al. (2006). Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer’s disease mouse models with abundant A beta in plasma. Neurobiol. Dis. 24 114–127. 10.1016/j.nbd.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Burgisser P., Matthieu J. M., Jeserich G., Waehneldt T. V. (1986). Myelin lipids: a phylogenetic study. Neurochem. Res. 11 1261–1272. 10.1007/bf00966121 [DOI] [PubMed] [Google Scholar]
- Butler R. N. (1994). ApoE: new risk factor for Alzheimer’s. Geriatrics 49 10–11. [PubMed] [Google Scholar]
- Butterfield D. A., Castegna A., Lauderback C. M., Drake J. (2002). Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer’s disease brain contribute to neuronal death. Neurobiol. Aging 23 655–664. 10.1016/s0197-4580(01)00340-2 [DOI] [PubMed] [Google Scholar]
- Button E. B., Gilmour M., Cheema H. K., Martin E. M., Agbay A., Robert J., et al. (2019). Vasoprotective functions of high-density lipoproteins relevant to Alzheimer’s disease are partially conserved in apolipoprotein B-depleted Plasma. Int. J. Mol. Sci. 20:462. 10.3390/ijms20030462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. D., Regina K. J., Kharasch E. D. (2014). Significance of lipid composition in a blood-brain barrier-mimetic PAMPA assay. J. Biomol. Screen 19 437–444. 10.1177/1087057113497981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cankurtaran M., Yesil Y., Kuyumcu M. E., Ozturk Z. A., Yavuz B. B., Halil M., et al. (2013). Altered levels of homocysteine and serum natural antioxidants links oxidative damage to Alzheimer’s disease. J. Alzheimers Dis. 33 1051–1058. 10.3233/jad-2012-121630 [DOI] [PubMed] [Google Scholar]
- Cantor R. S. (2018). Path to the desensitized state of ligand-gated ion channels: why are inhibitory and excitatory receptors different? J. Phys. Chem. B 122 5368–5374. 10.1021/acs.jpcb.7b10961 [DOI] [PubMed] [Google Scholar]
- Caporaso G. L., Takei K., Gandy S. E., Matteoli M., Mundigl O., Greengard P., et al. (1994). Morphologic and biochemical analysis of the intracellular trafficking of the Alzheimer beta/A4 amyloid precursor protein. J. Neurosci. 14 3122–3138. 10.1523/jneurosci.14-05-03122.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso S. M., Santos S., Swerdlow R. H., Oliveira C. R. (2001). Functional mitochondria are required for amyloid beta-mediated neurotoxicity. FASEB J. 15 1439–1441. 10.1096/fj.00-0561fje [DOI] [PubMed] [Google Scholar]
- Carvey P. M., Hendey B., Monahan A. J. (2009). The blood-brain barrier in neurodegenerative disease: a rhetorical perspective. J. Neurochem. 111 291–314. 10.1111/j.1471-4159.2009.06319.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro Dias M., Coisne C., Baden P., Enzmann G., Garrett L., Becker L., et al. (2019). Claudin-12 is not required for blood-brain barrier tight junction function. Fluids Barriers CNS 16:30. 10.1186/s12987-019-0150-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharan J. A., Sharma-Walia N. (2015). Lipoxins: nature’s way to resolve inflammation. J. Inflamm. Res. 8 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. Y., Ke D. S., Chen J. Y. (2009). Essential fatty acids and human brain. Acta Neurol. Taiwan. 18 231–241. [PubMed] [Google Scholar]
- Chang Y. T., Hsu S. W., Huang S. H., Huang C. W., Chang W. N., Lien C. Y., et al. (2019). ABCA7 polymorphisms correlate with memory impairment and default mode network in patients with APOEepsilon4-associated Alzheimer’s disease. Alzheimers Res. Ther. 11:103. 10.1186/s13195-019-0563-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappus-McCendie H., Chevalier L., Roberge C., Plourde M. (2019). Omega-3 PUFA metabolism and brain modifications during aging. Prog. Neuropsychopharmacol. Biol. Psychiatry 94:109662. 10.1016/j.pnpbp.2019.109662 [DOI] [PubMed] [Google Scholar]
- Cheignon C., Jones M., Atrian-Blasco E., Kieffer I., Faller P., Collin F., et al. (2017). Identification of key structural features of the elusive Cu-Abeta complex that generates ROS in Alzheimer’s disease. Chem. Sci. 8 5107–5118. 10.1039/c7sc00809k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. (2018). Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox. Biol. 14 450–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wei Y., Chen X., Jiao J., Zhang Y. (2017). Polyunsaturated fatty acids ameliorate aging via redox-telomere-antioncogene axis. Oncotarget 8 7301–7314. 10.18632/oncotarget.14236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Zhang J., Wu Y., Wang D., Feng G., Tang Y. P., et al. (2012). Monoacylglycerol lipase is a therapeutic target for Alzheimer’s disease. Cell. Rep. 2 1329–1339. 10.1016/j.celrep.2012.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Hui L., Geiger J. D. (2014). Role of LDL cholesterol and endolysosomes in amyloidogenesis and Alzheimer’s disease. J. Neurol. Neurophysiol. 5:236. 10.4172/2155-9562.1000236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng F., Cappai R., Lidfeldt J., Belting M., Fransson L. A., Mani K. (2014). Amyloid precursor protein (APP)/APP-like protein 2 (APLP2) expression is required to initiate endosome-nucleus-autophagosome trafficking of glypican-1-derived heparan sulfate. J. Biol. Chem. 289 20871–20878. 10.1074/jbc.m114.552810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini A., Andres-Lacueva C., Martin A., Lauretani F., Iorio A. D., Bartali B., et al. (2007). Low plasma N-3 fatty acids and dementia in older persons: the InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 62 1120–1126. 10.1093/gerona/62.10.1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs C. E., Romeu-Nadal M., Burdge G. C., Calder P. C. (2008). Gender differences in the n-3 fatty acid content of tissues. Proc. Nutr. Soc. 67 19–27. 10.1017/s0029665108005983 [DOI] [PubMed] [Google Scholar]
- Chiu C. C., Su K. P., Cheng T. C., Liu H. C., Chang C. J., Dewey M. E., et al. (2008). The effects of Omega-3 fatty acids monotherapy in Alzheimer’s disease and mild cognitive impairment: a preliminary randomized double-blind placebo-controlled study. Prog. Neuropsychopharmacol. Biol. Psychiatry 32 1538–1544. 10.1016/j.pnpbp.2008.05.015 [DOI] [PubMed] [Google Scholar]
- Chiurchiu V., Leuti A., Maccarrone M. (2018). Bioactive lipids and chronic inflammation: managing the fire within. Front. Immunol. 9:38. 10.3389/fimmu.2018.00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow V. W., Mattson M. P., Wong P. C., Gleichmann M. (2010). An overview of APP processing enzymes and products. Neuromol. Med. 12 1–12. 10.1007/s12017-009-8104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrast R., Saher G., Nave K. A., Verheijen M. H. (2011). Lipid metabolism in myelinating glial cells: lessons from human inherited disorders and mouse models. J. Lipid Res. 52 419–434. 10.1194/jlr.r009761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L. W., Li Y., Li Z., Tang A. Y., Cheung B. M., Leung R. Y., et al. (2007). A novel intronic polymorphism of ABCA1 gene reveals risk for sporadic Alzheimer’s disease in Chinese. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B 1007–1013. 10.1002/ajmg.b.30525 [DOI] [PubMed] [Google Scholar]
- Chun Y. S., Park Y., Oh H. G., Kim T. W., Yang H. O., Park M. K., et al. (2015). O-GlcNAcylation promotes non-amyloidogenic processing of amyloid-beta protein precursor via inhibition of endocytosis from the plasma membrane. J. Alzheimers. Dis. 44 261–275. 10.3233/jad-140096 [DOI] [PubMed] [Google Scholar]
- Chung S. J., Kim M. J., Kim Y. J., Kim J., You S., Jang E. H., et al. (2014). CR1, ABCA7, and APOE genes affect the features of cognitive impairment in Alzheimer’s disease. J. Neurol. Sci. 339 91–96. 10.1016/j.jns.2014.01.029 [DOI] [PubMed] [Google Scholar]
- Chung W. S., Verghese P. B., Chakraborty C., Joung J., Hyman B. T., Ulrich J. D., et al. (2016). Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc. Natl. Acad. Sci. U.S.A. 113 10186–10191. 10.1073/pnas.1609896113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavey V., Lestavel-Delattre S., Copin C., Bard J. M., Fruchart J. C. (1995). Modulation of lipoprotein B binding to the LDL receptor by exogenous lipids and apolipoproteins CI, CII, CIII, and E. Arterioscler. Thromb. Vasc. Biol. 15 963–971. 10.1161/01.atv.15.7.963 [DOI] [PubMed] [Google Scholar]
- Csernansky J. G., Dong H., Fagan A. M., Wang L., Xiong C., Holtzman D. M., et al. (2006). Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am. J. Psychiatry 163 2164–2169. 10.1176/ajp.2006.163.12.2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnane S. C., Schneider J. A., Tangney C., Tremblay-Mercier J., Fortier M., Bennett D. A., et al. (2012). Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J. Alzheimers Dis. 29 691–697. 10.3233/jad-2012-110629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutuli D. (2017). Functional and structural benefits induced by Omega-3 polyunsaturated fatty acids during aging. Curr. Neuropharmacol. 15 534–542. 10.2174/1570159x14666160614091311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiello L. A., Gongvatana A., Dunsiger S., Cohen R. A., Ott B. R. (2015). Association of fish oil supplement use with preservation of brain volume and cognitive function. Alzheimers Dement. 11 226–235. 10.1016/j.jalz.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R., Prat A. (2015). The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 7:a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P. K., Moore A. N. (1993). Inhibitors of endocytosis, endosome fusion, and lysosomal processing inhibit the intracellular proteolysis of the amyloid precursor protein. Neurosci. Lett. 164 183–186. 10.1016/0304-3940(93)90887-q [DOI] [PubMed] [Google Scholar]
- Davison A. N. (1972). Metabolism of myelin lipids in the developing brain. Biochem. J. 128 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chaves E. P., Narayanaswami V. (2008). Apolipoprotein E and cholesterol in aging and disease in the brain. Future Lipidol. 3 505–530. 10.2217/17460875.3.5.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries H. E., Kooij G., Frenkel D., Georgopoulos S., Monsonego A., Janigro D. (2012). Inflammatory events at blood-brain barrier in neuroinflammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia 53(Suppl. 6), 45–52. 10.1111/j.1528-1167.2012.03702.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wilde M. C., Vellas B., Girault E., Yavuz A. C., Sijben J. W. (2017). Lower brain and blood nutrient status in Alzheimer’s disease: results from meta-analyses. Alzheimers Dement. 3 416–431. 10.1016/j.trci.2017.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decsi T., Kennedy K. (2011). Sex-specific differences in essential fatty acid metabolism. Am. J. Clin. Nutr. 94(6 Suppl), 1914S–1919S. 10.3945/ajcn.110.000893 [DOI] [PubMed] [Google Scholar]
- Dehouck B., Fenart L., Dehouck M. P., Pierce A., Torpier G., Cecchelli R. (1997). A new function for the LDL receptor: transcytosis of LDL across the blood-brain barrier. J. Cell Biol. 138 877–889. 10.1083/jcb.138.4.877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeester N., Castro G., Desrumaux C., De Geitere C., Fruchart J. C., Santens P., et al. (2000). Characterization and functional studies of lipoproteins, lipid transfer proteins, and lecithin:cholesterol acyltransferase in CSF of normal individuals and patients with Alzheimer’s disease. J. Lipid. Res. 41 963–974. [PubMed] [Google Scholar]
- Denis I., Potier B., Heberden C., Vancassel S. (2015). Omega-3 polyunsaturated fatty acids and brain aging. Curr. Opin. Clin. Nutr. Metab. Care 18 139–146. [DOI] [PubMed] [Google Scholar]
- Derby C. A., Crawford S., Pasternak R. C., Sowers M., Sternfeld B., Matthews K. A. (2009). Lipid changes during the menopause transition in relation to age and weight. Am. J. Epidemiol. 169 1352–1361. 10.1093/aje/kwp043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M. K., Mastrangelo M. A., Ryan D. A., Sudol K. L., Narrow W. C., Bowers W. J. (2010). Early oligodendrocyte/myelin pathology in Alzheimer’s disease mice constitutes a novel therapeutic target. Am. J. Pathol. 177 1422–1435. 10.2353/ajpath.2010.100087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine M. J., Kittler J. T. (2018). Mitochondria at the neuronal presynapse in health and disease. Nat. Rev. Neurosci. 19 63–80. 10.1038/nrn.2017.170 [DOI] [PubMed] [Google Scholar]
- Dienel G. A., Cruz N. F., Adachi K., Sokoloff L., Holden J. E. (1997). Determination of local brain glucose level with [14C]methylglucose: effects of glucose supply and demand. Am. J. Physiol. 273 E839–E849. [DOI] [PubMed] [Google Scholar]
- Dimas P., Montani L., Pereira J. A., Moreno D., Trotzmuller M., Gerber J., et al. (2019). CNS myelination and remyelination depend on fatty acid synthesis by oligodendrocytes. eLife 8:e44702. 10.7554/eLife.44702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding R. B., Bao J., Deng C. X. (2017). Emerging roles of SIRT1 in fatty liver diseases. Int. J. Biol. Sci. 13 852–867. 10.7150/ijbs.19370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Couto F. S., de Mendonca A., Garcia C., Rocha L., Lechner M. C. (1998). Age of onset in patients with Alzheimer’s disease with different apoE genotypes. J. Neurol. Neurosurg. Psychiatry 64:817. 10.1136/jnnp.64.6.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodelet-Devillers A., Cayrol R., van Horssen J., Haqqani A. S., de Vries H. E., Engelhardt B., et al. (2009). Functions of lipid raft membrane microdomains at the blood-brain barrier. J. Mol. Med. 87 765–774. 10.1007/s00109-009-0488-6 [DOI] [PubMed] [Google Scholar]
