This cohort study examines the associations of dietary scores for 4 healthy eating patterns with risk of incident cardiovascular disease.
Key Points
Question
Are there associations of different healthy eating patterns with long-term risk of cardiovascular disease?
Findings
In this cohort study of individuals from the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-up Study (165 794 women and 43 339 men) with up to 32 years of follow-up, greater adherence to various healthy eating patterns was associated with lower risk of cardiovascular disease. The associations between dietary scores and risk of cardiovascular disease were consistent across different subgroups.
Meaning
These findings support the recommendations of the 2015-2020 Dietary Guidelines for Americans that multiple healthy eating patterns can be adapted to individual food traditions and preferences.
Abstract
Importance
The 2015-2020 Dietary Guidelines for Americans recommend multiple healthy eating patterns. However, few studies have examined the associations of adherence to different dietary patterns with long-term risk of cardiovascular disease (CVD).
Objective
To examine the associations of dietary scores for 4 healthy eating patterns with risk of incident CVD.
Design, Setting, and Participants
Prospective cohort study of initially healthy women from the Nurses’ Health Study (NHS) (1984-2016) and the NHS II (1991-2017) and men from the Health Professionals Follow-up Study (HPFS) (1986-2012). The dates of analysis were July 25 to December 4, 2019.
Exposures
Healthy Eating Index–2015 (HEI-2015), Alternate Mediterranean Diet Score (AMED), Healthful Plant-Based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI).
Main Outcomes and Measures
Cardiovascular disease events, including fatal and nonfatal coronary heart disease (CHD) and stroke.
Results
The final study sample included 74 930 women in the NHS (mean [SD] baseline age, 50.2 [7.2] years), 90 864 women in the NHS II (mean [SD] baseline age, 36.1 [4.7] years), and 43 339 men in the HPFS (mean [SD] baseline age, 53.2 [9.6] years). During a total of 5 257 190 person-years of follow-up, 23 366 incident CVD cases were documented (18 092 CHD and 5687 stroke) (some individuals were diagnosed as having both CHD and stroke). Comparing the highest with the lowest quintiles, the pooled multivariable-adjusted hazard ratios (HRs) of CVD were 0.83 (95% CI, 0.79-0.86) for the HEI-2015, 0.83 (95% CI, 0.79-0.86) for the AMED, 0.86 (95% CI, 0.82-0.89) for the HPDI, and 0.79 (95% CI, 0.75-0.82) for the AHEI (P for trend <.001 for all). In addition, a 25-percentile higher dietary score was associated with 10% to 20% lower risk of CVD (pooled HR, 0.80 [95% CI, 0.77-0.83] for the HEI-2015; 0.90 [95% CI, 0.87-0.92] for the AMED; 0.86 [95% CI, 0.82-0.89] for the HPDI; and 0.81 [95% CI, 0.78-0.84] for the AHEI). These dietary scores were statistically significantly associated with lower risk of both CHD and stroke. In analyses stratified by race/ethnicity and other potential risk factors for CVD, the inverse associations between these scores and risk of CVD were consistent in most subgroups.
Conclusions and Relevance
In 3 large prospective cohorts with up to 32 years of follow-up, greater adherence to various healthy eating patterns was consistently associated with lower risk of CVD. These findings support the recommendations of the 2015-2020 Dietary Guidelines for Americans that multiple healthy eating patterns can be adapted to individual food traditions and preferences.
Introduction
Dietary modifications have been established as one of the most important strategies for population prevention of cardiovascular disease (CVD),1,2 the primary cause of death in the United States and worldwide.3,4 Multiple studies have evaluated the associations of individual nutrients or foods with CVD risk.5 However, nutrients and foods are not consumed in isolation but in numerous and multifaceted combinations. Therefore, approaches that combine various nutrients and foods into “dietary patterns” could reflect real-world dietary practices and integrate potentially interactive and cumulative associations of different dietary components.6,7 In addition, dietary patterns more closely mimic real-world scenarios of nutrient and food combinations, which facilitates translation of findings into dietary recommendations.8
The 2015-2020 Dietary Guidelines for Americans9 highlight a shift from focusing on individual nutrients or foods to emphasizing healthy eating patterns as a whole and recommend multiple healthy dietary patterns to provide dietary choices for all Americans with diverse cultural and personal food traditions or preferences. However, few studies10,11,12,13 have comprehensively examined whether adherence to different dietary patterns could be associated with lower risk of incident CVD. Therefore, using 3 large prospective cohorts with up to 32 years of follow-up with data on repeated measures of dietary habits, we derived dietary scores for 4 healthy dietary patterns, including the Healthy Eating Index–2015 (HEI-2015), Alternate Mediterranean Diet Score (AMED), Healthful Plant-Based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI). We then examined their associations with risk of CVD, including coronary heart disease (CHD) and stroke.
Methods
Study Population
Data used in this prospective cohort study were from the Nurses’ Health Study (NHS) (1984-2016), NHS II (1991-2017), and Health Professionals Follow-up Study (HPFS) (1986-2012). The dates of analysis were July 25 to December 4, 2019. The NHS is a prospective cohort study of 121 700 female registered nurses aged 30 to 55 years that began in 1976. The NHS II was established in 1989 and consists of 116 671 younger female registered nurses, aged 25 to 42 years. The HPFS is a prospective cohort study of 51 529 male health professionals aged 40 to 75 years that began in 1986. The follow-up rates in all 3 cohorts exceed 90%.14,15,16,17 The institutional review boards of Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital approved the study protocol. The return of the questionnaires was considered to imply informed consent.
For the present analysis, baseline was defined as the year when the diet was first assessed with a validated semiquantitative food frequency questionnaire (FFQ) with more than 110 items in the cohorts (1984 in the NHS, 1991 in the NHS II, and 1986 in the HPFS). Of the participants who completed the baseline FFQ (84 199 in the NHS, 97 813 in the NHS II, and 51 529 in the HPFS), we excluded those participants who reported CVD, cancer, or diabetes at baseline (8127 in the NHS, 3792 in the NHS II, and 6547 in the HPFS) because the diagnoses of these conditions might have led to changes in diet. We also excluded participants with missing age at baseline (45 in the NHS, 224 in the NHS II, and 27 in the HPFS) and those who had daily energy intakes less than 600 or greater than 3500 kcal for women (1097 in the NHS and 2951 in the NHS II) and less than 800 or greater than 4200 kcal for men (1616 in the HPFS).
Assessment of Dietary Scores
Dietary information was collected every 2 to 4 years.18 Participants were asked how often, on average, they consumed a standard portion size of each food in the past year. The frequency responses ranged from never or less than 1 time per month to at least 6 times per day. The reproducibility and validity of the FFQs have been described in detail elsewhere,18,19,20,21 showing reasonably good correlation between nutrients assessed by the FFQs and multiple weeks of food records or biomarkers of diet. Using the nutrient and food components, we calculated the HEI-2015, AMED, HPDI, and AHEI to measure adherence to different dietary patterns. These dietary scores have been evaluated in previous studies and have been widely applied in numerous epidemiological studies on diet patterns associated with the risk of chronic diseases.22,23,24,25 The components and scoring criteria in detail for each dietary score are summarized in the eAppendix and eTables 1-4 in the Supplement. The HEI-2015 included 13 components, with the total score ranging from 0 to 100. The AMED included 9 components, with the total score ranging from 9 to 45. The HPDI included 18 components, with the total score ranging from 18 to 90. The AHEI included 10 components, with the total score ranging from 0 to 100. Higher dietary scores represented greater adherence to individual healthy eating patterns.
