Abstract
Over the past years, there is an increasing demand for healthy, natural foods. Due to the high content of betalains, beetroots are widely used in the food industry as a natural colorant. In this study, beetroot juices are shown as a great source of selenium compounds. The juices were purchased from a local store and the ecological one was purchased form organic street market. The content of organic selenium species, as well as betalains, were evaluated using hydrophilic interaction liquid chromatography (HILIC) chromatography. The concentrations of selenomethionine (SeMet) and methylselenocysteine (MeSeCys) in analyzed juices were comparable except for juice from ecological cultivation. In that case, the concentration of SeMet is the highest of all studied juices, but simultaneously the concentration of MeSeCys was the lowest one. No traces of major inorganic species of Se, such as Se(IV) and Se(VI) was detected. The reducing power of juices evaluated by Folin-Ciocalteu assay was in range 50.78–166.7 mg GA/L. Juices obtained from beetroot from ecological cultivation showed the highest ability to scavenge the 1,1-diphenyl-2- picrylhydrazyl (DPPH) radicals. There was a correlation between the yellow pigment content and the presence of selenocysteine in studied juices.
Keywords: Food science, Food analysis, Antioxidant activity, DPPH, Folin-ciocalteu method, Selenomethionine, Methylselenocysteine
Food science; Food analysis; Antioxidant activity; DPPH; Folin-ciocalteu method; Selenomethionine; Methylselenocysteine
1. Introduction
The red beetroot (Beta vulgaris L.) is a well known root vegetable cultivated in all temperate climates. It is consumed as an ingredient in salads and soups or as juice. Beetroots are also used in the food industry as a colorant because they are a great source of betalains, a group of chromoalkaloids, which can be divided into betacyanins (red-violet pigments) and betaxantins (yellow pigments) (Gengatharan et al., 2015; da Silva et al., 2019). It was reported that beetroots are a source of polyphenols, vitamins B and C (Georgiev et al., 2010; Kaźmierczak et al., 2014; Wruss et al., 2015; Sentkowska and Pyrzynska, 2018a; Sri Vidhya and Radhai, 2018). In the recent years, the interest in that vegetable has significantly increased due to the ability of red beets to accumulate nitrates, which play a significant role in blood pressure regulation and vascular control (Lundberg and Weitzberg, 2005; Sri Vidhya and Radhai, 2017; Balsalobre-Fernandez et al., 2018). Pilot studies showed that fifteen days of consumption of beetroot juice by elite runners resulted in improvements in the time to exhaustion (Balsalobre-Fernandez et al., 2018). Beetroots also exhibit antioxidant and anti-inflammatory activities (Czapski et al., 2009; Slavov et al., 2013). Data on the concentration of trace elements and minerals in juice prepared from different beetroot varieties are limited (Wruss et al., 2015; Sri Vidhya and Radhai, 2018) and there are no adequate reports concerning total selenium content in beetroot juice as well as the concentrations of its speciation forms.
Interest in selenium has been increasing over the past few decades with growing knowledge of its importance to overall health (Kieliszek and Błażejak, 2016; Tan et al., 2016). Selenium is essential for the proper course of many metabolic pathways, including thyroid hormone metabolism and many immune processes. (Weekley and Harris, 2012). Selenium deficiency has been proven to be associated with cardiovascular and inflammatory diseases and even cancer. (Misra et al., 2015; Fernandes and Gandin, 2015). The ability of plants to accumulate and transform inorganic selenium forms into its bioactive organic compounds has important implications for human nutrition and health as organic Se is more bioavailable and less toxic than the inorganic forms (Schiavon and Pilon-Smits, 2016; Adadi et al., 2019). The relatively low Se content in the soil in Europe does not always provide sufficient dietary intakes of this element. For this reason, food supplements are becoming popular (Constantinescu-Aruxandei et al., 2018).
This study aimed to determine the concentration of selenium species in commercially available beet juices using HPLC-MS. The concentrations of betanin and vulgaxanthin I, the most abundant betalain pigments, were also determined. The relationship between the content of selenium species and the antioxidant capacity of juice samples has been also investigated.
