Abstract
Objective
To determine the applicability of a minimally invasive diagnostic device to evaluate the quality of articular cartilage following autologous (OAT) and allogeneic (OCA) osteochondral graft transplantation in goat model.
Design
OAT grafts were harvested from lateral femoral condyles (LFCs) and transplanted into osteochondral defects created in medial femoral condyles (MFCs) of contralateral knees. OCA grafts were transplanted into MFC condyles after in vitro storage. Autologous platelet-rich plasma (PRP) was administered intraarticularly after the surgery and at 1 and 2 months postoperatively. OAT and OCA grafts were evaluated macroscopically (Oswestry arthroscopy score [OAS]), electromechanically (quantitative parameter, QP), and histologically (O’Driscoll score, safranin O staining intensity) at 3 and 6 months after transplantation. Results were compared with preoperative graft evaluation.
Results
Transplanted cartilage deteriorated within 6 months in all groups. Cartilage quality was better retained in OAT group compared with a decline in OCA group. QP and OAS scores were comparable in OAT and OCA groups at 3 months, but superior in OAT group at 6 months, according to all the methods applied. PRP injections significantly improved QP and OAS score at 6 months compared with 3 months in OAT group. QP moderately correlated with OAS, O’Driscoll score, and safranin O staining intensity.
Conclusions
Grafts did not retain preoperative quality parameters at 6 months follow-up; however, OAT were superior to OCA grafts. PRP may have a beneficial effect on macroscopic and electromechanical properties of cartilage; however, histological improvement is yet to be proved. Electromechanical diagnostic device enables reliable assessment of transplanted cartilage.
Keywords: osteochondral transplantation, autograft, allograft, cartilage electromechanical properties, platelet-rich plasma
Introduction
Traumatic osteochondral defects are burden to active people and challenge to orthopaedic surgeons.1,2 It is critical to restore articular cartilage to prevent subsequent development of osteoarthritis. Osteochondral autograft transplantation (OAT) is a well-described surgical procedure for cartilage surface restoration, unfortunately, donor site morbidity and scarcity of autologous tissue generally limits its application.3,4 Treatment outcome of osteochondral allograft transplantation (OCA) and OAT is comparable, when osteochondral defect location, trauma etiology, and concomitant knee pathology are addressed.5,6 Despite the favourable clinical outcome after OCA transplantation there are number of studies reporting inconsistent efficacy, especially in active patients.7,8 Recently, fresh allografts alone or in combination with autografts have been suggested as a better approach to treat large osteochondral defects, because of the larger pool of viable cells compared with cold-stored or frozen OCA. It has been shown that allograft freezing is associated with chondrocyte death; however, preclinical results are still acceptable and biomechanical properties of cold-stored or frozen grafts could be maintained even in acellular tissue.9-12 Glenn et al.5 showed that refrigerated osteochondral allografts can maintain their functionality in vitro and in vivo.
Assessing the viability and integration of the transplanted OCA into surrounding tissue is crucial to clinical outcome. It has been shown, that bone to bone integration is usually achieved, but cartilage integration is scant.13 Several studies have demonstrated that subchondral bone is critical for mechanical stability of the graft and plays an essential role in cartilage layer integrity and viability after osteochondral transplantation.14,15
Unfortunately, there are limitations in measuring the rate of success after these surgical procedures. Methods routinely used to evaluate treatment outcome after osteochondral transplantation procedures include functional, arthroscopic, and diagnostic imaging. Therefore, there is great need for more precise and objective diagnostic methods to assess the efficacy of osteochondral transplantation in a long-term follow-up.
Recently, evaluation of cartilage electromechanical properties with Arthro-BST device has been proposed as a new method to assess osteochondral graft quality. It has been indicated that electromechanical quantitative parameter (QP) measurements correlate with histological scores and biomechanical parameters.16 In addition, allograft storage conditions may influence electromechanical properties of cartilage. Nevertheless, electromechanical properties have not been thoroughly investigated after OAT and OCA transplantation and there is no clear evidence regarding the usefulness of this technique for evaluation of transplantation efficacy.
Platelet-rich plasma (PRP) also has been a subject of interest because of its potential to enhance osteochondral tissue regeneration after different cartilage repair procedures.17 Preclinical studies supported the use of PRP, however clinical reports indicated mixed outcomes after PRP application.17 In fact, only a few studies focused on the injective PRP treatment as an adjunct to the surgical management of osteochondral lesions, and the results are controversial.18,19
The objective of this study was to evaluate electromechanical, macroscopic, and histological properties of transplanted autologous and cold-stored allogeneic osteochondral grafts in goat knees at 3 and 6 months postoperatively. We also investigated the effect of autologous PRP application on osteochondral graft healing.
Materials and Methods
Osteochondral Graft Preparation and Animal Surgery
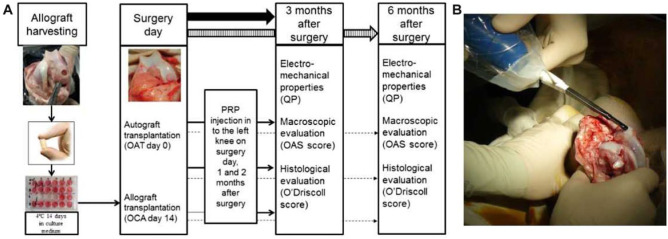
All experiments were approved by the Animal Health and Welfare Department, State Food and Veterinary Service. Our experiment was carried out in 2 stages ( Fig. 1 ). Twenty-six 5- to 6-month old male Saanen goats weighing 15 to 25 kg were used in the study. Lateral femoral condyles (LFCs) of 4 donor goats were used to harvest osteochondral plugs (6 mm diameter × 8 mm height) in a sterile fashion using Osteochondral Autograft Transfer System (OATS, Arthrex Inc, Naples, FL, USA). These grafts were assigned to OCA group and were cold-stored for 14 days as previously described.10
Figure 1.
