Abstract
OBJECTIVES:
To examine: (1) prevalence of fall risk–increasing drug (FRID) use among older adults with a fall-related injury, (2) which FRIDs were most frequently prescribed, (3) whether FRID use was reduced following the fall-related healthcare episode, and (4) which interventions have reduced falls or FRID use in older adults with a history of falls.
DESIGN:
Systematic review.
PARTICIPANTS:
Observational and intervention studies that assessed (or intervened on) FRID use in participants aged 60 years or older who had experienced a fall.
MEASUREMENTS:
PubMed and EMBASE were searched through June 30, 2019. Two reviewers independently extracted data and evaluated studies for bias. Discrepancies were resolved by consensus.
RESULTS:
Fourteen of 638 articles met selection criteria: 10 observational studies and 4 intervention studies. FRID use prevalence at time of fall-related injury ranged from 65% to 93%. Antidepressants and sedatives-hypnotics were the most commonly prescribed FRIDs. Of the 10 observational studies, only 2 used a design adequate to capture changes in FRID use after a fall-related injury, neither finding a reduction in FRID use. Three randomized controlled studies conducted in various settings (hospital, emergency department, and community pharmacy) with 12-month follow-up did not find a reduction in falls with interventions to reduce FRID use, although the study conducted in the community pharmacy setting was effective in reducing FRID use. In a nonrandomized (pre-post) intervention study conducted in an outpatient geriatrics clinic, falls were reduced in the intervention group.
CONCLUSIONS:
Limited evidence indicates high prevalence of FRID use among older adults who have experienced a fall-related injury and no reduction in overall FRID use following the fall-related healthcare encounter. There is a need for well-designed interventions to reduce FRID use and falls in older adults with a history of falls. Reducing FRID use as a stand-alone intervention may not be effective in reducing recurrent falls.
Keywords: fall-related injury, medications, older adults, systematic review
Falls and fall-related injuries constitute a critical, and growing, public health problem, as evidenced by the Centers for Disease Control and Prevention national initiative, Stopping Elderly Accidents, Deaths and Injuries (STEADI).1 One in three community-dwelling adults aged 65 years or older, and one in two aged 80 years or older, sustains a fall each year.2,3 Falls are the leading cause of unintentional injuries and injury-related deaths among adults aged 65 years and older.4 Evidence suggests that emergency department (ED) visits and hospitalizations for fall-related injury are on the rise.5 Treatment of injurious falls is expensive, costing over $50 billion annually in the United States.6
Medications are a key modifiable risk factor for falls7,8 and also important targets as a core component of the STEADI tool kit.1 Medications associated with increased fall risk have been termed fall risk–increasing drugs (FRIDs).9 Definitions of FRIDs vary, but commonly include benzodiazepines and nonbenzodiazepine hypnotics, antipsychotics, antidepressants, and opioids.10 Less frequently, certain cardiovascular medications and hypoglycemic agents may be included.9 As such, many of these medications are considered potentially inappropriate in older adults who have experienced a fall or hip fracture.10 Nonetheless, older adults hospitalized for fall-related injury are often discharged on one or more FRIDs—a potential missed opportunity for intervention to reduce drug-related harm.11 Despite its clinical importance, no systematic reviews, to our knowledge, have described prevalence of FRID use among older adults who have experienced a fall- or fracture-related hospitalization or ED visit (defined as fall-related injury hereafter) or interventions to reduce FRID use among older adults who have experienced a fall. We therefore undertook a systematic review of the published literature to examine: (1) the prevalence of FRID use among older adults with fall-related injury, (2) which FRIDs were most frequently prescribed, (3) whether FRID use was reduced following the fall-related healthcare episode, and (4) which interventions have reduced falls or FRID use in older adults with a history of falls.
METHODS
Study Eligibility
We included observational or intervention (eg, randomized controlled trials and quasi-experimental design) studies that assessed (or intervened on) FRID use in participants aged 60 years or older who had experienced a fall. For observational studies, we included those in whom FRID use was evaluated before and after a fall-related injury. For intervention studies, we included those in whom an intervention was undertaken to reduce or discontinue FRIDs in older adults presenting with a fall-related injury or a history of falls. Intervention studies were excluded if the intervention addressed other fall risk factors along with medications, because we were interested in effects of interventions that solely targeted FRID use.
Literature Search
In consultation with a librarian, we searched PubMed and EMBASE for studies published on or before June 30, 2019 (which was also the last search date). We limited our searches to studies published in English. We did not specify a start date because we wanted to find all possible published studies. The following search terms were used: older adults, falls, medications, fall risk increasing, central nervous system-active, psychotropic, emergency department, and hospitalization. We also included search terms for individual medication classes, including antidepressants, antipsychotics, benzodiazepines, hypnotics, and opioids. In addition, we manually reviewed the references of identified pertinent studies to identify additional studies that met our inclusion criteria. Two reviewers (L.H. and J.Y.) independently reviewed abstracts for study inclusion.
Because this review was conducted using existing published data, it is considered to be nonhuman subjects research and thus did not require approval from the University of Washington Institutional Review Board.
