Abstract
Purpose of Review:
Bone elongation is a complex process driven by multiple intrinsic (hormones, growth factors) and extrinsic (nutrition, environment) variables. Bones grow in length by endochondral ossification in cartilaginous growth plates at ends of developing long bones. This review provides an updated overview of the important factors that influence this process.
Recent Findings:
Insulin-like growth factor-1 (IGF-1) is the major hormone required for growth and a drug for treating pediatric skeletal disorders. Temperature is an underrecognized environmental variable that also impacts linear growth. This paper reviews the current state of knowledge regarding the interaction of IGF-1 and environmental factors on bone elongation.
Summary:
Understanding how internal and external variables regulate bone lengthening is essential for developing and improving treatments for an array of bone elongation disorders. Future studies may benefit from understanding how these unique relationships could offer realistic new approaches for increasing bone length in different growth-limiting conditions.
Keywords: growth plate, linear growth, endochondral ossification, temperature, insulin-like growth factor-1 (IGF-1), chondrocytes, vasculature
Introduction
Our understanding of the molecular events that control postnatal bone elongation in cartilage growth plates has burgeoned over the last several decades. Bone growth rate is almost entirely dependent on the dynamics of cartilaginous growth plates [1]. Long bones (such as humerus, radius/ulna, femur and tibia/fibula) lengthen as chondrocytes of the growth plate divide and expand, establishing a model for bone through the process of endochondral ossification. This process is regulated by local and systemic growth factors, including those of the somatotropic axis, which is the signaling between growth hormone (GH) and insulin-like growth factor-1 (IGF-1). IGF-1 is the major regulator of growth and controls bone elongation by promoting chondrocyte proliferation and hypertrophy [2,3]. Environmental factors, such as temperature, can also surprisingly alter bone and cartilage growth in both natural and laboratory settings [4].
The aim of this review is to critically examine the factors that influence bone elongation with respect to their effects on IGF-1 activity in the growth plate. This review focuses in particular on how further understanding of these factors is beneficial for developing methods to combat an array of bone elongation disorders when the processes of longitudinal bone growth are disrupted.
Processes of Bone Elongation
Endochondral Ossification and Growth Plate Morphology
The skeleton develops by means of two different mechanisms: (1) Intramembranous ossification, involving direct differentiation of mesenchyme cells to bone as with the development of the flat bones of the skull and (2) Endochondral ossification, occurring when mesenchyme cells condense and differentiate into cartilage tissue, which then is replaced by bone forming the vertebrae, ribs and limbs [5]. Endochondral ossification is initiated during fetal life and continues from infancy through adolescence (transitional period from childhood to adulthood). Cartilaginous growth plates situated at the ends of developing long bones function as the command center for bone lengthening (or linear growth in humans). Growth plate cartilage is comprised of cells known as chondrocytes that secrete extracellular matrix and are organized into functional zones (Figure 1).
Figure 1.
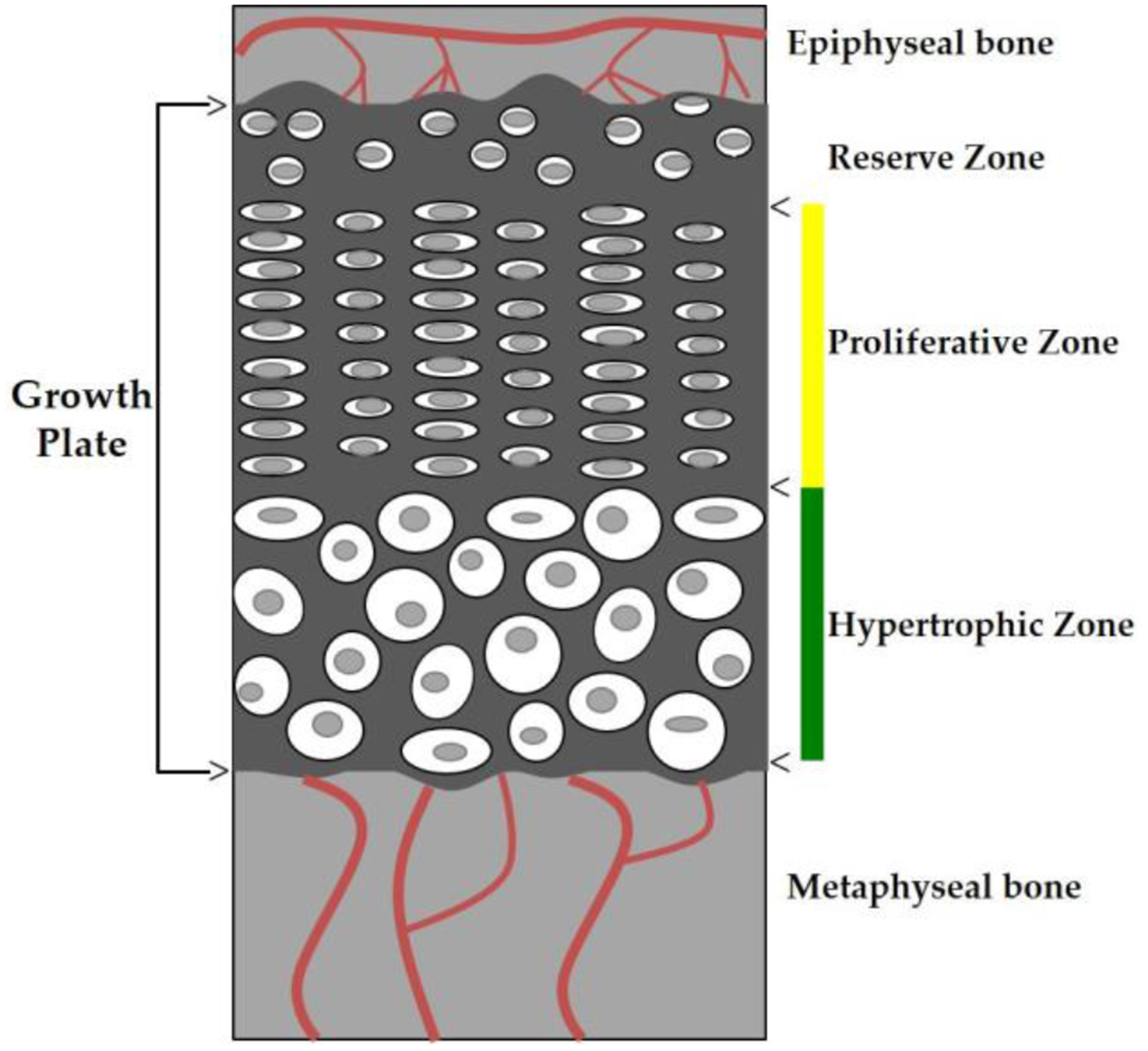
Diagram of a growth plate. Illustration represents the different cellular zones that comprise cartilaginous growth plates (dark gray). Arrows indicate the different zones. The vertical yellow line (top) denotes the proliferative zone (PZ) and the vertical green line (bottom) denotes the hypertrophic zone (HZ). The PZ is a region of actively dividing chondrocytes that are stacked and flattened in multicellular columns. The HZ is a region of enlarged chondrocytes. Epiphyseal and metaphyseal bone on each end of the growth plate contains blood vessels (red lines) that supply each region of bone.
Growth plates are anatomically located between the epiphysis and metaphysis of long bones. The end portion of the long bone is referred to as the epiphysis and the shaft is termed the diaphysis. Directly between the epiphysis and the diaphysis is the transitional zone, or metaphysis, which contains newly mineralized bone at the bone-cartilage interface (chondro-osseous junction, COJ). The region adjacent to the epiphysis is the reserve zone (RZ). The RZ consists of round quiescent chondrocytes. The role of the RZ has been debated but is most commonly reported to act as the coordinator for the organization and orientation of the neighboring proliferative zone (PZ) [6]. It has been suggested that the RZ contains stem-like cells that promote the production of proliferative chondrocytes and that this proliferative capacity decreases with age aiding to the closure of the growth plate associated with skeletal maturity [6–9]. Adjacent to the RZ is the PZ, which contains rapidly dividing chondrocytes that are stacked and flattened in multicellular columns. The PZ is a region of actively dividing cells that directs longitudinal growth along the long axis of the bone, in which the cells are aligned in columns.
The proliferating chondrocytes at the base of the columns make the transition from the PZ to the hypertrophic zone (HZ) where chondrocytes behave as terminally differentiated cells that begin to enlarge, secrete extracellular matrix and were once thought to ultimately undergo physiological death [10–13]. Hypertrophic chondrocytes induce vascular invasion and recruit osteogenic cells in the replacement of cartilage with bone at the chondro-osseous junction (the interface between growth cartilage and newly mineralized metaphyseal bone). A majority of the bone-forming osteoblasts are thought to be differentiated from bone marrow stromal cells. However, current research in the mammalian growth plate suggests that not all hypertrophic chondrocytes undergo apoptosis but instead transdifferentiate into osteoblasts during both bone development and repair [14–17]. Studies by Bahney et al. [14] used cartilage grafts to promote bone regeneration through endochondral ossification (analogous to long bone development). Their results from lineage tracing experiments showed that chondrocytes directly differentiated into osteoblasts during bone repair [14]. It is thought that since transdifferentiation occurs adjacent to the vasculature, that the vasculature may have a signaling role in the transformation of chondrocytes to osteoblasts [16]. Emerging research shows that this chondrocyte-to-osteoblast transdifferentiation is a critical component of bone elongation through the process of endochondral ossification [18].
Differential Growth Plate Activity
While the anatomical structure of the growth plate is comparable between the developing long bones, the rate at which these growth plates contribute to bone lengthening differs from bone to bone, and from proximal end to distal end. This phenomenon is referred to as differential growth [19,20]. The humerus, radius and ulna are the major long bones of the upper limb (excluding the hand and foot), and the femur and tibia comprise the lower limb. When comparing proximal to distal ends of these bones, the faster-growing sites are located at the proximal humerus (shoulder), distal radius and ulna (wrist), and distal femur and proximal tibia (knee) [1,8,21–27]. The most active developing growth plate in humans and rodents is the proximal tibia followed by the distal femur (knee), distal radius (wrist), and proximal humerus (shoulder) [28]. Wilsman et al. [27] have shown that the proximal tibial growth plate of 28-day-old rats (growth rate of 396 μM/day) is nearly nine times faster than the much slower proximal radial growth plate (growth rate of 47μM/day).
The main factors contributing to differential growth rate are the number of chondrocytes in the proliferative zone, the rate of proliferation, and the size of expanded cells in the hypertrophic zone [7,29–32]. Indeed, the faster growing sites are well known to have increased numbers of proliferative chondrocytes, increased rates of proliferation, and hypertrophic chondrocyte expansion [1,8,22,25–28,33–35].
Limb Length Discrepancy: Causes and Treatments
Limb length discrepancy (LLD), or anisomelia, is characterized by asymmetric length of lower extremities [36,37]. LLD usually emerges during childhood but the etiology varies and may be congenital, acquired or idiopathic. Prior to the development of the polio vaccine in the 1960s, one of the more common causes of LLD in children was poliomyelitis (viral infection causing paralysis) that resulted in shortening of the paralyzed limb [38,39]. While polio has been eliminated from the United States, treatments are still sought for adults suffering from long-term LLD as a result of poliomyelitis infection during childhood [40,41]. Other acquired causes of LLD include infection, tumors, and trauma [38,39,42]. LLD caused by trauma most commonly involves epiphyseal fractures, which typically result in shortening on the fractured side, and meta- and diaphyseal fractures, which usually lead to lengthening on the fractured side as a result of increased blood flow to the affected growth plate [38,39,42–46]. Regardless of the cause, untreated limb length inequalities underlie many painful musculoskeletal problems in adults [37,47] including lower back pain [48,49], gait abnormalities [50–54], scoliosis (abnormal curvature of the spine) [55], and osteoarthritis of the hip and knee [56–59].
Limb length discrepancy can vary in severity depending on the underlying cause. Limb length inequalities of 2 cm or more (about 2.4% of adult height [60–62]) typically involve surgical correction [37,63–65]. Limb shortening is recommended for limb length inequalities of 2–5 cm using epiphysiodesis procedures [63]. These methods involve shortening the longer limb by either permanently halting growth using a surgically formed bony bridge [66], or temporarily slowing elongation using guided growth techniques [63,67,68]. Limb lengthening is often recommended for inequalities over 5 cm [63] and includes methods based off the traditional Ilizarov technique using external fixators [69] or mechanical bone guidance [70]. One drawback to any type of invasive procedure, however, is that these surgeries can be associated with complications including pain, implant failure, angular deformities and infection [39,70].
The projected limb length discrepancy at the point of maturity varies based on the initial inequality and how much limb growth remains [71]. There have been numerous methods of predicting final limb length including the Green-Anderson method [72], Menelaus method [73], Moseley method [74], and Paley method [75]. These methods are based on mathematical modeling of growth patterns in the femur and tibia at different age points. While each method takes a slightly different approach, they are all based on growth charts developed by Green and Anderson [72] that show femoral and tibial lengths, along with stature, for boys and girls at different chronological and skeletal ages. The basic idea is to compare femoral and/or tibial length in a child to known growth charts to determine the proportion of adult limb length achieved, and thus the predicted amount of growth remaining. However, these predictions are subject to error because skeletal age can be more or less advanced than chronological age depending on the maturation rate of the child. Therefore, the predicated limb length discrepancy varies between these methods [76] and does not always match the actual limb length difference at maturity. Therefore, determining the most effective timing to start treatment is difficult and can lead to possible over- or under-compensation for unequal limb length. When the correction is not successful, surgical interventions may be repeated [63], putting a child at increased risk of post-surgical complications.
Although surgical interventions are typically only performed for a marked length inequality [37,77,78], studies have shown that discrepancies as small as 0.5–1 cm (0.6–1.2% limb length difference based on young adult height) may impact everyday walking [79], leading to problems such as back pain and knee osteoarthritis in adults [58]. Shoe lifts or prosthetics are inexpensive and noninvasive approaches, but they do not actually change bone length [39,47,70]. Alternative non-invasive methods for permanently lengthening limbs have been tested. A study by Zhang et al. [65] showed that applying intermittent 0.5 N lateral loads to the knee joints of mice (~8-weeks old) increased femoral (2.3%) and tibial length (3.7%). This increase in limb length is noteworthy because treatment occurred during slower rates of longitudinal bone growth when mice were close to skeletal maturity. Serrat et al. [80] were able to use once daily targeted limb heating (40°C for 40mins/day) to unilaterally increase femoral (1.3%) and tibial (1.5%) length of growing mice (3–5 weeks of age). At skeletal maturity (12 weeks of age), femoral (1.0%) and tibial (1.0%) lengths remained significantly increased on the heat-treated sides [80], suggesting that heat could be a realistic alternative, or at least supplement, to surgical limb lengthening in certain cases.
