Abstract
Introduction
Individuals with psychiatric conditions smoke at higher rates than the general population and may need more intensive treatment to quit. We examined whether or not extended treatment with nicotine patch, combined with behavior counseling, would disproportionally benefit smokers with versus without a lifetime psychiatric condition.
Methods
We conducted a secondary analysis of data from an effectiveness trial of treatment with 12 counseling sessions (48 weeks) and 21-mg nicotine patch (8, 24, or 52 weeks) among 525 adult daily smokers. A structured clinical interview assessed past and current psychiatric disorders (major depression, generalized anxiety disorder, alcohol abuse and/or dependence, and substance abuse and/or dependence), as described in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition). Abstinence was bioverified at week 52. Logistic regression evaluated the effect of the psychiatric status × treatment duration interaction on abstinence at week 52, covarying for sociodemographics, baseline psychological symptoms, and treatment adherence.
Results
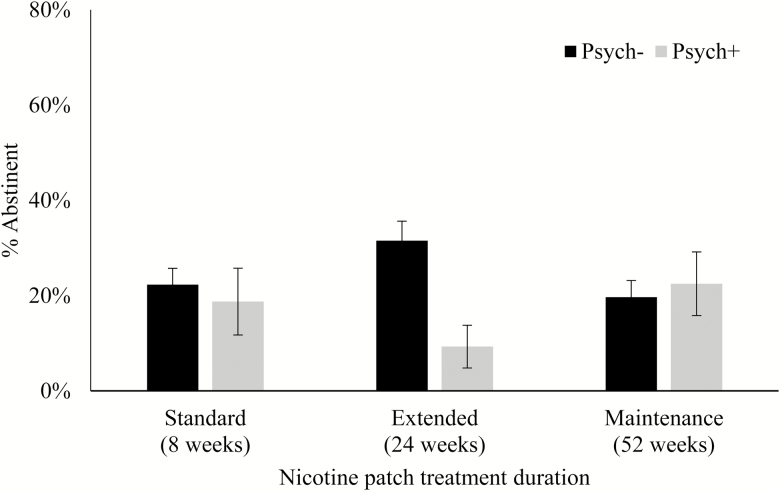
At baseline, 115 (21.9%) participants were diagnosed with one or more psychiatric conditions. The psychiatric status × treatment duration interaction was significant for week 52 abstinence (p = .027). Abstinence rates between smokers with versus without a psychiatric condition in the 24-week treatment arm (9.3% vs. 31.5% abstinent) significantly differed from the 8-week treatment arm (18.8% vs. 22.3%), p = .017. Abstinence rates for smokers with (22.5%) versus without a psychiatric condition (19.7%) in the 52-week treatment arm did not differ from those in the 8-week arm.
Conclusions
Targeted smoking cessation treatment, rather than extending treatment duration, may be especially warranted to optimize treatment for smokers with comorbid mood, anxiety, and substance use disorders.
Implications
Individuals with psychiatric conditions smoke at higher rates and have greater difficulty quitting compared to those in the general population, but little is known about how to best optimize treatment for this high tobacco burden population. The present study found that cessation response to extended duration treatment with the transdermal nicotine patch did not differ for smokers with versus without comorbid anxiety, mood, and substance use disorders in a large-scale clinical effectiveness trial. Development of targeted behavioral treatments may be required to optimize abstinence outcomes for this high-risk population, rather than simply extending the duration of pharmacotherapy treatments.
Introduction
As smoking prevalence continues to decline in the general population,1 those with psychiatric disorders are increasingly overrepresented among smokers and constitute an important tobacco use disparity group.2,3 Individuals with psychiatric disorders smoke at higher rates, smoke more heavily, and are less likely to quit than the general population.4–8 Accordingly, smokers with psychiatric disorders carry the highest burden of tobacco-related disease morbidity and mortality relative to their nonsmoking counterparts.9 As outlined in a recent statement from the Society for Research on Nicotine and Tobacco Treatment Network, although smokers with comorbid psychiatric conditions are motivated to engage in cessation treatment, evidence-based treatments for these individuals have yet to be established.10
US Public Health Service Clinical Practice Guidelines recommend a combination of behavioral counseling and a first-line pharmacotherapy (nicotine replacement therapy [NRT], bupropion, or varenicline) for smokers with and without psychiatric disorders.11 The safety and efficacy of first-line smoking cessation medications, including varenicline, have been demonstrated among smokers with psychiatric conditions,12–14 though in head-to-head comparisons, quit rates remain lower compared to those without lifetime psychopathology.14 It has been proposed that extending the duration of smoking cessation treatment for smokers with psychiatric disorders may improve these outcomes.15,16
Several NRT products are available over the counter at increasingly lower costs, making them highly available options for these smokers. Accordingly, smokers with psychiatric comorbidities are more likely to report using NRT for smoking cessation than those without psychiatric comorbidities.17,18 In practice, few smokers who engage smoking cessation services report using NRT for a prolonged period (up to 1 year) following an initially successful quit attempt.19 Evidence suggests that extended duration treatment with NRT may be particularly helpful for smokers with comorbid psychopathology, and this has been identified as an important area of study.20
To our knowledge, only one study has evaluated the effect of providing NRT for an extended duration to improve smoking cessation rates in a psychiatric population. Using a relapse prevention design, smokers with schizophrenia who had quit smoking using the nicotine patch for 3 months (n = 17) were randomized to receive an additional 6 months of treatment with either nicotine or placebo patches.21 Despite the small sample size, significantly more participants who received the additional nicotine patch therapy maintained abstinence (67%) compared to those in the placebo patch group (0%). However, this promising effect has not been tested in a larger and more diverse psychiatric population, it has not been implemented as a treatment approach for all smokers (only as a relapse prevention treatment among abstinent smokers), and there have not been direct comparisons between standard and extended duration NRT in a psychiatric population or direct comparison with a nonpsychiatric sample.