(A) Design of experimental setting. (B) In vivo evaluation of cartilage electromechanical properties with Arthro-BST.
Twenty-two goats underwent bilateral knee mini arthrotomies by medial parapatellar approach, in the second stage of a study. OAT grafts (n = 9) were harvested from LFC and transferred into medial femoral condyle (MFC) defects created in contralateral knees of the same goat. Cold-stored OCA grafts (n = 13) were transplanted into osteochondral defects created in MFC ( Fig. 1 ). Surgical incision was closed layer by layer and knee was moved through a full range of motion to ensure normal patellar tracking. Ten milliliters of venous blood were drawn from the jugular vein into double syringe (Arthrex ABS-10010) and centrifuged for 20 minutes at 2000 × g (Rotofix 32A centrifuge, Hettich, Beverly, MS, USA) to obtain PRP. Two milliliters of PRP was injected in to the left knee of each goat postoperatively and repeatedly at 1 and 2 months after surgery. Animals received antibiotics for 5 days after operation, were provided with food and water ad libitum, and were allowed to move freely.
Macroscopic Evaluation and Grading
Two independent researchers performed macroscopic evaluation and grading according to a modified Oswestry arthroscopy score (OAS) at 3 and 6 months after implantation. According to this scoring system graft level, integration with surrounding cartilage, appearance of the surface and color of the graft is assessed. Stiffness on probing was excluded from the original scoring system in our study, because cartilage electromechanical properties were evaluated using Arthro-BST (Biomomentum Inc., Laval, Quebec, Canada). The maximum score of 8 represents normal cartilage repair (Supplemental Data available in the online version of the article).
Electromechanical Evaluation
Electromechanical properties of cartilage were evaluated with Arthro-BST postmortem at 3 and 6 months after transplantation, as previously described.10 Briefly, positively charged mobile ions in the cartilage stroma are displaced with respect to the fixed, negatively charged proteoglycan molecules during cartilage compression. The resultant high QP parameter is a digital reflection of extracellular matrix disintegration, weak electromechanical properties and inferior load-bearing capacity of the cartilage, while low QP indicates strong electromechanical properties and superior load-bearing capacity.
QP measurements in each transplantation site were recorded 5 times to obtain median values. Measurements made on the day of surgery (OAT day 0 and OCA day 14) were used as a reference control.
Histological Evaluation and Grading
After macroscopic and QP examination, distal femurs were dissected, fixed in 10% neutral buffered formalin, decalcified, and embedded in paraffin. Five micrometers thick sagittal sections were stained with safranin O/fast green, as previously described.10 Light microscopy images were taken at 4× magnification with Olympus BX61 microscope equipped with Olympus DP72 CCD camera using CellSens Dimension imaging software (Olympus, Japan). Quality of the repaired cartilage was blindly evaluated by 2 investigators using histological grading score, as described by O’Driscoll et al.20 Higher score indicates superior cartilage repair (maximum score is 24). The images were analyzed using Image J software. The amount of glycosaminoglycan (GAG) distribution as represented by red color intensity of safranin O staining was quantified as previously described.10 Briefly, red color intensity (RCI) was calculated in the entire cross-sectional slice area by obtaining red, green, and blue image planes in a scale of 256 values (black = 0). The fraction of red (RF) was defined as the ratio of the R component to the sum of the R, G, and B components: RF = R/(R + G + B) and expressed as percentage.
Statistical Analysis
Data were analyzed using the SPSS version 19 (IBM Corp, Armonk, NY, USA). All results are presented as the mean ± standard deviation and include confidence intervals. Statistical differences between experimental groups at different time points were assessed using nonparametric Mann-Whitney-Wilcoxon test. Statistical difference between different experimental groups was assessed using independent-samples t test. P < 0.05 was considered statistically significant. The relationship between QP, histological grading, macroscopic evaluation, and GAG distribution was assessed by bivariate correlation analysis using Pearson’s correlation coefficient. A significant correlation was present when P < 0.05. A post hoc power analysis revealed value greater than 0.9 for all statistically significant comparisons.