2. Materials and methods
2.1. Reagents and samples
The commercial standards of selenium species (sodium selenite, sodium selenate, selenomethionine, methylselenocysteine), as well as betalains standards were purchased from Sigma (Steinheim, Germany). Methanol (MeOH) used for mobile phase preparation was purchased from Merck (Darmstadt, Germany). Water was obtained from a Mili-Q water purification system (Millipore, Bedford, MA, USA).
Freshly squeezed beet juices supplied by different manufacturers were purchased at the local market. Juices marked as S1, S2, S3, S4, and S5 were obtained from conventional cultivation. Juice marked as Seco came from beets from ecological cultivation. Organic cultivation of beet includes, among others, fertilization with the use of only natural fertilizers (manure, liquid manure, remains of green plants), co-cultivation of beetroot together with beans, peas or kohlrabi. It is also recommended to change the crop after 4–5 years of cultivation to specific plant species, e.g. onions, cucumbers, tomatoes. Before re-cultivating the beet, it is recommended to make medium plowing in the autumn before sowing the beet. All these treatments aimed at increasing the content of health-promoting compounds in beetroot are used on the farm where the organic juice used in the research was produced.
2.2. Chromatographic analysis
The amounts of selenium compounds and betalains were determined by the Shimadzu LC system coupled to an 8030 triple quadrupole mass spectrometer (Shimadzu, Japan). MS system was equipped with an ESI source operated in positive (organic selenium species) or negative (inorganic selenium) ion mode. The Electrospray Ionization ESI conditions involved capillary voltage 4.5 kV and temperature 400 °C. The gas flows were as follows: the source gas flow 3 Lmin-1 and drying gas flow 10 Lmin-1. Hydrophilic interaction liquid chromatography (HILIC) with a stationary silica phase was used for this purpose (Sentkowska and Pyrzynska, 2018b, Sentkowska and Pyrzynska, 2018a). The chromatographic separation was carried out using Atlantis silica column (100 × 2.1 mm, 3 μm) from Waters (Dublin, Ireland) at 30 °C (Sentkowska and Pyrzynska, 2018b). The mobile phase consisted of methanol and 8 mM ammonium acetate solution delivered in isocratic mode (85/15, MeOH/CH3COONH4, v/v). The eluent flow was 0.2 mL/min. The high content of methanol in the mobile phase under HILIC conditions enhances the efficiency of the ionization in the ion source of MS detector resulting in higher sensitivity of determination. Quantification was done from the calibration curve obtained in Selected Reaction Mode.
2.3. Antioxidant activity
The DPPH radical assay was performed according to the method previously described (Pyrzynska and Pękal, 2013). In detail, 0.1 mL of a sample was added to 2.4 mL of methanolic radical solution (9 × 10−5 mol L−1). The decrease in absorbance was measured 30 min after mixing reagents at 518 nm using Lambda 20 Perkin Elmer spectrophotometer. The results were expressed as a Trolox equivalent (TRE) in μM.
For Folin-Ciocalteu assay, 1 mL of sample was mixed with 0.1 mL of FC reagent and 0.9 mL of water. After 5 min, 1 mL of sodium carbonate (7% v/v) and 0.4 mL of water was added, and for another 30 min, the mixture was allowed for stabilization and formation of blue color. The absorbance against the blank was measured at 765 nm. The obtained results were expressed as gallic acid equivalent (GAE) in mM.
2.4. Statistical analysis
The experimental results were obtained from at least three parallel measurements and presented as average ± standard deviation. The significance of differences among means was carried out at 5% probability level using one way ANOVA and Tukey test. The correlation between the presented data was done based on the Pearson's correlation coeficients.
