Abstract
Background:
Cardiovascular disease is a major source of mortality in schizophrenia, and access to care after acute myocardial infarction (AMI) is poor for these patients.
Aims:
To understand the relationship between schizophrenia and access to coronary revascularization and the impact of revascularization on mortality among individuals with schizophrenia and AMI.
Method:
This study used a retrospective cohort of AMI in Ontario between 2008 and 2015. The exposure was a diagnosis of schizophrenia, and patients were followed 1 year after AMI discharge. The primary outcome was all-cause mortality within 1 year. Secondary outcomes were cardiac catheterization and revascularization (percutaneous coronary intervention or coronary artery bypass graft). Cox proportional hazard regression models were used to study the relationship between schizophrenia and mortality, and the time-varying effect of revascularization.
Results:
A total of 108,610 cases of incident AMI were identified, among whom 1,145 (1.1%) had schizophrenia. Schizophrenia patients had increased mortality, with a hazard ratio (HR) of 1.55 (95% CI, 1.37 to 1.77) when adjusted for age, sex, income, rurality, geographic region, and comorbidity. After adjusting for time-varying revascularization, the HR reduced to 1.38 (95% CI, 1.20 to 1.58). The impact of revascularization on mortality was similar among those with and without schizophrenia (HR: 0.42; 95% CI, 0.41 to 0.44 vs. HR: 0.40; 95% CI, 0.26 to 0.61).
Conclusions:
In this sample of AMI, mortality in schizophrenia is increased, and treatment with revascularization reduces the HR of schizophrenia. The higher mortality rate yet similar survival benefit of revascularization among individuals with schizophrenia relative to those without suggests that increasing access to revascularization may reduce the elevated mortality observed in individuals with schizophrenia.
Keywords: access to care, epidemiology, schizophrenia, health services research, health disparities
Abstract
Contexte :
La maladie cardiovasculaire est une source majeure de mortalité dans la schizophrénie, et l’accès aux soins après un infarctus aigu du myocarde (IAM) est médiocre pour ces patients.
Objectifs :
Comprendre la relation entre la schizophrénie et l’accès à la revascularisation coronarienne, ainsi que l’effet de la revascularisation sur la mortalité chez des personnes souffrant de schizophrénie et d’IAM.
Méthode :
Cette étude a utilisé une cohorte rétrospective d’IAM en Ontario entre 2008 et 2015. L’exposition était un diagnostic de schizophrénie, et les patients étaient suivis un an après le congé de l’IAM. Le résultat principal était la mortalité pour toutes causes confondues à l’intérieur d’un an. Les résultats secondaires étaient le cathétérisme cardiaque et la revascularisation percutanée (PCI) ou chirurgicale (CABG). Les modèles de régression aléatoire proportionnelle de Cox ont servi à étudier la relation entre la schizophrénie et la mortalité, et l’effet variable dans le temps de la revascularisation.
Résultats :
Nous avons identifié 108 610 cas d’épisodes d’IAM, parmi lesquels 1 145 (1,1%) souffraient de schizophrénie. Les patients souffrant de schizophrénie avaient une mortalité accrue, avec un rapport de risques de 1,55 (IC à 95%: 1,37 à 1,77) après ajustement pour l’âge, le sexe, le revenu, la ruralité, la région géographique et la comorbidité. Après ajustement pour l’effet variable dans le temps de la revascularisation, le rapport de risques diminuait à 1,38 (IC à 95%: 1,20 à 1,58). L’effet de la revascularisation sur la mortalité était semblable chez ceux souffrant de schizophrénie ou pas (RR:0,42; IC à 95%: 0,41 à 0,44 contre RR: 0,40; IC à 95%: 0,26 à 0,61).
Conclusions :
Dans cet échantillon d’IAM, la mortalité dans la schizophrénie augmente, et le traitement par revascularisation réduit le rapport de risques de la schizophrénie. Le taux plus élevé de mortalité et pourtant le bénéfice semblable de survie de la revascularisation chez les personnes souffrant de schizophrénie, relativement à celles qui n’en souffrent pas, suggère qu’accroître l’accès à la revascularisation peut réduire la mortalité élevée observée chez les personnes souffrant de schizophrénie.