Assessment of CVD
This study included incident cases of CVD, defined as fatal and nonfatal CHD (including nonfatal myocardial infarction and coronary artery bypass graft surgery) and fatal and nonfatal stroke. When a participant reported an incident event on each biennial questionnaire, permission was requested to examine medical records, which were reviewed by study investigators blinded to the participant’s risk factor status. Nonfatal myocardial infarction was confirmed according to the World Health Organization criteria,26 and nonfatal stroke was confirmed according to the National Survey of Stroke criteria.27 Information on coronary artery bypass graft surgery was based on unconfirmed self-reports.28 Death was identified from the next of kin, postal authorities, or a search of the National Death Index, and at least 98% of deaths could be ascertained in each cohort.29 Fatal CHD or stroke was defined as CHD or stroke listed as the cause of death on the death certificate.
Assessment of Covariates
Information on age, weight, physical activity, smoking status, multivitamin use, and aspirin use was assessed and updated every 2 to 4 years via the questionnaires throughout follow-up. Among women, information was also assessed on menopausal status, postmenopausal hormone use, and oral contraceptive use (NHS II only). Self-reported data on height and weight were used to calculate body mass index (BMI). Every 2 to 4 years, alcohol intake was updated on the FFQs. Information on Hispanic ethnicity was available in the NHS and the NHS II. If covariate information was missing, we imputed the mean values for continuous variables or used a missing indicator approach for categorical variables.
Statistical Analysis
This study calculated each individual’s person-years from the date of the return of the baseline questionnaire to the date of CVD diagnosis, death, or end of follow-up (June 2016 for the NHS, June 2017 for the NHS II, and January 2012 for the HPFS), whichever occurred first. We did not censor participants lost to active follow-up because fatal events were included in the outcomes.
The cumulative average dietary scores were calculated by averaging the repeated measurements to better represent long-term dietary habits and to minimize within-person variation. For instance, for the 1994-1995 risk set in the HPFS, dietary scores in 1986, 1990, and 1994 were averaged to estimate subsequent CVD risk. We stopped updating dietary scores on a report of incident cancer, diabetes, or angina because changes in diet after development of these conditions may confound the association between diet and chronic diseases. This study used Cox proportional hazards models with time-varying covariates and age as the underlying timescale, with stratification by calendar time (in 2-year intervals), to assess the association between the 4 dietary scores and the subsequent risk of CVD. The proportional hazards assumption was evaluated with a likelihood ratio test comparing the model with and without an interaction term between age and dietary scores. In multivariable analysis, we adjusted for the updated potential confounders, including age, race/ethnicity, BMI, physical activity, smoking status, alcohol intake, postmenopausal status and postmenopausal hormone use (NHS and NHS II), oral contraceptive use (NHS II), marital status, living alone or with others, family history of CHD, total energy intake, multivitamin use, and aspirin use. Tests for linear trend across quintiles were conducted by assigning a median value to each quintile of dietary score, producing a single continuous variable used in the model. All 3 cohorts had greater than 90% power to detect a hazard ratio (HR) of 0.90 with α = .05. In addition, a 25-percentile difference in each dietary score (25 points for the HEI-2015, 9 points for the AMED, 18 points for the HPDI, and 25 points for the AHEI) was calculated from the range of total dietary score (0-100 points for the HEI-2015, 9-45 points for the AMED, 18-90 points for the HPDI, and 0-100 points for the AHEI). Separate analyses were conducted for CVD, CHD, and stroke per a 25-percentile difference in each dietary score. All analyses were performed separately for each cohort and then were pooled with the use of fixed-effects meta-analysis with inverse-variance weighting. Heterogeneity was assessed with the I2 statistic, and low to moderate heterogeneity was observed (I2 < 60% for all). In addition, random-effects meta-analysis yielded similar results.
We conducted stratified analyses that were defined a priori by race/ethnicity (NHS and NHS II) and other potential risk modifiers, including age, sex, BMI, physical activity, smoking status, alcohol intake, menopausal status, multivitamin use, aspirin use, and history of hypertension and hypercholesterolemia. The interactions between covariates and the 4 dietary scores were examined using the likelihood ratio test. Given a large number of tests being performed for subgroup analyses, we adjusted the P value for multiple testing using Bonferroni correction, and statistical significance was set at P ≤ .001 (.05 ÷ [11 subgroups × 4 dietary scores]) to account for type I error. Several sensitivity analyses were performed to test the robustness of our findings. First, we did not include self-reported cases of coronary artery bypass graft surgery as an end point and reanalyzed the associations of the 4 dietary scores with the risk of total CVD and CHD. Second, to test whether our results were biased by selectively not updating dietary scores after an intermediate outcome, we continuously updated dietary scores until the end of follow-up. Third, instead of using repeated measures of dietary habits, we analyzed the associations of baseline dietary scores with the incidence of CVD. All analyses were performed with the SAS statistical package (version 9.4; SAS Institute Inc). All statistical tests were 2-sided, and P < .05 was considered to indicate statistical significance.
Results
The final study sample included 74 930 women in the NHS (mean [SD] baseline age, 50.2 [7.2] years), 90 864 women in the NHS II (mean [SD] baseline age, 36.1 [4.7] years), and 43 339 men in the HPFS (mean [SD] baseline age, 53.2 [9.6] years). Table 1 lists age and the age-adjusted characteristics of study participants at baseline according to quintiles of the 4 dietary scores. In all cohorts, participants with higher dietary scores tended to be older, have a lower BMI, be more likely to exercise, and be less likely to smoke (Table 1). Total energy intake was higher in participants with higher AMED but lower in those with higher HEI-2015, HPDI, and AHEI. The unadjusted Spearman rank correlation coefficients between the 4 dietary scores ranged from 0.35 to 0.75 (P < .001 for all), with the weakest correlation between the AMED and the HPDI and the strongest correlation between the HEI-2015 and the AHEI (eTable 5 in the Supplement).
Table 1. Baseline Characteristics of Participants According to Quintiles of the Healthy Eating Index–2015 (HEI-2015), Alternate Mediterranean Diet Score (AMED), Healthful Plant-Based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI)a.
