Abstract
Aims
Most cigarette smokers want to quit smoking and more than half make an attempt every year, but less than 10% remain abstinent for at least 6 months. Evidence-based tobacco use treatment improves the likelihood of quitting, but more than two-thirds of individuals relapse when provided even the most robust treatments. Identifying for whom treatment is effective will improve the success of our treatments and perhaps identify strategies for improving current approaches.
Methods
Two cohorts (training: N = 90, validation: N = 71) of cigarette smokers enrolled in group cognitive-behavioral therapy (CBT). Generalized estimating equations were used to identify baseline predictors of outcome, as defined by breath carbon monoxide and urine cotinine. Significant measures were entered as candidate variables to predict quit status. The resulting decision trees were used to predict cessation outcomes in a validation cohort.
Results
In the training cohort, the decision trees significantly improved on chance classification of smoking status following treatment and at 6-month follow-up. The first split of all decision trees, which was delay discounting, significantly improved on chance classification rates in both the training and validation cohort. Delay discounting emerged as the single best predictor of group CBT treatment response with an average baseline discount rate of ln(k) = −7.1, correctly predicting smoking status of 80% of participants at posttreatment and 81% of participants at follow-up.
Conclusions
This study provides a first step toward personalized care for smoking cessation though future work is needed to identify individuals that are likely to be successful in treatments beyond group CBT.
Implications
This study provides a first step toward personalized care for smoking cessation. Using a novel machine-learning approach, baseline measures of clinical and executive functioning are used to predict smoking cessation outcomes following group CBT. A decision point is recommended for the single best predictor of treatment outcomes, delay discounting, to inform future research or clinical practice in an effort to better allocate patients to treatments that are likely to work.
Introduction
Tobacco use is the single leading cause of preventable morbidity and mortality in the United States as well as one of the most costly health risk behaviors.(1–3) Most cigarette smokers want to quit smoking and more than half make a quit attempt every year, but less than 10% who attempt remain abstinent for at least 6 months.(4) Although evidence-based treatment for tobacco use greatly improves the likelihood that smokers achieve abstinence, more than two-thirds of individuals relapse when provided even the most robust treatments.(4–6) Identifying for whom treatment is most effective will improve the overall success of our tobacco treatments and perhaps identify strategies for improving current approaches.(7)
Proposals to improve smokers’ success at quitting include increasing the number of people who attempt, the frequency of quit attempts, and enhancing the effectiveness of current treatments.(8–10) Identifying factors that predict success will contribute to patient-centered treatment recommendations, treatment tailoring, and new therapeutic strategies designed to develop and support relevant clinical characteristics in preparation for or during treatment. Previous work has identified important factors for quitting tobacco that include both relatively stable characteristics, such as age of smoking initiation and degree of nicotine dependence, as well as factors that are more amenable to change, such as stress reactivity and rate of discounting the future.(11,12)
Generalized estimating equations (GEEs) are often used to identify predictors of treatment outcomes, whereas machine learning is a novel approach to explore predictors of treatment outcomes. Machine learning has been effectively applied in the addiction field to distinguish between smokers and nonsmokers,(13) individuals with and without substance use disorders,(14) and between individuals with different substance use disorders.(15) Connor et al.(16) used machine learning to predict outcomes following treatment for alcohol use and were able to accurately predict treatment outcomes for 77% of patients using decision trees. The advantage of using machine learning is that this method provides discrete decision rules to predict treatment outcomes and can provide clinically useful cutoff scores. This is the first study, to the best of our knowledge, to use GEE to inform a classification-based machine-learning approach (ie, classification and regression trees [CART]) to predict treatment outcomes among cohorts of cigarette smokers.
This study aims to identify clinical and psychosocial characteristics that predict long-term abstinence from smoking in two treatment-seeking cohorts of low-income smokers treated with multicomponent cognitive-behavioral therapy (CBT) for tobacco dependence in a parent study focused on the behavioral economics of relapse. An extensive assessment battery was collected at baseline and smokers were followed for 6 months after treatment. Consistent with prior work (e.g. Sheffer et al.11 ), GEEs were used to determine characteristics that predicted abstinence in the training cohort.These characteristics were then applied to a machine-learning framework to classify smokers as treatment responders (abstinent) or treatment nonresponders (relapsed). The resulting decision tree was then applied to the second independent cohort, the validation cohort, to determine if the same predictive structure would successfully classify responders in an independent sample.