Regulation of Postnatal Bone Lengthening
Endocrine and Paracrine Factors
The process of postnatal bone elongation is tightly controlled by 1) systemic endocrine molecules, such as hormones distributed through blood that act on target cells of the growth plate, and 2) locally produced paracrine/autocrine factors expressed in epiphyseal chondrocytes or surrounding perichondrium. Disruption of these regulators results in dysfunctional chondrocytes, abnormal longitudinal growth, and skeletal dysplasia, including short-limbed dwarfism [81]. Some of the primary endocrine factors that regulate longitudinal growth include growth hormone (GH), insulin-like growth factor 1 (IGF-1), thyroid hormone, estrogen, androgen, glucocorticoids, and vitamin D [2,81–86], all of which can have both coordinated and independent actions. For example, while circulating thyroid hormone has been shown to increase longitudinal bone growth indirectly by increasing systemic GH secretion [83], it also has been shown to directly interact with the epiphyseal chondrocytes and initiate terminal differentiation [87,88]. The actions of IGF-1 in the growth plate are discussed in detail below.
Locally acting autocrine/paracrine molecules in the growth plate include Indian hedgehog (Ihh), parathyroid hormone-related protein (PTHrP), fibroblast growth factors (FGFs) (including FGF1, −2, −9 and −18), bone morphogenic proteins (BMPs) (including BMP2–7), vascular endothelial growth factor (VEGF), IGF-1, and Wnt [2,11,81,85,86,89,90]. Each of these factors can regulate different regional zones of the growth plate. For instance, prehypertrophic chondrocytes at the maturational stage between active proliferation and hypertrophic expansion express signaling molecules responsible for the successful and timely transition from proliferative to hypertrophic phenotype. These include FGF receptors [91,92], BMPs [93,94], and Ihh/PTHrP receptors [95].
Bone lengthening is the ultimate result of the coordinated signaling of both endocrine and paracrine/autocrine factors that interact to promote chondrocyte proliferation and hypertrophy in the growth plate. In addition to circulating IGF-1, estrogen (endocrine) can regulate local expression of IGF-1 in growth plate chondrocytes through an autocrine/paracrine route [82]. While the impact of some factors, such as IGF-1 discussed below, have a dominant role in postnatal growth, normal bone elongation depends on the interaction of multiple regulatory inputs.
Nutritional Factors
In addition to hormones and growth factors, adequate nutrition is also an important facet of limb elongation. There are strong correlations between undernutrition (inadequate caloric/vitamin intake) and stunted growth [96–98], as well as overnutrition (high-fat/calorie diets) and accelerated linear growth [99–102]. In the absence of catch-up growth, the stunting associated with food restriction can lead to permanently shortened bones [103]. However, in cases of overnutrition (obesity-induced growth acceleration), it appears that an earlier onset of skeletal maturity during puberty counters the accelerated period of linear growth resulting in a final height within a normal range [101]. While inadequate nutrition can have some of the greatest impacts during the infancy phase of postnatal linear growth (the first 2 years of life), proper nutrition is essential for all stages of linear growth continuing from 2 years of life past the onset of puberty [104–106].
Nutrition can also alter endocrine/paracrine signaling in the growth plate [96]. Particularly, IGF-1, the major growth-stimulating hormone, is commonly involved in mechanisms of under- and overnutrition. Serrat et al. [107] found that a high-fat diet increases the delivery of IGF-1 to the growth plate and accelerates bone elongation in young mice. Others have shown, in both human and animal studies, that nutritional deficiency inhibits IGF-1 signaling in the growth plate and reduces final adult height [96,108,109].
Since there are multiple nutrient deficiencies in most food-restricted diets, it is difficult to determine the role of individual nutrients and their actions on longitudinal bone growth. Zinc (Zn), an essential trace mineral for linear growth, has been extensively studied. While the mechanism is unknown, it is thought that zinc reduces circulating IGF-1 levels [110,111], possibly due to an overall decrease in appetite. However, studies in rats suggest that zinc may also have a direct effect on the local action of IGF-1 in the growth plate [96,112,113]. Overall, key pathways for hormonal regulation of linear growth, including IGF-1, are clearly influenced by nutritional status.
Temperature Effects on Linear Growth
In addition to nutrition, other environmental factors such as altitude, climate and temperature can have a significant, yet often underrecognized, impact on bone elongation [114]. The classic effects of temperature on limb length have been described by Allen’s “extremity size rule,” which states that endotherms (warm-blooded animals) living in warm climates have relatively longer appendages (ears, limbs, tail) compared to their colder climate counterparts [115]. This correlation between temperature and limb length was long presumed to be a thermoregulatory adaptation by increasing surface area for heat dissipation in warmer environments, while reducing surface area relative to body mass for heat conservation in colder climates [116]. Allen’s rule is indeed still applied to a variety of different species in present day studies. In humans, Allen’s rule was used to suggest that shorter limbs (including those of Neanderthals, a group of archaic humans) are advantageous for survival in cold climates by reducing the metabolic cost of maintaining body temperature [117].
Other studies, however, have shown that the phenotype described by Allen’s rule can be replicated under laboratory conditions, suggesting that it might represent growth plasticity rather than a thermoregulatory adaptation to climate. In reptiles, for example, elevated incubation temperature (32°C) increased tibial length of embryonic crocodiles [118]. Serrat [4] reviewed the impact of temperature on extremity growth in various mammalian species, with a primary focus on rodents that had been most widely studied. Their group also experimentally demonstrated that mice housed at warm ambient temperature (27°C) during post-weaning growth have longer limbs than their cold-reared littermates (7°C) [24], with the most substantial differences occurring during the active period of postnatal growth. Al-Hilli and Wright [120] studied effects of warm ambient temperature (33°C) on tail growth in weanling mice and showed that heat-enhanced tail growth occurred during the first 3–4 weeks of exposure. Serrat [121] found that the “critical phase” of temperature sensitive growth occurs in the immediate post-weaning phase, followed by a later “maintenance phase,” whereby temperature-induced limb length differences are simply maintained through adulthood. Serrat et al. [80] later showed that targeted intermittent heat exposure (40°C on one side of the body for 40 minutes per day) unilaterally increased limb length on the heat-treated side growing mice, demonstrating a direct impact of temperature on bone elongation. While it is difficult to identify one single mechanism responsible for temperature-enhanced limb lengthening, it is likely the effect is driven by multiple direct and indirect mechanisms such as altered cell kinetics, gene expression, vascularization, transport of nutrients and growth factors [122], and systemic hormone concentrations [4].
Potential Function of IGF-1 in Heat-Enhanced Linear Growth
IGF-1 is the main regulator of linear growth and normal skeletal development does not occur without functional IGF-1 activity (see Table 1) [123–126]. In addition to liver-derived serum IGF-1 (endocrine), there is strong evidence that locally expressed IGF-1 (autocrine/paracrine) regulates longitudinal bone growth in the growth plate [87,127–132]. Therefore, a working hypothesis for heat-enhanced linear growth is that temperature increases activity of IGF-1, which increases rate of chondrocyte proliferation and hypertrophy to subsequently enhance limb growth. Heat-enhanced linear growth may occur: (1) indirectly by increasing IGF-1 access/delivery to the growth plate with temperature-enhanced blood flow (endocrine actions), (2) directly by increasing IGF-1 activity within the growth plate (autocrine/paracrine actions) by temperature-enhanced expression of local regulators, or (3) a combination of both direct and indirect mechanisms.
Table 1.
Mouse Models with Disrupted IGF-1 Regulation
| Model | Phenotype | Citation |
|---|---|---|
| IGF-1 null mice (global IGF-1 disruption) | 95% mice died prenatally; undetectable levels of IGF-1 in serum and tissues; significant decrease in tibial length (70%); abnormal chondrocyte proliferation and differentiation | [36,123,124,147] |
| Igf2 null mice (global IGF-2 disruption) | Mice are viable; reduced body growth (60%) at birth; normal postnatal growth | [148,149] |
| LID mice (systemic disruption of liver-derived IGF-1) | 75% reduction in serum IGF-1; no significant change in body length; no significant change in tibial length; slight decrease in femoral length (6%) | [149,150] |
| ALS knockout mice (systemic disruption ternary complexes (IGF-l/ALS/IGFBP-3) | 65% reduction in serum IGF-1; no significant change in body length; slight decrease in femoral length (7.5%) | [151] |
| LID+ALSKO mice (systemic disruption of liver-derived IGF-1 and ternary complexes (IGF-l/ALS/IGFBP-3)) | 85–90% reduction in serum IGF-1; significant reduction in body length (30%); significant decrease in femoral length (20%) | [152] |
| (Col2al)-driven Cre mice (local disruption of chondrocyte-derived IGF-1) | 40% reduction in chondrocyte IGF-1; Significant decrease in postnatal body length; no morphological differences in proliferative and hypertrophic regions | [153] |
| CartIGF-1r−/− mice (local disruption of cartilage-specific IGF-1R) | Mice died shortly after birth | [3] |
| TamCartIGF-1r−/− mice (local disruption of cartilage-specific IGF-1R induced by tamoxifen injections) | Significant decrease in body length (40%); disorganized growth plates associated with reduced chondrocyte proliferation and differentiation | [3,142,143] |
While blood flow and vascular transport of nutrients may play a role in heat-enhanced limb elongation (discussed in section 6), temperature may also directly alter growth through changes in IGF-1 expression. For example, eels (an ectotherm with endoskeleton made entirely of bone) reared in warmer temperatures (22°C) were significantly longer and had increased IGF-1 gene expression compared to those raised at colder temperatures (16°C) [133]. Broiler chicks had increased IGF-1 expression in skeletal muscle that was associated with increasing rearing temperature [134,135]. In addition to the suggested relationship between temperature and IGF-1 expression in developing tissues, elevated temperature has also been shown to weaken the affinity of IGF-1 for the acid labile subunit (ALS), which results in increased free circulating IGF-1 [136]. Further research is needed to elucidate the role of temperature in modulating IGF-1 action in bone and cartilage of mammals during development.
Growth Hormone and Insulin-Like Growth Factor Signaling
Evolution of the Somatomedin Hypothesis
GH and IGF-1 are integral for regulating normal growth and development. Our understanding of the means by which GH and IGF-1 regulate growth was set into motion after experiments conducted in 1957 by Salmon and Daughaday showed that a factor in the serum (“sulfation factor”) was able to stimulate sulfate incorporation into cartilage in vitro [137]. Later identified as somatomedin in the early 1970s, the somatomedin hypothesis described an intricate relationship in which pituitary-gland derived GH stimulated the liver to secrete an intermediate hormone (somatomedin), which in turn caused somatic growth [138]. The intermediates were termed insulin-like growth factor 1 (IGF-1) and insulin-like growth factor 2 (IGF-2) (also referred to as somatomedin C and A) later in the decade [139,140]. Both factors are important for growth and development at different stages of maturation as abnormal phenotypes result if either is disrupted (Table 1).
The introduction of the dual-effector theory in 1985 disputed the original somatomedin hypothesis and proposed an additional IGF-1-independent role of GH [141]. This revision came after reports of local IGF-1 production in non-hepatic tissues, including bone [142], demonstrating that IGF-1 acts as both an endocrine and autocrine/paracrine growth regulator. In the growth plate, GH directly stimulates cell differentiation in the precursor cells of the RZ, while local IGF-1 production mediates clonal expansion of PZ chondrocytes [87,127–131]. More recent studies support the direct contribution of GH acting on the growth plate independent of IGF-1 [143,144]. Thus since its inception, the somatomedin hypothesis has evolved to describe a more complex interplay between GH and IGF-1 (commonly referred to as the GH/IGF-1 axis) including negative feedback mechanisms where IGF-1 inhibits further GH production (Figure 2) [145].
Figure 2.
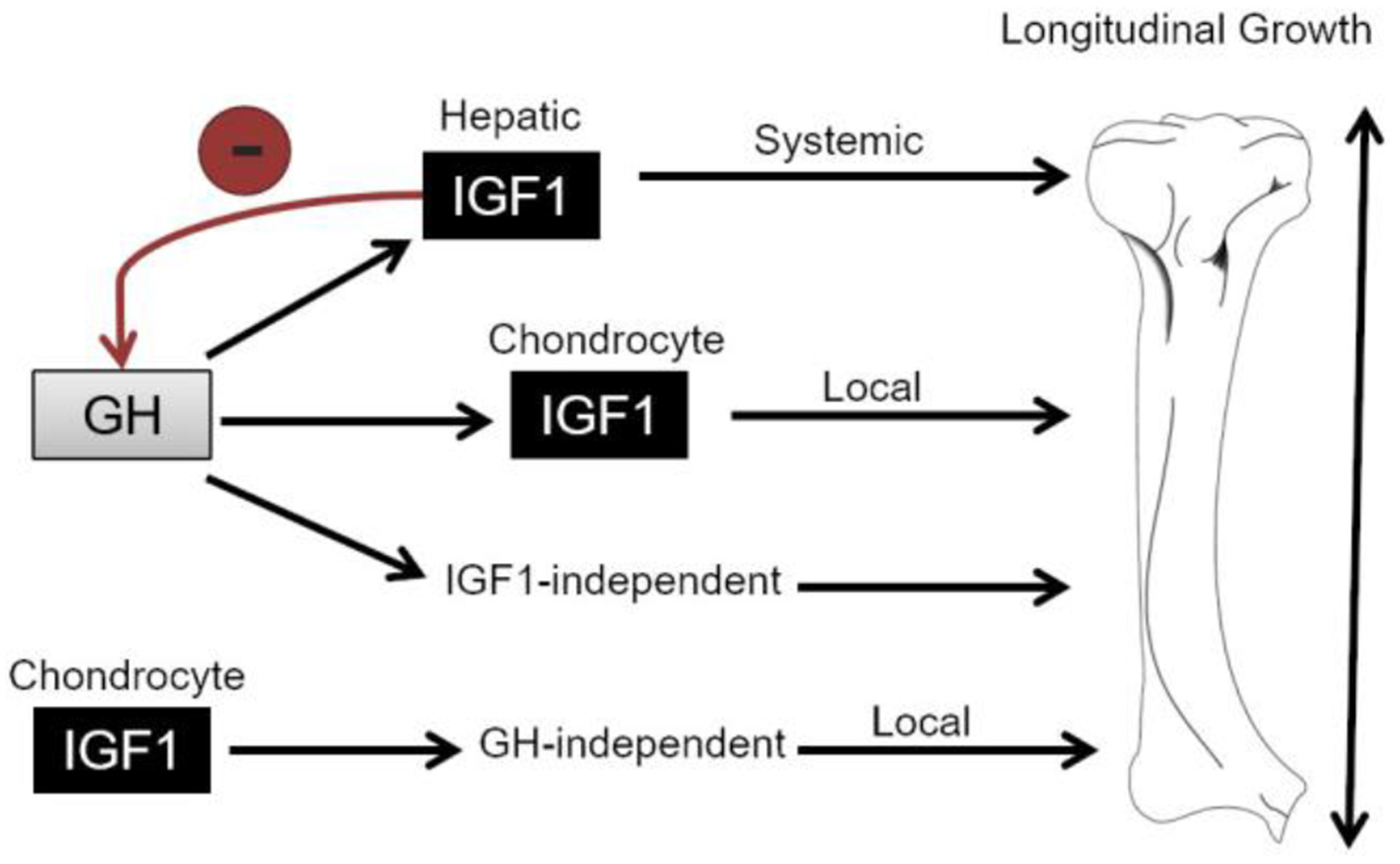
GH/IGF-1 Axis in Postnatal Limb Elongation. Flow diagram illustrates the basic pathways in which GH and IGF-1 interplay to promote longitudinal bone growth. In addition to stimulating IGF-1 production in the liver as originally proposed in the somatomedin hypothesis, GH also stimulates longitudinal growth (1) by promoting local production of IGF-1 in growth plate chondrocytes and (2) independent of IGF-1 as explained by the dual effector theory. IGF-1 also has autocrine/paracrine effects independent of GH by promoting longitudinal growth locally in growth plate chondrocytes. The red arrow demonstrates the negative feedback mechanism of IGF-1 inhibiting further GH synthesis and release.
