Abstract
The success rate of oral implants is lower in type 2 diabetes mellitus (T2DM) patients than in nondiabetic subjects; functional impairment of bone marrow-derived mesenchymal stem cells (BMSCs) is an important underlying cause. Many factors in the blood can act on BMSCs to regulate their biological functions and influence implant osseointegration, but which factors play important negative roles in T2DM patients is still unclear. This study is aimed at screening differentially expressed genes in the blood from T2DM and nondiabetic patients, identifying which genes impact the osteogenic differentiation potential of alveolar BMSCs in T2DM patients, exploring drug intervention regimens, and providing a basis for improving implant osseointegration. Thus, a whole-blood gene expression microarray dataset (GSE26168) of T2DM patients and nondiabetic controls was analyzed. Based on Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) results, differentially expressed genes and signaling pathways related to BMSC osteogenic differentiation were screened, and major risk genes were extracted based on the mean decrease Gini coefficient calculated using the random forest method. Bone morphogenetic protein-4 (BMP-4), with significantly low expression in T2DM blood, was identified as the most significant factor affecting BMSC osteogenic differentiation potential. Subsequently, metformin, a first-line clinical drug for T2DM treatment, was found to improve the osteogenic differentiation potential of BMSCs from T2DM patients via the BMP-4/Smad/Runx2 signaling pathway. These results demonstrate that low BMP-4 expression in the blood of T2DM patients significantly hinders the osteogenic function of BMSCs and that metformin is effective in counteracting the negative impact of BMP-4 deficiency.
1. Introduction
With the continuous development of oral implantology, implant restoration has become the preferred treatment option for patients with dentition defects [1]. However, type 2 diabetes mellitus (T2DM) has long been considered a relative contraindication for oral implant surgery [2, 3]. Although the risk of implant osseointegration failure in diabetic patients has decreased as implant surface treatment techniques improved [4], the healing-stage success rate and long-term survival rate of implants in diabetic patients are still significantly lower than those in nondiabetic patients [5]. Due to the complexity of the jawbone marrow microenvironment in diabetic patients, it is still challenging to clarify the causes and mechanisms of the aforementioned phenomena.
Bone marrow-derived mesenchymal stem cells (BMSCs) in jawbone marrow are adult stem cells that play important roles in implant osseointegration [6]. When an implant is placed into the jawbone, BMSCs begin to assemble around the surface of the implant. After the BMSCs adhere to the implant surface, the osteogenic differentiation process is initiated, and new bone gradually forms with the assistance of cells, blood, and related cytokines [7]. Previous studies have shown that BMSC proliferation, migration, differentiation, and mineralization in T2DM patients are significantly inferior to those in nondiabetic patients [8]. The weakened osteogenic differentiation potential of BMSCs in T2DM patients might be an important reason for the failure of implant osseointegration at the healing stage.
Sufficient blood supply is an indispensable factor in facilitating implant osseointegration [9]. The blood derived from the capillary bed of the bone marrow cavity not only provides the necessary cells and oxygen for bone tissue regeneration but also carries a large number of active proteins and cytokines [10]. Many proteins, including bone morphogenetic proteins (BMPs), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and transforming growth factor-β (TGF-β), can transport chemical signals to BMSCs by binding to surface receptors, thereby playing an important role in regulation of BMSC functions [11–14]. Among the BMP family, bone morphogenetic protein-4 (BMP-4) was recently shown to play a catalytic role in skeletal development and tooth formation [15, 16]. BMP-4 can bind bone morphogenetic protein receptor 1 (BMPR1) and further activate Smad signaling to affect the osteogenic differentiation of stem cells [17]. Previous studies have found that the blood of T2DM patients, in addition to alterations in sugar concentration, shows significantly different gene expression levels compared with the blood of nondiabetic individuals [18]. However, it remains unclear which differentially expressed genes in the blood significantly affect the osteogenic differentiation of BMSCs and potentially interfere with implant osseointegration in diabetic patients who undergo oral implantation. Furthermore, finding an effective and convenient way to improve the osteogenic differentiation function of BMSCs in T2DM patients is meaningful for decreasing the risk of implant osseointegration failure. The commonly used blood glucose-controlling drug, metformin, has been reported to improve bone metabolism and induce osteoblastic cell differentiation [19]. However, the mechanism of the effect of metformin on BMSCs still needs to be elucidated.
In the present study, bioinformatics analyses were performed using microarray data of whole blood from T2DM patients and nondiabetic controls to search for differentially expressed genes that are closely related to BMSC osteogenic differentiation, with the aim of providing markers for risk assessment prior to implant surgery for T2DM patients. These genes can also be used as intervention drug targets to improve the biological functions of alveolar BMSCs, providing a theoretical basis for drug selection for diabetic patients to control blood sugar and simultaneously promote implant osseointegration. Based on the findings in bioinformatics analyses, we planned to use recombinant human BMP-4 protein (rhBMP-4) and an inhibitor of downstream Smad to confirm the effect of BMP-4 on the biological function of BMSCs, as well as mechanism, in T2DM patients and to further explore whether the commonly used drug metformin is able to reverse the impairment of BMSC osteogenic function caused by the lack of BMP-4 in T2DM patient blood via the BMP/Smad pathway.
2. Materials and Methods
2.1. Dataset Source and Gene Set Enrichment Analysis
Gene expression microarray datasets of whole blood from T2DM patients and nondiabetic controls were downloaded from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/gds/?term=), with the accession code GSE26168 (type 2 diabetes mellitus: mRNA and miRNA profiling). A total of 24,526 standardized genetic data from nine T2DM patients (T2DM group, GSM532834-GSM532842) and eight nondiabetic controls (CON group, GSM532819-GSM532826) in the dataset were used for subsequent analysis. Based on the original matrix data of the microarray, gene set enrichment analysis (GSEA) was performed using GSEA v2.2.2 software (Broad Institute, USA) to identify the differences in biological functions between the two groups of blood samples. The analysis parameters were set to default values.
2.2. Identification of Differentially Expressed Genes
The online analysis tool GEO2R in the GEO website was used to screen differentially expressed genes in this study. The screening criteria were as follows: (1) fold change in upregulation or downregulation greater than 2 and (2) p values < 0.05. Origin 2019 software (OriginLab, USA) was used to construct volcano plots to intuitively represent gene expression differences in blood samples from the T2DM group and the CON group. Heat maps and cluster heat maps of differentially expressed genes in the two groups of blood samples were plotted using MeV 4.9.0 software (J. Craig Venter Institute, USA) to assess systematic differences in gene expression.
2.3. Gene Ontology Annotation and Kyoto Encyclopedia of Genes and Genomes Pathway Analysis
Gene Ontology (GO) annotation was performed to facilitate an understanding of the functions of differentially expressed genes, including three subontologies: biological process (BP), cellular component (CC), and molecular function (MF). Pathway analysis of differentially expressed genes using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database was employed to determine which biological pathways play an important role in the gene expression differences. The above analysis was conducted using the online tool DAVID v6.8 (https://david.ncifcrf.gov/). Significant GO terms and KEGG pathways were identified using Fisher's exact test, and the false discovery rate (FDR) was used to correct the p values. Bubble diagrams were created using the R v3.6.1 tool (https://www.r-project.org), and the pathway maps were generated with the online KEGG tool (https://www.kegg.jp/).
2.4. Analysis of Protein-Protein Interactions
To further investigate the functions of differentially expressed genes at the protein level, protein-protein interactions (PPIs) were analyzed using the online tool STRING v11.0 (https://string-db.org/), and network diagrams were generated using Cytoscape v3.7.1 software (National Resource for Network Biology, USA). The correlations among the differentially expressed proteins in each KEGG pathway were analyzed using the ClueGO v2.5.4 plug-in in Cytoscape v3.7.1 software. Venn diagrams were created using Origin 2019 software (OriginLab, USA) to reveal the number of overlapping proteins shared by each KEGG pathway.