- Doens D., Valiente P. A., Mfuh A. M., X T Vo A., Tristan A., Carreno L., et al. (2017). Identification of inhibitors of CD36-amyloid beta binding as potential agents for Alzheimer’s disease. ACS Chem. Neurosci. 8 1232–1241. 10.1021/acschemneuro.6b00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen M. R. (2012). Mitochondria, calcium-dependent neuronal death and neurodegenerative disease. Pflugers. Arch. 464 111–121. 10.1007/s00424-012-1112-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T., Tasker R., McGowan J. F. (2000). The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology 149 129–139. 10.1007/s002139900324 [DOI] [PubMed] [Google Scholar]
- Dunstan J. A., Simmer K., Dixon G., Prescott S. L. (2008). Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: a randomised controlled trial. Arch. Dis. Child. Fetal. Neonatal. Ed. 93 F45–F50. [DOI] [PubMed] [Google Scholar]
- Dyall S. C. (2015). Long-chain Omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 7:52. 10.3389/fnagi.2015.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert A., Schulz K. L., Rhein V., Gotz J. (2010). Convergence of amyloid-beta and tau pathologies on mitochondria in vivo. Mol. Neurobiol. 41 107–114. 10.1007/s12035-010-8109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehehalt R., Keller P., Haass C., Thiele C., Simons K. (2003). Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J. Cell. Biol. 160 113–123. 10.1083/jcb.200207113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Haj M., Antoine P., Amouyel P., Lambert J. C., Pasquier F., Kapogiannis D. (2016). Apolipoprotein E (APOE) epsilon4 and episodic memory decline in Alzheimer’s disease: a review. Ageing Res. Rev. 27 15–22. 10.1016/j.arr.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D. A., Weickert C. S., Garner B. (2010). Apolipoproteins in the brain: implications for neurological and psychiatric disorders. Clin. Lipidol. 51 555–573. 10.2217/clp.10.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S., Meyer-Lindenberg A., Opitz von Boberfeld C., Esslinger C., Schnell K., Kirsch P., et al. (2011). Hippocampal function in healthy carriers of the CLU Alzheimer’s disease risk variant. J. Neurosci. 31 18180–18184. 10.1523/jneurosci.4960-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estus S., Golde T. E., Kunishita T., Blades D., Lowery D., Eisen M., et al. (1992). Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science 255 726–728. 10.1126/science.1738846 [DOI] [PubMed] [Google Scholar]
- Evans B. A., Evans J. E., Baker S. P., Kane K., Swearer J., Hinerfeld D., et al. (2009). Long-term statin therapy and CSF cholesterol levels: implications for Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 27 519–524. 10.1159/000221835 [DOI] [PubMed] [Google Scholar]
- Evin G., Li Q. X. (2012). Platelets and Alzheimer’s disease: potential of APP as a biomarker. World J. Psychiatry 2 102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evin G., Zhu A., Holsinger R. M., Masters C. L., Li Q. X. (2003). Proteolytic processing of the Alzheimer’s disease amyloid precursor protein in brain and platelets. J. Neurosci. Res. 74 386–392. [DOI] [PubMed] [Google Scholar]
- Eyster K. M. (2007). The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv. Physiol. Educ. 31 5–16. 10.1152/advan.00088.2006 [DOI] [PubMed] [Google Scholar]
- Farooqui A. A., Horrocks L. A. (1998). Plasmalogen-selective phospholipase A2 and its involvement in Alzheimer’s disease. Biochem. Soc. Trans. 26 243–246. [DOI] [PubMed] [Google Scholar]
- Farooqui A. A., Liss L., Horrocks L. A. (1988). Stimulation of lipolytic enzymes in Alzheimer’s disease. Ann. Neurol. 23 306–308. 10.1002/ana.410230317 [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Grunfeld C. (2000). “Introduction to lipids and lipoproteins,” in Endotext, eds Feingold K. R., Anawalt B., Boyce A., Chrousos G., Dungan K., Grossman A., et al. (South Dartmouth, MA: MDText.com, Inc; ). [Google Scholar]
- Ferreira L. (2019). What human blood-brain barrier models can tell us about BBB function and drug discovery? Expert. Opin. Drug. Discov. 14 1113–1123. 10.1080/17460441.2019.1646722 [DOI] [PubMed] [Google Scholar]
- Fester L., Zhou L., Butow A., Huber C., von Lossow R., Prange-Kiel J., et al. (2009). Cholesterol-promoted synaptogenesis requires the conversion of cholesterol to estradiol in the hippocampus. Hippocampus 19 692–705. 10.1002/hipo.20548 [DOI] [PubMed] [Google Scholar]
- Fidani L., Goulas A., Crook R., Petersen R. C., Tangalos E., Kotsis A., et al. (2004). An association study of the cholesteryl ester transfer protein TaqI B polymorphism with late onset Alzheimer’s disease. Neurosci. Lett. 357 152–154. 10.1016/j.neulet.2003.11.071 [DOI] [PubMed] [Google Scholar]
- Filippov V., Song M. A., Zhang K., Vinters H. V., Tung S., Kirsch W. M., et al. (2012). Increased ceramide in brains with Alzheimer’s and other neurodegenerative diseases. J. Alzheimers Dis. 29 537–547. 10.3233/jad-2011-111202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filou S., Lhomme M., Karavia E. A., Kalogeropoulou C., Theodoropoulos V., Zvintzou E., et al. (2016). Distinct roles of apolipoproteins A1 and E in the modulation of high-density lipoprotein composition and function. Biochemistry 55 3752–3762. 10.1021/acs.biochem.6b00389 [DOI] [PubMed] [Google Scholar]
- Finean J. B., Robertson J. D. (1958). Lipids and the structure of myelin. Br. Med. Bull. 14 267–273. 10.1093/oxfordjournals.bmb.a069695 [DOI] [PubMed] [Google Scholar]
- Fishman J. B., Rubin J. B., Handrahan J. V., Connor J. R., Fine R. E. (1987). Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J. Neurosci. Res. 18 299–304. 10.1002/jnr.490180206 [DOI] [PubMed] [Google Scholar]
- Fonteh A. (2018). Reasons why Omega-3 polyunsaturated fatty acids produce mixed results in alzheimer’s disease. J. Glycom. Lipid. 7:1. [Google Scholar]
- Fonteh A. N., Chiang J., Cipolla M., Hale J., Diallo F., Chirino A., et al. (2013). Alterations in cerebrospinal fluid glycerophospholipids and phospholipase A2 activity in Alzheimer’s disease. J. Lipid. Res. 54 2884–2897. 10.1194/jlr.m037622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteh A. N., Cipolla M., Chiang A. J., Edminster S. P., Arakaki X., Harrington M. G. (2020). Polyunsaturated fatty acid composition of cerebrospinal fluid fractions shows their contribution to cognitive resilience of a pre-symptomatic Alzheimer’s disease cohort. Front. Physiol. 11:83. 10.3389/fphys.2020.00083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteh A. N., Cipolla M., Chiang J., Arakaki X., Harrington M. G. (2014). Human cerebrospinal fluid fatty acid levels differ between supernatant fluid and brain-derived nanoparticle fractions, and are altered in Alzheimer’s disease. PLoS One 9:e100519. 10.1371/journal.pone.0100519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteh A. N., Ormseth C., Chiang J., Cipolla M., Arakaki X., Harrington M. G. (2015). Sphingolipid metabolism correlates with cerebrospinal fluid Beta amyloid levels in Alzheimer’s disease. PLoS One 10:e0125597. 10.1371/journal.pone.0125597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster E. M., Dangla-Valls A., Lovestone S., Ribe E. M., Buckley N. J. (2019). Clusterin in Alzheimer’s disease: mechanisms, genetics, and lessons from other pathologies. Front. Neurosci. 13:164. 10.3389/fnins.2019.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank A. T., Zhao B., Jose P. O., Azar K. M., Fortmann S. P., Palaniappan L. P. (2014). Racial/ethnic differences in dyslipidemia patterns. Circulation 129 570–579. 10.1161/circulationaha.113.005757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank B., Gupta S. (2005). A review of antioxidants and Alzheimer’s disease. Ann. Clin. Psychiatry 17 269–286. [DOI] [PubMed] [Google Scholar]
- Frank M. G., Baratta M. V., Sprunger D. B., Watkins L. R., Maier S. F. (2007). Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav. Immun. 21 47–59. 10.1016/j.bbi.2006.03.005 [DOI] [PubMed] [Google Scholar]
- French H. M., Reid M., Mamontov P., Simmons R. A., Grinspan J. B. (2009). Oxidative stress disrupts oligodendrocyte maturation. J. Neurosci. Res. 87 3076–3087. 10.1002/jnr.22139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund Levi Y., Vedin I., Cederholm T., Basun H., Faxen Irving G., Eriksdotter M., et al. (2014). Transfer of Omega-3 fatty acids across the blood-brain barrier after dietary supplementation with a docosahexaenoic acid-rich Omega-3 fatty acid preparation in patients with Alzheimer’s disease: the OmegAD study. J. Intern. Med. 275 428–436. 10.1111/joim.12166 [DOI] [PubMed] [Google Scholar]
- Freund-Levi Y., Basun H., Cederholm T., Faxen-Irving G., Garlind A., Grut M., et al. (2008). Omega-3 supplementation in mild to moderate Alzheimer’s disease: effects on neuropsychiatric symptoms. Int. J. Geriatr. Psychiatry 23 161–169. 10.1002/gps.1857 [DOI] [PubMed] [Google Scholar]
- Freund-Levi Y., Eriksdotter-Jonhagen M., Cederholm T., Basun H., Faxen-Irving G., Garlind A., et al. (2006). Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch. Neurol. 63 1402–1408. [DOI] [PubMed] [Google Scholar]
- Frieden C., Wang H., Ho C. M. W. (2017). A mechanism for lipid binding to apoE and the role of intrinsically disordered regions coupled to domain-domain interactions. Proc. Natl. Acad. Sci. U.S.A. 114 6292–6297. 10.1073/pnas.1705080114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola K., Reeskamp L., van den Born B. J. (2017). Ethnicity, lipids and cardiovascular disease. Curr. Opin. Lipidol. 28 225–230. 10.1097/mol.0000000000000412 [DOI] [PubMed] [Google Scholar]
- Ghosh M., Garcia-Castillo D., Aguirre V., Golshani R., Atkins C. M., Bramlett H. M., et al. (2012). Proinflammatory cytokine regulation of cyclic AMP-phosphodiesterase 4 signaling in microglia in vitro and following CNS injury. Glia 60 1839–1859. 10.1002/glia.22401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilgun-Sherki Y., Melamed E., Offen D. (2001). Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 40 959–975. 10.1016/s0028-3908(01)00019-3 [DOI] [PubMed] [Google Scholar]
- Gilmore-Bykovskyi A. L., Jin Y., Gleason C., Flowers-Benton S., Block L. M., Dilworth-Anderson P., et al. (2019). Recruitment and retention of underrepresented populations in Alzheimer’s disease research: a systematic review. Alzheimers Dement. 19 751–770. 10.1016/j.trci.2019.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltay E. J., Gooren L., Toorians A. W., Katan M. B., Zock P. L. (2004). Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am. J. Clin. Nutr. 80 1167–1174. 10.1093/ajcn/80.5.1167 [DOI] [PubMed] [Google Scholar]
- Giulietti A., Vignini A., Nanetti L., Mazzanti L., Di Primio R., Salvolini E. (2016). Alzheimer’s disease risk and progression: the role of nutritional supplements and their effect on drug therapy outcome. Curr. Neuropharmacol. 14 177–190. 10.2174/1570159x13666150928155321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso C. A., Pfenning A. R., Lee S. S., Bennett D. A., Sibille E. L., Kellis M., et al. (2019). Rate of brain aging and APOE epsilon4 are synergistic risk factors for Alzheimer’s disease. Life Sci. Alliance 2 e201900303. 10.26508/lsa.201900303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M., Dolga A. M., Koepke J., Mengel D., Culmsee C., Dodel R., et al. (2014). alpha1-antitrypsin modulates microglial-mediated neuroinflammation and protects microglial cells from amyloid-beta-induced toxicity. J. Neuroinflammation 11:165. 10.1186/s12974-014-0165-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde T. E., Estus S., Younkin L. H., Selkoe D. J., Younkin S. G. (1992). Processing of the amyloid protein precursor to potentially amyloidogenic derivatives. Science 255 728–730. 10.1126/science.1738847 [DOI] [PubMed] [Google Scholar]
- Gong C. X., Liu F., Grundke-Iqbal I., Iqbal K. (2006). Impaired brain glucose metabolism leads to Alzheimer neurofibrillary degeneration through a decrease in tau O-GlcNAcylation. J. Alzheimers Dis. 9 1–12. 10.3233/jad-2006-9101 [DOI] [PubMed] [Google Scholar]
- Goozee K., Chatterjee P., James I., Shen K., Sohrabi H. R., Asih P. R., et al. (2017). Alterations in erythrocyte fatty acid composition in preclinical Alzheimer’s disease. Sci. Rep. 7:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska W., Sikora E., Bielak-Zmijewska A. (2017). Sirtuins, a promising target in slowing down the ageing process. Biogerontology 18 447–476. 10.1007/s10522-017-9685-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi S., Giussani P., Mauri L., Prioni S., Sonnino S., Prinetti A. (2019). Lipid rafts and neurodegeneration: structural and functional roles in physiologic aging and neurodegenerative diseases. J. Lipid. Res. 61 636–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm M. O., Haupenthal V. J., Mett J., Stahlmann C. P., Blumel T., Mylonas N. T., et al. (2016). Oxidized docosahexaenoic acid species and lipid peroxidation products increase amyloidogenic amyloid precursor protein processing. Neurodegener. Dis. 16 44–54. 10.1159/000440839 [DOI] [PubMed] [Google Scholar]
