Abstract
Background
About 50% of non-small cell lung cancer (NSCLC) patients have metastatic disease at initial diagnosis, which limits their treatment options and, consequently, the 5-year survival rate (15%). Immune checkpoint inhibitors (ICI), either alone or in combination with chemotherapy, have become standard of care (SOC) for most good performance status patients. However, most patients will not obtain long-term benefit and new treatment strategies are therefore needed. We previously demonstrated clinical safety of the tumour-selective immunocytokine L19-IL2, consisting of the anti-ED-B scFv L19 antibody coupled to IL2, combined with stereotactic ablative radiotherapy (SABR).
Methods
This investigator-initiated, multicentric, randomised controlled open-label phase II clinical trial will test the hypothesis that the combination of SABR and L19-IL2 increases progression free survival (PFS) in patients with limited metastatic NSCLC. One hundred twenty-six patients will be stratified according to their metastatic load (oligo-metastatic: ≤5 or poly-metastatic: 6 to 10) and randomised to the experimental-arm (E-arm) or the control-arm (C-arm). The C-arm will receive SOC, according to the local protocol. E-arm oligo-metastatic patients will receive SABR to all lesions followed by L19-IL2 therapy; radiotherapy for poly-metastatic patients consists of irradiation of one (symptomatic) to a maximum of 5 lesions (including ICI in both arms if this is the SOC). The accrual period will be 2.5-years, starting after the first centre is initiated and active. Primary endpoint is PFS at 1.5-years based on blinded radiological review, and secondary endpoints are overall survival, toxicity, quality of life and abscopal response. Associative biomarker studies, immune monitoring, CT-based radiomics, stool collection, iRECIST and tumour growth rate will be performed.
Discussion
The combination of SABR with or without ICI and the immunocytokine L19-IL2 will be tested as 1st, 2nd or 3rd line treatment in stage IV NSCLC patients in 14 centres located in 6 countries. This bimodal and trimodal treatment approach is based on the direct cytotoxic effect of radiotherapy, the tumour selective immunocytokine L19-IL2, the abscopal effect observed distant from the irradiated metastatic site(s) and the memory effect. The first results are expected end 2023.
Trial registration
ImmunoSABR Protocol Code: NL67629.068.18; EudraCT: 2018–002583-11; Clinicaltrials.gov: NCT03705403; ISRCTN ID: ISRCTN49817477; Date of registration: 03-April-2019.
Keywords: Immunotherapy, L19-IL2, Anti-PD-L1, Anti-PD-1, Radiotherapy, SABR, Phase 2, NSCLC, Stage IV, Multicentre
Background
Lung cancer is the leading cause of cancer-related death worldwide [1, 2], with an estimated mortality of 3.1 million in 2040 [3]. Non-small cell lung cancer (NSCLC) is the most common lung cancer type (85% of cases) and half of these patients have metastatic disease at initial diagnosis [4]. Immune checkpoint inhibitors (ICI), either alone for selected patients (Programmed Cell Death-ligand 1 (PD-L1) ≥ 50% EU and PD-L1 ≥ 1% in USA), or in combination with chemotherapy, have become the standard of care (SOC) for most good performance status (PS) patients with metastatic disease [5]. Metastasized NSCLC patients with oligo-metastatic disease showed a benefit in progression free survival (PFS) when local ablative therapy was added to systemic therapy (chemotherapy ([6–8]) or tyrosine kinase inhibitor ([7, 8])); one trial also demonstrated an improved overall survival (OS) [7]. Oligometastatic disease is usually defined as “limited metastasis” (NCCN guideline [9]), up to three metastases (ESMO guideline [5]) or up to five metastases (European Organization for the Research and Treatment of Cancer (EORTC) lung cancer group consensus definition [10–12] and most clinical trials [13–15]). These guidelines advise to treat these patients with a combination of systemic therapy and local ablative therapy, preferably within a clinical trial.
However, most patients with oligo-metastatic disease will not obtain long-term benefit due to resistance mechanisms. Several immunotherapy-based treatments have been developed to overcome this resistance and increase the long-term benefit. Most immunotherapies act on escape mechanisms like impaired antigen presentation, a decreased neoantigen repertoire and T-cell function, insensitivity to immune effector molecules, the tumour microenvironment and co-opting of alternative immune checkpoints [16]. In context of double ICI treatments, so far, the results in NSCLC are disappointing. The randomized phase III Checkmate 227 (NCT02477826) trial (nivolumab-ipilimumab) demonstrated prolonged 2-year OS compared to chemotherapy alone, independent of PD-L1 expression [17], albeit with a comparator arm (platinum doublet chemotherapy) which is now considered inferior [18]. On the other hand, the phase III MYSTIC (NCT02453282) and NEPTUNE (NCT02542293) trials (both durvalumab-tremelimumab) were reported negative for their primary endpoints [19, 20]. One option to improve OS is the addition of radiotherapy to ICI, as radiation might act synergistically with ICI on the immune system [21–23]. The added value of ICI has already been shown in stage III NSCLC, in which adjuvant durvalumab after concurrent chemoradiotherapy in patients with good PS resulted in an improved median PFS and OS, as well as an improved 3-year survival (66.3% versus 43.5%) [24, 25]. In stage IV NSCLC, early signals of efficacy have been observed. Albeit negative in the intention to treat population, the PEMBRO-RT phase II trial (NCT02492568) showed that combining pembrolizumab with stereotactic ablative radiotherapy (SABR) significantly increased the OS (12 months: 55% vs 36%, 18 months: 48% vs 28%) of PD-L1-negative NSCLC patients without increasing toxicity compared to pembrolizumab alone [26].
As the combination of radiotherapy and ICI still does not result in long-term benefit for most patients, the addition of new immunotherapy modalities to radiation should be explored. Based on personal communication, we know that L19-interleukin 2 (IL2) (darleukin; 15 MIO IU) has shown promising results in a phase I trial (NCT02086721) when combined with SABR in oligo-metastatic cancer patients. Two NSCLC patients (33%) are still without progression, respectively 3 and 4 years after treatment completion. Importantly, the combination did not result in grade 3 or higher toxicity. Based on these promising phase I data, we are performing a randomised phase II trial.