The purpose of this study was to evaluate the effectiveness of extended duration treatment with nicotine patches, combined with behavior counseling, among smokers with and without comorbid psychopathology in a secondary analysis of data from a clinical trial that recruited a community population of smokers randomized to receive 8, 24, or 52 weeks of nicotine patches.22 We hypothesized that participants who met criteria for one or more psychiatric conditions, compared to those who did not, would have proportionally higher abstinence rates at week 52 with extended duration treatment (24 or 52 weeks), as compared to standard treatment (8 weeks), with the nicotine patch.
Methods
Study Description
Data, collected between June 22, 2009, and April 15, 2014, were drawn from a randomized controlled trial of extended duration treatment with the 21-mg nicotine patch combined with up to 12 sessions of standard behavior counseling for smoking cessation in a community sample of smokers (NCT01047527); full details of the trial procedures are published elsewhere.22 Eligible participants were randomly assigned to receive standard (8-week), extended (24-week), or maintenance (52-week) treatment with the 21-mg transdermal nicotine patch. As placebo patches were not used, neither participants, nor study staff, nor counselors were blinded to nicotine patch treatment condition.
All participants, irrespective of nicotine patch treatment, were engaged in standardized behavioral smoking cessation counseling, including a prequit session (week-2) conducted in small groups (4–8 participants), and via telephone for the target quit day session (week 0; initiation of patches) and for 10 booster sessions (weeks 4, 8, 12, 16, 20, 24, 30, 36, 42, and 48). Counseling was consistent with the US Public Health Service Clinical Practice Guidelines,11 including skills-based and supportive strategies focused on managing cravings, withdrawal symptoms, and relapse prevention, and discussion of patch adherence and side effect management. Participants completed in-person visits at weeks 12, 24, 36, and 52 to bioverify abstinence. All participants provided written informed consent, and all procedures were approved by appropriate institutional review boards.
Participants
Participants were recruited via media sources, flyers, and word of mouth. Eligible participants were adults (≥18 years old) who reported smoking at least 10 cigarettes/day, were interested in quitting, and were able to safely use nicotine patches. Participants were excluded if they could not communicate fluently in English, if they met criteria for a lifetime psychotic disorder or manic episode, or if they reported current suicidality; women who were pregnant, lactating, or planning to become pregnant were also excluded.
Measures
Psychiatric Condition
The Mini-International Neuropsychiatric Interview (version 6.0)23 was administered at baseline to assess for lifetime major depressive disorder, past 6 months’ generalized anxiety disorder, past year alcohol abuse or dependence, and past year substance abuse or dependence, as described in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; the modules to assess lifetime bipolar disorder or psychotic disorder or current suicidality were also administered because these conditions were exclusionary. Staff training on the Mini-International Neuropsychiatric Interview included 3 hours of group didactics after which each staff member completed four standardized cases for proficiency. Initial training and ongoing monthly supervision were conducted by a doctoral-level clinical psychologist. For this study, participants who met criteria for one or more diagnoses were considered to be part of the psychiatric condition group (Psych+); those who did not meet criteria for any of the diagnoses were classified as Psych−.
Smoking Cessation Treatment Adherence
Adherence to nicotine patches was assessed via timeline followback24 of participants’ self-reported daily use of the patch, from which we calculated the average number of patches used per week of active treatment (average weekly patch use). As done previously, participants were classified as adherent if they reported using on average at least 6 patches per week.22,25 Counseling adherence was assessed by number of sessions attended of 12 sessions. Participants who attended at least 10 counseling sessions (>80%) were classified as adherent.
Abstinence
Bioverified 7-day point-prevalence abstinence was assessed at the week 52 in-person visit. Participants were classified as abstinent if they (1) reported not smoking any cigarettes, not even a puff, in the past 7 days and (2) provided a carbon monoxide reading of <10 ppm. Following an intention-to-treat model, participants were classified as nonabstinent if they (1) reported smoking a cigarette, even just a puff, in the past 7 days; (2) provided a carbon monoxide reading at least 10 ppm; or (3) could not be reached or were not able to provide a carbon monoxide sample.
Psychological Symptoms
Anxiety and depressive symptoms were assessed at baseline. Anxiety symptoms over the past 2 weeks were assessed using the 21-item Beck Anxiety Inventory.26 Each item on the Beck Anxiety Inventory is given a value from 0 to 3 (total range: 0–33), with higher scores indicating higher levels of anxiety. Depressive symptoms over the past week were assessed using the 30-item Inventory of Depressive Symptomatology (IDS).27 Each item on the IDS is given a value from 0 to 3 (total range: 0–30), with higher scores indicating higher levels of depressive symptomatology.
Tobacco Dependence
Degree of tobacco dependence was assessed using the Heaviness of Smoking Index,28 which assesses number of cigarettes smoked per day and time to first cigarette. Each item is scored on a scale from 0 to 3, for a total scores ranging from 0 to 6, with higher scores indicating higher tobacco dependence.