It is now well known that individuals with schizophrenia and other severe mental illnesses experience a “mortality gap” compared to the general population, and this gap has widened in recent years.1–3 One major contributor to this difference in mortality is the significantly increased morbidity and mortality from cardiovascular disease in this population.1,3–5 The causes of this cardiovascular disease–related elevated mortality risk are multifactorial and include the underlying biology of the mental illnesses; exposure to antipsychotic medications; behavioral factors such as diet, exercise, and smoking; and reduced access to medical care.6–8 Myocardial infarctions are also less likely to be recognized in patients with schizophrenia9 and are more likely to be fatal.10 For example, while ST-elevation myocardial infarction (STEMI) has decreased in the general population since 2003, this decrease has not occurred in those with severe mental illnesses.11
A number of studies have examined the differential treatment received by individuals with severe mental illnesses and in particular schizophrenia. These individuals are more likely to be readmitted for a cardiovascular condition after a cardiovascular event12 and have less access to specialist care after the myocardial infarction (MI).13 A number of studies have identified that cardiac procedures (percutaneous coronary intervention [PCI] and coronary artery bypass graft [CABG]) are reduced on the order of 50% in patients with schizophrenia compared to MI patients without schizophrenia.11,13–16 This differential lack of access to revascularization is significant, particularly since patients with mental illnesses have been found to have similar levels of comorbidities as other patients.17
The objectives of this study were to understand the relationship between a diagnosis of schizophrenia and mortality after acute myocardial infarction (AMI) and the relative impact of access to revascularization procedures on mortality among individuals with an incident AMI with or without schizophrenia.
Methods
Study Design
A retrospective cohort study was conducted to measure mortality and receipt of cardiac interventions among incident MI subjects with and without schizophrenia in the province of Ontario, Canada. Institute for Clinical Evaluative Sciences (ICES) is a prescribed entity under Section 45 of Ontario’s Personal Health Information Protection Act (PHIPA), which allows for personal information to be used for research without the need for patient consent.
Setting
This study used administrative data housed at ICES. ICES is an independent, nonprofit research organization that holds population-level data, including administrative data, for the purpose of evaluating health-care services and their effectiveness in Ontario. Through this system, patient records are linked using encoded identifiers, and this allows linkage across provincial health-care administrative databases. These databases include information for publicly insured physician and hospital services, which represent the majority of services provided in this region, since Ontario has a publicly funded single-payer health-care system.
The databases used included the Ontario Health Insurance Plan (OHIP) for physician billings and outpatient visits, the Canadian Institute for Health Information Discharge Abstract Database for acute hospitalizations, the National Ambulatory Care Reporting System for emergency department visits, the Registered Persons Database for demographic information and deaths (based on the census), the Ontario Drug Benefit Claims (ODB) for medication prescriptions, and the Ontario Mental Health Reporting System for inpatient mental health hospitalizations. The Ontario Myocardial Infarction Database (OMID) is an ICES-derived data set of MI discharges among patients aged 20 to 105 years and has been used in multiple previous studies. It contains information about AMI diagnosis, treatment, patient demographics, comorbidity, and mortality with a reported sensitivity and specificity of 95% and 88%, respectively.18–20 The methodology for the creation of this data set was introduced elsewhere.21 These data sets were linked using unique encoded identifiers and analyzed at ICES. The use of data in this project was authorized under Section 45 of Ontario’s PHIPA, which does not require review by a Research Ethics Board.
Participants
We included all patients discharged between April 1, 2008, and March 31, 2015, from OMID. Patients were excluded if they had a prior MI or had erroneous dates (were deceased prior to the recorded MI, for example). Patients were followed from the date of AMI admission to 1 year thereafter, or March 31, 2016, whichever came first, for outcomes.
Exposures
The main exposure was a diagnosis of schizophrenia, present at the time of the incident MI. The diagnosis of schizophrenia was determined based on an established algorithm,13,22 which uses physician billing data and inpatient hospitalization data: either 3 outpatient International Classification of Diseases (ICD)-9 codes for schizophrenia from outpatient physician billings within 3 years or a hospital-based diagnosis of schizophrenia (ICD-9 code—295.x; ICD-10 code F20 or F25) or a Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) Axis I diagnosis of schizophrenia (DSM-IV code—295.x). This is a specific measure of schizophrenia, yielding a prevalence of less than 1% and includes patients with a diagnosis of schizoaffective disorder, hereafter all referred to as, “schizophrenia” (sensitivity 96.5%, 95% CI, 92.5 to 100.0; specificity 57.1%, 95% CI, 50.2 to 64.1).
Main Outcome
The primary outcome was all-cause mortality within 1 year after the index MI admission. Secondary outcomes were receipt of, and time to, cardiac catheterization, revascularization (PCI and/or CABG), and recurrent MI, as defined by OHIP and CIHI codes (see Supplementary Table 3). Within the OMID database, catheterization, PCI, and CABG are defined outcomes, if they were received after admission or at specific time points (within 30 days of admission, within 90 days of admission, etc.). Dispensed prescriptions for angiotensin-converting enzyme inhibitor (ACE-I), angiotensin II receptor blocker (ARB), or HMG-CoA reductase inhibitor (statin) medications were tracked through the ODB database for individuals over the age of 65.