IGF Signaling Pathways
IGF binding protein-3 (IGFBP-3) is the major carrier of IGF-1 in serum. Most circulating IGF-1 (~75%) exist in a ternary complex consisting of IGF-1, IGFBP-3, and the acid labile subunit (ALS) [136,152]. This ternary complex prolongs the half-life of serum IGF-1 and regulates transport from the circulation to the target tissue [136,154,155]. Similar to IGF-1, IGF-2 binds to IGFBPs [154,155]. Upon release at the surface of the target tissue, both IGFs are capable of binding to the receptor tyrosine kinase type 1 IGF receptor (IGF-1R) [155] expressed in all regions of the growth plate [156,157]. The activation of the IGF-1R leads to a signaling cascade involving the phosphatidylinositide 3-kinase (P13K) and mitogen-activated protein kinase (MAPK) pathways that lead to cell survival and proliferation, which ultimately result in bone elongation (Figure 3). While IGF-2 is classically thought to function only during prenatal growth [148,149,158], IGF-1 is expressed at low levels during embryonic development but is essential for postnatal growth [36,123,125]. Evidence supports that IGF-1 is more critical to postnatal longitudinal bone growth compared to IGF-2 because the postnatal phenotype of the IGF-1 null is more severe than Igf2 knockouts (see Table 1) [36,125,148,149].
Figure 3.
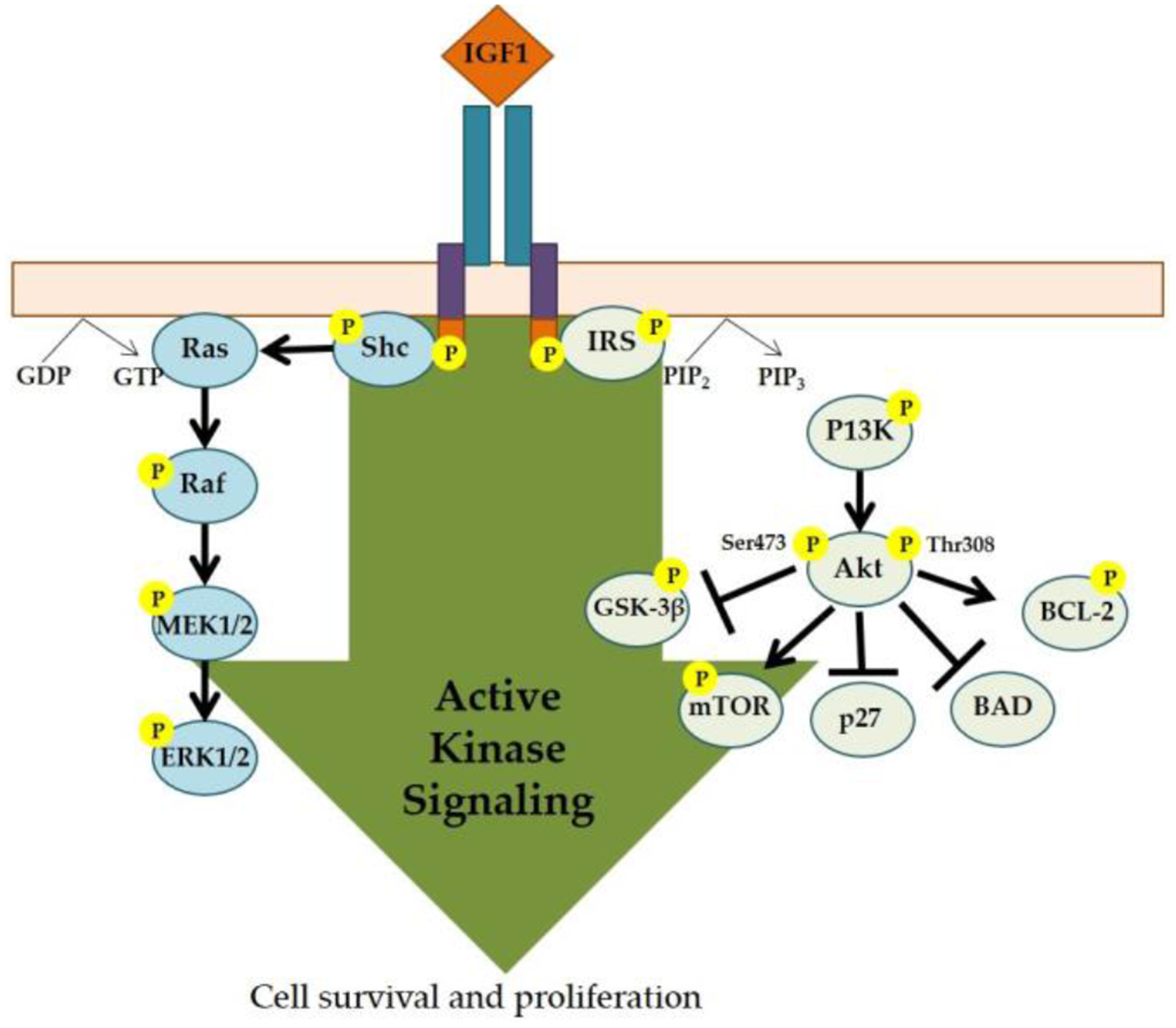
IGF-1 Induced Intracellular Signaling Pathway. The binding of IGF-1 to its receptor (IGF-1R) triggers downstream signaling cascades, which includes the phosphatidyl inositol-3 kinase (P13K) and mitogen-activated protein kinase (MAPK) pathways that ultimately lead to cell survival and proliferation. Illustration based on Crudden, Girnita, A, and Girnita, L [159].
Distinct and Overlapping Functions of GH and IGF-1 in Linear Growth
The growth hormone receptor knockout (GHR−/−) mouse model developed by the Kopchick laboratory in the 1990’s [160] has made important contributions to our understanding of the role of the GH/IGF-1 axis in postnatal bone elongation. The GHR−/− mouse is a model of human Laron Syndrome, a recessively inherited inactivating mutation(s) in the GHR. The disease is characterized by GH resistance, high serum GH, and low serum IGF-1 [160–163]. While at birth these mice are similar in size to their wild-type littermates (suggesting that prenatal growth is not dependent on GH), the significant size difference becomes apparent during postnatal development [160]. GHR−/− mice have a 30–40% reduction in body size [160,162–164] as well as a 65% reduction in tibial elongation rate [165,166] and decreased chondrocyte proliferation and hypertrophy [162]. Studies by Lupu et al. [31] found that GH and IGF-1 have distinct, yet overlapping, functions during mammalian linear growth. When comparing the GHR−/− mouse (lacking GH action) to double- Ghr/IGF-1 mutants (lacking GH and IGF action), the observed growth reduction of the double Ghr/IGF-1 mutants was more severe than that of either single mutant model [31]. With advancements in the field of endocrinology, investigators continue to refine the original somatomedin hypothesis to determine how GH and IGF-1 interact to regulate linear growth.
GH and IGF-1 Treatment of Multiple Endocrine Deficiencies
Endocrine disorders involving deficient or excessive production of the important growth-promoting hormones (GH, IGF-1, thyroid hormones, glucocorticoids, and sex steroids), can lead to abnormally short or tall stature. Short stature characterized by stunted linear growth may result from GH deficiency [31,130,167], IGF-1 deficiency [123–126,164,168], hypothyroidism [169], or hypercortisolism [170,171]. Thyroid hormone (T3) deficiency in children is associated with growth reduction [172] and studies in mice using knockout models have also shown that linear growth is impaired in thyroid hormone deficient mice [169,173] when levels are normal but the thyroid receptor (TRα1 and TRβ) is mutated [174,175].
A significant problem that results from short stature is the development of behavioral and emotional problems in children [81,176,177]. One common treatment option for children with stunted linear growth involves frequent subcutaneous injections of recombinant human GH until adult height is reached [130,178,179]. GH has also been used to treat short stature resulting from chromosomal disorders and genetic syndromes including Turner syndrome [167,178–180], Achondroplasia [181], Prader-Willi syndrome [178,182], and Noonan syndrome [178,183]. Other therapies to increase linear bone growth include oxandrolone (synthetic steroid similar to testosterone), estrogens, gonadotropin-releasing hormone (GnRH) and IGF-1 [179]. While GH therapy is more commonly used in clinical settings, IGF-1 can also effectively reverse skeletal growth discrepancies [31,163,184–192]. However, IGF-1 is not the optimal first-line of treatment because of adverse effects such as hypoglycemia (abnormally low levels of glucose in the bloodstream), resulting from IGF-1 acting on the insulin receptor [187,193–196]. In patients that already show symptoms of hypoglycemia, such as those with Laron’s Syndrome (GH insensitive), IGF-1 therapy can intensify these symptoms and can lead to loss of consciousness or seizures [186,197,198]. Other risks with IGF-1 treatment include headaches, intracranial hypertension, growth of the nasopharyngeal lymphoid tissues, hearing loss, and injection site lipohypertrophy [185,186,189,190]. Researchers continue to seek better delivery mechanisms using a lower dose of IGF-1 to avoid the adverse effects of increased systemic levels [199].
IGF-1 as a Primary Mediator of Longitudinal Bone Growth
Role of Locally Expressed IGF-1
Numerous studies have supported the importance of both GH and IGF-1 in mediating linear bone growth; however, IGF-1 appears to be the most critical regulator of postnatal growth. In mutant animal models where GH action is impaired [31,160–163,166], animals thrived despite being significantly smaller than the wild-type counterparts. In contrast, in mutant animals where local IGF-1 action is impaired, most animals died shortly after birth and those that did survive had severe growth defects [3,142,143] supporting the importance of locally produced IGF-1. The observed growth defects suggest that while a degree of circulating IGF-1 is necessary [152], local action of IGF-1 may be more critical for longitudinal bone growth (see Table 1).
The role of local IGF-1 in chondrocyte proliferation is still somewhat unclear. In the early to mid-1990s, investigators reported the expression of IGF-1 in the PZ of the epiphyseal growth plate measured by in situ hybridization [87,200,201], and these findings were replicated in a separate study a decade later [31]. However, these results differed from findings of two other groups that were both unable to detect IGF-1 mRNA in proliferating chondrocytes by in situ hybridization methods, and instead found IGF-2 mRNA [157,202]. Other investigators have also identified the expression of IGF-2 in the PZ [156,203]. These inconsistencies could be due to age of the study animals, however, because levels of IGF-2 in the PZ consistently decrease with age, whereas IGF-1 levels increase after birth [156]. Since several studies that did not detect IGF-1 mRNA in proliferating chondrocytes were done during the earliest stages of postnatal development [202,157], it is possible that these animals had not yet reached the postnatal age at which IGF-1 can be detected in the PZ.
Although once thought to only function during prenatal development, the significance of IGF-2 in early postnatal chondrocyte development has begun to emerge. IGF-2 has not been included in the classic GH-IGF-1 signaling axis [204,205] but the role of IGF-2 in postnatal bone growth has been revisited after a group discovered that human postnatal growth restriction was associated with nonsense IGF-2 mutations [206]. Uchimura et al. [207] found significantly reduced bone length in IGF2-null mice at postnatal periods prior to weaning (1–3 weeks of age) and their histological analysis suggests that IGF-2 may have a role in controlling the progression of chondrocytes from proliferation to hypertrophy. The mechanism and extent by which IGF-2 regulates longitudinal bone growth throughout the postnatal period remains unclear and is an important avenue for future investigation.
Regardless of its local versus systemic roles, IGF-1 is undisputedly a significant factor in epiphyseal cartilage development. Local regulation of IGF-1 has been studied in other regions of the growth plate aside from the PZ. Cell kinetic studies using hypophysectomized rats (pituitary gland removed reducing systemic levels of GH and IGF-1) have shown that IGF-1 regulates all phases of chondrocyte differentiation in the growth plate including chondrocyte precursors in the RZ of [208]. IGF-1 expression has also been observed in the HZ of epiphyseal growth plates [200,201] and local regulation by IGF-1 has been shown to augment chondrocyte hypertrophy [124,168,209,210]. An observed 35% reduction in HZ height was reported in an IGF-1 null mouse model [168]. IGF-1 has also been shown to induce collagen X production (made in the HZ) [168,211]. When local expression of IGF-1 was blocked by the binding of Wnt induced secreted protein 3 (WISP3), a protein involved in cell differentiation, IGF-1-induced collagen X expression was also reduced [211].
IGF-1 Antagonists
Mouse models designed to control the expression of IGF-1 through genetic manipulation have been critical in our understanding of IGF-1 regulation. Another approach for studying the role of IGF-1 in regulating linear bone growth is by using pharmaceutical inhibitors to block IGF-1 action. The major target of an IGF-1 antagonist is the IGF-1R, which serves as the gateway for IGF-1 action (see Figure 3). When activation of the IGF-1R is inhibited, the growth promoting effects of IGF-1 are blocked (Figure 4). Multiple types of antagonists of IGF-1 have been studied including small-molecule tyrosine kinase inhibitors (TKIs) and competitive antagonists such as monoclonal antibodies directed against the IGF-1R or the use of IGF-1 peptide analogs (Table 2). Since hormones and growth factors, including IGF-1, function to promote growth by inducing cell proliferation and survival, hormone antagonists are often investigated as means for treating abnormal cell growth such as carcinogenesis. However, many of these antagonists face scrutiny because of the reports of failure in clinical trials [212]. Therefore, investigators are continuing to seek a better understanding of these antagonists using animal models [145] and continue to research alternatives for IGF-1 antagonists to improve targeted therapies [212].
Figure 4.
