Graphical abstract
Keywords: SARS-CoV-2, COVID19, Ethanolamine, Hydroxychloroquine, ACEIs/ARBs inhibitors
Highlights
-
•
Various reports on antiviral drugs and their benefits against coronavirus disease.
-
•
Design strategies of some drugs from their active metabolites are discussed.
-
•
ACEIs/ARBs advantages and disadvantages, antibodies inhibiting interleukin-6 for are discussed.
-
•
Listing of drugs which are currently testing under clinical trials for COVID-19 virus and their mechanism of action are discussed.
Abstract
The inhibition of viral targets might provide new therapies for coronavirus disease abbreviated as COVID-19. The rational drug design identified as much of the recent discoveries of potent drugs molecule against any targets. This results in an improvement in bindings for better potency and selectivity. The drugs containing ethanolamine/propylamine fragments along with heterocycles have shown potential antiviral results. Similarly, there is the possibility of controlling the COVID-19 infection by nucleotide analogues. Here we also highlight drugs ACEIs/ARBs inhibitory discussing both their advantages and disadvantages. The class of compounds/antibodies inhibiting interleukin-6 works in antirheumatoid drugs are found useful in alleviating overactive inflammatory responses in the lungs of the patient. These inclusion based approaches counter some of the side-effects associated with the heterocycles and also potentiate the efficacy of the molecules. In this review article, design strategies for some of the drugs effective against SARS-CoV-2 are represented. The review also focuses on the listing of drugs that are currently testing under clinical trials for the COVID-19 virus with their mechanism of action. This conversation undertakes the opportunity to do a bit for the newer researchers working in this arena.
1. Introduction
Viruses are non-living having genetic material and an outer lipid envelope. The viral disease continued to be a serious issue to public health. The CoVs have become the major pathogens of emerging respiratory disease outbreaks. In the past major epidemic severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002 to 2003 (China), and H1N1 influenza in 2009, and recently the Middle East respiratory syndrome coronavirus (MERS-CoV) 2012 in Saudi Arabia was recorded. In December 2019 Wuhan China detected new viruses, very contagious and rapidly spread globally. It is first noticed in groups of people showing symptoms of pneumonia associated with the seafoods and live animal markets in the city of Wuhan. An epidemic later becomes pandemic known by name 2019-nCoV. They are a large family of single-stranded RNA viruses (+ssRNA) that can be isolated in different animal species. Because of its similarity with SARS outbreak (SARS-CoVs), it is known as SARS-CoV-2 [1]. SARS-CoV-2 is placed as a 7th member of the coronavirus family infecting human after severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV). Bats have been linked with these highly pathogenic viruses (SARS-CoV and MERS-CoV) and other hemorrhagic ebola, Marburg filoviruses, and paramyxoviruses such as Nipah virus [2]. SARS-CoV-2 is a novel coronavirus belonging to β-genus infecting mammals and humans. There is a 94.6% amino acid similarity between SARS-CoV-2 and SARS-CoV which suggests for the origin of these coronaviruses from the same species. Further, a similarity of 96% genome between the SARS-CoV-2 and bat sarbecovirus sampled from Rhinolophus affinis horseshoe bat in Yunan province (2013) Bat-CoV (RaTG13) indicates a homologous relationship between them [3], [4]. This makes to confirm that current outbreak of COVID-19 may have bat origin. A recent study in Myanmar was carried by Valitutto et al to identify coronavirus in bats. Free-ranging bats were captured and rectal and oral swabs were collected and screened for coronavirus by reactive consensus conventional polymerase chain reaction. Three novel alphacoronavirus, three novel beta coronavirus and one known alphacoronavirus were detected in bats in Myanmar [2]. The spike protein having receptor-binding domain (RBD) is the most variable part in the coronavirus genome. Two possible origins for the SARS-CoV-2 is concluded by: One possible conclusion is drawn on the basis of the previous outbreak (i.e. human is infected by virus after exposure to civets and camels for SARS and MERS). Thus, it is reasoned that SARS-CoV-2 is also evolved into a pathogenic state by natural selection first in the non-human host and then spill into humans. Another source of origin is that non-pathogenic viruses in animals jump to humans and then evolved into the current pathogenic states. The SARS-CoV-2 has very similar RBD to the earlier coronavirus from pangolins, armadillo-like mammals. It could have been transferred from Pangolins to humans with in-between hosts such as civets and ferrets [5].
As on April 15, 2020, there were more than 19.5 lakh cases and total death crosses more than 1.26 lakh worldwide. Among the most seriously affected countries are US, Spain, Italy and France [6].
COVID19 contains spike protein in the form of a crown (that’s why named corona) to have attachment to the specific receptors present in the epithelial cell and then multiply. There are several strategies to overcome viral infection; either blocking the receptors to avoid the entry of viruses, destroy the machinery i.e prevention of replication, prevention of release or shredding and activate the natural killer cells to kill the infected cells. Under each category, effective drugs are available [7], [8], [9]. Research efforts are focused on the influenza neuraminidase molecular targets, one of two major glycoproteins located on the influenza virus membrane envelope. This enzyme is responsible for the cleavage of terminal sialic acid residues from glycoconjugates and is essential for virus replication and infectivity [10], other hot areas include developing human neutralizing antibodies/monoclonal antibodies, virus-neutralizing antibodies, searching a library of compounds.
2. Drugs found to alleviate the symptoms of COVID 19 inhibitors
The COVID-19 caused disastrous effects leading to lakhs of death and affecting millions of people worldwide. It causes severe pneumonia and currently, no available antiviral therapy exists to treat SARS-CoV2 patients. A lot of clinical trials are undergoing to develop more targeted and effective viral drugs and vaccines and may take years [11]. However, some existing drugs were found to alleviate the symptoms of COVID-19 and are discussed.