Rationale
IL2 is a cytokine that plays an essential role in the activation of the innate and specific immune response [27, 28]. Unfortunately, the use of systemic administered IL2 is limited due to its serious acute toxicity profile which require intensive inpatient management [29]. Nevertheless, coupling IL2 to a tumour specific antibody, like L19, reduces the IL2 concentration in blood and increases the concentration in the tumour [30, 31], resulting in only low grade toxicity [32]. L19-IL2 is a fully human immunocytokine consisting of the human cytokine IL2 fused to the single-chain (scFv) human antibody fragment L19 targeting extra-domain B (ED-B) explicitly [33]. ED-B of fibronectin (FN) is a type III-FN domain, which can be inserted in the protein molecule by a mechanism of alternative splicing [34]. ED-Bcontaining fibronectin is a well-characterised marker of neo-angiogenesis and is expressed in the extracellular matrix surrounding newly formed blood vessels. As such, it is abundantly expressed around the vasculature of a variety of human cancers [35–37], while it is usually not present in healthy adult human tissues. It is known that in NSCLC, ED-B is expressed in the vast majority (~ 82%) of tumours [31, 38]. Darleukin represents a targeted form of IL2, capable of selective accumulation at the tumour site [39]. As such, this drug has the potential to boost selective anti-tumoural immune responses in patients with NSCLC. We have demonstrated in pre-clinical studies that L19-IL2, especially in combination with radiotherapy, results in improved disease control in lung and colorectal carcinoma models [21, 23]. Therapeutic responses were dependent on the irradiation dose, the target expression levels and are causally related to the presence of CD8+ cytotoxic T cells [21]. Best responses were found when administrating L19-IL2 after radiotherapy [40, 41]. Additionally, we have shown that this combination leads to improved local tumour control as well as tumour regression outside of the radiotherapy field and induces an immune-mediated memory effect preventing tumour-relapse after reinjection of tumour cells [23]. Furthermore, in pre-clinical Lewis Lung Carcinoma (LLC) models, we have observed that the synergy of radiotherapy with L19-IL2 is superior to the combination of radiotherapy with ICI. Interestingly, combining RT, L19-IL2 and ICI resulted in curative responses for this low immunogenic tumour model, which were associated with increased infiltration of NK and CD8+ T cells without any signs of toxicity [42]. Several trials with stage IV malignancies found 22.5 Mio IU as recommended L19-IL2 dose as monotherapy [32, 43, 44] or combined with dacarbazine [43, 44]. We recently received, based on personal communication, the safety results of L19-IL2 in the phase I trial (NCT02086721) given 15 Mio IU after SABR, as the combination of 22.5 Mio IU L19-IL2 with SABR resulted in more grade 3 toxicity. Therefore, the 15 Mio IU will be used in the current phase II trial protocol: ImmunoSABR (NCT03705403), designed to test the activity and efficacy of L19-IL2 (+ ICI treatment if SOC) following SABR in oligo-metastatic and conventional radiotherapy in poly-metastatic (maximum 10 metastatic sites) in NSCLC patients. A maximum of 10 lesions was defined, as patients without widespread metastases have low or acceptable toxicity when irradiating maximum 5 out of 10 lesions, and still have an active immune system to gain the best response on L19-IL2.
Methods/design
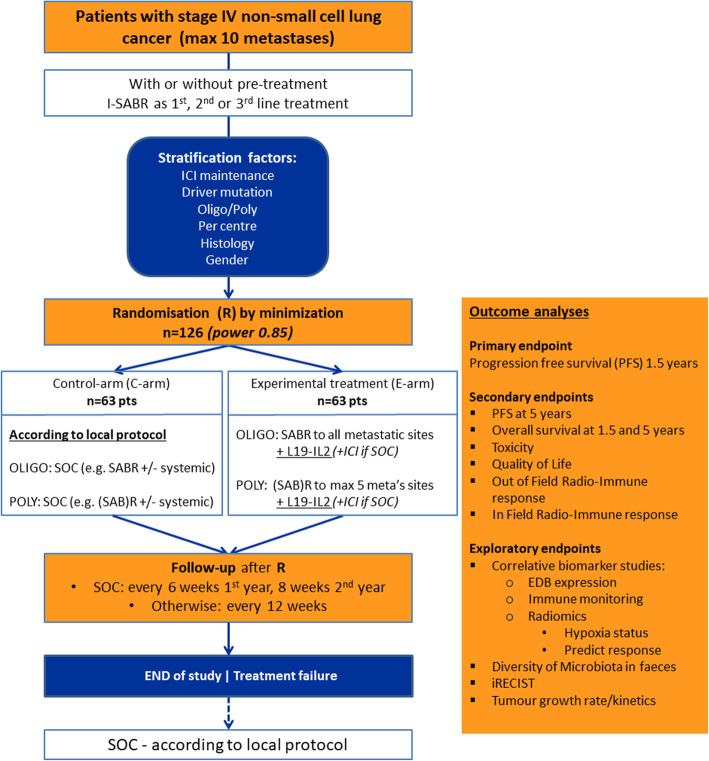
ImmunoSABR is a multicentre, randomised controlled open-label phase II clinical trial testing the hypothesis that the combination of (SAB)R and the immunocytokine L19-IL2 will increase the PFS in patients with limited metastatic NSCLC compared to SOC (including ICI in both arms if this is the SOC). After randomisation by minimisation, patients will be assigned either to the experimental arm (E-arm) or the control arm (C-arm) as described in Fig. 1.
Fig. 1.
Study design of ImmunoSABR phase II trial
Study population
The trial will consist of one cohort of 126 adult patients with stage IV NSCLC. ImmunoSABR will act as 1st, 2nd or maximum 3rd line treatment, and will be different for patients with oligo-metastatic NSCLC (max 5 metastases [11]) and patients with poly-metastatic NSCLC (6–10 metastases). As SOC and OS differ between oligo-metastatic and poly-metastatic patients [7], oligo versus poly will be used as a stratification factor for randomisation. Also, ICI maintenance treatment, centre, histology (squamous versus non-squamous), gender and driver mutation (equally divide previously TKI treated patients with oncogenic drivers) will be added as stratification factors. Patients will be randomised into 2 arms, using randomisation by minimisation (ALEA software): E-arm or C-arm. The algorithm uses a random factor to prevent the possibility of upfront knowledge about the randomisation result. All data collected for this trial will be entered in and stored on an online clinical data management platform containing pre-structured electronic case report forms per patient visit.
Sample size calculation
The expected 1.5-year PFS is minimally 15% in the C-arm and at least 35% in the E-arm. A sample size of 116 patients (58 patients per treatment arm) is needed to show this difference (20%) in PFS, using a Log-Rank test with a two-sided alpha of 0.05 and power of 85%. Patients will be evenly divided over the two arms during an accrual period of 2.5 years. Assuming a drop-out rate of 10%, a total of 126 patients (63 per arm) need to be included.