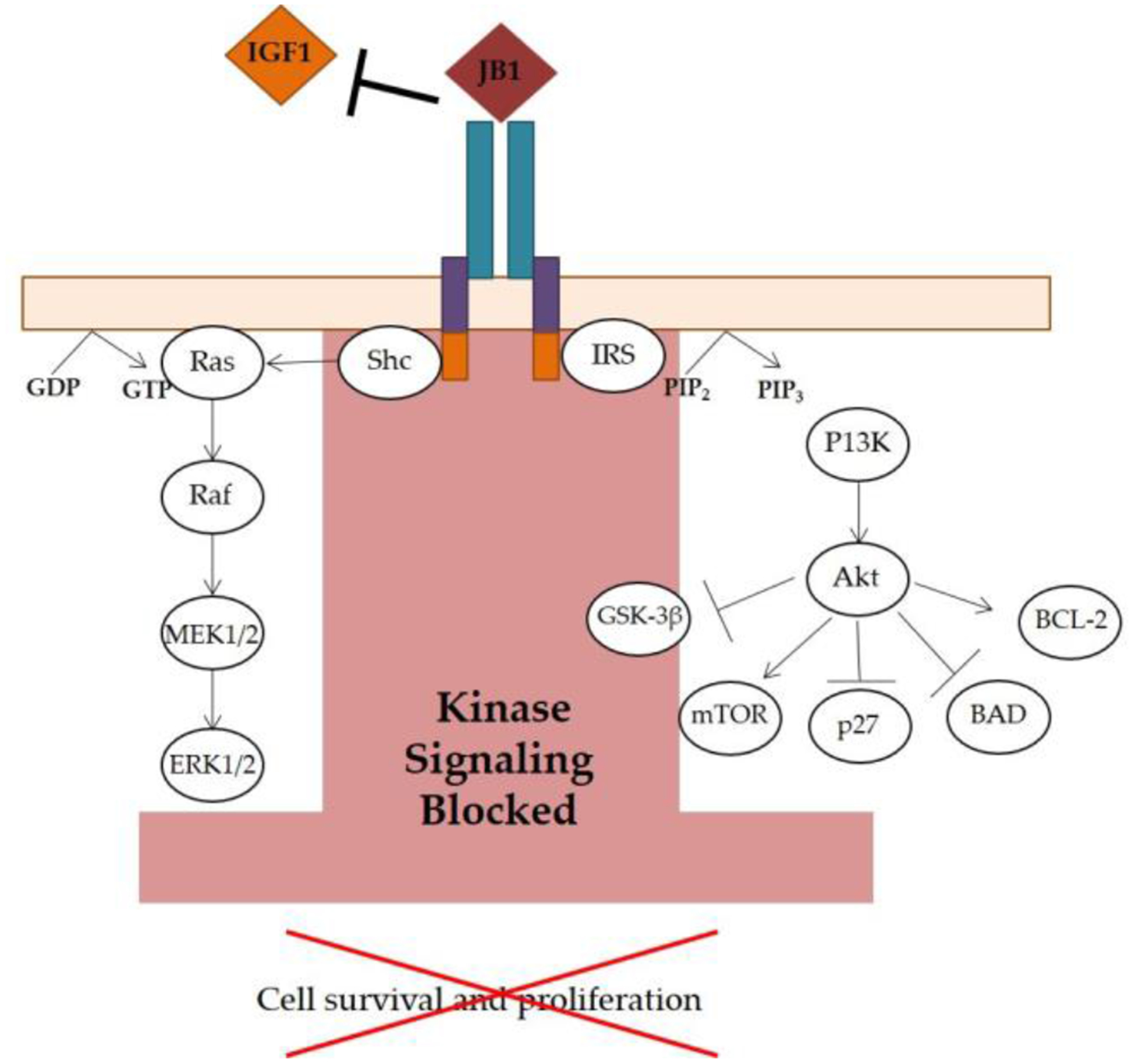
Inhibition of IGF-1R Signaling. The IGF-1 peptide analog, JB1, competitively binds to the IGF-1R. JB1 thus inhibits IGF-1 binding and prevents IGF-1R activation, ultimately blocking kinase signaling that would otherwise lead to cell survival and proliferation (repressed downstream effects indicated by the red “X”). Illustration based on Crudden, Girnita, A, and Girnita, L. [159].
Table 2.
Summary of IGF-1 Antagonists
| Antagonist | Advantage | Disadvantage | Citation |
|---|---|---|---|
| Anti-IGF-IR antibodies (i.e. ganitumab and αIR3) | Highly specific for the IGF-1R | Activates the insulin receptor and promotes unwanted growth; Large molecular weight (145–155kDa); Drug resistance | [145,216] |
| Small-molecule TKIs (i.e. Linsitinib and BMS-754807) | Targets both IGF-1R and insulin receptor; Small molecular weight (0.42-0.46kDa) | Less specific; Reported toxicity (i.e. diarrhea and myelosuppression); Drug resistance | [223–225] |
| IGF-1 peptide analogs (i.e. JB1, JB2 and JB3) | Highly specific for the IGF-1R; Small molecular weight (0.6–1.2kDa); Low toxicity | Drug resistance | [215–219] |
Apart from clinical studies using IGF-1 antagonists as anti-cancer drugs, testing antagonists for their ability to block IGF-1 action in growing bone is an important area of research. Since many of these drugs are administered systemically, the smaller sized antagonists (<0.9 kDa) are more soluble and better for transport out of the vasculature and especially into dense connective tissue [213,214]. As highlighted in Table 2, IGF-1 peptide analogs are effective because of their specificity, small molecular size (0.6–1.2 kDa) and low toxicity [215–219]. Multiple IGF-1 peptide analogs have been used to inhibit cellular proliferation including JB1, JB2 and JB3 [218]. JB1 is a commercially-available analog that competitively binds to the IGF-1R and blocks downstream IGF-1 activity [216,217,220–222] (Figure 4). As with many antagonists, resistance is possible. Haylor et al. [215] reported a bell-shaped curve with the dose response of JB3 and its successful inhibition of kidney growth in rats. In addition to determining an effective range of treatment, challenges still arise in eliciting a tissue specific response without affecting other systems dependent on IGF-1 for normal growth. Further investigation into methods of targeting small molecules will be beneficial in optimizing application of IGF-1 antagonists.
Role of Vasculature in Bone Elongation
Vascular Supply of the Growth Plate
The vasculature is crucial for transporting regulators that support the well-controlled processes of endochondral ossification. Although bone is a highly vascularized tissue, signaling molecules involved in bone elongation must overcome a challenge that is unique to the growth plate: cartilage does not have a penetrating blood supply. Systemic factors essential for bone elongation are delivered from surrounding blood vessels [226–228]. These vascular routes include: 1) epiphyseal vessels, 2) metaphyseal vessels, and 3) perichondral vessels [229–232], which arise from a ring vessel found in the encircling groove of Ranvier [229,233]. Studies have shown that the epiphyseal vasculature is essential for normal growth plate cartilage development [227,228,234,235]. Traditionally, epiphyseal vessels were thought to provide the means for normal chondrocyte proliferation, while chondrocyte hypertrophy was thought to be dependent on the metaphyseal vessels [236,237]. More recently, however, Farnum and colleagues found that small solutes reach growth plate chondrocytes from all three vascular routes [238], suggesting that both epiphyseal and metaphyseal vessels are crucial in bone elongation.
With the emergence of new imaging modalities, we now have a better understanding of the mechanisms of molecular transport to the growth plate from the surrounding vasculature. Multiphoton microscopy (MPM) as described by Zipfel, Williams, & Webb [239] is a minimally-invasive method of in vivo fluorescent imaging that can be used to study solute transport to the growth plate of live anesthetized animals at cellular-level resolution [238]. This imaging approach provides a new approach for tracking systemic fluorescent tracers through the vasculature into the growth plate in real time, in a way not possible using other techniques [119,122,235,238,240]. For example, dextrans larger than 40kDa are somewhat size-limited from entering the growth plate [122], but molecules less than 10kDa enter the growth plate through all three vascular routes [238]. Using these approaches to study the transport of molecules into the growth plate may ultimately provide a better understanding of how larger signaling molecules such as FGFs (FGF2, 18kDA; FGF18, 23kDa), PTHrP (9–23kDa), and BMPs (BMP-2, 26kDa) expressed in the perichondrium can regulate growth plate chondrogenesis [11].
Transport of Systemic IGF-1 into the Growth Plate
IGF-1 is the major circulating hormone of growth. At a molecular weight of 7.6kDa, IGF-1 falls within the range of molecules that readily enter the growth plate through the surrounding vasculature (less than 10kDa) [122]. To study the role of IGF-I uptake in bone elongation, Serrat and Ion [241] developed methods for visualizing the transport of fluorescently-labeled, biologically-active IGF-1 into the proximal tibial growth plate of live young mice using MPM. They showed that biologically active IGF-488 (IGF-1 conjugated with Alexa Fluor 488) entered the growth plate and localized to chondrocytes [241]. Since IGF-1 is used as a drug to treat short stature in GH insensitive children, such as Laron Syndrome patients [31,161,163,180,184–192], it is essential to understand how IGF-1 is transported through the vasculature. Real-time imaging could be a crucial step forward in developing approaches to better target IGF-1 delivery to the growth plate using mechanisms such as warm temperature [119,122,240].
Chondrocyte Expression of Angiogenic Factors
Angiogenesis (development of new blood vessels) is an essential part of normal linear bone growth. During endochondral ossification, hypertrophic chondrocytes secrete angiogenic factors that initiate vascular invasion and recruit bone absorbing and forming cells to replace mineralized cartilage with bone. The key regulator of angiogenesis during both prenatal and postnatal growth and development is vascular endothelial growth factor (VEGF). Survival is dependent on functional VEGF during both embryonic [242,243] and early postnatal life [244]. Different VEGF isoforms exist but only VEGF-A is expressed in growth plate cartilage and therefore is the most important for regulating longitudinal bone growth [245]. There are also additional splice isoforms of VEGF-A that vary between species. Hypertrophic chondrocytes in both human and murine growth plates secrete VEGF [7,81,234,246–249] to promote vascular invasion from the metaphyseal bone throughout postnatal limb elongation. In human growth plates, the most common splice isoforms are VEGF121, VEGF165 and VEGF189 [234,245]. The analogous isoforms in mice are VEGF120, VEGF164 and VEGF188 [234,250]. Interestingly, Maes et al. [234] discovered that different processes of vascularization require specific isoforms of VEGF-A and that the combined action of VEGF120 and VEGF188 is required for both epiphyseal and metaphyseal vascularization. Other local factors expressed by hypertrophic chondrocytes that promote angiogenesis include FGFs [251–253] and matrix metalloproteinase 9 (MMP-9) [81,247,250,254,255]. Systemic factors including estrogen [245] and IGF-1 [256,257]) have also been shown to induce vascular invasion by stimulation of VEGF in growth plate chondrocytes.
Normal longitudinal bone growth is dependent on VEGF expression in growth plate chondrocytes. Inhibition of VEGF in young mice (24 days old) suppressed blood vessel invasion, lengthened the hypertrophic zone and reduced bone growth, all of which was corrected after anti-VEGF treatment ended [247]. These results are clinically relevant to actively growing children that may require therapeutic intervention using angiogenesis inhibitors to prevent unwanted formation of new blood vessels, such as in pediatric cancers. A monoclonal antibody against VEGF, bevacizumab (Avastin®; Genentech, Inc), has been used in adults as an FDA approved anti-cancer agent [243] and is considered a promising treatment option for children [258,259]. It is especially important to improve chemotherapy in pediatric patients since children are undergoing an essential stage of bone development requiring growth factors, including IGF-1 and VEGF, for bone elongation. Therefore, as with IGF-1 antagonists, the development of non-invasive methods for targeting drugs directly to a tissue will be essential for preserving linear growth in children.
Conclusions
The goal of this review was to highlight factors that influence postnatal bone elongation with respect to their effects on IGF-1 activity in the growth plate, and in particular, the current state of knowledge regarding effects of IGF-1 and environmental variables, such as temperature, on bone elongation in cartilaginous growth plates. The available evidence demonstrates that environmental variables such as temperature and nutrition impact IGF-1 activity in the growth plate. While there are still gaps in our knowledge, there are many potential avenues of future studies to elucidate the complex mechanisms by which IGF-1 interacts with environmental variables to enhance bone elongation. By understanding the interplay between these important factors during postnatal linear growth, the goal is to ultimately develop better approaches for treating children with a range of bone elongation disorders.
Acknowledgments
This research was funded by the National Institute of General Medical Sciences (P20GM121299-01) and National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (R15AR067451-01). The NASA West Virginia Space Grant Consortium provided a research stipend to H.L.R. This work was made possible by support from the Marshall University Molecular and Biological Imaging Center and Appalachian Center for Cellular transport in Obesity Related Disorders (ACCORD). The authors thank J. Kopchick, C. Farnum, R. Williams, T. Salisbury, and G. Ion for helpful discussion and critical input while preparing drafts of this manuscript. George Vopal provided the tibia illustration in Figure 2.
Abbreviations
- ALS
acid labile subunit
- BMP
bone morphogenic protein
- COJ
chondro-osseous junction
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- GH
growth hormone
- GHR−/−
growth hormone receptor knockout
- GnRH
gonadotropin releasing hormone
- GP
growth plate
- HZ
hypertrophic zone of growth plate cartilage
- IGF-1
insulin-like growth factor 1
- IGF-1R
insulin-like growth factor 1 receptor
- IGF2
insulin-like growth factor 2
- IGFBP
insulin-like growth factor binding protein
- Ihh
Indian hedgehog
- LID
liver IGF-1-deficient
- LLD
limb length discrepancy
- MAPK
mitogen-activated protein kinase
- MMP-9
matrix metalloproteinase 9
- MPM
multiphoton microscopy
- PTHrP
parathyroid hormone-related protein
- PZ
proliferative zone of growth plate cartilage
- RZ
reserve zone of growth plate cartilage
- T3
triiodothyronine
- TKI
tyrosine kinase inhibitors
- TRα1
thyroid hormone receptor alpha 1
- TRβ
thyroid hormone receptor beta
- VEGF
vascular endothelial growth factor
- WISP3
Wnt induced secreted protein 3
- Wnt
wingless/integrated
- Zn
zinc
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest
The authors declare no conflict of interest.
Conflict of Interest
Holly Racine reports grants from NASA West Virginia Space Grant Consortium during the conduct of the study.
Maria Serrat reports grants from National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and grants from National Institute of General Medical Sciences, during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
••Of major importance
- 1.Farnum CE Postnatal growth of fins and limbs through endochondral ossification In Fins into limbs: evolution, development, and transformation; Hall BK, Ed.; University of Chicago Press: Chicago, 2007; pp. 118–151 ISBN 978–0-226-31337-5. [Google Scholar]
- 2.Lui JC; Nilsson O; Baron J Recent insights into the regulation of the growth plate. J. Mol. Endocrinol 2014, 53, T1–T9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.**.Wang Y; Cheng Z; ElAlieh HZ; Nakamura E; Nguyen M-T; Mackem S; Clemens TL; Bikle DD; Chang IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J. Bone Miner. Res 2011, 26, 1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]; Targeted knockout of the IGF-1 receptor in chondrocytes suggests that the IGF-1 receptor partly regulates cell proliferation and differentiation in the growth plate by suppressing PTHrP signaling.