3. Results and discussion
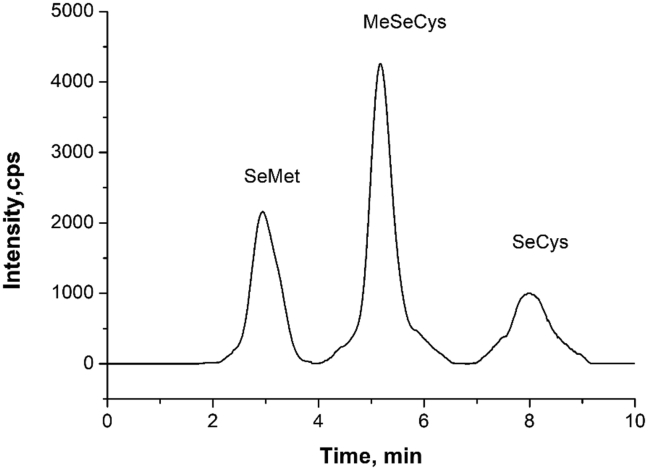
The content of Se forms in beetroots and juice prepared from them may be affected by variety, growing, and postharvest conditions. The samples used in this study were obtained from the Czerwona kula cultivar, which is the most popular beet cultivar in Poland. The determined concentrations of selenium species in studied beetroot juices are given in Table 1. The example chromatogram for S4 juice is presented in Figure 1. Generally, the concentrations of selenomethionine (SeMet) and methylselenocysteine (MeSeCys) in analyzed juices were comparable except for juice from ecological cultivation (sample Seco). In that case, the concentration of SeMet is the highest of all studied juices, but simultaneously the concentration of MeSeCys was the lowest one. These seleno-aminoacids are essential compounds in the human diet, being involved in antioxidant defense (Rahmanto and Davies, 2012; Sentkowska and Pyrzynska, 2019). Selenocysteine (SeCys) was detected only in S3 and S4 samples. We did not find any major inorganic species of Se, such as Se(IV) and Se(VI), for which the limit of detection by HILIC-MS is 0.15 μg L−1 (Sentkowska and Pyrzynska, 2018b). The retention times of Se (IV) and Se (VI) are approximately 1.2 and 1.8 min (Sentkowska and Pyrzynska, 2018b). In-plant foods, selenium exists mainly in organic forms. Thus, our results are consistent with those of the literature (Maneetong et al., 2013; Mechora et al., 2012; Pyrzynska, 2014; El-Ramady et al., 2014; Garousi et al., 2017; Wan et al., 2018). According to the EU regulations (Regulation (EC) 1170/2009), levels of inorganic selenium in dietary supplements should not exceed 1% of total Se content.
Table 1.
The content of selenium compounds and betalains in juice from beetroot (Beta vulgaris L.)∗.
| Compound | Seco | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|---|
|
Selenium compounds | ||||||
| SeMet | 0.56 ± 0.02a | 0.21 ± 0.01d | 0.33 ± 0.02b | 0.20 ± 0.01d | 0.26 ± 0. 01c | 0.20 ± 0.007d |
| MeSeCys | 0.08 ± 0.003c | 0.21 ± 0.01b | 0.22 ± 0.008b | 0.20 ± 0.01b | 0.28 ± 0.01a | 0.21 ± 0.011b |
| SeCys |
<LOD∗∗ |
<LOD |
<LOD |
0.11 ± 0.005b |
0.27 ± 0.02a |
<LOD |
|
Betalains | ||||||
| Betanin | 797 ± 24a | 535 ± 22b | 569 ± 21b | 406 ± 17c | 420 ± 14c | 541 ± 22b |
| Vulgaxantin I | 424 ± 16a | 417 ± 17a | 410 ± 18a | 311 ± 13b | 321 ± 11b | 410 ± 17a |
Data are expressed in mg L−1 (of juice) are expressed as the means ± SD of three independent experiments. Different letters in each row indicate a difference at a significance level of p = 0.05.
LOD- limit of detection- the lowest concentration of the analyte that can be detected by applied method, calculated as 3 time signal to noise ratio.
Figure 1.
The ESI-MS spectra of selenium compounds present in beetroot juice (sample S4). Column: Atlantis Silica (100 × 2.1 mm, 3 μm). Isocratic elution: 85% B, where A is 8 mM ammonium acetate solution, and B is methanol.