Covariates
Covariates and baseline characteristics were collected for participants using the databases listed previously. Demographic information included age, sex, rural residence, and income (based on neighborhood income quintiles). Patient preexisting health conditions were measured by Charlson comorbidity within 2 years before the index AMI admission and any previous diagnoses of congestive heart failure,23 hypertension,24 or diabetes,25 in the past, as identified in databases derived from the primary administrative databases. Since we measured congestive heart failure, hypertension, and diabetes separately, these comorbidities were removed from the Charlson comorbidity index for comparison.
Statistical Analysis
Categorical data were summarized using frequencies and percentages, and continuous data were summarized using means with standard deviations and medians with interquartile range. Chi-square tests were used to compare dichotomous or categorical variables, and t tests were used for continuous variables, between individuals with and without schizophrenia.
A series of cox proportional hazard models were used to estimate mortality with the main exposure for the first objective being a diagnosis of schizophrenia. For the second objective, we estimated the impact of receiving or not receiving revascularization interventions in the total cohort and in 2 cohorts stratified by a diagnosis of schizophrenia. For the first objective, a cox proportional hazard model was used to generate the hazard ratio (HR) of mortality within 1 year in patients with schizophrenia, compared to those without, adjusting for sex, age, neighborhood income quintile, rural residence, comorbidity (hypertension, diabetes, heart failure), Charlson comorbidity, and health region (Model 1). Model 2 then added time-varying receipt of revascularization (PCI or CABG) to Model 1, examining the impact of schizophrenia on mortality after adjusting for revascularization. Subsequently, Model 3 was reconstructed by further adding to Model 2 an interaction term between schizophrenia and receipt of revascularization (PCI or CABG). In addition, to further evaluate whether the impact of revascularization on mortality after AMI persists among both patients without and those with schizophrenia, we performed a stratified analysis by patient’s schizophrenia status, separately evaluating the effect of receipt of revascularization procedures on mortality within 1 year after AMI for patients without schizophrenia and patients with schizophrenia (Model 4 without schizophrenia and Model 5 with schizophrenia).
Results
Demographics
Between April 1, 2008, and March 31, 2015, there were 122,222 identified MI cases in OMID, of which 13,580 were excluded due to a previous MI, and 32 were excluded due to erroneous dates, yielding 108,610 cases of incident MI. A total of 1,145 (1.1%) individuals were identified as having schizophrenia at the time of the MI, with 107,465 (98.9%) identified as non-schizophrenia.
Compared to those without schizophrenia, patients with a diagnosis of schizophrenia were more likely to be female (44.9% vs. 36.3%; P < 0.001), younger (64.5 ± 13.8 years vs. 68.0 ± 14.4 years; P < 0.001), and much more likely to be in the lowest income quintile (37.9% vs. 21.7%; P < 0.001). They were less likely to live in a rural area (9.8% vs. 15.5%; P < 0.001).
Those with schizophrenia were more likely to have diabetes or congestive heart failure but were less likely to have hypertension. The Charlson comorbidity score was higher in individuals with schizophrenia (Table 1).
Table 1.
Demographic and Comorbidity Results.
| Characteristics | No schizophrenia (N = 107,465) | Schizophrenia (N = 1,145) | P |
|---|---|---|---|
| Demographics | |||
| Female | 39,030 (36.3%) | 514 (44.9%) | <0.001 |
| Age in years (mean 68.0 years, SD 14.4 years) | |||
| 20–54 | 21,525 (20.0%) | 303 (26.5%) | <0.001 |
| 55–64 | 24,025 (22.4%) | 302 (26.4%) | |