- 4.Serrat MA Environmental Temperature Impact on Bone and Cartilage Growth In Comprehensive Physiology; Terjung R, Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 621–655 ISBN 978–0-470-65071-4. [DOI] [PubMed] [Google Scholar]
- 5.Gilbert SF Development of the tetrapod limb In Developmental Biology; Sinauer Associates, Inc: Sunderland (MA), 2014; pp. 489–516. [Google Scholar]
- 6.Abad V; Meyers JL; Weise M; Gafni RI; Barnes KM; Nilsson O; Bacher JD; Baron J The Role of the Resting Zone in Growth Plate Chondrogenesis. Endocrinology 2002, 143, 1851–1857. [DOI] [PubMed] [Google Scholar]
- 7.Hunziker EB Mechanism of longitudinal bone growth and its regulation by growth plate chondrocytes. Microsc. Res. Tech 1994, 28, 505–519. [DOI] [PubMed] [Google Scholar]
- 8.Raimann A; Javanmardi A; Egerbacher M; Haeusler G A journey through growth plates: tracking differences in morphology and regulation between the spine and the long bones in a pig model. Spine J. 2017, 17, 1674–1684. [DOI] [PubMed] [Google Scholar]
- 9.Schrier L Depletion of resting zone chondrocytes during growth plate senescence. J. Endocrinol 2006, 189, 27–36. [DOI] [PubMed] [Google Scholar]
- 10.Mackie EJ; Ahmed YA; Tatarczuch L; Chen K-S; Mirams M Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol 2008, 40, 46–62. [DOI] [PubMed] [Google Scholar]
- 11.Kronenberg HM Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro IM; Adams CS; Freeman T; Srinivas V Fate of the hypertrophic chondrocyte: Microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate. Birth Defects Res. Part C Embryo Today Rev 2005, 75, 330–339. [DOI] [PubMed] [Google Scholar]
- 13.Ulici V; Hoenselaar KD; Gillespie JR; Beier F The PI3K pathway regulates endochondral bone growth through control of hypertrophic chondrocyte differentiation. BMC Dev. Biol 2008, 8, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.**.Bahney CS; Hu DP; Taylor AJ; Ferro F; Britz HM; Hallgrimsson B; Johnstone B; Miclau T; Marcucio RS Stem Cell-Derived Endochondral Cartilage Stimulates Bone Healing by Tissue Transformation. J. Bone Miner. Res 2014, 29, 1269–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]; Challenges traditional dogma that hypertrophic chondrocytes die by apoptosis and demonstrates that chondrocytes can directly transform into bone during fracture repair.
- 15.Enishi T; Yukata K; Takahashi M; Sato R; Sairyo K; Yasui N Hypertrophic Chondrocytes in the Rabbit Growth Plate Can Proliferate and Differentiate into Osteogenic Cells when Capillary Invasion Is Interposed by a Membrane Filter. PLoS ONE 2014, 9, e104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.**.Hu DP; Ferro F; Yang F; Taylor AJ; Chang W; Miclau T; Marcucio RS; Bahney CS Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development 2017, 144, 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identifies induction of pluripotency genes [Sox2, Oct4 (Pou5f1), Nanog] as a mechanism by which hypertrophic chondrocytes transdifferentiate into bone-forming osteoblasts during endochondral ossification.
- 17.Zhou X; von der Mark K; Henry S; Norton W; Adams H; de Crombrugghe B Chondrocytes Transdifferentiate into Osteoblasts in Endochondral Bone during Development, Postnatal Growth and Fracture Healing in Mice. PLoS Genet. 2014, 10, e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aghajanian P; Mohan S The art of building bone: emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 2018, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Digby KH The Measurement of Diaphysial Growth in Proximal and Distal Directions. J. Anat. Physiol 1916, 50, 187–188. [PMC free article] [PubMed] [Google Scholar]
- 20.Payton CG The Growth in Length of the Long Bones in the Madder-fed Pig. J. Anat 1932, 66, 414–425. [PMC free article] [PubMed] [Google Scholar]
- 21.Bisgard JD Longitudinal Growth of Long Bones. Arch. Surg 1935, 31, 568. [Google Scholar]
- 22.Krember NF Comparative patterns of cell division in epiphyseal cartilage plates in the rat. J. Anat 1972, 111, 137–142. [PMC free article] [PubMed] [Google Scholar]
- 23.Pritchett JW Longitudinal growth and growth-plate activity in the lower extremity. Clin. Orthop 1992, 274–279. [PubMed] [Google Scholar]
- 24.Serrat MA; King D; Lovejoy CO Temperature regulates limb length in homeotherms by directly modulating cartilage growth. Proc. Natl. Acad. Sci 2008, 105, 19348–19353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilsman NJ; Bernardini ES; Leiferman E; Noonan K; Farnum CE Age and pattern of the onset of differential growth among growth plates in rats. J. Orthop. Res 2008, 26, 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilsman NJ; Farnum CE; Green EM; Lieferman EM; Clayton MK Cell cycle analysis of proliferative zone chondrocytes in growth plates elongating at different rates. J. Orthop. Res 1996, 14, 562–572. [DOI] [PubMed] [Google Scholar]
- 27.Wilsman NJ; Leiferman EM; Fry M; Farnum CE; Barreto C Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J. Orthop. Res 1996, 14, 927–936. [DOI] [PubMed] [Google Scholar]
- 28.Rolian C Developmental basis of limb length in rodents: evidence for multiple divisions of labor in mechanisms of endochondral bone growth: Comparative endochondral bone growth in rodents. Evol. Dev 2008, 10, 15–28. [DOI] [PubMed] [Google Scholar]
- 29.Hunziker EB; Schenk RK; Cruz-Orive LM Quantitation of chondrocyte performance in growth-plate cartilage during longitudinal bone growth. J. Bone Joint Surg. Am 1987, 69, 162–173. [PubMed] [Google Scholar]
- 30.Kember N Cell kinetics and the control of bone growth. Acta Paediatr. 1993, 82, 61–65. [DOI] [PubMed] [Google Scholar]
- 31.Lupu F; Terwilliger JD; Lee K; Segre GV; Efstratiadis A Roles of Growth Hormone and Insulin-like Growth Factor 1 in Mouse Postnatal Growth. Dev. Biol 2001, 229, 141–162. [DOI] [PubMed] [Google Scholar]
- 32.Walker KVR; Kember NF Cell kinetics of growth cartilage in the rat tibia. Cell Prolif. 1972, 5, 401–408. [DOI] [PubMed] [Google Scholar]
- 33.**.Cooper KL; Oh S; Sung Y; Dasari RR; Kirschner MW; Tabin CJ Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 2013, 495, 375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that insulin-like growth factor-dependent hypertrophic cell enlargement is a primary mechanism for controlling site-specific elongation rate in growth plates. These results provide important insight into the evolution of limb proportions in mammals.
- 34.Hunziker EB; Schenk RK Physiological mechanisms adopted by chondrocytes in regulating longitudinal bone growth in rats. J. Physiol 1989, 414, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serrat MA; Lovejoy CO; King D Age- and site-specific decline in insulin-like growth factor-I receptor expression is correlated with differential growth plate activity in the mouse hindlimb. Anat. Rec. Adv. Integr. Anat. Evol. Biol 2007, 290, 375–381. [DOI] [PubMed] [Google Scholar]
- 36.Baker J; Liu JP; Robertson EJ; Efstratiadis A Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993, 75, 73–82. [PubMed] [Google Scholar]
- 37.Gurney B Leg length discrepancy. Gait Posture 2002, 15, 195–206. [DOI] [PubMed] [Google Scholar]
- 38.Morscher E Etiology and Pathophysiology of Leg Length Discrepancies In Leg Length Discrepancy The Injured Knee; Hungerford DS, Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 1977; Vol. 1, pp. 9–19 ISBN 978–3-642-66551-6. [Google Scholar]
- 39.Wilson PD; Thompson TC A clinical consideration of the methods of equalizing leg length. Ann. Surg 1939, 110, 992–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirienko A; Peccati A; Abdellatif I; Elbatrawy Y; Mostaf ZMA; Necci V Correction of poliomyelitis foot deformities with Ilizarov method. Strateg. Trauma Limb Reconstr 2011, 6, 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sonekatsu M; Sonohata M; Kitajima M; Kawano S; Mawatari M Total Hip Arthroplasty for Patients with Residual Poliomyelitis at a Mean Eight Years of Follow-up. Acta Med. Okayama 2018, 72, 17–22. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro F Developmental patterns in lower-extremity length discrepancies.: J. Bone Jt. Surg 1982, 64, 639–651. [PubMed] [Google Scholar]
- 43.Hansson LI; Stenström A; Thorngren K-G Effect of Fracture on Longitudinal Bone Growth in Rats. Acta Orthop. Scand 1976, 47, 600–606. [DOI] [PubMed] [Google Scholar]
- 44.Togrul E; Bayram H; Gulsen M; Kalacı A; Özbarlas S Fractures of the femoral neck in children: long-term follow-up in 62 hip fractures. Injury 2005, 36, 123–130. [DOI] [PubMed] [Google Scholar]
- 45.Truesdell ED Inequality of the lower extremities following fracture of the shaft of the femur in children. Ann. Surg 1921, 74, 498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wray JB; Goodman HO Post-Fracture Vascular Phenomena and Long-Bone Overgrowth in the Immature Skeleton of the Rat: J. Bone Jt. Surg 1961, 43, 1047–1055. [PubMed] [Google Scholar]
- 47.Campbell TM; Ghaedi BB; Tanjong Ghogomu E; Welch V Shoe Lifts for Leg Length Discrepancy in Adults With Common Painful Musculoskeletal Conditions: A Systematic Review of the Literature. Arch. Phys. Med. Rehabil 2018, 99, 981–993.e2. [DOI] [PubMed] [Google Scholar]
- 48.Friberg O Clinical symptoms and biomechanics of lumbar spine and hip joint in leg length inequality. Spine 1983, 8, 643–651. [DOI] [PubMed] [Google Scholar]
- 49.Defrin R; Benyamin SB; Aldubi RD; Pick CG Conservative Correction of Leg-Length Discrepancies of 10mm or Less for the Relief of Chronic Low Back Pain. Arch. Phys. Med. Rehabil 2005, 86, 2075–2080. [DOI] [PubMed] [Google Scholar]
- 50.Aiona M; Do KP; Emara K; Dorociak R; Pierce R Gait Patterns in Children With Limb Length Discrepancy: J. Pediatr. Orthop 2014, 1. [DOI] [PubMed] [Google Scholar]
- 51.Kaufman KR; Miller LS; Sutherland DH Gait asymmetry in patients with limb-length inequality. J. Pediatr. Orthop 1996, 16, 144–150. [DOI] [PubMed] [Google Scholar]
- 52.Khamis S; Carmeli E Relationship and significance of gait deviations associated with limb length discrepancy: A systematic review. Gait Posture 2017, 57, 115–123. [DOI] [PubMed] [Google Scholar]
- 53.Mahmood S; Huffman LK; Harris JG Limb-Length Discrepancy as a Cause of Plantar Fasciitis. J. Am. Podiatr. Med. Assoc 2010, 100, 452–455. [DOI] [PubMed] [Google Scholar]
- 54.Song KM; Halliday SE; Little DG The Effect of Limb-Length Discrepancy on Gait*: J. Bone Joint Surg. Am 1997, 79, 1690–1698. [DOI] [PubMed] [Google Scholar]
- 55.Papaioannou T; Stokes I; Kenwright J Scoliosis associated with limb-length inequality.: J. Bone Jt. Surg 1982, 64, 59–62. [PubMed] [Google Scholar]
- 56.Golightly YM; Tate JJ; Burns CB; Gross MT Changes in Pain and Disability Secondary to Shoe Lift Intervention in Subjects With Limb Length Inequality and Chronic Low Back Pain: A Preliminary Report. J. Orthop. Sports Phys. Ther 2007, 37, 380–388. [DOI] [PubMed] [Google Scholar]
- 57.Golightly YM; Allen KD; Renner JB; Helmick CG; Salazar A; Jordan JM Relationship of limb length inequality with radiographic knee and hip osteoarthritis. Osteoarthritis Cartilage 2007, 15, 824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harvey WF Association of Leg-Length Inequality With Knee Osteoarthritis: A Cohort Study. Ann. Intern. Med 2010, 152, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Resende RA; Kirkwood RN; Deluzio KJ; Morton AM; Fonseca ST Mild leg length discrepancy affects lower limbs, pelvis and trunk biomechanics of individuals with knee osteoarthritis during gait. Clin. Biomech 2016, 38, 1–7. [DOI] [PubMed] [Google Scholar]
- 60.Fredriks AM Nationwide age references for sitting height, leg length, and sitting height/height ratio, and their diagnostic value for disproportionate growth disorders. Arch. Dis. Child 2005, 90, 807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDowell MA; Fryar CD; Ogden CL Anthropometric reference data for children and adults: United States, 1988–1994. Vital Health Stat. 11 2009, 1–68. [PubMed] [Google Scholar]
- 62.Racine HL; Meadows CA; Ion G; Serrat MA Heat-Induced Limb Length Asymmetry Has Functional Impact on Weight Bearing in Mouse Hindlimbs. Front. Endocrinol 2018, 9, 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stevens PM The role of guided growth as it relates to limb lengthening. J. Child. Orthop 2016, 10, 479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vitale MA; Choe JC; Sesko AM; Hyman JE; Lee FY; Roye DP; Vitale MG The effect of limb length discrepancy on health-related quality of life: is the ‘2 cm rule’ appropriate?: J. Pediatr. Orthop. B 2006, 15, 1–5. [DOI] [PubMed] [Google Scholar]
- 65.Zhang P; Hamamura K; Turner CH; Yokota H Lengthening of mouse hindlimbs with joint loading. J. Bone Miner. Metab 2010, 28, 268–275. [DOI] [PubMed] [Google Scholar]
- 66.Phemister DB Operative arrestment of longitudinal growth of bones in the treatment of deformities. JBJS 1933, 15, 1. [Google Scholar]
- 67.Pendleton AM; Stevens PM; Hung M Guided Growth for the Treatment of Moderate Leg-length Discrepancy. Orthopedics 2013, 36, e575–e580. [DOI] [PubMed] [Google Scholar]
- 68.Sabharwal S; Nelson SC; Sontich JK What’s New in Limb Lengthening and Deformity Correction. J. Bone Joint Surg. Am 2015, 97, 1375–1384. [DOI] [PubMed] [Google Scholar]
- 69.Ilizarov GA The principles of the Ilizarov method. Bull. Hosp. Jt. Dis. Orthop. Inst 1988, 48, 1–11. [PubMed] [Google Scholar]
- 70.Hasler CC; Krieg AH Current concepts of leg lengthening. J. Child. Orthop 2012, 6, 89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelly PM; Diméglio A Lower-limb growth: how predictable are predictions? J. Child. Orthop 2008, 2, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson M; Green WT; Messner MB Growth and predictions of growth in the lower extremities. J. Bone Joint Surg. Am 1963, 45-A, 1–14. [PubMed] [Google Scholar]
- 73.Menelaus MB Correction of leg length discrepancy by epiphysial arrest. J. Bone Joint Surg. Br 1966, 48, 336–339. [PubMed] [Google Scholar]
- 74.Moseley CF A straight-line graph for leg-length discrepancies. JBJS 1977, 59, 174. [PubMed] [Google Scholar]
- 75.Paley D; Bhave A; Herzenberg JE; Bowen JR Multiplier Method for Predicting Limb-Length Discrepancy*: J. Bone Jt. Surg.-Am. Vol 2000, 82, 1432–1446. [DOI] [PubMed] [Google Scholar]
- 76.Monier BC; Aronsson DD; Sun M Percutaneous epiphysiodesis using transphyseal screws for limb-length discrepancies: high variability among growth predictor models. J. Child. Orthop 2015, 9, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gross RH Leg length discrepancy: how much is too much? Orthopedics 1978, 1, 307–310. [DOI] [PubMed] [Google Scholar]