Sociodemographic Variables
Sociodemographic information known to be associated with smoking cessation was collected at baseline, prior to treatment. These variables included sex (male vs. female), race (white vs. racial and/or ethnic minority), age (years), education (≤high school graduate vs. ≥some college), income (<$50 000/year vs. ≥$50 000/year), and sexual orientation (heterosexual vs. sexual minority).
Data Analysis
All analyses were conducted in SPSS, version 16.0. We first examined group differences (Psych+ vs. Psych−) on sociodemographic variables, tobacco dependence, psychological symptoms, treatment adherence, and abstinence using t tests (for continuous measures) and chi-square analyses (for categorical measures). In the primary analysis, we estimated a logistic regression model to evaluate the psychiatric condition × treatment duration interaction on 7-day point prevalence abstinence at week 52. We then assessed the effect of the interaction on treatment adherence by estimating separate logistic regression models to evaluate the psychiatric condition × treatment duration interaction on patch adherence and counseling adherence. All models controlled for the main effects of psychiatric condition (referent: Psych−) and treatment duration (referent: 8-week treatment). We then evaluated models adjusted for sociodemographic variables (sex, race, age, education, income, and sexual orientation), tobacco dependence (Heaviness of Smoking Index score), and baseline psychological symptoms (Beck Anxiety Inventory and IDS scores). The abstinence model was further adjusted for treatment adherence (patch adherence and counseling adherence).
Results
Sample Characteristics
The overall sample (N = 525) comprised 50% female and 52% racial and/or ethnic minorities; participants were 46-years-old on average. At baseline, 115 participants (22% of the sample) met criteria for one or more psychiatric conditions, as described in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition). Among the Psych+ group, 17 (15%) met criteria for current major depression, 87 (76%) for past major depression, 11 (10%) for generalized anxiety disorder, 13 (11%) for alcohol abuse, 9 (8%) for alcohol dependence, 16 (14%) for substance abuse, and 17 (15%) for substance dependence; 33 (29%) participants met criteria for two or more diagnoses. As shown in Table 1, group differences were observed on race, age, and baseline psychological symptoms, with those in the Psych+ group more likely to be white, younger, and report higher depressive and anxiety symptoms at baseline. No group differences were observed on treatment arm assignment, abstinence, treatment adherence (Table 1), or attrition rates (Supplementary Table). On average, participants reported using 4 patches per week (SD = 2.3, range = 0–6.9 patches) and attended 9 counseling sessions (SD = 3.6, range = 1–12). More participants were adherent to counseling than nicotine patch treatment, where 308 participants (59%) attended at least 10 counseling sessions and 206 participants (39%) reported using at least 6 patches per week on average.
Table 1.
Participant Characteristics (N = 525)
| Variable | Psych− (N = 410) | Psych+ (N = 115) | p value |
|---|---|---|---|
| Sociodemographic, smoking, and psychological symptoms | |||
| Sex, female, N (%) | 204 (50) | 62 (54) | .431 |
| Race, white, N (%) | 188 (46) | 66 (57) | .029 |
| Age, years, M (SD) | 47.0 (12.0) | 44.2 (12.1) | .028 |
| Education, HS graduate or less, N (%) | 130 (32) | 33 (29) | .537 |
| Income, <50 000/y, N (%) | 299 (73) | 81 (70) | .664 |
| Sexual orientation, sexual minority, N (%) | 29 (7) | 14 (12) | .115 |
| Tobacco dependence, HSI score, M (SD) | 3.1 (1.2) | 3.2 (1.3) | .585 |
| Depressive symptoms, baseline, IDS score, M (SD) | 10.2 (7.0) | 15.4 (9.2) | <.001 |
| Anxiety symptoms, baseline, BAI score, M (SD) | 3.8 (5.5) | 7.6 (7.9) | <.001 |
| Nicotine patch treatment | |||
| Treatment duration | .240 | ||
| Standard (8 wk), N (%) | 148 (36) | 32 (28) | |
| Extended (24 wk), N (%) | 130 (32) | 43 (37) | |
| Maintenance (52 wk), N (%) | 132 (32) | 40 (35) | |
| Week 52 smoking cessation treatment outcomes | |||
| 7-Day point prevalent abstinence, N (%) | 100 (24) | 19 (17) | .075 |
| Patch use, weekly average, M (SD) | 4.5 (2.7) | 4.1 (2.4) | .130 |
| Patch adherent, N (%) | 165 (40) | 41 (36) | .373 |
| Counseling sessions attended, M (SD) | 8.9 (3.6) | 8.7 (3.7) | .458 |
| Counseling adherent, N (%) | 242 (59) | 66 (57) | .417 |
HS = high school; HSI = Heaviness of Smoking Index; IDS = Inventory of Depressive Symptomatology; BAI = Beck Anxiety Inventory.
Values are mean (standard deviation) or number (percent of condition total). Bold indicates significant differences between Psych+ and Psych− groups. Patch adherent: averaged ≥6 of 7 patches per week. Counseling adherent: ≥10 of 12 smoking cessation counseling sessions.
Abstinence Outcomes
At week 52, 304 participants (57.9% of the baseline sample) attended the final session and provided a breath sample to bioverify their self-reported abstinence status, including 66 participants in the Psych+ group and 238 participants in the Psych− group. Abstinence among the Psych+ participants was bioverified for 6 (40.0%) in the 8-week treatment arm, 4 (16.0%) in the 24-week treatment arm, and 9 (34.6%) in the 52-week treatment arm. Abstinence among the Psych− participants was bioverified for 33 (42.3%) in the 8-week treatment arm, 41 (50.0%) in the 24-week treatment arm, and 26 (33.3%) in the 52-week treatment arm.