| 65–74 | 22,539 (21.0%) | 236 (20.6%) | |
| ≥75 | 39,376 (36.6%) | 304 (26.6%) | |
| Neighborhood income quintile | |||
| 1—lowest | 23,141 (21.7%) | 428 (37.9%) | <0.001 |
| 2 | 22,297 (20.9%) | 218 (19.3%) | |
| 3 | 21,668 (20.3%) | 188 (16.7%) | |
| 4 | 20,835 (19.5%) | 154 (13.6%) | |
| 5—highest | 18,918 (17.7%) | 141 (12.5%) | |
| Rural address | 16,599 (15.5%) | 112 (9.8%) | <0.001 |
| Medical history and cardiac risk factors | |||
| Previous hypertension | 70,928 (66.0%) | 700 (61.1%) | <0.001 |
| Duration of hypertension (years) | |||
| Hypertension duration mean ± SD | 11.74 ± 6.67 | 11.06 ± 6.83 | 0.007 |
| Hypertension duration, median (IQR) | 13 (8–17) | 13 (4-17) | 0.008 |
| Previous diabetes | 34,936 (32.5%) | 458 (40.0%) | <0.001 |
| Duration of diabetes (years) | |||
| Diabetes duration mean ± SD | 10.56 ± 6.74 | 10.17 ± 6.78 | 0.217 |
| Diabetes duration median (IQR) | 8 (4–17) | 8 (4-17) | 0.225 |
| Previous CHF | 15,623 (14.5%) | 192 (16.8%) | 0.033 |
| Duration of CHF (years) | |||
| CHF duration, mean ± SD | 5.20 ± 5.39 | 4.64 ± 5.12 | 0.149 |
| CHF duration, median (IQR) | 4 (0–8) | 4 (0-8) | 0.156 |
| Charlson comorbidity score in the 2 years before AMIa | <0.001 | ||
| 0 | 81,372 (75.7%) | 787 (68.7%) | |
| 1 | 15,409 (14.3%) | 198 (17.3%) | |
| 2 | 7,360 (6.8%) | 107 (9.3%) | |
| 3 | 2,517 (2.3%) | 38 (3.3%) | |
| ≥4 | 807 (0.8%) | 15 (1.3%) | |
| Duration of Schizophrenia | |||
| Mean ± SD | 11.00 ± 7.07 | ||
| Median (IQR) | 13 (4-17) | ||
| Days of follow-up | |||
| Mean ± SD | 318.21 ± 113.55 | 302.38 ± 129.33 | <0.001 |
| Median (IQR) | 365 (365–365) | 365 (365–365) | <0.001 |
Note. CHF = congestive heart failure; AMI = acute myocardial infarction; DM = diabetes mellitus; IQR = interquartile range.
a Charlson comorbidity excluding CHF, DM, and AMI.
Primary and Secondary Outcomes
Primary and secondary outcomes are shown in Table 2 with additional secondary outcomes in Supplementary Table 1. Mortality was significantly higher in patients with schizophrenia compared to those without schizophrenia, both at 30 days (12.4% vs. 8.6%, respectively; P < 0.001) and at 1 year (21.0% vs. 16.4%, respectively; P < 0.001). Revascularization occurred significantly less for schizophrenia patients at all time points, both by CABG (at 30 days, 8.1% of patients without schizophrenia vs. 3.7% with schizophrenia, P < 0.001) and PCI (52.4% of patients without schizophrenia vs. 42.4% with schizophrenia, P < 0.001).
Table 2.
Primary and Secondary Outcomes in Myocardial Infarction Patients with and without Schizophrenia.
| Outcome | No schizophrenia (N = 107,465) | Schizophrenia (N = 1,145) | P |
|---|---|---|---|
| Mortality | |||
| Died within 30 days after admission | 9,293 (8.6%) | 142 (12.4%) | <0.001 |
| Died within 1 year after admission | 17,600 (16.4%) | 241 (21.0%) | <0.001 |
| Revascularization | |||
| Catheterization | |||
| Received catheterization within 30 days | 80,867 (75.2%) | 710 (62.0%) | <0.001 |
| Received catheterization within 90 days | 81,916 (76.2%) | 720 (62.9%) | <0.001 |
| Received catheterization within 1 year | 82,930 (77.2%) | 736 (64.3%) | <0.001 |
| PCI | |||
| Received PCI within 30 days | 56,276 (52.4%) | 485 (42.4%) | <0.001 |
| Received PCI within 90 days | 57,084 (53.1%) | 494 (43.1%) | <0.001 |
| Received PCI within 1 year | 57,982 (54.0%) | 501 (43.8%) | <0.001 |
| CABG | |||
| Received CABG within 30 days | 8,670 (8.1%) | 42 (3.7%) | <0.001 |
| Received CABG within 90 days | 9,764 (9.1%) | 53 (4.6%) | <0.001 |
| Received CABG within 1 year | 10,834 (10.1%) | 59 (5.2%) | <0.001 |
| PCI or CABG | |||
| Received PCI or CABG within 30 days | 63,835 (59.4%) | 520 (45.4%) | <0.001 |
| Received PCI or CABG within 90 days | 65,325 (60.8%) | 539 (47.1%) | <0.001 |
| Received PCI or CABG within 1 year | 66,563 (61.9%) | 549 (47.9%) | <0.001 |
Note. PCI = percutaneous coronary intervention; CABG = coronary artery bypass graft.