Data Extraction
Two reviewers (S.G. and L.H.) independently extracted data from each study using a standardized data abstraction form developed by the authors. Data collected included study year, country, setting, study design, participants’ age and sex, FRIDs evaluated, and results. For intervention studies, the source of participants, key features of the intervention, primary and secondary outcome measures, and timing of data collection were also abstracted. Any disagreements during data extraction were resolved by consensus of the reviewer team. The original authors were contacted to clarify unclear data.
Study Quality and Bias Assessment
Two reviewers (Z.M. and L.H.) independently reviewed study quality and risk of bias. For observational studies, we used relevant items from the Newcastle-Ottawa Scale.12 In addition, we assessed whether the method of outcome assessment was able to capture a change in FRID use at a specific time point following discharge (rather than looking at change in use at discharge or within a period of time following discharge) following a fall-related injury. For randomized controlled trials, we used the Cochrane Risk of Bias Tool.13 Any disagreements were resolved by consensus of the reviewer team.
RESULTS
Description of Included Studies
Our search yielded 638 abstracts. After abstract review, a total of 20 full articles were reviewed. Ten observational studies14–23 and four intervention studies9,24–26 met inclusion criteria and were included in this review. The article selection process is summarized in Figure 1. Study characteristics are summarized in Tables 1 and 2. Studies were conducted in Europe,9,15,18–21,23,25,26 North America,16,17,22,24 and Australia.14 Sample sizes ranged from 100 to 2043 participants in observational studies14–23 and from 139 to 612 participants in intervention studies.9,24–26 Most participants were female. Participants in the observational studies tended to be older than those in the intervention studies.
Figure 1.
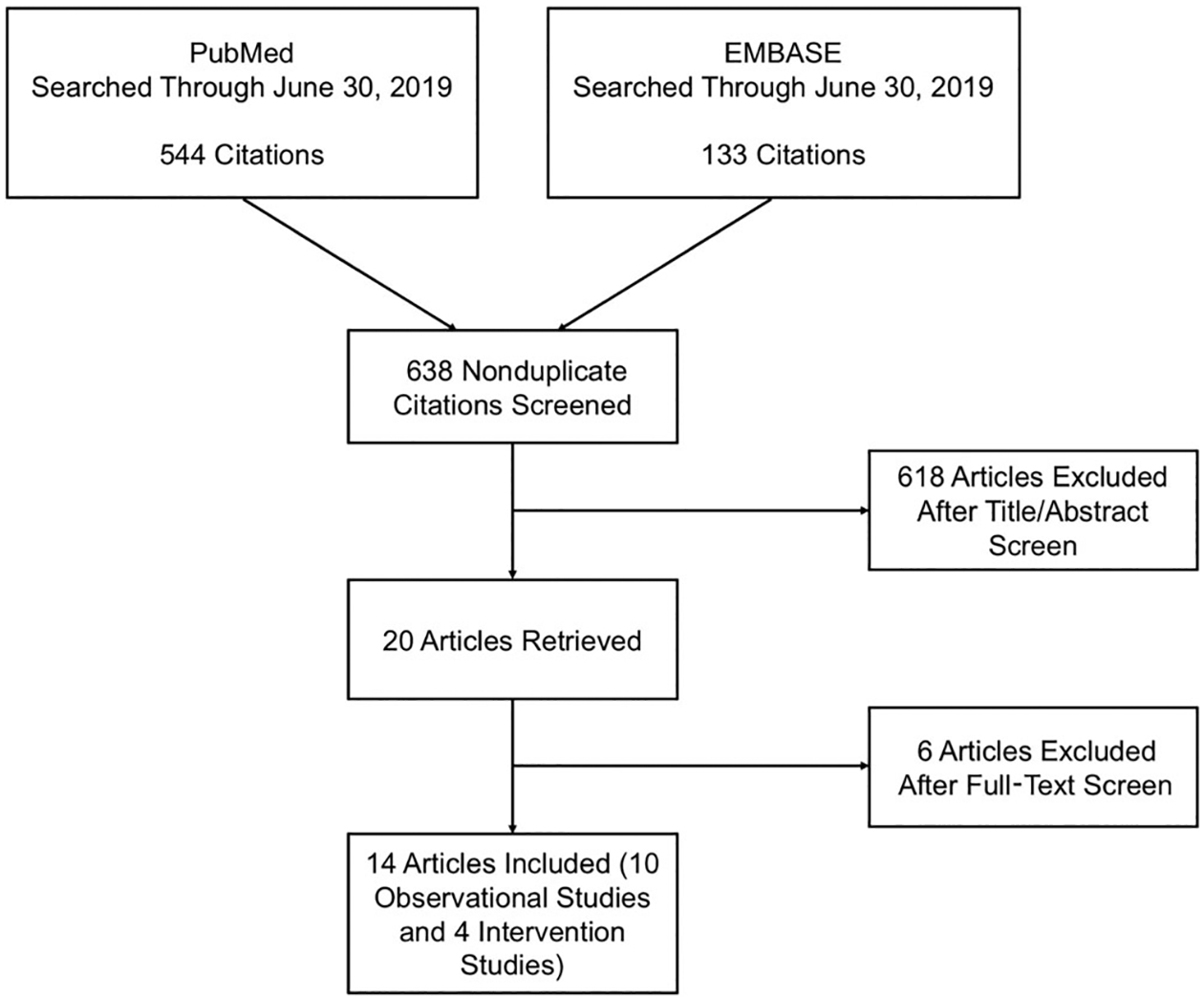
Article selection.