Study treatment
A complete overview of the inclusion and exclusion criteria is described in supplementary Table 1. Main inclusion criteria are NSCLC, ≤10 metastatic lesions at baseline, world health organization preformance status (WHO PS) 0–1 and adequate bone marrow, liver and renal function. Most important exclusion criteria are a systemic infection, pregnancy, history of organ transplant, autoimmune disease and impaired/uncontrolled cardiovascular function. All potentially eligible patients have to sign the informed consent (ICF) (see supplementary Table 2 for the ICF) and will subsequently be examined with respect to medical history, physical examination, WHO PS, vital signs, height and body weight, general and viral blood tests (e.g. Creatinine, total protein, albumin, ALAT, ASAT, alkalic phosphatase, γ-GT, bilirubin, Hb, WBC and differentiation, platelets, PT and aPTT, HIV, HBV and HCV) and a pregnancy test in women of child-bearing potential. The participants fulfilling the afore-mentioned criteria will undergo baseline imaging (e.g. fluordeoxyglucose Positron Emission Tomography/Computed Tomography (FDG PET/CT) and brain Magnetic Resonance Imaging (MRI)) as performed in respective institutes before being randomised (t = 0) into the C-arm or E-arm, see Figs. 1 and 2.
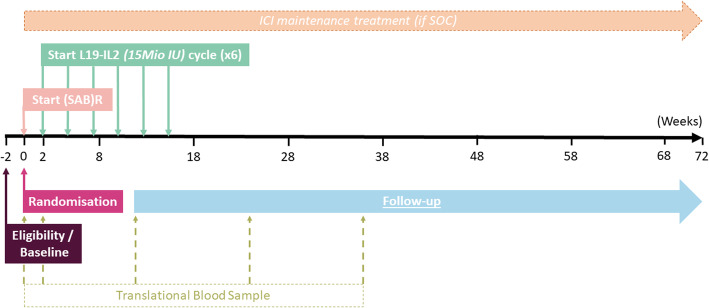
The participants fulfilling the eligibility criteria will undergo a SOC baseline imaging, translational blood sampling, and other baseline requirements before being randomised (t=0) into the C-arm or E-arm. Shortly after randomisation, the first fraction of (SAB)R will be planned. L19-IL2 will start within 72 hours after last fraction. Every patient will receive 6 cycles, which consist each of 21 days. Simultaneously with these cycles, ICI will be administered if SOC. Follow-up for a period of 1.5 years after randomisation.
Fig. 2.
Timeline of study treatment and follow-up
The SOC protocol will be applied for the patients within the C-arm, while having the same time points as the E-arm for translational samples and follow-up. Patients are allowed to have had front-line systemic therapy and/or treatment to the primary tumour prior to the trial, however, this is not obligatory. The participants in the E-arm will start with (SAB)R to maximum 5 metastatic lesions, according to a dose-fractionation regimen chosen by the research physician’s discretion. However, some study guidelines need to be taken into account: the minimum dose per fraction to the metastasis should be 7Gy for oligo-metastatic and 4Gy for poly-metastatic disease, with a relative biological effectiveness of in total 30-60Gy for oligo-metastatic and 8-35Gy for poly-metastatic disease, within every day or every second day between fractions [45–49]. The dose constraints for various critical organs suggested by the AAPM task group 101 should be respected [50]. To decrease the risk of radiation-induced lymphopenia, irradiation of large vessels (e.g. aorta), spleen and the heart should be avoided whenever possible [51]. Furthermore, irradiation scheduling should be organised to allow the first administration of L19-IL2 within 72 h after the last irradiation for patients randomised to the E-arm.
The participants in the E-arm will receive 6 cycles of L19-IL2 (and ICI maintenance treatment before every first L19-IL2 infusion of every cycle if SOC). Figure 2 and the supplementary Table 3 show a detailed timeline overview. One cycle L19-IL2 consists of three administrations of L19-IL2 at days 1, 3 and 5. Cycles are repeated every 3 weeks. At day 3 of each cycle, the quality of life (QoL) questionnaires EQ-5D, QLQ-C30 v3.0 and QLQ-LC13 will be filled out. Furthermore, before every L19-IL2 infusion, multiple tests will be performed, e.g. recording concurrent medication, blood tests, blood pressure, temperature, heart rate, WHO PS (0–1) and scoring adverse events with CTCAE v5.
During the first 3 h L19-IL2 infusion cycle, blood pressure, temperature and heart rate are assessed every 30 min, at the end of the administration and subsequently after 30 min, 1 h and 2 h following the administration. If no significant changes are seen in blood pressure, temperature and heart rate during the first 2 cycles, only end of infusion assessment will be required for subsequent cycles.
Follow-up
In each centre, both arms will receive follow-up on the same time points, based on their SOC (every 6 weeks versus every 12 weeks). For both arms, there will be follow-up CT-scan(s) with IV contrast (slice thickness of ≤ 3 mm), WHO PS and Quality of Life questionnaires to be completed at least every 12 weeks. Also, translational blood samples are taken simultaneously with the planned CT-scans in week 12, 24 and 36. Blinded local radiological review will be performed for every follow-up scan to assess tumour response using RECIST (version 1.1, [52]) and exploratory iRECIST [53].
Response criteria are based on a set of measurable lesions identified at baseline as target lesions for RECIST evaluation, and followed until disease progression. If all lesions are irradiated, RECIST is less accurate. In case of doubt, we propose a panel discussion. Follow-up and treatment for patients progressing will be done according to the local standard protocols. Patients will be followed every 3 months to record OS, toxicity, QoL and adverse events for 1.5 years. The PFS and OS will be collected for a total of 5 years.
Study parameters and endpoints
The main objective of the trial is to test if the combination of (SAB)R and the immunocytokine L19-IL2 (and ICI in case of SOC) will result in an increased PFS rate at 1.5 year after randomisation compared to the SOC, based on blinded radiological review (RECIST 1.1). The secondary objectives will be an assessment of 5-years PFS, 1.5-year and 5-year OS and 1.5-year CTCAE v5 toxicity grade, QoL, Out of Field Radio-Immune response (target(s) for RECIST analysis 1.1) and In-Field Radio-Immune response (non-target(s) for RECIST analysis). Exploratory analyses will be performed to investigate biomarkers (e.g. ED-B expression on tumour biopsies), diversity of the microbiota of faeces, CT radiomics and the changes of immunologic markers in repeated peripheral blood samples. The statistical methods are described in supplementary Table 4.