| Variable | HEI-2015 quintile | AMED quintile | HPDI quintile | AHEI quintile | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 1 | 3 | 5 | 1 | 3 | 5 | 1 | 3 | 5 | |
| NHS (1984) | ||||||||||||
| No. of participants | 15 279 | 14 977 | 14 588 | 16 116 | 14 887 | 13 590 | 15 829 | 15 560 | 13 550 | 15 238 | 14 956 | 14 743 |
| Dietary score, mean (SD) | 50.8 (4.8) | 64.4 (1.3) | 75.5 (3.3) | 19.5 (2.2) | 27.0 (0.8) | 35.3 (2.2) | 44.6 (3.1) | 54.4 (1.1) | 65.4 (3.2) | 29.3 (3.5) | 42.0 (1.5) | 57.8 (5.8) |
| Age, mean (SD), y | 48.2 (7.0) | 50.0 (7.1) | 52.6 (6.8) | 48.8 (7.1) | 50.1 (7.1) | 51.7 (7.0) | 48.1 (7.0) | 50.2 (7.1) | 52.4 (6.8) | 48.4 (7.1) | 50.2 (7.1) | 52.1 (6.8) |
| Non-Hispanic white race/ethnicity | 97.9 | 97.7 | 97.4 | 98.0 | 98.0 | 97.4 | 98.4 | 97.7 | 97.4 | 98.7 | 97.8 | 96.5 |
| Body mass index, mean (SD)b | 25.1 (5.2) | 25.0 (4.6) | 24.4 (4.1) | 25.1 (5.0) | 24.9 (4.6) | 24.6 (4.3) | 25.4 (5.2) | 24.9 (4.6) | 24.2 (4.0) | 25.1 (5.0) | 25.0 (4.7) | 24.4 (4.2) |
| Physical activity, mean (SD), MET-h/wk | 9.5 (16.1) | 12.9 (18.2) | 17.3 (24.3) | 9.5 (15.1) | 12.6 (19.0) | 17.7 (23.8) | 10.4 (15.8) | 12.7 (19.1) | 16.9 (24.4) | 9.4 (14.2) | 12.7 (19.0) | 18.0 (25.8) |
| Never | ||||||||||||
| Smoker | 40.4 | 45.9 | 44.2 | 40.7 | 44.5 | 45.6 | 45.4 | 44.9 | 41.3 | 43.7 | 44.2 | 42.7 |
| Drinker | 36.6 | 29.4 | 27.7 | 42.7 | 29.5 | 20.2 | 31.2 | 30.2 | 30.4 | 34.4 | 29.8 | 29.7 |
| Premenopausal | 41.2 | 42.1 | 40.9 | 41.3 | 41.6 | 41.4 | 42.7 | 41.5 | 40.3 | 42.1 | 42.0 | 40.8 |
| Married | 65.1 | 70.0 | 70.1 | 64.9 | 69.6 | 71.2 | 67.9 | 69.0 | 69.6 | 68.7 | 68.9 | 68.5 |
| Live alone | 9.9 | 9.3 | 10.5 | 10.4 | 9.6 | 9.8 | 9.6 | 9.5 | 10.6 | 8.7 | 9.5 | 11.3 |
| Family history of MI | 25.2 | 24.7 | 25.8 | 25.4 | 25.1 | 26.1 | 24.4 | 24.9 | 26.3 | 25.0 | 25.3 | 25.8 |
| Total energy intake, mean (SD), kcal/d | 2029 (566) | 1746 (490) | 1454 (413) | 1524 (488) | 1727 (506) | 2019 (525) | 2079 (504) | 1721 (495) | 1445 (434) | 1936 (492) | 1730 (536) | 1580 (505) |
| Multivitamin use | 29.2 | 37.6 | 45.1 | 30.4 | 36.0 | 45.1 | 32.2 | 36.9 | 42.6 | 30.7 | 36.0 | 44.9 |
| Aspirin use | 71.3 | 71.5 | 68.3 | 69.9 | 71.4 | 71.0 | 72.8 | 71.4 | 68.2 | 72.7 | 71.3 | 68.0 |
| NHS II (1991) | ||||||||||||
| No. of participants | 18 190 | 18 213 | 18 086 | 20 381 | 17 915 | 15 951 | 16 975 | 14 430 | 17 868 | 18 278 | 18 159 | 18 105 |
| Dietary score, mean (SD) | 49.5 (4.9) | 64.1 (1.4) | 75.6 (3.3) | 19.5 (2.2) | 27.0 (0.8) | 35.4 (2.2) | 44.6 (3.2) | 55.0 (0.8) | 65.6 (3.4) | 30.0 (3.9) | 43.5 (1.6) | 59.2 (5.4) |
| Age, mean (SD), y | 35.5 (4.7) | 36.1 (4.6) | 36.8 (4.5) | 35.7 (4.8) | 36.0 (4.7) | 36.6 (4.5) | 35.0 (4.7) | 36.1 (4.7) | 37.0 (4.4) | 35.3 (4.8) | 36.1 (4.7) | 36.9 (4.5) |
| Non-Hispanic white race/ethnicity | 95.8 | 96.5 | 96.7 | 96.5 | 96.6 | 96.3 | 96.4 | 96.3 | 96.6 | 97.4 | 96.6 | 95.5 |
| Body mass index, mean (SD)b | 25.2 (6.2) | 24.5 (5.1) | 23.9 (4.5) | 25.2 (5.8) | 24.6 (5.2) | 23.8 (4.7) | 25.3 (6.1) | 24.6 (5.2) | 23.9 (4.5) | 25.2 (6.0) | 24.6 (5.1) | 23.9 (4.7) |
| Physical activity, mean (SD), MET-h/wk | 15.2 (22.9) | 20.3 (25.6) | 27.7 (32.5) | 15.2 (22.0) | 20.3 (26.7) | 29.0 (34.2) | 16.2 (21.6) | 20.2 (26.6) | 27.5 (33.6) | 14.3 (19.6) | 19.8 (25.3) | 30.0 (35.3) |
| Never | ||||||||||||
| Smoker | 63.8 | 67.2 | 65.0 | 64.4 | 66.5 | 65.0 | 68.6 | 66.1 | 62.1 | 66.9 | 66.5 | 63.7 |
| Drinker | 49.9 | 58.5 | 62.4 | 43.0 | 57.6 | 72.6 | 53.1 | 57.1 | 61.4 | 49.4 | 58.4 | 62.9 |
| Premenopausal | 96.2 | 96.5 | 96.5 | 96.4 | 96.3 | 96.7 | 96.6 | 96.4 | 96.3 | 96.3 | 96.6 | 96.6 |
| Married | 77.8 | 79.6 | 74.4 | 76.9 | 79.3 | 76.6 | 79.8 | 78.8 | 74.5 | 81.9 | 79.1 | 71.6 |
| Live alone | 8.2 | 7.5 | 9.7 | 8.8 | 7.5 | 8.3 | 7.15 | 7.6 | 9.7 | 6.2 | 7.7 | 10.9 |
| Family history of MI | 43.1 | 42.0 | 41.9 | 43.1 | 42.3 | 41.5 | 42.5 | 41.9 | 42.5 | 43.0 | 42.8 | 41.9 |
| Total energy intake, mean (SD), kcal/d | 2024 (580) | 1795 (524) | 1536 (441) | 1503 (485) | 1774 (504) | 2131 (526) | 2146 (520) | 1759 (513) | 1517 (456) | 1969 (521) | 1765 (550) | 1677 (528) |
| Multivitamin use | 37.6 | 44.0 | 50.0 | 36.2 | 44.0 | 52.2 | 41.4 | 43.5 | 46.5 | 38.9 | 43.8 | 49.0 |
| Aspirin use | 12.4 | 11.2 | 10.2 | 11.6 | 11.2 | 11.0 | 11.5 | 11.0 | 11.2 | 12.0 | 11.1 | 10.6 |
| HPFS (1986) | ||||||||||||
| No. of participants | 9076 | 8700 | 8072 | 9658 | 10 642 | 8709 | 9985 | 8803 | 7313 | 9072 | 8714 | 8167 |
| Dietary score, mean (SD) | 52.5 (5.1) | 67.1 (1.4) | 79.1 (3.2) | 19.3 (2.4) | 27.5 (1.1) | 35.7 (2.5) | 44.5 (3.2) | 54.5 (1.1) | 65.6 (3.3) | 32.2 (4.2) | 46.7 (1.6) | 62.6 (5.2) |
| Age, mean (SD), y | 51.5 (9.3) | 53.3 (9.6) | 55.1 (9.5) | 51.7 (9.4) | 53.4 (9.5) | 54.5 (9.6) | 51.0 (9.3) | 53.3 (9.6) | 55.4 (9.4) | 51.3 (9.3) | 53.3 (9.6) | 55.1 (9.5) |
| Non-Hispanic white race/ethnicity | 91.0 | 90.3 | 91.2 | 90.9 | 90.4 | 91.8 | 92.0 | 90.5 | 90.7 | 92.0 | 90.6 | 91.2 |
| Body mass index, mean (SD)b | 25.7 (3.5) | 25.6 (3.4) | 24.8 (3.0) | 25.8 (3.4) | 25.5 (3.2) | 25.0 (3.3) | 25.6 (3.4) | 25.6 (3.3) | 25.1 (3.2) | 25.8 (3.5) | 25.6 (3.3) | 25.0 (3.2) |
| Physical activity, mean (SD), MET-h/wk | 14.7 (23.1) | 19.1 (26.5) | 24.7 (31.8) | 13.6 (20.2) | 18.7 (26.3) | 25.6 (30.5) | 16.5 (23.6) | 18.1 (22.4) | 23.9 (31.7) | 14.1 (21.1) | 18.5 (24.7) | 25.0 (31.0) |
| Never | ||||||||||||