Table 1.
Observational Study Characteristics and Main Results
| Author, Year, Country | Design | No. of Subjects (Mean Age, y) | Setting/Sample Description | Timing of FRID Use | Main Results |
|---|---|---|---|---|---|
| Studies evaluating FRID use at admission vs time point after discharge | |||||
| Benuza-Sola,15 2018, Spain | Prospective cohort, single site | 252 (82) | Hospital, admitted for fall-related fracture | T1: admission T2: 1 mo postfracture | No significant difference in mean number of FRIDs per subject from T1 to T2: 3.1 ± 1.9 vs 3.4 ±2 (P = .099) For FRID subgroups, from T1 to T2, a significant increase in mean number of FRIDs per patient was noted for hypnotics (0.266 ± 0.469 vs 0.389 ± 0.520; P = .003) and antidepressants (0.468 ± 0.640 vs 0.571 ± 0.696; P = .042) |
| Sjöberg,21 2010, Sweden | Prospective, single site | 100 (86) | Hospital, underwent surgery for hip fracture | T1: admission T2: discharge T3: 6 mo postdischarge | FRID use increased from T1 (93% of participants) to T2 (100% of participants) (P = .01) No significant change in FRID use from T1 (93%) to T3 (94%) (P = .41) |
| Studies evaluating FRID use at admission vs discharge | |||||
| Bennett,14 2014, Australia | Prospective cohort, single site | 204 (80.5) | Hospital, admitted for fall | T1: admission T2: discharge | No change in mean FRID use per patient from T1 (2.5 ± 2.1) to T2 (2.5 ± 1.9) (P value not reported) |
| Francis,16 2014, Canada | Retrospective convenience sample, single site | 148 (82) | Hospital, admitted for fall and taking at least one PIM | T1: admission T2: discharge | From T1 to T2, 27% of patients had dosage reduction or discontinuation of PIM (P < .001); 16% had greater number or increased dose of PIM (P value not reported) Mean number of PIMs decreased from 1.6 ± 0.8 to 1.4 ± 0.9 between T1 and T2 (P = .03) Benzodiazepines had highest rate of discontinuation/dosage reduction (26% of patients on benzodiazepine had discontinuation and 14% had dosage reduction between T1 and T2 [P values not reported]); antipsychotics were the most frequently added class, with 9% of patients having new prescription between T1 and T2 (P value not reported) |
| Marvin,19 2017, United Kingdom | Prospective cohort, single site | 100 (85) | Hospital, admitted for fall | T1: admission T2: discharge | Number of patients on ≥1 FRID at T1 vs T2: 65 vs 60 (P value not reported) Significant reduction in total number of FRIDs among all patients from T1 to T2 (112 vs 91; P = .004) Mean FRIDs for patients undergoing review (N = 82): 1.19 (T1) vs 0.94 (T2) (P value not reported) Decrease in mean FRIDs for patients undergoing review with pharmacist (N = 45): 1.44 (T1) vs 0.91 (T2) (P = .002) |
| Studies evaluating FRID use via pharmacy claims before admission and after discharge | |||||
| Hill-Taylor,17 2016, Canada | Retrospective cohort, Nova Scotia insurer | 1789 (81.6) | Fall-related hospitalization and prescription for zopiclone or benzodiazepine in T1 | T1: within 100 d preadmission T2: within 100 d postdischarge | Benzodiazepine use continued in 74.2% of subjects between T1 and T2 Long-acting benzodiazepine exposure decreased from T1 to T2: 6.6% to 5.03%; relative reduction = 23.8% (95% Cl = 0.5%−42.6%); absolute reduction = 1.57% (95% Cl = 0.03%−3.10%) |
| Kragh,18 2011, Sweden | Retrospective cohort, Swedish county | 2043 (83) | Experienced hip fracture | T1: within 6 mo before hip fracture T2: within 6 mo after hip fracture | From T1 to T2, use of all FRIDs increased significantly (P < .001), with percentage of patients treated with FRIDs increasing from 67.7% to 97.7% Opioids had largest increase between T1 and T2 (21.1% to 73.6%) |
| McMahon,20 2014, Ireland | Before-and-after design, single site | 1016 (82.7) | ED, admitted for fall | T1: within 12 mo before fall T2: within 12 mo after fall | No significant change in prevalence of PIMs using STOPP30 criteriaa: 42.2% (T1) vs 42.9% (T2) (P = .67) No significant change in prevalence of PIMs using STOPP33 criteriab: 53.1% (T1) vs 53.7% (T2) (P = .64) No significant change in prevalence of PIMs from AGS Beers Criteria®c: 44.0% (T1) vs 41.5% (T2) (P = .125) |
| Trenaman,22 2018, Canada | Before-and-after design, Nova Scotia insurer | 585 | Fall-related hospitalization and prescription for antipsychotic in T1 | T1: within 100 d preadmission T2: within 100 d postdischarge | 76.5% of participants surviving hospitalization had an antipsychotic drug dispensed at T2 |
| Walsh,23 2019, Ireland | Before-and-after design, 44 general practices | 927 (81.2) | Hospitalization due to fall, fracture, or syncope | T1: within 12 mo prehospitalization T2: within 12 mo posthospitalization | Significant increase in sedatived use from T1 (40% of participants) to T2 (45% of participants) (P < .01) No change in vasodilator use from T1 (54% of participants) to T2 (54% of participants) (P = .70) |
Abbreviations: CI, confidence interval; ED, emergency department; FRID, fall risk–increasing drug; PIM, potentially inappropriate medication; STOPP, screening tool of older persons’ prescriptions; T1, time 1; T2, time 2.