- Grimm M. O., Rothhaar T. L., Grosgen S., Burg V. K., Hundsdorfer B., Haupenthal V. J., et al. (2012). Trans fatty acids enhance amyloidogenic processing of the Alzheimer amyloid precursor protein (APP). J. Nutr. Biochem. 23 1214–1223. 10.1016/j.jnutbio.2011.06.015 [DOI] [PubMed] [Google Scholar]
- Growdon J. H., Hyman B. T. (2014). APOE genotype and brain development. JAMA Neurol. 71 7–8. [DOI] [PubMed] [Google Scholar]
- Guan Z., Wang Y., Cairns N. J., Lantos P. L., Dallner G., Sindelar P. J. (1999). Decrease and structural modifications of phosphatidylethanolamine plasmalogen in the brain with Alzheimer disease. J. Neuropathol. Exp. Neurol. 58 740–747. 10.1097/00005072-199907000-00008 [DOI] [PubMed] [Google Scholar]
- Guo X., Geng M., Du G. (2005). Glucose transporter 1, distribution in the brain and in neural disorders: its relationship with transport of neuroactive drugs through the blood-brain barrier. Biochem. Genet. 43 175–187. 10.1007/s10528-005-1510-5 [DOI] [PubMed] [Google Scholar]
- Hahn G., Ponce-Alvarez A., Deco G., Aertsen A., Kumar A. (2019). Portraits of communication in neuronal networks. Nat. Rev. Neurosci. 20 117–127. 10.1038/s41583-018-0094-0 [DOI] [PubMed] [Google Scholar]
- Halliday M. R., Rege S. V., Ma Q., Zhao Z., Miller C. A., Winkler E. A., et al. (2016). Accelerated pericyte degeneration and blood-brain barrier breakdown in apolipoprotein E4 carriers with Alzheimer’s disease. J. Cereb. Blood Flow Metab. 36 216–227. 10.1038/jcbfm.2015.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameister R., Kaur C., Dheen S. T., Lohmann C. H., Singh G. (2020). Reactive oxygen/nitrogen species (ROS/RNS) and oxidative stress in arthroplasty. J. Biomed. Mater. Res. B Appl. Biomater. 108 2073–2087. 10.1002/jbm.b.34546 [DOI] [PubMed] [Google Scholar]
- Han X., M Holtzman D., McKeel D. W., Jr., Kelley J., Morris J. C. (2002). Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J. Neurochem. 82 809–818. 10.1046/j.1471-4159.2002.00997.x [DOI] [PubMed] [Google Scholar]
- Hansen D. V., Hanson J. E., Sheng M. (2018). Microglia in Alzheimer’s disease. J. Cell Biol. 217 459–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. B. (2015). Lipid agonism: the PIP2 paradigm of ligand-gated ion channels. Biochim. Biophys. Acta 1851 620–628. 10.1016/j.bbalip.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S., Wang R., Zhang Y., Zhan H. (2018). Prediction of Alzheimer’s disease-associated genes by integration of gwas summary data and expression data. Front. Genet. 9:653. 10.3389/fgene.2018.00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harik S. I., Kalaria R. N. (1991). Blood-brain barrier abnormalities in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 640 47–52. [DOI] [PubMed] [Google Scholar]
- Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M. L., et al. (2009). Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 41 1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J. J., Jolivet R., Attwell D. (2012). Synaptic energy use and supply. Neuron 75 762–777. 10.1016/j.neuron.2012.08.019 [DOI] [PubMed] [Google Scholar]
- Hartmann D. (2012). A brief history of APP secretases, their substrates and their functions. Curr. Alzheimer Res. 9 138–139. 10.2174/156720512799361628 [DOI] [PubMed] [Google Scholar]
- Hasadsri L., Wang B. H., Lee J. V., Erdman J. W., Llano D. A., Barbey A. K., et al. (2013). Omega-3 fatty acids as a putative treatment for traumatic brain injury. J. Neurotrauma. 30 897–906. 10.1089/neu.2012.2672 [DOI] [PubMed] [Google Scholar]
- Hascalovici J. R., Vaya J., Khatib S., Holcroft C. A., Zukor H., Song W., et al. (2009). Brain sterol dysregulation in sporadic AD and MCI: relationship to heme oxygenase-1. J. Neurochem. 110 1241–1253. 10.1111/j.1471-4159.2009.06213.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Biebuyck J. F. (1979). Ketone bodies are selectively used by individual brain regions. Science 205 325–327. 10.1126/science.451608 [DOI] [PubMed] [Google Scholar]
- Hedqvist P., Raud J., Palmertz U., Kumlin M., Dahlen S. E. (1991). Eicosanoids as mediators and modulators of inflammation. Adv. Prostaglandin. Thromboxane. Leukot. Res. 21B 537–543. [PubMed] [Google Scholar]
- Helbecque N., Codron V., Cottel D., Amouyel P. (2008). An apolipoprotein A-I gene promoter polymorphism associated with cognitive decline, but not with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 25 97–102. 10.1159/000112176 [DOI] [PubMed] [Google Scholar]
- Heppner F. L., Ransohoff R. M., Becher B. (2015). Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 16 358–372. 10.1038/nrn3880 [DOI] [PubMed] [Google Scholar]
- Hering H., Lin C. C., Sheng M. (2003). Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J. Neurosci. 23 3262–3271. 10.1523/jneurosci.23-08-03262.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold C., Hooli B. V., Mullin K., Liu T., Roehr J. T., Mattheisen M., et al. (2016). Family-based association analyses of imputed genotypes reveal genome-wide significant association of Alzheimer’s disease with OSBPL6, PTPRG, and PDCL3. Mol. Psychiatry 21 1608–1612. 10.1038/mp.2015.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits A. Z., Guarente L. (2014). SIRT1 in neurodevelopment and brain senescence. Neuron 81 471–483. 10.1016/j.neuron.2014.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J. (2001). The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron 29 571–581. 10.1016/s0896-6273(01)00234-3 [DOI] [PubMed] [Google Scholar]
- Heverin M., Bogdanovic N., Lutjohann D., Bayer T., Pikuleva I., Bretillon L., et al. (2004). Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J. Lipid Res. 45 186–193. 10.1194/jlr.m300320-jlr200 [DOI] [PubMed] [Google Scholar]
- Higgs G. A., Moncada S., Vane J. R. (1984). Eicosanoids in inflammation. Ann. Clin. Res. 16 287–299. [PubMed] [Google Scholar]
- Hirsch-Reinshagen V., Wellington C. L. (2007). Cholesterol metabolism, apolipoprotein E, adenosine triphosphate-binding cassette transporters, and Alzheimer’s disease. Curr. Opin. Lipidol. 18 325–332. 10.1097/mol.0b013e32813aeabf [DOI] [PubMed] [Google Scholar]
- Hoglund K., Thelen K. M., Syversen S., Sjogren M., von Bergmann K., Wallin A., et al. (2005). The effect of simvastatin treatment on the amyloid precursor protein and brain cholesterol metabolism in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 19 256–265. 10.1159/000084550 [DOI] [PubMed] [Google Scholar]
- Hoofnagle A. N., Heinecke J. W. (2009). Lipoproteomics: using mass spectrometry-based proteomics to explore the assembly, structure, and function of lipoproteins. J. Lipid Res. 50 1967–1975. 10.1194/jlr.r900015-jlr200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper C., De Souto Barreto P., Pahor M., Weiner M., Vellas B. (2018). The relationship of Omega 3 polyunsaturated fatty acids in red blood cell membranes with cognitive function and brain structure: a review focussed on alzheimer’s disease. J. Prev. Alzheimers Dis. 5 78–84. [DOI] [PubMed] [Google Scholar]
- Hosseini M., Poljak A., Braidy N., Crawford J., Sachdev P. (2020). Blood fatty acids in Alzheimer’s disease and mild cognitive impairment: a meta-analysis and systematic review. Ageing Res. Rev. 60 101043. 10.1016/j.arr.2020.101043 [DOI] [PubMed] [Google Scholar]
- Hottman D. A., Chernick D., Cheng S., Wang Z., Li L. (2014). HDL and cognition in neurodegenerative disorders. Neurobiol. Dis. 72(Pt.A), 22–36. 10.1016/j.nbd.2014.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Xu B., Ge W. (2017). The role of lipid bodies in the microglial aging process and related diseases. Neurochem. Res. 42 3140–3148. 10.1007/s11064-017-2351-4 [DOI] [PubMed] [Google Scholar]
- Huang Y., Mahley R. W. (2014). Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol. Dis. 72(Pt. A), 3–12. 10.1016/j.nbd.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry E., Van Dam D., Kulik W., De Deyn P. P., Stet F. S., Ahouansou O., et al. (2010). Adeno-associated virus gene therapy with cholesterol 24-hydroxylase reduces the amyloid pathology before or after the onset of amyloid plaques in mouse models of Alzheimer’s disease. Mol. Ther. 18 44–53. 10.1038/mt.2009.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert A. J., Faulks S. C., Harper J. M., Miller R. A., Buffenstein R. (2006). Extended longevity of wild-derived mice is associated with peroxidation-resistant membranes. Mech. Ageing Dev. 127 653–657. 10.1016/j.mad.2006.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain G., Wang J., Rasul A., Anwar H., Imran A., Qasim M., et al. (2019). Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 18:26. 10.1186/s12944-019-0965-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson E. (2010). Blood-brain barrier: plugging the leak. Nat. Rev. Neurosci. 11:789. [DOI] [PubMed] [Google Scholar]
- Igarashi M., Ma K., Gao F., Kim H. W., Rapoport S. I., Rao J. S. (2011). Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer’s disease prefrontal cortex. J. Alzheimers Dis. 24 507–517. 10.3233/jad-2011-101608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M., Polvikoski T. M., Hall R., Slade J. Y., Perry R. H., Oakley A. E., et al. (2010). Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer’s disease, and dementia with Lewy bodies. Acta Neuropathol. 119 579–589. 10.1007/s00401-009-0635-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeshima-Kataoka H., Yasui M. (2016). Correlation between astrocyte activity and recovery from blood-brain barrier breakdown caused by brain injury. Neuroreport 27 894–900. 10.1097/wnr.0000000000000619 [DOI] [PubMed] [Google Scholar]
- Irizarry M. C., Deng A., Lleo A., Berezovska O., Von Arnim C. A., Martin-Rehrmann M., et al. (2004). Apolipoprotein E modulates gamma-secretase cleavage of the amyloid precursor protein. J. Neurochem. 90 1132–1143. [DOI] [PubMed] [Google Scholar]
- Ishiura S. (1991). Proteolytic cleavage of the Alzheimer’s disease amyloid A4 precursor protein. J. Neurochem. 56 363–369. 10.1111/j.1471-4159.1991.tb08160.x [DOI] [PubMed] [Google Scholar]
- Ito J., Nagayasu Y., Lu R., Kheirollah A., Hayashi M., Yokoyama S. (2005). Astrocytes produce and secrete FGF-1, which promotes the production of apoE-HDL in a manner of autocrine action. J. Lipid Res. 46 679–686. 10.1194/jlr.m400313-jlr200 [DOI] [PubMed] [Google Scholar]
- Ito J., Nagayasu Y., Miura Y., Yokoyama S., Michikawa M. (2014). Astrocytes endogenous apoE generates HDL-like lipoproteins using previously synthesized cholesterol through interaction with ABCA1. Brain Res. 1570 1–12. 10.1016/j.brainres.2014.04.037 [DOI] [PubMed] [Google Scholar]
- Iwamoto N., Kobayashi K., Kosaka K. (1989). The formation of prostaglandins in the postmortem cerebral cortex of Alzheimer-type dementia patients. J. Neurol. 236 80–84. 10.1007/bf00314401 [DOI] [PubMed] [Google Scholar]
- Iwasaki A., Medzhitov R. (2015). Control of adaptive immunity by the innate immune system. Nat. Immunol. 16 343–353. 10.1038/ni.3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyu D., Juttner M., Glenn J. R., White A. E., Johnson A. J., Fox S. C., et al. (2011). PGE1 and PGE2 modify platelet function through different prostanoid receptors. Prostaglandins Other Lipid Mediat. 94 9–16. 10.1016/j.prostaglandins.2010.11.001 [DOI] [PubMed] [Google Scholar]
- Janciauskiene S., Wright H. T. (1998). Inflammation, antichymotrypsin, and lipid metabolism: autogenic etiology of Alzheimer’s disease. Bioessays 20 1039–1046. [DOI] [PubMed] [Google Scholar]
- Janssen C. I., Kiliaan A. J. (2014). Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 53 1–17. 10.1016/j.plipres.2013.10.002 [DOI] [PubMed] [Google Scholar]
- Jean-Louis T., Rockwell P., Figueiredo-Pereira M. E. (2018). Prostaglandin J2 promotes O-GlcNAcylation raising APP processing by alpha- and beta-secretases: relevance to Alzheimer’s disease. Neurobiol. Aging 62 130–145. 10.1016/j.neurobiolaging.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre C., Nadjar A., Lebbadi M., Calon F., Laye S. (2014). n-3 LCPUFA improves cognition: the young, the old and the sick. Prostaglandins Leukot. Essent. Fatty Acids 91 1–20. 10.1016/j.plefa.2014.05.001 [DOI] [PubMed] [Google Scholar]
- Johnen A., Pawlowski M., Duning T. (2018). Distinguishing neurocognitive deficits in adult patients with NP-C from early onset Alzheimer’s dementia. Orphanet. J. Rare Dis. 13 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L., Holmans P. A., Hamshere M. L., Harold D., Moskvina V., Ivanov D., et al. (2010). Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS One 5:e13950. 10.1371/journal.pone.0013950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. S., Rebeck G. W. (2018). The synergistic effects of APOE genotype and obesity on Alzheimer’s disease risk. Int. J. Mol. Sci. 20:63. 10.3390/ijms20010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaether C., Haass C. (2004). A lipid boundary separates APP and secretases and limits amyloid beta-peptide generation. J. Cell Biol. 167 809–812. 10.1083/jcb.200410090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagedal K., Kim W. S., Appelqvist H., Chan S., Cheng D., Agholme L., et al. (2010). Increased expression of the lysosomal cholesterol transporter NPC1 in Alzheimer’s disease. Biochim. Biophys. Acta 1801 831–838. 10.1016/j.bbalip.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Kaiser H. J., Orlowski A., Rog T., Nyholm T. K., Chai W., Feizi T., et al. (2011). Lateral sorting in model membranes by cholesterol-mediated hydrophobic matching. Proc. Natl. Acad. Sci. U.S.A. 108 16628–16633. 10.1073/pnas.1103742108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaria R. N., Harik S. I. (1989). Abnormalities of the glucose transporter at the blood-brain barrier and in brain in Alzheimer’s disease. Prog. Clin. Biol. Res. 317 415–421. [PubMed] [Google Scholar]