The knowledge regarding the concentrations of selenium species in commercially available beetroot juices is important from the consumer point of view. However, it is difficult to compare the obtained results with other root vegetables. In the case of our study, it is impossible to calculate the results in mg per g as the amount of raw material used for the preparation of juice was not available, and there is no information about the content of Se in beets. Giri, Usha & Sunitha (1990) reported the content of Se in beetroot as 2.8 μg per 100 g of dry matter, similar to radish (3.2 μg per 100 g of dry matter). The determined concentration of selenium in tea infusion (2 g with 20 mL of boiling water, extraction three times for 5 min) was 14.3 μg L−1, and organic Se forms accounted for 80% (Chen et al., 2015). The used tea leaves originated from the Enshi region in China, where the soil is rich in selenium. The detected selenium concentration in several of green coffee infusions (extraction of 0.5 g with 20 mL of water for 15 min) of different geographical origin was in the range of 12–47 μg L−1 (Jeszka-Skowron et al., 2016).
The ecological cultivation of beetroots resulted in the high content of betanin in the Seco juice (Table 1). The content of vulgaxantin does not differ much between the ecological and conventional beetroots (except for S3 and S4). Slavov et al. (2013) determined the individual betalains in the pressed juice from untreated red beets (Beta vulgaris L. cv. Detroit dark red). They found that the most abundant betalain pigments were betanin (312.5 mg per 100 g of dry matter) and vulgaxanthin I (104.1 mg per 100 g of dry matter). In the pressed juice from microwaved red beets, the content of betacyanin was lower in comparison to untreated red beets, but the content of bataxanthins was twice as big (Slavov et al., 2013). The results suggested the correlation between the high content of SeCys and the low concentration of yellow pigments in beetroot juices. Because SeCys was detected in only two juices, further studies are needed to confirm this relationship.
The total reducing capacity of examined juices was evaluated using the Folin-Ciocalteu assay. This method is also called as total phenolic content, but it should be remembered, that FC reagent can also oxidise several non-phenolic organic compounds as well as some inorganic compounds which has an impact on the obtained results (Everette et al., 2010; Granato et al., 2016). The reducing power of beetroot juices was in the range of 50.78–166.7 mg GA L−1. The lowest value was obtained for Seco juice. It should be stressed out that juices in which SeCys was detected (samples S3 and S4) gave the highest result in FC assay (167 ± 8 and 99 ± 5 mg GA L−1, respectively).
The antioxidant activity of studied juices was also determined based on their scavenging effect on the stable 1,1-diphenyl- 2-picrylhydrazyl (DPPH) radical. Obtained results, expressed as Trolox equivalent, increased in the order: S5 < S1 < S2 < S3 < S4 < Seco (Table 2). The ecological juice showed the highest ability to scavenge free radicals, however juices S3 and S4 (where SeCys was detected) took second and third place in the DPPH assay. Betalains show antioxidant and radical scavenging activities as it was demonstrated in a wide range of assays Ravichandran et al., (2013); Slavov et al., (2013); Slimen et al. (2017). The high content of these red pigments in the ecological juice sample resulted in its high ability to scavenge the free radicals, which confirmed the findings reported by Czapski et al. (2009). On the other hand, significantly less correlation between the antioxidant activity of beet juices and yellow pigments content was found (Czapski et al. (2009). Thus, it was concluded that red betanin is responsible for the DPPH and FC antioxidant activity of red beets.
Table 2.
The antioxidant activities of beetroot juice (Beta vulgaris L.)∗.
| Juice | FC∗∗ (mg GA L−1) | DPPH (mmol TR L−1) |
|---|---|---|
| Seco | 51 ± 3e | 0.48 ± 0.02a |
| S1 | 59 ± 2d | 0.32 ± 0.01b |
| S2 | 71 ± 3c | 0.34 ± 0.01b |
| S3 | 99 ± 5b | 0.44 ± 0.02a |
| S4 | 167 ± 8a | 0.46 ± 0.02a |
| S5 | 60 ± 2d | 0.31 ± 0.01b |
Data are expressed in mg L−1 (of juice) are expressed as the means ± SD of three independent experiments. Different letters in each column indicate a difference at a significance level of p = 0.05.
FC- Folin-Ciocalteu assay.