Methods
Participants
This is a secondary data analysis of two cohorts of smokers who received group CBT for smoking cessation.(11,12) To participate, individuals in both cohorts needed to be 18 years or older, smoke 16 cigarettes or more per day, meet Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria for nicotine dependence, be not pregnant or lactating, be not using medications for smoking cessation, be free of psychiatric diagnoses that would likely interfere with participation in assessments or treatment, drinking 19 or less alcohol drinks per week, have no immediate plans to move out of the area, provide a carbon monoxide (CO) breath sample of 15 parts per million (ppm) or more (indicative of current cigarette smoking status), and have a negative urine drug screen (including amphetamine, benzodiazepine, cannabis, cocaine, opioids, and methadone). Data were included in initial analyses if participants completed at least one session of treatment and included two separate cohorts, a training cohort (N = 90) that was used to identify predictors and grow decision trees, and a validation cohort, which was used to test the out-of-sample predictive efficacy of the resulting decision trees (N = 71).
Procedure
This secondary data analysis study was approved by the Virginia Tech Institutional Review Board. The parent study was approved by the institutional review board at the University of Arkansas for Medical Sciences. In both cohorts, participants completed two 3-hour baseline assessment batteries. Participants smoked one cigarette before each pretreatment assessment session to standardize time since last cigarette and avoid withdrawal effects on cognitive and psychological measures. At the start of treatment, baseline CO and urine cotinine (COT) levels were collected to confirm current smoking status.
Tobacco Treatment
Participants received an evidence-based, intensive, manual-driven CBT for tobacco dependence consistent with the recommendations of the Public Health Service Clinical Practice Guideline(17,18) delivered by certified Tobacco Treatment Specialists. The treatment consisted of six 60-minute closed-group treatment sessions. The quit date was the day of the third treatment session. This manual has been used previously in a variety of research and clinical settings(19–21) and described in detail by Sheffer et al.(11) Consistent with the goals of the parent study, the training cohort was provided with 8 weeks of transdermal nicotine patches (4 weeks of 21 mg, 2 weeks of 14 mg, and 2 weeks of 7 mg nicotine patches). The validation cohort was treated without nicotine patches. In the parent study, we examined the behavioral economics of relapse after treatment with and without nicotine patches. Treatment without nicotine patches in this low-income, highly dependent sample of smokers resulted in very low abstinence rates. Patches were added to the second cohort to increase the proportion of participants who achieved long-term abstinence in the parent study.
Baseline Measures
Standard demographic and clinical characteristics were collected at baseline among both cohorts of participants. Clinical, executive function, and impulsivity measures were also collected during baseline assessment sessions. These measures are provided in Table 1 and described in the Supplementary Materials.
Table 1.
Demographic Characteristics of Each Cohort
| Training cohort | Validation cohort | |
|---|---|---|
| Age (y), mean (SD)a | 46.55 (12.69) | 51.71 (11.71) |
| Years of education, mean (SD) | 13.43 (1.98) | 13.27 (1.75) |
| Annual income, median (25th–75th quartile) | 25 100 (14 050–41 500) | 18 000 (11 197–26 750) |
| Sex (male), % | 53.77 | 59.30 |
aSignificant difference between cohorts.
Outcome Assessment
Abstinence was assessed with 7-day point prevalence abstinence and biochemically confirmed at the end of the six CBT sessions and 6 months after the quit date with exhaled CO and urine COT levels. Participants were considered abstinent in this per protocol analysis if they provided a CO level of 8 ppm or less using a Bedfont Smokerlyzer (Bedfont Scientific Ltd, Kent, England)(22) and/or a urine COT concentration of less than 100 ng/mL, or less using NicAlert Urine COT strips.(23) NicAlert strips are used for semiquantitative determination of COT in urine. As per the pharmaceutical package insert, the cutoff concentration for the NicAlert test is level 2, estimated to be 30–100 ng/mL. Because the training cohort continued to use nicotine patches for 2 weeks after the end of treatment, COT level was not used as the primary indicator of abstinence at the end of treatment for the training cohort.
Data Analysis
Descriptive statistics (means, SDs, medians, interquartile ranges) were used to characterize participants. Demographic differences between the two cohorts and CO and COT outcome response rates within the two cohorts were assessed with two-sample tests of significance (t tests, Mann–Whitney tests, and chi-square tests where appropriate).
GEE with an exchangeable correlation structure were used to filter candidate measures to include in the machine-learning algorithm (see Table 2 for baseline measures). GEE models are well suited for analyses of addiction treatment data as it allows for longitudinal data with correlations among observations within individual participants and makes group-level inferences about results instead of individual-level inferences provided by mixed model designs.(24) GEE incorporates all outcome data points into one model, corrects standard errors of estimates using the working correlation structure, and incorporates the effects of time. GEE handles missing data by simply entering the data point as missing; however, at least one outcome data point was necessary in order for a participant to be included in these analyses. Each measure was entered individually alongside time in a GEE model that included all assessment data points of CO or COT measures at baseline, posttreatment, and 6-month follow-up.(25) The feature selection threshold for identifying seed predictor variables that may be useful as a predictive algorithm of treatment outcome used an a priori cutoff of p < .10. The geepack package(26) in R(27) was used for fitting GEE models.