- Kamboh M. I., Minster R. L., Demirci F. Y., Ganguli M., Dekosky S. T., Lopez O. L., et al. (2012). Association of CLU and PICALM variants with Alzheimer’s disease. Neurobiol. Aging 33 518–521. 10.1016/j.neurobiolaging.2010.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky Y. G., Tikhonova L. A., Kosenko E. A. (2015). Critical analysis of Alzheimer’s amyloid-beta toxicity to mitochondria. Front Biosci 20:173–197. 10.2741/4304 [DOI] [PubMed] [Google Scholar]
- Kang J., Rivest S. (2012). Lipid metabolism and neuroinflammation in Alzheimer’s disease: a role for liver X receptors. Endocr. Rev. 33 715–746. 10.1210/er.2011-1049 [DOI] [PubMed] [Google Scholar]
- Kao Y. C., Ho P. C., Tu Y. K., Jou I. M., Tsai K. J. (2020). Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 21:1505 10.3390/ijms21041505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanos Y., Gosselet F., Dehouck M. P., Cecchelli R. (2014). Blood-brain barrier proteomics: towards the understanding of neurodegenerative diseases. Arch. Med. Res. 45 730–737. 10.1016/j.arcmed.2014.11.008 [DOI] [PubMed] [Google Scholar]
- Katt M. E., Mayo L. N., Ellis S. E., Mahairaki V., Rothstein J. D., Cheng L., et al. (2019). The role of mutations associated with familial neurodegenerative disorders on blood-brain barrier function in an iPSC model. Fluids Barriers CNS 16:20. 10.1186/s12987-019-0139-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaney J., Campbell M. (2015). The dynamic blood-brain barrier. FEBS J. 282 4067–4079. [DOI] [PubMed] [Google Scholar]
- Kelsch W., Sim S., Lois C. (2010). Watching synaptogenesis in the adult brain. Annu. Rev. Neurosci. 33 131–149. 10.1146/annurev-neuro-060909-153252 [DOI] [PubMed] [Google Scholar]
- Kennedy M. B. (2013). Synaptic signaling in learning and memory. Cold Spring Harb. Perspect. Biol. 8:a016824. 10.1101/cshperspect.a016824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A., Berrougui H., Pawelec G., Fulop T. (2012). Impairment of the ABCA1 and SR-BI-mediated cholesterol efflux pathways and HDL anti-inflammatory activity in Alzheimer’s disease. Mech. Ageing Dev. 133 20–29. 10.1016/j.mad.2011.11.008 [DOI] [PubMed] [Google Scholar]
- Kim M., Nevado-Holgado A., Whiley L., Snowden S. G., Soininen H., Kloszewska I., et al. (2017). Association between plasma ceramides and phosphatidylcholines and hippocampal brain volume in late onset Alzheimer’s disease. J. Alzheimers Dis. 60 809–817. 10.3233/jad-160645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y., Agranoff B. W., Radin N. S., Burton R. M. (1969). Comparison of the fatty acids of lipids of subcellular brain fractions. J. Neurochem. 16 397–404. 10.1111/j.1471-4159.1969.tb10380.x [DOI] [PubMed] [Google Scholar]
- Kitazume S., Tachida Y., Oka R., Shirotani K., Saido T. C., Hashimoto Y. (2001). Alzheimer’s beta-secretase, beta-site amyloid precursor protein-cleaving enzyme, is responsible for cleavage secretion of a Golgi-resident sialyltransferase. Proc. Natl. Acad. Sci. U.S.A. 98 13554–13559. 10.1073/pnas.241509198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knebl J., DeFazio P., Clearfield M. B., Little L., McConathy W. J., McPherson R., et al. (1994). Plasma lipids and cholesterol esterification in Alzheimer’s disease. Mech. Ageing Dev. 73 69–77. 10.1016/0047-6374(94)90039-6 [DOI] [PubMed] [Google Scholar]
- Kniewallner K. M., Ehrlich D., Kiefer A., Marksteiner J., Humpel C. (2015). Platelets in the Alzheimer’s disease brain: do they play a role in cerebral amyloid angiopathy? Curr. Neurovasc. Res. 12 4–14. 10.2174/1567202612666150102124703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama S. G., Rosene D. L., Sherman L. S. (2012). Age-related changes in human and non-human primate white matter: from myelination disturbances to cognitive decline. Age 34 1093–1110. 10.1007/s11357-011-9357-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi K., Wang G., Park L. (2016). Endothelial dysfunction and amyloid-beta-induced neurovascular alterations. Cell Mol. Neurobiol. 36 155–165. 10.1007/s10571-015-0256-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S., Omori M. (1992). Two-way cleavage of beta-amyloid protein precursor by multicatalytic proteinase. FEBS Lett. 304 57–60. 10.1016/0014-5793(92)80588-8 [DOI] [PubMed] [Google Scholar]
- Kosicek M., Zetterberg H., Andreasen N., Peter-Katalinic J., Hecimovic S. (2012). Elevated cerebrospinal fluid sphingomyelin levels in prodromal Alzheimer’s disease. Neurosci. Lett. 516 302–305. 10.1016/j.neulet.2012.04.019 [DOI] [PubMed] [Google Scholar]
- Kotani S., Sakaguchi E., Warashina S., Matsukawa N., Ishikura Y., Kiso Y., et al. (2006). Dietary supplementation of arachidonic and docosahexaenoic acids improves cognitive dysfunction. Neurosci. Res. 56 159–164. 10.1016/j.neures.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Kramer S. D., Schutz Y. B., Wunderli-Allenspach H., Abbott N. J., Begley D. J. (2002). Lipids in blood-brain barrier models in vitro II: influence of glial cells on lipid classes and lipid fatty acids. In Vitro Cell. Dev. Biol. Anim. 38 566–571. [DOI] [PubMed] [Google Scholar]
- Kumar A., Singh A. (2015). A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Front. Pharmacol. 6:206. 10.3389/fphar.2015.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H. J., Cha M. Y., Kim D., Kim D. K., Soh M., Shin K., et al. (2016). Mitochondria-targeting ceria nanoparticles as antioxidants for Alzheimer’s disease. ACS Nano 10 2860–2870. 10.1021/acsnano.5b08045 [DOI] [PubMed] [Google Scholar]
- Lamsa R., Helisalmi S., Herukka S. K., Tapiola T., Pirttila T., Vepsalainen S., et al. (2007). Study on the association between SOAT1 polymorphisms, Alzheimer’s disease risk and the level of CSF biomarkers. Dement. Geriatr. Cogn. Disord. 24 146–150. 10.1159/000105164 [DOI] [PubMed] [Google Scholar]
- Laughlin S. B., Sejnowski T. J. (2003). Communication in neuronal networks. Science 301 1870–1874. 10.1126/science.1089662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc V., Jasmin-Belanger S., Poirier J. (2010). APOE and cholesterol homeostasis in Alzheimer’s disease. Trends Mol. Med. 16 469–477. 10.1016/j.molmed.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Lee L. K., Shahar S., Chin A. V., Yusoff N. A. (2013). Docosahexaenoic acid-concentrated fish oil supplementation in subjects with mild cognitive impairment (MCI): a 12-month randomised, double-blind, placebo-controlled trial. Psychopharmacology 225 605–612. 10.1007/s00213-012-2848-0 [DOI] [PubMed] [Google Scholar]
- Leonard A. E., Kelder B., Bobik E. G., Chuang L. T., Parker-Barnes J. M., Thurmond J. M., et al. (2000). cDNA cloning and characterization of human Delta5-desaturase involved in the biosynthesis of arachidonic acid. Biochem J. 347(Pt. 3), 719–724. 10.1042/bj3470719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepara O., Valjevac A., Alajbegovic A., Zaciragic A., Nakas-Icindic E. (2009). Decreased serum lipids in patients with probable Alzheimer’s disease. Bosn. J. Basic Med. Sci. 9 215–220. 10.17305/bjbms.2009.2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepping R. J., Honea R. A., Martin L. E., Liao K., Choi I. Y., Lee P., et al. (2019). Long-chain polyunsaturated fatty acid supplementation in the first year of life affects brain function, structure, and metabolism at age nine years. Dev. Psychobiol. 61 5–16. 10.1002/dev.21780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuti A., Maccarrone M., Chiurchiu V. (2019). Proresolving lipid mediators: endogenous modulators of oxidative stress. Oxid. Med. Cell. Longev. 2019:8107265. 10.1155/2019/8107265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental I., Veatch S. (2016). The continuing mystery of lipid rafts. J. Mol. Biol. 428 4749–4764. 10.1016/j.jmb.2016.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. Z., Zheng L. J., Shen J., Li X. Y., Zhang Q., Bai X., et al. (2018). SIRT1 facilitates amyloid beta peptide degradation by upregulating lysosome number in primary astrocytes. Neural Regen. Res. 13 2005–2013. 10.4103/1673-5374.239449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Kan H. Y., Lavrentiadou S., Krieger M., Zannis V. (2002). Reconstituted discoidal ApoE-phospholipid particles are ligands for the scavenger receptor BI. The amino-terminal 1-165 domain of ApoE suffices for receptor binding. J. Biol. Chem. 277 21149–21157. 10.1074/jbc.m200658200 [DOI] [PubMed] [Google Scholar]
- Lim G. P., Yang F., Chu T., Chen P., Beech W., Teter B., et al. (2000). Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J. Neurosci. 20 5709–5714. 10.1523/jneurosci.20-15-05709.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q., Cao Y., Gao J. (2015). Decreased expression of the APOA1-APOC3-APOA4 gene cluster is associated with risk of Alzheimer’s disease. Drug. Des. Devel. Ther. 9 5421–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D., Simons K. (2010). Lipid rafts as a membrane-organizing principle. Science 327 46–50. 10.1126/science.1174621 [DOI] [PubMed] [Google Scholar]
- Lingwood D., Kaiser H. J., Levental I., Simons K. (2009). Lipid rafts as functional heterogeneity in cell membranes. Biochem. Soc. Trans. 37 955–960. 10.1042/bst0370955 [DOI] [PubMed] [Google Scholar]
- Liu K., Liu Y., Xu Y., Nandakumar K. S., Shen X., Lin J., et al. (2019). Regulatory role of Golgi brefeldin a resistance factor-1 in amyloid precursor protein trafficking, cleavage and Abeta formation. J. Cell Biochem. 120 15604–15615. 10.1002/jcb.28827 [DOI] [PubMed] [Google Scholar]
- Liu L., MacKenzie K. R., Putluri N., Maletic-Savatic M., Bellen H. J. (2017). The glia-neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab. 26 719–737e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loef M., Walach H. (2013). The Omega-6/Omega-3 ratio and dementia or cognitive decline: a systematic review on human studies and biological evidence. J. Nutr. Gerontol. Geriatr. 32 1–23. 10.1080/21551197.2012.752335 [DOI] [PubMed] [Google Scholar]
- Loera-Valencia R., Goikolea J., Parrado-Fernandez C., Merino-Serrais P., Maioli S. (2019). Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: potential novel targets for treatment. J. Steroid. Biochem. Mol. Biol. 190 104–114. 10.1016/j.jsbmb.2019.03.003 [DOI] [PubMed] [Google Scholar]
- Lohner S., Fekete K., Marosvölgyi T., Decsi T. (2013). Gender differences in the long-chain polyunsaturated fatty acid status: systematic review of 51 publications. Ann. Nutr. Metabol. 62 98–112. 10.1159/000345599 [DOI] [PubMed] [Google Scholar]
- Lopez L. B., Kritz-Silverstein D., Barrett Connor E. (2011). High dietary and plasma levels of the omega-3 fatty acid docosahexaenoic acid are associated with decreased dementia risk: the rancho bernardo study. J. Nutr. Health Aging 15 25–31. 10.1007/s12603-011-0009-5 [DOI] [PubMed] [Google Scholar]
- Louveau A., Smirnov I., Keyes T. J., Eccles J. D., Rouhani S. J., Peske J. D., et al. (2015). Structural and functional features of central nervous system lymphatic vessels. Nature 523 337–341. 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger D. M. (2008). Communication networks in the brain: neurons, receptors, neurotransmitters, and alcohol. Alcohol. Res. Health 31 196–214. [PMC free article] [PubMed] [Google Scholar]
- Lucatelli J. F., Barros A. C., Silva V. K., Machado Fda S., Constantin P. C., Dias A. A., et al. (2011). Genetic influences on Alzheimer’s disease: evidence of interactions between the genes APOE, APOC1 and ACE in a sample population from the South of Brazil. Neurochem. Res. 36 1533–1539. 10.1007/s11064-011-0481-7 [DOI] [PubMed] [Google Scholar]
- Luo C., Ren H., Yao X., Shi Z., Liang F., Kang J. X., et al. (2018). Enriched brain Omega-3 polyunsaturated fatty acids confer neuroprotection against microinfarction. EBioMedicine 32 50–61. 10.1016/j.ebiom.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald-Wicks L., McEvoy M., Magennis E., Schofield P. W., Patterson A. J., Zacharia K. (2019). Dietary long-chain fatty acids and cognitive performance in older australian adults. Nutrients 11:711. 10.3390/nu11040711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackic J. B., Stins M., McComb J. G., Calero M., Ghiso J., Kim K. S., et al. (1998). Human blood-brain barrier receptors for Alzheimer’s amyloid-beta 1- 40. asymmetrical binding, endocytosis, and transcytosis at the apical side of brain microvascular endothelial cell monolayer. J. Clin. Invest. 102 734–743. 10.1172/jci2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean F. L., Horne M. K., Williams R. J., Nisbet D. R. (2018). Review: biomaterial systems to resolve brain inflammation after traumatic injury. APL Bioeng. 2:021502 10.1063/1.5023709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W. (2016). Central nervous system lipoproteins: ApoE and regulation of cholesterol metabolism. Arterioscler. Thromb. Vasc. Biol. 36 1305–1315. 10.1161/atvbaha.116.307023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso C., Bates T. E., Butterfield D. A., Calafato S., Cornelius C., De Lorenzo A., et al. (2007). Natural antioxidants in Alzheimer’s disease. Expert. Opin. Investig. Drugs 16 1921–1931. [DOI] [PubMed] [Google Scholar]