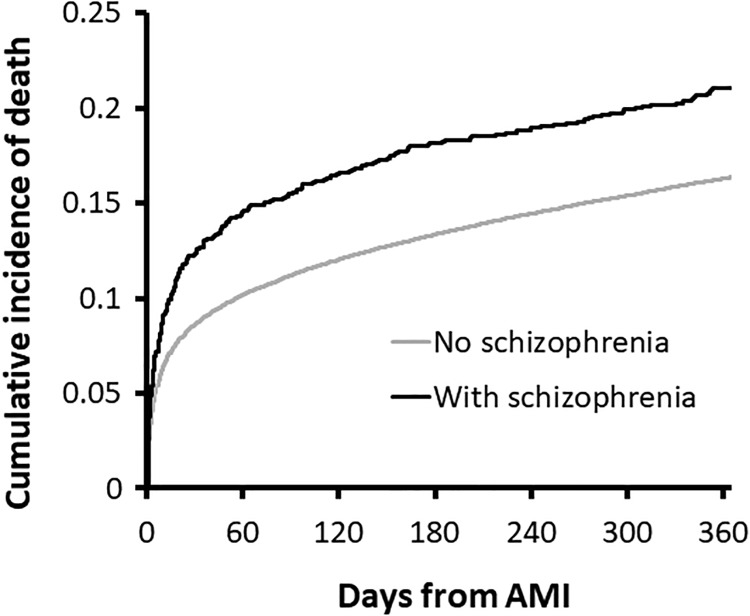
The cumulative probability of mortality during follow-up is shown in Figure 1. Most of the differential mortality occurs early after the incident MI. Supplementary Figure 1 shows the cumulative probability for receiving revascularization procedures after the AMI.
Figure 1.
Cumulative incidence of mortality within 1 year after acute myocardial infarction, by schizophrenia status, calculated from the Kaplan–Meier curve. Log-rank test: χ2 = 19.42, P value < 0.0001, DF = 1.
Cox Regression Models of Mortality and Procedures
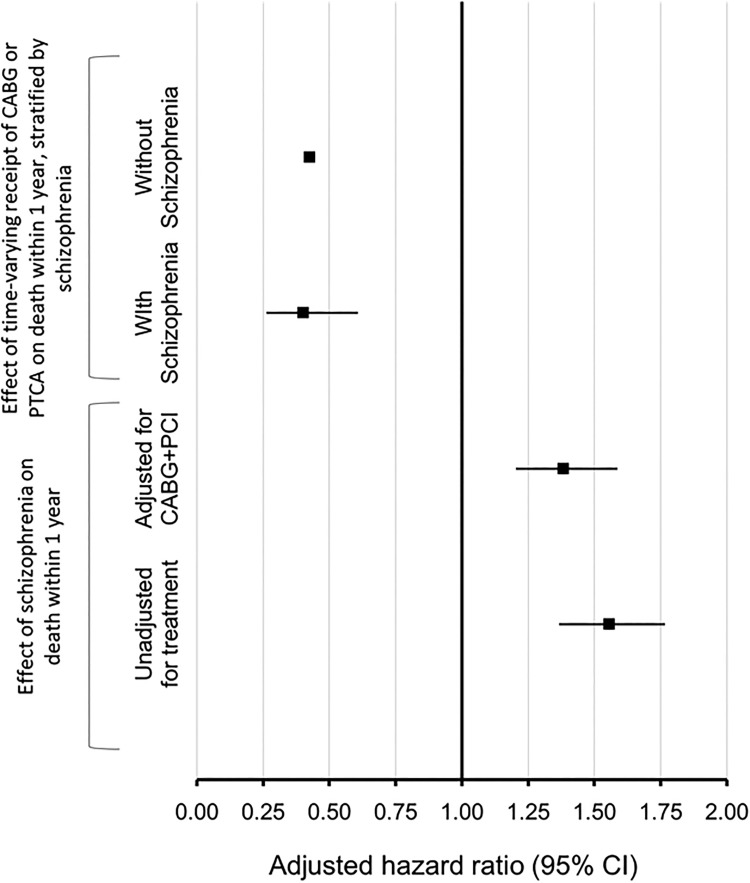
Results of cox regression models are shown in Figure 2. The initial Model 1 assessed the effect of schizophrenia on mortality within 1 year, adjusting for sex, age, neighborhood income quintile, rural residence, comorbidity (hypertension, diabetes, heart failure), Charlson comorbidity, and health region, without adjusting for receipt of CABG or PCI, and yielded a HR of 1.55 (1.37, 1.77) for schizophrenia. After adjusting for the time-varying receipt of coronary revascularization treatment (CABG or PCI), Model 2 shows a modestly reduced HR of 1.38 (1.20, 1.59). Model 3 studied the effect of schizophrenia and time-varying receipt of revascularization (PCI or CABG) on mortality, adjusted for the interaction between schizophrenia and revascularization. The interaction between schizophrenia and time-varying receipt of revascularization in Model 3 was not significant (P = 0.86), indicating that the effect of revascularization on mortality was not modified by schizophrenia status.