| Smoker | 45.0 | 50.8 | 54.9 | 46.0 | 50.1 | 53.1 | 51.2 | 50.0 | 47.9 | 46.4 | 51.1 | 52.4 |
| Drinker | 27.3 | 21.5 | 21.8 | 32.2 | 21.6 | 14.9 | 22.9 | 22.3 | 24.3 | 24.7 | 22.4 | 22.2 |
| Married | 88.5 | 91.4 | 89.7 | 88.5 | 90.5 | 90.9 | 89.6 | 90.1 | 90.3 | 89.9 | 90.4 | 90.4 |
| Live alone | 7.0 | 5.5 | 6.5 | 7.2 | 5.8 | 5.5 | 6.5 | 6.1 | 6.2 | 6.3 | 6.0 | 5.7 |
| Family history of MI | 29.6 | 32.4 | 34.9 | 30.0 | 32.3 | 34.2 | 30.5 | 31.3 | 34.8 | 30.0 | 32.2 | 34.9 |
| Total energy intake, mean (SD), kcal/d | 2290 (676) | 1982 (578) | 1700 (489) | 1769 (568) | 1983 (601) | 2270 (618) | 2331 (619) | 1943 (580) | 1705 (518) | 2119 (594) | 1981 (637) | 1895 (589) |
| Multivitamin use | 34.9 | 41.0 | 51.5 | 35.4 | 41.6 | 50.3 | 36.1 | 42.3 | 48.3 | 35.6 | 41.5 | 49.9 |
| Aspirin use | 27.4 | 26.5 | 26.2 | 25.4 | 28.0 | 28.1 | 26.9 | 26.7 | 27.3 | 27.4 | 26.5 | 25.9 |
Abbreviations: HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent task; MI, myocardial infarction; NHS, Nurses’ Health Study.
Data are percentages unless otherwise indicated. All variables, except age, are age standardized.
Calculated as weight in kilograms divided by height in meters squared.
During a total of 5 257 190 person-years of follow-up, 23 366 incident CVD cases were documented (18 092 CHD and 5687 stroke) (some individuals were diagnosed as having both CHD and stroke). After accounting for multiple potential confounding factors, the multivariable analyses showed statistically significant inverse associations across quintiles of the 4 dietary scores with risk of CVD in each cohort (eTable 6 in the Supplement). The tests for the proportional hazards assumption did not indicate a violation in any cohort. Comparing the highest with the lowest quintiles, the pooled multivariable-adjusted HRs of CVD were 0.83 (95% CI, 0.79-0.86) for the HEI-2015, 0.83 (95% CI, 0.79-0.86) for the AMED, 0.86 (95% CI, 0.82-0.89) for the HPDI, and 0.79 (95% CI, 0.75-0.82) for the AHEI (P for trend <.001 for all) (Table 2). In addition, a 25-percentile higher dietary score was associated with 10% to 20% lower risk of CVD (pooled HR, 0.80 [95% CI, 0.77-0.83] for the HEI-2015; 0.90 [95% CI, 0.87-0.92] for the AMED; 0.86 [95% CI, 0.82-0.89] for the HPDI; and 0.81 [95% CI, 0.78-0.84] for the AHEI) (Figure 1). We further examined the associations of dietary scores with CHD and stroke separately (Figure 1). For CHD, the pooled HRs per 25-percentile increment were 0.78 (95% CI, 0.74-0.82) for the HEI-2015, 0.89 (95% CI, 0.86-0.91) for the AMED, 0.84 (95% CI, 0.80-0.87) for the HPDI, and 0.79 (95% CI, 0.76-0.82) for the AHEI. For stroke, the pooled HRs per 25-percentile increment were 0.88 (95% CI, 0.81-0.96) for the HEI-2015, 0.90 (95% CI, 0.86-0.95) for the AMED, 0.92 (95% CI, 0.85-1.00) for the HPDI, and 0.90 (95% CI, 0.83-0.97) for the AHEI.
Table 2. Pooled Hazard Ratios of Cardiovascular Disease According to Quintiles of the Healthy Eating Index–2015 (HEI-2015), Alternate Mediterranean Diet Score (AMED), Healthful Plant-Based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI).
| Variablea | Quintile of dietary score | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| HEI-2015 | |||||
| Median score | |||||
| NHS | 55 | 62 | 67 | 71 | 76 |
| NHS II | 52 | 60 | 65 | 69 | 75 |
| HPFS | 55 | 63 | 68 | 72 | 78 |
| Deaths/person-years | 4864/1 023 862 | 4715/1 055 329 | 4734/1 065 029 | 4590/1 066 708 | 4463/1 046 262 |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.86 (0.83-0.90) | 0.81 (0.78-0.85) | 0.75 (0.72-0.78) | 0.68 (0.65-0.71) |
| Multivariable-adjusted HR (95% CI)b | 1 [Reference] | 0.93 (0.89-0.97) | 0.91 (0.87-0.95) | 0.86 (0.83-0.90) | 0.83 (0.79-0.86) |
| AMED | |||||
| Median score | |||||
| NHS | 20 | 24 | 27 | 30 | 34 |
| NHS II | 20 | 24 | 27 | 30 | 34 |
| HPFS | 20 | 24 | 27 | 31 | 35 |
| Deaths/person-years | 4858/1 034 832 | 4780/1 038 118 | 4839/1 076 122 | 4630/1 083 871 | 4259/1 024 246 |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.89 (0.85-0.92) | 0.83 (0.80-0.87) | 0.78 (0.75-0.82) | 0.71 (0.68-0.74) |
| Multivariable-adjusted HR (95% CI)b | 1 [Reference] | 0.94 (0.90-0.98) | 0.90 (0.87-0.94) | 0.88 (0.84-0.92) | 0.83 (0.79-0.86) |
| HPDI | |||||
| Median score | |||||
| NHS | 47 | 52 | 55 | 59 | 64 |
| NHS II | 47 | 52 | 55 | 59 | 64 |
| HPFS | 46 | 51 | 55 | 59 | 64 |
| Deaths/person-years | 4731/1 072 015 | 4829/1 109 660 | 4913/1 083 866 | 4740/1 055 658 | 4153/935 991 |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.92 (0.88-0.96) | 0.87 (0.83-0.90) | 0.85 (0.82-0.88) | 0.78 (0.75-0.81) |
| Multivariable-adjusted HR (95% CI)b | 1 [Reference] | 0.95 (0.91-0.99) | 0.91 (0.87-0.95) | 0.90 (0.86-0.94) | 0.86 (0.82-0.89) |
| AHEI | |||||
| Median score | |||||
| NHS | 34 | 41 | 46 | 51 | 59 |
| NHS II | 33 | 40 | 45 | 50 | 59 |
| HPFS | 35 | 42 | 48 | 53 | 61 |
| Deaths/person-years | 5110/1 063 578 | 4966/1 090 162 | 4840/1 077 328 | 4541/1 046 139 | 3909/979 984 |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.87 (0.84-0.90) | 0.82 (0.79-0.86) | 0.76 (0.73-0.79) | 0.68 (0.65-0.71) |
| Multivariable-adjusted HR (95% CI)b | 1 [Reference] | 0.91 (0.87-0.95) | 0.88 (0.85-0.92) | 0.84 (0.81-0.88) | 0.79 (0.75-0.82) |
Abbreviations: HPFS, Health Professionals Follow-up Study; HR, hazard ratio; NHS, Nurses’ Health Study.