Results
Macroscopic Evaluation
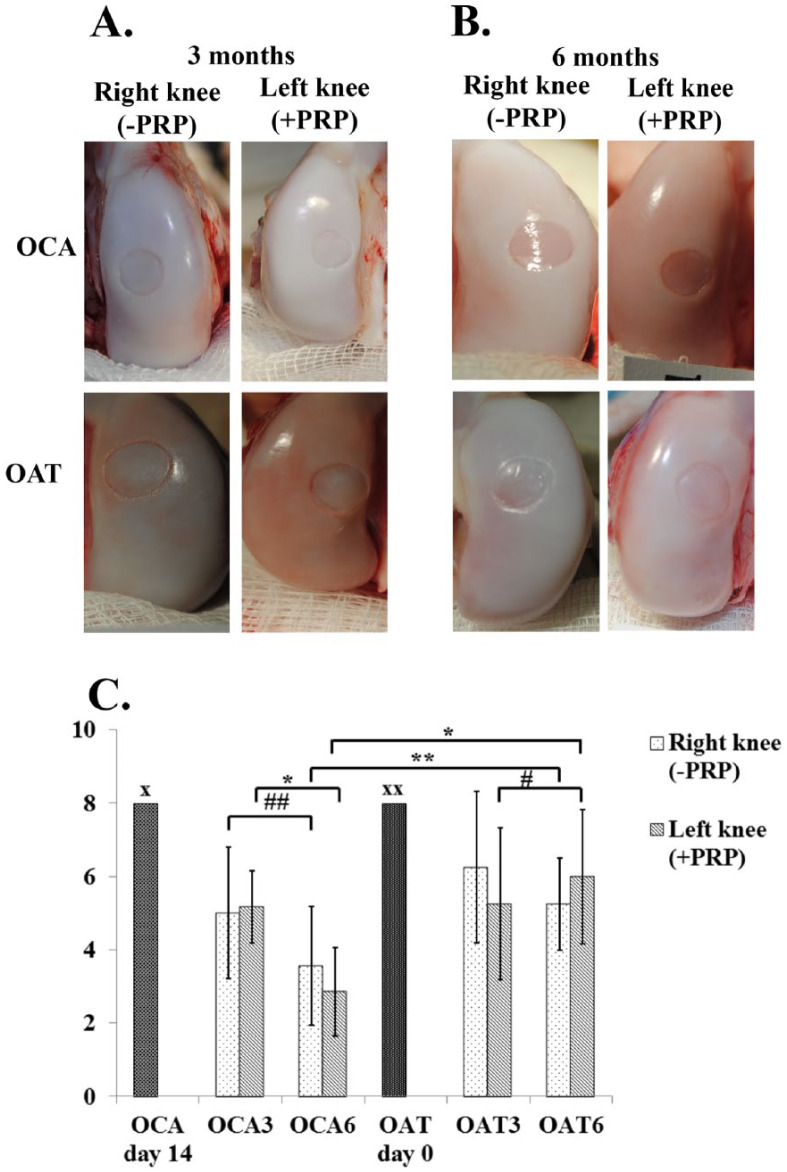
The OAS scores revealed that macroscopic appearance of transplanted cartilage in OAT group was numerically inferior than preoperative normal cartilage up to 6 months and almost significant (P = 0.059) if larger number of samples had been used. However, cartilage deterioration was noted in the OCA group after 3 and 6 months, as represented by impaired transplant integration to the surrounding cartilage and fine fronds on the surface.
OAS score in OCA group was lower than OAT group at 3 months postoperatively; however, the difference was nearly significant (P = 0.087, the difference could be significant if larger number of samples had been used). In contrast, OAS score was significantly inferior in OCA group when compared with the OAT group at 6 months postoperatively (P < 0.05).
PRP injections resulted in higher OAS score at 6 months postoperatively, when compared with 3 months in the OAT graft group (P < 0.05). This was mainly influenced by superior graft integration to the surrounding cartilage, smooth and white appearance of surface with isolated fronds on the graft. PRP administration had no positive effect on OAS scores in OCA group at 6 months ( Fig. 2 ). Mean OAS scores of the experimental groups are presented in Table 1 .
Figure 2.
Macroscopic appearance of osteochondral graft transplantation site on femoral condyles at 3 months (A) and 6 months (B) postoperatively. (C) OAS scores in each experimental group. OCA day 14, allogeneic graft transplantation day; OAT day 0, autologous graft transplantation day. OAT3, autologous graft after 3 months; OAT6, autologous graft after 6 months; OCA3, allogeneic graft after 3 months; OCA6, allogeneic graft after 6 months. OAS, Oswestry arthroscopy score; OCA, allogeneic graft; OAT, autologous graft; PRP, platelet-rich plasma. x and xx indicate maximum score for normal cartilage repair, #P=0.016, ##P = 0.004, *P < 0.001, **P = 0.01.
Table 1.
Macroscopic Scores According to a Modified OAS Score.
| Experimental Group | 3 Months |
6 Months | ||
|---|---|---|---|---|
| Right Knee (−PRP) | Left Knee (+PRP) | Right Knee (−PRP) | Left Knee (+PRP) | |
| OCA (n = 13) | ||||
| Mean ± SD | 5.00 ± 1.79 | 5.17 ± 0.98 | 3.57 ± 1.62 | 2.86 ± 1.21 |
| Range | 4.22-5.78 | 4.74-5.59 | 2.89-4.25 | 2.38-3.34 |
| OAT (n = 9) | ||||
| Mean ± SD | 6.25 ± 2.06 | 5.25 ± 2.06 | 5.25 ± 1.26 | 6.00 ± 1.83 |
| Range | 5.21-7.29 | 4.21-6.29 | 4.63-5.86 | 5.10-6.89 |
OCA = allogeneic graft; OAT = autologous graft; PRP = platelet-rich plasma; SD = standard deviation; OAS = Oswestry arthroscopy score.