- 78.Knutson GA Anatomic and functional leg-length inequality: a review and recommendation for clinical decision-making. Part I, anatomic leg-length inequality: prevalence, magnitude, effects and clinical significance. Chiropr. Osteopat 2005, 13, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.White SC; Gilchrist LA; Wilk BE Asymmetric Limb Loading with True or Simulated Leg-Length Differences: Clin. Orthop 2004, 421, 287–292. [DOI] [PubMed] [Google Scholar]
- 80.Serrat MA; Schlierf TJ; Efaw ML; Shuler FD; Godby J; Stanko LM; Tamski HL Unilateral heat accelerates bone elongation and lengthens extremities of growing mice: HEAT ACCELERATES BONE ELONGATION. J. Orthop. Res 2015, 33, 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Eerden BCJ; Karperien M; Wit JM Systemic and Local Regulation of the Growth Plate. Endocr. Rev 2003, 24, 782–801. [DOI] [PubMed] [Google Scholar]
- 82.Börjesson AE; Lagerquist MK; Liu C; Shao R; Windahl SH; Karlsson C; Sjögren K; Movérare-Skrtic S; Antal MC; Krust A; et al. The role of estrogen receptor α in growth plate cartilage for longitudinal bone growth. J. Bone Miner. Res 2010, 25, 2690–2700. [DOI] [PubMed] [Google Scholar]
- 83.Ohlsson C; Isgaard J; Törnell J; Nilsson A; Isaksson O; Lindahl A Endocrine regulation of longitudinal bone growth. Acta Paediatr. 1993, 82, 33–40. [DOI] [PubMed] [Google Scholar]
- 84.Simpson ME; Asling CW; Evans HM Some Endocrine Influences on Skeletal Growth and Differentiation. Yale J. Biol. Med 1950, 23, 1–27. [PMC free article] [PubMed] [Google Scholar]
- 85.Tryfonidou MA; Hazewinkel HAW; Riemers FM; Brinkhof B; Penning LC; Karperien M Intraspecies disparity in growth rate is associated with differences in expression of local growth plate regulators. Am. J. Physiol.-Endocrinol. Metab 2010, 299, E1044–E1052. [DOI] [PubMed] [Google Scholar]
- 86.Wang L; Shao YY; Ballock RT Thyroid hormone-mediated growth and differentiation of growth plate chondrocytes involves IGF-1 modulation of β-catenin signaling. J. Bone Miner. Res 2010, 25, 1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ohlsson C; Nilsson A; Isaksson O; Bentham J; Lindahl A Effects of triiodothyronine and insulin-like growth factor-I (IGF-I) on alkaline phosphatase activity, [3H]thymidine incorporation and IGF-I receptor mRNA in cultured rat epiphyseal chondrocytes. J. Endocrinol 1992, 135, 115–23. [DOI] [PubMed] [Google Scholar]
- 88.Stevens DA; Hasserjian RP; Robson H; Siebler T; Shalet SM; Williams GR Thyroid Hormones Regulate Hypertrophic Chondrocyte Differentiation and Expression of Parathyroid Hormone-Related Peptide and Its Receptor During Endochondral Bone Formation. J. Bone Miner. Res 2000, 15, 2431–2442. [DOI] [PubMed] [Google Scholar]
- 89.Karimian E; Chagin AS; Sävendahl L Genetic Regulation of the Growth Plate. Front. Endocrinol 2012, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maeda Y; Schipani E; Densmore MJ; Lanske B Partial rescue of postnatal growth plate abnormalities in Ihh mutants by expression of a constitutively active PTH/PTHrP receptor. Bone 2010, 46, 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lazarus JE; Hegde A; Andrade AC; Nilsson O; Baron J Fibroblast growth factor expression in the postnatal growth plate. Bone 2007, 40, 577–586. [DOI] [PubMed] [Google Scholar]
- 92.Su N; Jin M; Chen L Role of FGF/FGFR signaling in skeletal development and homeostasis: learning from mouse models. Bone Res. 2014, 2, 14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garrison P; Yue S; Hanson J; Baron J; Lui JC Spatial regulation of bone morphogenetic proteins (BMPs) in postnatal articular and growth plate cartilage. PLOS ONE 2017, 12, e0176752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nilsson O; Parker EA; Hegde A; Chau M; Barnes KM; Baron J Gradients in bone morphogenetic protein-related gene expression across the growth plate. J. Endocrinol 2007, 193, 75–84. [DOI] [PubMed] [Google Scholar]
- 95.Vortkamp A; Lee K; Lanske B; Segre GV; Kronenberg HM; Tabin CJ Regulation of Rate of Cartilage Differentiation by Indian Hedgehog and PTH-Related Protein. Science 1996, 273, 613–622. [DOI] [PubMed] [Google Scholar]
- 96.Millward DJ Nutrition, infection and stunting: the roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr. Res. Rev 2017, 30, 50–72. [DOI] [PubMed] [Google Scholar]
- 97.Cusick SE; Kuch AE Determinants of Undernutrition and Overnutrition among Adolescents in Developing Countries. Adolesc. Med. State Art Rev 2012, 23, 440–456. [PMC free article] [PubMed] [Google Scholar]
- 98.Ngari MM; Iversen PO; Thitiri J; Mwalekwa L; Timbwa M; Fegan GW; Berkley JA Linear growth following complicated severe malnutrition: 1-year follow-up cohort of Kenyan children. Arch. Dis. Child 2019, 104, 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Forbes GB Lean Body Mass and Fat in Obese Children. Pediatrics 1964, 34, 308–314. [PubMed] [Google Scholar]
- 100.Forbes GB Nutrition and growth. J. Pediatr 1977, 91, 40–42. [DOI] [PubMed] [Google Scholar]
- 101.He Q; Karlberg J BMI in Childhood and Its Association with Height Gain, Timing of Puberty, and Final Height. Pediatr. Res 2001, 49, 244–251. [DOI] [PubMed] [Google Scholar]
- 102.Johnson W; Stovitz SD; Choh AC; Czerwinski SA; Towne B; Demerath EW Patterns of linear growth and skeletal maturation from birth to 18 years of age in overweight young adults. Int. J. Obes 2012, 36, 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farnum CE; Lee AO; O’Hara K; Wilsman NJ Effect of short-term fasting on bone elongation rates: an analysis of catch-up growth in young male rats. Pediatr. Res 2003, 53, 33–41. [DOI] [PubMed] [Google Scholar]
- 104.Karlberg J; Albertsson-Wikland K Nutrition and linear growth in childhood In Recent Developments in Infant Nutrition: Scheveningen, 29 November – 2 December 1995; Bindels JG, Goedhart AC, Visser HKA, Eds.; Tenth Nutricia Symposium; Springer Netherlands: Dordrecht, 1996; pp. 112–127 ISBN 978-94-009-1790-3. [Google Scholar]
- 105.Roberts JL; Stein AD The Impact of Nutritional Interventions beyond the First 2 Years of Life on Linear Growth: A Systematic Review and Meta-Analysis. Adv. Nutr 2017, 8, 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prentice AM; Ward KA; Goldberg GR; Jarjou LM; Moore SE; Fulford AJ; Prentice A Critical windows for nutritional interventions against stunting. Am. J. Clin. Nutr 2013, 97, 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Serrat MA; Machnicki AL; Meadows CA; McCloud D; Thomas D; Hurley JD; Ion G Enhanced IGF-I Delivery to the Growth Plate and Accelerated Bone Elongation in a Mouse Model of Juvenile Obesity. FASEB J. 2019, 33, 774.23–774.23. [Google Scholar]
- 108.Savage MO Insulin-Like Growth Factors, Nutrition and Growth In World Review of Nutrition and Dietetics; Shamir R, Turck D, Phillip M, Eds.; S. KARGER AG: Basel, 2013; Vol. 106, pp. 52–59 ISBN 978-3-318-02265-0. [DOI] [PubMed] [Google Scholar]
- 109.Adriani M; Wirjatmadi B The effect of adding zinc to vitamin A on IGF-1, bone age and linear growth in stunted children. J. Trace Elem. Med. Biol 2014, 28, 431–435. [DOI] [PubMed] [Google Scholar]
- 110.D McNall A; D Etherton T; J Fosmire G The impaired growth induced by zinc deficiency in rats is associated with decreased expression of the hepatic IGF-I and the growth hormone receptor genes. J. Nutr 1995, 125, 874–9. [DOI] [PubMed] [Google Scholar]
- 111.Ninh NX; Thissen JP; Collette L; Gerard G; Khoi HH; Ketelslegers JM Zinc supplementation increases growth and circulating insulin-like growth factor I (IGF-I) in growth-retarded Vietnamese children. Am. J. Clin. Nutr 1996, 63, 514–519. [DOI] [PubMed] [Google Scholar]
- 112.MacDonald RS The Role of Zinc in Growth and Cell Proliferation. J. Nutr 2000, 130, 1500S–1508S. [DOI] [PubMed] [Google Scholar]
- 113.Browning JD; MacDonald RS; Thornton WH; O’Dell BL Reduced food intake in zinc deficient rats is normalized by megestrol acetate but not by insulin-like growth factor-I. J. Nutr 1998, 128, 136–142. [DOI] [PubMed] [Google Scholar]
- 114.Schell LM; Gallo MV; Ravenscroft J Environmental influences on human growth and development: Historical review and case study of contemporary influences. Ann. Hum. Biol 2009, 36, 459–477. [DOI] [PubMed] [Google Scholar]
- 115.Allen JA The influence of physical conditions in the genesis of species. In Radical Review; 1877; Vol. 1, pp. 108–140. [Google Scholar]
- 116.Newman MT The Application of Ecological Rules to the Racial Anthropology of the Aboriginal New World. Am. Anthropol 1953, 55, 311–327. [Google Scholar]
- 117.Tilkens MJ; Wall-Scheffler C; Weaver TD; Steudel-Numbers K The effects of body proportions on thermoregulation: an experimental assessment of Allen’s rule. J. Hum. Evol 2007, 53, 286–291. [DOI] [PubMed] [Google Scholar]
- 118.Pollard AS; Charlton BG; Hutchinson JR; Gustafsson T; McGonnell IM; Timmons JA; Pitsillides AA Limb proportions show developmental plasticity in response to embryo movement. Sci. Rep 2017, 7, 41926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Serrat MA; Williams RM; Farnum CE Exercise mitigates the stunting effect of cold temperature on limb elongation in mice by increasing solute delivery to the growth plate. J. Appl. Physiol 2010, 109, 1869–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Al-Hilli F; Wright EA The effects of changes in the environmental temperature on the growth of tail bones in the mouse. Br. J. Exp. Pathol 1983, 64, 34–42. [PMC free article] [PubMed] [Google Scholar]
- 121.Serrat MA Allen’s Rule Revisited: Temperature Influences Bone Elongation During a Critical Period of Postnatal Development: Temperature Impacts Bone in Critical Period. Anat. Rec 2013, 296, 1534–1545. [DOI] [PubMed] [Google Scholar]
- 122.Serrat MA; Efaw ML; Williams RM Hindlimb heating increases vascular access of large molecules to murine tibial growth plates measured by in vivo multiphoton imaging. J. Appl. Physiol 2014, 116, 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Powell-Braxton L; Hollingshead P; Warburton C; Dowd M; Pitts-Meek S; Dalton D; Gillett N; Stewart TA IGF-I is required for normal embryonic growth in mice. Genes Dev. 1993, 7, 2609–2617. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y; Nishida S; Sakata T; Elalieh HZ; Chang W; Halloran BP; Doty SB; Bikle DD Insulin-Like Growth Factor-I Is Essential for Embryonic Bone Development. Endocrinology 2006, 147, 4753–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu J-P; Baker J; Perkins AS; Robertson EJ; Efstratiadis A Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (IGF-1r). Cell 1993, 75, 59–72. [PubMed] [Google Scholar]
- 126.Mohan S; Richman C; Guo R; Amaar Y; Donahue LR; Wergedal J; Baylink DJ Insulin-Like Growth Factor Regulates Peak Bone Mineral Density in Mice by Both Growth Hormone-Dependent and -Independent Mechanisms. Endocrinology 2003, 144, 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Isaksson O; Jansson J; Gause I Growth hormone stimulates longitudinal bone growth directly. Science 1982, 216, 1237–1239. [DOI] [PubMed] [Google Scholar]
- 128.Isaksson OGP; Eden S; Jansson J Mode of Action of Pituitary Growth Hormone on Target Cells. Annu. Rev. Physiol 1985, 47, 483–499. [DOI] [PubMed] [Google Scholar]
- 129.Ohlsson C; Nilsson A; Isaksson O; Lindahl A Growth hormone induces multiplication of the slowly cycling germinal cells of the rat tibial growth plate. Proc. Natl. Acad. Sci 1992, 89, 9826–9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ohlsson C Growth Hormone and Bone. Endocr. Rev 1998, 19, 55–79. [DOI] [PubMed] [Google Scholar]
- 131.Schlechter NL; Russell SM; Greenberg S; Spencer EM; Nicoll CS A direct growth effect of growth hormone in rat hindlimb shown by arterial infusion. Am. J. Physiol.-Endocrinol. Metab 1986, 250, E231–E235. [DOI] [PubMed] [Google Scholar]
- 132.Isaksson OGP; Ohlsson C; Nilsson A; Isgaard J; Lindahl A Regulation of cartilage growth by growth hormone and insulin-like growth factor I. Pediatr. Nephrol 1991, 5, 451–453. [DOI] [PubMed] [Google Scholar]
- 133.Politis SN; Mazurais D; Servili A; Zambonino-Infante J-L; Miest JJ; Sørensen SR; Tomkiewicz J; Butts IAE Temperature effects on gene expression and morphological development of European eel, Anguilla anguilla larvae. PLOS ONE 2017, 12, e0182726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Al-Zghoul M; Al-Natour M; Dalab A; Alturki O; Althnaian T; Al-ramadan S; Hannon K; El-Bahr S Thermal Manipulation Mid-term Broiler Chicken Embryogenesis: Effect on Muscle Growth Factors and Muscle Marker Genes. Rev. Bras. Ciênc. Avícola 2016, 18, 607–618. [Google Scholar]
- 135.Halevy O; Krispin A; Leshem Y; McMurtry JP; Yahav S Early-age heat exposure affects skeletal muscle satellite cell proliferation and differentiation in chicks. Am. J. Physiol.-Regul. Integr. Comp. Physiol 2001, 281, R302–R309. [DOI] [PubMed] [Google Scholar]
- 136.Holman SR; Baxter RC Insulin-like growth factor binding protein-3: factors affecting binary and ternary complex formation. Growth Regul. 1996, 6, 42–47. [PubMed] [Google Scholar]
- 137.Salmon WD; Daughaday WH A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J. Lab. Clin. Med 1957, 49, 825–836. [PubMed] [Google Scholar]
- 138.Daughaday WH; Hall K; Raben MS; Salmon WD; Leo Van Den Brande J; Van Wyk JJ Somatomedin: Proposed Designation for Sulphation Factor. Nature 1972, 235, 107–107. [DOI] [PubMed] [Google Scholar]
- 139.Rinderknecht E; Humbel RE Primary structure of human insulin-like growth factor II. FEBS Lett. 1978, 89, 283–286. [DOI] [PubMed] [Google Scholar]
- 140.Rinderknecht E; Humbel RE The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J. Biol. Chem 1978, 253, 2769–2776. [PubMed] [Google Scholar]
- 141.Green H; Morikawa M; Mxon T A dual effector theory of growth-hormone action. Differentiation 1985, 29, 195–198. [DOI] [PubMed] [Google Scholar]
- 142.Tahimic CGT; Wang Y; Bikle DD Anabolic effects of IGF-1 signaling on the skeleton. Front. Endocrinol 2013, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.**.Wu S; Yang W; De Luca F Insulin-Like Growth Factor-Independent Effects of Growth Hormone on Growth Plate Chondrogenesis and Longitudinal Bone Growth. Endocrinology 2015, 156, 2541–2551. [DOI] [PubMed] [Google Scholar]; Using targeted excision of the IGF-1 receptor in the growth plate, this study demonstrates that growth hormone can directly promote bone elongation in the absence of locally acting IGF-1 and IGF-2.