2.5. Risk Gene Extraction Based on Random Forest Analysis
The random forest (RF) method is an integrated classifier composed of many decision trees, and each tree depends on the values of a random vector sampled independently [20]. In this study, differentially expressed genes were subjected to RF analysis using the R v3.6.1 tool (https://www.r-project.org) and ranked based on the mean decrease Gini (MDG) coefficient. MDG was used to quantify the contribution of the difference in the expression of each gene to the overall difference between the two groups [21], and comparison of MDGs provided a basis for extraction of the major risk genes.
2.6. Clinical Specimen Collection and Primary BMSC Culture
In this study, human alveolar BMSCs were isolated from wasted bone debris from the implant sockets of patients who underwent oral implantation. An informed consent form for the study was signed by each patient before surgery. The study was approved by the Ethics Committee of Beijing Stomatological Hospital, Capital Medical University (ethics approval: CMUSH-IRB-KJ-PJ-2017-01), and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
The primary BMSC culture method used in this study was similar to that described in our previous study [22]. During oral implant surgery, the implant sockets were prepared using a low-speed drilling technique (50 rpm, without irrigation), and the bone debris was then collected from the drill and placed in sterile tubes containing 0.5 ml phosphate-buffered saline (PBS) (HyClone, USA). An electronic balance (Sartorius, Germany) was used to weigh the tubes before and after the addition of the bone debris to enable quantification. After centrifugation, the bone debris was transferred into a 60 mm Petri dish (Corning, USA) with 5 ml of mesenchymal stem cell medium (MSCM) (ScienCell, USA) and placed in a 37°C and 5% CO2 incubator for 7 d. Thereafter, the medium was replaced every 3 d.
2.7. Flow Cytometric Analysis
Cells were cultured in 60 mm Petri dishes (Corning, USA) at a density of 106 cells/dish overnight and then fixed with 80% methanol. Primary anti-CD34, anti-CD44, anti-CD45, and anti-CD146 rabbit monoclonal antibodies (Abcam, UK) were incubated with the cells at a concentration of 1 μg/106 cells for 30 min. The samples were next incubated using a goat anti-rabbit fluorescent secondary antibody (ABclonal, China) at a concentration of 1 μg/106 cells for 1 h and assessed using FACSCalibur flow cytometry (BD Biosciences, USA).
2.8. Enzyme-Linked Immunosorbent Assay
After the bone debris samples of T2DM and nondiabetic patients were centrifuged, the supernatant in the centrifuge tube was collected. The bone morphogenetic protein-4 (BMP-4) content in the bone debris matrix was determined using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, USA) according to the manufacturer's protocol and was quantified per 100 mg of wet bone tissue for comparison.
2.9. Alkaline Phosphatase Assay and Alizarin Red S Staining
BMSCs were induced and cultured in osteogenic medium according to a StemPro Osteogenesis Differentiation Kit (Invitrogen, USA). Recombinant human BMP-4 protein (rhBMP-4) (R&D Systems, USA), a Smad inhibitor (LDN-193189) (Selleck, USA), and metformin (TargetMol, USA) were separately added to 0.5 ml medium in 24-well dishes (Corning, USA), and the final concentrations of each substance in the medium were as follows: rhBMP-4 (+: 10 ng/ml; ++: 50 ng/ml), LDN-193189 (+: 0.1 μM; ++: 0.5 μM), and metformin (+: 30 μM; ++: 100 μM). After 10 d of induction, cells were fixed in 70% ethanol for 1 h and stained using an ALP staining kit (Beyotime, China) according to the manufacturer's protocol. Intracellular ALP activity assays of BMSCs were performed at 3, 5, and 7 d of induction using an ALP Activity Assay Kit (Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer's protocol and were standardized based on protein concentration. After induction for 21 d, cells were fixed in 70% ethanol and stained with 2% alizarin red S staining solution (Sigma-Aldrich, USA) for 5 min. Then, 1 ml of isopropanol was added into each well to dissolve the red perylenequinone derivatives in the calcium nodules, and the optical density (OD) values were measured at a wavelength of 550 nm.
2.10. Western Blotting
Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer with 1 : 100 phenylmethylsulfonyl fluoride (PMSF) and 1 : 100 protease inhibitor cocktail (PIC) (Sigma-Aldrich, USA). Protein concentrations were determined using a Bicinchoninic Acid (BCA) Protein Quantitation Kit (Beyotime, China). Protein samples were separated using a premade 15% sodium dodecyl sulfate (SDS) polyacrylamide gel (Bio-Rad, USA) and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, USA) using a semidry transfer unit (Bio-Rad, USA). The membranes were blocked in 5% nonfat dry milk (Bio-Rad, USA) for 1 h. Primary antibodies were diluted to the recommended concentration in accordance with the manufacturer's instructions and then incubated with the membranes at 4°C overnight. Subsequently, the membranes were incubated with horseradish peroxidase-labeled anti-rabbit secondary antibody (ABclonal, China) for 1 h. Then, the membranes were immersed in electrochemiluminescence (ECL) solution (Bio-Rad, USA) for 3 min and imaged using a ChemiDoc MP Imaging System (Bio-Rad, USA). The primary antibodies included rabbit monoclonal anti-p-Smad1/5/8, anti-Smad1, and anti-Runx2 (Cell Signaling Technology, USA) and rabbit monoclonal anti-β-actin (ABclonal, China).
2.11. Statistical Analysis
SPSS 23.0 software was used for statistical analyses. All the data were acquired from at least three independent experiments. The data are expressed as the mean ± standard deviation (SD). Student's t-test or one-way analysis of variance (ANOVA) was used to determine statistical significance. The level of significance was defined by two p values (∗p < 0.05 and ∗∗p < 0.01).
3. Results
3.1. Differentially Expressed Genes and Gene Set Enrichment in the Blood Samples from T2DM Patients and Nondiabetic Controls
Heat map and volcano plots showed the genes differentially expressed with a fold change greater than 2 (p < 0.05) in blood samples from the T2DM group and the CON group (Figures 1(a) and 1(b)). A cluster heat map showed gene expression profiles with a fold change greater than 10 (p < 0.05) in the two groups (Figure 1(c)). In total, 2613 mRNAs were differentially expressed. Among them, 1032 mRNAs were upregulated, and 1581 mRNAs were downregulated in the T2DM group. In particular, 88 mRNAs were upregulated by more than 10-fold, and 117 mRNAs were downregulated by more than 10-fold.
Figure 1.
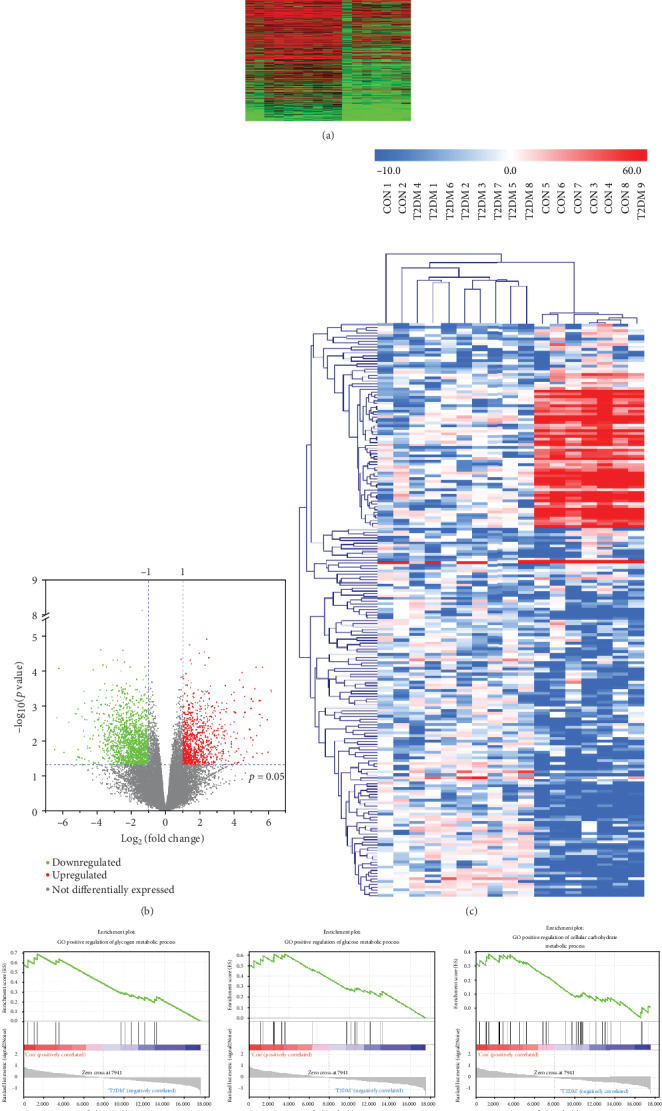
Gene expression differences in the blood from T2DM patients and nondiabetic controls and gene set enrichment analysis (GSEA). (a, b) Heat map and volcano plots showing significantly differentially expressed genes with a greater than twofold change in the blood from the two groups of patients. (c) Cluster heat map showing differentially expressed genes with a fold change greater than 10. (d) GSEA results showing the differences in glucose and lipid metabolism in the blood from T2DM patients and nondiabetic controls.