(i) Antimalarial drugs
-
•
Chloroquine/Hydroxychloroquine
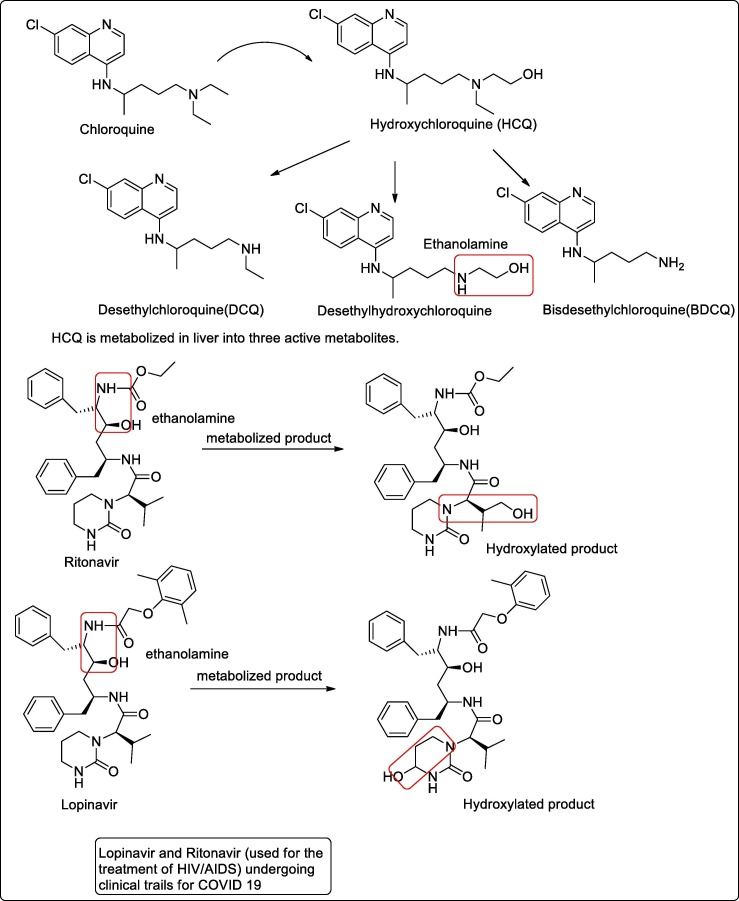
Chloroquine (CQ), a 4-aminoquinoline compound, has been used for the prophylaxis and treatment of malaria. Chloroquine now becomes ineffective for the prevention or treatment of malaria caused by P. falciparum. Hydroxychloroquine (HCQ) is an analogue of CQ in which one of the N-ethyl substituents of CQ is β-hydroxylated. HCQ is preferred over CQ when high doses are required because of the lower level of ocular toxicity of HCQ than of CQ. HCQ is metabolized in the liver into three active metabolites, Desethylchloroquine (DCQ), Desethylhydroxychloroquine (DHCQ) and bisdesethylchloroquine (BDCQ) [12]. Among the three DHCQ being the major metabolite. The absorption half-life was approximately 3–4 h and the terminal half-life ranged from 40 to 50 days [13]. Also, Desethylhydroxychloroquine is implicated early or established stage inflammatory diseases with the advantages that individual escapes retinal toxicity [14]. Clinical trials of hydroxychloroquine (used as effective antimalarial) treatment for COVID-19 pneumonia are underway in China (NCT04261517 and NCT04307693) [15]. The drugs chloroquine/hydroxychloroquine has shown positive results in COVID-19 positive patients. Further, it also suggests for the mechanism of interfering SARS-nCov-2 replication in some of the in-vitro studies [16]. Fig. 1 showing the metabolized product of hydroxychloroquine active against coronavirus.
Fig. 1.
Metabolized product of hydroxychloroquine and drug ritonavir/lopinavir active against coronavirus.
(ii) Antiviral drugs
The basis of antiviral therapy is preventive vaccines and antiviral agents which are effective against certain kinds of virus. The reemergence of new viruses or resistance to the currently available antiviral drugs/vaccines is major problems in current therapy.
-
•
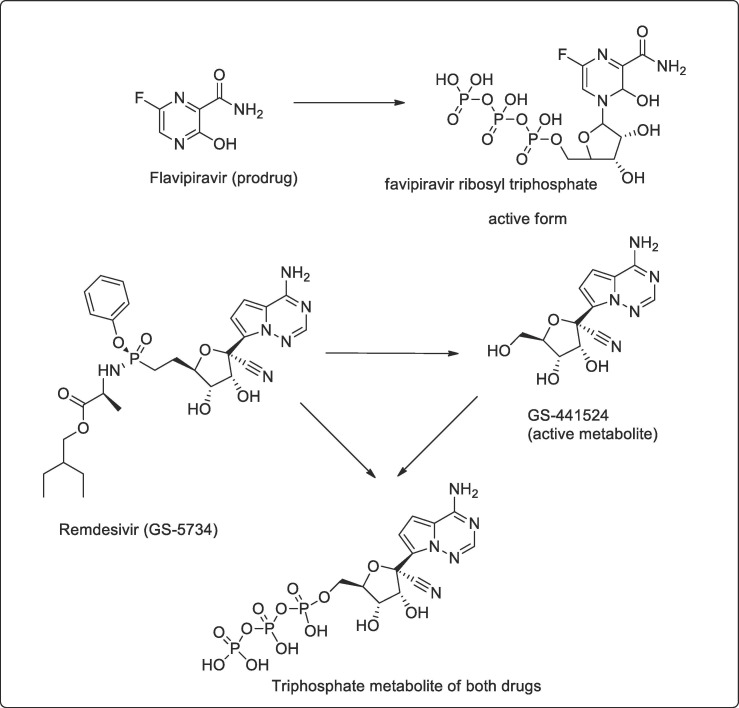
Favipiravir (T-705; 6-fluoro-3-hydroxy-2-pyrazine carboxamide) is an anti-viral agent that selectively and potently inhibits the RNA-dependent RNA polymerase (RdRp) of RNA viruses. It was synthesized by modifying pyrazine analog and effective against all subtypes of influenza viruses, including sensitive or resistant marketed neuraminidase and M2 inhibitors. The drugs are effective against not only the influenza virus but a wide range of viruses [17]. Favipiravir is phosphoribosylated by cellular enzymes to its active form, favipiravir-ribofuranosyl-5′-triphosphate (RTP) Fig. 2 . Ribavirin monophosphate inhibits the cellular enzyme inosine monophosphate dehydrogenase (IMPDH), resulting in reductions in intracellular guanosine triphosphate (GTP) pools. This effect leads to cell cytostasis and other manifestations of cytotoxicity, and likely contributes to the toxic effects of ribavirin in animals and humans [18]. In China, COVID-19 patients recovered by using a combination of existing anti-viral drugs (for SARS, Ebola and AIDS) drugs like Flavipiravir (developed by Japan-Avigan/Ebola), Remdesivir (Ebola) and a combination of Lopinavir and Ritonavir (anti-AIDS) along with Anti-malarial Chloroquine, and Azithromycin (antibiotics) and Pyrimidine (anti-TB). As per reports, out of these, a combination of Flavipiravir is most effective along with Chloroquine, Azithromycin and Pyrimidine coupled with standard care [19].