STOPP30: 30 STOPP criteria applied.
STOPP33: STOPP criteria plus three additional criteria.
Beers Criteria® included α blockers, doxazosin, tertiary tricyclic antidepressants, first- and second-generation antipsychotics (in dementia), benzodiazepines (short, intermediate, and long acting), nonbenzodiazepine hypnotics for longer than 90 days, and non–cyclooxygenase-selective nonsteroidal anti-inflammatory drug chronic use for longer than 3 months.
Sedatives included benzodiazepines, Z drugs (nonbenzodiazepine hypnotics), and antipsychotics
Table 2.
Intervention Study Characteristics and Main Results
| Author, Year, Country | Design, Setting, and Study Perioda | Participants | Intervention | Follow-Up | Main Results |
|---|---|---|---|---|---|
| Blalock,24 2010, United States | Randomized controlled trial Community pharmacy 2005–2007 | N = 186 community-dwelling subjects (mean age = 75.5 y) with fall within past year | Face-to-face medication consultations with community pharmacy resident
|
12 mo | Primary outcome:
|
| Boyé,25 2017, Netherlands | Randomized multicenter trial ED 2008 | N = 612 community-dwelling subjects (mean age = 76 y) with ED visit due to a fall | Fall-related assessment performed by research physician.
|
12 mo | Primary outcome:
|
| Sjöberg,26 2013, Sweden | Randomized controlled trial Hospital 2009 | N = 199 subjects (mean age = 85 y) having undergone surgery for hip fracture | Geriatrician performed overall assessment of falls risk and systematic medication review
|
12 mo | Primary outcome:
|
| van der Velde,9 2007, Netherlands | Pre-post Geriatric outpatient clinic 2003–2004 | N = 139 community-dwelling subjects (mean age = 78 y) newly referred to geriatric outpatient clinic with one or more falls in previous year | All potential FRIDs considered for withdrawal over a 1-mo period; prescribing physicians contacted if medication changes intended | 2 mo (after 1-mo FRID withdrawal period) | FRID withdrawal occurred in 75 of 139 patients (discontinuation in 67 patients, dose reduction in 8 patients); number of falls during follow-up was significantly lower among subjects with FRID withdrawal compared to those without (0.3 vs 3.6; P = .025) HR (95% Cl) for fall during follow-up: |
Abbreviations: C, control group; CI, confidence interval; CV, cardiovascular; ED, emergency department; FRID, fall risk–increasing drug; GP, general practitioner; HR, hazard ratio; I, intervention group; RR, relative risk.
Not all studies reported study period. Most studies provided enrollment period (Blalock, Sjöberg 2013, and van der Velde), whereas one provided study start date (Boyé).
Cardiovascular included: cardiac glycosides, class IA antiarrhythmics, vasodilators used in cardiac disease, antihypertensive agents, diuretics, β-blocker agents, calcium channel blockers, and agents acting on renin-angiotensin system.
Psychotropic included: opioids, anti–Parkinson’s disease agents, psycholeptics (antipsychotics, except lithium, anxiolytics, hypnotics, and sedatives), and antidepressants.
All FRIDs included: cardiovascular and psychotropic FRIDs plus α-adrenergic receptor antagonists.
Definition of FRIDs
Two studies focused on single medication classes (ie, benzodiazepines and zopiclone,17 antipsychotics22). All other studies included more than one central nervous system (CNS)–active medication class in their definition of FRIDs (Supplementary Table S1).9,14–16,18–21,23–26 All studies included benzodiazepines or sedatives/hypnotics as FRIDs, and all studies except two21,23 included antidepressants. Beyond these two classes, the definition of FRIDs varied by study. Some studies included cardiovascular medications,9,14,15,18,19,21,23,25,26 hypoglycemics,9,14,25 or α blockers9,15,19,21,25,26 in their definition of FRIDs.
Ascertainment of FRID Use and Study Quality
Observational studies ascertained FRID use from a variety of sources, including medical records,14–16,19,23 pharmacy claims,17,20,22 or national registries.18,21 Three studies selected patients based on FRID use before the fall-related injury.16,17,22 The timing and method of FRID measurement in relation to the fall-related injury varied. For studies using pharmacy claims or national registries, FRID use was ascertained within time periods before and after the fall-related injury (100 days, 6 months, or 12 months), as compared to studies using medical records, which captured FRID use at specific time points (hospital admission, discharge, and 1-month postdischarge). One study used national registry data to define current use at 6 months postdischarge based on timing of the medication fill21 (Figure 2).
Figure 2.