GSEA was conducted for the matrix data of all 24,526 genes in the T2DM and CON groups. The results showed that glycogen metabolic process, glucose metabolic process, cellular carbohydrate metabolic process, fatty acid transport, and lipid catabolic process were significantly downregulated in the T2DM group, and fatty acid biosynthetic process was stronger in the T2DM group than in the CON group (Figure 1(d)). The above results are consistent with the clinical features and pathological manifestations of T2DM.
3.2. Functional Analysis of Differentially Expressed Genes
GO analysis of differentially expressed genes helped identify the differences in blood functions between T2DM patients and nondiabetic individuals. The results showed enrichment of downregulated (Figures 2(a)–2(c)) and upregulated (Figures 2(d)–2(f)) differentially expressed genes in three subontologies: BP, CC, and MF. To explore the possible influences of abnormally expressed factors in the blood of T2DM patients on the osteogenic differentiation potential of BMSCs, we focused on the terms associated with this type of biological function. In the BP enrichment results of downregulated genes in T2DM patients, the fold enrichment of the term “positive regulation of osteoblast differentiation” was 2.49 (11 genes, p = 0.01). Follow-up analysis focused on the functions and effects of these 11 genes.
Figure 2.
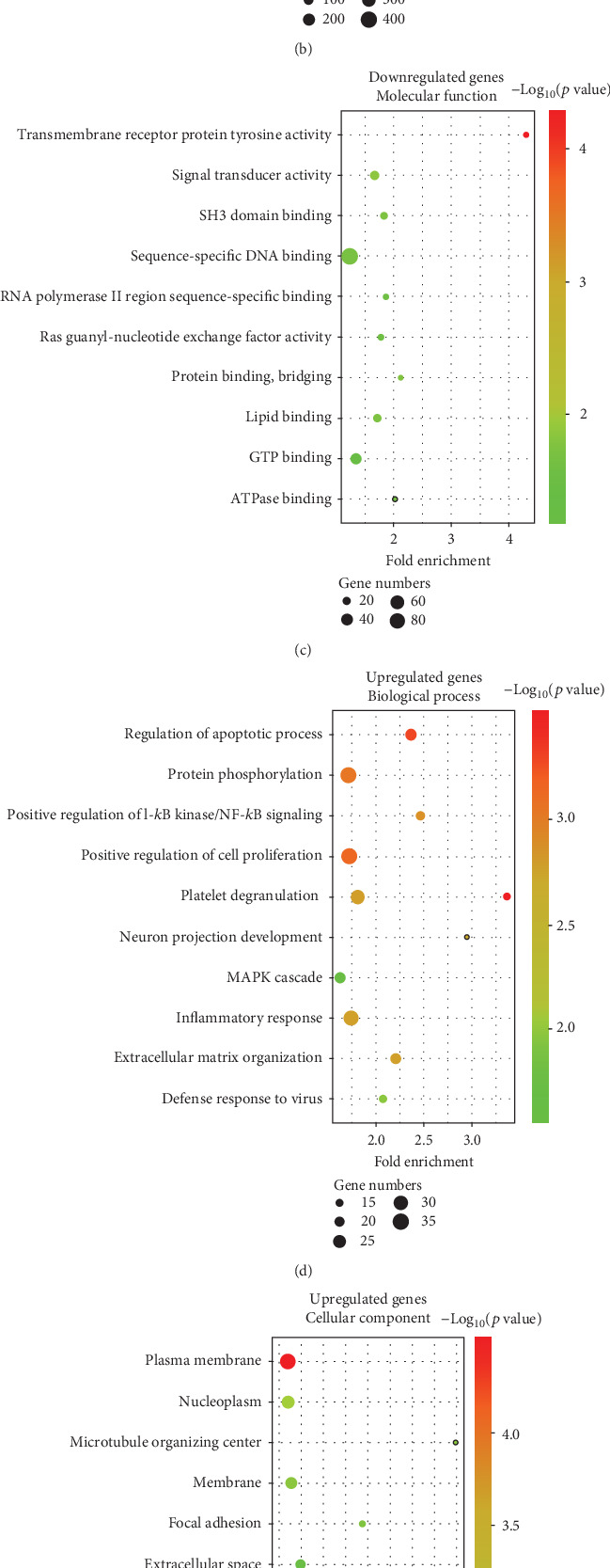
GO annotation of differentially expressed genes in the blood from T2DM patients and nondiabetic controls. Bubble diagrams showing the fold enrichment, gene numbers, and p value in the biological process, cellular component, and molecular function of (a–c) downregulated and (d–f) upregulated genes in blood samples from T2DM patients compared with nondiabetic controls.
3.3. Screening of Key Genes That Regulate Osteoblast Differentiation
The expression levels of the 11 genes that could regulate osteoblast differentiation in the blood were significantly higher in the CON group than in the T2DM group. The fold change and p value are shown in Figure 3(a). The RF analysis results showed that among the 11 genes, the MDG coefficient for BMP-4 was the highest (19.09), followed by RUNX2 (17.58) and BMP-7 (15.75) (Figure 3(b)). PPI analysis of BMP-4 showed that BMP-4 was closely associated with the Smad signaling proteins (Figure 3(c)). The KEGG pathway map of the TGF-β signaling pathway showed that BMP-4 can activate Smad1/5/8 phosphorylation by binding to the cell membrane receptors BMPR1 and BMPR2, thereby promoting osteoblast differentiation and other biological functions by activating IDs for regulation of DNA transcription (Figure 3(d)).
Figure 3.