Fig. 2.
Drugs showing metabolized product active of drug flavipiravir and remdesivir.
It also inhibits influenza strains resistant to current antiviral drugs, and shows a synergistic effect in combination with oseltamivir, thereby expanding influenza treatment options [20].
In one of the trials testing favipiravir with interferon in Shenzhen, results showed that patients treated with the combination had significantly reduced the duration of symptoms, as measured by viral load chest imaging, vs. a control group. In another study, clinical recovery rates were higher for COVID-19 patients treated with favipiravir vs. those in a control group [21].
-
•
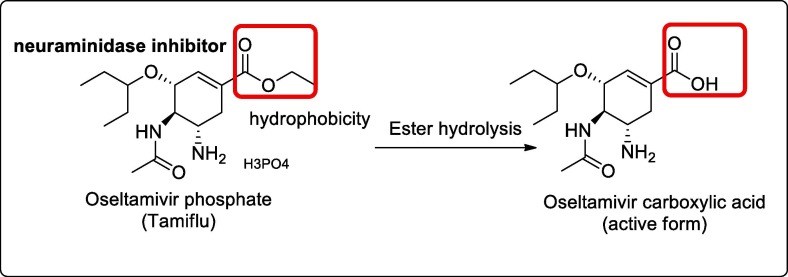
Oseltamivir is the first neuraminidase inhibitor available orally. The drug is used under the name Tamiflu for the treatment and prevention of influenza A and influenza B [22]. The drug is found to bind and inhibit the active site of neuraminidase enzymes required by the viral particle to release virions progeny. It thus reduces viral replication and reduces the viral load [23]. However, based on Cochrane review 2014, Osteltamivir does not reduce hospitalizations neither reduces the complications of influenza [24]. Further, it was also used in one of phase III clinical trials for COVID-19 in combination with ritonavir and protease inhibitor ASC09F [25], [26]. Fig. 3 .
-
•
Lopinavir and Ritonavir are effective antiretroviral of the protease inhibitor class (used in the treatment and prevention of AIDS/HIV) [27], undergoing clinical trials for COVID 19 [28]. These two drugs are antiretroviral of the protease inhibitor class and are given in fixed-dose combinations. The product of hydroxylation at the methine carbon of the terminal isopropyl moiety of ritonavir (propanolamine derived), was the only metabolite present in human plasma and made up 30.4% of the total dose recovered in human excreta over 6 days [29]. In the case of lopinavir, the predominant site of metabolism was found to be the carbon-4 of the cyclic urea moiety (propanolamine derived), with subsequent secondary metabolism occurring on the diphenyl core moiety [30]. Fig. 1 shows the metabolized product of drug Ritonavir/Lopinavir effective against coronavirus.
-
•
Remdesivir
Fig. 3.
Showing the structural features of oseltamivir and its active metabolite.
This drug is in phase III clinical trials in countries with a high prevalence of COVID19. Remdisiviral a broad-spectrum antiviral drugs target viral proteins and prevents copying, replication and decrease in viral production [31]. They are adenosine analogues and in some virus it insert into the viral chain and induces irreversible chain termination [32]. After its conversion into active triphosphate form the drugs, it competes for the adenosine triphosphate (natural nucleotide required for RNA synthesis) required for replication and thus terminate the RNA synthesis [33] Fig. 2.
-
•
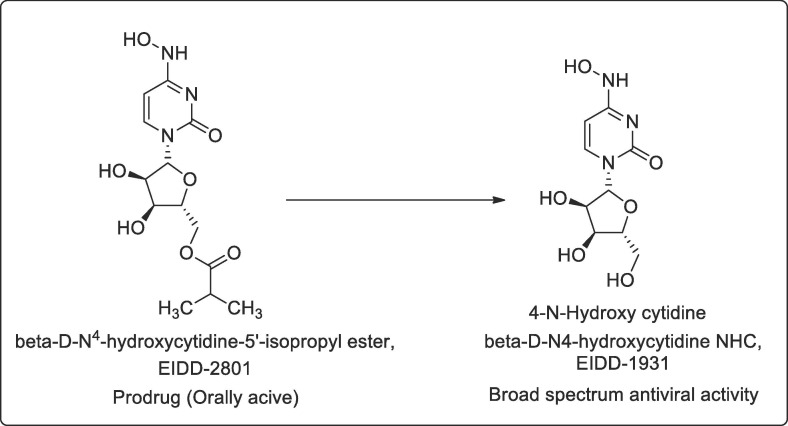
EIDD-1931
Another oral drug β-D-N4-hydroxycytidine NHC, EIDD-1931 a cytidine analogue showed promise in test-tube experiments with human lungs and airways cells. The article as described in Translational medicine April 6 a nucleoside analogues orally bioavailable prodrug (β-D-N4-hydroxycytidine-5′-isopropyl ester, EIDD-2801) possess broad-spectrum antiviral activity against SARS-CoV-2, MERS-CoV, SARS-CoV, and related zoonotic group 2b or 2c Bat-CoVs, as well as increased potency against a coronavirus bearing resistance mutations to the nucleoside analog inhibitor remdesivir [34] Fig. 4 .
-
•
Arbidol
Fig. 4.
Shows the metabolized active product of EIDD-2801.
Arbidol is used to treat influenza virus and administered orally at a dose of 200 mg for adults 3 times a day. In vitro studies inhibit SARS-CoV-2 infection at conc. of 10–30 μM [35]. A randomized clinical trial by Chen et al of drug Flavipiravir Vs. Arbidol was compared. The clinical recovery rate does not significantly differ between Flavipiravir and the Arbidol group whereas fever reduction and cough relief latency was shorter for Flavipiravir than Arbidol [36]. Another study by Zhu et al showed that Arbidol is superior to Lopinavir/Ritonavir in treating COVID-19. He evaluated the antiviral effects and safety of Lopinavir/Ritonavir against Arbidol in fifty patients of 2019-nCoV. The patient was divided into groups of 34 who received 400 mg/100 mg of Lopinavir/ritonavir twice a day for a week and 16 patient receives 0.2 g arbidol three times a day. No side-effects were observed in both the group and no signs of severe pneumonia. Viral load was not detected in the case of Arbidol group after 14 days whereas viral load was there in 15 patients (44.1%) who had received Lopinavir/ritonavir. Further, it was confirmed that patient on Arbidol drugs has a shorter positive RNA test compared to Lopinavir/ritonavir [37].