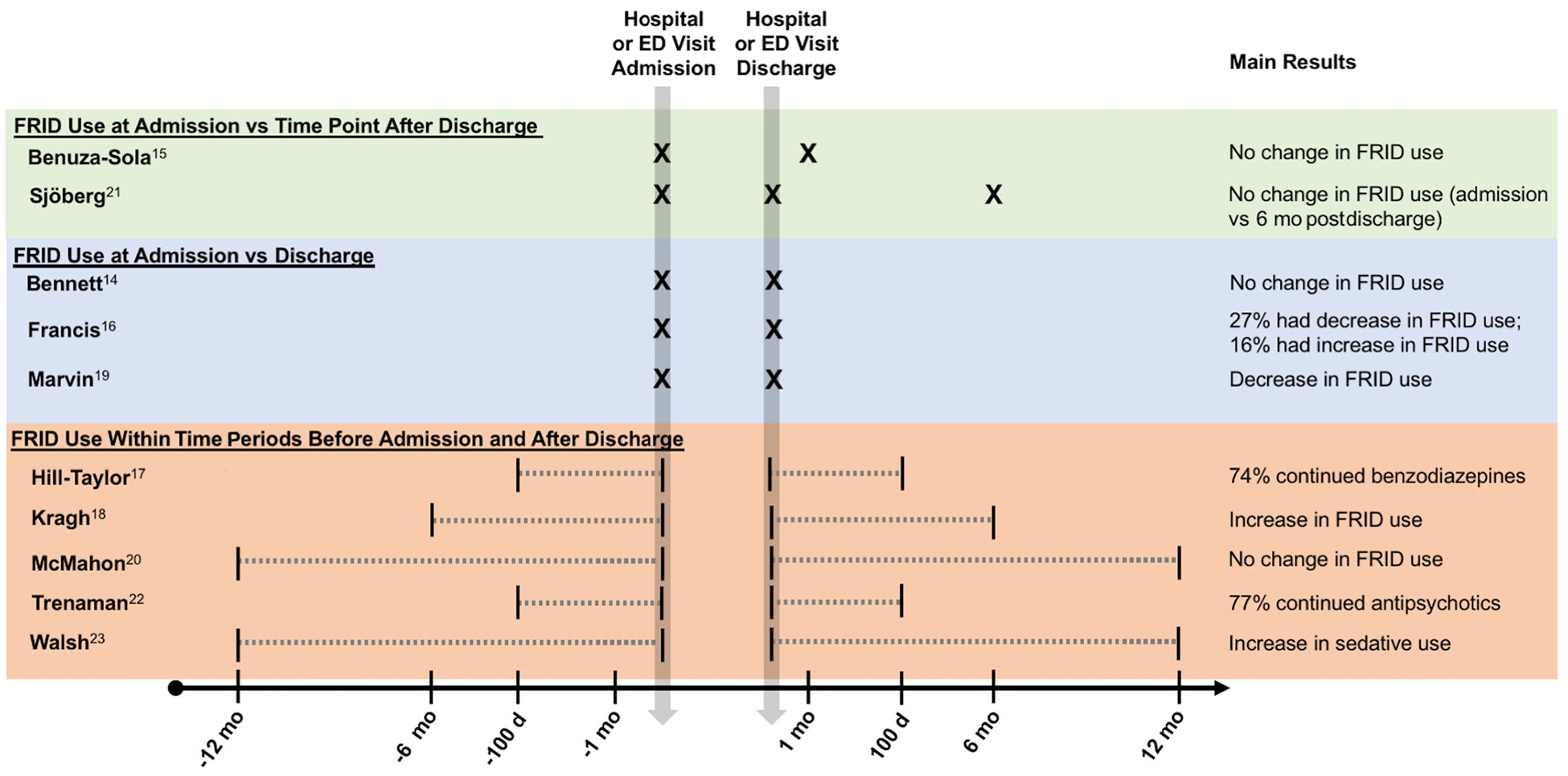
Summary of fall risk–increasing drug (FRID) measurement and main results among observational studies. ED indicates emergency department.
We categorized studies according to the timing of FRID ascertainment before and after the fall-related injury, ranked in decreasing order of strength of study design to evaluate change in FRID use after a fall-related injury: (1) studies that evaluated FRID use at admission, as well as time points following discharge (n = 2); (2) studies that evaluated FRID use at admission and discharge (n = 3); and (3) studies that evaluated FRID use via pharmacy claims during time periods before admission and after discharge (n = 5) (Table 1 and Figure 2).
Prevalence of FRIDs at Time of Fall-Related Injury
Only studies that ascertained FRIDs from medical records on admission provided information about prevalence of use at time of fall-related injury. The prevalence of any FRID use at the time of the fall-related injury ranged from 65% to 93%.15,19,21 One study did not report overall prevalence but instead reported average number of FRIDs per participant at admission,14 and another study selected participants based on FRID use at admission.16 Antidepressants were one of the most common FRID classes used at time of fall-related injury, with a range in prevalence from 15.0% to 40.0%14,15,21 (Supplementary Table S2). The prevalence for opioids ranged from 4.4% to 21.1%,14,15,21 and the prevalence for antipsychotics ranged from 3.0% to 14.0%.14,15,21 Anxiolytics, benzodiazepines, and sedatives-hypnotics were operationalized differently among studies, but for studies that evaluated sedatives-hypnotics, a range of 8.5% to 51.0%15,21 was found.