For intention-to-treat analyses, 119 participants (22.7% of the total sample) were bioverified abstinent, 19 (16.5%) in the Psych+ group and 100 (24.4%) in the Psych− group. The psychiatric condition × treatment duration interaction term was not significantly associated with week 52 abstinence in the unadjusted model (p = .057). After adjusting for sociodemographic variables, tobacco dependence, baseline psychological symptoms, and treatment adherence, the psychiatric condition × treatment duration interaction reached significance (p = .027; Table 2 and Figure 1).
Table 2.
Fully Adjusted Models Predicting Smoking Cessation and Treatment Adherence at Week 52
| Variable (unit or referent) | Abstinence | Patch adherence | Counseling adherence | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Sex (female) | 0.89 (0.51% to 1.54%) | .666 | 1.27 (0.85% to 1.90%) | .252 | 0.97 (0.65% to 1.45%) | .874 |
| Race (racial minority) | 0.86 (0.48% to 1.55%) | .616 | 0.75 (0.49% to 1.14%) | .176 | 0.57 (0.37% to 0.87%) | .009 |
| Age (years) | 1.00 (0.98% to 1.03%) | .909 | 1.02 (1.00% to 1.04%) | .017 | 1.04 (1.02% to 1.06%) | <.001 |
| Education (≤HS graduate) | 2.08 (1.09% to 3.95%) | .026 | 1.05 (0.67% to 1.66%) | .825 | 1.55 (0.98 to 2.47%) | .063 |
| Income (≤$50 000/y) | 1.82 (0.97% to 3.41%) | .062 | 0.81 (0.51% to 1.29%) | .366 | 0.98 (0.61% to 1.55%) | .914 |
| Sexual orientation (heterosexual) | 2.43 (1.03% to 5.75%) | .044 | 1.97 (1.00% to 3.90%) | .050 | 1.57 (0.78% to 3.17%) | .210 |
| Tobacco dependence (HSI score) | 0.76 (0.60% to 0.95%) | .016 | 1.11 (0.93% to 1.31%) | .243 | 1.02 (0.86% to 1.21%) | .799 |
| Anxiety symptoms (BAI score) | 0.99 (0.94% to 1.05%) | .703 | 0.97 (0.93% to 1.02%) | .224 | 1.02 (0.97% to 1.06%) | .465 |
| Depressive symptoms (IDS score) | 1.05 (1.01% to 1.09%) | .035 | 1.00 (0.97% to 1.04%) | .836 | 0.98 (0.95% to 1.01%) | .247 |
| Patch adherence (nonadherent) | 3.06 (1.74% to 5.36%) | <.001 | --- | --- | --- | --- |
| Counseling adherence (nonadherent) | 24.61 (8.48% to 71.38%) | <.001 | --- | --- | --- | --- |
| Psychiatric condition (Psych−) | 1.11 (0.32% to 3.88%) | .872 | 0.45 (0.16% to 1.21%) | .113 | 1.28 (0.52% to 3.07%) | .601 |
| Treatment duration (8 wk) | .204 | .002 | .018 | |||
| 24 wk | 1.55 (0.76% to 3.14%) | .230 | 1.67 (1.00% to 2.84%) | .050 | 2.20 (1.27%to 3.81%) | .005 |
| 52 wk | 0.80 (0.38% to 1.70%) | .570 | 0.61 (0.36% to 1.05%) | .074 | 1.46 (0.86% to 2.48%) | .158 |
| Psychiatric condition (Psych−) × treatment duration (8 wk) | .027 | .039 | .442 | |||
| Psych+ × 24 wk | 0.12 (0.02% to 0.68) | .017 | 1.61 (0.47% to 5.50%) | .450 | 0.60 (0.19%to 1.89%) | .381 |
| Psych+ × 52 wk | 0.81 (0.16% to 4.12) | .796 | 4.62 (1.31% to 16.31%) | .017 | 1.20 (0.37% to 3.88%) | .762 |
HS = high school; HSI = Heaviness of Smoking Index; IDS = Inventory of Depressive Symptomatology; BAI = Beck Anxiety Inventory.
Bold indicates significance at p <.05. Patch adherence was defined by self-reported use of ≥6 of 7 patches per week on average. Counseling adherence was defined by attendance at ≥10 of 12 smoking cessation counseling sessions. Abstinence was bioverified (CO ≤ 10 ppm) 7-day point prevalence.
Figure 1.
Abstinence at week 52 by psychiatric condition and treatment duration.
The interaction was driven primarily by the large difference in abstinence rates between Psych+ and Psych− participants in the 24-week treatment arm (9.3% vs. 31.5% abstinent) relative to similar abstinence rates in Psych+ and Psych− participants in the 8-week treatment arm (18.8% vs. 22.3% abstinent; 24-week vs. 8-week: OR = 0.12, 95% CI = 0.02 to 0.68, p = .017). No differences were observed between Psych+ and Psych− participants in the 52-week treatment arm (22.5% vs. 19.7%) compared to those in the 8-week treatment arm (p = .796). Other predictors of week 52 abstinence included education level (>high school graduate), sexual orientation (sexual minority), Heaviness of Smoking Index score (lower scores), IDS score (higher scores), patch use (adherent), and counseling attendance (adherent); all p’s <.05.