-
•
Galidesivir
Galidesivir is adenosine analogues that block RNA polymerase. RNA polymerase is involved in the viral replication process. Galidesivir is activated to active triphosphate form by cellular kinases and gets incorporated into the growing chains of RNA strand leading to chain termination. A Galidesivir drug is undergoing clinical trials and part 1 of the trial includes 24 hospitalized with three sequential cohorts of eight patients each. The patient receives intravenous (IV) galidesivir (n = 6) and placebo (n = 2) every 12 h for 7 days. After this dose regimen will be selected for part 2 of the trial based on the results of part 1 including safety, viral load, reduction in respiratory tract secretion and improvements in COVID-19 infection and mortality. A total of 42 hospitalized patients of COVID-19 will include in part 2 of the trial and will be randomized into 2:1 to receive IV galidesivir and placebo. The patient will remain hospitalized or release after symptoms allow the release and will be observed for mortality till day 56 [38].
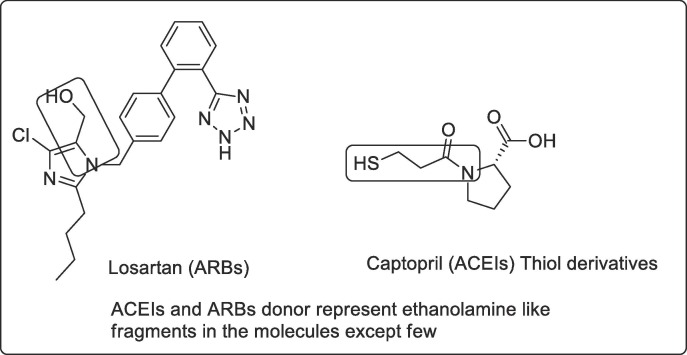
(iii) ACEIs/ARBs (Angiotensin converting enzyme inhibitors or Angiotensin-receptors blockers)
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and (SARS-CoV) uses the receptor angiotensin‐converting enzyme (ACE) 2 for entry into target cells. which is specifically highly expressed by epithelial cells in oral mucosa [39], [40], [41]. It is also argued that ACE2 could be directly inhibited by ACE inhibitors but ACE2 functions as a carboxypeptidase and is not inhibited by clinically prescribed ACE inhibitors [42]. ACE2 are increased in patients with hypertension or diabetes, and levels are further increased by different drugs, including ACEIs (Angiotensin-converting enzyme inhibitors e.g. captopril) and ARBs (Angiotensin-receptor blockers, e.g. Losartan) Fig. 5 [40]. Thus it increases the risk for the severity of SARS-CoV2. Conversely, some researchers also tell about benefits to the patient taking these mentioned drugs. They justified it as the upregulating of ACE2 will have benefits as it has vasodilatory and anti-inflammatory properties after ACE2 converts angiotensin II to angiotensin (1–7) [43]. There is preclinical studies in mice that justified that ARB treatment reverses lung injury through SARS-CoV-2 invasion by decreasing ACE2. Another way preclinical data also tells about the increase in ACE2 support for an effective treatment for viral lung injury. These preclinical studies do not support in clinical trials with the human subjects. One studies tells that it neither benefits by increasing ACE2 (done by infusing ACE2) nor ACEIs or ARBs decreases the severity by SARS-CoV-2 lung injury [44]. There is no such observational studies to confirm whether these ACEIs or ARBs could potentially harm or benefits the patient. Several European and American cardiology societies dispel this misinformation and advised not to stop these RAAS antagonists unless advised by physician [45], [46].
Fig. 5.
Drug losartan and captopril under the category of ARBs/ACEIs.
(iv) An immunosuppressant/arthritic drugs
-
•
Actemra (tocilizumab) - This drug is an IL-6 (interleukin 6) inhibitor (whole antibody) designed and was approved by the FDA for clinical trial III for the treatment of coronavirus in countries China and Japan and America. This drug is used in European countries for the treatment of Arthritis. This drug was permitted as earlier research suggested for increase in inflammatory response through increased interleukin-6 and causes the death of a patient suffering from community-acquired pneumonia [47]. More details of the drug tocilizumab is also discussed in antibodies under miscellaneous section.
-
•
Kevzara (sarilumab) – is also a whole human monoclonal antibody and work against Interleukin-6 receptors. This drug is FDA approved for rheumatoid arthritis. The drug is tested in COVID patients as IL-6 may play a role of inhibiting overactive inflammatory response in the lungs of severely and critically ill patients. It showed an improvement rapidly reducing fevers and the patient requires less oxygen support [48], [49].
-
•
Ruxolitinib
Phase I and phase II study was already carried out inpatient with COVID-19. Roxolitinib is an inhibitor of JAK ½ and is associated with multiple cellular signals including IL-6. The study intended to stop the dysregulated immune response caused by COVID-19. This drug work as an immunomodulator decreasing the respiratory or pneumonia symptoms caused by dysregulated immune response. It showed a good recovery of pneumonia in its primary outcome [50], [51].
-
•
Calquence (acalabrutinib):- The drug is used for the treatment of lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL) (blood cancer) and is Bruton’s tyrosine kinase (BTK) inhibitor. This was found to be useful in later stages of COVID-19 patients on ventilator support or intensive care unit. The drug was found to have some clinical benefits to the patient suffering from advanced lung diseases, but now it will be very early to say that it will provide benefits to the patients for recovery [52], [53].