Table 2.
The Training Cohort Baseline Measures and Characteristics
| Category | Measure | Mean (SD) | Median (interquartile range) | |
|---|---|---|---|---|
| Clinical measures | Fagerström Test for Nicotine Dependence (FTND) | 5.99 (1.92) | 6 (5–7) | |
| Motivation | 8.72 (1.49) | 9 (8–10) | ||
| Self-efficacy | 7.6 (2.21) | 8 (7–10) | ||
| Perceived Stress Scale | 32.4 (5.93) | 32 (28–36) | ||
| Positive and Negative Affect Scales (PANAS) | Positive Affect Scale | 33 (8.23) | 33 (28–40) | |
| Negative Affect Scale | 17 (7.15) | 14 (11–21) | ||
| Executive function and impulsivity measures | Barratt Impulsiveness Scale | Motor Impulsiveness | 15.1 (3.39) | 15 (13–17) |
| Cognitive Impulsiveness | 9.77 (2.92) | 9 (8–12) | ||
| Nonplanning Impulsiveness | 22.7 (4.88) | 23 (19–26) | ||
| Delay Discounting Task | −5.51 (3.72) | −5.15 (−7.37 to −2.89) | ||
| Eysenck Impulsiveness Scale | Impulsiveness | 7.27 (4.59) | 6 (4–10) | |
| Venturesomeness | 7.15 (3.88) | 7 (4–10) | ||
| Empathy | 12.8 (2.98) | 13 (11–15) | ||
| Frontal Systems Behavior Scales | Executive Dysfunction | 55.5 (13.5) | 54 (47–63) | |
| Apathy | 27.8 (7.44) | 26 (22–32) | ||
| Disinhibition | 55.7 (16.1) | 53 (45–63) | ||
| Total | 56.8 (15.7) | 54 (47–64) | ||
| MicroCog (MC) | Attention/Mental Control | 98.5 (13.2) | 103 (91–108) | |
| Memory | 105 (15.2) | 107 (95–116) | ||
| Spatial Processing | 100 (15.3) | 104 (93–110) | ||
| Reasoning/Calculation | 104 (11.6) | 106 (99–111) | ||
| Reaction Time | 94.2 (14.9) | 95 (85–108) | ||
| Information Processing Accuracy | 89.9 (15.7) | 107 (99–117) | ||
| Information Processing Speed | 107 (12.6) | 91 (79–102) | ||
| Cognitive Functioning | 114 (80.7) | 111 (96–123) | ||
| Cognitive Proficiency | 96.4 (14.3) | 96 (87–107) | ||
| Rotter’s Locus of Control Scale | 8.95 (3.83) | 9 (6–12) | ||
Selected measures for predicting smoking cessation from the GEE analysis were included in the machine-learning CART analysis. Decision trees were grown using the training sample and were then evaluated in an independent validation dataset. Separate decision trees were grown for posttreatment and 6 month follow-up timepoints and for each bioverification method (CO and COT). Participants were included in each tree if they had completed the specific timepoint-measurement combination.
CART is a machine-learning technique that uses recursive partitioning to segregate candidate variables through a decision tree composed of progressive binary splits. Every value of the candidate measures is considered as a potential split and the optimal split is selected based on the impurity criterion, that is, the reduction in the residual sum of squares that results from a binary split of the data at that singular tree node. The same variables can occur in a tree multiple times if it provides the lowest impurity criterion compared to the other candidate variables and splits. Missing values are ignored when considering a split and the probability and impurity values are calculated from the nonmissing values of that variable. Each parent node in a decision tree produces two child nodes, which in turn can become parent nodes producing more child nodes to increase overall tree fit. CART is a nonparametric approach that can handle numerical data that are highly skewed or multimodal, ordinal, and categorical predictors and is well suited for application to clinical decision making. In contrast to multivariate logistic regression that considers all parameters at once when making predictions, CART is uniquely suited to generate clinical decision tools because it provides a decision tree with specific parameters and cutoffs for each clinical choice.