Nature and extent of the burden and risks associated with participation, benefit and group relatedness
ICI, either alone for selected patients or in combination with chemotherapy, have become the SOC for most good PS patients with metastatic disease [5]. A specific population of metastasized NSCLC patients, namely those with oligo-metastatic disease, can obtain long-term survival with the addition of local ablative therapy [6, 7]. However, most patients (oligo- and poly-metastatic) do not obtain long-term survival and new treatment strategies are therefore needed. The ultimate aim of the combination of (SAB)R and L19-IL2 (with ICI if SOC) is to prolong PFS by inducing an immune response which would be able to control this systemic disease. Known/potential risks additional to the SOC treatment include:
L19-IL2 related side effects
Fever with chills, fatigue, nausea, vomiting, asthenia, (peripheral) oedema, skin rash, hyperhidrosis, chest pain, pruritus, elevated serum creatinine levels and pain at the tumour site. Signs of mild capillary leak syndrome and hypotension were found at the dose-limiting dosage level that is not administered in the current trial [32, 43, 54–58]. Most of the severe adverse events were seen in studies using a higher dose of L19-IL2 (22.5–30 Mio IU) compared to our phase I study dose of 15 Mio IU where no grade 3 or more toxicities occurred. We therefore expect, based on our experiences in the phase I trial, that the incidence and intensity of the adverse events are lower in the current phase II trial. Experiences from our phase 1 trial combined with published IL2-management guidelines [59–61] will be used as guidance when side effects occur.
(SAB)R related side effects
(SAB)R side effects are dependent on the location of the irradiated site. Toxicity is considered very low and the risk of radiation-induced lymphopenia will be decreased by avoiding, whenever conceivable, the irradiation of large vessels (e.g. aorta), spleen and the heart. Possible side effects are nausea, vomiting, diarrhoea for abdominal sites, local pain, discomfort and neuritis for soft tissue and bony sites and dyspnoea, cough, radiation pneumonitis and rib fractures for thoracic sites. Studies evaluating SABR to mixed oligo-metastatic sites report grade III toxicity rates below 12%, like bowel strictures, fatigue, dyspnoea, pain but also treatment-related death (e.g. radiation pneumonitis, pulmonary abscess) [62]. There might be an increased risk of immune-related toxicity for those patients receiving L19-IL2 and radiotherapy in combination with standard treatment pembrolizumab. Immune-related toxicity from standard of care pembrolizumab is rare, but can be serious and life-threatening [63]. Knowing that metastatic NSCLC is a mortal disease in the short-term, the potential burden seems proportional to the potential gain.
An independent Data Safety Monitoring Board (DSMB) has been established and will monitor patient recruitment, adverse effects reporting and data quality during the trial. A first safety analysis is planned after the first 15 patients treated with triple therapy. All Serious Adverse Events and Suspected Unexpected Serious Adverse Reactions (SUSAR) will be reported to the manufacture and following country specific and European Medicine Agency (EMA) guidelines.
Translational research
The translational research will focus on immunological marker evaluation oriented towards both finding prognostic and predictive biomarkers that will indicate sensitivity to (SAB)R/L19-IL2 treatment. Also, blood (e.g. neutrophil counts [64], lymphocyte counts [65–67], LIPI score [68], circulating tumour DNA [69], cell-free tumour DNA [70] and immune cell subsets), tissue (e.g. ED-B expression, somatic mutations [71], non-synonymous mutation load [72, 73], PD(L)-1 expression, neutrophil and macrophage type 2 levels, Immune cell subsets) and faeces (diversity in microbiota) markers related to the importance of immunological checkpoints for the L19-IL2 interaction will be assessed. The samples will be collected (only in case of patient informed consent) and stored for 15 years, supplementary Table 5.
Radiomics is one of the most promising techniques that have the potential to improve cancer prognostication [65, 67]. In Radiomics, large numbers (1500+) of quantitative features such as tumour image intensity, (multi-scale) texture and the shape and size of a tumour are extracted from standard medical images (CT, PET, MRI) using (semi)automatic software. Radiomics enables identification of quantitative imaging biomarkers (QIBs) to quantify and classify tumour phenotypes and other disease parameters non-invasively. An exploratory correlative analysis will be conducted on the available PET-CT / diagnostic and simulation CT / MRI data obtained prior and during this trial. All the scans will be de-identified and collected every year and at the end of the trial [74]. We have three main hypotheses: (A) Studies suggest that the mutational landscape in tumours influences the response to immunotherapy, as higher non-synonymous mutation burden in tumours was associated with an improved objective response, durable clinical benefit and PFS [46, 47]. However, analysing these mutations in tissues is currently a complex and laborious process. We hypothesise that these phenotypic differences in a tumour can be characterised non-invasively by applying radiomics; the heterogeneous tumours will be more sensitive to immunotherapy than less heterogeneous tumours. (B) Hypoxia immunological niche: Hypoxia is a negative prognostic factor possibly causing resistance to immunotherapy [75]. We hypothesise that radiomics can be applied to assess tumour hypoxia and thus can be used as a predictive factor for treatment response. (C) We hypothesise that radiomics can more accurately assess the progression of the disease than RECIST.
The tumour growth rate (TGR) before and during treatment and variation per period will give insights in tumour development but also more information regarding treatment specific response. All available scans prior to (incl diagnostic scan) and during this trial will be used to visualise the TGR and determine hyperprogressive disease, based on [76].
Discussion
A recent phase I study revealed the safety of the bimodal treatment but also indicated favorable treatment response in 2/6 patients being still progression-free for respectively 3 and 4 years. ImmunoSABR, a randomised phase II study, is the first randomised, investigator-initiated and intention to treat clinical trial that generates a reliable evidence base to change clinical practice from palliative to a curative treatment strategy in patients with limited metastatic NSCLC.
Supplementary information
Additional file 1: Supplementary Table 1. In- and exclusion criteria.
Additional file 2: Supplementary Table 2. The informed consent form used for ImmunoSABR phase 2 trial.
Additional file 3: Supplementary Table 3. Schematic overview of the study and timeline.
Additional file 4: Supplementary Table 4. Statistical analysis study parameters and subgroup analyses.
Additional file 5: Supplementary Table 5. Overview of the translational research samples.