-
•
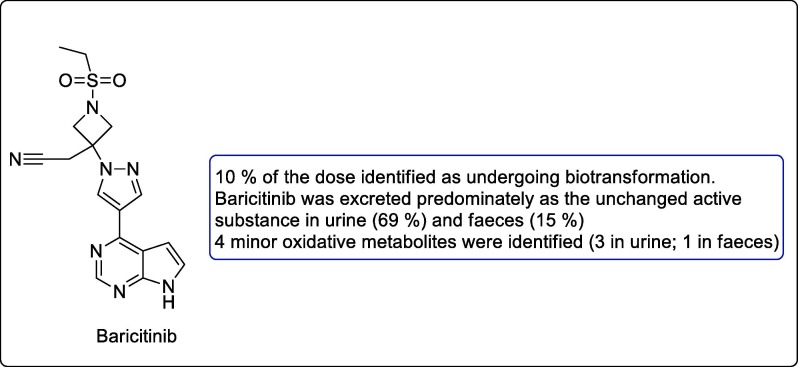
Baricitinib
Baricitinib is small molecule inhibitors and has potential effects in inhibiting JAK1 and JAK2 mediated cytokine release and thus may be regarded as helpful in inhibiting coronavirus to infect the cells Fig. 6 . The drug was approved for the treatment of rheumatoid arthritis, works by interfering STAT proteins and now undergoing clinical trials for COVID-19. This drug is used in combination with other antivirals as it has no potential drug-drug interaction [54], [55].
Fig. 6.
Structure of small molecule inhibitor baricitinib.
There is a general consensus that these JAK inhibitors may reduce the severity of patients infected with corona disease. However, some authors refuted the claims. One of the articles published claim that early stages of asymptomatic disease (80% cases) can clear virus through their endogenous antiviral mechanism (including interferon). However, these drugs may be helpful in the severe phase of the disease after the 7 days of onset of disease, where the severe inflammatory condition is seen, associated with high levels of interleukin-6, interferon α and β. These drugs may help the patient require hospital care where the JAK-STAT pathway largely mediated. Inhibition of the pathway may be considered as a potential strategy in alleviating the symptoms of coronavirus disease [56].
3. Miscellaneous
(i) Convalescent plasma therapy
Convalescent plasma therapy is used for recovery of SARS patients whose condition worsens despite all possible treatments. There is a report available that lower hospital stay and mortality rate is observed for the patient in convalescent plasma therapy than who are not treated with plasma therapy. The therapy is effective as antibodies from the convalescent therapy reduce the viral load and treat viremia. Viremia is in the highest peak in the first 10–14 days and primary immune response develops for virus clearance. Plasma therapy is more effective in the early stages of the disease. There is evidence that convalescent plasma may be used for the treatment of coronavirus patients without severe adverse effects. However there is a continuous need to evaluate for safety and efficacy of convalescent plasma therapy [57]. A pilot study was carried out by Duan et al for the feasibility of convalescent plasma therapy in severe COVID-19 patients. A dose of 200 mL convalescent plasma (CP) from freshly recovered patients of coronavirus with the neutralizing antibody titers above 1:640 was transfused to the patient. The clinical symptoms were significantly improved, increase in oxyhemoglobin saturation, increase lymphocyte count, decreased in C-reactive protein, and viral load was undetected in viremia patients without any severe side effects. The study confirmed for well-tolerated CP therapy in neutralizing viremia and needs further investigation [58]. Saghazadeh et al showed that plasma from patients recovered from COVID-19 has antibodies that showed good results in patients with severe COVID-19. Intravenous immunoglobulins and corticosteroids further improve results. Corticosteroids may help in the recovery of patients of COVID-19 however, there in no clinical trials results to support for corticosteroids therapy. Pro-inflammatory cytokines IL6 inhibitor especially monoclonal antibodies tocilizumab have the potential of clinical benefits to patients with COVID-19 severity [59]. Similarly, Shanmugaraj et al also displayed that monoclonal antibodies are a major class of effective passive immunotherapy against COVID-19 cases. The antibodies are isolated from the blood of infected patients. Immunotherapy by transferring convalescent sera helps in neutralizing the virus of infected patients [60]. Jawhara et al also showed that intravenous immunoglobulins (IVIg) may neutralize COVID-19 by boosting immune response in COVID-19 patients and were effective when they are taken from patients who have recovered from COVID-19 [61].
(ii) Antibodies
The spike (S) proteins of SARS-CoV-2 mediate entry through the receptor-binding domain (RBD) of ACE2. Antibodies to RBD are effective in neutralizing SARS-CoV-1S-protein-mediated entry. Quinlan et al showed immunization with SARS-CoV-2 RBD has a strong neutralizing response in rodents and was found to be comparable with 100 μg/ml of ACE2-Ig (potent SARS-CoV-2 entry inhibitors). Antibody-dependent enhancement (ADE) led to the pathogenicity of feline coronaviruses. Neutralizing antisera from immunized animals did not show the enhancement of the antibody in case of SARS-CoV-2 as was more commonly observed in Zika-virus antibody dependent enhancement. Thus it was further concluded that vaccines based on RBD against SARS-CoV-2 will be safe and effective [62]. S proteins present in the spike of coronavirus are major targets for the development of therapeutics. Wrapp et al isolate and characterized single domain antibodies (VHHs) from llama immunized with coronavirus spikes. The single-domain antibodies are potent neutralizing of both MERS-CoV and SARS-CoV-1 S pseudotyped viruses. The results showed cross-reactivity between SARS-CoV-1 S-directed single domain antibodies and SARS-CoV-2 S. This cross-reactivity single domain antibody (VHH) results in neutralizing SARS-CoV-2 S pseudotyped. This result showed that these VHH may serve as potential therapeutics against the neutralization of betacoronavirus [63]. Luo et al evaluated Tocilizumab treatment in 15 patients against COVID-19 for C-reactive protein (CRP) and IL6. Among them, 8 patients have received Tocilizumab in combination with methylprednisolone and another 5 patient received tocilizumab twice or more. Tocilizumab treatment improved the increased C-reactive protein in all patients and also decreased serum IL6. An increase in serum IL6 was found in patients who failed to receive tocilizumab. Thus it was concluded that tocilizumab is an effective treatment for COVID-19 patients with high cytokine storms [64]. Zhang et al showed through many data that cytokines storms in many patients are the cause of death and treatment to reduce these cytokines storms save COVID-19 patients. IL6 causes the release of cytokines and inhibition of IL6 pathway by the use of drugs such as tocilizumab blocks the IL6 pathway. Thus it was concluded tocilizumab to be effective drugs for patients with severe COVID-19 [65].