The rpart package(28) in R was used to analyze the grown decision trees using the GEE–selected baseline measures in the training dataset. Nodes in the tree were constrained to have a minimum size of 15 participants in parent nodes in efforts to minimize overfitting. CART analyses have been criticized for overfitting the training data, thus performing well in the training dataset but having limited utility outside of the sample dataset. To protect against this, 10-fold cross-validation was used to assess the predictive ability of the tree while minimizing the likelihood of overfitting. The predictive performance of these models was further evaluated using an independent validation dataset. The validation dataset was used to determine how the classification trees performed when applied to participant data that were not used to construct the trees. Correct classification rates, sensitivity (accurately identifying smokers), and specificity (accurately identifying quitters) were calculated for the complete tree and the first split of each tree to determine the accuracy of the full tree as well as the accuracy of the single best predictor. Whole tree and first split correct prediction rates were similarly computed for the validation cohort. Chi-square tests were used to evaluate if decision tree performance exceed a random assignment (flip of a coin) of smoking status for both the full tree and first split classifications in the training and validation samples.
Results
Sample Characteristics
Demographics of each of the cohorts are presented in Table 1. Significant differences between cohorts were observed for age (p < .01). No significant differences were detected between education (p = .6), income (p = .2), and sex (p = .3). CBT treatment adherence was high with a median of six sessions (interquartile range: 4–6) attended in the training cohort and five sessions (interquartile range: 4–6) attended in validation cohort. In the training cohort, CO was collected in 66 and COT in 65 of the 90 participants (73.3% and 72.2%, respectively) at the posttreatment assessment, and CO and COT were collected in 48 of the participants (53.3%) at the 6-month follow-up session. In the validation cohort, CO was collected in 42 and COT in 36 of the 71 participants (59.2% and 50.7%, respectively) at posttreatment, and CO was collected in 24 and COT in 22 of participants (33.8% and 31.0%, respectively) at the 6-month follow-up session. A significantly higher proportion of CO data was collected in the training cohort than the validation cohort at 6-month follow-up (χ2 = 3, df = 1, p = .02). Similarly, a significantly higher proportion of COT data was collected in the training cohort than the validation cohort at posttreatment and 6-month follow-up assessments (χ2 = 7, df = 1, p < .01 for both). No significant difference was detected between the proportion of participants with CO collected in the training and validation cohorts at the posttreatment assessment (χ2 = 3, df = 1, p = .08, NS).
Outcome Response Rate
In the training cohort, 65.1% of participants were abstinent based on CO and 24.6% of participants were abstinent based on COT at posttreatment. The decreased abstinence rates observed in COT compared to CO at the end of treatment are likely due to the continued use of the nicotine patch by the training cohort for 2 weeks after they completed the CBT sessions. Quit rates of 43.8% and 27.1% for CO and COT, respectively, were detected at the 6-month follow-up. Significant differences between CO- and COT-verified abstinence rates were detected directly following treatment (χ2 = 20.13, df = 1, p < .001). No significant differences between CO- and COT-verified abstinence were detected at 6-month follow-up in the training cohort (χ2 = 2.23, df = 1, p = .13).
In the validation cohort, the biologically verified quit rates at posttreatment assessment were 21.4% and 27.8% and the quit rates at 6-month follow-up were 25.0% and 22.7% for CO and COT, respectively. No significant differences in CO and COT quit rates were observed at the posttreatment for 6-month follow-up assessments in the validation cohort (χ2 = 0.15, df = 1, p = .70; χ2 < 0.001, df = 1, p = 1, respectively).
Generalized Estimating Equations
In all GEE models, time was a significant predictor so it will not be reported separately. The measures that met the feature selection threshold for predictive efficacy of either CO– or COT-verified abstinence are listed in Table 3. Of note, the Delay Discounting Task and Rotter’s Locus of Control Scale were significantly predictive of both CO and COT outcomes.
Table 3:
Significant Results (p < .10) From the Generalized Estimating Equations Analysis Applied to the Training Cohort
| Chi-square | p | ||
|---|---|---|---|
| CO | Delay Discounting Task | 5.83 | .016 |
| Eysenck Empathy Total | 5.30 | .021 | |
| MC Memory | 3.40 | .065 | |
| Rotter’s Locus of Control Scale | 3.38 | .066 | |
| COT | MC Information Processing Speed | 4.72 | .030 |
| Rotter’s Locus of Control Scale | 4.52 | .034 | |
| Delay Discounting Task | 4.16 | .041 | |
| FTND | 3.50 | .061 | |
| PANAS | 3.49 | .062 | |
| MC Reasoning/Calculation | 3.21 | .073 |
CO = carbon monoxide, COT = cotinine, FTND = Fagerström Test for Nicotine Dependence, MC = MicroCog, PANAS = Positive and Negative Affect Scale.