P for trend <.001 for all age-adjusted HR rows and multivariable-adjusted HR rows.
Multivariable adjusted for age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, or Hispanic [NHS and NHS II only]), body mass index (calculated as weight in kilograms divided by height in meters squared [<21, 21-24.9, 25-29.9, 30-34.9, or ≥35]), physical activity (quintile), smoking status (never, former, or current [1-14, 15-24, or ≥25 cigarettes per day]), alcohol intake (0, 0.1-4.9, 5.0-14.9, 15.0-19.9, 20.0-29.9, or ≥30 g/d), menopausal status (premenopausal or postmenopausal [never, past, or current postmenopausal hormone use]), oral contraceptive use (never, past, or current [NHS II only]), marital status (married, divorced/separated/single, or widowed), living alone or with others (alone or not), family history of myocardial infarction (yes or no), total energy intake (quintile), multivitamin use (yes or no), and aspirin use (yes or no).
Figure 1. Hazard Ratios of Cardiovascular Disease (CVD), Coronary Heart Disease (CHD), and Stroke per 25-Percentile Increment in the 4 Dietary Scoresa.
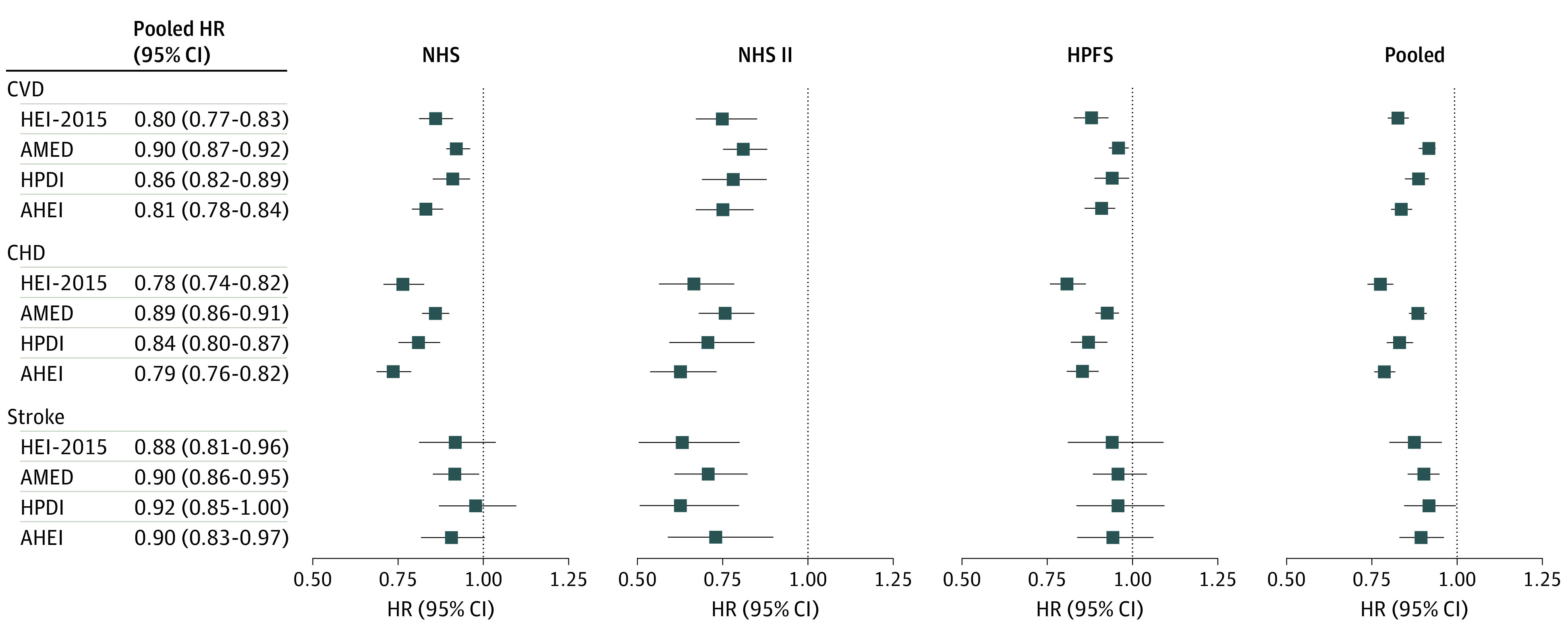
The multivariable analysis was adjusted for age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, or Hispanic [NHS and NHS II only]), body mass index (calculated as weight in kilograms divided by height in meters squared [<21, 21-24.9, 25-29.9, 30-34.9, or ≥35]), physical activity (quintile), smoking status (never, former, or current [1-14, 15-24, or ≥25 cigarettes per day]), alcohol intake (0, 0.1-4.9, 5.0-14.9, 15.0-19.9, 20.0-29.9, or ≥30 g/d), menopausal status (premenopausal or postmenopausal [never, past, or current postmenopausal hormone use]), oral contraceptive use (never, past, or current [NHS II only]), marital status (married, divorced/separated/single, or widowed), living alone or with others (alone or not), family history of myocardial infarction (yes or no), total energy intake (quintile), multivitamin use (yes or no), and aspirin use (yes or no). Results were pooled using the fixed-effect model with inverse-variance weighting. AHEI indicates Alternate Healthy Eating Index; AMED, Alternate Mediterranean Diet Score; HEI-2015, Healthy Eating Index–2015; HPDI, Healthful Plant-Based Diet Index; HPFS, Health Professionals Follow-up Study; HR, hazard ratio; and NHS, Nurses’ Health Study.
aCalculated per 25-percentile increment in the 4 dietary scores (25 points for the HEI-2015, 9 points for the AMED, 18 points for the HPDI, and 25 points for the AHEI).
Among women in the NHS and the NHS II, differences in dietary scores were observed by race/ethnicity over time (eFigure 1 and eFigure 2 in the Supplement). For example, the HEI-2015 was higher in Hispanic individuals than in other racial/ethnic groups. However, in stratified analyses by race/ethnicity, the associations between dietary patterns and risk of CVD did not differ statistically significantly; the HRs of CVD per 25-percentile difference in the HEI-2015 were 0.68 (95% CI, 0.64-0.72) in non-Hispanic white individuals and 0.71 (95% CI, 0.55-0.91) in overall minority racial and ethnic groups (P for interaction = .74) (Table 3). Similarly, there were no statistically significant differences in the associations of the other 3 dietary scores with CVD risk across racial/ethnic groups (Table 3). Consistent inverse associations were also observed in other subgroup analyses (Figure 2). In sensitivity analyses, the associations remained similar when we excluded self-reported cases of coronary artery bypass graft surgery as an end point (eFigure 3 in the Supplement). The inverse associations per 25-percentile increase in each dietary score with CVD risk were attenuated but remained statistically significant when the baseline dietary data were used and the dietary scores were continuously updated until the end of follow-up (eFigure 4 in the Supplement).