(iii) Anticoagulants
The COVID-19 disease includes life-threatening blood clots including coagulopathy called intravascular coagulation showing prothrombotic and venous thromboembolism. Initial data showed that anticoagulant therapy lessens the mortality rate in severe COVID-19 patients in sepsis-induced coagulopathy and elevated d-dimer. It is now recommended that COVID-19 hospitalized patients may receive thromboprophylaxis or full intensity anticoagulant therapy [66]. Song et al discussed that coagulation is associated with major death cause in COVID-19 patients. This led to expert consensus by the People’s Liberation Army Professional Committee of Critical Care Medicine and Chinese Society on Thrombosis and Haemostasis for the treatment of COVID associated dysfunction in COVID-19 patients. This includes anticoagulation therapy, test for coagulation, supporting therapy and preventions and others [67]. Comorbidities such as hypertension, diabetes and cardiovascular diseases are at higher risk for COVID-19. Cardiovascular manifestation of COVID-19 includes acute coronary syndrome, myocarditis, arrhythmias, cardiac arrest and cardiogenic shock. This has similarities with earlier SARS and MERS. Treatment of cardiovascular complications includes anticoagulation therapy, immunosuppressive, hemodynamic support, anti-arrhythmic, ACEIs and ARB [68]. Behnood Bikdeli et al concluded through their study that thrombotic disease may give a signal for patients with COVID-19. COVID-19 influences patients with thrombosis both arterial and venous circulations, platelet activation and endothelial dysfunctions. Some patients receiving antithrombotic therapy for thrombosis may develop COVID-19. Important consideration should be followed for the use of thrombotic drugs for the prevention or mitigation of thrombotic and hemorrhagic in high-risk patients [69]. Another study by Tang et al showed that anticoagulant treatment decreases mortality in COVID-19 patients with coagulopathy. The studies were conducted on 449 patients of whom 99 were received heparin (low molecular weight heparin LMWH). No differences were found in the mortality rate with heparin and nonheparin user after 28 days. However, the after 28 days mortality rate were lower in a patient with sepsis-induced coagulopathy (SIC) score or elevated d-dimer [70].
4. Outlook
Based on the most common drugs given to reduce the infection caused by SARS-CoV2, here we postulated that some of the drugs have the common features of ethanolamine/propylamine group present or activated to have that fragment present to decrease the severity of infection. One property of monoethyl amine is under carbon dioxide (hypoxia condition; carbonic acid formation) carbamate formation takes place which can be cyclized into oxazolidin-2-one [71]. The oxazolidin-2-one is known to possess antibacterial properties and is present in many antibacterial compounds such as Linezolid, Posizolid, Tedizolid, Redezolid (antibacterial), Cycloserine (antitubercular) and Contezolid (phase III) and Rivaroxaban approved by FDA for venous thromboembolism [27]. Most of the cases the immune system recover and kill the infected cells. Sometimes it becomes critical as lungs protective linings is gone and the patient further gets infected with bacteria and pneumonia occurs. The patient requires ventilator support for breathing. Bacteria multiplied and enter the bloodstream and death occurs. Pre-exposure prophylaxis and postexposure prophylaxis (PEP) with antimicrobial drugs are effective in preventing illness before potential exposure or after documented exposure to a variety of microbial pathogens, and in reducing the risk of secondary spread of infection [72]. Another major area covered in this review is antiviral drugs and the possibility of developing more potent inhibitors with better selectivity. Targetting through ACEIs/ARBs offers an interesting prospects for inhibiting virus entry. In the future, this could provide a successful design of potent inhibitors for coronavirus toppling its considerable risk. The growing benefits of interleukin inhibitors can be a step towards the right direction against coronavirus, but the prolems is that it is not more advantageous to the early detected patient with coronavirus. Hence the use is limited to the patient severely affected with lung infection. Convalescent plasma, antibodies and anticoagulant therapy lessen the mortality rate in severe COVID-19 patients, but it is quite early to predict the safety and efficacy of these therapy in SARS-CoV-2 infected patients.
5. Conclusion
Drug discovery is a challenging process due to the complexity of the biological system. Traditional randomized search procedure in the discovery of new drugs involved high expenditure in terms of time and money. The recent advance in organic, medicinal chemistry and computer science provides useful tools for the rational design of new chemical moieties or derivatives via mathematical models with predictive capabilities. Virus drug development has advanced in the past few years and resulted in some potent and selective antiviral drugs/vaccines. Still, these drugs are ineffective in treating completely SARS-CoV2. There is a chemical challenge to develop potent and selective antiviral drugs as viruses are clever enough to mutate and become resistant to the available drug very quickly. Current needs is the development of multi-targeted drugs through rational drug design rather than high throughput screening. Based on the current data ethanolamine/propylamine may play a major role along with heterocycles and expected to be active against COVID-19. Similarly, nucleotide analogues offered advantages by blocking the RNA replication. Targetting through ARBs/ACEIs, inhibiting interleukin offers another interesting opportunities. Such functionalities will not only target the viruses but also help medicinal chemists to design more effective drugs in the coming future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The author is thankful to the to Mr. Praveen Garg, Chairman and Prof. (Dr) G. D. Gupta, Director cum Principal, ISF College of Pharmacy, Moga, Punjab for his continuous support and encouragement.
References
- 1.M. Cascella, M. Rajnik, A. Cuomo, S.C. Dulebohn, R.D. Napoli, Features, Evaluation and Treatment Coronavirus (COVID-19), Treasure Island (FL); StatPearls Publishing, Jan 2020, https://www.ncbi.nlm.nih.gov/books/NBK554776/. [PubMed]
- 2.Valitutto M.T., Aung O., Tun K.Y.N. Detection of novel coronaviruses in bats in Myanmar. Plos One. 2020;15:1–11. doi: 10.1371/journal.pone.0230802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang P., Wang X. COVID-19: a new challenge for human beings. Cell. Mol. Immunol. 2020;17:555–557. doi: 10.1038/s41423-020-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.M.F. Boni, P. Lemey, X. Jiang, T. Tsan-Yuk Lam, B. Perry, T. Castoe, A. Rambaut, D.L. Robertson, Evolutionary origins of the SARS‐CoV‐2 sarbecovirus lineage responsible for the COVID-19 pandemic, bioRxiv, the preprint server for biology, 2020, pp. 1–25. https://doi.org/10.1101/2020.03.30.015008. [DOI] [PubMed]
- 5.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26 doi: 10.1038/s41591-020-0820-9. 450–455. www.nature.com/naturemedicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronavirus disease (COVID-19) pandemic, World Health Organization, https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
- 7.The Viral Life Cycle, Lumen Microbiology, https://courses.lumenlearning.com/microbiology/chapter/the-viral-life-cycle/.