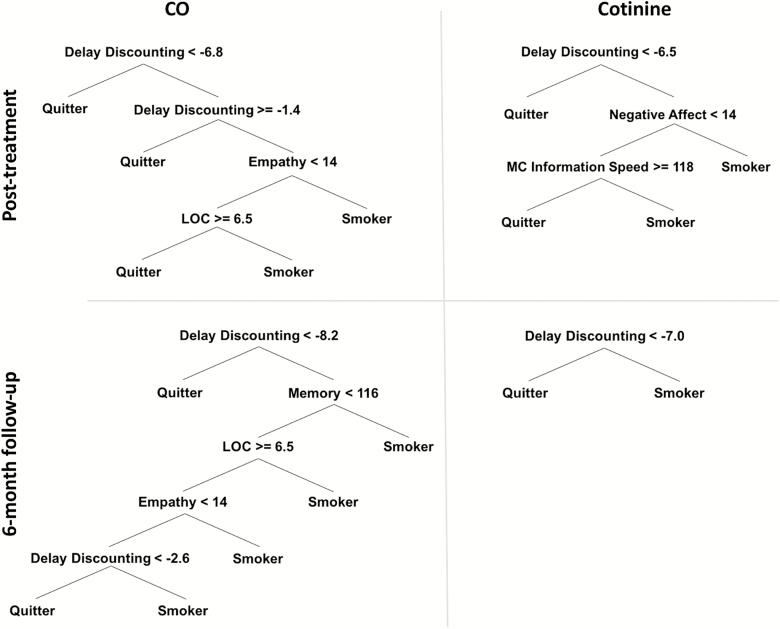
Decision Trees
The selected candidate measures were entered into the machine-learning algorithm to grow decision trees to predict CO and COT abstinence using the training dataset. This resulted in four decision trees obtained for two outcomes measures (ie, CO and COT) obtained at two timepoints (i.e. posttreatment and 6-month follow-up): posttreatment CO- and COT-verified abstinence in addition to 6-month follow-up CO- and COT-verified abstinence (see Figure 1). The first split in all four decision trees was the Delay Discounting Task, which provided the lowest impurity criterion. Thus, the Delay Discounting Task provides the most predictive power for identifying smoking abstinence. Across the four decision trees, the average classification accuracy using the first split alone was 69.53%. The full trees with the additional measures (see Table 3) on average correctly classified 81.88% of cases, an increased classification accuracy of 12.35% above using rate of delay discounting alone. The classification rates of the individual trees are shown in Table 4.
Figure 1.
Decision trees using the training cohort outcomes (CO or Cotinine) at posttreatment and 6-month follow-up assessment timepoints. CO = carbon monoxide, Delay Discounting = Delay Discounting Task, LOC = Rotter’s Locus of Control Scale, Empathy = Eysenck Empathy Subscale, Memory = MicroCog (MC) Memory Subscale, MC Information Speed = MC Information Processing Speed, Negative Affect = Positive and Negative Affect Scale (PANAS).
Table 4:
Decision Tree Characteristics
| Tree characteristics | Training cohort correct classification rate (%) | Validation cohort correct classification rate (%) | |||||
|---|---|---|---|---|---|---|---|
| Timepoint | Predicting | First split | First split point | First split | Full tree | First split | Full tree |
| Posttreatment | CO | Delay Discounting | −6.80 | 52.40 | 79.40 | 73.80 | 47.62 |
| COT | Delay Discounting | −6.50 | 76.40 | 81.80 | 66.70 | 63.90 | |
| 6-month follow-up | CO | Delay Discounting | −8.20 | 70.20 | 87.20 | 75.00 | 62.50 |
| COT | Delay Discounting | −7.00 | 79.10 | 79.10 | 81.80 | 81.80 | |
CO = carbon monoxide; COT = cotinine.