Treatment Adherence Outcomes
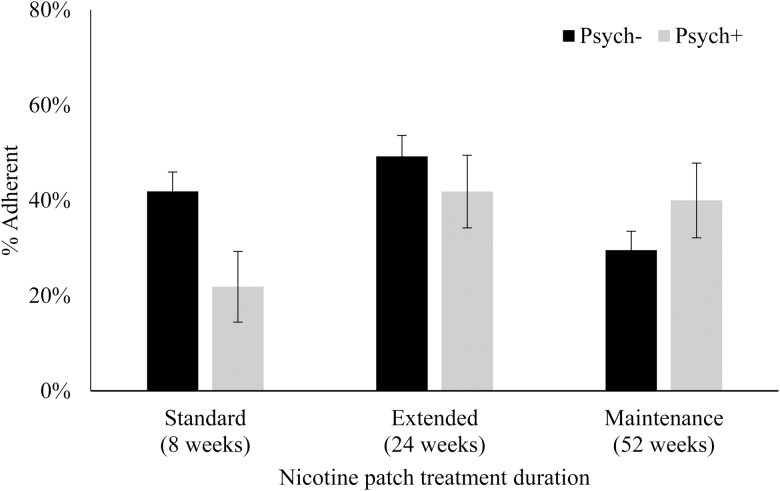
In the unadjusted model predicting patch adherence, the psychiatric condition × treatment duration interaction term was not significant (p = .054), but the interaction reached significance after adjusting for sociodemographic variables, tobacco dependence, and baseline psychological symptoms (p = .039; Table 2). Specifically, as shown in Figure 2, the Psych+ participants in the 52-week treatment arm had higher patch adherence than those in the 8-week treatment arm (40% vs. 22%), whereas the Psych− participants in the 52-week treatment arm had lower patch adherence than those in the 8-week treatment arm (30% vs. 42%; OR = 4.62, 95% CI = 1.31 to 16.31, p = .017). No interactive effects were observed by psychiatric condition in the 24-week treatment arm compared to the 8-week treatment arm (p = .450), even though the overall adherence rates were notably higher in both groups (Psych+, 42%; Psych−, 49%). Other predictors of patch adherence were age (older age), sexual orientation (sexual minority), and treatment duration (24-week compared to 8-week).
Figure 2.
Patch adherence by psychiatric condition and treatment duration.
For counseling adherence, the psychiatric condition × treatment duration interaction was not significant in the unadjusted model (p = .642), and it remained nonsignificant after adjusting for sociodemographic variables, tobacco dependence, and baseline psychological symptoms (p = .442; Table 2 and Supplementary Figure). Rather, the main effect of treatment duration was significantly associated with counseling adherence, specifically that participants in the 24-week treatment arm, but not the 52-week treatment arm, were more likely to be adherent to counseling compared to those in the 8-week treatment arm (OR = 2.20, 95% CI = 1.27 to 3.81, p = .005). The main effect of psychiatric condition was not associated with counseling adherence (p = .601). In the fully adjusted model, race (white) and age (older age) were associated with counseling adherence (p’s < .05).
Discussion
Though individuals with psychiatric disorders smoke at higher rates and have greater difficulty quitting than those in the general population,5,8 little is known about optimizing treatment for this population.10,11,15 In this study, we found the interaction between psychiatric condition and treatment duration was significant for week 52 abstinence. However, a dose–response relationship between treatment duration and abstinence rates among participants with comorbid mood, anxiety, or substance use disorders was not observed, consistent with the primary outcomes of this trial;22 thus, our overall hypothesis that smokers with comorbid psychopathology would selectively benefit from extended duration treatment with the nicotine patch was not supported.
Instead, this effect appeared to be largely driven by the disparity in abstinence rates among participants who received 24 weeks of nicotine patch treatment, in which only 9% of those participants with a psychiatric condition were abstinent compared to 32% of smokers without a psychiatric condition, relative to participants who only received 8 weeks of treatment, in which abstinence rates were similar between smokers with a psychiatric condition (19%) compared to those without (22%). This observed disparity in abstinence rates among participants in the 24-week treatment arm, but not those in the 52-week treatment arm (23% vs. 20% abstinent), may be related to the study design, in which the outcome was measured at the end of the 52-week treatment rather than followed up 10 or 6 months after finishing treatment, as was the case for the 8- and 24-week treatment arms, respectively.
Importantly, it does not appear as though treatment adherence accounts for the lack of benefit derived from extended duration treatment among smokers with a psychiatric condition, given that the abstinence models remained significant after adjusting for treatment adherence measures. This is especially notable given the high threshold set for counseling adherence in this study, attending at least 10 of 12 sessions over 48 weeks, which is a greater commitment than many behavioral treatments.11 This finding is consistent with a prior study involving this sample, which found that a higher level of anxiety symptoms at baseline, but not having a psychiatric diagnosis, was associated with poorer adherence to the first 8 weeks of patch treatment.29 Many previous studies have found that adults with psychiatric conditions have low adherence to their psychiatric or medical treatments30,31 and that they are less likely to adhere to smoking cessation treatments compared to smokers without psychiatric comorbidities.32,33 Consistent with previous studies,34,35 both pharmacotherapy and behavioral treatment adherence were strong predictors of abstinence in this sample; however, rates of treatment adherence did not significantly differ between smokers with versus without a psychiatric condition. The only difference we observed was in patch adherence between smokers with versus without a psychiatric condition in the 52-week treatment arm compared to the 8-week treatment arm. Specifically, smokers with a psychiatric condition were nearly twice as adherent to the extended, 52-week patch treatment compared to the 8-week treatment, whereas those without a psychiatric condition were approximately 40% more adherent to the standard, 8-week patch treatment compared to the 52-week treatment.