Change in FRID Use in Relation to Fall-Related Injury
The results of the observational studies categorized according to the strength of the study design to capture changes in FRID use after a fall-related injury are provided in Table 1. In the first group of studies, FRID use was measured at admission, as well as time points following discharge (1 month15 and 6 months21), and no change in FRID use was found. In the second group of studies that compared FRID use at admission and discharge, one study found no change in FRID use,14 whereas one found a reduction in FRID use.19 In a study that selected patients based on FRID use at hospital admission, 27% of patients had a dosage reduction or discontinuation of FRIDs between hospital admission and discharge (P < .001) and 16% of patients had an increase in dosage or number of FRIDs (P value not reported).16 The third group of studies evaluated FRID use over time periods before admission and after discharge and found an increase in overall FRID use18 or specific classes,23 or no change.20 Two studies that evaluated people with prescriptions for specific drug classes (benzodiazepines or zolpiclone,17 antipsychotics22) in the 100 days before admission found that use continued in roughly 75% of people in the 100 days following discharge.17,22
Interventions to Reduce FRIDs
Of the intervention studies, three were randomized controlled trials,24–26 and one used a single-group pre-post assessment.9 Interventions to reduce FRIDs varied and were conducted by a range of healthcare professionals in different settings, including a pharmacist at a community pharmacy,24 a research physician at an outpatient clinic,25 and a geriatrician in a hospital26 (Supplementary Figure S1). In one study conducted at an outpatient geriatrics clinic, the healthcare professional performing the intervention was not specified.9 The interventions evaluated in these studies consisted of a single interaction with the participant and involved an assessment with recommendations (written or verbal) back to the primary care provider, without an active role of the assessor/interventionist in implementing the recommendations. Three intervention studies (the randomized controlled trials)24–26 had a follow-up time of 12 months, and the pre-post intervention study9 had a follow-up time of 2 months.
The primary outcome measure in three of the intervention studies was falls, ascertained via falls calendars.9,24,25 In two of these studies, change in FRID use was evaluated as a secondary outcome.24,25 Reduction in FRIDs was the primary outcome and falls were a secondary outcome of the fourth intervention study.26
None of the randomized controlled trials found the intervention to be effective for preventing subsequent falls.24–26 However, one trial did find an effect on FRID use (CNS-active medications only): 14% (13/93) of participants in the intervention group experienced FRID discontinuation or dose reduction compared to 5% (5/93) of participants in the control group (P < .05).24 The single-group intervention study using a pre-post assessment found that withdrawal of FRIDs reduced falls, with the largest effect related to withdrawal of cardiovascular drugs.9
Risk of Bias
The risk of study bias is summarized in Supplementary Tables S3 and S4. For the observational studies, three of the eight criteria in the Newcastle-Ottawa Scale were related to selection of the nonexposed cohort (ie, control group not experiencing a fall-related injury), or comparability of these cohorts. None of the observational studies included a control group to evaluate changes in FRID use over time; thus, these criteria were considered not applicable and the studies were generally considered to have high risk of bias. Among intervention studies, the risk of bias varied; however, most criteria in the Cochrane Risk of Bias Tool were rated as low or unclear in the randomized controlled trials.24–26 In the single-group pre-post intervention study, the risk of bias was high for three of the six criteria given its single-group, nonrandomized design.9
DISCUSSION
To our knowledge, this is the first systematic review examining prevalence of and change in FRID use in older adults with hospitalization or ED visit for a fall (ie, fall-related injury), as well as interventions designed to reduce FRID use in those with a history of falls. Among older adults with fall-related injury, prevalence of FRID use at time of ED or hospital admission was high, ranging from 65% to 93%,15,19,21 with antidepressants and sedatives-hypnotics being the most commonly prescribed FRID classes. Of the 10 observational studies, only 2 used a design adequate to capture changes in FRID use after a fall-related injury. Despite FRIDs being known risk factors for falls and considered potentially inappropriate in this high-risk group,1,10,27 FRID use did not decrease at 1 and 6 months15,21 following the fall-related healthcare episode. Interventions to reduce FRID use (in the randomized controlled trials) were not effective in reducing falls,24–26 although one trial conducted in the community pharmacy setting was effective in reducing FRID use (CNS-active medications only).24
Overall, the prevalence of FRID use in older adults with a fall-related injury was high, with a considerable range (65%–93%). This may be because FRID definitions varied considerably across included studies. Variation in which medication classes were deemed FRIDs may be attributable in part to differences in prescribing guidance across organizations or continents. For example, the AGS Beers Criteria® were developed in the United States and include only CNS-active medications in their list of medications that may be potentially inappropriate in older adults with a history of falls or fractures.10 Other available criteria include CNS-active medications, as well as various other medication classes, such as vasodilator drugs,27 antihypertensives,1,28 and anticholinergics.1
While several observational studies have examined changes in FRID use after fall-related injury, an important limitation is that most studies did not assess FRID use at clinically meaningful time points following the injury to adequately capture discontinuation, because either change was measured at discharge only or medication use was assessed over a window of time rather than at a discrete time point. Measuring change at discharge is not adequate to capture changes in FRID use for several reasons. Several of the medications classified as FRIDs (eg, antidepressants, benzodiazepines, and cardiovascular drugs) require tapering over time; use of these medications may not easily be replaced with an alternative safer drug or nonpharmacological measures. Further, use of opioids for pain control after fracture may be clinically indicated. Therefore, no changes in FRID use or an increase in FRID use may be clinically justified. In addition, prescribers may be reluctant to make changes to chronic medications (including FRIDs) during the hospitalization or ED visit and, therefore, these changes are more likely to occur in the outpatient setting (eg, in primary care) by the patient’s prescriber(s) following discharge after the patient has stabilized from the acute injury, rather than at time of discharge. However, studies that looked out beyond the acute-care hospitalization or ED visit period15,21 where an outpatient provider might reasonably be expected to begin to address medication-related factors associated with an injurious fall, also found no change in FRID use. In the five studies that used pharmacy claims or national registry data to measure FRIDs over periods of time (eg, 12 months), use could have occurred at any point over the time period. In the preinjury period, FRID use assessed over a window of time may not reflect FRID use at the time of the injury. Related to this, FRID use assessed over a window of time after discharge (eg, 100 days, 6 months, or 12 months) is not able to distinguish between a person who filled a FRID prescription following discharge, but the medication was discontinued at the next outpatient visit (eg, a successful discontinuation outcome), compared with those who had multiple fills for a FRID prescription over the window (eg, unsuccessful discontinuation outcome). Other limitations of the observational studies included not assessing dose reductions (with the exception of one study16) and not including a control group to assess changes in FRID use.