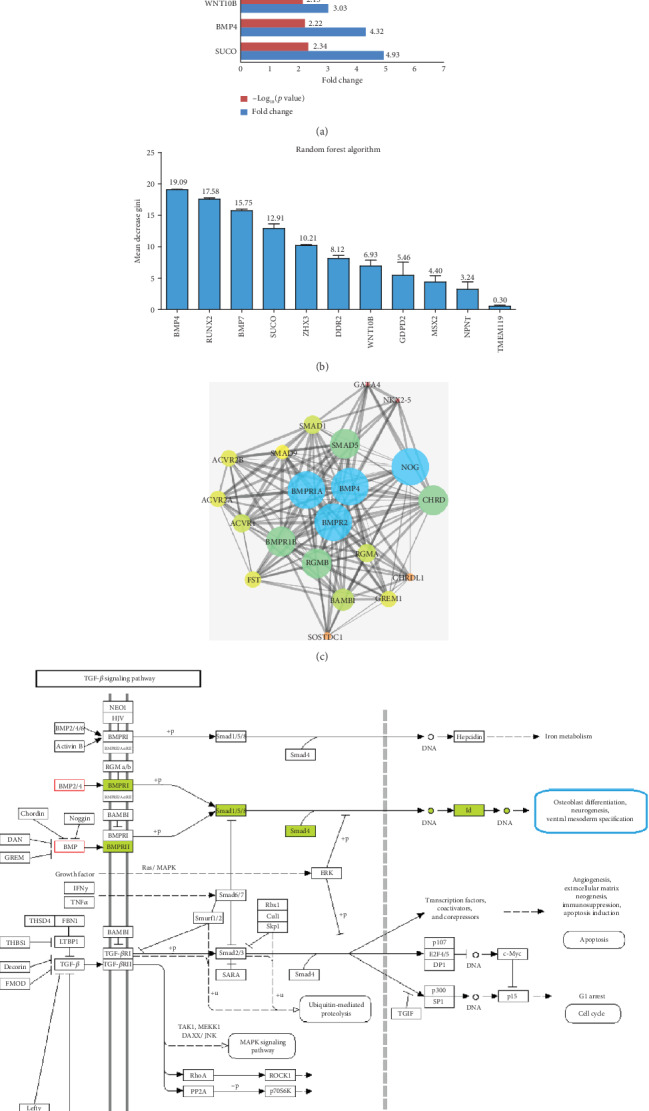
Differentially expressed genes associated with regulation of osteoblast differentiation and major risk gene screening. (a) Fold changes in downregulated genes enriched in the “positive regulation of osteoblast differentiation” term in blood samples from the T2DM group compared with the CON group. (b) The results of random forest analysis showing that BMP-4 has the highest mean decrease Gini coefficient and plays the most important role in “positive regulation of osteoblast differentiation.” (c) Protein-protein interaction analysis of BMP-4 biological function-related proteins showing that BMP-4 is closely related to Smad signaling. (d) Schematic diagram of the TGF-β signaling pathway showing that BMP-4 can gradually regulate cell biological functions, such as osteoblast differentiation, by activating Smad signaling.
3.4. Analysis of Biological Pathways of Differentially Expressed Genes
Figure 4(a) shows the KEGG pathway analysis results for differentially expressed genes in the blood from the T2DM group compared with that from the CON group. Among them, the focal adhesion pathway plays a critical role in the process of BMSC adhesion to the surface of biomaterials. A total of 45 genes were enriched in the focal adhesion pathway, with a fold enrichment of 1.72 (p < 0.01). In the focal adhesion pathway, extracellular matrix proteins can bind to integrins to activate the downstream Wnt signaling pathway and regulate the osteogenic differentiation ability of BMSCs (Figure 4(b)). The interaction analysis among differentially expressed genes in these KEGG pathways showed that the differentially expressed genes in the focal adhesion pathway have associations with the Ras signaling pathway, cAMP signaling pathway, Rap1 signaling pathway, and adrenergic signaling in cardiomyocytes (Figure 4(c)). The Venn diagram in Figure 4(d) shows the number of overlapping genes in these five important pathways.
Figure 4.
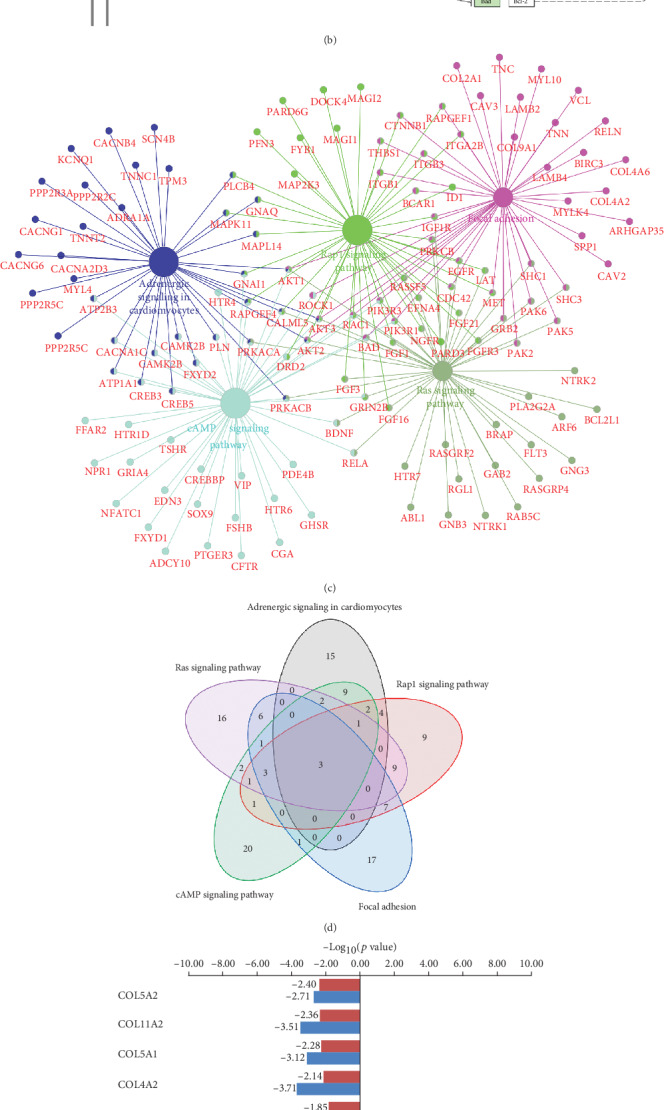
Analysis of biological pathways of differentially expressed genes in the blood of T2DM patients compared with nondiabetic controls. (a) The fold enrichment, gene numbers, and p value in KEGG pathway analysis of differentially expressed genes. (b) Schematic diagram of the focal adhesion signaling pathway showing that extracellular matrix proteins can regulate osteoblast differentiation by binding to integrins, increasing FAK phosphorylation, and activating the downstream Wnt signaling pathway. (c) Interaction analysis of differentially expressed genes in the differential KEGG pathways. (d) Venn diagram showing the number of overlapping genes among the KEGG pathways. (e) Differential expression status of the focal adhesion pathway-related collagen genes among the differentially expressed genes (the T2DM group compared with the CON group). (f) Random forest analysis of the aforementioned collagen genes.
Collagen represents an important class of extracellular matrix proteins and plays a key role in integrin binding and regulation of focal adhesion-associated signaling pathways. Among the 11 collagens associated with the focal adhesion pathway in the differentially expressed genes, nine were significantly downregulated in the T2DM group (Figure 4(e)), which could negatively affect the adhesion process of BMSCs. RF analysis showed that among the nine genes, COL5A1 had the highest MDG coefficient (29.06), followed by COL9A1 (19.93) and COL2A1 (19.61) (Figure 4(f)).
3.5. BMP-4 Expression in the Matrix of Alveolar Bone Debris from Patients Who Underwent Oral Implant Surgery
The major components of the alveolar bone debris supernatant samples after centrifugation represented diluted blood of diabetic and nondiabetic patients. Standardized ELISA results showed that the BMP-4 concentration in the matrix supernatant of alveolar bone debris from T2DM patients was significantly lower than that from nondiabetic patients (p < 0.05) (Figure 5(a)).
Figure 5.