Table 3. Pooled Hazard Ratios of Cardiovascular Disease According to the 4 Dietary Scores Across Racial/Ethnic Groups in the Nurses’ Health Study and Nurses’ Health Study IIa.
| Variableb | Cases/person-years | HR (95% CI) | P value for interactionc |
|---|---|---|---|
| HEI-2015 | |||
| Non-Hispanic white | 11 793/4 068 772 | 0.68 (0.64-0.72) | NA |
| Minority racial/ethnic group | 798/288 861 | 0.71 (0.55-0.91) | .74 |
| Non-Hispanic black | 144/52 982 | 0.62 (0.33-1.15) | .77 |
| Hispanic | 92/55 008 | 0.48 (0.22-1.07) | .39 |
| Other | 562/180 871 | 0.77 (0.58-1.03) | .41 |
| AMED | |||
| Non-Hispanic white | 11 793/4 068 772 | 0.80 (0.77-0.84) | NA |
| Minority racial/ethnic group | 798/288 861 | 0.79 (0.68-0.92) | .88 |
| Non-Hispanic black | 144/52 982 | 0.86 (0.59-1.27) | .71 |
| Hispanic | 92/55 008 | 0.74 (0.46-1.20) | .75 |
| Other | 562/180 871 | 0.78 (0.66-0.93) | .78 |
| HPDI | |||
| Non-Hispanic white | 11 793/4 068 772 | 0.78 (0.74-0.83) | NA |
| Minority racial/ethnic group | 798/288 861 | 0.70 (0.55-0.89) | .39 |
| Non-Hispanic black | 144/52 982 | 0.76 (0.41-1.40) | .93 |
| Hispanic | 92/55 008 | 0.82 (0.40-1.68) | .89 |
| Other | 562/180 871 | 0.67 (0.51-0.89) | .30 |
| AHEI | |||
| Non-Hispanic white | 11 793/4 068 772 | 0.70 (0.66-0.74) | NA |
| Minority racial/ethnic group | 798/288 861 | 0.72 (0.58-0.88) | .80 |
| Non-Hispanic black | 144/52 982 | 0.78 (0.45-1.34) | .70 |
| Hispanic | 92/55 008 | 0.50 (0.26-0.98) | .32 |
| Other | 562/180 871 | 0.74 (0.58-0.94) | .66 |
Abbreviations: AHEI, Alternate Healthy Eating Index; AMED, Alternate Mediterranean Diet Score; HEI-2015, Healthy Eating Index–2015; HPDI, Healthful Plant-Based Diet Index; HR, hazard ratio; NA, not applicable.
Results from the Nurses’ Health Study (NHS) and the NHS II were pooled using the fixed-effect model with inverse-variance weighting per 25-percentile increment in the 4 dietary scores (25 points for the HEI-2015, 9 points for the AMED, 18 points for the HPDI, and 25 points for the AHEI). Multivariable analysis adjusted for age, body mass index, physical activity, smoking status, alcohol intake, menopausal status, oral contraceptive use (NHS II only), calendar year marital status, living alone or with others, family history of myocardial infarction, total energy intake, multivitamin use, aspirin use, and family history of diabetes.
Non-Hispanic white includes non-Hispanic women with southern European/Mediterranean ancestry, Scandinavian ancestry, and other Caucasian ancestry. Minority racial/ethnic groups include non-Hispanic black, Hispanic, and other racial/ethnic women. Other includes women not classified as non-Hispanic white, non-Hispanic black, or Hispanic, such as Asian and American Indian.
Interaction between non-Hispanic white and total minority racial/ethnic group or individual racial/ethnic group.
Figure 2. Pooled Hazard Ratios of Cardiovascular Disease According to the 4 Dietary Scores Across Subgroups.
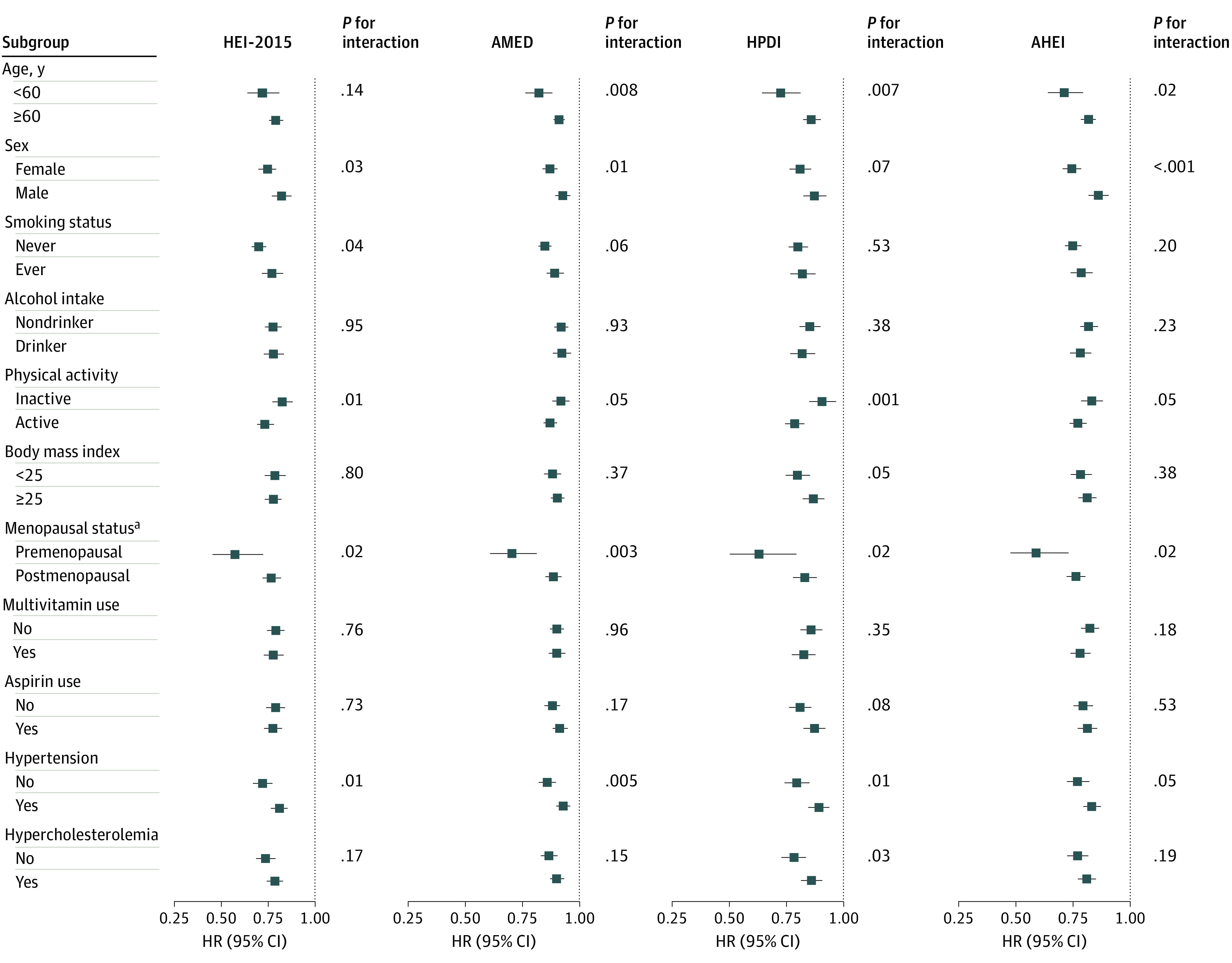
The multivariable analysis was adjusted for age (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black, or Hispanic [Nurses’ Health Study and Nurses’ Health Study II only]), body mass index (calculated as weight in kilograms divided by height in meters squared [<21, 21-24.9, 25-29.9, 30-34.9, or ≥35]), physical activity (quintile), smoking status (never, former, or current [1-14, 15-24, or ≥25 cigarettes per day]), alcohol intake (0, 0.1-4.9, 5.0-14.9, 15.0-19.9, 20.0-29.9, or ≥30 g/d), menopausal status (premenopausal or postmenopausal [never, past, or current postmenopausal hormone use]), oral contraceptive use (never, past, or current [Nurses’ Health Study II only]), marital status (married, divorced/separated/single, or widowed), living alone or with others (alone or not), family history of myocardial infarction (yes or no), total energy intake (quintile), multivitamin use (yes or no), and aspirin use (yes or no). Results were pooled using the fixed-effect model with inverse-variance weighting. AHEI indicates Alternate Healthy Eating Index; AMED, Alternate Mediterranean Diet Score; HEI-2015, Healthy Eating Index–2015; HPDI, Healthful Plant-Based Diet Index; and HR, hazard ratio.
aCalculated per 25-percentile increment in the 4 dietary scores (25 points for the HEI-2015, 9 points for the AMED, 18 points for the HPDI, and 25 points for the AHEI). These results are from the Nurses’ Health Study and Nurses’ Health Study II.