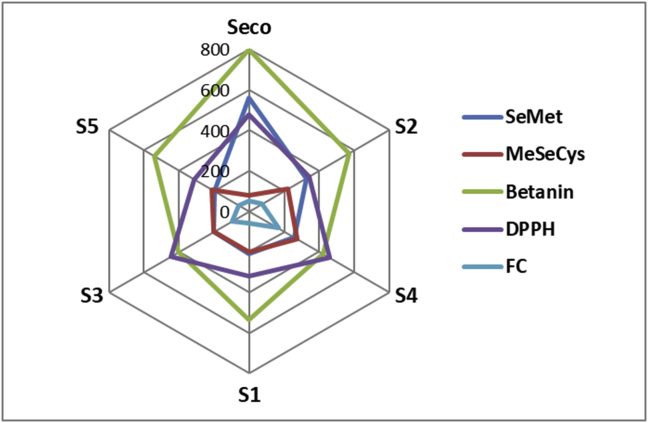
The correlation between the obtained results in this study for selenium species and betanin concentrations in studied beetroot juices as well as their antioxidant activity evaluated by the FC method and DPPH assay are presented in a graphical way in Figure 2 and in form of Pearson's correlation coefficients in Table 3. The highest value of correlation coefficient was observed for the SeMet and betanin (0.877). A moderate positive relationship was observed between the concentration of selenomethionine and DPPH antioxidant activity (0.532) and between MeSeCys and FC antioxidant activity (0.682). Generally, in many cases the values of correlation coefficients were negatives. That indicates that variables move in the opposite direction, when the increase in one value results in decrease in the second value. The highest opposite correlation coefficient was observed in case of MeSecys and betanin (-0.865).
Figure 2.
The comparison of total selenium, betalains content, and antioxidant properties of beetroot juices.
Table 3.
The values of Pearson's correlation coeficients between obtained results.
| SeMet | MeSeCys | Betanin | Vulgaxantin I | FC | |
|---|---|---|---|---|---|
| MeSeCys | -0.784∗ | ||||
| Betanin | 0.877∗ | -0.865∗ | |||
| Vulgaxantin I | 0.429ns | -0.513ns | 0.780∗ | ||
| FC | -0.298ns | 0.682∗ | -0.673ns | -0.834∗ | |
| DPPH | 0.532ns | -0.376ns | 0.126ns | -0.509ns | 0.472∗ |
∗Values are significant with P > 0.05.
ns- Values are significant with P > 0.05.
4. Conclusions
Selenoamino acids, such as SeMet and MeSeCys, were detected in all analyzed juices with no traces of inorganic selenium forms. Thus, beetroot juice can be a good source of organic selenium compounds, which can generate a Se reservoir and provide good bioavailability.
The total content of selenium in juices examined in this study was in the range of 43–87.5 μg in typical glass (250 mL). Assuming that the recommended daily allowance (RDA) of selenium is 55 μg, for an adult person, these juices can supply 78–159 % of that RDA value.
The obtained results provided data confirming protective role of beet juice against oxidative stress. The ecological cultivation of beetroots resulted in the highest afinity of Seco juice to scavenge DPPH radicals. On the other hand, other juices showed higher reductive properties obtained in Folin-Ciocalteu assay. The beetroot juice may be mixed with other vegetable or fruit juices to obtain ready-to-drink beverages with fresh-tasting, high nutritional value, and satisfactory organoleptic properties.
Declarations
Author contribution statement
A. Sentkowska: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
K. Pyrzynska: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Adadi P., Barakova N.V., Muravyov K.Y., Krivoshapkina E.F. Designing selenium functional foods and beverages: a review. Food Res. Int. 2019;120:708–725. doi: 10.1016/j.foodres.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Balsalobre-Fernandez C., Romero-Moraleda B., Cupeiro R., Peinado A.B., Butragueno J., Benito P.J. The effects of beetroot juice supplementation on exercise and running mechanics in elite distance runners: a double-blinded, randomized study. PloS One. 2018;13:1–10. doi: 10.1371/journal.pone.0200517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Zhu S., Lu D. Solidified floating organic drop microextraction for speciation of selenium and its distribution in selenium-rich tea leaves and tea infusions by electrothermal vapourisation inductively coupled plasma mass spectrometry. Food Chem. 2015;69:156–161. doi: 10.1016/j.foodchem.2014.07.147. [DOI] [PubMed] [Google Scholar]
- Commission Regulation (EC) No 1170/2009 of 30 November 2009 Amending Directive 2002/46/EC of the European Parliament and of Council and Regulation (EC) No 1925/2006 of the European Parliament and of the Council as Regards the Lists of Vitamin and Minerals and Their Forms that Can Be Added to Foods, Including Food Supplements.