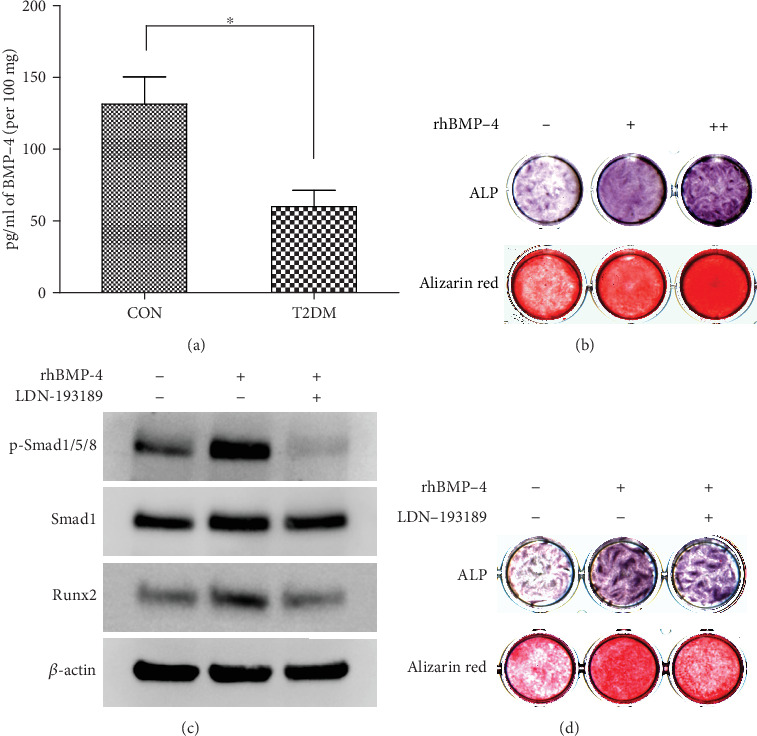
BMP-4/Smad/Runx2 signaling promotes the osteogenic differentiation of BMSCs from T2DM patients. (a) BMP-4 levels in the matrix supernatant of alveolar bone debris from implant sockets of T2DM patients who underwent oral implantation were significantly lower than those of nondiabetic patients. (b) rhBMP-4 dose-dependently enhanced ALP expression and mineralization in BMSCs from T2DM patients; “+” represents 10 ng/ml, and “++” represents 50 ng/ml. (c) Western blotting results showing that rhBMP-4 promoted Runx2 expression by stimulating p-Smad1/5/8 phosphorylation and that LDN-193189 significantly inhibited the aforementioned effects of rhBMP-4. (d) The promoting effect of rhBMP-4 on ALP expression and in vitro mineralization in BMSCs from T2DM patients can be inhibited by LDN-193189. The data are presented as the mean ± standard deviation. ∗p < 0.05; ∗∗p < 0.01.
3.6. Identification of Human BMSCs
Based on flow cytometric analysis, the cells expressed CD44 (positivity rate 98.2%) and CD146 (positivity rate 98.5%) but did not express CD34 (positivity rate 2.16%) or CD45 (positivity rate 1.06%) (Figure S1a), indicating that the cells express the surface markers of BMSCs. Alizarin red S staining results showed positive calcium node staining after 21 d of osteogenic induction (Figure S1b); this indicated that the cells had osteogenic differentiation potential. These findings confirmed that the cells obtained via the low-speed drilling technique exhibited the characteristics of BMSCs.
3.7. BMP-4/Smad/Runx2 Signaling Promotes Osteogenic Differentiation of BMSCs from T2DM Patients
ALP and alizarin red S staining showed that rhBMP-4 could increase ALP expression and the degree of mineralization in BMSCs from T2DM patients, and the effect of 50 ng/ml was more significant than the effect of 10 ng/ml (Figure 5(b)). According to the KEGG pathway map (Figure 3(d)), western blotting was used to detect changes induced in Smad signaling by rhBMP-4. The results showed that rhBMP-4 increased the phosphorylation level of Smad1/5/8 and further promoted Runx2 expression, and the Smad inhibitor LDN-193189 significantly inhibited the above functions of rhBMP-4 (Figure 5(c)). Furthermore, rhBMP-4 was confirmed to regulate ALP expression and in vitro mineralization of BMSCs via the above signaling pathways (Figure 5(d)).
3.8. Metformin Improves the Reduced Osteogenic Differentiation Ability of BMSCs from T2DM Patients Caused by Insufficient BMP-4 Combination
ELISA results showed that 30 μM metformin promoted BMP-4 secretion by BMSCs from T2DM patients (p < 0.05), and the promoting effect of 100 μM metformin was more significant (p < 0.01) (Figure 6(a)). Western blotting results showed that metformin increased the Smad1/5/8 phosphorylation level and thus Runx2 expression in BMSCs and that the activation effect of 100 μM metformin on the Smad signaling was similar to that of 50 ng/ml rhBMP-4 (Figure 6(b)). ALP and alizarin red S staining showed that metformin increased the osteogenic differentiation potential of BMSCs from T2DM patients. The effect of 100 μM metformin was more prominent than that of 30 μM, leading to an increase in BMSC ALP expression and in vitro mineralization to a similar extent as that induced by 50 ng/ml rhBMP-4 (Figure 6(c)). In summary, metformin improved the osteogenic differentiation potential of BMSCs from T2DM patients through the BMP-4/Smad/Runx2 signaling pathway (Figure 6(d)).
Figure 6.
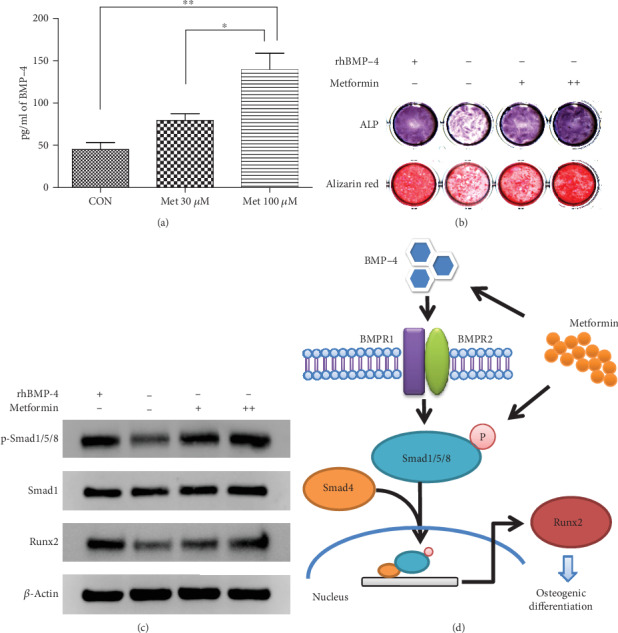
Metformin improves the osteogenic differentiation potential of BMSCs from T2DM patients. (a) Metformin dose-dependently promoted the secretion of BMP-4 by BMSCs from T2DM patients. (b) Western blotting results showing that metformin promoted Runx2 expression by stimulating Smad1/5/8 phosphorylation; “+” represents 30 μM, and “++” represents 100 μM. The effect of 100 μM metformin on Smad signaling activation was similar to that of 50 ng/ml rhBMP-4. (c) Metformin promoted ALP expression and in vitro mineralization in BMSCs from T2DM patients. Again, the effect of 100 μM metformin was similar to that of 50 ng/ml rhBMP-4. (d) Metformin regulates the osteogenic differentiation potential of BMSCs from T2DM patients through the BMP-4/Smad/Runx2 signaling pathway. The data are presented as the mean ± standard deviation. ∗p < 0.05; ∗∗p < 0.01.
4. Discussion
T2DM is a metabolic disease mainly characterized by hyperglycemia caused by islet dysfunction [23]. Clinically, the risk of oral implant failure in patients with T2DM is significantly higher than that in nondiabetic patients [2, 3, 5]. Abnormal BMSC biological function is an important cause of poor implant osseointegration [24]. Studies have shown that high glucose induction can significantly reduce the biological functions of human alveolar BMSCs, including proliferation, migration, differentiation, and mineralization [25, 26]. However, the etiology and pathology of diabetes are extremely complex, and hyperglycemia is only one of the pathological manifestations of T2DM. Recent clinical studies have also found that the stability of implants during the healing stage in T2DM patients with good glycemic control was still lower than that in nondiabetic patients [27], which indicated that in addition to blood glucose, other important factors exist that can seriously affect implant osseointegration in diabetic patients. Unfortunately, because these factors are still undetermined, there is currently no ideal treatment method to improve implant osseointegration in diabetic patients. In addition, recent meta-analyses and cohort studies have shown that T2DM is strongly associated with increased fracture risk [28]; T2DM is also considered a potential cause of secondary osteoporosis in the population [29]. The reduction in the osteogenic differentiation potential of BMSCs in diabetic patients has been reported to play a key role in reducing bone formation, impairing bone fracture healing, and increasing the degree of osteoporosis [30, 31]. Furthermore, osteoporosis is considered a potential risk factor for oral implant failure [32]. Thus, effectively improving the biological function of BMSCs is beneficial not only for oral implant treatment but also for curing other osteogenic-related health problems in T2DM patients.