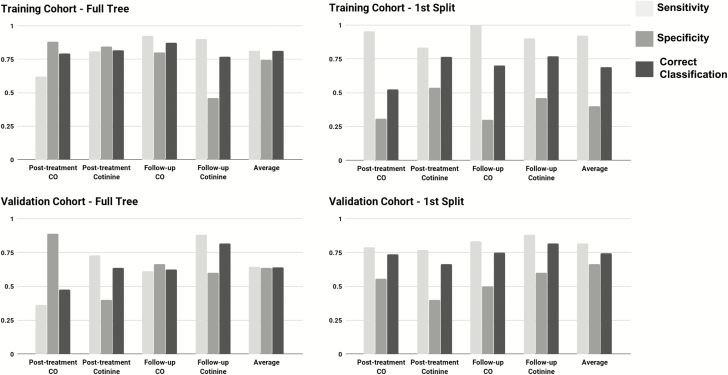
To assess the generalizability of the constructed decision trees, the four decision trees were fit to the validation cohort. The first split classification accuracy was 6.88% higher in the validation dataset (76.40%) than the training dataset (69.53%). A reduction in classification accuracy of 13.08% was observed when using the full tree (63.33%) as opposed to the first split (76.40%) to classify smoking outcomes in the validation dataset. The average full tree correct classification rate in the validation dataset (63.33%) had a reduced classification accuracy of 18.55% compared to the training dataset (81.88%). One possible reasons for the reduction in correct classification from the training to the validation cohorts may be that the trees are overfit to the training dataset and thus do not perform as well in the out-of-training-sample validation dataset. Another possible reason for the reduction in correct classification may be biological and psychological differences between the two cohorts, for example cohort differences in age or the methodological difference between the training and validation cohorts, namely the contribution of nicotine patch use to treatment outcomes in the training dataset, may influence the predictive accuracy of the trees. However, the improved correct classification rates observed using only the first split of the validation dataset suggested that using the first split alone may reduce overfitting compared to using the full tree. In addition to classification accuracy, the sensitivity and specificity of each tree is shown in Figure 2.
Figure 2.
Sensitivity, specificity, and correct classification of full tree and first split in the training and validation cohorts.
The accuracy of each decision tree was compared to a random classifier that predicted smoking cessation with probability of .5. The full decision trees and first splits of each decision tree in the training cohort all showed statistically significant improvements over a random classification (ps < .001). Only two of the full decision trees (posttreatment CO and 6-month follow-up COT) showed a significant improvement over a random classification in the validation cohort (p < .02), whereas all four of the first splits in the validation cohort significantly improved on random classification (ps < .05).
Delay discounting was the first split in all four decision trees and the classification accuracy was higher using the first split than the entire trees in the validation dataset. The rate of delay discounting at the first split averaged across the four decision trees, ln(k) = −7.1, may provide an estimate for an initial cutoff point for determining treatment response such that those with lower rates of discounting are likely to respond to group CBT for smoking cessation. In the current out-of-sample training cohort, this cutpoint correctly predicted smoking status at posttreatment in 80% of participants (82% based on CO and 78% based on COT) and correctly predicted smoking status at follow-up in 81% of participants (80% based on CO and 82% based on COT).
Discussion
This is the first study to use decision trees to predict treatment outcomes of group CBT for tobacco use disorders. The decision trees ubiquitously identified delay discounting rate as the first split for identifying treatment responders versus nonresponders. This finding is consistent with previous work showing that delay discounting is predictive of treatment outcomes in adolescent marijuana users,(29) cocaine users,(30) and cigarette smokers.(31,32) Across the four trees, an average rate of discounting of ln(k) = −7.1 is suggested as a preliminary cutoff in this population where lower rates of discounting are predictive of treatment response. Importantly, the decision trees also provided sensitivity and specificity that improves on chance prediction of treatment outcomes. In particular when using the first split alone, all four decision trees provided significantly improved correct classification in the independent validation dataset. These findings are discussed in more detail in the following paragraphs.
Decision trees have far-reaching applications in the clinical sciences with the opportunity to improve treatment selection and outcomes. The decision trees reported here (see Figure 1) illustrate the ability of the predictive measures to classify individuals as treatment responders (quitters) or nonresponders (smokers). The full decision trees correctly classified 81.3% of the training dataset. To assess for overfitting and to measure clinical utility, this treatment-outcome prediction tool was applied to other patients than those on which it was developed. Importantly, the validation dataset received the same psychotherapeutic treatment but was not provided with the nicotine patch. Using the independent validation dataset, the first split classification accuracy outperformed the full tree classification accuracy. The first split of each tree, which was delay discounting in all instances, on average correctly classified 74.3% of participants in the validation dataset. In contrast, the average full tree fit for the validation dataset was 63.9% or a reduction in classification accuracy of more than 17% compared to the average full tree classification accuracy in the training dataset and a 10% reduction in accuracy compared to the first split in the validation dataset. In addition, the rate of classification at the first split was significantly better than chance classification in all four decision trees, supporting the use of the first split alone for making predictions of smoking outcomes for new patients entering into group CBT regardless if nicotine patches are provided as part of treatment.