- Marchi C., Adorni M. P., Caffarra P., Ronda N., Spallazzi M., Barocco F., et al. (2019). ABCA1- and ABCG1-mediated cholesterol efflux capacity of cerebrospinal fluid is impaired in Alzheimer’s disease. J. Lipid Res. 60 1449–1456. 10.1194/jlr.p091033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquer C., Devauges V., Cossec J. C., Liot G., Lecart S., Saudou F., et al. (2011). Local cholesterol increase triggers amyloid precursor protein-Bace1 clustering in lipid rafts and rapid endocytosis. FASEB J. 25 1295–1305. 10.1096/fj.10-168633 [DOI] [PubMed] [Google Scholar]
- Martin R. E., Bazan N. G. (1992). Changing fatty acid content of growth cone lipids prior to synaptogenesis. J. Neurochem. 59 318–325. 10.1111/j.1471-4159.1992.tb08906.x [DOI] [PubMed] [Google Scholar]
- Martin T. F. (2000). Racing lipid rafts for synaptic-vesicle formation. Nat. Cell. Biol. 2 E9–E11. [DOI] [PubMed] [Google Scholar]
- Martinez-Frailes C., Di Lauro C., Bianchi C., de Diego-Garcia L., Sebastian-Serrano A., Bosca L., et al. (2019). Amyloid peptide induced neuroinflammation increases the p2x7 receptor expression in microglial cells, impacting on its functionality. Front. Cell Neurosci. 13:143. 10.3389/fncel.2019.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins M. J., Constancia M., Neves D., Simm A. (2017). Biomarkers of aging: from cellular senescence to age-associated diseases. Oxid. Med. Cell Longev. 2017:7280690. 10.1155/2017/7280690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K. A., Xu W., Gaglioti A. H., Holt J. B., Croft J. B., Mack D., et al. (2019). Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged =65 years. Alzheimers Dement. 15 17–24. 10.1016/j.jalz.2018.06.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch D. H., Nagler K., Schumacher S., Goritz C., Muller E. C., Otto A., et al. (2001). CNS synaptogenesis promoted by glia-derived cholesterol. Science 294 1354–1357. 10.1126/science.294.5545.1354 [DOI] [PubMed] [Google Scholar]
- Maulik M., Peake K., Chung J., Wang Y., Vance J. E., Kar S. (2015). APP overexpression in the absence of NPC1 exacerbates metabolism of amyloidogenic proteins of Alzheimer’s disease. Hum. Mol. Genet. 24 7132–7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E. A. (1993). Neuronal communication. Biol Signals 2 57–76. [DOI] [PubMed] [Google Scholar]
- Maysinger D., Ji J., Moquin A., Hossain S., Hancock M. A., Zhang I., et al. (2018). Dendritic polyglycerol sulfates in the prevention of synaptic loss and mechanism of action on glia. ACS Chem. Neurosci. 9 260–271. 10.1021/acschemneuro.7b00301 [DOI] [PubMed] [Google Scholar]
- McClean P. L., Jalewa J., Holscher C. (2015). Prophylactic liraglutide treatment prevents amyloid plaque deposition, chronic inflammation and memory impairment in APP/PS1 mice. Behav. Brain. Res. 293 96–106. 10.1016/j.bbr.2015.07.024 [DOI] [PubMed] [Google Scholar]
- McNamara R. K., Able J., Jandacek R., Rider T., Tso P., Eliassen J. C., et al. (2010). Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am. J. Clin. Nutr. 91 1060–1067. 10.3945/ajcn.2009.28549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara R. K., Asch R. H., Lindquist D. M., Krikorian R. (2018). Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: an update on neuroimaging findings. Prostaglandins Leukot. Essent. Fatty Acids 136 23–34. 10.1016/j.plefa.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecca A. P., Barcelos N. M., Wang S., Bruck A., Nabulsi N., Planeta-Wilson B., et al. (2018). Cortical beta-amyloid burden, gray matter, and memory in adults at varying APOE epsilon4 risk for Alzheimer’s disease. Neurobiol. Aging 61 207–214. 10.1016/j.neurobiolaging.2017.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo R. C., D’Avila H., Wan H. C., Bozza P. T., Dvorak A. M., Weller P. F. (2011). Lipid bodies in inflammatory cells: structure, function, and current imaging techniques. J. Histochem. Cytochem. 59 540–556. 10.1369/0022155411404073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergenthaler P., Lindauer U., Dienel G. A., Meisel A. (2013). Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 36 587–597. 10.1016/j.tins.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino-Zamorano C., Fernandez-de Retana S., Montanola A., Batlle A., Saint-Pol J., Mysiorek C., et al. (2016). Modulation of amyloid-beta1-40 transport by ApoA1 and ApoJ across an in vitro model of the blood-brain barrier. J. Alzheimers Dis. 53 677–691. 10.3233/jad-150976 [DOI] [PubMed] [Google Scholar]
- Mesa-Herrera F., Taoro-Gonzalez L., Valdes-Baizabal C., Diaz M., Marin R. (2019). Lipid and lipid raft alteration in aging and neurodegenerative diseases: a window for the development of new biomarkers. Int. J. Mol. Sci. 20:3810. 10.3390/ijms20153810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke M. (2018). Sex and gender differences in alzheimer’s disease dementia. Psychiatric. Time 35 14–17. [PMC free article] [PubMed] [Google Scholar]
- Mietelska-Porowska A., Wojda U. (2017). T lymphocytes and inflammatory mediators in the interplay between brain and blood in Alzheimer’s disease: potential pools of new biomarkers. J. Immunol. Res. 2017 4626540. 10.1155/2017/4626540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J., Reiner P. B. (1999). Regulation of amyloid precursor protein cleavage. J. Neurochem. 72 443–460. 10.1046/j.1471-4159.1999.0720443.x [DOI] [PubMed] [Google Scholar]
- Mobraten K., Haug T. M., Kleiveland C. R., Lea T. (2013). Omega-3 and omega-6 PUFAs induce the same GPR120-mediated signalling events, but with different kinetics and intensity in Caco-2 cells. Lipids Health. Dis. 12:101. 10.1186/1476-511X-12-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochel F. (2018). Lipids and synaptic functions. J. Inherit. Metab. Dis. 41 1117–1122. 10.1007/s10545-018-0204-1 [DOI] [PubMed] [Google Scholar]
- Mohaibes R. J., Fiol-deRoque M. A., Torres M., Ordinas M., Lopez D. J., Castro J. A., et al. (2017). The hydroxylated form of docosahexaenoic acid (DHA-H) modifies the brain lipid composition in a model of Alzheimer’s disease, improving behavioral motor function and survival. Biochim. Biophys. Acta Biomembr. 1859 1596–1603. 10.1016/j.bbamem.2017.02.020 [DOI] [PubMed] [Google Scholar]
- Molander-Melin M., Blennow K., Bogdanovic N., Dellheden B., Mansson J. E., Fredman P. (2005). Structural membrane alterations in Alzheimer brains found to be associated with regional disease development; increased density of gangliosides GM1 and GM2 and loss of cholesterol in detergent-resistant membrane domains. J. Neurochem. 92 171–182. 10.1111/j.1471-4159.2004.02849.x [DOI] [PubMed] [Google Scholar]
- Montani L., Suter U. (2018). Building lipids for myelin. Aging 10 861–862. 10.18632/aging.101458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanini I., Gatti C., Woelk H., Porcellati S. (1983). The influence of polyunsaturated phosphatidylcholine on brain lipid synthesis during aging. Farmaco. Sci. 38 376–382. [PubMed] [Google Scholar]
- Montoliu-Gaya L., Mulder S. D., Herrebout M. A. C., Baayen J. C., Villegas S., Veerhuis R. (2018). Abeta-oligomer uptake and the resulting inflammatory response in adult human astrocytes are precluded by an anti-Abeta single chain variable fragment in combination with an apoE mimetic peptide. Mol. Cell Neurosci. 89 49–59. 10.1016/j.mcn.2018.03.015 [DOI] [PubMed] [Google Scholar]
- Morris J. C., Schindler S., McCue L. M. (2019). Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 76 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. C., Tangney C. C. (2014). Dietary fat composition and dementia risk. Neurobiol. Aging 35(Suppl. 2), S59–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M. C., Evans D. A., Bienias J. L., Tangney C. C., Bennett D. A., Wilson R. S., et al. (2003). Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 60 940–946. [DOI] [PubMed] [Google Scholar]
- Moura R. P., Martins C., Pinto S., Sousa F., Sarmento B. (2019). Blood-brain barrier receptors and transporters: an insight on their function and how to exploit them through nanotechnology. Expert. Opin. Drug. Deliv. 16 271–285. 10.1080/17425247.2019.1583205 [DOI] [PubMed] [Google Scholar]
- Mukadam A. S., Breusegem S. Y., Seaman M. N. J. (2018). Analysis of novel endosome-to-golgi retrieval genes reveals a role for PLD3 in regulating endosomal protein sorting and amyloid precursor protein processing. Cell Mol. Life Sci. 75 2613–2625. 10.1007/s00018-018-2752-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoonm M. F., Ryan C. M., Sheu L., Yao J. K., Conklin S. M., Manuck S. B. (2010). Serum phospholipid docosahexaenonic acid is associated with cognitive functioning during middle adulthood. J. Nutr. 140 848–853. 10.3945/jn.109.119578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muszynski P., Kulczynska-Przybik A., Borawska R., Litman-Zawadzka A., Slowik A., Klimkowicz-Mrowiec A., et al. (2017). The relationship between markers of inflammation and degeneration in the central nervous system and the blood-brain barrier impairment in Alzheimer’s disease. J. Alzheimers Dis. 59 903–912. 10.3233/jad-170220 [DOI] [PubMed] [Google Scholar]
- Nägga K., Gustavsson A.-M., Stomrud E., Lindqvist D., van Westen D., Blennow K., et al. (2018). Increased midlife triglycerides predict brain β-amyloid and tau pathology 20 years later. Neurology 90 e73–e81. 10.1212/WNL.0000000000004749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasaruddin M. L., Holscher C., Kehoe P., Graham S. F., Green B. D. (2016). Wide-ranging alterations in the brain fatty acid complement of subjects with late Alzheimer’s disease as detected by GC-MS. Am. J. Transl. Res. 8 154–165. [PMC free article] [PubMed] [Google Scholar]
- Nasrabady S. E., Rizvi B., Goldman J. E., Brickman A. M. (2018). White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 6:22. 10.1186/s40478-018-0515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation D. A., Sweeney M. D., Montagne A., Sagare A. P., D’Orazio L. M., Pachicano M., et al. (2019). Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 25 270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlig A. (2004). Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot. Essent. Fatty Acids 70 265–275. 10.1016/j.plefa.2003.07.006 [DOI] [PubMed] [Google Scholar]
- Nelson A. R., Sagare A. P., Zlokovic B. V. (2017). Role of clusterin in the brain vascular clearance of amyloid-beta. Proc. Natl. Acad. Sci. U.S.A. 114 8681–8682. 10.1073/pnas.1711357114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A. R., Sweeney M. D., Sagare A. P., Zlokovic B. V. (2016). Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim. Biophys. Acta 1862 887–900. 10.1016/j.bbadis.2015.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D. G., Budd S. L. (2000). Mitochondria and neuronal survival. Physiol. Rev. 80 315–360. 10.1152/physrev.2000.80.1.315 [DOI] [PubMed] [Google Scholar]
- Ntambi J. M. (2019). Highlighting inflammation and lipid metabolism. Biochem. Biophys. Res. Commun. 520 688–689. 10.1016/j.bbrc.2019.10.014 [DOI] [PubMed] [Google Scholar]
- Nunan J., Small D. H. (2000). Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett. 483 6–10. 10.1016/s0014-5793(00)02076-7 [DOI] [PubMed] [Google Scholar]
- Nunes V. S., Cazita P. M., Catanozi S., Nakandakare E. R., Quintao E. C. R. (2018). Decreased content, rate of synthesis and export of cholesterol in the brain of apoE knockout mice. J. Bioenerg. Biomembr. 50 283–287. 10.1007/s10863-018-9757-9 [DOI] [PubMed] [Google Scholar]
- Nuutinen T., Huuskonen J., Suuronen T., Ojala J., Miettinen R., Salminen A. (2007). Amyloid-beta 1-42 induced endocytosis and clusterin/apoJ protein accumulation in cultured human astrocytes. Neurochem. Int. 50 540–547. 10.1016/j.neuint.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Nuutinen T., Suuronen T., Kyrylenko S., Huuskonen J., Salminen A. (2005). Induction of clusterin/apoJ expression by histone deacetylase inhibitors in neural cells. Neurochem. Int. 47 528–538. 10.1016/j.neuint.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Oberstein T. J., Taha L., Spitzer P., Hellstern J., Herrmann M., Kornhuber J., et al. (2018). Imbalance of circulating Th17 and regulatory T cells in Alzheimer’s disease: a case control study. Front. Immunol. 9:1213. 10.3389/fimmu.2018.01213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien J. S., Sampson E. L. (1965). Lipid composition of the normal human brain: gray matter, white matter, and myelin. J. Lipid. Res. 6 537–544. [PubMed] [Google Scholar]
- O’Brien R. J., Wong P. C. (2011). Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 34 185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donoghue M. C., Murphy S. E., Zamboni G., Nobre A. C., Mackay C. E. (2018). APOE genotype and cognition in healthy individuals at risk of Alzheimer’s disease: a review. Cortex 104 103–123. 10.1016/j.cortex.2018.03.025 [DOI] [PubMed] [Google Scholar]
- Ong W. Y., Farooqui T., Kokotos G., Farooqui A. A. (2015). Synthetic and natural inhibitors of phospholipases A2: their importance for understanding and treatment of neurological disorders. ACS Chem. Neurosci. 6 814–831. [DOI] [PubMed] [Google Scholar]
- Ouellet M., Emond V., Chen C. T., Julien C., Bourasset F., Oddo S., et al. (2009). Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: an in situ cerebral perfusion study. Neurochem. Int. 55 476–482. 10.1016/j.neuint.2009.04.018 [DOI] [PubMed] [Google Scholar]