- 8.The cycle of Infection, Encyclopaedia Britannica, https://www.britannica.com/science/virus/The-cycle-of-infection.
- 9.Viral Infection, Teach me physiology, https://teachmephysiology.com/immune-system/immune-responses/viral-infection/.
- 10.Lew W., Chen X., Kim C.U. Discovery and development of GS 4104 (oseltamivir): An orally active influenza neuraminidase inhibitor. Curr. Med. Chem. 2000;7:663–672. doi: 10.2174/0929867003374886. [DOI] [PubMed] [Google Scholar]
- 11.Tan E.L.C., Ooi E.E., Lin C., Tan H.C., Ling A.E., Lim B., Stanton L.W. Inhibition of SARS coronavirus infection in vitro with clinically approved antiviral drugs. Emerg. Infect. Dis. 2004;10:581–586. doi: 10.3201/eid1004.030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim H., Im J., Cho J. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by plasmodium vivax. Antimicrob. Agents. Chemother. 2009;53:1468–1475. doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plaquenil Hydroxychloroquine Sulfate Tablets, USP, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/009768s037s045s047lbl.pdf.
- 14.H. Robinson, W.H. Robinson, W. Sokolab, J. Wang, Chan, Desethylhydroxychloroquine for the treatment of diseases with inflammation. Patent JP2016518338A, 2013.
- 15.Efficacy and Safety of Hydroxychloroquine for Treatment of Pneumonia Caused by 2019-nCoV (HC-nCoV), https://clinicaltrials.gov/ct2/show/NCT04261517.
- 16.Effectiveness of Hydroxychloroquine in Covid-19 Patients (Covid), https://clinicaltrials.gov/ct2/show/NCT04328272.
- 17.Furuta Y., Komeno T., Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smee D.F., Hurst B.L., Egawa H., Takahashi K., Kadota T., Furuta Y. Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells. J. Antimicrob. Chemother. 2009;64:741–746. doi: 10.1093/jac/dkp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.A. Ghosh, U.S. stocks inched up Thu on coordinated global stimulus and hopes of an imminent Trump 'magic drug' (Chloroquine) to treat COVID-19; Dow slips early Fri on increasing U.S./global lockdown, Published 20th March 2020. https://www.iforex.in/news/us-stocks-inched-thu-coordinated-global-stimulus-and-hopes-imminent-trump-magic-drug-chloroquine-treat-covid-19-dow-slips-early-fri-increasing-usglobal-lockdown-67589.
- 20.Flavipiravir, Inxight: Drugs, National center for advancing Translational sciences, https://drugs.ncats.io/drug/EW5GL2X7E0.
- 21.M. Fitzhugh, Antiviral favipiravir effective against COVID-19, China says, Published 18th March 2020, https://www.bioworld.com/articles/433810-antiviral-favipiravir-effective-against-covid-19-china-says.
- 22.Oseltamivir phosphate monograph for professionals. The American Society of Health-System Pharmacists. Archived from the original on 13 May 2016. Retrieved 8 January 2017.
- 23.A Randomized, Open, Controlled Clinical Study to Evaluate the Efficacy of ASC09F and Ritonavir for 2019-nCoV Pneumonia, https://clinicaltrials.gov/ct2/show/NCT04261270.
- 24.Butler D. Tamiflu report comes under fire. Nat. 2014;508:439–440. doi: 10.1038/508439a. [DOI] [PubMed] [Google Scholar]
- 25.Davies B.E. pharmacokinetics of Oseltaminir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J. Antimicrob. Chemoth. 65. 2010;suppl 2:ii5-10. doi: 10.1093/jac/dkq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat. Biotech. 2020;38:379–381. doi: 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- 27.C.O. Gualerzi, L. Brandi, A. Fabbretti, C.L. Pon, Antibiotics: Targets, Mechanisms and Resistance. 2013, Online ISBN: 9783527659685, DOI: 10.1002/9783527659685.
- 28.M.D.B. Cao, M.D.Y. Wang, M.D.D. Wen, et al., A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19, N. Eng. J. Med. May 07, 2020, https://publons.com/publon/30788006/. [DOI] [PMC free article] [PubMed]
- 29.Denissen J.F., Grabowski B.A., Johnson M.K., Buko A.M., Kempf D.J., Thomas S.B., Surber B.W. Metabolism and disposition of the HIV-1 protease inhibitor ritonavir (ABT-538) in rats, dogs, and humans. Drug. Metab. Dispos. 1997;25:489–501. [PubMed] [Google Scholar]
- 30.Kumar G.N., Jayanti V.K., Johnson M.K., Uchic J., Thomas S., Lee R.D., Grabowski B.A., Sham H.L., Kempf D.J., Denissen J.F., Marsh K.C., Sun E., Roberts S.A. Metabolism and disposition of the HIV-1 protease inhibitor lopinavir (ABT-378) given in combination with ritonavir in rats, dogs, and humans. Pharm. Res. 2004;21:1622–1630. doi: 10.1023/b:pham.0000041457.64638.8d. [DOI] [PubMed] [Google Scholar]
- 31.Coronavirus treatments: what drugs might work against COVID-19? April 16, 2020. https://theconversation.com/coronavirus-treatments-what-drugs-might-work-against-covid-19-135352.
- 32.Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. Mechanism of inhibition of ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11:326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9 doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheahan T.P., Sims A.C., Zhou S. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020;12:1–15. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug. Discov. Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 36.Chen C., Huang J., Yin P. Favipiravir versus Arbidol for COVID-19: A randomized clinical trial. medRxiv. 2020:1–30. doi: 10.1101/2020.03.17.20037432. [DOI] [Google Scholar]
- 37.Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T., Lu J., Xue Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Inf. S. 2020;0163:30188–30192. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.A Study to Evaluate the Safety, Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever or COVID-19, https://clinicaltrials.gov/ct2/show/study/NCT03891420.