Electromechanical Evaluation
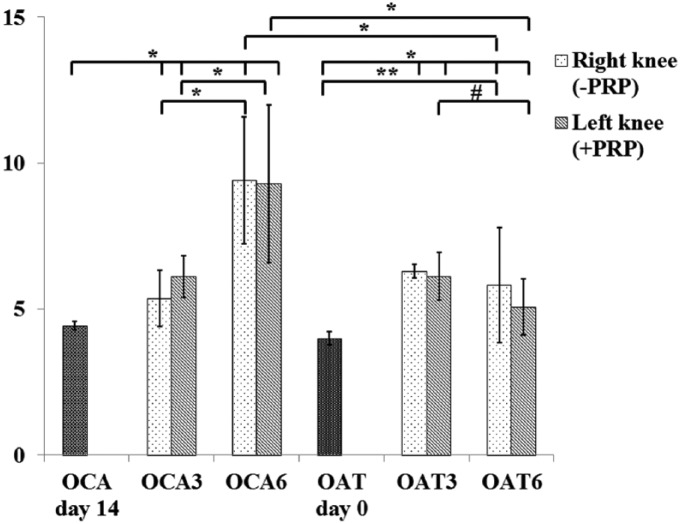
Electromechanical properties had a tendency to be superior in OAT group when compared with the OCA group after 3 months (P = 0.089, the difference could be significant if larger number of samples had been used), but significantly inferior compared with preoperative measurements, as assessed by increased QP values (P < 0.05). Cartilage quality was retained in the OAT group after 6 months (P = 0.498), whereas it worsened in the OCA group (P < 0.05) and was lower than that of the OAT group (P < 0.05), thus indicating subsequent deterioration of collagen and proteoglycans network.
PRP injections improved QP parameter in the OAT group at 6 months, when compared with 3 months (P < 0.05); however, such treatment did not influence cartilage electromechanical properties in the OCA group at any time ( Fig. 3 ). Mean QP values of experimental groups are presented in Table 2 .
Figure 3.
Quantitative parameter (QP) measurements of osteochondral grafts before and after transplantation. OAT day 0, autograft before implantation; OCA day 14, allograft after 14-day storage before implantation; OAT3, autologous graft after 3 months; OAT6, autologous graft after 6 months; OCA3, allogeneic graft after 3 months; OCA6, allogeneic graft after 6 months. OCA, allogeneic graft; OAT, autologous graft; PRP, platelet-rich plasma. #P = 0.002, *P < 0.001, **P = 0.001.
Table. 2.
Quantitative Parameter (QP) Values.
| Experimental Group | Control Measurements OCA Day 14 OAT Day 0 | After 3 Months |
After 6 Months | ||
|---|---|---|---|---|---|
| Right Knee (−PRP) | Left Knee (+PRP) | Right Knee (−PRP) | Left Knee (+PRP) | ||
| OCA (n = 13) | |||||
| Mean ± SD | 4.43 ± 0.15 | 5.37 ± 0.96 | 6.21 ± 0.71 | 9.41 ± 2.17 | 9.29 ± 2.69 |
| Range | 4.31-4.55 | 4.96-5.77 | 5.93-6.49 | 8.44-10.32 | 8.21-10.37 |
| OAT (n = 9) | |||||
| Mean ± SD | 4.00 ± 0.23 | 6.29 ± 1.99 | 6.13 ± 0.81 | 5.81 ± 1.97 | 5.07 ± 0.96 |
| Range | 3.68-4.31 | 5.34-7.24 | 5.73-6.53 | 4.89-6.73 | 4.63-5.51 |
OCA = allogeneic graft; OAT = autologous graft; OCA day 14 and OAT day 0 indicate day of surgery; PRP = platelet-rich plasma; SD = standard deviation.
Histological Evaluation
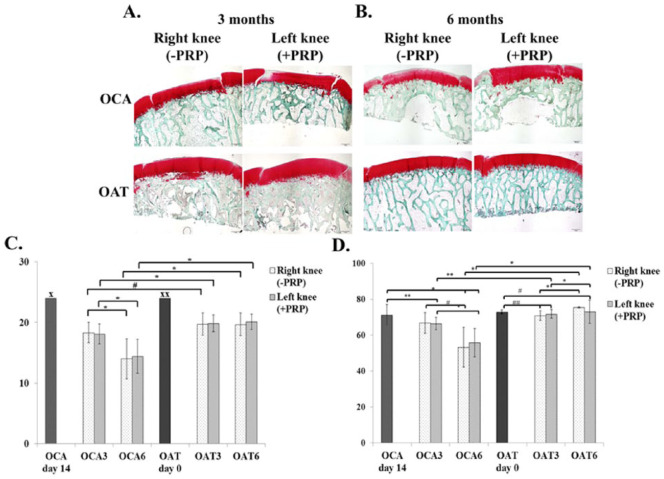
Histological properties of OAT and OCA groups were inferior at 3 months, compared with preoperative cartilage structure, as assessed by O’Driscoll score. However, osteochondral grafts were partially bonded to the adjacent cartilage and subchondral bone, and little clustering was detected at the border of native cartilage, thus showing active integration with adjacent cartilage.
Histological scores were retained in the OAT group up to 6 months (P = 0.897), however, decreased in OCA group (P < 0.05). This was represented by increased hypocellularity, clustering and reduced extracellular matrix deposition in transplanted cartilage. O’Driscoll score was lower in the OCA group at 3 months (P < 0.05) and was even lower at 6 months after transplantation (P < 0.05), when compared with the OAT group at the respective endpoint, thus indicating deterioration of cartilage histological structure in the OCA group at least up to 6 months.