- Palacios G., Palacios J. M., Mengod G., Frey P. (1992). Beta-amyloid precursor protein localization in the Golgi apparatus in neurons and oligodendrocytes. An immunocytochemical structural and ultrastructural study in normal and axotomized neurons. Brain Res. Mol. Brain Res. 15 195–206. 10.1016/0169-328x(92)90109-o [DOI] [PubMed] [Google Scholar]
- Palmisano B. T., Zhu L., Eckel R. H., Staffor J. M. (2018). Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 15 45–55. 10.1016/j.molmet.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloczi J., Varga Z. V., Hasko G., Pacher P. (2018). Neuroprotection in oxidative stress-related neurodegenerative diseases: role of endocannabinoid system modulation. Antioxid. Redox. Signal. 29 75–108. 10.1089/ars.2017.7144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Khalil H., Nicolazzo J. A. (2015). The impact of docosahexaenoic acid on Alzheimer’s disease: is there a role of the blood-brain barrier? Curr. Clin. Pharmacol. 10 222–241. 10.2174/157488471003150820151532 [DOI] [PubMed] [Google Scholar]
- Pan Y., Short J. L., Choy K. H., Zeng A. X., Marriott P. J., Owada Y., et al. (2016). Fatty acid-binding protein 5 at the blood-brain barrier regulates endogenous brain docosahexaenoic acid levels and cognitive function. J. Neurosci. 36 11755–11767. 10.1523/jneurosci.1583-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang C., Yang H., Hu B., Wang S., Chen M., Cohen D. S., et al. (2019). Identification and Analysis of Alzheimer’s Candidate Genes by an Amplitude Deviation Algorithm. J. Alzheimers Dis. Parkinsonism 9:460. 10.4172/2161-0460.1000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papassotiropoulos A., Wollmer M. A., Tsolaki M., Brunner F., Molyva D., Lutjohann D., et al. (2005). A cluster of cholesterol-related genes confers susceptibility for Alzheimer’s disease. J. Clin. Psychiatry 66 940–947. 10.4088/jcp.v66n0720 [DOI] [PubMed] [Google Scholar]
- Park L., Wang G., Moore J., Girouard H., Zhou P., Anrather J., et al. (2014). The key role of transient receptor potential melastatin-2 channels in amyloid-beta-induced neurovascular dysfunction. Nat. Commun. 5:5318. 10.1038/ncomms6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M. S., Johnson C. A., Rajan R., Owen O. E. (1975). The metabolism of ketone bodies in developing human brain: development of ketone-body-utilizing enzymes and ketone bodies as precursors for lipid synthesis. J. Neurochem. 25 905–908. 10.1111/j.1471-4159.1975.tb04428.x [DOI] [PubMed] [Google Scholar]
- Periyasamy S., Sathya M., Karthick C., Kandasamy M., Shanmugaapriya S., Tamilselvan J., et al. (2017). Association studies of specific cholesterol related genes (APOE, LPL, and CETP) with lipid profile and memory function: a correlative study among rural and tribal population of dharmapuri district, India. J. Alzheimers Dis. 60 S195–S207. [DOI] [PubMed] [Google Scholar]
- Phillips M. A., Childs C. E., Calder P. C., Rogers P. J. (2015). No effect of Omega-3 fatty acid supplementation on cognition and mood in individuals with cognitive impairment and probable alzheimer’s disease: a randomised controlled trial. Int. J. Mol. Sci. 16 24600–24613. 10.3390/ijms161024600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M., McEwen B. S. (2014). Mitochondria impact brain function and cognition. Proc. Natl. Acad. Sci. U.S.A. 111 7–8. 10.1073/pnas.1321881111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. (2012). A thickening network of lipids. Pain 153 3–4. 10.1016/j.pain.2011.09.026 [DOI] [PubMed] [Google Scholar]
- Piomelli D., Astarita G., Rapaka R. (2007). A neuroscientist’s guide to lipidomics. Nat. Rev. Neurosci. 8 743–754. [DOI] [PubMed] [Google Scholar]
- Piro J. R., Benjamin D. I., Duerr J. M., Pi Y., Gonzales C., Wood K. M., et al. (2012). A dysregulated endocannabinoid-eicosanoid network supports pathogenesis in a mouse model of Alzheimer’s disease. Cell Rep. 1 617–623. 10.1016/j.celrep.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas R. E., Boyles J. K., Lee S. H., Foss D., Mahley R. W. (1987). Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim. Biophys. Acta 917 148–161. 10.1016/0005-2760(87)90295-5 [DOI] [PubMed] [Google Scholar]
- Prasad M. R., Lovell M. A., Yatin M., Dhillon H., Markesbery W. R. (1998). Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem. Res. 23 81–88. [DOI] [PubMed] [Google Scholar]
- Prendecki M., Florczak-Wyspianska J., Kowalska M., Ilkowski J., Grzelak T., Bialas K., et al. (2018). Biothiols and oxidative stress markers and polymorphisms of TOMM40 and APOC1 genes in Alzheimer’s disease patients. Oncotarget 9 35207–35225. 10.18632/oncotarget.26184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J. E., Joan Abbott N., Begley D. J. (2014). Transcytosis of macromolecules at the blood-brain barrier. Adv. Pharmacol. 71 147–163. 10.1016/bs.apha.2014.06.001 [DOI] [PubMed] [Google Scholar]
- Prevost M., Kocher J. P. (1999). Structural characterization by computer experiments of the lipid-free LDL-receptor-binding domain of apolipoprotein E. Protein Eng. 12 475–483. 10.1093/protein/12.6.475 [DOI] [PubMed] [Google Scholar]
- Quinn J. F., Raman R., Thomas R. G., Yurko-Mauro K., Nelson E. B., Van Dyck C., et al. (2010). Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 304 1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla R. A., Dolan P. J., Jin Y. N., Johnson G. V. (2012). Truncated tau and Abeta cooperatively impair mitochondria in primary neurons. Neurobiol. Aging 33 e625–e635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj D., Yin Z., Breur M., Doorduin J., Holtman I. R., Olah M., et al. (2017). Increased white matter inflammation in aging- and Alzheimer’s disease brain. Front. Mol. Neurosci. 10:206. 10.3389/fnmol.2017.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy I. (2014). Recent advances in physiological lipoprotein metabolism. Clin. Chem. Lab. Med. 52 1695–1727. [DOI] [PubMed] [Google Scholar]
- Rangaraju V., Lewis T. L., Jr., Hirabayashi Y., Bergami M., Motori E., Cartoni R., et al. (2019). Pleiotropic mitochondria: the influence of mitochondria on neuronal development and Disease. J. Neurosci. 39 8200–8208. 10.1523/jneurosci.1157-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnakumar A., Zimmerman S. E., Jordan B. A., Mar J. C. (2019). Estrogen activates Alzheimer’s disease genes. Alzheimers Dement 5 906–917. 10.1016/j.trci.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C. (2012). Dyslipidemia and dementia: current epidemiology, genetic evidence and mechanisms behind the associations. J. Alzheimers Dis. 30 S127–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel B. C., Thompson P. M., Brinton R. D. (2016). Age, APOE and sex: triad of risk of Alzheimer’s disease. J. Steroid. Biochem. Mol. Biol. 160 134–147. 10.1016/j.jsbmb.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S. B., Ripellino J. A., Ingalls K. M., Robakis N. K., Felsenstein K. M. (1994). Non-amyloidogenic cleavage of the beta-amyloid precursor protein by an integral membrane metalloendopeptidase. J. Biol. Chem. 269 3111–3116. [PubMed] [Google Scholar]
- Robinson J. L., Lee E. B., Xie S. X., Rennert L., Suh E., Bredenberg C., et al. (2018). Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141 2181–2193. 10.1093/brain/awy146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A., Mangialasche F., Ngandu T., Solomon A., Kivipelto M. (2020). Multidomain interventions to prevent cognitive impairment, Alzheimer’s disease, and dementia: from FINGER to world-wide FINGERS. J. Prev. Alzheimers Dis. 7 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten B. P., Steinbusch H. W., Korr H., Schmitz C. (2002). Antioxidants and Alzheimer’s disease: from bench to bedside (and back again). Curr. Opin. Clin. Nutr. Metab. Care 5 645–651. [DOI] [PubMed] [Google Scholar]
- Saftig P., Peters C., von Figura K., Craessaerts K., Van Leuven F., De Strooper B. (1996). Amyloidogenic processing of human amyloid precursor protein in hippocampal neurons devoid of cathepsin D. J. Biol. Chem. 271 27241–27244. 10.1074/jbc.271.44.27241 [DOI] [PubMed] [Google Scholar]
- Salinas E. (2009). Neuronal communication: a detailed balancing act. Nat. Neurosci. 12 372–374. 10.1038/nn0409-372 [DOI] [PubMed] [Google Scholar]
- Sarrafpour S., Ormseth C., Chiang A., Arakaki X., Harrington M., Fonteh A. (2019). Lipid metabolism in late-onset Alzheimer’s disease differs from patients presenting with other dementia phenotypes. Int. J. Environ. Res. Public Health 16:1995. 10.3390/ijerph16111995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M., Walter J., Gentleman S. M. (2008). Interactions between APP secretases and inflammatory mediators. J. Neuroinflammation 5:25. 10.1186/1742-2094-5-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt F., Hussain G., Dupuis L., Loeffler J. P., Henriques A. (2014). A plural role for lipids in motor neuron diseases: energy, signaling and structure. Front. Cell Neurosci. 8:25. 10.3389/fncel.2014.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt S., Castelvetri L. C., Simons M. (2015). Metabolism and functions of lipids in myelin. Biochim. Biophys. Acta 1851 999–1005. 10.1016/j.bbalip.2014.12.016 [DOI] [PubMed] [Google Scholar]
- Schmitz G. E. J. (2008). The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 47 147–155. 10.1016/j.plipres.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Schmitz G., Muller G. (1991). Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J. Lipid Res. 32 1539–1570. [PubMed] [Google Scholar]
- Schonfeld P., Reiser G. (2017). Brain energy metabolism spurns fatty acids as fuel due to their inherent mitotoxicity and potential capacity to unleash neurodegeneration. Neurochem. Int. 109 68–77. 10.1016/j.neuint.2017.03.018 [DOI] [PubMed] [Google Scholar]
- Scialo F., Sriram A., Fernandez-Ayala D., Gubina N., Lohmus M., Nelson G., et al. (2016). Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab. 23 725–734. 10.1016/j.cmet.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiao A. M., Colino-Oliveira M., Assaife-Lopes N., Dias R. B., Ribeiro J. A. (2013). Lipid rafts, synaptic transmission and plasticity: impact in age-related neurodegenerative diseases. Neuropharmacology 64 97–107. 10.1016/j.neuropharm.2012.06.053 [DOI] [PubMed] [Google Scholar]
- Segi-Nishida E. (2014). Double function of MFSD2A transporter at the blood-brain barrier. Nihon Yakurigaku Zasshi 144:253. 10.1254/fpj.144.253 [DOI] [PubMed] [Google Scholar]
- Serhan C. N. (2010). Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am. J. Pathol. 177 1576–1591. 10.2353/ajpath.2010.100322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Chiang N., Dalli J. (2015). The resolution code of acute inflammation: novel pro-resolving lipid mediators in resolution. Semin. Immunol. 27 200–215. 10.1016/j.smim.2015.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Chiang N., Dalli J. (2018). New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol. Aspects. Med. 64 1–17. 10.1016/j.mam.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin B. (2012). Estrogen and cognitive functioning in women: lessons we have learned. Behav. Neurosci. 126 123–127. 10.1037/a0025539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Medway C., Bullock J., Brown K., Kalsheker N., Morgan K. (2010). Analysis of genome-wide association study (GWAS) data looking for replicating signals in Alzheimer’s disease (AD). Int. J. Mol. Epidemiol. Genet. 1 53–66. [PMC free article] [PubMed] [Google Scholar]
- Shibata M., Yamada S., Kumar S. R., Calero M., Bading J., Frangione B., et al. (2000). Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J. Clin. Invest. 106 1489–1499. 10.1172/jci10498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata N., Kawarai T., Lee J. H., Lee H. S., Shibata E., Sato C., et al. (2006). Association studies of cholesterol metabolism genes (CH25H, ABCA1 and CH24H) in Alzheimer’s disease. Neurosci. Lett. 391 142–146. 10.1016/j.neulet.2005.08.048 [DOI] [PubMed] [Google Scholar]
- Shibata N., Nagata T., Shinagawa S., Ohnuma T., Shimazaki H., Komatsu M., et al. (2013). Genetic association between APOA1 and APOD polymorphisms and Alzheimer’s disease in a Japanese population. J. Neural. Transm. 120 1599–1603. 10.1007/s00702-013-1036-7 [DOI] [PubMed] [Google Scholar]
- Shibuya Y., Chang C. C., Chang T. Y. (2015). ACAT1/SOAT1 as a therapeutic target for Alzheimer’s disease. Future Med. Chem. 7 2451–2467. 10.4155/fmc.15.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa M., Yanagisawa K., Nishiye H., Miyatake T. (1993). Identification of amyloid precursor protein in synaptic plasma membrane. Biochem. Biophys. Res. Commun. 196 240–244. 10.1006/bbrc.1993.2240 [DOI] [PubMed] [Google Scholar]
- Shinohara M., Tachibana M., Kanekiyo T., Bu G. (2017). Role of LRP1 in the pathogenesis of Alzheimer’s disease: evidence from clinical and preclinical studies. J. Lipid Res. 58 1267–1281. 10.1194/jlr.r075796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinto L., Quinn J., Montine T., Dodge H. H., Woodward W., Baldauf-Wagner S., et al. (2014). A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J. Alzheimers Dis. 38 111–120. 10.3233/jad-130722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai P., Liu Y., Lu W., Liu Q., Li T., Gong B. (2015). Genetic associations of CLU rs9331888 polymorphism with Alzheimer’s disease: a meta-analysis. Neurosci. Lett. 591 160–165. 10.1016/j.neulet.2015.02.040 [DOI] [PubMed] [Google Scholar]