Delay discounting rate was the first split in all four decision trees indicating that it provides the single best classification of treatment outcomes regardless of biochemical verification measure or timepoint. This finding adds to prior work identifying ways to use delay discounting to inform clinical practice and is consistent with suggestions that it be considered a marker of addiction processes.(33) Delay discounting is predictive of treatment outcomes in a variety of substance-using populations.(29–32) Moreover, similar machine-learning methods to those used in this article have identified delay discounting as a powerful measure to predict current stimulant and alcohol dependence from controls.(15,34)
This study goes beyond prior work to establish an estimated rate of delay discounting (ln[k] = –7.1) to use either to select participants for group CBT or to identify participants for additional interventions to improve the rate of delay discounting. As participants with higher discount rates were less likely to respond to treatment, decreasing discounting may improve treatment response. Two interventions, episodic future thinking(35,36) and repetitive transcranial magnetic stimulation,(37) have shown recent promise for improving rates of discounting in cigarette smokers. Adaptively assigning participants with discount rates above the discount rate cutoff for these adjunctive treatments may translate to increased smoking cessation following group CBT.
One limitation of this study is that the prediction of treatment responders is only as good as the set of parameters available. Although the assessment battery was quite extensive, if different subscales or individual items from the included measures were entered as candidates in the classification trees or if different variables were measured in the baseline battery then they may have outperformed those assessed and included here. In addition, as nicotine patches were provided to participants in the training but not the validation cohort, this design difference may have negatively influenced the fit of the trees in the validation cohort. This possibility is supported by the reduction in correct classification using the full trees to classify participants in the validation dataset. The case may be that if the decision trees grown here were applied to individuals that received both group CBT and nicotine patches like those in the training dataset then concerns about overfitting using the full trees may be alleviated.
This study is an early step in developing a tool to identify effective treatment regimens for cigarette smokers interested in cessation. Future work to identify predictive algorithms of treatment response for other smoking cessation interventions will help to elucidate characteristics that help to differentially select between treatment options. A tool of this sort will increase successful quit attempts by allocating people to treatments where they are likely to respond or provide a means to identify those individuals who would benefit from adjunctive, preparatory treatment prior to targeted smoking cessation interventions. Finally, implementation of predictive algorithms for patient treatment allocation may benefit patients by helping them to get treatment that works sooner and consequently have the added benefit of reducing treatment provider burden by reducing the proportion of treatment seekers that do not respond to a given treatment.
Funding
This work was supported by the National Institute on Drug Abuse (R01DA022386-01 to WKB) and (F31AA024368 to LNC).
Declaration of Interest
None declared.
Supplementary Material
References
- 1. Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291(10):1238–1245. [DOI] [PubMed] [Google Scholar]
- 2. Danaei G, Ding EL, Mozaffarian D, et al. . The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6(4):e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. [DOI] [PubMed] [Google Scholar]
- 4. Babb S, Malarcher A Schauer G, Asman K, Jamal A. Quitting smoking among adults—United States, 2000–2015. MMWR Morb Mortal Wkly Rep [Internet]. 2017;65 Available from: https://www.cdc.gov/mmwr/volumes/65/wr/mm6552a1.htm. Accessed September 09, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Fiore M. Treating Tobacco Use and Dependence: 2008 Update: Clinical Practice Guideline. Rockville, MD: Diane Publishing; 2008. 256p. [Google Scholar]
- 6. Anthenelli RM, Benowitz NL, West R, et al. . Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520. [DOI] [PubMed] [Google Scholar]
- 7. Golub RM, Fontanarosa PB. Comparative effectiveness research: relative successes. JAMA. 2012;307(15):1643–1645. [DOI] [PubMed] [Google Scholar]
- 8. Zhu SH, Lee M, Zhuang YL, Gamst A, Wolfson T. Interventions to increase smoking cessation at the population level: how much progress has been made in the last two decades?Tob Control. 2012;21(2):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abrams DB, Orleans CT, Niaura RS, Goldstein MG, Prochaska JO, Velicer W. Integrating individual and public health perspectives for treatment of tobacco dependence under managed health care: a combined stepped-care and matching model. Ann Behav Med. 1996;18(4):290–304. [DOI] [PubMed] [Google Scholar]
- 10. Orleans CT. Increasing the demand for and use of effective smoking-cessation treatments reaping the full health benefits of tobacco-control science and policy gains—in our lifetime. Am J Prev Med. 2007;33(6 suppl):S340–S348. [DOI] [PubMed] [Google Scholar]
- 11. Sheffer C, Mackillop J, McGeary J, et al. . Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. Am J Addict. 2012;21(3):221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sheffer CE, Christensen DR, Landes R, Carter LP, Jackson L, Bickel WK. Delay discounting rates: a strong prognostic indicator of smoking relapse. Addict Behav. 2014;39(11):1682–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pariyadath V, Stein EA, Ross TJ. Machine learning classification of resting state functional connectivity predicts smoking status. Front Hum Neurosci. 2014;8:425. doi:10.3389/fnhum.2014.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang L, Samaras D, Tomasi D. Machine Learning for Clinical Diagnosis From Functional Magnetic Resonance Imaging. Comput Vis Image Underst [Internet] 2005. Available from: http://ieeexplore.ieee.org/abstract/document/1467404/. Accessed September 09, 2017.