Randomized controlled trials that employed interventions to reduce FRIDs did not result in a significant reduction in falls risk. Although these interventions varied with regard to setting and healthcare professional performing the intervention, all were low intensity (ie, single contact) and consultative in nature (ie, the interventionist was not responsible for making any of the recommended changes). This suggests that more intensive and comprehensive interventions (eg, spanning longer time periods with multiple points of follow-up and/or integrated interventions that directly engage the patient and the prescriber) may be needed. For certain CNS-active medications, such as benzodiazepines and opioids, ongoing support may be needed for discontinuation given the risk of physiologic withdrawal and tapering failure.29 Patient-focused strategies hold promise in this regard: Direct-to-patient education about the risks of benzodiazepines has been found to be effective in reducing use.30 Further, the sample size for these studies was relatively small; therefore, they may have been under-powered to detect a difference in fall rates. Last, FRID use represents one of many risk factors for falls, and as such, FRID reduction represents one component of a multifactorial and comprehensive approach to decreasing fall risk. As such, medication-related interventions may work best in conjunction with other interventions, as has been found for other combinations of interventions.31
A strength of this review is that it was comprehensive and clinically pertinent in asking multiple research questions under the umbrella of FRID use in older adults presenting for medical attention for a fall. A limitation of this review was that it included only studies published in the English language and thus may have excluded relevant studies published in other languages.
In conclusion, our study finds a high prevalence of FRID use among older adults with hospitalization or ED visit for a fall or fall-related injury and a lack of reduction (or even an increase) in overall FRID use following the fall-related event. In some cases, this may be clinically justified, but in others, these results suggest that additional measures, such as increased monitoring and follow-up, may be needed to optimize prescribing following a fall-related healthcare episode. Given that interventions employing medication review with suggestions to the primary care provider as a one-time intervention were not effective in preventing falls, interventions that span longer periods of time and involve integration of the patient and the healthcare team need to be tested to address FRID use and fall risk in older adults with a history of falls. Further, interventions to reduce FRID use represent one part of multicomponent interventions that are often needed to reduce fall risk. Resources that highlight comprehensive strategies for reducing fall risk are available from the Centers for Disease Control and Prevention as part of the STEADI tool kit.1
Supplementary Material
Footnotes
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
Conflict of Interest:
None.
REFERENCES
- 1.Centers for Disease Control and Prevention. STEADI – Older Adult Fall Prevention. https://www.cdc.gov/steadi/index.html. Accessed May 20, 2019.
- 2.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. [DOI] [PubMed] [Google Scholar]
- 3.Hill K, Schwarz J, Flicker L, Carroll S. Falls among healthy, community-dwelling, older women: a prospective study of frequency, circumstances, consequences and prediction accuracy. Aust N Z J Public Health. 1999;23: 41–48. [DOI] [PubMed] [Google Scholar]
- 4.National Council on Aging. Falls Prevention Facts. https://www.ncoa.org/news/resources-for-reporters/get-the-facts/falls-prevention-facts. Accessed March 9, 2019.
- 5.Hartholt KA, Lee R, Burns ER, van Beeck EF. Increase in fall-related hospitalizations in the United States, 2001–2008. J Trauma. 2011;71:255–258. [DOI] [PubMed] [Google Scholar]
- 6.Florence CS, Bergen G, Atherly A, Burns E, Stevens J, Drake C. Medical costs of fatal and nonfatal falls in older adults. J Am Geriatr Soc. 2018;66: 693–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pit SW, Byles JE, Henry DA, Holt L, Hansen V, Bowman DA. A quality use of medicines program for general practitioners and older people: a cluster randomised controlled trial. Med J Aust. 2007;187:23–30. [DOI] [PubMed] [Google Scholar]
- 8.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Velde N, Stricker BH, Pols HA, van der Cammen TJ. Risk of falls after withdrawal of fall-risk-increasing drugs: a prospective cohort study. Br J Clin Pharmacol. 2007;63:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Geriatrics Society 2019 Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67: 674–694. [DOI] [PubMed] [Google Scholar]
- 11.Correa-Pérez A, Delgado-Silveira E, Martín-Aragón S, Rojo-Sanchíz AM, Cruz Jentoft AJ. Fall-risk increasing drugs and prevalence of polypharmacy in older patients dishcarged from an orthogeriatric unit after a hip fracture. Aging Clin Exp Res. 2019;31:969–975. [DOI] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 10, 2018.