- Siegel G. J. (1999). Basic Neurochemistry : Molecular, Cellular, and Medical Aspects, 6th Edn Philadelphia: Lippincott Williams & Wilkins. [Google Scholar]
- Simon A. K., Hollander G. A., McMichael A. (2015). Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 282:20143085. 10.1098/rspb.2014.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M., Nave K. A. (2015). Oligodendrocytes: myelination and axonal support. Cold Spring Harb. Perspect. Biol. 8:a020479. 10.1101/cshperspect.a020479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simopoulos A. P. (2006). Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed. Pharmacother. 60 502–507. 10.1016/j.biopha.2006.07.080 [DOI] [PubMed] [Google Scholar]
- Sinclair A. J., Begg D., Mathai M., Weisinger R. S. (2007). Omega 3 fatty acids and the brain: review of studies in depression. Asia Pac. J. Clin. Nutr. 16(Suppl. 1), 391–397. [PubMed] [Google Scholar]
- Sisodia S. S. (1992). Beta-amyloid precursor protein cleavage by a membrane-bound protease. Proc. Natl. Acad. Sci. U.S.A. 89 6075–6079. 10.1073/pnas.89.13.6075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper S. D., Di Marzo V. (2012). Endocannabinoids in nervous system health and disease: the big picture in a nutshell. Philos. Trans. R. Soc. Lond. B Biol. Sci. 367 3193–3200. 10.1098/rstb.2012.0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smach M. A., Edziri H., Charfeddine B., Ben Othman L., Lammouchi T., Ltaief A., et al. (2011). Polymorphism in apoA1 influences high-density lipoprotein cholesterol levels but is not a major risk factor of Alzheimer’s disease. Dement. Geriatr. Cogn. Dis. Extra. 1 249–257. 10.1159/000329910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden S. G., Ebshiana A. A., Hye A., An Y., Pletnikova O., O’Brien R., et al. (2017). Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: a nontargeted metabolomic study. PLoS Med 14:e1002266. 10.1371/journal.pmed.1002266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soininen H., Solomon A., Visser P. J., Hendrix S. B., Blennow K., Kivipelto M., et al. (2017). LipiDiDiet clinical study g: 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 16 965–975. 10.1016/s1474-4422(17)30332-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son G., Han J. (2018). Roles of mitochondria in neuronal development. BMB Rep. 51 549–556. 10.5483/bmbrep.2018.51.11.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Manku M. S., Horrobin D. F. (2008). Long-chain polyunsaturated fatty acids modulate interleukin-1beta-induced changes in behavior, monoaminergic neurotransmitters, and brain inflammation in rats. J. Nutr. 138 954–963. 10.1093/jn/138.5.954 [DOI] [PubMed] [Google Scholar]
- Song H., Hecimovic S., Goate A., Hsu F. F., Bao S., Vidavsky I., et al. (2004). Characterization of N-terminal processing of group VIA phospholipase A2 and of potential cleavage sites of amyloid precursor protein constructs by automated identification of signature peptides in LC/MS/MS analyses of proteolytic digests. J. Am. Soc. Mass Spectrom. 15 1780–1793. 10.1016/j.jasms.2004.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano S., Lu D. C., Chandra S., Pietrzik C. U., Koo E. H. (2001). The amyloidogenic pathway of amyloid precursor protein (APP) is independent of its cleavage by caspases. J. Biol. Chem. 276 29045–29050. 10.1074/jbc.m102456200 [DOI] [PubMed] [Google Scholar]
- Spiteller G. (2010). Is lipid peroxidation of polyunsaturated acids the only source of free radicals that induce aging and age-related diseases? Rejuvenation Res. 13 91–103. 10.1089/rej.2009.0934 [DOI] [PubMed] [Google Scholar]
- Spuch C., Ortolano S., Navarro C. (2012). New insights in the amyloid-Beta interaction with mitochondria. J. Aging Res. 2012 324968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R. A., Jain J. C. (2002). Scavenger receptor class B type I expression and elemental analysis in cerebellum and parietal cortex regions of the Alzheimer’s disease brain. J. Neurol. Sci. 196 45–52. 10.1016/s0022-510x(02)00026-6 [DOI] [PubMed] [Google Scholar]
- Stahl T., Reimers C., Johne R., Schliebs R., Seeger J. (2006). Viral-induced inflammation is accompanied by beta-amyloid plaque reduction in brains of amyloid precursor protein transgenic Tg2576 mice. Eur. J. Neurosci. 24 1923–1934. 10.1111/j.1460-9568.2006.05069.x [DOI] [PubMed] [Google Scholar]
- Stark K. D., Van Elswyk M. E., Higgins M. R., Weatherford C. A., Salem N., Jr. (2016). Global survey of the omega-3 fatty acids, docosahexaenoic acid andeicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 63 132–152. 10.1016/j.plipres.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Stassart R. M., Mobius W., Nave K. A., Edgar J. M. (2018). The axon-myelin unit in development and degenerative disease. Front Neurosci 12:467. 10.3389/fnins.2018.00467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. J., Austen B. M. (1996). Metabolites of the beta-amyloid precursor protein generated by beta-secretase localise to the trans-Golgi network and late endosome in 293 cells. J. Neurosci. Res. 46 211–225. [DOI] [PubMed] [Google Scholar]
- Stone D. J., Rozovsky I., Morgan T. E., Anderson C. P., Hajian H., Finch C. E. (1997). Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp. Neurol. 143 313–318. 10.1006/exnr.1996.6360 [DOI] [PubMed] [Google Scholar]
- Stonehouse W., Conlon C. A., Podd J., Hill S. R., Minihane A. M., Haskell C., et al. (2013). DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am. J. Clin. Nutr. 97 1134–1143. 10.3945/ajcn.112.053371 [DOI] [PubMed] [Google Scholar]
- Strike S. C., Carlisle A., Gibson E. L., Dyall S. C. (2016). A high Omega-3 fatty acid multinutrient supplement benefits cognition and mobility in older women: a randomized, double-blind, placebo-controlled pilot study. J. Gerontol. A Biol. Sci. Med. Sci. 71 236–242. 10.1093/gerona/glv109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasini D., Yalagala P. C. R., Goggin A., Tai L. M., Subbaiah P. V. (2019). Enrichment of brain docosahexaenoic acid (DHA) is highly dependent upon the molecular carrier of dietary DHA: lysophosphatidylcholine is more efficient than either phosphatidylcholine or triacylglycerol. J. Nutr. Biochem. 74 108231. 10.1016/j.jnutbio.2019.108231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana R., Newman S., Mohmmad-Abdul H., Keller J. N., Butterfield D. A. (2004). Protective effect of the xanthate, D609, on Alzheimer’s amyloid beta-peptide (1-42)-induced oxidative stress in primary neuronal cells. Free Radic. Res. 38 449–458. 10.1080/1071576042000206478 [DOI] [PubMed] [Google Scholar]
- Sumner A. (2009). Ethnic differences in triglyceride levels and high-density lipoprotein lead to underdiagnosis of the metabolic syndrome in black children and adults. J. Pediatr. 155 S7.e7–S7.e11. 10.1016/j.jpeds.2009.04.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. Y., Draczynska-Lusiak B., Sun G. Y. (2001). Oxidized lipoproteins, beta amyloid peptides and Alzheimer’s disease. Neurotox Res. 3 167–178. 10.1007/bf03033189 [DOI] [PubMed] [Google Scholar]
- Sun Y., Shi J., Zhang S., Tang M., Han H., Guo Y., et al. (2005). The APOC3 SstI polymorphism is weakly associated with sporadic Alzheimer’s disease in a Chinese population. Neurosci. Lett. 380 219–222. 10.1016/j.neulet.2005.01.038 [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Bostrom K., Jungbjer B., Olsson L. (1994). Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J. Neurochem. 63 1802–1811. 10.1046/j.1471-4159.1994.63051802.x [DOI] [PubMed] [Google Scholar]
- Sweeney M. D., Sagare A. P., Zlokovic B. V. (2018). Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14 133–150. 10.1038/nrneurol.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. H., Seah C., Pasternak S. H. (2014). The amyloid precursor protein is rapidly transported from the golgi apparatus to the lysosome and where it is processed into beta-amyloid. Mol. Brain 7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J. Z. A., Gleeson P. A. (2019). The role of membrane trafficking in the processing of amyloid precursor protein and production of amyloid peptides in Alzheimer’s disease. Biochim Biophys Acta Biomembr 1861 697–712. 10.1016/j.bbamem.2018.11.013 [DOI] [PubMed] [Google Scholar]
- Tchessalova D., Posillico C. K., Tronson N. C. (2018). Neuroimmune activation drives multiple brain states. Front. Syst. Neurosci. 12:39. 10.3389/fnsys.2018.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thinakaran G., Koo E. H. (2008). Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 283 29615–29619. 10.1074/jbc.r800019200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. H., Pelleieux S., Vitale N., Olivier J. L. (2016). Dietary arachidonic acid as a risk factor for age-associated neurodegenerative diseases: potential mechanisms. Biochimie 130 168–177. 10.1016/j.biochi.2016.07.013 [DOI] [PubMed] [Google Scholar]
- Tindale L. C., Leach S., Spinelli J. J., Brooks-Wilson A. R. (2017). Lipid and Alzheimer’s disease genes associated with healthy aging and longevity in healthy oldest-old. Oncotarget 8 20612–20621. 10.18632/oncotarget.15296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh W. H., Tan J. Z., Zulkefli K. L., Houghton F. J., Gleeson P. A. (2017). Amyloid precursor protein traffics from the Golgi directly to early endosomes in an Arl5b- and AP4-dependent pathway. Traffic 18 159–175. 10.1111/tra.12465 [DOI] [PubMed] [Google Scholar]
- Toledo J. B., Toledo E., Weiner M. W., Jack C. R., Jr., Jagust W., Lee V. M., et al. (2012). Alzheimer’s disease neuroimaging I: cardiovascular risk factors, cortisol, and amyloid-beta deposition in Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement 8 483–489. 10.1016/j.jalz.2011.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T., Wong P. C. (2011). Selectivity to amyloid-beta precursor protein cleavage provides hope against Alzheimer’s. Alzheimers Res. Ther. 3:7. 10.1186/alzrt66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M., Price S. L., Fiol-Deroque M. A., Marcilla-Etxenike A., Ahyayauch H., Barcelo-Coblijn G., et al. (2014). Membrane lipid modifications and therapeutic effects mediated by hydroxydocosahexaenoic acid on Alzheimer’s disease. Biochim. Biophys. Acta 1838 1680–1692. 10.1016/j.bbamem.2013.12.016 [DOI] [PubMed] [Google Scholar]
- Tremblay M. E., Stevens B., Sierra A., Wake H., Bessis A., Nimmerjahn A. (2011). The role of microglia in the healthy brain. J. Neurosci. 31 16064–16069. 10.1523/jneurosci.4158-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M. E., Zhang I., Bisht K., Savage J. C., Lecours C., Parent M., et al. (2016). Remodeling of lipid bodies by docosahexaenoic acid in activated microglial cells. J. Neuroinflammation 13:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulloch J., Leong L., Thomson Z., Chen S., Lee E. G., Keene C. D., et al. (2018). Glia-specific APOE epigenetic changes in the Alzheimer’s disease brain. Brain Res. 1698 179–186. 10.1016/j.brainres.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyurina Y. Y., Poloyac S. M., Tyurin V. A., Kapralov A. A., Jiang J., Anthonymuthu T. S., et al. (2014). A mitochondrial pathway for biosynthesis of lipid mediators. Nat. Chem. 6 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzioras M., Davies C., Newman A., Jackson R., Spires-Jones T. (2019). Invited Review: APOE at the interface of inflammation, neurodegeneration and pathological protein spread in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 45 327–346. 10.1111/nan.12529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M. S., Rahman M. M., Jakaria M., Rahman M. S., Hossain M. S., Islam A., et al. (2020). Estrogen signaling in Alzheimer’s Disease: molecular insights and therapeutic targets for Alzheimer’s dementia. Mol. Neurobiol. 10.1007/s12035-020-01911-8 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Umamaheswaran S., Dasari S. K., Yang P., Lutgendorf S. K., Sood A. K. (2018). Stress, inflammation, and eicosanoids: an emerging perspective. Cancer Metastasis Rev. 37 203–211. 10.1007/s10555-018-9741-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Rest O., Geleijnse J. M., Kok F. J., van Staveren W. A., Dullemeijer C., Olderikkert M. G., et al. (2008). Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology 71 430–438. 10.1212/01.wnl.0000324268.45138.86 [DOI] [PubMed] [Google Scholar]
- van den Kommer T. N., Dik M. G., Comijs H. C., Lutjohann D., Lips P., Jonker C., et al. (2012). The role of extracerebral cholesterol homeostasis and ApoE e4 in cognitive decline. Neurobiol. Aging 33 e617–e628. [DOI] [PubMed] [Google Scholar]
- Vance J. E., Hayashi H. (2010). Formation and function of apolipoprotein E-containing lipoproteins in the nervous system. Biochim. Biophys. Acta 1801 806–818. 10.1016/j.bbalip.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Vannucci S. J., Clark R. R., Koehler-Stec E., Li K., Smith C. B., Davies P., et al. (1998). Glucose transporter expression in brain: relationship to cerebral glucose utilization. Dev. Neurosci. 20 369–379. 10.1159/000017333 [DOI] [PubMed] [Google Scholar]