- 15. Bickel WK, Moody LN, Eddy CR, Franck CT. Neurocognitive dysfunction in addiction: testing hypotheses of diffuse versus selective phenotypic dysfunction with a classification-based approach. Exp Clin Psychopharmacol. 2017;25(4):322–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Connor JP, Symons M, Feeney GF, Young RM, Wiles J. The application of machine learning techniques as an adjunct to clinical decision making in alcohol dependence treatment. Subst Use Misuse. 2007;42(14):2193–2206. [DOI] [PubMed] [Google Scholar]
- 17. Fiore MC, Jaen CR, Baker T, et al. . Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services [Internet] 2008. Available from: http://www.rfpdb.com/process/download/name/Request-for-Proposal-Department-of-Public-Health_Public-Health-Initiatives-Branch-Telephone-QUITLINE-Services.pdf. Accessed September 09, 2017. [Google Scholar]
- 18. Chambless DL, David Klonsky E. Compendium of empirically supported treatments. In: Koocher GP, Norcross JC, Greene BA, eds. Psychologists’ Desk Reference. Oxford, UK: Oxford University Press; 2013: 160–166. [Google Scholar]
- 19. Schmitz JM, Rosenfarb IS, Payne TJ. Cognitive and affective responses to successful coping during smoking cessation. J Subst Abuse. 1993;5(1):61–72. [DOI] [PubMed] [Google Scholar]
- 20. Sheffer CE, Stitzer M, Payne TJ, Applegate BW, Bourne D, Wheeler JG. Treatment for tobacco dependence for rural, lower-income smokers: outcomes, predictors, and measurement considerations. Am J Health Promot. 2009;23(5):328–338. [DOI] [PubMed] [Google Scholar]
- 21. Sheffer CE, Bickel WK, Franck CT, et al. . Improving tobacco dependence treatment outcomes for smokers of lower socioeconomic status: a randomized clinical trial. Drug Alcohol Depend. 2017;181:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benowitz NL, Jacob P III, Ahijevych K, et al. . Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–59. [DOI] [PubMed] [Google Scholar]
- 23. Hukkanen J, Jacob P III, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 24. Hu FB, Goldberg J, Hedeker D, Flay BR, Pentz MA. Comparison of population-averaged and subject-specific approaches for analyzing repeated binary outcomes. Am J Epidemiol. 1998;147(7):694–703. [DOI] [PubMed] [Google Scholar]
- 25. Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 26. Højsgaard S, Halekoh U, Yan J.. geepack: Generalized Estimating Equation Package. R Package Version. 2014;1–2. [Google Scholar]
- 27. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2014. [Google Scholar]
- 28. Therneau T, Atkinson B, Ripley B.. rpart: Recursive Partitioning and Regression Trees. R Package Version 4.1-10. 2015. [Google Scholar]
- 29. Stanger C, Ryan SR, Fu H, et al. . Delay discounting predicts adolescent substance abuse treatment outcome. Exp Clin Psychopharmacol. 2012;20(3):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Washio Y, Higgins ST, Heil SH, et al. . Delay discounting is associated with treatment response among cocaine-dependent outpatients. Exp Clin Psychopharmacol. 2011;19(3):243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104(3):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krishnan-Sarin S, Reynolds B, Duhig AM, et al. . Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend. 2007;88(1):79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bickel WK, Koffarnus MN, Moody L, Wilson AG. The behavioral- and neuro-economic process of temporal discounting: a candidate behavioral marker of addiction. Neuropharmacology. 2014;76 (pt B):518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahn W-Y, Ramesh D, Moeller FG, Vassileva J. Utility of machine-learning approaches to identify behavioral markers for substance use disorders: impulsivity dimensions as predictors of current cocaine dependence. Front Psychiatry.2016; Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4785183/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stein JS, Wilson AG, Koffarnus MN, Daniel TO, Epstein LH, Bickel WK. Unstuck in time: episodic future thinking reduces delay discounting and cigarette smoking. Psychopharmacology (Berl). 2016;233(21-22):3771–3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stein JS, Tegge AN, Turner JK, Bickel WK. Episodic future thinking reduces delay discounting and cigarette demand: an investigation of the good-subject effect. J Behav Med [Internet]. 2017. Dec 21. Available from: 10.1007/s10865-017-9908-1. [DOI] [PubMed]
- 37. Sheffer CE, Mennemeier M, Landes RD, et al. . Neuromodulation of delay discounting, the reflection effect, and cigarette consumption. J Subst Abuse Treat. 2013;45(2):206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.