Previous studies have shown that many proteins and cytokines in the blood can play key regulatory roles in the biological functions of BMSCs [33]. For example, after BMP family members bind to their receptors on the cell surface, they can regulate osteogenic differentiation of BMSCs via the TGF-β signaling pathway [11, 34]; after COL family members bind integrins on the cell membrane, they can regulate the adhesion function of BMSCs via reconstruction of focal adhesions and cytoskeletons [7, 35]. For patients who undergo oral implantation, an abnormal content of the abovementioned cytokines in the blood will inevitably affect multiple biological functions of BMSCs, resulting in unstable implant osseointegration during the healing stage. In the present study, microarray data of blood samples from T2DM patients and nondiabetic controls (dataset GSE26168) were analyzed, the expression levels of many genes in these two groups were found to be significantly different, and the biological functions of a portion of the differentially expressed genes were enriched in osteogenic differentiation. Previous studies have also shown that osteogenic differentiation of alveolar BMSCs is a key step in the implant osseointegration process [6, 24]. Thus, we speculated that in addition to the changes in blood glucose levels, differences in the content of osteogenic differentiation-associated cytokines in the blood may be an important reason for the lower degree of implant osseointegration in T2DM patients than that in nondiabetic patients. However, the reason for such differences in the content of osteogenic differentiation-related cytokines in the blood between T2DM patients and normal controls is still unclear. These cytokines, such as the BMP family, can be synthesized and secreted into the circulation by various tissues and their cells, such as the bone marrow, adipose tissue, kidney, and liver [36–38]. Insulin resistance in T2DM and decreased insulin stimulation can impact metabolism in these tissues [39–41], which may affect the contents of the cytokines released by these tissues into the blood. However, this hypothesis needs to be confirmed by solid experimental research.
In this study, according to the comprehensive analysis of GO annotation and RF results, we found that the difference in the BMP-4 expression level in the blood of T2DM patients and nondiabetic controls may be a major risk factor for the difference in BMSC osteogenic differentiation between these two groups. The expression level of BMP-4 in the T2DM group was significantly lower than that in the CON group. Additionally, we examined the BMP-4 concentration in the matrix supernatant of alveolar bone debris from the implant sockets of T2DM and nondiabetic patients undergoing oral implant surgery, and the results also confirmed a significantly lower BMP-4 concentration in the T2DM group. BMP-4, an important secreted protein in the TGF-β superfamily, is widely known for its active functions in embryonic development and formation of the heart and other organs [42]. In recent years, BMP-4 has been considered a necessary regulatory factor of tooth development and bone tissue formation [43]. Binding of BMP-4 to the membrane receptor BMPR1 leads to phosphorylation of the intracellular signal transduction protein Smad1/5/8. p-Smad1/5/8 and Smad4 oligomerize to form a complex, which is transported into the nucleus to act as a transcription factor. Then, the expression of Runx2 and other osteogenic factors can be stimulated [44, 45]. In this study, we also found that rhBMP-4 had a significant promoting effect on osteogenic differentiation of BMSCs from T2DM patients, and this effect was achieved via the BMP-4/Smad/Runx2 axis. Therefore, the reduction in BMP-4 expression in the blood and bone matrix of T2DM patients is bound to affect the osteogenic differentiation potential of BMSCs and likely interferes with implant osseointegration during the healing stage.
Metformin is currently a commonly used and highly effective drug for the treatment of T2DM [46]. Metformin can reduce blood glucose levels by increasing peripheral glucose utilization, reducing intestinal absorption of glucose, and inhibiting hepatic gluconeogenesis [47]. Clinical studies have demonstrated that metformin has protective effects on bone tissue and can decrease the fracture risk in T2DM patients [48]. Metformin also has a potential effect on promoting osteogenic differentiation of BMSCs and preosteoblasts [49], and it enhances ALP expression and the in vitro mineralization ability of osteoblasts [50]. In vivo experiments have also shown that metformin can promote fracture healing and induce new bone formation in rats [51]. Studies on the mechanisms have reported that the ability of metformin to impact osteogenic differentiation is mainly caused via AMP-activated protein kinase (AMPK) signaling [52], whereby phosphorylated AMPK regulates expression of Runx2 to further promote osteogenic function in preosteoblasts [53]. In addition, Runx2 has been widely proven to be regulated by the BMP/Smad pathway in BMSCs [54], but whether metformin promotes Smad signaling is still unclear.
In the present study, we found that metformin could stimulate BMP-4 secretion by BMSCs from T2DM patients. Moreover, metformin was confirmed to activate the downstream target gene Runx2 via Smad signaling to enhance the osteogenic differentiation ability of BMSCs, and the results showed that 100 μM metformin had a promoting effect similar to that of rhBMP-4. Therefore, whether through stimulation of BMP-4 secretion or direct promotion of osteogenic differentiation via Smad/Runx2, metformin could compensate for the negative impact of low blood BMP-4 levels on the osteogenic potential of BMSCs in diabetic patients. These findings demonstrate another important application value of metformin in T2DM patients who undergo oral implantation: not only can the blood glucose levels be controlled by metformin, but it also has potential effect to improve implant osseointegration during the healing stage by enhancing the osteogenic differentiation of alveolar BMSCs.
In addition, based on the bioinformatics analysis results in this study, we found that a portion of the specific extracellular matrix (ECM) proteins in the blood, especially collagens, may play important roles in the process of BMSC-implant adhesion [9, 43]. Previous studies have shown that BMSC adhesion onto an implant surface is the initial step that precedes their subsequent functions [55]. After a biomaterial is implanted into the bone tissue, collagens in the blood will rapidly adsorb onto the surface of the biomaterial. Then, BMSCs recognize the tripeptide sequence Arg-Gly-Asp (RGD) in collagens through integrins, which promotes cell adherence onto the biomaterial surface and initiates subsequent cell spreading, proliferation, and osteogenic differentiation [56, 57]. In the present study, KEGG pathway analysis showed that the expression of focal adhesion signaling pathway-related genes was significantly different in the blood from T2DM patients and nondiabetic controls and that the expression of most collagen mRNAs was low in the T2DM group. The above results provide new ideas for future exploration of how to improve implant osseointegration in diabetic patients who undergo oral implantation. Increasing the adsorption of collagens by modifying the implant surface or using molecular agents to improve the adhesion ability of BMSCs in T2DM patients is an important direction for future research.
5. Conclusions
In summary, significant differences exist in the gene expression levels in the blood from T2DM patients and nondiabetic individuals. The low BMP-4 expression level in the blood from T2DM patients can affect the osteogenic differentiation potential of alveolar BMSCs. Metformin, a first-line clinical drug for treatment of T2DM, can significantly improve the osteogenic differentiation of BMSCs via the BMP-4/Smad/Runx2 signaling pathway, thereby compensating for the negative impact of BMP-4 deficiency on the functions of BMSCs from T2DM patients.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (Grant Nos. 61571311 to Wei Geng and 81371115 to Jun Li). The authors thank American Journal Experts (AJE) for professional English editing.
Contributor Information
Wei Geng, Email: gengwei717@163.com.