Red color intensity decreased in OAT group at 3 months and OCA group at 6 months; however, a significant increase was noted at 6 months when compared with 3 months in OAT group (P = 0.001).
PRP injections had no positive effect on histological structure in any of the groups at any time postoperatively. Images of histological sections stained with safranin O/fast green are presented in Figure 4 . O’Driscoll histological grading scores in each group are summarized in Table 3 .
Figure 4.
Histological sections of transplanted cartilage stained with safranin O/fast green at 3 months (A) and 6 months (B) after osteochondral transplantation. (C) Histological evaluation of transplantation site in different experimental groups with O’Driscoll histological grading score. x and xx indicate maximum score for normal cartilage repair, #P = 0.017, *P < 0.001. (D) Red color intensity (%) in different experimental groups. *P = 0.001, **P = 0.021, #P = 0.002, ##P = 0.043. OCA day 14, allogeneic graft transplantation day; OAT day 0, autologous graft transplantation day. OAT3, autologous graft after 3 months; OAT6, autologous graft after 6 months, OCA3, allogeneic graft after 3 months OCA6, allogeneic graft after 6 months; PRP, platelet-rich plasma (For interpretation of the references to colours in this figure legend, refer to the online version of this article).
Table 3.
O’Driscoll Histological Grading Scores.
| Experimental Group | 3 Months |
6 Months | ||
|---|---|---|---|---|
| Right Knee (−PRP) | Left Knee (+PRP) | Right Knee (−PRP) | Left Knee (+PRP) | |
| OCA (n = 13) | ||||
| Mean ± SD | 18.33 ± 1.66 | 18.09 ± 1.64 | 14.00 ± 3.29 | 14.42 ± 2.81 |
| Range | 17.64-19.02 | 17.43-18.75 | 12.63-15.37 | 13.29-15.54 |
| OAT (n = 9) | ||||
| Mean ± SD | 19.73 ± 1.85 | 19.83 ± 1.40 | 19.67 ± 1.87 | 20.11 ± 1.27 |
| Range | 19.02-20.43 | 19.27-20.39 | 18.76-20.56 | 19.49-20.72 |
OCA = allogeneic graft; OAT = autologous graft; PRP = platelet-rich plasma; SD = standard deviation.
Glycosaminoglycan Distribution
A decreased intensity of red color was noted in both OAT and OCA transplantation groups after 3 months compared with preoperative measurement. Intensity of red color significantly decreased in the OCA group, whereas it increased in the OAT group after 6 months. In addition, PRP injection slightly increased GAG staining intensity in the OAT group at 3 months and in the OCA group at 6 months ( Fig. 4 ). The measurements of red color intensity in each group are summarized in Table 4 .
Table 4.
Glycosaminoglycan Distribution as Represented by the Safranin O Staining Intensity.
| Experimental Group | Control Measurements OCA Day 14 OAT Day 0 | After 3 Months |
After 6 Months | ||
|---|---|---|---|---|---|
| Right Knee (−PRP) | Left Knee (+PRP) | Right Knee (−PRP) | Left Knee (+PRP) | ||
| OCA (n = 13) | |||||
| Mean ± SD | 71.48 ± 5.61 | 66.93 ± 5.69 | 66.54 ± 3.43 | 53.34 ± 11.15 | 55.95 ± 7.9 |
| Range | 65.87-77.09 | 61.24-72.62 | 63.11-69.97 | 42.19-64.49 | 48.05-63.85 |
| OAT (n = 9) | |||||
| Mean ± SD | 72.95 ± 1.06 | 70.93 ± 2.8 | 71.86 ± 2.44 | 75.55 ± 0.27 | 73.19 ± 6.51 |
| Range | 71.89-74.01 | 68.13-73.73 | 69.24-74.30 | 75.28-75.82 | 66.58-79.70 |
OCA = allogeneic graft; OAT = autologous graft; OCA day 14 and OAT day 0 indicate day of surgery; PRP = platelet rich plasma; SD = standard deviation.
Correlations
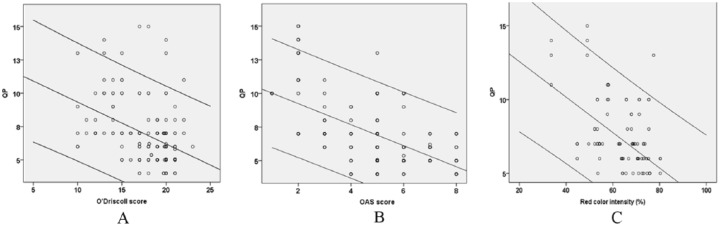
Statistical analysis revealed that electromechanical QP correlated inversely with the histological grading score (r = −0.596, P < 0.001), OAS score (r = −0.641, P < 0.001), and GAG distribution (r = −0.506, P = 0.001) ( Fig. 5 ). In addition, histological grading score correlated directly with the OAS score (r = 0.620, P < 0.001), GAG distribution correlated with O’Driscoll score (r = 0.533, P < 0.001) and OAS score (r = 0.623, P < 0.001) (data not shown).
Figure 5.