Figure 2.
Forest plot showing Cox proportional hazard models. The first two models at the bottom show the effect of schizophrenia on mortality at 1 year, adjusted for sex, age, neighborhood income quintile, rural residence, comorbidity (hypertension, diabetes, heart failure), Charlson comorbidity, and health region. The first model at the bottom (Model 1, unadjusted for treatment) is not adjusted for treatment received, and the one above it is adjusted for time-varying receipt of revascularization (Model 2, adjusted for coronary artery bypass graft [CABG] + percutaneous coronary intervention [PCI]). The two models at the top show the relationship between CABG and PCI and mortality, stratified by individuals with schizophrenia (Model 5) and individuals without schizophrenia (Model 4) after adjusting for sex, age, neighborhood income quintile, rural residence, comorbidity (hypertension, diabetes, heart failure), Charlson comorbidity, and health region.
Model 4 shows the effect of receipt of CABG or PCI decreased the risk of mortality within 1 year after AMI, among AMI patients without schizophrenia, with a HR of 0.42 (0.41, 0.44). Model 5 shows a very similar effect of receipt of CABG or PCI on mortality within 1 year after AMI, among AMI patients with schizophrenia, with a HR of 0.40 (0.26, 0.61).
Postdischarge Prescriptions
Within 3 months of the incident MI, patients with schizophrenia were significantly less likely to have received an ARB, β blocker, or statin but were similarly likely to have received an ACE-I (Supplementary Table 2). Although the majority of patients with or without schizophrenia received a prescription of at least one class of drug, those with schizophrenia did receive significantly less prescriptions at 1 year postdischarge (85.7% vs. 79.3%, P < 0.001).
Discussion
Our study shows that individuals with schizophrenia are more likely to die following AMI and to receive less treatment during and after myocardial infarctions, consistent with previous studies.16,18–23 We have also shown that the increased mortality associated with having a diagnosis of schizophrenia at the time of a first AMI is partially explained by reduced access to revascularization procedures and that the mortality reduction associated with revascularization procedures is equivalent whether or not an individual has a diagnosis of schizophrenia. Overall, patients with schizophrenia were very likely to receive evidence-based drug treatment for MI, although their receipt of prescriptions was reduced compared to those without the disorder.
This study replicates prior research showing the impact of schizophrenia on mortality after AMI.11,15,17,26–28 We believe this is the first study to demonstrate that revascularization has a similar beneficial impact on mortality in patients with schizophrenia. There is extensive evidence that individuals with mental illnesses and schizophrenia receive less invasive treatment after AMI, particularly revascularization. Our results show that at 1 month, individuals with schizophrenia are 23.6% less likely to receive revascularization as those without schizophrenia. Much of the difference in mortality also occurs early after the AMI, consistent with the literature as to the prognostic importance of an early invasive strategy post-MI. Taken together, the equivalent impact of revascularization on survival following AMI between individuals with and without schizophrenia in the face of significantly reduced access to revascularization procedures following AMI for individuals with schizophrenia suggests that efforts to improve the rate of revascularization for individuals with schizophrenia could have a substantial impact on the high mortality rate we have observed.
Many factors may contribute to reduced access to revascularization in patients with schizophrenia and other mental illnesses including concerns about informed consent, concerns about bias, and client adherence to follow-up after a procedure.11,17,27 A recent study found that among clients who underwent PCI for STEMI, those with schizophrenia had worse outcomes,29 although this study only looked at those individuals who rapidly received revascularization. However, in work by Druss et al., the incorporation of quality measures such as the receipt of reperfusion therapy improved the explanatory ability of the proportional hazard model for mortality,30 and other research has found that revascularization reduces the odds of mortality in individuals with severe mental illnesses.11 It may seem that there are obvious barriers to delivering care to individuals in this population, such as concerns about informed consent and adherence to post-revascularization follow-up care and prescriptions. This study cannot address these concerns. Rather, we show that when adjusting for relevant covariates, those individuals with schizophrenia who do receive revascularization do better than those who do not, despite these concerns about post-revascularization care, and revascularization access partially explains the increased mortality.
This article has a number of strengths and limitations. Ontario, Canada, provides a large and diverse population, and this database captures all AMIs and schizophrenia cases (including outpatients) in the population. Schizophrenia has been identified with a validated algorithm, and AMI is captured with a validated database which includes detailed information about processes of care and outcomes. There are also several limitations to the study. Given the low prevalence of schizophrenia found in our study, there are likely some schizophrenia cases that were misclassified as non-schizophrenia. It is also not possible to adjust for behavioral factors such as smoking, and other important psychiatric comorbidities such as depression, which also increase the risk of cardiovascular disease. The interplay between medication treatments (ACE-I, ARB, and HMG-CoA medications) and revascularization is not known. The psychiatric stability of patients at the time of AMI is not known and likely influences treatment decisions related to the AMI. Finally, this data set is only able to measure the receipt of revascularization procedures, which may have been offered but capably or incapably declined.