Discussion
In 3 large prospective cohorts, greater adherence to various dietary patterns was associated with lower CVD risk. Similar inverse associations were observed for incident CHD and stroke. The associations between dietary scores and risk of CVD were consistent across racial/ethnic and other subgroups.
Our results are broadly consistent with previous studies23,24,25 that reported inverse associations between healthy dietary scores and risk of incident CVD. To date, only 1 study30 has examined the associations between the HEI-2015 and risk of CVD among US adults. With data from the Atherosclerosis Risk in Communities Study, Hu and colleagues30 simultaneously investigated the associations of the HEI-2015 and other dietary scores with CVD risk and found similar inverse associations in direction and magnitude for the HEI-2015, AMED, and AHEI. The Dietary Patterns Methods Project also found that better diet quality (as assessed by the HEI-2010, AMED, AHEI, and other scores) was associated with 18% to 26% lower risk of all-cause and CVD mortality.12,13 These dietary scores share several components, including higher intake of whole grains, vegetables, fruits, legumes, and nuts,31 all of which have been associated with lower risk of CVD.32,33,34,35 The high correlations in the indexes, except between the AMED and the HPDI, also suggested a high degree of agreement. However, these dietary scores also differ in some specific components and scoring methods. None of the indexes were perfectly correlated, indicating that each dietary score represents a unique combination of dietary constituents. Our findings provide support for the recommendations of the current Dietary Guidelines for Americans9 that it is not necessary to conform to a single dietary plan to achieve healthy eating.
To facilitate a comparison of the associations between the 4 dietary scores of healthy eating patterns, we reported a 25-percentile increment in each dietary score as a common unit that was associated with a statistically significantly lower CVD risk (20% for the HEI-2015, 10% for the AMED, 14% for the HPDI, and 19% for the AHEI). The HEI-2015 and the AHEI appear to have stronger inverse associations with CVD than the AMED and the HPDI. These differences were mainly attributable to associations with CHD (22% for the HEI-2015, 11% for the AMED, 16% for the HPDI, and 21% for the AHEI) because all 4 dietary scores showed similar associations with risk of stroke (12% for the HEI-2015, 10% for the AMED, 8% for the HPDI, and 10% for the AHEI). These results were in line with previous findings of stronger associations for improved adherence to the AHEI than the AMED with subsequent risk of CHD incidence and CVD.36 Although the 4 dietary scores share several common healthy components, there are some differences in the specific components included in each dietary score, which may partly explain the differences in their associations with CVD risk. For example, fish intake, which has been associated with lower risk of CVD,37,38 was included as a positive contributor (higher intake receiving higher score) in the AMED but as a negative contributor (higher intake receiving lower score) in the HPDI. In addition, compared with the categorization scales used in the calculations of the AMED and the HPDI, the continuous scales used in the calculations of the HEI-2015 and the AHEI may have the advantage of capturing stronger associations. Because of the differences in the scoring systems, it is difficult to conclude that 1 healthy eating pattern is superior to another.
Previous studies39,40 with nationally representative data indicated differences across population groups in overall dietary patterns. In our study, the magnitude of the inverse associations between dietary scores and CVD risk was similar across racial/ethnic groups of women. These findings are consistent with those observed in the Multiethnic Cohort13 and the Atherosclerosis Risk in Communities Study,30 which showed a reduction in risk of CVD mortality according to higher HEI, AMED, and AHEI among several racial/ethnic groups. In addition, statistically significant inverse associations were consistently observed between these dietary scores and risk of CVD in subgroup analyses stratified by multiple potential risk factors for CVD, such as lifestyle factors (including physical activity, smoking status, and alcohol intake), aspirin use, and baseline prevalence of hypertension and hypercholesterolemia. We believe that the consistent associations in several sensitivity analyses also highlighted the robustness of our findings. These results support the notion that individuals could choose different healthy eating patterns based on their personal food traditions or preferences for prevention of CVD.
Strengths and Limitations
The strengths of this study include the prospective design, large sample size, long-term follow-up with a high retention rate, and repeated assessments of diet and lifestyle. However, several limitations should be noted. First, because the dietary assessment was based on self-reports, measurement error and misclassification were inevitable. However, the FFQs used in the study were extensively validated against diet records and biomarkers. Furthermore, repeated measures of dietary habits during the follow-up allowed us to calculate the cumulative average dietary scores, which reflect long-term dietary habits and reduce measurement errors. Second, although we controlled for several repeated measurements of lifestyle factors, the possibility of residual and unmeasured confounding could not be completely ruled out because of the observational nature of the study. Third, the dietary scores used in this study could not fully represent the healthy eating patterns. However, these scores were adapted in nutritional epidemiology studies22,23,24,25 to evaluate dietary adherence to healthy dietary patterns in free-living populations. Fourth, the generalizability of our findings may be limited because participants in our study were all health professionals and predominantly non-Hispanic white individuals. However, our findings are broadly consistent across different racial/ethnic groups and with results from other populations.
Conclusions
Greater adherence to various healthy eating patterns was consistently associated with lower risk of CVD. Our findings support the 2015-2020 Dietary Guidelines for Americans, which recommend multiple healthy eating patterns for individuals to adapt according to personal food traditions and preferences.
eAppendix. Assessment of Dietary Scores
eTable 1. Healthy Eating Index 2015 Components and Criteria for Scoring
eTable 2. Alternate Mediterranean Diet Score Components and Criteria for Scoring
eTable 3. Healthful Plant-Based Diet Index Components and Criteria for Scoring
eTable 4. Alternate Healthy Eating Index Components and Criteria for Scoring
eTable 5. Correlation Matrix of Four Dietary Scores
eTable 6. Hazard Ratios of Cardiovascular Disease According to Quintiles of the Healthy Eating Index-2015, Alternate Mediterranean Diet Score, Healthful Plant-Based Diet Index, and Alternate Healthy Eating Index in Individual Cohorts
eFigure 1. Trends in Healthy Eating Index-2015, Alternate Mediterranean Diet Score, Healthful Plant-Based Diet Index, and Alternate Healthy Eating Index by Race/Ethnicity in NHS
eFigure 2. Trends in Healthy Eating Index-2015, Alternate Mediterranean Diet Score, Healthful Plant-Based Diet Index, and Alternate Healthy Eating Index by Race/Ethnicity in NHSII
eFigure 3. Hazard Ratios of Cardiovascular Disease and Coronary Heart Disease According to Four Dietary Scores by Excluding Self-reported Cases of Coronary Artery Bypass Graft Surgery as an Endpoint
eFigure 4. Hazard Ratios of Cardiovascular Disease Based on Baseline and Updated Dietary Scores
eReferences.