Bivariate linear correlations assessed using Pearson’s correlation coefficient. (A) Inverse correlation between quantitative parameter (QP) and O’Driscoll histological grading score. Correlation coefficient (r = −0.545), corresponding P value (P < 0.001). (B) Inverse correlation between QP and Oswestry arthroscopy score (OAS). Correlation coefficient (r = −0.655), corresponding P value (P < 0.001). (C) Inverse correlation between quantitative QP and red color intensity (%) (glycosaminoglycan distribution). Correlation coefficient (r = −0.506), corresponding P value (P = 0.001) (For interpretation of the references to colours in this figure legend, refer to the online version of this article).
Discussion
In this in vivo study, we have evaluated electromechanical properties of articular cartilage after OAT and OCA transplantation in goat knee osteochondral defect model at 6 months’ follow-up. Arthro-BST enables accurate assessment of articular cartilage properties and thus could be used intraoperatively to evaluate surface of the joint and the quality of the repair tissue. To our knowledge, this is the first time that electromechanical properties have been adapted for the assessment of osteochondral transplantation efficacy.
Evaluation of electromechanical properties is a promising, new technique in cartilage research, which has already been shown to adequately reflect cartilage material properties.16 Schagemann et al.21 evaluated cartilage regeneration after bilayered scaffold implanation. Using Arthro-BST, they were able to detect early signs of degeneration of the cartilage bordering the repair site, that was supported by histological findings. It has been reported that electromechanical measurement of cartilage composition and structure is even more sensitive to changes than biomechanical testing.22 In addition, electromechanical QP has been shown to correlate directly with the Mankin histological score, apoptosis and inversely with chondrocyte viability.10,16 Similarly, we have also observed a correlation between electromechanical QP, histological O’Driscoll score, and GAG expression, which demonstrated that intrinsic properties of cartilage repair can be reflected by electromechanical changes. Thus, the demonstrated QP correlation with cartilage histological, biomechanical parameters, and cell viability, in conjunction with macroscopic analysis, can give basis for the decision on subsequent treatment approach.
Despite the valuable and quantifiable electromechanical results, recent studies have acknowledged limitations regarding the scatter of obtained data. A study by Sim et al.23 has shown that electromechanical properties are not patient-specific, even though it did not include important characteristics like participation in sport activities. Moreover, QP values are diverse and depend on the location of measurement throughout the cartilage surface, with significant differences even amongst knee cartilage compartments.23 Cartilage thinning can generate very low streaming potentials, resembling that of a healthy cartilage, but nevertheless is mainly due to the closely underlying bone. In addition, Becher et al.24 noted a significant learning curve and a need for training to employ electromechanical measurement routinely. This proves, that lack of specificity of the resultant cartilage quality value as expressed by a false-positive and false-negative data and additional cartilage evaluation, such as electromechanical grading, macroscopic scoring, and arthroscopic hook probing must be considered, to improve cartilage assessment, specify the lesion more accurately and employ electromechanical measurement as a routine diagnostic procedure.
Noninvasive techniques for cartilage quality evaluation have been employed in the past, including delayed gadolinium-enhanced magnetic resonance imaging (dGEMRIC) and T2-mapping of cartilage. dGEMRIC has been largely used in preclinical and clinical studies, because of the proven correlation to cartilage glycosaminoglycan content and cartilage thickness.25 In addition, T2-mapping is reported to reproducibly measure collagen content in the cartilage by the aberrant T2 relaxation times. Histological evaluation has shown a high correlation to T2 and ΔR1 values after allograft chondrocyte implantation in a rabbit model.26 However, despite the currently applied quantitative valuation of cartilage thickness, biochemical, and histological composition methods by noninvasive dGEMRIC and T2-mapping techniques, a study by Aroen et al.27 did not find a morphological difference between affected and healthy cartilage regions of interest. In addition, biomechanical parameters of cartilage, such as fibril, matrix modulus and permeability have been shown to correlate to the QP potential, as well. Electromechanical assessment might be a valuable addition to currently employed diagnostic methods, due to its effectiveness in detecting minor cartilage defects, especially in the absence of macroscopical changes, otherwise not seen during standard diagnostic procedures, such as MRI.
Because of the scarce availability of cartilage donor tissue, allografts have been widely used for the treatment of large osteochondral defects. Cell viability has long been considered as a significant predictor toward clinical success and increased osteochondral graft survival in previous studies.11 It requires an established protocol that should facilitate rapid processing of allografts for immediate implantation or prolonged storage. Protocols are varying, but this has been shown to be most effectively done at 4°C with subsequent graft deterioration past 14 days.10,11 However, Williams et al.28 have argued that chondrocyte viability exceeding even 80% was not able to protect from allograft failure. Therefore, other technical points related to allograft storage and implantation, such as timely harvesting, brief period of ex vivo handling, atraumatic implantation, and fixation are vital for a successful treatment outcome.
There is a lack of clinical data regarding the usefulness of autologous PRP on the outcome of OAT and OCA transplantation. Recently Altan et al.19 demonstrated that use of PRP concomitantly with OAT resulted in a better healing response and improved histological scores at 3 weeks postoperatively compared with the OAT-alone procedure. However, no significant difference was observed between the groups after 6 weeks. Guney et al.29 demonstrated similar improvements in clinical outcome after 42 months in patients treated with microfracture, microfracture plus PRP and mosaicplasty. Sánchez et al.30 debated a possible need for different formulations of PRP that could be tailored for each specific clinical situation.