- Constantinescu-Aruxandei D., Frincu R.M., Caprâ L., Oancea F. Selenium analysis and speciation in dietary supplements based on next-generation selenium ingredients. Nutrients. 2018;10:1466. doi: 10.3390/nu10101466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapski J., Mikołajczyk K., Kaczmarek M. Relationship between antioxidant capacity of red beet juice and contents of its betalain pigments. Pol. J. Food Nutr. Sci. 2009;59:119–122. [Google Scholar]
- El-Ramady H.R., Alla N.A., Fári M., Domokos-Szabolcsy É. Selenium enriched vegetables as biofortification alternative for alleviating micronutrient malnutrition. Int. J. Hortic. Sci. 2014;20:75–81. [Google Scholar]
- Everette J.D., Bryant Q.M., Green A.M., Abbey Y.A., Wangila G.W., Walker R.B. Throught study of reactivity of various compound classes toward the Folin-Ciocalteu reagent. J. Agric. Food Chem. 2010;58:8139–8144. doi: 10.1021/jf1005935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A.P., Gandin V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta. 2015;1850:1642–1660. doi: 10.1016/j.bbagen.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Garousi F., Domokos-Szabolesy E., Jánószky M., Kovác A.B., Veres S., Soós A., Kovác B. Selenoamino acids-enriched green pea as a value-added plant protein source for humans and livestock. Plant Foods Hum. Nutr. 2017;72:168–175. doi: 10.1007/s11130-017-0606-5. [DOI] [PubMed] [Google Scholar]
- Gengatharan A., Dykes G.A., Choo W.S. Betalains: natural plant pigments with potential application in functional foods. LWT – Food Sci. Technol. 2015;54:646–649. [Google Scholar]
- Georgiev V.G., Weber J., Kneschke E.M., Denev P.N., Bley T., Pavlov A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Foods Hum. Nutr. 2010;65:105–111. doi: 10.1007/s11130-010-0156-6. [DOI] [PubMed] [Google Scholar]
- Giri J., Usha K., Sunitha T. Evaluation of the selenium nd chromium content of plant foods. Plant Foods Hum. Nutr. 1990;40:49–59. doi: 10.1007/BF02193779. [DOI] [PubMed] [Google Scholar]
- Granato D., Santos J.S., Maciel L.G., Nunes D.S. Chemical perspective and criticism on selected analytical methods used to estimate the total content of phenolic compounds in food matrices. Trends Anal. Chem. 2016;80:266–279. [Google Scholar]
- Jeszka-Skowron M., Stanisz E., Paz de Pena M. Relationship between antioxidant capacity, chlorogenic acid and elemental composition of green coffee. LWT – Food Sci. Technol. 2016;73:243–250. [Google Scholar]
- Kaźmierczak R., Hallmann E., Lipowski J., Drela N., Kowalik A., Püssa T., Matt D., Luik A., Gozdowski D., Rembiałkowska E. Beetroot (Beta vulgaris L.) and naturally fermented beetroot juices from organic and conventional production: metabolomics, antioxidant levels and anticancer activity. J. Sci. Food Agric. 2014;94:2618–2629. doi: 10.1002/jsfa.6722. [DOI] [PubMed] [Google Scholar]
- Kieliszek M., Błażejak S. Current knowledge on the importance of selenium in food for living organisms: a review. Molecules. 2016;21:609–618. doi: 10.3390/molecules21050609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J.O., Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler. Thromb. Vasc. Biol. 2005;25:915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. [DOI] [PubMed] [Google Scholar]
- Maneetong S., Chookhampaeng S., Chantiratikul A., Chinrasri O., Thosaikham W., Sittipout R., Piyanete Chantiratikul P. Hydroponic cultivation of selenium-enriched kale (Brassica oleracea var. alboglabra L.) seedling and speciation of selenium with HPLC-ICP-MS. Microchem. J. 2013;108:87–91. [Google Scholar]
- Mechora S., Germ M., Stibilj V. Selenium compounds in selenium-enriched cabbage. Pure Appl. Chem. 2012;84:259–268. [Google Scholar]