Acknowledgements
The authors would like to thank the other members of the ImmunoSABR team: Floor Franssen, Natascha Thieme, Rianne Herben, Simone Moorman, Mieke Denys, Vanessa Parrein and Serena Bettarini, and acknowledge support from Janita van Timmeren, Evelyn de Jong and Cecile Wolfs.
Abbreviations
- AAMP
American Association of Physicists in Medicine
- AE
Adverse Event
- aPD-(L)1
Anti-programmed Cell Death (Ligand) 1
- ALAT
Alanine Amino Transferase
- ASAT
Aspartate Amino Transferase
- aPTT
activated partial thromboplastin time
- C-arm
Control arm
- CTCAE v5
Common Terminology Criteria for Adverse Events version 5
- DSMB
Data Safety Monitoring Board
- E-arm
Experimental arm
- ED-B
Extra Domain B (of fibronectin)
- EGFR
Epidermal Growth Factor
- EORTC
-
QLQ-C30
European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire C30
- EQ5D
EuroQol- 5 Dimension
- Fx
Fractions
- γ-GT
gamma- glutamytransferase
- Gy
Gray
- Hb
hemoglobin
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- HIV
Human immunodeficiency virus
- IFRI
In Field Radio-Immune
- iRECIST
immuno- Response Evaluation Criteria in Solid Tumors
- LC13
13 Lung cancer specific questions
- LVEF
Left ventricular ejection fraction
- MIO
IU million international unit
- NSCLC
Non-Small Cell Lung Cancer
- OFRI
Out of Field Radio-Immune
- OS
Overall Survival
- PCM
Paracetamol
- PI
Principal investigator
- PFS
Progression Free Survival
- PT
Prothrombin time
- PTV
Planning Target Volume
- QoL
Quality of Life
- RECIST
Response Evaluation Criteria in Solid Tumors
- RT
Radiotherapy
- SABR
Stereotactic Ablative Body Radiotherapy
- SAE
Serious Adverse Event
- SOC
Standard of Care
- SOP
Standard Operating Procedure
- SUSAR
Suspected Unexpected Serious Adverse Reaction
- TGR
Tumour growth rate
- WBC
White Blood Count
- WHO PS
World health organization preformance status
Authors’ contributions
PL, ET, DdR, DN, LD and EL were the initiators for the H2020 grant application and pre-designed the study. RL, CO, AMD, EL, ET, DR and PL were responsible for writing, adapting, and submitting the study protocol. AMD, FE, CH, ET, CD, YL, MJ, LH, JB, XG, VV, CB, AA, DP and PB are principal investigators (PI) of this trial in the respective participating institutes and have as such contributed in optimising and finalising the study protocol. RL and LD wrote this manuscript. All authors read and approved the final manuscript.
Funding
The authors would like to acknowledge financial support from the European Program H2020 (ImmunoSABR – n° 733008). Authors also acknowledge financial support from ERC advanced grant (ERC-ADG-2015, n° 694812 - Hypoximmuno). None of these funding bodies have and had influence on this study, protocol or manuscript. Their role was to make this one of a kind multicentric, investigator initiated, phase II trial possible.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
The study will be conducted according to the ICH Harmonised Tripartite Guideline for Good Clinical Practice and has been approved by the medical ethics committee of the Maastricht University Medical Centrum (MUMC)/Maastricht University (no: 18–068). All patients will receive oral and written information about the study. They are given sufficient time to consider participation before the informed consent will be signed. The sponsor/investigator has a liability insurance which is in accordance with article 7 of the WMO in the Netherlands. This insurance provides cover for damage to research subjects through injury or death caused by the study. The insurance applies to the damage that becomes apparent during the study or within 4 years after the end of the study.
Consent for publication
Not applicable.
Competing interests
PL reports, inside the submitted work, grants/sponsored research from Oncoradiomics, ptTheragnostic/DNAmito; advisor (SAB)/presentor fee from Oncoradiomics, Varian medical and Elekta. Furthermore, he is the inventor of two patents on radiomics and one non-patentable invention (software), licensed to Oncoradiomics and has (minority) shares in the company Oncoradiomics and MedC2. LH: none related to the current manuscript, outside of current manuscript: research funding Roche, Boehringer Ingelheim, AstraZeneca (all institution), the advisory board: Boehringer, BMS, Lilly, Takeda, Pfizer, MSD (all institution), travel reimbursement: Roche, BMS (self); mentorship program with key opinion leaders: funded by AstraZeneca; fees for educational webinars: Quadia (self). Philogen S.P.A supplies L19-IL2 (darleukin). Philogen had only influence on the drug-related topics in the study protocol, e.g. description, preparation, labelling, and justification of administration and dosage. DN: reports he is a co-Founder and Board Member in Philogen. DdR: none related to the current manuscript, outside of the current manuscript: advisory board of Bristol-Myers-Squibb, Astra Zeneca, Roche/Genentech, Merck/Pfizer and Celgene. Research grants have been received from Bristol-Myers Squibb and Boehringer Ingelheim. All income from the advisory board and from the research grants went integrally to the institution. AD: none related to the current manuscript, outside of the current manuscript: advisory board BMS, MSD, Roche, Eli Lilly, Takeda, Pfizer, Boehringer Ingelheim (all institution). Research grant: BMS (institution). YL: personal fees from Astra Zeneca, personal fees from RayStation, outside the submitted work. AA reports grants and other benefits from Merck, EMD and Fibrogen, and other benefits from BMS, BioMedX and Roche, outside the submitted work. All other authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12885-020-07055-1.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.The Global Cancer Observatory [https://gco.iarc.fr/]. Accessed 12 Feb 2020.