Jun Li, Email: lijun3021@aliyun.com.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Figure S1: identification of human BMSCs. (a) Flow cytometry analysis showed that the cells expressed CD44 and CD146 but did not express CD34 or CD45. (b) Alizarin red staining was positive after 21 d of osteogenic induction. These findings confirmed that the cells have characteristics consistent with those of BMSCs.
References
- 1.Papaspyridakos P., Chen C. J., Singh M., Weber H. P., Gallucci G. O. Success criteria in implant dentistry: a systematic review. Journal of Dental Research. 2012;91(3):242–248. doi: 10.1177/0022034511431252. [DOI] [PubMed] [Google Scholar]
- 2.Javed F., Romanos G. E. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: a systematic literature review. Journal of Periodontology. 2009;80(11):1719–1730. doi: 10.1902/jop.2009.090283. [DOI] [PubMed] [Google Scholar]
- 3.Eskow C. C., Oates T. W. Dental implant survival and complication rate over 2 years for individuals with poorly controlled type 2 diabetes mellitus. Clinical Implant Dentistry and Related Research. 2017;19(3):423–431. doi: 10.1111/cid.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naujokat H., Kunzendorf B., Wiltfang J. Dental implants and diabetes mellitus—a systematic review. International Journal of Implant Dentistry. 2016;2(1):p. 5. doi: 10.1186/s40729-016-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annibali S., Pranno N., Cristalli M. P., La Monaca G., Polimeni A. Survival analysis of implant in patients with diabetes mellitus: a systematic review. Implant Dentistry. 2016;25(5):663–674. doi: 10.1097/ID.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 6.Xu L., Zhang W., Lv K., Yu W., Jiang X., Zhang F. Peri-implant bone regeneration using rhPDGF-BB, BMSCs, and β-TCP in a canine model. Clinical Implant Dentistry and Related Research. 2016;18(2):241–252. doi: 10.1111/cid.12259. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z., Bachhuka A., Han S., et al. Tuning chemistry and topography of nanoengineered surfaces to manipulate immune response for bone regeneration applications. ACS Nano. 2017;11(5):4494–4506. doi: 10.1021/acsnano.6b07808. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Liu C. Y., Jiang Y. F., Wei X. Z., Li J. U. Proliferation and differentiation of human osteoblasts from a type 2 diabetic patient in vitro. Genetics and Molecular Research. 2015;14(3):11292–11299. doi: 10.4238/2015.September.22.23. [DOI] [PubMed] [Google Scholar]
- 9.Danesh-Sani S. A., Tarnow D., Yip J. K., Mojaver R. The influence of cortical bone perforation on guided bone regeneration in humans. International Journal of Oral and Maxillofacial Surgery. 2017;46(2):261–266. doi: 10.1016/j.ijom.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Przekora A., Benko A., Blazewicz M., Ginalska G. Hybrid chitosan/β-1,3-glucan matrix of bone scaffold enhances osteoblast adhesion, spreading and proliferation via promotion of serum protein adsorption. Biomedical Materials. 2016;11(4, article 045001) doi: 10.1088/1748-6041/11/4/045001. [DOI] [PubMed] [Google Scholar]
- 11.Lee J. S., Kim M. E., Seon J. K., et al. Bone-forming peptide-3 induces osteogenic differentiation of bone marrow stromal cells via regulation of the ERK1/2 and Smad1/5/8 pathways. Stem Cell Research. 2018;26:28–35. doi: 10.1016/j.scr.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W., Zhu C., Wu Y., et al. VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. European Cells & Materials. 2014;27:1–12. doi: 10.22203/eCM.v027a01. [DOI] [PubMed] [Google Scholar]
- 13.Peng W., Zhang J., Zhang H., Liu G., Dong W., Zhang F. Effects of lentiviral transfection containing bFGF gene on the biological characteristics of rabbit BMSCs. Journal of Cellular Biochemistry. 2018;119(10):8389–8397. doi: 10.1002/jcb.27034. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X., Huang B., Yang H., et al. TGF-β1 is involved in vitamin D-induced chondrogenic differentiation of bone marrow-derived mesenchymal stem cells by regulating the ERK/JNK pathway. Cellular Physiology and Biochemistry. 2017;42(6):2230–2241. doi: 10.1159/000479997. [DOI] [PubMed] [Google Scholar]
- 15.Ou M., Zhao Y., Zhang F., Huang X. Bmp2 and Bmp4 accelerate alveolar bone development. Connective Tissue Research. 2015;56(3):204–211. doi: 10.3109/03008207.2014.996701. [DOI] [PubMed] [Google Scholar]
- 16.Seki D., Takeshita N., Oyanagi T., et al. Differentiation of odontoblast-like cells from mouse induced pluripotent stem cells by pax9 and bmp4 transfection. Stem Cells Translational Medicine. 2015;4(9):993–997. doi: 10.5966/sctm.2014-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park E. S., Woods D. C., Tilly J. L. Bone morphogenetic protein 4 promotes mammalian oogonial stem cell differentiation via Smad1/5/8 signaling. Fertility and Sterility. 2013;100(5):1468–1475.e2. doi: 10.1016/j.fertnstert.2013.07.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grayson B. L., Wang L., Aune T. M. Peripheral blood gene expression profiles in metabolic syndrome, coronary artery disease and type 2 diabetes. Genes and Immunity. 2011;12(5):341–351. doi: 10.1038/gene.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molinuevo M. S., Schurman L., McCarthy A. D., et al. Effect of metformin on bone marrow progenitor cell differentiation: in vivo and in vitro studies. Journal of Bone and Mineral Research. 2010;25(2):211–221. doi: 10.1359/jbmr.090732. [DOI] [PubMed] [Google Scholar]
- 20.Pang H., Lin A., Holford M., et al. Pathway analysis using random forests classification and regression. Bioinformatics. 2006;22(16):2028–2036. doi: 10.1093/bioinformatics/btl344. [DOI] [PubMed] [Google Scholar]
- 21.Xin Z., Hua L., Yang Y. L., et al. A pathway analysis based on genome-wide DNA methylation of Chinese patients with Graves’ orbitopathy. BioMed Research International. 2019;2019:10. doi: 10.1155/2019/9565794.9565794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang C., Lin X., Wang S. L., Guo L. H., Wang X. Y., Li J. Osteogenic potential of three different autogenous bone particles harvested during implant surgery. Oral Diseases. 2017;23(8):1099–1108. doi: 10.1111/odi.12704. [DOI] [PubMed] [Google Scholar]
- 23.Hackett R. A., Steptoe A. Type 2 diabetes mellitus and psychological stress — a modifiable risk factor. Nature Reviews Endocrinology. 2017;13(9):547–560. doi: 10.1038/nrendo.2017.64. [DOI] [PubMed] [Google Scholar]
- 24.Xu R., Shi G., Xu L., et al. Simvastatin improves oral implant osseointegration via enhanced autophagy and osteogenesis of BMSCs and inhibited osteoclast activity. Journal of Tissue Engineering and Regenerative Medicine. 2018;12(5):1209–1219. doi: 10.1002/term.2652. [DOI] [PubMed] [Google Scholar]
- 25.Ying X., Chen X., Liu H., et al. Silibinin alleviates high glucose-suppressed osteogenic differentiation of human bone marrow stromal cells via antioxidant effect and PI3K/Akt signaling. European Journal of Pharmacology. 2015;765:394–401. doi: 10.1016/j.ejphar.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Chang T. C., Hsu M. F., Wu K. K. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS One. 2015;10(5, article e0126537) doi: 10.1371/journal.pone.0126537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundar G., Sridharan S., Sundaram R. R., Prabhu S., Rao R., Rudresh V. Impact of well-controlled type 2 diabetes mellitus on implant stability and bone biomarkers. The International Journal of Oral & Maxillofacial Implants. 2019;34(6):1441–1449. doi: 10.11607/jomi.7547. [DOI] [PubMed] [Google Scholar]