In our study, PRP injections significantly improved QP parameter, OAS score, and GAG expression after 6 months compared with 3 months in the OAT group, whereas it had no positive effect on any parameter in the OCA group. Different techniques used for PRP preparation, such as single or double centrifugation, length of centrifugation, and different methods of application, impede quality comparison amongst studies that reported superior clinical outcome.31,32 Custom formulation and preparation methods may be required to achieve clinical efficacy using allografts.
Conclusions
OAT and OCA grafts did not retain preoperative quality parameters at 6 months’ follow-up; however, OAT grafts were superior compared to OCA. Adjunct PRP therapy can be beneficial to OAT transplantation; however, OCA mosaicplasty may require different PRP application methods. Nondestructive intraoperative assessment of electromechanical QP can serve as an additional diagnostic method and give substantial input into qualitative valuation of damaged and repaired cartilage. However, a more sensitive measurement technique or complementary methods, such as improved electromechanical grading system, macroscopic scoring and arthroscopic hook probing might be needed to specify the lesion more accurately, assess the level of cartilage repair and employ electromechanical measurement as a routine diagnostic procedure.
Supplemental Material
Supplemental material, Supplementary_data_OAS for Nondestructive Assessment of Articular Cartilage Electromechanical Properties after Osteochondral Autologous and Allogeneic Transplantation in a Goat Model by Tomas Mickevicius, Alius Pockevicius, Audrius Kucinskas, Rimtautas Gudas, Justinas Maciulaitis and Arvydas Usas in CARTILAGE
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was funded by The European Social Fund under the Global Grant “Support to Research Activities of Scientists and Other Researcher (Global Grant)” No. VP1-3.1-SMM-07-K-03-078 “The effect of osteochondral allotransplantation on articular cartilage regeneration in experimental model of cartilage damage (CHONDRO)”
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: All experiments were approved by the Animal Health and Welfare Department, State Food and Veterinary Service.
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
Supplementary Material: Supplemental material for this article is available online.
References
- 1. Gracitelli GC, Meric G, Briggs DT, Pulido AA, McCauley JC, Belloti JC, et al. Fresh osteochondral allografts in the knee: comparison of primary transplantation versus transplantation after failure of previous subchondral marrow stimulation. Am J Sports Med. 2015;43(4):885-91. [DOI] [PubMed] [Google Scholar]
- 2. Mundi R, Bedi A, Chow L, Crouch S, Simunovic N, Enselman SE, et al. Cartilage restoration of the knee: a systematic review and meta-analysis of level 1 studies. Am J Sports Med. 2016;44(7):1888-95. [DOI] [PubMed] [Google Scholar]
- 3. Gudas R, Gudaitė A, Mickevičius T, Masiulis N, Simonaitytė R, Cekanauskas E, et al. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: a prospective study with a 3-year follow-up. Arthroscopy. 2013;29(1):89-97. [DOI] [PubMed] [Google Scholar]
- 4. Krych AJ, Harnly HW, Rodeo SA, Williams RJ., 3rd Activity levels are higher after osteochondral autograft transfer mosaicplasty than after microfracture for articular cartilage defects of the knee: a retrospective comparative study. J Bone Joint Surg Am. 2012;94(11):971-8. [DOI] [PubMed] [Google Scholar]
- 5. Glenn RE, Jr, McCarty EC, Potter HG, Juliao SF, Gordon JD, Spindler KP. Comparison of fresh osteochondral autografts and allografts: a canine model. Am J Sports Med. 2006;34(7):1084-93. [DOI] [PubMed] [Google Scholar]
- 6. Torrie AM, Kesler WW, Elkin J, Gallo RA. Osteochondral allograft. Curr Rev Musculoskelet Med. 2015;8(4):413-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pallante-Kichura AL, Cory E, Bugbee WD, Sah RL. Bone cysts after osteochondral allograft repair of cartilage defects in goats suggest abnormal interaction between subchondral bone and overlying synovial joint tissues. Bone. 2013;57(1):259-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shaha JS, Cook JB, Rowles DJ, Bottoni CR, Shaha SH, Tokish JM. Return to an athletic lifestyle after osteochondral allograft transplantation of the knee. Am J Sports Med. 2013;41(9):2083-9. [DOI] [PubMed] [Google Scholar]
- 9. Bae JY, Han D-W, Wakitani S, Nawata M, Hyon SH. Biological and biomechanical evaluations of osteochondral allografts preserved in cold storage solution containing epigallocatechin gallate. Cell Transplant. 2010;19(6):681-9. [DOI] [PubMed] [Google Scholar]
- 10. Mickevicius T, Pockevicius A, Kucinskas A, Gudas R, Maciulaitis J, Noreikaite A, et al. Impact of storage conditions on electromechanical, histological and histochemical properties of osteochondral allografts. BMC Musculoskelet Disord. 2015;16(1):314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pallante AL, Chen AC, Ball ST, Amiel D, Masuda K, Sah RL, et al. The in vivo performance of osteochondral allografts in the goat is diminished with extended storage and decreased cartilage cellularity. Am J Sports Med. 2012;40(8):1814-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosa SC, Gonçalves J, Judas F, Lopes C, Mendes AF. Assessment of strategies to increase chondrocyte viability in cryopreserved human osteochondral allografts: evaluation of the glycosylated hydroquinone, arbutin. Osteoarthritis Cartilage. 2009;17(12):1657-61. [DOI] [PubMed] [Google Scholar]