- 4.Zhang S, Bai X, Shan F. The progress and confusion of anti-PD1/PD-L1 immunotherapy for patients with advanced non-small cell lung cancer. Int Immunopharmacol. 2020;80:106247. doi: 10.1016/j.intimp.2020.106247. [DOI] [PubMed] [Google Scholar]
- 5.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 6.Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung Cancer: a Phase 2 randomized clinical trial. JAMA Oncol. 2018;4(1):e173501. doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez DR, Tang C, Zhang J, Blumenschein GR, Hernandez M, Lee JJ, Ye R, Palma DA, Louie AV, Camidge DR, et al. Local consolidative therapy Vs. maintenance therapy or observation for patients with Oligometastatic non–small-cell lung Cancer: long-term results of a multi-institutional, Phase II, randomized study. J Clin Oncol. 2019;37(18):1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrieta O, Barron F, Maldonado F, Cabrera L, Corona-Cruz JF, Blake M, Ramirez-Tirado LA, Zatarain-Barron ZL, Cardona AF, Garcia O, et al. Radical consolidative treatment provides a clinical benefit and long-term survival in patients with synchronous oligometastatic non-small cell lung cancer: a phase II study. Lung Cancer. 2019;130:67–75. doi: 10.1016/j.lungcan.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Ettinger DS, Wood DE, Aggarwal C, Aisner DL, Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, et al. NCCN guidelines insights: non-small cell lung Cancer, version 1.2020. J Natl Compr Cancer Netw. 2019;17(12):1464–1472. doi: 10.6004/jnccn.2019.0059. [DOI] [PubMed] [Google Scholar]
- 10.Levy A, Hendriks LEL, Berghmans T, Faivre-Finn C, GiajLevra M, GiajLevra N, Hasan B, Pochesci A, Girard N, Greillier L, et al. EORTC lung Cancer group survey on the definition of NSCLC synchronous oligometastatic disease. Eur J Cancer. 2019;122:109–114. doi: 10.1016/j.ejca.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Dingemans AC, Hendriks LEL, Berghmans T, Levy A, Hasan B, Faivre-Finn C, Giaj-Levra M, Giaj-Levra N, Girard N, Greillier L, et al. Definition of synchronous Oligometastatic non-small cell lung Cancer-a consensus report. J Thorac Oncol. 2019;14(12):2109–2119. doi: 10.1016/j.jtho.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Hendriks LEL, Dooms C, Berghmans T, Novello S, Levy A, De Ruysscher D, Hasan B, Giaj Levra M, Giaj Levra N, Besse B, et al. Defining oligometastatic non-small cell lung cancer: a simulated multidisciplinary expert opinion. Eur J Cancer. 2019;123:28–35. doi: 10.1016/j.ejca.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Giaj-Levra N, Giaj-Levra M, Durieux V, Novello S, Besse B, Hasan B, Hendriks LE, Levy A, Dingemans AC, Berghmans T, et al. Defining synchronous Oligometastatic non-small cell lung Cancer: a systematic review. J Thorac Oncol. 2019;14(12):2053–2061. doi: 10.1016/j.jtho.2019.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Giaj-Levra N, Giaj Levra M, Berghmans T, Novello S, Hendriks LE, Levy A, Besse B, Dingemans AC. Oligometastatic non-small cell lung cancer (NSCLC): does number of metastasis matter? Lung Cancer. 2020;139:216–218. doi: 10.1016/j.lungcan.2019.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, Mendez Romero A, Nevens D, Palma D, Park C, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Syn NL, Teng MWL, Mok TSK, Soo RA. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18(12):e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 17.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, et al. Nivolumab plus Ipilimumab in advanced non-small-cell lung Cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 18.Remon J, Passiglia F, Ahn MJ, Barlesi F, Forde PM, Garon EB, Gettinger S, Goldberg SB, Herbst RS, Horn L, et al. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol. 2020;15(6):914–47. [DOI] [PubMed]
- 19.Two analyses from the MYSTIC study show improved survival supporting the use of front-line durvalumab over chemotherapy in metastatic NSCLC. [https://www.esmo.org/Oncology-News/First-line-Durvalumab-Improves-Survival-Compared-to-Chemotherapy-in-Metastatic-NSCLC]. Accessed 11 Jan 2020.
- 20.Update on the Phase III NEPTUNE trial of Imfinzi plus tremelimumab in Stage IV non-small cell lung cancer. [https://www.astrazeneca.com/media-centre/press-releases/2019/update-on-the-phase-iii-neptune-trial-of-imfinzi-plus-tremelimumab-in-stage-iv-non-small-cell-lung-cancer-21082019.html]. Accessed 11 Jan 2020.
- 21.Zegers CM, Rekers NH, Quaden DH, Lieuwes NG, Yaromina A, Germeraad WT, Wieten L, Biessen EA, Boon L, Neri D, et al. Radiotherapy combined with the immunocytokine L19-IL2 provides long-lasting antitumor effects. Clin Cancer Res. 2015;21(5):1151–1160. doi: 10.1158/1078-0432.CCR-14-2676. [DOI] [PubMed] [Google Scholar]
- 22.Rekers NH, Zegers CM, Yaromina A, Lieuwes NG, Biemans R, Senden-Gijsbers BL, Losen M, Van Limbergen EJ, Germeraad WT, Neri D, et al. Combination of radiotherapy with the immunocytokine L19-IL2: additive effect in a NK cell dependent tumour model. Radiother Oncol. 2015;116(3):438–442. doi: 10.1016/j.radonc.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Rekers NH, Olivo Pimentel V, Yaromina A, Lieuwes NG, Biemans R, Zegers CML, Germeraad WTV, Van Limbergen EJ, Neri D, Dubois LJ, et al. The immunocytokine L19-IL2: an interplay between radiotherapy and long-lasting systemic anti-tumour immune responses. Oncoimmunology. 2018;7(4):e1414119. doi: 10.1080/2162402X.2017.1414119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, Cho BC, et al. Three-year overall survival with Durvalumab after Chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol. 2020;15(2):288–293. doi: 10.1016/j.jtho.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et al. Overall survival with Durvalumab after Chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 26.Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, Dumoulin DW, Bahce I, Niemeijer AN, de Langen AJ, et al. Effect of Pembrolizumab after stereotactic body radiotherapy vs Pembrolizumab alone on tumor response in patients with advanced non-small cell lung Cancer: results of the PEMBRO-RT Phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Heuvel MM, Verheij M, Boshuizen R, Belderbos J, Dingemans AM, De Ruysscher D, Laurent J, Tighe R, Haanen J, Quaratino S. NHS-IL2 combined with radiotherapy: preclinical rationale and phase Ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J Transl Med. 2015;13:32. doi: 10.1186/s12967-015-0397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phase I Clinical Study Combining L19-IL2 With SABR in Patients With Oligometastatic Solid Tumor (L19-IL2). [https://clinicaltrials.gov/ct2/show/NCT02086721]. Accessed 07 Apr 2020.