References
- 1.Yu E, Malik VS, Hu FB. Cardiovascular disease prevention by diet modification: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72(8):914-926. doi: 10.1016/j.jacc.2018.02.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187-225. doi: 10.1161/CIRCULATIONAHA.115.018585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics–2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 4.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet. 2019;393(10190):e44]. Lancet. 2018;392(10159):1789-1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2017 Diet Collaborators Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958-1972. doi: 10.1016/S0140-6736(19)30041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3-9. doi: 10.1097/00041433-200202000-00002 [DOI] [PubMed] [Google Scholar]
- 7.Schulze MB, Hu FB. Dietary patterns and risk of hypertension, type 2 diabetes mellitus, and coronary heart disease. Curr Atheroscler Rep. 2002;4(6):462-467. doi: 10.1007/s11883-002-0051-1 [DOI] [PubMed] [Google Scholar]
- 8.Cespedes EM, Hu FB. Dietary patterns: from nutritional epidemiologic analysis to national guidelines. Am J Clin Nutr. 2015;101(5):899-900. doi: 10.3945/ajcn.115.110213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Department of Health and Human Services; US Department of Agriculture Dietary Guidelines for Americans: 2015-2020. 8th ed. December 2015. Accessed January 2, 2020. https://health.gov/dietaryguidelines/2015/guidelines/
- 10.Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health. Circ Res. 2019;124(5):779-798. doi: 10.1161/CIRCRESAHA.118.313348 [DOI] [PubMed] [Google Scholar]
- 11.George SM, Ballard-Barbash R, Manson JE, et al. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol. 2014;180(6):616-625. doi: 10.1093/aje/kwu173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reedy J, Krebs-Smith SM, Miller PE, et al. Higher diet quality is associated with decreased risk of all-cause, cardiovascular disease, and cancer mortality among older adults. J Nutr. 2014;144(6):881-889. doi: 10.3945/jn.113.189407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harmon BE, Boushey CJ, Shvetsov YB, et al. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr. 2015;101(3):587-597. doi: 10.3945/ajcn.114.090688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790-797. doi: 10.1056/NEJMoa010492 [DOI] [PubMed] [Google Scholar]
- 15.Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the three Nurses’ Health Studies. Am J Public Health. 2016;106(9):1573-1581. doi: 10.2105/AJPH.2016.303338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464-468. doi: 10.1016/0140-6736(91)90542-W [DOI] [PubMed] [Google Scholar]
- 17.Willett WC, Green A, Stampfer MJ, et al. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med. 1987;317(21):1303-1309. doi: 10.1056/NEJM198711193172102 [DOI] [PubMed] [Google Scholar]
- 18.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51-65. doi: 10.1093/oxfordjournals.aje.a114086 [DOI] [PubMed] [Google Scholar]
- 19.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18(4):858-867. doi: 10.1093/ije/18.4.858 [DOI] [PubMed] [Google Scholar]
- 20.Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570-584. doi: 10.1093/aje/kww104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan C, Spiegelman D, Rimm EB, et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187(5):1051-1063. doi: 10.1093/aje/kwx328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reedy J, Lerman JL, Krebs-Smith SM, et al. Evaluation of the Healthy Eating Index–2015 [published correction appears in J Acad Nutr Diet. 2019;119(10):1759]. J Acad Nutr Diet. 2018;118(9):1622-1633. doi: 10.1016/j.jand.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women [published correction appears in Circulation. 2009;119(12):e379]. Circulation. 2009;119(8):1093-1100. doi: 10.1161/CIRCULATIONAHA.108.816736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satija A, Bhupathiraju SN, Spiegelman D, et al. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J Am Coll Cardiol. 2017;70(4):411-422. doi: 10.1016/j.jacc.2017.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009-1018. doi: 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendis S, Thygesen K, Kuulasmaa K, et al. ; Writing Group on Behalf of the Participating Experts of the WHO Consultation for Revision of WHO Definition of Myocardial Infarction . World Health Organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol. 2011;40(1):139-146. doi: 10.1093/ije/dyq165 [DOI] [PubMed] [Google Scholar]
- 27.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke: clinical findings. Stroke. 1981;12(2, pt 2)(suppl 1):I13-I44. [PubMed] [Google Scholar]
- 28.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123(5):894-900. doi: 10.1093/oxfordjournals.aje.a114319 [DOI] [PubMed] [Google Scholar]
- 29.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837-839. doi: 10.1093/oxfordjournals.aje.a113804 [DOI] [PubMed] [Google Scholar]
- 30.Hu EA, Steffen LM, Coresh J, Appel LJ, Rebholz CM. Adherence to the Healthy Eating Index–2015 and other dietary patterns may reduce risk of cardiovascular disease, cardiovascular mortality, and all-cause mortality. J Nutr. 2020;150(2):312-321. doi: 10.1093/jn/nxz218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118(1):74-100.e11. doi: 10.1016/j.jand.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 32.Aune D, Keum N, Giovannucci E, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. doi: 10.1136/bmj.i2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. doi: 10.1136/bmj.g4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guasch-Ferré M, Liu X, Malik VS, et al. Nut consumption and risk of cardiovascular disease. J Am Coll Cardiol. 2017;70(20):2519-2532. doi: 10.1016/j.jacc.2017.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller V, Mente A, Dehghan M, et al. ; Prospective Urban Rural Epidemiology (PURE) Study Investigators . Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390(10107):2037-2049. doi: 10.1016/S0140-6736(17)32253-5 [DOI] [PubMed] [Google Scholar]
- 36.Sotos-Prieto M, Bhupathiraju SN, Mattei J, et al. Changes in diet quality scores and risk of cardiovascular disease among US men and women. Circulation. 2015;132(23):2212-2219. doi: 10.1161/CIRCULATIONAHA.115.017158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chowdhury R, Stevens S, Gorman D, et al. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ. 2012;345:e6698. doi: 10.1136/bmj.e6698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jayedi A, Zargar MS, Shab-Bidar S. Fish consumption and risk of myocardial infarction: a systematic review and dose-response meta-analysis suggests a regional difference. Nutr Res. 2019;62:1-12. doi: 10.1016/j.nutres.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 39.Shan Z, Rehm CD, Rogers G, et al. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999-2016. JAMA. 2019;322(12):1178-1187. doi: 10.1001/jama.2019.13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang DD, Leung CW, Li Y, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174(10):1587-1595. doi: 10.1001/jamainternmed.2014.3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Assessment of Dietary Scores
eTable 1. Healthy Eating Index 2015 Components and Criteria for Scoring
eTable 2. Alternate Mediterranean Diet Score Components and Criteria for Scoring
eTable 3. Healthful Plant-Based Diet Index Components and Criteria for Scoring
eTable 4. Alternate Healthy Eating Index Components and Criteria for Scoring
eTable 5. Correlation Matrix of Four Dietary Scores
eTable 6. Hazard Ratios of Cardiovascular Disease According to Quintiles of the Healthy Eating Index-2015, Alternate Mediterranean Diet Score, Healthful Plant-Based Diet Index, and Alternate Healthy Eating Index in Individual Cohorts
eFigure 1. Trends in Healthy Eating Index-2015, Alternate Mediterranean Diet Score, Healthful Plant-Based Diet Index, and Alternate Healthy Eating Index by Race/Ethnicity in NHS
eFigure 2. Trends in Healthy Eating Index-2015, Alternate Mediterranean Diet Score, Healthful Plant-Based Diet Index, and Alternate Healthy Eating Index by Race/Ethnicity in NHSII
eFigure 3. Hazard Ratios of Cardiovascular Disease and Coronary Heart Disease According to Four Dietary Scores by Excluding Self-reported Cases of Coronary Artery Bypass Graft Surgery as an Endpoint
eFigure 4. Hazard Ratios of Cardiovascular Disease Based on Baseline and Updated Dietary Scores
eReferences.