Conclusions
In conclusion, individuals with schizophrenia continue to have worse outcomes after MI, and they receive significantly less evidence-based diagnostic tests and revascularization treatments. Moreover, revascularization has an equivalent reduction in mortality among individuals with and without schizophrenia. Failure to receive these treatments explains some, but not all, of the increased mortality observed among individuals with schizophrenia. The implication of these findings is 2-fold: we must do more to understand why these access differences exist, and we need to do what is necessary to ensure equitable access to these beneficial procedures for individuals with schizophrenia. Failure to improve access to procedures that are known to increase survival will perpetuate the very high mortality rates experienced by individuals with schizophrenia after AMI.
Supplemental Material
Hauck_SupplementaryMaterial for Mortality and Revascularization among Myocardial Infarction Patients with Schizophrenia: A Population-Based Cohort Study by Tanya S. Hauck, Ning Liu, Harindra C. Wijeysundera and Paul Kurdyak in The Canadian Journal of Psychiatry
Footnotes
Authors’ Note: ICES is a prescribed entity under Section 45 of Ontario’s Personal Health Information Protection Act (PHIPA), which allows for personal information to be used for research without the need for patient consent.
The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed herein are those of the author and not necessarily those of CIHI.
Tanya Hauck planned the study; performed the literature search; participated in study design, data interpretation, and writing and editing of the manuscript. Ning Liu participated in study design, performed statistical analyses, participated in editing of the manuscript. Paul Kurdyak planned the study and participated in study design, data interpretation, and writing and editing of the manuscript. Harindra C. Wijeysundera planned the study and participated in study design, data interpretation, and writing and editing of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Institute for Clinical Evaluative Sciences, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. This study was supported in part by the Medical Psychiatry Alliance, a collaborative health partnership of the University of Toronto, the Centre for Addiction and Mental Health, The Hospital for Sick Children, Trillium Health Partners, the Ontario Ministry of Health and Long-Term Care, and an anonymous donor.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry. 2010;196(2):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenbaum L. Closing the mortality gap—Mental illness and medical care. New Engl J Med. 2016;375(16):1585–1589. [DOI] [PubMed] [Google Scholar]
- 3. Gatov E, Rosella L, Chiu M, Kurdyak PA. Trends in standardized mortality among individuals with schizophrenia, 1993-2012: a population-based, repeated cross-sectional study. CMAJ. 2017;189(37):E1177–E1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin HC, Chen YH, Lee HC. Increased risk of acute myocardial infarction after acute episode of schizophrenia: 6 year follow-up study. Aust New Zealand J Psychiatry. 2010;44(3):273–279. [DOI] [PubMed] [Google Scholar]
- 5. Suvisaari J, Perälä J, Saarni SI, Kattainen A, Lönnqvist J, Reunanen A. Coronary heart disease and cardiac conduction abnormalities in persons with psychotic disorders in a general population. Psychiatry Res. 2010;175(1-2):126–132. [DOI] [PubMed] [Google Scholar]
- 6. Enger C, Weatherby L, Reynolds RF, Glasser DB, Walker AM. Serious cardiovascular events and mortality among patients with schizophrenia. J Nerv Ment Dis. 2004;192(1):19–27. [DOI] [PubMed] [Google Scholar]
- 7. Kilbourne AM, Morden NE, Austin K, et al. Excess heart-disease-related mortality in a national study of patients with mental disorders: identifying modifiable risk factors. Gen Hosp Psychiatry. 2009;31(6):555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Casey DE, Haupt DW, Newcomer JW, et al. Antipsychotic-induced weight gain and metabolic abnormalities: Implications for increased mortality in patients with schizophrenia. J Clin Psychiatry. 2004;65(Suppl. 7):4–18. [PubMed] [Google Scholar]
- 9. Nielsen J, Juel J, Al Zuhairi KSM, et al. Unrecognised myocardial infarction in patients with schizophrenia. Acta Neuropsychiatr. 2015;27(2):106–112. [DOI] [PubMed] [Google Scholar]
- 10. Bodén R, Molin E, Jernberg T, Kieler H, Lindahl B, Sundström J. Higher mortality after myocardial infarction in patients with severe mental illness: a nationwide cohort study. J Intern Med (GBR). 2015;277(6):727–736. [DOI] [PubMed] [Google Scholar]