- 29.Buchbinder EI, Dutcher JP, Daniels GA, Curti BD, Patel SP, Holtan SG, Miletello GP, Fishman MN, Gonzalez R, Clark JI, et al. Therapy with high-dose Interleukin-2 (HD IL-2) in metastatic melanoma and renal cell carcinoma following PD1 or PDL1 inhibition. J Immunother Cancer. 2019;7(1):49. doi: 10.1186/s40425-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.List T, Neri D. Immunocytokines: a review of molecules in clinical development for cancer therapy. Clin Pharmacol. 2013;5:29–45. doi: 10.2147/CPAA.S49231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santimaria M, Moscatelli G, Viale GL, Giovannoni L, Neri G, Viti F, Leprini A, Borsi L, Castellani P, Zardi L, et al. Immunoscintigraphic detection of the ED-B domain of fibronectin, a marker of angiogenesis, in patients with cancer. Clin Cancer Res. 2003;9(2):571–579. [PubMed] [Google Scholar]
- 32.Johannsen M, Spitaleri G, Curigliano G, Roigas J, Weikert S, Kempkensteffen C, Roemer A, Kloeters C, Rogalla P, Pecher G, et al. The tumour-targeting human L19-IL2 immunocytokine: preclinical safety studies, phase I clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer. 2010;46(16):2926–2935. doi: 10.1016/j.ejca.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 33.Lieverse RIY, Marcus D, van der Wiel AMA, et al. Human fibronectin Extra Domain B (ED-B) as a biomarker for targeted therapy in cancer [published online ahead of print, 2020 May 9]. Mol Oncol. 2020;10.1002/1878-0261.12705. 10.1002/1878-0261.12705. [DOI] [PMC free article] [PubMed]
- 34.Menrad A, Menssen HD. ED-B fibronectin as a target for antibody-based cancer treatments. Expert Opin Ther Targets. 2005;9(3):491–500. doi: 10.1517/14728222.9.3.491. [DOI] [PubMed] [Google Scholar]
- 35.Ebbinghaus C, Scheuermann J, Neri D, Elia G. Diagnostic and therapeutic applications of recombinant antibodies: targeting the extra-domain B of fibronectin, a marker of tumor angiogenesis. Curr Pharm Des. 2004;10(13):1537–1549. doi: 10.2174/1381612043384808. [DOI] [PubMed] [Google Scholar]
- 36.Carnemolla B, Balza E, Siri A, Zardi L, Nicotra MR, Bigotti A, Natali PG. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol. 1989;108(3):1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birchler MT, Milisavlijevic D, Pfaltz M, Neri D, Odermatt B, Schmid S, Stoeckli SJ. Expression of the extra domain B of fibronectin, a marker of angiogenesis, in head and neck tumors. Laryngoscope. 2003;113(7):1231–1237. doi: 10.1097/00005537-200307000-00023. [DOI] [PubMed] [Google Scholar]
- 38.Khan ZA, Caurtero J, Barbin YP, Chan BM, Uniyal S, Chakrabarti S. ED-B fibronectin in non-small cell lung carcinoma. Exp Lung Res. 2005;31(7):701–711. doi: 10.1080/01902140591007236. [DOI] [PubMed] [Google Scholar]
- 39.Tijink BM, Perk LR, Budde M, Stigter-van Walsum M, Visser GW, Kloet RW, Dinkelborg LM, Leemans CR, Neri D, van Dongen GA. (124)I-L19-SIP for immuno-PET imaging of tumour vasculature and guidance of (131)I-L19-SIP radioimmunotherapy. Eur J Nucl Med Mol Imaging. 2009;36(8):1235–1244. doi: 10.1007/s00259-009-1096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domagala-Kulawik J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl Lung Cancer Res. 2015;4(2):177–190. doi: 10.3978/j.issn.2218-6751.2015.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Limbergen EJ, De Ruysscher DK, Olivo Pimentel V, Marcus D, Berbee M, Hoeben A, Rekers N, Theys J, Yaromina A, Dubois LJ, et al. Combining radiotherapy with immunotherapy: the past, the present and the future. Br J Radiol. 2017;90(1076):20170157. doi: 10.1259/bjr.20170157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pimentel VO, Marcus D, Van der Wiel A, Biemans R, Lieuwes NG, Neri D, Theys J, Yaromina A, Dubois LJ, Lambin P. OC-0157 radiation and immunotherapy to fight cancer: a ‘pushing the gas and releasing the brakes’ approach. Radiother Oncol. 2019;133:S75–S76. [Google Scholar]
- 43.Eigentler TK, Weide B, de Braud F, Spitaleri G, Romanini A, Pflugfelder A, Gonzalez-Iglesias R, Tasciotti A, Giovannoni L, Schwager K, et al. A dose-escalation and signal-generating study of the immunocytokine L19-IL2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res. 2011;17(24):7732–7742. doi: 10.1158/1078-0432.CCR-11-1203. [DOI] [PubMed] [Google Scholar]
- 44.Weide B, Eigentler T, Catania C, Ascierto PA, Cascinu S, Becker JC, Hauschild A, Romanini A, Danielli R, Dummer R, et al. A phase II study of the L19IL2 immunocytokine in combination with dacarbazine in advanced metastatic melanoma patients. Cancer Immunol Immunother. 2019;68(9):1547–1559. doi: 10.1007/s00262-019-02383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, Lambert B, Delrue L, Bultijnck R, Claeys T, et al. Surveillance or metastasis-directed therapy for Oligometastatic prostate Cancer recurrence: a prospective, randomized, multicenter Phase II trial. J Clin Oncol. 2018;36(5):446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- 46.Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bergsma DP, Salama JK, Singh DP, Chmura SJ, Milano MT. Radiotherapy for Oligometastatic lung Cancer. Front Oncol. 2017;7:210. doi: 10.3389/fonc.2017.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomez DR, Blumenschein GR, Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, Doebele RC, Skoulidis F, Gaspar LE, Gibbons DL, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lancia A, Ingrosso G, Carosi A, Di Murro L, Giudice E, Cicchetti S, Morelli P, di Cristino D, Bruni C, Murgia A, et al. Oligometastatic cancer: stereotactic ablative radiotherapy for patients affected by isolated body metastasis. Acta Oncol. 2017;56(11):1621–1625. doi: 10.1080/0284186X.2017.1346383. [DOI] [PubMed] [Google Scholar]