- 39.Fang L., Karakiulakis G., Roth M. Antihypertensive drugs and risk of COVID-19? The Lancet. 2020;8:e32–e33. doi: 10.1016/S2213-2600(20)30159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sommerstein R., Kochen M.M., Messerli F.H., Gräni C. Coronavirus disease 2019 (COVID-19): Do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J. Am. Heart. Assoc. 2020;9 doi: 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet. Resp. Med. 2020;8 doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Y.L. Maxwell, ACE Inhibitors, ARBs, and COVID-19: New Insights, Advice, March 26, 2020. https://www.tctmd.com/news/ace-inhibitors-arbs-and-covid-19-new-insights-advice.
- 43.D. Rind, ACE Inhibitors and ARBs During the COVID-19 Pandemic, Clinical Spotlight, N. Eng. J. Med. April 09, 2020. https://www.jwatch.org/na51345/2020/04/09/ace-inhibitors-and-arbs-during-covid-19-pandemic.
- 44.A.B. Patel, A. Verma, COVID-19 and angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: what is the evidence? JAMA 323 (2020) 1769-1770. Published online March 24th 2020. doi:10.1001/jama.2020.4812. [DOI] [PubMed]
- 45.Cardiology societies recommend patients taking ACE inhibitors, ARBs who contract COVID-19 should continue treatment, Published March 17th 2020. https://www.healio.com/cardiology/vascular-medicine/news/online/%7Bfe7f0842-aecb-417b-9ecf-3fe7e0ddd991%7D/cardiology-societies-recommend-patients-taking-ace-inhibitors-arbs-who-contract-covid-19-should-continue-treatment.
- 46.A statement from the International Society of Hypertension on COVID-19, Published 16th march 2020. https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/.
- 47.FDA approves Roche’s Actemra COVID-19 trial, Published March 24th 2020. http://www.pmlive.com/pharma_news/fda_approves_roches_actemra_covid-19_trial_1329887.
- 48.Sanofi doses first patient in global Kevzara COVID-19 trial, Published March 30th 2020. https://www.pmlive.com/pharma_news/sanofi_doses_first_patient_in_global_kevzara_covid-19_trial_1331903.
- 49.See Sanofi Press Release, 30 March 2020, https://www.sanofi.com/en/media-room/press-releases/2020/2020-03-30-07-00-00.
- 50.Treatment of SARS Caused by COVID-19 With Ruxolitinib, https://clinicaltrials.gov/ct2/show/NCT04334044.
- 51.Ruxolitinib in Covid-19 Patients With Defined Hyperinflammation (RuxCoFlam), https://clinicaltrials.gov/ct2/show/NCT04338958.
- 52.Acalabrutinib Study With Best Supportive Care Versus Best Supportive Care in Subjects Hospitalized With COVID-19. (CALAVI), https://clinicaltrials.gov/ct2/show/NCT04346199.
- 53.L. Astor, Acalabrutinib to be Tested as Treatment of Exaggerated COVID-19-Related Immune Response, https://www.targetedonc.com/news/acalabrutinib-to-be-tested-as-treatment-of-exaggerated-covid19related-immune-response.
- 54.Baricitinib in Symptomatic Patients Infected by COVID-19: an Open-label, Pilot Study. (BARI-COVID), https://clinicaltrials.gov/ct2/show/NCT04320277.
- 55.Olumiant 2 mg Film-Coated Tablets, https://www.medicines.org.uk/emc/product/2434/smpc.
- 56.P.J. Richardson, M. Corbellino, J. Stebbing, lancet. Infect. Dis. Baricitinib for COVID-19: a suitable treatment? 2020, Published Online. April 3, 2020, https://doi.org/10.1016/S1473-3099(20)30270-X. [DOI] [PMC free article] [PubMed]
- 57.Chen Long, Xiong Jing, Bao Lei, Shi Yuan. Convalescent plasma as a potential therapy for COVID-19. The Lancet Infectious Diseases. 2020;20(4):398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duan K., Liu B., Li C. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. PNAS. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saghazadeha A., Rezaei N. Towards treatment planning of COVID-19: Rationale and hypothesis for the use of multiple immunosuppressive agents: Anti-antibodies, immunoglobulins, and corticosteroids. Int. Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian. Pac. J. Allergy. 2020;38:10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 61.Jawhara S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int. J. Mol. Sci. 2020;21:2272. doi: 10.3390/ijms21072272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.B.D. Quinlan, H. Mou, L. Zhang, Y. Guo, W. He, A. Ojha, M.S. Parcells, G. Luo, W. Li, G. Zhong, H. Choe, M. Farzan, The SARS-CoV-2 receptor-binding domain elicits a potent neutralizing response without antibody-dependent enhancement, bioRxiV (2020). https://doi.org/10.1101/2020.04.10.036418.
- 63.Wrapp D., Vlieger D.D., Corbett K.S., Torres G.M., Breedam W.V., Roose K., Schie L.V., Hoffmann M., Pöhlmann S., Graham P.B.S., Callewaert N., Schepens B., Saelens X., McLellan J.S. Structural basis for potent neutralization of betacoronaviruses by single-domain. Cell. 2020;181:1004–1015. doi: 10.1101/2020.03.26.010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020;92:1–5. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang C., Wu Z., Li J., Zhao H., Wang G. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: Emerging evidence and call for action. Br. J. Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. https://pubmed.ncbi.nlm.nih.gov/32304577/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song J., Wang G., Zhang W., Zhang Y., Li W., Zhou Z. Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil. Med. Res. 2020;7:1–10. doi: 10.1186/s40779-020-00247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Y. Kang, T. Chen, D. Mui, V. Ferrari, D. Jagasia, M. Scherrer-Crosbie, Y. Chen,Y. Han, Cardiovascular manifestations and treatment considerations in Covid-19 [published online ahead of print, 2020 Apr 30], Heart 2020; heartjnl-2020-317056. doi:10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed]
- 69.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. Am. J. Cardiol. 2020:1–59. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18:1094–1099. doi: 10.1111/JTH.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.P.E. Holub, J.E. Critchfield, W.Y. Su, Amine degradation chemistry CO2 service, in; Proceedings of the Laurance Reid Gas Conditioning Conference, University of Oklahoma Foundation, 1998, pp. 146–160.
- 72.Mitja O., Clotet B. Use of antiviral drugs to reduce COVID-19 transmission, Published Online. Lancet. Glob. Health. 2020;8:e639–e640. doi: 10.1016/S2214-109X(20)30114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]