- 13. De Caro F, Bisicchia S, Amendola A, Ding L. Large fresh osteochondral allografts of the knee: a systematic clinical and basic science review of the literature. Arthroscopy. 2015;31(4):757-65. [DOI] [PubMed] [Google Scholar]
- 14. Brown D, Shirzad K, Lavigne SA, Crawford DC. Osseous integration after fresh osteochondral allograft transplantation to the distal femur: a prospective evaluation using computed tomography. Cartilage. 2011;2(4):337-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Rechenberg B, Akens MK, Nadler D, Bittmann P, Zlinsky K, Kutter A, et al. Changes in subchondral bone in cartilage resurfacing—an experimental study in sheep using different types of osteochondral grafts. Osteoarthritis Cartilage. 2003;11(4):265-77. [DOI] [PubMed] [Google Scholar]
- 16. Sim S, Chevrier A, Garon M, Quenneville E, Yaroshinsky A, Hoemann CD, et al. Non-destructive electromechanical assessment (Arthro-BST) of human articular cartilage correlates with histological scores and biomechanical properties. Osteoarthritis Cartilage. 2014;22(11):1926-35. [DOI] [PubMed] [Google Scholar]
- 17. Vannini F, Di Matteo B, Filardo G. Platelet-rich plasma to treat ankle cartilage pathology—from translational potential to clinical evidence: a systematic review. J Exp Orthop. 2015;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smyth NA, Haleem AM, Murawski CD, Do HT, Deland JT, Kennedy JG. The effect of platelet-rich plasma on autologous osteochondral transplantation: an in vivo rabbit model. J Bone Joint Surg Am. 2013;95(24):2185-93. [DOI] [PubMed] [Google Scholar]
- 19. Altan E, Aydin K, Erkocak O, Senaran H, Ugras S. The effect of platelet-rich plasma on osteochondral defects treated with mosaicplasty. Int Orthop. 2014;38(6):1321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg Am. 1988;70(4):595-606. [PubMed] [Google Scholar]
- 21. Schagemann JC, Rudert N, Taylor ME, Sim S, Quenneville E, Garon M, et al. Bilayer implants: electromechanical assessment of regenerated articular cartilage in a sheep model. Cartilage. 2016;7(4):346-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Changoor A, Coutu JP, Garon M, Quenneville E, Hurtig MB, Buschmann MD. Streaming potential-based arthroscopic device is sensitive to cartilage changes immediately post-impact in an equine cartilage injury model. J Biomech Eng. 2011;133(6):61005. [DOI] [PubMed] [Google Scholar]
- 23. Sim S, Hadjab I, Garon M, Quenneville E, Lavigne P, Buschmann MD. Development of an electromechanical grade to assess human knee articular cartilage quality. Ann Biomed Eng. 2017;45(10):2410-21. [DOI] [PubMed] [Google Scholar]
- 24. Becher C, Ricklefs M, Willbold E, Hurschler C, Abedian R. Electromechanical assessment of human knee articular cartilage with compression-induced streaming potentials. Cartilage. 2016;7(1):62-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Crema MD, Hunter DJ, Burstein D, Roemer FW, Eckstein F, Krishnan N, et al. Association of changes in delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) with changes in cartilage thickness in the medial tibiofemoral compartment of the knee: a 2 year follow-up study using 3.0 T MRI. Ann Rheum Dis. 2014;73(11):1935-41. [DOI] [PubMed] [Google Scholar]
- 26. Endo J, Watanabe A, Sasho T, Yamaguchi S, Saito M, Akagi R, et al. Utility of T2 mapping and dGEMRIC for evaluation of cartilage repair after allograft chondrocyte implantation in a rabbit model. Osteoarthritis Cartilage. 2015;23(2):280-8. [DOI] [PubMed] [Google Scholar]
- 27. Årøen A, Brøgger H, Røtterud JH, Sivertsen EA, Engebretsen L, Risberg MA. Evaluation of focal cartilage lesions of the knee using MRI T2 mapping and delayed gadolinium enhanced MRI of cartilage (dGEMRIC). BMC Musculoskelet Disord. 2016;17:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85-A(11):2111-20. [DOI] [PubMed] [Google Scholar]
- 29. Guney A, Yurdakul E, Karaman I, Bilal O, Kafadar IH, Oner M. Medium-term outcomes of mosaicplasty versus arthroscopic microfracture with or without platelet-rich plasma in the treatment of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1293-8. [DOI] [PubMed] [Google Scholar]
- 30. Sánchez M, Delgado D, Sánchez P, Fiz N, Azofra J, Orive G, et al. Platelet rich plasma and knee surgery. Biomed Res Int. 2014;2014:890630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carneiro Mde O, Barbieri CH, Neto BJ. Platelet-rich plasma gel promotes regeneration of articular cartilage in knees of sheeps. Acta Ortop Bras. 2013;21(2):80-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958-65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_data_OAS for Nondestructive Assessment of Articular Cartilage Electromechanical Properties after Osteochondral Autologous and Allogeneic Transplantation in a Goat Model by Tomas Mickevicius, Alius Pockevicius, Audrius Kucinskas, Rimtautas Gudas, Justinas Maciulaitis and Arvydas Usas in CARTILAGE