Of note, we observed two unexpected predictors of abstinence in this sample. First, sexual minorities had 2.4 times greater odds of abstinence at the end of treatment, and nearly 2 times greater odds of patch adherence, compared to heterosexual participants; this finding was also observed in the main outcomes analysis.22 Despite higher rates of smoking among sexual minorities,36 many studies have shown similar cessation rates among sexual minorities compared to nonminorities in clinical trials, including in extended treatment for relapse prevention.37
Second, and of particular relevance to the present analysis, higher baseline depressive symptoms were associated with greater odds of abstinence, with each point increase on the IDS scale associated with a 5% increase in the odds of abstinence. Similarly, we previously found that anhedonic smokers in this sample were more than three times as likely to be abstinent after the first 8 weeks of treatment,38 possibly because these smokers selectively benefited from NRT, which has been shown to increase positive affect and decrease depressive symptoms during cessation.39,40 Another preliminary analysis in this sample demonstrated that, independent of depressive symptoms, when participants reported substituting their smoking behavior with alternative, positively reinforcing activities, they were more likely to achieve abstinence.41 Taken together, these trends may have contributed to the similar abstinence rates observed among those with and without a psychiatric condition, especially because the smokers with comorbid psychiatric conditions had higher levels of depressive symptoms at baseline.
Aside from one small study of relapse prevention among smokers with schizophrenia,21 this was the first study to test the effectiveness of extended duration NRT among smokers with a variety of psychiatric and substance use comorbidities (22% of the present sample) in a head-to-head comparison of extended duration treatment for individuals with and without psychiatric conditions. In a large sample, of whom 60% had a psychiatric diagnosis, Tulloch et al.42 demonstrated higher abstinence rates among smokers receiving extended use of dual-form NRT (up to 22 weeks) versus standard, monotherapy NRT (10 weeks), though it remains unknown whether it was the extended duration versus the added intensity of treatment (dual vs. monotherapy) that improved outcomes, and the results were not reported separately for the psychiatric group.
Alternate options that may be particularly beneficial for smokers with comorbid psychiatric disorders include non-NRT medications (ie, bupropion and varenicline).11 Despite ongoing concerns about neuropsychiatric side effects, the safety of varenicline and bupropion has been established for smokers with psychiatric and substance use diagnoses.14,33,43 Extending the use of these smoking cessation medications beyond the standard prescription (up to 1 year) increases abstinence rates in the general population,44,45 and a growing body of research supports the effectiveness of extended duration treatment with these medications for smokers with psychiatric diagnoses. For example, in the same study noted earlier, Tulloch et al.42 demonstrated higher abstinence rates among smokers (60% of whom had a psychiatric diagnosis) who used extended duration varenicline (24 weeks) compared to those who used standard duration, monotherapy NRT. Cox et al.46 found that continuing bupropion for up to 52 weeks was equally effective among smokers with and without a history of major depression, as both groups had significantly higher rates of abstinence at the end of treatment (52% and 56%, respectively) compared to those given a placebo (36% and 44%). Though not yet tested in clinical trials among smokers with psychiatric diagnoses, more intensive or novel medications that may increase abstinence among these highly dependent smokers may include combining bupropion and varenicline47 or greater uptake of medications currently identified as second-line treatments (eg, nortriptyline or clonidine).48
It may be that more flexible behavioral treatments, such as longer duration, greater intensity, or targeted content, are warranted to achieve higher rates of engagement in and success of smoking cessation treatment among smokers with psychiatric comorbidities.49 Case studies of smokers with severe mental illness illustrate the effectiveness of providing individualized, tailored smoking cessation treatment for these patients.50,51 This effect has also been observed in clinical trials, primarily among smokers with depression. In a staged care intervention conducted in mental health outpatient clinics, smokers with current depression were first engaged in motivational feedback to enhance their readiness to quit smoking and then, if and when the smokers reached the contemplation stage, they were engaged in an 8-week behavioral treatment with mood management; results indicate that those participants in the targeted behavioral treatment condition were more likely to make a quit attempt and ultimately achieve abstinence after 18 months than those in a brief contact and referral condition.52 A Cochrane review of smoking cessation interventions demonstrated that smokers with past or current depression who were enrolled in a behavioral treatment that included a mood management component, compared to the standard treatment alone, were 40%–50% more likely to achieve abstinence;12 notably, the mood management interventions varied widely across studies, which comprised primarily small sample sizes.