- 28.Moayeri A., Mohamadpour M., Mousavi S. F., Shirzadpour E., Mohamadpour S., Amraei M. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: a systematic review and meta-analysis. Therapeutics and Clinical Risk Management. 2017;13:455–468. doi: 10.2147/TCRM.S131945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Si Y., Wang C., Guo Y., Xu G., Ma Y. Prevalence of osteoporosis in patients with type 2 diabetes mellitus in the Chinese mainland: a systematic review and meta-analysis. Iranian Journal of Public Health. 2019;48(7):1203–1214. [PMC free article] [PubMed] [Google Scholar]
- 30.Marin C., Luyten F. P., Van der Schueren B., Kerckhofs G., Vandamme K. The impact of type 2 diabetes on bone fracture healing. Frontiers in Endocrinology. 2018;9:p. 6. doi: 10.3389/fendo.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo G., Liu H., Lu H. Glucagon-like peptide-1 (GLP-1) receptor agonists: potential to reduce fracture risk in diabetic patients? British Journal of Clinical Pharmacology. 2016;81(1):78–88. doi: 10.1111/bcp.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H., Liu N., Xu X., Qu X., Lu E. Smoking, radiotherapy, diabetes and osteoporosis as risk factors for dental implant failure: a meta-analysis. PLoS One. 2013;8(8, article e71955) doi: 10.1371/journal.pone.0071955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen C. C., Chen B., Gu J. T., et al. The angiogenic related functions of bone marrow mesenchymal stem cells are promoted by CBDL rat serum via the Akt/Nrf2 pathway. Experimental Cell Research. 2016;344(1):86–94. doi: 10.1016/j.yexcr.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Aquino-Martínez R., Artigas N., Gámez B., Rosa J. L., Ventura F. Extracellular calcium promotes bone formation from bone marrow mesenchymal stem cells by amplifying the effects of BMP-2 on SMAD signaling. PLoS One. 2017;12(5, article e0178158) doi: 10.1371/journal.pone.0178158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heino J. The collagen family members as cell adhesion proteins. BioEssays. 2007;29(10):1001–1010. doi: 10.1002/bies.20636. [DOI] [PubMed] [Google Scholar]
- 36.Katagiri T., Watabe T. Bone morphogenetic proteins. Cold Spring Harbor Perspectives in Biology. 2016;8(6, article a021899) doi: 10.1101/cshperspect.a021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salazar V. S., Gamer L. W., Rosen V. BMP signalling in skeletal development, disease and repair. Nature Reviews Endocrinology. 2016;12(4):203–221. doi: 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- 38.Fasshauer M., Blüher M. Adipokines in health and disease. Trends in Pharmacological Sciences. 2015;36(7):461–470. doi: 10.1016/j.tips.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Barazzoni R., Gortan Cappellari G., Ragni M., Nisoli E. Insulin resistance in obesity: an overview of fundamental alterations. Eating and Weight Disorders. 2018;23(2):149–157. doi: 10.1007/s40519-018-0481-6. [DOI] [PubMed] [Google Scholar]
- 40.Conte C., Epstein S., Napoli N. Insulin resistance and bone: a biological partnership. Acta Diabetologica. 2018;55(4):305–314. doi: 10.1007/s00592-018-1101-7. [DOI] [PubMed] [Google Scholar]
- 41.Artunc F., Schleicher E., Weigert C., Fritsche A., Stefan N., Häring H. U. The impact of insulin resistance on the kidney and vasculature. Nature Reviews Nephrology. 2016;12(12):721–737. doi: 10.1038/nrneph.2016.145. [DOI] [PubMed] [Google Scholar]
- 42.Chen D., Zhao M., Mundy G. R. Bone morphogenetic proteins. Growth Factors. 2009;22(4):233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 43.Yu M., Wang H., Fan Z., et al. BMP4 mutations in tooth agenesis and low bone mass. Archives of Oral Biology. 2019;103:40–46. doi: 10.1016/j.archoralbio.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hopkins A., Mirzayans F., Berry F. Foxc1 expression in early osteogenic differentiation is regulated by BMP4-SMAD activity. Journal of Cellular Biochemistry. 2016;117(7):1707–1717. doi: 10.1002/jcb.25464. [DOI] [PubMed] [Google Scholar]
- 45.Liu T., Li B., Zheng X. F., et al. Chordin-like 1 improves osteogenesis of bone marrow mesenchymal stem cells through enhancing BMP4-SMAD pathway. Frontiers in Endocrinology. 2019;10:p. 360. doi: 10.3389/fendo.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas I., Gregg B. Metformin; a review of its history and future: from lilac to longevity. Pediatric Diabetes. 2017;18(1):10–16. doi: 10.1111/pedi.12473. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Rangel E., Inzucchi S. E. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60(9):1586–1593. doi: 10.1007/s00125-017-4336-x. [DOI] [PubMed] [Google Scholar]
- 48.Hidayat K., Du X., Wu M. J., Shi B. M. The use of metformin, insulin, sulphonylureas, and thiazolidinediones and the risk of fracture: systematic review and meta-analysis of observational studies. Obesity Reviews. 2019;20(10):1494–1503. doi: 10.1111/obr.12885. [DOI] [PubMed] [Google Scholar]
- 49.Ma J., Zhang Z. L., Hu X. T., Wang X. T., Chen A. M. Metformin promotes differentiation of human bone marrow derived mesenchymal stem cells into osteoblast via GSK3β inhibition. European Review for Medical and Pharmacological Sciences. 2018;22(22):7962–7968. doi: 10.26355/eurrev_201811_16424. [DOI] [PubMed] [Google Scholar]
- 50.Zhen D., Chen Y., Tang X. Metformin reverses the deleterious effects of high glucose on osteoblast function. Journal of Diabetes and its Complications. 2010;24(5):334–344. doi: 10.1016/j.jdiacomp.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 51.La Fontaine J., Chen C., Hunt N., Jude E., Lavery L. Type 2 diabetes and metformin influence on fracture healing in an experimental rat model. The Journal of Foot & Ankle Surgery. 2016;55(5):955–960. doi: 10.1053/j.jfas.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 52.Jiating L., Buyun J., Yinchang Z. Role of metformin on osteoblast differentiation in type 2 diabetes. BioMed Research International. 2019;2019:6. doi: 10.1155/2019/9203934.9203934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim D. Y., Kim E. J., Jang W. G. Piperine induces osteoblast differentiation through AMPK-dependent Runx2 expression. Biochemical and Biophysical Research Communications. 2018;495(1):1497–1502. doi: 10.1016/j.bbrc.2017.11.200. [DOI] [PubMed] [Google Scholar]
- 54.Wang C. L., Xiao F., Wang C. D., et al. Gremlin2 suppression increases the BMP-2-induced osteogenesis of human bone marrow-derived mesenchymal stem cells via the BMP-2/Smad/Runx2 signaling pathway. Journal of Cellular Biochemistry. 2017;118(2):286–297. doi: 10.1002/jcb.25635. [DOI] [PubMed] [Google Scholar]
- 55.Ogle O. E. Implant surface material, design, and osseointegration. Dental Clinics of North America. 2015;59(2):505–520. doi: 10.1016/j.cden.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Mavropoulos E., Hausen M., Costa A. M., et al. The impact of the RGD peptide on osteoblast adhesion and spreading on zinc-substituted hydroxyapatite surface. Journal of Materials Science-Materials in Medicine. 2013;24(5):1271–1283. doi: 10.1007/s10856-013-4851-3. [DOI] [PubMed] [Google Scholar]
- 57.Wilson C. J., Clegg R. E., Leavesley D. I., Pearcy M. J. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Engineering. 2005;11(1-2):1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: identification of human BMSCs. (a) Flow cytometry analysis showed that the cells expressed CD44 and CD146 but did not express CD34 or CD45. (b) Alizarin red staining was positive after 21 d of osteogenic induction. These findings confirmed that the cells have characteristics consistent with those of BMSCs.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