- 50.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L, et al. Stereotactic body radiation therapy: the report of AAPM task group 101. Med Phys. 2010;37(8):4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 51.Gururangan S, Reap E, Schmittling R, Kocak M, Reynolds R, Grant G, Onar-Thomas A, Baxter P, Pollack IF, Phillips P, et al. Regulatory T cell subsets in patients with medulloblastoma at diagnosis and during standard irradiation and chemotherapy (PBTC N-11) Cancer Immunol Immunother. 2017;66(12):1589–1595. doi: 10.1007/s00262-017-2051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 53.Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litiere S, Dancey J, Chen A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greco C, Zelefsky MJ, Lovelock M, Fuks Z, Hunt M, Rosenzweig K, Zatcky J, Kim B, Yamada Y. Predictors of local control after single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases. Int J Radiat Oncol Biol Phys. 2011;79(4):1151–1157. doi: 10.1016/j.ijrobp.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 55.Kang JK, Kim MS, Kim JH, Yoo SY, Cho CK, Yang KM, Yoo HJ, Seo YS, Lee DH, Kang HJ, et al. Oligometastases confined one organ from colorectal cancer treated by SBRT. Clin Exp Metastasis. 2010;27(4):273–278. doi: 10.1007/s10585-010-9325-0. [DOI] [PubMed] [Google Scholar]
- 56.Milano MT, Katz AW, Muhs AG, Philip A, Buchholz DJ, Schell MC, Okunieff P. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008;112(3):650–658. doi: 10.1002/cncr.23209. [DOI] [PubMed] [Google Scholar]
- 57.Salama JK, Hasselle MD, Chmura SJ, Malik R, Mehta N, Yenice KM, Villaflor VM, Stadler WM, Hoffman PC, Cohen EE, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer. 2012;118(11):2962–2970. doi: 10.1002/cncr.26611. [DOI] [PubMed] [Google Scholar]
- 58.Stinauer MA, Kavanagh BD, Schefter TE, Gonzalez R, Flaig T, Lewis K, Robinson W, Chidel M, Glode M, Raben D. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34. doi: 10.1186/1748-717X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dutcher JP, Schwartzentruber DJ, Kaufman HL, Agarwala SS, Tarhini AA, Lowder JN, Atkins MB. High dose interleukin-2 (Aldesleukin) - expert consensus on best management practices-2014. J Immunother Cancer. 2014;2(1):26. doi: 10.1186/s40425-014-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartz RN, Stover L, Dutcher JP. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park) 2002;16(11 Suppl 13):11–20. [PubMed] [Google Scholar]
- 61.Marabondo S, Kaufman HL. High-dose interleukin-2 (IL-2) for the treatment of melanoma: safety considerations and future directions. Expert Opin Drug Saf. 2017;16(12):1347–1357. doi: 10.1080/14740338.2017.1382472. [DOI] [PubMed] [Google Scholar]
- 62.Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 63.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schernberg A, Blanchard P, Chargari C, Deutsch E. Neutrophils, a candidate biomarker and target for radiation therapy? Acta Oncol. 2017;56(11):1522–1530. doi: 10.1080/0284186X.2017.1348623. [DOI] [PubMed] [Google Scholar]
- 65.Fang P, Jiang W, Davuluri R, Xu C, Krishnan S, Mohan R, Koong AC, Hsu CC, Lin SH. High lymphocyte count during neoadjuvant chemoradiotherapy is associated with improved pathologic complete response in esophageal cancer. Radiother Oncol. 2018;128(3):584–590. doi: 10.1016/j.radonc.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 66.Fang P, Shiraishi Y, Verma V, Jiang W, Song J, Hobbs BP, Lin SH. Lymphocyte-sparing effect of proton therapy in patients with esophageal Cancer treated with definitive Chemoradiation. Int J Part Ther. 2018;4(3):23–32. doi: 10.14338/IJPT-17-00033.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014;148(3):467–476. doi: 10.1007/s10549-014-3185-2. [DOI] [PubMed] [Google Scholar]
- 68.Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L, Audigier-Valette C, et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in patients with advanced non-small cell lung Cancer. JAMA Oncol. 2018;4(3):351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lai J, Du B, Wang Y, Wu R, Yu Z. Next-generation sequencing of circulating tumor DNA for detection of gene mutations in lung cancer: implications for precision treatment. Onco Targets Ther. 2018;11:9111–9116. doi: 10.2147/OTT.S174877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adalsteinsson VA, Ha G, Freeman SS, Choudhury AD, Stover DG, Parsons HA, Gydush G, Reed SC, Rotem D, Rhoades J, et al. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat Commun. 2017;8(1):1324. doi: 10.1038/s41467-017-00965-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jao K, Tomasini P, Kamel-Reid S, Korpanty GJ, Mascaux C, Sakashita S, Labbe C, Leighl NB, Liu G, Feld R, et al. The prognostic effect of single and multiple cancer-related somatic mutations in resected non-small-cell lung cancer. Lung Cancer. 2018;123:22–29. doi: 10.1016/j.lungcan.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 72.Devarakonda S, Rotolo F, Tsao M-S, Lanc I, Brambilla E, Masood A, Olaussen KA, Fulton R, Sakashita S, McLeer-Florin A, et al. Tumor mutation burden as a biomarker in resected non–small-cell lung Cancer. J Clin Oncol. 2018;36(30):2995–3006. doi: 10.1200/JCO.2018.78.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, et al. First-line Nivolumab in stage IV or recurrent non-small-cell lung Cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Jong EEC, van Elmpt W, Hoekstra OS, Groen HJM, Smit EF, Boellaard R, Lambin P, Dingemans A-MC. Quality assessment of positron emission tomography scans: recommendations for future multicentre trials. Acta Oncol. 2017;56(11):1459–1464. doi: 10.1080/0284186X.2017.1346824. [DOI] [PubMed] [Google Scholar]
- 75.Salem A, Asselin M-C, Reymen B, Jackson A, Lambin P, West CML, O’Connor JPB, Faivre-Finn C. Targeting hypoxia to improve non–small cell lung Cancer outcome. JNCI. 2017;110(1):14–30. doi: 10.1093/jnci/djx160. [DOI] [PubMed] [Google Scholar]
- 76.Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, Mazieres J, Zalcman G, Brosseau S, Le Moulec S, et al. Hyperprogressive disease in patients with advanced non-small cell lung Cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4(11):1543–1552. doi: 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Table 1. In- and exclusion criteria.
Additional file 2: Supplementary Table 2. The informed consent form used for ImmunoSABR phase 2 trial.
Additional file 3: Supplementary Table 3. Schematic overview of the study and timeline.
Additional file 4: Supplementary Table 4. Statistical analysis study parameters and subgroup analyses.
Additional file 5: Supplementary Table 5. Overview of the translational research samples.
Data Availability Statement
Not applicable.