Several design considerations warrant comment. First, although we assessed smokers with a range of mental health conditions (including alcohol and substance use disorders), those with severe mental illness (ie, bipolar disorder, schizophrenia, and current suicidality) were excluded from the clinical trial, precluding our ability to draw conclusions about the effectiveness of extended duration treatment with NRT for smokers with those disorders. Second, as this study was a secondary analysis and the primary aims did not address psychiatric condition, participants were not recruited or stratified by psychiatric diagnostic status nor was participant engagement in either behavioral or pharmacological psychiatric treatment systematically assessed. Studies that are specifically designed and powered to test these hypotheses are needed. Despite this, the rate of lifetime psychiatric disorders in our community sample was relatively high (22%), with multiple comorbidity (29% of those with a psychiatric condition), and the proportion of participants who met criteria of a psychiatric condition did not differ between treatment arms. Finally, this sample was not powered to test the hypothesis that there is no difference between smokers with versus without a psychiatric condition, or between different psychiatric conditions. Rather, we can only conclude that smokers with psychiatric comorbidities did not selectively benefit from extended duration treatment with nicotine patch.
In sum, extended duration treatment with the nicotine patch produced similar outcomes among smokers with and without comorbid psychiatric conditions. It may be that extended duration or intensity of smoking cessation medications (eg, varenicline) or targeted behavioral treatment approaches will more effectively increase smoking cessation rates among smokers with mood, anxiety, and substance use disorders. With the increasing burden of tobacco use among smokers with psychiatric conditions,2,3 studies investigating treatments for smoking cessation among smokers with comorbid psychopathology will continue to be a crucial area of study.
Funding
This study was supported by the National Institute on Drug Abuse (grant number R01 DA025078 to RAS) and the National Cancer Institute (grant number R01 CA184211 to BH) at the National Institutes of Health.
Declaration of Interests
RAS and BH received varenicline and placebo free of charge from Pfizer for use in ongoing clinical trials supported by the National Institutes of Health. BH has provided consultation to Pfizer. RAS has provided consultation to Pfizer and GlaxoSmithKline.
Supplementary Material
References
- 1. Clarke TC, Ward BW, Freeman G, Schiller JS.. Early Release of Selected Estimates Based on Data From the January–September 2015 National Health Interview Survey. Atlanta, GA: National Center for Health Statistics; 2016. http://www.cdc.gov/nchs/nhis/releases/released201602.htm. Accessed February 23, 2016. [Google Scholar]
- 2. Steinberg ML, Williams JM, Li Y. Poor mental health and reduced decline in smoking prevalence. Am J Prev Med. 2015;49(3):362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cook BL, Wayne GF, Kafali EN, Liu Z, Shu C, Flores M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA. 2014;311(2):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(11):1107–1115. [DOI] [PubMed] [Google Scholar]
- 5. Smith PH, Homish GG, Giovino GA, Kozlowski LT. Cigarette smoking and mental illness: a study of nicotine withdrawal. Am J Public Health. 2014;104(2):e127–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hitsman B, Papandonatos GD, McChargue DE, et al. . Past major depression and smoking cessation outcome: a systematic review and meta-analysis update. Addiction. 2013;108(2):294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ziedonis D, Hitsman B, Beckham JC, et al. . Tobacco use and cessation in psychiatric disorders: national institute of mental health report. Nicotine Tob Res. 2008;10(12):1691–1715. [DOI] [PubMed] [Google Scholar]
- 8. Smith PH, Mazure CM, McKee SA. Smoking and mental illness in the U.S. population. Tob Control. 2014;23(e2):e147–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parks J, Svendsen D, Singer P, Foti M,eds. Morbidity and Mortality in People With Serious Mental Illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006. [Google Scholar]
- 10. Rojewski AM, Baldassarri S, Cooperman NA, et al. . Exploring issues of comorbid conditions in people who smoke. Nicotine Tob Res. 2016;18(8):1684–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fiore M, Jaén C, Baker T, et al. . Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 12. van der Meer RM, Willemsen MC, Smit F, Cuijpers P. Smoking cessation interventions for smokers with current or past depression. Cochrane Database Syst Rev. 2013;(8):CD006102. [DOI] [PubMed] [Google Scholar]
- 13. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. 2013;(2):CD007253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anthenelli RM, Benowitz NL, West R, et al. . Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520. [DOI] [PubMed] [Google Scholar]
- 15. Hitsman B, Moss TG, Montoya ID, George TP. Treatment of tobacco dependence in mental health and addictive disorders. Can J Psychiatry. 2009;54(6):368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kisely S, Campbell LA. Use of smoking cessation therapies in individuals with psychiatric illness: an update for prescribers. CNS Drugs. 2008;22(4):263–273. [DOI] [PubMed] [Google Scholar]
- 17. Morris CD, Burns EK, Waxmonsky JA, Levinson AH. Smoking cessation behaviors among persons with psychiatric diagnoses: results from a population-level state survey. Drug Alcohol Depend. 2014;136:63–68. [DOI] [PubMed] [Google Scholar]
- 18. Kerkvliet JL, Wey H, Fahrenwald NL. Cessation among state quitline participants with a mental health condition. Nicotine Tob Res. 2015;17(6):735–741. [DOI] [PubMed] [Google Scholar]
- 19. Shahab L, Dobbie F, Hiscock R, McNeill A, Bauld L. Prevalence and impact of long-term use of nicotine replacement therapy in UK stop-smoking services: findings from the ELONS study. Nicotine Tob Res. 2017;20(1):81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prochaska JJ. Nicotine replacement therapy as a maintenance treatment. JAMA. 2015;314(7):718–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dale Horst W, Klein MW, Williams D, Werder SF. Extended use of nicotine replacement therapy to maintain smoking cessation in persons with schizophrenia. Neuropsychiatr Dis Treat. 2005;1(4):349–355. [PMC free article] [PubMed] [Google Scholar]