- Misra S., Boylan M., Selvan A., Spallbolz J.E., Björnstedt M. Redox-active selenium compounds from toxicity and cell death to cancer treatment. Nutrients. 2015;7:3536–3556. doi: 10.3390/nu7053536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrzynska K., Pękal A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate antioxidant capacity of food samples. Anal. Methods. 2013;5:4288–4295. [Google Scholar]
- Pyrzynska K. Edible plants enriched with selenium. J. Agric. Sci. Technol. 2014;4:627–632. [Google Scholar]
- Rahmanto A.S., Davies M.J. Selenium-containing amino acids as direct and indirect antioxidants. Life. 2012;64:863–871. doi: 10.1002/iub.1084. [DOI] [PubMed] [Google Scholar]
- Ravichandran K., Thaw Saw N.M.,, Mohdaly A.A., Gabr A.M.M., Kastell A., Riedel H., Cai Z., Knorr D., Smetanska I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013;50:670–675. [Google Scholar]
- Schiavon M., Pilon-Smits E.A. The fascinating facets of plant selenium accumulation - biochemistry, physiology, evolution and ecology. New Phytol. 2016;213:1582–1596. doi: 10.1111/nph.14378. [DOI] [PubMed] [Google Scholar]
- Sentkowska A., Pyrzynska K. Zwitterionic hydrophilic interaction chromatography coupled to mass spectrometry for analysis of beetroot juice and antioxidant interactions between its bioactive compounds. LWT - Food Sci. Technol. 2018;93:641–648. [Google Scholar]
- Sentkowska A., Pyrzynska K. Hydrophilic interaction liquid chromatography in the speciation analysis of selenium. J. Chromtography B. 2018;1074–1075:8–15. doi: 10.1016/j.jchromb.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Sentkowska A., Pyrzynska K. Investigation of antioxidant activity of selenium compounds and their mixtures with tea polyphenols. Mol. Biol. Rep. 2019;46:3019–3029. doi: 10.1007/s11033-019-04738-2. [DOI] [PubMed] [Google Scholar]
- da Silva D.V.T., dos Santos Baião D., de Oliveira Silva F., Alves G., Perrone D., De, Aguila E.M., Paschoalin V.M.M. Betanin, a natural food additive: stability. bioavailability, antioxidant and preservative ability assessments. Molecules. 2019;24:2403058. doi: 10.3390/molecules24030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavov A., Kagyozov V., Denev P., Kratachaova M., Kratchanov C. Antioxidant activity of red beet juices obtained after microwave and thermal pretreatments. Czech J. Food Sci. 2013;31:130–147. [Google Scholar]
- Slimen I., Najar T., Abderrabba M. Chemical and antioxidant properties of betalains. J. Agric. Food Chem. 2017;65 doi: 10.1021/acs.jafc.6b04208. 675-668. [DOI] [PubMed] [Google Scholar]
- Sri Vidhya N.A.C., Radhai S.S. Efficacy on performance of athletes on supplementation of beetroot juice. Int. J. Food Sci. Nutr. 2017;2:181–184. [Google Scholar]
- Sri Vidhya N.A.C., Radhai S.S. Quality characteristics of developed beetroot juice. Int. J. Food Nutr. Sci. 2018;7:76–82. [Google Scholar]
- Tan L.C., Nancharaiah Y.V., van Hullebusch E.D., Lens P.N.J. Selenium: environmental significance, pollution, and biological treatment technologies. Biotechnol. Adv. 2016;34:886–907. doi: 10.1016/j.biotechadv.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Wan J., Zhang M., Adhikari B. Advances in selenium-enriched foods: from the farm to the fork. Trends Food Sci. Technol. 2018;70:1–5. [Google Scholar]
- Weekley C.M., Harris H.H. Which form is that ? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chem. Soc. Rev. 2012;42 doi: 10.1039/c3cs60272a. 8870-8804. [DOI] [PubMed] [Google Scholar]
- Wruss J., Waldenberger G., Huemer S., Uygun P., Lanzerstorfer P., Müller U., Höglinger O., Weghuber J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015;42:46–55. [Google Scholar]