- 144.Dobie R; Ahmed SF; Staines KA; Pass C; Jasim S; MacRae VE; Farquharson C Increased linear bone growth by GH in the absence of SOCS2 is independent of IGF-1: SOCS2 REGULATION OF GH INDUCED GROWTH. J. Cell. Physiol 2015, 230, 2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moody G; Beltran PJ; Mitchell P; Cajulis E; Chung Y-A; Hwang D; Kendall R; Radinsky R; Cohen P; Calzone FJ IGF-1R blockade with ganitumab results in systemic effects on the GH–IGF axis in mice. J. Endocrinol 2014, 221, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bikle D; Majumdar S; Laib A; Powell-Braxton L; Rosen C; Beamer W; Nauman E; Leary C; Halloran B The Skeletal Structure of Insulin-Like Growth Factor I-Deficient Mice. J. Bone Miner. Res 2001, 16, 2320–2329. [DOI] [PubMed] [Google Scholar]
- 148.DeChiara TM; Efstratiadis A; Robertsen EJ A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature 1990, 345, 78–80. [DOI] [PubMed] [Google Scholar]
- 149.Yakar S; Liu J-L; Stannard B; Butler A; Accili D; Sauer B; LeRoith D Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci 1999, 96, 7324–7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sjögren K; Liu JL; Blad K; Skrtic S; Vidal O; Wallenius V; LeRoith D; Törnell J; Isaksson OG; Jansson JO; et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc. Natl. Acad. Sci. U. S. A 1999, 96, 7088–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ueki I; Ooi GT; Tremblay ML; Hurst KR; Bach LA; Boisclair YR Inactivation of the acid labile subunit gene in mice results in mild retardation of postnatal growth despite profound disruptions in the circulating insulin-like growth factor system. Proc. Natl. Acad. Sci 2000, 97, 6868–6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yakar S; Rosen CJ; Beamer WG; Ackert-Bicknell CL; Wu Y; Liu J-L; Ooi GT; Setser J; Frystyk J; Boisclair YR; et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Invest 2002, 110, 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Govoni KE; Lee SK; Chung Y-S; Behringer RR; Wergedal JE; Baylink DJ; Mohan S Disruption of insulin-like growth factor-I expression in type IIαI collagenexpressing cells reduces bone length and width in mice. Physiol. Genomics 2007, 30, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baxter RC Insulin-like growth factor (IGF)-binding proteins: interactions with IGFs and intrinsic bioactivities. Am. J. Physiol.-Endocrinol. Metab 2000, 278, E967–E976. [DOI] [PubMed] [Google Scholar]
- 155.Le Roith D; Bondy C; Yakar S; Liu J-L; Butler A The Somatomedin Hypothesis: 2001. Endocr. Rev 2001, 22, 53–74. [DOI] [PubMed] [Google Scholar]
- 156.Parker EA; Hegde A; Buckley M; Barnes KM; Baron J; Nilsson O Spatial and temporal regulation of GH-IGF-related gene expression in growth plate cartilage. J. Endocrinol 2007, 194, 31–40. [DOI] [PubMed] [Google Scholar]
- 157.Wang E; Wang J; Chin E; Zhou J; Bondy CA Cellular patterns of insulin-like growth factor system gene expression in murine chondrogenesis and osteogenesis. Endocrinology 1995, 136, 2741–2751. [DOI] [PubMed] [Google Scholar]
- 158.Lund PK; Moats-Staats BM; Hynes MA; Simmons JG; Jansen M; D’Ercole AJ; Van Wyk JJ Somatomedin-C/insulin-like growth factor-I and insulin-like growth factor-II mRNAs in rat fetal and adult tissues. J. Biol. Chem 1986, 261, 14539–14544. [PubMed] [Google Scholar]
- 159.Crudden C; Girnita A; Girnita L Targeting the IGF-1R: The Tale of the Tortoise and the Hare. Front. Endocrinol 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou Y; Xu BC; Maheshwari HG; He L; Reed M; Lozykowski M; Okada S; Cataldo L; Coschigamo K; Wagner TE; et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc. Natl. Acad. Sci 1997, 94, 13215–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Laron Z Lessons from 50 years of study of Laron syndrome. Endocr. Pract 2015, 21, 1395–1402. [DOI] [PubMed] [Google Scholar]
- 162.List EO; Sackmann-Sala L; Berryman DE; Funk K; Kelder B; Gosney ES; Okada S; Ding J; Cruz-Topete D; Kopchick JJ Endocrine Parameters and Phenotypes of the Growth Hormone Receptor Gene Disrupted (GHR−/−) Mouse. Endocr. Rev 2011, 32, 356–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sims NA; Clément-Lacroix P; Da Ponte F; Bouali Y; Binart N; Moriggl R; Goffin V; Coschigano K; Gaillard-Kelly M; Kopchick J; et al. Bone homeostasis in growth hormone receptor–null mice is restored by IGF-I but independent of Stat5. J. Clin. Invest 2000, 106, 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yakar S; Isaksson O Regulation of skeletal growth and mineral acquisition by the GH/IGF-1 axis: Lessons from mouse models. Growth Horm. IGF Res 2016, 28, 26–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Davies JS; Gevers EF; Stevenson AE; Coschigano KT; El-Kasti MM; Bull MJ; Elford C; Evans BAJ; Kopchick JJ; Wells T Adiposity profile in the dwarf rat: an unusually lean model of profound growth hormone deficiency. Am. J. Physiol. - Endocrinol. Metab 2007, 292, E1483–E1494. [DOI] [PubMed] [Google Scholar]
- 166.Wang J; Zhou J; Cheng CM; Kopchick JJ; Bondy CA Evidence supporting dual, IGF-I-independent and IGF-I-dependent, roles for GH in promoting longitudinal bone growth. J. Endocrinol 2004, 180, 247–255. [DOI] [PubMed] [Google Scholar]
- 167.Tritos NA; Klibanski A Effects of Growth Hormone on Bone In Progress in Molecular Biology and Translational Science; Elsevier, 2016; Vol. 138, pp. 193–211 ISBN 978-0-12-804827-6. [DOI] [PubMed] [Google Scholar]
- 168.Wang J; Zhou J; Bondy CA IGF-1 promotes longitudinal bone growth by insulin-like actions augmenting chondrocyte hypertrophy. FASEB J. 1999, 13, 1985–1990. [DOI] [PubMed] [Google Scholar]
- 169.Bassett JHD; Williams GR Role of Thyroid Hormones in Skeletal Development and Bone Maintenance. Endocr. Rev 2016, 37, 135–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bello CE; Garrett SD Therapeutic issues in oral glucocorticoid use. Lippincotts Prim. Care Pract 1999, 3, 333–341; quiz 342–344. [PubMed] [Google Scholar]
- 171.Silvestrini G; Ballanti P; Patacchioli FR; Mocetti P; Di Grezia R; Martin Wedard B; Angelucci L; Bonucci E Evaluation of apoptosis and the glucocorticoid receptor in the cartilage growth plate and metaphyseal bone cells of rats after high-dose treatment with corticosterone. Bone 2000, 26, 33–42. [DOI] [PubMed] [Google Scholar]
- 172.Rivkees SA; Bode HH; Crawford JD Long-Term Growth in Juvenile Acquired Hypothyroidism: N. Engl. J. Med 1988, 318, 599–602. [DOI] [PubMed] [Google Scholar]
- 173.Friedrichsen S; Christ S; Heuer H; Schäfer MKH; Mansouri A; Bauer K; Visser TJ Regulation of Iodothyronine Deiodinases in the Pax8 −/− Mouse Model of Congenital Hypothyroidism. Endocrinology 2003, 144, 777–784. [DOI] [PubMed] [Google Scholar]
- 174.Gothe S; Wang Z; Ng L; Kindblom JM; Barros AC; Ohlsson C; Vennstrom B; Forrest D Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 1999, 13, 1329–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kindblom JM; Gevers EF; Skrtic SM; Lindberg MK; Göthe S; Törnell J; Vennström B; Ohlsson C Increased adipogenesis in bone marrow but decreased bone mineral density in mice devoid of thyroid hormone receptors. Bone 2005, 36, 607–616. [DOI] [PubMed] [Google Scholar]
- 176.Gordon M; Crouthamel C; Post EM; Richman RA Psychosocial aspects of constitutional short stature: Social competence, behavior problems, self-esteem, and family functioning. J. Pediatr 1982, 101, 477–480. [DOI] [PubMed] [Google Scholar]
- 177.Sandberg DE Should short children who are not deficient in growth hormone be treated? West. J. Med 2000, 172, 186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pfäffle R Hormone replacement therapy in children: The use of growth hormone and IGF-I. Best Pract. Res. Clin. Endocrinol. Metab 2015, 29, 339–352. [DOI] [PubMed] [Google Scholar]
- 179.Wit JM; Oostdijk W Novel approaches to short stature therapy. Best Pract. Res. Clin. Endocrinol. Metab 2015, 29, 353–366. [DOI] [PubMed] [Google Scholar]
- 180.Ranke MB Growth Hormone Therapy in Turner Syndrome. Horm. Res 1995, 44, 35–41. [DOI] [PubMed] [Google Scholar]
- 181.Harada D; Namba N; Hanioka Y; Ueyama K; Sakamoto N; Nakano Y; Izui M; Nagamatsu Y; Kashiwagi H; Yamamuro M; et al. Final adult height in long-term growth hormone-treated achondroplasia patients. Eur. J. Pediatr 2017, 176, 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Moix Gil E; Giménez-Palop O; Caixàs A Treatment with growth hormone in the Prader-Willi syndrome. Endocrinol. Diabetes Nutr. Engl. Ed 2018, 65, 229–236. [DOI] [PubMed] [Google Scholar]
- 183.Noonan JA; Kappelgaard A-M The Efficacy and Safety of Growth Hormone Therapy in Children with Noonan Syndrome: A Review of the Evidence. Horm. Res. Paediatr 2015, 83, 157–166. [DOI] [PubMed] [Google Scholar]
- 184.Azcona C; Preece MA; Rose SJ; Fraser N; Rappaport R; Ranke MB; Savage MO Growth response to rhIGF-I 80 mug/kg twice daily in children with growth hormone insensitivity syndrome: relationship to severity of clinical phenotype. Clin. Endocrinol. (Oxf.) 1999, 51, 787–792. [DOI] [PubMed] [Google Scholar]
- 185.Backeljauw PF; Kuntze J; Frane J; Calikoglu AS; Chernausek SD Adult and Near-Adult Height in Patients with Severe Insulin-Like Growth Factor-I Deficiency after Long-Term Therapy with Recombinant Human Insulin-Like Growth Factor-I. Horm. Res. Paediatr 2013, 80, 47–56. [DOI] [PubMed] [Google Scholar]
- 186.Chernausek SD; Backeljauw PF; Frane J; Kuntze J; Underwood LE; for the GH Insensitivity Syndrome Collaborative Group Long-Term Treatment with Recombinant Insulin-Like Growth Factor (IGF)-I in Children with Severe IGF-I Deficiency due to Growth Hormone Insensitivity. J. Clin. Endocrinol. Metab 2007, 92, 902–910. [DOI] [PubMed] [Google Scholar]
- 187.Laron Z; Klinger B Comparison of the growth-promoting effects of insulin-like growth factor I and growth hormone in the early years of life. Acta Paediatr. 2007, 89, 38–41. [DOI] [PubMed] [Google Scholar]
- 188.Laron Z Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol. Pathol 2001, 54, 311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Midyett LK; Rogol AD; Van Meter QL; Frane J; Bright GM Recombinant Insulin-Like Growth Factor (IGF)-I Treatment in Short Children with Low IGF-I Levels: First-Year Results from a Randomized Clinical Trial. J. Clin. Endocrinol. Metab 2010, 95, 611–619. [DOI] [PubMed] [Google Scholar]
- 190.Ranke MB; Savage MO; Chatelain PG; Preece MA; Rosenfeld RG; Blum WF; Wilton P Insulin-Like Growth Factor I Improves Height in Growth Hormone Insensitivity: Two Years’ Results. Horm. Res 1995, 44, 253–264. [DOI] [PubMed] [Google Scholar]
- 191.Ranke MB; Savage MO; Chatelain PG; Preece MA; Rosenfeld RG; Wilton P Long-Term Treatment of Growth Hormone Insensitivity Syndrome with IGF-I. Horm. Res. Paediatr 1999, 51, 128–134. [DOI] [PubMed] [Google Scholar]
- 192.Laron Z; Kauli R Fifty seven years of follow-up of the Israeli cohort of Laron Syndrome patients—From discovery to treatment. Growth Horm. IGF Res 2016, 28, 53–56. [DOI] [PubMed] [Google Scholar]
- 193.Clemmons DR Role of Insulin-Like Growth Factor Iin Maintaining Normal Glucose Homeostasis. Horm. Res. Paediatr 2004, 62, 77–82. [DOI] [PubMed] [Google Scholar]
- 194.Guevara-Aguirre J Two-Year Treatment of Growth Hormone (GH) Receptor Deficiency with Recombinant Insulin-Like Growth Factor I in 22 Children: Comparison of Two Dosage Levels and to GH-Treated GH Deficiency. J. Clin. Endocrinol. Metab 1997, 82, 629–633. [DOI] [PubMed] [Google Scholar]
- 195.Lindsey RC; Mohan S Skeletal effects of growth hormone and insulin-like growth factor-I therapy. Mol. Cell. Endocrinol 2016, 432, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ross JL; Lee PA; Gut R; Germak J Attaining genetic height potential: Analysis of height outcomes from the ANSWER Program in children treated with growth hormone over 5years. Growth Horm. IGF Res 2015, 25, 286–293. [DOI] [PubMed] [Google Scholar]
- 197.Cohen J; Blethen S; Kuntze J; Smith SL; Lomax KG; Mathew PM Managing the Child with Severe Primary Insulin-Like Growth Factor-1 Deficiency (IGFD): IGFD Diagnosis and Management. Drugs RD 2014, 14, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kovacs GT; Worgall S; Schwalbach P; Steichele T; Mehls O; Rosivall L Hypoglycemic Effects of Insulin-Like Growth Factor-1 in Experimental Uremia: Can Concomitant Growth Hormone Administration Prevent This Effect? Horm. Res. Paediatr 1999, 51, 193–200. [DOI] [PubMed] [Google Scholar]
- 199.**.Lui JC; Colbert M; Cheung CSF; Ad M; Lee A; Zhu Z; Barnes KM; Dimitrov DS; Baron J Cartilage-Targeted IGF-1 Treatment to Promote Longitudinal Bone Growth. Mol. Ther. J. Am. Soc. Gene Ther 2019, 27, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrated that systemic injections of cartilage-targeted IGF-1 fusion proteins had direct effects on the growth plate without off-target effects on the kidney that are seen with normal IGF-1.