- Veerhuis R., Nielsen H. M., Tenner A. J. (2011). Complement in the brain. Mol. Immunol. 48 1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermunt L., Sikkes S. A. M., van den Hout A., Handels R., Bos I., van der Flier W. M., et al. (2019). Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 15 888–898. 10.1016/j.jalz.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villasenor R., Schilling M., Sundaresan J., Lutz Y., Collin L. (2017). Sorting tubules regulate blood-brain barrier transcytosis. Cell Rep. 21 3256–3270. 10.1016/j.celrep.2017.11.055 [DOI] [PubMed] [Google Scholar]
- Viña J., Lloret A. (2010). Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-β peptide. J. Alzheimers Dis. 20 S527–S533. [DOI] [PubMed] [Google Scholar]
- Vina J., Lloret A., Orti R., Alonso D. (2004). Molecular bases of the treatment of Alzheimer’s disease with antioxidants: prevention of oxidative stress. Mol. Aspects. Med. 25 117–123. 10.1016/j.mam.2004.02.013 [DOI] [PubMed] [Google Scholar]
- Volmar C. H., Salah-Uddin H., Janczura K. J., Halley P., Lambert G., Wodrich A., et al. (2017). M344 promotes nonamyloidogenic amyloid precursor protein processing while normalizing Alzheimer’s disease genes and improving memory. Proc. Natl. Acad. Sci. U.S.A. 114 E9135–E9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. (2014). Lipid rafts: a signaling platform linking cholesterol metabolism to synaptic deficits in autism spectrum disorders. Front. Behav. Neurosci. 8:104. 10.3389/fnbeh.2014.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. F., Lu R., Wang Y. Z. (2010). Regulation of beta cleavage of amyloid precursor protein. Neurosci. Bull. 26 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhang X., Zhai L., Sheng X., Zheng W., Chu H., et al. (2018). Atorvastatin attenuates cognitive deficits and neuroinflammation induced by Abeta1-42 involving modulation of TLR4/TRAF6/NF-kappaB pathway. J. Mol. Neurosci. 64 363–373. 10.1007/s12031-018-1032-3 [DOI] [PubMed] [Google Scholar]
- Wang W. Y., Tan M. S., Yu J. T., Tan L. (2015a). Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 3:136. 10.3978/j.issn.2305-5839.2015.03.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhu M., Hjorth E., Cortes-Toro V., Eyjolfsdottir H., Graff C., et al. (2015b). Resolution of inflammation is altered in Alzheimer’s disease. Alzheimers Dement 11 e41–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. F., Cao Y. W., Feng Z. Z., Fu D., Ma Y. S., Zhang F., et al. (2013). Quantitative assessment of the effect of ABCA1 gene polymorphism on the risk of Alzheimer’s disease. Mol. Biol. Rep. 40 779–785. 10.1007/s11033-012-2115-9 [DOI] [PubMed] [Google Scholar]
- Wavrant-De Vrieze F., Compton D., Womick M., Arepalli S., Adighibe O., Li L., et al. (2007). ABCA1 polymorphisms and Alzheimer’s disease. Neurosci. Lett. 416 180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. J., Butt C. M., Mohajeri M. H. (2016). Docosahexaenoic acid and cognition throughout the Lifespan. Nutrients 8:99. 10.3390/nu8020099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L. (2014). Synaptic transmission: membrane lipids channel a message. Nat. Rev. Neurosci. 15:135. 10.1038/nrn3701 [DOI] [PubMed] [Google Scholar]
- Wender M., Adamczewska-Goncerzewicz Z., Szczech J., Godlewski A. (1988). Myelin lipids in aging human brain. Neurochem. Pathol. 8 121–130. [DOI] [PubMed] [Google Scholar]
- Wezyk M., Szybinska A., Wojsiat J., Szczerba M., Day K., Ronnholm H., et al. (2018). Overactive BRCA1 affects presenilin 1 in induced pluripotent stem cell-derived neurons in Alzheimer’s disease. J. Alzheimers Dis. 62 175–202. 10.3233/jad-170830 [DOI] [PubMed] [Google Scholar]
- Whelan J. (2008). (n-6) and (n-3) Polyunsaturated fatty acids and the aging brain: food for thought. J. Nutr. 138 2521–2522. 10.3945/jn.108.095943 [DOI] [PubMed] [Google Scholar]
- Whiley L., Sen A., Heaton J., Proitsi P., Garcia-Gomez D., Leung R., et al. (2014). Evidence of altered phosphatidylcholine metabolism in Alzheimer’s disease. Neurobiol. Aging 35 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington R. A., Planel E., Terrando N. (2017). Impaired resolution of inflammation in alzheimer’s disease: a review. Front. Immunol. 8:1464. 10.3389/fimmu.2017.01464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K. I., Higgs G. A. (1988). Eicosanoids and inflammation. J. Pathol. 156 101–110. [DOI] [PubMed] [Google Scholar]
- Willis L. M., Shukitt-Hale B., Joseph J. A. (2009). Dietary polyunsaturated fatty acids improve cholinergic transmission in the aged brain. Genes Nutr. 4 309–314. 10.1007/s12263-009-0141-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth M., Lange C., Huijbers W. (2019). Alzheimer’s disease neuroimaging I: plasma cortisol is associated with cerebral hypometabolism across the Alzheimer’s disease spectrum. Neurobiol. Aging 84 80–89. 10.1016/j.neurobiolaging.2019.08.003 [DOI] [PubMed] [Google Scholar]
- Wojsiat J., Zoltowska K. M., Laskowska-Kaszub K., Wojda U. (2018). Oxidant/antioxidant imbalance in Alzheimer’s disease: therapeutic and diagnostic prospects. Oxid. Med. Cell Longev. 2018:6435861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmer M. A., Sleegers K., Ingelsson M., Zekanowski C., Brouwers N., Maruszak A., et al. (2007). Association study of cholesterol-related genes in Alzheimer’s disease. Neurogenetics 8 179–188. [DOI] [PubMed] [Google Scholar]
- Wollmer M. A., Streffer J. R., Lutjohann D., Tsolaki M., Iakovidou V., Hegi T., et al. (2003a). ABCA1 modulates CSF cholesterol levels and influences the age at onset of Alzheimer’s disease. Neurobiol. Aging 24 421–426. 10.1016/s0197-4580(02)00094-5 [DOI] [PubMed] [Google Scholar]
- Wollmer M. A., Streffer J. R., Tsolaki M., Grimaldi L. M., Lutjohann D., Thal D., et al. (2003b). Genetic association of acyl-coenzyme A: cholesterol acyltransferase with cerebrospinal fluid cholesterol levels, brain amyloid load, and risk for Alzheimer’s disease. Mol. Psychiatry 8 635–638. 10.1038/sj.mp.4001296 [DOI] [PubMed] [Google Scholar]
- Wolozin B., Brown J., III, Theisler C., Silberman S. (2004). The cellular biochemistry of cholesterol and statins: insights into the pathophysiology and therapy of Alzheimer’s disease. CNS Drug. Rev. 10 127–146. 10.1111/j.1527-3458.2004.tb00009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. W. K., Braidy N., Crawford J., Pickford R., Song F., Mather K. A., et al. (2019). APOE genotype differentially modulates plasma lipids in healthy older individuals, with relevance to brain health. J. Alzheimers Dis. 72 703–716. 10.3233/jad-190524 [DOI] [PubMed] [Google Scholar]
- Wong M. W., Braidy N., Poljak A., Pickford R., Thambisetty M., Sachdev P. S. (2017). Dysregulation of lipids in Alzheimer’s disease and their role as potential biomarkers. Alzheimers Dement 13 810–827. 10.1016/j.jalz.2017.01.008 [DOI] [PubMed] [Google Scholar]
- Wood P. L. (2012). Lipidomics of Alzheimer’s disease: current status. Alzheimers Res. Ther. 4:5. 10.1186/alzrt103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. J., Zhuo M. (2008). Resting microglial motility is independent of synaptic plasticity in mammalian brain. J. Neurophysiol. 99 2026–2032. 10.1152/jn.01210.2007 [DOI] [PubMed] [Google Scholar]
- Xiao Z., Wang J., Chen W., Wang P., Zeng H., Chen W. (2012). Association studies of several cholesterol-related genes (ABCA1, CETP and LIPC) with serum lipids and risk of Alzheimer’s disease. Lipids Health Dis. 11:163. 10.1186/1476-511x-11-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Virassamy B., Vijayaraj S. L., Lim Y., Saadipour K., Wang Y. J., et al. (2013). The intracellular domain of sortilin interacts with amyloid precursor protein and regulates its lysosomal and lipid raft trafficking. PLoS One 8:e63049. 10.1371/journal.pone.0063049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Wang H., Wen J., Ma K., Chen D., Chen Z., et al. (2019). Regulation of microglial process elongation, a featured characteristic of microglial plasticity. Pharmacol. Res. 139 286–297. 10.1016/j.phrs.2018.11.028 [DOI] [PubMed] [Google Scholar]
- Yang X., Sheng W., Sun G. Y., Lee J. C. (2011). Effects of fatty acid unsaturation numbers on membrane fluidity and alpha-secretase-dependent amyloid precursor protein processing. Neurochem. Int. 58 321–329. 10.1016/j.neuint.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine H. N., Braskie M. N., Mack W. J., Castor K. J., Fonteh A. N., Schneider L. S., et al. (2017). Association of docosahexaenoic acid supplementation with alzheimer disease stage in apolipoprotein E epsilon4 carriers: a review. JAMA Neurol. 74 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates D. (2014). Synaptic plasticity: Microglial cell-mediated depression. Nat. Rev. Neurosci. 15:280. [DOI] [PubMed] [Google Scholar]
- Yatin S. M., Varadarajan S., Butterfield D. A. (2000). Vitamin E prevents Alzheimer’s Amyloid beta-peptide (1-42)-induced neuronal protein oxidation and reactive oxygen species production. J. Alzheimers Dis. 2 123–131. 10.3233/jad-2000-2212 [DOI] [PubMed] [Google Scholar]
- Yehuda S., Rabinovitz S., Carasso R. L., Mostofsky D. I. (2002). The role of polyunsaturated fatty acids in restoring the aging neuronal membrane. Neurobiol. Aging 23 843–853. 10.1016/s0197-4580(02)00074-x [DOI] [PubMed] [Google Scholar]
- Yin F., Sancheti H., Patil I., Cadenas E. (2016). Energy metabolism and inflammation in brain aging and Alzheimer’s disease. Free Radic. Biol. Med. 100 108–122. 10.1016/j.freeradbiomed.2016.04.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon I. S., Chen E., Busse T., Repetto E., Lakshmana M. K., Koo E. H., et al. (2007). Low-density lipoprotein receptor-related protein promotes amyloid precursor protein trafficking to lipid rafts in the endocytic pathway. FASEB J. 21 2742–2752. 10.1096/fj.07-8114com [DOI] [PubMed] [Google Scholar]
- Yu Q., Fang D., Swerdlow R. H., Yu H., Chen J. X., Yan S. S. (2016). Antioxidants rescue mitochondrial transport in differentiated Alzheimer’s disease trans-mitochondrial cybrid cells. J. Alzheimers Dis. 54 679–690. 10.3233/jad-160532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel M., Tacal O. (2019). Trafficking and proteolytic processing of amyloid precursor protein and secretases in Alzheimer’s disease development: an up-to-date review. Eur. J. Pharmacol. 856:172415. 10.1016/j.ejphar.2019.172415 [DOI] [PubMed] [Google Scholar]
- Zandl-Lang M., Fanaee-Danesh E., Sun Y., Albrecher N. M., Gali C. C., Cancar I., et al. (2018). Regulatory effects of simvastatin and apoJ on APP processing and amyloid-beta clearance in blood-brain barrier endothelial cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1863 40–60. 10.1016/j.bbalip.2017.09.008 [DOI] [PubMed] [Google Scholar]
- Zatorre R. J., Fields R. D., Johansen-Berg H. (2012). Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 15 528–536. 10.1038/nn.3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wang K., Yang L., Liu R., Chu Y., Qin X., et al. (2018). Lipid metabolism in inflammation-related diseases. Analyst 143 4526–4536. 10.1039/c8an01046c [DOI] [PubMed] [Google Scholar]
- Zhang Y. W., Thompson R., Zhang H., Xu H. (2011). APP processing in Alzheimer’s disease. Mol. Brain 4:3 10.1186/1756-6606-4-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B. (2009). Natural antioxidants protect neurons in Alzheimer’s disease and Parkinson’s disease. Neurochem. Res. 34 630–638. 10.1007/s11064-008-9900-9 [DOI] [PubMed] [Google Scholar]
- Zhao N., Liu C. C., Qiao W., Bu G. (2018). Apolipoprotein E, receptors, and modulation of Alzheimer’s disease. Biol. Psychiatry 83 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q. F., Yu J. T., Tan M. S., Tan L. (2015). ABCA7 in Alzheimer’s disease. Mol. Neurobiol. 51 1008–1016. [DOI] [PubMed] [Google Scholar]
- Zhao R. Z., Jiang S., Zhang L., Yu Z. B. (2019). Mitochondrial electron transport chain, ROS generation and uncoupling (review). Int. J. Mol. Med. 44 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Zlokovic B. V. (2014). Blood-brain barrier: a dual life of MFSD2A? Neuron 82 728–730. 10.1016/j.neuron.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Nelson A. R., Betsholtz C., Zlokovic B. V. (2015). Establishment and dysfunction of the blood-brain barrier. Cell 163 1064–1078. 10.1016/j.cell.2015.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng C., Zhou X. W., Wang J. Z. (2016). The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-alpha, TGF-beta and IFN-gamma. Transl. Neurodegener. 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Peng D., Yuan X., Lv Z., Pang S., Jiang W., et al. (2014). APOE and APOC1 gene polymorphisms are associated with cognitive impairment progression in Chinese patients with late-onset Alzheimer’s disease. Neural. Regen. Res. 9 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Liu X., Nemeth D. P., DiSabato D. J., Witcher K. G., McKim D. B., et al. (2019). Interleukin-1 causes CNS inflammatory cytokine expression via endothelia-microglia bi-cellular signaling. Brain Behav. Immun. 81 292–304. 10.1016/j.bbi.2019.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu R., Liu X., He Z. (2018). Association between CLU gene rs11136000 polymorphism and Alzheimer’s disease: an updated meta-analysis. Neurol. Sci. 39 679–689. 10.1007/s10072-018-3259-8 [DOI] [PubMed] [Google Scholar]
- Zlokovic B. V. (2008). The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57 178–201. 10.1016/j.neuron.2008.01.003 [DOI] [PubMed] [Google Scholar]