- 13.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett A, Gnjidic D, Gillett M, et al. Prevalence and impact of fall-risk-increasing drugs, polypharmacy, and drug-drug interactions in robust versus frail hospitalised falls patients: a prospective cohort study. Drugs Aging. 2014;31:225–232. [DOI] [PubMed] [Google Scholar]
- 15.Benuza-Sola M, Hidalgo-Ovejero ÁM, [notdef] Martí-Ayerdi J, Sánchez-Hernández JG, Menéndez-García M, García-Mata S. Study of fall risk-increasing drugs in elderly patients before and after a bone fracture. Postgrad Med J. 2018;94:76–80. [DOI] [PubMed] [Google Scholar]
- 16.Francis E, Dyks D, Kanji S. Influence of admission to a tertiary care hospital after a fall on use of potentially inappropriate medications among older patients. Can J Hosp Pharm. 2014;67:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill-Taylor B, Sketris IS, Gardner DM, Thompson K. Concordance with a STOPP (screening tool of older persons’ potentially inappropriate prescriptions) criterion in Nova Scotia, Canada: benzodiazepine and zoplicone prescription claims by older adults with fall-related hospitalizations. J Popul Ther Clin Pharmacol. 2016;23:e1–e12. [PubMed] [Google Scholar]
- 18.Kragh A, Elmståhl S, Atroshi I. Older adults’ medication use 6 months before and after hip fracture: a population-based cohort study. J Am Geriatr Soc. 2011;59(5):863–868. [DOI] [PubMed] [Google Scholar]
- 19.Marvin V, Ward E, Poots AJ, Heard K, Rajagopalan A, Jubraj B. Deprescribing medicines in the acute setting to reduce the risk of falls. Eur J Hosp Pharm. 2017;24:10–15.28184303 [Google Scholar]
- 20.McMahon CG, Cahir CA, Kenny RA, Bennett K. Inappropriate prescribing in older fallers presenting to an Irish emergency department. Age Ageing. 2014;43:44–50. [DOI] [PubMed] [Google Scholar]
- 21.Sjöberg C, Bladh L, Klintberg L, Mellström D, Ohlsson C, Wallerstedt SM. Treatment with fall-risk-increasing and fracture-preventing drugs before and after a hip fracture: an observational study. Drugs Aging. 2010;27: 653–661. [DOI] [PubMed] [Google Scholar]
- 22.Trenaman SC, Hill-Taylor BJ, Matheson KJ, et al. Antipsychotic drug dispensations in older adults, including continuation after a fall-related hospitalization: identifying adherence to screening tool of older persons’ potentially inappropriate prescriptions criteria using the Nova Scotia Seniors’ Pharmacare Program and Canadian Institute for Health’s discharge databases. Curr Ther Res Clin Exp. 2018;89:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh ME, Boland F, Moriarty F, Fahey T. Modification of potentially inappropriate prescribing following fall-related hospitalizations in older adults. Drugs Aging. 2019;36:461–470. [DOI] [PubMed] [Google Scholar]
- 24.Blalock SJ, Casteel C, Roth MT, Ferreri S, Demby KB, Shankar V. Impact of enhanced pharmacologic care on the prevention of falls: a randomized controlled trial. Am J Geriatr Pharmacother. 2010;8:428–440. [DOI] [PubMed] [Google Scholar]
- 25.Boyé ND, van der Velde N, de Vries OJ, et al. Effectiveness of medication withdrawal in older fallers: results from the Improving Medication Prescribing to reduce Risk Of FALLs (IMPROveFALL) trial. Age Ageing. 2017;46:142–146. [DOI] [PubMed] [Google Scholar]
- 26.Sjöberg C, Wallerstedt SM. Effects of medication reviews performed by a physician on treatment with fracture-preventing and fall-risk-increasing drugs in older adults with hip fracture-a randomized controlled study. J Am Geriatr Soc. 2013;61:1464–1472. [DOI] [PubMed] [Google Scholar]
- 27.O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44:213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milos V, Bondesson Å, Magnusson M, Jakobsson U, Westerlund T, Midlöv P. Fall risk-increasing drugs and falls: a cross-sectional study among elderly patients in primary care. BMC Geriatr. 2014;14:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paquin AM, Zimmerman K, Rudolph JL. Risk versus risk: a review of benzodiazepine reduction in older adults. Expert Opin Drug Saf. 2014;13: 919–934. [DOI] [PubMed] [Google Scholar]
- 30.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174:890–898. [DOI] [PubMed] [Google Scholar]
- 31.Tricco AC, Thomas SM, Veroniki AA, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