- 200.Nilsson A; Carlsson B; Isgaard J; Isaksson OGP; Rymo L Regulation by GH of insulin-like growth factor-I mRNA expression in rat epiphyseal growth plate as studied with in-situ hybridization. J. Endocrinol 1990, 125, 67–NP. [DOI] [PubMed] [Google Scholar]
- 201.Lazowski DA; Fraher LJ; Hodsman A; Steer B; Modrowski D; Han VK Regional variation of insulin-like growth factor-I gene expression in mature rat bone and cartilage. Bone 1994, 15, 563–576. [DOI] [PubMed] [Google Scholar]
- 202.Shinar DM; Endo N; Halperin D; Rodan GA; Weinreb M Differential expression of insulin-like growth factor-I (IGF-I) and IGF-II messenger ribonucleic acid in growing rat bone. Endocrinology 1993, 132, 1158–1167. [DOI] [PubMed] [Google Scholar]
- 203.Reinecke M; Schmid AC; Heyberger-Meyer B; Hunziker EB; Zapf J Effect of Growth Hormone and Insulin-Like Growth Factor I (IGF-I) on the Expression of IGF-I Messenger Ribonucleic Acid and Peptide in Rat Tibial Growth Plate and Articular Chondrocytes in Vivo 1. Endocrinology 2000, 141, 2847–2853. [DOI] [PubMed] [Google Scholar]
- 204.Humbel RE Insulin-like growth factors I and II. Eur. J. Biochem 1990, 190, 445–462. [DOI] [PubMed] [Google Scholar]
- 205.Pierce AL; Dickey JT; Felli L; Swanson P; Dickhoff WW Metabolic hormones regulate basal and growth hormone-dependent igf2 mRNA level in primary cultured coho salmon hepatocytes: effects of insulin, glucagon, dexamethasone, and triiodothyronine. J. Endocrinol 2010, 204, 331–339. [DOI] [PubMed] [Google Scholar]
- 206.Begemann M; Zirn B; Santen G; Wirthgen E; Soellner L; Büttel H-M; Schweizer R; van Workum W; Binder G; Eggermann T Paternally Inherited IGF2 Mutation and Growth Restriction. N. Engl. J. Med 2015, 373, 349–356. [DOI] [PubMed] [Google Scholar]
- 207.Uchimura T; Hollander JM; Nakamura DS; Liu Z; Rosen CJ; Georgakoudi I; Zeng L An essential role for IGF2 in cartilage development and glucose metabolism during postnatal long bone growth. Development 2017, 144, 3533–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hunziker EB; Wagner J; Zapf J Differential effects of insulin-like growth factor I and growth hormone on developmental stages of rat growth plate chondrocytes in vivo. J. Clin. Invest 1994, 93, 1078–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Abbaspour A; Takata S; Matsui Y; Katoh S; Takahashi M; Yasui N Continuous infusion of insulin-like growth factor-I into the epiphysis of the tibia. Int. Orthop 2008, 32, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mushtaq T; Bijman P; Ahmed SF; Farquharson C Insulin-Like Growth Factor-I Augments Chondrocyte Hypertrophy and Reverses Glucocorticoid-Mediated Growth Retardation in Fetal Mice Metatarsal Cultures. Endocrinology 2004, 145, 2478–2486. [DOI] [PubMed] [Google Scholar]
- 211.Repudi SR; Patra M; Sen M WISP3-IGF-1 interaction regulates chondrocyte hypertrophy. J. Cell Sci 2013, 126, 1650–1658. [DOI] [PubMed] [Google Scholar]
- 212.Guha M Anticancer IGF-1R classes take more knocks. Nat. Rev. Drug Discov 2013, 12, 250–250. [DOI] [PubMed] [Google Scholar]
- 213.Hadacek F Low-molecular-weight metabolite systems chemistry. Front. Environ. Sci 2015, 3. [Google Scholar]
- 214.Macielag MJ Chemical properties of antimicrobials and their uniqueness In Antibiotic discovery and development; Dougherty TJ, Pucci MJ, Eds.; Springer: New York, 2012; pp. 793–820 ISBN 978-1-4614-1399-8. [Google Scholar]
- 215.Haylor J; Hickling HL; Eter EAE; Moir AJG; Oldroyd SD; Hardisty CA; Nahas AME JB3, an IGF-I receptor antagonist, inhibits early renal growth in diabetic and uninephrectomized rats. J. Am. Soc. Nephrol. JASN 2000, 11, 2027–2035. [DOI] [PubMed] [Google Scholar]
- 216.Huang BK; Golden LA; Tarjan G; Madison LD; Stern PH Insulin-Like Growth Factor I Production Is Essential for Anabolic Effects of Thyroid Hormone in Osteoblasts. J. Bone Miner. Res 2010, 15, 188–197. [DOI] [PubMed] [Google Scholar]
- 217.Kleinridders A Deciphering Brain Insulin Receptor and Insulin-Like Growth Factor 1 Receptor Signalling. J. Neuroendocrinol 2016, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pietrzkowski Z; Wernicke D; Porcu P; Jameson BA; Baserga R Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor 1. Cancer Res. 1992, 52, 6447–6451. [PubMed] [Google Scholar]
- 219.Smith LEH; Shen W; Perruzzi C; Soker S; Kinose F; Xu X; Robinson G; Driver S; Bischoff J; Zhang B; et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat. Med 1999, 5, 1390–1395. [DOI] [PubMed] [Google Scholar]
- 220.Brock RS; Gebrekristos BH; Kuniyoshi KM; Modanlou HD; Falcao MC; Beharry KD Biomolecular Effects of Jb1 (an IGF-I Peptide Analog) in a Rat Model of Oxygen-Induced Retinopathy. Pediatr. Res 2011, 69, 135–141. [DOI] [PubMed] [Google Scholar]
- 221.Todd BJ; Fraley GS; Peck AC; Schwartz GJ; Etgen AM Central Insulin-Like Growth Factor 1 Receptors Play Distinct Roles in the Control of Reproduction, Food Intake, and Body Weight in Female Rats1. Biol. Reprod 2007, 77, 492–503. [DOI] [PubMed] [Google Scholar]
- 222.Wen Z; Zeng W; Dai J; Zhou X; Yang C; Duan F; Liu Y; Yang H; Yuan L Paravertebral fascial massage promotes brain development of neonatal rats via the insulin-like growth factor 1 pathway. Neural Regen. Res 2012, 7, 1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li R; Pourpak A; Morris SW Inhibition of the Insulin-like Growth Factor-1 Receptor (IGF-1R) Tyrosine Kinase as a Novel Cancer Therapy Approach. J. Med. Chem 2009, 52, 4981–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Pillai RN; Ramalingam SS Inhibition of insulin-like growth factor receptor: end of a targeted therapy? Transl. Lung Cancer Res 2013, 2, 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Refolo MG; D’Alessandro R; Lippolis C; Carella N; Cavallini A; Messa C; Carr BI IGF-1R tyrosine kinase inhibitors and Vitamin K1 enhance the antitumor effects of Regorafenib in HCC cell lines. Oncotarget 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Brodin H Longitudinal Bone Growth, the Nutrition of the Epiphyseal Cartilages and the Local Blood Supply: An Experimental Study in the Rabbit. Acta Orthop. Scand 1955, 26, 3–92. [PubMed] [Google Scholar]
- 227.Maes C Role and Regulation of Vascularization Processes in Endochondral Bones. Calcif. Tissue Int 2013, 92, 307–323. [DOI] [PubMed] [Google Scholar]
- 228.Trueta J Studies of the development and decay of the human frame. Br. J. Surg 1968, 55, 564–564. [Google Scholar]
- 229.Brookes M; Revell WJ Growth Cartilages In Blood Supply of Bone; Springer London: London, 1998; pp. 152–176 ISBN 978-1-4471-1545-8. [Google Scholar]
- 230.Brighton CT Structure and Function of the Growth Plate: Clin. Orthop. Rel. Res 1978, 136, 22–32. [PubMed] [Google Scholar]
- 231.Spira E; Farin I The Vascular Supply to the Epiphyseal Plate Under Normal and Pathological Conditions. Acta Orthop. Scand 1967, 38, 1–22. [DOI] [PubMed] [Google Scholar]
- 232.Wirth T; Syed Ali MM; Rauer C; Süß D; Griss P; Syed Ali S The Blood Supply of the Growth Plate and the Epiphysis: A Comparative Scanning Electron Microscopy and Histological Experimental Study in Growing Sheep. Calcif. Tissue Int 2002, 70, 312–319. [DOI] [PubMed] [Google Scholar]
- 233.Walzer SM; Cetin E; Grübl-Barabas R; Sulzbacher I; Rueger B; Girsch W; Toegel S; Windhager R; Fischer MB Vascularization of primary and secondary ossification centres in the human growth plate. BMC Dev. Biol 2014, 14, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Maes C; Stockmans I; Moermans K; Van Looveren R; Smets N; Carmeliet P; Bouillon R; Carmeliet G Soluble VEGF isoforms are essential for establishingepiphyseal vascularization and regulating chondrocyte development and survival. J. Clin. Invest 2004, 113, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Williams RM; Zipfel WR; Tinsley ML; Farnum CE Solute Transport in Growth Plate Cartilage: In Vitro and In Vivo. Biophys. J 2007, 93, 1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Trueta J; Amato VP The vascular contribution to osteogenesis. III. Changes in the growth cartilage caused by experimentally induced ischaemia. J. Bone Joint Surg. Br 1960, 42-B, 571–587. [DOI] [PubMed] [Google Scholar]
- 237.Zoetis T; Tassinari MS; Bagi C; Walthall K; Hurtt ME Species comparison of postnatal bone growth and development. Birth Defects Res. B. Dev. Reprod. Toxicol 2003, 68, 86–110. [DOI] [PubMed] [Google Scholar]
- 238.Farnum CE; Lenox M; Zipfel W; Horton W; Williams R In vivo delivery of fluoresceinated dextrans to the murine growth plate: Imaging of three vascular routes by multiphoton microscopy. Anat. Rec. A. Discov. Mol. Cell. Evol. Biol 2006, 288A, 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zipfel WR; Williams RM; Webb WW Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotechnol 2003, 21, 1369–1377. [DOI] [PubMed] [Google Scholar]
- 240.Serrat MA; Williams RM; Farnum CE Temperature alters solute transport in growth plate cartilage measured by in vivo multiphoton microscopy. J. Appl. Physiol 2009, 106, 2016–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Serrat MA; Ion G Imaging IGF-I uptake in growth plate cartilage using in vivo multiphoton microscopy. J. Appl. Physiol 2017, 123, 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Carmeliet P; Ferreira V; Breier G; Pollefeyt S; Kieckens L; Gertsenstein M; Fahrig M; Vandenhoeck A; Harpal K; Eberhardt C; et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 1996, 380, 435–439. [DOI] [PubMed] [Google Scholar]
- 243.Ferrara N; Carver-Moore K; Chen H; Dowd M; Lu L; O’Shea KS; Powell-Braxton L; Hillan KJ; Moore MW Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 1996, 380, 439–442. [DOI] [PubMed] [Google Scholar]
- 244.Gerber HP; Hillan KJ; Ryan AM; Kowalski J; Keller GA; Rangell L; Wright BD; Radtke F; Aguet M; Ferrara N VEGF is required for growth and survival in neonatal mice. Dev. Camb. Engl 1999, 126, 1149–1159. [DOI] [PubMed] [Google Scholar]
- 245.Emons J; Chagin AS; Malmlöf T; Lekman M; Tivesten Å; Ohlsson C; Wit JM; Karperien M; Sävendahl L Expression of vascular endothelial growth factor in the growth plate is stimulated by estradiol and increases during pubertal development. J. Endocrinol 2010, 205, 61–68. [DOI] [PubMed] [Google Scholar]
- 246.Filipowska J; Tomaszewski KA; Niedźwiedzki Ł; Walocha JA; Niedźwiedzki T The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis 2017, 20, 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Gerber H-P; Vu TH; Ryan AM; Kowalski J; Werb Z; Ferrara N VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med 1999, 5, 623–628. [DOI] [PubMed] [Google Scholar]
- 248.Horner A; Bishop NJ; Bord S; Beeton C; Kelsall AW; Coleman N; Compston JE Immunolocalisation of vascular endothelial growth factor (VEGF) in human neonatal growth plate cartilage. J. Anat 1999, 194, 519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zelzer E; Olsen BR Multiple Roles of Vascular Endothelial Growth Factor (VEGF) in Skeletal Development, Growth, and Repair In Current Topics in Developmental Biology; Elsevier, 2004; Vol. 65, pp. 169–187 ISBN 978-0-12-153165-2. [DOI] [PubMed] [Google Scholar]
- 250.Maes C; Carmeliet P; Moermans K; Stockmans I; Smets N; Collen D; Bouillon R; Carmeliet G Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech. Dev 2002, 111, 61–73. [DOI] [PubMed] [Google Scholar]
- 251.Baron J; Klein KO; Yanovski JA; Novosad JA; Bacher JD; Bolander ME; Cutler GB Induction of growth plate cartilage ossification by basic fibroblast growth factor. Endocrinology 1994, 135, 2790–2793. [DOI] [PubMed] [Google Scholar]
- 252.Hung IH; Yu K; Lavine KJ; Ornitz DM FGF9 regulates early hypertrophic chondrocyte differentiation and skeletal vascularization in the developing stylopod. Dev. Biol 2007, 307, 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Liu Z; Lavine KJ; Hung IH; Ornitz DM FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Dev. Biol 2007, 302, 80–91. [DOI] [PubMed] [Google Scholar]
- 254.Engsig MT; Chen Q-J; Vu TH; Pedersen A-C; Therkidsen B; Lund LR; Henriksen K; Lenhard T; Foged NT; Werb Z; et al. Matrix Metalloproteinase 9 and Vascular Endothelial Growth Factor Are Essential for Osteoclast Recruitment into Developing Long Bones. J. Cell Biol 2000, 151, 879–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Vu TH; Shipley JM; Bergers G; Berger JE; Helms JA; Hanahan D; Shapiro SD; Senior RM; Werb Z MMP-9/Gelatinase B Is a Key Regulator of Growth Plate Angiogenesis and Apoptosis of Hypertrophic Chondrocytes. Cell 1998, 93, 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Ahmed SF; Farquharson C The effect of GH and IGF-1 on linear growth and skeletal development and their modulation by SOCS proteins. J. Endocrinol 2010, 206, 249–259. [DOI] [PubMed] [Google Scholar]
- 257.Álvarez-García Ó; García-López E; Loredo V; Gil-Peña H; Rodríguez-Suárez J; Ordóñez FÁ; Carbajo-Pérez E; Santos F Rapamycin induces growth retardation by disrupting angiogenesis in the growth plate. Kidney Int 2010, 78, 561–568. [DOI] [PubMed] [Google Scholar]
- 258.Barone A; Rubin JB Opportunities and Challenges for Successful Use of Bevacizumab in Pediatrics. Front. Oncol 2013, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Han K; Peyret T; Quartino A; Gosselin NH; Gururangan S; Casanova M; Merks JHM; Massimino M; Grill J; Daw NC; et al. Bevacizumab dosing strategy in paediatric cancer patients based on population pharmacokinetic analysis with external validation: Bevacizumab dosing strategy in paediatric cancer patients. Br. J. Clin. Pharmacol 2016, 81, 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]