- 22. Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. . Long-term nicotine replacement therapy: a randomized clinical trial. JAMA Intern Med. 2015;175(4):504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sheehan DV, Lecrubier Y, Sheehan KH, et al. . The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- 24. Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12(2):101–112. [Google Scholar]
- 25. Schnoll RA, Patterson F, Wileyto EP, et al. . Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med. 2010;152(3):144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–897. [DOI] [PubMed] [Google Scholar]
- 27. Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The inventory of depressive symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–486. [DOI] [PubMed] [Google Scholar]
- 28. Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–799. [DOI] [PubMed] [Google Scholar]
- 29. Handschin J, Hitsman B, Blazekovic S, et al. . Factors associated with adherence to transdermal nicotine patches within a smoking cessation effectiveness trial. J Smok Cessat. 2017;13(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Julius RJ, Novitsky MA Jr, Dubin WR. Medication adherence: a review of the literature and implications for clinical practice. J Psychiatr Pract. 2009;15(1):34–44. [DOI] [PubMed] [Google Scholar]
- 31. Grenard JL, Munjas BA, Adams JL, et al. . Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gariti P, Alterman AI, Mulvaney FD, Epperson L. The relationship between psychopathology and smoking cessation treatment response. Drug Alcohol Depend. 2000;60(3):267–273. [DOI] [PubMed] [Google Scholar]
- 33. McClure JB, Swan GE, Catz SL, et al. . Smoking outcome by psychiatric history after behavioral and varenicline treatment. J Subst Abuse Treat. 2010;38(4):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. 2008;30(10):1852–1858. [DOI] [PubMed] [Google Scholar]
- 35. Ferguson SG, Shiffman S, Gitchell JG. Nicotine replacement therapies: patient safety and persistence. Patient Relat Outcome Meas. 2011;2:111–117. https://www.dovepress.com/nicotine-replacement-therapies-patient-safety-and-persistence-peer-reviewed-article-PROM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2005–2014. MMWR Morb Mortal Wkly Rep. 2015;64(44):1233–1240. [DOI] [PubMed] [Google Scholar]
- 37. Grady ES, Humfleet GL, Delucchi KL, Reus VI, Muñoz RF, Hall SM. Smoking cessation outcomes among sexual and gender minority and nonminority smokers in extended smoking treatments. Nicotine Tob Res. 2014;16(9):1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Powers JM, Carroll AJ, Veluz-Wilkins AK, et al. . Is the effect of anhedonia on smoking cessation greater for women versus men?Nicotine Tob Res. 2017;19(1):119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Strasser AA, Kaufmann V, Jepson C, et al. . Effects of different nicotine replacement therapies on postcessation psychological responses. Addict Behav. 2005;30(1):9–17. [DOI] [PubMed] [Google Scholar]
- 40. Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. J Consult Clin Psychol. 1996;64(4):791–798. [DOI] [PubMed] [Google Scholar]
- 41. Goelz PM, Audrain-McGovern JE, Hitsman B, et al. . The association between changes in alternative reinforcers and short-term smoking cessation. Drug Alcohol Depend. 2014;138:67–74. https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S0376871614000623?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0376871614000623%3Fshowall%3Dtrue&referrer=https:%2F%2Fwww.ncbi.nlm.nih.gov%2F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tulloch HE, Pipe AL, Els C, Clyde MJ, Reid RD. Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial. BMC Med. 2016;14:80 https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-016-0626-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nahvi S, Wu B, Richter KP, Bernstein SL, Arnsten JH. Low incidence of adverse events following varenicline initiation among opioid dependent smokers with comorbid psychiatric illness. Drug Alcohol Depend. 2013;132(1–2):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laude JR, Bailey SR, Crew E, et al. . Extended treatment for cigarette smoking cessation: a randomized control trial. Addiction. 2017;112(8): 1451–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hall SM, Humfleet GL, Muñoz RF, Reus VI, Prochaska JJ, Robbins JA. Using extended cognitive behavioral treatment and medication to treat dependent smokers. Am J Public Health. 2011;101(12):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cox LS, Patten CA, Niaura RS, et al. . Efficacy of bupropion for relapse prevention in smokers with and without a past history of major depression. J Gen Intern Med. 2004;19(8):828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rose JE, Behm FM. Combination varenicline/bupropion treatment benefits highly dependent smokers in an adaptive smoking cessation paradigm. Nicotine Tob Res. 2017;19(8):999–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gómez-Coronado N, Walker AJ, Berk M, Dodd S. Current and emerging pharmacotherapies for cessation of tobacco smoking. Pharmacotherapy. 2018;38(2):235–258. [DOI] [PubMed] [Google Scholar]
- 49. Aubin HJ, Rollema H, Svensson TH, Winterer G. Smoking, quitting, and psychiatric disease: a review. Neurosci Biobehav Rev. 2012;36(1):271–284. [DOI] [PubMed] [Google Scholar]
- 50. Williams JM, Zimmermann MH, Steinberg ML, et al. . A comprehensive model for mental health tobacco recovery in New Jersey. Adm Policy Ment Health. 2011;38(5):368–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Filia SL, Baker AL, Kulkarni J, Williams JM. Sequential behavioral treatment of smoking and weight control in bipolar disorder. Transl Behav Med. 2012;2(3):290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hall SM, Tsoh JY, Prochaska JJ, et al. . Treatment for cigarette smoking among depressed mental health outpatients: a randomized clinical trial. Am J Public Health. 2006;96(10):1808–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.