- 11. Schulman-Marcus J, Goyal P, Swaminathan RV, et al. Comparison of trends in incidence, revascularization, and in-hospital mortality in ST-elevation myocardial infarction in patients with versus without severe mental illness. Am J Cardiol. 2016;117(9):1405–1410. [DOI] [PubMed] [Google Scholar]
- 12. Callaghan RC, Boire MD, Lazo RG, McKenzie K, Cohn T. Schizophrenia and the incidence of cardiovascular morbidity: a population-based longitudinal study in Ontario, Canada. Schizophr Res. 2009;115(2-3):325–332. [DOI] [PubMed] [Google Scholar]
- 13. Kurdyak P, Vigod S, Calzavara A, Wodchis WP. High mortality and low access to care following incident acute myocardial infarction in individuals with schizophrenia. Schizophr Res. 2012;142(1-3):52–57. [DOI] [PubMed] [Google Scholar]
- 14. Wu SI, Chen SC, Juang JJM, et al. Diagnostic procedures, revascularization, and inpatient mortality after acute myocardial infarction in patients with schizophrenia and bipolar disorder. Psychosom Med. 2013;75(1):52–59. [DOI] [PubMed] [Google Scholar]
- 15. Laursen TM, Munk-Olsen T, Agerbo E, Gasse C, Mortensen PB. Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry. 2009;66(7):713–720. [DOI] [PubMed] [Google Scholar]
- 16. Kisely S, Campbell LA, Wang Y. Treatment of ischaemic heart disease and stroke in individuals with psychosis under universal healthcare. Br J Psychiatry. 2009;195(6):545–550. [DOI] [PubMed] [Google Scholar]
- 17. Druss BG, Bradford DW, Rosenheck RA, Radford MJ, Krumholz HM. Mental disorders and use of cardiovascular procedures after myocardial infarction. J Am Med Assoc. 2000;283(4):506–511. [DOI] [PubMed] [Google Scholar]
- 18. Tu JV, Naylor CD, Austin P. Temporal changes in the outcomes of acute myocardial infarction in Ontario, 1992-1996. CMAJ. 1999;161(10):1257–1261. [PMC free article] [PubMed] [Google Scholar]
- 19. Ko DT, Austin PC, Chan BTB, Tu JV. Quality of care of international and Canadian medical graduates in acute myocardial infarction. Arch Intern Med. 2005;165(4):458–463. [DOI] [PubMed] [Google Scholar]
- 20. Cox JL, Melady MP, Chen E, Naylor CD. Towards improved coding of acute myocardial infarction in hospital discharge abstracts: a pilot project. Can J Cardiol. 1997;13(4):351–358. [PubMed] [Google Scholar]
- 21. Ko DT, Wijeysundera HC, Jackevicius CA, Yousef A, Wang J, Tu JV. Diabetes mellitus and cardiovascular events in older patients with myocardial infarction prescribed intensive-dose and moderate-dose statins. Circ Cardiovasc Qual Outcomes. 2013;6(3):315–322. [DOI] [PubMed] [Google Scholar]
- 22. Kurdyak P, Lin E, Green D, Vigod S. Validation of a population-based algorithm to detect chronic psychotic illness. Can J Psychiatry. 2015;60(8):362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schultz SE, Rothwell DM, Chen Z, Tu K. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can. 2013;33(3):160–166. [PubMed] [Google Scholar]
- 24. Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1(1):e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 25. Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512–516. [DOI] [PubMed] [Google Scholar]
- 26. Young JK, Foster DA, Shander D, Druss BG. Cardiovascular procedures in patients with mental disorders [1] (multiple letters). J Am Med Assoc. 2000;283(24):3198–3199. [Google Scholar]
- 27. Mitchell AJ, Lord O. Do deficits in cardiac care influence high mortality rates in schizophrenia? A systematic review and pooled analysis. J Psychopharmacol (Oxford). 2010;24(4 Suppl):69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mitchell AJ, Lawrence D. Revascularisation and mortality rates following acute coronary syndromes in people with severe mental illness: comparative meta-analysis. Br J Psychiatry. 2011;198(6):434–441. [DOI] [PubMed] [Google Scholar]
- 29. Jakobsen L, Terkelsen CJ, Christiansen EH, et al. Severe mental illness and clinical outcome after primary percutaneous coronary intervention. Am J Cardiol. 2017;120(4):550–555. [DOI] [PubMed] [Google Scholar]
- 30. Druss BG, Bradford WD, Rosenheck RA, Radford MJ, Krumholz HM. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565–572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hauck_SupplementaryMaterial for Mortality and Revascularization among Myocardial Infarction Patients with Schizophrenia: A Population-Based Cohort Study by Tanya S. Hauck, Ning Liu, Harindra C. Wijeysundera and Paul Kurdyak in The Canadian Journal of Psychiatry